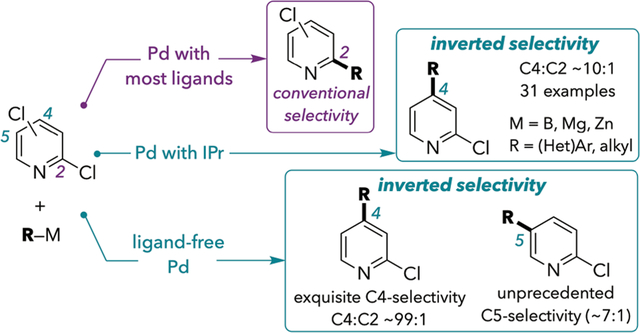
Abstract
Halides adjacent to nitrogen are conventionally more reactive in Pd-catalyzed cross-couplings of dihalogenated N-heteroarenes. However, a very sterically hindered N-heterocyclic carbene ligand is shown to promote room-temperature cross-coupling at C4 of 2,4-dichloropyridines with high selectivity (~10:1). This work represents the first highly-selective method with broad scope for C4-coupling of these substrates where selectivity is clearly under ligand-control. Under the optimized conditions, a broad scope of substituted 2,4-dichloropyridines and related compounds undergo cross-coupling to form C4—C(sp2) and C4—C(sp3) bonds using organoboron, -zinc, and -magnesium reagents. The synthetic utility of this method is highlighted in multistep syntheses that combine C4-selective cross-coupling with subsequent nucleophilic aromatic substitution reactions. The majority of the products herein (71%) have not been previously reported, emphasizing the ability of this methodology to open up underexplored chemical space. Remarkably, we find that ligand-free “Jeffery” conditions enhance the C4-selectivity of Suzuki coupling by an order of magnitude (>99:1). These ligand-free conditions enable the first C5-selective cross-couplings of 2,5-dichloropyridine and 2,5-dichloropyrimidine.
Keywords: 2,4-dichloropyridine; site-selectivity; N-heterocyclic carbene; Suzuki-Miyaura coupling; ligand-free cross-coupling
Graphical Abstract
INTRODUCTION
Nitrogen-containing heterocycles are privileged scaffolds in discovery-oriented synthesis such as medicinal chemistry. Heteroaromatic cores can be elaborated through cross-coupling reactions, which are among the most widely used methods in organic synthesis.1 Heteroarene starting materials containing multiple halogen leaving groups are practical building blocks since cross-coupling often occurs with predictable selectivity to give a product that retains one halide. Halides, especially chlorides, are valuable structural features of drug-like molecules. In fact, 15% of the unique small molecule drugs approved by the FDA from 1994 to 2012 are chlorinated.2 For 6-membered nitrogen-containing heteroarenes, cross-coupling typically takes place at a C—X bond adjacent to nitrogen, if present.3 It is generally very challenging to invert the conventional site preference to react at a C—X bond distal to nitrogen. As such, innate substrate biases leave significant chemical space underexplored by organic synthesis.
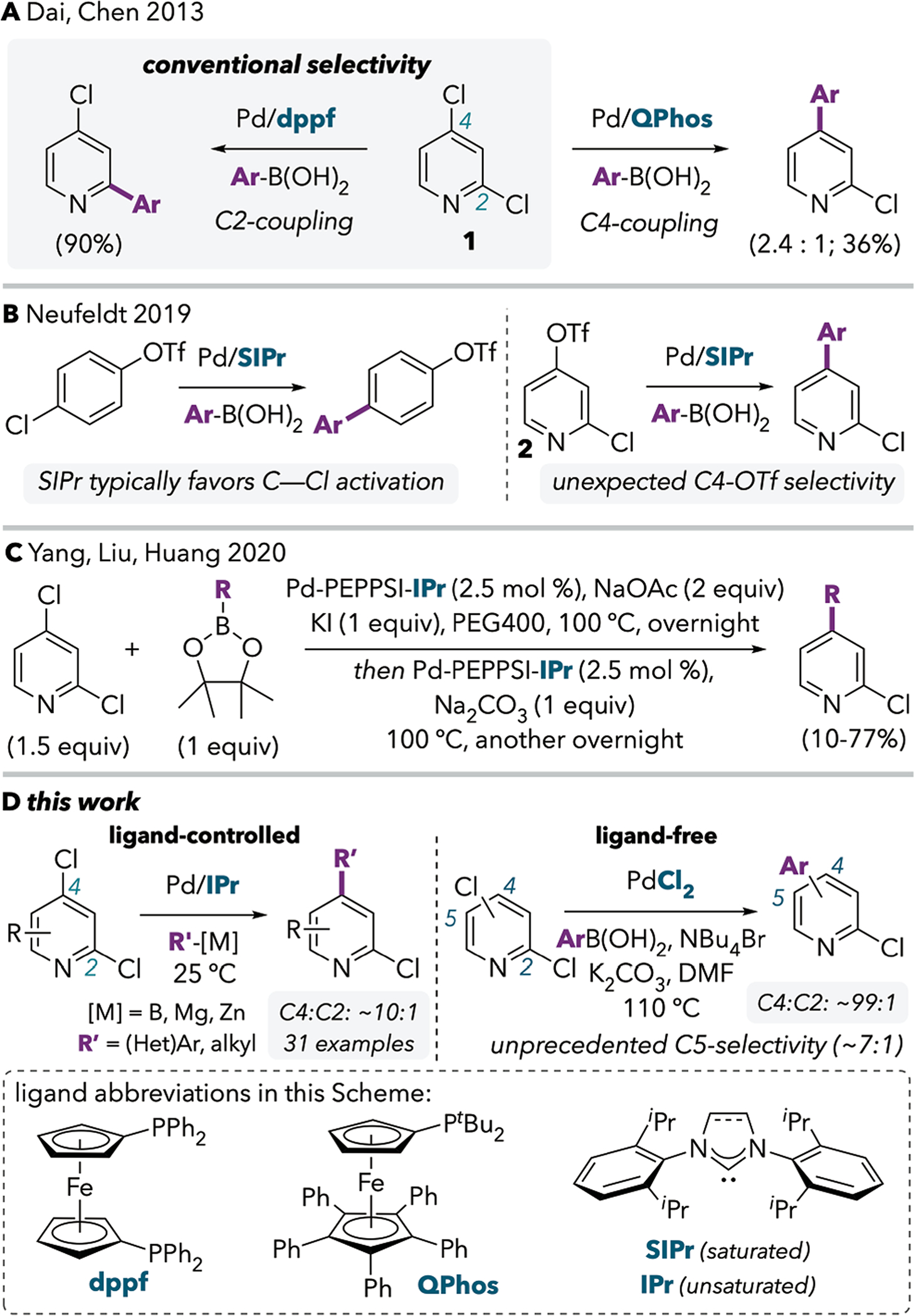
Like other nitrogen-containing heterocycles, 2,4-dichloropyridines nearly always undergo cross-coupling adjacent to nitrogen (at C2, example in Scheme 1A, left).4,5,6,7 Houk and Merlic explained this selectivity based on a distortion-interaction model: the C2—Cl bond is weaker, and is therefore easier to distort into the transition state geometry.8 C2 is also more positively charged due to its proximity to nitrogen, which may enhance attractive interactions with a reductant such as Pd(0). Preferential cross-coupling at C4 to leave a C2-chloride intact is possible if C4 is substituted by bromide or iodide, but heteroarenes containing mixed halogens are often much less accessible than dichlorinated substrates.
Scheme 1.
Ligand Effects on the Cross-Coupling Selectivity of 2,4-Dichloropyridines
Dai, Chen, and coworkers provided the first evidence that ‘innate’ selectivity could be inverted by judicious ligand choice.9 In the presence of the bulky monophosphine QPhos, 1 undergoes Suzuki-Miyaura cross-coupling preferentially at C4, albeit with modest selectivity and yield (Scheme 1A). In contrast, the diphosphine dppf promotes conventional selectivity leading exclusively to the C2-coupled product. In 2019, we reported that the room-temperature Pd/SIPr-catalyzed cross-coupling of 2 gives exclusive reaction at the C4-triflate (Scheme 1B, right).10 This result was surprising, not only because the C2 site of di(pseudo)halogenated pyridines is normally more reactive toward cross-coupling, but also because Pd/SIPr is usually chemoselective for chloride over triflate(Scheme 1B, left). Based on the anomolous selectivity for the reaction of 2 in Scheme 1B, we began exploring the use of the N-heterocyclic carbene (NHC) ligand SIPr and its unsaturated analog IPr for C4-selective cross-coupling of dichloropyridines. During the course of our investigations, Yang and coworkers reported the use of a Pd(PEPPSI)(IPr) catalyst for C4-selective Suzuki-Miyaura cross-coupling of 2,4-dichloropyridines.11 A series of standard optimization reactions using 1 at 60–100 °C revealed that the Pd(PEPPSI)(IPr) catalyst provides C4-selectivity ranging from 2.5 : 1 to 10.4 : 1, varying with solvent and base. Intriguingly, a ten-fold improvement in selectivity was found upon switching to unusual conditions that employ KI, NaOAc, and PEG400, together with the addition of a second batch of Pd(PEPPSI)(IPr) and a second base (Na2CO3) after ~12 h (Scheme 1C).
Here, we report that the use of IPr under mild conditions (often room temperature) enables a broader scope of C4-selective cross-couplings of 2,4-dichloropyridines and related substrates (Scheme 1D, left). Control studies demonstrate that site-selectivity is ligand-controlled under the conditions that we report. However, we further disclose that ligand is not necessary to achieve high C4-selectivity under some high temperature conditions, including those previously reported by Yang et al. We find that ligand-free conditions analogous to those used in the Jeffery-Heck reaction lead to much higher C4-selectivity than can be achieved under ligand-control (~99:1 versus ~10:1 for reactions of 1, Scheme 1D, right). These ligand-free conditions enable unprecedented C5-selectivity in the cross-coupling of 2,5-dichloropyridine and 2,5-dichloropyrimidine. At present, the ligand-free conditions are less general than the ligand-controlled system. Preliminary evidence suggests that the unprecedented selectivity in the absence of strongly coordinating ligands (e.g., phosphines or NHCs) may be a consequence of multinuclear Pd species.
RESULTS AND DISCUSSION
Initial Discovery of C4-Selective Suzuki Coupling.
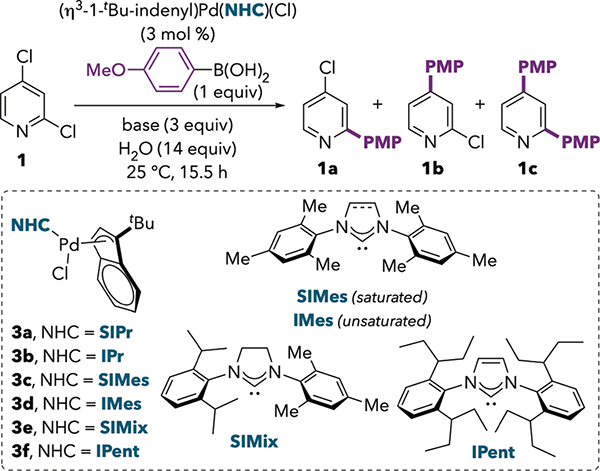
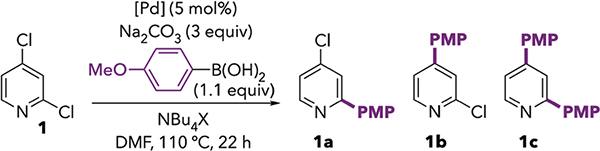
The unexpected C4-OTf selectivity shown in Scheme 1B led us to apply our previously reported reaction conditions10 to the Suzuki-Miyaura cross-coupling of 2,4-dichloropyridine (1). The Hazari precatalyst (η3-1-tBu-indenyl)Pd(SIPr)(Cl)12 (3a) was used with KF as a base in THF at room temperature (Table 1, entry 1). Gratifyingly, these conditions afforded 1b as the major product, resulting from reaction at C4. The crude ratio of 1a : 1b = 1 : 8 reflects much higher C4-selectivity than had been previously reported with QPhos (Scheme 1A), and is similar to the selectivity later reported by Yang during most of their optimization studies.11 The yield of 1b increased when K2CO3 was used as the base (entry 2), and selectivity and yield improved slightly upon switching to catalyst 3b containing the unsaturated analogue IPr (entry 3). Similar results were obtained in benzene (entry 5), but the polar solvents DMF and propylene carbonate led to an erosion of selectivity (entries 6–7).13 The steric bulk of IPr appears to be important for selectivity: when using the smaller ligands SIMes and IMes, much more cross-coupling at C2 was observed (entries 9–10). Interestingly, the unsaturated ligand IMes is slightly more selective than its saturated analog, SIMes. This observation parallels the trend seen with IPr versus SIPr (compare entries 5 and 8). Use of a ligand whose sterics are intermediate between SIPr and SIMes (SIMix) affords intermediate selectivity (entry 11). Finally, the ligand IPent, which bears longer alkyl ortho-substituents, promotes overarylation leading to a mixture of products 1b and 1c (entry 12).14 None of 1b and only trace 1a was detected when the ligand was omitted (entries 4 and 13), demonstrating the critical role of the NHC ligand in both the selectivity and reactivity of the catalyst under these conditions. The mechanistic origin of the observed ligand-controlled selectivity is the topic of further study in our group and will be reported in the near future. In brief, our mechanistic studies indicate that IPr favors oxidative addition at 12e− Pd(0), whereas reaction at 14e− Pd(0) is more prominent with other ligands.
Table 1.
Optimization of the Pd/NHC-Catalyzed C4-Selective Suzuki-Miyaura Couplinga
| ||||||
|---|---|---|---|---|---|---|
| entry | NHC | solvent | 1a (%) |
1b (%) |
1c (%) |
1a : 1b |
| 1b | SIPr | THF | 5 | 39 | 5 | 1 : 8 |
| 2 | SIPr | THF | 7 | 66 | 10 | 1 : 9 |
| 3 | IPr | THF | 7 | 69 | 8 | 1 : 10 |
| 4c | -- | THF | 1 | <1 | 0 | -- |
| 5 | IPr | C6H6 | 8 | 70 | 6 | 1 : 9 |
| 6 | IPr | DMF | 8 | 47 | 15 | 1 : 6 |
| 7 | IPr | PCd | 9 | 45 | 17 | 1 : 5 |
| 8 | SIPr | C6H6 | 9 | 64 | 8 | 1 : 7 |
| 9 | SIMes | C6H6 | 24 | 35 | 3 | 1 : 1.5 |
| 10 | IMes | C6H6 | 23 | 40 | 3 | 1 : 1.7 |
| 11 | SIMix | C6H6 | 8 | 34 | 2 | 1 : 4 |
| 12 | IPent | C6H6 | 1 | 34 | 23 | 1 : 34 |
| 13e | -- | C6H6 | 2 | 0 | 0 | -- |
GC yields calibrated against undecane as the internal standard. Average of two trials. PMP = para-methoxyphenyl.
KF was used instead of K2CO3.
No NHC ligand; Pd source was PdCl2 (3 mol %).
PC = propylene carbonate.
No NHC ligand; Pd source was (η3-1-tBu-indenyl)2(μ-Cl)2Pd2 (1.5 mol %).
Scope of the C4-Selective Suzuki Coupling.
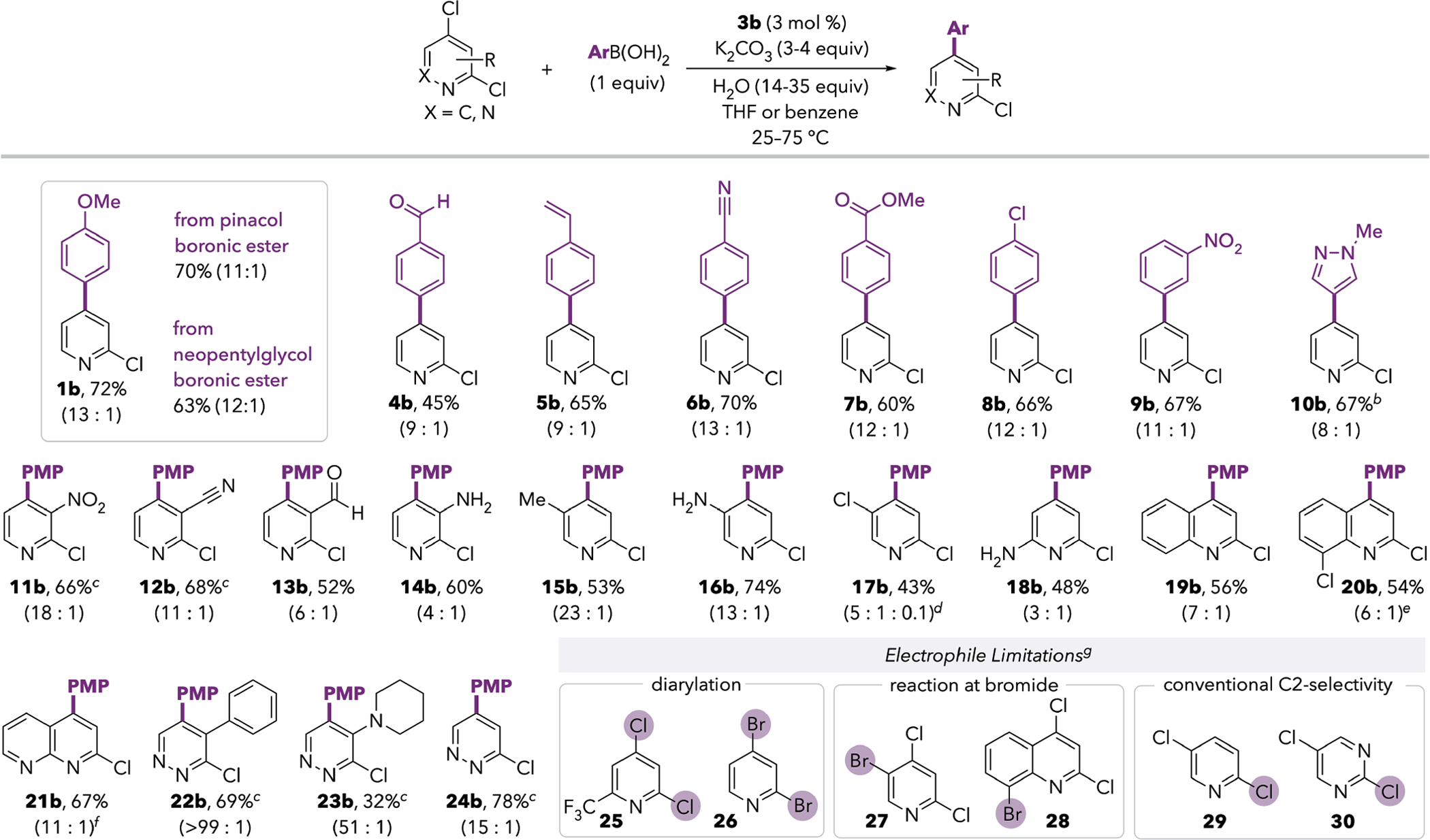
With optimized conditions in hand (Table 1, entries 3 and 5), we next examined the scope of aryl boronic acids in the Suzuki coupling. Substrate 1 undergoes Pd/IPr-catalyzed cross-coupling with diverse arylboronic acids (Scheme 2), affording the C4-coupled products in moderate to good isolated yields. Boronic esters are also competent cross-coupling partners in the reaction to form 1b. The conditions are tolerant of aldehyde, nitrile, and alkene functional groups on the boronic acid coupling partner (4b-6b). Using 4-chlorophenylboronic acid, the major product 8b results from reaction of the 4-pyridyl chloride, leaving both the 2-pyridyl and the aryl chloride intact. A pyrazoleboronic acid reacts well (10b), but poor conversions were observed with other heteroarylboron reagents (see SI). This limitation may be addressed through the use of heteroarylzinc or magnesium reagents instead of boronic acids (vide infra).
Scheme 2.
Scope of C4-Selective Suzuki-Miyaura Cross-Couplings Catalyzed by Pd/IPra
aIsolated yields of products shown. Ratios are the crude ratio of C4:C2-coupled product prior to purification, based on GC signal integrations. ~5–20% diarylation was observed in all cases unless otherwise indicated (see SI for isolated yields when available). Reactions were conducted at room temperature unless otherwise indicated. bReaction conducted at 75 °C. cReaction conducted at 60 °C. dRatio represents C4:C2:C5-coupled products. eNo cross-coupling at C8 was detected. f3f (3 mol %) was used as the catalyst instead of 3b. gSee SI for details.
The optimized conditions enable Suzuki-Miyaura coupling of 2,4-dichloropyridines with diverse substituents at the 3-, 5-, and 6-positions. Aldehyde, nitrile, and unprotected amines are well-tolerated, and no competing amination products were detected in the reactions of aminopyridines. Interestingly, the C4-selectivity is eroded when there is an amino substituent at the 3- or 6-position (14b and 18b), but high selectivity is retained when the amino group is at the 5-position (16b). This observation suggests that the ability of substituents to coordinate to palladium might play a role in the observed site selectivity. A limitation of this method is the substrate with a 6-trifluoromethyl group (25). Whereas diarylation is seen in ~5%–20% yield for most of the other reactions in Scheme 2, the major product of the reaction of 25 corresponds to diarylation, despite the use of only 1 equivalent of arylboronic acid (see SI). In addition to dichloropyridines, the analogous 2,4-dichloroquinoline and 2,4-dichloro-1,8-naphthyridine substrates undergo C4-selective coupling (19b and 21b). These conditions are also effective for cross-coupling at the conventionally disfavored C5-site of 3,5-dichloropyridazines (22b-24b).9 Additional chloride substituents at less electrophilic sites are well-tolerated, including at the 5-position of pyridine and at the 8-position of quinoline (17b and 20b). However, bromide substituents react more quickly than C4-chloride (27–28). 2,4-Dibromopyridine (26) does not display the same behavior as its dichloro analogue; 26 leads to both C4-arylated and diarylated compounds as major products. Finally, these conditions are not effective for cross-coupling distal to nitrogen using substrates 29 or 30. With these substrates, the major product results from reaction at C2—Cl, representing conventional selectivity.
C4-Selective Kumada and Negishi Couplings.
The Pd/IPr catalytic system is also effective for promoting C4-selective cross-coupling with organozinc and organomagnesium nucleophiles. Although the Suzuki coupling is mostly limited to arylboronic acids, Negishi and Kumada conditions enable installation of heteroaryl and alkyl groups in good yield (Scheme 3). For example, a 2-pyridyl group was introduced at the C4 position of 1 using the corresponding Grignard reagent (product 31b); this group is notoriously challenging to install through Suzuki coupling methods.15 Secondary and primary alkyl Grignard reagents were effective coupling partners (32b and 33b), and C4-methylation and benzylation could be achieved using zinc reagents (35b and 36b). Interestingly, the selectivity of these reactions appears to be influenced by the identity of the coupling partner. Products 31b and 33b were the only products observed by GCMS from their respective reactions, whereas the C2-coupled compounds were minor products in the other reactions. This phenomenon may reflect decomposition of some C2-coupled products, leading to artificially high C4:C2 ratios (see SI).
Scheme 3.
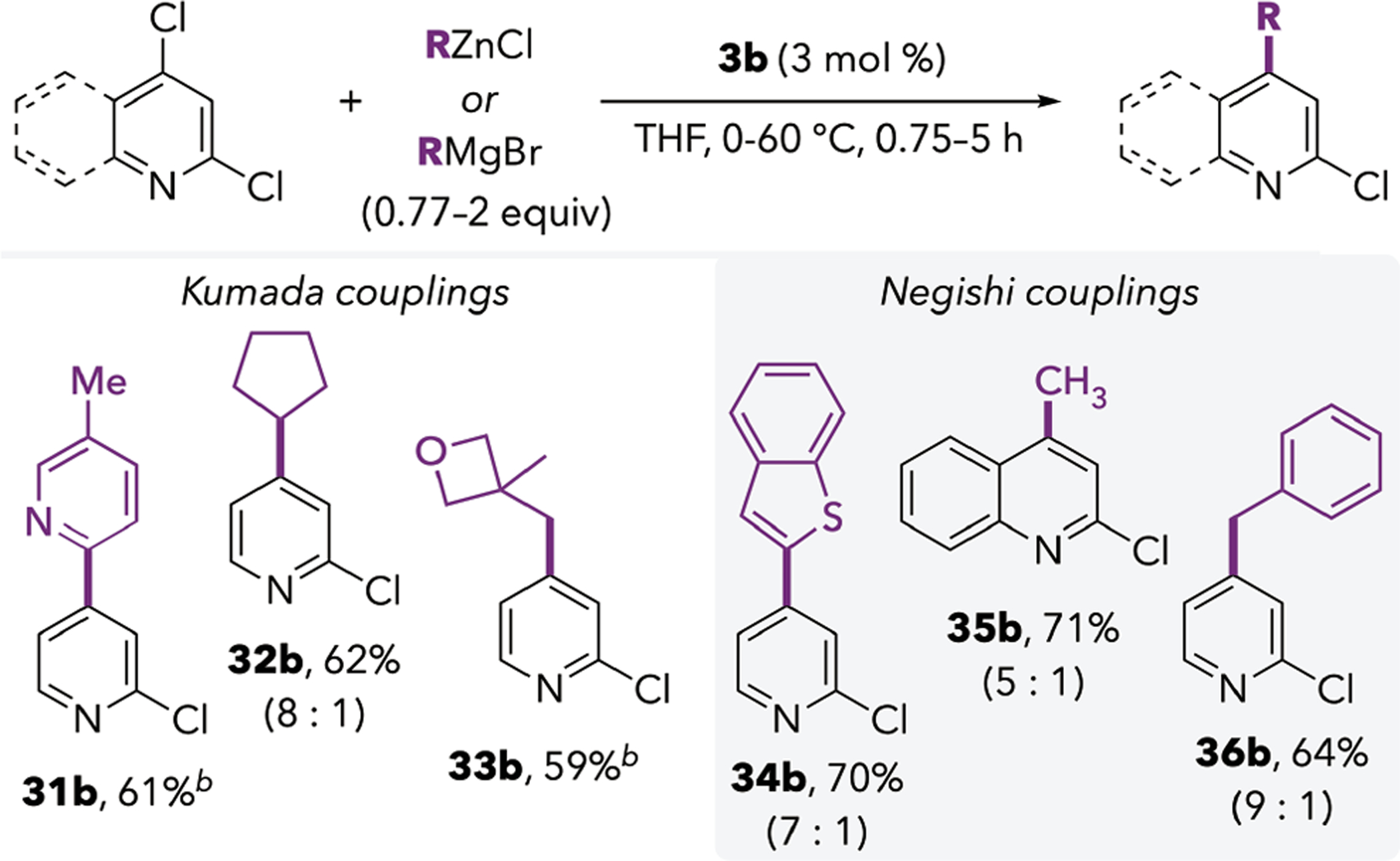
C4-Selective Negishi and Kumada Couplings of 2,4-Dichloropyridine and 2,4-Dichloroquinolinea
aIsolated yields of product shown. Ratios are the crude ratio of C4:C2-coupled product prior to purification, based on GC signal integrations. bProduct shown was the only product observed.
Application to Multi-Step Syntheses.
We envisioned that the C4-selective cross-coupling methods could be particularly useful for accessing pyridine motifs with a C—C bond at C4 and a C—heteroatom bond at C2 (“substitution pattern B”, Scheme 4A). Nucleophilic aromatic substitution (SNAr) reactions are a commonly used strategy for introducing heteroatom substituents onto pyridine. For 2,4-dihalopyridines, transition metal-free SNAr reactions tend to proceed at C4.16 Conversely, as discussed above, the conventional site for cross-coupling reactions of these substrates is C2. As such, sequential cross-coupling and SNAr reactions are useful for generating products with substitution pattern A based on typical selectivity, independent of the order of these steps. However, the complementary pattern B is not generally accessible by conventional methods.
Scheme 4.
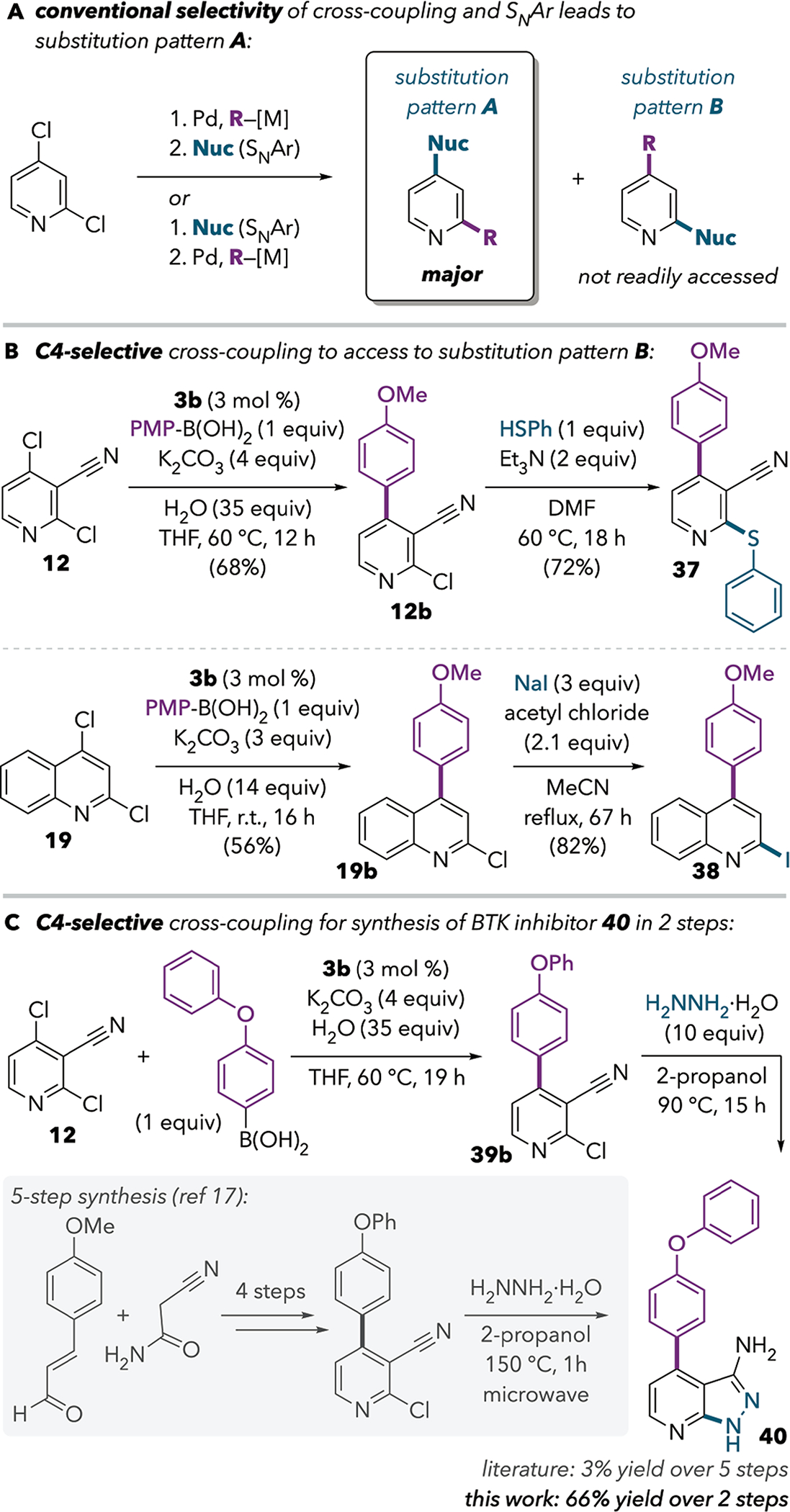
Sequential C4-Selective Cross-Coupling and SNAr Enable Access to a Challenging Substitution Pattern
Three compounds with substitution pattern B were prepared by Pd/IPr-catalyzed C4-selective cross-coupling followed by SNAr reactions. The use of thiolate and iodide nucleophiles for the SNAr steps enabled synthesis of products 37 and 38 (Scheme 4B). We were also able to apply this strategy to a novel synthesis of the Bruton’s tyrosine kinase inhibitor 40. The previously reported synthesis of this compound required 5 synthetic steps providing an overall yield of 3%.17 However, our 2-step synthesis enables access to 40 in 66% overall yield.
Role of Catalyst Speciation in C4-Selectivity.
We were intrigued by the exceptional ~99:1 selectivity achieved under Yang et al.’s unusual conditions, as our own experience suggested that this high selectivity was uncharacteristic of a Pd/IPr catalyst. We hypothesized that selectivity might not be under ligand-control under the final optimized conditions that were previously reported.
Upon conducting ligand-free control reactions, we found that IPr is not necessary for the C4-selectivity in the system reported by Yang et al. (Scheme 5B). The C4-coupled product is formed in excellent selectivity using PdCl2 in the absence of added ligands. As such, the selectivity in Yang’s high-temperature reaction is not controlled by IPr and its mechanistic origin is not the same as that of the room temperature Pd/IPr-catalyzed conditions that we report here. Yang et al. suggest that KI might initially react with 1 to form 2-chloro-4-iodopyridine, which then undergoes selective cross-coupling at C4—I. However, inspired by a recent report by Fairlamb,18,19 we were intrigued by the possibility that the high C4-selectivity in the Yang system might be attributed to a change in catalyst speciation from mono- to multi-nuclearity. Thus, we turned to alternative cross-coupling conditions that have been well-established to promote formation of palladium nanoparticles by applying “Jeffery-Heck” conditions toward the Suzuki coupling of 1 (Table 2). These so-called “Jeffery” conditions are characterized by the use of simple palladium salts, inorganic bases, and tetraalkylammonium additives.20,21
Scheme 5.
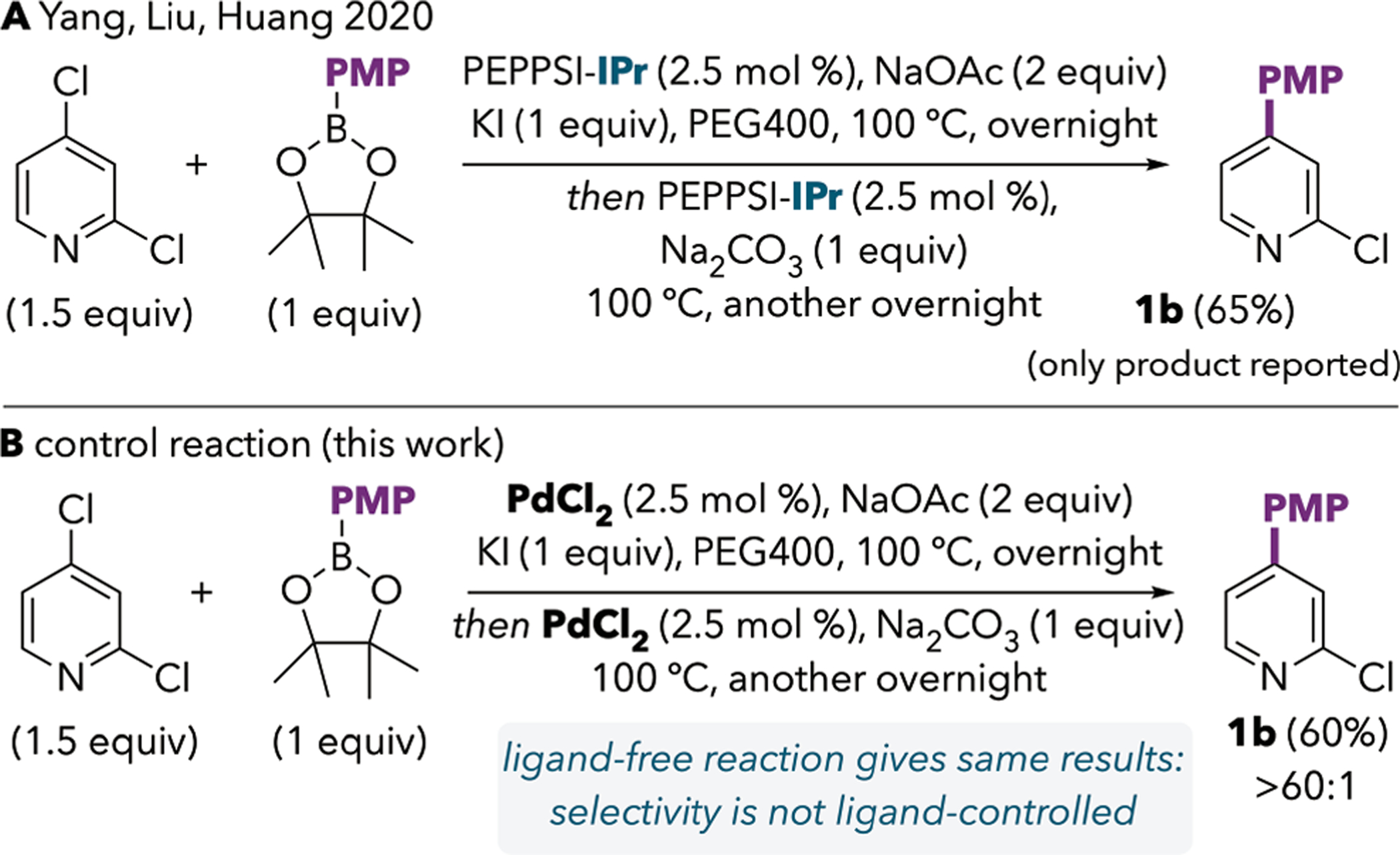
Control Reaction Based on Previously Reported C4-Selective Coupling Shows that Selectivity is Ligand-Independent Under the Reported Conditions
Table 2.
Optimization of the Ligand-Free C4-Selective Suzuki Couplinga
| ||||||
|---|---|---|---|---|---|---|
| entry | [Pd] | NBu4X (equiv) |
1a (%) |
1b (%) |
1c (%) |
1a : 1b (%) |
| 1 | PdCl2 | Br (3) | 0.5 | 81 | 0.4 | 1 : >99 |
| 2 | PdCl2 | Br (5) | 0.5 | 84 | 0.4 | 1 : >99 |
| 3 | Pd(OAc)2 | Br (3) | 0.7 | 73 | 0.4 | 1 : >99 |
| 4 | Pd2dba3 | Br (3) | 0.5 | 57 | 0.2 | 1 : >99 |
| 5 | PdCl2 | Cl (3) | 1.1 | 72 | 0.5 | 1 : 65 |
| 6 | PdCl2 | Br (1) | 1.2 | 69 | 0.7 | 1 : 58 |
| 7 | PdCl2 | PF6 (3) | 3.0 | 48 | 3.1 | 1 : 16 |
| 8 | PdCl2 | -- | 3.5 | 56 | 3.1 | 1 : 16 |
| 9b | PdCl2 | -- | 2.1 | 69 | 1.6 | 1 : 33 |
| 10c | PdCl2 | Br (3) | 0.9 | 79 | 0.6 | 1 : 88 |
| 11d | PdCl2 | Br (3) | 1.1 | 83 | 0.7 | 1 : 75 |
| 12e | PdCl2 | Br (3) | 0.8 | 45 | 0.5 | 1 : 56 |
| 13f | PdCl2 | Br (3) | 0.3 | 9 | 0.5 | 1 : 29 |
| 14c | PdCl2 | Br (5) | 0.8 | 85 | 0.5 | 1 : >99 |
GC yields calibrated against undecane as the internal standard. Average of two trials.
With KBr (3 equiv).
With H2O (5 equiv).
With H2O (10 equiv).
With 1 mol % of PdCl2.
Reaction run at 25 °C.
Gratifyingly, ligand-free Jeffery-type conditions using PdCl2, Na2CO3, and 3–5 equivalents of NBu4Br were found to afford remarkable C4-selectivity for the Suzuki-Miyaura coupling of 1 (Table 2, entries 1–2, 1a : 1b = 1 : >99 by GC analysis). Comparable selectivity but somewhat lower conversion was observed with Pd(OAc)2 or Pd2dba3 (entries 3 and 4). Similar results were obtained using NBu4Cl instead of the bromide salt (compare entries 1 and 5), indicating that selectivity cannot be explained by initial Cl/Br exchange at C4. The use of only 1 equivalent of NBu4Br (entry 6), replacing the halide with a noncoordinating anion such as PF6 (entry 7), or complete omission of the quaternary ammonium salt (entries 8–9) results in diminished selectivity and yield of 1b, consistent with the known roles of both coordinating anions and non-coordinating cations on stabilizing nanoparticles.22 Added water has a marginally detrimental effect on selectivity when using 3 equiv of NBu4Br (entries 10–11), but the combination of H2O (5 equiv) and NBu4Br (5 equiv, entry 14) appeared to improve conversion for some of the reactions in Scheme 6. Finally, lower catalyst loading (entry 12) or lower temperature (entry 13) adversely affect selectivity and yields of 1b.
Scheme 6.
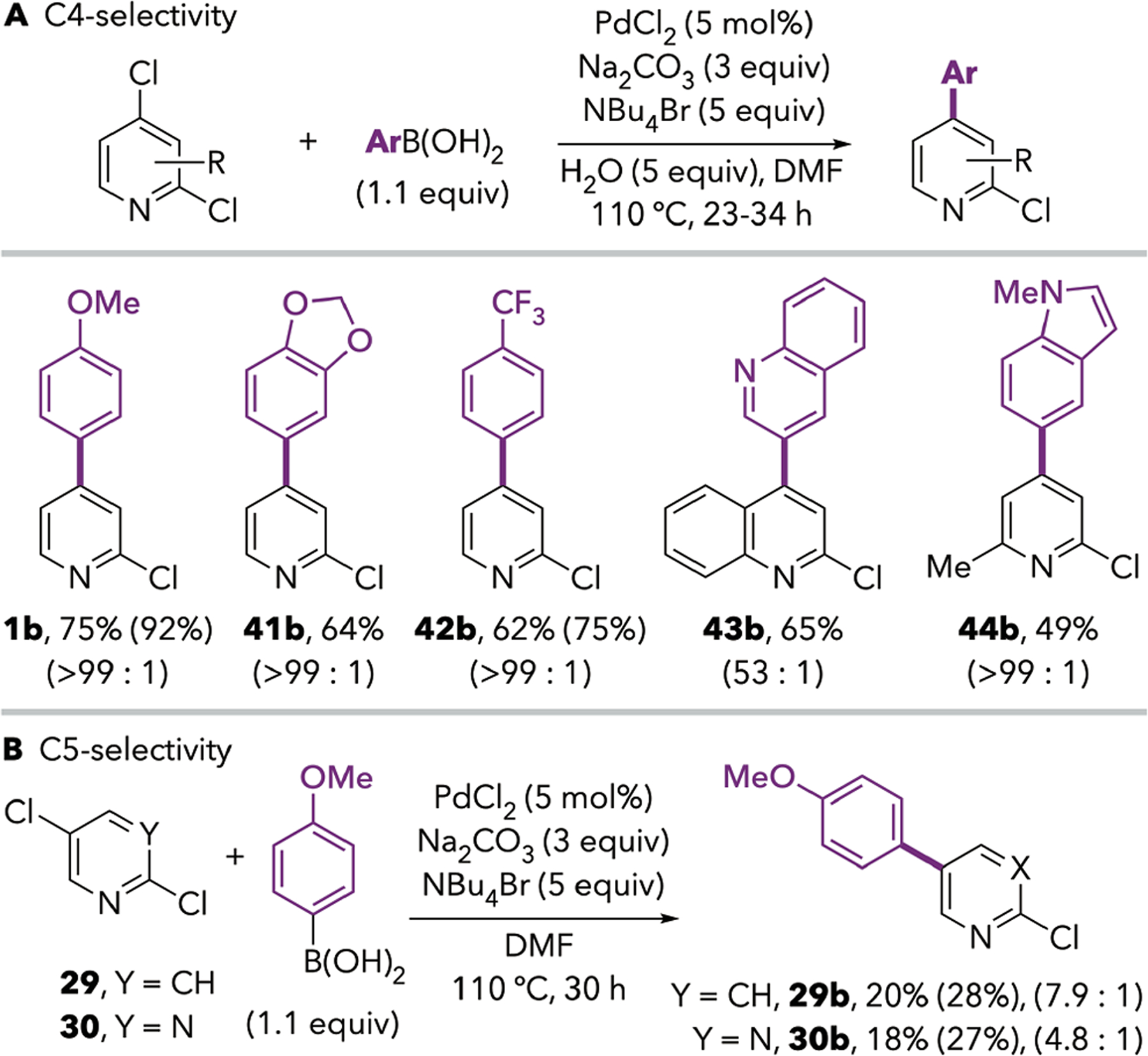
Scope of C4-Selective Suzuki-Miyaura Cross-Couplings Under Ligand-Free Catalysisa
aIsolated yields are reported. Yields in parentheses are the calibrated GC yields of the indicated product prior to purification. Ratios are the crude ratio of C4:C2- or C5:C2-coupled products prior to purification, based on GC signal integrations.
The optimized Jeffery-type ligand-free conditions are general to coupling of 1 and 19 with boronic acids bearing both electron-donating and electron-withdrawing functional groups (Scheme 6). In all cases, C2- and diarylation products were absent or observed in only trace quantities. As such, product purification is more facile compared to the Pd/IPr conditions. Remarkably, 2,5-dichloropyridine and 2,5-dichloropyrimidine undergo preferential coupling at C5 to afford products 29b and 30b. This selectivity is unprecedented: all previously reported cross-couplings of these substrates with spectroscopically-supported structural assignments proceed through C2—Cl cleavage.23,24,25 Yields are only modest with these substrates as well as with many substituted 2,4-dichloropyridines and related electrophiles. Current work in our group aims to uncover the mechanistic origin of the unique selectivity under ligand-free conditions. We anticipate that mechanistic insight will enable improvements to the reaction scope.
CONCLUSION
Ligand-controlled selectivity is demonstrated with a Pd/IPr catalytic system. This system is effective for C4-selective Suzuki, Kumada, and Negishi cross-couplings of a variety of substituted 2,4-dichloropyridines and related substrates. This methodology allows installation of aryl, heteroaryl, and alkyl groups at the C4 position of pyridine while retaining a chloride substituent at C2. If desired, the chloride can subsequently be replaced with a different heteroatom through an SNAr reaction. This synthetic sequence comprising C4-selective cross-coupling followed by SNAr provides products with a substitution pattern that can be challenging to access through more conventional methods. The mechanistic origin of ligand-controlled inversion of typical site-selectivity is the focus of an upcoming report.
Intriguingly, ligand-free “Jeffery” conditions are found to increase the C4-selectivity in a Suzuki coupling of 2,4-dichloropyridine by an order of magnitude. For the first time, C5-selective cross-coupling of 2,5-dichloropyridine and 2,5-dichloropyrimidine is achieved through the use of these ligand-free conditions. By analogy to the Jeffery-Heck reaction, it is likely that palladium nanoparticles are involved under these ligand-free conditions. Ongoing work in our group examines the mechanistic basis for this atypical selectivity.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institute of General Medical Sciences (NIGMS) of the NIH under Award Number R35GM137971. Support for MSU’s NMR Center was provided by the NSF (Grant No. NSF-MRI:CHE-2018388 and NSF-MRI:DBI-1532078), MSU, and the Murdock Charitable Trust Foundation (2015066:MNL). Funding for the mass spectrometry facility was provided in part by NIH NIGMS (P20GM103474 and S10OD28650), the Murdock Charitable Trust Foundation, and MSU. We are grateful to Umicore for a gift of (η3-1-tBu-indenyl)2(μ-Cl)2Pd2 and Pd precatalysts (η3-1-tBu-indenyl)Pd(IPr)(Cl) and (η3-1-tBu-indenyl)Pd(IPent)(Cl).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental details, materials, methods, NMR spectra, and characterization data including regiochemical assignments of products (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.Roughley SD; Jordan AM The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates J. Med. Chem 2011, 54, 3451. [DOI] [PubMed] [Google Scholar]
- 2.Smith BR; Eastman CM; Njardarson JT Beyond C, H, O, and N! Analysis of the Elemental Composition of U.S. FDA Approved Drug Architectures. J. Med. Chem 2014, 57, 9764–9773. [DOI] [PubMed] [Google Scholar]
- 3.Reviews:; (a) Schröter S; Stock C; Bach T Regioselective cross-coupling reactions of multiple halogenated nitrogen-, oxygen-, and sulfur-containing heterocycles. Tetrahedron 2005, 61, 2245–2267; [Google Scholar]; (b) Fairlamb IJS Regioselective (site-selective) functionalisation of unsaturated halogenated nitrogen, oxygen and sulfur heterocycles by Pd-catalysed cross-couplings and direct arylation processes. Chem. Soc. Rev 2007, 36, 1036–1045; [DOI] [PubMed] [Google Scholar]; (c) Manabe K; Yamaguchi M; Catalyst-Controlled Site-Selectivity Switching in Pd-Catalyzed Cross-Coupling of Dihaloarenes. Catalysts 2014, 4, 307–320; [Google Scholar]; (d) Almond-Thynne J; Blakemore DC; Pryde DC; Spivey AC Site-selective Suzuki–Miyaura coupling of heteroaryl halides – understanding the trends for pharmaceutically important classes. Chem. Sci 2017, 8, 40–62; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Palani V; Perea MA; Sarpong R Site-Selective Cross-Coupling of Polyhalogenated Arenes and Heteroarenes with Identical Halogen Groups. Chem. Rev 2021, Article ASAP. DOI: 10.1021/acs.chemrev.1c00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Cruskie MP Jr; Zoltewicz JA; Abboud KA; Revised Structure and Convergent Synthesis of Nemertelline, the Neurotoxic Quaterpyridine Isolated from the Hoplonemertine Sea Worm J. Org. Chem 1995, 60, 7491–7495; [Google Scholar]; (b) Norman MH; Chen N; Chen Z; Fotsch C; Hale C; Han N; Hurt R; Jenkins T; Kincaid J; Liu L; Lu Y; Moreno O; Santora VJ; Sonnenberg JD; Karbon W Structure-Activity Relationships of a Series of Pyrrolo[3,2-d]pyrimidine Derivatives and Related Compounds as Neuropeptide Y5 Receptor Antagonists. J. Med. Chem 2000, 43, 4288–4312; [DOI] [PubMed] [Google Scholar]; (c) Murray JI; Woscholskiab R; Spivey AC Highly efficient and selective phosphorylation of amino acid derivatives and polyols catalysed by 2-aryl-4-(dimethylamino)pyridine-N-oxides – towards kinase-like reactivity. Chem. Commun 2014, 50, 13608–13611; [DOI] [PubMed] [Google Scholar]; (d) Young IS; Glass AL; Cravillion T; Han C; Zhang H; Gosselin F Palladium-Catalyzed Site-Selective Amidation of Dichloroazines. Org. Lett 2018, 20, 3902–3906. [DOI] [PubMed] [Google Scholar]
- 5.(a) Shiota T; Yamamori T Regioselective Reactions of Organozinc Reagents with 2,4-Dichloroquinoline and 5,7-Dichloropyrazolo[1,5-a]pyrimidine. J. Org. Chem 1999, 64, 453–457; [Google Scholar]; (b) Legros J-Y; Primault G; Fiaud J-C Syntheses of acetylquinolines and acetylisoquinolines via palladium-catalyzed coupling reactions. Tetrahedron 2001, 57, 2507–2514. [Google Scholar]
- 6.Blaise E; Kümmerle AE; Hammoud H; De Araújo Júnior, D. X; Bihel F; Bourguignon J; Schmitt M Access to 4-Alkylaminopyridazine Derivatives via Nitrogen-Assisted Regioselective Pd-Catalyzed Reactions. J. Org. Chem 2014, 79, 10311–10322. [DOI] [PubMed] [Google Scholar]
- 7.(a) Dai Y; Har-tandi K; Ji Z; Ahmed AA; Albert DH; Bauch JL; Bouska JJ; Bousquet PF; Cunha GA; Glaser KB; Harris CM; Hickman D; Guo J; Li J; Marcotte PA; Marsh KC; Moskey MD; Martin RL; Olson AM; Osterling DJ; Pease LJ; Soni NB; Stewart KD; Stoll VS; Tapang P; Reuter DR; Davidsen SK; Michaelides MR Discovery of N-(4-(3-amino-1H-indazol-4-yl)phenyl)-N′-(2-fluoro-5-methylphenyl)urea (ABT-869), a 3-aminoindazole-based orally active multitargeted receptor tyrosine kinase inhibitor. J. Med. Chem 2007, 50, 1584–1597; [DOI] [PubMed] [Google Scholar]; (b) Khoje AD; Gundersen L-L Reactivity and regioselectivity in Stille couplings of 3-substituted 2,4-dichloropyridines. Tetrahedron Lett. 2011, 52, 523–525. [Google Scholar]
- 8.(a) Legault CY; Garcia Y; Merlic CA; Houk KN Origin of Regioselectivity in Palladium-Catalyzed Cross-Coupling Reactions of Polyhalogenated Heterocycles. J. Am. Chem. Soc 2007, 129, 12664–12665. [DOI] [PubMed] [Google Scholar]; (b) Garcia Y; Schoenebeck F; Legault CY; Merlic CA; Houk KN Theoretical Bond Dissociation Energies of Halo-Heterocycles: Trends and Relationships to Regioselectivity in Palladium-Catalyzed Cross-Coupling Reactions. J. Am. Chem. Soc 2009, 131, 6632–6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai X; Chen Y; Garrell S; Liu H; Zhang L-K; Palani A; Hughes G; Nargund R Ligand-Dependent Site-Selective Suzuki Cross-Coupling of 3,5-Dichloropyridazines. J. Org. Chem 2013, 78, 7758–7763. [DOI] [PubMed] [Google Scholar]
- 10.Reeves EK; Humke JN; Neufeldt SR N-Heterocyclic Carbene Ligand-Controlled Chemodivergent Suzuki−Miyaura Cross Coupling, J. Org. Chem 2019, 84, 11799–11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang M; Chen J; He C; Hu X; Ding Y; Kuang Y; Liu J; Huang Q Palladium-Catalyzed C-4 Selective Coupling of 2,4-Dichloropyridines and Synthesis of Pyridine-Based Dyes for Live-Cell Imaging. J. Org. Chem 2020, 85, 6498–6508. [DOI] [PubMed] [Google Scholar]
- 12.Melvin PR; Nova A; Balcells D; Dai W; Hazari N; Hruszkewycz DP; Shah HP; Tudge MT Design of a Versatile and Improved Precatalyst Scaffold for Palladium- Catalyzed Cross-Coupling (η3-1-tBu-indenyl)2(μ-Cl)2Pd2. ACS Catal. 2015, 5, 3680–3688. [Google Scholar]
- 13. We hypothesize that polar solvents can promote a more polar “displacement” mechanism for oxidative addition, compared to a 3-centered mechanism in nonpolar solvents. These two different mechanisms are biased toward C2 versus C4 selectivity, respectively (a detailed mechanistic study is forthcoming).
- 14.Pd/IPent has been shown to promote exhaustive arylation in other contexts. Although the origin of IPent’s effect has not been studied, it is likely that its steric bulk disfavors dissociation of Pd from the product after reductive elimination.; Groombridge BJ; Goldup SM; Larrosa I Selective and general exhaustive cross-coupling of di-chloroarenes with a deficit of nucleophiles mediated by a Pd–NHC complex. Chem. Commun 2015, 51, 3832–3834. [DOI] [PubMed] [Google Scholar]
- 15.Cook XAF; de Gombert A; McKnight J; Pantaine LRE; Willis MC The 2-Pyridyl Problem: Challenging Nucleophiles in Cross-Coupling Arylations. Angew. Chem., Int. Ed 2021, 60, 11068–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Examples: [Google Scholar]; (a) Banks RE; Burgess JE; Cheng WM; Haszeld-ine RN 93. Heterocyclic polyfluoro-compounds. Part IV. Nucleophilic substitution in pentafluoropyridine: the preparation and properties of some 4-substituted 2,3,5,6-tetrafluoropyridines. J. Chem. Soc 1965, 575–581; [Google Scholar]; (b) Mittelbach M An Easy and Convenient Synthesis of 6-Methyl-4(1H)-pyridone-3-carboxylic Acid. Synthesis 1988, 6, 479–480; [Google Scholar]; (c) Schlosser M; Rausis T; Bobbio C Re-routing Nucleophilic Substitution from the 4-Position to the 2- or 6-Position of 2,4-Dihalopyridines and 2,4,6-Trihalopyridines: The Solution to a Long-Standing Problem. Org. Lett 2005, 7, 127–129; [DOI] [PubMed] [Google Scholar]; (d) Schlosser M; Bobbio C; Rausis T Regiochemically Flexible Substitutions of Di-, Tri-, and Tetrahalopyridines: The Trialkylsilyl Trick. J. Org. Chem 2005, 70, 2394–2502. [DOI] [PubMed] [Google Scholar]
- 17.Duan J; Jian B; Lu Z Azaindoles as BTK Kinase Modulators and Use Thereof. International Patent WO 2011/019780 A1, February 17, 2011. (pages 63–64)
- 18.(a) Scott NWJ; Ford MJ; Jeddi N; Eyles A; Simon L; Whitwood AC; Tanner T; Willans CE; Fairlamb IJS A Dichotomy in Cross-Coupling Site Selectivity in a Dihalogenated Heteroarene: Influence of Mononuclear Pd, Pd Clusters, and Pd Nanoparticles—the Case for Exploiting Pd Catalyst Speciation. J. Am. Chem. Soc 2021, 143, 9682–9693. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Appleby KM; Dzotsi E; Scott NWJ; Dexin G; Jeddi N; Whitwood AC; Pridmore NE; Hart S; Duckett SB; Fairlamb IJS Bridging the Gap from Mononuclear PdII Precatalysts to Pd Nanoparticles: Identification of Intermediate Linear [Pd3(XPh3)4]2+ Clusters as Catalytic Species for Suzuki–Miyaura Couplings (X = P, As). Organometallics 2021, 40, 35603570. [Google Scholar]
- 19. Fairlamb’s conditions employ PPh3 in a low ligand:Pd ratio, which promote formation of clusters and nanoparticles. These conditions were shown to effect C4-selective cross-coupling of 2,4-dibromopyridine (C2 : C4 ~ 1 : 10). Notably, Fairlamb’s conditions are not effective for C4-selective cross-coupling of 2,4-dichloropyridine (see SI).
- 20.(a) Jeffery T Palladium-catalysed Vinylation of Organic Halides under Solid-Liquid Phase Transfer Conditions. J. Chem. Soc., Chem. Commun, 1984, 1287–1289. [Google Scholar]; (b) Jeffery T Highly stereospecific palladium-catalysed vinylation of vinylic halides under solid-liquid phase transfer conditions. Tetrahedron Lett. 1985, 26, 2667–2670. [Google Scholar]; (c) Jeffery T On the efficiency of tetraalkylammonium salts in Heck type reactions. Tetrahedron 1996, 52, 10113–10130. [Google Scholar]
- 21.Chernyshev VM; Khazipov OV; Eremin DB; Denisova EA; Ananikov VP Formation and stabilization of nanosized Pd particles in catalytic systems: Ionic nitrogen compounds as catalytic promoters and stabilizers of nanoparticles. Coord. Chem. Rev 2021, 437, 212860. [Google Scholar]
- 22.de Vries JG When Does Catalysis with Transition Metal Complexes Turn into Catalysis by Nanoparticles? in Selective Nanocatalysts and Nanoscience (2011) Wiley:VCH, pp 73–103 [Google Scholar]
- 23.For selected examples of typical selectivity with 2,5-dihalopyrimidine see:; (a) Hughes G; Wang C; Batsanov AS; Fern M; Frank S; Bryce MR; Perepichka IF; Monkman AP; Lyons BP New pyrimidine- and fluorene-containing oligo(arylene)s: synthesis, crystal structures, optoelectronic properties and a theoretical study Org. Biomol. Chem, 2003, 1, 3069–3077. [DOI] [PubMed] [Google Scholar]; (b) Malik I; Ahmed Z; Reimann S; Ali I; Villinger A; Langer P Synthesis and Photophysical Properties of Alkynylated Pyrimidines by Site-Selective Sonogashira Reactions of 2,4,5,6-Tetrachloropyrimidine; First Synthesis of Tetraalkynyl-pyrimidines. Eur. J. Org. Chem 2011, 2088–2093. [Google Scholar]
- 24.For selected examples of typical selectivity with 2,5-dichloropyridines see:; (a) Brumfield S; Matasi JJ; Tulshian D; Czarniecki M; Greenlee W; Garlisi C; Qiu H; Devito K; Chen S; Sun Y; Bertorelli R; Ansell J; Geiss W; Le V; Martin GS; Vellekoop SA; Haber J; Allard ML Synthesis and SAR development of novel P2X7 receptor antagonists for the treatment of pain: Part 2. Bioorganic & Med. Chem. Lett 2011, 24, 7287–7290. [DOI] [PubMed] [Google Scholar]; (b) Curreli F; Ahmed S; Benedict Victor SM; Iusupov IR; Belov DS; Markov PO; Kurkin AV; Altieri A; Debnath AK Preclinical Optimization of gp120 Entry Antagonists as anti-HIV-1 Agents with Improved Cytotoxicity and ADME Properties through Rational Design, Synthesis, and Antiviral Evaluation. J. Med. Chem 2020, 63, 1724–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.For reported exceptions to conventional selectivity of 2,5-dichloropyridine and -pyrimidine in direct arylations and Sonogashira couplings, albeit without spectroscopic support for structural assignment of the products, see; (a) Zheng L-L; Yin B; Tian X-C; Yuan M-Y; Li X-H; Gao F Pd/Cu bimetallic co-catalyzed direct 2-arylation of benzoxazole with aryl chloride. Tetrahedron Lett. 2019, 60, 151316; [Google Scholar]; (b) Al-Khawaldeh I; Yasiri MJA; Aldred GG; Basmadjian C; Bordoni C; Harnor SJ; Heptinstall AB; Hobson SJ; Jennings CE; Khalifa S; Lebraud H; Martin MP; Miller DC; Shrives HJ; Souza JV; Stewart HL; Temple M; Thomas HD; Totobenazara J; Tucker JA; Tudhope SJ; Wang LZ; Bronowska AK; Cano C; Endicott JA; Golding BT; Hardcastle IR; Hickson I; Wedge SR; Willmore E; Noble MEM; Waring MJ An Alkynylpyrimidine-Based Covalent Inhibitor That Targets a Unique Cysteine in NF-κB-Inducing Kinase. J. Med. Chem 2021, 64, 10001–10018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


