Abstract
Background:
ATP-binding cassette membrane transporter G2 (ABCG2) gene is one of transporter family and well characterized for their association with chemoresistance. Promoter methylation is a mechanism for regulation of gene expression. O6-Methyl guanine DNA methyl transferase (MGMT) gene plays a fundamental role in DNA repair. MGMT has the ability to remove alkyl adducts from DNA at the O6 position of guanine. Alkylating agents exert their function through adding these alkyls adducts to DNA leading to cell death unless it is repaired by MGMT. MGMT promoter was found to be methylated in several malignancies. The aim of the present work is to study the relation of MGMT and ABCG2 promoter methylation status in advanced breast cancer patients to response to cyclophosphamide–doxorubicin (AC) based therapeutic regime.
Methods:
This retrospective study included Forty-two female patients with advanced breast cancer assessed before receiving chemotherapy and after the completion of regimens. They were grouped into responders and non-responders according to RECIST criteria. Methylation analysis of MGMT and ABCG2 genes were performed on breast cancer tissues.
Results:
MGMT promoter was methylated in 40.5% of the cases. ABCG2 promoter was methylated in 14.3% of cases. There was no statistically significant association between MGMT and ABCG2 promoter methylation status and clinicopathological parameters. There was statistically significant association between methylation status of both promoters and response to AC when followed by Taxane.
Conclusion:
Methylation of MGMT and ABCG2 promoters combined could be a potential predictive factor for response to cyclophosphamide-doxorubicin based therapeutic regime.
Key Words: ABCG2, Breast cancer, Chemoresistance, DNA methylation, MGMT
Introduction
Breast cancer is a complex and heterogeneous disease. Several factors have implications in prognosis and clinical management decisions (1). Although treatment modalities for breast cancer have evolved in the past few years, yet survival rates in advanced breast cancer are modest. Nonetheless, combining several therapeutic agents to overcome drug resistance and tumor heterogeneity did not achieve great results (2).
Epigenetic modification plays an important role in the pathogenesis of cancer (3,4) and responsiveness to therapeutic regimens (5). Cancer specific methylated loci referred to as “CpG island methylator phenotype” (CIMP) is one of the epigenetic modifications that has been suggested to characterize certain phenotypes within tumors (6). In breast cancer, CIMP has been identified in several studies (7,8).
MGMT is a direct DNA repair gene that is ubiquitously expressed. It is responsible for the removal of alkyl adducts from O6 position on guanine induced by chemotherapeutic alkylating agents (such as cyclophosphamide). Acrolein, a metabolite of cyclophosphamide, is believed to add alkyl adduct to O6 guanine causing cytotoxic antitumor effects (9). MGMT promoter methylation had been correlated with low expression of its protein and was found to have a predictive value for gliomas response to alkylating agent, tenozolomide (5).
ABCG2, an ATP binding cassette membrane transporter, or breast cancer resistance protein (BCRP) is responsible for the export of compounds outside the cell (10). Different molecules have been identified as substrates for it including: doxorubicin, 5’fluorouracil (5 Flu) (11) and paclitaxel (Taxane) (12). It is expressed in blood brain barrier, placenta and breast. Its over expression had been linked to chemoresistance due to decreased intracellular accumulation of drugs (13). Methylation of its promoter is linked to down expression of the transporter (14).
We hypothesized that methylation of MGMT would render the cells vulnerable to cyclophosphamide enhancing its cytotoxic action, while methylation of ABCG2 would lead to accumulation of doxorubicin / 5 Flu/ taxane intracellular enhancing their action.
Materials and Methods
Ethics approval and consent to participate
An informed written consent was taken from all subjects included in this study according to the rules approved by the ethical committee of the Medical Research Institute IORG #: IOR G0008812 and according to the Helsinki declaration (World medical declaration of Helsinki, 2014).
To test our hypothesis, we studied correlation of MGMT and ABCG2 promoters’ methylation status with advanced breast cancer patients’ response to cyclophosphamide –doxorubicin based therapeutic regimen.
After the approval of the Ethical Committee of the Medical Research Institute IORG#: IORG0008812 and according to the Helsinki declaration (World medical declaration of Helsinki, 2014), retrospective study was conducted in the Department of Cancer Management and Research, Alexandria University Alexandria, Egypt from the period of December 2014 to December 2017. 584 patients attended the clinic for evaluation: workup and treatment, about 20% of them fit the criteria of the study. Only 42 female patients with advanced breast cancer (stage III and IV), approved to the analysis of the breast tissue samples, were reachable, and were followed up completely by the department. Consents were taken from patients for samples analysis and data collection.
Clinical and radiological evaluations
Clinical and radiological evaluations were done to form a baseline for comparison after receiving therapy. Evaluation was repeated after 4 cycles of AC and 6 cycles of FAC (cyclophosphamide–doxorubicin-5FU) regimen to assess the response to treatment. Response was then reassessed after intake of paclitaxel (Taxane). Responses after AC and FAC were evaluated in 42 cases. As for Taxane response, only 28 cases were assessed as some cases did not receive the drug or response could not be reached. They were subgrouped according to response into responders: patients who achieved complete or partial response and non-responders: patients who had a stable or progressive disease course according to RECIST criteria version 1.1. (15). Patients concurrently receiving anti HER2 (Human epidermal growth factor receptr2) therapy was excluded from the study. Some patients undergone surgical operation prior to therapy and were not considered in the study as well.
Pathological examination to biopsies taken from patients
Core biopsies, excisional biopsies in formalin fixed paraffin embedded (FFPE) and fine needle aspiration-stained slides of breast tissue were included in the study. The biopsies undertook histopathological examination and graded according to Nottingham modification of the Bloom–Richardson system, (16) as well as immunohistochemistry for HER2 (17)/PR/ER (Progesterone and estrogen receptors) (18) for FFPE breast tissue. The patients are classified into three molecular subtypes: luminal A, B (Luminal), HER2 overexpressing and basal like (triple negative). Staging of patients was done at presentation according to the American Joint Committee on Cancer (AJCC) 8th manual of cancer staging (19).
Analysis of methylation status of promoters of MGMT and ABCG2 genes by methyl specific PCR (MSP)
DNA was extracted using QIAamp® DNA FFPE Tissue extraction kit. Samples concentration ranged from 5 ng/ μl to 560 ng/μl on nanodrop. Bisulfite conversion was then done using EpiTect® Fast Bisulfite Conversion kit. Methyl specific PCR (MSP) was performed using previously published primers (20,21) that were validated using online Bisearch software tool (http://bisearch.enzim.hu/?m=genompsearch), their sequence, target location and amplicon size are in Table 1. MyTaq Hot start red mastermix (bioline) was used for target amplification.
Table 1.
Primers sequences and amplified target’s location and size.
| Primer | Sequence 5’-3’ | Location | Amplicon size | |
|---|---|---|---|---|
| MGMT (M) | F | TTTCGACGTTCGTAGGTTTTCGC | 129467251- | 81 |
| R | GCACTCTTCCGAAAACGAAACG | 129467332 | ||
| MGMT (U) | F | TTTGTGTTTTGATGTTTGTAGGTTTTTGT | 129467245- | 93 |
| R | AACTCCACACTCTTCCAAAAACAAAACA | 129467338 | ||
| ABCG2 (M) | F | TGTCGCGTTGAGTCGTTA | 88159033- | 235 |
| R | AACGTCCCCGATACTTCG | 88159268 | ||
| ABCG2 (U) | F | TGTGTTTTGTTGTGTTGAGTTGT | 88159026- | 135 |
| R | TCACTCTAATTCATTCCATTCAATC | 88159161 |
The primers were validated using online Bisearch software tool (http://bisearch.enzim.hu/?m=genompsearch).
EpiTech® control, fully methylated and unmethylated bisulfite converted DNA controls (10 ng/ul) were used for testing the specificity of primers. They were also included in all PCR runs; methylated control PCR was done along with methylated PCR reactions and unmethylated control PCR was done along with unmethylated PCR reactions.
PCR conditions were set as follows: 3 minutes of initial DNA denaturation at 95 °C followed by 40-45 cycles of denaturation for 20 seconds (sec) at 95 °C, annealing: for rmethylated MGMT prime target 66 °C for 35 sec, unmethylated MGMT primer target 60 °C for 35 sec, methylated ABCG2 primer target 61 °C for 45 sec, unmethylated ABCG2 primer target 55 °C for 20 sec, all followed by extension for 30 sec at 72 °C. A final extension at 72 °C for 7 minutes was done for all runs.
For analysis of results, DNA extract and PCR products (Figs. 1 and 2), were run on agarose gel under electrophoresis. Positive and negative (Nuclease free water) controls were included in each PCR run. 10 μL of PCR product was applied on a 2% agarose gel containing 3 μL ethidium bromide along with a 50 – 1000 bp DNA ladder. Electrophoresis was done at 110 V for 41 minutes. Bands were detected using UV transilluminator.
Fig. 1.
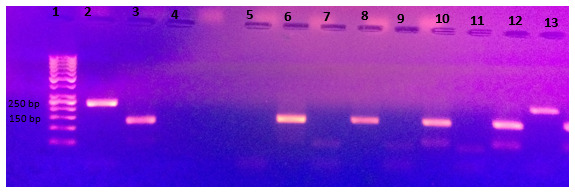
Shows amplified bisulfite converted DNA after MSP using methylated and unmethylated primers for ABCG2 on 2% agarose gel. Cases are run each methylated and unmethylated side by side. Lane 1: DNA ladder Lane 2: positive control, methylated converted DNA EpiTech control, showing target band at 235 bp. Lane 3: positive control, unmethylated converted DNA EpiTech control, showing target band at 135 bp. Lane 4: negative control for methylated primers, nuclease free water, showing no band. Lane 5: negative control for unmethylated primers, nuclease free water, showing no band. Lane 6: unmethylated band for case 1, Lane 7: no methylated band for case 1. Lane 12: unmethylated band at target location (at 135 bp) for case 4. Lane 13: methylated band at target location (at 235 bp) for case 4.
Fig. 2.
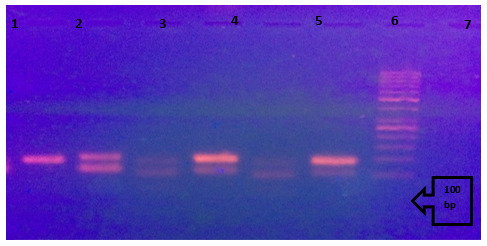
Shows amplified bisulfite converted DNA after MSP using methylated and unmethylated primers for MGMT on 2% agarose gel. Cases are run each methylated and unmethylated side by side. Lane 1: positive control, methylated converted DNA EpiTech control, showing target band at 81bp. Lane 2: positive control, unmetbylated converted DNA EpiTech control, showing target band at 93 bp. Lane 3: methylated band at target location (at 81 bp) for case 1. Lane 4: unmethylated band at target location (at 93 bp) for case 1. Lane 5: methylated band at target location (at 81 bp) for case 2. Lane 6: unmethylated band at target location (at 93 bp) for case 2. Lane 7: DNA ladder.
Statistical analysis of the data
Data was analyzed using IBM SPSS software package version 20.0. Comparison between different groups regarding categorical variables was tested using Chi-square test. When more than 20% of the cells have expected count less than 5, correction for chi-square was conducted using Fisher’s Exact. Monte carlo test was used for categories more than two. Logistic regression was done to test the independent predictive role for variables in response to therapy. Significant test results were quoted as two-tailed probabilities. Significance of the obtained results was judged at the 5% level (22).
Results
The present study is a retrospective cohort study conducted using data from patients ‘medical records. The study included 42 patients that were assessed for response to AC or FAC regimens and 28 were assessed for response to Taxane (20 received AC prior to Taxane and 8 received FAC). Age of the included cases at presentation ranged from 30-74 years with a mean age of 48.33±12.28 and median age of 48.
Distribution of clinicopathological data among the studied patients is shown in (Table 2)
Table 2.
Clinicopathological data among studied patients (n= 42).
| Variable | No (%) | |
|---|---|---|
| Stage | III | 27 (64.3) |
| IV | 15 (35.7) | |
| Grade | 2 | 28 (66.7) |
| 3 | 7 (16.7) | |
| Not assessed | 7 (16.7) | |
| Molecular subtypes | Luminal (A and B) | 31 73.8 |
| HER2 expressing | 4 (9.5) | |
| Triple negative | 7 (16.7) | |
| Promoter methylation | MGMT | 17 (40.5) |
| ABCG2 | 6 (14.3) | |
| Chemotherapy protocol | AC | 29 (69) |
| FAC | 13 (31) | |
| AC plus Taxane | 20 (71) | |
| FAC plus Taxane | 8 (29) | |
| Responders to regimens | AC (29 cases) | 14 (48) |
| FAC (13 cases) | 2 (15.4) | |
| AC followed by Taxane (20 cases) | 10 (50) | |
| FAC followed by Taxane (8 cases) | 2 (25) | |
Luminal subtypes were mostly of grade 2 tumors. Also, most patients with stage III at presentation had grade 2 tumors. However, no statistically significant association was present between grade of tumor and clinical stage of patients at presentation or molecular subtype.
MGMT promoter was found to be methylated in 40.5% (17/42) of the cases, while ABCG2 promoter was methylated in only 14.3% (6/42). No association was observed between age and grade, stage, or methylation status of MGMT or ABCG2 in our study.
MGMT was methylated in 35.5% (11/31) of luminal, 71.4% (5/7) of triple negative and 25% (1/4) of HER2 expressing breast cancer cases included in our study, but no statistically significant association between MGMT methylation and molecular subtypes could be detected as well. ABCG2 and MGMT methylation statuses were not associated with each other.
Association between response to chemotherapeutic regimens and age, tumor grade, molecular subtypes, or stage
No association was observed with age, tumor grade or subtype and response to different chemotherapeutic regimens.
On the other hands, Stage III was significantly associated with response to different therapeutic regimens, having 7 folds response rate than stage IV to AC/FAC therapy, OR 95% CI= 7 (1.32-37.15) (p= 0.014) and 8 folds response rate than stage IV to sequential AC/FAC- Taxane therapy, OR 95% CI= 8.3(1.34-51.67) (p= 0.015).
Association between response to chemotherapy regimens and methylation status of MGMT and ABCG2 promoters
Although unmethylated MGMT promoter was frequently observed among responding patients to sequential AC-Taxane the methylation status was not statistically associated with response (P=0.141). Moreover, the methylation status of MGMT was not statistically associated with response to other regimens (AC – FAC or AC /FAC - Taxane) (p= 0.889, p= 0.401 respectively).
ABCG2 showed marginal association with response to anthracyclin – cyclophosphamide- based regimens (AC and FAC) (p= 0.067), and when AC was followed by Taxane (p= 0.087), all responders had unmethylated ABCG2.
Association between response to chemotherapy regimens and methylation status of both MGMT and ABCG2 promoters
When we combined the methylation status for both genes (MGMT and ABCG2) and correlated it with response to different regimens, statistically significant association was obtained only between response to AC followed by Taxane regimen and unmethylation of both genes (p= 0.030) Table 3.
Table 3.
. Association between response to AC- Taxane and promoters methylation statuses of MGMT and ABCG2.
| Response to AC- Taxane | p value | MCp | |||||
|---|---|---|---|---|---|---|---|
| No response (n= 10) | Response (n= 10) | ||||||
| No. | % | No. | % | ||||
| MGMT and ABCG2 methylation | Both methylated | 2 | 20.0 | 0 | 0.0 | 8.000* | 0. 030* |
| Both unmethylated | 3 | 30.0 | 9 | 90.0 | |||
| MGMT methylated only | 3 | 30.0 | 1 | 10.0 | |||
| ABCG2 methylated only | 2 | 20.0 | 0 | 0.0 | |||
X2, p: X2 and p values for Chi square test for comparing between the two categories
MCp: p value for Monte Carlo for Chi square test for comparing between the two categories
: Statistically significant at p≤ 0.05
Multivariate analysis logistic regression for response to AC and FAC
Logistic regression analysis of multivariate (stage- methylation of MGMT promoter) in response to AC and FAC regimens shows only stage was found to be the most significant independent predictor for the response with Odds ratio (OR) of 6.96, 95% confidence interval (1.3-37.2).
Discussion
Breast cancer behavior remains diverse and current prognostic factors do not suffice the individualization of treatment and prediction of response and outcomes (23). Nevertheless, current chemotherapeutic regimens show modest rate of response in advanced breast cancer as reported by Nabholtz et al (24), in metastatic breast cancer patients receiving AC regimen, where only 47% of patients achieved response, complete and partial responses. FAC regimen did not achieve greater response in metastatic breast cancer patients either, with an ORR reaching 55% as reported in multicenter trial by Jassem et al (25). The relatively low rate of response was reflected in our study as well, where response rate ranged from 25 to 50% to different regimens.
Variability in response to chemotherapeutic regimens is attributed to several factors, among them is age. Young age is generally considered to have unfavorable prognosis in terms of overall survival (26), despite that Huober et al (27) in the GeparTrio study reported that younger age (below 40) has higher pathological response rate after anthracyclin-Taxane based neoadjuvant chemotherapy especially those with triple negative tumor. However, we did not find statistically significant association between age and response to any regimen/drug (AC-FAC-Taxane) alone or combined. Our data were in accordance with Liedtke et al (28).
It is worth mentioning that age of the included cases in our study ranged from 30-74 years at presentation. Interestingly, more than half (23/42) of the cases were diagnosed with advanced breast cancer at age below 50, which raises an alarm for intervention to be taken from healthcare communities to conduct awareness campaigns urging women to undergo early screening for breast cancer.
When we examined the predictive effect of grade and molecular subtypes on response to doxorubicin- cyclophosphamide based regimen, no association was observed between them and response to different regimens. In contrast, tumor grade 3 was found to have complete pathological response rate higher than lower grades in patients receiving anthracyclin-Taxane based neoadjuvant therapy in GeparTrio study (27). Such finding could be attributed to the high mitotic count in higher grade tumors rendering them more susceptible to chemotherapeutic drugs (16). Absence of association between grade and response to neoadjuvant therapy in our study might be limited by the small number of grade 3 tumors included.
Stage III in our study was significantly associated with response to different regimens/drugs this finding was in accordance with Goorts et al, who reported strong association between early stage and pathological complete response (pCR) to neoadjuvant chemotherapy in univariate and multivariate analysis with grade, hormonal and HER2 statuses as cofounders. They identified stage as the most important predictive factor for pCR (29). Moreover, early stages of breast cancer were associated with high overall survival rate and disease-free survival in several studies (26, 29, 30). Such data infer the need for spreading awareness for early screening of breast cancer for better outcomes in terms of disease course and response to therapy.
MGMT promoter was found to be methylated in 40.5% (17/42) of the cases. Methylation frequency of MGMT in other studies on breast cancer tissue were similar to our work; Sharma et al (32) found that MGMT was methylated in 32% of the cases and Asiaf et al (33) reported a similar MGMT promoter methylation frequency; 39.8%. Methylation of MGMT was also reportedly higher in breast cancer tissue than in their normal counterpart suggesting an important role for MGMT in carcinogenesis (34,35).
Although no statistically significant association between MGMT methylation and molecular subtypes could be detected in our study as previously reported (31). Fumagalli et al (36) reported a high rate of methylation of MGMT in TN cases 83.1%(74/89), which was in accordance with the high methylation rate observed in TN cases included in our study. Given the fact that TN tumors are also known for their high rate of BRCA mutations, it could be a distinct feature for this subtype to have frequent abnormal dysfunction of multiple repair genes (37).
Analysis of ABCG2 promoter revealed methylated in only 14.3% (6/42)of cases. ABCG2 methylation was not associated with age, tumor grade, stage, or molecular subtypes.
Evaluation of the predictive role of the methylation status of MGMT and ABCG2 promoters in patients receiving AC based regimens (AC-FAC-Sequential AC/Taxane- sequential FAC/Taxane) was conducted. Neither of them was associated with response to the regimens when examined alone. Our results for MGMT methylation alone in response to neoadjuvant therapy were consistent with Fumagalli et al study conducted on 84 TN breast cancer patients. They found no correlation between methylation of MGMT and pCR response to neoadjuvant chemotherapy including and not limited to cyclophosphamide, anthracyclins and Taxane (37). Unfortunately, we were not able to compare our results as regards ABCG2 methylation with response to anthracyclin – cyclophosphamide-based regimens in breast cancer due to paucity of published data about ABCG2 methylation especially in breast cancer.
On the other hand, when we analyzed the methylation status for both genes (MGMT and ABCG2) altogether in regards with the response to different regimens, statistically significant association was obtained between the response to Sequential AC - Taxane therapy and unmethylation of both genes. Although individual genes had no role in chemoresistance to the studied regimen their combined effect was detectable in the therapy. This might be attributed to lack of direct role in the given chemotherapeutic regimen’s pathways while the status of methylation per se is unfavorable as regards disease progression and response to certain regimens as AC- Taxane. We would recommend that larger sample size with more homogenous therapeutic regimen would be conducted to examine the predictive role of MGMT and ABCG2 methylation status to response to therapy as we acknowledge it is a limitation of our study.
Having said that, still, stage was the most independent predictor for response after applying multivariate analysis using logistic regression for stage and methylation in response to different therapeutic regimens. Again, this highlights the importance of early screening programs for breast cancer and how they should be conducted nationwide along with awareness campaigns emphasizing the great benefits of early detection of cancer.
Our work aimed at studying the relation of MGMT and ABCG2 promoters’ methylation status in advanced breast cancer patients to response to cyclophosphamide –doxorubicin based therapeutic regimen.
Although there was lack of significant association between the methylation status of each gene and the response to therapy when assessed separately, a significant association of unmethylation of both genes combined with response to AC followed by Taxane therapy was found. Such finding could indicate that methylation event of both genes together could be a potential bad predictive factor for response to AC/Taxane therapy.
Classical prognostic factors such as grade, age and molecular subtypes were not predictive of response to doxorubicin-cyclophosphamide based therapy
Stage is the most significant independent factor for prediction of response to anthracyclin-cyclophosphamide based therapy, which highlights the great need for raising awareness for early breast cancer detection.
Acknowledgements
Authors acknowledged all breast cancer patients enrolled in the study.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Vidal M, Paré L, Prat A. In: Molecular Classification of Breast Cancer. In: Management of Breast Diseases. 2nd ed . Jatoi I, Rody A (eds), editors. Switzerland: Springer International Publishing; 2016. pp. 203–20. [Google Scholar]
- 2.Di Leo A, Curigliano G, Diéras V, Malorni L, Sotiriou C, Swanton C, et al. New approaches for improving outcomes in breast cancer in Europe. Breast. 2015;24(4):321–30. doi: 10.1016/j.breast.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Klutstein M, Moss J, Kaplan T, Cedar H. Contribution of epigenetic mechanisms to variation in cancer risk among tissues. PNAS. 2017;114(9):2230–4. doi: 10.1073/pnas.1616556114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nourolahzadeh Z, Houshmand M, Mohammad FM, Ghorbian S. Correlation between Lsp1 (Rs3817198) and Casc (Rs4784227) Polymorphisms and the Susceptibility to Breast Cancer. Rep Biochem Mol Biol. 2020;9(3):291–296. doi: 10.29252/rbmb.9.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Yan W, Zhang S, Gu Y, Wang Y, Wei Y, et al. Survival differences of CIMP subtypes integrated with CNA information in human breast cancer. Oncotarget. 2017;8(30):48807–48819. doi: 10.18632/oncotarget.16178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang F, Turcan S, Rimner A, Kaufman A, Giri D, Morris LG, et al. Breast cancer methylomes establish an epigenomic foundation for metastasis. Sci Transl Med. 2011;3(75):75ra25. doi: 10.1126/scitranslmed.3001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roessler J, Ammerpohl O, Gutwein J, Steinemann D, Schlegelberger B, Weyer V, et al. The CpG island methylator phenotype in breast cancer is associated with the lobular subtype. Epigenomics. 2015;7(2):187–99. doi: 10.2217/epi.14.74. [DOI] [PubMed] [Google Scholar]
- 9.Friedman HS, Pegg AE, Johnson SP, Loktionova NA, Dolan ME, Modrich P, et al. Modulation of cyclophosphamide activity by O 6-alkylguanine-DNA alkyltransferase. Cancer Chemother Pharmacol. 1999;43(1):80–5. doi: 10.1007/s002800050866. [DOI] [PubMed] [Google Scholar]
- 10.Doyle L, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. 2003;22(47):7340–58. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 11.Mo W, Zhang JT. Human ABCG2: structure, function, and its role in multidrug resistance. Int J Biochem Mol Biol. 2012;3(1):1–27. [PMC free article] [PubMed] [Google Scholar]
- 12.Natarajan K, Xie Y, Baer MR, Ross DD. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem Pharmacol. 2012;83(8):1084–1103. doi: 10.1016/j.bcp.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan ST, Li ZL, He ZX, Qiu JX, Zhou SF. Molecular mechanisms for tumour resistance to chemotherapy. Clin Exp Pharmacol Physiol. 2016;43(8):723–37. doi: 10.1111/1440-1681.12581. [DOI] [PubMed] [Google Scholar]
- 14.Moon HH, Kim SH, Ku JL. Correlation between the promoter methylation status of ATP-binding cassette sub-family G member 2 and drug sensitivity in colorectal cancer cell lines. Oncol Rep. 2016;35(1):298–306. doi: 10.3892/or.2015.4342. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Rakha E, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12(4):207. doi: 10.1186/bcr2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J Clin Oncol. 2013;31:31, 3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 18.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J Oncol Pract. 2010;6(4):195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast Cancer-Major Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin. 2017;67(4):290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 20.Liu T, Xu F, Du X, Lai D, Liu T, Zhao Y, et al. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem. 2010;340(1-2):265–73. doi: 10.1007/s11010-010-0426-5. [DOI] [PubMed] [Google Scholar]
- 21.Uno M, Oba-Shinjo SM, Camargo AA, Moura RP, Aguiar PH, Cabrera HN, et al. Correlation of MGMT promoter methylation status with gene and protein expression levels in glioblastoma. Clinics (Sao Paulo). 2011;66(10):1747–1755. doi: 10.1590/S1807-59322011001000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field A. Discovery Statistics Using IBM SPSS Statistics. 4th Ed. London: SAGE Publications Ltd; 2013. [Google Scholar]
- 23.Soosanabadi M, Mirfakhraie R, Atanesyan L, Biglarian A, Moghadam FA, Rahimi M, et al. Application of Multiplex Ligation-Dependent Probe Amplification in Determining the Copy Number Alterations of HER Gene Family Membersin Invasive Ductal Breast Carcinoma. Rep Biochem Mol Biol. 2019;8(2):91–101. [PMC free article] [PubMed] [Google Scholar]
- 24.Nabholtz JM, Falkson C, Campos D, Szanto J, Martin M, Chan S, et al. Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol. 2003;21(6):968–75. doi: 10.1200/JCO.2003.04.040. [DOI] [PubMed] [Google Scholar]
- 25.Jassem J, Pieńkowski T, Płuzańska A, Jelic S, Gorbunova V, Mrsic-Krmpotic Z, et al. Doxorubicin and paclitaxel versus fluorouracil, doxorubicin, and cyclophosphamide as first-line therapy for women with metastatic breast cancer: final results of a randomized phase III multicenter trial. J Clin Oncol. 2001;19(6):1707–15. doi: 10.1200/JCO.2001.19.6.1707. [DOI] [PubMed] [Google Scholar]
- 26.Biganzoli L. Prognostic and predictive factors. . Cancer Treat Res. 2009;151:13–30. doi: 10.1007/978-0-387-75115-3_2. [DOI] [PubMed] [Google Scholar]
- 27.Huober J, von Minckwitz G, Denkert C, Tesch H, Weiss E, Zahm DM, et al. Effect of neoadjuvant anthracycline-Taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat. 2010;124(1):133–40. doi: 10.1007/s10549-010-1103-9. [DOI] [PubMed] [Google Scholar]
- 28.Liedtke C, Rody A, Gluz O, Baumann K, Beyer D, Kohls EB, et al. The prognostic impact of age in different molecular subtypes of breast cancer. Breast Cancer Res Treat. 2015;152(3):667–73. doi: 10.1007/s10549-015-3491-3. [DOI] [PubMed] [Google Scholar]
- 29.Goorts B, van Nijnatten TJ, de Munck L, Moossdorff M, Heuts EM, de Boer M, et al. Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2017;163(1):83–91. doi: 10.1007/s10549-017-4155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saadatmand S, Bretveld R, Siesling S, Tilanus-Linthorst MM. Influence of tumour stage at breast cancer detection on survival in modern times: population based study in 173,797 patients. BMJ. 2015;351:h4901. doi: 10.1136/bmj.h4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darwish AD, Helal AM, Aly El-Din NH, Solaiman LL, Amin A. Breast cancer in women aging 35 years old and younger: The Egyptian National Cancer Institute (NCI) experience. Breast. 2017;31:1–8. doi: 10.1016/j.breast.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Sharma G, Mirza S, Parshad R, Srivastava A, Gupta SD, et al. Clinical significance of promoter hypermethylation of DNA repair genes in tumor and serum DNA in invasive ductal breast carcinoma patients. Life Sci. 2010;87(3-4):83–91. doi: 10.1016/j.lfs.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Asiaf A, Ahmad ST, Malik AA, Aziz SA, Rasool Z, Masood A, et al. Protein expression and methylation of MGMT, a DNA repair gene and their correlation with clinicopathological parameters in invasive ductal carcinoma of the breast. Tumour Biol. 2015;36(8):6485–96. doi: 10.1007/s13277-015-3339-9. [DOI] [PubMed] [Google Scholar]
- 34.An N, Shi Y, Ye P, Pan Z, Long X. Association Between MGMT Promoter Methylation and Breast Cancer: a Meta-Analysis. Cell Physiol Biochem. 2017;42(6):2430–2440. doi: 10.1159/000480196. [DOI] [PubMed] [Google Scholar]
- 35.Chen R, Zheng Y, Zhuo L, Wang S. Association between MGMT Promoter Methylation and Risk of Breast and Gynecologic Cancers: A Systematic Review and Meta-Analysis. Sci Rep. 2017;7(1):12783. doi: 10.1038/s41598-017-13208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fumagalli C, Pruneri G, Possanzini P, Manzotti M, Barile M, Feroce I, et al. Methylation of O6-methylguanine-DNA methyltransferase (MGMT) promoter gene in triple-negative breast cancer patients. Breast Cancer Res Treat. 2012;134(1):131–7. doi: 10.1007/s10549-011-1945-9. [DOI] [PubMed] [Google Scholar]
- 37.Eliyatkın N, Yalç⌉n E, Zengel B, Aktaş S, Vardar E. Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to A New Age, and A New Way. J Breast Health. 2015;11(2):59–66. doi: 10.5152/tjbh.2015.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fumagalli C, Pasqua SD, Bagnardi V, Cardillo A, Sporchia A, Colleoni M, et al. Prevalence and Clinicopathologic Correlates of O6-Methylguanine-DNA Methyltransferase Methylation Status in Patients with Triple-Negative Breast Cancer Treated Preoperatively by Alkylating Drugs. Clin Breast Cancer. 2014;14(4):285–90. doi: 10.1016/j.clbc.2014.02.010. [DOI] [PubMed] [Google Scholar]


