Abstract
Objective:
To examine the evidence for efficacy of phosphatidylserine for symptoms of attention-deficit/hyperactivity disorder (ADHD) in children.
Methods:
Medline, Cochrane Library, and ClinicalTrials.gov were searched from inception through August 2020. Studies of any design that assessed phosphatidylserine supplementation for children aged ≤18 years with a diagnosis of ADHD were included in the systematic review; only randomized clinical trials were included in the meta-analysis. Standardized mean differences and 95% confidence intervals (CIs) were calculated, and the heterogeneity of the studies was estimated using I2. The overall quality of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation tool.
Results:
Four studies met the inclusion criteria for the narrative review (n = 344) and three for the meta-analysis (n = 216). Results of the meta-analysis showed a statistically significant effect of 200–300 mg/day of phosphatidylserine on symptoms of inattention relative to placebo (effect size [ES] 0.36; 95% CI: 0.07 to 0.64; p = 0.01). The effects of phosphatidylserine on overall symptoms of ADHD (ES 0.76; 95% CI: −0.07 to 1.60; p = 0.07) and hyperactivity-impulsivity (ES 0.24; 95% CI: −0.04 to 0.53; p = 0.09) were not statistically significant.
Conclusions:
Preliminary evidence suggests that phosphatidylserine may be effective for reducing symptoms of inattention in children with ADHD, although the quality of the evidence is low and additional research in this area is warranted.
Keywords: ADHD, pediatrics, integrative medicine
Introduction
Rationale
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by a persistent pattern of hyperactivity, impulsivity, or inattention, which interferes with daily functioning.1 It is associated with many academic and health issues, including obesity, peer problems, unintentional injuries or poisoning, emergency department admission, lower grades, and expulsion from school.2–8 Children with ADHD are more likely than other children to have psychiatric comorbidities, including oppositional defiant disorder, conduct disorder, depression, and anxiety.9 ADHD can persist into adulthood, and adults with ADHD are at increased risk of substance use disorders, tobacco use, speeding violations, divorce, homelessness, and suicide.6,10–12 In the United States, 11% of children 4–17 years old have been diagnosed with ADHD, contributing to an annual societal cost of $36–$52 billion.13 This widespread condition, with its many associated adverse effects, is a clinical and public health concern.
Standard-of-care treatment for ADHD includes stimulant medication with or without behavioral therapy; however, it is estimated that fewer than 1 in 3 children receive this treatment.14,15 Medication may be avoided or discontinued due to side effects, concerns about its long-term use, or simply because it is not effective in reducing many children's symptoms.16 Early identification and treatment may prevent or mitigate some of the negative side effects of the disorder, and thus, alternative interventions are needed.
Recently, interest in phosphatidylserine as an intervention for ADHD has grown. Children with ADHD have lower levels of phosphatidylserine in serum, as well as the basal ganglia and prefrontal cortex.17,18 These brain regions are targeted by stimulant medications used to treat ADHD, suggesting that low levels of phosphatidylserine in these regions may contribute to symptoms of the disorder.19
Phosphatidylserine contains omega-3 fatty acids, which are also found in fish oil. Omega-3 fatty acids have been extensively studied as treatment for ADHD, and six recent meta-analyses were conducted, with mixed yet promising results.20–25 Several trials have been conducted examining phosphatidylserine supplementation in children and adolescents with ADHD, but the authors were unable to identify any systematic review or meta-analysis on this subject. Therefore, they systematically evaluated studies of phosphatidylserine supplementation in children ≤18 years old with ADHD. They conducted a meta-analysis to assess the effect of supplementation on ADHD symptoms.
Methods
Protocol # and registration
This review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) and PRISMA guidelines.26,27 The protocol was published in the International Prospective Register of Systematic Reviews database before the review began (#CRD 42018093188).
Inclusion criteria
Studies were included if they assessed supplementation of phosphatidylserine (any dose or duration) for children and adolescents ≤18 years with a diagnosis of ADHD. Studies with participants both older than and younger than 18 years were included if the data were able to be stratified by age. If data were not presented in such a way, three attempts were made to contact the study authors and obtain raw data. Only English language articles were included. All study designs were eligible to be included in the systematic review, but only randomized-controlled trials (RCTs) were included in the meta-analysis.
Outcome measures
Studies were included only if they assessed symptoms of ADHD. Primary outcome measures included scales which assessed ADHD or symptom domains of ADHD (inattention, hyperactivity, impulsivity). If a study used multiple measures of ADHD, objective measures of attention were selected over subjective measures for use in the meta-analysis. Objective assessments can be computerized or noncomputerized, and involve measuring a child's response time and errors when asked to complete certain tasks.
Subjective measures are questionnaires about a child's behavior that are completed by an observer (e.g., parent, teacher, or clinician). For this meta-analysis, computerized objective measures were prioritized for inclusion over noncomputerized objective measures. When multiple subjective scales were used, the most psychometrically validated and/or relevant scale was chosen. Secondary outcomes included adverse events and attrition rate.
Search strategy
Search strategies were developed by one researcher (A.B.) with the assistance of a professional librarian (Appendix A1). In brief, the authors searched a combination of terms including attention, hyperactivity, and phosphatidylserine. The search was conducted from the inception dates of each of the databases to August 31, 2020, for Medline/PubMed, Medline/OVID, Cochrane database (both systematic reviews and CENTRAL), and ClinicalTrials.gov.
Attempts were made to find published reports for relevant trials registered on CENTRAL and ClinicalTrials.gov. If reports were not found, authors were contacted up to three times to attempt to locate the report. In addition, the reference lists of studies included in the review (primary studies) were examined. A citation search on the primary studies was completed using the Scopus database.
Study selection and data extraction
Two investigators conducted the search in the PubMed database and compared results (A.B., J.N.). One investigator (A.B.) ran the searches in the other databases with the help of a professional librarian. Forms for screening study eligibility were created by one investigator and piloted by three investigators (A.B., J.N., M.G.). Eligibility assessment was conducted by two independent investigators in duplicate using the DistillerSR software, a data management tool from Evidence Partners, with κ = 0.93 (A.B., J.N.).28
Data to be extracted were detailed in the protocol (Appendix A2). Data extraction forms were created by one investigator (A.B.) and piloted by three investigators (A.B., J.N., M.G.). Data for the meta-analyses were extracted independently in duplicate by two investigators (A.B., A.S.); all other data were extracted by one investigator (A.B.). When trials included an open-label extension, the open-label data were not included in the analysis. If articles appeared to be missing information, authors were contacted up to three times to attempt to clarify.
Data synthesis and statistical analysis
The RevMan statistical tool (version 5.3) from the Cochrane Collaboration was used to conduct the meta-analyses.29 Hyperactivity/impulsivity and inattention were the only symptom domains assessed across all three studies, and a meta-analysis was conducted on each outcome. Because assessment measures varied across studies, the authors computed standardized mean differences (SMDs) in the meta-analysis of continuous outcomes. Percent attrition was reported for each study group. Adverse events per group were only reported in one study and were not included in the meta-analysis.
A random-effects model was used for the meta-analysis, based on the recommendations of DerSimonian and Laird.30 I2 was used as a measure of heterogeneity.31 The authors did not assess publication bias with a funnel plot because only three studies were identified for meta-analysis.32
Methodological quality assessment
The Cochrane Risk of Bias (CROB) tool was used to assess risk of bias of individual studies.33 Two investigators (A.B., J.N.) independently completed the CROB tool in duplicate and any disagreements were resolved by discussion. Reports were compared with their protocols, when available, to assess for selective outcome reporting within studies.
The GRADE tool, a product of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group, was used to assess the quality of the evidence for each outcome included in the meta-analysis.34 Evidence from RCTs is given a starting rating of “high quality” and is downgraded for serious concerns about risk of bias, inconsistency, indirectness, imprecision, or publication bias. A GRADE determination of very low/low quality suggests a large degree of uncertainty with regard to effect estimates, and future research may substantially change findings. A determination of moderate/high quality suggests that future research is unlikely to substantially change findings.
Results
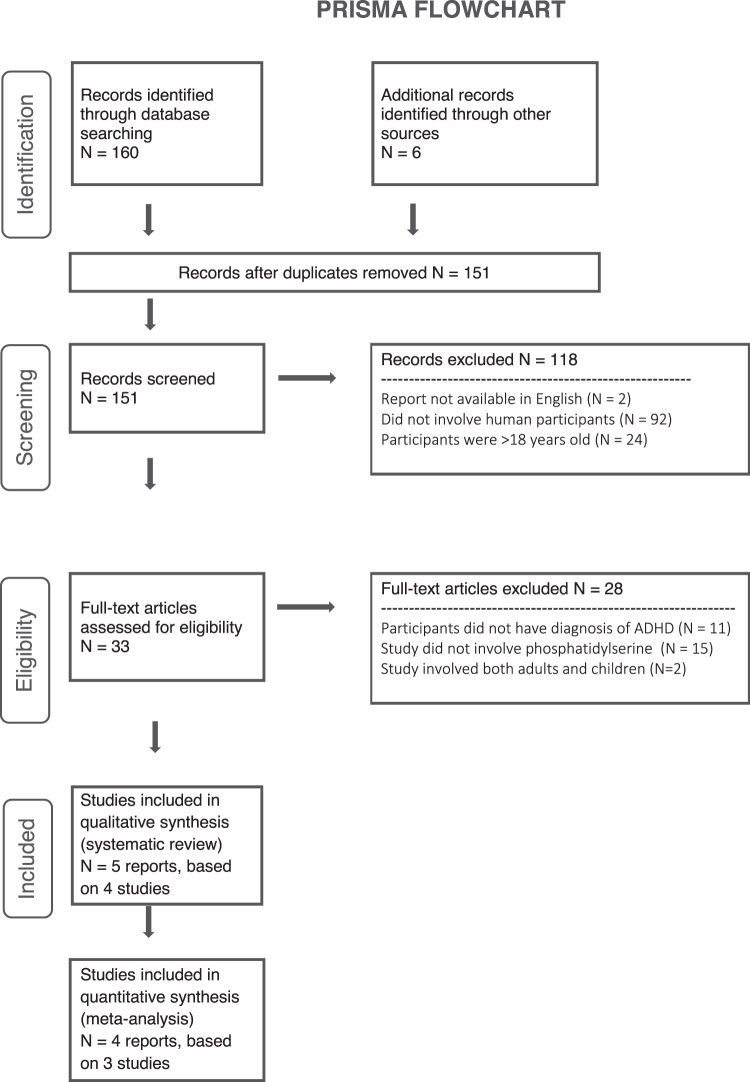
A total of 166 reports were identified via database searching and hand-searching the reference lists of primary studies (Fig. 1). After duplicates were removed, 151 reports remained. Of these, 92 were excluded for not involving human participants, and 24 were excluding for involving only adults. Two studies were excluded for not being available in English, although neither of them appeared to involve phosphatidylserine supplementation. Thirty-three full texts were assessed for eligibility, four studies met the inclusion criteria for the narrative review, and three RCTs were included in the meta-analysis. One study published efficacy and safety data in two separate reports.
FIG. 1.
Preferred Reporting Items for Systematic Review and Meta-analysis flowchart. ADHD, attention-deficit/hyperactivity disorder.
Characteristics of included studies
Study size varied from 21 to 200 participants; the combined total of participants was n = 344 (Table 1).35–39 Three studies were RCTs and one study was a pilot trial with no control group. Only the RCTs were included in the meta-analysis, with a combined sample size of n = 216. All studies required a diagnosis of ADHD by a qualified health care provider for inclusion, except for the pilot study, which included children with suspected, but not confirmed, ADHD.35 All studies excluded children currently using medication for ADHD, except the pilot study, which included several children on stimulant medication.
Table 1.
Characteristics of Included Studies
| Study | Study design | M/F | Dose of phosphatidylserine/day (brand) | Control | Duration of intervention (weeks) | Attrition % (proportion) | Objective outcome measure | Subjective outcome measure | Results | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hirayama and Masuday35 | Pilot study (n = 21, age 6–12 years) | 13/2a | 200 mg (Lipamin-PS 90) | None | 8 | 29% (6/21) | DTVP, short-term memory test, CPT | DSM-IV-Q, learning disorder questionnaire | Significant improvements in both inattention and hyperactivity (p < 0.01, p < 0.05) via DSM-IV-Q; improved mean number of correct answers to DTVP (p < 0.001); no significant results with memory test, CPT, or learning disorder questionnaire |
| 2 | Hirayama et al.36 | RCT (n = 40, age 4–14 years) | 34/2a | 200 mg, chewable | Chewable placebo matched in taste | 8 | Intervention: 5% (1/20) Control: 15% (3/20) | WISC-III DS, Go/No-Go | DSM-IV-TR-Q | Significant improvement in ADHD symptoms, assessed via DSM-IV-TR-Q, reported in intervention group over time (p = 0.01); auditory memory domain of the WISC-III improved in intervention group over time (p < 0.05); no significant change in mean response times on the Go/No-Go test, but intervention group had significant improvement in several domains over time (p < 0.05 for all domains). Between-group assessments not reported for DSM-IV-TR-Q and WISC-III |
| 3 | Manor et al.37 | RCT + open label (n = 200, age 6–13 years) | 133/67 | 300 mg plus 80 mg EPA and 40 mg DHA, encapsulated (PS-Omega-3) | Encapsulated cellulose | 15 | Intervention: 20% (27/133) Control: 16% (11/67) | None | CRS-T, CRS-P, SDQ,b CHQb | Per-protocol analysis found significant improvements in the restless/impulsive domain of the CRS-P (p = 0.047) in the intervention group compared with control; no significant changes on the CRS-T. A priori subgroup analysis revealed significant improvements on 4 domains of CRS-P (p = 0.020, p = 0.014, p = 0.027, p = 0.044) and 2 domains of the CRS-T (p = 0.036, p = 0.039) in intervention vs. control |
| 4 | Vaisman et al.39 | RCT with two placebo groups (n = 83, age 8–13 years) | 63/20 | 300 mg plus 156 mg EPA and 95 mg DHA, in an emulsified chocolate spread | Active control of fish oil with 153 mg EPA and 96 mg DHA; control of canola oil, both in emulsified chocolate spreads | 12 | Intervention: 40% (11/29) Active control: 25% (7/28) Control: 19% (5/26) | TOVA; blood lipid analysis | CRS-P, CBCb | Significant improvement in 3/4 domains of the TOVA in intervention group compared with control, as well as total TOVA score (p < 0.001, p = 0.046, p = 0.036 for domains; and p = 0.002 for total score) |
Gender of dropouts not reported.
Did not assess ADHD symptoms, will not be discussed.
ADHD, attention-deficit/hyperactivity disorder; CBC, Child Behavior Checklist-Hebrew Version; CHQ, Child Health Questionnaire; CPT, continuous performance test (computerized); CRS-P, Conners' Parent Rating Scale Revised Long-Hebrew Version; CRS-T, Conners' Teacher Rating Scale Revised Long-Hebrew Version; DHA, docosahexaenoic acid; DSM, Diagnostic and Statistical Manual; DSM-IV-Q, DSM-IV questionnaire; DSM-IV-TR-Q, DSM-IV, text revision, questionnaire; DTVP, Developmental Test of Visual Perception; EPA, eicosapentaenoic acid; PS, phosphatidylserine; RCT, randomized-controlled trial; SDQ, Strengths and Difficulties Questionnaire; TOVA, Test of Variables of Attention; WISC-III DS, Wechsler Intelligence Scale 3rd edition, digit span category.
The dosage of phosphatidylserine across studies varied from 200 to 300 mg/day and the duration of intervention ranged from 8 to 15 weeks. Phosphatidylserine formulations varied; some studies used commercial products (Lipamin-PS 9035 or PS-Omega-337), which were ingested as capsules. One study emulsified phosphatidylserine with a chocolate spread,39 while another used phosphatidylserine in a chocolate-flavored chewable tablet.36 Placebo controls included cellulose capsules,37 chewable chocolate tablets,36 and, in one study, canola oil as placebo.39 In addition to a placebo control, one study also used fish oil as an active comparator.39 Three studies reported attrition ranging from 20% to 40%.
Risk of bias within studies
Using the CROB tool, all three of the randomized studies were found to have substantial risk of bias (Table 2). Attempts to contact authors for clarification regarding the study methods in question were unsuccessful.36,37,39 The risk of bias tool only allows assessment of randomized trials, and so, the pilot study could not be assessed. It is considered at high risk of bias due to its design.
Table 2.
Risk-of-Bias Assessment for Randomized Clinical Trials
| Study | Random sequence generation | Allocation concealment | Blinding of participants/personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Hirayama et al.36 | High | Unclear | Low | High | Low | Unclear | Low |
| Manor et al.37 | Low | Low | Low | Low | Low | Low | Higha |
| Vaisman et al.39 | Low | Unclear | Low | Low | High | Low | Low |
Rated as high bias due to unplanned subgroup analysis.
Meta-analyses
Three meta-analyses were conducted for the following comparisons:
-
1.
phosphatidylserine versus placebo for overall ADHD symptomology,
-
2.
phosphatidylserine versus placebo for symptoms of hyperactivity/impulsivity, and
-
3.
phosphatidylserine versus placebo for symptoms of inattention. All three meta-analyses were conducted using three RCTs.
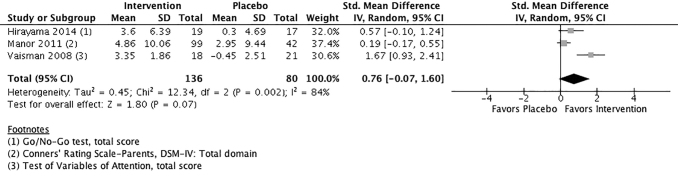
The effect of phosphatidylserine versus placebo for overall ADHD symptomology is presented in Figure 2; the effect size (ES) of phosphatidylserine supplementation is expressed as an SMD with a 95% confidence interval (CI). The pooled ES was 0.76 (95% CI: −0.07 to 1.60), suggesting that participants who received the intervention experienced a mean improvement in ADHD symptoms compared with participants who received placebo. The estimated intervention ES was of moderate magnitude, but not statistically significant (p = 0.07). An I2 = 84% implies substantial heterogeneity between studies, and pooled results should be interpreted with caution.
FIG. 2.
Phosphatidylserine versus placebo for overall ADHD symptomology. CI, confidence interval; DSM, Diagnostic and Statistical Manual; SD, standard deviation.
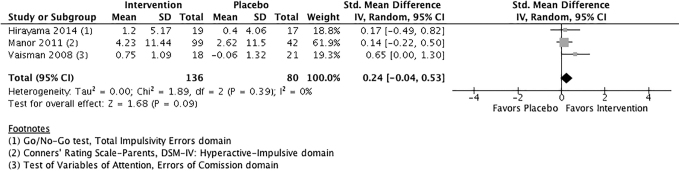
The effect of phosphatidylserine versus placebo for symptoms of hyperactivity/impulsivity is presented in Figure 3. The pooled ES was 0.24 (95% CI: −0.04 to 0.53), suggesting that phosphatidylserine may be more effective than placebo than reducing symptoms of hyperactivity/impulsivity, with a small ES. The pooled ES was not statistically significant (p = 0.09), however, although heterogeneity was 0%.
FIG. 3.
Phosphatidylserine versus placebo for symptoms of hyperactivity/impulsivity.
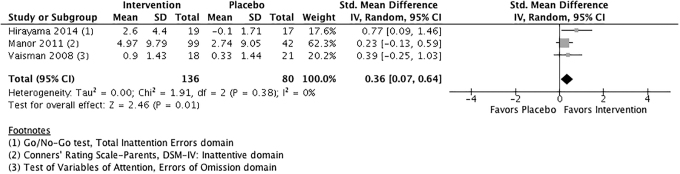
The effect of phosphatidylserine versus placebo for symptoms of inattention is presented in Figure 4. The pooled ES was 0.36 (95% CI: 0.07 to 0.64), suggesting that phosphatidylserine is more effective than placebo in reducing symptoms of inattention. The pooled effect is statistically significant (p = 0.01), and I2 = 0%, indicating very little heterogeneity among studies.
FIG. 4.
Phosphatidylserine versus placebo for symptoms of inattention.
Sensitivity analysis
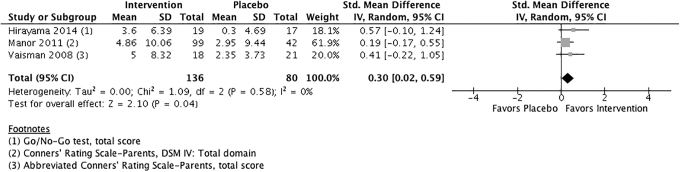
Because the heterogeneity for comparison 1 was so high (84%), the authors conducted an additional unplanned meta-analysis. To explore the potential impact of pooling objective and subjective outcome measures on heterogeneity, they replaced the objective, computerized measure of Test of Variables of Attention (TOVA) score in the Vaisman et al. study with the subjective measure of the abbreviated Conners' Rating Scale-Parent questionnaire (CRS-P) (Fig. 5).39
FIG. 5.
Phosphatidylserine versus placebo for overall ADHD symptomology using only noncomputerized outcome measures.
For the outcome of overall ADHD symptomology, this reduced heterogeneity to I2 = 0%. The pooled ES was 0.30 (95% CI: 0.02 to 0.59), suggesting that phosphatidylserine may have a small effect over placebo in reducing the overall symptoms of ADHD (p = 0.04). The authors were unable to conduct a similar sensitivity analysis for the outcomes of hyperactivity/impulsivity or inattention as Vaisman et al. reported only mean change in the total score of the CRS-P, and not individual symptom domains.
Synthesis of results
Using the GRADE criteria, the confidence in the moderate ES of phosphatidylserine to improve overall measures of ADHD symptomology was very low (Table 3).40 The quality of the evidence was downgraded for risk of bias, inconsistency, and imprecision. The unplanned meta-analysis using only subjective scales to assess total symptoms of ADHD was downgraded for risk of bias and imprecision, resulting in a low quality of evidence.
Table 3.
Grading of Recommendations Assessment, Development and Evaluation Tool for Assessing the Quality of the Evidence
| Certainty assessment |
No. of patients |
Effect |
Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Outcome | Phosphatidylserine | Placebo | Absolute (95% CI) | |
| 3 | Randomized trials | Seriousa | Seriousb | Not serious | Seriousc | Total symptoms of ADHD | 136 | 80 | SMD 0.76 SD higher (0.07 lower to 1.6 higher) | ⊕○○○ VERY LOW |
| 3 | Randomized trials | Seriousa | Not serious | Not serious | Seriousc | Total symptoms of ADHD, noncomputerized measures only | 136 | 80 | SMD 0.3 SD higher (0.02 higher to 0.59 higher) | ⊕⊕○○ LOW |
| 3 | Randomized trials | Seriousa | Not serious | Not serious | Seriousc | Hyperactivity/impulsivity | 136 | 80 | SMD 0.24 SD higher (0.04 lower to 0.53 higher) | ⊕⊕○○ LOW |
| 3 | Randomized trials | Seriousa | Not serious | Not serious | Seriousc | Inattention | 136 | 80 | SMD 0.36 SD higher (0.07 higher to 0.64 higher) | ⊕⊕○○ LOW |
Explanations:
a. Cochrane Risk-of-Bias tool found all three studies at high risk of bias.
b. Heterogeneity = 84%.
c. Total participant data included in meta-analysis <400.
CI, confidence interval; SD, standard deviation; SMD, standardized mean difference.
The quality of evidence for the analysis on symptoms of hyperactivity/impulsivity was found to be low due to risk of bias and imprecision. The quality of evidence for the analysis on symptoms of inattention was also found to be low due to risk of bias and imprecision. Low-quality evidence suggests that the estimated ES of phosphatidylserine on ADHD may not reflect the true ES, and future research may have different findings.
Discussion
The authors found three studies with very low- and low-quality evidence, mostly due to methodological limitations and heterogeneity between studies. Pending further studies, there is low-quality evidence that 200–300 mg/day of phosphatidylserine may have a small but statistically significant effect on symptoms of inattention, but not on symptoms of hyperactivity/impulsivity or overall ADHD symptoms. Wide variation in interventions, placebos, and outcome measures among included trials limited the utility of pooling findings across studies and the heterogeneity contributed to a downgrading of the evidence.
Placebo types varied substantially across studies. In one study, the control group was given canola oil, which contains omega-3 and omega-6 fatty acids, and can also contain trans-fats.39,41,42 These fats are not inert and may have interfered with the study results. Omega-6s and trans-fats can compete with omega-3s for inclusion in the cell membrane, potentially widening the gap between the intervention and placebo groups.43
Including omega-6s and trans-fats could have led to overestimation of the intervention effect, whereas including omega-3s in the placebo group could have resulted in an underestimation of the treatment effect. The complex interactions of these fats make it difficult to determine what their combined effect in this study could have been. For optimal transparency and accuracy, placebo ingredients must be both carefully chosen and reported to avoid interference with the intervention.
Outcome measures also varied across studies, which contributed to heterogeneity. The computerized TOVA used in Vaisman et al. resulted in substantially higher SMDs and skewed the forest plot.39 Manor et al.37 and Hirayama et al.36 both used parent-report and teacher-report scales. Parent- and teacher-report measures are subjective and are vulnerable to recall bias, and parents may not detect subtle changes in cognition and processing speed.
To fully capture changes in ADHD symptoms over time, future studies should use a variety of outcome measures, including proxy reports as well as objective noncomputerized and objective computerized tasks. The use of objective computerized tasks could result in more precise estimates of treatment effect. Hirayama et al.36 and the pilot study conducted by Hirayama and Masuday35 both used a Diagnostic and Statistical Manual symptom questionnaire as an outcome measure, which is not validated for this use. Future studies should use only measures validated for tracking symptom change in children with ADHD over time.
Rates of attrition were higher in the intervention group in two of three studies.37,39 Reasons for this difference could include higher adverse events in the intervention group, or that the intervention was less tolerable in some way; however, adverse event data were not provided in two studies, limiting the ability to assess reasons for attrition.36,39 Careful documentation of attrition in future studies, including differences in prognostic factors among those who left the study or were lost to follow-up, would allow readers to better assess the potential impact of selection bias.
These pooled results should be interpreted in the context of additional limitations of included studies. Because of the short duration of the interventions (≤15 weeks), the effect on ADHD symptoms over the long term cannot be determined. All three studies included in the meta-analysis performed per protocol (PP) analyses, one of them after performing an intention-to-treat (ITT) analysis, which yielded nonsignificant results.37 Studies that do not use ITT analysis can overestimate treatment effects when compared with those that do use ITT analysis.40 In addition, PP analysis can break randomization schemes and may lead to unbalanced prognostic factors across groups. In future studies, protocols should be published online to allow evaluation of selective outcome reporting, and should include details about planned analyses (PP, ITT, or subgroup analyses, with rationale).
Despite the limited quality of included studies, these results are consistent with other studies of omega-3 supplementation in children with ADHD. Six recent meta-analyses on omega-3 supplementation had mixed results, including one that found it to be effective in reducing symptoms of hyperactivity more than inattention compared with controls, although both were significantly reduced post-treatment.20 Another meta-analysis found improvements only in certain categories of symptoms, including emotional lability and oppositional behavior in a subgroup analysis.25 This subgroup analysis was not possible in this review because emotional lability and oppositional behavior were not evaluated in all studies.
A third meta-analysis found a similar effect on symptoms of inattention and hyperactivity and a fourth found reduction only following supplementation of both omega-3s and omega-6s.22,23 Two other meta-analyses found no difference in symptoms after omega-3 supplementation compared with placebo.21,24 Pooled SMD ESs in these six reviews ranged from 0.16 (95% CI: 0.01 to 0.31; p = 0.01)23 to 0.31 (95% CI: 0.16 to 0.47; p < 0.0001).22 Each meta-analysis included studies with various scales used to measure symptom improvement; scales varied widely, but the majority were subjective scales. Only one of the six meta-analyses mentioned that data from objective scales were included in the data analysis, and none mentioned using computerized scales such as the TOVA.23 ESs of 0.16–0.31 are consistent with this ES of 0.30 when using only noncomputerized scales in this analysis. These ESs were 0.24, 0.36, and 0.76 when including the computerized TOVA scale in the data analysis, suggesting that the computerized scale may capture symptom changes that subjective measures do not.
Stimulant medication, the standard of care for ADHD, has shown standardized ESs in children ranging from 0.56 to 1.02 depending on the specific medication used.44 This pooled ES for phosphatidylserine is smaller than this ES, but the fewer side effects associated with phosphatidylserine may make it an option for children who do not tolerate stimulants, or possibly as adjunctive therapy alongside stimulant medication.
A recent study examined an omega-3/6 supplement, methylphenidate (MPH, a stimulant medication), and both omega-3/6 and MPH in children with ADHD.45 The combined group required a reduced dose of MPH for symptom reduction [monotherapy, 1.0 mg/(kg·d); combination, 0.8 mg/(kg·d), p < 0.001]. Symptom reduction by group varied between the different scales used, and the mixed results did not suggest that the adjunctive therapy had a greater ES than MPH monotherapy. However, the combined group had fewer adverse events than the MPH group, including less appetite suppression, a common side effect of stimulant medication, and fewer adverse events overall (p = 0.009 and p = 0.001, respectively, for the difference between groups). The combined group had more responders, defined as >30% symptom reduction from baseline to month 12, than either monotherapy group (93% combined group; 80% MPH group; 60% omega-3/6 group; p = 0.008 for the difference between groups).
These results suggest that adjunctive therapy with omega-3/6s may increase response rates and decrease adverse events in children on stimulant medication, which may in turn increase medication adherence. Although no studies exist examining stimulant medication in conjunction with phosphatidylserine, this is an important area of future research, as it also may lead to reduced dosage of medication and fewer adverse events. A retrospective study on adjunctive phosphatidylserine supplementation in adults with ADHD (n = 518) reported that 31% of participants reduced the dose of their ADHD medication, further supporting this concept.46
Limitations of this review
This review was limited by the small number of studies that have been conducted, the small sample size of these studies, and the low quality of the evidence. Included studies did not stratify results by age and the authors were unable to evaluate the efficacy of the intervention in different age groups. Although they had planned subgroup analysis by medication status, all children in the randomized studies were not on medication, so this was not possible. A subgroup analysis by medication status may have yielded important information on the efficacy of phosphatidylserine used as adjunctive therapy alongside stimulant medication for ADHD.
Conclusion
This review provides preliminary low-quality evidence that phosphatidylserine supplementation could reduce symptoms of inattention in children. Due to the small number of studies included in this review and the heterogeneity of methods across studies, these estimates of effect are likely to change with future studies. Additional rigorous research is warranted to investigate phosphatidylserine as a low-cost and likely low-risk intervention for children with ADHD.
Acknowledgment
The authors are grateful to Noelle Stello, professional research librarian, for her assistance in designing the search strategy for this review.
Appendix
Appendix A1. Search Strategies
PubMed/Medline Search Strategy
-
1
attention defic* [tw]
-
2
attention disorder* [tw]
-
3
attention dysfunc* [tw]
-
4
hyperactiv* [tw]
-
5
hyper-activ* [tw]
-
6
hyperkin* [tw]
-
7
hyper-kin* [tw]
-
8
impulsiv* [tw]
-
9
inattentiv* [tw]
-
10
inattention* [tw]
-
11
minimal brain damage [tw]
-
12
minimal brain disorder [tw]
-
13
minimal brain dysfunction [tw]
-
14
adhd [tw]
-
15
addh [tw]
-
16
ad/hd [tw]
-
17
adhs [tw]
-
18
attention deficit disorder with hyperactivity [mh]
-
19
hkd [tw]
-
20
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19
-
21
Efalex* [tw]
-
22
PCSO-524* [tw]
-
23
phosphatidylserine [tw]
-
24
Vayarin* [tw]
-
25
Virtiva* [tw]
-
26
phosphatidylserines [mh]
-
27
Lyprinol* [tw]
-
28
21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27
-
29
20 AND 28
OVID/Medline Search Strategy
OVID/Medline search strategy was based on the PubMed/Medline search strategy, with only minor changes due to differences in syntax between the two user interfaces.
Cochrane Database Search Strategy
(attention defic* or attention disorder* or attention dysfunc* or hyperactiv* or hyper-activ* or impulsiv* or inattentiv* or inattention* or hyperkin* or hyper-kin* or minimal brain damage or minimal brain disorder or minimal brain dysfunction or add or adds or ADHD or HKD) and (Efalex*or PCSO-524* or phosphatidylserine* or Vayarin* or Virtiva* or Lyprinol*)
ClinicalTrials.gov Search Strategy
The ClinicalTrials.gov database did not have advanced search options; therefore, a simple search was performed with Boolean operators, as follows: (attention OR adhd) [DISEASE] AND (Phosphatidyl Serine OR phosphatidylserines) [TREATMENT]
Appendix A2. Data for Extraction
Data sought were listed in the protocol including the following: aim of the study, study design, study location, method of recruitment, inclusion criteria, exclusion criteria, total number randomized, baseline imbalances, withdrawal and exclusions, duration of study, mode of data collection, incentive for participation, intervention(s) of interest, definition of intervention, dosage of intervention, outcome(s) of interest, definition of outcome, measurement of outcome, type of placebo, covariates, definition of covariates, measurement of covariates, strengths of study, limitations of study, medication status, comorbidities, adverse events, attrition rate, funding source, and database the study was found in.
Authors' Contributions
A.B. conceptualized this review, and it was her master's thesis. The protocol was designed by A.B., J.N., M.G., and D.H. The literature search was performed by A.B. and J.N. Data analysis was performed by A.B., D.H., and A.S. A.B. drafted the article, and J.N., M.G., D.H., and A.S. all critically revised the work.
Ethical Approval
This review did not require approval from an institutional review board (IRB) because it only accessed data that were already published. During its design and implementation, the main author (A.B.) was a student at the National University of Natural Medicine and had oversight by its Scientific Review Committee and IRB.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this review.
References
- 1. American Psychiatric Association. DSM-V: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Association, 2013. [Google Scholar]
- 2. LeFever GB, Villers MS, Morrow AL, Vaughn ES. Parental perceptions of adverse educational outcomes among children diagnosed and treated for ADHD: A call for improved school/provider collaboration. Psychol Sch 2002;39:63–71. [Google Scholar]
- 3. Weiss G, Hechtman L, Perlman T, et al. . Hyperactives as young adults. Arch Gen Psychiatry 1979;36:675. [DOI] [PubMed] [Google Scholar]
- 4. Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up. Arch Gen Psychiatry 1985;42:937. [DOI] [PubMed] [Google Scholar]
- 5. Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. J Pediatr Psychol 2007;32:643–654. [DOI] [PubMed] [Google Scholar]
- 6. Nigg JT. Attention-deficit/hyperactivity disorder and adverse health outcomes. Clin Psychol Rev 2013;33:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruiz-Goikoetxea M, Cortese S, Aznarez-Sanado M, et al. . Risk of unintentional injuries in children and adolescents with ADHD and the impact of ADHD medications: A systematic review and meta-analysis. Neurosci Biobehav Rev 2018;84:63–71. [DOI] [PubMed] [Google Scholar]
- 8. Ruiz-Goikoetxea M, Cortese S, Magallón S, et al. . Risk of poisoning in children and adolescents with ADHD: A systematic review and meta-analysis. Sci Rep 2018;8:7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cuffe SP, Visser SN, Holbrook JR, et al. . ADHD and psychiatric comorbidity: Functional outcomes in a school-based sample of children. J Atten Disord 2020;24:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychol Med 2006;36:159–165. [DOI] [PubMed] [Google Scholar]
- 11. Murphy K, Barkley RA. Attention deficit hyperactivity disorder adults: Comorbidities and adaptive impairments. Compr Psychiatry 1996;37:393–401. [DOI] [PubMed] [Google Scholar]
- 12. García Murillo L, Ramos-Olazagasti MA, Mannuzza S, et al. . Childhood attention-deficit/hyperactivity disorder and homelessness: A 33-year follow-up study. J Am Acad Child Adolesc Psychiatry 2016;55:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention website. Data and Statistics: Attention-Deficit/Hyperactivity Disorder (ADHD). October 12, 2017. Online document at: https://www.cdc.gov/ncbddd/adhd/data.html, accessed November 2, 2017.
- 14. Wolraich ML, Hagan JF, Allan C, et al. . Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2019;144:e20192528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention website. Key findings: Treatment of attention-deficit/hyperactivity disorder (ADHD) among children with special health care needs. 2017. Online document at: https://www.cdc.gov/ncbddd/adhd/features/adhd-keyfindings-treatment-special-needs-children.html, accessed November 5, 2017.
- 16. Mueller AK, Fuermaier ABM, Koerts J, Tucha L. Stigma in attention deficit hyperactivity disorder. Atten Defic Hyperact Disord 2012;4:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spahis S, Vanasse M, Bélanger SA, et al. . Lipid profile, fatty acid composition and pro- and anti-oxidant status in pediatric patients with attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fat Acids 2008;79:47–53. [DOI] [PubMed] [Google Scholar]
- 18. Stanley JA, Kipp H, Greisenegger E, et al. . Regionally specific alterations in membrane phospholipids in children with ADHD: An in vivo 31P spectroscopy study. Psychiatry Res Neuroimaging 2006;148:217–221. [DOI] [PubMed] [Google Scholar]
- 19. Czerniak SM, Sikoglu EM, King JA, et al. . Areas of the brain modulated by single-dose methylphenidate treatment in youth with ADHD during task-based fMRI: A systematic review. Harv Rev Psychiatry 21:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hawkey E, Nigg JT. Omega-3 fatty acid and ADHD: Blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev 2014;34:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gillies D, Sinn JK, Lad SS, et al. . Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev 2012;7:CD007986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: Systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 2011;50:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sonuga-Barke EJS, Brandeis D, Cortese S, et al. Nonpharmacological interventions for ADHD: Systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry2013;170:275–289. [DOI] [PubMed] [Google Scholar]
- 24. Catalá-López F, Hutton B, Núñez-Beltrán A, et al. . The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. Gluud C, ed. PLoS One 2017;12:e0180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cooper RE, Tye C, Kuntsi J, et al. . The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behaviour and conduct problems in ADHD: A systematic review and meta-analysis. J Affect Disord 2016;190:474–482. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Shamseer L, Clarke M, et al. . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, et al. . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DistillerSR. Ottawa, Canada: Evidence Partners [computer program].
- 29. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- 30. Dersimonian R, Laird N. Meta-Analysis in Clinical Trials*. Online document at: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.331.2969&rep=rep1&type=pdf, accessed September 15, 2018.
- 31. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Higgins JPT, Altman DG, Gøtzsche PC, et al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guyatt GH, Oxman AD, Vist GE, et al. . GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirayama S, Masuday RR. Effect of phosphatidylserine administration on symptoms of attention-deficit/hyperactivity disorder in children. AgroFOOD Ind hi-tech 2006;17:32–36. [Google Scholar]
- 36. Hirayama S, Terasawa K, Rabeler R, et al. . The effect of phosphatidylserine administration on memory and symptoms of attention-deficit hyperactivity disorder: A randomised, double-blind, placebo-controlled clinical trial. J Hum Nutr Diet 2014;27(Suppl. 2):284–291. [DOI] [PubMed] [Google Scholar]
- 37. Manor I, Magen A, Keidar D, et al. . The effect of phosphatidylserine containing Omega3 fatty-acids on attention-deficit hyperactivity disorder symptoms in children: A double-blind placebo-controlled trial, followed by an open-label extension. Eur Psychiatry 2012;27:335–342. [DOI] [PubMed] [Google Scholar]
- 38. Manor I, Magen A, Keidar D, et al. . Safety of phosphatidylserine containing omega3 fatty acids in ADHD children: A double-blind placebo-controlled trial followed by an open-label extension. Eur Psychiatry 2013;28:386–391. [DOI] [PubMed] [Google Scholar]
- 39. Vaisman N, Kaysar N, Zaruk-Adasha Y, et al. . Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: Effect of dietary n-3 fatty acids containing phospholipids. Am J Clin Nutr 2008;87:1170–1180. [DOI] [PubMed] [Google Scholar]
- 40. McMaster University. Gradepro GDT: GRADEpro Guideline Development Tool. 2015. Online document at: https://gradepro.org, accessed October 20, 2018.
- 41. Azizian H, Kramer JKG. A rapid method for the quantification of fatty acids in fats and oils with emphasis on trans fatty acids using Fourier Transform near infrared spectroscopy (FT-NIR). Lipids 2005;40:855–867. [DOI] [PubMed] [Google Scholar]
- 42. Gunstone F. Vegetable Oils in Food and Technology: Composition, Properties and Uses. Oxford, 2002. Online document at: http://health120years.com/cn/pdf/hd_Vegetable.Oils.pdf, accessed January 9, 2020.
- 43. Ibarguren M, López DJ. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim Biophys Acta Biomembr 2014;1838:1518–1528. [DOI] [PubMed] [Google Scholar]
- 44. Cortese S, Adamo N, Del Giovane C, et al. . Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018;5:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barragán E, Breuer D, Döpfner M. Efficacy and safety of omega-3/6 fatty acids, methylphenidate, and a combined treatment in children with ADHD. J Atten Disord 2017;21:433–441. [DOI] [PubMed] [Google Scholar]
- 46. Ivanir E, Richter Y, Artzi G, et al. . Short & long-term impact of a nutritional approach for the management of ADHD: A real world study. Washington, D.C., USA: American Professional Society of Attention-Related Disorders (APSARD) Annual Meeting, 2016;75:2016. [Google Scholar]