Abstract
Mood disorders can be considered among the most common and debilitating mental disorders. Major depression, as an example of mood disorders, is known to severely reduce the quality of life as well as psychosocial functioning of those affected. Its impact on the burden of disease worldwide has been enormous, with the World Health Organisation projecting depression to be the leading cause of mental illness by 2030. Despite several studies on the subject, little has been done to contextualise the condition in Africa, coupled with the fact that there is still much to be understood on the subject. This review attempts to shed more light on the prevalence of depression in Sub-Saharan Africa (SSA), its pathophysiology, risk factors, diagnosis and the experimental models available to study depression within the sub-region. It also evaluates the contribution of the sub-region to the global research output of depression as well as bottlenecks associated with full exploitation of the sub region’s resources to manage the disorder.
Keywords: Major depressive disorder, Antidepressants, Sub-Saharan Africa, Medicinal plants
Introduction
Mood disorders are common mental disorders in Sub-Saharan African (SSA) that usually go undiagnosed and underreported. This subject was the theme of discussion at the International Brain Research Organization –Africa Regional Committee (IBRO-ARC) Advanced School on Depression and Mood Disorders in Sub-Saharan Africa, which took place at the University of Ilorin in the lead up to the bi-annual international meeting of the Society of Neuroscientists of Africa organised jointly with the Neuroscience Society of Nigeria. Although almost 10% of the overall disease burden in SSAa can be traced to neuropsychiatric disorders (Fekadu et al., 2017), there is currently a paucity of literature on depression epidemiology, research and management in Sub-Saharan Africa. Data available is scattered in several places and, at best, not contextualised to the sub-region. This review on depression in SSA is a product of the attendees of the IBRO-ARC advanced school that seeks to provide a comprehensive body of work, giving an overview of depression in SSA to spur on further contextualised work. We have explored the epidemiology, pathophysiology, risk factors, diagnosis, and experimental approach to studying depression in the context of Africa. We have additionally identified contributions of the sub-region in the study of the disorder and emerging management approaches, as well as necessary gaps to be filled. To identify relevant articles for this review, a systematic review of the PUBMED central, PsycINFO, and Google scholar databases, for articles published before 2020 was conducted using the following key terms: (1) mood disorders (2) depression; (3) each sub-Saharan African country; and (4) epidemiology, pathophysiology, and treatment. Additional studies were identified through the bibliographic references used in the reviewed articles. The search was limited to articles published in the English language.
Epidemiology of depression in Sub-Saharan Africa (SSA)
Mood disorders are one of the most common and debilitating mental illnesses. Generally, psychiatric mood disorders may be classified into various categories including; depression (extreme low emotional state), mania (extreme high emotional state), bipolar disorder I and II (alternating periods of depression and mania), cyclothymia, depression with or without psychosis, recurrent mania with or without psychosis and dysthymia (Delgardo and Morena, 2006, Gureje, 2007, Kessler, 2007, Marvel and Paradiso, 2008, Phillips and Kupfer, 2013). Depression has been identified to have a ‘chameleon presentation’ as it cuts across all age groups (Safeekh, 2017, Adewuya, 2018, Adebayo Adebisi, 2019).
Epidemiological data on depression in SSA countries is poor. In many cases, country-specific data are absent and rather replaced by point prevalence. Only a few countries in Africa present epidemiological data for depression, and where available, there exists methodological inconsistencies and high variabilities across countries. This, in turn, limits the generalisation and synthesis of data across studies (Baxter, 2013, Esan and Esan, 2016).
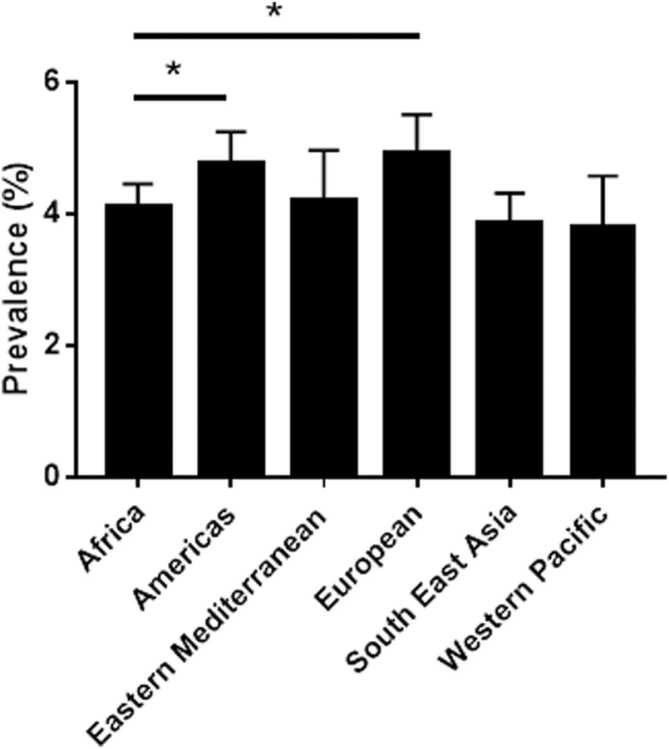
The absence of data is a significant burden, nonetheless, incomplete data on the prevalence of depression is available for limited countries (Table 1). These countries include South Africa, Nigeria, Cameroon, Ethiopia, Sudan, Kenya, Uganda, Ghana, the Democratic Republic of Congo, Zambia, and Botswana. For the main part, data from these countries are obtained from cross-sectional or cohort studies in hospitals, schools or prisons, increasing the burden of missing data and misrepresentation. Despite the gaps and limited knowledge on the epidemiology of depression in SSA, available data suggests prevalence is only lower than that reported in the Americas and European regions as defined by the World Health Organisation(WHO) (Fig. 1) (Depression and Other Common Mental Disorders Global Health Estimates, 2017) (Fig. 2).
Table 1.
Depression rates recorded in the general population versus small controlled populations.
| Country | % Depression rate in general populationa | % Depression rate in controlled population |
|---|---|---|
| Angola | 3.6 | 38 – 67 (Xavier and Peixoto, 2015) (Bernatsky et al., 2007) |
| Benin | 3.9 | 11.6 – 89 ( Melgar and Rossi 2010; Nubukpo et al., 2004) |
| Botswana | 4.7 | 15–38 (Lawler et al., 2011) |
| Burkina Faso | 3.6 | 4.3 – 11 (Ouedraogo et al., 2019; Duthe et al., 2016) |
| Burundi | 4.2 | 15–43 (Tol et al., 2014; Ventevogel et al., 2014) |
| Cameroon | 3.9 | 7 – 31 (Gaynes, 2012, Ngasa, 2017) |
| Cape Verde | 4.9 | 6 (Conde et al., 2019) |
| Central African Republic | 4.2 | 5.1 – 88.6 (Fouchier and Kedia 2018; Mbelesso et al., 2014) |
| Chad | 3.5 | 3.9 – 13.1 (Koyanagi et al., 2010) |
| Democratic Republic of Congo | 3.9 | 3.8–27 (Maj et al., 1994; Koyanagi et al., 2010) |
| Cote D’Ivoire | 3.8 | 2–48.9 (Ulanja et al., 2019; Bindt et al., 2012; Koyanagi et al., 2010) |
| Equatorial Guinea | 4.2 | – |
| Eritrea | 4.3 | 6.2–81.6 (Kelifa et al., 2020; Netsereab et al., 2018; Nakash et al., 2016) |
| Ethiopia | 4.7 | 4.8–57 (Duko et al., 2018; Bitew, 2014) |
| Gabon | 4.3 | 48.4 (Camara et al., 2018) |
| Gambia | 3.9 | 7.5 – 73.9 (Sherwood et al., 2015; Bartram 2018) |
| Ghana | 4.2 | 6.2 – 62 (Cristobal-Narvaez et al., 2020; Alhassan et al., 2014) |
| Guinea | 3.9 | 13 – 34 (Secor et al., 2019; Camara et al., 2014) |
| Guinea-Bissau | 4 | – |
| Kenya | 4.4 | 6.3 – 72.9 (Cristobal-Narvaez et al., 2020; Kamau et al., 2012) |
| Lesotho | 4.8 | 8–28 (Stahlman et al., 2015; Cerutti et al., 2016) |
| Liberia | 3.5 | 12.6–40 (Vinck et al., 2013; Johnson et al., 2008) |
| Madagascar | 4.4 | 21.5 (Melgar and Rossi 2010) |
| Malawi | 4.1 | 4.2–30.3 (Stewart et al., 2009; Udedi M, 2014) |
| Mali | 3.6 | 3.3 – 26.7 (Cristobal-Narvaez et al., 2020; Zoungrana et al., 2017) |
| Mauritania | 4.1 | 2.7 – 12.4 (Melgar and Rossi, 2010; Cristobal-Narvaez et al., 2020;) |
| Mauritius | 4.4 | 6.9 – 51.8 (Cristobal-Narvaez et al., 2020; Duval et al., 2010) |
| Mozambique | 4.1 | 7.03–34 (Melgar and Rossi 2010; Zacarias et al., 2012) |
| Namibia | 4.4 | 5.6 – 30 (Cristobal-Narvaez et al., 2020; Kalomo 2018) |
| Niger | 3.4 | 75.62 (John et al., 2019) |
| Nigeria | 3.9 | 7.8 – 30.6 (Amoran et al., 2007, Abasiubong, 2011, Uche et al., 2015) |
| Rwanda | 3.8 | 5 – 75 (Binagwaho et al., 2016; Bolton et al., 2002) |
| Senegal | 3.9 | 4.5 – 57.8 (Rai et al., 2013; Diale et al., 2015; Sy et al., 2019) |
| Sierra Leone | 3.9 | 7.1 – 80 (Bah et al., 2020; Secor et al., 2019) |
| South Africa | 4.6 | 1.9 – 38 (Cooper et al., 2009; Flutterman et al., 2010) |
| South Sudan | 4.4 | 6.4 – 47.5 (Ayazi et al., 2012; Assil and Zeidan, 2013) |
| Swaziland | 4.2 | 1.7 – 22.7 (Murray and Burnham, 2009; Malgvist et al., 2016;) |
| Tanzania | 4.1 | 2.7 – 15.5 (Gaynes et al., 2012; Marwick and Kaaya 2010) |
| Togo | 3.9 | 4.4 – 39 (Nubukpo et al, 2004; Kakpovi et al., 2017) |
| Uganda | 4.6 | 8.1 – 21 (Kinyanda et al., 2011; Amone-P’Olak et al., 2013) |
| Zambia | 4 | 5.1 – 53.7 (Chipimo and Fylkesnes, 2010; Mbewe et al., 2013) |
| Zimbabwe | 4 | 2.3 – 33 (Chibanda et al., 2010; Abas and Broadhead 1997) |
Adapted from the WHO Global Burden of Disease Study 2015 (Depression and Other Common Mental Disorders Global Health Estimates, 2015)
Fig. 1.
Depression rates in designated WHO regions. Countries in the WHO Africa region, which represent the SSA countries, have a depression rate (4.1 ± 0.05%) which is significantly lower than that recorded in the Americas (4.8 ± 0.09%) and the European region (4.9 ± 0.1%) *p < 0.0001. One-Way ANOVA Kruskal-Wallis Post-hoc test. Primary data obtained from the WHO Global Burden of Disease Study 2015 (Depression and Other Common Mental Disorders Global Health Estimates, 2015).
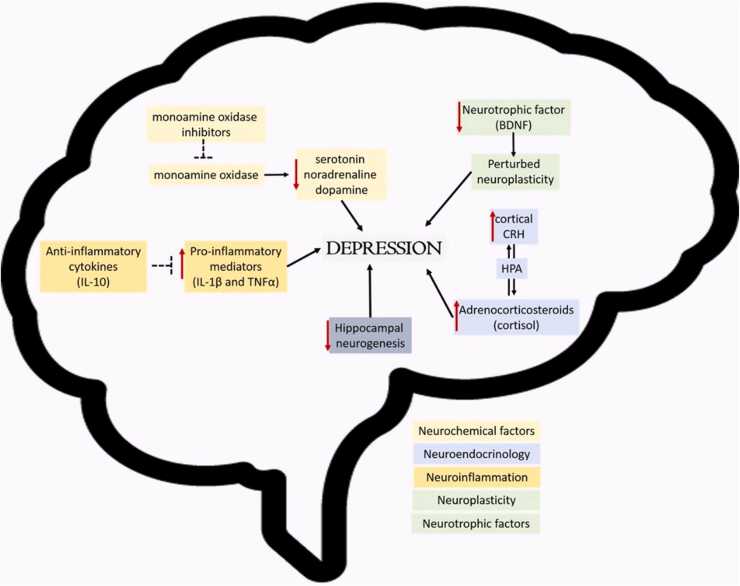
Fig. 2.
Pathophysiology of Depression. Schematic diagram classifying the known pathophysiological mechanisms underlying depression. These are Neurochemical factors, Neuroendocrinology, Neuroinflammation, Neuroplasticity and Neurotrophic factors.
Altogether there are over 300 million cases of mood disorders recorded worldwide. Between 2005 and 2015, there was an increase of 18.4%, alluding to a continual yearly increase in the worldwide prevalence (Ferrari, 2013, Depression and Other Common Mental Disorders Global Health Estimates, 2017).
Depression, which is often associated with indifference, low spirits and misery, is the most common of all the mood disorders, having an estimated prevalence of 4.4% with a higher incidence in females (5.1%) compared to males (3.6%) (Ferrari et al., 2013). The burden of depressive disorders varies by WHO regions, ranging from 2.6% in males located within the Pacific to 5.9% in females located within Africa. In addition, prevalence rates also vary by age, peaking in older adulthood (55 – 74 years) with a 7.5% rate among females and 5.5% among males (Kessler, 2007, Depression and Other Common Mental Disorders Global Health Estimates, 2017).
In Africa, about 29.19 million people (9% of 322 million) suffer from depression, with over 7 million in Nigeria (3.9% of 322 million). Estimates place the lifetime prevalence of depressive disorders from 3.3% to 9.8% (Esan and Esan, 2016). The co-occurrence of stroke and depression is high, with 1 in every 3 stroke patients being diagnosed as clinically depressed (Ojagbemi et al., 2017). This high co-incidence has been linked to functional dependency and reduced quality of life after stroke within the region. Similarly, depression is two- to three times more prevalent in people living with HIV (PLHIV) than in the general population (Bernard et al., 2017). This goes to further support the link between the reduced quality of life and depression as previously stated.
In northern Nigeria, depression is the most prevalent mood disorder, with an incidence of 54.5% in patients attending clinics in Northern Nigerian Tertiary Institutions (Aiyelero et al.,2011). A reported 33% of women attending mental health clinics in the Niger₋Delta region of Nigeria were diagnosed with depression. In this instance, common associations with depression included childhood adversity, sexual violence, and substance misuse in both sexes (Nduna et al., 2013). Another study conducted in Yoruba-speaking communities of Nigeria, Gureje et al. (2007) reported the lifetime and 12-month prevalence estimates of major depressive disorder to be 26.2% (95% CI 24.3–28.2) and 7.1% (5.9–8.3) respectively in persons aged 65-years and above (Gureje et al., 2007). However, in another large community-based study comprising 6752 adults aged 18 and above selected from 21 out of the 36 states in Nigeria, lifetime and 12-month estimates of major depressive episodes were 3.1 ± 0.3% and 1.1 ± 0.1% respectively (Gureje et al., 2006). A total of 721 (65.2%) were from urban communities, while 384 (34.8%) were from the rural community. The overall prevalence of depression was found to be 5.2%. Again, depression was found to be more prevalent among women than men (5.7% vs 4.8%). Furthermore, depression is more common in the rural areas of Nigeria than in the urban areas (7.3% vs 4.2%) (Amoran et al., 2007). This ties in well with the previously established relationship between quality of life and depression.
South Africa reports similarly high cases of depression, with the prevalence of depressive symptoms being 20.5% in females and 13.5% in males. Studies among HIV-positive patients in South Africa, Botswana, Zambia, and Uganda have also reported a high prevalence of depression, with the prevalence ranging from 11.5% in Uganda (Kinyanda et al., 2019) to nearly 30% in South Africa, Zambia, Sudan and Botswana (Esan and Esan, 2016, Opondo, 2018, van den Heuvel, 2013, Assil and Zeidan, 2013); (Stein et al., 2008). In Ethiopia alone, the prevalence of depression ranges between 11% and 38%, dependent on the population of interest (Bitew, 2014, Duko, 2018). Cross-sectional studies among medical students in Cameroon have revealed that depression was high in that group and associated with chronic medical conditions and major life events (Ngasa et al., 2017). From that study, it was reported that about one-third of women (30.6%, 95% CI: 22.8–36.7) had major depressive disorder. With regards to the severity of depression, 34.6%, 26.4%, 3.4% and 0.80% students were classified as having mild, moderate, moderately severe and severe depression respectively (Ngasa et al., 2017). Postpartum complications, ageing, imprisonment, unwanted pregnancy, cardiovascular diseases, HIV, drug abuse, and conflicts make up the main risk factors of depression in SSA.
A recent WHO’s Global health estimate for depression and other common mental disorders reports that the African region represents about 10% of the global burden of mental disorders (5.4% and 3.2% for depression and anxiety, respectively) (Depression and Other Common Mental Disorders Global Health Estimates, 2017). Unfortunately, these statistics are not representative of the real extent of depression in African countries due to factors including misdiagnosis, undiagnosis, and unpublished data. The data on Africa presented as % depression rates in Table 1 is certain to be a misrepresentation of the real number of patients but serves as a starting point to assess the gravity of depression on the continent. In most countries where data could be retrieved, there was a sharp contrast with % depression rates reported in small controlled populations ranging from hospital settings (HIV patients, pregnant women, etc.) to prison populations. For many of these populations, depression was not the primary health concern for which they attended a health facility. As such, in the absence of comorbidity, which moves patients to report to the hospital, many cases would have gone unreported. Therefore, although it is expected that smaller population studies will yield higher prevalence rates in comparison to the national prevalence rate, the vast number of undiagnosed cases must be acknowledged.
Pathophysiology of depression
Several factors contribute to the pathophysiology of depression. Some of the most common pathophysiological pathways are associated with neurochemical factors, neuroendocrinology, neuroinflammation, neuroplasticity, and neurotrophic factors.
The contributions of neurochemicals such as serotonin, noradrenaline, dopamine, and others in depression have been widely studied and reported on, as such will only be briefly reviewed here (El Yacoubi, 2003, Torrey, 2005). The monoamine hypothesis describes one of the more popular pathophysiological mechanisms underlying depression. It proposes that depression occurs as a result of decreased levels of monoamines such as noradrenaline, serotonin, and dopamine found in the anterior cingulated cortex, ventral tegmentum and the Brodmann area 25 regions of the brain (Amthor, 2014). There are several studies that have provided evidence in support of the monoamine hypothesis based on their location, behaviours they mediate and consequences of controlled depletions. (Maes and Meltzer, 1995, Asberg, 1997, Placidi, 2001, Stockmeier, 2003). The monoamine hypothesis has for many years served as the foundation for the production of antidepressant drugs, specifically monoamine oxidase inhibitors, to restore the normal levels of monoamines (Thomsen and Sorensen, 2007; Ramesh et al., 2017; Jesulola et al., 2018).
Though depression is regarded as a stress disorder, there is usually no damage to the Hypothalamus-Pituitary-Adrenal (HPA) axis (Belmaker and Agam, 2008). This is despite the fact that cortical brain regions implicated in psychological stress induce the release of corticotropin-releasing hormone (CRH), which acts via the hypothalamic-pituitary-adrenal (HPA) axis to release cortisol among other adrenocorticosteroids from the adrenal gland. However, there are a few cases of depression where there is evidence of abnormalities to the HPA axis and the CRH release/control system (Pariante and Lightman, 2008). High CRH levels are implicated in some depressed symptoms such as reduced appetite, disturbed sleep, as well as decreased libido (Nemeroff, 1996). Furthermore, high cortisol levels may be the connection between depression and associated long term diseases such as coronary heart disease, osteoporosis and type II diabetes (Gold and Chrousos, 1999). High CRH levels are observed in the cerebrospinal fluid (CSF) of depressed patients (Hasler, 2010), and post-mortem studies have demonstrated increased CRH secreting neurons in limbic brain regions in depressed patients (Hasler, 2010).
Neuroinflammation mediated by cytokines has also been implicated in the pathophysiology of depression (Hurley and Tizabi, 2013). Several cytokines are associated with depression; notable amongst these are interleukins (IL), interferon-gamma and tumour necrosis factors (TNF) (Strawbridge et al., 2017). Studies suggest that markers of neuroinflammation underlie both the development of depression and chronic stress (Dhabhar, 2000, Mc Ewan, 2000). Pro-inflammatory mediators such as IL-1β and TNFα may be responsible for behavioural responses such as hyperthermia, loss of appetite, sleep disruptions, anhedonia, nausea, fatigue and loss of interest in physical and social environments as observed in depression (Dantzer et al., 2007, Jesulola et al., 2018). Anti-inflammatory cytokines such as IL-10 prevents an excess building and signalling of pro-inflammatory cytokines. Therefore, an imbalance of the pro and anti-inflammatory cytokines is likely to play a role in the pathogenesis of depression (Dantzer et al., 2007).
Reduced hippocampal neurogenesis and volume have been identified as an underlying correlate of depression (Hayley and Litteljohn, 2013). Decreased activity of neurotrophic factors such as the brain-derived neurotrophic factor (BDNF) leading to disturbed neuroplasticity has also been implicated in depression (Sacher et al., 2012; Cattaneo et al., 2010). The underlying mechanism could be due to a missense polymorphism with substitution of methionine which causes disruptions in the maturation of the protein leading to reduced BDNF action (Chen et al., 2004). This polymorphism in BDNF action is related to the start of depressive disorder (Schumacher, 2005, Frielingsdorf, 2010). All this contributes towards aberrant neuroplasticity which may play a role in some depressive cases (Hayley and Litteljohn, 2013).
In addition to the action of BDNF in adults, there is enough evidence to show the link between depression and disturbances of normal neurogenesis during the early development of the brain (Jacobs et al., 2000, Monteggia, 2004, Duman and Monteggia, 2006). The widespread action of BDNF in the nervous system and the inter-connections with depression prevents it from being fully exhausted here but has been handled elsewhere (Borroni, 2009, Castrén and Rantamäki, 2010, Yu and Chen, 2011).
Diagnosis of depression and mood disorders in Sub-Saharan Africa
Diagnosis of depression and mood disorders is a challenging science, giving that depression is characterized by a myriad of symptoms and syndromes that mimic other disorders. Often, depression does not occur alone but coexists with other conditions which tend to confound an accurate diagnosis. According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), the diagnosis of a Major Depression Episode (MDE) requires five or more symptoms recurring in one or more episodes for at least a period of 2-weeks (Shaffery et al., 2003, American Psychiatric Association, 2013, Tolentino and Schmidt, 2018). One of the primary symptoms should be either a depressed mood or anhedonia (loss of interest or pleasure), while the secondary symptoms are changes in appetite or weight, sleep disturbances (insomnia or hypersomnia), psychomotor agitation or retardation, fatigue or loss of energy, diminished ability to think or concentrate, feelings of worthlessness or excessive guilt, and recurrent thoughts of suicide (Shaffery et al., 2003, Tolentino and Schmidt, 2018). The symptoms must be significant enough to; (1) cause impairment in social, educational or occupational activities, (2) must not be due to any other medical condition or substance,(3) must not be due to bereavement unless beyond a period of 2 months and (4) must not be due to mania (Shaffery et al., 2003).
Depression is often episodic with specific classifications including mild, moderate, or severe, with or without psychotic features, in full or partial remission, chronic (at least two years in duration); with catatonic, melancholic, or atypical features; or with postpartum onset (American Psychiatric Association, 2013, Tolentino and Schmidt, 2018). Diagnosis of depression is largely by clinical interview-based instruments, and in a few cases, laboratory tests exist for depression screening (Smith et al., 2013). In SSA, where medical personnel with expertise in mental health are not as abundant as observed in Europe, many do not have the training to effectively administer diagnostic tools. Where skilled practitioners do exist, they do not have the time and/or resources to administer diagnostic tools to all individuals at risk of mood disorders (Ali et al., 2016). This makes the identification of individuals with depression intractable, coupled with cultural and social stigma associated with the disorder (Adewuya, 2006, Adewuya et al., 2006). Despite this setback, a frantic effort is being made to increase awareness of depression in SSA. In many high-income countries, depression screening is standard routine practice during primary care using several validated instruments (Smith et al., 2013). In settings with few mental health specialists, it is often difficult to achieve diagnostic goals with these same tools due to vast differences in cultural definitions of the disorder and unavailability of validated instruments which are context-specific (Abiodun, 1994, Adewuya, 2006, Adewuya et al., 2006). Regardless of these challenges, several validated screening instruments are still used across SSA in addition to unstructured interviews to diagnose depression cases (Table 2). Among all these tools, the specific ability of an instrument to correctly identify “true cases” (sensitivity) from “non-cases” (specificity) is paramount (Sweetland et al., 2014).
Table 2.
The mst common diagnostic tools used in clinical settings and the SSA countries where use of these tools have been reported.
| Diagnostic Tool | Countries Reported | References |
|---|---|---|
| Self-Reporting Questionnaire (SRQ) | Burundi, Cote D’Ivoire, Ethiopia, Eritrea, Gambia, Ghana, Guinea-Bissau, Malawi, Mauritius, Mozambique, Nigeria, Rwanda, Uganda, Zambia, Zimbabwe | (De Jong, 1996, Rumble, 1996, Patel, 1997, Uwakwe, 2000, Youngmann, 2008, Hanlon et al., 2008, Stewart et al., 2009, Chipimo and Fylkesnes, 2010, Scholte, 2011, Rivet-Duval et al., 2011, Nakimuli-Mpungu, 2012, Bahadur Thapa, 2014, Weobong, 2014, Familiar, 2016, Netsereab, 2018, Slekiene and Mosler, 2019) |
| Mini International Neuropsychiatric Interview (MINI) | Burkina Faso, Cote D’Ivoire, Democratic Republic of Cong, Ethiopia, Kenya, Namibia, Nigeria, Rwanda, Sierra Leone, Uganda, Zambia, Zimbabwe | (Adewuya, 2008, Kinyanda, 2011, Kamau, 2012, Igwe, 2013, Umubyeyi, 2014, Dialé, 2015, Duthé, 2016, Kwobah, 2017, Howlett, 2018, Tang, 2019) |
| Composite International Diagnostic Interview (CIDI), | Benin, Burkina Faso, Cameroon, Chad, Cote D’Ivoire, Democratic Republic of Congo, Ethiopia, Gambia, Ghana, Kenya, Malawi, Mali, Mauritania, Mauritius, Morocco, Namibia, Nigeria, Senegal, South Africa, Swaziland, Tanzania, Zambia, Zimbabwe, | (Bertschy et al., 1992, Ndetei, 2009, Gaynes, 2012, Koyanagi, 2017, Cristóbal-Narváez et al., 2020, Mughal, 2020) |
| General Health Questionnaire (GHQ) | Angola, Benin, Central African Republic, Ethiopia,Gambia, Kenya, Liberia, Namibia, Nigeria, Rwanda, Zambia | (Nubukpo and Preux, 2004, Bernatsky et al., 2007, Kessler, 2007, Chipimo and Fylkesnes, 2010, Scholte, 2011, Bitew, 2014; Mwape et al., 2016; Dabilgou et al.,2019; Rodriques and Pieters, 2019) |
| Hopkins Symptom Checklist (HCSL) | Benin, Botswana, Central African Republic, Democratic Republic of Congo, Ethiopia, Ghana, Liberia, Nigeria, Rwanda, Siwerra Leone, Tanzania, Zambia | (Bolton, 2001, Bolton et al., 2002, Kaaya, 2002, Adewuya et al., 2006, Ventevogel, 2007, Bass, 2008; Weobong et al., 2009; Vinck and Pham, 2010; Guptaet al., 2010; Ertl et al., 2011; Sheikh et al., 2016; Bah et al., 2020) |
| Becks Depression and Anxiety Scales | Botswana, Ethiopia, Gambia, Ghana, Kenya, Namibia, Nigeria, Senegal, Togo, Uganda, Zambia | (Berard et al., 1998, Ndetei, 2009, Lawler, 2011, Dialé, 2015, Mossie et al., 2016, Musyimi, 2017, Tang, 2019, Mughal, 2020) |
| PHQ-9 | Botswana, Cameroon, Democratic Republic of Congo, Cote D’Ivoire, Ethiopia, Ghana, Guinea, Kenya, Lesotho, Liberia, Mozambique, Namibia, Nigeria, Rwanda, Sierra Leone, | (Bindt et al.,; Kaaya et al., 2002; Adewuya, 2006, Adewuya et al., 2006; Weobong et al., 2009; Ertl et al., 2011; Gaynes et al., 2012; Gelaye et al., 2013; Yotebieng et al., 2017; Audet et al., 2018; Duko et al., 2018; Osok et al., 2018; Halsted et al., 2019; Secor et al., 2020; Ngasa et al., 2017) |
| Centre for Epidemiological Studies Depression Scale (CES-D) | Benin, Burundi, Gabon, Gambia, Ghana, Guuinea, South Africa, Zambia, Gambia, Kenya, Senegal, Namibia, Rwanda, Senegal, South Africa, Zambia | (Myer, 2008, Chishinga, 2011, Klis, 2011, Nakimuli-Mpungu, 2012, Othieno, 2014, Mughal, 2020) |
Although these instruments have been validated for use across diverse settings, methods for establishing their confidence vary widely, and there is an overall lack of consensus about best practices for ensuring cross-cultural equivalence (Sweetland et al., 2014). Many of the best performing diagnostic tools have been those that were locally adapted. The validity of a screening tool can always be improved for local adoption through focus group discussions with representatives of the population in which the screening tool is to be implemented (Ali et al., 2016). The main aim of these discussions should be geared towards standardizing these instruments to the greatest extent possible to enhance cross-cultural comparison and facilitate their usage (Sweetland et al., 2014). This will also allow the incorporation of local idioms of distress into the diagnostic tool to better account for the local experience and expression of mood disorders.
The problem with diagnosis of depression is further complicated by the rich diversity of languages and cultures across SSA; an instrument used satisfactorily in one cultural setting may or may not have the same applicability in another setting, or within a different population, even in the same country. Many of the western derived diagnostic tools often require careful contextualisation to maintain sensitivity. Five essential features such as establishing equivalence across language, content, concept, techniques and criteria have been described by Flaherty et al. as essential for a particular instrument to be considered “culture-free” (Flaherty et al., 1988, Sweetland et al., 2014). Notwithstanding the inconsistencies in the prominence, manifestation and expression of symptoms across the socio-cultural divide in SSA, there is also considerable evidence that supports universality in the experience of depression, anxiety and other mood disorders. Attention should, therefore, be geared towards standardizing these instruments for use while considering the diverse socio-cultural and linguistic settings within the sub-region.
Pharmacological management of depression
Based on the neurobiology of depression and other mood disorders, varying hypotheses have been postulated, which have driven the development of effective therapeutic interventions. The earliest pharmacological agents introduced for the management of mania were barbiturates (Lopez-Munoz et al., 2018). Management of depression has evolved into interventions that involved the use of monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), noradrenaline reuptake inhibitors (NRIs) and mood stabilizers (Quello et al., 2005, Delaloye and Holtzheimer, 2014). These classes of agents seek to either modulate the activity of the neurotransmitters such as serotonin, noradrenaline, dopamine, γ-aminobutyric acid, and glutamate, as well as their receptors and/or enhance activity BDNF (Li et al., 2012, Mathews et al., 2012, Wang and Si, 2013, Jun, 2014, Ashok, 2017). Antidepressants in these categories include fluoxetine, olanzapine, mirtazapine, imipramine, risperidone, lamotrigine and nefazodone (Youdim and Bakhle, 2006, Bauer, 2010, Li et al., 2012, Shim, 2017).
Worldwide, most guidelines recommend new-generation antidepressant, especially SSRIs, as 1st line of agents for the management of depression (Excellence, 2009). This is attributed to the fact that although they are of comparative efficacy among other antidepressants, they have the most favourable risk-benefit ratio (Cipriani et al., 2018). Other antidepressants like TCAs are associated with an increased risk of overdose and likelihood to discontinue use due to side effects (Kato et al., 2018). In SSA, SSRIs are registered for use in 12 countries where data could be retrieved (Table 3). The relatively high-cost of SSRIs to TCAs limits their use even though they are of a better risk-benefit ratio. Within the SSRIs, fluoxetine is the most widely available in the sub-region and the most widely prescribed. Other SSRIs like sertraline which are widely used in developed nations due to acceptability and balance of benefit, is only available in a few African countries and seldom prescribed.
Table 3.
Nationally approved antidepressants in selected SSA countries by their respective drug regulatory authorities.
| Country | Approved Antidepressants | References |
|---|---|---|
| Botswana | Amitriptyline, fluoxetine, trimipramine | (Ministry of Health, 2012) |
| Ethiopia | Fluoxetine, amitriptyline | (Ethiopia Food Medicine and Healthcare Administration and Control Authority, 2010) |
| Ghana | Fluoxetine, sertraline, citalopram, imipramine, amitriptyline | (Ministry of Health, 2017) |
| Kenya | Amitriptyline, fluoxetine, venlafaxine | (Ministry of Health) |
| Nigeria | Fluoxetine, amitriptyline | (Federal Ministry of Health, 2018) |
| South Africa | Fluoxetine, citalopram, amitriptyline | (National Department of Health, 2015) |
| Sudan | Imipramine, sertraline | (Federal Ministry of Health, D. G. of P. 2014) |
| Uganda | Amitriptyline, fluoxetine, imipramine | (Ministry of Health) |
| Zambia | Sertraline, clomipramine, fluoxetine | (Zambia Ministry of Health, 2019) |
| Malawi | Amitriptyline, fluoxetine, | (Ministry of Health, (Government of Malawi), 2015) |
| Rwanda | Amitriptyline, fluoxetine, flupenthixol | (MINISTERE DE LA SANTE REPUBLIQUE DU RWANDA, 2010) |
| Zimbabwe | Amitriptyline, fluoxetine, sertraline, imipramine, citalopram, venlafaxine, duloxetine, mianserin | (The National Medicine and Therapeutics Policy Advisory Committee, 2015) |
Emerging pharmacotherapy for depression
Several challenges plague current treatments that limit the extent of the use of current antidepressants. Studies have shown there are little differences between placebo and some antidepressants in current use (Bear et al., 2019). There is also a significant time-lapse between the initiation of therapy and the onset of therapeutic actions. The time-lapse, in certain antidepressants, from the onset of medication to effective therapeutic benefits, can result in exacerbation of depressive symptoms. These shortcomings are driving the search for novel antidepressants to produce superior and rapid results (Machado-Vieira et al., 2010). These challenges have led to the development of alternative hypotheses and mechanisms of depression because traditional hypotheses like monoamine theories cannot completely explain the treatment shortfalls. With new mechanism comes newer approaches to managing depression. Prominent among these is the glutamate approach. Selective NMDA receptor subtype 2B antagonists (Ibrahim et al., 2012), glutamate release inhibitors, partial agonists of glycine-site modulators of NMDA (Preskorn et al., 2015) and metabotropic glutamate receptor antagonist antagonists (Dwyer et al., 2013) are all being evaluated for antidepressant effects. Negatively modulating GABAA with the use of selective GABAAα5 inverse agonist is also being explored (Fischell et al., 2015). These approaches have been shown to produce rapid-onset and long-lasting antidepressant effects, as such, are envisioned to be game-changers.
Additionally, reduced neuroplasticity and the upstream signalling pathways that contribute to neuroplasticity and neurogenesis have also been implicated in depression (Huang et al., 2017) and are currently areas of active research and experimentation for novel therapeutics (Duman et al, 2016). There are also emerging brain stimulation applications that target resistant/refractory depression. Methods such as transcranial magnetic stimulation (TMS) (Harel, 2014, Rachid, 2018), Transcranial Direct Current Stimulation (tDCS) (Loo, 2012, Loo, 2018), Cranial Electrotherapy Stimulation (CES) (Gilula and Kirsch, 2005, Yennurajalingam, 2018) and newer alternatives like Magnetic Seizure Therapy (MST) (Sun, 2018, Daskalakis, 2020). Each of these is increasingly being evaluated and applied in investigational treatment settings. They are believed to exert antidepressant effects mediated by glutamate interruption that ultimately results in enhanced neurotransmitter transport trafficking and increased synaptic plasticity and synaptogenesis.
It is important to note that SSA is behind with regards to research and development of these emerging approaches owing to challenges such as access to adequate funding and the lower number of available scientists required to contribute to these areas of research (Maina et al., 2020). Except for South Africa, no other SSA country is currently using or investigating the use of brain stimulation approaches to treat depression. The same scenario pertains to other emerging approaches where the research has been contributed by researchers in the Americas, Europe and Asia.
Indigenous therapies for depression management in Sub-Saharan Africa
Africans have used natural products for managing a host of diseases since ancient times. The use of several medicinal plants for the management of mood disorders has been reported in several ethnobotanical studies (Stafford, 2008, Teixeira et al., 2016, Ior, 2017, Amoateng, 2018, Wubetu et al., 2018). Some of the plants identified in ethnobotanical studies have been evaluated preclinically to access their efficacy in a simple test for antidepressant activity, such as the tail suspension and forced swim tests and were found to reduce immobility (Table 4). However, few have been extensively evaluated for their mechanisms of antidepressant action. Indeed none of the studies identified used transgenic animal models of depression for evaluation of mechanisms of action. This finding is in line with a recent report which highlights the absence of advanced techniques in neuroscience studies carried out in Africa (Maina et al., 2020). The use of molecular-based techniques to identify molecular markers of antidepressant action are also few in number. It is important to note that clinical trials involving natural products or standard herbal extracts from SSA evaluating in depression management are non-existent. Moving forward, work must be done to the point of standardized herbal extracts, which can be used clinically. In this aspect, some strides have been made with regards to Sceletium tortuosum (Terburg et al., 2013). The standardized herbal extract will pave the way for much more feasible clinical trials to prove the efficacy of these medicinal plants.
Table 4.
Emerging novel therapeutic agents for managing depression.
| Plant (Family) | Country of Study | Plant part Evaluated | Mechanism (s) of anti-depressant activity | References |
|---|---|---|---|---|
| Adansonia digitata (Bombacaceae) | Nigeria | Stem bark | Dopaminergic, muscarinic and noradrenaergic pathways | (Murtala and Akindele, 2020) |
| Agapanthus campanulatus (Alliaceae) | South Africa | Leaves, Flowers, Roots | Inhibition of SERT, NET and DAT | (Pedersen et al., 2008) |
| Boophone distica (Amaryllidaceae) | South Africa | Bulb | Inhibition of SERT, NET and DAT | (Pedersen et al., 2008) |
| Cymbopogon citratus | Nigeria | Leaves | Via serotonergic and noradrenergic pathways | (Umukoro et al., 2017) |
| Gladiolus dalenii (Iridaceae) | Cameroon | Corms | Via NMDA, serotonin and noradrenergic systems | (Ngoupaye et al., 2014a) |
| Kalankoe integra (Crassulaceae) | Ghana | Leaves | Via serotoninergic, opioidergic, and noradrenergic systems | (Kukuia et al., 2015) |
| Mondia whitei (Asclepiadeace) | South Africa | Inhibition of SERT | (Pedersen et al., 2008) | |
| Newbouldia laevis (Bignoniaceae) | Nigeria | Leaves | Via D2 receptor | (Murtala and Akindele, 2020) |
| Palisuta hirsute (Commelinaceae) | Ghana | Leaves | Potentiation of noradrenergic neurotransmission | (Woode et al., 2010) |
| Pseudospondias microcarpa (Anacardiaceae) | Ghana | Leaves | Via NMDA, nitric oxide and serotonin pathways | (Adongo et al., 2015) |
| Ruta graveolens (Rutaceae) | South Africa | Leaves | Monoamine oxidase inhibition | (Stafford et al., 2007) |
| Sceletium tortuosum (Aizoaceae) | South Africa | Monoamine oxidase inhibition | (Coetzee et al., 2016) | |
| Trichilia monadelpha (Meliaceae) | Ghana | Stem bark | Serotonergic and Opiodergic mechanisms | (Kukuia et al., 2018) |
| Xylopia aethiopica | Ghana | Fruits | Enhancement of 5-HT neurotransmission | (Biney et al., 2016) |
Risk Factors for depression and mood disorders in Sub-Saharan Africa
There are many known and unknown risk factors that increase one’s chances of developing depression and other mood disorders. Risk factors are largely genetic or environmental, with some of the risk factors associated with poor health, low quality of life, socio-economics and relationships.
Poor health management as a result of poor patronage and utilization of medical services is a major cause of depression among the aged (Padayachey et al., 2017). It was also found to contribute to disability, increased morbidity and mortality among a similar population (Padayachey et al., 2017). Treatment of conditions such as Alzheimer’s disease, Parkinson’s disease and dementia which were found to be risk factors of depression, are typically present in the elderly (Felicia et al., 2013).
The quality of life in the family characterized by parental warmth is very important for healthy living in adolescents. These include quality time from a parent, family routine activities, maternal support and improved communication. However, the lack of these can lead to major depressive disorder. The amount of time spent with parents has been known to improve depressive symptoms in adolescents already diagnosed with depression (Manczak, 2019). Nonetheless, a high incidence of depression is reported among the older generation when compared with the younger generation, and this higher incidence is often associated with functional disability, social, economic and political factors (Akosile, 2017, Pannetier, 2017).
Again, the geographical location and socio-economic environment of individuals has been shown to affect the rate of depression. For instance, a recent report indicates a higher rate of depression among elderly persons in low- and middle-income countries compared to those in high income countries (Ojagbemi et al., 2020). Unsurprisingly, the Ibadan Study of Ageing which was carried out in a low- and middle-income country (Nigeria) reported one of the highest rates of major depressive disorder in the literature with a 12-month prevalence of about 7% (Gureje et al., 2007) compared to an average rate of about 3% reported in high-income countries (De La Torre-Luque and Ayuso-Mateos, 2020). Additional reports suggest that elderly persons in low-middle-income countries may be at elevated risk of depression compared to their peers in high income countries (Gureje, 2020).
Another factor that is known to increase the risk of depression is the disease state of people. One of such diseases is HIV/AIDs. Unsurprisingly, it has been shown that almost half of persons living with HIV are affected by depression (Osok et al., 2018). Taking a sample population in South Africa, it was discovered that people living with HIV had higher rates of mood disorders when compared to the general population (Spies et al., 2018). Most people living with HIV show signs and symptoms of depression when they were screened for HIV testing regardless of whether the result comes back positive or not (Kagee et al., 2017; Spies et al., 2018). It is worthy of note that depression has an undesirable consequence on the progression of treatment of HIV patients, especially as it concerns attitude to regular use of medication (Lima et al., 2007; Mayston et al., 2012; Spies et al., 2018). In line with this, depression has been associated with worsening of clinical outcomes among people living with HIV due to poorer virologic response, reduction in physical functioning and medication adherence. This effectively creates a positive feedback loop, with other factors such as bereavements, low economic status, insufficient social support, poor educational background and substance abuse, further worsening both disease conditions (Brown, 2013, Kaneez, 2016). Apart from HIV, maternal depression in low and middle – income countries have been reported to be unusually high (antenatal depression) (Brittain, 2015, Huang, 2016). For example, pregnant youths in rural areas of Kenya, who are HIV positive and at a very young age were found to be at higher risk of exhibiting depressive symptoms when compared to pregnant adolescents at a much older age (Osok et al., 2018). Other chronic diseases such as arthritis and hypertension are also known to increase the risk of depression (Meng, 2012, Beurel et al., 2020).
Experimental modelling and evaluating depression and mood disorders
Over the years, several researchers have developed various experimental models for the study of depression and associated mood disorders. The wide range of behavioural deficits associated with depression makes it a challenging task to create these disorders in a controlled environment for study (Benedikz et al., 2009). However, the development of suitable experimental models has gone a long way in accelerating mood disorder research by increasing the understanding of the mechanism underlying the pathophysiology of the disorder and providing the possibility of early-onset screening (El-Mallakh et al, 2003).
Animal models have given many insights into the underlying mechanisms of mood changes, neurodegenerative symptoms and attention deficit in depression, but human depression is complex, thereby making the generation of insightful and valid models less straightforward when compared to other conditions (Miller and Raison, 2016). Advancement in genetic and pharmacological methodologies have aided in the creation of more relevant experimental animal models. These advancements have provided a clearer insight into some intrinsic intracellular mechanisms that play critical roles in the pathophysiology of depression (Valvassori et al., 2013).
It has been proposed from an evolutionary perspective that depression is an analogue of the involuntary defeat strategy, which is initiated when struggling for limited resources. (Sloman, 2008). However, modelling detailed psychiatric features of depression in rodents or other primitive primates is impossible because behaviours such as suicidality and guilt are uniquely human features and cannot be replicated in animal models. However, the features that can be modelled include anhedonia, neurovegetative changes, measures of helplessness, alterations in sleep, behavioural despair and change in appetite patterns. These available models and their use in Africa are discussed in subsequent sections and summarised in (Table 5).
Table 5.
Evidence of proven capacity in the use of animal models of depression.
Animal models of depression and mood disorder
Animals are commonly used to recreate features that mimic depression, particularly to identify new therapeutic targets and preclinical screening of novel therapeutic agents (Valvassori et al., 2013) (Krishnan and Nestler, 2011). Over the years, different researchers have made a series of attempts to create animal models that mirror some possible number of physiological and behavioural changes common in depression and mood disorders. Behavioural tests are commonly used to assess the face validity of an animal model, which demonstrates its ability to mirror the symptoms of depression (Valvassori et al., 2013). Several behavioural tests have been widely used in much experimental work to assess drugs with antidepressant activities (Réus, 2011, Budni, 2012), but such drugs are unable to provide deep insight into the neurobiological aspects of depression (Dzirasa and Covington, 2012).
Behaviour is often regarded as the final output of CNS; thereby, behavioural tests serve as the primary basis of depression evaluation, along with genetics, electrophysiology, and histology. Altogether, these techniques serve as major tools for understanding underlying mechanisms in depression and screening for novel therapeutic targets/agents (Hånell and Marklund, 2014). Behavioural paradigms that are employed in animal models of depression are discussed below, with an indication of African countries with a proven ability to perform these tests (Table 5).
Forced swimming test (FST)
This is the most common test in evaluating depression-like behaviour in animal models of depression. It was first described by (Porsolt et al., 1977) in rats and subsequently in mice. It mostly determines the period of immobility in rats or mice, as immobility is interpreted to manifest as a result of negative mood, hopelessness, and lack of motivation. All these are established symptoms of depression (Chenu, 2005).
The procedure involves two exposures to a deep cylindrical tank in which the rats cannot touch the tank bottom, hence, forced to swim for survival. The first exposure is termed ‘pre-test session’, it’s for 15 min. The second exposure is termed “ test session”, takes place after 24 h, and it is for 5 min (Belovicova, 2017, Porsolt et al., 1977; J.C.B., Sáenz et al., 2006).
The tail suspension test (TST)
This test is mainly used in mice, and as the name implies, the mouse is hung upside down by the tail. It was first described by (Steru et al., 1985). It manifests similar behaviours to the FST but has the advantage of being able to eliminate the risk of hypothermia brought on by the forced swim test. The procedure is such that animals are supposed to struggle to escape the stressful condition, but after a period, the animal ceases to struggle, leading to immobility. The earlier the animal ceases to struggle, and as such, the longer the immobility period, the greater the likelihood of depression (Belovicova et al, 2017).
Sucrose consumption preference test
Sucrose consumption preference test assesses the sensitivity of animals to reward (Belovicova et al., 2017). The animals are provided with water without and with different concentrations of sucrose, and the preference is analyzed. Reduction of interest in sucrose is seen as the manifestation of depression (Belovicova et al., 2017). It is a very reliable test for depression as seen in the work of (Willner et al., 1987), where prolonged administration of tricyclic antidepressants (TCA) resulted in a renewed preference for sucrose in a rat model of depression (Willner et al., 1987).
The open-field test
Hall & Ballachey developed this test in 1932. It provides a qualitative and quantitative measurement of a rodent's locomotive activity and exploratory drive, thereby testing the rodent’s emotionality. The apparatus is an open field divided into squares and surrounded by high walls to prevent escape. Rodent’s activities are assessed using the numbers of square crosses, rearing frequency and time spent moving. To assess anxiety, other parameters taken into consideration are; defecation, time spent in the centre of the apparatus as well as first, few minutes of activities (Berggren et al., 1978, Can, 2012, Gould et al., 2001, Slattery and Cryan, 2012). Currently, there are automatic open-field apparatuses that allow for easier and accurate processes through the use of software-linked infrared beams or video cameras.
Foot shock escape task
Réus et al. (2011) and Maier and Watkins (1984), proposed foot shock escape task as a model of learned helplessness in mice and an essential preclinical test. The foot shock test evaluates the ability of animals to escape when subjected to an escapable foot shock after having previously been exposed to an inescapable footshock. It has since become one of the important preclinical tests for evaluating learned helplessness that is behavioural evidence of depression (Maier and Watkins, 1984).
Three-chamber sociability and social novelty test
The test assesses cognition in the form of general sociability and interest in social novelty in rodent models of CNS disorders. It is well established that rodents usually socialize by spending more time with another rodent, and they investigate a novel intruder more so than a familiar one (social novelty). Based on these inclinations, rodents with deficits in sociability and/or social novelty (potentially due to depression)can be easily identified using the Three Chamber Test (Lundberg et al., 2019).
After a period of acclimation in the empty box, the subject encounters a never-before-met intruder under one pencil cup and an empty pencil cup in the “sociability” session. The subject then encounters the first intruder as well as a second never-before-met intruder under another pencil cup in the “social novelty” session. The time spent sniffing each pencil cup, the time spent in each chamber, and the number of entries into each chamber is recorded. The data obtained from this test is useful for quantifying deficits in social behaviour of rats such as that experienced during depression as well as evaluating novel chemical entities for their effect on social behaviour (Lundberg et al., 2019).
The multivariate concentric square field (MCSF) test
The Multivariate concentric square field (MCSF) test is used to assess depression which manifests as deficits in risk assessment, elevated anxiety and shelter seeking behaviour in rodents. It consists of a large square field of 100 cm by 100 cm, surrounding a smaller central square field of 70 cm by 70 cm with walls of 25 cm height. The arena is divided into zones: which include risk areas and shelter/ hoard areas (Meyerson, 2006, Ekmark-Lewén, 2010, Kaidanovich-beilin, 2011). Furthermore, MCSF allows the subjects free choice with respect to choosing the area to explore, Unlike other behavioural paradigms for assessment of depression, the MCSF is a forced exploration task involving inescapable novelty that evokes risk performance and also allows for investigation of different variables of behaviour in the same apparatus in the same session. The test is carried out using a ceiling mounted video and tracking software. Usually, the experiment is initiated by placing the subject in the central arena. The recording is started immediately, and the session lasts for at least 20 min. Data can be collected for the following parameters: Latency to leave the central arena, Latency to visit a certain zone, Frequency of visits, Total duration of time spent in each zone, Duration of time spent per visit in each zone, Distance moved, Velocity of the subject and Number of animals visiting each zone.
Recommendations
As previously stated, the reported prevalence of depression in SSA is most likely an under-estimation. It can also be noted that only thirteen African countries report the use of basic animal behavioural tests typically used in depression research. This highights the limitation in research capacity needed to drive the developmental of contextual solutions. The following recommendations which focus on clinical interventions rather than basic science, could potentially bridge the knowledge gap that has been exposed.
-
1.
Researchers and clinicians working in each country could collaborate towards the development of contextually relevant tools for depression diagnosis. The formation of consortiums across countries could allow lessons learnt in the development of such tools to be easily transferred from one setting to another. It is expected that, the development of such tools within a country, will allow easy deployment across the country especially where national health agencies are involved in the development process.
-
2.
As mobile and digital technology continues to experience high penetration rates across the continent, this could be leveraged to collect, analyse and store data. Such tools have the potential to collect live data from multiple locations which is then housed in a centralized database. Such a database may then be queried by researchers for analysis and reporting purposes. This could be combined with the use of the aforementioned diagnostic tools.
-
3.
African governments could work towards investing 1% of their GDP in research as recommended by the African Union. This could make available more funds to support research teams in the collection and reporting of data from all parts of their respective countries. Such funds could also go towards training of front line staff or health professionals in remote parts of their respective countries to identify “at risk” patients and flag them up for further investigation.
-
4.
There is the need for African neuroscientists to engage in education and advocacy campaigns. This will shed light on the realities of depression and create room for conversations around mental health. It will also empower people to identify signs of depression and by so doing, be encouraged to seek help either for themselves or their family. Lastly, such campaigns could be leveraged to raise funds from local philanthropic organisations for the purposes of supporting research.
Conclusion
Although different studies give varying reports of the prevalence of depression, a recent WHO’s Global health estimate for depression reports the highest prevalence of 5.4% (Depression and Other Common Mental Disorders Global Health Estimates, 2015). The SSA region, as a result, represents about 10% of the global burden of mental disorders. These reports are fraught with challenges of misdiagnosis, underdiagnoses, and unpublished data, which results in under-estimation of the real number of patients. The region remains behind in the search for novel approaches to manage the disorder. However, the region has a huge potential that can be harnessed to enhance our understanding and management of the disorder as well as contribute to the global drug discovery process.
Ethical statement
The authors affirm that the above is an original piece of work which has not been submitted anywhere else for publication.
Conflict of interest
There are no conflicts of interest to declare.
Acknowledgements
The authors of this review wish to express their profound gratitude to the International Brain Research Organisation for availing them the opportunity to attend the pre conference IBRO-ARC school on Depression and Mood disorder in Sub-Saharan Africa.
References
- Abasiubong F., et al. Assessing the psychological well-being of caregivers of people living with HIV/AIDS in Niger Delta region, Nigeria. AIDS Care. 2011;23(4):494–500. doi: 10.1080/09540121.2010.516340. [DOI] [PubMed] [Google Scholar]
- Abiodun O.A. A validity study of the hospital anxiety and depression scale in general hospital units and a community sample in Nigeria. Br. J. Psychiatry. 1994;165:669–672. doi: 10.1192/bjp.165.5.669. [DOI] [PubMed] [Google Scholar]
- Adebayo Adebisi Y., et al. Depression Among Pharmacy Students in Nigeria: Is It a Neglected Issue? Strides Dev. Med. Educ. 2019;16(1):97918. doi: 10.5812/sdme.97918. [DOI] [Google Scholar]
- Adewuya A.O., et al. Validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in late pregnancy among Nigerian women. J. Psychosom. Obstet. Gynecol. 2006;27:267–272. doi: 10.1080/01674820600915478. [DOI] [PubMed] [Google Scholar]
- Adewuya A.O., et al. Relationship between depression and quality of life in persons with HIV infection in Nigeria. Int. J. Psychiatry. Med. 2008;38(1):43–51. doi: 10.2190/PM.38.1.d. [DOI] [PubMed] [Google Scholar]
- Adewuya A.O., et al. Gender difference in the point prevalence, symptoms, comorbidity, and correlates of depression: findings from the Lagos State Mental Health Survey (LSMHS), Nigeria. Arch. women’s Ment. Health. 2018;21(6):591–599. doi: 10.1007/S00737-018-0839-9. [DOI] [PubMed] [Google Scholar]
- Adewuya A.O., Ola B.A., Afolabi O.O. Validity of the patient health questionnaire (PHQ-9) as a screening tool for depression amongst Nigerian university students. J. Affect. Disord. 2006;96:89–93. doi: 10.1016/j.jad.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Adongo D.W., et al. Antidepressant-like effect of the leaves of Pseudospondias microcarpa in mice: evidence for the involvement of the serotoninergic system, NMDA receptor complex, and nitric oxide pathway. BioMed. Res. Int. 2015:2015. doi: 10.1155/2015/397943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinpelu L.A., et al. Antidepressant activity and mechanism of aqueous extract of vigna unguiculata ssp. Dekindtiana (L.) walp dried aerial part in mice. Int. J. Neurosci. Behav. Sci. 2017;5(1):7–18. doi: 10.13189/ijnbs.2017.050102. [DOI] [Google Scholar]
- Akosile C.O., et al. Depression, functional disability and quality of life among Nigerian older adults: prevalences and relationships. Arch. Gerontol. Geriatr. 2017 doi: 10.1016/j.archger.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Ali G.-C., Ryan G., De Silva M.J. Validated screening tools for common mental disorders in low and middle income countries: a systematic review. PLoS One. 2016 doi: 10.1371/journal.pone.0156939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaghnouje A., et al. Subacute assessment of the toxicity and antidepressant-like effects of origanum majorana L. Polyphenols in swiss albino mice. Molecules. 2020;25:5653. doi: 10.3390/MOLECULES25235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . 2013. ‘Diagnostic and statistical manual of mental disorders: 5th Edn.’
- Amin S.N., et al. Hippocampal and cerebellar changes in acute restraint stress and the impact of pretreatment with ceftriaxone. Brain Sci. 2020;10(4):193. doi: 10.3390/BRAINSCI10040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoateng P., et al. Extract of Synedrella nodiflora (L) Gaertn exhibits antipsychotic properties in murine models of psychosis. BMC Complement. Altern. Med. 2017;17(1):1–14. doi: 10.1186/S12906-017-1901-2/FIGURES/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoateng P., et al. Medicinal plants used in the treatment of mental and neurological disorders in Ghana. Evid.-Based Complement. Altern. Med. 2018:2018. doi: 10.1155/2018/8590381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoran O., Lawoyin T., Lasebikan V. Prevalence of depression among adults in Oyo State, Nigeria: a comparative study of rural and urban communities. Aust. J. Rural Health. 2007;15(3):211–215. doi: 10.1111/J.1440-1584.2006.00794.X. [DOI] [PubMed] [Google Scholar]
- Amthor, F. .2014. Neurobiology For Dummies. 111 River Street, Hoboken, NJ 07030–5774: John Wilet and Sons, Inc.
- Asberg M. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Ann. N. Y. Acad. Sci. 1997;836:158–181. doi: 10.1111/j.1749-6632.1997.tb52359.x. [DOI] [PubMed] [Google Scholar]
- Ashok A.H., et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol. Psychiatry. 2017;22(5):666–679. doi: 10.1038/mp.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assil S.M., Zeidan Z.A. Prevalence of depression and associated factors among elderly Sudanese: a household survey in Khartoum State. East. Mediterr. Health J. 2013:435–440. doi: 10.26719/2013.19.5.435. [DOI] [PubMed] [Google Scholar]
- Audet C.M., et al. Depression among female heads-of-household in rural Mozambique: a cross-sectional population-based survey. J. Affect. Disord. 2018;227:48–55. doi: 10.1016/j.jad.2017.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bah A.J., et al. Prevalence of anxiety, depression and post-traumatic stress disorder among Ebola survivors in northern Sierra Leone: a cross-sectional study. BMC Public Health. 2020;20(1):1391. doi: 10.1186/s12889-020-09507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadur Thapa S. Depression and Its correlates in South Africa and Ghana among people aged 50 and above: findings from the WHO study on global ageing and adult health. J. Psychiatry. 2014;17(06) doi: 10.4172/1994-8220.1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J.K., et al. Post-partum depression in Kinshasa, Democratic Republic of Congo: validation of a concept using a mixed-methods cross-cultural approach. Trop. Med. Int. Health. 2008:1534–1542. doi: 10.1111/j.1365-3156.2008.02160.x. [DOI] [PubMed] [Google Scholar]
- Bauer M., et al. Lithium’s emerging role in the treatment of refractory major depressive episodes: augmentation of antidepressants. Neuropsychobiology. 2010;62(1):36–42. doi: 10.1159/000314308. [DOI] [PubMed] [Google Scholar]
- Baxter A.J., et al. Global epidemiology of mental disorders: what are we missing? PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear H.A., et al. Systematic review and meta-analysis: outcomes of routine specialist mental health care for young people with depression and/or anxiety’. J. Am. Acad. Child Adolesc. Psychiatry. 2019 doi: 10.1016/j.jaac.2019.12.002. [DOI] [PubMed] [Google Scholar]
- Belmaker R., Agam G. Major depressive disorder. N. Engl. J. Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Belovicova K., et al. Animal tests for anxiety-like and depression-like behavior in rats’. Interdiscp. Toxicol. 2017;10(1):40–43. doi: 10.1515/intox-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Azu B., et al. Evaluation of the neurobehavioral properties of Naringin in Swiss Mice. Drug Res. 2018:465–474. doi: 10.1055/a-0575-3730. [DOI] [PubMed] [Google Scholar]
- Benedikz E., Kloskowska E., Winblad B. The rat as an animal model of Alzheimer’s disease. J. Cell. Mol. Med. 2009:1034–1042. doi: 10.1111/j.1582-4934.2009.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard R., Boermeester F., Viljoen G. Depressive disorders in an out-patient oncology setting: prevalence, assessment, and management. Psycho. Oncol. 1998;7:112–120. doi: 10.1002/(SICI)1099-1611(199803/04)7:2<112::AID-PON300>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Berggren U., Tallstedt L., Ahlenius S. Psychopharmacology. 1978;9(45):41–45. doi: 10.1007/BF00428028. [DOI] [PubMed] [Google Scholar]
- Bernard C., Dabis F., de Rekeneire N. Prevalence and factors associated with depression in people living with HIV in sub-Saharan Africa: a systematic review and meta-analysis’. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0181960. e0181960–e0181960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatsky S., Souza R., de Jong K. Mental health in HIV-positive pregnant women: Results from Angola. AIDS Care. Taylor & Francis Group. 2007;19(5):674–676. doi: 10.1080/09540120601012705. [DOI] [PubMed] [Google Scholar]
- Bertschy G., Viel J.F., Ahyi R.G. Depression in Benin: an assessment using the comprehensive psychopathological rating scale and the principal component analysis’. J. Affect. Disord. 1992;25(3):173–180. doi: 10.1016/0165-0327(92)90003-O. [DOI] [PubMed] [Google Scholar]
- Beurel E., Toups M., Nemeroff C.B. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107(2):234–256. doi: 10.1016/J.NEURON.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikomo E., Ebuehi O., Magbagbeola O. Antidepressant activity of ethanol leaf extract of Annona muricata L., in Sprague-Dawley Rats. Am. J. Biochem. 2017;7(1):1–5. doi: 10.5923/j.ajb.20170701.01. [DOI] [Google Scholar]
- Bindt, C. et al. (no date) ‘Antepartum Depression and Anxiety Associated with Disability in African Women: Cross-Sectional Results from the CDS Study in Ghana and Cô te d’Ivoire’. doi:10.1371/journal.pone.0048396. [DOI] [PMC free article] [PubMed]
- Biney R.P., et al. Xylopia aethiopica fruit extract exhibits antidepressant-like effect via interaction with serotonergic neurotransmission in mice. J. Ethnopharmacol. 2016;184:49–57. doi: 10.1016/j.jep.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Bitew T. Prevalence and risk factors of depression in Ethiopia: a review. Ethiop. J. Health Sci. 2014;24(2):161–169. doi: 10.4314/ejhs.v24i2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton P. Cross-cultural validity and reliability testing of a standard psychiatric assessment instrument without a gold standard. J. Nerv. Ment. Dis. 2001;189:238–242. doi: 10.1097/00005053-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Bolton P., Neugebauer R., Ndogoni L. Prevalence of depression in rural Rwanda based on symptom and functional criteria. J. Nerv. Mental Dis. 2002;190(9):631–637. doi: 10.1097/00005053-200209000-00009. [DOI] [PubMed] [Google Scholar]
- Borroni B., et al. Role of BDNF Val66Met functional polymorphism in Alzheimer’s disease-related depression. Neurobiol. Aging. 2009:1406–1412. doi: 10.1016/j.neurobiolaging.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Brittain K., et al. Risk factors for antenatal depression and associations with infant birth outcomes: results from a South African Birth Cohort Study’. Paediatr Perinat Epidemiol. 2015:505–514. doi: 10.1111/ppe.12216. [DOI] [PubMed] [Google Scholar]
- Brown C.H., et al. ‘A computational future for preventing HIV in minority communities: How advanced technology can improve implementation of effective programs’. J. Acquired Immune Defic. Syndr. 2013;SUPPL. 1:S72. doi: 10.1097/QAI.0b013e31829372bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budni J., et al. ‘Role of potassium channels in the antidepressant-like effect of folic acid in the forced swimming test in mice’. Pharmacol. Biochem. Behav. 2012:148–154. doi: 10.1016/j.pbb.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Can, A. et al. (2012) ‘The Tail Suspension Test’, (January), pp. 3–7. doi:10.3791/3769.
- Castrén E., Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Devel. Neurobiol. 2010:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- Cattaneo A., et al. ‘Reduced peripheral brain-derived neurotrophic factor mRNA levels are normalized by antidepressant treatment. Int. J. Neuropsychopharmacol. 2010;13(1):103–108. doi: 10.1017/S1461145709990812. [DOI] [PubMed] [Google Scholar]
- Chen Z., et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J. Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenu B.P.F. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology. 2005:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Chipimo P.J., Fylkesnes K. Comparative Validity of Screening Instruments for Mental Distress in Zambia. Clin. Pract. Epidemiol. Ment. Health. 2010:4–15. doi: 10.2174/1745017901006010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishinga N., et al. ‘Validation of brief screening tools for depressive and alcohol use disorders among TB and HIV patients in primary care in Zambia.’. BMC Psychiatry. 2011;11:75. doi: 10.1186/1471-244X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A., et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Focus. 2018;16(4):420–429. doi: 10.1176/appi.focus.16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee D.D., López V., Smith C. High-mesembrine Sceletium extract (TrimesemineTM) is a monoamine releasing agent, rather than only a selective serotonin reuptake inhibitor’. J. Ethnopharmacol. 2016;177:111–116. doi: 10.1016/j.jep.2015.11.034. [DOI] [PubMed] [Google Scholar]
- Cristóbal-Narváez P., Haro J.M., Koyanagi A. ‘Perceived stress and depression in 45 low- and middle-income countries’. J. Affective Disord. 2020;274:799–805. doi: 10.1016/j.jad.2020.04.020. [DOI] [PubMed] [Google Scholar]
- Dabilgou A.A., et al. Symptoms of depression and associated risk factors in patients with epilepsy in Burkina Faso. Open J. Depress. Sci. Res. 2019;08(01):29–40. doi: 10.4236/ojd.2019.81004. [DOI] [Google Scholar]
- Dallé E., Daniels W.M.U., Mabandla M.V. Fluvoxamine maleate normalizes striatal neuronal inflammatory cytokine activity in a Parkinsonian rat model associated with depression. Behav. Brain Res. 2017:189–196. doi: 10.1016/j.bbr.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., et al. From information to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2007;9:45–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis Z.J., et al. Magnetic seizure therapy (MST) for major depressive disorder. Neuropsychopharmacology. 2020;45(2):276–282. doi: 10.1038/s41386-019-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong J.T. A comprehensive public mental health programme in Guinea-Bissau: a useful model for African, Asian and Latin-American countries. Psychol. Med. 1996:97–108. doi: 10.1017/s0033291700033742. [DOI] [PubMed] [Google Scholar]
- De La Torre-Luque A., Ayuso-Mateos J.L. The course of depression in late life: a longitudinal perspective. Epidemiol. Psychiatr. Sci. 2020:29. doi: 10.1017/S204579602000058X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloye, S. and Holtzheimer, P.E. (2014) ‘DialoguesClinNeurosci-16–83.xml’, pp. 83–91.
- Delgardo P., Morena F. In: The Textbook of Mood Disorders. Stein D.K., Kupfer D.J., Schatzberg A.F., editors. American Psychiatric Publishing Inc; Washington DC: 2006. Neurochemistry of mood disorders’; pp. 101–116. [Google Scholar]
- Depression and Other Common Mental Disorders Global Health Estimates (2015).
- Depression and Other Common Mental Disorders Global Health Estimates 2017. Available at: 〈https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf〉 (Accessed: 25 July 2019).
- Dhabhar F. Acute stress enhances while chronic stress suppresses skin immunity: the role of stress hormones and leukocyte trafficking. Ann. N. Y. Acad. Sci. 2000;917:876–893. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- Dialé N.N.N., et al. Factors related to depression in patients undergoing hemodialysis due to renal failure in senegal. Psychol. Sci. Res. 2015;06(04):409–414. doi: 10.4236/psych.2015.64038. [DOI] [Google Scholar]
- Duko, B. et al. (2018) ‘Prevalence and associated factors of depression among patients with HIV/AIDS in Hawassa, Ethiopia, cross-sectional study’, Annals of General Psychiatry. BioMed Central Ltd., 17(1). doi:10.1186/s12991–018-0215–1. [DOI] [PMC free article] [PubMed]
- Duman R., Monteggia L. A neurotrophic model for stressrelated mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duman R.S., et al. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 2016;22(3):238. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthé G., et al. Mental health and urban living in sub-Saharan Africa: major depressive episodes among the urban poor in Ouagadougou, Burkina Faso. Popul. Health Metrics. BioMed. 2016;14(1):18. doi: 10.1186/s12963-016-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J.M., Lepack A.E., Duman R.S. mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J. Mol. Psychiatry. 2013;1(1):15. doi: 10.1186/2049-9256-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzirasa K., Covington H.E. Increasing the validity of experimental models for depression. Ann. N. Y. Acad. Sci. 2012:36–45. doi: 10.1111/j.1749-6632.2012.06669.x. [DOI] [PubMed] [Google Scholar]
- Hailu A.E., Engidawork E. Screening of the antidepressant-like activity of two hypericum species found in Ethiopia. Ethop. Pharm. J. 2014;30:21–32. [Google Scholar]
- Ekmark-Lewén S., et al. The multivariate concentric square field test reveals behavioral profiles of risk taking, exploration, and cognitive impairment in mice subjected to traumatic brain injury’. J. Neurotrauma. 2010;27(9):1643–1655. doi: 10.1089/NEU.2009.0953. [DOI] [PubMed] [Google Scholar]
- El Brouzi M.Y. Intrahippocampal effects of nickel injection on the affective and cognitive response in wistar rat: potential role of oxidative stress. Biol. Trace Element Res. 2020:3382–3392. doi: 10.1007/S12011-020-02457-5. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc. Natl. Acad. Sci. USA. 2003;100(10):6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El zahaf N.A., Elhwuegi A.S. The effect of GABAmimetics on the duration of immobility in the forced swim test in albino mice. Libyan. J. Med. 2014;9(1) doi: 10.3402/LJM.V9.23480. http://dx.doi.org/10.3402/ljm.v9.23480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mallakh R.S., et al. Intracerebroventricular administration of ouabain as a model of mania in rats. Bipolar Disord. 2003:362. doi: 10.1034/j.1399-5618.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- El-Marasy S.A., et al. Anti-depressant effect of cerebrolysin in reserpine-induced depression in rats: behavioral, biochemical, molecular and immunohistochemical evidence. Chem. Biol. Interact. 2021 doi: 10.1016/J.CBI.2020.109329. [DOI] [PubMed] [Google Scholar]
- Ertl V., et al. Validation of a mental health assessment in an African conflict population. Psychol. Assess. 2011;22:318–324. doi: 10.1037/a0018810. [DOI] [PubMed] [Google Scholar]
- Esan O., Esan A. Epidemiology and burden of bipolar disorder in Africa: a systematic review of data from Africa. Eur. Psychiatry. 2016;51(1):93–100. doi: 10.1007/s00127-015-1091-5. [DOI] [PubMed] [Google Scholar]
- Ethiopia Food Medicine and Healthcare Administration and Control Authority 2010 ‘LIST OF ESSENTIAL MEDICINES FOR ETHIOPIA’, (September).
- Excellence, N. I. for C. (2009) Depression:the treatment and management of depression in adults (update), Clinicalguidelines, CG90. National Institute for Clinical Excellence.Familiar, I. et al. (2016)‘Exploring Psychological Distress in Burundi During and After the ArmedConflict’, Community Mental Health Journal. Springer New York LLC,52(1), pp. 32–38. doi: 10.1007/s10597-015-9902-4. [DOI] [PubMed]
- Familiar I., et al. Exploring psychological distress in Burundi during and after the armed conflict. Community Ment. Health J. 2016:32–38. doi: 10.1007/s10597-015-9902-4. [DOI] [PubMed] [Google Scholar]
- Federal Ministry of Health, D. G. of P.. 2014. ‘Sudan National Standard Treatment Guidelines’
- Federal Ministry of Health . 2018. ‘Nigeria Essential Medicines List 6th Ed with Addendum’.
- Fekadu, N.. 2014. Evaluation of the Antidepressant-like Activity of the Crude Extract and Solvent Fractions of Rosa abyssinica Lindley (Rosaceae) Using Rodent Models of Depression.
- Fekadu N., Shibeshi W., Engidawork E. Major depressive disorder: pathophysiology and clinical management. J. Depress Anxiety. 2017;6(1):255–257. [Google Scholar]
- Felicia A., Paul G., Hausdorff J.M. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013:51–61. doi: 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Ferrari A.J., et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11) doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischell J., et al. Rapid antidepressant action and restoration of excitatory synaptic strength after chronic stress by negative modulators of alpha5-containing GABA A receptors. Neuropsychopharmacology. 2015;40(11):2499–2509. doi: 10.1038/npp.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty J.A., Gaviria M., Pathak D. Developing instruments for cross-cultural psychiatric research. J. Nerv. Ment. Dis. 1988;176:257–263. [PubMed] [Google Scholar]
- Frielingsdorf H., et al. Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 2010;1208:150–157. doi: 10.1111/j.1749-6632.2010.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes B.N., et al. ‘Prevalence and predictors of major depression in HIV-infected patients on antiretroviral therapy in Bamenda, a semi-urban center in Cameroon’. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelaye B., et al. Diagnostic validity of the composite international diagnostic interview (CIDI) depression module in an east African population. Int. J. Psychiatry Med. 2013;46(4):387–405. doi: 10.2190/PM.46.4.e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula M.F., Kirsch D.L. Cranial electrotherapy stimulation review: a safer alternative to psychopharmaceuticals in the treatment of depression. J. Neurother. 2005;9(2):7–26. [Google Scholar]
- Gold P.W., Chorousos G.P. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc. Assoc. Am. Phys. 1999 doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- Gould T.J., Keith R.A., Bhat R.V. Differential sensitivity to lithium’s reversal of amphetamine-induced open-field activity in two inbred strains of mice. Behav. Brain Res. 2001;118:95–105. doi: 10.1016/s0166-4328(00)00318-1. [DOI] [PubMed] [Google Scholar]
- Gupta R., et al. Depression and HIV in Botswana: a population-based study on gender-specific socioeconomic and behavioral correlates. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0014252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O., et al. Lifetime and 12-month prevalence of mental disorders in the Nigerian Survey of Mental Health and Well-Being. Br. J. Psychiatry. 2006:465–471. doi: 10.1192/BJP.188.5.465. [DOI] [PubMed] [Google Scholar]
- Gureje O. Psychiatry in Africa: the myths, the exotic, and the realities. Afr. J. Psychiatry. 2007;10(1):11–14. doi: 10.4314/ajpsy.v10i1.30225. [DOI] [Google Scholar]
- Gureje O. Epidemiology and Psychiatric Sciences. Cambridge University Press; 2020. Late-life depression in socially and culturally diverse settings; p. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O., Kola L., Afolabi E. Epidemiology of major depressive disorder in elderly Nigerians in the Ibadan study of ageing: a community-based survey. Lancet. 2007:957–964. doi: 10.1016/S0140-6736(07)61446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsted S., et al. Depressive symptoms, suicidal ideation, and mental health care-seeking in central Mozambique. Soc. Psychiatry Psychiatr. Epidemiol. 2019:1519–1533. doi: 10.1007/s00127-019-01746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon C., Medhin G., Alem A. Detecting perinatal common mental disorders in Ethiopia: validation of the self-reporting questionnaire and Edinburgh Postnatal depression scale. J. Affect. Disord. 2008;108:251–262. doi: 10.1016/j.jad.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Harel E.V., et al. H-coil repetitive transcranial magnetic stimulation for treatment resistant major depressive disorder: an 18-week continuation safety and feasibility study. World J. Biol. Psychiatry. 2014:298–306. doi: 10.3109/15622975.2011.639802. [DOI] [PubMed] [Google Scholar]
- Hasler G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians ? World Psychiatry. 2010;9:155–161. doi: 10.1002/j.2051-5545.2010.tb00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S., Litteljohn D. Neuroplasticity and the next wave of antidepressant strategies. Front. Cell Neurosci. 2013;7:218. doi: 10.3389/fncel.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel L., et al. Frequency and correlates of anxiety and mood disorders among TB- and HIV-infected Zambians. AIDS Care. 2013:1527–1535. doi: 10.1080/09540121.2013.793263. [DOI] [PubMed] [Google Scholar]
- Howlett P.J., et al. Case series of severe neurologic sequelae of ebola virus disease during epidemic, Sierra Leone. Emerg. Infect. Dis. 2018;24(8):1412–1421. doi: 10.3201/eid2408.171367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. Parental depression and associations with parenting and children’s physical and mental health in a Sub-Saharan African setting. Child Psychiatry Hum. Dev. 2016 doi: 10.1007/s10578-016-0679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-J., Lane H.-Y., Lin C.-H. New treatment strategies of depression: based on mechanisms related to neuroplasticity. Neural plast. 2017:2017. doi: 10.1155/2017/4605971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley L.L., Tizabi Y. Neuroinflammation, neurodegeneration, and depression. Neurotox. Res. 2013:131–144. doi: 10.1007/s12640-012-9348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L., et al. A randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J. Clin. Psychopharmacol. 2012;32(4):551. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igwe M.N., et al. Factors associated with depression and suicide among patients with diabetes mellitus and essential hypertension in a Nigerian teaching hospital. Afr. Health Sci. 2013;13(1):68–77. doi: 10.4314/ahs.v13i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ior L.D. Ethnobotanical survey of plants used in the management of mental illnesses in some selected local government areas of Plateau State, Nigeria. J. Pharm. Phytother. 2017 [Google Scholar]
- Jacobs B.L., van Praag H., Gage F. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol. Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Jesulola E., Micalos P., Baguley I.J. Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model - are we there yet? Behav. Brain Res. 2018:79–90. doi: 10.1016/j.bbr.2017.12.025. [DOI] [PubMed] [Google Scholar]
- Jun C., et al. Disturbance of the Glutamatergic system in mood disorders. Exp. Neurobiol. 2014;23(1):28. doi: 10.5607/en.2014.23.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaya S.F., et al. Validity of the hopkins symptom checklist-25 amongst HIV-positive pregnant women in Tanzania. Acta Psychiatr. Scand. 2002;106:9–19. doi: 10.1034/j.1600-0447.2002.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O. Assessment of social interaction behaviors. J. Vis. Exp. 2011 doi: 10.3791/2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamau J.W., et al. Psychiatric morbidity among HIV-infected children and adolescents in a resource-poor Kenyan urban community. AIDS Care. 2012;24(7):836–842. doi: 10.1080/09540121.2011.644234. [DOI] [PubMed] [Google Scholar]
- Kaneez S. Depression and coping mechanism among HIV/AIDS patients under anti-retroviral therapy. Indian J. Soc. Psychiatry. 2016;32(2):149. doi: 10.4103/0971-9962.181098. [DOI] [Google Scholar]
- Kato T., et al. Optimising first- and second-line treatment strategies for untreated major depressive disorder - the SUN☺D study: a pragmatic, multi-centre, assessor-blinded randomised controlled trial. BMC Med. BioMed. Cent. 2018;16(1):103. doi: 10.1186/s12916-018-1096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World psychiatry: Off. J. World Assoc. 2007:168–176. 〈http://www.ncbi.nlm.nih.gov/pubmed/18188442〉 [PMC free article] [PubMed] [Google Scholar]
- Kinyanda E., et al. Prevalence and risk factors of major depressive disorder in HIV/AIDS as seen in semi-urban Entebbe district, Uganda. BMC Psychiatry BioMed. Cent. 2011;11(1):205. doi: 10.1186/1471-244X-11-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyanda E., et al. Rates, types and co-occurrence of emotional and behavioural disorders among perinatally HIV-infected youth in Uganda: the CHAKA study. Soc. Psychiatry Psychiatr. Epidemiol. 2019;54(4):415–425. doi: 10.1007/s00127-019-01675-0. [DOI] [PubMed] [Google Scholar]
- Klis S., et al. Posttraumatic stress and depressive symptoms among people living with HIV in the Gambia’. AIDS Care. 2011;23(4):426–434. doi: 10.1080/09540121.2010.507756. [DOI] [PubMed] [Google Scholar]
- Koyanagi A., et al. Depression comorbid with tuberculosis and its impact on health status: cross-sectional analysis of community-based data from 48 low- and middle-income countries. BMC Med. BioMed. Cent. 2017;15(1):209. doi: 10.1186/s12916-017-0975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. Animal models of depression: Molecular perspectives. Curr. Topics Behav. Neurosci. 2011:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukuia K., et al. Phytotherapy of experimental depression: Kalanchoe integra Var. Crenata (Andr.) Cuf Leaf Extract. J. Pharm. Bioallied Sci. 2015;7(1):26–31. doi: 10.4103/0975-7406.148785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukuia K.K.E., et al. Antidepressant Potentials of Components from Trichilia monadelpha (Thonn.) J.J. de Wilde in Murine Models. Evid. Based Complement. Altern. Med. 2018;2018:6863973. doi: 10.1155/2018/6863973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwobah E., et al. PREVALENCE of psychiatric morbidity in a community sample in Western Kenya. BMC Psychiatry BioMed. Cent. 2017;17(1):30. doi: 10.1186/s12888-017-1202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler K., et al. Depression among HIV-positive individuals in Botswana: a behavioral surveillance. AIDS Behavi. 2011;15(1):204–208. doi: 10.1007/s10461-009-9622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Frye M.A., Shelton R.C. Review of pharmacological treatment in mood disorders and future directions for drug development’. Neuropsychopharmacol. Nat. 2012;37(1):77–101. doi: 10.1038/npp.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo C.K., et al. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. British J. Psychiatry. 2012:52–59. doi: 10.1192/bjp.bp.111.097634. [DOI] [PubMed] [Google Scholar]
- Loo C.K., et al. International randomized-controlled trial of transcranial direct current stimulation in depression. Brain stimul. 2018:125–133. doi: 10.1016/j.brs.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Lundberg S., et al. Adolescent exploratory strategies and behavioral types in the multivariate concentric square field TM test. Front. Behav. Neuroci. 2019;13(March):1–18. doi: 10.3389/fnbeh.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R., et al. The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals. 2010;3(1):19–41. doi: 10.3390/ph3010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Meltzer H. In: Bloom F.E., Kupfer D.J., editors. Wiley- Liss, Inc.; 1995. The serotonin hypothesis of major depression’; pp. 933–944. (Psychopharmacology: The Fourth Generation of Progress. Raven, New York). [Google Scholar]
- Maier S.F., Watkins L. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 1984:30100. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maina, M. et al., 2020. ‘20 years of African Neuroscience: Waking a sleeping giant’. doi: 10.1101/2020.06.03.131391. [DOI]
- Manczak E.M. Time Spent with Parents Predicts Change in Depressive Symptoms in Adolescents with Major Depressive Disorder. J. Abnorm Chid Psychol. 2019 doi: 10.1007/s10802-019-00526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-SayI J., Leonard B.E. Studies on the nötropic potential of some angiotensin converting enzyme inhibitors in the mouse. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1989:953–962. doi: 10.1016/0278-5846(89)90046-8. [DOI] [PubMed] [Google Scholar]
- Mante P.K., et al. Anxiolytic-like effect of the leaves of Pseudospondias microcarpa (A. Rich.) Engl. in mice. J. Basic Clin. Physiol. Pharmacol. 2016 doi: 10.1515/jbcpp-2015-0067. [DOI] [PubMed] [Google Scholar]
- Marvel C.L., Paradiso S. Cognitive and neurological impairment in mood disorders Cherie. Psychiatry. 2008;183(1):118–122. doi: 10.1016/S0193-953X(03)00106-0.Cognitive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews D.C., Henter I.D., Zarate C.A. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72(10):1313–1333. doi: 10.2165/11633130-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Ewan B. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- Meng L., et al. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J. Hypertens. 2012;30(5):842–851. doi: 10.1097/HJH.0B013E32835080B7. [DOI] [PubMed] [Google Scholar]
- Merzoug S., Toumi M.L., Tahraoui A. Quercetin mitigates Adriamycin-induced anxiety- and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats. Naunyn-Schmiedebergs Arch. Pharmacol. 2014:921–933. doi: 10.1007/S00210-014-1008-Y. [DOI] [PubMed] [Google Scholar]
- Mesfin M., Asres K., Shibeshi W. Evaluation of anxiolytic activity of the essential oil of the aerial part of Foeniculum vulgare Miller in mice. BMC Complement. Altern. Med. BioMed Cent. 2014;14(1):1–7. doi: 10.1186/1472-6882-14-310/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson B.J., et al. The Concentric Square Field: A multivariate test arena for analysis of explorative strategies. Behav. Brain Res. 2006:100–113. doi: 10.1016/j.bbr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Miguel L.M., et al. Effets de l’huile des fruits de Dacryodes edulis sur les symptômes de l’anxiété et les performances cognitives chez la souris, après administration prolongée. L’Encéphale. 2019:397–404. doi: 10.1016/J.ENCEP.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target’. Nat. Rev. Immunol. 2016:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINISTERE DE LA SANTE REPUBLIQUE DU RWANDA (2010) ‘No Title LA LISTE NATIONALE DES MEDICAMENTS ESSENTIELS, 5 ème édition’, p. 400.
- Ministry of Health. 2016a. ‘Essential Medicines And Health Supplies List For Uganda’, pp. 1–209.
- Ministry of Health.2016b. ‘Kenya Essential Medicines List’, pp. 1–72.
- Ministry of Health. 2012. ‘Essential Medicine List’.
- Ministry of Health, (Government of Malawi) . 2015. ‘Malawi Standard Treatment Guidelines (MSTG) 5 th Edition 2015 Guidelines (MSTG)’, p. 687.
- Ministry of Health, G. N. D. P. 2017. ‘Standard Treatment Guidelines, 7th Edition’.
- Monteggia L., et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. USA. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossie A., Kindu D., Negash A. Prevalence and severity of depression and its association with substance use in Jimma Town, Southwest Ethiopia. Depress. Res. Treat. 2016:2016. doi: 10.1155/2016/3460462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughal A., et al. A systematic review of validated screening tools for anxiety disorders and PTSD in low to middle income countries. BMC Psychiaty. 2020 doi: 10.21203/rs.3.rs-15809/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtala A.A., Akindele A.J. Anxiolytic- and antidepressant-like activities of hydroethanol leaf extract of Newbouldia laevis (P.Beauv.) Seem. (Bignoniaceae) in mice. J. Ethnopharmacol. 2020;249 doi: 10.1016/j.jep.2019.112420. [DOI] [PubMed] [Google Scholar]
- Musyimi C.W., et al. Mental health outcomes of psychosocial intervention among traditional health practitioner depressed patients in Kenya. Cult. Med. Psychiatry. 2017:453–465. doi: 10.1007/s11013-017-9527-x. [DOI] [PubMed] [Google Scholar]
- Mutiu Ajao A.A. Anxiolytic and sedative properties of hydroethanolic extract of Telfairia occidentalis leaves in mice. Revista Brasileira de Farmacognosia. 2012;25:15–25. [Google Scholar]
- Mwape L., Katowa-Mukwato P., +Prudencia Mweemba ‘Psychological Distress during the Perinatal Period: Using Edinburgh Postnatal Depression Scale (EPDS) General Health Questionnaire (GHQ-12) and the Study Specific Measure of Perinatal Distress (SSMPD) on a Zambian Cohort’. J. Prevent. Rehab. Med. 2016;1(2):44–52. doi: 10.21617/jprm.2016.0102.10. [DOI] [Google Scholar]
- Myer L., et al. Common mental disorders among HIVinfected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care. 2008;22:147–158. doi: 10.1089/apc.2007.0102. [DOI] [PubMed] [Google Scholar]
- Nakimuli-Mpungu E. Cross-cultural adaptation and validation of the self-reporting questionnaire among HIV+ individuals in a rural ART program in southern Uganda. HIV AIDS. 2012;4:51. doi: 10.2147/HIV.S29818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakimuli-Mpungu E., et al. Depression, alcohol use and adherence to antiretroviral therapy in sub-Saharan Africa: a systematic review. AIDS Behav. 2012:2101–2118. doi: 10.1007/s10461-011-0087-8. [DOI] [PubMed] [Google Scholar]
- National Department of Health (2015) Standard Treatment Guidelines And Essential Medicines List for South Africa: Hospital Level 2012 Edition. doi: 10.1017/CBO9781107415324.004. [DOI]
- Ndetei D.M., et al. The prevalence of mental disorders in adults in different level general medical facilities in Kenya: a cross-sectional study. Ann. Gen. Psychiatry BioMed. Cent. 2009;8(1):1. doi: 10.1186/1744-859X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nduna M., et al. Prevalence and factors associated with depressive symptoms among young women and men in the Eastern Cape Province, South Africa. J. Child Adolesc. Ment. Health. 2013:43–54. doi: 10.2989/17280583.2012.731410. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol. Psychiatry. 1996;1:336–342. [PubMed] [Google Scholar]
- Netsereab T.B., et al. Validation of the WHO self-reporting questionnaire-20 (SRQ-20) item in primary health care settings in Eritrea 11 medical and health sciences 1117 public health and health services. Int. J. Men. Health Syst. 2018:61. doi: 10.1186/s13033-018-0242-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hånell A., Marklund N. Structured evaluation of rodent behavioral tests used in drug discovery research. Front. Behav. Neurosci. 2014;8:1–13. doi: 10.3389/fnbeh.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngasa S.N., et al. Prevalence and factors associated with depression among medical students in Cameroon: A cross-sectional study. BMC Psychiatry. BioMed. Cent. 2017;17(1) doi: 10.1186/s12888-017-1382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoupaye G.T., et al. Antidepressant properties of aqueous acerate from Gladiolus dalenii corms. Afr. J. Tradit. Complement. Altern. Med. 2014:53–61. [PMC free article] [PubMed] [Google Scholar]
- Ngoupaye G.T., et al. Antidepressant properties of aqueous macerate from gladiolus dalenii corms. Afr. J. Tradit. Complement. Altern. Med. 2014:53. doi: 10.4314/ajtcam.v11i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoupaye G.T., Ngo Bum E., Daniels W.M.U. ‘Antidepressant-like effects of the aqueous macerate of the bulb of Gladiolus dalenii Van Geel (Iridaceae) in a rat model of epilepsy-associated depression’. BMC Complement. Altern. Medicine. BioMed. Cent. 2013;13(1):272. doi: 10.1186/1472-6882-13-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nubukpo P., Houinato D., et al. Anxiété et dépression chez les épileptiques en population générale au Bénin (Afrique de l’Ouest) L’Encephale. 2004;30(3):214–219. doi: 10.1016/S0013-7006(04)95432-2. [DOI] [PubMed] [Google Scholar]
- Nubukpo P., Preux P.M., et al. Psychosocial issues in people with epilepsy in Togo and Benin (West Africa) I. Anxiety and depression measured using Goldberg’s scale. Epilepsy Behav. 2004:722–727. doi: 10.1016/j.yebeh.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Ojagbemi A., et al. Depression after stroke in Sub-Saharan Africa: a systematic review and meta-analysis. Behav. Neurol. 2017;2017 doi: 10.1155/2017/4160259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojagbemi A., Bello T., Gureje O. Late-life depression in sub-Saharan Africa: lessons from the Ibadan Study of Ageing. Epidemiol. Psychiatr. Sci. 2020:29. doi: 10.1017/S2045796020000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoli C.O., et al. Anticonvulsant and anxiolytic evaluation of leaf extracts of Ocimum gratissimum, a culinary herb. Pharmacogn. Res. 2010:36–40. doi: 10.4103/0974-8490.60580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omotoso G.O., et al. Moringa oleifera phytochemicals protect the brain against experimental nicotine-induced neurobehavioral disturbances and cerebellar degeneration. Pathophysiology. 2018:57–62. doi: 10.1016/j.pathophys.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Opondo P.R., et al. HIV prevalence among hospitalized patients at the main Psychiatric Referral Hospital in Botswana. AIDS Behav. 2018:1503–1516. doi: 10.1007/s10461-017-1878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osok J., et al. Depression and its psychosocial risk factors in pregnant Kenyan adolescents: a cross- sectional study in a community Health Centre of Nairobi. BMC Psychiatry. 2018:1–10. doi: 10.1186/s12888-018-1706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othieno C.J., et al. Depression among university students in Kenya: prevalence and sociodemographic correlates. J. Affect. Disord. 2014;165:120–125. doi: 10.1016/j.jad.2014.04.070. [DOI] [PubMed] [Google Scholar]
- Padayachey U., Ramlall S., Chipps J. Depression in older adults: prevalence and risk factors in a primary health care sample Depression in older adults: prevalence and risk factors in a primary health care sample. South Afr. Fam. Pract. 2017:61–66. doi: 10.1080/20786190.2016.1272250. [DOI] [Google Scholar]
- Pannetier J., et al. Mental health of sub-saharan african migrants: the gendered role of migration paths and transnational ties. SSM - Popul. Health. 2017;3(December 2016):549–557. doi: 10.1016/j.ssmph.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante C., Lightman S. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Patel V., et al. The Shona Symptom Questionnaire: the development of an indigenous measure of common mental disorders in Harare. Acta Psychiatr. Scand. 1997:469–475. doi: 10.1111/j.1600-0447.1997.tb10134.x. [DOI] [PubMed] [Google Scholar]
- Pedersen M.E., et al. Effects of South African traditional medicine in animal models for depression. J. Ethnopharmacol. 2008;119(3):542–548. doi: 10.1016/j.jep.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Phillips L.M., Kupfer J.D. Bipolar disorder diagnosis: challenges and future directions. Lancet. 2013;381(9878):1663–1671. doi: 10.1016/S0140-6736(13)60989-7.Bipolar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placidi G.P., et al. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol. Psychiatry. 2001;50:783–791. doi: 10.1016/s0006-3223(01)01170-2. [DOI] [PubMed] [Google Scholar]
- Porsolt, R.D., Le Pichon, M., & Jalfre, M. (1977) ‘© 1977 Nature Publishing Group’. [DOI] [PubMed]
- Preskorn S., et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J. Psychiatr. Pract. 2015;21(2):140–149. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- Quello, B.S., Brady, T.K. and Sonne, C.S.. 2005. ‘<Quelloetal2005-SciencePracticePerspectives.pdf>’.
- Rachid F. Maintenance repetitive transcranial magnetic stimulation (rTMS) for relapse prevention in with depression: a review. Psychiatry Res. 2018:363–372. doi: 10.1016/j.psychres.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Ramesh M., Dokurugu Y.M., Thompson M., et al. Therapeutic, molecular and computational aspects of novel monoamine oxidase (MAO) inhibitors. Comb. Chem. High Throughput Screen. 2017;20(6):492–509. doi: 10.2174/1386207320666170310121337. [DOI] [PubMed] [Google Scholar]
- Réus G.Z., et al. Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behav. Brain Res. 2011:166–171. doi: 10.1016/j.bbr.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Rivet-Duval E., Heriot S., Hunt C. Preventing adolescent depression in Mauritius: a universal school-based program. Child Adolesc. Ment. Health. 2011;16(2):86–91. doi: 10.1111/j.1475-3588.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- Rodriques I., Pieters W.R. Assessing the predictors of satisfaction with life of employees in windhoek, Namibia. SA J. Human Resour. Manag. 2019;17(0):9. doi: 10.4102/sajhrm.v17i0.1145. [DOI] [Google Scholar]
- Rumble S., et al. Prevalence of psychiatric morbidity in the adult population of a rural South African village. Psychol. Med. 1996;26:997–1007. doi: 10.1017/s0033291700035327. [DOI] [PubMed] [Google Scholar]
- Sáenz J.C.B., Villagra O.R., Trías J.F. Factor analysis of Forced Swimming test, Sucrose Preference test and Open Field test on enriched, social and isolated reared rats. Behav. Brain Res. 2006;169:57–65. doi: 10.1016/j.bbr.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Sabry M.F., Hamed M.R., El sayed M.E. Effect of biphenyl dimethyl dicarboxylate and fluoxetine on performance of normally-fed and protein malnourished psychologically stressed mice in elevated plus maze. Bull. Fac. Pharm. Cairo Univ. 2014:51–61. doi: 10.1016/j.bfopcu.2014.04.004. [DOI] [Google Scholar]
- Sacher J., et al. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J. Affect. Disord. 2012;140(2):142–148. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Safeekh A. Understanding depression: An overview’. Arch. Med. Health Sci. 2017;5(1):107. doi: 10.4103/AMHS.AMHS_52_17. [DOI] [Google Scholar]
- Scholte W.F., et al. Psychometric properties and longitudinal validation of the self-reporting questionnaire (SRQ-20) in a Rwandan community setting: a validation study. BMC Med. Res. Methodol. BioMed. Cent. 2011;11(1):116. doi: 10.1186/1471-2288-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J., et al. Evidence for a relationship between genetic variants at the brainderived neurotrophic factor (BDNF) locus and major depression. Biol. Psychiatry. 2005;58:307–314. doi: 10.1016/j.biopsych.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Secor A., et al. Mental health among Ebola survivors in Liberia, Sierra Leone and Guinea: Results from a cross-sectional study. BMJ Open. 2020 doi: 10.1136/bmjopen-2019-035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffery J., Hoffmann R., Armitage R. The neurobiology of depression: perspectives from animal and human sleep studies. Neuroscientist. 2003;9(1):82–98. doi: 10.1177/1073858402239594. [DOI] [PubMed] [Google Scholar]
- Sheikh T.L., et al. Descriptive characterization of psycho-trauma, psychological distress, and post-traumatic stress disorder among children and adolescent internally displaced persons in Kaduna, Nigeria. Front. Psychiatry. 2016;7(OCT):28. doi: 10.3389/fpsyt.2016.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherif F., Oreland L. Effect of the GABA-transaminase inhibitor vigabatrin on exploratory behaviour in socially isolated rats. Behav. Brain Res. 1995:135–140. doi: 10.1016/0166-4328(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Shim I.H., et al. Antidepressants and mood stabilizers: Novel research avenues and clinical insights for bipolar depression. Int. J. Mol. Sci. 2017;18(11) doi: 10.3390/ijms18112406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery D.A., Cryan J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012;7(6):1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- Slekiene J., Mosler H.J. The link between mental health and safe drinking water behaviors in a vulnerable population in rural Malawi. BMC Psychology. BioMed. Cent. 2019;7(1) doi: 10.1186/s40359-019-0320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloman L. A new comprehensive evolutionary model of depression and anxiety. J. Affect. Disord. 2008:219–228. doi: 10.1016/j.jad.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Smach M.A., et al. Maillard reaction products and phenolic compounds from roasted peanut flour extracts prevent scopolamine-induced amnesia via cholinergic modulation and antioxidative effects in mice. J. Med. Food. 2021:645–652. doi: 10.1089/jmf.2020.0028. 〈https://home.liebertpub.com/jmf〉 [DOI] [PubMed] [Google Scholar]
- Smith K.M., Renshaw P.F., Bilello J. The diagnosis of depression: current and emerging methods. Comp. Psychiatry. 2013;54(1):1–6. doi: 10.1016/j.comppsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford G.I., et al. Monoamine oxidase inhibition by Southern African traditional medicinal plants. South Afr. J. Bot. 2007;73(3):384–390. doi: 10.1016/j.sajb.2007.03.001. [DOI] [Google Scholar]
- Stafford G.I., et al. Review on plants with CNS-effects used in traditional South African medicine against mental diseases’. J. Ethnopharmacol. 2008;119(3):513–537. doi: 10.1016/j.jep.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Stein D.J., et al. Lifetime prevalence of psychiatric disorders in South Africa. Br. J. Psychiatry. 2008;192(2):112–117. doi: 10.1192/bjp.bp.106.029280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steru L., et al. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology. 1985:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stewart R.C., Kauye F., Umar E. Validation of a Chichewa version of the self-reporting questionnaire (SRQ) asa brief screening measure for maternal depressive disorder in Malawi, Africa. J. Affect. Disord. 2009;112:126–134. doi: 10.1016/j.jad.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Stockmeier C.A. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J. Psychiatr. Res. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Strawbridge R., Young A.H., Cleare A. Biomarkers for depression: recent insights, current challenges and future prospects. Neuropsychiatr. Dis. Treat. 2017;13:1245–1262. doi: 10.2147/NDT.S114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., et al. Magnetic seizure therapy reduces suicidal ideation and produces neuroplasticity in treatment-resistant depression. Transl. Psychiatry. 2018;8(1):1–11. doi: 10.1038/s41398-018-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetland A.C., Belkin G.S., Verdeli H. Measuring depression and anxiety in sub-saharan africa. Depress. Anxiety. 2014:223–232. doi: 10.1002/da.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A.M., et al. Introduction of an Alcohol-Related Electronic Screening and Brief Intervention (eSBI) Program to Reduce Hazardous Alcohol Consumption in Namibia’s Antiretroviral Treatment (ART) Program. AIDS Behav. 2019:3078–3092. doi: 10.1007/s10461-019-02648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira G., Martins E.S., Catarino L. Antidepressant Plant Species from the Portuguese-Speaking African Countries (PALOP) Herbal Med. Depress. 2016:433–481. [Google Scholar]
- Terburg D., et al. Acute effects of sceletium tortuosum (Zembrin), a dual 5-HT reuptake and PDE4 inhibitor, in the human amygdala and its connection to the hypothalamus. Neuropsychopharmacology. 2013;38(13):2708–2716. doi: 10.1038/npp.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Medicine and Therapeutics Policy Advisory Committee . National Medicine and Therapeutics Policy Advisory Committee; 2015. EDLIZ 2015: 7th Essential Medicines List and Standard Treatment Guidelines for Zimbabwe. [Google Scholar]
- Thomsen P.H., Sorensen M.J. ‘Evidence for psychopharmacological treatment of depression in children and adolescents?’. Ugeskr Laeger. 2007;169(14):1289–1294. [PubMed] [Google Scholar]
- Tolentino J.C., Schmidt S.L. DSM-5 criteria and depression severity: Implications for clinical practice. Front. Psychiatry. 2018;9(OCT):1–9. doi: 10.3389/fpsyt.2018.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey E.F., et al. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol. Psychiatry. 2005:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Uche N., Nwaopara U., Stanley P. Citation: Nwaopara U, Stanley P (2015) Prevalence of Depression in Port Harcourt Prison. J. Psychiatry. 2015;18(6):340. 10.472/2378-5756.1000340. [Google Scholar]
- Umubyeyi A., et al. Intimate partner violence and its contribution to mental disorders in men and women in the post genocide Rwanda: Findings from a population based study. BMC Psychiatry BioMed. Cent. 2014;14(1) doi: 10.1186/s12888-014-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umukoro S., et al. Evidence for the involvement of monoaminergic pathways in the antidepressant-like activity of cymbopogon citratus in mice. Drug Res. 2017;67(7):419–424. doi: 10.1055/s-0043-106586. [DOI] [PubMed] [Google Scholar]
- Uwakwe R. Psychiatric morbidity in elderly patients admitted to non-psychiatric wards in a general/teaching hospital in Nigeria. Int. J. Geriatr. Psychiatry. 2000;15(4):346–354. doi: 10.1002/(sici)1099-1166(200004)15:4<346::aid-gps125>3.0.co;2-9. 10.1002/(SICI)1099-1166(200004)15:4<346::AID-GPS125>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Valvassori S.S., et al. Contributions of animal models to the study of mood disorders. Revista Brasileira de Psiquiatria. 2013:S121–S131. doi: 10.1590/1516-4446-2013-1168. [DOI] [PubMed] [Google Scholar]
- Ventevogel P., et al. Properties of the Hopkins symptom checklist-25 (HSCL-25) and the self-reporting questionnaire (SRQ-20) as screening instruments used in primary care in Afghanistan. Soc. Psychiatry Psychiatric Epidemiol. 2007:328–335. doi: 10.1007/s00127-007-0161-8. [DOI] [PubMed] [Google Scholar]
- Vinck P., Pham P.N. Association of exposure to violence and potential traumatic events with self-reported physical and mental health status in the Central African Republic. JAMA - J. Am. Med. Assoc. Am. Med. Assoc. 2010;304(5):544–552. doi: 10.1001/jama.2010.1065. [DOI] [PubMed] [Google Scholar]
- Wang P., Si T. Use of antipsychotics in the treatment of depressive disorders. Shanghai Arch. Psychiatry. 2013;30(1):103–109. doi: 10.3969/j.issn.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weobong B., et al. Prevalence and determinants of antenatal depression among pregnant women in a predominantly rural population in Ghana: The DON population-based study. J. Affect. Disord. 2014;165:1–7. doi: 10.1016/j.jad.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Weobong B., Akpalu B., Doku V. The comparative validity of screening scales for postnatal common mental disorder in Kintampo, Ghana. J. Affect. Disord. 2009;113:109–117. doi: 10.1016/j.jad.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Willner P., et al. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. ‘Psychopharmacology. 1987:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Woode E., et al. Anxiolytic and antidepressant effects of a leaf extract of Palisota hirsuta K. Schum.(Commelinaceae) in mice. Int. J. Pharmacol. 2010;6(1):1–17. [Google Scholar]
- Wubetu M., Sintayehu M., Aeta M.A. Ethnobotany of medicinal plants used to treat various mental illnesses in Ethiopia: a systematic review Muluken Wubetu1, Mezinew Sintayehu2, Mohammedbrhan Abdelwuhab Aeta2, Haimanot Reta2 and Dagninet Derebe2. Asian J. Plant Sci. Res. 2018;8(1):9–33. [Google Scholar]
- Xavier P.B., Peixoto B. Emotional distress in angolan patients with several types of tuberculosis. Afr. Health Sci. 2015;15(2):378–384. doi: 10.4314/ahs.v15i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yennurajalingam S., et al. Cranial electrotherapy stimulation for the management of depression, anxiety, sleep disturbance, and pain in patients with advanced cancer: a preliminary study. J. Pain Symptom Manag. 2018;55(2):198–206. doi: 10.1016/j.jpainsymman.2017.08.027. [DOI] [PubMed] [Google Scholar]
- Yotebieng K.A., Fokong K., Yotebieng M. Depression, retention in care, and uptake of PMTCT service in Kinshasa, the Democratic Republic of Congo: a prospective cohort. AIDS Care. 2017:285–289. doi: 10.1080/09540121.2016.1255708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim M.B.H., Bakhle Y.S. Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006;147(SUPPL. 1) doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngmann R., et al. Adapting the SRQ for Ethiopian Populations: a culturally-sensitive psychiatric screening instrument. Transcult. Psychiatry. 2008;45(4):566–589. doi: 10.1177/1363461508100783. [DOI] [PubMed] [Google Scholar]
- Yu H., Chen Z.Y. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sinica. 2011:3–11. doi: 10.1038/aps.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambia Ministry of Health . 2019. ‘Republic of Zambia MINISTRY OF HEALTH’, Office, (May), pp. 2009–2015.