Abstract
Background:
Oncogenic activation of mitogen-activated protein kinase (MAPK) signaling is associated with radioiodine refractory (RAIR) thyroid cancer. Preclinical models suggest that activation of the receptor tyrosine kinase erbB-3 (HER3) mitigates the MAPK pathway inhibition achieved by BRAF inhibitors in BRAFV600E mutant thyroid cancers. We hypothesized that combined inhibition of BRAF and HER3 using vemurafenib and the human monoclonal antibody CDX-3379, respectively, would potently inhibit MAPK activation and restore radioactive iodine (RAI) avidity in patients with BRAF-mutant RAIR thyroid cancer.
Methods:
Patients with BRAFV600E RAIR thyroid cancer were evaluated by thyrogen-stimulated iodine-124 (124I) positron emission tomography–computed tomography (PET/CT) at baseline and after 5 weeks of treatment with oral vemurafenib 960 mg twice daily alone for 1 week, followed by vemurafenib in combination with 1000 mg of intravenous CDX-3379 every 2 weeks. Patients with adequate 124I uptake on the second PET/CT then received therapeutic radioactive iodine (131I) with vemurafenb+CDX-3379. All therapy was discontinued two days later. Treatment response was monitored by serum thyroglobulin measurements and imaging. The primary endpoints were safety and tolerability of vemurafenib+CDX-3379, as well as the proportion of patients after vemurafenb+CDX-3379 therapy with enhanced RAI incorporation warranting therapeutic 131I.
Results:
Seven patients were enrolled; six were evaluable for the primary endpoints. No grade 3 or 4 toxicities related to CDX-3379 were observed. Five patients had increased RAI uptake after treatment; in 4 patients this increased uptake warranted therapeutic 131I. At 6 months, 2 patients achieved partial response after 131I and 2 progression of disease. Next-generation sequencing of 5 patients showed that all had co-occurring telomerase reverse transcriptase promoter alterations. A deleterious mutation in the SWItch/Sucrose Non-Fermentable (SWI/SNF) gene ARID2 was discovered in the patient without enhanced RAI avidity after therapy and an RAI-resistant tumor from another patient that was sampled off-study.
Conclusions:
The endpoints for success were met, providing preliminary evidence of vemurafenib+CDX-3379 safety and efficacy for enhancing RAI uptake. Preclinical data and genomic profiling in this small cohort suggest SWI/SNF gene mutations should be investigated as potential markers of resistance to redifferentiation strategies. Further evaluation of vemurafenib+CDX-3379 as a redifferentiation therapy in a larger trial is warranted (ClinicalTrials.gov: NCT02456701).
Keywords: BRAF mutant, CDX-3379, radioiodine refractory thyroid cancer, redifferentiation, vemurafenib
Introduction
BRAFV600E is associated with radioactive iodine (RAI; 131I)–refractoriness in follicular cell-derived thyroid cancers. In RAI-refractory (RAIR) preclinical models, mutant BRAF abolishes RAI uptake through activation of the mitogen-activated protein kinase (MAPK) signaling pathway and suppression of the transcriptional program required for iodine concentration (1,2). Pharmacologic MAPK pathway inhibition can restore thyroid-specific gene expression and tumor RAI avidity (“redifferentiation”).
In a proof-of-principle clinical trial with the MEK1/2 inhibitor selumetinib and quantitative iodine-124 (124I) positron emission tomography–computed tomography (PET/CT) lesional dosimetry, we demonstrated that RAI uptake and efficacy could be restored in a subset (8/20) of patients with RAIR thyroid cancer (3). However, only one of nine participants with BRAFV600E tumors benefited (3). With the BRAF inhibitor vemurafenib, we observed enhanced 124I uptake in 4 of 10 patients; after 131I, 2 patients achieved partial response (PR) and 2 stable disease (SD) (4). Paired biopsies showed that the tumor with the most robust MAPK pathway inhibition, and restoration of thyroid gene expression also had the highest enhancement of RAI avidity (4). Similar clinical outcomes were reported in a trial with the BRAF inhibitor dabrafenib and in retrospective series (5–7).
Preclinical data suggest that only incremental increases in MAPK inhibition (∼10–15%) is necessary to evince significantly enhanced redifferentiation (2). Inhibitors that selectively inhibit monomeric BRAFV600E release negative feedback signals leading to increased expression of epidermal growth factor receptor 3 (ERBB3; or HER3), which in the context of high neuregulin-1 (HER3 ligand) levels heterodimerizes with ERBB2 (HER2) to induce MAPK and PI3K/Akt pathway reactivation (8). Lapatinib, an inhibitor of ERBB2/ERBB3, extinguishes these rebound signaling events and enhances vemurafenib antitumor effects (8).
We hypothesized that potent inhibition of MAPK signaling through combined targeting of BRAF and ERBB3 would enhance iodine avidity in BRAFV600E RAIR thyroid cancer patients. CDX-3379 (formerly KTN3379; Celldex), a human monoclonal antibody with specificity for the extracellular domain of ERBB3, locks the protein into an inactive conformation to inhibit both ligand-dependent and ligand-independent pathway activation (9). We conducted a pilot trial to evaluate the safety, tolerability, and redifferentiation potential of vemurafenib plus CDX-3379 in BRAFV600E RAIR patients.
Materials and Methods
Patients
The Memorial Sloan Kettering Cancer Center (MSK) Institutional Review Board (IRB) approved this research. All patients provided written informed consent. Eligible patients had RAIR thyroid carcinoma of follicular cell origin with BRAFV600E and measurable disease defined by the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1). Tumors were classified according to the World Health Organization classification of endocrine tumors (10), except for poorly differentiated thyroid carcinoma (PDTC), which was diagnosed using MSK criteria (11). RAIR disease was defined as: (a) a non-RAI-avid metastatic lesion on a diagnostic scan ≤2 years before enrollment; (b) an RAI-avid lesion of stable size or which progressed despite therapeutic RAI ≥6 months before study entry; or (c) ≥1 18F-fluorodeoxyglucose-avid lesion(s) with SUVmax ≥5. Full eligibility criteria are given in Supplementary Figure S1.
Study design
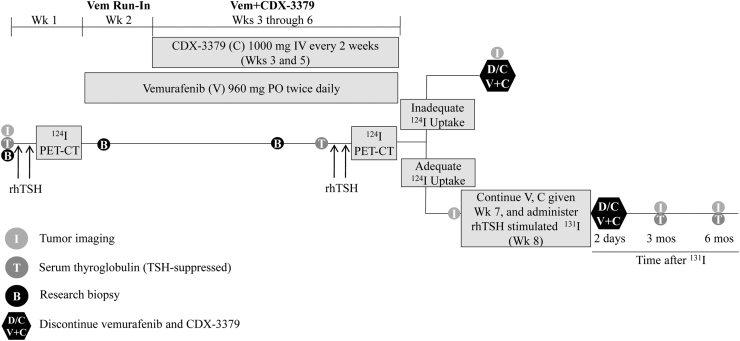
This was a single-arm, single-center trial. Figure 1 provides the study schema. Patients underwent thyrogen-stimulated (Sanofi Genzyme) 124I PET/CT for lesional dosimetry on a low-iodine diet and then received oral vemurafenib 960 mg twice daily. After a 1-week vemurafenib run-in, 1000 mg CDX-3379 was concurrently administered intravenously (IV) during weeks 3 and 5 (vem+CDX-3379). During week 6, patients underwent a second (on-treatment) thyrogen-stimulated 124I PET/CT scan; if lesional dosimetry met the threshold to warrant therapeutic 131I (≥2000 cGy could be delivered to ≥1 tumor using ≤300 mCi of 131I), patients received a third dose of CDX-3379 during week 7 while continuing vemurafenib. These patients underwent whole body and blood 131I dosimetry to determine 131I maximum tolerated activity (MTA). Each patient's clinical data, 124I PET lesional dosimetry results, and MTA were reviewed in a multidisciplinary meeting to determine the 131I activity to be administered. 131I was given on week 8. Vemurafenib was discontinued two days after 131I or discontinued after the second 124I PET/CT for patients who did not meet the lesional dosimetry threshold.
FIG. 1.
Study schema. Vem or V, vemurafenib; C, CDX-3379; D/C, discontinue; B, research biopsy; I, tumor imaging; 124I PET-CT, iodine-124 positron emission tomography–computed tomography; 131I, radioactive iodine; IV, intravenous; mos, months; PO, orally once a day; rhTSH, recombinant human thyrotropin or thyrogen; T, serum thyroglobulin (TSH-suppressed); Wk, week.
Tumor imaging (CT without contrast or MRI) was performed at baseline, before therapeutic 131I, and 3 months (±1 month) and 6 months (±1 week) post-131I. Patients who did not receive 131I had imaging performed ≤3 weeks after the second 124I PET/CT scan. Analyses of research biopsies and associated tumor genetic data for two patients obtained in this trial have been published previously (12).
Next-generation sequencing
Five cases were evaluated using the next-generation sequencing (NGS) MSK-IMPACT (MSK-Integrated Mutation Profiling of Actionable Cancer Targets) platform, an FDA-approved assay for evaluating genetic alterations in >300 cancer-related genes (13,14). Genomic data were analyzed through the cBioPortal for Cancer Genomics (15,16).
Data analysis
The primary objectives were to determine the proportion of BRAF-altered RAIR thyroid cancer patients who had sufficiently increased tumoral iodine incorporation after vem+CDX-3379 to warrant therapeutic 131I as determined by 124I PET/CT lesional dosimetry, and to evaluate the safety and tolerability of vem+CDX-3379. Secondary objectives were to evaluate whether vem+CDX-3379 enhanced 131I activity as assessed by overall response and progression-free survival rates at six months by RECIST v1.1, and to evaluate changes in serum thyroglobulin in patients treated with 131I. Toxicity was assessed through 30 days following the last dose of CDX-3379 using the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0).
Statistical considerations
Target accrual for the study was 10 patients. For the redifferentiation primary objective, the prespecified threshold for a positive study was 2 of 10 patients with sufficiently increased tumoral iodide incorporation to warrant therapeutic 131I. If the true response rate in the population was <5%, the probability of seeing ≥2 responses in 10 patients would be <10%, making the threshold of ≥2 responses indicative of study drug activity. For the safety primary objective, a run-in phase was planned such that if ≥2 of the first 6 patients developed a grade 3 or 4 toxicity attributed to CDX-3379, then the regimen would be deemed unsafe and accrual would be halted.
Results
Patient baseline characteristics
After enrolling seven patients with RAIR BRAFV600E thyroid cancer between August 2015 and June 2016 (Supplementary Fig. S2), the study was terminated before target accrual was met owing to sponsor decision. Table 1 summarizes the clinicopathological characteristics of patients enrolled. The median age was 67 years (range, 50–83 years); 6 patients were male and 1 was a female patient. Four patients had papillary thyroid carcinoma (PTC; two classical variant, two tall cell variant); three had PDTC (11). Six patients had prior 131I (one treatment each; range, 29.4–241.9 mCi). One patient who had locally aggressive tumor with tracheal and esophageal invasion had been treated with surgical resection followed by doxorubicin plus intensity-modulated radiation. Only one other patient was previously treated with doxorubicin chemoradiation, and none had prior exposure to tyrosine kinase inhibitors. The trial did not require progressive disease (PD) for enrollment, although all seven patients had evidence of increased tumor growth or appearance of new tumor deposits within one year of baseline study imaging.
Table 1.
Baseline Clinicopathological Characteristics of All Patients (n = 7)
| Characteristic | n (%)a |
|---|---|
| Age, years | |
| Median | 67 |
| Range | 50–83 |
| Sex | |
| Male | 6 (86) |
| Female | 1 (14) |
| Tumor histology | |
| Classical variant papillary thyroid cancer | 2 (29) |
| Tall cell variant papillary thyroid cancer | 2 (29) |
| Poorly differentiated thyroid cancerb | 3 (42) |
| Tumor genotype | |
| BRAFV600E mutation | 7 (100) |
| Prior treatments of 131I per patient | |
| 0 | 1 (14) |
| 1 | 6 (86) |
| Other prior treatments for thyroid cancer | |
| Doxorubicin (concurrently with RT) | 2 (29) |
| VEGFR-targeted TKI | 0 (0) |
| Sites of disease | |
| Local (thyroid bed, neck nodes or mediastinal extension/nodes) | 7 (100) |
| Lungs | 6 (86) |
| Distant nodes (hilar, paraaortic, subcarinal) | 2 (29) |
| Soft tissue nodules | 1 (14) |
| Bone | 1 (14) |
Values are given as number of patients (%) unless otherwise specified; percentages are calculated based on the total number of patients enrolled (n = 7).
Poorly differentiated thyroid carcinoma was defined by mitoses and tumor necrosis (11).
I, radioactive iodine; RT, radiation therapy; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor.
Safety analysis
All seven patients were treated with vemurafenib. One patient had a grade 3 maculopapular rash on vemurafenib alone in the context of an infectious respiratory illness and was removed from the study before receiving CDX-3379 (Patient 6); 3 received three doses of CDX-3379 and therapeutic 131I (Patients 2, 4, and 5); 1 received four doses of CDX-3379 and therapeutic 131I because of a vemurafenib-related toxicity delay (Patient 1); and 2 did not qualify for therapeutic 131I and received only two doses of CDX-3379 (Patients 3 and 7). Vemurafenib dosing was modified for three patients, including a hold followed by reduction to 720 mg twice daily for grade 3 maculopapular rash/mucositis and grade 2 hand-foot syndrome (HFS; Patient 1); reduction to 720 mg twice daily for grade 3 diarrhea (Patient 4); and a hold for grade 1 fever but later resumed at full dose (Patient 3) (Table 2). The most frequent CDX-3379-related toxicities were diarrhea, nausea, arthralgia, HFS, alkaline phosphatase elevation, and maculopapular rash (Table 3). No dose reductions or delays were required for CDX-3379-related toxicity, and no grade 3 or 4 toxicities were attributable to CDX-3379. The most common toxicities overall were maculopapular rash, diarrhea, arthralgia, HFS, nausea, and alkaline phosphatase elevation.
Table 2.
Toxicity Attributable to Vemurafenib
| Toxicity possibly, probably, or definitely related to vemurafenib | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| Maculopapular rash | 3 | 2 | 5 | |||
| Nausea | 4 | 4 | ||||
| Diarrhea | 2 | 1 | 3 | |||
| Arthralgia | 2 | 1 | 3 | |||
| Alanine aminotransferase elevation | 2 | 2 | ||||
| Alkaline phosphatase elevation | 2 | 2 | ||||
| Fatigue | 2 | 2 | ||||
| Plantar-palmar erythrodysesthesia syndromea | 1 | 1 | 2 | |||
| Pruritus | 2 | 2 | ||||
| Acneiform rash | 2 | 2 | ||||
| Actinic keratosis | 1 | 1 | ||||
| Alopecia | 1 | 1 | ||||
| Anemia | 1 | 1 | ||||
| Anorexia | 1 | 1 | ||||
| Aspartate aminotransferase elevation | 1 | 1 | ||||
| Bloating | 1 | 1 | ||||
| Creatinine increased | 1 | 1 | ||||
| Dehydration | 1 | 1 | ||||
| Dry skin | 1 | 1 | ||||
| Dysgeusia | 1 | 1 | ||||
| Elevated bilirubin | 1 | 1 | ||||
| Fever | 1 | 1 | ||||
| Hoarseness | 1 | 1 | ||||
| Hypophosphatemia | 1 | 1 | ||||
| Mucositis | 1 | 1 | ||||
| Myalgia | 1 | 1 | ||||
| Pain with swallowing | 1 | 1 | ||||
| Watering eyes | 1 | 1 | ||||
| Weight loss | 1 | 1 | ||||
| Hypokalemia | 1 | 1 |
Plantar-palmar erythrodysesthesia syndrome is also known as hand-foot syndrome.
Table 3.
Toxicity Attributable to CDX-3379
| Toxicity possibly, probably, or definitely related to CDX-3379 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| Diarrhea | 2 | 1 | 3 | |||
| Nausea | 3 | 3 | ||||
| Arthralgia | 2 | 2 | ||||
| Plantar-palmar erythrodysesthesia syndromea | 1 | 1 | 2 | |||
| Alkaline phosphatase elevation | 2 | 2 | ||||
| Maculopapular rash | 2 | 2 | ||||
| Acneiform rash | 1 | 1 | ||||
| Actinic keratosis | 1 | 1 | ||||
| Anorexia | 1 | 1 | ||||
| Dehydration | 1 | 1 | ||||
| Dysgeusia | 1 | 1 | ||||
| Fever | 1 | 1 | ||||
| Hoarseness | 1 | 1 | ||||
| Pain with swallowing | 1 | 1 | ||||
| Weight loss | 1 | 1 | ||||
| Bloating/PT = abdominal distension | 1 | 1 | ||||
| Hypokalemia | 1 | 1 |
Plantar-palmar erythrodysesthesia syndrome is also known as hand-foot syndrome.
Efficacy
All seven enrolled subjects had multiple tumors negative for 124I uptake at baseline, including three patients without any detectable RAI avidity, verifying RAIR disease status (Table 4). Six patients were evaluable for redifferentiation and response to vem+CDX-3379 with or without 131I (Table 4). Imaging was repeated in three patients on treatment before therapeutic 131I; all had minor tumor regressions (−10%, −13%, and −21%) after vem+CDX-3379 alone. Of the 5 patients (83%) with increased 124I avidity after vem+CDX-3379 alone, 4 (67%) had sufficient change to warrant therapeutic 131I (Fig. 2); they underwent whole-body and blood dosimetry and were treated with a range of 131I doses below the calculated MTA (range, 197–299 mCi; 30–97% of MTA) (Table 4).
Table 4.
Redifferentiation and 131I Efficacy Outcomes Achieved for All Patients (N = 7)
| Patient no. | Thyroid pathology | Metastatic disease sitesa | Lifetime 131I received before study, mCi (no. doses given) | Baseline 124I avidity (avid organ site) | Increased 124I avidity with V+C/lesional dosimetry threshold for 131I met? | 131I given/MTA, mCi (% of MTA given) | Tumor size change with V + C alone relative to baseline, % | Tumor size change 3 months post-tx with 131I relative to baseline, % | Tumor size change 6 months post-tx with 131I relative to baseline, % | Best overall response at 6 months |
|---|---|---|---|---|---|---|---|---|---|---|
| 131I treated | ||||||||||
| 1 | PDTC | L, TB, ML | 29 (1) | + (ML, TB) | Yes/Yes | 284/293 (97) | −21% | −42% | −42% | PR |
| 2 | PTC (TCV) | TB, L, NL | 200 (1) | None | Yes/Yes | 197/588 (34) | −13% | −10% | — | PD |
| 4 | PTC | TB, NL, PT, L | 125 (1) | None | Yes/Yes | 299/571 (52) | −10% | −30% | −50% | PR |
| 5 | PDTC | TB, PT, HL, ML, OL. STb | 0 | + (PT, TB) | Yesb/Yes | 201/668 (30) | — | 6%b | — | PDb |
| No131I given | ||||||||||
| 3 | PTC | L, TB | 242 (1) | + (TB, L) | Yesc/No | — | −8%d | Brain metastasis detected/treated almost 3 years after study treatment | ||
| 6 | PTC (TCV) | TB, NL, ML, L | 175 (1) | None | — | — | 5%e | Did not receive CDX-3379, treated with vem only on study; initiated systemic therapy ∼3 years after vem | ||
| 7 | PDTC | TB, NL, L, B, ST | 102 (1) | + (TB, L) | No/No | — | −31%f | Initiated systemic therapy immediately after coming off study | ||
Italicized metastatic disease site indicates metastatic sites identified by 124I PET/CT only.
Heterogeneous response with increased 124I was noted in the thyroid bed and paratracheal disease, but not in the hilar, mediastinal, and obturator lymph nodes or the soft tissue deposit. Tumor growth after three months of 131I was noted at sites lacking 131I avidity, resulting in a RECIST designation of PD.
New, increased 124I uptake with study therapy that met lesional dosimetry criteria for 131I therapy but was not treated because a structural correlate for the former could not be identified on imaging.
Imaging performed ∼7 months after last dose of study drugs.
Imaging performed ∼7 months after last dose of vemurafenib; did not receive CDX-3379.
Comparison of neck disease was carried out between MRI at baseline and CT performed within ∼1 month of drug therapy. Lung nodules that were measured were compared between CT scans.
I PET-CT, iodine-124 positron emission tomography–computed tomography; B, bone; HL, hilar lymph node; L, lung; ML, mediastinal lymph node; MTA, maximum tolerable activity (calculated by blood dosimetry); NL, neck lymph node; OL, obturator lymph node; PD, progression of disease; PDTC, poorly differentiated thyroid carcinoma; PR, partial response; PT, paratracheal disease; PTC, papillary thyroid carcinoma; post-tx, post-treatment; ST, soft tissue deposit; TB, thyroid bed; TCV, tall cell variant; V+C, vemurafenib plus CDX-3379.
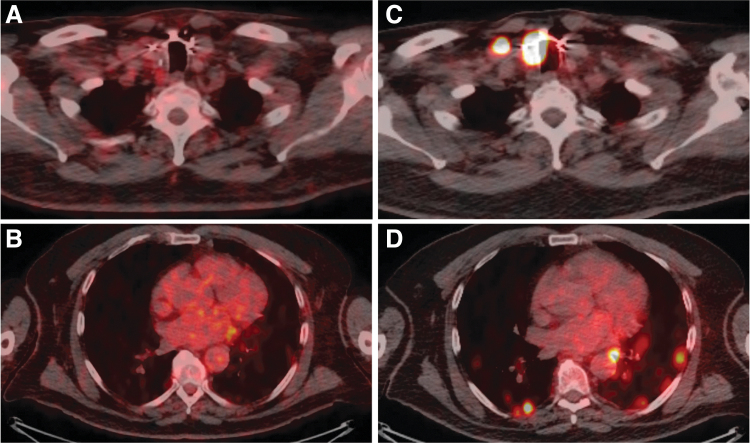
FIG. 2.
Imaging of 124I avidity in Patient 4 at baseline and after combination vemurafenib plus CDX-3379. Baseline imaging of Patient 4 from the first 124I PET-CT lesional dosimetry showed no avidity in either the (A) thyroid bed, metastatic lymph node, or (B) lung metastases. Imaging from the second 124I PET-CT lesional dosimetry showed increased avidity after vemurafenib plus CDX-3379 in both the (C) thyroid bed, metastatic lymph node, and (D) lung metastases.
Two of these patients had a confirmed PR 6 months after receiving therapeutic 131I (−42%, −50%) while remaining off other therapy for >3 years (Patient 1, > 36 months [lost to follow-up]; Patient 4, >47 months [surveillance ongoing]). Patient 2 had SD at three months post-therapeutic 131I but was designated PD owing to initiating new thyroid cancer treatment before the six-month response assessment. Patient 5 had PD at three months; details of that case are described below. Patient 3 had increased 124I avidity, including a thyroid bed focus that met criteria for therapeutic 131I and new/increased iodine uptake in lung metastases, but was not treated because a structural correlate for the former could not be identified on CT imaging. Outcomes for all three patients not treated with 131I are detailed in Table 4. Table 5 provides the changes in serum thyroglobulin and thyrotropin values for all vem+CDX-3379-treated patients.
Table 5.
Serum Thyroglobulin and Thyrotropin Levels in Six Patients Who Received Vemurafenib Plus CDX-3379
| Patient no. | Tg at baseline (TSH) | Tg on W6D1 V+C (TSH) | Tg at ∼5 weeks post-tx with 131I (TSH) | Tg at ∼3 months post-tx with 131I (TSH) | Tg at ∼6 months post-tx with 131I (TSH) |
|---|---|---|---|---|---|
| 131I treated | |||||
| 1 | 18.7 (2.30) | 22.9 (6.22) | 75.6 (17.89) | 19.8 (0.87) | 11.3 (0.02) |
| 2 | 101.3 (0.25) | 79.1 (1.56) | 118.4 (0.47) | 132.3 (0.4) | NA |
| 4 | 196.8 (0.88) | 11.5 (0.53) | 25.8 (5.44) | 6.7 (2.40) | 3.7 (0.05) |
| 5 | 0.2 (0.14) | 0.1 (3.48) | 0.1 (0.26) | <0.1 (0.19) | NA |
| No 131I given | |||||
| 3 | 549.0 (0.02) | 176.3 (0.19) | NA | NA | 323.8 (0.02)b |
| 7 | <0.1* (0.09) | <0.1* (0.40) | NA | <0.1* (3.87)c | NA |
Levels of serum Tg (TSH) are expressed as the following units: ng/mL (mIU/mL).
Checked ∼7 months after study.
Checked ∼3 months after study.
Patient 7 Tg was undetectable owing to the presence of thyroglobulin antibodies.
NA, not available; post-tx, post-treatment; Tg, thyroglobulin; TSH, thyrotropin; V+C, vemurafenib plus CDX-3379; W6D1, week 6 day 1.
NGS analysis and redifferentiation
Genetic analysis of primary thyroid samples from Patients 2, 3, 5, and 6 detected three genetic mutations per patient, whereas analysis of a neck lymph node sample from Patient 7 detected seven genetic alterations (Table 6). All five patients evaluated by NGS had telomerase reverse transcriptase (TERT) promoter alterations, which portend poor prognosis in patients with the BRAFV600E sequence variant (17,18). The two patients who did not receive 131I had noteworthy genetic alterations: Patient 3 had an inactivating RMB10Q915* nonsense variant, which has been associated with increased risk of death related to thyroid cancer (19); Patient 7 had an inactivating ARID2Q1462* variant, which is a SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin remodeling gene mutation that mediates insensitivity to MAPK inhibitor–induced redifferentiation in mouse models (12).
Table 6.
Genomic Profiles of Enrolled Patients
| Patient no. | BRAF | TERT promoter mutation | Other mutations |
|---|---|---|---|
| 2 | V600E | C228T | CD274 L190V |
| 3 | V600E | C228T | RBM10 Q915* |
| 5 | V600E | C228T | 9p21 loss (CDKN2B, CDKN2Ap16INK4A, CDKN2Ap14ARF), SMO D255N |
| 6 | V600E | C228T | DNMT3A F752L |
| 7 | V600E | C228T | ARID2 (A1318*, Q1462*), APC P119A, HIST1H3D E98Q, MDC1 P342S, TSHR V558M |
The bolded genes are alterations with known implications re: patient prognostics and impact on thyroid differentiation, as discussed in the text.
TERT, telomerase reverse transcriptase.
Serial genomic analysis of tumor samples from Patient 5 further demonstrated the potential role for ARID2 inactivation in mediating resistance to MAPK inhibitor redifferentiation (Table 7). Enrolled on study <1 year after undergoing resection of an invasive primary tumor into the trachea/esophagus and requiring tracheal stent placement with chemoradiation, Patient 5 received vem+CDX-3379 and showed a heterogeneous but promising response, including intense locoregional uptake and shrinkage of 124I-negative left flank, soft tissue, and obturator lymph node metastases. Specifically, this patient had, enhanced 124I uptake in paratracheal and thyroid bed tumors relative to baseline, but no detectable avidity in mediastinal/hilar/obturator lymph node, lung, and left flank soft tissue metastases.
Table 7.
Genomic Profiles of the Poorly Differentiated Thyroid Carcinoma and Anaplastic Thyroid Cancer Tumors from Patient 5
| Tumor site profiled | Total no. of alterations | BRAFV600E | TERT C228T | 9p21 loss | SMO D255N | ARID2 E108* | Other mutations |
|---|---|---|---|---|---|---|---|
| PDTC (RAI-positive primary) | 4 | X | X | X | X | None | |
| ATC (RAI-negative pelvic lymph node) | 20 | X | X | X | X | X | RB1 X738_splice, SOX9 K398N, APC D1570N, CSF1R *973Sext*43, EPHA5 E657K, IDH1 R343K, JAK1 S397C, PTCH1 E1428Q, KMT2D E4781K, NCOA3 H618D, RAD54L E297K, NUP93 E19Q, CDK12 D962H, PBRM1 Q170E, ASXL1 S62C |
ATC, anaplastic thyroid carcinoma; PDTC, poorly differentiated thyroid carcinoma; RAI, radioactive iodine.
As predicted by 124I PET/CT lesional dosimetry, three months after therapy was discontinued Patient 5 had a mixed response, including neck disease regression (36 mm →30 mm) and off-therapy growth of 131I nonavid tumors (soft tissue metastatic lesion: 14 mm →25 mm); growth of the nontarget lesions resulted in the overall PD designation. Patient 5 received another drug treatment that produced tumor regression except in the obturator node; biopsy of this RAI-resistant node >7 months after study completion revealed anaplastic thyroid cancer (ATC) transformation, including emergence of new alterations such as an inactivating ARID2-truncating mutation not previously detected in the primary tumor. These findings and preclinical modeling suggest that inactivating SWI/SNF mutated genes, such as mutant ARID2, may contribute to resistance to MAPK inhibitor redifferentiation.
Discussion
The premise of this study is that intrinsic resistance of thyroid cancers to BRAF inhibitors is mediated by adaptive HER3 upregulation, which leads to HER3/HER2-mediated reactivation of MAPK signaling (8). We hypothesized that combined BRAF/HER3 targeting with vem+CDX-3379 would potently inhibit MAPK signaling to enhance 131I efficacy. Despite early termination of accrual, the study met the prespecified threshold for safety and redifferentiation. None of the six patients who received ≥1 dose(s) of combination therapy developed grade ≥3 CDX-3379-related toxicity, and 4 patients (67%) met 124I lesional dosimetry criteria for 131I. These outcomes compare favorably with the 40% of patients who qualified for 131I with vemurafenib alone using the same 124I lesional dosimetry criterion (4).
Another trial with the BRAF inhibitor dabrafenib reported that 60% of patients with RAIR tumors developed new 131I uptake on whole-body scintigraphy (20). Results from a redifferentiation trial of dabrafenib plus the MEK inhibitor trametinib (MERAIODE trial; ClinicalTrials.gov: NCT03244956) has reported promising results in abstract form: ∼65% redifferentiation rate and 6-month 38% overall response rate (21). The vem+CDX-3379 study results here should be considered preliminary given the small sample size; potential selection biases make cross-trial comparisons with other small studies difficult and precludes adopting these results as definitive evidence of efficacy. The promising findings do justify conducting a full phase II evaluation to establish efficacy. A randomized trial comparing BRAF inhibition alone versus in combination with either MEK or HER3 targeting will be required to establish the advantage of combination therapy for redifferentiation.
While all patients had multiple RAI nonavid tumors on the baseline 124I PET to verify RAIR disease status, four of seven patients also had at least one lesion with some evidence of RAI uptake before drug therapy. Susceptibility to redifferentiation may differ between RAIR patients with completely RAI nonavid tumors versus those with a mix of RAI avid and nonavid disease. This also highlights a need to revisit how RAIR disease is defined in the context of our evolving understanding of the clinical and biologic basis of RAI susceptibility.
In addition to more conventional RAIR criteria, this study allowed RAIR disease to be defined as having a tumor with an FDG PET SUVmax ≥5 based on the inverse correlation between FDG and RAI tumor avidity (22). Of the two patients who were enrolled based only on the FDG-PET criterion, both had RAI nonavid tumors that failed to redifferentiate on study: one failed to qualify for 131I (Patient 7) and the other experienced ATC transformation in the refractory tumors (Patient 5). Further study is required to guide how FDG avidity may be used as a marker of RAIR disease, and if SUVmax thresholds should be used.
Among the four patients who met lesional dosimetry criteria and received vem+CDX-3379 and 131I, 50% (2/4) had a confirmed PR at 6 months post-therapy; the other 2 patients had PD at 3 months (Patient 5) and initiation of systemic therapy after the 3-month scans (Patient 2). Both patients received <50% of the determined 131I MTA (34% and 30%), while the two responders received higher percentages (97% and 52%). Higher 131I doses relative to predicted MTA may mediate better clinical responses by overcoming the potentially short effective half-lives of 131I. Alternatively, serial administration of redifferentiation therapy followed by 131I may be more effective than giving just one high-activity bolus of 131I.
Another important consideration is tumor-to-tumor heterogeneity. This is illustrated by the genomic profiles of two tumors from Patient 5, which revealed that the RAI-negative ATC tumor possessed a higher mutation burden and an inactivating ARID2 mutation that was not present in the PDTC primary tumor. Patient 7, the only patient whose tumors failed to demonstrate any enhanced 124I uptake on vem+CDX-3379, had an ARID2 mutation that was identified before trial enrollment (Table 6). Mutated SWI/SNF genes such as ARID2 are enriched in PDTC and ATC, which suggests that these alterations may lead to dedifferentiation. Studies in BrafV600E-mutant thyroid cancer mouse models showed that concomitant SWI/SNF loss-of-function rendered tumors refractory to the redifferentiation effects of MAPK pathway inhibition (12). This preclinical observation is consistent with an analysis of serial research biopsies obtained from a responder (Patient 2, wild-type ARID2) and nonresponder (Patient 7, ARID2 alteration) to vem+CDX-3379 on this study; despite achieving MAPK pathway inhibition with study treatment in both patients, thyroid differentiation (quantified with the enhanced Thyroid Differentiation Score) increased only in the responder (12). Hence, some SWI/SNF mutations may mediate resistance to redifferentiation and serve as biomarkers to identify patients who will not benefit from this strategy. Analysis of larger datasets is necessary to validate this hypothesis.
TERT promoter alterations are associated with increased mortality when co-occurring with BRAF alterations in patients with PTC (23); all five patients whose tumors were analyzed using the NGS platform had TERT promoter mutations. Of interest, Patient 3, who did not qualify for 131I, had a truncating alteration in RBM10, a tumor suppressor that regulates alternative mRNA splicing. RBM10 loss-of-function mutations are enriched in fatal forms of non-ATC thyroid cancer (19), suggesting that they could contribute to increased thyroid cancer virulence. While it is not known if mutant RBM10 would alter mRNA splicing programs to prevent MAPK pathway inhibitors from restoring the thyroid-specific gene expression programs requisite for redifferentiation, mutant RBM10 has been associated with vemurafenib resistance in vitro (24).
In summary, data from this small pilot trial suggest that HER3-targeting combined with vemurafenib is a promising redifferentiation approach, although the findings are preliminary given the small sample size. A phase II and/or randomized clinical trial is necessary to definitively demonstrate the advantage of this combination. These data also illustrate that beyond optimizing the redifferentiation approach, determining the optimal level of 131I incorporation needed for efficacy and identifying molecular markers to predict redifferentiation success will be critical for maximizing the benefit of this treatment strategy for patients.
Data Sharing Statement
Memorial Sloan Kettering Cancer Center supports the international committee of medical journal editors (ICMJE) and the ethical obligation of responsible sharing of data from clinical trials. Data collected for the study are available in this article and the Supplementary Data. The protocol summary, a statistical summary, and informed consent form will be made available on ClinicalTrials.gov when required as a condition of Federal awards, other agreements supporting the research, and/or as otherwise required.
Supplementary Material
Acknowledgments
Editorial support was provided by Crystal Tran, BS, on behalf of the Memorial Sloan Kettering Cancer Center Editorial Service.
Authors' Contributions
Study conception and design: A.L.H. and J.A.F.; Drafting of article: V.T. and A.L.H.; PET/CT analysis: K.S.P. and R.K.G.; RECIST interpretation/analysis: S.H.; Genomic data analysis: A.L.H., J.A.F., and J.K.; Study investigators: L.D., E.S., S.S.B., S.M.L., R.K.G., K.S.P., S.H., R.M.T., M.M.S., S.F., L.B., J.W., R.A.G., and D.G.P.; Interpretation of data: all authors; Review and approval of article: all authors.
Author Disclosure Statement
V.T. reports holding stock in Infinity Pharmaceuticals. L.D. has an advisory board role for Pfizer, Regeneron, Cue Biopharma, and Eisai; and reports research support from Cue Biopharma, Merck, and Regeneron. E.S. serves on the advisory board or as a consultant for Bristol-Myers Squibb, Eisai, Loxo Oncology, Novartis, Lily, and Regeneron, and reports research funding from Eisai, Plexxikon, Roche/Genentech, Lily, and Regeneron. S.S.B. is an employee of Flatiron Health and reports holding stock in Roche, prior consulting fees from Bristol Myers Squibb and AstraZeneca, and prior research support from AstraZeneca. R.K.G., K.S.P., S.H., R.M.T., M.M.S., S.F., L.B., J.W., R.A.G., V.E.S., J.A.K., and D.G.P. have no competing financial interests. S.M.L. reports receiving commercial research grants from Y-mAbs Therapeutics, Inc., Genentech, Inc., WILEX AG, Telix Pharmaceuticals Limited, and Regeneron Pharmaceuticals, Inc.; and holding ownership/equity interests in Elucida Oncology, Inc., Voreyda Theranostics Inc., Y-mAbs Therapeutics, Inc., and ImaginAb, Inc., S.M.L. is the inventor of issued patents both currently unlicensed and licensed by Memorial Sloan Kettering Cancer Center to Samus Therapeutics, Inc., Elucida Oncology, Inc., and Y-mAbs Therapeutics, Inc., S.M.L. serves or has served as a consultant both compensated and/or uncompensated for Cardinal Health, Fonds de recherche du Quebec, Cynvec LLC, Eli Lilly and Company, Prescient Therapeutics Limited, Advanced Innovative Partners, Inc., Gerson Lehrman Group, Progenics Pharmaceuticals, Inc., EXINI Diagnostics AB, and Janssen Pharmaceuticals, Inc. J.A.F. has an advisory board role for Loxo Oncology/Lilly and reports research support from Eisai. A.L.H. has served on the advisory boards/consulting for Eisai Pharmaceuticals, Sanofi Genzyme, Novartis, Kura Oncology, AstraZeneca, Merck, Bristol-Myers Squibb, Genentech/Roche, Sun Pharmaceuticals, Ayala Pharmaceuticals, Regeneron, CureVac, Prelude Therapeutics, Rgenta, Exelixis, Inxmed, Cellestia. He serves on the DSMC of an Affyimmune clinical trial. Koltan Pharmaceuticals provided funding for this clinical trial, including support for A.L.H.'s effort. He also serves as the Principal Investigator on clinical trials funded by AstraZeneca, Genentech/Roche, Bayer, Novartis, Bristo-Myers Squibb, Merck, Pfizer, Eisai, Ayala Pharmaceuticals, Elevar Therapeutics, Astellas, Kura Oncology.
Funding Information
Kolltan Pharmaceuticals (now Celldex) provided KTN3379 (now CDX-3379) and funding. Kolltan, along with the contracted CRO Chiltern, monitored the study, managed the study database, and ran study analyses including the final tables and listings. Celldex is currently the study sponsor of record. The study was also funded in part through the National Institutes of Health/National Cancer Institute (NIH/NCI) Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748, NIH/NCI R01 CA184724, NIH/NCI R01 CA201250, NIH/NCI R01 CA249663, and NIH/NCI SPORE in Thyroid Cancer Grant P50 CA172012. Support was also provided by the Geoffrey Beene Cancer Research Center and Cycle for Survival at Memorial Sloan Kettering Cancer Center. The ASCO Conquer Cancer Foundation provided support for L.D. through a Young Investigator Award.
Supplementary Material
References
- 1. Chakravarty D, Santos E, Ryder M, Knauf JA, Liao X-H, West BL, Bollag G, Kolesnick R, Thin TH, Rosen N, Zanzonico P, Larson SM, Refetoff S, Ghossein R, Fagin JA. 2011. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest 121:4700–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nagarajah J, Le M, Knauf JA, Ferrandino G, Montero-Conde C, Pillarsetty N, Bolaender A, Irwin C, Krishnamoorthy GP, Saqcena M, Larson SM, Ho AL, Seshan V, Ishii N, Carrasco N, Rosen N, Weber WA, Fagin JA. 2016. Sustained ERK inhibition maximizes responses of BrafV600E thyroid cancers to radioiodine. J Clin Invest 126:4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, Ghossein RA, Ricarte-Filho JC, Domínguez JM, Shen R, Tuttle RM, Larson SM, Fagin JA. 2013. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 368:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunn LA, Sherman EJ, Baxi SS, Tchekmedyian V, Grewal RK, Larson SM, Pentlow KS, Haque S, Tuttle RM, Sabra MM, Fish S, Boucai L, Walters J, Ghossein RA, Seshan VE, Ni A, Li D, Knauf JA, Pfister DG, Fagin JA, Ho AL. 2019. Vemurafenib redifferentiation of BRAF mutant, RAI-refractory thyroid cancers. J Clin Endocrinol Metab 104:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iravani A, Solomon B, Pattison DA, Jackson P, Ravi Kumar A, Kong G, Hofman MS, Akhurst T, Hicks RJ. 2019. Mitogen-activated protein kinase pathway inhibition for redifferentiation of radioiodine refractory differentiated thyroid cancer: an evolving protocol. Thyroid 29:1634–1645. [DOI] [PubMed] [Google Scholar]
- 6. Jaber T, Waguespack SG, Cabanillas ME, Elbanan M, Vu T, Dadu R, Sherman SI, Amit M, Santos EB, Zafereo M, Busaidy NL. 2018. Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive iodine. J Clin Endocrinol Metab 103:3698–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rothenberg SM, Daniels GH, Wirth LJ. 2015. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib-response. Clin Cancer Res 21:5640–5641. [DOI] [PubMed] [Google Scholar]
- 8. Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, Ryder M, Ghossein RA, Rosen N, Fagin JA. 2013. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov 3:520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee S, Greenlee EB, Amick JR, Ligon GF, Lillquist JS, Natoli EJ Jr., Hadari Y, Alvarado D, Schlessinger J. 2015. Inhibition of ErbB3 by a monoclonal antibody that locks the extracellular domain in an inactive configuration. Proc Natl Acad Sci U S A 112:13225–13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lloyd RV, Osamura RY, Klöppel G, Rosai J. 2017. WHO Classification of Tumours of Endocrine Organs. Vol 10. Fourth edition. World Health Organization (IARC), Lyon, France. [Google Scholar]
- 11. Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, Shah JP, Singh B, Ghossein RA. 2006. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer 106:1286–1295. [DOI] [PubMed] [Google Scholar]
- 12. Saqcena M, Leandro-Garcia LJ, Maag JLV, Tchekmedyian V, Krishnamoorthy GP, Tamarapu PP, Tiedje V, Reuter V, Knauf JA, de Stanchina E, Xu B, Liao X-H, Refetoff S, Ghossein R, Chi P, Ho AL, Koche RP, Fagin JA. 2021. SWI/SNF complex mutations promote thyroid tumor progression and insensitivity to redifferentiation therapies. Cancer Discov 11:1158–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, Brannon AR, O'Reilly C, Sadowska J, Casanova J, Yannes A, Hechtman JF, Yao J, Song W, Ross DS, Oultache A, Dogan S, Borsu L, Hameed M, Nafa K, Arcila ME, Ladanyi M, Berger MF. 2015. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schrader KA, Cheng DT, Joseph V, Prasad M, Walsh M, Zehir A, Ni A, Thomas T, Benayed R, Ashraf A, Lincoln A, Arcila M, Stadler Z, Solit D, Hyman DM, Zhang L, Klimstra D, Ladanyi M, Offit K, Berger M, Robson M. 2016. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol 2:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cerami E, Gao JJ, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. 2012. The cBio Cancer Genomics Portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao JJ, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun YC, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. 2013. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. 2016. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 126:1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, Pai S, Bishop J. 2014. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol 32:2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. 2019. Poorly differentiated carcinoma of the thyroid gland: current status and future prospects. Thyroid 29:311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. 2015. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res 21:1028–1035. [DOI] [PubMed] [Google Scholar]
- 21. Leboulleux S, Cao CD, Zerdoud S, Attard M, Bournaud C, Benisvy D, Taieb D, Bardet S, Terroir-Cassou-Mounat M, Betrian S, Lion G, Schiazza A, Sajous C, Garcia ME, Schlumberger MJ, Godbert Y, Borget I 2021 MERAIODE: a redifferentiation phase II trial with trametinib and dabrafenib followed by radioactive iodine administration for metastatic radioactive iodine refractory differentiated thyroid cancer patients with a BRAFV600E mutation (NCT 03244956) J Endocr Soc 5:A876. [Google Scholar]
- 22. Wang W, Larson SM, Tuttle RM, Kalaigian H, Kolbert K, Sonenberg M, Robbins RJ. 2001. Resistance of [18f]-fluorodeoxyglucose-avid metastatic thyroid cancer lesions to treatment with high-dose radioactive iodine. Thyroid 11:1169–1175. [DOI] [PubMed] [Google Scholar]
- 23. Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M. 2017. Mortality risk stratification by combining BRAF V600E and TERT promoter mutations in papillary thyroid cancer: genetic duet of BRAF and TERT promoter mutations in thyroid cancer mortality. JAMA Oncol 3:202–208. [DOI] [PubMed] [Google Scholar]
- 24. Antonello ZA, Hsu N, Bhasin M, Roti G, Joshi M, Van Hummelen P, Ye E, Lo AS, Karumanchi SA, Bryke CR, Nucera C. 2017. Vemurafenib-resistance via de novo RBM genes mutations and chromosome 5 aberrations is overcome by combined therapy with palbociclib in thyroid carcinoma with BRAFV600E. Oncotarget 8:84743–84760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.