Abstract
Acellular adipose matrix (AAM) has received increasing attention for soft tissue reconstruction, due to its abundant source, high long-term retention rate and in vivo adipogenic induction ability. However, the current decellularization methods inevitably affect native extracellular matrix (ECM) properties, and the residual antigens can trigger adverse immune reactions after transplantation. The behavior of host inflammatory cells mainly decides the regeneration of AAM after transplantation. In this review, recent knowledge of inflammatory cells for acellular matrix regeneration will be discussed. These advancements will inform further development of AAM products with better properties.
Keywords: acellular adipose matrix, transplantation, immunogenicity, inflammatory cells, regeneration
Introduction
Soft tissue defect remains a challenge for esthetic and reconstructive surgery. Trauma, congenital anomalies or iatrogenic causes can lead to this defect, which affects psychological functioning and patient’s quality of life. Plastic surgeons typically utilize autologous tissue flap transfer for reconstruction of soft tissue defects (Vaienti et al., 2005; Zhang et al., 2016). However, the tenuous nature of microsurgical tissue transfer and donor-site morbidity increases the difficulty of surgical operation, which requires an adequately trained surgeon. Fat grafting is demonstrated to be effective and safe for the treatment of small- and mid-sized volume deficiencies. However, clinicians continue to face challenges such as donor site morbidity, graft loss, calcification and oil cysts (Nguyen et al., 1990; Coleman, 2006; Khouri et al., 2012; Kølle et al., 2013). Hyaluronic acid, collagen and polymethyl methacrylate are all common implantable biomaterials. These biomaterials have the advantages of easy access and no injury to the donor area over autologous fat transplantation. However, they have the problems of foreign body reaction and inflammation, distortion and repeated injections due to biomaterial absorption (Alam et al., 2008; Spector and Lim, 2016; Van Nieuwenhove et al., 2017).
The extracellular matrix (ECM) is a cell-secreted three-dimensional structure that provides mechanical and structural supports to the tissues, which can also serve as a reservoir and a place for active ion, nutrient, water, metabolite and signal exchange (Gattazzo et al., 2014; Mendibil et al., 2020). Therefore, ECM acts as the microenvironment in which tissue-resident cells attach, communicate and interact, thereby regulating cell dynamics and behavior as well as maintaining tissue-specific functions and phenotypes. Similarly, decellularized ECM scaffold allows the seeding and proliferation of specific cells, while being degraded by the host tissue and replaced with new tissue (Massaro et al., 2021). Acellular adipose matrix (AAM) has received increasing attention for reconstruction of soft tissue defects, due to its abundant source, high long-term retention rate and in vivo adipogenic induction ability. The processing techniques for AAM generally include the removal of cellular components and lipids, while maintaining the basic ECM architecture and biological activity. Collagen type I, collagen type IV and laminin are the most essential ECM proteins in adipose tissues (Mendibil et al., 2020), (Costa et al., 2017). In addition, adipogenesis can be induced by extracellular proteins such as fibroblast growth factor, insulin-like growth factor, transforming growth factor beta and bone morphogenetic proteins in the AAM (Kayabolen et al. 2017).
Immune reaction can be triggered when the biomaterials are introduced to the human body (Cravedi et al., 2017). Although the use of decellularized biomaterials for regenerative medicine and tissue engineering reduces transplantation-related risks (Crapo et al., 2011; Sart et al., 2020; Peña et al., 2021), the current decellularization approaches are not able to completely eliminate all immunogenic antigens (Crapo et al., 2011), (Brown et al., 2011; Kawecki et al., 2018). Typically, the immune reaction consists of adaptive and innate responses (Hillebrandt et al., 2019). The innate response is dependent on the body’s ability to recognize potentially harmful pathogens and rapidly recruit immune cells to diminish them through inflammatory reactions. The adaptive response is manifested by antigen-specific reactions. This is the case of allo/xenograft rejection caused by the dysregulation of major histocompatibility complex (MHC), which is the most serious risk associated with transplantation (Massaro et al., 2021), (Wong and Griffiths, 2014). According to our previous study, immunocamouflage MHC by methoxy polyethylene glycol could effectively improve the regeneration properties of xenogeneic AAM (Liu et al., 2021). On the other hand, orderly infiltration and transformation of host inflammatory cells are highly essential for adipose tissue regeneration in fat transplantation (Cai et al., 2017a; Cai et al., 2017b; Cai et al., 2018; Liu et al., 2018). Appropriate infiltration of inflammatory cells (e.g., neutrophils and monocytes) at the early stage of transplantation and timely polarization of macrophages from M1 to M2 could promote adipogenic and retention rates in xenogeneic AAM (Liu et al., 2021). Therefore, the reaction of host immune system to AAM can determine the regeneration outcome. In this review, we highlight recent advancements in inflammatory cell behavior and their interaction with AAM after transplantation.
Immunogenicity of Acellular Adipose Matrix
Generally, immunogenicity is the ability of a substance or molecule to induce a specific immune response (Mahanty et al., 2015). Incomplete decellularization is the main source of immunogenicity in AAM (Crapo et al., 2011; Keane et al., 2012). The expression of MHC antigens is intrinsically linked to immunogenicity. MHC is composed of a cluster of genes that can determine whether the cells, tissues or biological products are accepted or rejected. DNA fragments are the most common residual cellular materials that have been associated with AAM immunogenicity (Massaro et al., 2021). Although a certain amount of DNA (<50 ng/mg AAM dry weight with a fragment length of <200 bp) is considered acceptable (Dalgliesh et al., 2018; Chakraborty et al., 2020; Massaro et al., 2021), there are no definitive data on the actual effect of DNA residue on the degradation and remodeling of AAM grafts. The alpha-Gal (α-Gal; Gal1-3Gal1-4GlcNAc-R) epitope persisting on the cytomembrane is another essential factor in xenogeneic AAM rejection (Chen et al., 1999; Naso et al., 2011). This epitope is expressed on the surface of most mammalian tissues, except for humans and primates. In humans, the xenograft with α-Gal expression triggers the activation of specific antibodies, thus resulting in xenograft rejection (Stahl et al., 2018). Although AAM products have entered the stage of clinical trials and shown their safety (Kokai et al., 2019; Gold et al., 2020; Kokai et al., 2020; Anderson et al., 2022), the actual remaining antigens in AAM are unclear and in vivo immunological studies are still very limited. At least 92% lipid antigen removal is the threshold level of residual antigenicity necessary to overcome recipient graft-specific adaptive humoral immune response in rabbit transplant model (Dalgliesh et al., 2018). However, the exact data of α-Gal, MHC and DNA residues in the products under this threshold are still unclear.
After decellularization or proteolytic digestion processes, alterations in the structures of functional proteins, glycoproteins and glycosaminoglycans in the native ECM are unavoidable. The ECM protein, such as hyaluronan, collagen types I and IV, versican, aggrecan, laminin and α1β1 integrin, are still integrated with the ECM after decellularization or proteolytic digestion processes (Dziki et al., 2017; Chakraborty et al., 2020). These motifs help maintain the secondary immunity, B cell differentiation, antibody production, and chemokine receptor (CXCR)-1 and 2-regulated neutrophil attraction, (Partington et al., 2013; Youngstrom et al., 2013). Meanwhile, ECM components such as hyaluronan fragments, tenascins, and sulfated proteoglycans following decellularization or proteolytic digestion processes could amplify inflammation (Gaudet and Popovich, 2014; Dziki et al., 2018). However, some ECM damage may be tolerated by the innate immune response, provided that macromolecular structure is maintained (Dalgliesh et al., 2018).
Despite the above-mentioned potential immunogenicity, an immune response is less likely to be provoked in the AAM (Massaro et al., 2021). Considering the genetic similarities among species, the presence of xenogeneic proteins can be revealed if they are functionally homologous to their human counterparts. Xenogeneic ECM proteins can effectively interact with human binding receptors, especially in decellularized scaffolds, through binding to peptide motifs or matrix bound proteins that are highly conserved across multiple species (Massaro et al., 2021).
From the Very Beginning After Transplantation
The host response following biomaterial implantation can be classified as three phases: protein adsorption, inflammation, and foreign body reaction. The spontaneous adsorption of blood components, such as sugars, lipids and proteins, onto the graft surface occurs within seconds after implantation (Wilson et al., 2005; Mariani et al., 2019). Simultaneously, at the biomaterial interface, the surrounding tissue forms a provisional matrix (e.g., seroma, blood clot and thrombus) that releases bioactive agents (e.g., PDGF, TGF-β, CXCL4 and LTB4) to govern the subsequent phases of inflammation (Anderson et al., 2008; Franz et al., 2011; Brown et al., 2012; Bryers et al., 2012; Anderson, 2015). Changes in the conformation and composition of the adsorbed proteins trigger inflammatory responses and a series of processes including the recruitment and adhesion of innate immune cells (e.g., monocytes and neutrophils) (Anderson, 2015; Chung et al., 2017; Selders et al., 2017; Zhou and Groth, 2018). During the inflammation phase, acute inflammation is predominantly determined by the appearance of neutrophils that enter the implant sites through damaged vessels (Anderson et al., 2008; Zhou and Groth, 2018). Later, the chronic inflammatory response at the graft is initiated by mononuclear cells (e.g., lymphocytes and monocytes), which are recruited by the signals released by the activated and apoptotic neutrophils (Anderson et al., 2008). More importantly, the activation of an immune response cascade has been proven to be a positive phenomenon because inflammation is a physiological process after transplantation (Kawecki et al., 2018). Therefore, early post-transplant inflammatory response plays a vital role in regulating AAM regeneration.
Neutrophils-Mediated Immune Reaction and Implant Remodeling
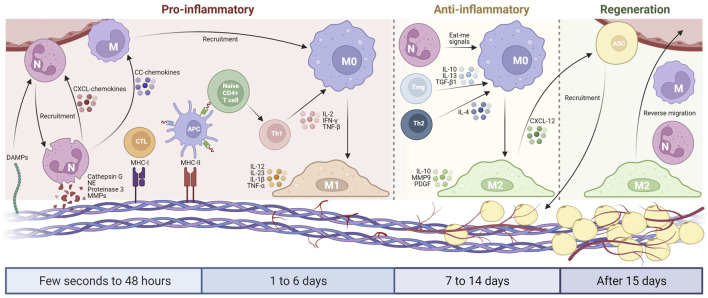
Neutrophils are the first cells that appear at the implant sites, and they play a critical role during the early graft reaction. During the leukocyte adhesion cascade process, neutrophils are recruited from the bloodstream to a site of injury via chemotaxis (Ley et al., 2007; Rosales, 2018). After adhesion to the graft surface, they are activated for matrix reprogramming, angiogenesis and regeneration (López-Boado et al., 2004; Dupré-Crochet et al., 2013). Within 24–48 h, neutrophils infiltrate the graft and generate peptides, enzymes and neutrophil extracellular traps, which subsequently recruit immune cells (e.g., monocytes and lymphocytes) and affect surrounding tissue (Anderson et al., 2008; Björnsdottir et al., 2015; Perobelli et al., 2015). Afterward, they will undergo apoptosis, and subsequently being engulfed, phagocytozed and digested by the attracted macrophages (Figure 1).
FIGURE 1.
Schematic diagram of the host inflammatory cell implantation in AAM. From a few seconds to 48 h, DAMPs cause the initial recruitment of neutrophils, which secrete serine proteases and MMPs to cleave AAM components. Neutrophils produce CXCL-chemokines and CC-chemokines, which recruit more neutrophils and monocytes from the circulation. The remaining MHC molecules in AAM activate lymphocytes that promote M1 macrophage polarization via pro-inflammatory cytokines during 1–6 days after implantation. From day 7–14, the host blood vessels begin to grow in the AAM, thereby inducing adipogenesis. Neutrophils, Treg cells, and Th2 lymphocytes subsequently promote M2 macrophage polarization that translates pro-inflammatory into an anti-inflammatory environment. After 15 days, the inflammation level of AAM gradually decreased, the neovascularization further developed, and the adipogenesis process continued with the participation of M2 macrophages. Created with BioRender.com.
Neutrophils migrate to the graft in three phases: forward migration, recruitment amplification, and reverse migration (de Oliveira et al., 2016). Damage-associated molecular patterns (DAMPs) are recognized by specific pattern recognition receptors, which causes the initial recruitment of neutrophils (de Oliveira et al., 2016). DAMPs include N-formyl peptides, ECM components and DNA proteins, all of which can serve as the products of tissue destruction during the decellularization process. CXCL8 family of chemokines (CXCL8 chemokines) can attract more distant neutrophils (de Oliveira et al., 2016). Following transmigration, the recruitment of neutrophils can be amplified via CXCL8 chemokines and leukotriene B4 (Zhang et al., 2015). The third phase is characterized by the removal of neutrophils from the graft via macrophage phagocytosis, apoptosis or reverse migration.
Angiogenesis is a tightly coupled spatiotemporal process that requires cells to degrade and remodel the surrounding ECM (Hanjaya-Putra et al., 2012; Zhang et al., 2015; Crosby and Zoldan, 2019). Firstly, the basement membrane is degraded to allow the capillary sprout to grow in an existing blood vessel (Crosby and Zoldan, 2019). This is followed by further ECM degradation that allows the sprouting endothelial cells to invade the host tissue, thus creating a zone for the sprouting vascular lumen (Crosby and Zoldan, 2019). Human neutrophils express a specific set of neutrophil serine proteases, namely, proteinase 3, neutrophil elastase (NE) and cathepsin G (Owen and Campbell, 1995; Kessenbrock et al., 2011). These three serine proteases can cleave ECM components such as collagen, fibronectin, elastin, proteoglycans and laminin (Pipoly and Crouch, 1987; Rao et al., 1991; Owen and Campbell, 1995). Neutrophils also produce matrix metalloproteinases (MMPs) that can induce tissue regeneration, angiogenesis and matrix remodeling (Selders et al., 2017). MMPs can function as collagenases and gelatinases to break down connective tissues. However, in an appropriate amount, MMPs can contribute to tissue remodeling, angiogenesis and matrix reprogramming, which are consistent with the early resolution of immune-mediated responses (Mantovani et al., 2011).
Neutrophils also release CC chemokines to promote monocyte chemotaxis (Selders et al., 2017), and regulate the activation and recruitment of dendritic cells and natural killer cells (Kumar and Sharma, 2010; Mantovani et al., 2011; Brinkmann and Zychlinsky, 2012; Chistiakov et al., 2015). In addition, when the neutrophils give off eat-me signals, a specific phenotype is triggered in the engulfing macrophages, and the macrophages polarize into the M2 phenotype to activate a regenerative pathway, thereby negatively modulating inflammatory response (Mantovani et al., 2011). These functions are particularly important for AAM, as the M2-like macrophages can facilitate resolution and matrix remodeling, which in turn improve graft integration. The M2 macrophage polarization transition characterizes the resolution of inflammation, which is additionally triggered by the presence of apoptotic neutrophils (Boni et al., 2019). In contrast, a sustainable number of neutrophils may increase the M1 macrophage recruitment, which induces the fusion of these cells into foreign body giant cells and ultimately results in persistent inflammation (Boni et al., 2019).
Acute inflammation is a normal and essential reaction of the innate immune system, which can lead to a persistent inflammatory response that accelerates biomaterial degradation and tissue damage (Selders et al., 2017). Considering the above-mentioned functions of neutrophils, it is necessary to develop an AAM that can result in limited self-activation of neutrophils to maintain their biodegradability at an appropriate level. However, the exact role of neutrophils in AAM grafts is still unknown, and awaits further investigation.
Macrophages-Mediated Immune Reaction and Implants Remodeling
Monocyte-derived macrophage plays a critical role in regulating inflammatory responses during the implantation of biomaterials. It determines whether the graft becomes encapsulated, causes persistent inflammation or is completely integrated into the body, thus allowing for tissue regeneration. The two phenotypes of macrophages, M1 and M2, are responsible for cell-biomaterial interaction (Figure 1). Generally, M1 macrophages are involved in the pro-inflammatory response that leads to the development of chronic inflammation, whereas M2 macrophages promote a regenerative response in the tissue that is favourable for successful transplantation (Mirmalek-Sani et al., 2013; Wong and Griffiths, 2014). The fate of the AAM is closely dependent on the monocyte-derived macrophages that exist in the graft (Huleihel et al., 2017).
Within 1–6 days following transplantation, M1 macrophage initiates vascularization in the graft via pro-inflammatory signaling, which involves the upregulated levels of IL-1β, IL-6, IL-12, IL-23 and TNF-α (Brown et al., 2009). Later, M2 subtype can inhibit the fibrous or granuloma encapsulation by releasing IL-10 with rapid iron transport for positive tissue remodeling (Chakraborty et al., 2020). M2 macrophages directly contribute to adipogenesis in adipogenic induction models by promoting angiogenesis and adipogenesis (Han et al., 2015; Li et al., 2016). M2 macrophages also promote stem cell recruitment, preadipocyte survival and vessel remodeling during adipose tissue regeneration by releasing platelet-derived growth factor, MMP-9 and chemokine ligand 12 (Cai et al., 2018; Spiller et al., 2014).
The phenotype and function of macrophages can be altered by physicochemical stimuli, including biomaterial surface topography and roughness (McWhorter et al., 2015; Zhou and Groth, 2018). The fundamental mechanism by which an AAM maintains a dynamic balance between M1 and M2 macrophages is currently unknown (Spiller et al., 2015; Swinehart and Badylak, 2016). The timely transformation of M1 into M2 macrophages is conducive to the adipogenesis of the graft in xenogeneic AAM transplantation model (Liu et al., 2021). Optimization of the decellularization process can generate specific ECM peptides in the bio-scaffold, which leads to cell migration and integration with the host tissue.
Previous studies have shown that a successful ECM-derived biomaterial can effectively promote the plasticity of macrophages, thus allowing them to polarize from M1 to M2 within 7–14 days (Brown et al., 2014; Julier et al., 2017). However, the differences in the functional plasticity of macrophages as well as the roles of their intermediate subtypes (M2a-c) in the immune modulation process remain largely unclarified (Qiu et al., 2018; Robb et al., 2018). These specific groups can play potential roles in tissue remodeling, immune regulation and wound healing. Therefore, future studies on infiltrated macrophages in AAM should refine the classification of M2 macrophages, rather than categorizing them as M2 phenotype. The resolution phase of inflammation begins after 15 days of transplantation, while macrophage infiltration and adipogenesis are still visible 12 weeks after transplantation (He et al., 2018; Lin et al., 2019; Liu et al., 2021). Therefore, it is required to further extend the terminal observation time point of the AAM graft in future studies.
Lymphocytes-Mediated Immune Reaction and Implant Remodeling
In addition to the monocyte-driven macrophages, lymphocytes may emerge at the inflammatory sites after AAM implantation, thereby recognizing the antigenic fragments on the AAM and activating dendritic cells and macrophages (Chakraborty et al., 2020). Lymphocytes are involved in the B and T cell-mediated adaptive responses by interacting with the MHC molecules expressed on the surface of antigen-presenting cells (APCs) and are identified by the receptors on the T cell surface (Bach et al., 1997). T cells can be used to identify the difference between the peptides (e.g., versican, aggrecan, laminin and integrins) of host cells and the peptides on APCs (Figure 1) (Chakraborty et al., 2020).
MHC class I molecules refer to the peptides on the antigen surface that replicate simultaneously and those proteins present in the cytosolic fractions of the cells, which have shown to activate cytotoxic CD8+ T cells (Ghosh et al., 2005). MHC class II molecules are restricted to specific cells called APCs, including dendritic cells, macrophages and B cells. The presentation of peptides by MHC class II on APCs can activate CD4+ T cells (Wieczorek et al., 2017). Upon activation, cytotoxic CD8+ T cells and CD4+ Th1 cells migrate to the graft, where they can activate monocyte-driven and resident macrophages to combat the antigenic motifs (Allman et al., 2001).
T-helper 1 (Th1) lymphocytes produce tumor necrosis factor-β, interferon-γ and interleukin-2, resulting in macrophage activation, stimulation of complement-fixing antibody isotypes and differentiation of CD8+ cells to a cytotoxic phenotype (Irwin et al., 1989; Chen et al., 1996). T-helper 2 (Th2) lymphocytes generate cytokines, IL-4, -5, -6 and -10, leading to the production of non-complement-fixing antibody isotypes (Pattison and Krensky, 1997; Sadtler et al., 2016). Regulatory T (Treg) cells are immune regulatory cells that play a crucial role in maintaining immune homeostasis (Wang et al., 2008). Generally, Th1 polarization can contribute to proinflammatory responses, while Th2/Treg polarization is involved in transplant acceptance and constructive remodeling response. The decreased level of Th1:Th2 in grafts may be beneficial to the in vivo retention and regeneration of acellular matrix (Allman et al., 2001; Bayrak et al., 2010; Melgar-Lesmes et al., 2017). Our recent study has demonstrated that the increased level of Treg cells from methoxy polyethylene glycol-modified AMM is associated with adipogenic induction and M2 polarization (Liu et al., 2021). mPEG-modified antigens (e.g., MHC molecules) often exhibit a reduced affinity for receptors compared to the unmodified antigens (Webster et al., 2007). The strength of the interactions between MHC and T cell receptor (TCR) is closely associated with the fate of T cells (Kim and Williams, 2010; Josefowicz et al., 2012). Low-affinity antigen-TCR engagement can lead to a decrease in intracellular TCR signaling events that enhance the differentiation of naive T cells into Treg cells (Sauer et al., 2008; Turner et al., 2009). In addition, Treg cells could promote M2 macrophage polarization through the secretion of IL-10, IL-13 and TGF-β1 (Liu et al., 2021). The behavior of T cells in AAM play a key role in graft retention and regeneration, but the exact mechanism needs to be explored further.
It is worth noting that the acellular biomaterial’s properties can have an impact on lymphocyte differentiation. As mentioned above, ECM serves as a reservoir of growth factors and cytokines that persist after decellularization. Hence, the detection and quantification of ECM are of immense importance. After decellularization, surface molecules and peptides (e.g., hyaluronan, collagen fragments, versican, aggrecan, laminin and α1β1 integrin) are retained on the scaffolds, which can regulate immune responses (Thomas et al., 2007; Bollyky et al., 2011). However, the mechanism by which decellularized scaffold components mediate the exact host immune responses remains unknown.
Conclusion
The innate and adaptive immune responses to AAM, as well as the role of inflammatory cell degradation and remodeling, are still poorly understood. Different molecular structures and individual components of the proteins in AAM elicit specific immune responses in the host tissues after transplantation. These responses can modulate host cell functions, such as inflammatory response, cell migration and progenitor cell differentiation, in the AAM under pathological and physiological conditions, which can facilitate tissue regeneration. Therefore, it is necessary to further elucidate the inflammatory process of AAM after transplantation, in order to enhance the regeneration of AAM in vivo.
Author Contributions
KL: Writing-Original Draft; YH: Writing-Review & Editing; FL: Project administration.
Funding
This work was supported by the National Nature Science Foundation of China (81772101, 81801933), the Natural Science Foundation of Guangdong Province of China (2017A030313900), and the Administrator Foundation of Nanfang Hospital (2016Z010, 2017C008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Alam M., Gladstone H., Kramer E. M., Murphy J. P., Nouri K., Neuhaus I. M., et al. (2008). ASDS Guidelines of Care: Injectable Fillers. Dermatol Surg. 34 Suppl 1 (Suppl. 1), S115–S148. 10.1111/j.1524-4725.2008.34253.x [DOI] [PubMed] [Google Scholar]
- Allman A. J., McPherson T. B., Badylak S. F., Merrill L. C., Kallakury B., Sheehan C., et al. (2001). Xenogeneic Extracellular Matrix Grafts Elicit a TH2-Restricted Immune Response. Transplantation 71, 1631–1640. 10.1097/00007890-200106150-00024 [DOI] [PubMed] [Google Scholar]
- Anderson A. E., Wu I., Parrillo A. J., Wolf M. T., Maestas D. J., Graham I., et al. (2022). An Immunologically Active, Adipose-Derived Extracellular Matrix Biomaterial for Soft Tissue Reconstruction: Concept to Clinical Trial. J. Article 7, 610–1038. 10.1038/s41536-021-00197-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M. (2015). Exploiting the Inflammatory Response on Biomaterials Research and Development. J. Mater Sci. Mater Med. 26, 121–1007. 10.1007/s10856-015-5423-5 [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Rodriguez A., Chang D. T. (2008). Foreign Body Reaction to Biomaterials. Semin. Immunol. 20, 86–100. 10.1016/j.smim.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach F. H., Ferran C., Hechenleitner P., Mark W., Koyamada N., Miyatake T., et al. Accommodation of Vascularized Xenografts: Expression of "protective Genes" by Donor Endothelial Cells in a Host Th2 Cytokine Environment. Nat. Med. 1997, 3, 196.1038, 204. 10.1038/nm0297-196 [DOI] [PubMed] [Google Scholar]
- Bayrak A., Tyralla M., Ladhoff J., Schleicher M., Stock U. A., Volk H. D., et al. (2010). Human Immune Responses to Porcine Xenogeneic Matrices and Their Extracellular Matrix Constituents In Vitro . Biomaterials 31, 3793–3803. 10.1016/j.biomaterials.2010.01.120 [DOI] [PubMed] [Google Scholar]
- Björnsdottir H., Welin A., Michaëlsson E., Osla V., Berg S., Christenson K., et al. (2015). Neutrophil NET Formation Is Regulated from the inside by Myeloperoxidase-Processed Reactive Oxygen Species. Free Radic. Biol. Med. 89, 1024–1035. 10.1016/j.freeradbiomed.2015.10.398 [DOI] [PubMed] [Google Scholar]
- Bollyky P. L., Wu R. P., Falk B. A., Lord J. D., Long S. A., Preisinger A., et al. (2011). ECM Components Guide IL-10 Producing Regulatory T-Cell (TR1) Induction from Effector Memory T-Cell Precursors. Proc. Natl. Acad. Sci. U. S. A. 108, 7938–7943. 10.1073/pnas.1017360108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni B. O. O., Lamboni L., Souho T., Gauthier M., Yang G. (2019). Immunomodulation and Cellular Response to Biomaterials: the Overriding Role of Neutrophils in Healing. J. Article 6, 1122–1137. 10.1039/C9MH00291J [DOI] [Google Scholar]
- Brinkmann V., Zychlinsky A. (2012). Neutrophil Extracellular Traps: Is Immunity the Second Function of Chromatin?. J. Cell Biol. 198, 773–783. 10.1083/jcb.201203170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. N., Freund J. M., Han L., Rubin J. P., Reing J. E., Jeffries E. M., et al. (2011). Comparison of Three Methods for the Derivation of a Biologic Scaffold Composed of Adipose Tissue Extracellular Matrix. Tissue Eng. Part C Methods 17, 411–421. 10.1089/ten.TEC.2010.0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. N., Ratner B. D., Goodman S. B., Amar S., Badylak S. F. (2012). Macrophage Polarization: an Opportunity for Improved Outcomes in Biomaterials and Regenerative Medicine. Biomaterials 33, 3792–3802. 10.1016/j.biomaterials.2012.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. N., Valentin J. E., Stewart-Akers A. M., McCabe G. P., Badylak S. F. (2009). Macrophage Phenotype and Remodeling Outcomes in Response to Biologic Scaffolds with and without a Cellular Component. Biomaterials 30, 1482–91. 10.1016/j.biomaterials.2008.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. N., Sicari B. M., Badylak S. F. (2014). Rethinking Regenerative Medicine: a Macrophage-Centered Approach. Front. Immunol. 5, 510. 10.3389/fimmu.2014.00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryers J. D., Giachelli C. M., Ratner B. D. (2012). Engineering Biomaterials to Integrate and Heal: the Biocompatibility Paradigm Shifts. Biotechnol. Bioeng. 109, 1898–911. 10.1002/bit.24559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Feng J., Liu K., Zhou S., Lu F. (2018). Early Macrophage Infiltration Improves Fat Graft Survival by Inducing Angiogenesis and Hematopoietic Stem Cell Recruitment. Plast. Reconstr. Surg. 141, 376–386. 10.1097/PRS.0000000000004028 [DOI] [PubMed] [Google Scholar]
- Cai J., Li B., Liu K., Feng J., Gao K., Lu F. (2017). Low-dose G-CSF Improves Fat Graft Retention by Mobilizing Endogenous Stem Cells and Inducing Angiogenesis, whereas High-Dose G-CSF Inhibits Adipogenesis with Prolonged Inflammation and Severe Fibrosis. Biochem. Biophys. Res. Commun. 491, 662–667. 10.1016/j.bbrc.2017.07.147 [DOI] [PubMed] [Google Scholar]
- Cai J., Li B., Liu K., Li G., Lu F. (2017). Macrophage Infiltration Regulates the Adipose ECM Reconstruction and the Fibrosis Process after Fat Grafting. Biochem. Biophys. Res. Commun. 490, 560–566. 10.1016/j.bbrc.2017.06.078 [DOI] [PubMed] [Google Scholar]
- Chakraborty J., Roy S., Ghosh S. (2020). Regulation of Decellularized Matrix Mediated Immune Response. Biomater. Sci. 8, 1194–1215. 10.1039/c9bm01780a [DOI] [PubMed] [Google Scholar]
- Chen N., Gao Q., Field E. H. (1996). Prevention of Th1 Response Is Critical for Tolerance. Transplantation 61, 1076–83. 10.1097/00007890-199604150-00016 [DOI] [PubMed] [Google Scholar]
- Chen R. H., Mitchell R. N., Kadner A., Adams D. H. (1999). Differential Galactose Alpha(1,3) Galactose Expression by Porcine Cardiac Vascular Endothelium. Xenotransplantation 6, 169–72. 10.1034/j.1399-3089.1999.00024.x [DOI] [PubMed] [Google Scholar]
- Chistiakov D. A., Bobryshev Y. V., Orekhov A. N. (2015). Neutrophil's Weapons in Atherosclerosis. Exp. Mol. Pathol. 99, 663–671. 10.1016/j.yexmp.2015.11.011 [DOI] [PubMed] [Google Scholar]
- Chung L., Maestas D. R., Housseau F., Elisseeff J. H. (2017). Key Players in the Immune Response to Biomaterial Scaffolds for Regenerative Medicine. Adv. Drug Deliv. Rev. 114, 184–192. 10.1016/j.addr.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Coleman S. R. (2006). Structural Fat Grafting: More Than a Permanent Filler. Plast. Reconstr. Surg. 118, 108S–120S. 10.1097/01.prs.0000234610.81672.e7 [DOI] [PubMed] [Google Scholar]
- Costa A., Naranjo J. D., Londono R., Badylak S. F. (2017). Biologic Scaffolds. Cold Spring Harb. Perspect. Med. 7, a025676. 10.1101/cshperspect.a025676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo P. M., Gilbert T. W., Badylak S. F. (2011). An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 32, 3233–3243. 10.1016/j.biomaterials.2011.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravedi P., Farouk S., Angeletti A., Edgar L., Tamburrini R., Duisit J., et al. (2017). Regenerative Immunology: the Immunological Reaction to Biomaterials. Transpl. Int. 30, 1199–1208. 10.1111/tri.13068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby C. O., Zoldan J. (2019). Mimicking the Physical Cues of the ECM in Angiogenic Biomaterials. Regen. Biomater. 6, 61–73. 10.1093/rb/rbz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgliesh A. J., Parvizi M., Lopera-Higuita M., Shklover J., Griffiths L. G. (2018). Graft-specific Immune Tolerance Is Determined by Residual Antigenicity of Xenogeneic Extracellular Matrix Scaffolds. Acta Biomater. 79, 253–264. 10.1016/j.actbio.2018.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira S., Rosowski E. E., Huttenlocher A. (2016). Neutrophil Migration in Infection and Wound Repair: Going Forward in Reverse. Nat. Rev. Immunol. 16, 378–391. 10.1038/nri.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré-Crochet S., Erard M., Nüβe O. (2013). ROS Production in Phagocytes: Why, when, and where? J. Leukoc. Biol. 94, 657–670. 10.1189/jlb.1012544 [DOI] [PubMed] [Google Scholar]
- Dziki J. L., Huleihel L., Scarritt M. E., Badylak S. F. (2017). Extracellular Matrix Bioscaffolds as Immunomodulatory Biomaterials. Tissue Eng. Part A 23, 1152–1159. 10.1089/ten.TEA.2016.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziki J. L., Hussey G., Badylak S. F. (2018). Alarmins of the Extracellular Space. Semin. Immunol. 38, 33–39. 10.1016/j.smim.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Franz S., Rammelt S., Scharnweber D., Simon J. C. (2011). Immune Responses to Implants - a Review of the Implications for the Design of Immunomodulatory Biomaterials. Biomaterials 32, 6692–6709. 10.1016/j.biomaterials.2011.05.078 [DOI] [PubMed] [Google Scholar]
- Gattazzo F., Urciuolo A., Bonaldo P. (2014). Extracellular Matrix: a Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta 1840, 2506–2519. 10.1016/j.bbagen.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet A. D., Popovich P. G. (2014). Extracellular Matrix Regulation of Inflammation in the Healthy and Injured Spinal Cord. Exp. Neurol. 258, 24–34. 10.1016/j.expneurol.2013.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Rosenthal R., Zajac P., Weber W. P., Oertli D., Heberer M., et al. (2005). Culture of Melanoma Cells in 3-dimensional Architectures Results in Impaired Immunorecognition by Cytotoxic T Lymphocytes Specific for Melan-A/mart-1 Tumor-Associated Antigen. Ann. Surg. 242, 851–858. 10.1097/01.sla.0000189571.84213.b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. H., Kinney B. M., Kaminer M. S., Rohrich R. J., D'Amico R. A. (2020). A Multi-Center, Open-Label, Pilot Study of Allograft Adipose Matrix for the Correction of Atrophic Temples. J. Cosmet. Dermatol 19, 1044–1056. 10.1111/jocd.13363 [DOI] [PubMed] [Google Scholar]
- Han T. T., Toutounji S., Amsden B. G., Flynn L. E. (2015). Adipose-derived Stromal Cells Mediate In Vivo Adipogenesis, Angiogenesis and Inflammation in Decellularized Adipose Tissue Bioscaffolds. Biomaterials 72, 125–137. 10.1016/j.biomaterials.2015.08.053 [DOI] [PubMed] [Google Scholar]
- Hanjaya-Putra D., Wong K. T., Hirotsu K., Khetan S., Burdick J. A., Gerecht S. (2012). Spatial Control of Cell-Mediated Degradation to Regulate Vasculogenesis and Angiogenesis in Hyaluronan Hydrogels. Biomaterials 33, 6123–6131. 10.1016/j.biomaterials.2012.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Lin M., Wang X., Guan J., Dong Z., Lu F., et al. (2018). Optimized Adipose Tissue Engineering Strategy Based on a Neo-Mechanical Processing Method. Wound Repair Regen. 26, 163–171. 10.1111/wrr.12640 [DOI] [PubMed] [Google Scholar]
- Hillebrandt K. H., Everwien H., Haep N., Keshi E., Pratschke J., Sauer I. M. (2019). Strategies Based on Organ Decellularization and Recellularization. Transpl. Int. 32, 571–585. 10.1111/tri.13462 [DOI] [PubMed] [Google Scholar]
- Huleihel L., Dziki J. L., Bartolacci J. G., Rausch T., Scarritt M. E., Cramer M. C., et al. (2017). Macrophage Phenotype in Response to ECM Bioscaffolds. Semin. Immunol. 29, 2–13. 10.1016/j.smim.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M. J., Heath W. R., Sherman L. A. (1989). Species-restricted Interactions between CD8 and the Alpha 3 Domain of Class I Influence the Magnitude of the Xenogeneic Response. J. Exp. Med. 170, 1091–101. 10.1084/jem.170.4.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz S. Z., Lu L. F., Rudensky A. Y. (2012). Regulatory T Cells: Mechanisms of Differentiation and Function. Annu. Rev. Immunol. 30, 531–64. 10.1146/annurev.immunol.25.022106.141623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julier Z., Park A. J., Briquez P. S., Martino M. M. (2017). Promoting Tissue Regeneration by Modulating the Immune System. Acta Biomater. 53, 13–28. 13 10.1016/j.actbio.2017.01.056 [DOI] [PubMed] [Google Scholar]
- Kayabolen A., Keskin D., Aykan A., Karslioglu Y., Zor F., Tezcaner A. (2017). Native Extracellular Matrix/Fibroin Hydrogels for Adipose Tissue Engineering With Enhanced Vascularization. Biomed. Mater. 12, 035007. 10.1088/1748-605X/aa6a63 [DOI] [PubMed] [Google Scholar]
- Kawecki M., Łabuś W., Klama-Baryla A., Kitala D., Kraut M., Glik J., et al. (2018). A Review of Decellurization Methods Caused by an Urgent Need for Quality Control of Cell-free Extracellular Matrix' Scaffolds and Their Role in Regenerative Medicine. J. Biomed. Mater Res. B Appl. Biomater. 106, 909–923. 10.1002/jbm.b.33865 [DOI] [PubMed] [Google Scholar]
- Keane T. J., Londono R., Turner N. J., Badylak S. F. (2012). Consequences of Ineffective Decellularization of Biologic Scaffolds on the Host Response. Biomaterials 33, 1771–1781. 10.1016/j.biomaterials.2011.10.054 [DOI] [PubMed] [Google Scholar]
- Kessenbrock K., Dau T., Jenne D. E. (2011). Tailor-made Inflammation: How Neutrophil Serine Proteases Modulate the Inflammatory Response. J. Mol. Med. Berl. 89, 23–8. 10.1007/s00109-010-0677-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouri R. K., Eisenmann-Klein M., Cardoso E., Cooley B. C., Kacher D., Gombos E., et al. (2012). Brava and Autologous Fat Transfer Is a Safe and Effective Breast Augmentation Alternative: Results of a 6-year, 81-patient, Prospective Multicenter Study. Plast. Reconstr. Surg. 129, 1173–1187. 10.1097/PRS.0b013e31824a2db6 [DOI] [PubMed] [Google Scholar]
- Kim C., Williams M. A. (2010). Nature and Nurture: T-Cell Receptor-dependent and T-Cell Receptor-independent Differentiation Cues in the Selection of the Memory T-Cell Pool. Immunology 131, 310–7. 10.1111/j.1365-2567.2010.03338.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokai L. E., Schilling B. K., Chnari E., Huang Y. C., Imming E. A., Karunamurthy A., et al. (2019). Injectable Allograft Adipose Matrix Supports Adipogenic Tissue Remodeling in the Nude Mouse and Human. Plast. Reconstr. Surg. 143, 299e–309e. 10.1097/PRS.0000000000005269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokai L. E., Sivak W. N., Schilling B. K., Karunamurthy A., Egro F. M., Schusterman M. A., et al. (2020). Clinical Evaluation of an Off-The-Shelf Allogeneic Adipose Matrix for Soft Tissue Reconstruction. Plast. Reconstr. Surg. Glob. Open 8, e2574. 10.1097/GOX.0000000000002574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kølle S. F., Fischer-Nielsen A., Mathiasen A. B., Elberg J. J., Oliveri R. S., Glovinski P. V., et al. (2013). Enrichment of Autologous Fat Grafts with Ex-Vivo Expanded Adipose Tissue-Derived Stem Cells for Graft Survival: a Randomised Placebo-Controlled Trial. Lancet 382, 1113–1120. 10.1016/S0140-6736(13)61410-5 [DOI] [PubMed] [Google Scholar]
- Kumar V., Sharma A. (2010). Neutrophils: Cinderella of Innate Immune System. Int. Immunopharmacol. 10, 1325–34. 10.1016/j.intimp.2010.08.012 [DOI] [PubMed] [Google Scholar]
- Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007). Getting to the Site of Inflammation: the Leukocyte Adhesion Cascade Updated. Nat. Rev. Immunol. 7, 678–689. 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- Li Z., Xu F., Wang Z., Dai T., Ma C., Liu B., et al. (2016). Macrophages Undergo M1-To-M2 Transition in Adipose Tissue Regeneration in a Rat Tissue Engineering Model. Artif. Organs 40–E167. 10.1111/aor.12756 [DOI] [PubMed] [Google Scholar]
- Lin M., Ge J., Wang X., Dong Z., Xing M., Lu F., et al. (2019). Biochemical and Biomechanical Comparisions of Decellularized Scaffolds Derived from Porcine Subcutaneous and Visceral Adipose Tissue. J. Tissue Eng. 10, 2041731419888168. 10.1177/2041731419888168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Cai J., Li H., Feng J., Feng C., Lu F. (2018). The Disturbed Function of Neutrophils at the Early Stage of Fat Grafting Impairs Long-Term Fat Graft Retention. Plast. Reconstr. Surg. 142, 1229–1238. 10.1097/PRS.0000000000004882 [DOI] [PubMed] [Google Scholar]
- Liu K., He Y., Yao Y., Zhang Y., Cai Z., Ru J., et al. (2021). Methoxy Polyethylene Glycol Modification Promotes Adipogenesis by Inducing the Production of Regulatory T Cells in Xenogeneic Acellular Adipose Matrix. J. Article 12, 10016110. 10.1016/j.mtbio.2021.100161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Boado Y. S., Espinola M., Bahr S., Belaaouaj A. (2004). Neutrophil Serine Proteinases Cleave Bacterial Flagellin, Abrogating its Host Response-Inducing Activity. J. Immunol. 172, 509–15. 10.4049/jimmunol.172.1.509 [DOI] [PubMed] [Google Scholar]
- Mahanty S., Prigent A., Garraud O. (2015). Immunogenicity of Infectious Pathogens and Vaccine Antigens. BMC Immunol. 16–31. 10.1186/s12865-015-0095-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Cassatella M. A., Costantini C., Jaillon S. (2011). Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 11, 519–531. 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- Mariani E., Lisignoli G., Borzì R. M., Pulsatelli L. (2019). Biomaterials: Foreign Bodies or Tuners for the Immune Response? Ijms 20, 636. 10.3390/ijms20030636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro M. S., Palek R., Rosendorf J., Cervenkova L., Liska V., Moulisova V. (2021). Decellularized Xenogeneic Scaffolds in Transplantation and Tissue Engineering: Immunogenicity versus Positive Cell Stimulation. J. Article 127, 11220310. 10.1016/j.msec.2021.112203 [DOI] [PubMed] [Google Scholar]
- McWhorter F. Y., Davis C. T., Liu W. F. (2015). Physical and Mechanical Regulation of Macrophage Phenotype and Function. Cell Mol. Life Sci. 72, 1303–16. 10.1007/s00018-014-1796-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgar-Lesmes P., Balcells M., Edelman E. R. (2017). Implantation of Healthy Matrix-Embedded Endothelial Cells Rescues Dysfunctional Endothelium and Ischaemic Tissue in Liver Engraftment. Gut 66, 1297–1305. 10.1136/gutjnl-2015-310409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendibil U., Ruiz-Hernandez R., Retegi-Carrion S., Garcia-Urquia N., Olalde-Graells B., Abarrategi A. (2020). Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Ijms 21, 5447. 10.3390/ijms21155447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmalek-Sani S. H., Sullivan D. C., Zimmerman C., Shupe T. D., Petersen B. E. (2013). Immunogenicity of Decellularized Porcine Liver for Bioengineered Hepatic Tissue. Am. J. Pathol. 183, 558–565. 10.1016/j.ajpath.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naso F., Gandaglia A., Iop L., Spina M., Gerosa G. (2011). First Quantitative Assay of Alpha-Gal in Soft Tissues: Presence and Distribution of the Epitope before and after Cell Removal from Xenogeneic Heart Valves. Acta Biomater. 7, 1728–1734. 10.1016/j.actbio.2010.11.030 [DOI] [PubMed] [Google Scholar]
- Nguyen A., Pasyk K. A., Bouvier T. N., Hassett C. A., Argenta L. C. (1990). Comparative Study of Survival of Autologous Adipose Tissue Taken and Transplanted by Different Techniques. J. Article 85 (378), 387. 10.1097/00006534-199003000-00008 [DOI] [PubMed] [Google Scholar]
- Owen C. A., Campbell E. J. (1995). Neutrophil Proteinases and Matrix Degradation. The Cell Biology of Pericellular Proteolysis. Semin. Cell Biol. 6, 367–76. 10.1016/s1043-4682(05)80007-8 [DOI] [PubMed] [Google Scholar]
- Partington L., Mordan N. J., Mason C., Knowles J. C., Kim H. W., Lowdell M. W., et al. (2013). Biochemical Changes Caused by Decellularization May Compromise Mechanical Integrity of Tracheal Scaffolds. Acta Biomater. 9, 5251–5261. 10.1016/j.actbio.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Pattison J. M., Krensky A. M. (1997). New Insights into Mechanisms of Allograft Rejection. Am. J. Med. Sci. 313, 257–263. 10.1097/00000441-199705000-00002 [DOI] [PubMed] [Google Scholar]
- Peña A. N., Peña J. A. G. J., Garcia J. A., Elisseeff J. H. (2021). Translational Considerations for Adipose-Derived Biological Scaffolds for Soft Tissue Repair. Curr. Opin. Biomed. Eng. 20, 100321. 10.1016/j.cobme.2021.100321 [DOI] [Google Scholar]
- Perobelli S. M., Galvani R. G., Gonçalves-Silva T., Xavier C. R., Nóbrega A., Bonomo A. (2015). Plasticity of Neutrophils Reveals Modulatory Capacity. Braz J. Med. Biol. Res. 48, 665–675. 10.1590/1414-431X20154524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipoly D. J., Crouch E. C. (1987). Degradation of Native Type IV Procollagen by Human Neutrophil Elastase. Implications for Leukocyte-Mediated Degradation of Basement Membranes. Biochemistry 26, 5748–5754. 10.1021/bi00392a025 [DOI] [PubMed] [Google Scholar]
- Qiu X., Liu S., Zhang H., Zhu B., Su Y., Zheng C., et al. (2018). Mesenchymal Stem Cells and Extracellular Matrix Scaffold Promote Muscle Regeneration by Synergistically Regulating Macrophage Polarization toward the M2 Phenotype. Stem Cell Res. Ther. 9, 88–1186. 10.1186/s13287-018-0821-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N. V., Wehner N. G., Marshall B. C., Gray W. R., Gray B. H., Hoidal J. R. (1991). Characterization of Proteinase-3 (PR-3), a Neutrophil Serine Proteinase. Structural and Functional Properties. J. Biol. Chem. 266, 9540–9548. 10.1016/s0021-9258(18)92854-1 [DOI] [PubMed] [Google Scholar]
- Robb K. P., Shridhar A., Flynn L. E. (2018). Decellularized Matrices as Cell-Instructive Scaffolds to Guide Tissue-specific Regeneration. J. Article 4, 3627–3643. 10.1021/acsbiomaterials.7b00619 [DOI] [PubMed] [Google Scholar]
- Rosales C. (2018). Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types?. Front. Physiol. 9–113. 10.3389/fphys.2018.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadtler K., Estrellas K., Allen B. W., Wolf M. T., Fan H., Tam A. J., et al. (2016). Developing a Pro-regenerative Biomaterial Scaffold Microenvironment Requires T Helper 2 Cells. Science 352, 366–370. 10.1126/science.aad9272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sart S., Jeske R., Chen X., Ma T., Li Y. (2020). Engineering Stem Cell-Derived Extracellular Matrices: Decellularization, Characterization, and Biological Function. Tissue Eng. Part B Rev. 26, 402–422. 10.1089/ten.TEB.2019.0349 [DOI] [PubMed] [Google Scholar]
- Sauer S., Bruno L., Hertweck A., Finlay D., Leleu M., Spivakov M., et al. (2008). T Cell Receptor Signaling Controls Foxp3 Expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U. S. A. 105, 7797–802. 10.1073/pnas.0800928105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selders G. S., Fetz A. E., Radic M. Z., Bowlin G. L. (2017). An Overview of the Role of Neutrophils in Innate Immunity, Inflammation and Host-Biomaterial Integration. Regen. Biomater. 4, 55–68. 10.1093/rb/rbw041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector M., Lim T. C. (2016). Injectable Biomaterials: a Perspective on the Next Wave of Injectable Therapeutics. Biomed. Mater 11, 014110. 10.1088/1748-6041/11/1/014110 [DOI] [PubMed] [Google Scholar]
- Spiller K. L., Anfang R. R., Spiller K. J., Ng J., Nakazawa K. R., Daulton J. W., et al. (2014). The Role of Macrophage Phenotype in Vascularization of Tissue Engineering Scaffolds. Biomaterials 35, 4477–4488. 10.1016/j.biomaterials.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller K. L., Freytes D. O., Vunjak-Novakovic G. (2015). Macrophages Modulate Engineered Human Tissues for Enhanced Vascularization and Healing. Ann. Biomed. Eng. 43, 616–27. 10.1007/s10439-014-1156-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl E. C., Bonvillain R. W., Skillen C. D., Burger B. L., Hara H., Lee W., et al. (2018). Evaluation of the Host Immune Response to Decellularized Lung Scaffolds Derived from α-Gal Knockout Pigs in a Non-human Primate Model. Biomaterials 187, 93–104. 10.1016/j.biomaterials.2018.09.038 [DOI] [PubMed] [Google Scholar]
- Swinehart I. T., Badylak S. F. (2016). Extracellular Matrix Bioscaffolds in Tissue Remodeling and Morphogenesis. Dev. Dyn. 245, 351–360. 10.1002/dvdy.24379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. H., Edelman E. R., Stultz C. M. (2007). Collagen Fragments Modulate Innate Immunity. Exp. Biol. Med. (Maywood) 232, 406–411. [PubMed] [Google Scholar]
- Turner M. S., Kane L. P., Morel P. A. (2009). Dominant Role of Antigen Dose in CD4+Foxp3+ Regulatory T Cell Induction and Expansion. J. Immunol. 183, 4895–4903. 10.4049/jimmunol.0901459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaienti L., Soresina M., Menozzi A. (2005). Parascapular Free Flap and Fat Grafts: Combined Surgical Methods in Morphological Restoration of Hemifacial Progressive Atrophy. Plast. Reconstr. Surg. 116, 699–711. 10.1097/01.prs.0000177449.12366.48 [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhove I., Tytgat L., Ryx M., Blondeel P., Stillaert F., Thienpont H., et al. (2017). Soft Tissue Fillers for Adipose Tissue Regeneration: From Hydrogel Development toward Clinical Applications. Acta Biomater. 63, 37–49. 10.1016/j.actbio.2017.09.026 [DOI] [PubMed] [Google Scholar]
- Wang S., Jiang J., Guan Q., Lan Z., Wang H., Nguan C. Y., et al. (2008). Reduction of Foxp3-Expressing Regulatory T Cell Infiltrates during the Progression of Renal Allograft Rejection in a Mouse Model. Transpl. Immunol. 19, 93–102. 10.1016/j.trim.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Webster R., Didier E., Harris P., Siegel N., Stadler J., Tilbury L., et al. (2007). PEGylated Proteins: Evaluation of Their Safety in the Absence of Definitive Metabolism Studies. Drug Metab. Dispos. 35, 9–16. 10.1124/dmd.106.012419 [DOI] [PubMed] [Google Scholar]
- Wieczorek M., Abualrous E. T., Sticht J., Álvaro-Benito M., Stolzenberg S., Noé F., et al. (2017). Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 8, 292–3389. 10.3389/fimmu.2017.00292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. J., Clegg R. E., Leavesley D. I., Pearcy M. J. (2005). Mediation of Biomaterial-Cell Interactions by Adsorbed Proteins: a Review. Tissue Eng. 11, 1–18. 10.1089/ten.2005.11.1 [DOI] [PubMed] [Google Scholar]
- Wong M. L., Griffiths L. G. (2014). Immunogenicity in Xenogeneic Scaffold Generation: Antigen Removal vs. Decellularization. Acta Biomater. 10, 1806–16. 10.1016/j.actbio.2014.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom D. W., Barrett J. G., Jose R. R., Kaplan D. L. (2013). Functional Characterization of Detergent-Decellularized Equine Tendon Extracellular Matrix for Tissue Engineering Applications. PLoS One 8, e64151. 10.1371/journal.pone.0064151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Hubenak J., Iyyanki T., Alred E., Turza K. C., Davis G., et al. (2015). Engineering Vascularized Soft Tissue Flaps in an Animal Model Using Human Adipose-Derived Stem Cells and VEGF+PLGA/PEG Microspheres on a Collagen-Chitosan Scaffold with a Flow-Through Vascular Pedicle. Biomaterials 73, 198–213. 10.1016/j.biomaterials.2015.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Hayakawa T. J., Levin L. S., Hallock G. G., Lazzeri D. (2016). The Economy in Autologous Tissue Transfer: Part 1. The Kiss Flap Technique. Plast. Reconstr. Surg. 137, 1018–30. 10.1097/01.prs.0000479971.99309.21 [DOI] [PubMed] [Google Scholar]
- Zhou G., Groth T. (2018). Host Responses to Biomaterials and Anti-inflammatory Design-A Brief Review. Macromol. Biosci. 18, 1800112. 10.1002/mabi.201800112 [DOI] [PubMed] [Google Scholar]