Abstract
Recombinant sucrose-6-phosphate synthase (SpsA) was synthesized in Escherichia coli BL21DE3 by using the spsA gene of the cyanobacterium Synechocystis sp. strain PCC 6803. Transformants exhibited a 10,000-fold increase in survival compared to wild-type cells following either freeze-drying, air drying, or desiccation over phosphorus pentoxide. The phase transition temperatures and vibration frequencies (P⩵O stretch) in phospholipids suggested that sucrose maintained membrane fluidity during cell dehydration.
Loss of even a small fraction of intracellular water is lethal for most cells. Nevertheless, some cells, including many microbial pathogens of humans, survive extreme desiccation, often for protracted periods (1, 22). The maximal longevity of microorganisms in a metabolically inactive, desiccated state is unknown (16, 22, 25), but there have been controversial (10, 21, 24) reports of ancient, yet viable bacteria in 25 million- to 40-million-year-old Dominican amber (2, 17).
Understanding the mechanisms that some organisms use to withstand the removal of virtually all of their water is an important problem in cell biology. The nonreducing disaccharides sucrose and trehalose protect membranes and proteins in vitro from dehydration damage, as described by the “water replacement hypothesis” (3). Survival of dehydration damage in a variety of organisms is correlated with intracellular accumulation of one of these disaccharides, and even the addition of exogenous trehalose or sucrose to cells that are sensitive to drying can increase survival (18, 22).
It has been proposed that the ability to survive desiccation may be conferred by transfection of desiccation-sensitive cells with genes which permit synthesis of trehalose or sucrose (4). This seems eminently practical since synthesis of either disaccharide requires only two steps, involves only two gene products, a synthase (reaction 1) and a phosphatase (reaction 2), and requires substrates found in all cells:
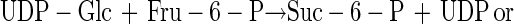 |
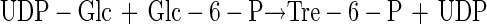 |
1 |
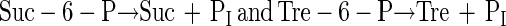 |
2 |
An attempt to develop this approach in plants (13) was challenged because in this system survival of dehydration is not easily estimated and direct effects of a disaccharide on physical properties of dry tissue cannot be detected readily (9). In addition, trehalases are widespread in plants, which complicates the issue further (20). In the present study we used desiccation-sensitive Escherichia coli as a model to address these problems.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
E. coli BL21DE3 (Invitrogen, Carlsbad, Calif.) has a chromosomal copy of the T7 RNA polymerase gene under control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible lacUV5 promoter. Derivative strain BL21DE3(pT7-7) contains expression vector pT7-7, which has a T7 promoter that permits high-level gene expression after induction with IPTG. Strain BL21DE3(pSpsA) contains the sucrose-6-phosphate synthase (spsA) gene from the cyanobacterium Synechocystis sp. strain PCC 6803 cloned in pT7-7.
Cloning of spsA.
The following two primers were used to amplify spsA from genomic DNA of Synechocystis sp. strain PCC 6803 (7, 15): 5′ AGAGCGCATATGAGCTATTCATCAAAATACA 3′ (the translation start codon is in boldface type) and 5′ GAGACGGTCGACTTAAACGGGGTCTAACAACTCA 3′ (the translation stop codon is in boldface type). These primers had 5′-terminal NdeI and SalI sites, respectively, which aided subcloning in pT7-7. Assays were performed in 50-μl mixtures containing pH 9.0 buffer (Promega, Madison, Wis.), 12.5 nmol of each deoxynucleoside triphosphate (final concentration of each deoxynucleoside triphosphate, 250 μM), 1 mM Mg2+, and 2.5 U of Taq DNA polymerase (Promega). The annealing temperature used for the first cycle (60 s) was 72°C, and the annealing temperature was then decreased 0.8C° per cycle for the next nine cycles (to 65°C) and was kept constant at 65°C for the remaining 30 cycles. Each cycle included denaturation at 95°C for 1 min and elongation at 72°C for 90 s. The assay began with denaturation at 95°C for 2 min and ended with elongation for 10 min at 72°C. A 2,184-bp DNA amplification product was purified, ligated to pCRR2.1-TOP (Invitrogen) to obtain pDWSPS1, and subcloned in E. coli TOP10. A DNA sequence analysis was performed to confirm the identity of spsA (data not shown). pDWSPS1 was digested with NdeI and SalI to remove the spsA fragment, which was then cloned in pT7-7 (which had been digested previously with NdeI and SalI) to obtain pSpsA.
Growth of strains and expression of spsA.
Strain BL21DE3 was grown in Luria-Bertani (LB) medium, while strains BL21DE3(pT7-7) and BL21DE3(pSpsA) were grown in LB medium containing ampicillin (final concentration, 100 μg ml−1). For each experiment single colonies of the different strains were grown in 3 ml of LB medium or LB medium containing 100 μg of ampicillin per ml for 3 h at 37°C. The 3-ml cultures were used to inoculate 50-ml portions of fresh medium (with 100 μg of ampicillin per ml), and after 2 h the optical density at 600 nm (OD600) of each cell suspension was adjusted to 0.08 with LB medium. Cells were harvested by low-speed centrifugation, resuspended to a final concentration of about 107 cells ml−1 in 100 ml of M9 medium (with ampicillin), and, after 0.1 mM (final concentration) IPTG was added, incubated for an additional 3 h. The relationships between OD600 and cell densities of the strains used in this study were determined empirically by performing multiple trials in which we counted colonies after cultures were serially diluted. The cell density of BL21DE3(pSpsA) used for calculations in the series of experiments described here was 5 × 107 cells ml−1. M9 medium contained (per liter) 6 g of Na2HPO4, 3 g of KH2PO4, 0.5 g of NaCl, and 1 g of NH4Cl as the basal salts. The basal medium was autoclaved and the pH was adjusted to 7.4, after which 2 ml of MgSO4 (1 M stock solution), 0.1 ml of CaCl2 (1 M stock solution), and 10 ml of glucose (20% [wt/vol], filter sterilized) were added. In replicate experiments glucose was omitted from M9 medium.
Cell extracts.
To detect sucrose 6-phosphate (sucrose-6-P), cell extracts were prepared by the method of Germer et al. (11). Cell pellets obtained from 200-ml cultures of strains BL21DE3(pSpsA) and BL21DE3(pT7-7) after induction in M9 medium (see above) were freeze-dried (5 μm of Hg, −75°C) by using a Labconco model 8 system, resuspended in 800 μl of ice-cold 15 mM trichloroacetic acid (TCA), and incubated on ice for 20 min. After centrifugation at 10,000 × g for 5 min, the total (350-μl) supernatant fraction was recovered for analysis (note that the dried cells imbibed water during rehydration). Cell pellets obtained from 500-ml cultures of BL21DE3(pSpsA) and BL21DE3(pT7-7) induced in M9 medium without glucose were resuspended in 800 μl of ice-cold 15 mM TCA as described above, and after centrifugation the total (250-μl) supernatant fraction was recovered.
Identification of sucrose-6-P in BL21DE3(pSpsA).
The presence of sucrose-6-P in cell extracts was first evaluated by thin-layer chromatography (TLC). Two-microliter aliquots of cell extracts from 200-ml cultures of BL21DE3(pSpsA) and BL21DE3(pT7-7) induced in M9 medium and 20-μl aliquots of cell extracts from 500-ml cultures of the same strains induced in M9 medium without glucose were separated on silica gel plates (type G/UV; Whatman International Ltd., Maidstone, Kent, England). The solvent system used was acetonitrile-water-ethyl acetate-isopropyl alcohol-acetic acid (85:20:30:30:10, vol/vol/vol/vol/vol). The sugars were visualized by dipping the plates in a naphthoresorcinol-ethanol-sulfuric acid solution (200 mg of naphthoresorcinol, 100 ml of 95% [vol/vol] ethanol, 4 ml of concentrated sulfuric acid) for a few seconds and drying the plates with gentle heating. Solutions of sucrose and sucrose 6-P (3 mM each) were used as standards. After drying, the TLC plates were scanned with the digital television camera of a model 4000 AlphaInnotech ChemiImager low-light imaging system operated with AlphaEase 3.3 software, a Dell Pentium computer, and an Optiquest color monitor. Spot densities were then determined for samples of interest with automatic background correction.
To confirm the identity of sucrose-6-P in recombinant E. coli, 100-μl aliquots of cell extracts (see above) prepared from BL21DE3(pSPsA) and BL21DE3(pT7-7) cells were incubated with 4 U of calf intestinal alkaline phosphatase (CIP) (Sigma Chemical Co., St. Louis, Mo.) in 50 mM Tris-HCl (pH 9.0)–1 mM MgCl2–0.1 mM ZnCl2 at 37°C overnight.
Enzyme assay for sucrose and sucrose-6-P.
The amounts of sucrose and sucrose-6-P in induced BL21DE3(pSPsA) were determined by a discontinuous spectrophotometric assay based on coupling invertase-catalyzed sucrose hydrolysis with oxidation of the liberated fructose catalyzed by fructose dehydrogenase. The reaction generated reducing equivalents that were transferred to a tetrazolium salt with a concomitant increase in the absorbance at 570 nm (12). After CIP treatment of the BL21DE3(pSPsA) and BL21DE3(pT7-7) cell extracts, 20-μl aliquots were incubated with 100 μl of test reagent and 100 μl of blank reagent prepared as previously described (12). Solutions containing 0.5, 5, and 25 mM sucrose were used as standards. Samples were incubated at 37°C, and absorbance at 570 mn was monitored every 15 min over a 30-min incubation period; after this the volumes of the samples were adjusted to 1 ml.
Subjecting cells to water stress.
After induction in M9 medium or M9 medium without glucose (see above), cell suspensions were subjected to acute water stress by using three techniques. For freeze-drying, 100-μl samples were frozen in liquid nitrogen and dried with a freeze-drier (5 μm of Hg, −75°C) for 24 h. For air drying, 100-μl samples were dried under sterile air at room temperature at a water activity (aw) of 0.25 (−200 MPa) for 72 h. For chemical desiccation, 10-μl samples were equilibrated under matric conditions (22, 23) in a nitrogen atmosphere over phosphorus pentoxide (aw, 0) for 72 h. Replicates were dried in the light and in the dark.
Desiccated cells were rehydrated in 10 or 100 μl of sterile water (based on the initial volume of the cell suspension) and plated immediately (or after dilution with M9 medium) onto 1.5% (wt/vol) agar plates containing LB medium and 100 μg of ampicillin per ml. Viable colonies were counted after overnight growth at 37°C in the light, and data were recorded as percentages of nondesiccated controls.
Immobilization and storage of clones.
Cultures of BL21DE3(pT7-7) and BL21DE3(pSpsA) were grown to an OD600 of 0.155 and diluted 1:1.5 with LB medium, and cells were harvested from 3-ml aliquots. Cell pellets were resuspended in M9 medium containing ampicillin (100 μg ml−1) and were induced with 0.1 mM IPTG for 4 h at 37°C. Uncharged microporous Nylon66 membranes (pore size, 1.5 μm) with a surface coating consisting of 50% amino groups and 50% carboxyl groups on a polyester support (Boehringer GmbH, Mannheim, Germany) were used for immobilization studies. The membranes either were used directly or were first wetted with a filter-sterilized 100 mM trehalose solution and blotted to dampness between sterile filter paper. Aliquots (2 μl) of cell suspensions were spotted onto the membranes in replicate, dried overnight at room temperature in the dark under a stream of sterile air, and then stored frozen in the dark at −20°C. After 72 h the desiccated cells were rehydrated by placing the membranes on fresh LB agar plates containing ampicillin (100 μg ml−1) and then incubating the plates at 37°C overnight in the light.
Determination of phase transitions.
Phase transitions were measured by Fourier transform infrared (FTIR) spectroscopy as described previously (5, 8). This method directly monitors changes in the frequency of CH2 bands, which correspond to the conformation of the acyl chains of membrane lipids. Spectra were obtained by using a Perkin-Elmer model 2000 optical bench, a microcomputer, and Perkin-Elmer Spectrum 2000 software. Data processing consisted of baseline flattening of the 3,000- to 2,800-cm−1 region by using three fixed points for each spectrum and normalization of absorbance by using the abex routine in the Spectrum software. The temperature was controlled with a Peltier device and was monitored with a fine thermocouple on the FTIR spectroscope window, as described previously (5, 8).
RESULTS
Expression of spsA in E. coli.
A prominent, approximately 75-kDa polypeptide was present in total-protein extracts obtained from BL21DE3(pSpsA) induced with 0.1 mM IPTG for 3 h. The polypeptide was synthesized in BL21DE3(pSpsA) after induction in either M9 medium or M9 medium without glucose. The 75-kDa peptide was not detected in BL21DE3(pT7-7) following induction under the same conditions, nor was it detected in extracts of BL21DE3(pSpsA) obtained prior to induction (data not shown). The sucrose phosphate synthase of Synechocystis sp. strain PCC6803 has a 750-amino-acid sequence (15).
Synthesis of sucrose.
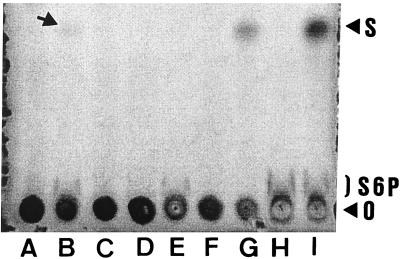
The sucrose-6-P standard produced a characteristic comet-shaped band at and above the origin on TLC plates (Fig. 1, lanes H and I). Cell extracts of BL21DE3(pSpsA) induced for SpsA synthesis produced a band like the sucrose-6-P standard band on TLC plates (Fig. 1, lanes B and E). Resolution of cell extracts obtained from induced BL21DE3(pSpsA) resulted in a faint spot (Fig. 1, lane B, arrow) with an Rf value (0.64) identical to that of the sucrose standard (Fig. 1, lane I). When the same extracts were treated with CIP prior to TLC, the intensity of the spot increased significantly concomitant with disappearance of sucrose-6-P (Fig. 1, lane G). The spot was not produced when we used extracts obtained from BL21DE3(pSpsA) that was induced in the absence of glucose but contained sucrose-6-P (Fig. 1, lane E), and it was not produced when the BL21DE3(pT7-7) extracts were used. When BL21DE3(pSpsA) was induced in the absence of glucose, it was necessary to increase the concentration of cell extract loaded onto TLC plates 30-fold in order to detect sucrose-6-P (Fig. 1, lane E). Sucrose-6-P was not detected in cell extracts obtained from BL21DE3(pT7-7) induced in the presence or absence of glucose (Fig. 1, lanes A and D). One transformant, which was constructed by using independent PCR amplification of spsA, was not able to synthesize sucrose-6-P (Fig. 1, lane C) and did not survive desiccation or freeze-drying (see below).
FIG. 1.
E. coli BL21DE3(pSpsA) synthesizes sucrose-6-P and sucrose, as determined by TLC analysis of cell extracts and carbohydrate standards. Lanes A, B, and C, 2-μl aliquots of 350-μl cell extracts obtained from 200-ml M9 medium-induced cultures of BL21DE3(pT7-7) (lane A), BL21DE3(pSpsA) clone 1 (used throughout this study) (lane B), and BL21DE3(pSpsA) clone 2 (a desiccation-sensitive clone) (lane C); lanes D and E, 20-μl aliquots of 250-μl cell extracts obtained from 500-ml cultures of BL21DE3(pT7-7) (lane D) and BL21DE3(pSpsA) (lane E) induced in medium M9 without glucose; lanes F and G, 2-μl aliquots of CIP-treated cell extracts from BL21DE3(pT7-7) (lane F) and BL21DE3(pSpsA) (lane G) cultures induced in M9 medium; lane H, 2 μl of sucrose-6-P (3 mg ml−1); lane I, 2 μl of sucrose 6-P and sucrose (3 mg ml−1 each). S, sucrose; S6P, sucrose-6-P; O, origin. The arrow in lane B indicates the position of sucrose.
The continuous spectrophotometric assay revealed that the sucrose content of cell extracts (20-μl aliquots) of BL21DE3(pSpsA) was 7 mM (4.21 × 1021 molecules liter−1). Because 350 μl of cell extract was obtained from each 200-ml culture (1010 cells) of BL21DE3(pSpsA) (see above), the sucrose content was therefore approximately 1.4 × 108 sucrose molecules per cell. This value included sucrose molecules which were derived from sucrose-6-P after CIP treatment prior to the assay (see above). From scanning and direct quantification of standards and samples on TLC plates before and after CIP treatment (e.g., a comparison of the sucrose bands in Fig. 1, lanes B and G), the ratio of sucrose to sucrose-6-P in cell extracts was calculated to be approximately 1:3.3, which was equivalent to 2.3 × 107 and 7.7 × 107 molecules of sucrose and sucrose-6-P, respectively, per cell.
Survival of E. coli during water stress.
The ability of bacteria to survive freeze-drying or desiccation is enhanced at high cell densities and in stationary-phase cells (1, 22). Therefore, to study the specific contribution of spsA to desiccation tolerance, cell densities were kept low (107 cells ml−1; OD600, 0.08) and only cells in the exponential phase of growth were used in the experiments described below.
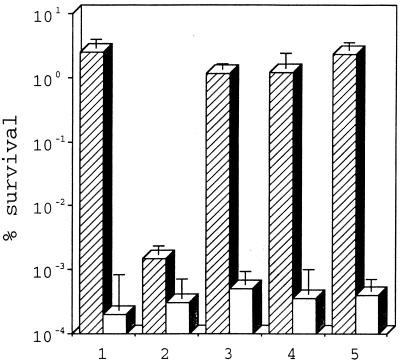
Expression of spsA resulted in a substantial increase in survival after drying (Fig. 2). The level of survival of BL21DE3(pSpsA) after freeze-drying was about 2.5% (i.e., 2.5 × 105 viable cells ml−1 of the original 107 cells ml−1), while the level of survival of BL21DE3(pT7-7) was 104-fold lower (0.0003%; approximately 30 viable cells ml−1 of the original 107 cells ml−1). After desiccation and 3 days of storage in air in the light, the survival values for BL21DE3(pSpsA) and BL21DE3(pT7-7) were 0.001 and 0.0001%, respectively. Survival of BL21DE3(pSpsA) increased 103-fold (to 1%) if desiccated cells were stored in the dark prior to rehydration. Survival of BL21DE3(pSpsA) was enhanced further when desiccated cells were stored over phosphorus pentoxide (aw, 0) for 3 days prior to rehydration. In this case the survival values for cells stored in the light and in the dark were 1.2 and 2.3%, respectively. These values were 104-fold higher than the values obtained with BL21DE3(pT7-7). The presence of glucose during induction of spsA did not influence the results of these experiments. In preliminary trials light and dark did not have significant effects on survivability following freeze-drying (data not shown); only the responses of cells to freeze-drying in the light were investigated subsequently and are reported below.
FIG. 2.
spsA enhances survival of E. coli BL21DE3(pSpsA). The data points are means of three values, and the error bars indicate standard deviations based on more than 20 trials. Open bars, BL21DE3(pT7-7); cross-hatched bars, BL21DE3(pSpsA). Data set 1, freeze-drying in the light; data set 2, air drying in the light; data set 3, air drying in the dark; data set 4, chemical desiccation in the light; data set 5, chemical desiccation in the dark.
Mechanism of enhanced desiccation tolerance.
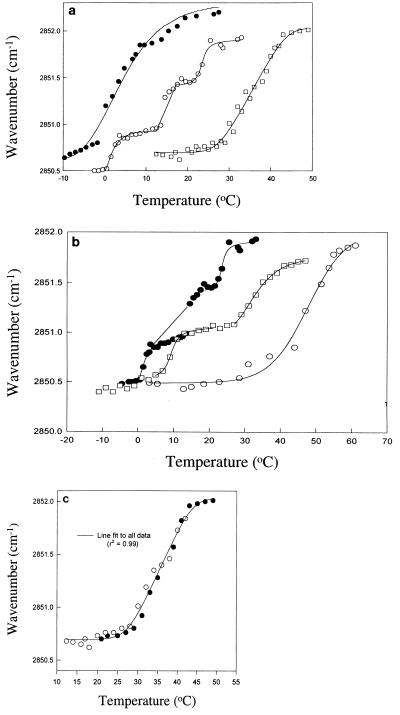
Fully hydrated wild-type BL21DE3 cells had a characteristic melting curve (melting temperature [Tm], 6°C) (Fig. 3a). The P⩵O stretch in the phospholipids of these hydrated cells was centered at about 1,230 cm−1 (data not shown). As expected, there was a marked increase in the Tm of BL21DE3(pT7-7) cells when they were freeze-dried (Tm, 35°C) (Fig. 3a), and consistent with these data, there was a P⩵O stretch at about 1,250 cm−1 (removal of the water around the P⩵O groups increased the frequency of vibration since P was no longer H bonded to water). The phosphate frequency was depressed in phospholipids dried with trehalose or sucrose due to hydrogen bonding between the −OH groups of the sugar and the phosphate of the phospholipid (26). Because of the presence of sucrose phosphate in BL21DE3(pSpsA), it was not possible to assign the phosphate stretch unambiguously to the membrane phospholipids. Thus, we cannot be sure that sucrose phosphate interacted directly with the polar head group. However, freeze-dried cells of BL21DE3(pSpsA) exhibited clear, but complex, depression of Tm (Tm, 15°C) (Fig. 3a). This depression depended on the thermal history of the cells (Fig. 3b). When desiccated BL21DE3(pSpsA) cells were first heated, they had a high Tm, 45°C. However, after chain melting, when the sample was cooled rapidly (50°C min−1) to 0°C and then immediately reheated, a complex transition with three components was observed at a significantly lower temperature. When the same sample was then chilled to −10°C and kept at this temperature for 45 min before analysis, the low-temperature transition began to revert to the high-temperature transition (Fig. 3b). The low-temperature transition was not identified after cells were stored at −20°C overnight, and the original high-temperature curve was restored (data not shown). The single melting curve for freeze-dried BL21DE3(T7-7) had a high Tm (35°C) that was not affected by the thermal history of the cells, including repeated melting of the same sample (Fig. 3c).
FIG. 3.
FTIR spectroscopy of membrane phospholipids. (a) Symbols: ●, fully hydrated wild-type strain BL21DE3; ○, freeze-dried BL21DE3(pSpsA); □, freeze-dried BL21DE3(pT7-7). (b) BL21DE3(pSpsA). Symbols: ○, first heating; ●, second heating; □, third heating after incubation for 45 min at −10°C. (c) BL21DE3(pT7-7). Symbols: ○, first heating; ●, second heating. The line was fit to all of the data (R2 = 0.99).
Storage of recombinant clones.
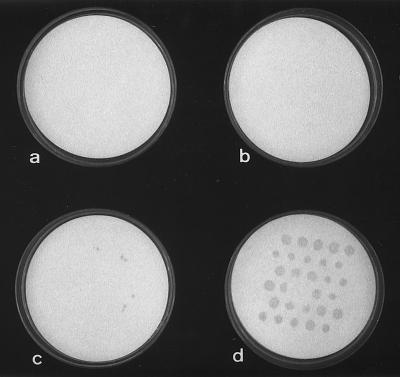
Strain BL21DE3(pT7-7) did not survive immobilization on nylon membranes (with or without trehalose), storage at −20°C for 72 h, and rehydration at 37°C (Fig. 4a and b). Few colonies of strain BL21DE3(pSpsA) survived the same treatment on untreated membranes (Fig. 4c), while full recovery occurred on membranes that had been pretreated with 100 mM trehalose (Fig. 4d).
FIG. 4.
Enhanced survival of E. coli BL21DE3(pSpsA) after immobilization on nylon membranes, storage at −20°C, and rehydration with LB medium at 37°C. (a) BL21DE3(pT7-7) spotted onto untreated membrane. (b) BL21DE3(pT7-7) spotted onto treated (100 mM trehalose) membrane. (c) BL21DE3(pSpsA) spotted onto untreated membrane. (d) BL21DE3(pSpsA) spotted onto treated (100 mM trehalose) membrane.
DISCUSSION
Desiccation and freeze-drying are severe stresses; they affect gene expression and gene regulation markedly, and multiple targets are damaged (1, 22). Different factors influence inactivation of dried cells; these factors include the growth phase, the cell concentration, the drying method, and the storage conditions (19). Also, the rates of survival for recombinant strains of E. coli following desiccation are lower than the rates of survival for their untransformed counterparts (14). Our goal was to synthesize sucrose in desiccation-sensitive cells in order to test the water replacement hypothesis. We demonstrated that in vivo sucrose synthesis had a marked protective effect in E. coli that is sensitive to drying in air. Furthermore, we did this under conditions under which any inherent capacity of the cells to offer some resistance to drying was minimized.
Sucrose and trehalose protect membranes by depressing the Tm of the phase transition when water is removed from the phospholipid bilayer and thus maintain the dry membranes in a physical state similar to that of fully hydrated membranes (3). Our FTIR data indicate that the sucrose synthesized in BL21DE3(pSpsA) protects membrane phospholipids. However, the heterogeneity of the melting curve suggests that sucrose may not be present in sufficient amounts to fully depress the Tm of all of the phospholipid (which is consistent with sucrose concentration data).
In experiments in which extracellular trehalose was used to protect E. coli from the effects of freeze-drying, Israeli et al. calculated that theoretically 5.6 × 107 molecules of trehalose per cell were needed to fully saturate the interphospholipid spaces in the outer membrane (14). This value is very similar to the value calculated in this study for the intracellular content of sucrose (2.3 × 107 molecules of sucrose per cell). However, it should be emphasized that trehalose is generally more effective than sucrose in protecting membranes from desiccation and freeze-drying (1, 18, 22).
The sucrose present in BL21DE3(pSpsA) may be in contact with only one face of the bilayer (depression of the Tm of the inner monolayer could conceivably affect the outer monolayer). An increase in the P⩵O stretch value similar to that observed in BL21DE3(pT7-7) was observed for dried BL21DE3(pSpsA). After heating, however, the P⩵O stretch value was depressed and was similar to the value obtained for hydrated BL21DE3 (data not shown). We concluded that when the phospholipid in BL21DE3(pSpsA) is stored at temperatures below the Tm, sucrose is forced out of the bilayer, an effect similar to the effect observed previously with phospholipid vesicles (6). When the chains are melted, however, the sucrose again has access to the headgroup and forms H bonds with P⩵O groups, and the P⩵O stretch value is close to the P⩵O stretch value for hydrated cells. The fact that the P⩵O stretch value for dried BL21DE3(pSpsA) was not lower than the value obtained with hydrated BL21DE3 is consistent with the proposed heterogeneous (nonsaturating) distribution of sucrose.
In general, the effects of desiccation are more pronounced in cells stored in the light than in cells stored in the dark because of photooxidative reactions catalyzed by residual water (1, 22). The damage occurs at the level of enzyme activity (e.g., nitrogenase), in proteins (e.g., phycobiliproteins), or in DNA (22). When cells of BL21DE3(pSpsA) were dried at −200 MPa, the residual water, even though the water content was low, may have competed with sucrose for sites in the phospholipid membrane and may have contributed to the light sensitivity of the cells (Fig. 2, data set 2). In contrast, storage over phosphorus pentoxide resulted in complete desiccation of the membranes, which enhanced the protective effect of sucrose (Fig. 2, data sets 4 and 5). Complete desiccation also alleviated the differential sensitivity due to light (Fig. 2, data sets 4 and 5).
The presence of nonreducing sugars in vivo and in vitro (i.e., overexpression of spsA) and addition of 100 mM trehalose resulted in full recovery of cells following desiccation, freezing, and rehydration (Fig. 4d). Immobilization of cells in this way can be used for construction and storage of high-density clones, such as the clones in gene libraries. In vivo synthesis of sucrose may also be useful for stabilization of other cell lines, including eukaryote cell lines.
ACKNOWLEDGMENTS
This study was supported by grant N00173-98-1-G005-LOG from the Defense Advanced Research Programs Agency—Naval Research Laboratories and by grant IBN 9513157 from the National Science Foundation.
REFERENCES
- 1.Billi, D., and M. Potts. Desiccation. In K. B. Storey and J. M. Storey (ed.), Cell and molecular responses to stress, in press. Elsevier Science, Amsterdam, The Netherlands.
- 2.Cano R, Borucki M K. Revival and identification of bacterial spores in 25-million-year-old to 40-million-year-old Dominican amber. Science. 1995;268:1060–1064. doi: 10.1126/science.7538699. [DOI] [PubMed] [Google Scholar]
- 3.Crowe J H, Crowe L M, Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 4.Crowe J H, Crowe L M. Membrane integrity in anhydrobiotic organisms: toward a mechanism for stabilizing dry cells. In: Somero G N, Osmond C B, Bolis C L, editors. Water in life: comparative analysis of water relationships at the organismic, cellular, and molecular level. Berlin, Germany: Springer-Verlag; 1992. pp. 87–103. [Google Scholar]
- 5.Crowe J H, Hoekstra F A, Crowe L M, Anchordoguy T J, Drobnis E. Lipid phase transitions measured in intact-cells with fourier-transform infrared spectroscopy. Cryobiology. 1989;26:76–78. doi: 10.1016/0011-2240(89)90035-7. [DOI] [PubMed] [Google Scholar]
- 6.Crowe J H, Hoekstra F A, Nguyen K H N, Crowe L M. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim Biophys Acta. 1996;1280:187–196. doi: 10.1016/0005-2736(95)00287-1. [DOI] [PubMed] [Google Scholar]
- 7.Curatti L, Folco E, Desplats P, Abratti G, Limones V, Herrera-Estrella L, Salerno G. Sucrose-phosphate synthase from Synechocystis sp. strain PCC 6803: identification of the spsA gene and characterization of the enzyme expressed in Escherichia coli. J Bacteriol. 1998;180:6776–6779. doi: 10.1128/jb.180.24.6776-6779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drobnis E Z, Crowe L M, Berger T, Anchordoguy T J, Overstreet J W, Crowe J H. Cold shock damage is due to lipid phase-transitions in cell-membranes—a demonstration using sperm as a model. Exp Zool. 1993;265:432–437. doi: 10.1002/jez.1402650413. [DOI] [PubMed] [Google Scholar]
- 9.Gaff D. Tobacco-plant desiccation tolerance. Nature. 1996;382:502. [Google Scholar]
- 10.Gerhardt P. Survival of ancient bacteria desiccated within amber: believe it or not? ASM News. 1998;64:68–69. [Google Scholar]
- 11.Germer J, Muffler A, Hengge-Aronis R. Trehalose is not relevant for in vivo activity of ς-containing RNA polymerase in Escherichia coli. J Bacteriol. 1998;180:1603–1606. doi: 10.1128/jb.180.6.1603-1606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes E W. Coupled enzymatic assay for the determination of sucrose. Anal Biochem. 1997;244:103–109. doi: 10.1006/abio.1996.9865. [DOI] [PubMed] [Google Scholar]
- 13.Holmström K-O, Mäntylä E, Welin B, Mandal A, Palva E T, Tunnela O E, Londesborough J. Drought tolerance in tobacco. Nature. 1996;379:683–684. [Google Scholar]
- 14.Israeli E, Shaffer B T, Lighthart B. Protection of freeze-dried Escherichia coli by trehalose upon exposure to environmental conditions. Cryobiology. 1993;30:519–523. doi: 10.1006/cryo.1993.1052. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hiroswa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Osumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. I. Sequence features in the 1 Mb region from map positions 64% to 92% of the genome. DNA Res. 1996;3:109–136. [Google Scholar]
- 16.Kennedy M J, Reader S L, Swierczynski L M. Preservation records of micro-organisms: evidence of the tenacity of life. Microbiology. 1994;140:2513–2529. doi: 10.1099/00221287-140-10-2513. [DOI] [PubMed] [Google Scholar]
- 17.Lambert L H, Cox T, Mitchell K, Rossello-Mora R A, Del Cueto C, Dodge D E, Orkand P, Cano R J. Staphylococcus succinus sp. nov., isolated from Dominican amber. Int J Syst Bacteriol. 1998;48:511–518. doi: 10.1099/00207713-48-2-511. [DOI] [PubMed] [Google Scholar]
- 18.Leslie S B, Israeli E, Lighthart B, Crowe J H, Crowe L M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol. 1995;61:3592–3597. doi: 10.1128/aem.61.10.3592-3597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lievense L C, van't Riet K. Convective drying of bacteria. II. Factors influencing survival. Adv Biochem Eng. 1993;50:71–89. [PubMed] [Google Scholar]
- 20.Müller J, Boller T, Wiemken A. Trehalose and trehalase in plants: recent developments. Plant Sci. 1995;112:1–9. [Google Scholar]
- 21.Postgate J, Priest F G. Putative oligocene spores. Microbiology. 1995;141:2763–2764. [Google Scholar]
- 22.Potts M. Desiccation tolerance of prokaryotes. Microbiol Rev. 1994;58:755–805. doi: 10.1128/mr.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potts M, Bowman M A, Morrison N S. Control of matric water potential (Ψm) in immobilized cultures of cyanobacteria. FEMS Microbiol Lett. 1984;24:193–196. [Google Scholar]
- 24.Priest F G. Age of bacteria from amber. Science. 1995;270:2015. [PubMed] [Google Scholar]
- 25.Sneath P H A. Longevity of microorganisms. Nature. 1962;4842:643–646. doi: 10.1038/195643a0. [DOI] [PubMed] [Google Scholar]
- 26.Tsvetkova N M, Phillips B L, Crowe L M, Crowe J H, Risbud S H. Effect of sugars on headgroup mobility in freeze dried dipalmitoylphosphatidylcholine bilayers: solid state 31P NMR and FTIR studies. Biophys J. 1998;75:2947–2955. doi: 10.1016/S0006-3495(98)77736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]