Abstract
Background:
Prostate cancer is known as one of the most prevalent health disorders in the male population globally. The aim of the current study was to evaluate the effects of separate and concomitant use of MK-2206 and salinomycin on prostate cancer cell line.
Methods:
The antitumor potential of separate and concomitant use of MK-2206 and salinomycin was evaluated in a panel of prostate cancer cell line (PC-3). To get insights into the underlying mechanism of action, different assays including the rate of apoptosis, cell viability, and gene expression were performed in treated prostate cancer cells.
Results:
A significant reduction was detected in the viability percentage of prostate cancer cells (p< 0.001) and the rate of Akt expression (p< 0.001) in all salinomycin, MK-2206, and salinomycin+MK-2206 groups compared to the negative control group. Furthermore, in comparison with the negative control group, there was a notable increase in both the rate of Bad expression (p< 0.001) and prostate cancer cells apoptosis after salinomycin, MK-2206, and salinomycin+MK-2206 treatments. Moreover, the concomitant use of salinomycin+MK-2206 revealed synergistic improvements regarding the viability of prostate cancer cells and the rate of the Akt and Bad expressions compared to the separate administration of salinomycin and MK-2206 (all p< 0.05)
Conclusion:
The findings of the present study may contribute to improving the efficacy of the therapies regarding the management of prostate cancer and providing a beneficial strategy in clinical trials.
Key Words: Apoptosis, Gene Expression, MK 2206, Prostatic Neoplasms, Salinomycin
Introduction
Prostate cancer (PC) is the most frequent health disorder among men, representing one of the foremost causes of cancer-related morbidity and mortality worldwide (1). Several factors have been suggested to be involved in the pathogenesis of PC such as age, genetics, environmental toxins, hazardous chemicals, and radiation, but the exact mechanism remains unknown (2). Considering the severity of PC, it can be localized or progressive. Lymphatic system may play an important role in penetrating the metastasized version of this cancer into the bones (3, 4). Different treatment procedures including chemotherapy, radical prostatectomy, radiotherapy, and hormone therapy have been practiced providing recommendations for the management of this disease (5-7). Yet, the efficacy of all available treatment protocols is limited due to their major side effects such as erectile impairment, obesity, and the loss of bone mass density (8). Hence, finding natural alternatives with fewer adverse effects is necessary in order to overcome the negative health impacts which are associated with PC treatment (9).
The irregular activation of phosphatidylinositol 3 kinase (PI3K) has been confirmed as a critical condition in cancer onset and progression (10). Furthermore, Akt also known as protein kinase B (PKB), is a principal serine-threonine kinase downstream of PI3K which involved in promoting cell growth and preventing the apoptosis (11). MK-2206 is a small orally active allosteric Akt inhibitor which binds to Akt protein through a site in the second pleckstrin-homology domain leading to Akt deformation and preventing its localization to the plasma membrane (12). According to the previous studies, this novel compound has been reported as an effective anticancer agent in both adult and pediatric tumors (13, 14).
Salinomycin is a monocarboxylic ionophore antibiotic isolated from the bacterium Streptomyces albus (15). Also, there are convincing evidences regarding the antifungal, antiparasitic, antiviral and anti-inflammatory properties of this compound (15). Lately, the anticancer effect of salinomycin has been highlighted due to its potential in apoptosis induction and down-regulating the growth of various types of chemotherapeutic-resistant cancer cells (16). Moreover, it has been reported that treating cancer cells with high concentrations of salinomycin would reduce the total Akt protein activity in the body (17). Therefore, in the present study, it was hypothesized that the separate and concomitant use of MK-2206 and salinomycin might be helpful treatment procedures in PC. This study was approved by the Ethics Committee in Biomedical Research of Shahid Sadoughi University of Medical Sciences (IR.SSU.MEDICINE.REC.1399.204).
Materials and Methods
Cell line and culture
The human PC cell line (PC-3) was purchased from Pastor Institute (Tehran, Iran). These cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; INOCLON, LOT number: C1419005) accompanied respectively with 10% fetal bovine serum (FBS), 2 mM of L-glutamine, 100 U/ml of penicillin and 100 µg/ml of streptomycin (WelGENE) and maintained in a humidified incubator at 37 °C in 5% CO2. All experiments were performed in the logarithmic phase, in which the cells are actively proliferating, and the number of cells increases logarithmically.
Cell viability
In order to evaluate cell survival, PC-3 cells were seeded in a 96-well plate at the density of 3 × 104/well. After 24 h incubation, cells were treated with 1.13 μM salinomycin (Sigma-Aldrich Chemical Co., Cat. No. S4526) or 11 μM MK-2206 (Cayman Chemical Company, USA, Item No. 11593) or 10 μM compound C (as the positive control for the inhibition of PI3K/Akt signaling pathway; Sigma-Aldrich Chemical Co., Cat. No. 171260) or 7.8 μM salinomycin+MK-2206 for 72 h. Then, these cells were added with 0.5 mg/ml of the MTT solution (Sigma-Aldrich Chemical Co., LOT number: MKCL9866) and the precipitates were dissolved in dimethyl sulfoxide (DMSO, as the negative control; Lifebiolab, Germany, Cat. No. LB17067). A colorimetric analysis was implemented at 570 nm with an ELIZA Microplate Reader (Lx800, Biotech, Germany). Cell viability was assessed as relative percentage of the treated cells to untreated cells by comparing optical densities.
Apoptosis Assessment
To measuring apoptosis, the 4,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich Chemical Co., LOT number: 066M4053V) was employed to counterstain the nuclei. PC-3 cells were seeded in six-well plates and treated with 5 μM salinomycin or 10 μM MK-2206 or 12 μM compound C or 7 μM salinomycin+MK-2206 salinomycin for 24 h. Then, cells were washed three times with PBS (pH 7.4) and incubated for 5 minutes with 0.5 µg/mL DAPI staining. All images were observed under a fluorescence microscope (Carl Zeiss, Jena, Germany).
Total RNA isolation
The total RNA was extracted using RNX- Plus kit (SinaClon BioScience, Tehran, Iran, Cat. No. EX6101) based on the manufacturer’s instructions. Then, concentration and absorbance of the extracted RNA were measured at 260/280 nm by a nanodrop. Additionally, 28S, 18S, and 5S rRNAs bands were observed on 2% agarose gel, representing optimum extraction quality of RNA. Finally, the RNAs were stored in 0.2 ml sterile microtubes at -80 °C.
cDNA Synthesis
After the procedure of RNA extraction, the cDNA synthesis was done using Easy cDNA Synthesis Kit Parstous (Tehran, Iran) based on the manufacturer’s instructions. After normalization, the aliquots were stored at -80 °C until performing the real-time polymerase chain reaction (RT-PCR).
Real Time PCR (RT-PCR)
Real time PCR is known as the gold standard for gene expression assessment in terms of its high assay specificity, high detection sensitivity and extensive linear dynamic range (18). To analysis of gene expression, β-Actin was considered as the reference/housekeeping gene and the expression levels of target genes including Akt and pro-apoptotic protein Bad (BCL2 associated agonist of cell death) were assessed in comparison to this reference gene. It is worth mentioning that the housekeeping gene is expressed in all body cells and external variables have no effect on its expression rate (19). Primer Blast, Gene Runner and UCSC gene sites were selected for checking non-primer binding to the non-specific genomic locus or non-formation homodimer and heterodimer. Besides, the “Tm Calculate site” was used for checking the primers Tm. The primer sequence of β-Actin, Akt, and Bad genes were purchased from the BONmiR company (Stem Cell Technology, Iran) (Table 1). In the present study, PCR procedure was done using an Opticon I thermal cycler (MJ Research PTC-200) with YTA SYBR Green qPCR MasterMix 2X Kit (Yektatajhiz, Tehran, Iran, Cat No. YT2551). Thermal cycling settings were as follows: 95 °C for 15 min, followed by 40 cycles of 95 °C for 15 s, 55 °C for 15 s, and 72 °C for 30 s. Also, a final extension of 72 °C for 5 min was performed. All PCR reactions were done in duplicate. Reproducibility of SYBR Green real-time PCR was assessed by running samples independently on different days.
Table 1.
Primer sequences of AKT, Bad, and β-Actin.
| Gene name | Forward primer (5′→3′) | Reverse primer (5′→3′) | Product size (bp) |
|---|---|---|---|
| AKT | AGCGACGTGGCTATTGTGAAG | GCCATCATTCTTGAGGAGGAAGT | 96 |
| Bad | CCCAGAGTTTGAGCCGAGTG | CCCATCCCTTCGTCGTCCT | 249 |
| β-Actin | CGCGAGAAGATGACCCAGATC | GATAGCACAGCCTGGATAGCAAC | 77 |
Statistical analysis
The Statistical Package for Social Sciences (version 22.0; SPSS Inc) was used for statistical analyses. One-way ANOVA, followed by Tukey's multiple range post-hoc tests were used to compare the study quantitative variables. P-values< 0.05 were statistically significant.
Results
The viability of PC cells
The effects of treatments on the viability of PC cells have been shown in (Fig. 1). The total amount of PC cells viability was significantly decreased in cells treated by compound C compared to the negative control (p< 0.001). Also, there was a significant reduction in the percentage of cell viability in salinomycin, MK-2206, and salinomycin+MK-2206 treated cells when compared to those in the negative control group (all p< 0.001). Although salinomycin and MK-2206 showed significant differences in comparison with the compound C (p= 0.03, p= 0.03, respectively), there was no statistically significant difference detected between the salinomycin+Mk-2206 group and the compound C in terms of the cell viability (p= 0.07). Furthermore, the concomitant use of salinomycin+MK-2206 showed further decrease in cancer cells viability in comparison with the separate administration of salinomycin and MK-2206 (p< 0.05).
Fig. 1.
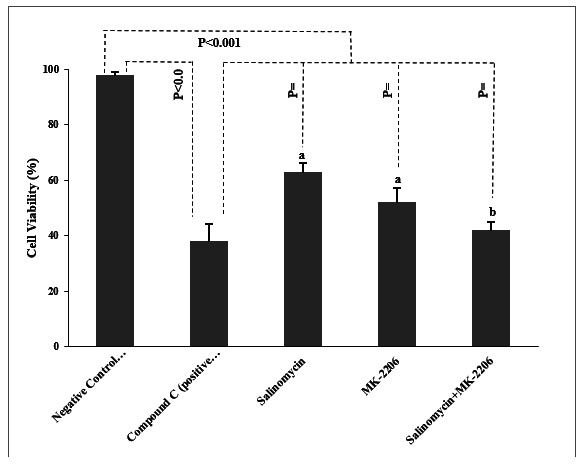
The viability percentage of prostate cancer cells. The viability percentage of prostate cancer cells Data presented as Mean±SD. *P-values obtained from the One-way ANOVA, followed by Tukey's multiple range post-hoc tests. p-value< 0.05 was considered as significant. Graph columns bearing different superscripts are significantly different at p-value< 0.05.
Apoptosis assessment
According to the macroscopic results of DAPI-stained PC-3 cells, salinomycin, MK- 2206, and salinomycin+MK-2206 inhibited the proliferation and induced apoptosis in the study cell line (Fig. 2).
Fig. 2.
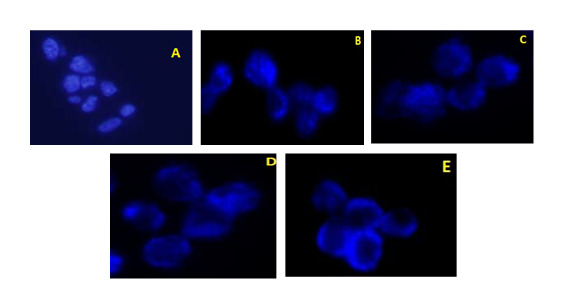
Macroscopic images of DAPI-stained prostate cancer cells. Macroscopic images of DAPI-stained prostate cancer cells. A) Negative control, B) Compound C treated cells, C) Salinomycin treated cells, D) MK-2206 treated cells, E) Salinomycin+MK-2206 treated cells.
Gene Expression The expression of Akt
The results of the study treatments regarding the expression of Akt in PC-3 cells have been presented in (Fig. 3).
Fig. 3.
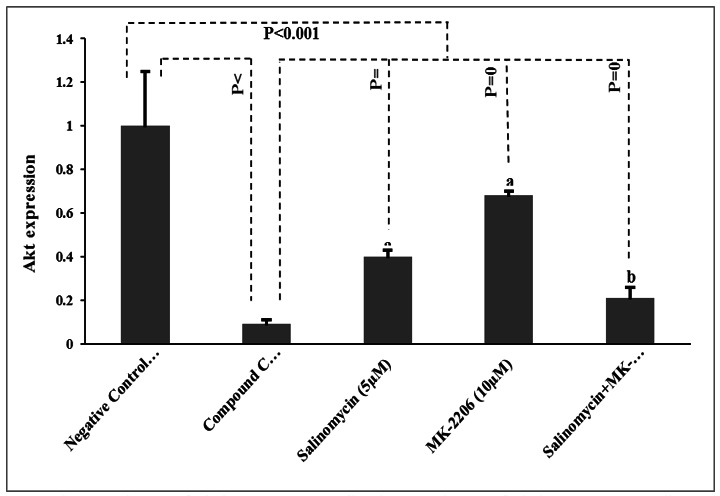
The expression rate of Akt in prostate cancer cells. The expression rate of Akt in prostate cancer cells. Data presented as Mean±SD. P-values obtained from the One-way ANOVA, followed by Tukey's multiple range post-hoc tests. P-value< 0.05 was considered as significant. Graph columns bearing different superscripts are significantly different at p-value< 0.05.
By the end of the study, lower expression of Akt was detected in the positive control group compared to the negative control group (p< 0.001). Likewise, there was a significant decrease regarding the rate of Akt expression in salinomycin, MK-2206, and salinomycin+MK-2206 groups when compared to the negative control group (p< 0.001). Meanwhile, salinomycin, MK-2206, and salinomycin+MK-2206 treatments revealed significant differences compared to the compound C (p= 0.03, p= 0.009, and p= 0.04, respectively). Moreover, the reduction in Akt expression was notably higher in the salinomycin+MK-2206 treated cells in comparison with salinomycin and MK-2206 (both p< 0.05).
3.3.1. The expression of Bad
The findings of the study treatments relating to the expression of Bad in PC-3 cells have been shown in (Fig. 4).
Fig. 4.
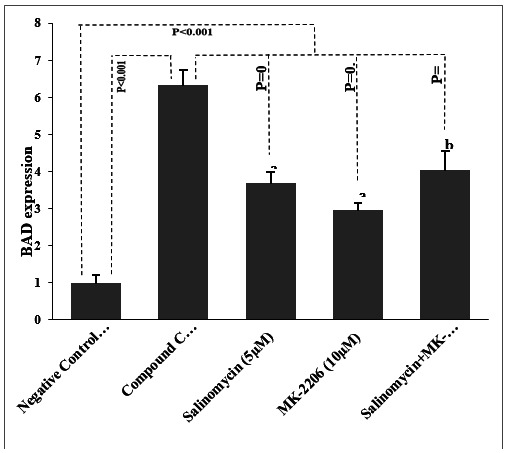
The expression rate of Bad in prostate cancer cells. The expression rate of Bad in prostate cancer cells. Data presented as Mean±SD. P-values obtained from the One-way ANOVA, followed by Tukey's multiple range post-hoc tests. P-value< 0.05 was considered as significant. Graph columns bearing different superscripts are significantly different at p-value< 0.05.
The higher expression of Bad was found in the positive control group in comparison with the negative control group (p< 0.001). As well, a significant enhancement was observed regarding the rate of Bad expression in salinomycin, MK-2206, and salinomycin + MK-2206 groups when compared to the negative control group (p< 0.001). Besides, compared to the compound C, salinomycin, MK-2206, and salinomycin+MK-2206 showed significant differences associated with the Bad expression progression (p= 0.008, p= 0.002, and p= 0.01, respectively). In addition, the up regulation in Bad expression was notably higher in the salinomycin+MK-2206 treated cells compared to salinomycin and MK-2206 (both p< 0.05).
Discussion
To the best of our knowledge, this study was the first effort to investigate the effects of separate and concomitant use of MK-2206 and salinomycin on PC cell line. The results of the current study revealed that single and concomitant use of MK-2206 and salinomycin caused viability reduction, improved apoptosis, higher expression of the Bad together with lower expression of the Akt in PC-3 cell line.
It has been documented that abnormal activation of the PI3K/Akt signaling pathway may play as the survival mechanism of cancer cells due to apoptosis inhibition through phosphorylation of the pro-apoptotic protein Bad (20). Therefore, it would not be unexpected if cancer cells decrease Akt and Bad expression as the crucial characters of apoptosis regulation (21).
Consistent with the results of the present study, Choi et al. (17) described lower total Akt protein levels after salinomycin and MK-2206 treatment in breast cancer cells. Also, the researchers suggested that co-treatment with salinomycin and MK-2206 increased apoptosis of the cancer cells by reducing total Akt expression. There are convincing reports regarding the synergistic effect of MK-2206 with several therapeutic drugs through enhancement of apoptotic cell death and autophagy (22-24). In one study by Hirai et al. (10), they have indicated that MK-2206 suppressed the Akt activation procedure in human cancer cell lines. Furthermore, MK-2206 synergistically inhibited Akt pathway in combination with cytotoxic drugs such as topoisomerase inhibitors, antimetabolites, anti-microtubule agents, and DNA cross-linkers in lung NCI-H460 or ovarian A2780 tumor cells. In one study, Sangai et al. (11) also documented the Akt signaling and cell-cycle progression inhibition in a dose-dependent manner of MK-2206 treatment in breast cancer cell lines. In 2014, Neri et al. (25) found that a combination of MK-2206 with one more mTOR inhibitor (RAD001) induced a caspase-dependent apoptosis in B-precursor acute lymphoblastic leukemia cells. The researchers described this synergistic effect through the extrinsic and intrinsic molecular pathways which involved in increase cleaved caspase-8, caspase-9, and poly adenosine diphosphate-ribose polymerase (PARP). Similarly, in 2013, Parajuli et al. (26) showed a remarkable reduction in cisplatin resistant ovarian cancer cell viability in a dose dependent manner. The investigators also found salinomycin as an inhibitor factor for the Akt and Bad expression, which are all in agreement with our results. Moreover, Kim et al reported that concomitant use of salinomycin with other PI3K inhibitors may result in higher apoptosis in comparison with single treatment (27). It has also been documented that salinomycin may decrease oxidative stress and cell migration as well as downregulating the expression of MYC, AR and ERG in PC cells (28). Although the exact action mechanism of salinomycin is still uncertain, different experiments have suggested that salinomycin may prevent cancer stem cell proliferation as a result of the autophagy activation and interfering with Wnt/β-catenin signaling pathway which is involved in the mainstenance of progenitor cells in numerous cancer cells (29, 30).
Our findings should be interpreted while considering the main limitations. As a result of limited funding, the comparative effects of other important cytotoxic medications including doxorubicin, gemcitabine, docetaxel, and carboplatin were not evaluated. Therefore, future well-designed studies are needed to simultaneously evaluate other specific agents which may clarify the beneficial underlying mechanisms related to the effects of MK-2260 and salinomycin on PC-3 cells.
The findings of the current study may contribute to improving the efficacy of the therapies regarding the PC management and also providing a beneficial strategy in clinical trials.
Acknowledgements
The authors would like to thank the department of clinical biochemistry, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran.
The authors report no conflict of interest.
References
- 1.Barani M, Sabir F, Rahdar A, Arshad R, Kyzas GZ. Nanotreatment and nanodiagnosis of prostate cancer: recent updates. . Nanomaterials (Basel). 2020;10(9):1696. doi: 10.3390/nano10091696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujita K, Hayashi T, Matsushita M, Uemura M, Nonomura N. Obesity, inflammation, and prostate cancer. J Clin Med. 2019;8(2):201. doi: 10.3390/jcm8020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Huang V, Xu X, Livingstone J, Soares F, Jeon J, et al. Widespread and functional RNA circularization in localized prostate cancer. . Cell. 2019;176(4):831–843. doi: 10.1016/j.cell.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Mei W, Lin X, Kapoor A, Gu Y, Zhao K, Tang D. The contributions of prostate cancer stem cells in prostate cancer initiation and metastasis. . Cancers (Basel). . 2019;11(4):434. doi: 10.3390/cancers11040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-Gracia JL, Gúrpide A, de Fata Chillón F, Villacampa F. The role of chemotherapy in the treatment of hormone sensitive metastatic prostate cancer. . Arch Esp Urol. . 2018;71(3):276–280. [PubMed] [Google Scholar]
- 6.Dyrstad SW, Shah P, Rao K. Chemotherapy for prostate cancer. . Current pharmaceutical design. . 2006;12(7):819–37. doi: 10.2174/138161206776056100. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo A, Mollica V, Cimadamore A, Santoni M, Scarpelli M, Giunchi F, et al . Is there a role for immunotherapy in prostate cancer? . Cells. . 2020;9(9):2051. doi: 10.3390/cells9092051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oun R, Moussa YE, Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. . 2018;47(19):6645–6653. doi: 10.1039/c8dt00838h. [DOI] [PubMed] [Google Scholar]
- 9.Lawania RD, Mishra A. Anticancer potential of plants and natural products: a review. . Journal of Diagnostic Techniques and Biomedical Analysis. . 2017;2013 [Google Scholar]
- 10.Hirai H, Hirai H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. . Mol Cancer Ther. . 2010;9(7):1956–67. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 11.Sangai T, Akcakanat A, Chen H, Tarco E, Wu Y, Do K-A, et al. Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. . Clin Cancer Res. . 2012;18(20):5816–28. doi: 10.1158/1078-0432.CCR-12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y, Gong W, Hua Z, Chen B, Zhao G, Liu Z , et al. Combination of Rapamycin and MK-2206 induced cell death via autophagy and Necroptosis in MYCN-amplified neuroblastoma cell lines. . Front Pharmacol. . 2020;11:31. doi: 10.3389/fphar.2020.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djuzenova CS, Fiedler V, Memmel S, Katzer A, Sisario D, Brosch PK, et al. Differential effects of the Akt inhibitor MK-2206 on migration and radiation sensitivity of glioblastoma cells. . BMC cancer. 2019;19(1):1–18. doi: 10.1186/s12885-019-5517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorlick R, Maris JM, Houghton PJ, Lock R, Carol H, Kurmasheva RT, et al. Testing of the Akt/PKB inhibitor MK-2206 by the pediatric preclinical testing program. Pediatric blood & cancer. . 2012;59(3):518–24. doi: 10.1002/pbc.23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Yang Y, Li S, Meng P. Salinomycin triggers prostate cancer cell apoptosis by inducing oxidative and endoplasmic reticulum stress via suppressing Nrf2 signaling. Exp Ther Med. 2021;22(3):946. doi: 10.3892/etm.2021.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K-Y, Yu S-N, Lee S-Y, Chun S-S, Choi Y-L, Park Y-M, et al. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. . Biochemical and biophysical research communications. . 2011;413(1):80–86. doi: 10.1016/j.bbrc.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 17.Choi A-R, Kim J-H, Yoon S. Sensitization of cancer cells through reduction of total Akt and downregulation of salinomycin-induced pAkt, pGSk3β, pTSC2, and p4EBP1 by cotreatment with MK-2206. . BioMed research international. . 2014;2014 doi: 10.1155/2014/295760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arikawa E, Sun Y, Wang J, Zhou Q, Ning B, Dial SL, et al. Cross-platform comparison of SYBR® Green real-time PCR with TaqMan PCR, microarrays and other gene expression measurement technologies evaluated in the MicroArray Quality Control (MAQC) study. . BMC genomics. . 2008;9(328) doi: 10.1186/1471-2164-9-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nozari E, Moradi A, Samadi M. Effect of Atorvastatin, Curcumin, and Quercetin on miR-21 and miR-122 and their correlation with TGFβ1 expression in experimental liver fibrosis. . Life Sci. . 2020;259:118293. doi: 10.1016/j.lfs.2020.118293. [DOI] [PubMed] [Google Scholar]
- 20.Al-Bazz YO, Underwood JC, Brown BL, Dobson PR. Prognostic significance of Akt, phospho-Akt and BAD expression in primary breast cancer. . Eur J Cancer. . 2009;45(4):694–704. doi: 10.1016/j.ejca.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Smith AJ, Karpova Y, D'Agostino Jr R, Willingham M, Kulik G. Expression of the Bcl-2 protein BAD promotes prostate cancer growth. . PLoS One. . 2009;4(7):e6224. doi: 10.1371/journal.pone.0006224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balasis ME, Forinash KD, Chen YA, Fulp WJ, Coppola D, Hamilton AD, et al. Combination of farnesyltransferase and Akt inhibitors is synergistic in breast cancer cells and causes significant breast tumor regression in ErbB2 transgenic mice. . Clin Cancer Res. 2011;17(9):2852–2862. doi: 10.1158/1078-0432.CCR-10-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y, Zhang Y, Zhang L, Ren X, Huber-Keener KJ, Liu X, et al. MK-2206, a novel allosteric inhibitor of Akt, synergizes with gefitinib against malignant glioma via modulating both autophagy and apoptosis. Mol Cancer Ther. 2012;11(1):154–64. doi: 10.1158/1535-7163.MCT-11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen K-F, Chen H-L, Tai W-T, Feng W-C, Hsu C-H, Chen P-J, et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. . J Pharmacol Exp Ther. . 2011;337(1):155–61. doi: 10.1124/jpet.110.175786. [DOI] [PubMed] [Google Scholar]
- 25.Neri LM, Cani A, Martelli A, Simioni C, Junghanss C, Tabellini G, et al. Targeting the PI3K/Akt/mTOR signaling pathway in B-precursor acute lymphoblastic leukemia and its therapeutic potential. . Leukemia. 2014;28(4):739–48. doi: 10.1038/leu.2013.226. [DOI] [PubMed] [Google Scholar]
- 26.Parajuli B, Lee H-G, Kwon S-H, Cha S-D, Shin S-J, Lee G-H, et al. Salinomycin inhibits Akt/NF-κB and induces apoptosis in cisplatin resistant ovarian cancer cells. . Cancer Epidemiol. . 2013;37(4):512–7. doi: 10.1016/j.canep.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Kim J-H, Choi A-R, Kim YK, Kim HS, Yoon S. Low amount of salinomycin greatly increases Akt activation, but reduces activated p70S6K levels. . Int J Mol Sci. 2013;14(9):17304–18. doi: 10.3390/ijms140917304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ketola K, Hilvo M, Hyötyläinen T, Vuoristo A, Ruskeepää A-L, Orešič M, et al. Salinomycin inhibits prostate cancer growth and migration via induction of oxidative stress. . British journal of cancer. . 2012;106(1):99–106. doi: 10.1038/bjc.2011.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, et al. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. . Cell death & disease. 2014;5(1):e1039–e. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li T, Su L, Zhong N, Hao X, Zhong D, Singhal S, et al. Salinomycin induces cell death with autophagy through activation of endoplasmic reticulum stress in human cancer cells. . Autophagy. . 2013;9(7):1057–68. doi: 10.4161/auto.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]


