Abstract
Introduction
Rural communities bear a disproportionate share of the opioid and methamphetamine use disorder epidemics. Yet, rural people who use drugs (PWUD) are rarely included in trials testing new drug use prevention and treatment strategies. Numerous barriers impede rural PWUD trial engagement and advancing research methods to better retain rural PWUD in clinical trials is needed. This paper describes the Peer-based Retention Of people who Use Drugs in Rural Research (PROUD-R2) study protocol to test the effectiveness of a peer-driven intervention to improve study retention among rural PWUD.
Methods and analysis
The PROUD-R2 study is being implemented in 21 rural counties in three states (Kentucky, Ohio and Oregon). People who are 18 years or older, reside in the study area and either used opioids or injected any drug to get high in the past 30 days are eligible for study inclusion. Participants are allocated in a 1:1 ratio to two arms, stratified by site to assure balance at each geographical location. The trial compares the effectiveness of two retention strategies. Participants randomised to the control arm provide detailed contact information and receive standard retention outreach by study staff (ie, contacts for locator information updates, appointment reminders). Participants randomised to the intervention arm are asked to recruit a ‘study buddy’ in addition to receiving standard retention outreach. Study buddies are invited to participate in a video training and instructed to remind their intervention participant of follow-up appointments and encourage retention. Assessments are completed by intervention, control and study buddy participants at 6 and 12 months after enrolment.
Ethics and dissemination
The protocol was approved by a central Institutional Review Board (University of Utah). Results of the study will be disseminated in academic conferences and peer-reviewed journals, online and print media, and in meetings with community stakeholders.
Trial registration number
Keywords: PUBLIC HEALTH, Substance misuse, STATISTICS & RESEARCH METHODS
Strengths and limitations of this study.
The intervention design uses a combination of elements of successful peer navigator models for treatment retention and peer-driven models of recruitment to improve study retention.
The multisite nature of the study representing geographically diverse rural areas of the US strengthens potential generalisability.
Because the intervention is embedded in the participant retention protocol, participants and site staff cannot be blinded to arm assignment.
A limitation of the protocol is that participants may refer study buddies with whom they have weak social connections making it difficult to leverage the relationships for retention.
Relationships between intervention participants and study buddies may dissolve over time and present challenges for leveraging those ties for continued retention in the intervention arm.
Introduction
Opioid use disorder (OUD) is a national crisis in the USA.1 Emergence of illicit fentanyl, which is 50–100 times more potent than morphine, as an adulterant of heroin, methamphetamine and other drugs has precipitously increased overdose. Rural communities bear a disproportionate share of the opioid and methamphetamine use disorder epidemics.2 3 Clinical trials testing new drug use prevention and treatment strategies are urgently needed. Yet, rural communities are rarely included in such trials and numerous barriers may impede retention for rural participants.4 5 Advancing research methods to better retain rural people who use drugs (PWUD) in clinical trials addresses a major gap in development of new interventions to address drug use and other conditions in rural America and improves generalisability of inferences from clinical trials.
Poor retention is a common threat to validity in substance use disorder (SUD) research,6–8 particularly in rural communities.9 Barriers include perceived stigma, scheduling difficulties, transportation difficulties, community-level distrust of the research process and limited investigator experience working in non-urban communities.9 The National Drug Abuse Treatment Clinical Trials Network (CTN) identified individual-level and site-level facilitators of retention.10–15 A review of 24 completed CTN trials including 11 000 participants seeking treatment for SUD found that gender and race/ethnicity did not affect retention. Younger participants, however, had lower retention.12 Length of trial and participant burden were not strong predictors of retention, suggesting a need to identify key variables that influence trial participation.15 Few trials, however, enrolled rural participants.
Peer navigators may be an untapped resource for improving clinical trial retention. Peers share characteristics with participants, such as demographics and drug use history, which may facilitate rapport with PWUD.16 Peers are effective at engaging and retaining hard-to-reach urban populations in SUD treatment and other clinical care.17 18 A systematic review of nine studies in mainly urban settings reported that peer-delivered recovery support services in SUD treatment improved outcomes,17 but their potential in rural settings is less clear.
Respondent-driven sampling (RDS)19 20 is a common peer-based strategy that has been used to recruit participants in biological and behavioural studies in over 80 countries,21 22 including SUD studies.23 24 RDS is a network-based sampling technique that forms chains of respondents, where purposively sampled initial participants, or ‘seeds’, are identified and given a limited number of coupons to recruit drug-using peers, who then recruit their peers. The value of peers in facilitating recruitment of PWUD into research is well established, but its effectiveness in improving study retention needs more rigorous evaluation.
The Peer-based Retention Of people who Use Drugs in Rural Research (PROUD-R2) study tests the effectiveness of a peer-driven intervention to improve study retention among PWUD in rural communities. PROUD-R2 overcomes two historical roadblocks in clinical trials research: (1) widely dispersed rural populations that complicate clinical trials implementation and (2) inclusion of individuals with SUD whose social circumstances impede clinical trial participation. PROUD-R2 began with a formative survey on factors, motivations and barriers influencing research participation and retention among rural PWUD,5 followed by the launch of the trial described in this manuscript. The PROUD-R2 intervention focuses on optimising study retention in this special population and prepares a cohort of rural PWUD for participation in clinical trials. We hypothesise that participants who receive a peer-based retention strategy will be more likely to be retained in the study at 6 and 12 months compared with participants who receive standard retention approaches.
Methods
Study Setting
PROUD-R2 leverages the National Rural Opioids Initiative (NROI),25 a multistate consortium assessing interventions to increase access to care and reduce opioid overdose deaths and comorbidities in rural America. The three sites involved in PROUD-R2 include 21 rural counties in Ohio (n=6), Oregon (n=3) and Kentucky (n=12).
The Oregon counties lie in the southwest coastal and mountainous areas of the state, areas with high overdose rates. These counties have a rural population from 38% to 45%.26 The Ohio and Kentucky counties fall within Appalachia. These counties have been designated b as ‘Distressed’ or ‘At-Risk’ counties based on several economic indicators, including unemployment, per capita income and poverty rates.27 All six Ohio counties and 10 of Kentucky’s twelve counties are within the top 5% of counties in the USA most vulnerable to continued high HCV transmission and the potential for an HIV outbreak among people who inject drugs.28 One Ohio county and 10 Kentucky counties are 100% rural with the remaining counties ranging from 54% to 81%.26
Eligibility criteria
People who are 18 years or older, reside in the study area, and either used opioids or injected any drug to get high in the past 30 days are eligible for study inclusion. There are no exclusion criteria. Investigators may remove a participant from the study if worsening health precludes participation; participant poses a safety risk to study staff; participation is determined to be due to external pressure; or the study is terminated by the institutional review board (IRB), data safety monitoring board (DSMB) or funder. Participants are not prohibited from concurrent research or care as a condition of PROUD-R2 participation.
Randomisation
Participants are allocated in a 1:1 ratio to two arms, stratified by site to assure balance at each geographical location. Research staff at each site log into a REDcap randomisation module hosted at Oregon Health and Science University. Randomisation is performed following completion of baseline data collection. Study buddies (described below) are not randomised or enrolled in the trial as intervention or control participants.
Trial arms
The trial compares the effectiveness of two retention strategies (table 1). Participants randomised to the control arm receive standard retention outreach by study staff. Participants randomised to the intervention arm are asked to recruit a ‘study buddy’ in addition to receiving standard retention outreach. Study buddies remind their intervention participant of follow-up appointments and encourage attendance. Intervention participants and study buddies are advised against interacting if pressure/coercion to participate arises, they do not feel safe, or they feel the interaction could trigger unwanted drug use. Assessments are completed at 6 and 12 months after enrolment. Table 2 summarises the activities planned for participant contact in the intervention and control arms.
Table 1.
Description of staff tasks in the PROUD-R2 participant retention protocol by study arm
| Intervention participants | Control participants | |
| Within 3 weeks*† | Remind participant weekly to refer study buddy for a US$10 incentive | Not Applicable |
| 1 month*† | Remind participant weekly to refer study buddy for a US$10 incentive | Contact participant to verify or update locator information (US$10 incentive) |
| 2 months | Remind participant to refer study buddy if they have not done so already | Not applicable |
| 3 months* | Contact participant to verify/update locator information (US$10 incentive if completed before 5 months) | |
| 5 months | Contact participant to remind them of their appointment | |
| Contact study buddy to remind them that their peer is due for follow-up on (date) | Not applicable | |
| 1 week* | For those who are unreachable, contact participant to remind them of their appointment | |
| 2 weeks* | Follow-up PROUD-R survey window opens. For those who are unreachable, contact participant to remind them of their appointment and contact participants’ contacts | |
| 3 weeks* | For those who are unreachable, contact participant to remind them of their appointment and staff conduct home visit‡ | |
| 24 hours prior to appointment | Contact participant to remind them of their appointment | |
| 6 months | Follow-up PROUD-R survey | |
| 15 min after appointment time* | For those who miss appointment, contact participant to remind them of their appointment | |
| 1 week* | For those who miss appointment, contact to ask them to reschedule and mail letter to the participant | |
| 2 weeks* | For those who miss appointment, contact for appointment reminder and conduct home visit‡ for those who are unreachable Intervention only: Contact study buddy to remind them that their peer is due for follow-up by (date) |
|
| 3 weeks* | For those who miss appointment, contact participant’s contacts to remind them of participant’s appointment and mail a letter to participant’s contacts | |
| 24 hours before 7 months* | For those who miss appointment, contact participant to remind them that survey window is closing | |
| 7 months | Follow-up PROUD-R survey window closes | |
| 9 months - 13 months | 3-month to 7-month process described above repeats | |
*Jail logs are searched to identify if participant is in jail.
†Oregon and Ohio also encourage participants coenrolled in their Rural Opioid Initiative (ROI) studies and PROUD-R2 (intervention, control and study buddy participants) to refer peers for respondent-driven sampling.
‡Suspended due to COVID-19 restrictions.
PROUD-R2, Peer-based Retention Of people who Use Drugs in Rural Research.
Table 2.
Participant timeline
| Time point | Study period | |||||
| Enrolment | Allocation | Postallocation | Closeout | |||
| −t1 | 0 months | 0 months | 6 months | 12 months | 13 months | |
| Enrolment | ||||||
| Eligibility screen | X | |||||
| Informed consent | X | |||||
| Randomisation | X | |||||
| Interventions | ||||||
| Standard retention (control) | X | X | X | |||
| Standard retention+peer retention (intervention) | X | X | X | |||
| Assessments | ||||||
| Baseline survey | X | |||||
| Follow-up survey | X | X | ||||
| Analysis | X | |||||
Control condition
Following standard procedures used in longitudinal research with PWUD, participants provide detailed locator form information (online supplemental appendix A) to assist with retention and/or contact for future research including names, pseudonyms, phone numbers, addresses, email addresses, social media contact information, employer contact information, probation/parole officer information if applicable and contact information for up to three people who should know how to reach the participant if contact information changes. Participants are contacted at the mid-point of each follow-up interval (ie, at 3-month postenrolment and 9 months postenrolment) to update locator information and remind them about their follow-up appointment date. Participants receive US$10 for updating or verifying locator information at each interval.
bmjopen-2022-064400supp001.pdf (269.3KB, pdf)
Study staff contact the participant using provided information and, if unsuccessful, attempt to reach one of their contacts from the locator form. Staff send another reminder by phone, text or private message on social media at predefined intervals (table 2). If participants cannot be reached via phone or text messaging, staff attempt contact by email and mail. Sites use the same phone/text message script for appointment reminders and record information on the number of attempted contacts, number of successful contacts, intervals between contacts and type of contact with each participant. In Kentucky and Oregon, staff may offer transportation or accompany participants to the field office if COVID-19 precautions allow. Offering transportation is infeasible in Ohio. In Kentucky, participants who are incarcerated and consented to be contacted for follow-up while incarcerated may complete follow-up surveys from jail (with permission from jail administrative staff). In Oregon, staff are permitted to maintain contact while participants are incarcerated, and schedule postrelease follow-up visits. In Ohio, study staff routinely check jail and prison records to identify participants who may be unable to attend follow-up appointments.
Intervention condition
In addition to receiving the same retention strategies given to control participants, intervention participants are also provided a ‘golden ticket’ at the time of randomisation to refer a study buddy. They receive US$10 for referral of an eligible study buddy who enrols in the study. The ticket includes a unique number to link the study buddy and intervention participant; if tickets are lost, names and demographic data are used to link individuals.
Study buddies
Study buddies who enrol in the study receive a 6 min peer retention training video suggesting ways to encourage peer participation in the study without coercion; the messages build on those used in CTN trials12 14 29 and previous research.30–35 During the iterative development process, we sought feedback on scripts and preliminary video clips from Community Advisory Boards of PWUD and/or peer support specialists at each site. The final video, available online,36 describes the study, discusses the importance of retention and shows a mock peer-to-peer interaction demonstrating a study buddy reminding a peer about their follow-up appointment, inquiring about best contact methods and briefly discussing barriers. When possible, staff show videos to study buddies in person following completion of their baseline survey. Due to social distancing measures during COVID-19, staff administer some surveys by phone and send a url for the video via text message, email or social media. The video is book-ended by a short series of questions that allow staff to determine whether a study buddy watched the video and their duration of viewing. Following the video, staff ask participants about their understanding of the video and clarify content for those who did not fully understand.
Staff contact the study buddy 1 month prior to their peer’s follow-up appointment date and 2 weeks afterward if the appointment is missed. In these contacts, the staff encourage the study buddy to inform the peer (ie, the intervention arm enrollee) that they are due for a follow-up but do not provide the peer’s contact information to the study buddy. Study buddies are not members of the control or intervention arms but do complete the same data collection procedures and receive the same retention protocol as the control participants. Study buddies are required to meet the same eligibility criteria as other participants except for the drug use criterion, which was removed for study buddies in February 2022.
Outcomes
The primary and secondary outcomes are to compare the proportion of participants retained in intervention and control arms at 12 and 6 months postbaseline, respectively. Participants are considered ‘retained’ if they at least partially complete the follow-up survey during the 164–210 days postbaseline assessment window for 6-month retention and 344–390 days window for 12-month retention.
Data collection
NROI sites use a harmonised RDS strategy to recruit PWUD.25 Issues of stigma, mobility, legality and absence of adequate sampling frames for high-risk populations37 create challenges for recruitment in these populations. RDS helps to address these challenges.20 23 38 Each PROUD-R2 site recruited rural PWUD for the NROI using RDS. In Kentucky and Oregon, RDS for NROI commenced in February and March 2018, respectively, and in Ohio, began in November 2019. Participants who enrol in the NROI studies are invited to participate in the proposed PROUD-R2 study. In addition, staff conduct outreach at syringe service programmes and other community venues to recruit. The total proposed sample size for PROUD-R2 is 700 participants.
Community-based field staff conduct surveys that are programmed in REDCap web-based data collection system.39 Staff administer surveys in-person or by phone based on participant preference. Each site uses the same survey, which collects detailed information on demographic characteristics, sexual and drug-related risk behaviour, criminal justice involvement, SUD treatment, medical care access and attitudes toward clinical trials. Staff administer follow-up surveys at 6 months and 12 months postbaseline. In Ohio, participants receive US$40 for completing each survey, and in Kentucky and Oregon, participants receive US$15 for each survey. The amount is higher in Ohio to improve comparability given that Kentucky and Oregon participants also have a chance to enrol in other ongoing projects wherein they receive additional incentives.
Follow-up surveys ask participants whether participants received encouragement from peers to attend their follow-up assessment; if so, from whom (name and demographic data) and whether they have a coupon referral code (see paragraph above). Information provided about who encouraged them to follow-up determines whether an incentive is owed to the study buddy. Study buddies receive US$10 for successfully encouraging their intervention participant’s retention at each follow-up.
Relationships between intervention participants and study buddies may evolve over time as people become unreachable due to incarceration, hospitalisation, movement outside of the study area or achieve different stages of recovery. Therefore, at the end of each follow-up survey, participants in the intervention arm are asked about the nature of their relationship with their study buddy. Items assess frequency of contact and where (city, state) the study buddy currently lives and whether they are homeless, incarcerated, hospitalised or in inpatient treatment. Participants are also asked whether they think this person is in treatment and/or recovery from substance use and how that affected their interactions. Similar dyadic questions were used for network research among rural PWUD.40 The data on the relationship between participant/study buddy will be investigated as a possible factor associated with retention in exploratory analyses.
Blinding, fidelity and contamination
Investigators remain blinded through recruitment and follow-up until completion of primary and secondary analyses, using uninformative participant labels. Study biostatisticians will also use these labels during analyses. Due to the nature of the interventions, participants and site staff administering the intervention are not blinded. These staff are instructed to use uninformative labels when discussing participants with blinded investigators.
We will use descriptive analyses to evaluate fidelity. Among individuals in the intervention arm, we will report the number and percentage of participants who did not recruit a study buddy, whose study buddy did not watch the training video, and who were not encouraged or actively discouraged by their study buddy to remain in the study. Fidelity assessment will be used to help inform the Per-Protocol Population. We will also use data from a retention task tracker programmed in REDCap that field staff use to record whether they successfully completed each task described in table 1.
Contamination is possible given participants in different study arms may be connected socially. Participants are asked whether they communicated with any peers about their follow-up visit and, if so, which peers(s) (eg, first name, nickname, first initial of last name, approximate age, gender, race and referral code (if known)) and the nature of the communication (ie, appointment reminder, received or gave encouragement to stay enrolled, messages about the importance of the research). With this detail, analysts will cross-reference information, identify potential contamination across study conditions, and conduct sensitivity analyses as appropriate.41
Data management
Data are stored in a secure REDCap database, separate from the randomisation database behind a firewall. The data manager assesses transferred data for completeness, queries sites regarding any inconsistencies, and code merged data files for analysis. The list linking participants to their unique identifier is password protected and maintained at research sites. To protect participant confidentiality, only de-identified data are shared for analysis. Data transmitted between sites contain only a unique identifier and no protected health information.
Statistical methods
The intention-to-treat (ITT) population will contain all randomised participants according to their assigned study arm. The per-protocol population will include participants who complete the trial as originally allocated. For instance, participants from the intervention arm who do not recruit a study buddy or lose contact with their study buddy during follow-up as well as those from the control arm who are determined to have received support from an enrolled study buddy will be excluded from this population. We hypothesise that the proportion of participants retained in the intervention arm will be greater than the control arm at 12 (primary outcome) and 6 (secondary outcome) month follow-up in the ITT population. Participants who do not attend their 6-month or 12-month visit will be considered ‘not retained’. There should be no missingness in the outcome in the way it has been defined and we will not conduct imputation for covariates with missing data. Type 1 error (α) will be set to 0.05 in primary and secondary outcome analyses, while α=0.1 will be used for subgroup and exploratory analyses except where specified. All tests will be two tailed.
We will use a generalised linear mixed model (GLMM) with a logit link (ie, mixed effects logistic regression) with a fixed effect for study arm and random effects for site and RDS chain nested within site. We will include covariates: gender; age at enrolment; race/ethnicity; education; drug of choice and unstable housing status. Covariates will be measured at baseline except for drug of choice and unstable housing status, which may be updated from follow-up surveys. If a covariate causes collinearity or convergence problems when modelling, it will be excluded.
We will report exponentiated coefficients (ORs) for the study arm variable from models with and without covariates along with corresponding 95% CIs based on bootstrapped standard errors. For reporting and dissemination, we will transform ORs to relative risk,42 43 which are recommended for prospective studies. Relative risk estimated from the model including covariates will represent intervention effectiveness. Sensitivity analyses will be performed repeating this analytic strategy with (1) a measure of the intervention intensity in lieu of study arm in the ITT population and (2) the per-protocol population. Supplementary preplanned analyses will explore intervention effects separately for each site as well as known or hypothesised associations with retention within groups, for example, specific drug use (opioids vs methamphetamine, opioids alone vs polysubstance use) and those experiencing unstable housing.
Another exploratory assessment will be performed using similar methods to other analyses but following a purposeful risk factor model building strategy. Among participants in the intervention arm, we will explore factors associated with retention at 12 months. Participant characteristics, study buddy characteristics, relationship between participant and study buddy, and study buddy contact characteristics will be assessed as fixed effects in univariable GLMM; the random effects used in primary analyses will be included in all models. Variables associated with retention at 12 months at a p<0.25 threshold in univariable models will be considered for inclusion in a multivariable model. Factors significantly associated with the outcome at p<0.10 as well as confounding variables will be retained in the final model. A confounder will be defined as a covariate from the list above resulting in a 10% change or greater in the estimated ORs when included in the model. Adjusted ORs and corresponding 95% CIs will be calculated from the final model.
Power calculation
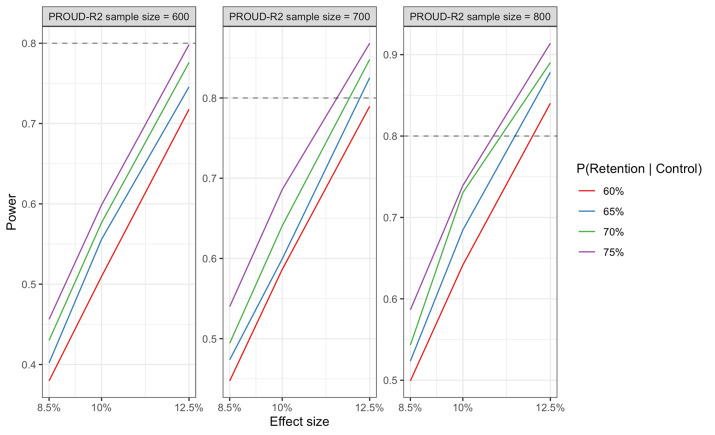
Assuming a target sample size of 700 PROUD-R2 participants, we estimate that we will have at least 80% power to detect an approximately 11%–12.5% increase in retention attributable to the intervention (figure 1). Power estimates assume >65% retention in the control arm, between-RDS chain variance of 0.05, and two-sided hypothesis test at α=0.05. We simulated RDS chain structures based on NROI recruitment data collected prior to the start of PROUD-R2. Power calculations are based on simulations using GLMM with a logit link; simulated models included a fixed effect term for study group and random intercept for RDS chain. Calculations were performed using R.
Figure 1.
PROUD-R2 study power. PROUD-R2, Peer-based Retention Of people who Use Drugs in Rural Research.
Data monitoring
A DSMB comprising an addiction medicine physician, a statistician and two behavioural scientists with expertise in research among PWUD oversees the study. The DSMB is independent of the sponsor and competing interests. The DSMB will meet at least annually to review emerging data, and make recommendations about the trial’s conduct, including stopping the trial. At each meeting, the DSMB will determine whether a change in the protocol is warranted, there are safety concerns and formally vote to allow the study to continue. A report on DSMB meetings and activities will be sent to the funding agency within a month after each meeting. No formal interim analyses are planned.
Social harms
Social harms related to participation will be actively assessed and documented. For this study, social harms are defined as any intended or unintended cause of physical; emotional; or psychosocial injury or hurt from one participant to another, a participant to themselves, or an institution to a participant, occurring as a result of study participation.44 Participants will complete a social harms questionnaire at each study visit. Study staff are trained to provide appropriate care, counselling and referral as needed. Any identified social harms are reported to study investigators who determine severity and provide details to the IRB as required.
Auditing
A PROUD-R2 staff person assesses REDCap data for missingness and data quality and provides feedback to site coordinators regarding any issues that need to be addressed. Investigators in Kentucky, Ohio and Oregon review study consent materials to assure appropriate documentation of consent at least semiannually.
Patient and public Involvement
Participants nor the public were directly involved in the development of the research question, outcome measures or conduct of the trial. However, elements of PROUD-R2’s design were informed by a formative survey of rural PWUD on factors influencing study retention.5 Also, during the iterative process of developing the intervention training video for study buddies, we sought feedback from Community Advisory Boards of PWUD and/or peer support specialists at each site about burden (ie, length) and content. Longitudinal follow-up of participants will end so that analysis can begin, making it impossible to disseminate results directly to each cohort member. However, results will be distributed via study social media pages, websites and to community partners who serve PWUD.
Ethics and dissemination
Sites participating in PROUD-R2 rely on a Single IRB (University of Utah IRB) for human subjects oversight; local IRBs cede responsibility. Modifications to the protocol (eg, changes to eligibility criteria, outcomes or analyses, changes in study procedures) and revisions to consent forms and other participant-facing documents are submitted to the Single IRB for approval. Study staff complete human subjects training and are approved as personnel by the Single IRB. Protocol modifications are submitted to the Single IRB prior to implementation and reflected in ClinicalTrials.gov. Approval from the funding agency will be sought for major protocol modifications such as changes in inclusion criteria or aims prior to submitting those changes to the Single IRB.
All participants enrolling in PROUD-R2 complete an informed consent process. Potential participants’ understanding of study procedures are assessed using a comprehension tool included within the informed consent document. In addition to describing the protocol, risks and benefits, this consent form states that the participant, if randomised to the intervention arm, will provide permission for staff to reveal their name to the study buddy whom they referred so that person can help encourage their retention in the study. Participants are advised that they can refuse to engage with their study buddy, which may be important should different stages of recovery or interpersonal conflicts arise. Consent procedures are completed over the phone or in a private area with only the participant and study staff present.
Findings will be disseminated to the public and healthcare professionals in peer-reviewed journals, professional conferences and community forums. Authorship eligibility guidelines follow ICMJE criteria. We will submit manuscripts to NIHMS to be made publicly available no later than 12 months after the official date of publication in compliance with the funder’s open access policy. Deidentified data will be made available to interested parties on submission and approval of a written request describing data security protocols and intended use.
Supplementary Material
Acknowledgments
We would like to thank the study participants’ willingness to share their experiences and time with us. We would also like to acknowledge the valuable contributions of the study staff, including Sean Farrell, Skylar Gross, Josh Haynes, Kelly Jones and Lisa Kennedy, as well as the study’s coinvestigators Hannah LF Cooper, Vivian F. Go, William C. Miller and Sharon Walsh. We also appreciate the individuals who contributed to our training video, including actors Brandi Taylor and Tony Vezina and filming and graphics experts Christi Hildebran and Eric Martin.
Footnotes
Contributors: The following individuals contributed to the design of the protocol described in this manuscript: AMY, KL, SB, MRE, RRC, GL, EF, ATE, MB, JL and PTK. The following individuals made substantial contributions to the implementation of the protocol: SB, GL, EF, ATE, MB, RA, CB, KC, RE, LM, RM and CN. The following individuals made substantial contributions to the drafted work and/or substantively revised it: AMY, KL, SB, MRE, RRC, GL, EF, ATE, MB, RA, CB, KC, RE, LM, RM, CN, JL, ENW and PTK. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work.
Funding: PROUD-R2 is funded by the National Institutes of Health through NCATS U01TR002631 (MPIs: Korthuis, Young). The Rural Opioid Initiative studies that are integrated with PROUD-R2 are funded by UG3/UH3 DA044798 (PIs: Young, Cooper), UH3DA044831 (PI: Korthuis), UH3DA044822 (PI: Miller, Go). Kathy Lancaster was supported by K01DA048174 (PI: Lancaster). The project is also supported by UL1TR002369 (Oregon Clinical & Translational Research Institute), UL1TR001998 (University of Kentucky Center for Clinical and Translational Science), and UL1TR001070 (The Ohio State University Center for Clinical and Translational Science) from the National Institutes of Health and K12 HS026370 from AHRQ/PCORI.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The study sponsor had no role in study design, collection, management, interpretation of data, writing of this manuscript, or decision to submit this manuscript for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Macrae J, Hyde P. HHS Launches Multi-pronged Effort to Combat Opioid Abuse U.S. Department of Health & Human Services, 2015. Available: http://www.hhs.gov/blog/2015/07/27/hhs-launches-multi-pronged-effort-combat-opioid-abuse.html [Accessed 03 Nov 2015].
- 2.Jones CM, Compton WM, Mustaquim D. Patterns and Characteristics of Methamphetamine Use Among Adults - United States, 2015-2018. MMWR Morb Mortal Wkly Rep 2020;69:317–23. 10.15585/mmwr.mm6912a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn KE, Barrett FS, Yepez-Laubach C, et al. Opioid overdose experience, risk behaviors, and knowledge in drug users from a rural versus an urban setting. J Subst Abuse Treat 2016;71:1–7. 10.1016/j.jsat.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health 2011;2011:1–10. 10.1155/2011/948789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hetrick AT, Young AM, Elman MR, et al. A cross-sectional survey of potential factors, motivations, and barriers influencing research participation and retention among people who use drugs in the rural USA. Trials 2021;22:948. 10.1186/s13063-021-05919-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Northrup TF, Greer TL, Walker R, et al. An ounce of prevention: a pre-randomization protocol to improve retention in substance use disorder clinical trials. Addict Behav 2017;64:137–42. 10.1016/j.addbeh.2016.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paquette DM, Bryant J, De Wit J. Use of respondent-driven sampling to enhance understanding of injecting networks: a study of people who inject drugs in Sydney, Australia. Int J Drug Policy 2011;22:267–73. 10.1016/j.drugpo.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 8.Young AM, Rudolph AE, Quillen D, et al. Spatial, temporal and relational patterns in respondent-driven sampling: evidence from a social network study of rural drug users. J Epidemiol Community Health 2014;68:792–8. 10.1136/jech-2014-203935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman L, Hammerback K, Snowden M. It could be a pearl to you: exploring recruitment and retention of the program to encourage active, rewarding lives (pearls) with hard-to-reach populations. Gerontologist 2015;55:667–76. 10.1093/geront/gnt137 [DOI] [PubMed] [Google Scholar]
- 10.Burlew K, Larios S, Suarez-Morales L, et al. Increasing ethnic minority participation in substance abuse clinical trials: lessons learned in the National Institute on drug abuse's clinical trials network. Cultur Divers Ethnic Minor Psychol 2011;17:345–56. 10.1037/a0025668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll KM, Ball SA, Jackson R, et al. Ten take home lessons from the first 10 years of the ctn and 10 recommendations for the future. Am J Drug Alcohol Abuse 2011;37:275–82. 10.3109/00952990.2011.596978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korte JE, Rosa CL, Wakim PG, et al. Addiction treatment trials: how gender, race/ethnicity, and age relate to ongoing participation and retention in clinical trials. Subst Abuse Rehabil 2011;2:205–18. 10.2147/SAR.S23796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magruder KM, Miller S, et al. Retention of under-represented minorities in drug abuse treatment studies. Clin Trials 2009;6:252–60. 10.1177/1740774509105224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto RM, Campbell ANC, Hien DA, et al. Retention in the National Institute on drug abuse clinical trials network women and trauma study: implications for posttrial implementation. Am J Orthopsychiatry 2011;81:211–7. 10.1111/j.1939-0025.2011.01090.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakim PG, Rosa C, Kothari P, et al. Relation of study design to recruitment and retention in CTN trials. Am J Drug Alcohol Abuse 2011;37:426–33. 10.3109/00952990.2011.596972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallum-Montes R, Morgan S, Rovito HM, et al. Linking Peers, patients, and providers: a qualitative study of a peer integration program for hard-to-reach patients living with HIV/AIDS. AIDS Care 2013;25:968–72. 10.1080/09540121.2012.748869 [DOI] [PubMed] [Google Scholar]
- 17.Bassuk EL, Hanson J, Greene RN, et al. Peer-Delivered recovery support services for addictions in the United States: a systematic review. J Subst Abuse Treat 2016;63:1–9. 10.1016/j.jsat.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 18.Bradford JB, Coleman S, Cunningham W. HIV system navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS 2007;21:S-49–S-58. 10.1089/apc.2007.9987 [DOI] [PubMed] [Google Scholar]
- 19.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl 1997;44:174–99. 10.2307/3096941 [DOI] [Google Scholar]
- 20.Heckathorn DD. Respondent-driven sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Soc Probl 2002;49:11–34. 10.1525/sp.2002.49.1.11 [DOI] [Google Scholar]
- 21.Malekinejad M, Johnston LG, Kendall C, et al. Using respondent-driven sampling methodology for HIV biological and behavioral surveillance in international settings: a systematic review. AIDS Behav 2008;12:105–30. 10.1007/s10461-008-9421-1 [DOI] [PubMed] [Google Scholar]
- 22.Lu X. Respondent-Driven Sampling: Theory, Limitations & Improvements. Solna, Sweden: Karolinska Institutet, 2013. [Google Scholar]
- 23.Magnani R, Sabin K, Saidel T, et al. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS 2005;19 Suppl 2:S67–72. 10.1097/01.aids.0000172879.20628.e1 [DOI] [PubMed] [Google Scholar]
- 24.Gallagher KM, Sullivan PS, Lansky A, et al. Behavioral surveillance among people at risk for HIV infection in the U.S.: the National HIV behavioral surveillance system. Public Health Rep 2007;122 Suppl 1:32–8. 10.1177/00333549071220S106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute on Drug Abuse . Grants awarded to address opioid crisis in rural regions, 2017. Available: https://www.drugabuse.gov/news-events/news-releases/2017/08/grants-awarded-to-address-opioid-crisis-in-rural-regions; [Accessed 15 Feb 2018].
- 26.US Census Bureau . Decennial census, table P2, 2010, updated decennially, 2010. [Google Scholar]
- 27.Appalachian Regional Commission . County economic status and distressed areas by state, FY 2021. Appalachian Regional Commission, 2021. [Google Scholar]
- 28.Van Handel MM, Rose CE, Hallisey EJ, et al. County-level vulnerability assessment for rapid dissemination of HIV or HCV infections among persons who inject drugs, United States. J Acquir Immune Defic Syndr 2016;73:323–31. 10.1097/QAI.0000000000001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruglass LM, Miele GM, Hien DA, et al. Helping alliance, retention, and treatment outcomes: a secondary analysis from the NIDA clinical trials network women and trauma study. Subst Use Misuse 2012;47:695–707. 10.3109/10826084.2012.659789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batista P, Deren S, Banfield A, et al. Challenges in recruiting people who use drugs for HIV-related biomedical research: perspectives from the field. AIDS Patient Care STDS 2016;30:379–84. 10.1089/apc.2016.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bower P, Brueton V, Gamble C, et al. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials 2014;15:399. 10.1186/1745-6215-15-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ickovics JR, Meisler AW. Adherence in AIDS clinical trials: a framework for clinical research and clinical care. J Clin Epidemiol 1997;50:385–91. 10.1016/S0895-4356(97)00041-3 [DOI] [PubMed] [Google Scholar]
- 33.Oransky M, Fisher CB, Mahadevan M, et al. Barriers and opportunities for recruitment for nonintervention studies on HIV risk: perspectives of street drug users. Subst Use Misuse 2009;44:1642–59. 10.1080/10826080802543671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barratt MJ, Norman JS, Fry CL. Positive and negative aspects of participation in illicit drug research: implications for recruitment and ethical conduct. Int J Drug Policy 2007;18:235–8. 10.1016/j.drugpo.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 35.Fry C, Dwyer R. For love or money? an exploratory study of why injecting drug users participate in research. Addiction 2001;96:1319–25. 10.1046/j.1360-0443.2001.969131911.x [DOI] [PubMed] [Google Scholar]
- 36.Peer-based retention of people who use drugs in rural research (PROUD-R2) team and Comagine health. PROUD-R2 study: research, recruitment, and retention, 2020. YouTube. Available: https://youtu.be/KzD3y5uXYnk
- 37.Heckathorn DD, Semaan S, Broadhead RS, et al. Extensions of respondent-driven sampling: a new approach to the study of injection drug users aged 18-25. AIDS Behav 2002;6:55–67. 10.1023/A:1014528612685 [DOI] [Google Scholar]
- 38.Malekinejad M, Johnston LG, Kendall C, et al. Using respondent-driven sampling methodology for HIV biological and behavioral surveillance in international settings: a systematic review. AIDS Behav 2008;12:105–30. 10.1007/s10461-008-9421-1 [DOI] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young AM, Jonas AB, Mullins UL, et al. Network structure and the risk for HIV transmission among rural drug users. AIDS Behav 2013;17:2341–51. 10.1007/s10461-012-0371-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young AM, Rudolph AE, Su AE, et al. Accuracy of name and age data provided about network members in a social network study of people who use drugs: implications for constructing sociometric networks. Ann Epidemiol 2016;26:802–9. 10.1016/j.annepidem.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Yu KF, Kai FY. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1. 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 43.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 2014;348:f7450. 10.1136/bmj.f7450 [DOI] [PubMed] [Google Scholar]
- 44.Kumwenda MK, Johnson CC, Choko AT, et al. Exploring social harms during distribution of HIV self-testing kits using mixed-methods approaches in Malawi. J Int AIDS Soc 2019;22 Suppl 1:e25251. 10.1002/jia2.25251 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-064400supp001.pdf (269.3KB, pdf)