Abstract
Semaglutide is a glucagon-like peptide-1 receptor agonist that was recently approved by the US Food and Drug Administration for chronic weight management. This paper reviews data on the mechanism of action, weight-loss and cardiometabolic efficacy, and safety of semaglutide 2.4 mg/week for obesity. Semaglutide has demonstrated the largest weight loss of any obesity medication to date with reductions of approximately 15% of initial weight at 68 weeks, accompanied by improvements in cardiovascular risks factors and physical functioning. The approval of this medication provides patients with greater options for weight management.
Introduction
Obesity is a multifactorial chronic disease that affects 650 million adults globally.(1) It is associated with a number of physical and mental health complications including risk factors for cardiovascular disease (e.g., plasma lipids, blood pressure, glucose, inflammation), coronary heart disease, heart failure, hypertension, and atrial fibrillation.(2) Weight losses of 5% or more of initial weight help to ameliorate these complications, with larger losses producing greater health benefits.(3) Comprehensive lifestyle intervention remains the cornerstone of obesity treatment, but suboptimal weight losses and weight regain are common with this approach.
Anti-obesity medications (AOM) help facilitate weight management when used in conjunction with a reduced calorie diet and increased physical activity by individuals with a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with an obesity-related co-morbidity such as type 2 diabetes, hypertension, or dyslipidemia. Patients who are unable to achieve weight loss goals with a comprehensive weight loss (e.g., 5% of initial weight loss in 3–6 months) may pursue adjunct treatment with AOM. In the US, an estimated 53.5% of adults are eligible for a weight loss medication.(4) Five medications are currently approved for weight management in the US (liraglutide, orlistat, phentermine, naltrexone/bupropion, phentermine/topiramate, and semaglutide), three in Europe (liraglutide, orlistat, and naltrexone/bupropion) and one in China (orlistat). These medications have various mechanisms of action that target diverse pathways related to weight regulation.
One such pathway is the incretin hormone system. Glucagon-like peptide 1 (GLP-1) is a hormone released from the proglucagon gene in L-cells of the distal small intestine and colon in response to oral nutrient intake.(5) The hormone binds to GLP-1 receptors expressed in tissues such as the pancreatic beta cells, gastric mucosa, kidney, heart, and hypothalamus.(6, 7) It stimulates insulin release and secretion in hyperglycemic states, inhibits glucagon release in hyperglycemic or euglycemic states, slows gastric emptying, and reduces food intake.(6, 7) The half-life of GLP-1 is 1 to 2 minutes due to N-terminal degradation by the enzyme dipeptidyl peptidase 4 (DPP-4).(8, 9)
Synthetic GLP-1 agonists have variable resistance to enzymatic degradation and therefore have a longer half-life, facilitating therapeutic use. Liraglutide 3.0 mg, injected subcutaneously daily, was the first GLP-1 agonist approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for chronic weight management. Another medication in this class, semaglutide 2.4 mg administered subcutaneously once weekly, was approved by the FDA in 2021 as an adjunct to reduced calorie intake and increased physical activity for chronic weight management in adults with obesity (initial BMI≥30 kg/m2) or overweight (initial BMI≥27 kg/m2) with at least one weight-related comorbidity.(10) In this review we briefly examine the weight loss efficacy of liraglutide 3.0 mg/day. We then review data on the mechanism of action, weight-loss and cardiometabolic efficacy, and safety of semaglutide 2.4 mg/week for the treatment of obesity.
Liraglutide
Liraglutide is a GLP-1 receptor agonist that has been modified to noncovalently bind to serum albumin, resulting in slower degradation and a half-life of 11 to 15 hours.(11) The medication, as an injectable dose of 1.8 mg daily, was approved for the treatment of type 2 diabetes in Europe in 2009(12) and in the US in 2010.(13) Liraglutide, as an injectable dose of 3.0 mg daily, was approved in 2014 by the FDA(14) and in 2015 by the EMA(15) for chronic weight management in adults with an initial BMI of at least 30 kg/m2 (or at least 27 kg/m2 in the presence of at least one weight-related comorbid condition). Dosing begins at 0.6 mg daily for one week and is then titrated up weekly at 0.6 mg intervals until the recommended dose of 3 mg daily is reached.
Liraglutide induces weight loss by reducing energy intake.(16) It has little known effect on energy expenditure.(16) Compared to placebo, liraglutide lowers ad libitum energy intake by 16%, as shown with a laboratory meal paradigm.(16) The efficacy of liraglutide 3 mg daily for weight loss was demonstrated in several large, multicenter trials. The first was a 20-week study that compared the effects of liraglutide at doses of 1.2 mg, 1.8 mg, 2.4 mg, and 3.0 mg, injected subcutaneously once daily, with placebo and an open-label active comparator, orlistat 120 mg taken three times/day.(17) The mean weight loss was significantly greater with all doses of liraglutide relative to placebo (4.8 kg, 5.5 kg, 6.3 kg, and 7.2 kg for liraglutide 1.2 mg, 1.8 mg, 2.4 mg, and 3.0 mg, respectively, versus 2.8 kg for placebo). Participants who received liraglutide 2.4 mg or 3.0 mg also lost significantly more weight than those assigned to orlistat (4.1 kg). An extension of the trial showed that from randomization to year 1, participants on liraglutide 3.0 mg lost 5.8 kg more than placebo-treated participants and 3.8 kg more than those on orlistat.(18) At 2 years, participants who were on liraglutide 2.4 or 3.0 mg lost 3 kg more than those on orlistat.
Four randomized, controlled, multicenter phase 3a trials further tested liraglutide for obesity treatment. These included: Satiety and Clinical Adiposity- Liraglutide Evidence in individuals with and without diabetes (SCALE); SCALE Maintenance; SCALE Diabetes; and SCALE Sleep Apnea.(19–22) A meta-analysis showed that liraglutide produced a mean 5.2 kg placebo-subtracted weight loss at 1 year, with 63% of participants achieving a ≥5% weight loss, inclusive of 34% of participants who lost ≥10% of initial weight.(23) Liraglutide, relative to placebo, resulted in declines of 15.7 mg/dL in fasting blood glucose, 0.5% in hemoglobin A1c, and 2.8 mmHg in systolic blood pressure.(24)
Semaglutide
Once-weekly subcutaneous semaglutide 1.0 mg was approved by the US Food and Drug Administration in 2017 and the European Medicines Agency in 2018 for the treatment of type 2 diabetes. A once-daily oral version of the medication, at a maximum dose of 14 mg, was approved for treating type 2 diabetes in the US in 2019 and in Europe in 2020.(13) The dose of semaglutide for weight management is 2.4 mg injected subcutaneously once weekly (on the same day each week, with or without meals). This dose was selected based on a phase 2 clinical trial in which greater weight loss was achieved with once-daily semaglutide, 0.4 mg (equal to 2.8 mg weekly), than with placebo or liraglutide 3.0 mg/day.(25) It is available in single-use, prefilled pens. The initial dose is 0.25 mg, administered by subcutaneous injection in the abdomen, thigh, or upper arm once weekly for 4 weeks. The dose is increased in 4-week intervals (over a total of 16 weeks) until a dose of 2.4 mg per week is reached.
Mechanisms of Action for Weight Loss
Semaglutide is a long-acting GLP-1 receptor agonist with 94 percent homology with native human GLP-1. It has structural modifications to allow for reversible albumin binding, reducing renal clearance and decreasing degradation by DPP-4, while also allowing for sufficiently high GLP-1R affinity.(11, 26) This formulation results in slower degradation and a half-life 155 to 184 hours, allowing for once-weekly subcutaneous dosing without compromising weight loss efficacy.(11, 26)
Figure 1 shows theoretical and empirically supported mechanisms of action of semaglutide. Semaglutide promotes insulin secretion from beta cells in the pancreas and decreases glucagon secretion in a glucose-dependent manner.(27, 28) Semaglutide results in weight loss via reduced energy intake with minimal effects on energy expenditure.(29) Compared to placebo, semaglutide 2.4 mg (i.e., once-weekly for 20 weeks) reduced ad libitum energy intake by 35%, as shown with a laboratory meal paradigm.(30) Semaglutide, relative to placebo, also resulted in larger reductions in hunger and food cravings, increased fullness and satiety, and better control of eating. Studies in rodents have also shown that semaglutide has direct and indirect effects on neutral pathways involved in hedonic and homeostatic appetite control.(31)
Figure 1.
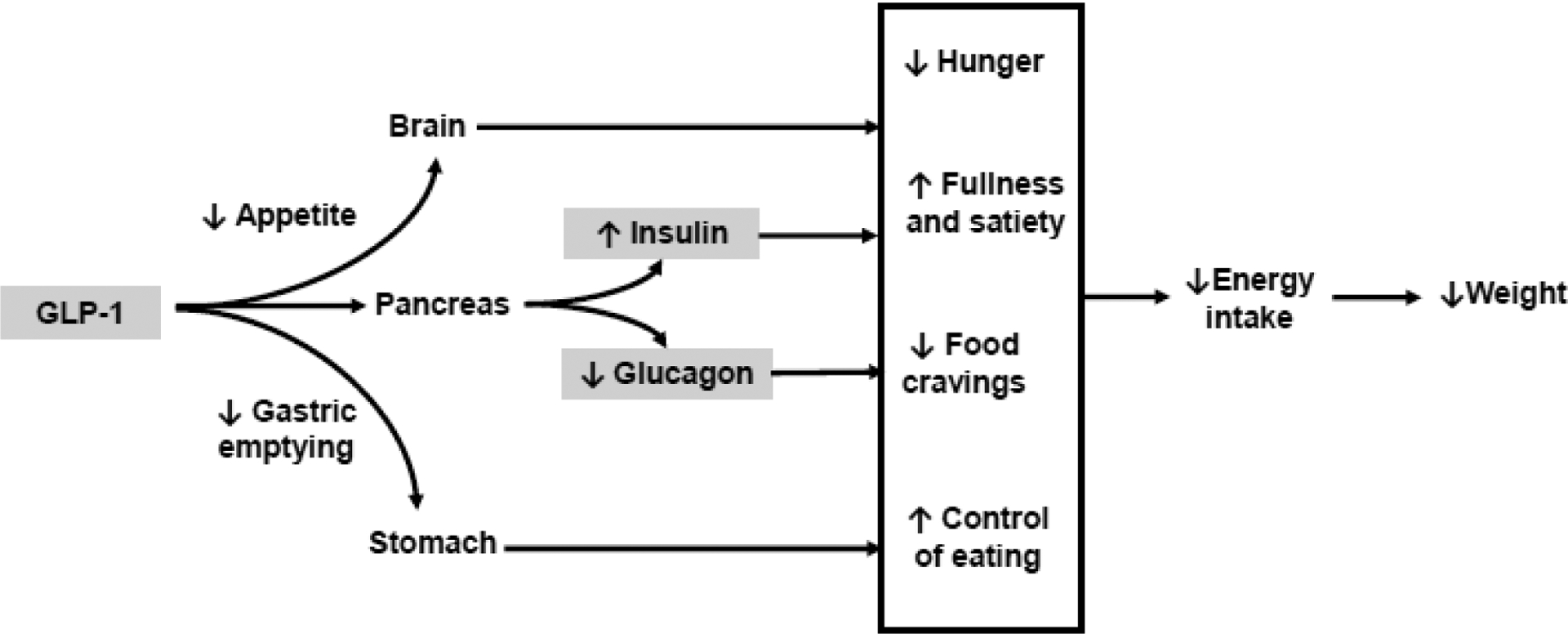
Theoretical and empirically supported mechanisms of action of semaglutide for obesity
Gastrointestinal effects have been hypothesized to mediate the effects of GLP-1 receptor agonists on weight loss. However, only a very small proportion (<1 percentage point) of weight loss is explained by nausea or vomiting.(32) Delayed gastric emptying could hypothetically contribute to the weight loss effects. However, studies have shown that semaglutide tends to have minimal effects on gastric emptying.(30)
Weight-Loss and Cardiometabolic Efficacy
Semaglutide Treatment Effect in People with obesity (STEP) was a pivotal, global phase 3 clinical development program that evaluated semaglutide 2.4 mg once weekly for weight management.(33) We summarize here the published results from the STEP 1–4 trials (Figures 2 and 3; Table 1) and update the status of ongoing studies.
Figure 2.
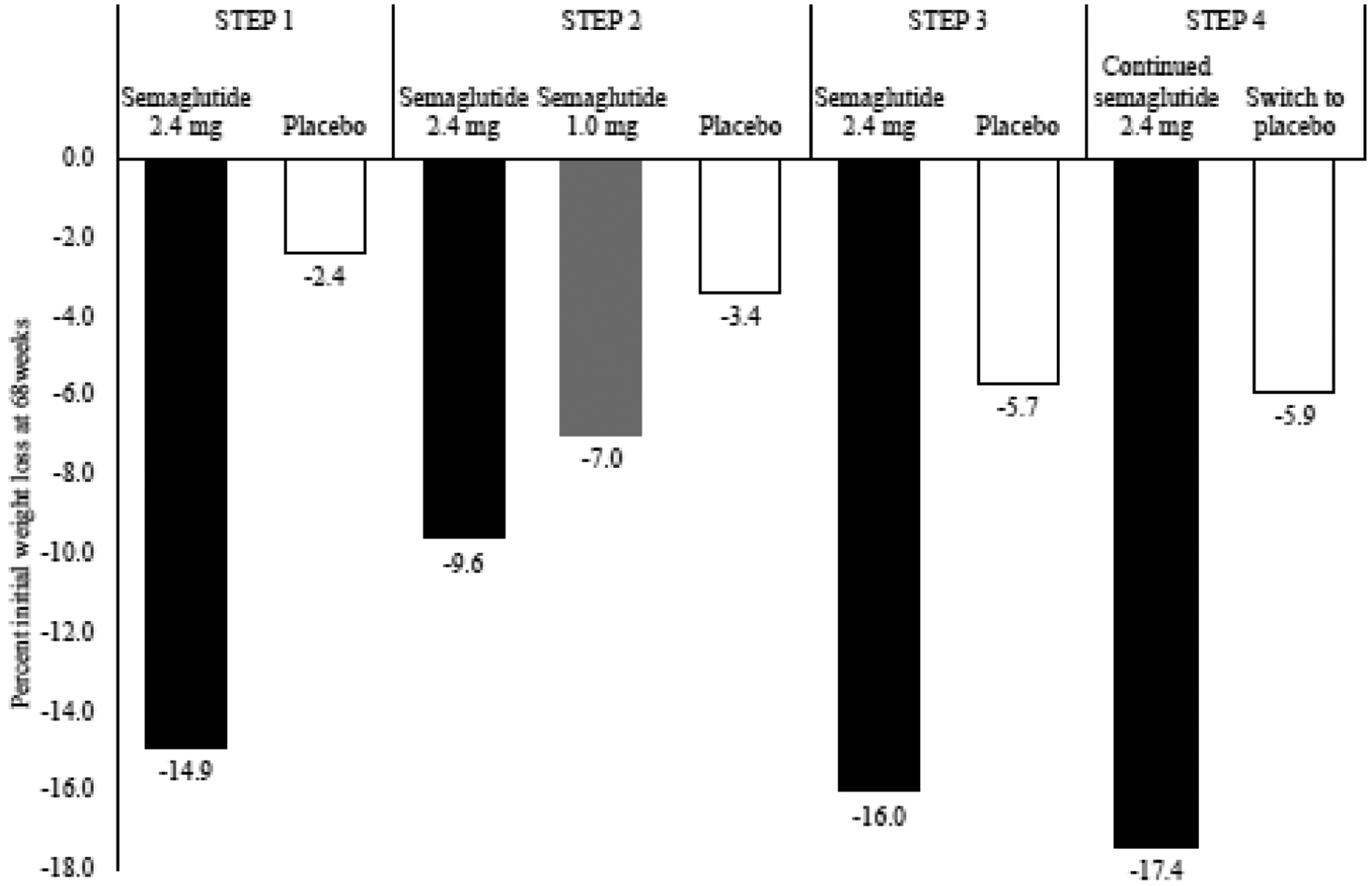
Percent initial weight loss at 68 weeks for STEP trials 1–4
Figure 3.
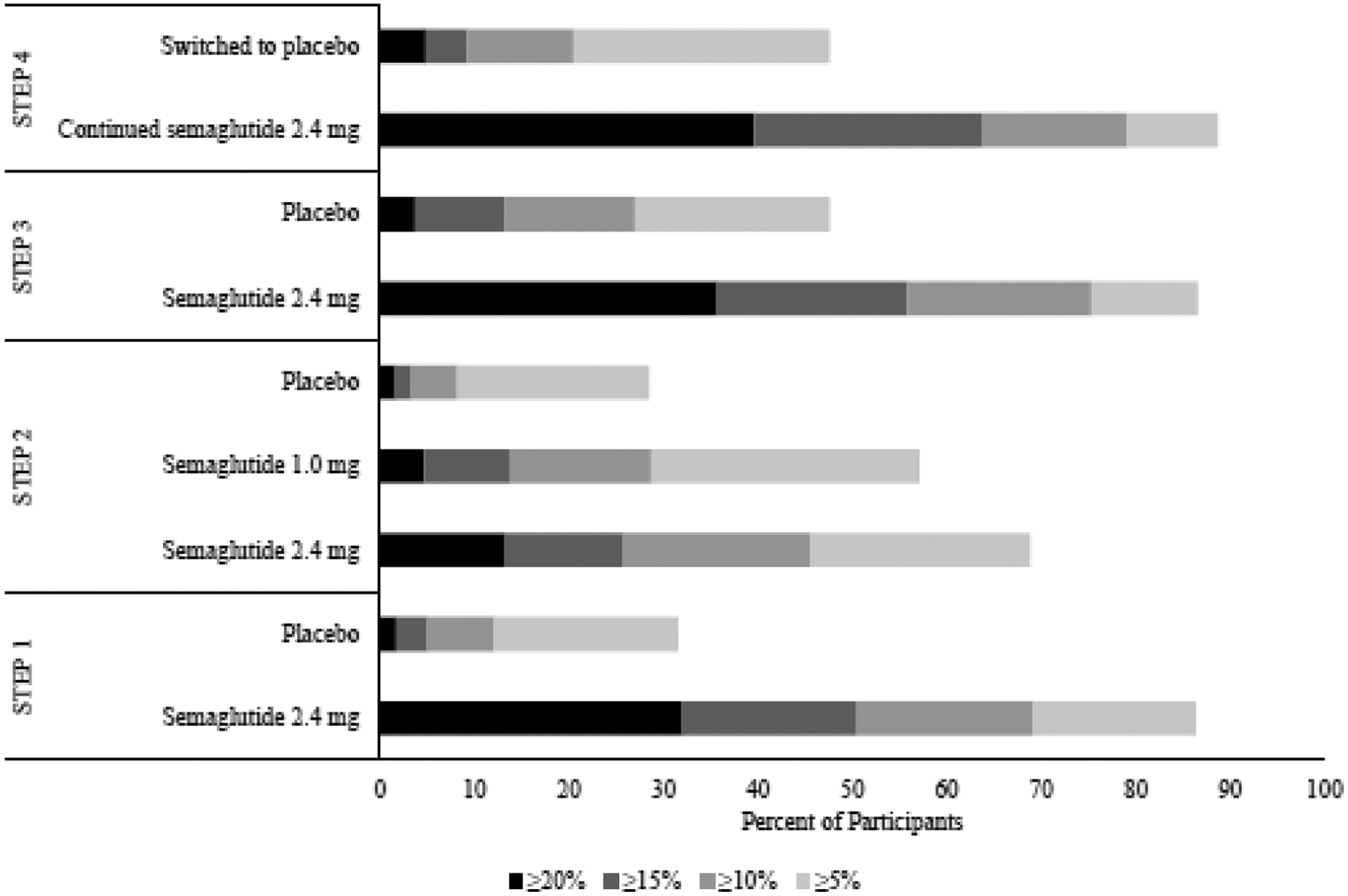
Categorical weight loss at 68 weeks for STEP trials 1–4
Table 1.
Summary of Semaglutide Treatment Effect in People with Obesity (STEP) Trials 1 to 4
| Trial name | Treatment arms (N) | SBP, mm Hg | DBP, mm Hg | HbA1c, percentage points | Fasting plasma glucose, mg/dL | Total cholesterola | Triglyceridesa | LDLa | HDLa | C-reactive proteina | Waist circumference, cm | SF-36 physical function score | SF-36 Mental health score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STEP 1 | Semaglutide 2.4 mg + lifestyle intervention (1306) | −6.16* | −2.83* | −0.45* | −8.35* | 0.97* | 0.78* | 0.97* | 1.05* | 0.47* | −13.54* | 2.21* | Favors semaglutide*b |
| Placebo + lifestyle intervention (655) | −1.06 | −0.42 | −0.15 | −0.48 | 1.00 | 0.93 | 1.01 | 1.01 | 0.85 | −4.13 | 0.41 | NR | |
| STEP 2 | Semaglutide 2.4 + lifestyle intervention (404) | −3.9* | −1.6 | −1.6* | −2.1*c | 0.99* | 0.78* | 1.00* | 1.07* | 0.51* | −9.4* | 2.5* | NSb |
| Semaglutide 1.0 + lifestyle intervention (403)^ | −2.9 | −0.6 | −1.5 | −1.8*c | 0.98 | 0.83* | 0.99 | 1.05 | 0.58* | −6.7* | 2.4 | NSb | |
| Placebo + lifestyle intervention (403) | −0.5 | −0.9 | −0.4 | −0.1c | 0.99 | 0.91 | 1.00 | 1.04 | 0.83 | −4.5 | 1 | ||
| STEP 3 | Semaglutide 2.4 + low calorie diet for the first 8 weeks + intensive behavioral therapy (407) | −5.6* | −3.0* | −0.51* | −6.73* | −3.8* | −22.5* | −4.7* | 6.5 | 59.6* | −14.6* | 3 | −0.8* |
| Placebo + low calorie diet for the first 8 weeks + intensive behavioral therapy (204) | −1.6 | −0.8 | −0.27 | −0.65 | 2.1 | −6.5 | 2.6 | 5.0 | −22.9% | −6.3 | 2.3 | −2.9 | |
| STEP 4d | Continued semaglutide 2.4 + lifestyle intervention (535) | 0.5* | 0.3 | −0.1* | −0.8* | 5* | −6* | 1* | 18 | NR | −6.4* | 1.0* | 0.1* |
| Switched to placebo + lifestyle intervention (268) | 4.4 | 0.9 | 0.1 | 6.7 | 11 | 15 | 8 | 18 | NR | 3.3 | −1.5 | −3.4 |
Note. SBP=systolic blood pressure. DBP=diastolic blood pressure.
p<0.05 with placebo.
Formal statistical comparisons were not performed between semaglutide 1.0 mg and semaglutide 2.4 mg.
For STEP trials 1 and 2, values presented are a ratio of week 68 to baseline. For STEP trial 3, values presented are absolute change scores, and c-reactive protein is the percent change at week 68. For STEP trial 4, values presented are relative percent change.
Individual change scores not reported.
mmol/L.
All participants were treated with semaglutide from baseline to 20 weeks. Results presented are changes from weeks 20–68.
STEP 1
STEP 1 was a 68-week randomized, placebo-controlled trial that assessed weight management in 1961 adults with a BMI of ≥30 kg/m2 (or ≥27 kg/m2 with ≥1 weight-related comorbidity) and without type 2 diabetes.(34) Participants were randomly assigned to once-weekly subcutaneous 2.4 mg semaglutide or placebo, against a background of individual lifestyle counseling sessions every 4 weeks. Semaglutide-treated patients lost 14.9% of baseline weight compared with 2.4% for placebo (estimated treatment difference = −12.4 percentage points, 95% CI −13.4 to −11.5; Table 1). Significantly more participants in the semaglutide than in the placebo group achieved reductions of ≥5%, ≥10%, and ≥15% (Figure 3). Fully 32.0% of semaglutide-treated participants lost 20% or more of baseline weight, compared with only 1.7% of the placebo group. Greater weight loss was associated with greater improvements in cardiometabolic risk factors. Semaglutide-treated participants, relative to placebo, achieved larger reductions in waist circumference, systolic and diastolic blood pressure, hemoglobin A1c, fasting blood glucose, total cholesterol, LDL cholesterol, triglycerides, and c-reactive protein. They also experienced greater improvements in HDL cholesterol, and in physical functioning as assessed by the 36-item Short Form Health Survey (SF-36) and by the Impact of Weight on Quality of Life–Lite Clinical Trials Version (IWQOL-Lite-CT) questionnaires (Table 1).
STEP 2
In STEP 2, 1210 patients with type 2 diabetes, a glycated hemoglobin of 7–10%, and a BMI≥27 kg/m2 were randomized to once weekly subcutaneous semaglutide 2.4 mg, semaglutide 1.0 mg (i.e., the dose approved for type 2 diabetes), or placebo.(35) Treatment lasted for 68 weeks, as in the STEP 1 trial, to allow for a full 52 weeks at the 2.4 mg dose, after up titration for the first 16 weeks. Participants received lifestyle counseling sessions every 4 weeks. The semaglutide 2.4 mg group lost 9.6% of initial body weight which was significantly more than the semaglutide 1.0 mg and placebo groups which lost 7.0% and 3.4%, respectively (Figure 2). The estimated treatment difference for semaglutide 2.4 mg vs placebo was −6.2 percentage points (95% CI −7.3 to −5.2, p<0.001), and for semaglutide 2.4 mg vs semaglutide 1.0 mg was 2.7 percentage points (95% CI −3.7 to −1.6, p<0.0001). More participants in the semaglutide 2.4 mg group than the semaglutide 1.0 mg or placebo groups achieved a weight reduction of ≥5%, ≥10%, and ≥15% (Figure 3). The semaglutide 2.4 mg group, compared with placebo, had significantly greater reductions in waist circumference, A1c, fasting glucose, systolic blood pressure, triglycerides, c-reactive protein, and physical functioning scores on the SF-36 and IWQOL-Lite-CT (Table 1).
STEP 3
STEP 3 was a 68-week, randomized, placebo-controlled study of 611 adults with a BMI≥30 kg/m2 or ≥27 kg/m2 with ≥1 weight related comorbidity.(36) Participants were excluded if they had diabetes. Compared to STEP 1, STEP 3 used a more intensive lifestyle intervention, which included an initial 8-week meal replacement diet, as well as 30 individual lifestyle counseling visits. Investigators wished to explore whether adding this more intensive behavioral therapy would increase the total weight loss achieved with semaglutide 2.4 beyond that observed in STEP 1. Participants who received semaglutide with intensive behavioral therapy lost 16.0% of initial weight at week 68, compared with 5.7% for placebo (estimated treatment difference = −10.3 percentage points; 95% CI=−12.0% to −8.6%, p<0.001; Figure 2). Semaglutide-treated participants were more likely than placebo to lose ≥5%, ≥10%, ≥15%, and ≥20% of initial weight, with 35.7% and 3.7% of participants, respectively, meeting the last criterion (Figure 3). Participants in the semaglutide group, relative to placebo, had greater reductions in waist circumference, systolic and diastolic blood pressure, c-reactive protein, hemoglobin A1c, and lipids (with the exception of HDL; Table 1). Groups did not differ significantly in physical function.
STEP 4
STEP 4 examined the effect of continuing versus withdrawing semaglutide treatment after 20 weeks of initial therapy in adults with a BMI ≥30 kg/m2 (or ≥27 kg/m2 with ≥1 weight-related comorbidity) and without diabetes.(37) A total of 902 participants received semaglutide once-weekly during the run-in. After 20 weeks, 803 participants achieved the 2.4 mg/week maintenance dose, lost an average of 10.6% of initial weight, and agreed to continue in a 48-week randomized trial in which they were assigned in double-blind fashion to continued semaglutide or switched to placebo. Those who remained on semaglutide lost 7.9 percentage points of their randomization weight from weeks 20 to 68, whereas those switched to placebo gained 6.9 percentage points (estimated treatment differences = −14.8; 95% CI=−16.0 to −13.5 percentage points; p<0.001). As measured from the start of the run-in period, the two groups achieved mean cumulative weight losses at week 68 of 17.4% and 5.7%, respectively (see Figures 2 and 3). Continued semaglutide, relative to the switch to placebo, was associated with better outcomes for waist circumference, systolic blood pressure, hemoglobin A1c, fasting plasma glucose, fasting serum insulin, lipid profile, and physical functioning on the SF-36 (Table 1).
Safety
As with other GLP-1 receptor agonists, the most common side effects with semaglutide are gastrointestinal. In STEP trials 1–3, the percent of participants in the semaglutide 2.4 mg vs placebo groups who experienced nausea was 33.7–58.2% versus 9.2–22.1%; diarrhea was 21.3–36.1% vs 11.9–22.1%; vomiting was 21.8–27.3% vs 2.7–10.8%; and constipation was 17.4–36.9% vs 5.5–24.5%. Most gastrointestinal events were mild-to-moderate in severity, transient, and occurred during the first 20 weeks of treatment during dose escalation. For example, in STEP 3, the median event duration of nausea was 5 days in both groups, vomiting was 2 days in both groups, diarrhea was 3 days in both groups, and constipation was 27 days with semaglutide vs 16 days with placebo.(36) The proportion of patients with premature discontinuation because of adverse events was 5.9–7.0% in the semaglutide groups and 2.9–3.5% in the placebo groups. Gastrointestinal effects were the main adverse-event-related cause of drug discontinuation, accounting for 3.4 to 4.5% of discontinuations in the semaglutide group versus 0 to 1% in the placebo group. Gallbladder-related disorders (primarily cholelithiasis) were reported in 2.6% vs 1.2% of participants on semaglutide vs placebo in STEP 1, 0.2% vs 0.7% of participants in STEP 2, and 4.9% vs 1.5% in STEP 3.
Semaglutide is contraindicated in pregnancy and in patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia syndrome type 2, or known hypersensitivity to semaglutide or any of the components. Rare cases of acute pancreatitis, hypoglycemia, acute kidney injury, diabetic retinopathy in patients with type 2 diabetes, angioedema and anaphylaxis have been reported with semaglutide.
Cardiovascular Safety
As with other GLP-1 receptor agonists, semaglutide is associated with increases in heart rate of 1 to 4 beats per minute.(34–36, 38) Pulse rate should be monitored routinely in patients taking semaglutide, and the medication should be stopped in those with sustained increases. The small mean increase in pulse rate does not appear to adversely affect cardiovascular outcomes, as revealed by results of the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6).(39) In this multicenter, double-blind, cardiovascular outcomes study, 3297 adults with type 2 diabetes and established cardiovascular disease were randomized to receive semaglutide (0.5 mg or 1.0 mg) daily or volume-matched placebo for 104 weeks. The median observation time was 2.1 years. The primary composite outcome -- death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke -- occurred in significantly fewer participants treated by semaglutide (6.6%) than by placebo (8.9%) (HR, 0.74; 95% CI, 0.58 to 0.95; p<0.001 for noninferiority; p=0.02 for superiority).
Cardiovascular outcomes currently are being investigated in people with obesity with established cardiovascular disease (but not diabetes) in the Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity (SELECT) trial.(40) Participants are randomized to semaglutide 2.4 mg or placebo for 31 to 59 months of treatment. The trial’s primary outcome is time to first occurrence of a composite endpoint consisting of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke. An observational, non-interventional survey study, called SELECT-LIFE, will examine the long-term effects of participants taking part in the SELECT trial. SELECT-LIFE will last up to 10 years after the SELECT trial ends.
Ongoing STEP Trials
Several additional STEP trials are ongoing or planned. STEP 5 (NCT03693430) enrolled 304 adults with a BMI ≥30 kg/m2 (or ≥27 kg/m2 with ≥1 obesity-related comorbidity) who were randomized to semaglutide 2.4 mg or placebo for 104 weeks. In STEP 1–4, the trial duration was 68 weeks, and weight loss seemed to plateau, on average, close to the end of each trial. STEP 5 was designed to assess the durability of weight loss with semaglutide and will be able to better show the longer-term trajectory of weight loss with the medication. This trial was completed in March 2021. STEP 6 (NCT03811574) enrolled 401 East Asian adults from Japan and Korea with a BMI≥27 kg/m2 and ≥2 weight-related comorbidities or ≥35 kg/m2 with ≥1 comorbidity (following guidelines from the Japan Society for the Study of Obesity). Participants were randomized to semaglutide 1.7 mg, semaglutide 2.4 mg, or placebo for 68 weeks. The study was completed in November 2020. STEP 7 (NCT04251156) is a randomized controlled trial of 375 people with or without type 2 diabetes which is being conducted primarily in China. STEP 8 (NCT04074161) is comparing the weight loss efficacy of semaglutide versus liraglutide 3.0 mg/day versus placebo for individuals with overweight/obesity. STEP TEENS (NCT04102189) is evaluating the efficacy and safety of semaglutide in adolescents with a BMI equal to or above the 95th percentile (on gender and age-specific growth charts (CDC.gov)) or equal to or above 85th percentile with ≥1 weight-related comorbidity. A study is also ongoing investigated how well semaglutide works in people living with heart failure and obesity (STEP-HFpEF; NCT04788511).
Discussion
Taken together, the first four STEP trials have revealed several critical findings concerning the induction and maintenance of weight loss. Prior to semaglutide, other FDA-approved AOM produced placebo-subtracted weight losses of 2.6 kg to 8.8 kg at 1 year.(23) STEP 1 found that semaglutide increased this value to 12.5 kg, with a mean 14.9% reduction in baseline weight at 68 weeks, compared with to 7%−8% for most other AOM. More than half of participants in STEP 1 lost 15% or more of baseline weight, and 30% of participants lost 20% of initial weight, a loss that approaches that produced by sleeve gastrectomy. Greater weight loss reliably produces greater improvements in several cardiometabolic risk factors,(3) as well as quality of life,(41) as was observed in the STEP trials. This should be welcomed news to patients and their practitioners.
Participants in STEP 1 received only approximately monthly, brief lifestyle counseling visits, which produced a loss of 2.4% of baseline weight at week 68 (as combined with placebo). STEP 3, with the provision of meal replacement products (for the first 8 weeks) and intensive behavioral therapy(42) (IBT, 30 counseling sessions over 68 weeks), increased weight loss in the placebo group to 5.7% at week 68. (The loss approached 8% at week 24 before participants regained weight in the remainder of the study.) Study investigators had thought that the addition of semaglutide to meal replacements and IBT might boost total weight loss to 18% to 20% of initial weight. However, this combined intervention produced a 16% loss at week 68, only 1 percentage point greater than that observed in STEP 1. Thus, the study did not suggest the occurrence of fully additive weight loss, which had been observed when IBT was combined with AOM that produced a mean reduction of only 5%−6% of initial weight.(43) Further study is needed to determine the frequency of lifestyle counseling required to achieve a 15% reduction in weight with semaglutide 2.4 mg. The medication may be so effective in reducing appetite and unplanned eating episodes– the traditional targets of IBT – that less intensive dietary and behavioral counseling are required. This, however, would not eliminate the need for education on eating a health-promoting diet or on increasing physical activity, both of which contribute to reductions in cardiovascular risk factors.(3)
STEP 2 demonstrated the efficacy of semaglutide 2.4 mg in inducing clinically meaningful weight loss in patients with overweight/obesity and type 2 diabetes. However, the week-68 loss of 9.6% was approximately one-third less than that observed in participants without diabetes in the three other STEP trials reviewed. Similar attenuation in weight loss in patients with vs without type 2 diabetes has been observed with other AOM, including orlistat,(44) naltrexone-bupropion,(45) and liraglutide 3.0 mg,(46) as well as with some behavioral interventions.(47, 48) Mechanisms responsible for smaller weight losses in patients with diabetes may include reductions in resting metabolic rate, which occur with improved glycemic control (accompanying weight loss), decreased dietary adherence and “defensive snacking” related to fears of hypoglycemia, as well as reduced glycosuria.(49, 50) STEP 2 also demonstrated that semaglutide 2.4 mg induced significantly greater weight loss than semaglutide 1.0 mg, the dose approved for the treatment of type 2 diabetes (9.6% vs 7.0%). HbA1c improved markedly in both groups – by 1.5–1.6% - but did not differ significantly, affirming the different doses prescribed for the management of obesity vs type 2 diabetes.
STEP 4 revealed two very important findings, the first which is that patients will lose substantial amounts of weight during the 16-week upward titration period but will not achieve their maximum weight loss until taking the full dose for 1 year. This continued weight loss, after the typical plateau that occurs at approximately 6 months with other AOM and behavioral interventions, is welcomed by patients and likely reinforces adherence to the medication. The second finding concerns the relatively rapid weight regain that occurred in participants who were randomly assigned to discontinue medication after the 20-week run-in. Such regain is common following the termination of AOM (and behavioral interventions) and underscores the need to prescribe these medications on a long-term basis with the recognition that obesity, for most individuals, is a chronic condition that can be managed by not cured. This method is comparable to the management of hypertension, type 2 diabetes, hypercholesterolemia, sleep apnea, and related diseases. Patients will need to understand that, following the first year of treatment, continued use of semaglutide will help them principally in maintaining their lost weight rather than in losing additional weight (which many will desire because they will still have excess weight). Concerns after 1 year that the medication “is not working any longer” can be addressed by showing how the medication prevented weight regain at a maintenance dose of 2.4 mg in participants in STEP 4.
As with all GLP-1 medications, gastrointestinal side effects were common in participants who took semaglutide 2.4 mg, though most were mild-to-moderate, and the majority of participants recovered without treatment discontinuation. The mechanisms behind the gastrointestinal side effects are not fully understood. They could be the result of the effect of the medication on gastrointestinal functions such as gastric emptying, intestinal motility, secondary changes in the secretion of other gastrointestinal peptide hormones, and/or direct or indirect effects on the central nervous system.(51) Patients may be advised to eat slowly, decrease portions, stop eating when satiated, and to avoid high-fat foods. Flexible titration may be necessary for patients who are experiencing side effects, and adjunctive therapies (e.g., antiemetic medications) may be helpful. Apart from gradual dose titration, data on how to prevent or treat gastrointestinal side effects are needed.
Similar to other GLP1-RA medications,(52) semaglutide is associated with an increased risk of gallbladder events relative to placebo. The exact mechanism of development of cholelithiasis in GLP1-RAs is unknown but may be attributable to large, rapid weight losses experienced by some patients.(53) Cholelithiasis and cholecystectomy are common in patients who achieve rapid weight loss with very-low-calorie diets(54) or bariatric surgery.(55) Cholelithiasis formation may also be a result of the direct action of GLP-1 and its agonist on biliary secretion and/or drug-induced modification of gallbladder motility.(56) If cholelithiasis is suspected, gallbladder imaging studies and appropriate clinical follow-up are indicated. Studies are needed to identify individuals who may be at most risk for development of gallstones with semaglutide, as well as the effectiveness of possible prophylactic treatment options (e.g., ursodeoxycholic acid 500 mg/day; ensuring at least 7 grams per day of fat intake).(57)
Several promising additional AOM are in the development pipeline. Semaglutide is approved as a subcutaneous injection for obesity treatment. While participants only need to inject themselves once a week, this may limit use for some individuals who are reluctant to inject a medication. An oral version of semaglutide is currently approved for type 2 diabetes and is being tested in a trial for obesity treatment. The oral formulation may improve convenience, acceptance, and adherence. Several combination medications are also in development, such as amylin/semaglutide and glucose-dependent insulinotropic polypeptide (GIP, formerly known as gastric inhibitory polypeptide)/GLP-1. For example, tirzepatide, a GIP/GLP-1 co-agonist, is being tested for type 2 diabetes (SURPASS program) and for obesity (SURMOUNT program). With the large anticipated weight losses from these medications, studies examining the effects on body composition will be necessary.
Economic evaluations are needed to help elucidate potential short- and long-term cost-savings with AOM including those generated by improvements in weight and prevention and treatment of weight-related comorbidities. Several studies in patients with type 2 diabetes (in different countries) have shown absolute cost savings with semaglutide 1.0 mg, as well as the cost effectiveness of semaglutide 1.0 mg compared to other diabetes pharmacotherapies.(58, 59) However, few cost evaluations have been conducted for AOM specifically for weight management; one study suggested that semaglutide may not be cost-effective because of its high price.(60) However, these findings were calculated before results from the STEP trials were published. Further analyses are needed to compare the costs and benefits of semaglutide relative to those for behavioral and surgical approaches.
Semaglutide has demonstrated the highest percent weight loss of any obesity medication to date. Several trials of injectable semaglutide are in process, which may expand the global reach of obesity pharmacotherapies, as well as the options for weight management in youth. Increased knowledge of the physiology of weight regulation has provided opportunities to improve the treatment of this chronic condition. A greater number of effective pharmacotherapeutics also may improve the precision of obesity treatment. Future studies will be necessary to directly compare different medications, and to identify biological and behavioral phenotypes that may help to tailor treatments.
Funding
AMC was supported, in part, by the National Institute of Nursing Research of the National Institutes of Health under Award Number K23NR017209.
Disclosures
Dr. Chao reports grants from WW International Co and Eli Lilly and Co, outside the submitted work. Dr. Tronieri has served as a consultant to Novo Nordisk. Dr. Wadden reports serving on advisory boards for Novo Nordisk and WW International Co and receiving grants from Novo Nordisk. Dr. Amaro reports receiving grants from Fractyl Laboratories and serving on advisory boards for Novo Nordisk and Pfizer. No funding was received to assist with the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Obesity and overweight 2018. [Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 2.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–70. [DOI] [PubMed] [Google Scholar]
- 3.Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. NEJM. 2013;369(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal R, Vaduganathan M, Chiu N, Bhatt DL. Potential implications of the FDA approval of semaglutide for overweight and obese adults in the United States. Prog Cardiovasc Dis. 2021. [DOI] [PubMed] [Google Scholar]
- 5.Koliaki C, Doupis J. Incretin-based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2011;2(2):101–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YS, Jun HS. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism. 2014;63(1):9–19. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly D The structure and function of the glucagon‐ like peptide‐ 1 receptor and its ligands. Br J Pharmacol. 2012;166(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilsbøll T, Agersø H, Krarup T, Holst J. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab. 2003;88(1):220–4. [DOI] [PubMed] [Google Scholar]
- 9.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. [DOI] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014 2021. [Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014.
- 11.Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne). 2019;10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Medicines Agency. Victoza 2009. [Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/victoza.
- 13.Food and Drug Administration. Victoza® (liraglutide [rDNA origin] injection), solution for subcutaneous use 2010. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022341lbl.pdf.
- 14.Food and Drug Administration. SAXENDA (liraglutide [rDNA origin] injection), solution for subcutaneous use 2014. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206321Orig1s000lbl.pdf.
- 15.European Medicines Agency. Saxenda 2015. [Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/saxenda.
- 16.Van Can J, Sloth B, Jensen C, Flint A, Blaak E, Saris W. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes. 2014;38(6):784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. The Lancet. 2009;374(9701):1606–16. [DOI] [PubMed] [Google Scholar]
- 18.Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean M, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36(6):843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. NEJM. 2015;373(1):11–22. [DOI] [PubMed] [Google Scholar]
- 20.Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale P, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes. 2013;37(11):1443–51. [DOI] [PubMed] [Google Scholar]
- 21.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–99. [DOI] [PubMed] [Google Scholar]
- 22.Blackman A, Foster G, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes. 2016;40(8):1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315(22):2424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khera R, Pandey A, Chandar AK, Murad MH, Prokop LJ, Neeland IJ, et al. Effects of weight-loss medications on cardiometabolic risk profiles: a systematic review and network meta-analysis. Gastroenterology. 2018;154(5):1309–19. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637–49. [DOI] [PubMed] [Google Scholar]
- 26.Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58(18):7370–80. [DOI] [PubMed] [Google Scholar]
- 27.Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–66. [DOI] [PubMed] [Google Scholar]
- 28.Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–54. [DOI] [PubMed] [Google Scholar]
- 29.Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, et al. Effects of once‐ weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes and Metab. 2017;19(9):1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes and Metab. 2021;23(3):754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wharton S, Calanna S, Davies M, Dicker D, Goldman B, Lingvay I, et al. Gastrointestinal tolerability of once‐ weekly semaglutide 2.4 mg in adults with overweight or obesity, and the relationship between gastrointestinal adverse events and weight loss. Diabetes Obes and Metab. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushner RF, Calanna S, Davies M, Dicker D, Garvey WT, Goldman B, et al. Semaglutide 2.4 mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5. Obesity. 2020;28(6):1050–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilding JP, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. NEJM. 2021. [DOI] [PubMed] [Google Scholar]
- 35.Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2· 4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–84. [DOI] [PubMed] [Google Scholar]
- 36.Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wegovy (semaglutide) injection 2.4 mg [Available from: https://www.novopi.com/wegovy.pdf.
- 39.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. NEJM. 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 40.Ryan DH, Lingvay I, Colhoun HM, Deanfield J, Emerson SS, Kahn SE, et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J. 2020;229:61–9. [DOI] [PubMed] [Google Scholar]
- 41.Pearl RL, Wadden TA, Tronieri JS, Berkowitz RI, Chao AM, Alamuddin N, et al. Short‐ and long‐ term changes in health‐ related quality of life with weight loss: results from a randomized controlled trial. Obesity. 2018;26(6):985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wadden TA, Tsai AG, Tronieri JS. A protocol to deliver intensive behavioral therapy (IBT) for obesity in primary care settings: the MODEL‐ IBT program. Obesity. 2019;27(10):1562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. NEJM. 2005;353(20):2111–20. [DOI] [PubMed] [Google Scholar]
- 44.Kelley DE, Bray GA, Pi-Sunyer FX, Klein S, Hill J, Miles J, et al. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin-treated type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2002;25(6):1033–41. [DOI] [PubMed] [Google Scholar]
- 45.Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–99. [DOI] [PubMed] [Google Scholar]
- 47.Wing RR, Marcus MD, Epstein LH, Salata R. Type II diabetic subjects lose less weight than their overweight nondiabetic spouses. Diabetes Care. 1987;10(5):563–6. [DOI] [PubMed] [Google Scholar]
- 48.Antoun G, Nikpay M, McPherson R, Harper M-E, Dent R. Is type 2 diabetes in adults associated with impaired capacity for weight loss? Canadian Journal of Diabetes. 2018;42(3):313–6. e1. [DOI] [PubMed] [Google Scholar]
- 49.Carlson MG, Campbell PJ. Intensive insulin therapy and weight gain in IDDM. Diabetes. 1993;42(12):1700–7. [DOI] [PubMed] [Google Scholar]
- 50.Brown A, Guess N, Dornhorst A, Taheri S, Frost G. Insulin‐ associated weight gain in obese type 2 diabetes mellitus patients: What can be done? Diabetes Obes and Metab. 2017;19(12):1655–68. [DOI] [PubMed] [Google Scholar]
- 51.Baraboi ED, St-Pierre DH, Shooner J, Timofeeva E, Richard D. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1011–R24. [DOI] [PubMed] [Google Scholar]
- 52.Nreu B, Dicembrini I, Tinti F, Mannucci E, Monami M. Cholelithiasis in patients treated with Glucagon-Like Peptide-1 Receptor: An updated meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2020;161:108087. [DOI] [PubMed] [Google Scholar]
- 53.Erlinger S Gallstones in obesity and weight loss. Eur J Gastroenterol Hepatol. 2000;12(12):1347–52. [DOI] [PubMed] [Google Scholar]
- 54.Kamrath RO, Plummer LJ, Sadur CN, Adler MA, Strader W, Young R, et al. Cholelithiasis in patients treated with a very-low-calorie diet. Am J Clin Nutr. 1992;56(1):255S–7S. [DOI] [PubMed] [Google Scholar]
- 55.Manatsathit W, Leelasinjaroen P, Al-Hamid H, Szpunar S, Hawasli A. The incidence of cholelithiasis after sleeve gastrectomy and its association with weight loss: a two-centre retrospective cohort study. Int J Surg. 2016;30:13–8. [DOI] [PubMed] [Google Scholar]
- 56.Nexøe‐ Larsen CC, Sørensen PH, Hausner H, Agersnap M, Baekdal M, Brønden A, et al. Effects of liraglutide on gallbladder emptying: A randomized, placebo‐ controlled trial in adults with overweight or obesity. Diabetes Obes and Metab. 2018;20(11):2557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lammert F, Acalovschi M, Ercolani G, van Erpecum KJ, Gurusamy K, van Laarhoven CJ, et al. EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. 2016. [DOI] [PubMed]
- 58.Viljoen A, Hoxer CS, Johansen P, Malkin S, Hunt B, Bain SC. Evaluation of the long‐ term cost‐ effectiveness of once‐ weekly semaglutide versus dulaglutide for treatment of type 2 diabetes mellitus in the UK. Diabetes Obes and Metab. 2019;21(3):611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkinson L, Hunt B, Johansen P, Iyer NN, Dang-Tan T, Pollock RF. Cost of achieving HbA1c treatment targets and weight loss responses with once-weekly semaglutide versus dulaglutide in the United States. Diabetes Ther. 2018;9(3):951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee M, Lauren BN, Zhan T, Choi J, Klebanoff M, Abu Dayyeh B, et al. The cost‐ effectiveness of pharmacotherapy and lifestyle intervention in the treatment of obesity. Obesity Science & Practice. 2020;6(2):162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


