Abstract
The process of cellular senescence is rapidly emerging as a modulator of organismal aging and disease. Targeting the development and removal of senescent cells is considered a viable approach to achieving improved organismal healthspan and lifespan. Nutrition and health are intimately linked and an appropriate dietary regimen can greatly impact organismal response to stress and diseases including during aging. With a renewed focus on cellular senescence, emerging studies demonstrate that both primary and secondary nutritional elements such as carbohydrates, proteins, fatty acids, vitamins, minerals, polyphenols, and probiotics can influence multiple aspects of cellular senescence. The present review describes the recent molecular aspects of cellular senescence-mediated understanding of aging and then studies available evidence of the cellular senescence modulatory attributes of major and minor dietary elements. Underlying pathways and future research directions are deliberated to promote a nutrition-centric approach for targeting cellular senescence and thus improving human health and longevity.
Keywords: Cellular senescence, Aging, Nutrition, Carbohydrates, Vitamins, Polyphenols, Fatty acids
Introduction
Human health is a culmination of dynamic interactions and regulations by several genetic, non-genetic, and environmental factors. The regulatory systems of the body work intricately and coordinately to maintain functional homeostasis in cells and tissues by sensing and regulating nutrient availability and response, cell division and regeneration, thwarting off foreign and infectious agents as well as keeping memory and swiftness of the neurological responses. These systems are invariably deregulated during aging and diseases, and any preventive or therapeutic approach attempts to reinstate this ‘healthy’ state of homeostasis (van Beek et al., 2016). Aging is recognized as the single most influential risk factor that dramatically enhances the frequency and susceptibility of the elderly to several critical illnesses including the defining disorders of the twenty-first century, i.e., cancer and diabetes as well as the ongoing COVID-19 pandemic (Chen et al., 2021a; Fulop et al., 2019; Niccoli and Partridge, 2012). Organismal aging seems inevitable, and yet understanding of the underlying cause(s) and mechanisms of aging have long remained ambiguous and perplexing largely due to its stochastic and multifaceted nature. However, advances in our current molecular concepts of aging are beginning to answer some of the fundamental questions related to the evolutionary significance of aging as well as its predisposition to age-associated disorders. Unlike growth and development, aging is not considered a programmed process, (Blagosklonny, 2013), and accumulating evidence suggests that the observable macro phenotype of aging is essentially a culmination of microscopic cellular damage that builds up over time (Rattan, 2008; Yin and Chen, 2005). In fact, a strong view is now developing that understanding aging itself should be considered central for comprehending various age-related diseases and the development of common mitigative strategies (Blagosklonny, 2012; Hayflick, 2021; Le Bourg, 2022). In this regard, the process of cellular senescence is emerging as an over-arching phenomenon that seemingly links cellular aging to organismal aging, and therefore, a cellular senescence-centric view of aging is rapidly gaining attention (Borghesan et al., 2020; Jeyapalan and Sedivy, 2008). Perhaps even more strikingly, cellular senescence is also considered a common underlying causative factor in the pathogenesis of distinct age-related human disorders including but not limited to arthritis, diabetes, neurodegenerative disorders, sarcopenia, cancer, and cardiovascular diseases thereby enabling gerontologists to study age-related diseases through a novel and integrative approach (Borghesan et al., 2020; Kaur and Farr, 2020).
Targeting cellular senescence and its phenotype is rapidly gaining attention as a highly useful strategy in expanding organismal healthspan and lifespan (Soto-Gamez and Demaria, 2017; Yuan et al., 2020). This also paves way for developing probable anti-aging or healthy aging therapies such as the identification and development of ‘geroprotectors’ (Aliper et al., 2016). While anti-aging may seem a far-fetched and philosophically contentious phenomenon; the notion of healthy aging appears to be a valid strategy that may prevent the aggravation or frequency of chronic or fatal diseases, thereby resulting in improved healthspan and/or lifespan (Kritchevsky, 2016). Amongst the non-genetic factors, exercise, lifestyle, and nutrition are the only known modulators that can favorably influence health and aging. Further, nutrition is considered the single most potent factor that can mitigate some of the deleterious aspects of aging including predisposition to diseases, and thus a novel discipline called ‘nutrigerontology’ has recently been emphasized (Verburgh, 2015). The present narrative review delineates the current cellular senescence-centric molecular understanding of aging and its interdependent effects on the fundamental regulatory systems of the body. We then discuss the influence of primary and secondary nutritional components in modulating different aspects of cellular senescence and disease and deliberate probable research opportunities.
Cellular senescence in aging: molecular mechanisms and systemic effects
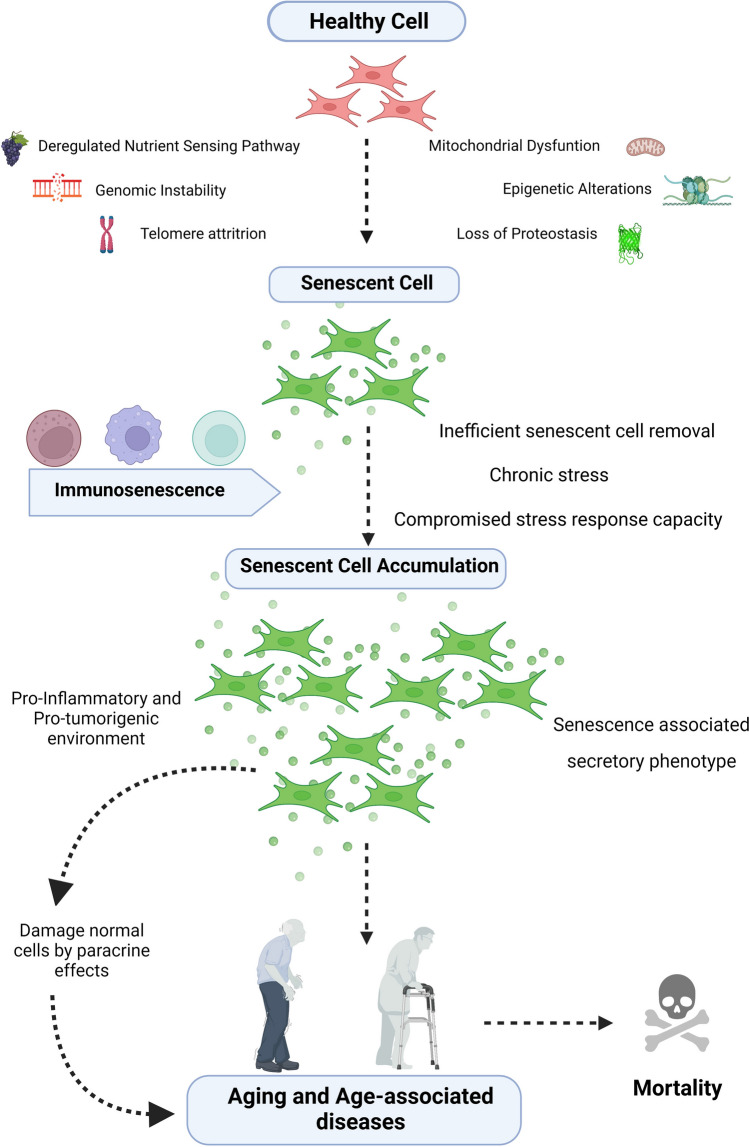
Aging has long remained a philosophical and scientific mystery (Medawar, 1952). From a biological perspective, the aging organismal phenotype is essentially considered a time-dependent accumulation of a variety of molecular and cellular damage that hampers tissue/organ functions ultimately predisposing the elderly to morbidity and mortality (Rattan, 2008; Yin and Chen, 2005). Although several theories of aging have been put forward, an all-encompassing theory explaining the what, why, where, and how of aging has remained elusive. Notwithstanding this, accumulating studies are now beginning to uncover some of the fundamental aspects of aging, and in the process have also highlighted the intricacies of the process. In particular, the role of cellular senescence as the causal nexus explaining various facets of aging is gaining attention amongst gerontologists (Borghesan et al., 2020). Cellular senescence was first identified in fibroblasts by Hayflick and Moorhead back in 1961; (Hayflick and Moorhead, 1961), however, it was initially perceived as an in vitro artifact, and its acceptance as a mainstream aging theory was dismissed for a long time (Cristofalo et al., 2004). Observations in the last decade have now shown conclusive evidence that cellular senescence is indeed a ‘hallmark of aging’ which may potentially serve as a connecting factor among other known hallmarks of aging (López-Otín et al., 2013). Cellular senescence describes a morphologically, biochemically, and metabolically distinct state wherein the cells permanently lose the capacity to divide and enter a stable cell cycle arrest. These cells are then referred to as senescent cells (SC) and are characterized by shortened telomeres, hypertrophy, altered chromatin structure, accumulation of DNA damage and reactive oxygen species (ROS), activation of cell cycle inhibitory pathways (p53, p16Ink4a, and/or p21CIP1), senescence-associated β-galactosidase (SA-β-gal) activity, resistance to apoptotic cell death, and development of senescence-associated heterochromatic foci (Fig. 1) (Campisi, 2013; Kim and Kim, 2019). In addition, SC are also accompanied by a chronic pro-inflammatory behavior known as senescence-associated secretory phenotype (SASP) which consists of increased expression of a cell-specific battery of pro-inflammatory cytokines and growth factors (Birch and Gil, 2020). Development of SC is a complex process but is invariably liked to chronic exposure to cellular stress (Ben-Porath and Weinberg, 2004). Cellular and metabolic stressors can activate either apoptosis or cellular senescence program depending upon the type and duration of the stressor. Although the exact dichotomy of this cellular fate under stress is still debatable, (Childs et al., 2014) it is accepted that chronic stress conditions can propel the cells towards a senescence program. During the course of organismal lifespan, cells are threatened by various internal and external stressors, and indeed, a link between dysregulated organismal stress response capacity and the development of SC has been observed (Zhang et al., 2017). Inherent deficiencies in the replication of telomeric regions of the chromosomes with each cell division result in ‘replicative senescence’ (Campisi, 1997) while premature senescence can also be induced in cells through acute or chronic stress exposure (stress-induced premature senescence) (Toussaint et al., 2000; Toussaint et al., 2002). Further, cellular senescence is no longer considered a phenomenon related only to somatic proliferative cells, as terminally differentiated cells such as immune cells or adipocytes are also known to undergo senescence (von Zglinicki et al., 2021). It is pertinent to note that SC are not necessarily undesirable by themselves as these cells are important mediators of certain physiological processes such as wound healing (Demaria et al., 2014) and even embryonic development (Muñoz-Espín et al., 2013). However, it is the age-associated gradual accumulation of SC in various tissues and organs which has been correlated with an increased risk of disease and death (Fig. 1) (Idda et al., 2020; Krishnamurthy et al., 2004; Yousefzadeh et al., 2020). Indeed, studies have shown that targeted removal of SC through agents called ‘senolytics’ can enhance the lifespan, alleviate systemic inflammation, improve organ functions, and also mitigate characteristic age-related disorders such as diabetes (Aguayo-Mazzucato et al., 2019; Baker et al., 2011; Baker et al., 2016; Xu et al., 2018). Conversely, the addition of SC to healthy tissues can induce premature aging and disease-like phenotype suggesting that SC are sufficient to drive age-related pathologies (Kim et al., 2020; Xu et al., 2016). As SC accumulate in tissues and organs, the chronic presence of SASP becomes increasingly detrimental as it affects nearby healthier cells through paracrine effects resulting in pro-inflammatory and pro-tumorigenic environment (Birch and Gil, 2020). There is also evidence that the rate of development of SC is not linear with age and is also tissue-dependent suggesting variable predisposition to biological aging depending upon the tissue type (Karin et al., 2019). We have also recently reported that adipose tissue is more vulnerable to the development of age-associated senescence-like features which were predominant from the age of 14 months in experimental mice (Sharma et al., 2022). A startling common link between SC and various age-related diseases is also rapidly gaining attention. There is evidence that SC accumulation might play a direct role in the pathogenesis and/or exacerbation of disorders such as type I and type II diabetes (Aguayo-Mazzucato et al., 2019; Thompson et al., 2019), osteoarthritis (Xu et al., 2016), cognitive functional decline (Lye et al., 2019), as well as cancer (Liu and Hornsby, 2007). It is conceivable that age-related disorders may be linked to the basic process of aging, and thus strategies aimed at targeting these disorders within the purview of cellular senescence and aging may provide a new therapeutic as well as economic perspective to the traditional disease-specific research focus (Blagosklonny, 2018; Boccardi and Mecocci, 2021; Scott et al., 2021).
Fig. 1.
Schematic representation of development of senescent cells and their deleterious effects with age. Chronic intrinsic and extrinsic stressors augment cellular and macromolecular damage resulting in deregulation of cell signalling and activation of cellular senescence pathways. The age-related impairment of the immune system contributes to the inefficient systemic clearance of senescent cells thereby aiding in increased senescent cell burden in tissues. The SASP of accumulating senescent cells promotes pro-inflammatory and pro-tumorigenic environment thereby predisposing elderly to increased morbidity and mortality
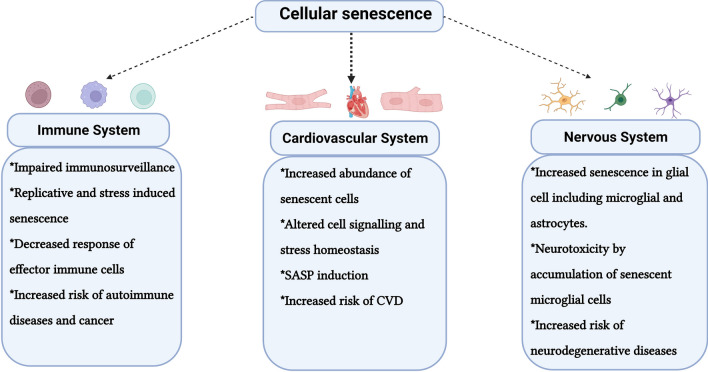
The systemic effects of cellular senescence on key regulatory bodily systems and their related disorders are increasingly being deciphered (Fig. 2). The immune system is gaining central attention in this regard and a bidirectional relationship between cellular senescence and the immune system is rapidly emerging (Sharma, 2021). The immune system is considered to play a critical role in regulating the accumulation of SC in tissues and it is speculated that loss of immune functions with age could impair systemic SC clearance and therefore increase SC burden (Kale et al., 2020; Sharma, 2021). This is because SC are immunogenic and are recognized and removed by cells of the immune system such as NK cells in young healthier organisms (Kale et al., 2020). The chemotactic factors in the SASP of young organisms attract immune cells to the location of accumulating SC which ultimately results in their removal. However, as we age, the gradual restructuring of the immune system through the process of immunosenescence appears to deter their immunosurveillance and phagocytic potential which may contribute to hampered identification and removal of SC. For example, it was demonstrated that in vivo deficiency in cytotoxic response of effector immune cells can enhance the accumulation of SC in tissues accompanied with chronic inflammation (Ovadya et al., 2018). Further, a recent study observed that DNA damage and senescence in murine hematopoietic cells are sufficient to drive systemic effects of cellular senescence thereby implying the critical role of the immune system in driving organismal aging (Yousefzadeh et al., 2021). It is thus not surprising that senescent immunotherapy is considered a promising anti-aging strategy (Burton and Stolzing, 2018). On the other hand, similar to other cells, immune cells are also liable to undergo cellular senescence, and thus, together with immunosenescence, immune cells are significantly impacted with age although this dichotomy remains to be completely resolved (Sharma, 2021). Another dimension to this intricate association was revealed when it was observed that SC can actively develop strategies to evade their immune system-mediated clearance similar to cancer cells (Pereira et al., 2019). Thus, the immune system and cellular senescence are interlinked, and exploring their interrelationships and biological effects is an active area of research. In addition to the immune system, cells of the cardiovascular system such as cardiomyocytes and endothelial cells have been shown to present characteristic features of age-associated cellular senescence that have been implicated in the development of age-related cardiovascular disorders (CVD) (Fig. 2). Although the molecular mechanisms governing CVD are largely unknown, accumulating evidence suggests a key role of cellular senescence in the pathogenesis of CVD (Shakeri et al., 2018), and targeting SC has been argued as a potential therapeutic opportunity against various deleterious aspects of CVD (Childs et al., 2018). For instance, in response to toxic agents, cardiomyocytes exhibit increased ROS levels and persistent DNA damage that upregulates characteristic senescence markers such as p16INK4a, p21CIP1, and SA-β-gal expression (Mitry et al., 2020). It was recently demonstrated that human and murine cardiomyocytes acquire a senescent‐like phenotype characterized by overexpression of p21CIP1 and p16INK4a resulting in the development of pro‐fibrotic and pro‐hypertrophic environments and thus contributing to age-related myocardial dysfunction (Anderson et al., 2019). Crucially, pharmacological clearance of SC in mice alleviated some of the deleterious aspects of cardiac aging, including myocardial hypertrophy and fibrosis (Walaszczyk et al., 2019). Vascular endothelial cell senescence is also emerging as a prominent contributor to CVD as it may affect vascular permeability, repair, and angiogenesis (Jia et al., 2019). Aged endothelial cells show characteristics of SC such as reduced telomere length, increased DNA damage foci formation, SASP induction, and elevated intracellular ROS production (Hohensinner et al., 2016; Khan et al., 2017). A recent report has shown that EC senescence is not only detrimental to CVD but can also induce metabolic disorders by impairing insulin sensitivity through the senescence-associated secretory phenotype (Barinda et al., 2020). Similar to cardiovascular disorders, cellular senescence is also considered a key player in regulating age-dependent neurodegenerative diseases (Fig. 2) (Si et al., 2021). For example, senescent astrocytes accumulate in Alzheimer’s patients wherein they promote inflammation through the SASP (Bhat et al., 2012; Walker et al., 2020) while attenuation of cellular senescence has been shown to alleviate neuroinflammation associated with Alzheimer’s (Hou et al., 2021). In addition, a recent study revealed that senescent neurons with tau neuropathology are also prevalent in patients with AD (Dehkordi et al., 2021) while removal of accumulated senescent glial cells attenuated cognitive decline and age-related neurogenerative disorders (Bussian et al., 2018). Explicatively senescent glial cells have been observed during aging which contribute to the pathology of AD (Hu et al., 2021). During aging, brain microglia show characteristic expression of senescence markers such as telomere shortening, SA-β-gal activity, altered metabolic profile, and increased oxidative stress (Greenwood and Brown, 2021). In-state of senescence, microglia are neurotoxic and become detrimental in many neurodegenerative diseases by producing inflammation, inflammatory cytokines, superoxide anions, and nitric oxide (Nakajima and Kohsaka, 2004; Streit, 2002). Taken together, these observations suggest that cellular senescence is an important determinant in regulating the functional efficacy of major regulatory systems of the body with age and thus is a promising therapeutic target.
Fig. 2.
Effects of cellular senescence on major regulatory systems of body during aging
Primary diet constituents and cellular senescence
Carbohydrates
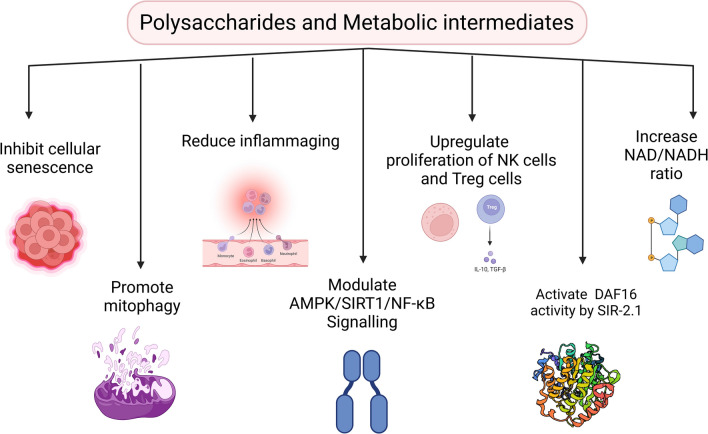
Carbohydrates are primary sources of cellular energy although their role in cell structure and signaling is also known. Carbohydrate metabolism is of great significance during aging as an association between carbohydrate consumption and chronic disorders such as obesity and diabetes is well recognized (Kelly et al., 2020). Besides, diets rich in glucose and fructose have been shown to accelerate aging in model organisms while a reduction in carbohydrate intake is often associated with reduced severity of disorders such as diabetes (Feinman et al., 2015). However, low carbohydrate diets and their significance during aging are still controversial and contradictory (Mooradian, 2020). Regardless, it is crucial to consider that complex carbohydrates and/or their derivatives have been demonstrated to suppress cellular senescence and augment healthy aging. For instance, a recent study showed that a heteropolysaccharide derived from the medicinal herb Astragalus membranaceus alleviated hepatocyte senescence by inhibiting the development of cellular senescence and promoting mitophagy via mTOR pathway both in vitro and in vivo (Yao et al., 2021). Another recent report demonstrated that Astragalus polysaccharides can reduce glucose-induced premature senescence and inflammasome activation in rat aortic endothelial cells (Miao et al., 2022). Using a d-galactose induced aging mice model, the application of Aronia melanocarpa heteropolysaccharides successfully ameliorated inflammation and aging in mice by modulating the AMPK/SIRT1/NF-κB signaling pathway and gut microbiota (Zhao et al., 2021). Studies on polysaccharides isolated from the herb Angelica sinensis have revealed anti-cellular senescence and antioxidant attributes in haematopoietic cells and endothelial progenitor cells while in vivo suppression of cellular senescence and improvement in brain senescence in a murine model of d-galactose induced aging was also observed (Cheng et al., 2019; Lai and Liu, 2015; Mu et al., 2017; Xiao et al., 2017). Similarly, the polysaccharide TLH-3 isolated from the mushroom Tricholoma lobayense alleviated premature cellular senescence in vitro and improved in vivo markers of senescence and SASP in premature aging mice (Pan et al., 2018). Also, polysaccharides extracted from the medicinal plant Lycium barbarum prevented the augmentation of oxidative stress-induced epithelial senescence and apoptosis in human lens epithelial cells in vitro (Qi et al., 2014). Another report revealed that marine sulphated polysaccharide Fucoidan can rescue endothelial cells from cellular senescence, and improve their survival, proliferation, and functional response which was implicated in enhanced neovasculogenic potential in vivo (Lee et al., 2015). Daily administration of polysaccharides isolated from Korean ginseng berry to old C57BL/6J mice resulted in improved indices of immunosenescence and inflamm-aging characterized by increased proliferation of Treg and NK cells, reduced systemic inflammatory molecules, and attenuation of thymic involution (Kim et al., 2018). Previous studies have also identified the role of metabolic carbohydrate intermediates in extending lifespan in model organisms by modulating nutrient signalling pathways. For instance, Caenorhabditis elegans (C. elegans) when treated with trehalose extends lifespan by lowering insulin/insulin growth factor-1 signaling and suppressed aging by offsetting stressors (Honda et al., 2010). In aged Saccharomyces cerevisiae cells, trehalose accumulation elicited an anti-aging response and increased ethanol production (Trevisol et al., 2011). Another study demonstrated the role of pyruvate in extending the lifespan of C. elegans by improved tolerance to oxidative stress via amplified mitochondrial pyruvate metabolism (Mouchiroud et al., 2011). Tricarboxylic acid cycle metabolites like malate and fumarate are also linked with lifespan extension in C. elegans via regulation of transcription factor DAF-16/FOXO, histone deacetylase SIR-2.1 and increasing the amount of oxidized NAD and FAD cofactors (Edwards et al., 2013; Sun et al., 2017). Oligosaccharides like N-glycan and N-acetylglucosamine supplementation reduced aggregation of proteins via ER-associated protein degradation, proteasomal activity, and autophagy consequently extending lifespan in C. elegans (Denzel et al., 2014). In a pre-clinical study, chitosan oligosaccharides have been utilized as a therapeutic agent against age-related illnesses (Kong et al., 2018). Together, these findings suggest that complex carbohydrates and intermediates of carbohydrate metabolism can regulate aging by modulating cellular senescence, proteostasis, and inflammation. However, adequate carbohydrate intake must be monitored since a high carbohydrate diet is associated with an increased risk of mortality in clinical studies (Dehghan et al., 2017). Together, it is reasonable to assert that although carbohydrates are often neglected concerning their bioactivity, especially with regard to aging, a carefully curated carbohydrate-rich diet could be potentially useful in mitigating cellular senescence which should be explored further (Fig. 3).
Fig. 3.
Modulation of cellular senescence by dietary polysaccharides and metabolic intermediates of carbohydrate metabolism
Dietary proteins and amino acids
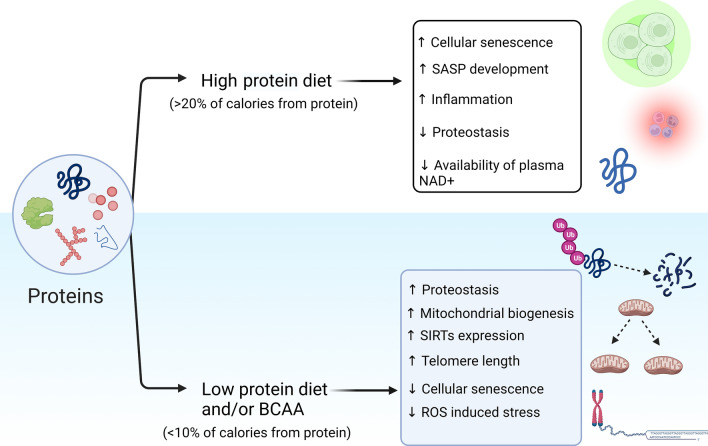
Proteins and amino acids are major structural and functional constituents of cells. In addition to carbohydrates, proteins are an essential part of the human diet and attempts have been made to ascertain a suitable carbohydrate to protein ratio in diets for augmenting health and aging. In this regard, a low protein (< 10% of calories from protein) and high carbohydrate diet, often in the ratio of 1:10, has scientific evidence of improving health during aging and extending the lifespan (Le Couteur et al., 2016; Levine et al., 2014). It was observed that even a short-term low protein and high carbohydrate diet regimen in mice can improve indices of metabolic health indicated by levels of insulin, glucose, lipids, and homeostatic model assessment (HOMA), and surprisingly these effects were similar to the stricter calorie restriction diet despite an increase in total energy intake (Solon-Biet et al., 2015). On the other hand, consumption of a high protein diet (> 20% of calories from protein) amongst the elderly augmented all-cause mortality incidences by 75% and increased the risk of cancers by 400% suggesting the detrimental effects of high protein consumption during aging (Levine et al., 2014). In addition, impaired protein metabolism appears to be intimately linked to cellular senescence and organismal longevity. A recent study compared the proteomic profile of fibroblast cells across species and reported that long-lived animals tend to have lower turnover rates of highly abundant cellular proteins which eventually results in lower oxidative stress and efficient energy management (Swovick et al., 2021). Further, restriction of protein synthesis suppressed cellular senescence both at the cellular and organismal levels (Takauji et al., 2016). Moreover, the balance between cellular protein synthesis, folding, and degradation (proteostasis) is also impaired in SC, and maintenance of proteostasis is considered a key therapeutic mechanism regulating senescence (Joy et al., 2021; Sabath et al., 2020). These observations augment the rationale that protein consumption, cellular metabolism, and energy homeostasis are intimately linked which can affect organismal longevity. However, studies examining the impact of dietary proteins and specific amino acids on cellular and organismal senescence are limited. A recent study observed that consumption of protein-rich diets can accelerate tissue senescence and SASP development in mice and thus promote the deleterious effects of aging (Nehme et al., 2021). Another study observed that protein-rich diets are associated with reduced availability of plasma NAD+ levels and inflammation in healthy middle-aged adults indicating that protein-deficient diets might promote longevity by improving cellular energy expenditure and expression of enzymes such as SIRTs (Seyedsadjadi et al., 2018). In terms of specific amino acids, it has been observed that amino acids can both promote and limit organismal lifespan and senescence, and thus amino acids can be used as markers of longevity (Rallis et al., 2020). In particular, the metabolism of branched-chain amino acids (BCAA) is associated with the regulation of human aging (Mansfeld et al., 2015). It was previously reported that supplementation of BCAA can improve mitochondrial biogenesis, alleviate ROS-induced stress, and thus augment lifespan in aging mice (D’Antona et al., 2010). In terms of cellular senescence, it was reported that cellular supplementation with BCAA can augment senescence-induced tumor suppression in liver cancer cells (Nakano et al., 2013) while a recent study has observed that higher circulatory levels of BCAA are positively associated with longer telomere lengths and thus suppressed systemic cellular senescence in middle-aged subjects (Fig. 4) (Zhang et al., 2020). In addition to the quantity of proteins consumed, the source of proteins (animals or plants) in diet also appears to strongly impact organismal lifespan although deeper studies relating these aspects to cellular senescence are warranted (Song et al., 2016). Further, information on SASP modulatory and senolytic attributes of proteins and dietary amino acids is rare and needs further exploration.
Fig. 4.
Influence of dietary protein intake and specific amino acids on different aspects of cellular senescence
Fatty acids
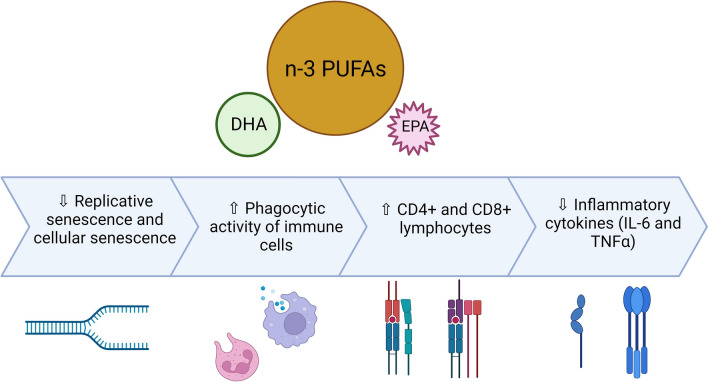
Fatty acids are essential structural and signaling molecules in cells. Essential fatty acids must be supplied in the diet and their critical role in maintaining growth and including aging is well recognized (Lai et al., 2018). In particular, omega-3-polyunsaturated fatty acids (PUFA) have shown several beneficial effects in ameliorating age-related insults including inflammation, osteopenia, type II diabetes, and imparting vasodilatory properties (Cugno et al., 2021; Simopoulos, 1999). In fact, a recent study has demonstrated that higher circulating levels of marine n-3 PUFA are associated with a lower risk of premature death (Harris et al., 2021). In addition, immunomodulatory and anti-immunosenescence activities of omega-3-fatty acids rich fish oil have also been reported. For instance, the consumption of fish oil can accelerate the phagocytic activity of immune cells, increase CD4+ and CD8+ lymphocytes, reduce inflammatory cytokines, and can increase muscle strength in the elderly (de Lourdes Nahhas Rodacki et al., 2015, Rodacki et al., 2012). Supplementation of fish oil ameliorated rosiglitazone-induced osteopenia in aging C57BL/6 mice resulting in a higher bone density, reduced pro-inflammatory cytokines, and increased anti-inflammatory cytokines in aging mice (Cugno et al., 2021). Furthermore, research proposes that supplementation of eicosapentaenoic acid may decrease NK cell activity in older individuals (Thies et al., 2001). Fish oil rich in omega-3 fatty acids attenuated various cardiac dysfunctions from ventricular hypertrophy to cardiac remodeling, as seen in aging mice (Halade et al., 2011). In addition, a central role of lipids in regulating both replicative and stress-induced cellular senescence is also rapidly emerging (Millner and Atilla-Gokcumen, 2020). In general, SC display characteristics increase in cellular lipid accumulation indicating deregulated lipid metabolism (Flor et al., 2017). Further research has revealed that lipid composition undergoes global changes in SC resulting in the remodeling of cell membranes which is particularly implicated in the development of SASP (Lizardo et al., 2017). Another recent study has indicated an essential role of fatty acid synthase in the development and initiation of the senescence program in mouse hepatic stellate cells and human primary fibroblasts (Fafián-Labora et al., 2019). It is therefore not surprising that the application of fatty acids has shown promise in suppressing cellular senescence as well as improving the aging immune responses. For example, a recent clinical trial has documented that consumption of marine n-3 PUFAs in subjects with renal transplantation reduces the risk of cellular senescence and SASP damage thereby resulting in improved recovery (Chan et al., 2021). Consumption of omega-3-fatty acids has been associated with decreased replicative senescence in human immune cells by preserving their telomere length (Farzaneh-Far et al., 2010; Kiecolt-Glaser et al., 2013). Supplementation of omega-3-fatty acids for 4 months in middle-aged subjects resulted in maintenance of telomerase activity while attenuating markers of stress and inflammation (Madison et al., 2021). Consumption of n-3 PUFA by d-galactose-induced aging mice reduced cellular DNA damage and protected the liver and testes of animals against telomere shortening (Chen et al., 2017). In a study on pigs, it was observed that supplementation with linseed oil for 9 weeks counteracted the age-related increase in the expression of TRF-1 which could be implicated in telomere length-promoting effects of PUFAs (Ogłuszka et al., 2020). Using in vitro model of stress-induced senescence in vascular endothelial cells, it was observed that supplementation with EPA and DHA can attenuate cellular senescence and its biomarkers by primarily inhibiting DNA damage and augmentation of cellular antioxidant potential (Sakai et al., 2017). Together, these observations assert that fatty acids are key mediators of the development of SC which also signifies their therapeutic potential (Fig. 5).
Fig. 5.
Cellular senescence and immunosenescence modulatory attributes of omega-3-fatty acids during aging
Vitamins and minerals
An important role of certain vitamins in regulating cellular senescence is also emerging. In particular, vitamin D appears to influence several facets of cellular senescence both in vitro and in vivo. A recent study suggests that vitamin D deficiency and cellular senescence are related which together influence the pathogenesis of obesity in experimental animals (Bima et al., 2021). Another recent study observed that vitamin D supplementation could rescue against doxorubicin-induced cellular senescence in human endothelial cells by upregulation of IL-10 and FOXO3a expression mediated by the modulation of pAMPKα/SIRT1/FOXO3a complex activity (Chen et al., 2021b). These observations are largely attributed to the ability of vitamin D to regulate cell cycle and proliferation as also reported previously (Samuel and Sitrin, 2008). Moreover, relatively higher vitamin D levels are also linked to increased telomere length and thus suppression of senescence. For instance, it was reported that subjects with higher levels of 25-hydroxyvitamin D exhibited significantly higher telomere length in whole blood cells (Mazidi et al., 2017). Similarly, two independent studies on elderly subjects also reported that serum 25-Hydroxyvitamin D is positively associated with mean telomere length suggesting that levels of 25-Hydroxyvitamin D could be predictors of telomere length and longevity (Beilfuss et al., 2017; Richards et al., 2007). Another recent study suggests that higher levels of vitamin D are positively related to longer telomere length but negatively associated with indications of type II diabetes as monitored through levels of Hb1Ac suggesting a deeper correlation between vitamin D, and cellular senescence, and age-related diseases (Akash et al., 2021). In addition to vitamin D, vitamin E is also reported to have anti-cellular senescence attributes which could be attributed to its strong antioxidant activity. For example, it was observed that treatment with vitamin E could suppress the progression of cellular senescence in human endothelial and fibroblast cells through the inhibition of cell cycle inhibitors (La Fata et al., 2015). Moreover, inadequate consumption of vitamin E was associated with shorter telomere lengths in leucocytes of humans suggesting that appropriate dietary consumption of vitamin E can mitigate cellular senescence (Corina et al., 2019). In addition, a recent study has identified vitamin B2 as a suppressor of senescence by promoting mitochondrial energetic homeostasis indicating that dietary riboflavin could also impact aging (Nagano et al., 2021). In addition to vitamins, certain minerals also appear to be associated with cellular senescence. The mineral magnesium is reportedly active in modulating cellular senescence and aging. Chronic magnesium deficiency in cultured fibroblasts results in an accelerated senescence program characterized by increased expression of cell cycle inhibitors and SA-β-gal activity as well as reduced telomere length (Killilea and Ames, 2008). Conversely, dietary supplementation of magnesium enhanced the mitochondrial functions and prevented oxidative stress in tissues resulting in enhanced murine lifespan (Villa-Bellosta, 2020). However, there are inconsistent reports on magnesium levels and telomere length in leucocytes which warrant further exploration (O'Callaghan et al., 2014; Yu et al., 2020). Zinc is another important mineral that is actively involved in regulating aging through modulation of the immune system (Haase and Rink, 2009) as well as through general suppression of systemic cellular stress (Giacconi et al., 2018). Zinc metabolism is impaired in SC and evidence suggests that zinc deficiency can contribute to the accumulation of SC and vascular pathology (Malavolta et al., 2017). Further, impaired zinc metabolism is also linked to shortened telomeres and increased inflammation in PBMCs (Cipriano et al., 2009) while accumulation of zinc is associated with increased ROS production and senescence induction in vascular smooth muscle cells (Salazar et al., 2017). Iron is another important mineral that has been implicated in driving aging. In particular, blocking iron availability through chelation is considered an important lifespan-extending mechanism of several dietary natural molecules such as EGCG, berberine, and curcumin (Mangan, 2021). This is because although iron deficiency anemia is often observed in the elderly, iron stores in tissues gradually increase with age which is implicated in the inhibition of ferroptosis and thus augmentation of age-related pathologies (Mazhar et al., 2021). Further, it has been observed that iron rapidly accumulates in SC causing inhibition of iron-induced cell death and thus aiding in the survival of SC (Killilea et al., 2004; Masaldan et al., 2018). In fact, augmentation of ferroptosis and iron metabolism is emerging as a novel therapy for removing the accumulation of SC in vivo which should be further explored (Go et al., 2021).
Secondary diet constituents and cellular senescence
Polyphenols
Plant polyphenols are a diverse group of phytomolecules that are considered important constituents of a healthy diet due to their well-documented role in modulating human health. Polyphenols have been reported to confer cytoprotective and health beneficial effects through the modulation of several cell signaling pathways such as NRF2, NF-κB, mTOR, Sirtuins as well as key processes such as autophagy, immunomodulation, cell proliferation, and apoptosis (Cory et al., 2018; Vauzour et al., 2010). In addition, studies suggest that long term consumption of dietary polyphenols confers a protective role in abating a multitude of age-related degenerative diseases like cancer (Lee and Lee, 2006), cardiovascular diseases (Khurana et al., 2013), muscular atrophy (Nikawa et al., 2021), neurodegenerative diseases (Rossi et al., 2008), arthritis (Behl et al., 2021), and even organismal longevity (Queen and Tollefsbol, 2010). Current research in this domain is now focused on understanding whether and how polyphenols can modulate cellular senescence and SASP thereby impacting organismal aging (Sharma and Padwad, 2020). We and others have previously observed anti-cellular senescence attributes of isolated dietary polyphenols such as green tea EGCG (Kumar et al., 2019; Kumar et al., 2020a), berberine (Dang et al., 2020), resveratrol (Giovannelli et al., 2011), quercetin (Sohn et al., 2018), kaempferol (Yao et al., 2019), tocotrienol (Khee et al., 2014), genistein (Wu et al., 2021), pterostilbene (Jiang et al., 2021), and apigenin (Li et al., 2021) in various in vitro and in vivo settings (Table 1). Further, anti-SASP effects of dietary flavonoids apigenin and kaempferol in bleomycin-induced senescence in fibroblasts were also reported that involved inhibition of the NF-κB pathway via IRAK1/IκBα signaling (Lim et al., 2015). In addition, cellular senescence suppressive attributes of polyphenol-rich fractions isolated from fruits such as lemons (Shimizu et al., 2019), grape seed extract (Wan et al., 2021; Xu et al., 2021), as well as red wine (Botden et al., 2012) have also been documented. In addition, a growing interest amongst polyphenols is the identification of novel senolytics that may selectively induce apoptosis in SC and thus alleviate SC burden in tissues with age (Li et al., 2019; Wang et al., 2021). In fact, quercetin was the first non-synthetic molecule identified with a senolytic activity (Zhu et al., 2015) and since then the combination of dasatinib and quercetin has shown promising results in alleviating SC burden in both preclinical and clinical studies resulting in improved organ functions and lifespan (Hickson et al., 2019; Novais et al., 2021). Our lab has previously identified that tea polyphenol EGCG can also act as a senolytic and can extend murine lifespan by decreasing SC burden in multiple tissues (Kumar et al., 2019; Sharma et al., 2022). Similarly, other polyphenols such as fisetin (Zhu et al., 2017) and piperlongumine (Wang et al., 2016) as well as polyphenols-rich fractions of Silybum marianum flower (Woo et al., 2021) have also been reported as senolytic agents. Moreover, polyphenols are known for their immunomodulatory activities and there is evidence that polyphenol consumption can also stimulate the aging immune system and prevent inflamm-aging (Baeza et al., 2010; Sharma et al., 2017). Together, polyphenols appear to confer cytoprotective and pro-longevity attributes through the inhibition of cellular senescence and improving immune responses, and therefore novel and traditional polyphenols-rich medicinal plants should be investigated for developing a nutrition-oriented holistic anti-senescence and senescence immunotherapies (Luo et al., 2021; Sharma and Padwad, 2020). Mechanistically, dietary polyphenols have shown the ability to modulate nutrient-sensing pathways (NSP) such as the mTOR and sirtuins which are implicated in their observed anti-cellular senescence effects (Davinelli et al., 2012). The NSPs act as metabolic sensors for stress and energy which can affect downstream targets to either promote or suppress cellular growth and differentiation. As such, the coordinated activation and functioning of these pathways are necessary and their molecular targeting is recognized in anti-aging therapies including calorie restriction (Pignatti et al., 2020). In terms of cellular senescence, these pathways are even more significant since SC are inherently under redox and metabolic stress and yet being stable, their NSP profile is largely deregulated (Carroll and Korolchuk, 2018). In general, SC display increased glycolysis (James et al., 2015), overactivated mTOR signaling (Kumar et al., 2019), and suppressed Sirtuins activity (Xu et al., 2020). The mTOR pathway is of particular significance in this regard as this evolutionarily conserved signaling system is considered the basal driving force of cellular senescence as it promotes the growth of non-dividing senescent cells (Blagosklonny, 2008; Liu and Sabatini, 2020). The cellular senescence modulatory effects of various dietary constituents involve interactions with these pathways to confer their cytoprotective effects. For example, we and others have observed that the anti-cellular senescence attributes of EGCG are mediated by the inhibition of mTOR pathway (Kumar et al., 2019) and activation of Sirtuins pathway (Lilja et al., 2020). Similarly, resveratrol (Demidenko and Blagosklonny, 2009) and berberine (Zhao et al., 2013) have also shown anti-cellular senescence effects mediated by the suppression of mTOR activity.
Table 1.
Anti-cellular senescence effects of selected polyphenols
| Compound | Model | Senescence type | Observations | References |
|---|---|---|---|---|
| EGCG | Human articular chondrocytes-knee (NHAC-kn) | IL-1β-stimulated human chondrocytes | EGCG (10 μM) resulted in downregulation of expression of IL-1, COX-2, MMP-13, and p16Ink4a | Huang et al. (2021) |
| Carvacrol | NIH 3T3 cell line | Acrylamide- and H2O2-induced cellular senescence | Carvacrol (100 μM) significantly reduced SA-β-gal activity, lipid peroxidation, and increased glutathione activity | Evazalipour et al. (2021) |
| Tocotrienol | Primary cultures of HDFs (Human diploid fibroblasts) | Replicative senescence | Tocotrienol-rich fraction TRF (0.5 mg/mL) resulted in modulation of senescence associated-miRNA and prevented cellular senescence | Gwee Sian Khee et al. (2014) |
| Pterostilbene | Mice | Ethanol-triggered hepatocyte senescence | Pterostillbene (10–20 μM) reduced SASP, cellular communication network factor 1 (CCN1) reduction via p62-mediated selective autophagy | Yiming Jiang et al. (2021) |
| Genistein (GEN) | Human umbilical vein endothelial cells | H2O2-induced senescence | GEN (40 and 80 μg/mL) downregulated the expression of p16Ink4a, p21, and TXNIP, NLRP3 | Wu et al. (2021) |
| Dasatinib + Quercetin | C57BL/6 mice | Senescent cell-transplantation | Dasatinib + Quercetin (1 μM + 20 μM) selective elimination of senescent cells, reduced mortality by 65% | Xu et al. (2018) |
| (-)-Epicatechin | Rats | Endothelial cells isolated from aged rats | Epicatechin (1 μM) decreased SA-β-gal activity, NO production, and increased Sirt1 expression | Ramirez-Sanchez et al. (2018) |
| Apigenin | Human fibroblast strains | Stress-induced senescence | Apigenin (10 μM) suppressed SASP phenotype and its paracrine effects | Perrott et al. (2017) |
| Anthocyanins rich extract of Ribes meyeri | Murine neural stem cells | Natural replicative senescence in isolated cells | Anthocyanins rich extract of Ribes meyeri (100 pg/mL) improved proliferation response, increased telomere lengths, and decreased p16Ink4a expression | Gao et al. (2020) |
| Ferulic acids | Human dermal fibroblasts | UVA-induced cell senescence | Ferulic acids (10–20 µM) increased proliferation response and antioxidant capacity | Hahn et al. (2016) |
| Gallic acids | Rat embryonic fibroblast | H2O2-induced senescence | Gallic acids (554.25 µM) decreased β-galactosidase activity, reduced inflammatory cytokines, and oxidative stress markers | Rahimifard et al. (2020) |
| Naringenin | Myocardial cells | H2O2-induced senescence | Naringenin (40 μM) decreased expression of cell cycle inhibitors and arrested cells, improved redox homeostasis | Da Pozzo et al. (2017) |
Probiotics
It is now acknowledged that dietary consumption of probiotic bacteria can affect several facets of human health including maturation of the immune system, nutrition, and metabolism, brain development, as well as in the pathogenesis of chronic disorders such as cancer and diabetes (Cerdó et al., 2017; George Kerry et al., 2018; Maldonado Galdeano et al., 2019; Taherian et al., 2019). In fact, probiotic-derived functional foods are of attractive consumer interest and several traditional and novel probiotic fermented functional foods are available (Marco et al., 2017; Melini et al., 2019). Moreover, there is evidence that modulation of the gut microbiota could be a critical intermediate process governing the purported health beneficial effects of several dietary elements. This is primarily attributed to the fact that nutritional components first interact with the gut bacteria and their microbiota-mediated biotransformation in the gut has the potential to qualitatively and quantitatively change the physiological effects of parent molecules (Sallam et al., 2021; Wang et al., 2018). The role of gut microbiota is increasingly being emphasized in longevity and gut microbial signatures are emerging as predictors of human lifespan (Galkin et al., 2020; Wilmanski et al., 2021). Further, gut microbiota undergoes structural and compositional changes with age, dietary habits, or during disease (gut dysbiosis) and therefore supplementation with specific probiotics has been shown to improve gut dysbiosis and alleviate several deleterious aspects of organismal aging physiology such as immunosenescence (Sharma et al., 2014), neurodegeneration (Lye et al., 2018), and chronic diseases (Buford, 2017). In fact, a functional term called ‘gerobiotics’ has been recently proposed to identify new probiotics with the ability to counter aging and age-related disorders (Tsai et al., 2021). In terms of cellular senescence, it has been shown that a dysbiotic gut is a source of potential novel metabolites that can augment cellular senescence and SASP in vivo and thus augment aging and disease phenotype (Yoshimoto et al., 2013). Conversely, we have demonstrated that secretory metabolites of probiotic Lactobacillus fermentum can inhibit stress-induced development of senescence and SASP in preadipocytes by improving cellular and metabolic stress (Kumar et al., 2020b). Previous studies also showed that consumption of probiotics in aged mice could prevent intestinal senescence and inflamm-aging thereby extending organismal healthspan (Jeong et al., 2015; Jeong et al., 2016). In addition, it appears that the observed anti-cellular senescence effects of dietary constituents, including phytomolecules such as quercetin, could be mediated through the modulation of the gut microbiota composition in vivo (Saccon et al., 2021). This is an exciting new area of research as the amalgamation of probiotics and bioactive phytomolecules such as polyphenols is considered viable and has been shown to confer cytoprotective and anti-aging effects (Banerjee and Dhar, 2019; Sharma et al., 2019).
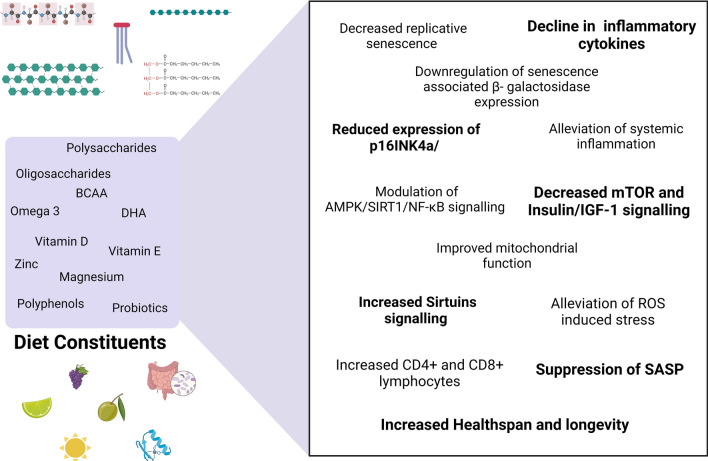
In conclusion, cellular senescence-mediated understanding of aging and age-dependent disorders is rapidly gaining attention as a viable therapeutic target (Soto-Gamez and Demaria, 2017). It is increasingly being realized that cellular senescence could be central to developing anti-aging strategies as evidence of its integration with other established age-related phenomena such as immunosenescence and gut dysbiosis is also emerging (Budamagunta et al., 2021; Sharma, 2022). Besides, the striking presence of cellular senescence in pathophysiologically distinct human disorders renews hope of a single targetable approach to disease management during aging. Nutritional elements are essential for our survival and growth and thus their role in positively modulating cellular senescence and aging seems unsurprising. As highlighted in this manuscript, all forms of nutrition, albeit with varying degrees, have shown some potency to alleviate the different facets of cellular senescence and improve cellular functions (Fig. 6). It is exciting to note that essential nutritional components such as carbohydrates, fats, and proteins can affect the different facets of cellular senescence. However, detailed knowledge of their specific effects is still limited, and further in vivo studies are required to truly assess their anti-cellular senescence relevance. The role of minerals is also of particular interest as their impaired metabolism appears to be specific markers of cellular senescence and yet their therapeutic potential is little explored. It would be interesting to assess how specific food items rich in certain minerals could add affect the progression and development of cellular senescence. Secondary dietary elements are also of great interest as polyphenols such as quercetin have already shown promising clinical results in alleviating SC burden and improving age-related pathologies (Hickson et al., 2019). Similarly, we are only beginning to understand how probiotics can be useful in mitigating cellular senesce and aging which requires further investigations. Apart from individual components, whole diets composed of carefully curated dietary components should be assessed for their global effects on cellular senescence as also noted previously in a preliminary study (Leung et al., 2018). More such concerted efforts on different dietary regimens must be pursued for a better understanding of whole diets and their influence on cellular senescence and aging. It has long been argued that regular exercise and a healthy diet regimen is key to improving both the healthspan and lifespan. Emerging evidence now suggests that indeed both exercise and a healthy diet (e.g., Mediterranean diet) can improve the indices of general health and longevity through specific targeting of cellular senescence and its deleterious effects (Englund et al., 2021; Marin et al., 2012; Shannon et al., 2021). It is desirable that dietary constituents should be studied individually as well as in combinations of whole diets with a specific aim of identifying nutritional geroprotectors through the purview of cellular senescence that may enable a better pharmacological understanding of nutrition as a regulator of aging and diseases.
Fig. 6.
Potential biological effects and targets of various dietary constituents in influencing the markers of cellular senescence and related pathologies
Acknowledgements
The authors acknowledge the funding received by a grant from the Department of Science and Technology, Government of India under the INSPIRE Faculty scheme (IFA17-LSPA79).
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bhawna Diwan, Email: diwanbhawna@gmail.com.
Rohit Sharma, Email: rohit25sharma@gmail.com.
References
- Aguayo-Mazzucato C, Andle J, Lee TB, Midha A, Talemal L, Chipashvili V, Hollister-Lock J, van Deursen J, Weir G, Bonner-Weir S. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metabolism. 2019;30:129–142.e4. doi: 10.1016/j.cmet.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akash C, Prabhu M, Maldar A, Akash P, Mishra S, Madhura TK, Kumar S, Patil RS, Piplani S, Smitha KS. Association of telomere length and serum vitamin D levels with type 2 diabetes mellitus and its related complications: a possible future perspective. Genome Integrity. 2021;12:2. doi: 10.4103/genint.genint_3_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliper A, Belikov AV, Garazha A, Jellen L, Artemov A, Suntsova M, Ivanova A, Venkova L, Borisov N, Buzdin A, Mamoshina P, Putin E, Swick AG, Moskalev A, Zhavoronkov A. In search for geroprotectors: in silico screening and in vitro validation of signalome-level mimetics of young healthy state. Aging. 2016;8:2127–2152. doi: 10.18632/aging.101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik M, Jurk D, Proctor C, Correia-Melo C, Victorelli S, Fielder E, Berlinguer-Palmini R, Owens A, Greaves LC, Kolsky KL, Parini A, Douin-Echinard V, LeBrasseur NK, Arthur HM, Tual-Chalot S, Schafer MJ, Roos CM, Miller JD, Robertson N, Mann J, Adams PD, Tchkonia T, Kirkland JL, Mialet-Perez J, Richardson GD, Passos JF. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO Journal. 2019 doi: 10.15252/embj.2018100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeza I, De Castro NM, Arranz L, De la Fuente M. Soybean and green tea polyphenols improve immune function and redox status in very old ovariectomized mice. Rejuvenation Research. 2010;13:665–674. doi: 10.1089/rej.2010.1049. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Dhar P. Amalgamation of polyphenols and probiotics induce health promotion. Critical Reviews in Food Science and Nutrition. 2019;59:2903–2926. doi: 10.1080/10408398.2018.1478795. [DOI] [PubMed] [Google Scholar]
- Barinda AJ, Ikeda K, Nugroho DB, Wardhana DA, Sasaki N, Honda S, Urata R, Matoba S, Hirata K-i, Emoto N. Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype. Nature Communications. 2020;11:1–13. doi: 10.1038/s41467-019-13993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl T, Mehta K, Sehgal A, Singh S, Sharma N, Ahmadi A, Arora S, Bungau S. Exploring the role of polyphenols in rheumatoid arthritis. Critical Reviews in Food Science and Nutrition. 2021 doi: 10.1080/10408398.2021.1924613. [DOI] [PubMed] [Google Scholar]
- Beilfuss J, Camargo CA, Jr, Kamycheva E. Serum 25-hydroxyvitamin D has a modest positive association with leukocyte telomere length in middle-aged US adults. Journal of Nutrition. 2017;147:514–520. doi: 10.3945/jn.116.244137. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Weinberg RA. When cells get stressed: an integrative view of cellular senescence. The Journal of Clinical Investigation. 2004;113:8–13. doi: 10.1172/JCI200420663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE. 2012;7:e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bima A, Eldakhakhny B, Nuwaylati D, Alnami A, Ajabnoor M, Elsamanoudy A. The interplay of vitamin D deficiency and cellular senescence in the pathogenesis of obesity-related co-morbidities. Nutrients. 2021;13:4127. doi: 10.3390/nu13114127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes and Development. 2020;34:1565–1576. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–3354. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. American Journal of Pathology. 2012;181:1142–1146. doi: 10.1016/j.ajpath.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging is not programmed: genetic pseudo-program is a shadow of developmental growth. Cell Cycle. 2013;12:3736–3742. doi: 10.4161/cc.27188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Disease or not, aging is easily treatable. Aging (Albany NY) 2018;10:3067–3078. doi: 10.18632/aging.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi V, Mecocci P. Senotherapeutics: targeting senescent cells for the main age-related diseases. Mechanisms of Ageing and Development. 2021;197:111526. doi: 10.1016/j.mad.2021.111526. [DOI] [PubMed] [Google Scholar]
- Borghesan M, Hoogaars WMH, Varela-Eirin M, Talma N, Demaria M. A senescence-centric view of aging: implications for longevity and disease. Trends in Cell Biology. 2020;30:777–791. doi: 10.1016/j.tcb.2020.07.002. [DOI] [PubMed] [Google Scholar]
- Botden IP, Oeseburg H, Durik M, Leijten FP, Van Vark-Van Der Zee LC, Musterd-Bhaggoe UM, Garrelds IM, Seynhaeve AL, Langendonk JG, Sijbrands EJ, Danser AH, Roks AJ. Red wine extract protects against oxidative-stress-induced endothelial senescence. Clinical Science (Lond) 2012;123:499–507. doi: 10.1042/CS20110679. [DOI] [PubMed] [Google Scholar]
- Budamagunta V, Foster TC, Zhou D. Cellular senescence in lymphoid organs and immunosenescence. Aging (Albany NY) 2021;13:19920–19941. doi: 10.18632/aging.203405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5:80. doi: 10.1186/s40168-017-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DGA, Stolzing A. Cellular senescence: immunosurveillance and future immunotherapy. Ageing Research Reviews. 2018;43:17–25. doi: 10.1016/j.arr.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. The biology of replicative senescence. European Journal of Cancer. 1997;33:703–709. doi: 10.1016/S0959-8049(96)00058-5. [DOI] [PubMed] [Google Scholar]
- Campisi J. Aging, cellular senescence, and cancer. Annual Review of Physiology. 2013;75:685. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll B, Korolchuk VI. Nutrient sensing, growth and senescence. The FEBS Journal. 2018;285:1948–1958. doi: 10.1111/febs.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdó T, Ruíz A, Suárez A, Campoy C. Probiotic, prebiotic, and brain development. Nutrients. 2017;9:1247. doi: 10.3390/nu9111247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Eide IA, Tannæs TM, Waldum-Grevbo B, Jenssen T, Svensson M. Marine n-3 polyunsaturated fatty acids and cellular senescence markers in incident kidney transplant recipients: the omega-3 fatty acids in renal transplantation (orentra) randomized clinical trial. Kidney Medicine. 2021;3:1041–1049. doi: 10.1016/j.xkme.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wei Y, Chen X, Jiao J, Zhang Y. Polyunsaturated fatty acids ameliorate aging via redox-telomere-antioncogene axis. Oncotarget. 2017;8:7301–7314. doi: 10.18632/oncotarget.14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, Li T, Margolick JB, Pawelec G, Leng SX. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Research Reviews. 2021;65:101205. doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Holder R, Porter C, Shah Z. Vitamin D3 attenuates doxorubicin-induced senescence of human aortic endothelial cells by upregulation of IL-10 via the pAMPKα/Sirt1/Foxo3a signaling pathway. PLoS ONE. 2021;16:e0252816. doi: 10.1371/journal.pone.0252816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Yao H, Xiang Y, Chen L, Xiao M, Wang Z, Xiao H, Wang L, Wang S, Wang Y. Effect of Angelica polysaccharide on brain senescence of Nestin-GFP mice induced by d-galactose. Neurochemistry International. 2019;122:149–156. doi: 10.1016/j.neuint.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Reports. 2014;15:1139–1153. doi: 10.15252/embr.201439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Li H, van Deursen JM. Senescent cells: a therapeutic target for cardiovascular disease. Journal of Clinical Investigation. 2018;128:1217–1228. doi: 10.1172/JCI95146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriano C, Tesei S, Malavolta M, Giacconi R, Muti E, Costarelli L, Piacenza F, Pierpaoli S, Galeazzi R, Blasco M, Vera E, Canela A, Lattanzio F, Mocchegiani E. Accumulation of cells with short telomeres is associated with impaired zinc homeostasis and inflammation in old hypertensive participants. The Journals of Gerontology: Series A. 2009;64A:745–751. doi: 10.1093/gerona/glp048. [DOI] [PubMed] [Google Scholar]
- Corina A, Rangel-Zúñiga OA, Jiménez-Lucena R, Alcalá-Díaz JF, Quintana-Navarro G, Yubero-Serrano EM, López-Moreno J, Delgado-Lista J, Tinahones F, Ordovás JM, López-Miranda J, Pérez-Martínez P. Low intake of vitamin E accelerates cellular aging in patients with established cardiovascular disease: the CORDIOPREV Study. The Journals of Gerontology: Series A. 2019;74:770–777. doi: 10.1093/gerona/gly195. [DOI] [PubMed] [Google Scholar]
- Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The role of polyphenols in human health and food systems: a mini-review. Frontiers in Nutrition. 2018;5:87–87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofalo VJ, Lorenzini A, Allen RG, Torres C, Tresini M. Replicative senescence: a critical review. Mechanisms of Ageing and Development. 2004;125:827–848. doi: 10.1016/j.mad.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Cugno C, Kizhakayil D, Calzone R, Rahman SM, Halade GV, Rahman MM. Omega-3 fatty acid-rich fish oil supplementation prevents rosiglitazone-induced osteopenia in aging C57BL/6 mice and in vitro studies. Scientific Reports. 2021;11:10364. doi: 10.1038/s41598-021-89827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Pozzo E, Costa B, Cavallini C, Testai L, Martelli A, Calderone V, Martini C. The citrus flavanone naringenin protects myocardial cells against age-associated damage. Oxidative Medicine and Cellular Longevity. 2017;2017:9536148–9536148. doi: 10.1155/2017/9536148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, An Y, He J, Huang B, Zhu J, Gao M, Zhang S, Wang X, Yang B, Xie Z. Berberine ameliorates cellular senescence and extends the lifespan of mice via regulating p16 and cyclin protein expression. Aging Cell. 2020;19:e13060. doi: 10.1111/acel.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metabolism. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Davinelli S, Willcox DC, Scapagnini G. Extending healthy ageing: nutrient sensitive pathway and centenarian population. Immunity & Ageing. 2012;9:9. doi: 10.1186/1742-4933-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lourdes Nahhas Rodacki C, Rodacki ALF, Coelho I, Pequito D, Krause M, Bonatto S, Naliwaiko K, Fernandes LC. Influence of fish oil supplementation and strength training on some functional aspects of immune cells in healthy elderly women. British Journal of Nutrition. 2015;114:43–52. doi: 10.1017/S0007114515001555. [DOI] [PubMed] [Google Scholar]
- Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, Iqbal R, Kumar R, Wentzel-Viljoen E, Rosengren A, Amma LI, Avezum A, Chifamba J, Diaz R, Khatib R, Lear S, Lopez-Jaramillo P, Liu X, Gupta R, Mohammadifard N, Gao N, Oguz A, Ramli AS, Seron P, Sun Y, Szuba A, Tsolekile L, Wielgosz A, Yusuf R, Hussein Yusufali A, Teo KK, Rangarajan S, Dagenais G, Bangdiwala SI, Islam S, Anand SS, Yusuf S. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390:2050–2062. doi: 10.1016/S0140-6736(17)32252-3. [DOI] [PubMed] [Google Scholar]
- Dehkordi SK, Walker J, Sah E, Bennett E, Atrian F, Frost B, Woost B, Bennett RE, Orr TC, Zhou Y, Andhey PS, Colonna M, Sudmant PH, Xu P, Wang M, Zhang B, Zare H, Orr ME. Profiling senescent cells in human brains reveals neurons with CDKN2D/p19 and tau neuropathology. Nature Aging. 2021;1:1107–1116. doi: 10.1038/s43587-021-00142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko ZN, Blagosklonny MV. At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence. Cell Cycle. 2009;8:1901–1904. doi: 10.4161/cc.8.12.8810. [DOI] [PubMed] [Google Scholar]
- Denzel MS, Storm NJ, Gutschmidt A, Baddi R, Hinze Y, Jarosch E, Sommer T, Hoppe T, Antebi A. Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell. 2014;156:1167–1178. doi: 10.1016/j.cell.2014.01.061. [DOI] [PubMed] [Google Scholar]
- Edwards CB, Copes N, Brito AG, Canfield J, Bradshaw PC. Malate and fumarate extend lifespan in Caenorhabditis elegans. PLoS ONE. 2013;8:e58345. doi: 10.1371/journal.pone.0058345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund DA, Sakamoto AE, Fritsche CM, Heeren AA, Zhang X, Kotajarvi BR, Lecy DR, Yousefzadeh MJ, Schafer MJ, White TA, Atkinson EJ, LeBrasseur NK. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell. 2021;20:e13415. doi: 10.1111/acel.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evazalipour M, Safarzadeh Kozani P, Safarzadeh Kozani P, Shabani S, Rezaei Soufi B, Zamani E. Acrylamide induced oxidative cellular senescence in embryonic fibroblast cell line (NIH 3T3): a protection by carvacrol. Jundishapur Journal of Natural Pharmaceutical Products. 2021 doi: 10.5812/jjnpp.109399. [DOI] [Google Scholar]
- Fafián-Labora J, Carpintero-Fernández P, Jordan SJD, Shikh-Bahaei T, Abdullah SM, Mahenthiran M, Rodríguez-Navarro JA, Niklison-Chirou MV, O’Loghlen A. FASN activity is important for the initial stages of the induction of senescence. Cell Death & Disease. 2019;10:318–318. doi: 10.1038/s41419-019-1550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, Accurso A, Frassetto L, Gower BA, McFarlane SI, Nielsen JV, Krarup T, Saslow L, Roth KS, Vernon MC, Volek JS, Wilshire GB, Dahlqvist A, Sundberg R, Childers A, Morrison K, Manninen AH, Dashti HM, Wood RJ, Wortman J, Worm N. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2015;31:1–13. doi: 10.1016/j.nut.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Flor AC, Wolfgeher D, Wu D, Kron SJ. A signature of enhanced lipid metabolism, lipid peroxidation and aldehyde stress in therapy-induced senescence. Cell Death Discovery. 2017;3:17075. doi: 10.1038/cddiscovery.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Khalil A, Cohen AA, Witkowski JM. Are we ill because we age? Frontiers in Physiology. 2019;10:1508. doi: 10.3389/fphys.2019.01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin F, Mamoshina P, Aliper A, Putin E, Moskalev V, Gladyshev VN, Zhavoronkov A. Human gut microbiome aging clock based on taxonomic profiling and deep learning. iScience. 2020;23:101199. doi: 10.1016/j.isci.2020.101199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wu Y, He D, Zhu X, Li H, Liu H, Liu H. Anti-aging effects of Ribes meyeri anthocyanins on neural stem cells and aging mice. Aging. 2020;12:17738–17753. doi: 10.18632/aging.103955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George Kerry R, Patra JK, Gouda S, Park Y, Shin H-S, Das G. Benefaction of probiotics for human health: a review. Journal of Food and Drug Analysis. 2018;26:927–939. doi: 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacconi R, Costarelli L, Piacenza F, Basso A, Bürkle A, Moreno-Villanueva M, Grune T, Weber D, Stuetz W, Gonos ES, Schön C, Grubeck-Loebenstein B, Sikora E, Toussaint O, Debacq-Chainiaux F, Franceschi C, Hervonen A, Slagboom E, Ciccarone F, Zampieri M, Caiafa P, Jansen E, Dollé MET, Breusing N, Mocchegiani E, Malavolta M. Zinc-induced metallothionein in centenarian offspring from a large European population: the MARK-AGE Project. The Journals of Gerontology: Series A. 2018;73:745–753. doi: 10.1093/gerona/glx192. [DOI] [PubMed] [Google Scholar]
- Giovannelli L, Pitozzi V, Jacomelli M, Mulinacci N, Laurenzana A, Dolara P, Mocali A. Protective effects of resveratrol against senescence-associated changes in cultured human fibroblasts. The Journals of Gerontology: Series A. 2011;66A:9–18. doi: 10.1093/gerona/glq161. [DOI] [PubMed] [Google Scholar]
- Go S, Kang M, Kwon SP, Jung M, Jeon OH, Kim BS. The senolytic drug jq1 removes senescent cells via ferroptosis. Tissue Engineering and Regenerative Medicine. 2021;18:841–850. doi: 10.1007/s13770-021-00346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood EK, Brown DR. Senescent microglia: the key to the ageing brain? International Journal of Molecular Sciences. 2021;22:4402. doi: 10.3390/ijms22094402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwee Sian Khee S, Mohd Yusof YA, Makpol S. Expression of senescence-associated micrornas and target genes in cellular aging and modulation by tocotrienol-rich fraction. Oxidative Medicine and Cellular Longevity. 2014;2014:725929. doi: 10.1155/2014/725929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase H, Rink L. The immune system and the impact of zinc during aging. Immunity & Ageing. 2009;6:9. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn HJ, Kim KB, Bae S, Choi BG, An S, Ahn KJ, Kim SY. Pretreatment of ferulic acid protects human dermal fibroblasts against ultraviolet a irradiation. Annals of Dermatology. 2016;28:740–748. doi: 10.5021/ad.2016.28.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade GV, Williams PJ, Lindsey ML, Fernandes G. Fish oil decreases inflammation and reduces cardiac remodeling in rosiglitazone treated aging mice. Pharmacological Research. 2011;63:300–307. doi: 10.1016/j.phrs.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WS, Tintle NL, Imamura F, Qian F, Korat AVA, Marklund M, Djoussé L, Bassett JK, Carmichael P-H, Chen Y-Y, Hirakawa Y, Küpers LK, Laguzzi F, Lankinen M, Murphy RA, Samieri C, Senn MK, Shi P, Virtanen JK, Brouwer IA, Chien K-L, Eiriksdottir G, Forouhi NG, Geleijnse JM, Giles GG, Gudnason V, Helmer C, Hodge A, Jackson R, Khaw K-T, Laakso M, Lai H, Laurin D, Leander K, Lindsay J, Micha R, Mursu J, Ninomiya T, Post W, Psaty BM, Risérus U, Robinson JG, Shadyab AH, Snetselaar L, Sala-Vila A, Sun Y, Steffen LM, Tsai MY, Wareham NJ, Wood AC, Wu JHY, Hu F, Sun Q, Siscovick DS, Lemaitre RN, Mozaffarian D, The Fatty A, Outcomes Research C Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nature Communications. 2021;12:2329. doi: 10.1038/s41467-021-22370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. The greatest risk factor for the leading cause of death is ignored. Biogerontology. 2021;22:133–141. doi: 10.1007/s10522-020-09901-y. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Experimental Cell Research. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL, Kellogg TA, Khosla S, Koerber DM, Lagnado AB, Lawson DK, LeBrasseur NK, Lerman LO, McDonald KM, McKenzie TJ, Passos JF, Pignolo RJ, Pirtskhalava T, Saadiq IM, Schaefer KK, Textor SC, Victorelli SG, Volkman TL, Xue A, Wentworth MA, Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohensinner PJ, Kaun C, Buchberger E, Ebenbauer B, Demyanets S, Huk I, Eppel W, Maurer G, Huber K, Wojta J. Age intrinsic loss of telomere protection via TRF1 reduction in endothelial cells. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2016;1863:360–367. doi: 10.1016/j.bbamcr.2015.11.034. [DOI] [PubMed] [Google Scholar]
- Honda Y, Tanaka M, Honda S. Trehalose extends longevity in the nematode Caenorhabditis elegans. Aging Cell. 2010;9:558–569. doi: 10.1111/j.1474-9726.2010.00582.x. [DOI] [PubMed] [Google Scholar]
- Hou Y, Wei Y, Lautrup S, Yang B, Wang Y, Cordonnier S, Mattson MP, Croteau DL, Bohr VA. NAD supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS–STING. Proceedings of the National Academy of Sciences of the United States of America. 2021;118:e2011226118. doi: 10.1073/pnas.2011226118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Fryatt GL, Ghorbani M, Obst J, Menassa DA, Martin-Estebane M, Muntslag TAO, Olmos-Alonso A, Guerrero-Carrasco M, Thomas D, Cragg MS, Gomez-Nicola D. Replicative senescence dictates the emergence of disease-associated microglia and contributes to Aβ pathology. Cell Reports. 2021;35:109228. doi: 10.1016/j.celrep.2021.109228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H-T, Cheng T-L, Yang C-D, Chang C-F, Ho C-J, Chuang S-C, Li J-Y, Huang S-H, Lin Y-S, Shen H-Y, Yu T-H, Kang L, Lin S-Y, Chen C-H. Intra-articular injection of (−)-epigallocatechin 3-gallate (EGCG) ameliorates cartilage degeneration in Guinea pigs with spontaneous osteoarthritis. Antioxidants. 2021;10:178. doi: 10.3390/antiox10020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idda ML, McClusky WG, Lodde V, Munk R, Abdelmohsen K, Rossi M, Gorospe M. Survey of senescent cell markers with age in human tissues. Aging (Albany NY) 2020;12:4052–4066. doi: 10.18632/aging.102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James EL, Michalek RD, Pitiyage GN, de Castro AM, Vignola KS, Jones J, Mohney RP, Karoly ED, Prime SS, Parkinson EK. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. Journal of Proteome Research. 2015;14:1854–1871. doi: 10.1021/pr501221g. [DOI] [PubMed] [Google Scholar]
- Jeong JJ, Kim KA, Jang SE, Woo JY, Han MJ, Kim DH. Orally administrated Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa B signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS ONE. 2015;10:e0116533. doi: 10.1371/journal.pone.0116533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JJ, Kim KA, Hwang YJ, Han MJ, Kim DH. Anti-inflammaging effects of Lactobacillus brevis OW38 in aged mice. Beneficial Microbes. 2016;7:707–718. doi: 10.3920/BM2016.0016. [DOI] [PubMed] [Google Scholar]
- Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mechanisms of Ageing and Development. 2008;129:467–474. doi: 10.1016/j.mad.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Aroor AR, Jia C, Sowers JR. Endothelial cell senescence in aging-related vascular dysfunction. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2019;1865:1802–1809. doi: 10.1016/j.bbadis.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhou Y, Xu W, Wang X, Jin H, Bao X, Lu C. Induction of Sestrin2 by pterostilbene suppresses ethanol-triggered hepatocyte senescence by degrading CCN1 via p62-dependent selective autophagy. Cell Biology and Toxicology. 2021 doi: 10.1007/s10565-021-09635-8. [DOI] [PubMed] [Google Scholar]
- Joy J, Barrio L, Santos-Tapia C, Romão D, Giakoumakis NN, Clemente-Ruiz M, Milán M. Proteostasis failure and mitochondrial dysfunction leads to aneuploidy-induced senescence. Developmental Cell. 2021;56:2043–2058.e7. doi: 10.1016/j.devcel.2021.06.009. [DOI] [PubMed] [Google Scholar]
- Kale A, Sharma A, Stolzing A, Desprez P-Y, Campisi J. Role of immune cells in the removal of deleterious senescent cells. Immunity & Ageing. 2020;17:16. doi: 10.1186/s12979-020-00187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin O, Agrawal A, Porat Z, Krizhanovsky V, Alon U. Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nature Communications. 2019;10:5495. doi: 10.1038/s41467-019-13192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Farr JN. Cellular senescence in age-related disorders. Translational Research. 2020;226:96–104. doi: 10.1016/j.trsl.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T, Unwin D, Finucane F. Low-carbohydrate diets in the management of obesity and type 2 diabetes: a review from clinicians using the approach in practice. International Journal of Environmental Research and Public Health. 2020;17:2557. doi: 10.3390/ijerph17072557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SY, Awad EM, Oszwald A, Mayr M, Yin X, Waltenberger B, Stuppner H, Lipovac M, Uhrin P, Breuss JM. Premature senescence of endothelial cells upon chronic exposure to TNFα can be prevented by N-acetyl cysteine and plumericin. Scientific Reports. 2017;7:1–13. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khee SG, Yusof YA, Makpol S. Expression of senescence-associated microRNAs and target genes in cellular aging and modulation by tocotrienol-rich fraction. Oxidative Medicine and Cellular Longevity. 2014;2014:725929. doi: 10.1155/2014/725929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, Venkataraman K, Hollingsworth A, Piche M, Tai TC. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients. 2013;5:3779–3827. doi: 10.3390/nu5103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Jaremka LM, Derry HM, Glaser R. Telomere length: a marker of disease susceptibility? Brain, Behavior, and Immunity. 2013;34:29–30. doi: 10.1016/j.bbi.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea DW, Ames BN. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5768–5773. doi: 10.1073/pnas.0712401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea DW, Wong SL, Cahaya HS, Atamna H, Ames BN. Iron accumulation during cellular senescence. Annals of the New York Academy of Sciences. 2004;1019:365–367. doi: 10.1196/annals.1297.063. [DOI] [PubMed] [Google Scholar]
- Kim EC, Kim JR. Senotherapeutics: emerging strategy for healthy aging and age-related disease. BMB Reports. 2019;52:47. doi: 10.5483/BMBRep.2019.52.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Yi YS, Kim J, Han SY, Kim SH, Seo DB, Cho JY, Shin SS. Effect of polysaccharides from a Korean ginseng berry on the immunosenescence of aged mice. Journal of Ginseng Research. 2018;42:447–454. doi: 10.1016/j.jgr.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Jiang K, Ferguson CM, Tang H, Chen X, Zhu X, Hickson LJ, Tchkonia T, Kirkland JL, Lerman LO. Transplanted senescent renal scattered tubular-like cells induce injury in the mouse kidney. American Journal of Physiology-Renal Physiology. 2020;318:F1167–F1176. doi: 10.1152/ajprenal.00535.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong SZ, Li JC, Li SD, Liao MN, Li CP, Zheng PJ, Guo MH, Tan WX, Zheng ZH, Hu Z. Anti-aging effect of chitosan oligosaccharide on d-galactose-induced subacute aging in mice. Marine Drugs. 2018;16:181. doi: 10.3390/md16060181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. Journal of Clinical Investigation. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky SB. Nutrition and healthy aging. The Journals of Gerontology: Series A. 2016;71:1303–1305. doi: 10.1093/gerona/glw165. [DOI] [PubMed] [Google Scholar]
- Kumar R, Sharma A, Kumari A, Gulati A, Padwad Y, Sharma R. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology. 2019;20:171–189. doi: 10.1007/s10522-018-9785-1. [DOI] [PubMed] [Google Scholar]
- Kumar R, Sharma A, Padwad Y, Sharma R. Preadipocyte secretory factors differentially modulate murine macrophage functions during aging which are reversed by the application of phytochemical EGCG. Biogerontology. 2020;21:325–343. doi: 10.1007/s10522-020-09861-3. [DOI] [PubMed] [Google Scholar]
- Kumar R, Sharma A, Gupta M, Padwad Y, Sharma R. Cell-free culture supernatant of probiotic Lactobacillus fermentum protects against H2O2-induced premature senescence by suppressing ROS-Akt-mTOR axis in murine preadipocytes. Probiotics and Antimicrobial Proteins. 2020;12:563–576. doi: 10.1007/s12602-019-09576-z. [DOI] [PubMed] [Google Scholar]
- La Fata G, Seifert N, Weber P, Mohajeri MH. Vitamin E supplementation delays cellular senescence in vitro. Biomed Research International. 2015;2015:563247. doi: 10.1155/2015/563247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai P, Liu Y. Angelica sinensis polysaccharides inhibit endothelial progenitor cell senescence through the reduction of oxidative stress and activation of the Akt/hTERT pathway. Pharmaceutical Biology. 2015;53:1842–1849. doi: 10.3109/13880209.2015.1027779. [DOI] [PubMed] [Google Scholar]
- Lai HT, de Oliveira Otto MC, Lemaitre RN, McKnight B, Song X, King IB, Chaves PH, Odden MC, Newman AB, Siscovick DS, Mozaffarian D. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: prospective cohort study. BMJ. 2018;363:k4067. doi: 10.1136/bmj.k4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourg E. Geroscience: the need to address some issues. Biogerontology. 2022 doi: 10.1007/s10522-022-09951-4. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, Solon-Biet S, Cogger VC, Mitchell SJ, Senior A, de Cabo R, Raubenheimer D, Simpson SJ. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cellular and Molecular Life Sciences. 2016;73:1237–1252. doi: 10.1007/s00018-015-2120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Lee HJ. The roles of polyphenols in cancer chemoprevention. Biofactors. 2006;26:105–121. doi: 10.1002/biof.5520260202. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee SH, Choi SH, Asahara T, Kwon SM. The sulfated polysaccharide fucoidan rescues senescence of endothelial colony-forming cells for ischemic repair. Stem Cells. 2015;33:1939–1951. doi: 10.1002/stem.1973. [DOI] [PubMed] [Google Scholar]
- Leung CW, Fung TT, McEvoy CT, Lin J, Epel ES. Diet quality indices and leukocyte telomere length among healthy US adults: data from the National Health and Nutrition Examination Survey, 1999–2002. American Journal of Epidemiology. 2018;187:2192–2201. doi: 10.1093/aje/kwy124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metabolism. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Qin L, Feng R, Hu G, Sun H, He Y, Zhang R. Emerging senolytic agents derived from natural products. Mechanisms of Ageing and Development. 2019;181:1–6. doi: 10.1016/j.mad.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Li BS, Zhu RZ, Lim SH, Seo JH, Choi BM. Apigenin alleviates oxidative stress-induced cellular senescence via modulation of the SIRT1-NAD CD38 Axis. The American Journal of Chinese Medicine. 2021;49:1235–1250. doi: 10.1142/S0192415X21500592. [DOI] [PubMed] [Google Scholar]
- Lilja S, Oldenburg J, Pointner A, Dewald L, Lerch M, Hippe B, Switzeny O, Haslberger A. Epigallocatechin gallate effectively affects senescence and anti-sasp via SIRT3 in 3T3-L1 preadipocytes in comparison with other bioactive substances. Oxidative Medicine and Cellular Longevity. 2020;2020:4793125. doi: 10.1155/2020/4793125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Park H, Kim HP. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochemical Pharmacology. 2015;96:337–348. doi: 10.1016/j.bcp.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Research. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nature Reviews Molecular Cell Biology. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizardo DY, Lin YL, Gokcumen O, Atilla-Gokcumen GE. Regulation of lipids is central to replicative senescence. Molecular BioSystems. 2017;13:498–509. doi: 10.1039/C6MB00842A. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Si H, Jia Z, Liu D. Dietary anti-aging polyphenols and potential mechanisms. Antioxidants. 2021;10:283. doi: 10.3390/antiox10020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye HS, Lee YT, Ooi SY, Teh LK, Lim LN, Wei LK. Modifying progression of aging and reducing the risk of neurodegenerative diseases by probiotics and synbiotics. Frontiers in Bioscience (Elite Ed) 2018;10:344–351. doi: 10.2741/e826. [DOI] [PubMed] [Google Scholar]
- Lye JJ, Latorre E, Lee BP, Bandinelli S, Holley JE, Gutowski NJ, Ferrucci L, Harries LW. Astrocyte senescence may drive alterations in GFAPα, CDKN2A p14ARF, and TAU3 transcript expression and contribute to cognitive decline. GeroScience. 2019;41:561–573. doi: 10.1007/s11357-019-00100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison AA, Belury MA, Andridge R, Renna ME, Rosie Shrout M, Malarkey WB, Lin J, Epel ES, Kiecolt-Glaser JK. Omega-3 supplementation and stress reactivity of cellular aging biomarkers: an ancillary substudy of a randomized, controlled trial in midlife adults. Molecular Psychiatry. 2021;26:3034–3042. doi: 10.1038/s41380-021-01077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavolta M, Costarelli L, Giacconi R, Basso A, Piacenza F, Pierpaoli E, Provinciali M, Ogo OA, Ford D. Changes in Zn homeostasis during long term culture of primary endothelial cells and effects of Zn on endothelial cell senescence. Experimental Gerontology. 2017;99:35–45. doi: 10.1016/j.exger.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, Vélez E, Perdigón G. Beneficial effects of probiotic consumption on the immune system. Annals of Nutrition and Metabolism. 2019;74:115–124. doi: 10.1159/000496426. [DOI] [PubMed] [Google Scholar]
- Mangan D. Iron: an underrated factor in aging. Aging (Albany NY) 2021;13:23407–23415. doi: 10.18632/aging.203612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfeld J, Urban N, Priebe S, Groth M, Frahm C, Hartmann N, Gebauer J, Ravichandran M, Dommaschk A, Schmeisser S, Kuhlow D, Monajembashi S, Bremer-Streck S, Hemmerich P, Kiehntopf M, Zamboni N, Englert C, Guthke R, Kaleta C, Platzer M, Sühnel J, Witte OW, Zarse K, Ristow M. Branched-chain amino acid catabolism is a conserved regulator of physiological ageing. Nature Communications. 2015;6:10043. doi: 10.1038/ncomms10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligné B, Gänzle M, Kort R, Pasin G, Pihlanto A, Smid EJ, Hutkins R. Health benefits of fermented foods: microbiota and beyond. Current Opinion in Biotechnology. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Marin C, Delgado-Lista J, Ramirez R, Carracedo J, Caballero J, Perez-Martinez P, Gutierrez-Mariscal FM, Garcia-Rios A, Delgado-Casado N, Cruz-Teno C, Yubero-Serrano EM, Tinahones F, Malagon Mdel M, Perez-Jimenez F, Lopez-Miranda J. Mediterranean diet reduces senescence-associated stress in endothelial cells. Age (Dordr) 2012;34:1309–1316. doi: 10.1007/s11357-011-9305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaldan S, Clatworthy SAS, Gamell C, Meggyesy PM, Rigopoulos AT, Haupt S, Haupt Y, Denoyer D, Adlard PA, Bush AI, Cater MA. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biology. 2018;14:100–115. doi: 10.1016/j.redox.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazhar M, Din AU, Ali H, Yang G, Ren W, Wang L, Fan X, Yang S. Implication of ferroptosis in aging. Cell Death Discovery. 2021;7:149. doi: 10.1038/s41420-021-00553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazidi M, Michos ED, Banach M. The association of telomere length and serum 25-hydroxyvitamin D levels in US adults: the National Health and Nutrition Examination Survey. Archives of Medical Science. 2017;13:61–65. doi: 10.5114/aoms.2017.64714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar PB. An unsolved problem of biology. Published for the College by H.K. Lewis, London (1952)
- Melini F, Melini V, Luziatelli F, Ficca AG, Ruzzi M. Health-promoting components in fermented foods: an up-to-date systematic review. Nutrients. 2019;11:1189. doi: 10.3390/nu11051189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao XY, Zhu XX, Gu ZY, Fu B, Cui SY, Zu Y, Rong LJ, Hu F, Chen XM, Gong YP, Li CL. Astragalus polysaccharides reduce high-glucose-induced rat aortic endothelial cell senescence and inflammasome activation by modulating the mitochondrial Na(+)/Ca(2+) exchanger. Cell Biochemistry and Biophysics. 2022 doi: 10.1007/s12013-021-01058-w. [DOI] [PubMed] [Google Scholar]
- Millner A, Atilla-Gokcumen GE. Lipid players of cellular senescence. Metabolites. 2020 doi: 10.3390/metabo10090339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitry MA, Laurent D, Keith BL, Sira E, Eisenberg CA, Eisenberg LM, Joshi S, Gupte S, Edwards JG. Accelerated cardiomyocyte senescence contributes to late-onset doxorubicin-induced cardiotoxicity. American Journal of Physiology-Cell Physiology. 2020 doi: 10.1152/ajpcell.00073.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooradian AD. The merits and the pitfalls of low carbohydrate diet: a concise review. The Journal of Nutrition, Health & Aging. 2020;24:805–808. doi: 10.1007/s12603-020-1417-1. [DOI] [PubMed] [Google Scholar]
- Mouchiroud L, Molin L, Kasturi P, Triba MN, Dumas ME, Wilson MC, Halestrap AP, Roussel D, Masse I, Dallière N, Ségalat L, Billaud M, Solari F. Pyruvate imbalance mediates metabolic reprogramming and mimics lifespan extension by dietary restriction in Caenorhabditis elegans. Aging Cell. 2011;10:39–54. doi: 10.1111/j.1474-9726.2010.00640.x. [DOI] [PubMed] [Google Scholar]
- Mu X, Zhang Y, Li J, Xia J, Chen X, Jing P, Song X, Wang L, Wang Y. Angelica sinensis polysaccharide prevents hematopoietic stem cells senescence in d-galactose-induced aging mouse model. Stem Cells International. 2017;2017:3508907. doi: 10.1155/2017/3508907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Nagano T, Awai Y, Kuwaba S, Osumi T, Mio K, Iwasaki T, Kamada S. Riboflavin transporter SLC52A1, a target of p53, suppresses cellular senescence by activating mitochondrial complex II. Molecular Biology of the Cell. 2021;32:br10. doi: 10.1091/mbc.E21-05-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Kohsaka S. Microglia: neuroprotective and neurotrophic cells in the central nervous system. Current Drug Targets. Cardiovascular & Haematological Disorders. 2004;4:65–84. doi: 10.2174/1568006043481284. [DOI] [PubMed] [Google Scholar]
- Nakano M, Nakashima A, Nagano T, Ishikawa S, Kikkawa U, Kamada S. Branched-chain amino acids enhance premature senescence through mammalian target of rapamycin complex I-mediated upregulation of p21 protein. PLoS ONE. 2013;8:e80411. doi: 10.1371/journal.pone.0080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme J, Yang D, Altulea A, Varela-Eirin M, Wang L, Hu S, Wu Y, Togo J, Niu C, Speakman JR, Demaria M. High dietary protein and fat contents exacerbate hepatic senescence and SASP in mice. FEBS Journal. 2021 doi: 10.1111/febs.16292. [DOI] [PubMed] [Google Scholar]
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Current Biology. 2012;22:R741–R752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Nikawa T, Ulla A, Sakakibara I. Polyphenols and their effects on muscle atrophy and muscle health. Molecules. 2021;26:4887. doi: 10.3390/molecules26164887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novais EJ, Tran VA, Johnston SN, Darris KR, Roupas AJ, Sessions GA, Shapiro IM, Diekman BO, Risbud MV. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nature Communications. 2021;12:5213. doi: 10.1038/s41467-021-25453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan NJ, Bull C, Fenech M. Elevated plasma magnesium and calcium may be associated with shorter telomeres in older South Australian women. The Journal of Nutrition Health and Aging. 2014;18:131–136. doi: 10.1007/s12603-013-0401-4. [DOI] [PubMed] [Google Scholar]
- Ogłuszka M, Te Pas MFW, Poławska E, Nawrocka A, Stepanow K, Pierzchała M. Omega-3 alpha-linolenic fatty acid affects the level of telomere binding protein TRF1 in porcine skeletal muscle. Animals (Basel) 2020;10:1090. doi: 10.3390/ani10061090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, Windheim J, Tsoory M, Schirmbeck R, Amit I, Geiger H, Krizhanovsky V. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nature Communications. 2018;9:5435. doi: 10.1038/s41467-018-07825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W-J, Ding Q-Y, Wang Y, Wang D-D, Lu Y-M, Yang W-W, Cai Z-N, Cheng X-D, Zhang W-N, Chen Y. A bioactive polysaccharide TLH-3 isolated from Tricholoma lobayense protects against oxidative stress-induced premature senescence in cells and mice. Journal of Functional Foods. 2018;42:159–170. doi: 10.1016/j.jff.2017.12.070. [DOI] [Google Scholar]
- Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers ES, Subramanian P, Patel N, Virasami A, Sebire NJ, Kinsler V, Valdovinos A, LeSaux CJ, Passos JF, Antoniou A, Rustin MHA, Campisi J, Akbar AN. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nature Communications. 2019;10:2387. doi: 10.1038/s41467-019-10335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrott KM, Wiley CD, Desprez PY, Campisi J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience. 2017;39:161–173. doi: 10.1007/s11357-017-9970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatti C, D’Adamo S, Stefanelli C, Flamigni F, Cetrullo S. Nutrients and pathways that regulate health span and life span. Geriatrics (Basel) 2020;5:95. doi: 10.3390/geriatrics5040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G, Wang G, Zhong J. Lycium barbarum polysaccharides protect human lens epithelial cells against oxidative stress-induced apoptosis and senescence. PLoS ONE. 2014;9:e110275. doi: 10.1371/journal.pone.0110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen BL, Tollefsbol TO. Polyphenols and aging. Current Aging Science. 2010;3:34–42. doi: 10.2174/1874609811003010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimifard M, Baeeri M, Bahadar H, Moini-Nodeh S, Khalid M, Haghi-Aminjan H, Mohammadian H, Abdollahi M. Therapeutic effects of gallic acid in regulating senescence and diabetes; an in vitro study. Molecules (Basel, Switzerland) 2020;25:5875. doi: 10.3390/molecules25245875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallis C, Mülleder M, Smith G, Au YZ, Ralser M, Bähler J. Amino acids whose intracellular levels change most during aging alter chronological life span of fission yeast. The Journals of Gerontology: Series A. 2020;76:205–210. doi: 10.1093/gerona/glaa246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Sanchez I, Mansour C, Navarrete-Yañez V, Ayala-Hernandez M, Guevara G, Castillo C, Loredo M, Bustamante M, Ceballos G, Villarreal FJ. (−)-Epicatechin induced reversal of endothelial cell aging and improved vascular function: underlying mechanisms. Food & Function. 2018;9:4802–4813. doi: 10.1039/C8FO00483H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan SI. Increased molecular damage and heterogeneity as the basis of aging. Biological Chemistry. 2008;389:267–272. doi: 10.1515/BC.2008.030. [DOI] [PubMed] [Google Scholar]
- Richards JB, Valdes AM, Gardner JP, Paximadas D, Kimura M, Nessa A, Lu X, Surdulescu GL, Swaminathan R, Spector TD, Aviv A. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. The American Journal of Clinical Nutrition. 2007;86:1420–1425. doi: 10.1093/ajcn/86.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodacki CLN, Rodacki ALF, Pereira G, Naliwaiko K, Coelho I, Pequito D, Fernandes LC. Fish-oil supplementation enhances the effects of strength training in elderly women. The American Journal of Clinical Nutrition. 2012;95:428–436. doi: 10.3945/ajcn.111.021915. [DOI] [PubMed] [Google Scholar]
- Rossi L, Mazzitelli S, Arciello M, Capo CR, Rotilio G. Benefits from dietary polyphenols for brain aging and Alzheimer's disease. Neurochemical Research. 2008;33:2390–2400. doi: 10.1007/s11064-008-9696-7. [DOI] [PubMed] [Google Scholar]
- Sabath N, Levy-Adam F, Younis A, Rozales K, Meller A, Hadar S, Soueid-Baumgarten S, Shalgi R. Cellular proteostasis decline in human senescence. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:31902–31913. doi: 10.1073/pnas.2018138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccon TD, Nagpal R, Yadav H, Cavalcante MB, Nunes ADC, Schneider A, Gesing A, Hughes B, Yousefzadeh M, Tchkonia T, Kirkland JL, Niedernhofer LJ, Robbins PD, Masternak MM. Senolytic combination of Dasatinib and Quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2021 doi: 10.1093/gerona/glab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai C, Ishida M, Ohba H, Yamashita H, Uchida H, Yoshizumi M, Ishida T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE. 2017;12:e0187934. doi: 10.1371/journal.pone.0187934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G, Huang J, Feresin RG, Zhao Y, Griendling KK. Zinc regulates Nox1 expression through a NF-κB and mitochondrial ROS dependent mechanism to induce senescence of vascular smooth muscle cells. Free Radical Biology and Medicine. 2017;108:225–235. doi: 10.1016/j.freeradbiomed.2017.03.032. [DOI] [PubMed] [Google Scholar]
- Sallam IE, Abdelwareth A, Attia H, Aziz RK, Homsi MN, von Bergen M, Farag MA. Effect of gut microbiota biotransformation on dietary tannins and human health implications. Microorganisms. 2021;9:965. doi: 10.3390/microorganisms9050965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S, Sitrin MD. Vitamin D's role in cell proliferation and differentiation. Nutrition Reviews. 2008;66:S116–S124. doi: 10.1111/j.1753-4887.2008.00094.x. [DOI] [PubMed] [Google Scholar]
- Scott AJ, Ellison M, Sinclair DA. The economic value of targeting aging. Nature Aging. 2021;1:616–623. doi: 10.1038/s43587-021-00080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedsadjadi N, Berg J, Bilgin AA, Braidy N, Salonikas C, Grant R. High protein intake is associated with low plasma NAD+ levels in a healthy human cohort. PLoS ONE. 2018;13:e0201968. doi: 10.1371/journal.pone.0201968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeri H, Lemmens K, Gevaert AB, De Meyer GRY, Segers VFM. Cellular senescence links aging and diabetes in cardiovascular disease. American Journal of Physiology-Heart and Circulatory Physiology. 2018;315:448–462. doi: 10.1152/ajpheart.00287.2018. [DOI] [PubMed] [Google Scholar]
- Shannon OM, Ashor AW, Scialo F, Saretzki G, Martin-Ruiz C, Lara J, Matu J, Griffiths A, Robinson N, Lillà L, Stevenson E, Stephan BCM, Minihane AM, Siervo M, Mathers JC. Mediterranean diet and the hallmarks of ageing. European Journal of Clinical Nutrition. 2021;75:1176–1192. doi: 10.1038/s41430-020-00841-x. [DOI] [PubMed] [Google Scholar]
- Sharma R. Perspectives on the dynamic implications of cellular senescence and immunosenescence on macrophage aging biology. Biogerontology. 2021 doi: 10.1007/s10522-021-09936-9. [DOI] [PubMed] [Google Scholar]
- Sharma R. Emerging interrelationship between the gut microbiome and cellular senescence in the context of aging and disease: perspectives and therapeutic opportunities. Probiotics & Antimicrobial Proteins. 2022 doi: 10.1007/s12602-021-09903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Padwad Y. Perspectives of the potential implications of polyphenols in influencing the interrelationship between oxi-inflammatory stress, cellular senescence and immunosenescence during aging. Trends in Food Science & Technology. 2020;98:41–52. doi: 10.1016/j.tifs.2020.02.004. [DOI] [Google Scholar]
- Sharma R, Kapila R, Dass G, Kapila S. Improvement in Th1/Th2 immune homeostasis, antioxidative status and resistance to pathogenic E. coli on consumption of probiotic Lactobacillus rhamnosus fermented milk in aging mice. AGE. 2014;36:9686. doi: 10.1007/s11357-014-9686-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sharma R, Sharma A, Kumari A, Kulurkar PM, Raj R, Gulati A, Padwad YS. Consumption of green tea epigallocatechin-3-gallate enhances systemic immune response, antioxidative capacity and HPA axis functions in aged male Swiss albino mice. Biogerontology. 2017;18:367–382. doi: 10.1007/s10522-017-9696-6. [DOI] [PubMed] [Google Scholar]
- Sharma R, Kumari M, Kumari A, Sharma A, Gulati A, Gupta M, Padwad Y. Diet supplemented with phytochemical epigallocatechin gallate and probiotic Lactobacillus fermentum confers second generation synbiotic effects by modulating cellular immune responses and antioxidant capacity in aging mice. European Journal of Nutrition. 2019;58:2943–2957. doi: 10.1007/s00394-018-01890-6. [DOI] [PubMed] [Google Scholar]
- Sharma R, Kumar R, Sharma A, Goel A, Padwad Y. Long term consumption of green tea EGCG enhances healthspan and lifespan in mice by mitigating multiple aspects of cellular senescence in mitotic and post-mitotic tissues, gut dysbiosis and immunosenescence. The Journal of Nutritional Biochemistry. 2022 doi: 10.1016/j.jnutbio.2022.109068. [DOI] [PubMed] [Google Scholar]
- Shimizu C, Wakita Y, Inoue T, Hiramitsu M, Okada M, Mitani Y, Segawa S, Tsuchiya Y, Nabeshima T. Effects of lifelong intake of lemon polyphenols on aging and intestinal microbiome in the senescence-accelerated mouse prone 1 (SAMP1) Scientific Reports. 2019;9:3671. doi: 10.1038/s41598-019-40253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Z, Sun L, Wang X. Evidence and perspectives of cell senescence in neurodegenerative diseases. Biomedicine & Pharmacotherapy. 2021;137:111327. doi: 10.1016/j.biopha.2021.111327. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Essential fatty acids in health and chronic disease. American Journal of Clinical Nutrition. 1999 doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- Sohn EJ, Kim JM, Kang SH, Kwon J, An HJ, Sung JS, Cho KA, Jang IS, Choi JS. Restoring effects of natural anti-oxidant quercetin on cellular senescent human dermal fibroblasts. The American Journal of Chinese Medicine. 2018;46:853–873. doi: 10.1142/S0192415X18500453. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, Mitchell SJ, Coogan SC, Cogger VC, Gokarn R, McMahon AC, Raubenheimer D, de Cabo R, Simpson SJ, Le Couteur DG. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Reports. 2015;11:1529–1534. doi: 10.1016/j.celrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, Giovannucci EL. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Internal Medicine. 2016;176:1453–1463. doi: 10.1001/jamainternmed.2016.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Gamez A, Demaria M. Therapeutic interventions for aging: the case of cellular senescence. Drug Discovery Today. 2017;22:786–795. doi: 10.1016/j.drudis.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Sun X, Chen W-D, Wang Y-D. DAF-16/FOXO transcription factor in aging and longevity. Frontiers in Pharmacology. 2017 doi: 10.3389/fphar.2017.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swovick K, Firsanov D, Welle KA, Hryhorenko JR, Wise JP, George C, Sformo TL, Seluanov A, Gorbunova V, Ghaemmaghami S. Interspecies differences in proteome turnover kinetics are correlated with life spans and energetic demands. Molecular & Cellular Proteomics. 2021;20:100041. doi: 10.1074/mcp.RA120.002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taherian M, Mahin Samadi P, Rastegar H, Faramarzi MA, Rostami-Nejad M, Yazdi MH, Rezaei-Tavirani M, Yazdi Z. An overview on probiotics as an alternative strategy for prevention and treatment of human diseases. Iranian Journal of Pharmaceutical Research. 2019;18:31–50. doi: 10.22037/ijpr.2020.112232.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takauji Y, Wada T, Takeda A, Kudo I, Miki K, Fujii M, Ayusawa D. Restriction of protein synthesis abolishes senescence features at cellular and organismal levels. Scientific Reports. 2016;6:18722. doi: 10.1038/srep18722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. American Journal of Clinical Nutrition. 2001;73:539–548. doi: 10.1093/ajcn/73.3.539. [DOI] [PubMed] [Google Scholar]
- Thompson PJ, Shah A, Ntranos V, Van Gool F, Atkinson M, Bhushan A. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metabolism. 2019;29:1045–1060.e10. doi: 10.1016/j.cmet.2019.01.021. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Experimental Gerontology. 2000;35:927–945. doi: 10.1016/S0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Remacle J, Dierick JF, Pascal T, Frippiat C, Zdanov S, Magalhaes JP, Royer V, Chainiaux F. From the Hayflick mosaic to the mosaics of ageing. Role of stress-induced premature senescence in human ageing. The International Journal of Biochemistry & Cell Biology. 2002;34:1415–29. doi: 10.1016/S1357-2725(02)00034-1. [DOI] [PubMed] [Google Scholar]
- Trevisol ETV, Panek AD, Mannarino SC, Eleutherio ECA. The effect of trehalose on the fermentation performance of aged cells of Saccharomyces cerevisiae. Applied Microbiology and Biotechnology. 2011;90:697–704. doi: 10.1007/s00253-010-3053-x. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Cheng LH, Liu YW, Jeng OJ, Lee YK. Gerobiotics: probiotics targeting fundamental aging processes. Bioscience of Microbiota, Food and Health. 2021;40:1–11. doi: 10.12938/bmfh.2020-026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek JHGM, Kirkwood TBL, Bassingthwaighte JB. Understanding the physiology of the ageing individual: computational modelling of changes in metabolism and endurance. Interface Focus. 2016;6:20150079–20150079. doi: 10.1098/rsfs.2015.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JPE. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2:1106–1131. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburgh K. Nutrigerontology: why we need a new scientific discipline to develop diets and guidelines to reduce the risk of aging-related diseases. Aging Cell. 2015;14:17–24. doi: 10.1111/acel.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Bellosta R. Dietary magnesium supplementation improves lifespan in a mouse model of progeria. EMBO Molecular Medicine. 2020;12:e12423. doi: 10.15252/emmm.202012423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T, Wan T, Miwa S. Senescence in post-mitotic cells: a driver of aging? Antioxidants & Redox Signaling. 2021;34:308–323. doi: 10.1089/ars.2020.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaszczyk A, Dookun E, Redgrave R, Tual-Chalot S, Victorelli S, Spyridopoulos I, Owens A, Arthur HM, Passos JF, Richardson GD. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell. 2019;18:e12945–e12945. doi: 10.1111/acel.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L, Jacobs E, McAleese KE, Johnson M, Attems J. Do senescent cells play a role in Alzheimer’s disease? Alzheimer's & Dementia. 2020;16:e043820. [Google Scholar]
- Wan W, Zhu W, Wu Y, Long Y, Liu H, Wan W, Wan G, Yu J. Grape seed proanthocyanidin extract moderated retinal pigment epithelium cellular senescence through NAMPT/SIRT1/NLRP3 pathway. Journal of Inflammation Research. 2021;14:3129–3143. doi: 10.2147/JIR.S303540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, Zhou D, Zheng G. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY) 2016;8:2915–2926. doi: 10.18632/aging.101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Pan TM, Tsai TY. Lactic acid bacteria-fermented product of green tea and Houttuynia cordata leaves exerts anti-adipogenic and anti-obesity effects. Journal of Food and Drug Analysis. 2018;26:973–984. doi: 10.1016/j.jfda.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, He Y, Rayman MP, Zhang J. Prospective selective mechanism of emerging senolytic agents derived from flavonoids. Journal of Agricultural and Food Chemistry. 2021;69:12418–12423. doi: 10.1021/acs.jafc.1c04379. [DOI] [PubMed] [Google Scholar]
- Wilmanski T, Diener C, Rappaport N, Patwardhan S, Wiedrick J, Lapidus J, Earls JC, Zimmer A, Glusman G, Robinson M, Yurkovich JT, Kado DM, Cauley JA, Zmuda J, Lane NE, Magis AT, Lovejoy JC, Hood L, Gibbons SM, Orwoll ES, Price ND. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nature Metabolism. 2021;3:274–286. doi: 10.1038/s42255-021-00348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Shin S, Cho E, Ryu D, Garandeau D, Chajra H, Fréchet M, Park D, Jung E. Senotherapeutic-like effect of Silybum marianum flower extract revealed on human skin cells. PLoS ONE. 2021;16:e0260545. doi: 10.1371/journal.pone.0260545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Li S, Qu G, Hua J, Zong J, Li X, Xu F. Genistein alleviates H2O2-induced senescence of human umbilical vein endothelial cells via regulating the TXNIP/NLRP3 axis. Pharmaceutical Biology. 2021;59:1388–1401. doi: 10.1080/13880209.2021.1979052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Xiong L, Song X, Jin P, Chen L, Chen X, Yao H, Wang Y, Wang L. Angelica sinensis polysaccharides ameliorate stress-induced premature senescence of hematopoietic cell via protecting bone marrow stromal cells from oxidative injuries caused by 5-fluorouracil. International Journal of Molecular Sciences. 2017;18:2265. doi: 10.3390/ijms18112265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson KO, Lowe V, Tchkonia T, Westendorf JJ, Kirkland JL. Transplanted senescent cells induce an osteoarthritis-like condition in mice. The Journals of Gerontology: Series A. 2016;72:780–785. doi: 10.1093/gerona/glw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nature Medicine. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wang L, Fozouni P, Evjen G, Chandra V, Jiang J, Lu C, Nicastri M, Bretz C, Winkler JD, Amaravadi R, Garcia BA, Adams PD, Ott M, Tong W, Johansen T, Dou Z, Berger SL. SIRT1 is downregulated by autophagy in senescence and ageing. Nature Cell Biology. 2020;22:1170–1179. doi: 10.1038/s41556-020-00579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Fu Q, Li Z, Liu H, Wang Y, Lin X, He R, Zhang X, Ju Z, Campisi J, Kirkland JL, Sun Y. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nature Metabolism. 2021;3:1706–1726. doi: 10.1038/s42255-021-00491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Jiang H, Li YH, Gao Q, Xu YN, Kim NH. Kaempferol alleviates the reduction of developmental competence during aging of porcine oocytes. Animal Science Journal. 2019;90:1417–1425. doi: 10.1111/asj.13280. [DOI] [PubMed] [Google Scholar]
- Yao T, Chen J-M, Shen L-E, Yu Y-S, Tang Z-H, Zang G-Q, Zhang Y, Chen X-H. Astragalus polysaccharide alleviated hepatocyte senescence via autophagy pathway. The Kaohsiung Journal of Medical Sciences. 2021 doi: 10.1002/kjm2.12495. [DOI] [PubMed] [Google Scholar]
- Yin D, Chen K. The essential mechanisms of aging: irreparable damage accumulation of biochemical side-reactions. Experimental Gerontology. 2005;40:455–465. doi: 10.1016/j.exger.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Zhao J, Bukata C, Wade EA, McGowan SJ, Angelini LA, Bank MP, Gurkar AU, McGuckian CA, Calubag MF, Kato JI, Burd CE, Robbins PD, Niedernhofer LJ. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell. 2020;19:e13094. doi: 10.1111/acel.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, Cui Y, Angelini L, Lee KA, McGowan SJ, Burrack AL, Wang D, Dong Q, Lu A, Sano T, O'Kelly RD, McGuckian CA, Kato JI, Bank MP, Wade EA, Pillai SPS, Klug J, Ladiges WC, Burd CE, Lewis SE, LaRusso NF, Vo NV, Wang Y, Kelley EE, Huard J, Stromnes IM, Robbins PD, Niedernhofer LJ. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594:100–105. doi: 10.1038/s41586-021-03547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Liu H, He S, Li P, Ma C, Ma M, Liu Y, Lv L, Ping F, Zhang H, Li W, Sun Q, Xu L, Li Y. Dietary magnesium intake and leukocyte telomere attrition in adults: the regulatory role of serum tumor necrosis factor α. Mediators of Inflammation. 2020;2020:7610436. doi: 10.1155/2020/7610436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Alexander PB, Wang XF. Cellular senescence: from anti-cancer weapon to anti-aging target. Science China Life Sciences. 2020;63:332–342. doi: 10.1007/s11427-019-1629-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Unnikrishnan A, Deepa SS, Liu Y, Li Y, Ikeno Y, Sosnowska D, Van Remmen H, Richardson A. A new role for oxidative stress in aging: the accelerated aging phenotype in Sod1−/− mice is correlated to increased cellular senescence. Redox Biology. 2017;11:30–37. doi: 10.1016/j.redox.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou Q, Yang R, Hu C, Huang Z, Zheng C, Liang Q, Gong R, Zhu X, Gong H, Yuan H, Chen C, Li X, Zhang N, Yang Z, Sun L. Serum branched-chain amino acids are associated with leukocyte telomere length and frailty based on residents from Guangxi longevity county. Scientific Reports. 2020;10:10252. doi: 10.1038/s41598-020-67010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Halicka HD, Li J, Darzynkiewicz Z. Berberine suppresses gero-conversion from cell cycle arrest to senescence. Aging. 2013;5:623–636. doi: 10.18632/aging.100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Liu X, Zheng Y, Liu W, Ding C. Aronia melanocarpa polysaccharide ameliorates inflammation and aging in mice by modulating the AMPK/SIRT1/NF-κB signaling pathway and gut microbiota. Scientific Reports. 2021;11:20558. doi: 10.1038/s41598-021-00071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD, Tchkonia T, Kirkland JL. New agents that target senescent cells: the flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging (Albany NY) 2017;9:955–963. doi: 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]