Abstract
Introduction
Children and adolescents with autism spectrum disorder (ASD) appear to be at greater risk of excess weight gain. The aim of this systematic review and meta-analysis was to examine whether children with ASD have a greater prevalence of obesity and whether the prevalence of ASD is higher in children with obesity.
Methods
We conducted a systematic search on PubMed, Scopus, and PsychINFO until May 21, 2021. We used the meta package in the R in order to calculate the pooled prevalence and relative risk of obesity in children with ASD.
Results
We found 20 eligible studies investigating the prevalence of obesity in children with ASD, with the prevalence ranging from 7.9 to 31.8% and from 1.4 to 23.6% among controls. All but three studies originated from the USA. The proportion of children with obesity in ASD populations was 17% (95% confidence interval [CI]: 13–22). The relative risk of obesity in children with ASD compared with control children was 1.58 (95% CI: 1.34–1.86). There were no controlled studies reporting on the prevalence of ASD in children with obesity.
Conclusion
Children and adolescents with ASD have a higher prevalence of obesity than healthy controls. There is a need for further prevalence studies of obesity in children with ASD, especially outside the USA, since the few European studies carried out have failed to show a significant difference between obesity prevalence in children with and without ASD. There is no knowledge at all regarding the prevalence of ASD among children with obesity.
Keywords: Obesity, Autism, Children, Review
Introduction
Autism spectrum disorder (ASD) is characterized by abnormalities in social interactions, social communication, and repetitive behavior and interests (Diagnostic and Statistical Manual, 5th edition; DSM-5) [1]. The social deficits include lack of social-emotional reciprocity and difficulty of developing relationships. The repetitive behaviors and interests can be characterized by extreme reluctance to change daily routines, and intense interests [1].
The prevalence of ASD worldwide is estimated by the DSM-5 to be 1% [1]. There are some indications that the prevalence of ASD is increasing [2], due to changes in the diagnostic criteria, more refined screening and diagnostic tools, and an increased awareness of ASD [3, 4]. ASD is four times more often diagnosed in males than in females [1].
Childhood obesity is a growing public health problem with a negative impact on both physical and mental status. It is associated with an elevated risk of cardiovascular disease and a reduced life span, both related to obesity later in life but unrelated to adult weight status [5, 6, 7, 8, 9]. The social and emotional consequences of childhood obesity include impaired quality of life [10], difficulties forming relationships with peers, being bullied, and depression [11, 12]. Additionally, weight seems to be programmed during childhood and adolescence, which makes prevention early in life important in order to reduce the risk of mental and physical sequelae, including premature death [13]. The definition of obesity in children, recommended by The Institute of Medicine and American Academy of Pediatrics, corresponds to BMI ≥95th percentile based on growth charts specific to the child's age and sex [14]. An alternative and equivalent definition of childhood obesity are ≥ two standard deviations of BMI for age and sex, recommended by the WHO [15].
The prevalence of childhood obesity in the USA has been estimated at 17% in a study of typically developed (TD) children and adolescents aged 2–19 in 2014 [16]. Other countries seem to have lower prevalence numbers than the USA. A Dutch study that assessed children aged 4–16 found the obesity prevalence in boys to be 2.6% and in girls 3.3% [17].
Children and adolescents with developmental disabilities, including ASD, appear to be at greater risk of excess weight gain [18]. The reasons for developing obesity are multifactorial. Typical autistic eating habits in children with ASD, including selective eating, are one of these factors [19, 20, 21, 22]. Children with ASD exhibit a preference for energy-dense foods, sweetened beverages, and snack foods [23] and have a low intake of vegetables [19, 23, 24] compared with their TD peers.
The most effective and commonly used interventions for ASD are educational and behavioral therapies. No drug has so far been able to improve the social deficits included in the ASD diagnosis. However, second-generation antipsychotic drugs, such as risperidone, have been shown to reduce effectively the disruptive behaviors typically seen in these children [4]. A negative side effect of second-generation antipsychotic drugs is substantial weight gain [4, 25], which probably contributes to the overrepresentation of obesity in children with ASD.
The first study that investigated the prevalence of obesity in children with ASD was published in 1997. Forty-three percent of the children with ASD were suffering from obesity, and the severity of the obesity correlated positively with the severity of the ASD [26]. During the following 2 decades, a plethora of studies have continued to report prevalence rates of obesity in ASD populations; however, the vast majority of studies have been conducted in the USA.
Based on the above findings, we aimed at conducting a systematic review and meta-analysis to examine the prevalence and relative risk of obesity in children diagnosed with ASD as well as the prevalence of ASD in children with obesity. We hypothesized that there is an overrepresentation of obesity in children aged 2–18 diagnosed with ASD compared with children without ASD. We further assumed that the prevalence of ASD in children aged 2–18 with obesity would be higher than the ASD prevalence among children without obesity.
Methods
All procedures were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [27].
Information Sources
The search included PubMed, Scopus, and PsychINFO databases and was conducted between May 7 and May 21, 2021.
Search Process
The search terms to cover ASD were autism, autism spectrum disorder, Asperger syndrome, and autistic disorder. To make sure the reports would include at least one of the terms representing ASD, the searches were designed by using the coded word “OR” between the terms in the following way, “Autism” OR “Autism Spectrum Disorder” OR “Asperger Syndrome” OR “Autistic Disorder.” The term Pervasive Developmental Disorder was later included in the search to cover the earlier term for ASD from the DSM-IV. “Obesity” OR “Overweight” was added to the search as well as “Child” OR “Adolescent” to fit the patient group we were examining. The three different parts of the search were combined with the coded term “AND” to make sure that all of the segments of the search were included in the process. The first search had all these elements put together as follows: (“Autism” OR “Autism Spectrum Disorder” OR “Asperger Syndrome” OR “Autistic Disorder”) AND (“Obesity” OR “Overweight”) AND (“Child” OR “Adolescent”). The same search was made in all three databases. In PsychINFO, we excluded books and actively included “Children” and “Adolescents” under their age group tab.
The second search was made in order to include the earlier term for ASD and was composed as follows: (“Pervasive Developmental Disorder”) AND (“Obesity” OR “Overweight”) AND (“Child” OR “Adolescent”). The same search was performed in all three databases. In line with the first search, we also excluded books and actively included “Children” and “Adolescents” under their age group tab in PsychINFO.
Eligibility Criteria
For eligibility criteria, see Table 1. We did not exclude papers based on publication date, ethnicity, sex, country of publication, journal of publication or level of care.
Table 1.
Eligibility criteria for the systematic review
| Inclusion criteria | Exclusion criteria |
|---|---|
| Control group | Language other than English |
| Age of patients in the study group and in the control group: 2–18 years old | No full-text availability |
| ≥50 people in the study population and control group, respectively | Reviews |
| ASD diagnoses consistent with the guidelines of the time | Books |
| Obesity defined and reported on in the study | Case studies |
| Reported prevalence of obesity/ASD in study group and control group | Theses |
ASD, autism spectrum disorder.
Study Selection
The selection process followed the PRISMA protocol [28]. The studies found in the databases were first screened by title and abstract. Studies which measured the prevalence of obesity in children and/or adolescents who were diagnosed with ASD were included, as well as studies including children and/or adolescents with obesity who had been screened for an ASD diagnosis. Since the search was conducted in three different databases some studies could be included more than once. Duplicates were excluded using EndNote.
A secondary screening was made on the selected articles in full text according to the eligibility criteria. This screening was conducted by the first author and by at least one coauthor (all PhDs; one postdoc and two senior researchers), ensuring that all articles were read by at least two authors.
Data Collection Process
The relevant data were extracted from the selected studies by the first author and one of the coauthors in order to minimize selection bias.
Data Items
The variables extracted from the studies were year of publication, age range of patients, number of study participants, number of control participants, definition of obesity in the study, how the ASD diagnosis was assigned, characteristics of the control group (healthy controls or patients with other psychiatric diagnoses than ASD), prevalence of obesity/ASD in the study group, prevalence of obesity/ASD in the control group, study type (e.g., national surveys, retrospective chart reviews, etc.), and other comments. Whether weight and height had been measured or self-reported was also noted.
Statistical Analyses
The odds ratios (OR) of having obesity among children and/or adolescents with ASD versus the control group were calculated for each of the studies selected for this review. The ORs were calculated using the following formula: OR = ad/cb. The 95% confidence intervals (CIs) of these ORs were calculated using the following formula: 95% CI = e^[ln (OR) ± 1.96√(1/a + 1/b + 1/c + 1/d)]. In these formulas, a = (n) of exposed cases, b = (n) of exposed non-cases, c = (n) of unexposed cases, d = (n) of unexposed non-cases [29].
Meta-Analyses
We used the meta package in the R version 3.6.2 software to calculate the pooled prevalence of obesity in children with ASD and the OR and relative risk compared with controls. We conducted (a) a meta-analysis of the proportion of children with obesity in populations with ASD, (b) a meta-analysis of the OR for children with obesity in populations with ASD and children with obesity in populations without ASD, and (c) a meta-analysis of the relative risk of obesity among children with ASD compared with children without ASD. We used both the fixed effect model, assuming homogeneity, which means no heterogeneity, and the random effects model, assuming heterogeneity.
The random effects model assumes that the parameter that we want to estimate is normally distributed. This model always results in wider CIs, which take smaller studies into account to a greater extent. The DerSimonian and Laird method was used for random effects and the inverse variance method was used for fixed effects when estimating the OR and the relative risk. The Freeman-Tukey transformation was used to calculate the weighted summary proportion of obesity in children with ASD under the fixed and random effect model.
The extent of inconsistency in the study results was quantified by calculating I2, defined as (Q − df) × 100%/Q, where Q is the χ2 statistic and df its degree of freedom. I2 describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error. I2 values vary between 0% and 100%, and over 75% means considerable heterogeneity. Results from both the fixed effect and the random effects model will be presented for all models. The central estimates will often be the same for the fixed effect and the random effects models. However, the CIs are always wider for the random effects model. If the central estimates are the same according to the two models, this does not indicate no heterogeneity. If there is no heterogeneity, the fixed effect and the random effects model will yield the same result, with regard both to the central estimate and the 95% CI. The publication bias was estimated for the meta-analyses using a funnel plot.
Results
Study Selection
Regarding the search terms autism, autism spectrum disorder, Asperger syndrome, and autistic disorder, 1,065 records were found in the three databases. When searching for pervasive developmental disorder, 43 additional records were identified. The articles were screened by title and abstract. After duplicates and articles not available in full text were removed, 85 articles remained. These full-text papers were screened according to the eligibility criteria by the first author and one of the coauthors. Sixty-five papers were excluded leaving 20 studies to be included in the review. The study selection is thoroughly reported in the PRISMA flow diagram (Fig. 1) and the excluded articles that were screened by full text are reported with the reasons for exclusion in online supplementary Table S1 (for all online suppl. material, see www.karger.com/doi/10.1159/000523943).
Fig. 1.
PRISMA flow diagram. After screening by title and abstract, 168 records remained. Duplicates (same studies in several databases) were then excluded (n = 71 articles). Ninety-seven articles remained after the duplicates had been removed. When articles not available in full text were removed, 85 articles remained. The full-text papers were screened according to the eligibility criteria, and 65 papers were excluded leaving 20 studies to be included in the review. Eleven of the remaining 20 studies were based on data from the National Survey of Children's Health (NSCH). Only one study per NSCH survey year was included in the meta-analysis, and the selected study should comprise the broadest age span and the largest number of individuals with autism. Six NSCH studies were therefore excluded (1 from NSCH year 2004, 3 from NSCH year 2012, and 2 from NSCH year 2017), leaving 14 studies that were included in the meta-analysis.
Characteristics of the Included Studies
Table 2 shows the nine studies where weight and height had been measured by a health professional. In all but two of these studies, ASD diagnoses had been either assigned in the study or a previous ASD diagnosis was confirmed in the study.
Table 2.
Summary of studies included in the review
| Author | Country | Study design | Ages, years | Sample (ASD/controls) | Gender % male (ASD/controls) | Characteristics of control group | Obesity definition | ASD diagnosis |
|---|---|---|---|---|---|---|---|---|
| Barnhill et al. [58] | USA | Cross-sectional case-control study | 2–13 | 86/57 | 92%/82% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Before the study by trained clinicians and confirmed by psychologist. DSM-IV and 5 |
|
| ||||||||
| de Hoogd et al. [43] | The Netherlands | Retrospective chart review | 4–18 | 256/336 | 85.9%/80.1 % | Psychiatric controls | ≥95th percentile of the sex-specific BMI for age growth charts | Clinical assessment based on DSM-IV-TR by treating physician |
|
| ||||||||
| Evans et al. [23] | USA | Cross-sectional study | 3–11 | 53/58 | 83%/78% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Confirmed by autism diagnostic interveiew − revised |
|
| ||||||||
| Esteban-Figuerola et al. [42] | Spain | Epidemologic | 3–12 | 79/350 | 83.5% in ASD | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Confirmed by DSM-V, ADOS-2 and ADI-R |
|
| ||||||||
| Healy et al. [45] | Ireland | Cross-sectional study | 13 | 67/74 | 79.1%/52.7% | Healthy controls | Standardized by age and sex by international obesity task force, based on estimated BMI at age 18 | Diagnosis reported by primary caregiver |
|
| ||||||||
| Hill et al. [48] | USA and Canada | Cross-sectional study | 2–17 | 5,053/8,844 | 84.5% in ASD | Healthy controls from NHANES | ≥95th percentile of the sex-specific BMI for age growth charts | Confirmed by DSM-IV, and ADOS |
|
| ||||||||
| Hyman et al. [49] | USA and Canada | Cross-sectional study | 2–11 | 362/559 | 86% in ASD | Healthy controls from NHANES | ≥95th percentile of the sex-specific BMI for age growth charts | Confirmed by DSM-IV and ADOS |
|
| ||||||||
| Ptomey et al. [44] | USA | Retrospective database study | 2–18 | 1,073/274 | 80.8% in ASD | Controls diagnosed with IDD or Down's syndrome | ≥95th percentile of the sex-specific BMI for age growth charts | Diagnosis reported by primary caregiver |
|
| ||||||||
| Shedlock et al. [40] | USA | Retrospective case-control study | 2–18 | 48,762/243,810 | 80%/80% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | ICD-9 codes at 2 separate encounters |
Weight and height were clinically measured. NHANES, National Health and Nutrition Examination Survey; ADOS, autism diagnostic obeservation schedule.
Table 3 presents the eleven remaining studies lacking measurements but reporting anthropometric data. A common denominator for all these studies were that weight, height, and ASD diagnoses were based on parental reports and not assessed by the research teams (see also the paragraph entitled “National Survey of Children's Health” below).
Table 3.
Summary of studies included in the review
| Author | Country | Study design | Ages, years | Sample (ASD/controls) | Gender % male (ASD/controls) | Characsteristics of control group | Obesity definition | ASD diagnosis |
|---|---|---|---|---|---|---|---|---|
| Chen et al. [30] | USA | Cross-sectional national survey, NSCH (2004) | 10–17 | 247/22,713 | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Reported by guardian | |
|
| ||||||||
| Corvey et al. [32] | USA | Cross-sectional national survey, NSCH (2012) | 6–17 | 1,385/64,259 | 82.8%/50.6% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Reported by guardian |
|
| ||||||||
| Curtin et al. [31] | USA | Cross-sectional national survey, NSCH (2004) | 3–17 | 454/85,272 | 79%/51% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Reported by guardian |
|
| ||||||||
| Healy et al. [38] | USA | Cross-sectional national survey, NSCH (2017) | 10–17 | 750/25,173 | 80.6%/50.3% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Reported by guardian |
|
| ||||||||
| Mccoy et al. [33] | USA | Cross-sectional national survey, NSCH (2012) | 10–17 | 915/41,879 | 81%/51.5% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Reported by guardian |
|
| ||||||||
| Mccoy and Morgan [37] | USA | Cross-sectional national survey, NSCH (2017) | 10–17 | 1,144/31,168 | 80.4%/49.9% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Reported by guardian |
|
| ||||||||
| Must et al. [34] | USA | Cross-sectional national survey, NSCH (2012) | 10–17 | 925/42,852 | 83.7%/50.5% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Reported by guardian |
|
| ||||||||
| Philips et al. [39] | USA | Cross-sectional national survey, NHIS (2010) | 12–17 | 93/8,141 | 79.7%/47% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Reported by guardian |
|
| ||||||||
| Rimmer et al. [59] | USA | Cross-sectional online survey (2009) | 12–18 | 159/12,973 | Healthy controls from youth risk behavior survey 2007 | ≥95th percentile of the sex-specific BMI for age growth charts | Reported by guardian | |
|
| ||||||||
| Tybor et al. [36] | USA | Cross-sectional national survey, NSCH (2017) | 10–17 | 699/23,552 | 80.1%/50.3% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Reported by guardian |
|
| ||||||||
| Voulgarakis et al. [35] | USA | Cross-sectional national survey, NSCH (2012) | 2–17 | 2,041/2,041 | 79.3%/51.7% | Healthy controls | ≥95th percentile of the sex-specific BMI for age growth charts | Reported by guardian |
Weight and height reported by parent/guardian. NSCH, National survey of Children's health; NHIS, National Health Interview Survey.
Of all 20 papers, 17 were written in the USA alone or in collaboration with Canada. The other three were from the Netherlands, Ireland, and Spain, respectively. Sixteen papers presented a gender distribution in the ASD group, and all of them comprised more males than females. The percentage of males varied between 79% and 92%. Eight papers included only children above age 10. Four papers only looked at children in the lower end of the age range (2–13 years). The number of participants in the studies ranged between 111 and 292,572 children in total, of which the number of children with ASD varied between 53 and 48,762. Only two studies had control groups consisting of patients with other psychiatric disorders (i.e., mood disorder, tic disorder, schizophrenia spectrum disorder, disruptive behavior disorder, Down syndrome, intellectual disability).
National Survey of Children's Health
The National Survey of Children's Health (NSCH) consists of cross-sectional national surveys conducted by the National Center for Health and Statistics at the Centers for Disease Control and Prevention under the direction of the Maternal and Child Health Bureau in the USA. The surveys are population-based and include interviews conducted with a parent of children aged 0–17 years. If the household includes multiple children, only one child is randomly selected to participate. The purpose of the NSCH is to obtain prevalence estimates for health problems in a nationally representative sample of noninstitutionalized children, including children with ASD. Until 2016, the interviews were conducted by trained interviewers [30, 31, 32, 33, 34, 35], but since 2016, the survey consists of internet-based parental questionnaires [36, 37, 38]. In the present review, nine papers are based on different surveys from the NSCH from the years 2003, 2012, and 2016, and some of these studies are based on NSCH data originated from the same year. Only one study per survey year was included in the analyses, and the study selected should comprise the broadest age span, and the largest number of individuals with autism. One article from 2010 used a similar survey from the same organization, called the National Health Interview Survey (NHIS) with the same principal method [39]. In all these eleven studies, weight and height are based on parental reports, and no reliability assessments of the reports had been performed. Based on the parental report, BMIs and the obesity prevalence were calculated. Regarding the ASD diagnosis, the parent was asked “Has a doctor or other health care provider ever told you that your child has autism, Asperger's disorder, pervasive developmental disorder (PDD), or other autism spectrum disorder?” [35]. In 2003, 102,353 interviews were performed with a response rate of 55.3% [31], in 2007 the response rate was 46.7% (91,642 interviews) [34], in 2012, 95,677 surveys were conducted (response rate: 23%) [35], and in 2016, 95,677 interviews were made with a completion rate of 68.7% [38].
Obesity Prevalence in Children with ASD, including Meta-Analyses
In the present review, 20 articles were analyzed regarding prevalence estimates for obesity in children with ASD (Table 4). The prevalence of obesity in the children with ASD ranged between 7.9% and 31.8%, and between 1.4% and 23.6% in the control group. The prevalence of obesity was statistically significantly higher in the ASD group in eleven of the studies (Table 4). The prevalence of obesity was between 7.9% and 22.8% among children with ASD in the three European studies; prevalence rates that were not statistically significantly higher than the rates among the control children without ASD.
Table 4.
Obesity prevalence in the studies included in the review
| Author | Prevalence in ASD group, % | Prevalence in control group, % | Ages, years | p value (for %) | OR (95% CI) |
|---|---|---|---|---|---|
| Barnhill et al. [58] | 9.0 | 7.0 | 13 | 0.773 | 1.36 (0.39, 4.47) |
| Chen et al. [30] | 23.4ab | 12.2ab | 10–17 | <0.001 | 2.21 (1.64, 2.97) |
| Corvey et al. [32] | 16.4 | 9.9 | 6–17 | <0.001 | 1.79 (1.76, 1.81) |
| Curtin et al. [31] | 30.4 | 23.6 | 3–17 | 0.075 | 1.41 (1.16, 1.73) |
| de hoogd et al. [43] | 15.2 | 10.3 | 2–17 | 0.079 | 1.55 (0.95, 2.52) |
| Evans et al. [23] | 17.0 | 9.0 | 2–18 | 0.09 | 2.17 (0.68, 6.94) |
| Esteban-Figuerola et al. [42] | 22.8 | 14 | 3–12 | 0.060 | 1.81 (0.99, 3.32) |
| Healy et al. [45] | 7.9 | 1.4 | 2–13 | 0.073 | 5.89 (0.67, 51.75) |
| Healy et al. [38] | 23.1 | 15.9 | 10–17 | <0.001 | 1.58 (1.33, 1.88) |
| Hill et al. [48] | 18.0 | 16.7 | 2–11 | 0.12 | 1.09 (1.00, 1.20) |
| Hyman et al. [49] | 14.0 | 5.4 | 4–18 | 0.079 | 1.59 (0.94, 2.69) |
| McCoy et al. [33] | 22.2 | 14.1 | 10–17 | <0.001 | 1.74 (1.48, 2.03) |
| Mccoy and Morgan [37] | 23.3 | 12.5 | 10–17 | <0.001 | 2.13 (1.85, 2.45) |
| Must et al. [34] | 23.1 | 14.1 | 10–17 | <0.001 | 1.83 (1.57, 2.14) |
| Philips et al. [39] | 31.8 | 13.1 | 12–17 | <0.001 | 3.16 (2.04, 4.90) |
| Ptomey et al. [44] | 24.4 | 21.9 | >2 | 0.428 | 1.15 (0.84, 1.58) |
| Rimmer et al. [59] | 24.6c | 13.0c | 12–18 | <0.001 | 2.18 (1.51, 3.13) |
| Shedlock et al. [40] | 8.2 | 4.7 | 3–11 | <0.001 | 1.81 (1.74, 1.88) |
| Tybor et al. [36] | 23.0 | 15.9 | 10–17 | 0.0049 | 1.58 (1.32, 1.89) |
| Voulgarakis et al. [35] | 12.6 | 7.2 | 2–17 | 0.015 | 1.86 (1.50, 2.30) |
Adjusted for age, sex/gender, race/ethnicity.
Adjusted for household income/poverty-to-income ratio, household education/parental education/maternal education, geographic location, family structure.
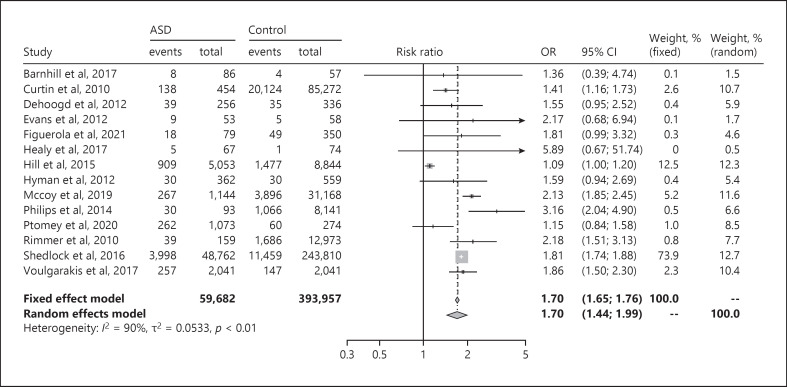
The OR for children with obesity in populations with ASD and children with obesity in populations without ASD was 1.70 (95% CI: 1.44–1.99) (See Fig. 2). The I2 value was over 75% (I2 = 90%), signaling high heterogeneity, i.e., highly inconsistent study results. When dividing the data into two forest plots based on whether the participants had been measured or not, the parent-reported studies still showed high heterogeneity (I2 = 90%) (online suppl. Fig. S1a, b).
Fig. 2.
Forest plot of OR between children with ASD and obesity and control children with obesity. ASD, autism spectrum disorder; OR, odds ratio; CI, confidence interval. The two left-hand columns (“events” and “total”) correspond to the ASD group, and the next two columns (“events” and “total”) represent the control group.
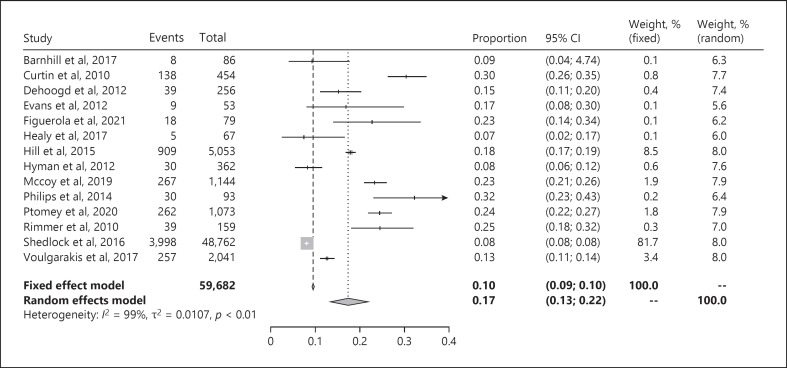
Figure 3 illustrates the forest plot of the proportion of children with obesity in ASD populations. The pooled prevalence of obesity in children with ASD was 17% (95% CI: 13–22). The study by Shedlock et al. [40], which includes the largest number of participants by far, dominates the results in this analysis. The separate results from the fixed effect model and the random effects model confirm the skewed representation (Fig. 3).
Fig. 3.
Forest plot of proportion of children with obesity in ASD populations. ASD, autism spectrum disorder; CI, confidence interval.
Heterogeneity, I2, was 98% when all studies were pooled together (Fig. 3). When the studies were divided into forest plots based on whether weight and height were measured (online suppl. Fig. S2a) or reported by parents (online suppl. Fig. S2b), none of the plots showed an I2 value below 75%.
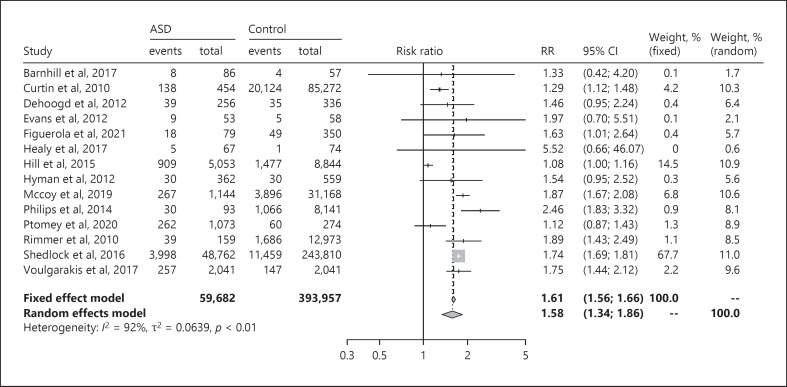
The relative risk of obesity among children with ASD compared with children without ASD was 1.58 (95% CI: 1.34–1.86), according to the forest plot shown in Figure 4 I2 was 92%, implying high heterogeneity (Fig. 4). The I2 value in the studies with measured weight and height was 92% (online suppl. Fig. S3a), which was equivalent with the result in the subgroup of studies where parents had reported weight and height (online suppl. Fig. S3b). The relative risk of obesity among the children with ASD in the three studies from Europe varied between 1.46 and 5.52. The funnel plot showed no publication bias (online suppl. Fig. S4), indicating that the findings were robust.
Fig. 4.
Forest plot of the relative risk of obesity among children with ASD compared with children without ASD. ASD, autism spectrum disorder; RR, risk ratio; CI, confidence interval.
Sensitivity Analyses
The sensitivity analysis was performed by replacing the NSCH study from 2004 with the largest sample of children with autism and the broadest age span, 3–17 years, by Curtin et al. [31], with the NSCH study conducted the same year with the second largest sample of autism and the second broadest age span, 6–17 years, by Chen et al. [30]. The same procedure was performed regarding the NSCH study from 2012, where Voulgarakis et al. [35] (age span 2–17 years) was replaced by Corvey et al. [32] (age span 6–17 years), and the NSCH study from 2017, where McCoy and Morgan [37] (age span 10–17; number of children with autism: 1,144) was replaced by Healy et al. [38] (age span 10–17; number of children with autism: 750). The sensitivity analysis showed that the OR, obesity prevalence and relative risk were on a par with the findings in the original analyses (online suppl. Table S2, sensitivity analysis 1).
According to Akinbami and Ogden [41], parents may overestimate overweight among children below the age of ten. A sensitivity analysis was thus performed where studies with children below 10 years of age, in combination with parent-reported anthropometric data, were excluded. According to the sensitivity analysis, the proportion of obesity among the children with autism, the OR, and the relative risk were almost the same as in the original analyses (online suppl. Table S2, sensitivity analysis 2).
In order to prevent a mix of different study designs, one study with case-control design by Shedlock et al. [40] and one study with an epidemiological design by Esteban-Figuerola et al. [42] were excluded. A sensitivity analysis implied a slightly lower OR, 1.68 (95% CI: 1.39–2.04; I2: 88%) versus 1.70 (95% CI: 1.44–1.99; I2: 90%), a higher proportion of obesity among the children with autism, 18% (95% CI: 14–23; I2: 94%) compared with 17% (95% CI: 13–22; I2: 99%), and a lower relative risk, 1.56 (95% CI: 1.28–1.91; I2: 89%) versus 1.58 (95% CI: 1.34–1.86; I2: 92%) (online suppl. Table S2; sensitivity analysis 3).
Two studies used a control group where the participants had another psychiatric disorder than ASD; in the study by de Hoogd et al. [43], the control group was recruited from the psychiatric department, and Ptomey et al. [44] included children with an intellectual developmental disorder and Down's syndrome in the control group. A sensitivity analysis, where the two studies with a psychiatric control group were excluded, was therefore performed. The OR and the relative risk were marginally increased compared with the original analysis (online suppl. Table S2; sensitivity analysis 4).
Among the studies where weight and height had been measured (Table 2), there were two studies, one by Healy et al. [45] and one by Ptomey et al. [44], where the ASD diagnoses had only been reported by the parents and not confirmed by the researchers. In a sensitivity analysis, these two studies were excluded. The heterogeneity was not affected by excluding the two studies (online suppl. Table S2; sensitivity analysis 5).
A subgroup analysis of population-based versus hospital-based studies was conducted to investigate whether the heterogeneity (I2) could be reduced. Regarding the population-based subgroup heterogeneity pertaining to the OR was 76%, the proportion of children with obesity 97%, and the relative risk was 84%. With respect to the hospital-based subgroup heterogeneity regarding the OR was 93%, the proportion of children with obesity 99%, and the relative risk was 94%. Hence, the heterogeneity remained after the two subgroups were analyzed separately (online suppl. Table S2; subgroup analysis 1 + 2).
ASD Prevalence in Children with Obesity
Only one of the 85 articles that were read in full text to test for eligibility looked at the ASD prevalence in children with obesity. The study published by Wentz et al. [46] was excluded from the review due to the lack of a control group. The study reported an ASD prevalence of 13.2% among children with obesity (8.1% among females and 17.9% among males).
Potential Causes of Obesity Comorbidity Presented in the Included Studies
Obesity Prevalence in Children with ASD by Sex
All of the papers that reported sex distribution in children with ASD showed a male preponderance. This is in line with ASD prevalence studies in general [47]. No studies reported a statistically significant association between obesity prevalence and the gender of the children with ASD [38, 48].
Obesity Prevalence in Children with ASD Based on Age
Some articles investigated the relationship between age and prevalence of obesity in children with ASD. Two studies found a stabilizing period between 6 and 11 years among children with ASD, when obesity was not overrepresented compared with TD children [48, 49]. However, Healy et al. [38] found no association between age and prevalence of obesity in children with ASD.
Obesity Prevalence in Children with ASD Based on ASD Severity
Several papers investigated the relationship between obesity and the severity of the ASD diagnosis. Healy et al. [38], and McCoy and Morgan [37] found that the obesity prevalence increased with increasing severity; however, Hill et al. [48] found no such association.
Eating Behavior, Physical Activity, and Sedentary Behavior in ASD Children
Evans et al. [23] reported that children with ASD consumed significantly more sweetened beverages, energy-dense snacks, and fewer meals with vegetables than TD children. Three studies found children with ASD to be less likely to participate in physical activity compared with TD children [33, 37, 45]. This association increased with age [33, 37]. Several studies reported that children with ASD were not more likely to participate in sedentary behavior, e.g., computer use, than TD children [33, 37, 45]. However, sedentary behavior increased with age according to one report [33]. These results imply that with increasing age, children with ASD are less physically active, which may be due to several sports being less tailored to children with ASD.
Obesity Prevalence in ASD Based on Medication
Approximately 35% of the children with ASD were on medication [36, 37, 38]. Five studies found an association between being medicated for ASD and suffering from obesity [32, 36, 40, 43, 48]. One study found no such association [38].
Discussion
The major findings of the present systematic review and meta-analysis were that the prevalence estimates of obesity among children with ASD was 17%, and the children with ASD had a 58% greater risk of developing obesity compared with TD children. When the studies were divided into subgroups, based on whether anthropometric data were measured or reported by parents, the prevalence estimates and relative risk did not differ between the subgroup where weight and height had been measured compared with the subgroup where anthropometric data had been reported by the parents.
Other meta-analyses published between 2017 and 2020 are in accordance with the findings of the present study. Li et al. [50] found the pooled estimate of the prevalence of obesity to be 21.8% for all age groups, including adults. They observed an increase in prevalence from 14.8% in age group 2–5 to 23.5% in age group 6–12, 29.9% in age group 13–17, and to 31.3% among those over age 18. The lower prevalence estimates in the present study are partly explained by only individuals with ASD below the age of 18 being investigated, while Li et al. [50] also included adults in their meta-analysis. Kahathuduwa et al. [51] reported a significantly increased risk of 41% among children with ASD to develop obesity compared with healthy control children, and Zheng's et al. [52] found a significantly higher prevalence of obesity among children with ASD than among controls, with an OR of 1.84. Kahathuduwa et al. [51] excluded all studies with self-reported or parent-reported anthropometric data. We agree with their reasoning and therefore decided to divide the studies into two groups: studies where the children were measured and studies where the anthropometric data were based on parental reports. In the present meta-analysis, many of the studies that had not measured the children included the largest amounts of participants, in one case more than a thousand children with ASD and comorbid obesity [40]. Among the studies where weight and height had been measured, the relative risk in the present study was higher than the findings by Kahathuduwa's et al.[51] (Kahathuduwa et al. [51]: 41%; present study: 58%). However, in line with Kahathuduwa et al. [51], we found a very high heterogeneity (Kahathuduwa et al. [51]: 93%; present study: 92%), and due to this inconsistency, these data must be interpreted with caution.
Obesity Prevalence in Children with ASD
The prevalence of obesity in children and adolescents in the present review ranged between 7.9% and 31.8%. The prevalence of obesity was statistically significantly higher in the ASD groups than in the control groups in twelve of the 20 studies. All but 3 of the 20 studies were conducted in North America, which limits our chances of drawing any general conclusions regarding the prevalence of obesity in children with ASD in other countries. There were indications of a relationship between the prevalence of obesity and age; the older the children with ASD, the higher the prevalence of obesity. A systematic review published in 2020 [50] corroborates our observation. That review showed a higher prevalence of obesity in the age group 13–17 than in younger age groups. The use of psychotropic medication, less physical activity, and an excess intake of high-calorie foods and drinks were factors in the present review that contributed to the higher prevalence of obesity in the young populations with ASD.
Out of the 20 studies in the systematic review, there were seven studies with the lower end of the OR CIs below 1 (Table 4). These seven studies had the smallest number of study participants with ASD, ranging between 79 and 1,073. This may imply that these studies had too few participants to reach a significant difference between the index groups and the controls. However, the report by Hill et al. [48], with a nonsignificant difference of obesity prevalence between participants with ASD and healthy controls, included a large study population (n = 13,897). Hill et al. [48] used a control group consisting of healthy controls from the National Health and Nutrition Examination Survey. The study by Hyman et al. [49], which used the same control group as Hill et al. [48], also presented a nonsignificant difference between groups. The control group used by the two studies [48, 49] had the second highest obesity prevalence of all control groups (16.7%), which may explain the nonsignificant results.
Only three of the studies in the present review were performed outside North America. They were performed on children living in Ireland [45], the Netherlands [43], and Spain [42]. None of the studies showed a significant difference between the ASD group and the control group. The study made in Ireland had the overall lowest prevalence of obesity, both in the ASD group and in the control group (7.9% vs. 1.4%, OR 5.89 [0.67, 51.75]). The number of participants was the second smallest (n = 141) of all studies, which may have yielded inadequate power to attain significant results. The study from the Netherlands [43] was one out of two studies [44] with a psychiatric control group, which may be a reason for the nonsignificant result, indicating a higher risk for children with psychiatric diagnoses in general to suffer from obesity compared with TD children. However, since the Irish, the Dutch, and the Spanish reports are the only three studies made outside the USA, we still lack enough data to draw any conclusions regarding obesity prevalence in other parts of the world than the USA. Moreover, the three European prevalence studies tended to have generally lower prevalence rates than the studies from the USA. One may surmise that there would be higher rates of obesity in children with ASD in the USA compared with children with ASD in other countries, since childhood obesity in general is more prevalent in the USA than in other western countries [53]. In addition, a review of children with comorbid ASD and overweight/obesity noted that living in the USA was a positive moderator of the association between ASD and overweight/obesity [51].
National Survey of Children's Health
Some of the studies made by the NSCH were performed during the same years and therefore from exactly the same study population. All NSCH studies were eligible for the systematic review, but only one study per year was included in the meta-analysis.
Four studies originated from the NSCH in 2012. Must et al. [34] and McCoy et al. [33] had an age range between 10 and 17 years, Corvey et al. [32] between 6 and 17 years and Voulgarakis et al. [35] between 2 and 17 years. In the two studies with the same age range (10–17 years), the number of participants was very similar, McCoy et al. [33] having 915 participants with ASD and 41,879 healthy controls, and Must et al. [34] having 925 children with ASD and 42,852 healthy controls. Must et al. [34] had more males in their sample (83.7% vs. 81%). The obesity prevalence in the two studies was the same in the two control groups (14.1%), but the obesity prevalence among the children with ASD was slightly higher in the study by Must et al. [34] (23.1% vs. 22.2%), as well as the ORs (1.83 [1.57, 2.14] vs. 1.74 [1.48, 2.03]). The differences may be explained by a higher rate of males in the ASD group in the study by Must et al. [34]. However, other differences between the two study populations may also have affected the results. The highest OR among the four studies performed in 2012 was reported by Voulgarakis et al. [35]. They investigated children with the widest age range of the four surveys, which contradicts the theory that studies with older children/adolescents result in higher ORs.
The age range of the three NSCH studies performed in 2017 was 10–17 years, and two of the studies had almost the same results. The two studies by Healy et al. [38] and Tybor et al. [36] had 750 versus 699 participants with ASD, and 25,173 versus 23,552 healthy controls, respectively. Both studies reported the obesity prevalence of the ASD group and the control group to be 23% and 15.9%, respectively. These similarities suggest that the two studies contain practically the same participants. Therefore, when scrutinizing the NSCH studies this phenomenon should be kept in mind when comparing data and drawing conclusions. The third NSCH study from 2017, performed by McCoy and Morgan [37], had more participants, a lower obesity prevalence in the control group and a higher OR (2.13 [1.85, 2.45]) than the two other NSCH studies from that year.
Strengths and Limitations
In this review, all full-text papers were read by two researchers independently to decide about eligibility and extract relevant data from the eligible articles. However, only the first author took responsibility for the identification step of the PRISMA procedure, and therefore a selection bias cannot be excluded. Even so, a quality marker for the screening process was the discovery of the high number of duplicates in the different searches. In addition, a meticulous search in reference lists of previous reviews showed a great overlap with the included papers in the present review and no further eligible articles were found. Previous reviews have, as opposed to the present review, included prevalence studies of both overweight and obesity in children with ASD. Since obesity is associated with more extensive physical and psychiatric consequences than overweight, we decided to focus solely on the group of children with ASD and comorbid obesity [51]. Compared with previous systematic reviews and meta-analyses we have narrowed the aim and focused only on children and adolescents, in contrast to Li et al. [50] and Zheng et al. [52], where all ages were analyzed. Furthermore, as mentioned above, we aimed to concentrate on children with obesity instead of those with either overweight or obesity. In addition, as opposed to Kahathuduwa et al. [51], we included both studies with measured weight and those where weight was reported by the parent but analyzed the two subgroups separately. Interestingly, the prevalence estimates and the relative risk did not differ between the two subgroups, and for this reason, this finding may indicate that both studies that rely on parental report and studies with measured anthropometric information can contribute reliable data. The present study also aimed at investigating the prevalence of ASD in children with obesity. However, no systematic review or meta-analysis could be performed since no controlled prevalence studies were found. This negative finding is still of great interest due to the knowledge gap that has been disclosed. Despite a relatively large number of prevalence studies of obesity in ASD, there has been a total lack of incentive to explore whether ASD could be overrepresented in individuals with obesity.
We decided to exclude dissertations, non-English written papers and studies without a control group, which may have affected the results, e.g., studies with an origin outside North America may have been overlooked. One non-English study was found, but the study did not meet the inclusion criteria due to the lack of a control group [54]. The number of databases used was, however, limited, and more studies could probably be found by searching additional databases such as Google scholar, Web of Science, etc. This may be considered a weakness. We still believe that our findings are generalizable since our data largely corroborate the results of previous systematic reviews and meta-analyses.
The included studies all used BMI when defining obesity. It is however important to note that BMI does not provide information on percentage of fat or distribution of fat. Most of the studies included used a cross-sectional design, resulting in a limited ability to draw conclusions about causality [30, 32, 34]. Most of the papers did not include institutionalized children with ASD, meaning that the most severe cases of ASD may not be represented in the data.
The survey-based studies included were based on parental reports which come with limitations. First, height and weight reported by parents may not be entirely accurate. A study by Goodman et al. [55] found that regarding teenagers defined as obese by clinicians, reports of height and weight from parents could be used when studying teen obesity and correlates. Akinbami and Ogden [41] found that parents overestimated the prevalence of overweight among younger children to the degree that the researchers advised against using parental reports with respect to children under the age of 10. Weden et al. [56] found similar results when looking at children aged 2–13; underestimating weight increased with age and underestimating height decreased with age. The researchers also recommended caution when studying parental-reported data on height regarding younger children [56]. Based on these findings, some of the studies included in this review were based on surveys with parental-reported anthropometric data, where only children above the age of 10 were included. However, the papers by Curtin et al. [31] included children aged 3–17, Corvey et al. [32] 6–17 and Voulgarakis et al. [35] 2–17 year-old individuals. Their results may be less accurate due to the non-trustworthy parental report pertaining to the children's anthropometric data under the age of 10. Children with ASD may consume more health care and may therefore be more frequently measured than TD children. We cannot find any other reason for parents of children with ASD to be better or worse at estimating their child's height and weight compared with parents of TD children. Kahathaduwa et al. [51] published a systematic review in 2019 pertaining to children with ASD and comorbid overweight or obesity. They made, on the other hand, another decision than in the present study and excluded all studies based on parental reports regarding weight and height.
A second limitation of the survey-based studies is that the ASD diagnoses had to be supplied by a health provider prior to the survey. This condition may have excluded some children not yet assigned an ASD diagnosis, due to, e.g., living in an area with limited access to healthcare or poor health knowledge. The low response rates of the surveys may also lead to an exclusion of the children with the least attention from healthcare.
A final limitation concerns our predefined exclusion criteria; we decided to exclude studies with less than 50 participants. Smaller studies, with less than 50 individuals, may indicate less representativeness, and the exclusion of smaller studies is noted in other systematic reviews [57]. Zheng et al. [52] published a meta-analysis on individuals with ASD and comorbid overweight and/or obesity, and the sample sizes of individuals with ASD ranged between 40 and 986,352 participants. They found 14 studies, of which 12 studies corresponded to the same age group as in the current study, which included 14 studies. The meta-analysis by Zheng et al. [52] found OR between children with obesity in populations with ASD and children with obesity in populations without ASD to be 1.84, which is in accordance with our results (OR 1.75). Kahathuduwa et al.'s [51] meta-analysis presented 31 reports of prevalence of obesity in children with ASD, and 9 of the studies had less than 50 participants with ASD (4 studies had less than 30 participants with ASD). Despite the inclusion of several studies with small sample sizes in the paper by Kahathuduwa et al. [51], the prevalence of obesity was fairly similar to our own findings (Kahathuduwa et al. [51]: 22.2% [CI: 18.1–26.9]; present study: 17% [CI: 13–22]). We therefore believe that our decision to exclude small samples did not cause skewed results. The results of the funnel plot in the present study confirm that our findings are robust.
A further predefined exclusion criterion was to exclude studies without a control group. This decision affected one of the three meta-analyses that we performed: the meta-analysis of the proportion of children with obesity in populations with ASD. As mentioned in the paragraph above, the proportion of children with obesity in the present study was in agreement with the findings by Kahathuduwa et al. [51], where studies without a comparison group were allowed.
Conclusions and Implications
The prevalence estimates and relative risk of obesity in children and adolescents with ASD are increased compared with TD children and adolescents. Publications with more accurate study design, reporting measured weight and height, show evidence of a difference between populations with ASD and TD populations in accordance with studies relying on parental reports. However, the meta-analyses showed considerable heterogeneity, which calls for a conscientious interpretation of the results. Older age, psychotropic medication, less physical activity and high consumption of high caloric foods and drinks seem to increase the risk of developing obesity among children with ASD. The present results suggest that we need to take further action in terms of preventing the emergence of obesity in children with ASD, at an early age. In addition, we have to intervene among those who have already developed obesity. To include these children in regular physical activity and guide families towards more healthy dietary habits are examples of interventions based on research data in the present review. Future prevalence studies must focus on collecting data also outside the USA; the few studies that have been conducted elsewhere have failed to show a statistically significant difference between obesity prevalence in children with and without ASD. The present review found no controlled studies on the prevalence of ASD in children with obesity. This signals a remarkable knowledge gap. We cannot exclude that a significant minority of children with obesity may suffer from ASD, and should be treated accordingly.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
Dr. Wentz received support from Swedish State Support for Clinical Research (#ALFGBG-813401). Dr. Dahlgren received support from (#ALFGBG-427731 and # ALFGBG-719711).
Author Contributions
All the authors contributed to the ideas and methods used in the study. All also contributed to interpretation of results, writing the paper, and approved the submitted version. All the authors collected the data. Olivia Sammels and Elisabet Wentz performed the data analyses.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
Our warmest gratitude to the staff at HTA center, Sahlgrenska University hospital for introducing the PRISMA methodology and to the personnel at the Gothenburg University Library for helping the first author with the literature search strategies. Independent statistical analysis was performed by Nils-Gunnar Pehrsson and Christopher Backström.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM-5) 5th ed. Washington DC: American Psychiatric Press; 2013. [Google Scholar]
- 2.Data & statistics on autism spectrum disorder [Internet] [cited 2019 Dec 26]. Available from: https://www.cdc.gov/ncbddd/autism/data.html.
- 3.Fact sheets: autism spectrum disorders. 2019. [cited 2019 Dec 26]. Available from: https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders.
- 4.Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383((9920)):896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 5.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999;23(Suppl 2):S2–11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 6.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76((3)):653–8. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 7.Thompson AE. JAMA PATIENT PAGE. Childhood obesity. JAMA. 2015;314((8)):850. doi: 10.1001/jama.2015.6674. [DOI] [PubMed] [Google Scholar]
- 8.Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32((6)):731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedemann C, Heneghan C, Mahtani K, Thompson M, Perera R, Ward AM. Cardiovascular disease risk in healthy children and its association with body mass index: systematic review and meta-analysis. BMJ. 2012;345:e4759. doi: 10.1136/bmj.e4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes AR, Farewell K, Harris D, Reilly JJ. Quality of life in a clinical sample of obese children. Int J Obes. 2007;31((1)):39–44. doi: 10.1038/sj.ijo.0803410. [DOI] [PubMed] [Google Scholar]
- 11.Gortmaker SL, Must A, Perrin JM, Sobol AM, Dietz WH. Social and economic consequences of overweight in adolescence and young adulthood. N Engl J Med. 1993;329((14)):1008–12. doi: 10.1056/NEJM199309303291406. [DOI] [PubMed] [Google Scholar]
- 12.Janssen I, Craig WM, Boyce WF, Pickett W. Associations between overweight and obesity with bullying behaviors in school-aged children. Pediatrics. 2004;113((5)):1187–94. doi: 10.1542/peds.113.5.1187. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization Obesity: preventing and managing the global epidemic − report of a WHO consultation on obesity, Geneva, 3–5 June 1997. 1998. [PubMed]
- 14.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 15.Childhood overweight and obesity. [Internet]. [cited 2019 Dec 26]. Available from: https://www.who.int/dietphysicalactivity/childhood/en/
- 16.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315((21)):2292–9. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Hurk K, Van Dommelen P, Buuren S, Verkerk P, Hirasing R. Prevalence of overweight and obesity in the Netherlands in 2003 compared to 1980 and 1997. Arch Dis Child. 2007;92:992–5. doi: 10.1136/adc.2006.115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matheson BE, Douglas JM. Overweight and obesity in children with autism spectrum disorder (ASD): a critical review investigating the etiology, development, and maintenance of this relationship. Rev J Autism Dev Disord. 2017;4((2)):142–56. [Google Scholar]
- 19.Bandini LG, Anderson SE, Curtin C, Cermak S, Evans EW, Scampini R, et al. Food selectivity in children with autism spectrum disorders and typically developing children. J Pediatr. 2010;157((2)):259–64. doi: 10.1016/j.jpeds.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cermak SA, Curtin C, Bandini LG. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J Am Diet Assoc. 2010;110((2)):238–46. doi: 10.1016/j.jada.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreck KA, Williams K, Smith AF. A comparison of eating behaviors between children with and without autism. J Autism Dev Disord. 2004;34((4)):433–8. doi: 10.1023/b:jadd.0000037419.78531.86. [DOI] [PubMed] [Google Scholar]
- 22.Råstam M. Eating disturbances in autism spectrum disorders with focus on adolescent and adult years. Clin Neuropsychiatry. 2008;5((1)):31–42. [Google Scholar]
- 23.Evans EW, Must A, Anderson SE, Curtin C, Scampini R, Maslin M, et al. Dietary patterns and body mass index in children with autism and typically developing children. Res Autism Spectr Disord. 2012;6((1)):399–405. doi: 10.1016/j.rasd.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiteley PRJ, Shattock P. Feeding patterns in autism. Autism. 2000;4((2)):207–11. [Google Scholar]
- 25.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302((16)):1765–73. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho HH, Eaves LC, Peabody D. Nutrient intake and obesity in children with autism. Focus Autism Other Dev Disabl. 1997;12((3)):187–92. [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339((7)):b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6((7)):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szumilas M. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry. 2010;19((3)):227–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Chen AY, Kim SE, Houtrow AJ, Newacheck PW. Prevalence of obesity among children with chronic conditions. Obesity. 2010;18((1)):210–3. doi: 10.1038/oby.2009.185. [DOI] [PubMed] [Google Scholar]
- 31.Curtin C, Anderson SE, Must A, Bandini L. The prevalence of obesity in children with autism: a secondary data analysis using nationally representative data from the National Survey of Children's Health. BMC Pediatr. 2010;10:11. doi: 10.1186/1471-2431-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corvey K, Menear KS, Preskitt J, Goldfarb S, Menachemi N. Obesity, physical activity and sedentary behaviors in children with an autism spectrum disorder. Matern Child Health J. 2016;20((2)):466–76. doi: 10.1007/s10995-015-1844-5. [DOI] [PubMed] [Google Scholar]
- 33.McCoy SM, Jakicic JM, Gibbs BB. Comparison of obesity, physical activity, and sedentary behaviors between adolescents with autism spectrum disorders and without. J Autism Dev Disord. 2016;46((7)):2317–26. doi: 10.1007/s10803-016-2762-0. [DOI] [PubMed] [Google Scholar]
- 34.Must A, Eliasziw M, Phillips SM, Curtin C, Kral TVE, Segal M, et al. The effect of age on the prevalence of obesity among US youth with autism spectrum disorder. Child Obes. 2017;13((1)):25–35. doi: 10.1089/chi.2016.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voulgarakis H, Bendell-Estroff D, Field T. Prevalence of obesity and autism spectrum disorder. Behav Dev Bull. 2017;22((1)):209–14. [Google Scholar]
- 36.Tybor DJ, Eliasziw M, Kral TVE, Segal M, Sherwood NE, Sikich L, et al. Parental concern regarding obesity in children with autism spectrum disorder in the United States: National Survey of Children's Health 2016. Disabil Health J. 2019;12((1)):126–30. doi: 10.1016/j.dhjo.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 37.McCoy SM, Morgan K. Obesity, physical activity, and sedentary behaviors in adolescents with autism spectrum disorder compared with typically developing peers. Autism. 2020;24((2)):387–99. doi: 10.1177/1362361319861579. [DOI] [PubMed] [Google Scholar]
- 38.Healy S, Aigner CJ, Haegele JA. Prevalence of overweight and obesity among US youth with autism spectrum disorder. Autism. 2019;23((4)):1046–50. doi: 10.1177/1362361318791817. [DOI] [PubMed] [Google Scholar]
- 39.Phillips KL, Schieve LA, Visser S, Boulet S, Sharma AJ, Kogan MD, et al. Prevalence and impact of unhealthy weight in a national sample of US adolescents with autism and other learning and behavioral disabilities. Matern Child Health J. 2014;18((8)):1964–75. doi: 10.1007/s10995-014-1442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shedlock K, Susi A, Gorman GH, Hisle-Gorman E, Erdie-Lalena CR, Nylund CM. Autism spectrum disorders and metabolic complications of obesity. J Pediatr. 2016;178:183–7.e1. doi: 10.1016/j.jpeds.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 41.Akinbami LJ, Ogden CL. Childhood overweight prevalence in the United States: the impact of parent-reported height and weight. Obesity. 2009;17((8)):1574–80. doi: 10.1038/oby.2009.1. [DOI] [PubMed] [Google Scholar]
- 42.Esteban-Figuerola P, Morales-Hidalgo P, Arija-Val V, Canals-Sans J. Are there anthropometric and body composition differences between children with autism spectrum disorder and children with typical development? Analysis by age and spectrum severity in a school population. Autism. 2021;25((5)):1307–20. doi: 10.1177/1362361320987724. [DOI] [PubMed] [Google Scholar]
- 43.de Hoogd S, Overbeek WA, Heerdink ER, Correll CU, de Graeff ER, Staal WG. Differences in body mass index z-scores and weight status in a Dutch pediatric psychiatric population with and without use of second-generation antipsychotics. J Child Adolesc Psychopharmacol. 2012;22((2)):166–73. doi: 10.1089/cap.2011.0079. [DOI] [PubMed] [Google Scholar]
- 44.Ptomey LT, Walpitage DL, Mohseni M, Dreyer Gillette ML, Davis AM, Forseth B, et al. Weight status and associated comorbidities in children and adults with Down syndrome, autism spectrum disorder and intellectual and developmental disabilities. J Intellect Disabil Res. 2020;64((9)):725–37. doi: 10.1111/jir.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Healy S, Haegele JA, Grenier M, Garcia JM. Physical activity, screen-time behavior, and obesity among 13-year olds in Ireland with and without autism spectrum disorder. J Autism Dev Disord. 2017;47((1)):49–57. doi: 10.1007/s10803-016-2920-4. [DOI] [PubMed] [Google Scholar]
- 46.Wentz E, Björk A, Dahlgren J. Neurodevelopmental disorders are highly over-represented in children with obesity: a cross-sectional study. Obesity. 2017;25((1)):178–84. doi: 10.1002/oby.21693. [DOI] [PubMed] [Google Scholar]
- 47.Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators. Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years: autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63((2)):1–21. [PubMed] [Google Scholar]
- 48.Hill AP, Zuckerman KE, Fombonne E. Obesity and autism. Pediatrics. 2015;136((6)):1051–61. doi: 10.1542/peds.2015-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hyman SL, Stewart PA, Schmidt B, Cain U, Lemcke N, Foley JT, et al. Nutrient intake from food in children with autism. Pediatrics. 2012;130((Suppl 2)):S145–53. doi: 10.1542/peds.2012-0900L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li YJ, Xie XN, Lei X, Li YM, Lei X. Global prevalence of obesity, overweight and underweight in children, adolescents and adults with autism spectrum disorder, attention-deficit hyperactivity disorder: a systematic review and meta-analysis. Obes Rev. 2020;21((12)):e13123. doi: 10.1111/obr.13123. [DOI] [PubMed] [Google Scholar]
- 51.Kahathuduwa CN, West BD, Blume J, Dharavath N, Moustaid-Moussa N, Mastergeorge A. The risk of overweight and obesity in children with autism spectrum disorders: a systematic review and meta-analysis. Obes Rev. 2019;20((12)):1667–79. doi: 10.1111/obr.12933. [DOI] [PubMed] [Google Scholar]
- 52.Zheng Z, Zhang L, Li S, Zhao F, Wang Y, Huang L, et al. Association among obesity, overweight and autism spectrum disorder: a systematic review and meta-analysis. Sci Rep. 2017;7((1)):11697. doi: 10.1038/s41598-017-12003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384((9945)):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zurita Ortegaa F, Martinez Porcelb R, Ali Morelic OJ, Fernández Garciad R. Some contributions to the determination of obesity prevalence among children with special educational needs. Pediatr Aten Primaria. 2009;12((45)):15–31. [Google Scholar]
- 55.Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106((1 Pt 1)):52–8. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- 56.Weden MM, Brownell PB, Rendall MS, Lau C, Fernandes M, Nazarov Z. Parent-reported height and weight as sources of bias in survey estimates of childhood obesity. Am J Epidemiol. 2013;178((3)):461–73. doi: 10.1093/aje/kws477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berkman ND, Lohr KN, Bulik CM. Outcomes of eating disorders: a systematic review of the literature. Int J Eat Disord. 2007;40((4)):293–309. doi: 10.1002/eat.20369. [DOI] [PubMed] [Google Scholar]
- 58.Barnhill K, Gutierrez A, Ghossainy M, Marediya Z, Marti CN, Hewitson L. Growth status of children with autism spectrum disorder a case-control study. J Hum Nutr Diet. 2017;30((1)):59–65. doi: 10.1111/jhn.12396. [DOI] [PubMed] [Google Scholar]
- 59.Rimmer JH, Yamaki K, Davis Lowry BM, Wang E, Vogel LC. Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities. J Intellect Disabil Res. 2010;54((9)):787–94. doi: 10.1111/j.1365-2788.2010.01305.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.