Abstract
Understanding the mechanisms controlling forest carbon storage is crucial to support “nature-based” solutions for climate change mitigation. We used a dataset of 892 Atlantic Forest inventories to assess the direct and indirect effects of environmental conditions, human impacts, tree community proprieties, and sampling methods on tree above-ground carbon stocks. We showed that the widely accepted drivers of carbon stocks, such as climate, soil, topography, and forest fragmentation, have a much smaller role than the forest disturbance history and functional proprieties of the Atlantic Forest. Specifically, within-forest disturbance level was the most important driver, with effect at least 30% higher than any of the environmental conditions individually. Thus, our findings suggest that the conservation of tropical carbon stocks may be dependable on, principally, avoiding forest degradation and that conservation policies focusing only on carbon may fail to protect tropical biodiversity.
The human-induced disturbance is two- to sixfold more important than other drivers of tropical forest carbon stocks.
INTRODUCTION
Tropical forests play a central role in the carbon cycle on earth, regarding not only carbon flows but also terrestrial carbon stocks (1). We currently know that forest carbon stocks are determined by tree species proprieties, environmental conditions, and anthropic disturbances. Different tree community proprieties can increase carbon stocks through a more efficient use of the available resources (e.g., species niche complementarity) (2) and through the carbon storage potential of the most dominant species (e.g., functional traits) (3). Climate and soil conditions (e.g., temperature and soil fertility) can not only directly affect forest carbon (4, 5) but also control species composition, which in turn can affect the carbon storage potential of the forest (6, 7). Moreover, topography can influence carbon stocks through its influence on local soil conditions and sunlight incidence (8). Last, forest degradation and fragmentation not only cause the direct removal of biomass but also shift in species’ demography and functional tree composition [e.g., wood density (WD) and maximum height], which can translate into long-term losses of carbon storage potential (9–11).
Although different studies have evaluated the effects of tree community proprieties, environmental conditions, and human impacts on carbon stocks, the existing evidence is more concentrated in relatively undisturbed forests [see, e.g., (12, 13)] and it is inconsistent [see, e.g., (4, 5, 14, 15)]. Such inconsistency may be explained by differences in the role played by each driver across biogeographic contexts or in the methods used across studies, such as field protocols or carbon allometric equations (16). Furthermore, most studies have evaluated one or few drivers, which prevent more comprehensive assessments of their relative roles and of possible interactive effects among candidate drivers (17). Regardless of the reason, a better understanding of what drives forest carbon storage, especially in highly altered tropical forests, may anticipate the outcomes of global changes in more intact forests (e.g., Amazon) (18), optimize the efficiency of carbon conservation and restoration projects (19), and support nature-based solutions for climate change mitigation (19).
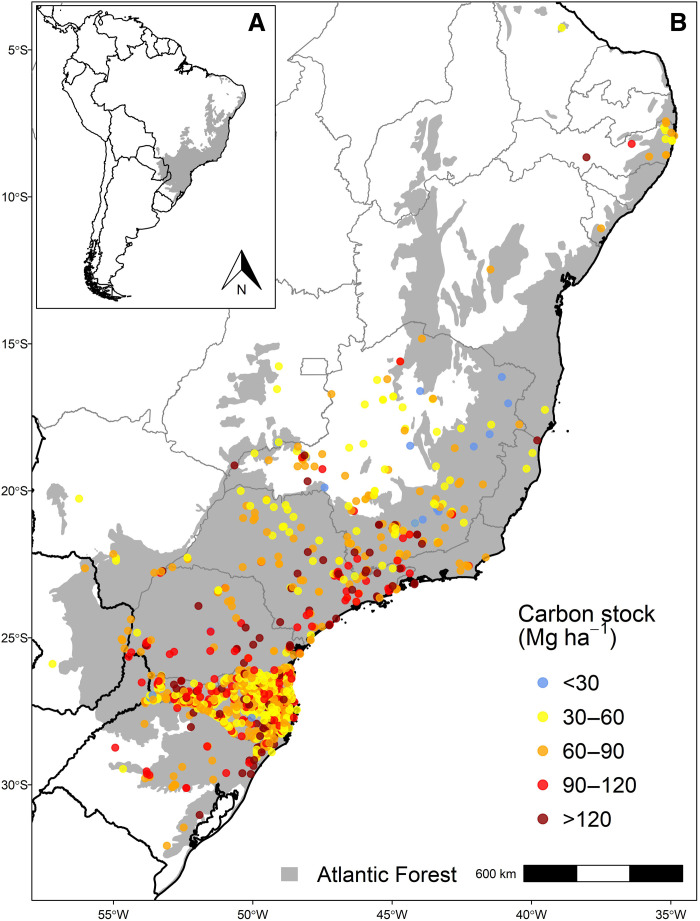
Here, we use a large dataset with 892 forest inventories to assess the relative role of tree community proprieties, environmental conditions, and human impacts as drivers of carbon stocks. We also assess the effect of field sampling methods on the estimation of above-ground carbon (AGC) stocks. We focus on the highly threatened Atlantic Forest (Fig. 1) (20, 21), which comprises a wide spectrum of environmental conditions and biogeographic and human intervention histories (22) and represents the present or the future of other tropical forests, providing a good testing ground to answer our questions. We use a causal mediation analysis, which allows for the simultaneous quantification of many different drivers, and the separation into their direct and indirect effects (23). On the basis of the present knowledge on forest carbon drives, we quantify the direct effects of tree community proprieties, environment, human impacts, and field methods and also the indirect effects of the environment and human impacts on carbon stocks through their effects on tree community proprieties. We address two questions: (i) what are the main drivers of the Atlantic Forest carbon stocks? and (ii) what are the direct and indirect effects of the different drivers? Last, we explore the implications of our results for the future of carbon stocks, projecting carbon losses and gains in scenarios of climate and human impacts changes.
Fig. 1. The distribution of the Atlantic Forest inventories included in this study.
For each inventory (points), we present the estimated AGC stock separated by classes (colors). The inventory data were obtained from the Neotropical Tree Communities database (24).
RESULTS
The relative roles of carbon stock direct drivers
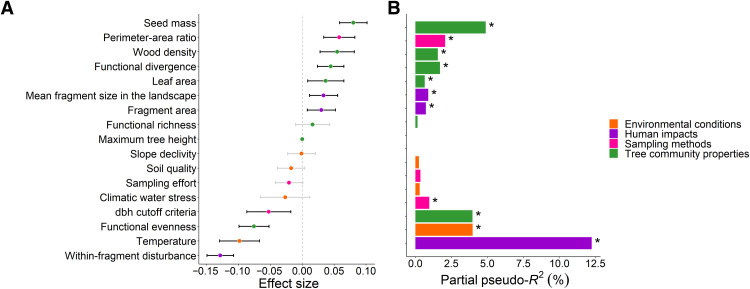
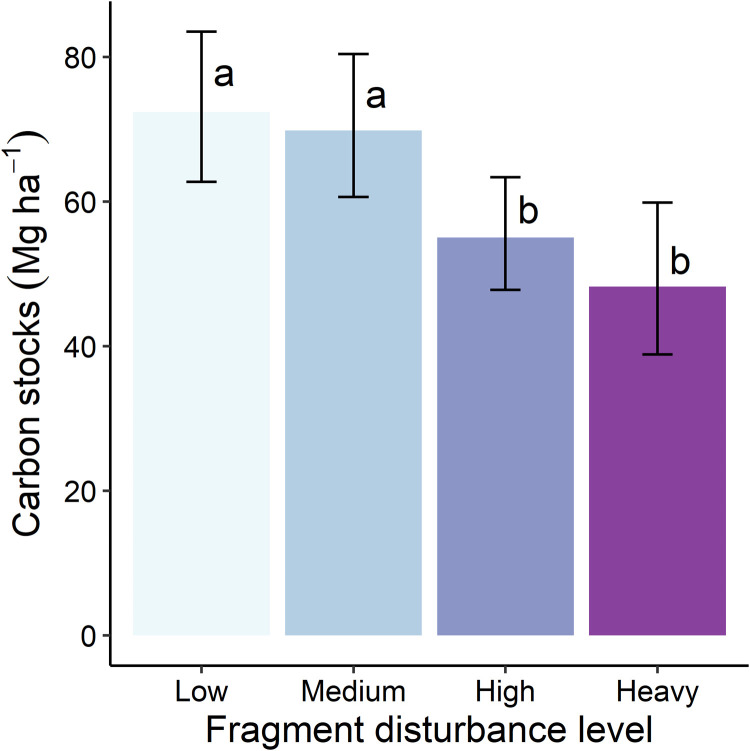
Human impacts, tree community proprieties, environmental conditions, and field sampling methods (i.e., fixed effects) explained 34.76% of the total variance of the forest AGC stocks in the Atlantic Forest, with relative contributions of 39.95, 37.36, 13.05, and 9.76%, respectively (Fig. 2 and table S1). The within-forest disturbance level was the main driver of carbon stocks, with an effect that was at least 30% greater than the climatic driver with the greatest effect, namely, temperature (Fig. 2 and table S1). Forests with heavy, high, and medium levels of human disturbance showed approximately 66, 76, and 96% of the carbon stocks found in fragments with low levels of human disturbance (Fig. 3 and tables S2 and S3).
Fig. 2. The main drivers of carbon stocks in the Atlantic Forest.
(A) Standardized estimates of the coefficients from averaged models containing the effects of environmental conditions, human impacts, tree community proprieties, and sampling methods on carbon stocks. (B) Bars show the partial pseudo-R2 values for each of the covariables (table S1) included in the averaged models (n = 892 inventories). Drivers with significant effects (P < 0.05) are shown with asterisks. The error bars show standard errors for 95% confidence intervals of the mean parameter estimates.
Fig. 3. Individual covariable with the greatest effect on the Atlantic Forest carbon stocks.
The effect of within-fragment disturbance level on AGC stocks (tables S2 and S3) was fitted with the optimum model (table S1). Within-fragment disturbance level is shown as a categorical variable (i.e., without ridit score transformation). Different letters are significantly different group means (P < 0.05). Error bars represent the 95% confidence intervals (n = 892 inventories).
The community-weighted mean (CWM) of seed mass (SM) was the second variable with the greatest effect size and partial pseudo-R2 on carbon stocks (Fig. 2). Carbon stocks increased with the abundance of large-seeded, large-leaved, hardwood species (Fig. 2 and table S1). Species functional evenness (FEve) had a negative effect on carbon stocks, while the functional divergence (FDiv) had a positive one. Potential maximum tree height and functional richness (FRic) had not displayed substantial effects on the carbon stocks (Fig. 2 and table S1). Carbon stocks decreased with temperature but were not affected by the climatic water stress (Fig. 2 and table S1). Soil quality and slope declivity did not have a significant direct effect on Atlantic Forest carbon stocks (Fig. 2 and table S1). The reduction of fragment size (at the local scale) and mean fragment size (at the landscape scale) both decreased the carbon stocks (Fig. 2 and table S1). Last, it is noteworthy that the effects of the dbh (diameter at breast height) cutoff criteria and particularly of the perimeter-area ratio of the sampling units were equal or greater than some of the environmental, tree community, and human-related variables (Fig. 2 and table S1). The sampling effort did not have a significant effect on carbon stock estimates (Fig. 2 and table S1).
Indirect effects of environment and human impacts on carbon stocks
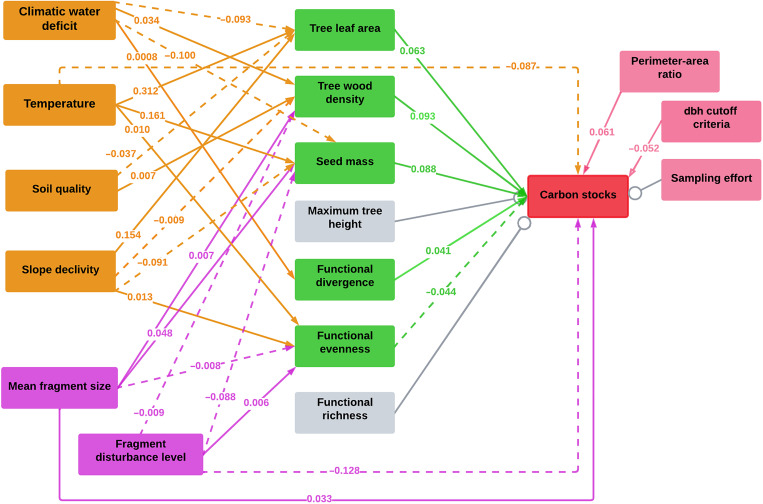
We found a considerable influence of the environmental conditions and human impacts on carbon stocks via effects on the tree community proprieties (Fig. 4 and Table 1). Therefore, in addition to their direct effects, temperature, mean fragment size, and within-fragment disturbance level also presented indirect effects on carbon stocks. The stocks increased with mean fragment size, while they decreased with temperature and within-fragment disturbance level (Fig. 2). The indirect effects of within-fragment disturbance level on carbon stocks were predominantly negative (via SM, WD, and FEve), whereas the indirect effects of temperature and mean fragment size in the landscape [via leaf area (LA), WD, FEve, and FDiv] were predominantly positive (Fig. 4 and Table 1). Although climatic water stress, soil quality, and slope declivity showed no significant direct effect on carbon stocks (Fig. 2), they affected the tree community proprieties, resulting in significant but indirect effects on carbon stocks (Fig. 4 and Table 1). Climatic water stress (via SM, WD, LA, and FDiv), soil quality (via WD and LA), and slope declivity (via SM, WD, LA, FEve, and FDiv) indirectly decreased the Atlantic Forest carbon stocks (Fig. 4 and Table 1).
Fig. 4. Causal mediation analysis describing the direct and indirect effects of multiple drivers on the carbon stocks (n = 892).
Solid lines indicate positive significant effects (P < 0.05), whereas dotted lines indicate negative significant effects (P < 0.05). Environmental conditions with significant effects are indicated in orange, human impacts in purple, tree community properties in green, and field sampling methodology aspects in pink. Variables with nonsignificant effects on carbon stocks are indicated in gray. All model estimates are presented in table S4.
Table 1. Indirect effects of environmental conditions and human impacts on carbon stocks.
Standardized coefficients with a significance level (significant; P < 0 0.05) are given for all relationships. WD, CWM wood density; Seed mass, CWM seed mass; Leaf area, CWM leaf area; FEve, functional evenness; FDiv, functional divergence (n = 892). Climatic water deficit was −1 transformed, and WD, Seed mass, and Leaf area were transformed in the natural logarithmic scale. All models were fitted with scaled drivers.
| Driver | Via | Indirect effect | P value |
| Climatic water deficit | FDiv | 0.00031 | 0.044 |
| Climatic water deficit | FEve | ||
| Climatic water deficit | WD | 0.00222 | <0.0001 |
| Climatic water deficit | Seed mass | −0.00723 | 0.006 |
| Climatic water deficit | Leaf area | −0.00358 | 0.01 |
| Slope declivity | FDiv | ||
| Slope declivity | FEve | −0.00099 | <0.0001 |
| Slope declivity | WD | −0.00058 | <0.0001 |
| Slope declivity | Seed mass | −0.00661 | <0.0001 |
| Slope declivity | Leaf area | 0.00591 | 0.01 |
| Within-fragment disturbance level | FDiv | ||
| Within-fragment disturbance level | FEve | −0.00046 | 0.006 |
| Within-fragment disturbance level | WD | −0.00059 | 0.004 |
| Within-fragment disturbance level | Seed mass | −0.00637 | <0.0001 |
| Within-fragment disturbance level | Leaf area | ||
| Soil quality | FDiv | ||
| Soil quality | FEve | ||
| Soil quality | WD | 0.00050 | 0.002 |
| Soil quality | Seed mass | ||
| Soil quality | Leaf area | −0.00144 | 0.048 |
| Mean annual temperature | FDiv | ||
| Mean annual temperature | FEve | −0.00076 | 0.002 |
| Mean annual temperature | WD | ||
| Mean annual temperature | Seed mass | 0.01180 | <0.0001 |
| Mean annual temperature | Leaf area | 0.01200 | 0.004 |
| Mean fragment size | FDiv | ||
| Mean fragment size | FEve | 0.00067 | <0.0001 |
| Mean fragment size | WD | 0.00047 | 0.008 |
| Mean fragment size | Seed mass | 0.00346 | 0.038 |
| Mean fragment size | Leaf area |
Carbon gains and losses to climate and forest human disturbance changes
Projecting the carbon losses, we found that an increase of the within-forest disturbance level in 100, 50, and 25% of the Atlantic Forest fragments with low to medium levels of disturbance to heavy and high levels would represent losses of 15.24, 8.09, and 4.20% of Atlantic Forest carbon stocks (Table 2), respectively. If temperature increases 1.5°C without changes in precipitation, the regional carbon losses could be 5.12%, while an increase of 4°C in global temperatures may result in a decrease of 13.11% of carbon stocks across Atlantic Forest (Table 2). Projecting the carbon gains, if 100, 50, and 25% of fragments with heavy and high levels of human disturbance advance in their successional trajectory to fragments with medium and low levels of human disturbance, the regional carbon gains would be 17.44, 8.42, and 4.03%, respectively (Table 2).
Table 2. Carbon gains and losses from forest human disturbance and climate changes.
The predictions were obtained from the direct and indirect effects provided by mediation causal analysis (Fig. 3 and Table 1). Carbon change estimates are shown according to the difference between the current carbon stock estimates and the projected carbon stock estimates. Current carbon stock: 68.9 (Mg ha−1). MAT, mean annual temperature.
| Human impacts scenarios | |||
| AGC stocks (Mg ha−1) | AGC stocks changes (%) | AGC stocks (Mg ha−1) | |
| Optimistic scenario | |||
| 100% of fragments with high and heavy levels of disturbances are recovered to medium and low levels of disturbances |
80.92 | 17.44 | 12.02 |
| 50% of fragments with high and heavy levels of disturbances are recovered to medium and low levels of disturbances |
74.70 | 8.42 | 5.80 |
| 25% of fragments with high and heavy levels of disturbances are recovered to medium and low levels of disturbances |
71.79 | 4.20 | 2.896 |
| Pessimistic scenario | |||
| 100% of fragments with low and medium levels of disturbances are degraded to high and heavy levels of disturbances |
58.39 | −15.24 | −10.50 |
| 50% of fragments with low and medium of disturbances are degraded to high and heavy levels of disturbances |
63.32 | −8.09 | −5.57 |
| 25% of fragments with low and medium of disturbances are degraded to high and heavy levels of disturbances |
66.12 | −4.03 | −2.77 |
| Climate impacts scenarios | |||
| AGC stocks (Mg ha−1) | AGC stocks changes (%) | AGC stocks (Mg ha−1) | |
| MAT increases by 2°C | 65.36 | 5.12 | −3.53 |
| MAT increase by 4°C | 59.86 | 13.11 | −9.03 |
DISCUSSION
Given the global urgency to mitigate climate change, understanding the drivers of carbon stocks in tropical forests is increasingly important (24). Here, we found that widely accepted drivers of carbon stocks, such as climate, soil, topography, and forest fragmentation, have a much smaller role than forest human disturbances and tree functional proprieties. In the Atlantic Forest, the greatest driver of the variation in carbon stocks was the level of human disturbances within fragments, with a role two- to sixfold greater than any other variable included in the analysis (Fig. 2 and Table 1). The greater accessibility to forest fragments increases their exposure to fire, selective logging, and fuelwood extraction (25). In addition, the opening of the fragment canopy and microclimatic changes created by disturbances can increase tree damage and mortality even after the disturbance has ceased (26, 27). Along with fragment disturbance, reductions in the fragment size and mean fragment size in the landscape also played an important role (Fig. 2 and Table 1). In small fragments, tree mortality is usually induced by stronger edge effects, which alter the fragment microclimate and increase wind turbulence (26). Furthermore, logging and forest ground fires can be aggravating sources of tree mortality in landscapes marked by high levels of forest fragmentation (28).
Tree community proprieties were also important, with larger carbon stocks found in forests dominated by heavy-seeded, large-leaved, hardwood trees (Fig. 2 and Table 1). Hardwood species can accumulate not only more carbon per unit of biomass but also more carbon over time due to the lower inherent mortality, turnover, and stem breakage (29). SM does not directly affect species’ carbon storage potential, but it correlates well with species longevity (30) and carbon storage potential (31). In addition, if water availability is not a limiting factor, species with larger LAs can be more efficient to intercept light (32), an essential resource for the growth and thus carbon assimilation. It has been well documented that carbon stocks are determined by these functional traits. However, our result contrasts with those of other studies conducted in Amazonia, where WD was the main trait controlling the variation in carbon stocks (33–35). In Atlantic Forest, the functional trait with the greatest effect on carbon stocks was SM, with an effect at the least 40% greater than WD (Fig. 2 and table S1).
We also found that how species concentrate and diverge in their functional niche spaces matters for carbon storage. In the Atlantic Forest, the negative effect of FEve was substantially greater than the positive effect of FDiv on carbon stocks. Thus, we found more evidence supporting the mass ratio hypothesis, which proposes that carbon stocks are determined by the characteristics (traits) of the most dominant species in the community (3), than the niche complementarity hypothesis, which predicts that the dominant species in the community have opposite functional trait values (i.e., different ecological strategies) allowing to accumulate more carbon due to a more efficient use of the available resources (2). Therefore, we learned that not only the abundance of heavy-seeded, large-leaved, hardwood tree species (36) but also the concentration of species exhibiting similar strategies of resource use (i.e. lower FEve) (37) are important for determining higher carbon stocks.
We found smaller carbon stocks in sites with higher temperatures, where the rates of most of the ecophysiological processes that control primary productivity (i.e. photosynthesis and respiration) are higher (38). In boreal and temperate forests, increases in temperature allow plants to come close to the maximum photosynthetic threshold, increasing their carbon assimilation (39). However, in the warmer conditions of tropical and subtropical forests, the increase in temperature leads to higher maintenance costs for trees (e.g., higher respiration costs) and, thus, to a decrease in their carbon storage potential (4, 38).
The effects of field sampling methodology on forest carbon estimates are poorly documented (40) and, as far as we know, this is the first time that their effects are weighted against well-known drivers of carbon storage. Here, we found that field methods can be as important as some environmental and biological variables to explain the variation in carbon stock estimates. The effect of the dbh cutoff criteria was expected (due to the inclusion of more or fewer trees), and we reveal that the shape of the sampling units (i.e., plots) also plays an important role in carbon storage. Studies carried out using more elongated sampling units (i.e., higher perimeter-area ratio) tended to overestimate AGC stocks, which is probably related to the inclusion or exclusion of larger trees close to the plot limits (41). Therefore, we reinforce here that larger and less elongated sampling units (e.g. 20 × 20 m or higher) should be used to improve the accuracy of carbon stocks estimation (40, 42). More generally, sampling aspects other than the total sampling area should be accounted for while modeling biomass stocks, particularly when using data from datasets using different sampling strategies. Our results suggest that the simple standardization of carbon estimates by the sampling effort alone (e.g. (14, 15, 43) is not enough to guarantee unbiased interpretations of carbon stocks drivers.
Indirect effects of environment and human impacts on carbon stocks
While it has been documented that environmental conditions and human impacts affect forest carbon stocks, most studies have focused exclusively on direct effects, overlooking their indirect effects (43). Here, we found that indirect effects of human impacts on carbon stocks via changes in species functional proprieties were predominantly negative (Table 1). The heterogeneity of disturbed fragments or landscapes may exclude competitively dominant species (i.e., high FEve) (44) and favor the proliferation of pioneer species, which have relatively low WD and light SM, contributing to further reductions in carbon (9).
Temperature also presented both direct and indirect effects on carbon stocks. The abundance of heavy-seeded and large-leaved trees increased in warmer temperatures, resulting in a positive indirect effect on carbon stocks. The positive relationship between temperature and SM is considered an adaptation to improve germination rates in higher temperatures (45). On the other hand, warmer climates tend to present a greater dominance of small-leaved species due to the increase in transpiration rates (46). In the Atlantic Forest, there probably is enough water available for an effective transpiration cooling, allowing large-leaved species to assimilate carbon even in warmer climates (47). Furthermore, the greater FEve among dominant species slightly decreased carbon stocks in warmer climates. But if we consider together all indirect effects, the overall effect of temperature on carbon stocks remained positive (Table 1 and table S1).
Climatic water stress [i.e., CWD (climatic water deficit)] decreased the abundance of heavy-seeded and large-leaved trees, while it increased the FDiv and the abundance of hardwood species (Fig. 4). Because the effect of the decrease in the abundance of heavy-seeded species was three times higher than any other indirect effects, the overall indirect effect of CWD on carbon stocks was negative (Table 1). Water stress conditions may favor the reduction of the functional similarity between co-occurring abundant species (increases in FDiv), probably due to the higher influence of resource competition (9). Moreover, because of the fibers and thick-walled vessels, higher WD protects against vessel implosion and allows species survival during periods of water deficit (48). However, these positive indirect effects on carbon stocks were compensated by the higher abundance of light-seeded and small-leaved trees in forests under drier climates.
Although soil quality and slope declivity showed no significant direct effect on carbon stocks (Fig. 2), they affected the tree community property variables (Fig. 4), which resulted in significant indirect effects on carbon stocks (Table 1). Soil quality increased the abundance of hard-wood trees and decreased the abundance of large-leaved ones, resulting in an overall negative indirect effect on carbon stocks (Table 1). Although we showed that low-fertility soils have higher carbon stocks, as many findings of several other studies conducted in Amazonia (5), unlike many others, this negative effect is due to the greater dominance of species with greater LA in low-fertility soils. Steeper slopes had smaller carbon stocks (Table 1), mainly driven by the decrease in the abundance of species WD, LA, and SM and by the increase in FEve (Fig. 4). On steep slopes, soil leaching, water runoff, higher light incidence, and wind exposure filter out some plant strategies that are not already adapted to these conditions (49) and favor the proliferation and survival of individuals with a short life span (i.e., lighter WD and SM) (50).
Implications for carbon protection policies
Under the current global change scenario, the conservation and restoration of forest carbon have attracted unprecedented attention. Here, we provided a comprehensive assessment of the main drivers of carbon stocks for the Atlantic Forest with important implications for nature-based solutions to mitigate climate changes. First, the conservation of the Atlantic Forest carbon stocks is highly dependent on avoiding forest degradation, which can generate carbon losses at least 30% higher than any future climate change. Moreover, emissions from forest degradation can jeopardize efforts of conservation planning and climate change mitigation agreements (e.g., REDD+ and AICHI targets). For instance, the intensification of within-fragment disturbances could lead to carbon losses of up to 10.50 Mg ha−1 (−15.24%), while the carbon protection and enhancement could achieve carbon gains by 12.02 Mg ha−1 (+17.44%) (Table 2). Second, the Atlantic Forest carbon stocks are also threatened by climate changes, specifically increases in temperature and water stress. If global warming were constrained to 1.5°C above preindustrial levels, as suggested by Intergovernmental Panel on Climate Change [(IPCC-54) (51)], 3.53 Mg ha−1 (−5.12% carbon loss) of carbon would be released only from the Atlantic Forest. If global warming continues at its current rate, carbon emissions can exceed 9.03 Mg ha−1 (−13.11% carbon loss) (Table 2). Third, initiatives aimed at mitigating climate change through the restoration of forest ecosystems could benefit from including species with greater WD, heavier seeds masses, and larger leaves. Fourth, the relationship between the taxonomic and functional diversity and carbon stocks was weak in Atlantic Forest, revealing that conservation policies focusing only on carbon may fail to protect biodiversity and highlighting the importance of separate add-on incentive mechanisms to achieve biodiversity conservation as well (52). Last, policies of carbon conservation should take into account the sampling methodology aspects across inventories, which can lead to biases in carbon estimation and, consequently, the misinterpretation of the efficiency of climate mitigation actions. Thus, the use of “good measurement practices to carbon stocks estimation” across forests (53) should be accounted for in carbon stock reporting.
MATERIALS AND METHODS
Forest inventories
We used forest inventory data stored in the Neotropical Tree Communities database [TreeCo (20, 21)] continuous effort to compile and organize plant community data in eastern South America (http://labtrop.ib.usp.br/doku.php?id=projetos:treeco:start). Here, we selected data from forest inventories with any type of stand biomass estimate [i.e., basal area (BA) or above-ground biomass (AGB)] conducted in any Atlantic Forest types. Aiming to reduce the noise in the dataset, we applied four filters to the inventory data: (i) a total sampling area equal or larger than 0.25 ha; (ii) a cutoff criterion of stem dbh above 4.8 cm (e.g., dbh ≥ 5.0 cm and dbh ≥10.0 cm); (iii) data on species names, abundance, and biomass fully available and extractable; (iv) inventories with species trait data (see details below) that together made up at least 80% of the total community abundance. The first filter was applied to reduce common overestimation biases of AGC related to small sample sizes (16). The second filter was applied to avoid including tree regeneration and shrubs data. The third and fourth were necessary to calculate biotic metrics and ensure that they were representative of the community (54). In the end, we performed data analysis using a subset of 892 forest inventories (Fig. 1).
AGC stocks
Among the 892 inventories considered here, 365 contained only estimates of the stand BA (m2 ha−1) and 527 contained estimates of both BA and AGB (Mg ha−1). To make the most out of the available data, we built an equation based on the inventories that had both BA and AGB to obtain AGB from BA estimates (Eq. 1). To do this, we first calculated the AGC stocks (i.e., AGC or carbon stocks) by multiplying AGB by the conversion of 0.456 g of carbon per gram of AGB (55). Then, because inventories used 20 different allometric equations to estimate biomass (table S5), we converted the AGC values obtained using each of the equations to the value expected using a single and common allometric equation, which here was the one proposed by Chave et al. (18). To perform this correction, we used individual tree dbh and height measurements available from 109 Atlantic Forest inventories available from the Minas Gerais Forest Inventory (56) to estimate the relationships between each of the 20 allometric equations and the one equation proposed by Chave et al. (18). A simple linear regression model was used to describe the relationship between each pair of allometric equations and for all pairs of equations; the variance explained by the model was above 93% (fig. S1). After obtaining AGC values using a common allometric equation for the 527 inventories with both BA and AGC (or AGB), we compared the performance of 2 linear and 12 nonlinear candidate equations to select the one that best described the relationship between BA and AGC. The selection was based on the visual assessment of the residues and the lowest value of Akaike information criterion (AIC) (fig. S2 and table S6). The Gompertz equation had the best performance (fig. S3), and it was used to obtain AGC from BA for the all 892 of inventories
| (1) |
where AGC is given in Mg ha−1 using the allometric equation of (18) and BA is given in m2 ha−1.
Preselection of covariables
A wide range of covariables associated with each inventory could be combined to explain the variation of AGC stocks, generating thousands of possible models. To limit the number of possible models, we performed model construction and selection in two steps. First, we separated the available covariables in groups based on our a priori hypotheses (i.e., climatic, topographic, soil, biological, human-related, and methodological covariables; fig. S4 and data S1). We then performed a preselection of the candidate covariables within each of these groups based on their individual contribution to model performance (i.e., AIC and R-squared of the models) and based on their biological meaning and ease of interpretation in the context of global changes. We constructed 54 candidate models including several possible combinations of the preselected candidate covariables related to environmental conditions (17 covariables), human-related impacts (7 covariables), tree community diversity (8 covariables), and field sampling methodology (3 covariables) (data S1). To select the best candidate covariables, each covariable was included individually in the model, and its additional contribution to improving the model performance was evaluated by the decrease in the model AIC value, and in case of minimal change in the AIC value, we evaluated the increase in the full model explained variance, R2. At this point, the collinearity was not evaluated because our intention was only to select candidate covariables and not to estimate the regression coefficients. The final candidate covariables selected for modeling carbon stocks included four environmental [i.e., mean annual temperature (MAT), CWD, slope declivity, and soil quality], three human-related (i.e., within-fragment disturbance level, mean fragment size in the landscape, and fragment size), seven related to tree community proprieties and diversity (i.e., CWM of tree maximum height, WD, LA, SM, FRic, FEve, and FDiv), and three related to the field sampling methodology used in the inventories (i.e., dbh cutoff criteria, perimeter-area ratio, and sampling effort).
Site descriptors
To describe local climate conditions, we extracted the MAT (°C) and CWD (mm) from the geographical coordinates of each inventory. MAT was obtained from the maps provided by Alvares et al. (57, 58) at 100-m resolution and ranged in our dataset from 11.5° to 25.6°C. For inventories from Paraguay and Argentina, not covered by Alvares et al. (57, 58), MAT was obtained from WorldClim 2 at 1-km resolution (59). The long-term CWD was obtained from maps provided by Chave et al. (18) at ~4.5-km resolution (available at https://chave.ups-tlse.fr/pantropical_allometry.htm). This variable is calculated as the total rainfall minus evapotranspiration during the dry months, when evapotranspiration is equal or exceeds precipitation and is commonly used to reflect seasonal water stress. As CWD is by definition negative, we decided to multiply it by −1 to facilitate its interpretation. Thus, sites with higher CWD values are more seasonally water-stressed.
Slope declivity (°) was the selected covariable to describe site topography (range in the dataset: 0° to 44°), and it was calculated from the shuttle radar topography mission elevation data (~30-m resolution) (60). To represent the soil conditions, we used the soil quality index, which takes into account the soil depth, fertility, drainage, and aluminum toxicity for plant growth (21). Each soil attribute (depth, fertility, drainage, and toxicity of aluminum) was classified with a value of 0 (worst quality for plant growth) to 4 (better quality for plant growth). Thus, this index ranged from 0 (worst quality) to 16 (best quality). The soil type information necessary for the construction of this index was extracted from the original publication or, when absent, from local, state, and/or national soil maps. We used the database of soil profiles provided by Benedetti et al. (61) to obtain mean soil physical properties.
To describe human impacts–related covariables, three were selected because they explained better the variation of carbon stocks in our dataset (see data S1): within-fragment disturbance level, fragment size, and mean fragment size in the landscape. The within-disturbance level of fragment was classified on the basis of the information about the type, intensity, and timing of human disturbances (i.e., selective logging, fire, hunting, and thinning) and/or forest successional stage (e.g., initial, early, and late secondary or advanced and old growth) provided by the authors of the inventories. Although we recognize that natural disturbance events can alter the carbon stocks of forest fragments, these events are rare, so we assumed that the major drivers of forest succession are related to human disturbances (e.g., clear-cutting, logging, and fire). Then, we considered four levels of within-fragment disturbance: heavy (N = 11), high (N = 423), medium (N = 285), and low (N = 173 inventories). Heavy levels are represented by early secondary forest regrowing after clear-cut 10 to 20 years before the inventory, locally known as “capoeiras.” The high level represents chronically disturbed fragments, typically disturbed less than 50 years before the inventory. The medium level represents lightly or sporadically disturbed fragments, and/or forests disturbed 50 to 80 years before the inventory. Last, the low level represents fragments without records of disturbances or those undisturbed for at least 80 years. We recognize that this is a rather coarse classification, with substantial variation in forest conditions within levels; however, we were unable to refine this classification any further because of a lack of more objective and detailed information in the original publications. Still, this classification has support in the legal classification of the Atlantic Forest (62) and is the best information available to take into account within-fragment disturbances across the entire Atlantic Forest (21).
The size of the inventoried fragment was extracted from the original publications and double-checked by comparing it with other sources of information (63). The mean fragment size is the mean area of all fragments present in 4 × 4–km landscape subset centered on the coordinate of each inventory. Landscapes were obtained from vegetation cover maps [30-m resolution (64)], and a 70% canopy closure threshold was used to classify landscapes into fragment or nonfragment pixels. Classified landscapes were then used to calculate mean fragment size and other landscape metrics not used for analyses (21). Smaller mean fragment sizes are indicative of higher forest loss and lower habitat amount. Landscape metrics were extracted in R version 4.2.0 (2022) using the contributed packages “raster” and SDMTools (65).
To assess effects of tree community properties on AGC stocks, we used three types of metrics: (i) CWM of species trait values; (ii) functional trait diversity indices; and (iii) a taxonomic diversity index, namely, the Shannon-Wiener index. CWM represents the central tendency of the species traits in the community and was calculated as the mean of each trait weighted by the abundance of the species in each community. The abundance was chosen as a weighting factor because BA is strongly related to community biomass/carbon. We computed the CWM of four species-level traits that are considered important for carbon accumulation: maximum height (Hmax, m), WD (g cm−3), SM (g), and LA (cm2). The species maximum height was calculated as the 90th percentile height of all trees of the species, and species WD was obtained from the Global Wood Density database [filtered by Tropical South America (36)]. SM and LA values were obtained in the literature (21). For the species with no available WD, SM, and LA, we used the genera or family mean values. In the end, 100% of the species had values of maximum height and WD, 76% had values of SM, and 42% had values of LA. For WD, CWM was obtained after removing palms, palmoids, cacti, and tree ferns. For maximum height, we removed shrubs before the calculation of CWM. For SM, we removed tree ferns before the calculation of CWM.
We used three measures of functional diversity: FRic, FEve, and FDiv. Functional indices were calculated for each inventory based on Hmax, WD, SM, LA, and other species traits (i.e., leaf type and dispersion syndrome) available from the TreeCo database [see (21) for the full list of trait sources stored in TreeCo].
We included as many traits as available for computing indices so we could better describe the functional composition of the community, and not regarding those traits that are related to species carbon storage potential. FRic represents the amount of niche space filled by species in the community. FEve represents the regularity in the distribution of species dominance and reflects how thoroughly the resources available are being exploited by the plant community and is higher when the functional strategies of co-occurring species are evenly distributed in relation to resource use (37). FDiv represents the functional distance among the most dominant species and is higher when the dominant species have high functional trait differentiation (37). For FEve and FDiv, species abundances were used as weights to generate species multivariate-trait spaces. CWMs and functional and diversity indices were calculated in R version 4.2.2 (2022) using packages “FD” and “vegan” (66).
Last, we obtained for each inventory the sampling methods used to estimate carbon stocks. The dbh cutoff criteria were obtained from the original publications and ranged in our dataset from 4.8 to 20 cm. The perimeter-area ratio of the sampling units was obtained from the dimensions of sampling units (range, 0.025 to 0.92), and it provides a simple quantitative description of the shape of the sampling unit: The greater the ratio, the more elongated the plot sample units are. Sampling effort was obtained from the original publications, and it ranged from 0.25 to 26 ha.
Statistical analysis
The statistical analysis was divided into two parts. First, we assessed the relative role and direct effects of environmental conditions, human impacts, tree community proprieties, and sampling methods on carbon stocks using an approach based on model selection and multimodel inference. In the second part, we assessed the indirect effects of environmental conditions and human impacts on carbon stocks mediated by tree community proprieties using causal mediation analysis. Although both analyses were based on regression models and causal mediation analysis may also provide the direct effects, the separation was necessary because it would be possible neither to achieve the variation in carbon stocks explained by each covariable nor to compare the effect of important but highly correlated covariables (Spearman’s Rho coefficient < 0.6), as fragment size and mean fragment size (fig. S5), avoiding multicollinearity issues.
Model selection and multimodel inference were performed with all the candidate covariables selected in preselection (data S1). We constructed candidate models using all possible combinations of the covariables and ranked them based on the model AIC and performed a model selection based on the lowest AIC values (ΔAICc ≤ 4) followed by model averaging to infer about the relative effects of individual variables. The selected models were constrained to have covariables with Spearman’s coefficients lower than 0.6 (fig. S5) and low variance inflation values (VIF ≤ 4) (67). As no individual model had support from the data to be considered as the single best model (AIC weights < 0.10), the first 40 equally plausible candidate models (i.e., delta AIC ≤ 4 and AIC weights sum > 0.77) were averaged to address the uncertainty in the selection of the best candidate covariables (68). Last, to describe the relative variable importance of each covariable, we calculated the partial pseudo-R2 (table S1), which represents the variance explained by each covariable taking into account the effects of other covariables present in the model.
Causal mediation analysis was performed because it allows the simultaneous computation of multiple paths [see, e.g., (23, 69)]. Linear mixed-effects models were created as the basis of the mediation analysis: first, models expressing variation in tree community property variables (i.e., mediator) in relation to environmental conditions and human impacts (the “mediator model”; table S4, A to E), and then a model expressing variation in carbon stocks in relation to the mediators, environmental conditions, and human impacts, considering the effects of all covariables (the “outcome model”; table S4F). Last, we constructed mediation models (effects of X via M on Y; fig. S5) to identify how much of the effect of environmental conditions and human impacts were direct and how much were indirect, mediated by tree community property variables. Indirect effects represent the expected difference in the potential outcome when the mediator took the value that would realize under the treatment condition as opposed to the control condition, while the treatment status itself is held constant (70, 71). Given the impossibility to constrain highly correlated covariables (>0.6) in causal mediation analysis, mean fragment size in the landscape was included in this analysis rather than fragment size (fig. S5).
For the mixed-effects models and causal mediation analysis, the biogeographical subregions of the Atlantic Forest (72) were defined as random effects (to account a possible lack of independence between sites within the same biogeographical region). The carbon stocks of each inventory were ln-transformed to (i) achieve the residual normality and homoscedasticity assumptions, (ii) reduce the effect of outliers, and (iii) account for possible nonlinear relationships between variables. We also ln-transformed the CWM of functional traits before analyses. The within-fragment disturbance level, the only ordinal variable, was transformed into a continuous variable using “ridit scores” by assigning values of 0 (bottom of hierarchy, heavy level of within-fragment disturbance) to 1 (top of hierarchy, low level of within-fragment disturbance), reflecting the relative ranking of each level (73). All covariables were standardized to a mean of zero and an SD of one to allow comparisons of the strength of the effects among variables of the model.
Residual diagnostic plots were used to examine all model residual normality and homoscedasticity assumptions (fig. S7). We also used correlograms of Moran’s I to assess the spatial autocorrelation of model residuals. When the presence of spatial autocorrelation was significant, we added spatial filters to the models [Moran’s eigenvector maps (74)]. The “mediation” package does not provide a validation function to assess the goodness of fit of mixed regression models. In this way, the validation of the causal mediation analyses was achieved by ensuring the fit of all models included in the analyses (fig. S7 and table S4). Analyses and graphs were performed using R version 4.2.0 (2022) and the following packages: mediation (75), lme4 (76), MuMIn (77), and ggplot2 (78). The Moran’s I tests and correlograms were performed using the spDep (79) and ncf (80) packages.
Predicting carbon gains and losses to climate and forest human disturbance changes
We used the direct and indirect effects provided by mediation causal analysis (Fig. 4 and Table 1) to predict the impact of changes in climate and fragment disturbance on the future carbon stocks across Atlantic Forest. Predictions of fragment human disturbances were made for two different scenarios: an optimistic and a pessimistic scenario. In the optimistic scenario, we assumed a widespread decrease in fragment disturbance and projected carbon gains related to the advance of fragments with heavy and high levels of disturbance to medium and low levels of disturbance, respectively. In the pessimist scenario, we assumed an increase of fragment disturbances so that fragments with low and medium levels of disturbance are disturbed to heavy and high levels, respectively. Future climate changes were simulated on the basis of the IPCC Special Report (81), and as above, we simulated different scenarios: a stringent mitigation scenario [RCP2.6 (81)] and scenarios without additional efforts to constrain emissions (“baseline scenarios”) [RCP6.0 and RCP8.5 (81)]. The first scenario (RCP2.6) aims to keep global warming below 2°C of preindustrial temperatures (1850 to 1900), and in the second scenario, we assume an increase in mean temperature by 4°C of preindustrial levels (81).
Acknowledgments
Funding: TreeCo database was funded by grant 2013/08722-5, São Paulo Research Foundation (FAPESP). L.F.S.M. was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant 308575/2019-9; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grants 308575/2019-9, 309764/2019, and 311303/2020; and São Paulo Research Foundation (FAPESP) grant 2013/08722-5.
Author contributions: This study was conceived and designed by M.V.P., L.F.S.M., and R.A.F.L. Data were compiled and/or validated by R.A.F.L., G.P., A.L.d.G., and A.C.V. Data analysis was conducted by M.V.P. with the support of V.A.M. M.V.P. drafted the paper, and all authors have revised the subsequent drafts. All authors have contributed substantially to the interpretation and discussion of the results.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S7
Tables S1 to S6
Other Supplementary Material for this manuscript includes the following:
Data S1
REFERENCES AND NOTES
- 1.Keith H., Vardon M., Obst C., Young V., Houghton R. A., Mackey B., Evaluating nature-based solutions for climate mitigation and conservation requires comprehensive carbon accounting. Sci. Total Environ. 769, 144341 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Chen H. Y. H., Reich P. B., Forest productivity increases with evenness, species richness and trait variation: A global meta-analysis. J. Ecol. 100, 742–749 (2012). [Google Scholar]
- 3.Grime J. P., Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 86, 5 (2002). [Google Scholar]
- 4.Stegen J. C., Swenson N. G., Enquist B. J., White E. P., Phillips O. L., Jørgensen P. M., Weiser M. D., Monteagudo Mendoza A., Núñez Vargas P., Variation in above-ground forest biomass across broad climatic gradients. Glob. Ecol. Biogeogr. 20, 744–754 (2011). [Google Scholar]
- 5.Quesada C. A., Phillips O. L., Schwarz M., Czimczik C. I., Baker T. R., Patiño S., Fyllas N. M., Hodnett M. G., Herrera R., Almeida S., Alvarez Dávila E., Arneth A., Arroyo L., Chao K. J., Dezzeo N., Erwin T., di Fiore A., Higuchi N., Honorio Coronado E., Jimenez E. M., Killeen T., Lezama A. T., Lloyd G., Löpez-González G., Luizão F. J., Malhi Y., Monteagudo A., Neill D. A., Vargas P. N., Paiva R., Peacock J., Peñuela M. C., Cruz A. P., Pitman N., Priante Filho N., Prieto A., Ramírez H., Rudas A., Salomão R., Santos A. J. B., Schmerler J., Silva N., Silveira M., Vásquez R., Vieira I., Terborgh J., Lloyd J., Basin-wide variations in Amazon forest structure and function are mediated by both soils and climate. Biogeosciences 9, 2203–2246 (2012). [Google Scholar]
- 6.Engelbrecht B. M. J., Comita L. S., Condit R., Kursar T. A., Tyree M. T., Turner B. L., Hubbell S. P., Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447, 80–82 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Toledo M., Peña-Claros M., Bongers F., Alarcón A., Balcázar J., Chuviña J., Leaño C., Licona J. C., Poorter L., Distribution patterns of tropical woody species in response to climatic and edaphic gradients. J. Ecol. 100, 253–263 (2012). [Google Scholar]
- 8.Lin D., Anderson-Teixeira K. J., Lai J., Mi X., Ren H., Ma K., Traits of dominant tree species predict local scale variation in forest aboveground and topsoil carbon stocks. Plant Soil 409, 435–446 (2016). [Google Scholar]
- 9.Magnago L. F. S., Edwards D. P., Edwards F. A., Magrach A., Martins S. V., Laurance W. F., Functional attributes change but functional richness is unchanged after fragmentation of Brazilian Atlantic forests. J. Ecol. 102, 475–485 (2014). [Google Scholar]
- 10.Andrade R. B., Balch J. K., Parsons A. L., Armenteras D., Roman-Cuesta R. M., Bulkan J., Scenarios in tropical forest degradation: Carbon stock trajectories for REDD+. Carbon Balance Manag. 12, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Avila A. L., van der Sande M. T., Dormann C. F., Peña-Claros M., Poorter L., Mazzei L., Ruschel A. R., Silva J. N. M., de Carvalho J. O. P., Bauhus J., Disturbance intensity is a stronger driver of biomass recovery than remaining tree-community attributes in a managed Amazonian forest. J. Appl. Ecol. 55, 1647–1657 (2018). [Google Scholar]
- 12.Malhi Y., Wood D., Baker T. R., Wright J., Phillips O. L., Cochrane T., Meir P., Chave J., Almeida S., Arroyo L., Higuchi N., Killeen T. J., Laurance S. G., Laurance W. F., Lewis S. L., Monteagudo A., Neill D. A., Vargas P. N., Pitman N. C. A., Quesada C. A., Salomão R., Silva J. N. M., Lezama A. T., Terborgh J., Martínez R. V., Vinceti B., The regional variation of aboveground live biomass in old-growth Amazonian forests. Glob. Chang. Biol. 12, 1107–1138 (2006). [Google Scholar]
- 13.O. L. Phillips, S. L. Lewis, Recent changes in tropical forest biomass and dynamics (2014); www.rainfor.org.
- 14.Poorter L., van der Sande M. T., Arets E. J. M. M., Ascarrunz N., Enquist B., Finegan B., Licona J. C., Martínez-Ramos M., Mazzei L., Meave J. A., Muñoz R., Nytch C. J., de Oliveira A. A., Pérez-García E. A., Prado-Junior J., Rodríguez-Velázques J., Ruschel A. R., Salgado-Negret B., Schiavini I., Swenson N. G., Tenorio E. A., Thompson J., Toledo M., Uriarte M., van der Hout P., Zimmerman J. K., Peña-Claros M., Biodiversity and climate determine the functioning of Neotropical forests. Glob. Ecol. Biogeogr. 26, 1423–1434 (2017). [Google Scholar]
- 15.Mensah S., Veldtman R., Assogbadjo A. E., Glèlè Kakaï R., Seifert T., Tree species diversity promotes aboveground carbon storage through functional diversity and functional dominance. Ecol. Evol. 6, 7546–7557 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chave J., Condit R., Aguilar S., Hernandez A., Lao S., Perez R., Error propagation and scaling for tropical forest biomass estimates. Philos. Trans. R. Soc. B Biol. Sci. 359, 409–420 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holdaway R. J., Easdale T. A., Carswell F. E., Richardson S. J., Peltzer D. A., Mason N. W. H., Brandon A. M., Coomes D. A., Nationally representative plot network reveals contrasting drivers of net biomass change in secondary and old-growth forests. Ecosystems 20, 944–959 (2017). [Google Scholar]
- 18.Chave J., Réjou-Méchain M., Búrquez A., Chidumayo E., Colgan M. S., Delitti W. B. C., Duque A., Eid T., Fearnside P. M., Goodman R. C., Henry M., Martínez-Yrízar A., Mugasha W. A., Muller-Landau H. C., Mencuccini M., Nelson B. W., Ngomanda A., Nogueira E. M., Ortiz-Malavassi E., Pélissier R., Ploton P., Ryan C. M., Saldarriaga J. G., Vieilledent G., Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 20, 3177–3190 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Popkin G., How much can forests fight climate change? Nature 565, 280–282 (2019). [DOI] [PubMed] [Google Scholar]
- 20.de Lima R. A. F., Mori D. P., Pitta G., Melito M. O., Bello C., Magnago L. F., Zwiener V. P., Saraiva D. D., Marques M. C. M., de Oliveira A. A., Prado P. I., How much do we know about the endangered Atlantic Forest? Reviewing nearly 70 years of information on tree community surveys. Biodivers. Conserv. 24, 2135–2148 (2015). [Google Scholar]
- 21.de Lima R. A. F., Oliveira A. A., Pitta G. R., de Gasper A. L., Vibrans A. C., Chave J., ter Steege H., Prado P. I., The erosion of biodiversity and biomass in the Atlantic Forest biodiversity hotspot. Nat. Commun. 11, 6347 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro M. C., Metzger J. P., Martensen A. C., Ponzoni F. J., Hirota M. M., The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 142, 1141–1153 (2009). [Google Scholar]
- 23.Aung M. T., Song Y., Ferguson K. K., Cantonwine D. E., Zeng L., McElrath T. F., Pennathur S., Meeker J. D., Mukherjee B., Application of an analytical framework for multivariate mediation analysis of environmental data. Nat. Commun. 11, 5624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris N. L., Gibbs D. A., Baccini A., Birdsey R. A., de Bruin S., Farina M., Fatoyinbo L., Hansen M. C., Herold M., Houghton R. A., Potapov P. V., Suarez D. R., Roman-Cuesta R. M., Saatchi S. S., Slay C. M., Turubanova S. A., Tyukavina A., Global maps of twenty-first century forest carbon fluxes. Nat. Clim. Chang. 11, 234–240 (2021). [Google Scholar]
- 25.Laurance W. F., Goosem M., Laurance S. G. W., Impacts of roads and linear clearings on tropical forests. Trends Ecol. Evol. 24, 659–669 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Magnago L. F. S., Rocha M. F., Meyer L., Martins S. V., Meira-Neto J. A. A., Microclimatic conditions at forest edges have significant impacts on vegetation structure in large Atlantic forest fragments. Biodivers. Conserv. 24, 2305–2318 (2015). [Google Scholar]
- 27.Balch J. K., Nepstad D. C., Curran L. M., Brando P. M., Portela O., Guilherme P., Reuning-Scherer J. D., de Carvalho O., Size, species, and fire behavior predict tree and liana mortality from experimental burns in the Brazilian Amazon. For. Ecol. Manage. 261, 68–77 (2011). [Google Scholar]
- 28.Barlow J., Peres C. A., Lagan B. O., Haugaasen T., Large tree mortality and the decline of forestbiomass following Amazonian wildfires. Ecol. Lett. 6, 6–8 (2003). [Google Scholar]
- 29.Prado-Junior J. A., Schiavini I., Vale V. S., Arantes C. S., van der Sande M. T., Lohbeck M., Poorter L., Conservative species drive biomass productivity in tropical dry forests. J. Ecol. 104, 817–827 (2016). [Google Scholar]
- 30.Mittelman P., Dracxler C. M., Santos-Coutinho P. R. O., Pires A. S., Sowing forests: A synthesis of seed dispersal and predation by agoutis and their influence on plant communities. Biol. Rev. 96, 2425–2445 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Bello C., Galetti M., Pizo M. A., Magnago L. F. S., Rocha M. F., Lima R. A. F., Peres C. A., Ovaskainen O., Jordano P., Defaunation affects carbon storage in tropical forests. Sci. Adv. 1, e1501105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright I. J., Dong N., Maire V., Prentice I. C., Westoby M., Díaz S., Gallagher R. V., Jacobs B. F., Kooyman R., Law E. A., Leishman M. R., Niinemets Ü., Reich P. B., Sack L., Villar R., Wang H., Wilf P., Global climatic drivers of leaf size. Science 357, 917–921 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Cavanaugh K. C., Gosnell J. S., Davis S. L., Ahumada J., Boundja P., Clark D. B., Mugerwa B., Jansen P. A., O’Brien T. G., Rovero F., Sheil D., Vasquez R., Andelman S., Carbon storage in tropical forests correlates with taxonomic diversity and functional dominance on a global scale. Glob. Ecol. Biogeogr. 23, 563–573 (2014). [Google Scholar]
- 34.Fauset S., Johnson M. O., Gloor M., Baker T. R., Monteagudo M A., Brienen R. J. W., Feldpausch T. R., Lopez-Gonzalez G., Malhi Y., ter Steege H., Pitman N. C. A., Baraloto C., Engel J., Pétronelli P., Andrade A., Camargo J. L. C., Laurance S. G. W., Laurance W. F., Chave J., Allie E., Vargas P. N., Terborgh J. W., Ruokolainen K., Silveira M., Aymard C G. A., Arroyo L., Bonal D., Ramirez-Angulo H., Araujo-Murakami A., Neill D., Hérault B., Dourdain A., Torres-Lezama A., Marimon B. S., Salomão R. P., Comiskey J. A., Réjou-Méchain M., Toledo M., Licona J. C., Alarcón A., Prieto A., Rudas A., van der Meer P. J., Killeen T. J., Junior B. H. M., Poorter L., Boot R. G. A., Stergios B., Torre E. V., Costa F. R. C., Levis C., Schietti J., Souza P., Groot N., Arets E., Moscoso V. C., Castro W., Coronado E. N. H., Peña-Claros M., Stahl C., Barroso J., Talbot J., Vieira I. C. G., van der Heijden G., Thomas R., Vos V. A., Almeida E. C., Davila E. Á., Aragão L. E. O. C., Erwin T. L., Morandi P. S., de Oliveira E. A., Valadão M. B. X., Zagt R. J., van der Hout P., Loayza P. A., Pipoly J. J., Wang O., Alexiades M., Cerón C. E., Huamantupa-Chuquimaco I., di Fiore A., Peacock J., Camacho N. C. P., Umetsu R. K., de Camargo P. B., Burnham R. J., Herrera R., Quesada C. A., Stropp J., Vieira S. A., Steininger M., Rodríguez C. R., Restrepo Z., Muelbert A. E., Lewis S. L., Pickavance G. C., Phillips O. L., Hyperdominance in Amazonian forest carbon cycling. Nat. Commun. 6, 6857 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Souza F. C., Dexter K. G., Phillips O. L., Pennington R. T., Neves D., Sullivan M. J. P., Alvarez-Davila E., Alves Á., Amaral I., Andrade A., Aragao L. E. O. C., Araujo-Murakami A., Arets E. J. M. M., Arroyo L., Aymard C G. A., Bánki O., Baraloto C., Barroso J. G., Boot R. G. A., Brienen R. J. W., Brown F., Camargo J. L. C., Castro W., Chave J., Cogollo A., Comiskey J. A., Cornejo-Valverde F., da Costa A. L., de Camargo P. B., di Fiore A., Feldpausch T. R., Galbraith D. R., Gloor E., Goodman R. C., Gilpin M., Herrera R., Higuchi N., Honorio Coronado E. N., Jimenez-Rojas E., Killeen T. J., Laurance S., Laurance W. F., Lopez-Gonzalez G., Lovejoy T. E., Malhi Y., Marimon B. S., Marimon-Junior B. H., Mendoza C., Monteagudo-Mendoza A., Neill D. A., Vargas P. N., Peñuela Mora M. C., Pickavance G. C., Pipoly J. J., Pitman N. C. A., Poorter L., Prieto A., Ramirez F., Roopsind A., Rudas A., Salomão R. P., Silva N., Silveira M., Singh J., Stropp J., ter Steege H., Terborgh J., Thomas-Caesar R., Umetsu R. K., Vasquez R. V., Célia-Vieira I., Vieira S. A., Vos V. A., Zagt R. J., Baker T. R., Evolutionary diversity is associated with wood productivity in Amazonian forests. Nat. Ecol. Evol. 3, 1754–1761 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Chave J., Coomes D., Jansen S., Lewis S. L., Swenson N. G., Zanne A. E., Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Kraft N. J. B., Godoy O., Levine J. M., Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. U.S.A. 112, 797–802 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark D. B., Clark D. A., Oberbauer S. F., Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2. Glob. Chang. Biol. 16, 747–759 (2010). [Google Scholar]
- 39.Keith H., Mackey B. G., Lindenmayer D. B., Re-evaluation of forest biomass carbon stocks and lessons from the world’s most carbon-dense forests. Proc. Natl. Acad. U.S.A. 106, 11635–11640 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chave J., Condit R., Lao S., Caspersen J. P., Foster R. B., Hubbell S. P., Spatial and temporal variation of biomass in a tropical forest: Results from a large census plot in Panama. J. Ecol. 91, 240–252 (2003). [Google Scholar]
- 41.Stovall A. E. L., Shugart H., Yang X., Tree height explains mortality risk during an intense drought. Nat. Commun. 10, 4385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mauya E. W., Hansen E. H., Gobakken T., Bollandsås O. M., Malimbwi R. E., Næsset E., Effects of field plot size on prediction accuracy of aboveground biomass in airborne laser scanning-assisted inventories in tropical rain forests of Tanzania. Carbon Balance Manag. 10, 1–14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durán S. M., Sánchez-Azofeifa G. A., Rios R. S., Gianoli E., The relative importance of climate, stand variables and liana abundance for carbon storage in tropical forests. Glob. Ecol. Biogeogr. 24, 939–949 (2015). [Google Scholar]
- 44.Connell J. H., Diversity in tropical rain forests and coral reefs: High diversity of trees and corals is maintained only in a nonequilibrium state. Science 199, 1302–1310 (1978). [DOI] [PubMed] [Google Scholar]
- 45.Zhang C., Ma Z., Zhou H., Zhao X., Long-term warming results in speciesspecific shifts in seed mass in alpine communities. PeerJ 2019, e7416 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tozer W. C., Rice B., Westoby M., Evolutionary divergence of leaf width and its correlates. Am. J. Bot. 102, 367–378 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Kirschbaum M. U. F., McMillan A. M. S., Warming and elevated CO2 have opposing influences on transpiration. Which is more important? Curr. For. Rep. 4, 51–71 (2018). [Google Scholar]
- 48.Hacke U. G., Sperry J. S., Pockman W. T., Davis S. D., McCulloh K. A., Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126, 457–461 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Werner F. A., Homeier J., Is tropical montane forest heterogeneity promoted by a resource-driven feedback cycle? Evidence from nutrient relations, herbivory and litter decomposition along a topographical gradient. Funct. Ecol. 29, 430–440 (2015). [Google Scholar]
- 50.Hofhansl F., Chacón-Madrigal E., Fuchslueger L., Jenking D., Morera-Beita A., Plutzar C., Silla F., Andersen K. M., Buchs D. M., Dullinger S., Fiedler K., Franklin O., Hietz P., Huber W., Quesada C. A., Rammig A., Schrodt F., Vincent A. G., Weissenhofer A., Wanek W., Climatic and edaphic controls over tropical forest diversity and vegetation carbon storage. Sci. Rep. 10, 5066 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.IPCC, 2014: Climate Change 2014: Synthesis Report, in Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Core Writing Team, R. K. Pachauri, L. A. Meyer, Eds. (IPCC, Geneva, Switzerland), pp. 151. [Google Scholar]
- 52.Phelps J., Webb E. L., Adams W. M., Biodiversity co-benefits of policies to reduce forest-carbon emissions. Nat. Clim. Chang. 2, 497–503 (2012). [Google Scholar]
- 53.R. E. McRoberts, G. Ståhl, C. Vidal, M. Lawrence, E. Tomppo, K. Schadauer, G. Chirici, A. Bastrup-Birk, in National Forest Inventories: Pathways for Common Reporting (Springer, 2010), pp. 33–43. [Google Scholar]
- 54.Pakeman R. J., Quested M. H., Sampling plant functional traits: What proportion of the species need to be measured? Appl. Veg. Sci. 1, 91–96 (2007). [Google Scholar]
- 55.Martin A. R., Doraisami M., Thomas S. C., Global patterns in wood carbon concentration across the world’s trees and forests. Nat. Geosci. 11, 915–920 (2018). [Google Scholar]
- 56.J. R. Scolforo, Inventário Florestal de Minas Gerais: Monitoramento da Flora Nativa (Universidade Federal de Lavras, 2008). [Google Scholar]
- 57.Alvares C. A., Stape J. L., Sentelhas P. C., de Moraes Gonçalves J. L., Modeling monthly mean air temperature for Brazil. Theor. Appl. Climatol. 113, 407–427 (2013). [Google Scholar]
- 58.Alvares C. A., Stape J. L., Sentelhas P. C., de Moraes Gonçalves J. L., Sparovek G., Köppen’s climate classification map for Brazil. Meteorol. Z. 22, 711–728 (2013). [Google Scholar]
- 59.Fick S. E., Hijmans R. J., WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017). [Google Scholar]
- 60.Farr T. G., Rosen P. A., Caro E., Crippen R., Duren R., Hensley S., Kobrick M., Paller M., Rodriguez E., Roth L., Seal D., Shaffer S., Shimada J., Umland J., Werner M., Oskin M., Burbank D., Alsdorf D. E., The shuttle radar topography mission. Rev. Geophys. 45, (2007). [Google Scholar]
- 61.M. M. Benedetti, N. Curi, G. Sparovek, A. De, C. Filho, S. Henrique, G. Silva, Updated Brazilian’s georeferenced soil database—An improvement for international scientific information exchanging, in Principles, Application and Assessment in Soil Science, B. E. Ozkaraova Gungor, Ed. (InTech, 2011). [Google Scholar]
- 62.Conama, RESOLUÇÃO CONAMA No 10, DE 1o DE OUTUBRO DE 1993 (1993).
- 63.R. Técnico, Atlas dos Remanescentes Florestais da Mata Atlântica (2017).
- 64.Hansen M. C., Potapov P. V., Moore R., Hancher M., Turubanova S. A., Tyukavina A., Thau D., Stehman S. V., Goetz S. J., Loveland T. R., Kommareddy A., Egorov A., Chini L., Justice C. O., Townshend J. R. G., High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013). [DOI] [PubMed] [Google Scholar]
- 65.J. Vanderwal, L. Falconi, S. Januchowski, L. Shoo, C. S. Maintainer, Package ‘SDMTools’ type package title species distribution modelling tools: Tools for processing data associated with species distribution modelling exercises (2013); www.rforge.net/SDMTools/.
- 66.J. Oksanen, F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. Mcglinn, P. R. Minchin, R. B. O’hara, G. L. Simpson, P. Solymos, M. Henry, H. Stevens, E. Szoecs, H. W. Maintainer, Package ‘vegan’ Title Community Ecology Package (2017).
- 67.Dormann C. F., Elith J., Bacher S., Buchmann C., Carl G., Carré G., Marquéz J. R. G., Gruber B., Lafourcade B., Leitão P. J., Münkemüller T., Mcclean C., Osborne P. E., Reineking B., Schröder B., Skidmore A. K., Zurell D., Lautenbach S., Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46 (2013). [Google Scholar]
- 68.Burnham K. P., Anderson D. R., Huyvaert K. P., AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–35 (2011). [Google Scholar]
- 69.Haughian S. R., Frego K. A., Does CWD mediate microclimate for epixylic vegetation in boreal forest understories? A test of the moisture-capacitor hypothesis. For. Ecol. Manag. 389, 341–351 (2017). [Google Scholar]
- 70.Imai K., Tingley D., Yamamoto T., Experimental designs for identifying causal mechanisms. J. R. Stat. Soc. A Stat. Soc. 176, 5–51 (2012). [Google Scholar]
- 71.Imai K., Keele L., Tingley D., A general approach to causal mediation analysis. Psychol. Methods 15, 309–334 (2010). [DOI] [PubMed] [Google Scholar]
- 72.Olson D. M., Dinerstein E., The Global 200: Priority ecoregions for global conservation. Ann. Mo. Bot. Gard. , 199–224 (2002). [Google Scholar]
- 73.Chen H. C., Wang N. S., The assignment of scores procedure for ordinal categorical data. Sci. World J. 2014, 1–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dray S., Legendre P., Peres-Neto P. R., Spatial modelling: A comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol. Model. 196, 483–493 (2006). [Google Scholar]
- 75.T. Dustin, Y. Teppei, H. Kentaro, K. Luke, I. Kosuke, T. Minh, W. Weihuang, Package “mediation” (2019).
- 76.Bates D., Mächler M., Bolker B. M., Walker S. C., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 77.K. Barton, Package ‘MuMIn’ Title Multi-Model Inference (2022).
- 78.Package ‘ggplot2’ title create elegant data visualisations using the grammar of graphics (2021); https://ggplot2.tidyverse.org.
- 79.R. Bivand, R packages for analyzing spatial data: A comparative case study with areal data. Geogr. Anal. (2022).
- 80.N. Bjornstad, J. Cai, ncf: ncf: Spatial Covariance Functions. R package version 1.2 (2018); https://cran.r-project.org/package=ncf.
- 81.V. Masson-Delmotte, P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, T. Waterfield, Global warming of 1.5°C: An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty (Intergovernmental Panel on Climate Change, 2019); www.environmentalgraphiti.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S7
Tables S1 to S6
Data S1