Abstract
Background:
Human exposures to bisphenol A (BPA) are widespread. The current study addresses uncertainties regarding human pharmacokinetics of BPA following dermal exposure.
Objective:
To examine the absorption, distribution, metabolism and excretion of BPA in humans following dermal administration.
Methods:
We dermally administered deuterated BPA (d6-BPA) to 10 subjects (6 men and 4 women) at a dose of 100 μg/kg over a 12-hour period and conducted blood and urine analysis from the beginning of dosing through a three- or six-day period. We present time-course serum and urine concentrations of total and unconjugated (“free”) d6-BPA and used this information to calculate terminal half-life and area under the curve.
Results and Conclusions:
Detectable serum levels of total d6-BPA were observed at 1.4 h after the start of dosing, and a maximum serum concentration (Cmax) of 3.26 nM was observed. Free d6-BPA was detectable in serum 2.8 h after start of dermal administration, with Cmax of 0.272 nM. Beginning at approximately seven hours and continuing to 12 h (which corresponds to cessation of exposure), the concentration of free and total serum d6-BPA plateaued. The terminal half-lives of total d6-BPA and free d6-BPA in the body were 21.4 ± 9.81 h and 17.6 ± 7.69 h, respectively. Elimination from the body was rate-limited by kinetics in the dermal compartment. Free d6-BPA was a greater percentage of the area under the curve of total serum BPA (8.81%) compared to the0.56% observed in our previously published oral study. Recovery of total d6-BPA in urine was < 2% of the applied dose after six days. Analysis of the area under the curve for dermal and oral administration revealed that 2.2% of the dermal dose became systemically available. These data are in line with prior studies indicating how pharmacokinetics of BPA differ following oral and dermal exposures. Dermal exposure resulted in a longer apparent half-life and higher free:total d6-BPA ratio compared to oral.
Keywords: Absorption, Distribution, Bioavailability, Endocrine disruptor, Excretion, Metabolism
1. Introduction
Bisphenol A (BPA) is considered a synthetic estrogen with the potential to disrupt the human endocrine system leading to adverse health effects (FAO/WHO, 2011; Rubin, 2011; Shelby, 2008). Biomonitoring data demonstrating widespread exposure to BPA in general populations around the world has made the assessment of human health risk from BPA exposure a public health concern (Bushnik et al., 2010; Calafat et al., 2008; CDC, 2019; EFSA, 2017; EFSA, 2015; Koch et al., 2012; NIEHS, 2019; Zhang et al., 2011).
Understanding pharmacokinetic behavior of BPA following oral and dermal exposures is critical to evaluating human health risks associated with BPA. BPA in food packaging is a primary source of human exposure in the general population (Cao et al., 2009; Carwile et al., 2011; EFSA, 2015). Rapid and efficient conjugation via first pass metabolism following oral exposure to BPA greatly reduces the portion of the biologically active unconjugated (“free”) compound that enters the systemic circulation where it can potentially reach target tissues and lead to biological effects (EFSA, 2015, FAO/WHO, 2011; Thayer et al., 2015; Volkel et al., 2002). Human studies indicate that dermal exposure can also occur in the general population and in occupational settings due in part to the presence of BPA in thermal printing papers such as those used in cash register receipts (Biedermann et al., 2010; ECHA, 2015; Hormann et al., 2014; Liu and Martin, 2017; Thayer et al., 2016). Without the highly efficient first pass effect unique to the ingestion route, the pharmacokinetics following dermal exposure potentially differ substantially from that of oral exposure. Indeed, using a realistic dermal exposure scenario based on the handling of simulated receipt paper, Liu and Martin (2017) showed that free BPA was detected in a higher proportion of urinary samples when compared to oral exposure. As a result, dermal exposures could be of higher toxicological relevance than the oral exposure to BPA from diet.
Several in vitro studies have measured dermal penetration of BPA in human skin (Demierre et al., 2012; Marquet et al., 2011; Morck et al., 2010; Toner et al., 2018; Zalko et al., 2011). Quantitative results were highly variable between studies due to differences in the skin samples (thickness, anatomical origin) and experimental conditions (exposure duration, concentration, vehicle, experimental apparatus) (EFSA (2015), Table 52 Appendix D). Of the five in vitro studies identified, four reported estimates of percent percutaneous absorption being 1.7–3.9% over 24 h (Toner et al., 2018), 8.6% over 24 h (Demierre et al., 2012), 13% over 48 h (Morck et al., 2010), and 46% over 72 h (Zalko et al., 2011).
Three in vitro studies used viable human skin and also tested dermal metabolism with 14C-BPA using different experimental conditions (Marquet et al., 2011; Toner et al., 2018; Zalko et al., 2011). Estimates of dermal metabolism as a percentage of applied dose from these studies were 2.5% (non-glucuronide metabolites) over 24 h (Marquet et al., 2011), 14–19% (glucuronide, sulfate, and additional polar metabolites) over 24 h (Toner et al., 2018), and 14–39% (glucuronide and sulfate) over 72 h (Zalko et al., 2011). Limited information is available from animal models. Marquet et al. (2011) analyzed the 14C mass balance after dermal application of 200 μg/cm2 14C-BPA in acetone under occlusive conditions in male Sprague-Dawley rats. After 24 h of exposure, approximately 26% of the applied dose was absorbed, based on recovery from urine, feces, and the carcass.
Estimates of daily uptake of BPA from handling thermal receipt paper vary widely (see Supplementary B-4 Tables S5-7). They are strongly dependent on the assumptions (e.g., dermal absorption with or without skin deposition) and parameters (e.g., percentage absorption, maximum flux using the permeability coefficient) considered in the estimate. Other important factors in these estimates include paper handling time and frequency, concentration of BPA in thermal paper (typical concentration for functional use: 1–2% by weight (Eckardt and Simat, 2017)), skin area in contact with paper, length of time between contacts, and hand washing. Assuming a rather low concentration of BPA in thermal paper of 0.03%, Liao and Kannan (2011) estimated intakes of 0.00022 μg/kg/d for the general population and 0.0162 μg/kg/d for occupationally exposed individuals. Using different parameter assumptions and a BPA concentration of 1.15%, Lassen et al. (2011) estimated 4 μg/kg/d for consumers and 21.5 μg/kg/d for cashiers. Both studies assumed the transfer of BPA from thermal paper to the skin to be proportional with the duration of dermal contact. EFSA (2015) assumed a BPA concentration of 1.15% and fixed dermal transfer amount per event, and estimated consumer uptake of 0.0059–0.0542 μg/kg/d. The Liu and Martin (2017) study examining handling of simulated receipts (soft notebook paper containing 2.5% d16-BPA) derived uptakes of 0.002–0.046 μg/kg/d from the cumulative urinary excretion of BPA. While human studies involving real-world scenarios are important for providing realistic estimates of internal exposure, they are less suitable for deriving robust estimates for pharmacokinetic parameters.
To address the limitations of the in vitro studies with human skin and of the human studies with uncontrolled dermal exposure to BPA from thermal paper, we undertook a toxicokinetic study in humans with dermal administration of a controlled dose of deuterated BPA (d6-BPA) by measuring blood and urine concentrations of free and conjugated d6-BPA in 10 volunteers who were each exposed via dermal administration. The aim of this approach was to reduce uncertainties concerning the extent of absorption, distribution, metabolism and excretion of BPA in humans following controlled dermal exposure. This study represents the dermal arm of a previously published oral study (Thayer et al., 2015), permitting comparison across arms of the same study design and more generally for oral versus dermal absorption and distribution.
2. Materials and methods
Study methods are summarized below and laboratory analysis procedures are described in Supplemental Materials Part A. The NIEHS Institutional Review Board (IRB) approved protocol, blood, and urine collection methods.
2.1. Subject eligibility and recruitment
Men and non-pregnant women were recruited by the National Institute of Environmental Health Sciences Clinical Research Unit (NIEHS CRU) in 2014 and 2017 from the Raleigh Durham region of North Carolina using standard flyer advertisements. Additionally, subjects who participated in the oral exposure arm of this study (Thayer et al., 2015) were invited for participation in the dermal arm. The time commitment needed to complete the study visits and lack of flexibility with the day that they can be scheduled were limiting factors for recruitment. Only four previous oral participants agreed to and completed participation in the carboxymethylcellulose (CMC) dermal phase in 2014. Four additional participants were enrolled that had not completed the oral phase to pilot the ethanol dermal phase. For the extended CMC and ethanol dermal phases in 2017 all the participants that had previously participated were re-contacted and five were able to participate and four completed all visits. After a pre-screening interview and examination, healthy volunteers meeting the inclusion criteria were considered eligible for study (see Supplemental Materials Part A). Criteria included age (25–45), ability to fast overnight, and agreement to avoid conceiving a child and not to donate eggs or sperm for six months following their participation. Criteria for exclusion included specified pre-existing health conditions, pregnancy, and use of medications which may affect glucuronidation of BPA (Verner et al., 2010). Blood samples used to determine eligibility status were collected at the time of the initial screening visit. Each participant completed a short questionnaire (see Supplemental Materials Part A for the questionnaire and the “Supplemental_Questionnaire” Excel data sheet file for results) to collect demographic information including age, ethnicity, gender, and information about possible BPA exposure at home and work. Although the questionnaire included information about BPA exposure at home, the use of d6-BPA in the study minimized issues of background interference and the need to have subjects alter normal eating patterns and other daily activities. All human subject research activities were conducted in accordance at the NIEHS CRU with protocols approved by the National Institute of Health Clinical Center Institutional Review Board (protocol number 12-E-0089; clinicaltrials.gov identifier: NCT01573429). In addition, the participation of the National Center for Toxicological Research (NCTR) laboratory in an initial 2014 protocol using a 0.3% CMC suspension vehicle (described below and Supplemental Materials Part A) was reviewed and approved by the Research in Human Subjects Committee of the U.S. Food and Drug Administration (RIHSC# 12-036T).
2.1.1. Dosing
Neither the 0.3% CMC suspension nor the 95% ethanol solution were ideal models of real-world human exposure. However, they were considered representative of the potential range of dermal penetration, with less dermal penetration expected with use of a 0.3% CMC suspension vs. the 95% ethanol vehicle. Thus, both the 0.3% CMC suspension and the 95% ethanol solution vehicles continued to be utilized after the 2014 study.
The solution for dermal application (25 mg/mL) was prepared by dissolving a weighed portion of d6-BPA (dimethyl-d6; 98.6% isotopic purity and > 99.7% chemical purity, CDN Isotopes, Pointe-Claire, Quebec)” (Twaddle et al., 2010) in USP grade 95% ethanol (Decon Laboratories, King of Prussia, PA) or in an aqueous suspension containing 0.3% CMC. The d6-BPA solutions were stored as 0.5 mL individual aliquots in 2-mL tubes and stored frozen at −20 °C until the day before the participant’s dosing visit when they were thawed in the refrigerator.
For most participants, the nominal d6-BPA concentration (25 mg/mL) was used to calculate the appropriate amount X (mL) of the dosing solution/suspension from the participant body weight Y (kg) measured on the day of the study and the target dose (100 μg/kg BW) (see below). The d6-BPA dosing concentration was validated for four selected participants using HPLC-MS/MS, and this analytical value was used in the calculation instead of the nominal concentration (e.g., a 70 kg subject will receive an application of 0.28 mL of the dosing solution):
Generally, one application site was used per subject. For the 2014-CMC study arm, two or more application sites were used (with simultaneous application) depending on the subject’s weight. This was done because a practice session indicated that larger individuals with correspondingly larger dose formulation volumes would have the solution run off their arms, so the formulation was divided to prevent this runoff.
Dermal administration started in the morning after a fasting period beginning at 12 midnight on the day of the visit. Locations on the skin of the volar forearm were identified and a circle drawn using a Hill Top® Chamber (Hill Top Research, Inc., Cincinnati, OH) as a template. A pipette with a polypropylene pipet tip was used to apply the d6-BPA solution (ethanol) or suspension (CMC) to the unshaven volar forearm of the participant, choosing an area with the least amount of hair and with no visible scratches or open wounds. For both the CMC and ethanol experiments the solution was allowed to dry for approximately five minutes, using a gentle stream of air from a hair dryer if needed. Drying time was noted in the participant’s record. The beginning of application of the d6-BPA solution or suspension was considered to be time zero for data-recording purposes. After the solution dried, the application area(s) were encircled with petroleum jelly and each area was then covered with the Hill Top Chamber (HTC) without a pad covering the area. The chamber provided a viewing window for study staff to monitor for possible skin irritation. The area under the HTC was 4.91 cm2. The chamber was secured with tape to prevent spreading of the dried d6-BPA layer/film on the skin. At 12 h after start of administration, the HTC(s) were removed and skin was rinsed and/or wiped with rinsates and wipe materials saved for analysis.
2.2. Study design
2.2.1. 2014 Protocol versus 2017 protocol
The main goal of the study was to assess measurable levels of deuterated BPA (d6-BPA) and d6-BPA conjugates in blood and urine after administration of a single dose of 100 μg/kg body weight of dermally applied d6-BPA over the course of 12 h. The target dermal dose of 100 μg/kg was selected in order to match the administered dose used in an oral arm of this study (Thayer et al., 2015). A dermal arm was initially conducted in 2014 to evaluate the suitability of the oral arm sample collection protocol to evaluate pharmacokinetics following dermal exposure (Fig. 1), and to help select a vehicle to best model the nature of human exposure (i.e., handling thermal paper). More specifically, the 2014 protocol was conducted to verify that detectable levels of d6-BPA could be measured using either a 0.3% aqueous carboxymethylcellulose (CMC) suspension vehicle or a 95% ethanol solution vehicle, and whether follow-up for 3 days after dermal administration was sufficiently long to observe complete (or nearly complete) urinary elimination.
Fig. 1.
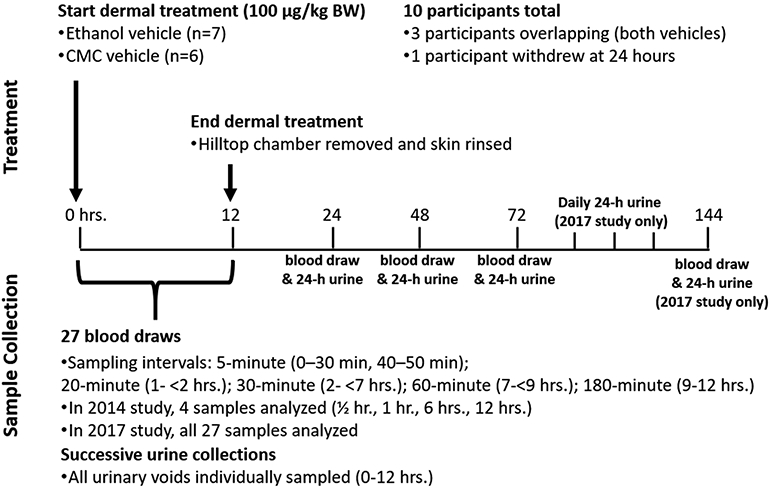
Treatment and sample collection timelines for the 2014 and 2017 protocols. For all studies, 27 blood samples were collected at baseline and during the 12-hour period after start of dermal administration. Only four of these samples were analyzed in 2014 to evaluate analytical detection and for method development (0.5, 1, 6 and 12-hours). All samples from the 2017 protocol were analyzed to capture both the early and late-stage kinetics.
Preliminary results of the 2014 study on four subjects using 0.3% CMC suspension vehicle (Supplemental Materials Part B-3) indicated that a longer follow-up period post-treatment was warranted. The protocol was subsequently amended to allow for extending the follow-up period to six days for subjects participating in the 2017 studies (see Fig. 1).
The preliminary analysis of the 2014 CMC data also included estimates of free d6-BPA serum concentrations. These results are presented in Supplemental Materials Part B-3 but are not incorporated into the data analysis due to inter-laboratory differences in the analytical chemistry methodology. For the current study, urine and serum samples from the 2014 preliminary analysis were re-analyzed using the method described below to better compare across the 2014 and 2017 experiments. Analysis of free d6-BPA in serum was limited to samples from the ethanol study arms. This was done to complement the 2014 CMC analysis by expanding the database to include free d6-BPA measures for the ethanol vehicle, and to focus post-2014 analyses on the vehicle believed to facilitate higher absorption.
It should be noted that this study was designed to evaluate the pharmacokinetics of dermal uptake, which required prolonged exposure at a high surface concentration. Ethanol and CMC vehicles on the forearm are not representative of typical exposures (such as thermal receipts on the hands), and the use of a Hill Top chamber caused occlusive conditions which likely promoted sweat layer formation on the covered skin. This administration regimen might have enhanced dermal absorption relative to what might be expected following skin contact to thermal receipt paper. The aim was to ensure that dermal uptake would indeed occur, so that kinetic parameters can be evaluated and compared against other controlled exposure scenarios.
2.2.2. Recovery of unabsorbed d6-BPA after dermal administration
Following dermal administration and removal of the HTC at 12 hrs, the application area(s) were rinsed with a polypropylene squeeze bottle filled with 95% ethanol to recover unabsorbed d6-BPA for quantification. A volume of 1–2 mL rinsing solution was squirted on the exposed skin area, and a sponge or gauze was used to wipe the exposed skin with the rinsing solution. For the 2014 protocol using CMC, no wiping material was used. The site rinsate was collected in a specimen cup. The rinsing step was typically repeated several times, and the total volume of site rinsate was recorded (see Supplemental Materials B-1 Table S4 and “Supplemental_Rinsate_Data” Excel data sheet). Rinsing of the HTC with ethanol and recording of the total volume of HTC rinsate was done in a similar manner. Polypropylene vials with 1–2 mL aliquots of the rinsates were sent for d6-BPA analysis. The HTC and the sponge/gauze were also sent to the analytical lab for the recovery and determination of any residual d6-BPA adsorbed to these materials.
2.2.3. Blood collection
Pre-dose fasting blood level determinations of d6-BPA and d6-BPA conjugates were made after the insertion of an indwelling IV cannula/saline lock (Moore Medical, Part 66408; Insyte™ Autoguard™ shielded IV catheters made of a polyurethane based BD Vialon™ biomaterial). Samples were collected in red-top, additive-free, silicone-coated glass tubes. The supplier varied across participants because of changes in availability. Tubes were purchased from Becton Dickinson (Part 366,431 for a 7 mL tube or Part 366,430 for a 10 mL tube) or from Fisher Scientific (BD 366,430 for a 10 mL tube). See Supplemental Materials Part A for the specific tubes used in the 2014 and 2017 protocols.
After the start of administration, blood was collected for 12 h via an indwelling IV cannula/saline lock at: 0, 5, 10, 15, 20, 25, 30, 40, 45, 50, 60, 80 and 100 (± 5 min); 120, 150, 180, 210, 240, 270 and 300 (± 10 min); 330, 360, 390, 420, 480, 540 min (± 15 min); and 720 min (± 20 min). Additional blood samples were collected by peripheral blood draw at 24/48/72 h for all participants remaining in the study, and at 144 h for 2017 participants. Blood was sampled by trained phlebotomists via the indwelling catheter into glass tubes. After clotting at room temperature for at least 30 min, samples were centrifuged, and serum was stored at −80 °C in polypropylene microcentrifuge tubes (Sarstedt, Part #76.690). Serum was analyzed as the preferred matrix since it was used in previous studies to represent circulating concentrations in blood. The blood:plasma partition ratio for BPA and BPA-glucuronide is close to 1 (Edginton and Ritter, 2009) meaning that the compounds are outside the blood cells. Polystyrene pipettes (CoStar part# 4051) were used to transfer serum. Vital signs were monitored for 12 h following dosing. Blood samples for kidney and liver function testing (e.g., ALT, AST, ALP, creatinine, and blood urea nitrogen) were collected at the initial screening visit and on the last day of the study.
2.2.4. Urine collection
The clinic staff provided a collection container (Thermo Scientific™ Samco™ SW-3000 24-hour Urine Collection Containers from Fisher Scientific) to the participant at the screening visit, and an ice chest with frozen ice packs to collect baseline 24-hour urine prior to dosing visit. A fasting urine specimen was collected prior to dosing using polypropylene urine collection cups (Moore Medical-AMSure® Urine Specimen Container Part# 69716). Following the start of administration of d6-BPA, participants collected all unscheduled urine voids in separate containers from 0 to 2, 2 to 4, 4 to 8, and 8 to 12 h. At the end of the d6-BPA dosing day (day 0), the clinic staff again provided participants with a urine collection container and ice chest (see above). Participants of the 2014 protocol performed a 24-hour collection for 3 days post-dose, while 2017 participants performed these collections for 6 days post-dose. Participants were provided a new collection container at each follow up visit. Because the 6-day collections extended over a weekend, 2017 participants were provided three 24-hour collection containers and a temperature-controlled portable cooler (Koolatron model # EC-0432 with 12-volt power adapter) with ice packs on day 3. Urine samples were kept on ice until frozen for storage at −80 °C until analysis. Creatinine concentration and volume were measured for all urine samples.
All samples (urine, serum, experimental material and rinsates) were shipped on dry ice to the Senator Frank R. Lautenberg Laboratory at the Icahn School of Medicine at Mount Sinai in New York City, New York for d6-BPA analysis. The Lautenberg Laboratory is part of the Human Health Exposure Analysis Resource (HHEAR), a continuation of Children’s Health Exposure Analysis Resource (CHEAR), which has participated and qualified in proficiency testing programs for BPA conducted by G-EQUAS (http://www.g-equas.de/) (FAO/WHO, 2011; Rubin, 2011; Shelby, 2008), OSEQAS (https://www.inspq.qc.ca/en/ctq/eqas/oqesas/description) (FAO/WHO, 2011; Rubin, 2011; Shelby, 2008), and CHEAR inter-laboratory Round Robin.
2.3. d6-BPA measurements
2.3.1. Standards and reagents
Deuterated d6-BPA (dimethyl-d6; 98% isotopic purity and 99% chemical purity; product # 588806-1G) was purchased from Sigma Aldrich (St. Louis, MO) and the corresponding labeled internal standard 13C12-BPA (99%) was obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA). LC/MS grade acetic acid (≥99.7%), acetonitrile (≥99.9%), ammonium acetate (≥99.0%), ethyl acetate (≥99.9%), formic acid (≥ 99.0%), methanol (≥ 99.9%), and water were purchased from Fisher Scientific (Hampton, NH). β-Glucuronidase/arylsulfatase enzyme from Helix pomatia was purchased from Sigma-Aldrich (BGALA-RO, product # 10127698001, Roche Life Science through Sigma Aldrich). All solvents, reagents, and experimental labware were tested for d6-BPA and 13C12-BPA, and none was detected.
2.3.2. Analytical methods
Sample preparation and quantification of total and free d6-BPA in urine and serum were based on a previously described method (Thayer et al., 2015; Twaddle et al., 2010) with some modifications. In brief, 13C12-BPA was added to an individual urine or serum sample (0.2 mL) for isotope-dilution high performance liquid chromatography-tandem mass spectrometry (HPLC–MS/MS) analysis. The conjugated metabolites d6-BPA glucuronide and d6-BPA sulfate were digested and cleaved enzymatically with the β-glucuronidase/arylsulfatase enzyme from Helix pomatia that has an approximate specific activity of 100,000 units/mL of β-glucuronidase and 47,500 units/mL of sulfatase (Dwivedi et al., 2018). The resulting total d6-BPA was extracted by solid phase extraction (SPE) using an Oasis HLB hydrophilic-lipophilic balanced reversed-phase 96-well plate (30 mg sorbent per well, 30 μm particle size; Waters Corporation, Milford, MA). The procedure was automated using a liquid handler (epMotion 5075vtc; Eppendorf, Hauppauge, NY). Enzymatic digestates of urine or serum were loaded onto the 96-well SPE plate preconditioned with 1 mL each of methanol and water. Each well in the SPE plate was washed with 1 mL water and eluted with 0.75 mL methanol for 3 min at 300 mbar twice. The two eluates for each sample were pooled and evaporated to dryness under a gentle nitrogen stream with a SPE Dry 96 evaporator (Biotage, LLC; Charlotte, NC), reconstituted with 0.2 mL of acetonitrile:water (50:50, vol:vol), and a 20 μL injection volume was subjected to analysis. The HPLC-MS/MS (Nexera XR, Shimadzu and Sciex 6500 triple quadruple MS, AB Sciex; Framingham, MA) was operated in electrospray negative mode for ionization and multiple reaction monitoring (MRM) for data acquisition. Most prominent ion transition was used for quantitation and the most intense second ion transition was used for confirmation (MRM transition of 233 → 138, 215 for d6-BPA and 239 → 224, 139 for 13C12-BPA). Chromatographic separation was achieved on a Betasil C18, 5 μm, 100 × 2.1 mm analytical column with 10 × 2.1 mm guard column (Thermo Scientific, Waltham, MA) using a mobile phase gradient with LC-MS grade water and acetonitrile. To measure unconjugated d6-BPA in serum, the same methodology was applied to the same samples but omitted the enzymatic deconjugation step by replacing the enzyme volume with 1 M ammonium acetate buffer (pH 5.0) solution.
HTC and site rinsates were diluted 100 to 10,000-fold (vol:vol) and a 0.2 mL aliquot was sampled and spiked with 13C12-BPA as internal standard. The aliquots were subjected to a sequential liquid–liquid extraction (LLE) with ethyl acetate (Liao and Kannan, 2012), and the pooled supernatant organic phase was evaporated to dryness prior to reconstitution with 0.2 mL of acetonitrile:water (50:50, vol:vol). The HTC and the sponge/gauze were subjected to repeated washing with methanol for dissolving d6-BPA sorbed to these surfaces; collected wash fractions were pooled and measured for total volume. Aliquots (0.2 mL) were subjected to LLE extraction and LC-MS/MS analysis for d6-BPA quantification taking corresponding dilution factors into consideration. Methanol wash and LLE with ethyl acetate was repeated until d6-BPA was not observed in the resulting extracts indicating a complete recovery. The LOQ of d6-BPA is the same for SPE and LLE methods.
2.3.3. Laboratory quality control
Quality controls (QCs) included in each batch were procedural, instrumental, and matrix pool blanks, in-house urine or serum matrix spikes of d6-BPA at low (0.005–0.05 ng/mL), medium (0.1–1 ng/mL) and upper range (10–100 ng/mL) of assay validation with both enzyme de-conjugation and no-enzyme matrix for both total and free d6-BPA. Efficiency of the deconjugation step was assessed and optimized using 4-methyl-umbelliferone and its conjugates as test substrates (Grignon et al., 2017). The limits of detection (LOD) and quantification (LOQ) were calculated as 3S (three times standard deviation) and 10S, respectively, of ten replicate analyses of matrix pool blank spiked with 0.01 ng/mL of d6-BPA. The calculated LOD and LOQ were 0.0005 ng/mL (0.002 nM) and 0.001 ng/mL (0.004 nM), respectively. The matrix effect on d6-BPA was assessed by calculating recoveries from the difference between known spiked amounts in matrix, extracted matrix, and solvent QCs that were between 80% and 120%. Batchwise relative standard deviations (RSDs) of QCs during analysis of the study specimens were < 10% for d6-BPA in the fortified matrix material, except for the spikes at the LOQ where RSDs up to 20% were accepted. Intra-batch precision, expressed as percent coefficient of variation (CV), was below 10% and inter-batch precision was below 30% for d6-BPA in the QC samples.
With respect to the re-analysis of samples collected in 2014, analysis of archived urine samples from the two proficiency testing programs (G-EQUAS and OSEQAS) have been repeatedly analyzed since 2016. The analysis outcome is within the suggested tolerance range for these archived samples that are stored at −80 °C and without an additional freeze–thaw cycle since aliquoting.
Additional information related to analytical methods are available in Supplemental Materials Part C.
2.4. Pharmacokinetic analysis
Analyses and data visualizations were performed using the R programming language (R Core Team, 2019). Plots of serum concentrations of total and free d6-BPA at each time point were generated at the individual-level and are provided in Supplemental Materials Part B-2. The terminal half-life in serum (t1/2) was determined for each dataset by fitting an exponential curve, beginning at the peak concentration (typically the 12-hour timepoint). A single terminal phase was observed, and all data from the peak concentration through the end of data collection (3–6 days, depending on protocol) were fit to a single exponential curve by least-squares regression of log-transformed concentration vs. time. The observed t1/2 represents both serum elimination kinetics and diffusion kinetics of absorption from the dermal compartment (which may be a rate-limiting step). If the absorption process would indeed be the rate-limiting step that governs the disappearance from the body, this kinetics would then be described as a flip-flop situation (Derendorf and Rowland, 2020; Gabrielsson and Weiner, 2016). To estimate the area-under-the-curve from time zero to infinity (AUC0−∞) for serum concentrations, the log-linear trapezoidal rule was applied using t1/2 and the serum concentration at the last time point.
3. Results and discussion
Results are summarized below. Both individual-level data, and mean data grouped by different study characteristics and vehicles, are available in Supplemental Materials Part B-1.
3.1. Subject characteristics
The average age of male (n = 6) and female (n = 4) volunteers was 33 years (range 25–42) and 31 years (range 26–35), respectively. Half of the subjects were non-Hispanic whites (n = 5; Table 1), while the remainder were black or African-American (n = 4) and mixed-race (n = 1). The average BMI of male and female volunteers was 27.2 (22.4–34.2) and 28.7 (21.6–34), respectively. The average body weight of male and female volunteers was 91.2 kg (67.7–124.0) and 80.0 kg (70.0–95.4), respectively. Three of the 10 subjects (two males and one female) participated in both experimental protocols (2014 and 2017) and received a different dosing vehicle (CMC or ethanol) at each visit. Additional details on the HTC treatment and length of follow-up are presented in Supplemental Materials Part B-1. One participant reported work as a cashier and others reported consumption of food from metal or plastic containers in the 24 h prior to the administration. However, sub-analyses based on this information was not pursued given the small sample size and use of d6-BPA to minimize background interference.
Table 1.
Population characteristics by subject and by experiment*
| Characteristics | Number of Subjects | Number of Experiments |
|---|---|---|
| Year | ||
| 2014 & 2017* | 3 | 6 |
| 2017 only | 2 | 2 |
| 2014 only | 5 | 5 |
| Total | 10 | 13 |
| Sex | ||
| Male | 6 | 8 |
| Female | 4 | 5 |
| Race | ||
| White | 5 | 6 |
| Black or African-American | 4 | 5 |
| Multiple Races | 1 | 2 |
| BMI | ||
| < 25 | 3 | 4 |
| 25–29.9 | 4 | 6 |
| ≥30 | 3 | 3 |
In three subjects, dosing and urine collection was conducted in both 2014 and 2017. All repeat participants received two different dosing vehicles (CMC at one visit, ethanol at the other).
3.2. Pharmacokinetic analysis
3.2.1. BPA pharmacokinetics after dermal administration
A summary of estimated pharmacokinetic parameters is presented in Table 2. As noted earlier, serum concentrations of free d6-BPA were only analyzed for the ethanol data. There was no discernable difference in total d6-BPA kinetics between the CMC and ethanol vehicles given the extent of interindividual variability, and so the data for both vehicles were combined. Based on data collected under the 2017 protocol, detectable serum levels of total d6-BPA were observed at approximately 1.4 h (ranging from 0.6 to 3.7 h) following the start of dermal administration. Detectable free serum d6-BPA appeared approximately 1.8 h (1.5–2.2 h) after the appearance of total d6-BPA at the individual-level, or approximately 2.8 h (2.5–3.1 h) after start of dermal administration. Serum concentrations of free and total d6-BPA increased rapidly for seven hours (Fig. 2). Free d6-BPA was a significantly greater percentage of the total serum BPA, with 10.9% (6.6–17%) at Cmax compared to the 0.39% observed in the oral arm (Thayer et al., 2015). Beginning at approximately seven hours and continuing to 12 h (which corresponds to cessation of exposure), the concentration of free and total serum d6-BPA plateaued (Fig. 2 inset). After cessation of dermal exposure, elimination from the serum was slow, with t1/2 values for free and total BPA estimated to be approximately 15–20 h.
Table 2.
Pharmacokinetic parameters for total and free d6-BPA in serum and urine of human subjects administered d6-BPA via 12-hour dermal application.
| Serum |
Urine |
|||||
|---|---|---|---|---|---|---|
| Cmax (nM) | % free Cmax | AUC0−∞ (nM × h) | % free AUC | t1/2 (h) | Cumulative excreted (μg/kg BW) | |
| 2017 protocol | ||||||
| Total d6-BPA | 2.63 ± 1.69 (5) | 9.66 ± 3.39 (3) | 72.4 ± 45.7 (4) | 8.41 ± 1.67 (2) | 20.0 ± 6.84 (4) | 1.16 ± 0.572 (4) |
| Free d6-BPA | 0.282 ± 0.0583 (3) | – | 8.68 ± 1.35 (2) | – | 12.2 ± 5.16 (2) | – |
| 2014 protocol | ||||||
| Total d6-BPA | 3.66 ± 2.65 (8) | 11.9 ± 4.15 (4) | 107 ± 57.4 (8) | 9.02 ± 1.85(4) | 22.1 ± 11.4 (8) | 0.919 ± 0.554 (8) |
| Free d6-BPA | 0.265 ± 0.193 (4) | – | 6.93 ± 3.18 (4) | – | 20.3 ± 7.77 (4) | – |
| Combined | ||||||
| Total d6-BPA | 3.26 ± 2.31 (13) | 10.9 ± 3.73 (7) | 95.6 ± 54.4 (12) | 8.81 ± 1.65 (6) | 21.4 ± 9.81 (12) | 0.998 ± 0.546 (12) |
| Free d6-BPA | 0.272 ± 0.141 (7) | – | 7.51 ± 2.69 (6) | – | 17.6 ± 7.69 (6) | – |
All values shown as mean ± SD (n), where n is the number of datasets used to estimate the parameter. AUC0−∞ Estimated by log-linear trapezoidal rule from time zero to infinity (using t1/2 and the final serum concentration). Cmax was directly obtained from the data for all datasets. t1/2 estimated for individuals I and G of the 2014 protocol omitted data for t = 12 h, since concentrations increased to t = 24 h. AUC, t1/2 and cumulative amount excreted could not be estimated for individual D due to early withdrawal from the study. Time to Cmax (i.e., tmax) is not applicable because serum concentration increases to Cmax during exposure, and predictably declines when exposure is stopped at 720 min. There was no discernable difference between vehicles (CMC or ethanol) given the interindividual variability, and data for free d6-BPA were only analyzed for the ethanol results. A pilot analysis was performed on the 2014 CMC data, which included an estimate of free d6-BPA (Supplemental Materials Part B-3). These preliminary results are not incorporated in this analysis due to inter-laboratory differences. Results separated by vehicle are available in Supplemental B-1 Table S1.
Fig. 2.
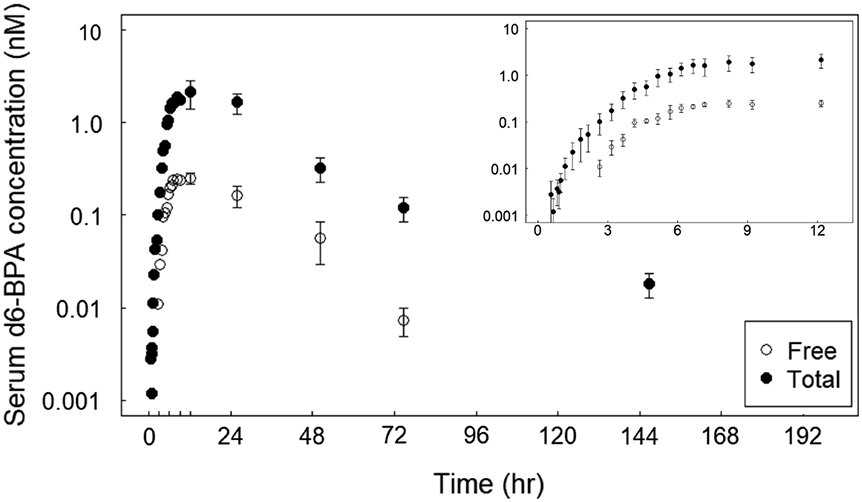
Serum concentration of free and total d6-BPA during and after 12 h of dermal administration under the 2017 protocol. 100 μg/kg BW of d6-BPA was applied as either an ethanol solution or a CMC suspension. There was no discernable difference between vehicle given the interindividual variability, and so the data for both vehicles were combined for this figure. The log plots represent the average concentrations ± SE (n = 5 for data up to 24 h, n = 4 for remaining data). The inset expands the first 12 h.
Total d6-BPA was detected in the urine at first urinary void for all individuals (within 2 h). Approximately 1% of the target dose was collected in urine by three days post-dose (Fig. 3). Concentration of total urinary d6-BPA increased rapidly during 8–12 h, and then decreased continuously beginning around 24 h post-dose. For all individuals, there remained detectable levels of total urinary d6-BPA at the end of the experiment (three days post-dose for the 2014 study participants, and six days post-dose for the 2017 study participants). However, the daily urinary excretion of total d6-BPA was a negligible percent of the dose after about three days. As shown in Fig. 3, there is high interindividual variability in the cumulative excreted dose (which did not plateau at three days post-dose). Individual-level concentrations and cumulative amounts excreted are available in Supplemental Materials Part B-1 and “Supplemental_Serum_Data” and “Supplemental_Urine_Data” Excel data sheet files.
Fig. 3.
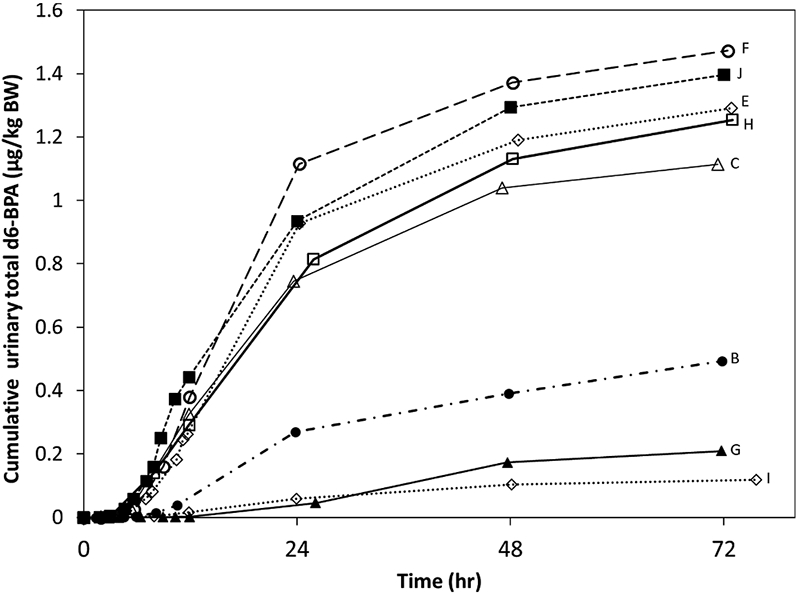
Cumulative urinary excretion of total d6-BPA in human subjects. Cumulative excretion is expressed in units of μg eliminated per kilogram body weight. Data for individuals dosed under the 2014 protocol are shown (n = 8). Data for both vehicles were included in this figure. There was no discernable pattern with respect to body weight or body mass index, however the individuals with the lowest urinary values were African American (B, G, and I), while all others were either white or mixed-race (C). A cumulative data plot for four individuals dosed under the 2017 protocol is available in “Supplemental_Urine_Data.xlsx”.
The plateau of serum unconjugated and total d6-BPA indicates that a steady-state between absorption and elimination processes had been reached (Fig. 2 inset). Given the serum elimination half-life of ~ 6 h for unconjugated d6-BPA (observed in Thayer et al., 2015), steady-state is achieved faster than expected. If the dermal absorption rate of d6-BPA into blood was constant (as in case of intravenous infusion), it would take approximately five elimination half-lives (30 h) to reach a quasi-steady state serum concentration of unconjugated d6-BPA (97% of the final level). In the current dermal study, however, the rate of uptake from the dermis into the blood is not constant over time but dependent on a series of concentration driving forces defined by Fick’s laws of diffusion. As the concentration of d6-BPA in the stratum corneum increases and approaches equilibrium with the d6-BPA in vehicle on the surface of the skin, mass transfer from the surface to the dermal layers is slowed. Additionally, the serum level of d6-BPA rapidly increases during the initial phase until steady state, reducing the concentration gradient between dermis and plasma. As a result, the total systemic uptake rate decreases from a transient/high value to a low/steady-state value. In effect, the initially high and transient uptake rate accelerates the process of reaching steady state.
The terminal half-lives for both total and free serum d6-BPA following dermal application of 100 μg/kg (Table 2) were over 3 times greater (21.4 and 17.6 h) than those from our earlier (Thayer et al. (2015) oral study (which estimated t1/2 of 6.4 and 5.6 h for total and free d6-BPA, respectively). Such a large discrepancy indicates a slow release of d6-BPA from a skin depot into the circulatory system, continuing/proceeding in parallel to the elimination process. This is in contrast with the fast absorption and distribution observed in the oral arm of the study (Thayer et al., 2015). The absorption half-lives in the oral study were 0.26 and 0.52 h for total and free BPA, respectively, and the distribution half-life for free BPA was 1.2 h. There was minimal interaction or overlap between absorption/distribution processes and the elimination process after ~ 6 h following an oral bolus dose. Whereas the terminal half-lives from the oral bolus study of Thayer et al. (2015) reflect the elimination of total and free BPA from serum, those from the present study should be interpreted as the time required to eliminate half of the applied dose from the skin (the rate-limiting step).
The mean Cmax following oral administration of 100 μg/kg (427 nmol/kg) of d6-BPA was over 1700 nM for total d6-BPA (> 600 times greater than after the dermal application, 3.26 nM) and 6.5 nM for free d6-BPA (> 20 times greater, 0.272 nM). This variation was due to multiple factors. The fractional absorbed dose following oral administration of d6-BPA in a cookie (~100%) was much greater than by dermal application of a d6-BPA formulation over 12 h (< 10%). For a more detailed account on the fractional absorbed dose, see Section “Estimates of absorbed d6-BPA After Dermal Administration”. Additionally, an acute oral bolus dose is generally expected to result in a higher peak concentration than an equivalent dose continuously absorbed over 12 h. This is compounded by the more rapid systemic uptake following oral exposure when compared to dermal exposure.
Differences in serum AUC between the oral and dermal studies were not as great as the differences in peak serum concentration due to the longer exposure duration and longer terminal half-life observed from the dermal data (Table 2). The AUC following oral administration (see Thayer et al. (2015)) was approximately 44 times greater than that following dermal application for total d6-BPA (4263 versus 95.6 nM × h), and just three times greater for free d6-BPA (23 versus 7.51 nM × h). There was a notable difference between the oral and dermal studies with regards to the percentage of total d6-BPA present as free. Following oral administration, free d6-BPA was 0.39 ± 0.17% and 0.56 ± 0.16% of the total based on the Cmax and AUC, respectively (Thayer et al., 2015). A higher proportion of free d6-BPA following dermal administration (10.9 ± 3.73% based on Cmax and 8.81 ± 1.65% based on AUC) is due to the lack of hepatic first-pass effect.
There was variation between the 2014 and 2017 dermal studies. Serum data under the 2014 protocol indicated higher Cmax and AUC for total d6-BPA than did the 2017 data (Table 2). Because of both the study variation and interindividual variation, it was difficult to estimate the effect of vehicle differences on pharmacokinetics. Similarly, the effect of age, sex, race, or body weight could not be determined because sample sizes were small once the individuals’ data were stratified by either vehicle or study year. With the exception of individual A, participants identifying as African-American exhibited lower cumulative urinary recovery, and lower Cmax and AUC (see Supplemental Material Part B-1, Table S2). The impact of vehicle (ethanol solution vs. CMC suspension) on serum kinetics of total d6-BPA would ideally be assessed by comparing matched pairs (individuals who underwent experiments using both vehicles). However, only three matched-pair datasets are available, and analysis is complicated by the differences between the 2014 and 2017 protocols. No matched individuals underwent the same protocol for both vehicles. As a result, a rigorous analysis to determine a vehicle effect was not possible at the individual matched-pair level. However, there did not appear to be a consistent difference between CMC and ethanol datasets (see Supplemental Materials B-1, Table S1).
3.3. Recovery of unabsorbed d6-BPA after dermal administration
A substantial amount of the applied dose was recovered from the application site at 12 h following dermal administration of d6-BPA in ethanol. For the ethanol experiments under both the 2014 and 2017 protocols, 71–99% of the applied dermal dose remained unabsorbed on the skin or on the HTC (Fig. 4C/D). This is in good agreement with the experiments using CMC conducted in 2017, which found an unabsorbed dose of 75–79% (Fig. 4B). For the 2014 CMC data, a lower percentage (19–21%) of the applied dose was recovered (Fig. 4A). For this dataset, no sponge or gauze was used to wipe exposed skin of the rinsing solution. This likely had a negative impact on the effectiveness of surface recovery.
Fig. 4.
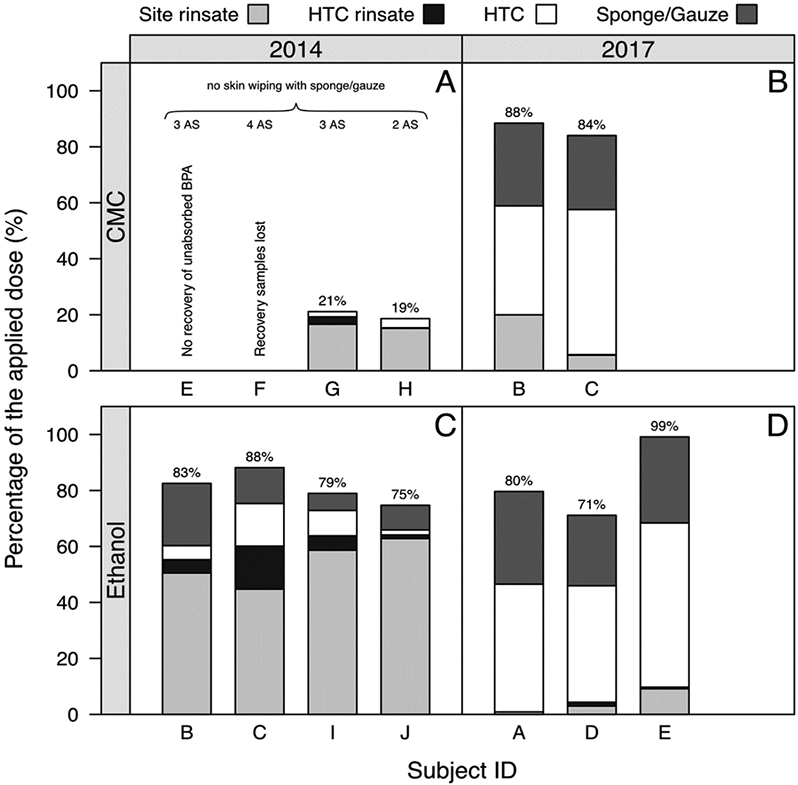
Recovery of unabsorbed d6-BPA after dermal administration. The stacked bar chart shows the percentage of the applied d6-BPA dose that was recovered in the skin rinsate and the Hill Top Chamber (HTC) rinsate, and in the extracts of the HTC and sponge/gauze. The data for the individual participants are grouped in subfigures according to the vehicle (carboxymethylcellulose [CMC], ethanol) and the year in which the experiment was conducted. The percentage numbers on top the stacked bar charts indicate the unabsorbed dose. One application site (AS) per participant was used, unless otherwise indicated (see 2–4 AS in Subfigure A). The HTC was removed at 12 h post-dose. The sponge/gauze was used for wiping the exposed skin with rinsate solution, unless otherwise indicated (see Subfigure A).
In all experiments conducted in 2014, the largest proportion of the unabsorbed dose was found on the skin whereas only a minor portion was recovered from the HTC (Fig. 4A/C). The opposite is true for the experiments conducted in 2017, where the largest proportion of the unabsorbed dose was recovered from the HTC and a minor portion from the skin rinsate (Fig. 4B/C/D). Multiple differences between the 2014 and 2017 experiments were documented, and these could have contributed to the recovery variations. As noted earlier, there was a lack of skin wiping for the 2014 CMC experiment. In addition, the 2014 CMC study used 2–4 application sites rather than a single application site (Fig. 4A). Distributing the dermal dose (100 μg/kg BW) across a larger skin surface area would enable the applied BPA to diffuse into a larger portion of skin surface to build up a skin depot. Different nurses implemented the surface and HTC rinsing protocol between 2014 and 2017, which could have also impacted surface recovery. Irrespective of these differences, there was good agreement in the unabsorbed dose (71–88%) across the subjects when excluding the implausible value of 99% from one participant (where 1.4% of the dose was still collected in urine) and excluding the low values from the 2014 study using CMC where no skin wiping was performed.
3.4. Estimates of absorbed d6-BPA after dermal administration
The dermal and HTC recovery mass balance implies that up to 12–29% of the applied dermal dose penetrated the skin over a 12-hour period. This is likely an over-estimate due to the experimental variation in rinsate recovery as well as unaccounted amounts lost to the environment. The percentage of total d6-BPA recovered in urine was significantly lower (approximately 1% of the target dose). This is likely an under-estimate of total absorbed dose because detectable levels of d6-BPA were measured in urine and serum at the end of the experiment for all individuals. This indicates that not all d6-BPA had been cleared from the subjects at the time the last observations were made. Comparing the average serum AUC for total d6-BPA between Thayer et al. (2015) (where the target oral 100 μg/kg of d6-BPA dose was almost completely absorbed) and the current work, the dermal AUC for a target 100 μg/kg dose was 2.2% of the oral AUC. This may be one estimate for the average percent dose absorbed into systemic circulation following 12-hour dermal application, but there is some uncertainty due to interindividual variability, and oral/dermal toxicokinetic differences. Given the high approximate dermal absorption of up to 12–29% as implied from surface recovery, and the fact that detectable levels were measured in serum at the end of the experiment, it is possible that a portion of the administered dose that penetrated the skin remained in the dermis and epidermis throughout the post-dose period and beyond six days. This depot of d6-BPA in the skin would have continued to diffuse into the blood until depleted or lost by other removal processes (contact with other surfaces, skin washing, and desquamation).
To further reduce the uncertainty surrounding the dermal absorption of BPA, a plausibility check was conducted by which the three estimates for the fraction dermally absorbed (Fabs) were used to calculate the serum clearance (CL) of unconjugated BPA for a human subject with a body weight (BW) of 80 kg (Table 3). Apart from Fabs, the absolute dermal dose (D) and the AUC for unconjugated BPA entered into the calculation (Table 3). The calculated serum clearances were then compared with the clearance predicted by allometric scaling based on animal data with intravenous administration of BPA.
Table 3.
Plausibility check of the fraction dermally absorbed (Fabs), as obtained by three different approaches, in terms of serum clearance of unconjugated BPA.
| Approach | Fabs (%) | Dose D (nmol) | AUC (nM × h) | CL (L/h) |
|---|---|---|---|---|
| Recovery of total BPA in urine | 1 | 34,141 | 7.51 | 45 |
| Serum AUCs of total BPA after oral and dermal administration | 2.2 | 95.5 | ||
| Mass-balance considerations based on recovery of unabsorbed BPA | 12–29 | 545–1318 |
The absolute dermal dose (D, nmol) derives from the per-body-weight dose of 100 μg/kg BW multiplied by a body weight of 80 kg and divided by the molecular mass of 234.32 g/mol for d6-BPA. The assumed body weight is representative for the six subjects from which the estimate for the mean serum AUC of 7.51 nM × h for unconjugated BPA was obtained. The serum clearance for unconjugated BPA (CL) was calculated as: CL = Fabs/100% × D/AUC.
The Fabs values derived from the three approaches (1%, 2.2%, and 12–29%) translate into serum clearances of 45 L/h, 95.5 L/h, and 545–1318 L/h (Table 3). Allometric scaling approaches predict serum clearances of 140 L/h (= 5.264 × BW0.749; Cho et al. (2002)) and 122 L/h (= 2.34 × BW0.9014; Collet et al. (2015)), respectively, for an 80-kg human. The Fabs value of 1%, which was obtained by considering the recovery of total BPA in urine, translates into a serum clearance of half that predicted by allometric scaling. The Fabs value of 2.2%, which was calculated from the serum AUCs of total BPA after oral and dermal administration, translates into a serum clearance which is physiologically plausible as it is consistent with allometric scaling estimates. This conclusion holds true even assuming a small portion of the dermally absorbed BPA is metabolized in the skin before reaching systemic circulation. The Fabs value of 12–29%, which derives from mass-balance considerations based on the recovery of unabsorbed BPA, yields a serum clearance of 545–1318 L/h which is physiologically implausible as it grossly exceeds not only the values predicted by allometric scaling but also the hepatic blood flow (Collet et al., 2015). This strongly suggests that the 12–29%, being the complement of the portion recovered from the application site, was mostly lost to the environment rather than absorbed.
3.5. Comparison with in vitro skin permeation data
The AUC-derived estimate of 2.2% accounts for dermal absorption occurring during the 12-hour administration period and the post-exposure period where skin-to-systemic transfer occurs. This estimate is in good agreement with in vitro studies using excised human skin after performing a time-adjustment to normalize the reported values to a 12-hour duration (assuming proportionality of the uptake amounts). 12-hour time-adjusted estimates for the percentage of an applied dose reaching a receptor compartment were 0.85–1.95% (Toner et al., 2018), 3.25% (Morck et al., 2010), 4.3% (Demierre et al., 2012), and 7.7% (Zalko et al., 2011). A 12-hour adjusted value for percent dose reaching the receptor of approximately 21% can be obtained from the study of Liu and Martin (2019). However, this study used a reconstructed human epidermal model with a thickness of approximately 120 μm (20–30% of the total thickness is stratum corneum), whereas the excised human skin samples consisting of native, complete epidermis and portions of dermis were 200–500 μm thick.
The consistency with in vitro data is also shown when comparing the steady-state flux of BPA through the human body with that through excised human skin. The serum and urinary data of this study revealed the presence of a transient period of steady-state flux of d6-BPA through the human body. Three in vitro studies (Demierre et al., 2012; Marquet et al., 2011; Toner et al., 2018) provided information on the steady-state flux through human skin. Achieving steady-state under in vitro and in vivo conditions should make the study results comparable, provided that the data are scaled to a common dose. Dividing the steady-state flux by the concentration of the dosing solution yields the permeability coefficient (Kp), which is a dose-independent measure that enables such a comparison.
Supplementary Materials Part B-5 Table S8 summarizes the information on the experimental conditions, the steady-state flux, and the permeability coefficient for the three in vitro studies and the present study. The volume per unit area of the applied dosing solution was quite comparable across all studies (5-fold variation). In contrast, the applied concentration in the present study was 6–10,000-fold higher than in the in vitro studies. This translated into corresponding differences in the amount of BPA applied per unit area.
Evidence for a transient period of steady-state flux of d6-BPA through the human body comes from the serum and urine data. As noted earlier, a quasi steady-state condition was achieved in serum between 7 and 12 h (Fig. 2 inset). Consistent with this observation is the steep linear increase in cumulative urinary excretion between ~5 and 24 h after the start of dermal application (Fig. 3).
Of the eight individuals dosed under the 2014 protocol, five subjects showed a consistent cumulative urinary excretion of total d6-BPA of 0.7–1.1 μg/kg BW at 24 h (Fig. 3). The data of these individuals were used to calculate the steady-state flux of d6-BPA through the body. This was done by multiplying the slope of the steep linear increase in cumulative urinary excretion with body weight and dividing the results by the exposed skin area. This yields a mean steady-state flux of 632 ng/cm2/h. Dividing this value by the concentration of the dosing solution (25,000 mg/L) yields a Kp value of 2.5 × 10−5 cm/h, which agrees with the values of 0.8–1.6 × 10−5 cm/h (Toner et al., 2018) and 3.0 × 10−5 cm/h (Marquet et al., 2011). In contrast, the Kp value of Demierre et al. (2012) was several fold higher, which might be explained by the use of thinner skin samples (thickness: 200 μm instead of 350–500 μm). Liu and Martin (2019) reported a considerably higher Kp value of 0.033–0.036 cm/h), which can be explained by the specific characteristics of the reconstructed human epidermal model used (see above).
3.6. Comparison with other human studies involving the dermal exposure to isotope-labeled bisphenols
The pharmacokinetics of BPA following dermal administration differs substantially from oral administration. The results of the two study arms, Thayer et al. (2015) and the current work, showed that dermal exposure caused a longer terminal serum half-life and higher free:total d6-BPA ratios in serum and urine compared to oral, which is consistent with findings of a study involving the handling of simulated receipt paper containing relevant levels (2.5%) of BPA (Liu and Martin, 2017), and a toxicokinetic study with bisphenol S (BPS) (Khmiri et al., 2020).
The longer terminal serum half-life resulted from continuing systemic uptake from a diminishing skin depot after cessation of dermal exposure. This is supported by data of urinary excretion, which showed total d6-BPA continued to be measured at six days post-dose. Other studies observed similar results. Khmiri et al. (2020) applied a d8-BPS suspension containing 1% CMC at a dose of 1000 μg/kg BW on the forearm of volunteers for six hours. An oral arm of the study was also performed by administering 100 μg/kg BW in a cookie. Following dermal administration, plasma and urine measurements of BPS and its glucuronide were below the lower limit of quantification for most time points, which was due to the very low dermal absorption fraction (< 0.1%). However, based on cumulative urinary excretion data, that study indicated slower dermal absorption of BPS when compared to oral administration, and a longer residence time after dermal absorption (elimination was not complete at 72 h).
Similarly, Liu and Martin (2017) observed much slower elimination of d16-BPA following dermal exposure when compared to oral ex-posure. Incidentally, the labeled BPA was detected in the pre-exposure urine samples of dietary study volunteers, one week after these individuals participated in the dermal arm of the study. In the dermal arm, participants handled simulated thermal receipts (soft notebook paper containing 20 mg d16-BPA) for 5 min and then placed a nitrile glove on the exposed hand for 2 h. Unabsorbed BPA was then recovered from the exposed skin using hand wipes (residual BPA in the gloves was not tested for), and the participants then washed their hands with soap. Data were presented for five participants. Between 0.07 and 0.87 μg d16-BPA was collected from the hand-wipes, and serum concentrations of free and total d16-BPA (measured at three time points over 7.5 h post-dosing) were below the LOD. Cumulative urinary excretion was collected up to 46 h and showed the steepest slope between 12 and 36 h. At the end of the collection period, 0.1–2.7 μg d16-BPA was excreted via urine. This was comparable in shape with that of the present study.
A second dermal trial with one participant exhibited different kinetics in comparison to the first trial: 3 μg d16-BPA was recovered by the hand-wipe, indicating a considerably higher transfer from the simulated paper to the skin. Cumulative urinary excretion was close to linear from 12 h up to 116 h post-dose, at which a total of 12 μg was excreted via urine. Serum samples, taken 21 and 51 h, had detectable levels of free and total d16-BPA being within the range of 1–2 times the LOD (0.015 ng/mL). Greater than 50% of the total BPA concentration was measured as free BPA at these time points (whereas for the oral study, all serum data for free BPA were below the LOD), which again highlights the importance of the first-pass effect.
Taken together, data indicate the presence of a skin depot of considerable capacity. This also relates to an additional study design difference between Liu and Martin (2017) and the current study. In Liu and Martin (2017), volunteers were exposed via the palm of the hand whereas volunteers of the present study were exposed via the inner forearm. The stratum corneum (SC) of palm skin is thicker than that of the forearm which has an influence on the dermal absorption kinetics. BPA is lipophilic and therefore easily partitions into the SC, which consists of SC lipids and dead corneocytes. The SC of palm skin can therefore establish a larger skin depot for BPA than that of the forearm skin. The longer diffusion distance in the SC of palm skin could also explain a slower/delayed appearance in the blood. The consequence is a much longer sustained constant urinary excretion over several days as observed in the second dermal trial of Liu and Martin (2017).
4. Conclusions
The present study for the first time provides estimates for dermal absorption of BPA in humans being exposed to a controlled dose of deuterated BPA over 12 h. Approximately 1% of the dermal dose was excreted in urine by three days post-dose. This figure underestimates the total absorbed dose, because detectable levels of d6-BPA were still measured in urine and serum at this time point. Analysis of dermal:oral serum AUC profiles for total d6-BPA revealed that approximately 2.2% of the dermal dose may have reached the systemic circulation. This AUC-derived estimate accounts for dermal absorption occurring during the 12-hour administration period and the post-exposure period where skin-to-systemic transfer continues. This estimate is in good agreement with in vitro studies when adjusting the results for an exposure duration of 12 h. Time-adjusted estimates for the percentage of applied dose reaching a receptor compartment were 0.85–1.95% (Toner et al., 2018), 3.25% (Morck et al., 2010), 4.3% (Demierre et al., 2012), and 7.7% (Zalko et al., 2011). The consistency with in vitro studies is also shown by the permeability coefficient (Kp). The Kp value of 2.5 × 10−5 cm/h for the present study agrees with the values of 0.8–1.6 × 10−5 cm/h (Toner et al., 2018) and 3.0 × 10−5 cm/h (Marquet et al., 2011).
Both the percent dermal absorption and the permeability coefficient are crucial parameters for the estimation of daily uptake of BPA from consumer products, including thermal receipt paper. In the Liu and Martin (2017) study, participants handled simulated receipt paper containing isotope-labeled BPA at a typical concentration of 2.5% for 5 min, followed by hand washing two hours later. From the slope of the cumulative urinary excretion curves following dermal exposure, daily intakes of BPA of 0.002–0.046 μg/kg/d could be estimated. These results suggest that alternative estimates of ~20 μg/kg/d, as estimated by Lassen et al. (2011) for cashiers under worst-case assumptions (i.e., 50% dermal absorption), likely exceeds the absorption capacity of the skin on the fingertips.
The results of the present study could form a basis to achieve a more mechanistic understanding of the dermal absorption of BPA and, thereby, to better inform risk assessment.
Supplementary Material
Acknowledgements
The authors would like to thank Michael Devito (US EPA) and Brad Collins (NTP) for internal review.
Funding and disclaimer
Authors declare no financial conflict of interest. Funding was provided through author’s employment and institutional resources for analytical chemistry analysis to Mt. Sinai. Mount Sinai CHEAR/HHEAR laboratory hub acknowledges Dhavalkumar Patel, Divya Pulivarthi, and Anil Meher who performed the measurements of bisphenol A biomarkers in urine and serum, and native form in the experimental material and rinsates. The views expressed are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency (US EPA).
Footnotes
CRediT authorship contribution statement
Alan F. Sasso: Formal analysis, Software, Data curation, Writing - original draft, Writing - review & editing, Visualization. Ralph Pirow: Formal analysis, Software, Data curation, Writing - original draft, Writing - review & editing, Visualization. Syam S. Andra: Conceptualization, Writing - original draft, Writing - review & editing, Formal analysis, Investigation, Resources, Methodology, Data curation. Rebecca Church: Writing - original draft, Investigation, Resources, Methodology, Data curation. Rebecca Nachman: Writing - original draft, Writing - review & editing. Susanne Linke: Writing - original draft, Formal analysis, Validation, Data curation. Dustin F. Kapraun: Formal analysis, Validation, Software, Data curation, Writing - review & editing. Shepherd H. Schurman: Formal analysis, Investigation, Resources, Methodology. Manish Arora: Conceptualization, Writing - original draft, Writing - review & editing, Formal analysis, Investigation, Resources, Methodology, Data curation. Kristina A. Thayer: Conceptualization, Methodology, Writing - review & editing. John R. Bucher: Conceptualization, Methodology, Resources. Linda S. Birnbaum: Conceptualization, Methodology, Writing - review & editing, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.106031.
References
- Biedermann S, Tschudin P, Grob K, 2010. Transfer of bisphenol A from thermal printer paper to the skin. Anal. Bioanal. Chem 398, 571–576. [DOI] [PubMed] [Google Scholar]
- Bushnik T, Haines D, Levallois P, Levesque J, Van Oostdam J, Viau C, 2010. Lead and bisphenol A concentrations in the Canadian population. Health Rep. 21, 7–18. [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, 2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-oetylphenol: 2003-2004. Environ. Health Perspect 116, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XL, Corriveau J, Popovic S, 2009. Migration of bisphenol A from can coatings to liquid infant formula during storage at room temperature. J. Food Prot 72, 2571–2574. [DOI] [PubMed] [Google Scholar]
- Carwile JL, Ye X, Zhou X, Calafat AM, Michels KB, 2011. Canned soup consumption and urinary bisphenol A: a randomized crossover trial. JAMA 306, 2218–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2019. National report on human exposure to environmental chemicals: updated tables, January 2019. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf.
- Cho CY, Shin BS, Jung JH, Kim DH, Lee KC, Han SY, Kim HS, Lee BM, Yoo SD, 2002. Pharmacokinetic scaling of bisphenol A by species-invariant time methods. Xenobiotica 32, 925–934. [DOI] [PubMed] [Google Scholar]
- Collet SH, Picard-Hagen N, Lacroix MZ, Puel S, Viguie C, Bousquet-Melou A, Toutain PL, Gayrard V, 2015. Allometric scaling for predicting human clearance of bisphenol A. Toxicol. Appl. Pharmacol 284, 323–329. [DOI] [PubMed] [Google Scholar]
- Demierre AL, Peter R, Oberli A, Bourqui-Pittet M, 2012. Dermal penetration of bisphenol A in human skin contributes marginally to total exposure. Toxicol. Lett 213, 305–308. [DOI] [PubMed] [Google Scholar]
- Derendorf H, Rowland Schmidt S., 2020. Rowland and Tozer’s clinical pharmacokinetics and pharmacodynamics: concepts and applications, fifth ed. Wolters Kluwer, Philadelphia. [Google Scholar]
- Dwivedi P, Zhou X, Powell TG, Calafat AM, Ye X, 2018. Impact of enzymatic hydrolysis on the quantification of total urinary concentrations of chemical biomarkers. Chemosphere 199, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHA, 2015. Opinion on Annex XV dossier proposing restrictions on Bisphenol A (https://www.echa.europa.eu/documents/10162/209030fc-ca4b-4745-97b6-98bfc4d6bdd3, accessed 3 December 2019). European Chemicals Agency, Helsinki. [Google Scholar]
- Eckardt M, Simat TJ, 2017. Bisphenol A and alternatives in thermal paper receipts - a German market analysis from 2015 to 2017. Chemosphere 186, 1016–1025. [DOI] [PubMed] [Google Scholar]
- Edginton AN, Ritter L, 2009. Predicting plasma concentrations of bisphenol A in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model. Environ. Health Perspect 117, 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, 2017. Bisphenol A (BPA) hazard assessment protocol. EFSA Support. Publ 14 (12), 1354E. [Google Scholar]
- EFSA, 2015. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 13, 3978. [Google Scholar]
- FAO/WHO, 2011. Toxicological and Health Aspects of Bisphenol A. Food and Agriculture Organization of the United Nations and the World Health Organization, Switzerland: ISBN 978 92 14 156427 4. [Google Scholar]
- Gabrielsson J, Weiner D, 2016. Pharmacokinetic and pharmacodynamic data analysis, fifth ed. Apotekarsocieteten, Sweden. [Google Scholar]
- Grignon C, Dupuis A, Albouy-Llaty M, Condylis M, Barrier L, Carato P, Brunet B, Migeot V, Venisse N, 2017. Validation of a probe for assessing deconjugation of glucuronide and sulfate phase II metabolites assayed through LC-MS/MS in biological matrices. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 1061–1062, 72–78. [DOI] [PubMed] [Google Scholar]
- Hormann AM, Vom Saal FS, Nagel SC, Stahlhut RW, Moyer CL, Ellersieck MR, Welshons WV, Toutain PL, Taylor JA, 2014. Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA). PLoS ONE 9 (10), e110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khmiri I, Cote J, Mantha M, Khemiri R, Lacroix M, Gely C, Toutain PL, Picard-Hagen N, Gayrard V, Bouchard M, 2020. Toxicokinetics of bisphenol-S and its glucuronide in plasma and urine following oral and dermal exposure in volunteers for the interpretation of biomonitoring data. Environ. Int 138, 105644. [DOI] [PubMed] [Google Scholar]
- Koch HM, Kolossa-Gehring M, Schroter-Kermani C, Angerer J, Bruning T, 2012. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: a retrospective exposure evaluation. J. Eposure Sci. Environ. Epidemiol 22, 610–616. [DOI] [PubMed] [Google Scholar]
- Lassen C, Mikkelsen SH, Brandt UK, 2011. Migration of bisphenol A from cash register receipts and baby dummies. Survey of Chemical Substances in Consumer Products, No. 110 2011. Danish Ministry of the Environment. http://www2.mst.dk/udgiv/publications/2011/04/978-87-92708-93-9.pdf (accessed 2 December 2019). [Google Scholar]
- Liao C, Kannan K, 2011. Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure. Environ. Sci. Technol 45, 9372–9379. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K, 2012. Determination of free and conjugated forms of bisphenol A in human urine and serum by liquid chromatography-tandem mass spectrometry. Environ. Sci. Technol 46, 5003–5009. [DOI] [PubMed] [Google Scholar]
- Liu J, Martin JW, 2017. Prolonged exposure to bisphenol A from single dermal contact events. Environ. Sci. Technol 51, 9940–9949. [DOI] [PubMed] [Google Scholar]
- Liu J, Martin JW, 2019. Comparison of Bisphenol A and Bisphenol S Percutaneous Absorption and Biotransformation. Environ. Health Perspect 127, 67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet F, Payan JP, Beydon D, Wathier L, Grandclaude MC, Ferrari E, 2011. In vivo and ex vivo percutaneous absorption of [14C]-bisphenol A in rats: a possible extrapolation to human absorption? Arch. Toxicol 85, 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morck TJ, Sorda G, Bechi N, Rasmussen BS, Nielsen JB, Ietta F, Rytting E, Mathiesen L, Paulesu L, Knudsen LE, 2010. Placental transport and in vitro effects of Bisphenol A. Reprod. Toxicol 30, 131–137. [DOI] [PubMed] [Google Scholar]
- NIEHS, 2019. BPA CLARITY Project. https://ntp.niehs.nih.gov/whatwestudy/topics/bpa/index.html.
- R Core Team, 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- Rubin BS, 2011. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol 127, 27–34. [DOI] [PubMed] [Google Scholar]
- Shelby MD, 2008. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. NTP CERHR Mon v, vii-ix, 1–64 passim. [PubMed] [Google Scholar]
- Thayer KA, Doerge DR, Hunt D, Schurman SH, Twaddle NC, Churchwell MI, Garantziotis S, Kissling GE, Easterling MR, Bucher JR, Birnbaum LS, 2015. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int 83, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Taylor KW, Garantziotis S, Schurman SH, Kissling GE, Hunt D, Herbert B, Church R, Jankowich R, Churchwell MI, Scheri RC, Birnbaum LS, Bucher JR, 2016. Bisphenol A, Bisphenol S, and 4-Hydroxyphenyl 4-Isoprooxyphenylsulfone (BPSIP) in Urine and Blood of Cashiers. Environ. Health Perspect 124, 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner F, Allan G, Dimond SS, Waechter JM Jr., Beyer D, 2018. In vitro percutaneous absorption and metabolism of Bisphenol A (BPA) through fresh human skin. Toxicol. in vitro 47, 147–155. [DOI] [PubMed] [Google Scholar]
- Twaddle NC, Churchwell MI, Vanlandingham M, Doerge DR, 2010. Quantification of deuterated bisphenol A in serum, tissues, and excreta from adult Sprague-Dawley rats using liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom 24, 3011–3020. [DOI] [PubMed] [Google Scholar]
- Verner MA, Magher T, Haddad S, 2010. High concentrations of commonly used drugs can inhibit the in vitro glucuronidation of bisphenol A and nonylphenol in rats. Xenobiotica 40, 83–92. [DOI] [PubMed] [Google Scholar]
- Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W, 2002. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol 15, 1281–1287. [DOI] [PubMed] [Google Scholar]
- Zalko D, Jacques C, Duplan H, Bruel S, Perdu E, 2011. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere 82, 424–430. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Alomirah H, Cho HS, Li YF, Liao C, Minh TB, Mohd MA, Nakata H, Ren N, Kannan K, 2011. Urinary bisphenol A concentrations and their implications for human exposure in several Asian countries. Environ. Sci. Technol 45, 7044–7050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


