Abstract
Study design
Cohort study.
Objectives
To investigate and critique different methods for aerobic exercise intensity prescription in adults with spinal cord injury (SCI).
Setting
University laboratory in Loughborough, UK.
Methods
Trained athletes were split into those with paraplegia (PARA; n = 47), tetraplegia (TETRA; n = 20) or alternate health condition (NON-SCI; n = 67). Participants completed a submaximal step test with 3 min stages, followed by graded exercise test to exhaustion. Handcycling, arm crank ergometry or wheelchair propulsion were performed depending on the sport of the participant. Oxygen uptake (V̇O2), heart rate (HR), blood lactate concentration ([BLa]) and ratings of perceived exertion (RPE) on Borg’s RPE scale were measured throughout. Lactate thresholds were identified according to log-V̇O2 plotted against log-[BLa] (LT1) and 1.5 mmol L−1 greater than LT1 (LT2). These were used to demarcate moderate (<LT1), heavy (>LT1, < LT2) and severe (>LT2) exercise intensity domains.
Results
Associations between percentage of peak V̇O2 (%V̇O2peak) and HR (%HRpeak) with RPE differed between PARA and TETRA. At LT1 and LT2, %V̇O2peak and %HRpeak were significantly greater in TETRA compared to PARA and NON-SCI (P < 0.05). The variation in %V̇O2peak and %HRpeak at lactate thresholds resulted in large variability in the domain distribution at fixed %V̇O2peak and %HRpeak.
Conclusions
Fixed %V̇O2peak and %HRpeak should not be used for aerobic exercise intensity prescription in adults with SCI as the method does not lead to uniform exercise intensity domain distribution.
Subject terms: Physiology, Health occupations
Introduction
For adults with spinal cord injury (SCI), aerobic exercise is beneficial for improving indices of physical [1] and mental [2] health. On this theme, scientific guidelines published in 2018 describe the dose of aerobic exercise required to improve cardiorespiratory fitness and cardiometabolic health in adults with SCI [3]. Central to the guidelines is information on the frequency (e.g., 3 times per week) and duration (e.g., 30 min) of the exercise, both of which are simple to define and monitor. The final important aspect of the guidelines is the exercise intensity. If aerobic exercise is performed at too low an intensity, without sufficient exercise volume, it will not lead to beneficial physiological adaptations [4]. Despite this, the guidelines provide no clear prescription of the exercise intensity, other than to say that aerobic exercise should be of a “moderate to vigorous” intensity [3]. The lack of clarity with the exercise intensity terminology is a hindrance to adults with SCI using the guidelines to inform their exercise habits; practitioners actively prescribing exercise training; and researchers investigating the effects of exercise training interventions on markers of health in adults with SCI. There is, therefore, an urgent need to better understand aerobic exercise intensity prescription in adults with SCI.
Guidelines for non-disabled adults define thresholds for five intensity zones (very light, light, moderate, vigorous, near-maximal/maximal) according to many physiological variables [5]. These variables include percentage maximum oxygen uptake (%V̇O2max) and heart rate (%HRmax), oxygen uptake and heart rate reserve (%V̇O2R, %HRR), and ratings of perceived exertion (RPE) [5]. However, despite the SCI guidelines adopting the “moderate” and “vigorous” descriptives, there is no equivalent resource published for adults with SCI regarding the physiological thresholds coinciding with these descriptors. Furthermore, given the physiological consequences of SCI on cardiovascular and respiratory responses to exercise [6], there is no justification for simply adopting the percentage thresholds utilised for non-disabled adults.
An alternative approach to exercise intensity prescription is to consider whether different methods result in participants exercising in the same of three exercise domains (moderate, heavy, severe) [7]. This is because of the similar V̇O2 and blood lactate responses between individuals exercising in these domains [7]. Specifically, the moderate intensity domain (below lactate threshold (LT)) is characterised by steady state responses for V̇O2 and blood lactate concentration ([BLa]) [8]. In the heavy intensity domain (between LT and critical power/speed (CP/CS)) there is a delayed steady state response due to the V̇O2 “slow component”, whilst in the severe domain (above CP/CS) no steady state response is observed [8].
To satisfy the aim of producing a homogenous exercise intensity, the fixed percentage approach is only valid if it is demonstrated that equal relative intensities result in individuals exercising in the same intensity domain [7]. However, in a recent study of non-disabled participants, no fixed %V̇O2max or %HRmax, typically used for exercise prescription, resulted in all participants being in the same intensity domain [9]. This has led to assertions that using fixed %V̇O2max or %HRmax for prescribing exercise intensity is inaccurate and will lead to significant inter-individual physiological responses, precluding homogenous exercise intensity prescription [7, 9]. Furthermore, with evidence that individual participant %V̇O2R:%HRR relationships diverges from the assumed linear trajectory, there are also questions over how appropriate %V̇O2R and %HRR are for prescribing exercise intensity at the individual level [10].
For adults with SCI there is currently nothing more to inform aerobic exercise intensity prescription than the arbitrary use of “moderate to vigorous” intensity [3]. Furthermore, evidence in non-disabled adults would suggest a need to rethink the traditional use of fixed percentages [7, 9, 10]. Therefore, this study aimed to investigate and critique potential methods for prescribing aerobic exercise intensity in adults with SCI.
Methods
This study was performed via a retrospective analysis of athlete data collected in the author’s laboratory. All procedures were approved by the Human participants ethical sub-committee at Loughborough University, and participants provided written, informed consent.
Participants
Data were available for 134 individuals (male: 98; female: 36). Participants were split into those with paraplegia (PARA), tetraplegia (TETRA), or alternate health condition (NON-SCI), see Table 1. Examples of health conditions for NON-SCI included spina bifida, limb deficiency, cerebral palsy, and arthrogryposis. Participants were competitive athletes, competing at a national or international level, from one of the following sports: handcycling, para-alpine ski, paratriathlon, wheelchair basketball, wheelchair rugby or wheelchair tennis.
Table 1.
Participant characteristics by group.
| PARA | TETRA | NON-SCI | ||
|---|---|---|---|---|
| Sample size (n) | 47 | 20 | 67 | |
| Sex (M/F) | 30/17 | 18/2 | 50/17 | |
| Age (years) | 33 ± 8a | 32 ± 7a | 27 ± 7 | |
| Body mass (kg) | 70.9 ± 14.1a | 70.8 ± 12.9 | 64.5 ± 12.8 | |
| Neurological level of injury | T4-L2 | C3-C7 | - | |
| Injury completeness | Complete: 21 | Complete: 8 | - | |
| Incomplete: 23 | Incomplete: 4 | |||
| Unavailable: 3 | Unavailable: 8 | |||
| Time since injury (years) | 12 ± 9 | 12 ± 6 | - | |
| Peak oxygen uptake | (L·min−1) | 2.5 ± 0.6b | 1.7 ± 0.5 | 2.6 ± 0.7b |
| (ml·kg−1·min−1) | 35.1 ± 8.2b | 23.4 ± 5.8 | 39.9 ± 8.3b | |
| Peak heart rate | (beats·min−1) | 188 ± 9b | 134 ± 20 | 187 ± 10 b |
| Sport (n) | Handcycling | 8 | 0 | 3 |
| Paratriathlon | 11 | 0 | 8 | |
| Para alpine ski | 2 | 1 | 2 | |
| Wheelchair basketball | 20 | 1 | 31 | |
| Wheelchair rugby | 0 | 15 | 13 | |
| Wheelchair tennis | 6 | 3 | 10 | |
| Test mode (n) | Arm crank ergometry | 11 | 1 | 7 |
| Handcycling | 10 | 0 | 6 | |
| Wheelchair propulsion | 26 | 19 | 54 | |
a: significantly greater than NON-SCI; b: significantly greater than TETRA, P < 0.05.
Exercise testing
Participants completed a submaximal step test followed by graded exercise test (GXT) to exhaustion. Handcycle (HC) tests were performed in the participants own handcycle attached to a Cyclus 2 ergometer (Avantronic Richter, Leipzig, Germany). For some Paratriathlon, and all para-alpine ski athletes, arm crank ergometry (ACE) was used (Lode Angio, Lode B. V., Groningen, the Netherlands). The ergometer was positioned vertically so the crank axis centre was level with the shoulder, and horizontally to allow slight elbow flexion at the furthest point of the crank cycle. Wheelchair basketball, rugby and tennis players performed a wheelchair propulsion (WCP) test using a motorised treadmill (HP Cosmos, Traunstein, Germany) and their own custom sports wheelchair.
Submaximal tests were individualised based on the sport, sex, training status and level of impairment of the participant, with the goal of completing 6-8 stages (average: 6; range: 4–10). HC and ACE tests started at 15–60 W, with 10–20 W increments every 3 min. WCP tests started at 0.7–2.8 m s−1 and were increased by 0.2-0–4 m s−1 every 3 min. V̇O2 (Metalyzer 3B, Cortex, Leipzig, Germany) and HR (RS400, Polar, Kempele, Finland) were continually monitored throughout. The Metalyzer was calibrated before each participant against ambient air and a mix of 15% O2, 5% CO2, with the volume calibrated using a 3 L syringe. RPE was verbally reported in the final minute of each stage using Borg’s 6–20 RPE scale [11]. A capillary blood sample from the ear lobe was collected at the end of each stage for measurement of [BLa] (Biosen C-line, EKF Diagnostics, Barleben, Germany). HC and ACE tests were continuous, however, WCP tests were discontinuous as the treadmill needed to be slowed between stages to facilitate blood sampling. For discontinuous tests, the typical interval between stages was 45–60 s. Submaximal tests continued until [BLa] exceeded 4 mmol L−1 or RPE was rated as 17. The RPE criteria was used in TETRA where there may have been blunted lactate responses [12].
Following the submaximal test, participants received 15 min of active recovery or rest before performing a GXT to exhaustion. The starting workload was set to that from the preceding test when [BLa] increased by 0.5 mmol L−1 above rest. Participants performed 1 min at this load, before the exercise intensity were increased in a stepwise manner by 10–20 W min−1 (HC/ACE) or 0.1 m s−1 min−1 (WCP) until participants reached volitional exhaustion. This was defined as an inability to maintain their preferred cadence at the required PO for HC/ACE, or the required speed of the treadmill, despite verbal encouragement. V̇O2 and HR were again monitored throughout, with RPE and [BLa] measured at the end of the test.
Data processing
V̇O2 and HR data were subjected to a 30 s rolling average, with the greatest of these from the GXT recorded as peak values (V̇O2peak, HRpeak). V̇O2 and HR in the final 30 s of each submaximal stage were extracted and calculated as percentages of peak (%V̇O2peak, %HRpeak). Using the submaximal data, the lactate thresholds were identified as the intersection of the horizontal and ascending sections of the plot of log-[BLa] against log-V̇O2 (LT1) [13], and at [BLa] equal to LT1 plus 1.5 mmol L−1 (LT2) [14]. The inverse of the log-V̇O2 at these points were calculated to give the V̇O2 at LT1 and LT2. HR at LT1 and LT2 was identified by interpolation of the linear V̇O2:HR relationship for each participant. RPE was modelled against [BLa] using a quadratic function for each participant, with the resultant coefficients used to calculate the RPE at LT1 and LT2 [15]. Exercise intensity domains were defined as moderate (<LT1), heavy (between LT1 and LT2) and severe (>LT2).
Statistical analyses
Analyses were performed using IBM SPSS Statistics Version 23.0 (IBM Corp., Armonk, NY) and MLWiN Version 3.05 [16]. Data are presented as mean (standard deviation) with statistical significance accepted at P < 0.05. Data were checked for normal distribution using the Shapiro Wilk statistic.
All individual RPE data points were modelled against the corresponding %V̇O2peak and %HRpeak using dynamic multilevel models with lagged independent variable, whilst accounting for the initial condition. Separate models were created for %V̇O2peak and %HRpeak, which served as the independent variable, with RPE as the dependent variable. Models were multilevel to adjust for the repeated stages performed by each participant and were used due to their ability to characterise group- and individual-level effects [17]. Stage was defined as the first, and participant as the second level. Models accounted for the initial condition (e.g., stage (i) = 1) as it was thought that RPE would depend on the %V̇O2peak and %HRpeak when i = 1. The need for them to be dynamic and incorporate a lagged independent variable was required as it was thought RPE at subsequent measurement occasions (when i > 1) would be dependent on %V̇O2peak and %HRpeak for that, as well as the previous, measurement occasion (i.e., i–1). Potential confounding variables were added to the models to assess whether they improved the model fit with fixed effects, or random effects for between- and within-individual variation. Confounding variables were sex (male/female), group (PARA/TETRA/NON-SCI) and exercise mode (ACE/HC/WCP). The resultant models were used to calculate the %V̇O2peak and %HRpeak corresponding to each value on Borg’s RPE scale.
Differences in V̇O2 (L min−1, ml kg−1 min−1, %V̇O2peak), HR (beats·min−1, %HRpeak) and RPE between groups at LT1 and LT2 were assessed via one-way analysis of variance with Bonferroni post-hoc correction for multiple comparisons. Standardised effect sizes (ES) were calculated to describe the magnitude of differences and categorised as trivial (< 0.2), small (0.2–0.6), moderate (0.6–1.2), large (1.2–2.0) and very large (> 2.0) [18]. For each group, the percentage of participants in each intensity domain (moderate, heavy, severe) were calculated at 5% intervals from 35 to 95% V̇O2peak and %HRpeak.
Results
The associations between RPE and both %V̇O2peak and %HRpeak were not significantly affected by sex or exercise mode, so stratification based on these variables was not needed. The %V̇O2peak and %HRpeak coinciding with each rating on Borg’s RPE scale for PARA and TETRA can be found in Table 2. The full RPE models against %V̇O2peak and %HRpeak can be found in the Supplementary Material.
Table 2.
Resultant calculations of percentage peak oxygen uptake and heart rate by group based on multilevel modelling.
| RPE | %V̇O2peak | %HRpeak | ||
|---|---|---|---|---|
| PARA | TETRA | PARA | TETRA | |
| 6 | 16 | 22 | 40 | 43 |
| 7 | 22 | 28 | 44 | 48 |
| 8 | 28 | 34 | 49 | 52 |
| 9 | 34 | 40 | 54 | 57 |
| 10 | 40 | 46 | 58 | 62 |
| 11 | 46 | 52 | 63 | 66 |
| 12 | 52 | 58 | 68 | 71 |
| 13 | 58 | 64 | 73 | 76 |
| 14 | 64 | 70 | 77 | 81 |
| 15 | 70 | 76 | 82 | 85 |
| 16 | 76 | 82 | 87 | 90 |
| 17 | 82 | 88 | 91 | 95 |
| 18 | 88 | 94 | 96 | 99 |
| 19 | 94 | 100 | 100 | |
| 20 | 100 | |||
RPE and %V̇O2peak model
RPE was significantly affected by the initial %V̇O2peak (i = 1) (P < 0.01), by %V̇O2peak at subsequent occasions when i > 1 (P < 0.01), as well as by the lagged %V̇O2peak (i.e., i–1) (P < 0.01). Each of these variables also showed significant between-individual variation, which was incorporated into the model. There was also an effect of Group at occasions when i > 1 (P = 0.01). TETRA showing significantly greater within-individual variation for the effect of %V̇O2peak on RPE compared to PARA and NON-SCI. As such, PARA and NON-SCI remained grouped, as there was no difference between these groups.
RPE and %HRpeak model
RPE was significantly affected by the initial %HRpeak (i = 1) (P < 0.01), by %HRpeak at subsequent occasions when i > 1 (P < 0.01), as well as by the lagged %HRpeak (i.e., i–1) (P < 0.01). These effects were fixed and showed no significant between- or within-individual variation. There was a fixed effect for Group, with the association between RPE and %HRpeak being significantly different for PARA (P = 0.03). There was no difference between TETRA and NON-SCI, so these remained grouped in this model.
Responses at LT1 and LT2
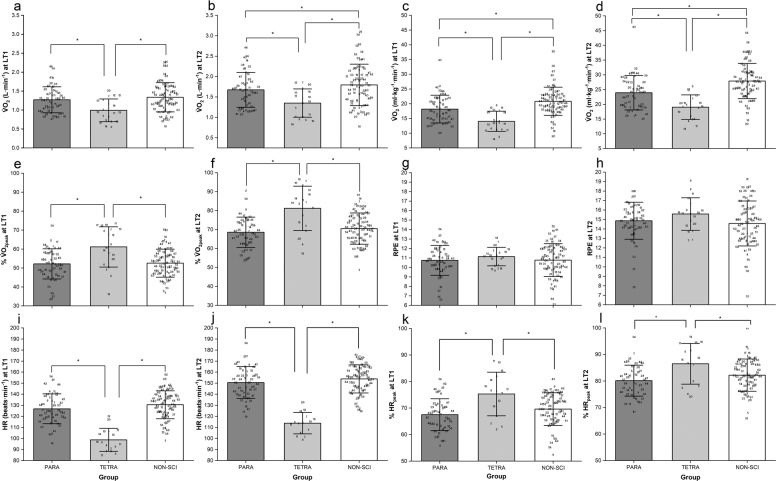
The V̇O2, HR and RPE at LT1 and LT2 are shown in Fig. 1. At LT1 there was a significant group effect for absolute (F2 = 7.11, P < 0.01; Fig. 1a) and relative V̇O2 (F2 = 17.65, P < 0.01; Fig. 1c), %V̇O2peak (F2 = 9.86, P < 0.01; Fig. 1e), HR (F2 = 42.79, P < 0.01; Fig. 1i) and %HRpeak (F2 = 7.94, P < 0.01; Fig. 1k). LT1 occurred at a significantly smaller absolute and relative V̇O2 in TETRA compared to PARA (ES = 0.86, 1.00) and NON-SCI (ES = 1.00, 1.62). However, %V̇O2peak at LT1 was significantly greater in TETRA compared to PARA (ES = 0.96) and NON-SCI (ES = 0.94). Similarly, HR at LT1 was smaller in TETRA compared to PARA (ES = 2.33) and NON-SCI (ES = 2.74), whereas %HRpeak was greater in TETRA compared to PARA (ES = 1.00) and NON-SCI (ES = 0.69). There was no significant difference between groups for RPE (F2 = 0.48, P = 0.62) at LT1 (Fig. 1g).
Fig. 1. Group physiological responses at lactate thresholds.
a Absolute V̇O2 at LT1. b Absolute V̇O2 at LT2. c Relative V̇O2 at LT1. d Relative V̇O2 at LT2. e %V̇O2peak at LT1. f %V̇O2peak at LT2. g RPE at LT1. h RPE at LT2. i HR at LT1. j HR at LT2. k %HRpeak at LT1. l %HRpeak at LT2. Data are presented at mean (SD) with individual points overlaid. Within each group, numbers refer to the same participant in each figure. Asterisk (*) indicates significantly greater than the identified group, P < 0.05.
There was also a significant group effect at LT2 for absolute (F2 = 9.96, P < 0.01; Fig. 1b) and relative V̇O2 (F2 = 19.75, P < 0.01; Fig. 1d), %V̇O2peak (F2 = 14.80, P < 0.01; Fig. 1f), HR (F2 = 58.99, P < 0.01; Fig. 1i) and %HRpeak (F2 = 6.10, P < 0.01; Fig. 1l). Absolute and relative V̇O2 at LT2 were significantly smaller in TETRA compared to PARA (ES = 0.83, 0.97) and NON-SCI (ES = 1.03, 1.72). However, %V̇O2peak at LT2 was significantly greater in TETRA compared to PARA (ES = 1.26) and NON-SCI (ES = 1.06). Furthermore, HR at LT2 was significantly smaller in TETRA than in PARA (ES = 3.00) and NON-SCI (ES = 3.55), while %HRpeak was significantly greater in TETRA compared to PARA (ES = 0.93) and NON-SCI (ES = 0.62). There was no significant difference between groups in RPE (F2 = 2.18, P = 0.19) at LT2 (Fig. 1h).
Intensity classification
Thresholds for %V̇O2peak and %HRpeak corresponding with intensity classifications used in non-disabled exercise guidelines are shown in Table 3. These data suggest there are differences between non-disabled individuals, PARA and TETRA in the thresholds for intensity classifications. Frequency distribution of individuals within moderate, heavy and severe intensity domains for discrete percentages of %V̇O2peak and %HRpeak are shown in Figs. 2 and 3, respectively. These show that no %V̇O2peak or %HRpeak typically used for exercise prescription purposes leads to all participants being in the same domain, with many %V̇O2peak including participants spread across all three domains.
Table 3.
Classification of exercise intensity for individuals with paraplegia and tetraplegia, compared to non-disabled guidelines.
| Very light (RPE ≤ 8)a | Light (RPE 9–11)a | Moderate (RPE 12–13)a | Vigorous (RPE 14–17)a | Near maximal-maximal (RPE ≥ 18)a | |
|---|---|---|---|---|---|
| %V̇O2peak | |||||
| Non-disableda | ≤36% V̇O2peak | 37–45% V̇O2peak | 46–63% V̇O2peak | 64–90% V̇O2peak | ≥91% V̇O2peak |
| Paraplegia | ≤31% V̇O2peak | 32–49% V̇O2peak | 50–61% V̇O2peak | 62–85% V̇O2peak | ≥86% V̇O2peak |
| Tetraplegia | ≤37% V̇O2peak | 38-55% V̇O2peak | 56–67% V̇O2peak | 68–91% V̇O2peak | ≥92% V̇O2peak |
| %HRpeak | |||||
| Non-disableda | ≤56% HRpeak | 57–63% HRpeak | 64-76% HRpeak | 77–95% HRpeak | ≥96% HRpeak |
| Paraplegia | ≤51% HRpeak | 52–65% HRpeak | 66-75% HRpeak | 76–93% HRpeak | ≥94% HRpeak |
| Tetraplegia | ≤54% HRpeak | 55–68% HRpeak | 69–78% HRpeak | 79–97% HRpeak | ≥98% HRpeak |
a data from Riebe et al. [5].
Fig. 2. Distribution of individuals in moderate, heavy, and severe intensity domains at fixed %V̇O2peak.
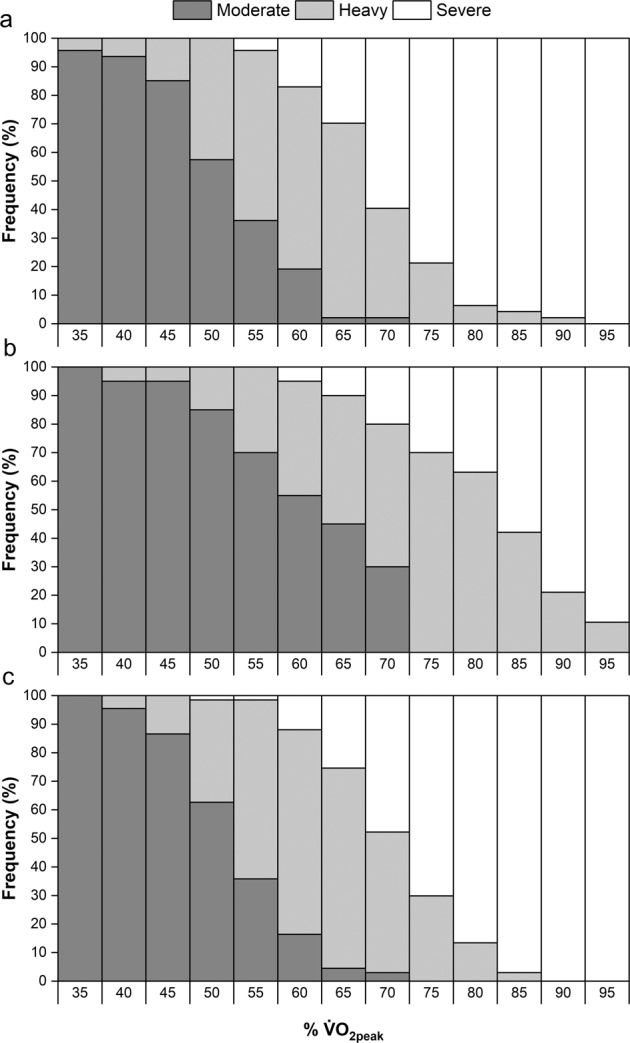
a PARA group. b TETRA group. c NON-SCI group.
Fig. 3. Distribution of individuals in moderate, heavy and severe intensity domains at fixed %HRpeak.
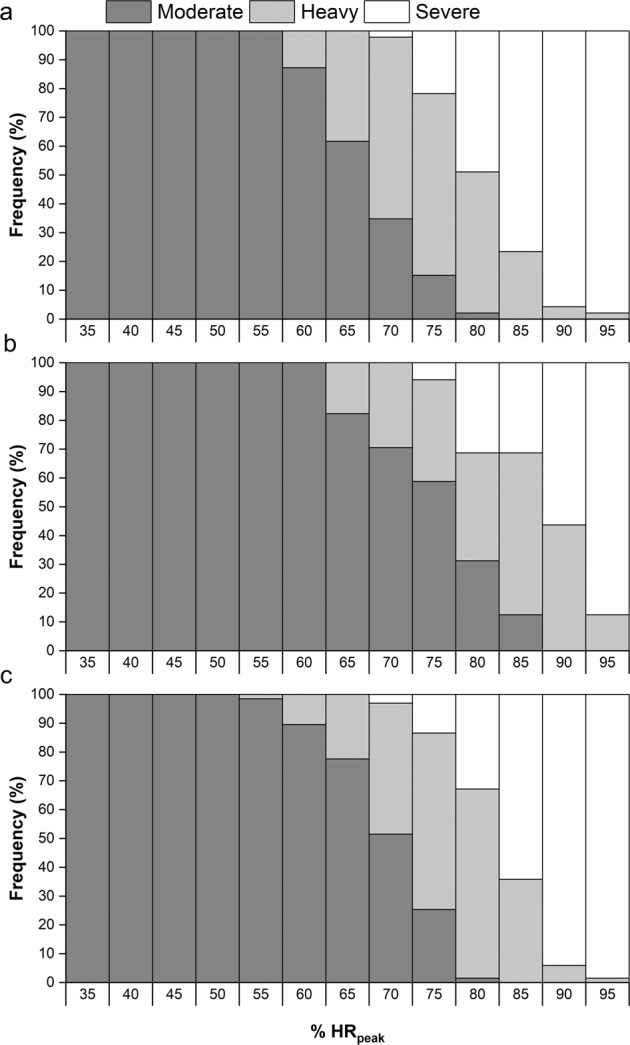
a PARA group. b TETRA group. c NON-SCI group.
Discussion
This study aimed to investigate potential methods of aerobic exercise intensity prescription in adults with SCI. Findings demonstrate that there are differences between PARA and TETRA for the %V̇O2peak and %HRpeak corresponding with the descriptions of “moderate” and “vigorous” exercise intensity, as used by the exercise guidelines for adults with SCI [1, 3]. However, the findings also show that using fixed %V̇O2peak or %HRpeak cannot guarantee a homogenous domain-specific exercise intensity prescription for adults with SCI.
Fixed percentages and intensity domains for exercise intensity prescription
The finding of adults with SCI being in different intensity domains, as defined in this study according to LT1 and LT2, despite being at the same %V̇O2peak or %HRpeak supports similar evidence in non-disabled participants [9]. The domain-specific distribution is also arguably more variable in adults with SCI. For V̇O2peak, Iannetta et al. [9]. report participants in moderate, heavy, and severe domains only at 70% V̇O2peak, whereas in this study this was shown at several fixed percentages, 55-70% V̇O2peak in PARA and 60-70% V̇O2peak in TETRA. It would, therefore, given recent calls to stop using fixed percentages for exercise intensity prescription in non-disabled participants [7, 9, 10], seem appropriate that this is expanded to apply to adults with SCI performing aerobic exercise. This would apply to all adults with SCI, and not just the athletic population utilised in this study. Inter-individual variation will exist in sedentary or low-active participants as much, if not more, than in athletic groups, further limiting the use of fixed percentages for exercise intensity prescription.
Instead, more attention should be given to methods that can lead to participants exercising within the same exercise intensity domain, each of which are characterised by distinct V̇O2 kinetic and blood lactate profiles [19, 20]. This study utilised LT1 to identify the transition between moderate and heavy intensity domains, in accordance with the literature [19, 20]. However, a limitation within the current study was the use of LT2 to identify the heavy-severe domain transition, due to a lack of evidence supporting this, as well as the number of different methods used to measure and identify LT2 [7, 21]. As such, firm conclusions cannot be made regarding the heavy-severe domain transition from this study. It would have been more appropriate to measure CP/CS for this purpose [19–21], however, only data from a GXT were available in this study.
This highlights an important limitation to the widespread implementation of intensity domain-related exercise prescription. Specifically, the suitability of different testing protocols for identifying different threshold concepts, as well as data collection and threshold identification methods used [21]. This poses the challenge of how to simply prescribe domain-specific exercise intensity. Data from the present study may support the use of RPE for this purpose, as no difference in RPE was found between groups at LT1 (Fig. 1g) and LT2 (Fig. 1h), in support of previous findings [15]. Mean RPE at LT1 and LT2 in this study were found to be 11 and 15, respectively, suggesting that these values could be used to guide exercise prescription using a simple and easy to implement method. However, the SD for these values ranged from 1 to 2 units dependent on group. This inter-individual variation serves, therefore, as a limitation to the use of fixed RPE values for exercise intensity prescription and highlights the importance of individualisation in this context. Individualisation, though, poses a further challenge, due to the trade-off between the precision required for research purposes versus the simple messaging for population-level exercise guidelines.
Implications for research
Our results highlight the need to prescribe exercise intensity in a way that ensures a homogenous intensity domain distribution between participants within both acute and longitudinal study designs. Previously, studies have conducted training interventions with an intensity of either a target range, or fixed value, using variables such as %V̇O2peak, %HRpeak and RPE, e.g. [22–26]., which will have led to significant domain heterogeneity. This means the dose of exercise stimulus would not have been controlled between participants. Moving forwards, researchers should identify the domain transitions and prescribe intensity in relation to these. That being said, the authors are not aware of any investigations into CP/CS using participants with SCI, so initial studies need to investigate the validity and reliability of identifying domain transitions in participants in SCI. Furthermore, V̇O2 kinetic responses to exercise in each domain should be investigated in participants with SCI, due to potential differences in V̇O2 kinetics between non-disabled participants and those with SCI [27]. Finally, these investigations must also account for any differences based on the mode of exercise [28].
Our findings also emphasise the need to individualise the exercise intensity prescription. In non-disabled participants, standardised intensity prescription (e.g., 55–75% V̇O2peak), has been shown to lead to significant heterogeneity in responsiveness [29] leading to participants being described as either “responders” or “non-responders” to the intervention. However, an individualised exercise prescription (relative to ventilatory threshold) in non-disabled participants resulted in 100% responsiveness, compared to 60% in the standardised intervention [30]. Subsequently, future exercise research in participants with SCI should individualise the intensity prescription according to intensity domains, whilst also report individual responsiveness to an intervention. This will improve methodological control while also increasing confidence in conclusions made based on the data.
Implications for exercise guidelines using “moderate to vigorous” exercise intensity
Exercise guidelines must balance the precision required for a specific intensity prescription, against the need for a simple population-level recommendation. Furthermore, scientific guidelines must undergo a knowledge translation process to ensure that the scientific integrity of the guidelines are maintained, whilst also incorporating the varied needs of all potential end-users [31].
In adults with SCI, the scientific guidelines [1, 3] recommend performing aerobic exercise at a “moderate to vigorous” intensity, without providing any specific details on what this means. The same intensity prescription is also used in a community and clinical-practice version of the guidelines [31]. Results from the current study would suggest the scientific integrity of such an intensity prescription is questionable. Table 3 shows equivalent thresholds for PARA and TETRA for “moderate” and “vigorous” intensity, based on the guidelines for non-disabled adults [5]. Combining these with the intensity domain distributions in Fig. 2, shows participants were spread across moderate, heavy, and severe domains at both “moderate” and “vigorous” intensities. It should also be noted that participants in the present study were competitive athletes, and that responses would likely be even more variable for sedentary or low-active populations. This shows how the use of “moderate” to “vigorous” intensity will not lead to anything close to resembling a uniform exercise intensity prescription between individuals. It also shows how someone expecting to perform “moderate” intensity exercise may actually be much closer to their maximum capacity. This will likely decrease the pleasure the person feels during the exercise, which could ultimately impact on whether they decide to continue doing it [32].
As it is operationally more difficult to define compared to frequency (e.g., 3 times a week) and duration (e.g., 30 min), it is possible that exercise intensity becomes an ignored piece of exercise guidelines. Perhaps research could seek to understand end-user perceptions of “exercise intensity”, or needs for interpreting and monitoring intensity, before using that information alongside physiological principles to underpin an evidence-informed intensity prescription. Alternatively, maybe the focus for exercise guidelines should shift from exercise intensity to also acknowledge factors that might help individuals become and stay active, such as their pleasure when performing exercise [32].
Conclusion
Prescribing a “moderate to vigorous” exercise intensity will not lead to a uniform intensity domain distribution in adults with SCI. Neither will the use of fixed percentages of V̇O2peak or HRpeak, or generic values of RPE, due to inter-individual variation. Such methods of exercise intensity prescription should not be used in this population. Future research should individualise the intensity prescription to ensure a homogenous inter-individual domain distribution. However, the accurate testing required to ensure an individualised intensity prescription poses a challenge to exercise guidelines aimed at informing behaviour at the population-level.
Data archiving
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Supplementary information
Acknowledgements
We would like to thank the various members of the Peter Harrison Centre for Disability Sport who were involved in the collection of data used in this study. These include Dr John Lenton, Dr Christof Leicht, Dr Tom Paulson, Dr Terri Paulson, Dr Katy Griggs, Dr Ben Stephenson, Dr Ben Stone, Tom O’Brien, and Conor Murphy.
Author contributions
MJH and VLGT were both responsible for developing the concept of the study and were both involved in the data collection. MJH performed the data analysis, with MJH and VLGT involved in the interpretation of the data. MJH drafted the original version of the manuscript, with VLGT providing comments. MJH and VLGT were both responsible for reviewing and approving the final version of the manuscript.
Funding
This work was supported by funding from the Peter Harrison Foundation.
Statement of ethics
We certify that all applicable institutional regulations concerning the ethical use of human volunteers were followed during the course of this research.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41393-021-00733-2.
References
- 1.van der Scheer JW, Martin Ginis KA, Ditor DS, Goosey-Tolfrey VL, Hicks AL, West CR, et al. Effects of exercise on fitness and health of adults with spinal cord injury. Neurology. 2017;89:736–45. doi: 10.1212/WNL.0000000000004224. [DOI] [PubMed] [Google Scholar]
- 2.Kim DI, Lee J, Park H, Jeon JY. The relationship between physical activity levels and mental health in individuals with spinal cord injury in South Korea. Int J Environ Res Public Health. 2020;17:1–9. doi: 10.3390/ijerph17124423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin Ginis KA, van der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P, et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 2018;56:308–21. doi: 10.1038/s41393-017-0017-3. [DOI] [PubMed] [Google Scholar]
- 4.van der Scheer JW, de Groot S, Tepper M, Faber W, ALLRISC Group. Veeger DH, et al. Low-intensity wheelchair training in inactive people with long-term spinal cord injury: a randomized controlled trial on fitness, wheelchair skill performance and physical activity levels. J Rehabil Med. 2016;48:33–42. doi: 10.2340/16501977-2037. [DOI] [PubMed] [Google Scholar]
- 5.Riebe D, Ehrman JK, Liguori G, Magal M ACSM’s guidelines for exercise testing and prescription. Tenth edit. (Wolters Kluwer: Philadelphia, PA, 2018).
- 6.Theisen D. Cardiovascular determinants of exercise capacity in the Paralympic athlete with spinal cord injury. Exp Physiol. 2012;97:319–24. doi: 10.1113/expphysiol.2011.063016. [DOI] [PubMed] [Google Scholar]
- 7.Jamnick NA, Pettitt RW, Granata C, Pyne DB, Bishop DJ. An examination and critique of current methods to determine exercise intensity. Sports Med. 2020;50:1729–56. doi: 10.1007/s40279-020-01322-8. [DOI] [PubMed] [Google Scholar]
- 8.Burnley M, Jones AM. Power–duration relationship: physiology, fatigue, and the limits of human performance. Eur J Sport Sci. 2018;18:1–12. doi: 10.1080/17461391.2016.1249524. [DOI] [PubMed] [Google Scholar]
- 9.Iannetta D, Inglis EC, Mattu AT, Fontana FY, Pogliaghi S, Keir DA, et al. A critical evaluation of current methods for exercise prescription in women and men. Med Sci Sport Exerc. 2020;52:466–73. doi: 10.1249/MSS.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 10.Ferri Marini C, Sisti D, Leon AS, Skinner JS, Sarzynski MA, Bouchard C, et al. HRR and VO2R fractions are not equivalent: is it time to rethink aerobic exercise prescription methods? Med Sci Sport Exerc. 2021;53:174–82. doi: 10.1249/MSS.0000000000002434. [DOI] [PubMed] [Google Scholar]
- 11.Borg GA Borg’s Perceived Exertion and Pain Scales. (Human Kinetics: Champaign, IL, 1998).
- 12.Hutchinson MJ, Kilgallon JW, Leicht CA, Goosey-Tolfrey VL. Perceived exertion responses to wheelchair propulsion differ between novice able-bodied and trained wheelchair sportspeople. J Sci Med Sport. 2019;23:403–7. doi: 10.1016/j.jsams.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Beaver WL, Wasserman K, Whipp BJ. Improved detection of lactate threshold during exercise using a log-log transformation. J Appl Physiol. 1985;59:1936–40. doi: 10.1152/jappl.1985.59.6.1936. [DOI] [PubMed] [Google Scholar]
- 14.Dickhuth H-H, Huonker M, Münzel T, Drexler H, Berg A, Keul J Individual anaerobic threshold for evaluation of competitive athletes and patients with left ventricular dysfunction. In: Bachl N, Graham TE, Lollgen H (eds). Advances in Ergometry. (Springer: Berlin Heidelberg, 1991), pp 173-9.
- 15.Hutchinson MJ, Kouwijzer I, de Groot S, Goosey-Tolfrey VL Comparison of two Borg exertion scales for monitoring exercise intensity in able-bodied participants, and those with paraplegia and tetraplegia. Spinal Cord. 2021. 10.1038/s41393-021-00642-4. [DOI] [PMC free article] [PubMed]
- 16.Charlton C, Rasbash J, Browne WJ, Healy M, Cameron B MLwiN Version 3.05. 2020.
- 17.Kristjansson SD, Kircher JC, Webb AK. Multilevel models for repeated measures research designs in psychophysiology: an introduction to growth curve modeling. Psychophysiology. 2007;44:728–36. doi: 10.1111/j.1469-8986.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- 18.Batterham AM, Hopkins WG. Making meaningful inferences about magnitudes. Int J Sports Physiol Perform. 2006;1:50–57. doi: 10.1123/ijspp.1.1.50. [DOI] [PubMed] [Google Scholar]
- 19.Poole DC, Jones AM. Oxygen uptake kinetics. Compr Physiol. 2012;2:933–96. doi: 10.1002/cphy.c100072. [DOI] [PubMed] [Google Scholar]
- 20.Poole DC, Burnley M, Vanhatalo A, Rossiter HB, Jones AM. Critical power: an important fatigue threshold in exercise physiology. Med Sci Sport Exerc. 2016;48:2320–34. doi: 10.1249/MSS.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole DC, Rossiter HB, Brooks GA, Gladden LB. The anaerobic threshold: 50+ years of controversy. J Physiol. 2021;599:737–67. doi: 10.1113/JP279963. [DOI] [PubMed] [Google Scholar]
- 22.Bakkum AJ, de Groot S, Stolwijk-Swuste JM, van Kuppevelt DJ, ALLRISC. van der Woude LH, et al. Effects of hybrid cycling versus handcycling on wheelchair-specific fitness and physical activity in people with long-term spinal cord injury: a 16-week randomized controlled trial. Spinal Cord. 2015;53:395–401. doi: 10.1038/sc.2014.237. [DOI] [PubMed] [Google Scholar]
- 23.Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, et al. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord. 2003;41:34–43. doi: 10.1038/sj.sc.3101389. [DOI] [PubMed] [Google Scholar]
- 24.Kim D-I, Lee H, Lee B-S, Kim J, Jeon JY. Effects of a 6-week indoor hand-bike exercise program on health and fitness levels in people with spinal cord injury: a randomized controlled trial study. Arch Phys Med Rehabil. 2015;96:2033–40. doi: 10.1016/j.apmr.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Pelletier CA, Totosy de Zepetnek JO, MacDonald MJ, Hicks AL. A 16-week randomized controlled trial evaluating the physical activity guidelines for adults with spinal cord injury. Spinal Cord. 2015;53:363–7. doi: 10.1038/sc.2014.167. [DOI] [PubMed] [Google Scholar]
- 26.Valent LJM, Dallmeijer AJ, Houdijk H, Slootman HJ, Janssen TW, Post MWM, et al. Effects of hand cycle training on physical capacity in individuals with tetraplegia: a clinical trial. Phys Ther. 2009;89:1051–60. doi: 10.2522/ptj.20080340. [DOI] [PubMed] [Google Scholar]
- 27.Gollie JM, Herrick JE, Keyser RE, Chin LMK, Collins JP, Shields RK, et al. Fatigability, oxygen uptake kinetics and muscle deoxygenation in incomplete spinal cord injury during treadmill walking. Eur J Appl Physiol. 2017;117:1989–2000. doi: 10.1007/s00421-017-3685-y. [DOI] [PubMed] [Google Scholar]
- 28.Barstow TJ, Scremin AME, Mutton DL, Kunkel CF, Cagle TG, Whipp BJ. Peak and kinetic cardiorespiratory responses during arm and leg exercise in patients with spinal cord injury. Spinal Cord. 2000;38:340–5. doi: 10.1038/sj.sc.3101014. [DOI] [PubMed] [Google Scholar]
- 29.Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, et al. Familial aggregation of V̇O(2max) response to exercise training: Results from the HERITAGE family study. J Appl Physiol. 1999;87:1003–8. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 30.Weathermax RM, Harris NK, Kilding AE, Dalleck LC. Incidence of V˙O2max responders to personalized versus standardized exercise prescription. Med Sci Sport Exerc. 2019;51:681–91. doi: 10.1249/MSS.0000000000001842. [DOI] [PubMed] [Google Scholar]
- 31.Hoekstra F, McBride CB, Borisoff J, Fetterly MJ, Ginis S, Latimer-Cheung AE, et al. Translating the international scientific spinal cord injury exercise guidelines into community and clinical practice guidelines: a Canadian evidence-informed resource. Spinal Cord. 2020;58:647–57. doi: 10.1038/s41393-019-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekkekakis P, Parfitt G, Petruzzello SJ. The pleasure and displeasure people feel when they exercise at different intensities: decennial update and progress towards a tripartite rationale for exercise intensity prescription. Sports Med. 2011;41:641–71. doi: 10.2165/11590680-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.