Abstract
Post-operative skeletal fibrosis is considered one of the major complications causing dysfunction of the skeletal system and compromising the outcomes of clinical treatment. Limited success has been achieved using current therapies; more effective therapies to reduce post-operative skeletal fibrosis are needed. Stem cells possess the ability to repair and regenerate damaged tissue. Numerous studies show that stem cells serve as a promising therapeutic approach for fibrotic diseases in tissues other than the skeletal system by inhibiting the inflammatory response and secreting favorable cytokines through activating specific signaling pathways, acting as so-called medicinal signaling cells. In this review, current therapies are summarized for post-operative skeletal fibrosis. Given that stem cells are used as a promising therapeutic approach for fibrotic diseases, little effort has been undertaken to use stem cells to prevent post-operative skeletal fibrosis. This review aims at providing useful information for the potential application of stem cells in preventing post-operative skeletal fibrosis in the near future.
Keywords: Epidural fibrosis, Intraarticular fibrosis, Peritendinous fibrosis, Post-operative skeletal fibrosis, Stem cell
1. Introduction
Fibrosis, a progressive fatal disease which accounts for more than one third of deaths worldwide, is considered the end-stage of many diseases which can occur in numerous organ systems, including but not limited to lung, skin and kidney 1, 2. Of course, the skeletal system is no exception. Post-operative skeletal fibrotic diseases, including epidural fibrosis, intraarticular fibrosis and peritendinous fibrosis, often occur after surgery, trauma or injury of the skeletal system 3-5. Post-operative skeletal fibrosis is considered a major complication which can cause skeletal system dysfunction, increase medical costs for patients, reduce the satisfaction rate of clinical treatment and even lead to patient depression. Due to these potential complications, post-operative skeletal fibrosis has drawn great attention. Following the elucidation of in-depth cellular and molecular mechanisms underlying the formation and progression of post-operative skeletal fibrosis, many therapeutic strategies have been applied to reduce this condition, such as microsurgical techniques, effective hemostasis, various drugs and biomaterial mediated strategies 3, 4. However, side effects of the current approaches diminish the efficacy of treatments and more effective therapeutic strategies are needed.
With the development of regenerative medicine, stem cells have proven to possess great potential as a therapy for various diseases6. Increasing evidence indicates that stem cells have shown anti-fibrotic effects in numerous fibrotic tissues, including kidney, lung, heart, liver and skin, in both animal models and human studies. For example, jugular vein injection of bone marrow-derived stem cells (BMSCs) could ameliorate bleomycin-induced lung fibrosis in a rat model 7 and tail injection of BMSCs could reduce interstitial fibrosis in the Col4α3−/− deficient mouse model 8. Stem cells are also proven to be a therapy for fibrotic diseases of the skin 9, cardiovascular system 10 and liver 11. Recently, stem cells have also been found to have an anti-fibrotic effect in skeletal fibrotic diseases. For instance, resident stem cells could attenuate muscle fibrosis by intronic polyadenylation of platelet-derived growth factor alpha (PDGFα) 12 and adipose-derived stem cells (ADSCs) delivered by poly lactic-co-glycolic acid (PLGA) scaffold could prevent epidural fibrosis adhesion by reconstructing epidural fat 13.
The above-mentioned studies indicate that stem cells show anti-fibrotic effects by secreting immunomodulatory and trophic factors, which is in line with a new term for mesenchymal stem cells, Medicinal Signaling Cells coined by Caplan14. Since several kinds of stem cells are discussed throughout the article, “stem cells” are used to indicate the broad scope of this concept while tissue-specific stem cells were detailed in individual studies. The primary aim of this review is to illustrate the current understanding and achievements of stem cells as an approach for anti-fibrotic therapy, which might provide useful information to develop a therapeutic strategy for skeletal fibrotic diseases.
2. Definition of post-operative skeletal fibrosis and common mechanisms
Post-operative skeletal fibrotic disease mainly consists of three subtypes: epidural fibrosis, intraarticular fibrosis and peritendinous fibrosis. Epidural fibrosis after laminectomy is a major reason for failed back surgery syndrome which is defined by persistent chronic pain of the spinal nerve root and lower back. The progressive formation of fibrotic scar between the dura mater and surrounding muscles can result in poor outcomes after spine surgery, which is a challenge for physicians. Intraarticular fibrosis is a serious post-operative complication of knee surgery and has gained the attention of adult reconstruction surgeons. Progressive scar formation limits the function of the knee joint by altering the range of motion, resulting in side effects such as knee joint pain, cartilage degeneration and muscle atrophy. Peritendinous fibrosis adhesion occurs after tendon repair, finger fracture fixation and other hand injuries, and limits the glide of the tendon and interphalangeal joint range of motion.
Despite no uniform mechanism that causes post-operative skeletal fibrosis, several potential mechanisms exist for each type of skeletal fibrosis. Fibroblast activation, which is considered the major cause of post-operative skeletal fibrosis, can be recognized as the common mechanism. During the repair process, damaged cells release inflammatory cells followed by a change in inflammatory response from the acute to the chronic phase. Following secretion of growth factors, cytokines and chemokines to the damaged area during chronic inflammation 15, 16, the fibroblasts activated by the secreted factors migrate to the damaged skeletal area to produce collagen and form fiber to assist in healing, during which collagen formation and collagen catabolism maintain a balance due to the existence of a fibrinolytic enzyme. Unfortunately, the newly formed collagen exceeds the degraded collagen and thus there is excess collagen deposited in the remodeling process 16, 17. The formed collagen develops into fibrosis tissue followed by adhesion to adjacent tissues, such as tendon, joint, skin, dura and nerve. The adhesion between fibrosis tissue and adjacent tissue decreases the range of motion of the joint and limits the movement of tendons; it also can lead to radicular low back pain and loss of muscle function.
3. Current therapies for post-operative skeletal fibrosis
Given the above-mentioned common mechanisms, additional therapies have been undertaken to prevent post-operative skeletal fibrosis through inhibiting fibroblast proliferation and suppressing the pro-inflammatory response. In this section, we summarize current therapeutic strategies to prevent post-operative skeletal fibrosis in animals and humans, including topical application of anti-tumor drugs, immunosuppressant drugs, biomaterials and other anti-fibrotic drugs (Table.S1). The disadvantages of each approach are also highlighted.
3.1. Anti-tumor drugs
Owing to the anti-fibrotic effect of anti-tumor drugs, positive results have been achieved in reducing post-operative skeletal fibrosis. Previous studies have shown that topical application of anti-tumor drugs, including mitomycin C (MMC) 18-24, 10-hydroxycamptothecine (HCPT) 4, 25-30, colchicine 31, 32 and 5-fluorouracil (5-Fu) 18, 33, could prevent post-operative skeletal fibrosis in animal models by inhibiting the inflammatory response, suppressing fibroblast proliferation and reducing collagen deposition. However, more research is needed to determine the exact effect of 5-Fu 34, 35. Interestingly, a previous study has shown that topical application of 0.5 mg/mL MMC could prevent epidural fibrosis after microendoscopic discectomy surgery in 37 patients 36. Further evidence showed that anti-tumor drugs could reduce post-operative skeletal fibrosis by regulating miR-200b and RhoE expression, upregulating NOXA expression, decreasing vascular endothelial growth factor (VEGF) expression, blocking the transforming growth factor beta (TGF-β) pathway and activating the endoplasmic reticulum (ER) stress signaling pathway 29, 30.
3.2. Immunosuppressant drugs
Local immune response is considered critical for each type of post-operative skeletal fibrosis and some immunosuppressant drugs used to prevent post-operative skeletal fibrosis have achieved success, including rapamycin 5, 37, 38, tacrolimus 39-41, pimercrolimus 42, azithromycin 43, methotrexate 44 and cyclosporin A 18. Results showed that they could prevent post-operative skeletal fibrosis by inhibiting fibroblast proliferation 37-39, suppressing interleukin 2 (IL-2) and TGF-β1 expression 40, regulating miR-429 and RhoE expression 41 and activating autophagy 5 and the ER stress signaling pathway 44.
3.3. Biomaterials
More evidence indicates that some biomaterials have been successfully used to prevent post-operative skeletal fibrosis in animal models, including hyaluronic acid (HA) and derivatives 45-50, chitosan and derivatives 51-53 and amniotic membrane and derivatives 54-57. Specifically, topical application of HA derivatives could prevent peritendinous fibrosis in patients 58, 59. The underlying mechanisms include acting as a barrier, reducing inflammatory response and suppressing fibroblast proliferation. Other biomaterials, such as ADCON-L 60, 61, collagen 62, 63, seprafilm 64 and silk-polyethylene glycol gel 65, have also been applied to prevent skeletal fibrosis.
3.4. Other methods
Given the anti-fibrotic effect, traditional Chinese drugs have achieved positive effects in preventing post-operative fibrosis, including salvianolic acid B 66, angelica sinensis 67, safflower yellow 68 and daidzein 69. Non-steroidal anti-inflammatory drug (NSAIDs), including ketoprofen 70, aceclofenac 71, indomethacin 72 and ibuprofen 73, could also modify local inflammation response and prevent post-operative fibrosis.
To help alleviate the side effects of drugs, controlled release systems have been developed to reduce post-operative skeletal fibrosis, including MMC controlled-release films 74, 75, liposome-encapsulated HCPT 76 and gelatin combined with dexamethasone 77, decorin 78 and parecoxib 79. Moreover, other drugs and proteins are used to prevent post-skeletal fibrosis, including simvastatin 80, rosuvastatin 81, resveratrol 82 and all-trans retinoic acid 83, verapamil 84, 85, melatonin 86, taurine 87, citicoline 88, 89, pentoxifylline 90 and transduction with lentivirus carrying extracellular signal-regulated kinase 2 91-93. Additionally, oral application of pentoxifylline 94 and percutaneous lysis of epidural fibrosis 95 have achieved success in patients.
3.5. Disadvantages of current therapies for skeletal fibrosis
Despite some promising results from current approaches for the treatment of skeletal fibrosis, unexpected side effects still exist. For example, MMC could reduce epidural fibrosis in animal studies, but no clinically meaningful benefit was observed 36. In addition, as an anti-tumor drug, MMC might cause dose-related complications after topical application, such as in a previous report of ophthalmologic surgery including epithelial defects, delayed wound healing, corneal perforation, necrotizing keratitis and persistent hypotony maculopathy 18. Another case is that ADCON has been proven to prevent post-operative skeletal fibrosis in animal models, but failed to prevent peritendinous fibrosis in patients 96. Moreover, some unexpected adverse effects were found in the application of ADCON-L for patients, including back pain, spontaneous intracranial hypotension and subdural hematoma 97. In additional to these serious side effects, most of these therapies achieved success in animals but less success was obtained in patients, indicating of the limited benefits of these therapies clinically.
4. Current stem cell therapy for tissue fibrosis
More evidence indicates that stem cells play a significant role in on preventing fibrosis in many animal models, as well as achieving good clinical outcomes. In this section, the current literature on stem cells as a therapy for fibrotic diseases is summarized, including kidney, lung, cardiac, liver and skin fibrosis (Table.S2).
4.1. Kidney fibrosis
Kidney fibrosis commonly appearing interstitially is considered a feature of chronic kidney disease. In a recent study, stem cells were tested for the effect on kidney fibrosis models, including Col4α3−/− and Col4α5−/− 8, 98, 99, unilateral ureteral obstruction (UUO) 100-102, remnant kidney 103, 104, renal artery stenosis 105, 106, transplantation 107, unilateral ischemia-reperfusion (IR) injury 108 and nephrectomy IR cyclosporine 109. Evidence indicates that stem cells could reduce renal fibrosis through some potential mechanisms, including inhibition of the renin-angiotensin system 99, suppression of the inflammatory response 100, 102, 103, 105-108, activation of the paracrine system 104 and regeneration of podocytes 98. Interestingly, stem cells reducing renal fibrosis and promoting survival do not delay the progression of kidney failure 8.
4.2. Lung fibrosis
Lung fibrosis is a serious progressive lung disease which leads to respiratory failure. Bleomycin-induced lung fibrosis is used as a classic animal model for lung fibrosis research 7, 110-113. Other frequently used models include hyperoxia-induced 114, silica-induced 115 and irradiate-induced 116 lung fibrosis. Stem cells have proven to reduce lung fibrosis through suppressing T-cell function 112, inhibiting expression of IL-1β 113, IL-6 113, 114, TGF-β 110, 111, 113, 114, tumor necrosis factor alpha (TNF-α) 110, 113, 114 and alpha smooth muscle actin (α-SMA) 114, 115, but increase matrix metalloproteinase (MMP) expression 110. Moreover, tail vein injection of BMSCs transfected with FLK1 (VEGF receptor-2) reduced irradiation-induced fibrosis by promoting differentiation into functional lung cells 116.
4.3. Cardiac fibrosis
Cardiac fibrosis is defined as an over-deposition of collagen in the heart. Many causes may contribute to the fibrosis formation process, including hypertrophic cardiomyopathy, toxic insults and metabolic disturbances. The left anterior descending artery (LAD) fibrosis model is widely used for evaluation of stem cell treatment 10, 117-119. Increasing evidence indicates that stem cells have the ability to improve cardiac function by reducing cardiac fibrosis 10, 117-120. Interestingly, VEGF, a pro-angiogenic factor, which contributes to fibrosis formation, is upregulated after stem cell application 118, 119.
4.4. Liver fibrosis
Liver fibrosis is a common stage for secondary liver disease, including chronic hepatitis B, chronic hepatitis C and alcoholism. A CCL4-induced liver fibrosis model is often used for assessing the effect of stem cells on liver fibrosis 11, 121-126. Stem cells could act as an anti-fibrotic agent on liver fibrosis by decreasing collagen deposition 123, 124, inhibiting expression of VEGF 121 and TGF-β1 122, preventing the formation of epithelial to mesenchymal transition (EMT) 122 and increasing expression of MMPs 11, 124, 126. Encouraging reports have shown that human umbilical cord derived mesenchymal stem cells (uMSCs) could reduce hepatitis B virus (HBV)-induced liver fibrosis and promote liver function by inhibiting the serum laminin level and increasing hepatocyte growth factor (HGF) level in patients 127.
4.5. Skin fibrosis
Skin fibrosis is a pathological outcome of various injuries including wound injuries, exogenous-induced fibrosis and autoimmune skin diseases. There are three fibrosis models, including bleomycin-induced fibrosis 9, 128, radiation-induced fibrosis 129-131 and excisional-induced fibrosis 132, 133. These models are used to detect the effect of stem cells on skin fibrosis. Results have revealed that stem cells could reduce skin fibrosis and promote skin healing by decreasing collagen deposition 9, 133, suppressing macrophage and neutrophil infiltration 9, 130, inhibiting expression levels of TGF-β1 9, 128, 129, 133, IL-1 130, VEGF 128, α-SMA 9, 133 and TNF-α 130, 133 and increasing expression levels of MMPs 9 and IL-10 130.
5. Potential mechanisms underlying anti-fibrosis of stem cells
As summarized above, stem cells have shown an anti-fibrotic effect in animal models and patients (Figure 1). Therefore, stem cells could be potential therapies for post-operative skeletal fibrosis. In this section, we propose several potential mechanisms, which may contribute to forthcoming stem cell therapy of post-operative skeletal fibrosis (Figure 2).
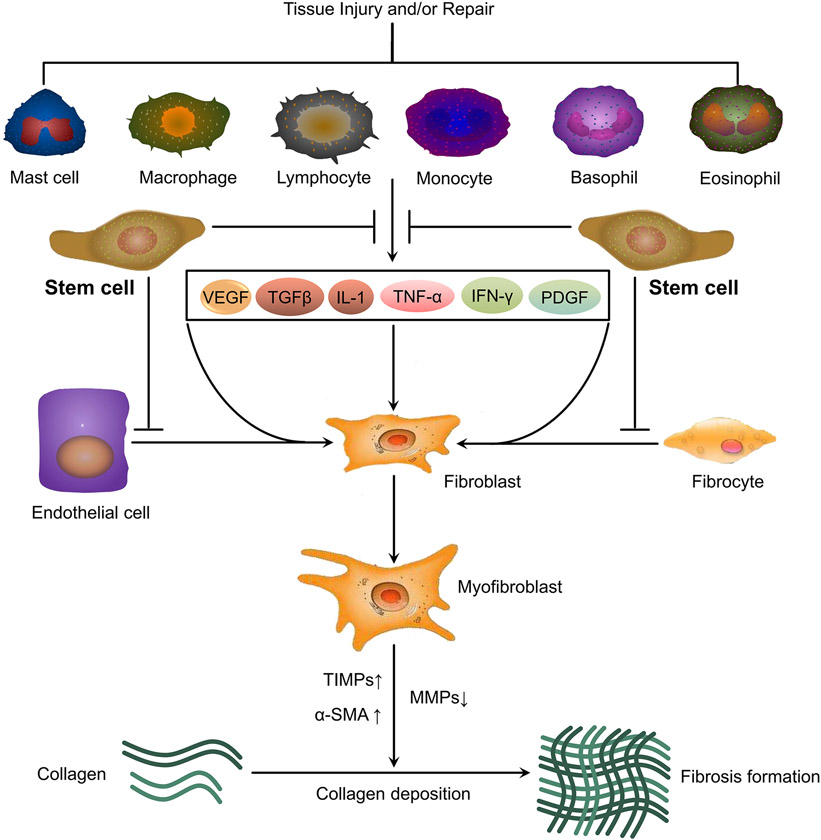
Figure 1.
Potential interference of stem cells on fibrosis formation. Cellular and molecular pathways include tissue injury and/or repair triggering secretion of cytokines (VEGF, TGF-β, IL-1, TNF-α, IFN-γ, PDGF) by mast cells, macrophages, lymphocytes, monocytes, basophils and eosinophils that lead to activation of fibroblasts via trans-differentiation of epithelial cells and fibrocytes and further into myofibroblasts. Cytokines and exosomes secreted by stem cells could inhibit fibroblast activation and other cell types to trans-differentiate into myofibroblasts, resulting in upregulation of MMPs and downregulation of TIMPs and α-SMA which leads to inhibition of fibrosis formation.
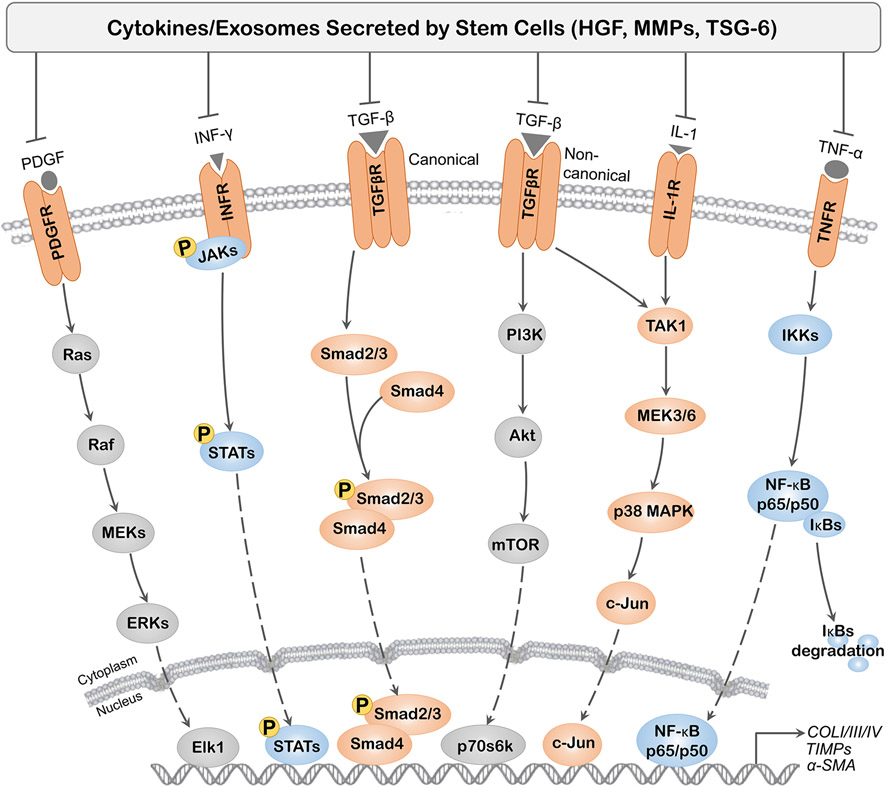
Figure 2.
Main cytokine pathways regulating tissue fibrosis. Cytokines (TGF-β, IL-1, TNF-α, IFN-γ, PDGF) secreted by inflammatory cells mediate the activation of several intracellular signaling pathways. As a major fibrogenic cytokine, TGF-β activates the canonical and noncanonical pathway resulting in induction of fibrogenic genes, such as collagen I/III/IV, TIMPs and α-SMA. Other cytokines, such as PDGF, IFN-γ, IL-1 and TNF-α, are also involved in regulating fibrosis formation primarily through their binding to specific receptors. Cytokines secreted by stem cells, including but not limited to HGF, IL-1Ra, MMPs and TSG-6, could inhibit profibrotic effect of fibrogenic cytokines.
Abbreviation: α-SMA: alpha-smooth muscle actin; HGF: hepatocyte growth factor; IFN-γ: interferon gamma; IGF-I: insulin-like growth factor I; IL-1: interleukin 1; IL-1Ra: interleukin 1 receptor antagonist; TSG-6: tumor necrosis factor-stimulated gene-6; MMP: matrix metalloproteinase; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor κB; PDGF: platelet-derived growth factor; PI3K: phosphoinositol 3-kinase; Ras: renal artery stenosis; TGF-β: transforming growth factor beta; TIMPs: tissue inhibitor of metalloproteinases; TNF-α: tumor necrosis factor alpha.
5.1. Suppression of inflammation response
Chronic inflammation is considered the major factor contributing to fibrosis. During the chronic inflammation period, newly generated lymphocytes and macrophages migrate to the target organ followed by the pathological fibrosis process triggered by activated myofibroblasts. Stem cell application could modify organ microenvironment, including promotion of anti-inflammatory cytokine secretion and suppression of pro-inflammatory cytokine secretion, to prevent fibrosis formation. In the fibrosis model, use of stem cells suppressed infiltration of T lymphocytes and neutrophils 9, 112 and macrophage function, including inhibiting pro-inflammatory CD68- and CD80-positive macrophages and increasing anti-inflammatory CD163-positive macrophages 9, 130.
TNF-α is a pro-inflammatory cytokine which plays a role in the fibrosis process. Numerous studies have shown that TNF-α expression, at both mRNA and protein levels, is decreased after application of stem cells 100, 103, 105, 110, 113, 114, 130, 133. In the same manner as TNF-α, interleukins, including IL-1α 130, IL-1β 113, 130 and IL-6 103, 107, 113, 114, are suppressed following stem cell application in several fibrosis models. As a cytotoxic factor, interferon gamma (IFN-γ) could also be diminished after stem cell application 110.
EMT is proven to be a central role for the relationship between inflammation and fibrosis formation 134. Previous reports showed that stem cells could reduce renal and liver fibrosis by inhibiting EMT 100, 108, 122. Vimentin, a marker of EMT, is proven to be involved in post-operative skeletal fibrosis and, when downregulated, fibrosis is prevented. Increasing evidence shows that vimentin is downregulated by stem cell application in fibrosis models 103, 122, indicative of suppression of inflammation by stem cells through inhibition of EMT.
5.2. Inhibition of the TGF-β1 pathway
TGF-β1 is considered the major profibrotic cytokine which contributes to fibrosis formation. TGF-β1 promotes profibrotic cell proliferation and induces extracellular matrix (ECM) formation 135. Moreover, TGF-β1 mRNA level was reported to be significantly higher in 28-day scar tissue 136 and in the tissue of the post-operative skeletal fibrosis model 25, 40, 66, 84. α-SMA, a myofibroblast marker, could be upregulated by TGF-β1. Downregulation of the α-SMA expression level was found in a fibrosis model treated by stem cells 9, 114, 115, 124, 126, 133, suggesting that stem cells can inhibit proliferation of myofibroblasts. Furthermore, inhibition of the TGF-β pathway has proven to be responsible for the effect of stem cells on fibrotic diseases 103, 110, 113, 128, 129. A similar effect has also been found in transplanted exosomes isolated from stem cell-conditioned medium 122. Stem cell-conditioned medium from in vitro studies could suppress fibroblast differentiation and activity by inhibiting the TGF-β/Smad pathway 137, 138.
HGF secreted by stem cells was reported to inhibit fibroblast proliferation and reduce collagen deposition 117, 120, 127. Moreover, stem cells overexpressing HGF exhibited a better anti-fibrotic effect than non-transfected cells in the bleomycin-induced lung fibrosis model 139. Further studies indicate that HGF could inhibit TGF-β1 expression and promote fibrosis scar remodeling by increasing MMP-1 expression 140.
Tumor necrosis factor-stimulated gene-6 (TSG-6) secreted from stem cells functions as a TNF-α inhibitor. Recent reports indicate that stem cells could prevent peritoneal fibrosis by secreting TSG-6; the same effect was found in reducing wound fibrosis and renal fibrosis by inhibiting TGF-β1 expression 133, 141, 142.
5.3. Promotion of tissue remodeling
ECM has proven to play a critical role in fibrosis formation and to possess active TGF-β1 ability. Therefore, inhibiting ECM formation and inducing degradation are possible mechanisms to prevent fibrosis. Insufficiency of MMPs, the major regulator of ECM degradation, or an imbalance of MMPs/TIMPs (tissue inhibitor of metalloproteinases) fails to promote ECM degradation. In previous studies, stem cells have proven to reduce fibrosis by inducing degradation of ECM components, such as hydroxyproline content and collagen deposition 7, 9, 110, 115, 120, 122, 124 as well as laminin and fibronectin 107, 127. Exosomes and microvesicles released from transplanted stem cells also possess the ability to degenerate ECM 115, 122.
In fibrosis model studies, expression levels of MMP-2, MMP-9 and MMP-13 increased following stem cell application 9, 11, 117, 124. MMP-2 and MMP-9 are gelatinases that have high activity against gelatin, the main component of ECM. Intriguingly, some studies found that expression levels of MMP-2 and MMP-9 were downregulated after stem cell application according to some in vivo studies 7, 120. TIMPs, including TIMP-1, TIMP-2, TIMP-3 and TIMP-4, are suppressed by application of stem cells 110. Suppression of TIMP2 by stem cell-conditioned medium also suggests that stem cells possess a paracrine effect 117. The TIMP/MMP ratio was downregulated by application of stem cells indicating that there was a balance between degradation and synthesis of ECM 103.
5.4. Other potential mechanisms
In addition to the above mentioned mechanisms, others include the p38/MAPK pathway 132, regulation of miR-let7c 101 and the ER stress pathway 106. In particular, ER stress has been proven to be linked to post-operative skeletal fibrosis 30, 44, 143. Induction of fibroblast apoptosis is considered a mechanism of preventing post-operative skeletal fibrosis. Interestingly, the anti-apoptotic effect of stem cells on fibrosis prevention was found in several fibrosis models, possibly suggesting that application of stem cells could modify the microenvironment to protect resident cells from damage and promote recovery 98, 103. Furthermore, VEGF is considered a profibrotic factor for fibrosis; expression of VEGF is inhibited by stem cell application in some fibrosis models 121, 128. However, other reports found that VEGF expression increased after stem cell application in fibrosis models 105, 118, 119, indicating that stem cells could reduce fibrosis by promoting angiogenesis and vascular stability.
6. Conclusion and Perspective
Post-operative skeletal fibrosis contributes to poor clinical outcomes and current therapeutic strategies have provided unsatisfactory results in reducing clinical post-operative skeletal fibrosis. In this review, we assess current therapies for post-operative skeletal fibrosis and discuss the effect and mechanisms of stem cells on preventing fibrotic diseases in the kidney, lung, heart, liver and skin. Most current therapies for post-operative skeletal fibrosis can inhibit fibroblast proliferation to a certain degree but interfere with the physiological function of surrounding tissues. Fortunately, increasing evidence indicates that stem cells can contribute not only to a local tissue regeneration but also to modulation of a regional microenvironment when stem cells are applied as a therapy for anti-fibrosis. Therefore, we hypothesize that stem cells might be a better choice for reducing post-operative skeletal fibrosis. More experiments should be undertaken to help us gain a better understanding of the effectiveness of stem cells as a therapy for post-operative skeletal fibrosis, including but not limited to stem cell source, amount, delivery approach and treatment timing.
Supplementary Material
Acknowledgement
We thank Suzanne Danley for editing the manuscript. This work was supported by Research Grants from the Musculoskeletal Transplant Foundation (MTF) and the National Institutes of Health (1R01AR067747) to M.P., the National Natural Science Foundation of China (NO.81772332) and Jiangsu Provincial Medical Innovation Team (CXTDB2017004) to J.W., the National Natural Science Foundation of China (NO.81501870) and Jiangsu Province Youth Medical Talents (QNRC2016343) to X.L., and the National Natural Science Foundation of China (81601889) to S.C.
Footnotes
Disclosure statement
No completing financial interest exist
References
- 1.Rockey DC, Bell PD, Hill JA. 2015. Fibrosis--a common pathway to organ injury and failure. The New England journal of medicine 372:1138–1149. [DOI] [PubMed] [Google Scholar]
- 2.Neary R, Watson CJ, Baugh JA. 2015. Epigenetics and the overhealing wound: the role of DNA methylation in fibrosis. Fibrogenesis & tissue repair 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemos DR, Babaeijandaghi F, Low M, et al. 2015. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nature medicine 21:786–794. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Yan L, Wang J, et al. 2013. Comparison of the effects of mitomycin C and 10-hydroxycamptothecin on an experimental intraarticular adhesion model in rabbits. European journal of pharmacology 703:42–45. [DOI] [PubMed] [Google Scholar]
- 5.Zheng W, Qian Y, Chen S, et al. 2018. Rapamycin Protects Against Peritendinous Fibrosis Through Activation of Autophagy. Frontiers in pharmacology 9:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segers VF, Lee RT. 2008. Stem-cell therapy for cardiac disease. Nature 451:937–942. [DOI] [PubMed] [Google Scholar]
- 7.Ortiz LA, Gambelli F, McBride C, et al. 2003. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences of the United States of America 100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ninichuk V, Gross O, Segerer S, et al. 2006. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney international 70:121–129. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Huang S, Enhe J, et al. 2014. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. International wound journal 11:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M, Wang Y, Wani MA, et al. 2003. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. Journal of molecular and cellular cardiology 35:1113–1119. [DOI] [PubMed] [Google Scholar]
- 11.Sakaida I, Terai S, Yamamoto N, et al. 2004. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology 40:1304–1311. [DOI] [PubMed] [Google Scholar]
- 12.Mueller AA, van Velthoven CT, Fukumoto KD, et al. 2016. Intronic polyadenylation of PDGFRalpha in resident stem cells attenuates muscle fibrosis. Nature 540:276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Chen Y, Yue Y, et al. 2012. Reconstruction of epidural fat with engineered adipose tissue from adipose derived stem cells and PLGA in the rabbit dorsal laminectomy model. Biomaterials 33:6965–6973. [DOI] [PubMed] [Google Scholar]
- 14.Caplan AI. 2017. Mesenchymal Stem Cells: Time to Change the Name! Stem cells translational medicine 6:1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomasek JJ, Gabbiani G, Hinz B, et al. 2002. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature reviews Molecular cell biology 3:349–363. [DOI] [PubMed] [Google Scholar]
- 16.Wynn TA. 2008. Cellular and molecular mechanisms of fibrosis. The Journal of pathology 214:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn TA. 2007. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. The Journal of clinical investigation 117:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yildiz KH, Gezen F, Is M, et al. 2007. Mitomycin C, 5-fluorouracil, and cyclosporin A prevent epidural fibrosis in an experimental laminectomy model. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 16:1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JY, Stenzel W, Lohr M, et al. 2006. The role of mitomycin C in reducing recurrence of epidural fibrosis after repeated operation in a laminectomy model in rats. Journal of neurosurgery Spine 4:329–333. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Ge Y, Fu Y, et al. 2015. Mitomycin C induces fibroblasts apoptosis and reduces epidural fibrosis by regulating miR-200b and its targeting of RhoE. European journal of pharmacology 765:198–208. [DOI] [PubMed] [Google Scholar]
- 21.Su C, Yao C, Lu S, et al. 2010. Study on the optimal concentration of topical mitomycin-C in preventing postlaminectomy epidural adhesion. European journal of pharmacology 640:63–67. [DOI] [PubMed] [Google Scholar]
- 22.Kocaoglu B, Akgun U, Nalbantoglu U, et al. 2011. Adhesion reduction after knee surgery in a rat model by mitomycin C. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA 19:94–98. [DOI] [PubMed] [Google Scholar]
- 23.Yan L, Sun Y, Wang J, et al. 2010. The effect of mitomycin C in reducing intraarticular adhesion after knee surgery in rabbits. European journal of pharmacology 643:1–5. [DOI] [PubMed] [Google Scholar]
- 24.Kocaoglu B, Agir I, Nalbantoglu U, et al. 2010. Effect of mitomycin-C on post-operative adhesions in tendon surgery: an experimental study in rats. The Journal of bone and joint surgery British volume 92:889–893. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Wang L, Sun S, et al. 2008. The effect of 10-hydroxycamptothecine in preventing fibroblast proliferation and epidural scar adhesion after laminectomy in rats. European journal of pharmacology 593:44–48. [DOI] [PubMed] [Google Scholar]
- 26.Dai J, Sun Y, Yan L, et al. 2017. Upregulation of NOXA by 10-Hydroxycamptothecin plays a key role in inducing fibroblasts apoptosis and reducing epidural fibrosis. PeerJ 5:e2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan L, Sun Y, Li X, et al. 2015. The Effect of Hydroxycamptothecin on Wound Healing Following Reduction of the Knee Intra-Articular Adhesion in Rabbits. Cell biochemistry and biophysics 73:221–227. [DOI] [PubMed] [Google Scholar]
- 28.Liang Y, Sun Y, Li X, et al. 2014. The optimal concentration of topical hydroxycamptothecin in preventing intraarticular scar adhesion. Scientific reports 4:4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Sun Y, Chen H, et al. 2016. Hydroxycamptothecin induces apoptosis of fibroblasts and prevents intraarticular scar adhesion in rabbits by activating the IRE-1 signal pathway. European journal of pharmacology 781:139–147. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Chen H, Sun Y, et al. 2017. Hydroxycamptothecin prevents intraarticular scar adhesion by activating the PERK signal pathway. European journal of pharmacology 810:36–43. [DOI] [PubMed] [Google Scholar]
- 31.Ozdemir O, Calisaneller T, Sonmez E, et al. 2010. Topical use of colchicine to prevent spinal epidural fibrosis in rats. Neurological research 32:1117–1120. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Liang Y, Hu J, et al. 2014. Reduction of intraarticular adhesion by topical application of colchicine following knee surgery in rabbits. Scientific reports 4:6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duci SB, Arifi HM, Ahmeti HR, et al. 2015. Biomechanical and Macroscopic Evaluations of the Effects of 5-Fluorouracil on Partially Divided Flexor Tendon Injuries in Rabbits. Chinese medical journal 128:1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozkan U, Osun A, Samancioglu A, et al. 2014. The effect of bevacizumab and 5-Fluorouracil combination on epidural fibrosis in a rat laminectomy model. European review for medical and pharmacological sciences 18:95–100. [PubMed] [Google Scholar]
- 35.Sun Y, Wang LX, Wang L, et al. 2007. A comparison of the effectiveness of mitomycin C and 5-fluorouracil in the prevention of peridural adhesion after laminectomy. Journal of neurosurgery Spine 7:423–428. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Sui T, Hong X, et al. 2013. Inhibition of epidural fibrosis after microendoscopic discectomy with topical application of mitomycin C: a randomized, controlled, double-blind trial. Journal of neurosurgery Spine 18:421–427. [DOI] [PubMed] [Google Scholar]
- 37.Zhao S, Sun Y, Li X, et al. 2016. Reduction of intraarticular adhesion of knee by local application of rapamycin in rabbits via inhibition of fibroblast proliferation and collagen synthesis. Journal of orthopaedic surgery and research 11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Zhao S, Li X, et al. 2016. Local application of rapamycin reduces epidural fibrosis after laminectomy via inhibiting fibroblast proliferation and prompting apoptosis. Journal of orthopaedic surgery and research 11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismailoglu O, Albayrak B, Gulsen I, et al. 2011. Topical application of tacrolimus prevents epidural fibrosis in a rat postlaminectomy model: histopathological and ultrastructural analysis. Turkish neurosurgery 21:630–633. [PubMed] [Google Scholar]
- 40.Yan L, Li X, Wang J, et al. 2013. Immunomodulatory effectiveness of tacrolimus in preventing epidural scar adhesion after laminectomy in rat model. European journal of pharmacology 699:194–199. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Chen H, Wang S, et al. 2017. Tacrolimus induces fibroblasts apoptosis and reduces epidural fibrosis by regulating miR-429 and its target of RhoE. Biochemical and biophysical research communications 490:1197–1204. [DOI] [PubMed] [Google Scholar]
- 42.Cemil B, Tun K, Kaptanoglu E, et al. 2009. Use of pimecrolimus to prevent epidural fibrosis in a postlaminectomy rat model. Journal of neurosurgery Spine 11:758–763. [DOI] [PubMed] [Google Scholar]
- 43.Emmez H, Borcek AO, Durdag E, et al. 2011. Immunomodulatory effectiveness of azithromycin in prevention of postlaminectomy epidural fibrosis. Neurological research 33:344–348. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, Yan L, Wang J, et al. 2017. Methotrexate prevents epidural fibrosis through endoplasmic reticulum stress signalling pathway. European journal of pharmacology 796:131–138. [DOI] [PubMed] [Google Scholar]
- 45.Isik S, Taskapilioglu MO, Atalay FO, et al. 2015. Effects of cross-linked high-molecular-weight hyaluronic acid on epidural fibrosis: experimental study. Journal of neurosurgery Spine 22:94–100. [DOI] [PubMed] [Google Scholar]
- 46.Wu CY, Huang YH, Lee JS, et al. 2016. Efficacy of topical cross-linked hyaluronic acid hydrogel in preventing post laminectomy/laminotomy fibrosis in a rat model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 34:299–306. [DOI] [PubMed] [Google Scholar]
- 47.Chen JM, Lee SH, Tsai TT, et al. 2014. Anti-adhesive effect of hyaluronate in a rabbit laminectomy model. Biomedical journal 37:218–224. [DOI] [PubMed] [Google Scholar]
- 48.Gaughan EM, Nixon AJ, Krook LP, et al. 1991. Effects of sodium hyaluronate on tendon healing and adhesion formation in horses. American journal of veterinary research 52:764–773. [PubMed] [Google Scholar]
- 49.Isik S, Ozturk S, Gurses S, et al. 1999. Prevention of restrictive adhesions in primary tendon repair by HA-membrane: experimental research in chickens. British journal of plastic surgery 52:373–379. [DOI] [PubMed] [Google Scholar]
- 50.Brunelli G, Longinotti C, Bertazzo C, et al. 2005. Adhesion reduction after knee surgery in a rabbit model by Hyaloglide, a hyaluronan derivative gel. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 23:1377–1382. [DOI] [PubMed] [Google Scholar]
- 51.Rajiv S, Drilling A, Bassiouni A, et al. 2017. Chitosan Dextran gel as an anti adhesion agent in a postlaminectomy spinal sheep model. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 40:153–156. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Yan L, Sun Y, et al. 2012. A comparative study of the preventive effects of mitomycin C and chitosan on intraarticular adhesion after knee surgery in rabbits. Cell biochemistry and biophysics 62:101–105. [DOI] [PubMed] [Google Scholar]
- 53.Chen SH, Chen CH, Fong YT, et al. 2014. Prevention of peritendinous adhesions with electrospun chitosan-grafted polycaprolactone nanofibrous membranes. Acta biomaterialia 10:4971–4982. [DOI] [PubMed] [Google Scholar]
- 54.Choi HJ, Kim KB, Kwon YM. 2011. Effect of amniotic membrane to reduce postlaminectomy epidural adhesion on a rat model. Journal of Korean Neurosurgical Society 49:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao H, Fan H. 2009. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 18:1202–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozgenel GY, Samli B, Ozcan M. 2001. Effects of human amniotic fluid on peritendinous adhesion formation and tendon healing after flexor tendon surgery in rabbits. The Journal of hand surgery 26:332–339. [DOI] [PubMed] [Google Scholar]
- 57.Demirkan F, Colakoglu N, Herek O, et al. 2002. The use of amniotic membrane in flexor tendon repair: an experimental model. Archives of orthopaedic and trauma surgery 122:396–399. [DOI] [PubMed] [Google Scholar]
- 58.Ozgenel GY, Etoz A. 2012. Effects of repetitive injections of hyaluronic acid on peritendinous adhesions after flexor tendon repair: a preliminary randomized, placebo-controlled clinical trial. Ulusal travma ve acil cerrahi dergisi = Turkish journal of trauma & emergency surgery : TJTES 18:11–17. [DOI] [PubMed] [Google Scholar]
- 59.Riccio M, Battiston B, Pajardi G, et al. 2010. Efficiency of Hyaloglide in the prevention of the recurrence of adhesions after tenolysis of flexor tendons in zone II: a randomized, controlled, multicentre clinical trial. The Journal of hand surgery, European volume 35:130–138. [DOI] [PubMed] [Google Scholar]
- 60.Einhaus SL, Robertson JT, Dohan FC Jr., et al. 1997. Reduction of peridural fibrosis after lumbar laminotomy and discectomy in dogs by a resorbable gel (ADCON-L). Spine 22:1440–1446; discussion 1446-1447. [DOI] [PubMed] [Google Scholar]
- 61.Petrie JL, Ross JS. 1996. Use of ADCON-L to inhibit postoperative peridural fibrosis and related symptoms following lumbar disc surgery: a preliminary report. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 5 Suppl 1:S10–17. [DOI] [PubMed] [Google Scholar]
- 62.Tatsui CE, Martinez G, Li X, et al. 2006. Evaluation of DuraGen in preventing peridural fibrosis in rabbits. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2005. Journal of neurosurgery Spine 4:51–59. [DOI] [PubMed] [Google Scholar]
- 63.Zhao H, Guan HG, Gu J, et al. 2013. Collagen membrane alleviates peritendinous adhesion in the rat Achilles tendon injury model. Chinese medical journal 126:729–733. [PubMed] [Google Scholar]
- 64.Yilmaz E, Avci M, Bulut M, et al. 2010. The effect of seprafilm on adhesion formation and tendon healing after flexor tendon repair in chicken. Orthopedics 33:164–170. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Liang M, Zheng Z, et al. 2015. Adhesion Prevention after Laminectomy Using Silk-Polyethylene Glycol Hydrogels. Advanced healthcare materials 4:2120–2127. [DOI] [PubMed] [Google Scholar]
- 66.Chen F, Zuo Z, Wang K, et al. 2014. Study on salvianolic acid B in the reduction of epidural fibrosis in laminectomy rats. BMC musculoskeletal disorders 15:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C, Kong X, Zhou H, et al. 2013. An Experimental Novel Study: Angelica sinensis Prevents Epidural Fibrosis in Laminectomy Rats via Downregulation of Hydroxyproline, IL-6, and TGF- beta 1. Evidence-based complementary and alternative medicine : eCAM 2013:291814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu B, Luo C, Ouyang L, et al. 2011. An experimental study on the effect of safflower yellow on tendon injury-repair in chickens. The Journal of surgical research 169:e175–184. [DOI] [PubMed] [Google Scholar]
- 69.Liu X, Jia H, Xia H. 2017. Reduction Of Intra-Articular Adhesion by Topical Application Of Daidzein Following Knee Surgery In Rabbits. African journal of traditional, complementary, and alternative medicines : AJTCAM 14:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He Y, Revel M, Loty B. 1995. A quantitative model of post-laminectomy scar formation. Effects of a nonsteroidal anti-inflammatory drug. Spine 20:557–563; discussion 579-580. [DOI] [PubMed] [Google Scholar]
- 71.Sandoval MA, Hernandez-Vaquero D. 2008. Preventing peridural fibrosis with nonsteroidal anti-inflammatory drugs. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 17:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Szabo RM, Younger E. 1990. Effects of indomethacin on adhesion formation after repair of zone II tendon lacerations in the rabbit. The Journal of hand surgery 15:480–483. [DOI] [PubMed] [Google Scholar]
- 73.Liu S, Pan G, Liu G, et al. 2017. Electrospun fibrous membranes featuring sustained release of ibuprofen reduce adhesion and improve neurological function following lumbar laminectomy. Journal of controlled release : official journal of the Controlled Release Society 264:1–13. [DOI] [PubMed] [Google Scholar]
- 74.Wang BB, Xie H, Wu T, et al. 2017. Controlled-release mitomycin C-polylactic acid film prevents epidural scar hyperplasia after laminectomy by inducing fibroblast autophagy and regulating the expression of miRNAs. European review for medical and pharmacological sciences 21:2526–2537. [PubMed] [Google Scholar]
- 75.Liu J, Ni B, Zhu L, et al. 2010. Mitomycin C-polyethylene glycol controlled-release film inhibits collagen secretion and induces apoptosis of fibroblasts in the early wound of a postlaminectomy rat model. The spine journal : official journal of the North American Spine Society 10:441–447. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Ni B, Liu J, et al. 2011. Application of liposome-encapsulated hydroxycamptothecin in the prevention of epidural scar formation in New Zealand white rabbits. The spine journal : official journal of the North American Spine Society 11:218–223. [DOI] [PubMed] [Google Scholar]
- 77.Tian F, Dou C, Qi S, et al. 2015. Preventive effect of dexamethasone gelatin sponge on the lumbosacral epidural adhesion. International journal of clinical and experimental medicine 8:5478–5484. [PMC free article] [PubMed] [Google Scholar]
- 78.Turkoglu E, Dinc C, Tuncer C, et al. 2014. Use of decorin to prevent epidural fibrosis in a post-laminectomy rat model. European journal of pharmacology 724:86–91. [DOI] [PubMed] [Google Scholar]
- 79.Sae-Jung S, Jirarattanaphochai K. 2013. Prevention of peridural fibrosis using a cyclooxygenase-2 inhibitor (nonsteroidal anti-inflammatory drug) soaked in absorbable gelatin sponge: an experimental comparative animal model. Spine 38:E985–991. [DOI] [PubMed] [Google Scholar]
- 80.Sun Y, Yan LQ, Liang Y, et al. 2015. Reduction of epidural scar adhesion by topical application of simvastatin after laminectomy in rats. European review for medical and pharmacological sciences 19:3–8. [PubMed] [Google Scholar]
- 81.Gurer B, Kahveci R, Gokce EC, et al. 2015. Evaluation of topical application and systemic administration of rosuvastatin in preventing epidural fibrosis in rats. The spine journal : official journal of the North American Spine Society 15:522–529. [DOI] [PubMed] [Google Scholar]
- 82.Sun P, Miao B, Xin H, et al. 2014. The effect of resveratrol on surgery-induced epidural fibrosis in laminectomy rats. Evidence-based complementary and alternative medicine : eCAM 2014:574236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang C, Kong X, Ning G, et al. 2014. All-trans retinoic acid prevents epidural fibrosis through NF-kappaB signaling pathway in post-laminectomy rats. Neuropharmacology 79:275–281. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z, Wang Y, Xie P, et al. 2014. Calcium channel blockers in reduction of epidural fibrosis and dural adhesions in laminectomy rats. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie 24 Suppl 1:S293–298. [DOI] [PubMed] [Google Scholar]
- 85.Li Y, Ma X, Yu P, et al. 2014. Intra-articular adhesion reduction after knee surgery in rabbits by calcium channel blockers. Medical science monitor : international medical journal of experimental and clinical research 20:2466–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erol FS, Kavakli A, Ilhan N, et al. 2010. Efects of melatonin and octreotide on peridural fibrosis in an animal model of laminectomy. Turkish neurosurgery 20:50–56. [PubMed] [Google Scholar]
- 87.Yang L, Tang J, Chen H, et al. 2016. Taurine Reduced Epidural Fibrosis in Rat Models after Laminectomy via Downregulating EGR1. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 38:2261–2271. [DOI] [PubMed] [Google Scholar]
- 88.Ozay R, Bekar A, Kocaeli H, et al. 2007. Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scarring after peripheral nerve surgery in rats. Surgical neurology 68:615–622; discussion 622. [DOI] [PubMed] [Google Scholar]
- 89.Savran M, Bekar A, Cansev M, et al. 2012. Prevention of epidural fibrosis in rats by local or systemic administration of citicoline. Turkish neurosurgery 22:634–640. [DOI] [PubMed] [Google Scholar]
- 90.Kelten B, Erdogan H, Antar V, et al. 2016. Pentoxifylline Inhibits Epidural Fibrosis in Post-Laminectomy Rats. Medical science monitor : international medical journal of experimental and clinical research 22:840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li F, Ruan H, Fan C, et al. 2010. Efficient inhibition of the formation of joint adhesions by ERK2 small interfering RNAs. Biochemical and biophysical research communications 391:795–799. [DOI] [PubMed] [Google Scholar]
- 92.Zhang C, Kong X, Liu C, et al. 2014. ERK2 small interfering RNAs prevent epidural fibrosis via the efficient inhibition of collagen expression and inflammation in laminectomy rats. Biochemical and biophysical research communications 444:395–400. [DOI] [PubMed] [Google Scholar]
- 93.Li F, Liu S, Fan C. 2013. Lentivirus-mediated ERK2 siRNA reduces joint capsule fibrosis in a rat model of post-traumatic joint contracture. International journal of molecular sciences 14:20833–20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Georges C, Lefaix JL, Delanian S. 2004. Case report: resolution of symptomatic epidural fibrosis following treatment with combined pentoxifylline-tocopherol. The British journal of radiology 77:885–887. [DOI] [PubMed] [Google Scholar]
- 95.Chun-jing H, Hao-xiong N, jia-xiang N. 2012. The application of percutaneous lysis of epidural adhesions in patients with failed back surgery syndrome. Acta cirurgica brasileira 27:357–362. [DOI] [PubMed] [Google Scholar]
- 96.Golash A, Kay A, Warner JG, et al. 2003. Efficacy of ADCON-T/N after primary flexor tendon repair in Zone II: a controlled clinical trial. Journal of hand surgery 28:113–115. [DOI] [PubMed] [Google Scholar]
- 97.Kim SB, Lim YJ. 2010. Delayed Detected Unexpected Complication of ADCON-L(R) Gel in Lumbar Surgery. Journal of Korean Neurosurgical Society 48:268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prodromidi EI, Poulsom R, Jeffery R, et al. 2006. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem cells 24:2448–2455. [DOI] [PubMed] [Google Scholar]
- 99.Sedrakyan S, Da Sacco S, Milanesi A, et al. 2012. Injection of amniotic fluid stem cells delays progression of renal fibrosis. Journal of the American Society of Nephrology : JASN; 23:661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Asanuma H, Vanderbrink BA, Campbell MT, et al. 2011. Arterially delivered mesenchymal stem cells prevent obstruction-induced renal fibrosis. The Journal of surgical research 168:e51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang B, Yao K, Huuskes BM, et al. 2016. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Molecular therapy : the journal of the American Society of Gene Therapy 24:1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huuskes BM, Wise AF, Cox AJ, et al. 2015. Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fibrosis in obstructive nephropathy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 29:540–553. [DOI] [PubMed] [Google Scholar]
- 103.Semedo P, Correa-Costa M, Antonio Cenedeze M, et al. 2009. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem cells 27:3063–3073. [DOI] [PubMed] [Google Scholar]
- 104.He J, Wang Y, Sun S, et al. 2012. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology 17:493–500. [DOI] [PubMed] [Google Scholar]
- 105.Ebrahimi B, Eirin A, Li Z, et al. 2013. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PloS one 8:e67474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu XY, Urbieta-Caceres V, Krier JD, et al. 2013. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem cells 31:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Franquesa M, Herrero E, Torras J, et al. 2012. Mesenchymal stem cell therapy prevents interstitial fibrosis and tubular atrophy in a rat kidney allograft model. Stem cells and development 21:3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Du T, Zou X, Cheng J, et al. 2013. Human Wharton's jelly-derived mesenchymal stromal cells reduce renal fibrosis through induction of native and foreign hepatocyte growth factor synthesis in injured tubular epithelial cells. Stem cell research & therapy 4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alfarano C, Roubeix C, Chaaya R, et al. 2012. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell transplantation 21:2009–2019. [DOI] [PubMed] [Google Scholar]
- 110.Moodley Y, Atienza D, Manuelpillai U, et al. 2009. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. The American journal of pathology 175:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao F, Zhang YF, Liu YG, et al. 2008. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplantation proceedings 40:1700–1705. [DOI] [PubMed] [Google Scholar]
- 112.Jun D, Garat C, West J, et al. 2011. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem cells 29:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee SH, Jang AS, Kim YE, et al. 2010. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respiratory research 11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang YS, Oh W, Choi SJ, et al. 2009. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell transplantation 18:869–886. [DOI] [PubMed] [Google Scholar]
- 115.Choi M, Ban T, Rhim T. 2014. Therapeutic use of stem cell transplantation for cell replacement or cytoprotective effect of microvesicle released from mesenchymal stem cell. Molecules and cells 37:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yan X, Liu Y, Han Q, et al. 2007. Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Experimental hematology 35:1466–1475. [DOI] [PubMed] [Google Scholar]
- 117.Mias C, Lairez O, Trouche E, et al. 2009. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem cells 27:2734–2743. [DOI] [PubMed] [Google Scholar]
- 118.Das H, George JC, Joseph M, et al. 2009. Stem cell therapy with overexpressed VEGF and PDGF genes improves cardiac function in a rat infarct model. PloS one 4:e7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chacko SM, Khan M, Kuppusamy ML, et al. 2009. Myocardial oxygenation and functional recovery in infarct rat hearts transplanted with mesenchymal stem cells. American journal of physiology Heart and circulatory physiology 296:H1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li L, Zhang Y, Li Y, et al. 2008. Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transplant international : official journal of the European Society for Organ Transplantation 21:1181–1189. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Lian F, Li J, et al. 2012. Adipose derived mesenchymal stem cells transplantation via portal vein improves microcirculation and ameliorates liver fibrosis induced by CCl4 in rats. Journal of translational medicine 10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li T, Yan Y, Wang B, et al. 2013. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem cells and development 22:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abdel Aziz MT, Atta HM, Mahfouz S, et al. 2007. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clinical biochemistry 40:893–899. [DOI] [PubMed] [Google Scholar]
- 124.Rabani V, Shahsavani M, Gharavi M, et al. 2010. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell biology international 34:601–605. [DOI] [PubMed] [Google Scholar]
- 125.Chang YJ, Liu JW, Lin PC, et al. 2009. Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life sciences 85:517–525. [DOI] [PubMed] [Google Scholar]
- 126.Meier RP, Mahou R, Morel P, et al. 2015. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. Journal of hepatology 62:634–641. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Z, Lin H, Shi M, et al. 2012. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. Journal of gastroenterology and hepatology 27 Suppl 2:112–120. [DOI] [PubMed] [Google Scholar]
- 128.Chen W, Xia ZK, Zhang MH, et al. 2017. Adipose tissue-derived stem cells ameliorates dermal fibrosis in a mouse model of scleroderma. Asian Pacific journal of tropical medicine 10:52–56. [DOI] [PubMed] [Google Scholar]
- 129.Zheng K, Wu W, Yang S, et al. 2015. Bone marrow mesenchymal stem cell implantation for the treatment of radioactivityinduced acute skin damage in rats. Molecular medicine reports 12:7065–7071. [DOI] [PubMed] [Google Scholar]
- 130.Horton JA, Hudak KE, Chung EJ, et al. 2013. Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem cells 31:2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Francois S, Mouiseddine M, Mathieu N, et al. 2007. Human mesenchymal stem cells favour healing of the cutaneous radiation syndrome in a xenogenic transplant model. Annals of hematology 86:1–8. [DOI] [PubMed] [Google Scholar]
- 132.Li Y, Zhang W, Gao J, et al. 2016. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem cell research & therapy 7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qi Y, Jiang D, Sindrilaru A, et al. 2014. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. The Journal of investigative dermatology 134:526–537. [DOI] [PubMed] [Google Scholar]
- 134.Zavadil J, Bottinger EP. 2005. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24:5764–5774. [DOI] [PubMed] [Google Scholar]
- 135.Klingberg F, Chow ML, Koehler A, et al. 2014. Prestress in the extracellular matrix sensitizes latent TGF-beta1 for activation. The Journal of cell biology 207:283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kryger ZB, Sisco M, Roy NK, et al. 2007. Temporal expression of the transforming growth factor-Beta pathway in the rabbit ear model of wound healing and scarring. Journal of the American College of Surgeons 205:78–88. [DOI] [PubMed] [Google Scholar]
- 137.Zhou Y, Zhang Q, Gao Y, et al. 2018. Induced pluripotent stem cell-conditioned medium suppresses pulmonary fibroblast-to-myofibroblast differentiation via the inhibition of TGF-beta1/Smad pathway. International journal of molecular medicine 41:473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang X, Ma Y, Gao Z, et al. 2018. Human adipose-derived stem cells inhibit bioactivity of keloid fibroblasts. Stem cell research & therapy 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gazdhar A, Susuri N, Hostettler K, et al. 2013. HGF Expressing Stem Cells in Usual Interstitial Pneumonia Originate from the Bone Marrow and Are Antifibrotic. PloS one 8:e65453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taniyama Y, Morishita R, Aoki M, et al. 2002. Angiogenesis and antifibrotic action by hepatocyte growth factor in cardiomyopathy. Hypertension 40:47–53. [DOI] [PubMed] [Google Scholar]
- 141.Wang N, Li Q, Zhang L, et al. 2012. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PloS one 7:e43768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu HJ, Yiu WH, Li RX, et al. 2014. Mesenchymal stem cells modulate albumin-induced renal tubular inflammation and fibrosis. PloS one 9:e90883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li X, Wang S, Dai J, et al. 2017. Homoharringtonine prevents surgery-induced epidural fibrosis through endoplasmic reticulum stress signaling pathway. European journal of pharmacology 815:437–445. [DOI] [PubMed] [Google Scholar]
- 144.Richards PJ, Turner AS, Gisler SM, et al. 2010. Reduction in postlaminectomy epidural adhesions in sheep using a fibrin sealant-based medicated adhesion barrier. Journal of biomedical materials research Part B, Applied biomaterials 92:439–446. [DOI] [PubMed] [Google Scholar]
- 145.Ross JS, Obuchowski N, Modic MT. 1999. MR evaluation of epidural fibrosis: proposed grading system with intra- and inter-observer variability. Neurological research 21 Suppl 1:S23–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.