Despite increased awareness of the potential risks of electronic cigarette (e-cigarette) use [1], vaping e-liquids containing nicotine continues to be thought of as a less harmful alternative to smoking combustible cigarettes. Recent evidence points to nicotine-containing e-cigarettes, either alone or in combination with nicotine patches, as being effective for smoking cessation [2, 3]. Although both e-cigarettes and nicotine replacement therapy (NRT) provide a replacement source of nicotine, e-cigarettes are often vaped over the long term while NRT is applied mostly for a discrete quitting period [2].
Short abstract
As opposed to smoking cessation with nicotine-replacement therapy and/or varenicline, nicotine-containing e-cigarette use does not improve some airway inflammatory markers. https://bit.ly/3FyqIt9
To the Editor:
Despite increased awareness of the potential risks of electronic cigarette (e-cigarette) use [1], vaping e-liquids containing nicotine continues to be thought of as a less harmful alternative to smoking combustible cigarettes. Recent evidence points to nicotine-containing e-cigarettes, either alone or in combination with nicotine patches, as being effective for smoking cessation [2, 3]. Although both e-cigarettes and nicotine replacement therapy (NRT) provide a replacement source of nicotine, e-cigarettes are often vaped over the long term while NRT is applied mostly for a discrete quitting period [2]. A recent study showed that 63 (80%) out of 79 participants who successfully switched from tobacco cigarettes to nicotine-containing e-cigarettes continued to use e-cigarettes after 52 weeks compared to only four (9%) out of 44 participants who continued to use NRT [2]. Thus, harm reduction by switching from tobacco cigarettes to e-cigarettes must be examined. Here, we report that expression of tumour necrosis factor (TNF)-α mRNA remained elevated and levels of matrix metalloproteinase (MMP)-9 activity were significantly increased in the upper airways of smokers who switched from combustible cigarettes to nicotine-containing e-cigarettes as compared to smokers who abstained from combustible cigarettes with the aid of NRT and/or varenicline.
Veterans with ≥5-pack-years smoking history who actively smoked were recruited from the Miami Veterans Affairs Medical Center (Miami, FL, USA) and trained to vape flavouring-free e-liquid containing 50%/50% (w/v) propylene glycol and vegetable glycerine with freebase nicotine (12 mg·mL−1) using a second-generation eVic Supreme (Joyetech, Shenzen, China) e-cigarette device (www.clinicaltrials.gov identifier NCT03251053) [4]. Volunteers who exclusively used nicotine-containing e-cigarettes during a 4-week replacement phase (weeks 1–4) were then asked to continue exclusive e-cigarette use for an additional 12-week maintenance phase (weeks 5–16). Compliance was biochemically confirmed by exhaled levels of carbon monoxide (<6 ppm) and venous carboxyhaemoglobin values (<1.6%). Seven participants successfully switched to exclusive e-cigarette use and abstained from smoking combustible cigarettes for the duration of the 16-week study period (e-cigarette group). Seven active smokers with ≥5-pack-years smoking history who continued to smoke combustible cigarettes (cigarette group) and eight active smokers with ≥5-pack-years smoking history who quit smoking (biochemically confirmed with exhaled levels of CO ≤5 ppm) with the aid of NRT and/or varenicline (NRT/VAR group) served as comparators, and were recruited from the Miami Veterans Affairs Medical Center and Swope Health (Kansas City, MO, USA), respectively.
The mean±sd age of study participants was 55±11 years in the NRT/VAR group, 55±12 years in the cigarette group and 57±13 years in the e-cigarette group (p=0.69, Kruskal–Wallis test). The percentage of males was 38% (three out of eight) in the NRT/VAR group, 100% (seven out of seven) in the cigarette group and 100% (seven out of seven) in the e-cigarette group (p=0.004, Chi-squared test). Most participants were of black ethnicity: 100% (eight out of eight) in the NRT/VAR group, 57% (four out of seven) in the cigarette group and 71% (five out of seven) in the e-cigarette (p=0.28, Chi-squared test). Mean±sd smoking history was 21±14 pack-years in the NRT/VAR group, 27±14 pack-years in the cigarette group and 36±21 pack-years in the e-cigarette group (p=0.24, one-way ANOVA followed by Tukey test).
To assess inflammation in the upper airways, we collected nasal epithelial cells (NECs) and nasal epithelial lining fluid (ELF) from participants in both the cigarette and e-cigarette groups at the beginning of week 5 and the end of week 16. NECs and nasal ELF were collected from the NRT/VAR group at the beginning of the study (visit 1) and at the end of any consecutive 12-week period (within 18 weeks of visit 1) in which volunteers were able to successfully abstain from combustible cigarettes, as assessed by exhaled CO levels. NECs were collected using a 5.5-mm diameter nylon brush (Doft AB, Östhammar, Sweden) and nasal ELF was collected in the nostrils using precut, absorbent Leukosorb paper (Pall Corporation, Port Washington, NY, USA). Sampling of the nasal mucosa is minimally invasive and nasal samples can be reliably assayed for markers of airway inflammation as a well-accepted surrogate for the lower airways [5, 6].
TNF-α, transforming growth factor (TGF)-β1 and MMP-9 were the major focus of this study because they are well-known mediators of smoking-related airway inflammation and play important roles in the progression of COPD pathogenesis [7–10]. Furthermore, expression of these markers is known to be elevated in nasal samples from smokers [10, 11]. TNF-α also decreases in subjects quitting with NRT within a 12-week period [12]. We therefore measured expression levels of TNF-α, TGF-β1, and MMP-9 mRNAs from NECs by droplet digital PCR with TaqMan assays (TNF-α, Hs00174128_m1; TGF-β1, Hs00998133_m1; MMP-9, Hs00234579_m1; Thermo Fisher Scientific, Waltham, MA, USA) and levels of MMP-9 activity from nasal ELF collections using a Human Active MMP-9 Fluorokine E Kit (#F9M00; R&D Systems, Minneapolis, MN, USA). The study protocol was reviewed and approved by the Miami Veterans Affairs Medical Center and the University of Kansas Medical Center Institutional Review Boards.
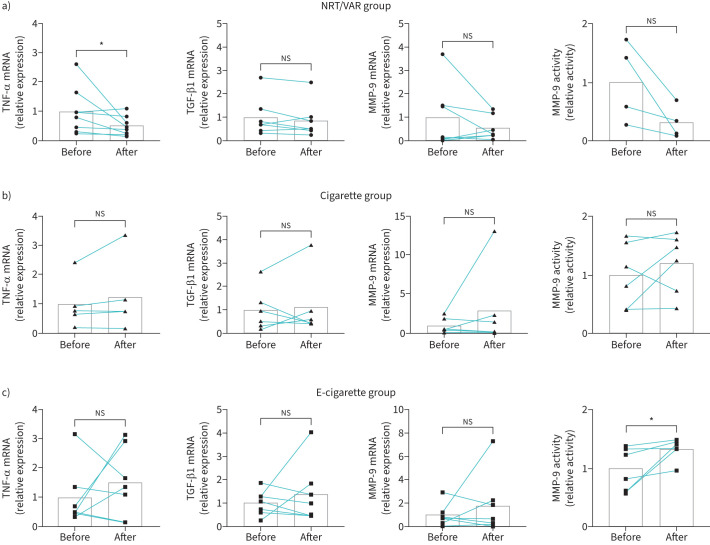
The NRT/VAR group showed a significant decrease in expression levels of TNF-α mRNA after 12 weeks (figure 1a), consistent with a recent report showing reduced systemic levels of TNF-α in participants who quit smoking with NRT or varenicline after 12 weeks [12]. However, TNF-α mRNA expression levels were unchanged in both the cigarette and e-cigarette groups after the 12-week period (figure 1b and c). No group showed significant changes in expression levels of TGF-β1 or MMP-9 mRNAs, although trends were observed (figure 1). As MMP-9 activity, rather than expression, is likely to be a better indicator of an inflammatory environment, we measured MMP-9 activity from nasal ELF samples. Required adjustments in nasal ELF collection procedures due to COVID-19 (no nasal spray application before collection) limited the amount of material for MMP-9 activity assays in the NRT/VAR group for some participants. Despite the small sample size, levels of MMP-9 activity showed a trending decrease in the NRT/VAR group after 12 weeks, correlating with the reduction in MMP-9 mRNA expression (figure 1a). Although the cigarette group did not show a significant change in MMP-9 activity levels (figure 1b), the e-cigarette group showed a significant increase in levels of MMP-9 activity after the 12-week period (figure 1c).
FIGURE 1.
Changes in expression levels of tumour necrosis factor (TNF)-α, transforming growth factor (TGF)-β1 and matrix metalloproteinase (MMP)-9 mRNAs and activity levels of MMP-9 in the upper airways of study participants. a) Relative expression levels of TNF-α, TGF-β1, and MMP-9 mRNAs in nasal epithelial cells (NECs), and relative activity levels of MMP-9 in nasal epithelial lining fluid (ELF), collected from smokers who abstained from smoking with the aid of nicotine-replacement therapy or varenicline (NRT/VAR group) measured at baseline (before) and after a 12-week period of successful abstinence from combustible cigarettes (after). b) Relative expression levels of TNF-α, TGF-β1 and MMP-9 mRNAs in NECs, and relative activity levels of MMP-9 in nasal ELF, collected from smokers who continued to smoke combustible cigarettes measured at week 5 (before) and week 16 (after). c) Relative expression levels of TNF-α, TGF-β1, and MMP-9 mRNAs in NECs, and relative activity levels of MMP-9 in nasal ELF, collected from smokers who switched exclusively to electronic cigarettes (e-cigarettes) measured at week 5 (before) and week 16 (after). Measurements from some participants were not included because mRNA levels were below the limit of detection. Bars show mean values. All data were analysed using Prism software (GraphPad, San Diego, CA, USA). ns: not significant. *: p<0.05 by Wilcoxon test.
In conclusion, the finding of persistent inflammation in the upper airways of e-cigarette users that successfully abstained from smoking should be carefully considered in the context of e-cigarettes as a tobacco smoking cessation tool designed for long-term use. COPD is a pathophysiological consequence of persistent airway inflammation linked to increased TNF-α signalling and protease activity, including MMP-9 [7, 8]. MMP-9 was recently found to be equally elevated in bronchoalveolar lavage from both smokers and users of nicotine-containing e-cigarettes, including both previous smokers and never-smokers, compared to healthy nonsmokers [13].
An important limitation of this study is that only second-generation e-cigarette devices containing e-liquids with freebase nicotine were used. Newer generation e-cigarettes may be more or less toxic than the e-cigarette device used in the present study. It will therefore be important to determine whether airway inflammation persists in smokers who switch to newer-generation e-cigarettes. Another limitation is the low overall subject number and significant difference in the sex of participants among the three groups given differences in nicotine metabolism between males and females [14]. Unfortunately, our study was not powered to examine sex differences. However, a recent study found that circulating levels of endothelin-1 and TNF-α were significantly reduced in smokers who quit with NRT or varenicline after 12 weeks with no significant differences based on sex [12]. Despite these limitations, our study provides evidence that NRT or varenicline as cessation methods can lead to improvements in some airway inflammatory markers compared to nicotine-containing e-cigarettes after only 12 weeks of abstinence.
Acknowledgements
We would like to thank Tricia Snow, Michael Arnold and Terri Tapp for their help monitoring abstinence and collecting nasal samples from study participants at Swope Health.
Provenance: Submitted article, peer reviewed.
This study is registered at www.clinicaltrials.gov with identifier number NCT03251053.
Author contributions: M.D. Kim, N. Baumlin, M. Campos and M. Salathe conceived and designed the study. M.D. Kim, N. Baumlin and M. Salathe contributed to data acquisition, data analysis and interpretation. A. Guerrero-Cignarella, A. Schmid, C. Aguiar, N. Nollen, E.L. Leavens and J.S. Ahluwalia contributed to study design, study participant enrolment and sample collection. M. Mohiuddin and J.S. Dennis contributed to data acquisition and analysis. M.D. Kim and M. Salathe wrote the manuscript with intellectual input from all authors. All authors read and approved the final manuscript.
Conflicts of interest: The authors declare no conflict of interest.
Support statement: This study was supported by the NIH/NHLBI (R01 HL139365 to M. Salathe), the James and Esther King Florida Biomedical Research Program (grant number 5JK02 to M. Salathe and M. Campos), and the Flight Attendant Medical Research Institute (CIA 160011 to M. Salathe). A portion of this work was also supported by the NIH/NIDA (R01 DA046576 to N. Nollen), Frontiers: The Heartland Institute for Clinical and Translational Research which is supported by a CTSA grant to the University of Kansas Medical Center from the NIH National Center for Advancing Translational Science (grant number UL1TR000001), and by the National Cancer Institute Cancer Center Support Grant P30 CA168524 and used the Clinical Pharmacology and Biospecimen Repository Shared Resources. J.S. Ahluwalia was supported in part by P20GM130414, a NIH-funded Center of Biomedical Research Excellence. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Marques P, Piqueras L, Sanz MJ. An updated overview of e-cigarette impact on human health. Respir Res 2021; 22: 151. doi: 10.1186/s12931-021-01737-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of E-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019; 380: 629–637. doi: 10.1056/NEJMoa1808779 [DOI] [PubMed] [Google Scholar]
- 3.Walker N, Parag V, Verbiest M, et al. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. Lancet Respir Med 2020; 8: 54–64. doi: 10.1016/S2213-2600(19)30269-3 [DOI] [PubMed] [Google Scholar]
- 4.Guerrero-Cignarella A, Luna Diaz LV, Balestrini K, et al. Differences in vaping topography in relation to adherence to exclusive electronic cigarette use in veterans. PloS One 2018; 13: e0195896. doi: 10.1371/journal.pone.0195896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDougall CM, Blaylock MG, Douglas JG, et al. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol 2008; 39: 560–568. doi: 10.1165/rcmb.2007-0325OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebuli ME, Speen AM, Clapp PW, et al. Novel applications for a noninvasive sampling method of the nasal mucosa. Am J Physiol Lung Cell Mol Physiol 2017; 312: L288–L296. doi: 10.1152/ajplung.00476.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 2007; 87: 1047–1082. doi: 10.1152/physrev.00048.2006 [DOI] [PubMed] [Google Scholar]
- 8.Churg A, Zhou S, Wright JL. Matrix metalloproteinases in COPD. Eur Respir J 2012; 39: 197–209. doi: 10.1183/09031936.00121611 [DOI] [PubMed] [Google Scholar]
- 9.Takizawa H, Tanaka M, Takami K, et al. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med 2001; 163: 1476–1483. doi: 10.1164/ajrccm.163.6.9908135 [DOI] [PubMed] [Google Scholar]
- 10.Kim MD, Baumlin N, Dennis JS, et al. Losartan reduces cigarette smoke-induced airway inflammation and mucus hypersecretion. ERJ Open Res 2021; 7: 00394-02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues FM, Ramos D, Xavier RF, et al. Nasal and systemic inflammatory profile after short term smoking cessation. Respir Med 2014; 108: 999–1006. doi: 10.1016/j.rmed.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 12.Derella CC, Tingen MS, Blanks A, et al. Smoking cessation reduces systemic inflammation and circulating endothelin-1. Sci Rep 2021; 11: 24122. doi: 10.1038/s41598-021-03476-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh A, Coakley RD, Ghio AJ, et al. Chronic E-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am J Respir Crit Care Med 2019; 200: 1392–1401. doi: 10.1164/rccm.201903-0615OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benowitz NL, Lessov-Schlaggar CN, Swan GE, et al. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther 2006; 79: 480–488. doi: 10.1016/j.clpt.2006.01.008 [DOI] [PubMed] [Google Scholar]