Abstract
Background:
NMDA receptors regulate synaptic plasticity and modulate a wide variety of behaviors. Mammalian NMDA receptors are inhibited by ethanol even at low concentrations. In mice, the F639A mutation in transmembrane domain 3 of the NR1 subunit reduces ethanol sensitivity of the receptor and in some paradigms, reduces behavioral ethanol sensitivity and increases ethanol consumption. We tested the fly equivalent of the F639A and K544Q mutations for effects on ethanol sensitivity. Drosophila shows a high degree of behavioral and mechanistic conservation in its responses to ethanol.
Methods:
Homologous recombination and CRISPR/Cas9 genome editing were used to generate amino acid changes in the Drosophila NMDAR1 gene, yielding F654A and K558Q alleles. Animals were tested for the degree of ethanol sensitivity, the ability to acquire tolerance to ethanol, ethanol drinking preference, circadian rhythmicity, learning, and locomotor defects.
Results:
We observed that mutating the NMDAR1 channel also reduces ethanol sensitivity in adult flies. However, in flies, it was the K558Q mutation (orthologous to K544Q in mice) that reduces ethanol sensitivity in a recovery-from-sedation assay. The effects of the F654A mutation (orthologous to F639A in mice) were substantially different in flies than in mammals. In flies, F654A mutation produces phenotypes opposite those in mammals. In flies, the mutant allele is homozygous viable, does not seem to affect health, and increases ethanol sensitivity. Both mutations increased feeding but did not alter the animal’s preference for 5% ethanol food. F654A depressed circadian rhythmicity and the capacity of males to court, but it did not depress the capacity for associative learning. K554Q, on the other hand, has little effect on circadian rhythmicity, only slightly suppresses male courtship, and is a strong learning mutant.
Conclusions:
Mutations in transmembrane domain 3 and in the extracellular vestibule calcium binding site of the NR1 NMDA subunit affect ethanol sensitivity in Drosophila.
Keywords: Drosophila, ethanol, alcohol, alcoholism, NMDA receptor, tolerance, ethanol preference, circadian rhythms, learning and memory
Introduction
NMDA receptors (NMDARs) are highly conserved ligand-gated ion channels that mediate synaptic plasticity (reviewed in Cull-Candy and Leszkiewicz, 2004). Drosophila NMDARs have the electrophysiological hallmarks of mammalian NMDARs and are important for learning, memory, habituation, phototaxis preference, olfactory avoidance, aggression, touch sensation, and sleep (Xia et al., 2005; Wu et al., 2007; Chen et al., 2012; Edwards et al., 2009; Larkin et al., 2010; Sambandan et al., 2006; Tsubouchi et al., 2012; Zhou et al., 2010; Tomita et al., 2015).
In mammals, chronic ethanol consumption can also promote NMDAR-dependent excitotoxic neuronal cell death after ethanol clearance. This is caused by ethanol-induced HMGB1-TLR4–mediated phosphorylation of the NR2B subunit of the NMDAR that increases migration of NMDARs to the synapse, enhancing neural excitability and sensitivity to glutamate-induced excitotoxicity (reviewed in Crews and Vetreno, 2014). In flies, this has been explored in the adult’s antennal neurons where prolonged exposure to high doses of ethanol vapor causes excitotoxic cell neuronal death. Either synaptically silencing these neurons or reducing NMDAR1 expression in these neurons was shown to be protective for this type of ethanol-induced neuronal cell death (French and Heberlein, 2009).
Ethanol inhibits human recombinant NMDARs at concentrations as low as 10 mM (Smothers et al., 2001), and site-directed mutagenesis has shown that residues in TM3 influence ethanol sensitivity (Ronald et al., 2001). For the NR1 subunit encoded by the rat GRIN1 gene, it was found that an F639A mutation reduced inhibition by ethanol, suggesting that ethanol directly interacts with the F639 residue (Ronald et al., 2001). In a second report, ten different amino acid substitutions at F639 were tested for effects on ethanol inhibition of NMDAR function, but only F639A and F639S were found to significantly reduce inhibition by ethanol. Both substitutions were also found to affect the glycine EC50 (Smothers and Woodward, 2006).
Furthermore, den Hartog et al. (2013) described the effects of the NMDAR1F639A mutation in knock-in mice. Receptors from the F639A mice were physiologically less sensitive to inhibition by ethanol. Mice homozygous for F639A die within three weeks of birth, so only heterozygotes were tested for behavioral effects. Injection of a low dose of ethanol that induces hyperactivity in wild-type mice had no effect on F639A heterozygotes. In the rotarod test of motor impairment, F639A heterozygotes recovered from a high dose of ethanol faster than wild type, but no difference was seen at low doses. Presence of the F639A allele also reduced the anxiolytic effect of ethanol. No differences in duration of loss-of-righting reflex, hypothermia, or metabolism responses were seen. The F639A mutation also affected drinking behaviors. In a two-hour limited-access paradigm, mutant mice drank less ethanol than wild-type controls, but in response to an every-other-day intermittent-access protocol, mutant mice drank more ethanol than wild type (den Hartog et al., 2013).
Our interest in the NMDAR was to see whether a mutation that suppressed ethanol sensitivity of the channel would also suppress the acquisition of ethanol tolerance in flies. We used CRISPR/Cas9 to generate the analogous mutation in flies (F639A in mammals and F654A in flies) and examined a variety of ethanol-induced behaviors to catalog the scope of behaviors affected by this mutation. During the course of constructing the F639A orthologous mutation in flies, we also generated two other unique alleles and characterized their behavioral phenotypes.
Results
Studies of mammalian NMDARs identified residue F639 in TM3 as important for ethanol modulation. Drosophila NMDAR1 residue F654 aligns to the mammalian F639 residue. In our pursuit of generating this allele in Drosophila, we inadvertently generated two additional mutant alleles. The positions of all amino acid substitutions are shown in Figures 1 and 2. We first used ends-out homologous recombination to knock the F654A mutation into the Drosophila NMDAR1 gene (Gong and Golic, 2003). However, the cloned DNA proved highly unstable in bacteria. Repetitively, rearranged or insert-less plasmids were obtained. These technical issues were eventually thought to have been overcome, and a knock-in fly was generated. However, this fly carried the F654A mutation and a second mutation in the gene. In light of our difficulty in propagating NMDAR1-containing clones in bacteria, we concluded that the receptor DNA behaved as if it was toxic to bacteria and that the second mutation suppressed this toxicity. The second mutation, K558Q, is equivalent to K544 residue in the mouse nomenclature used in the papers from the Woodward lab.
Figure 1.
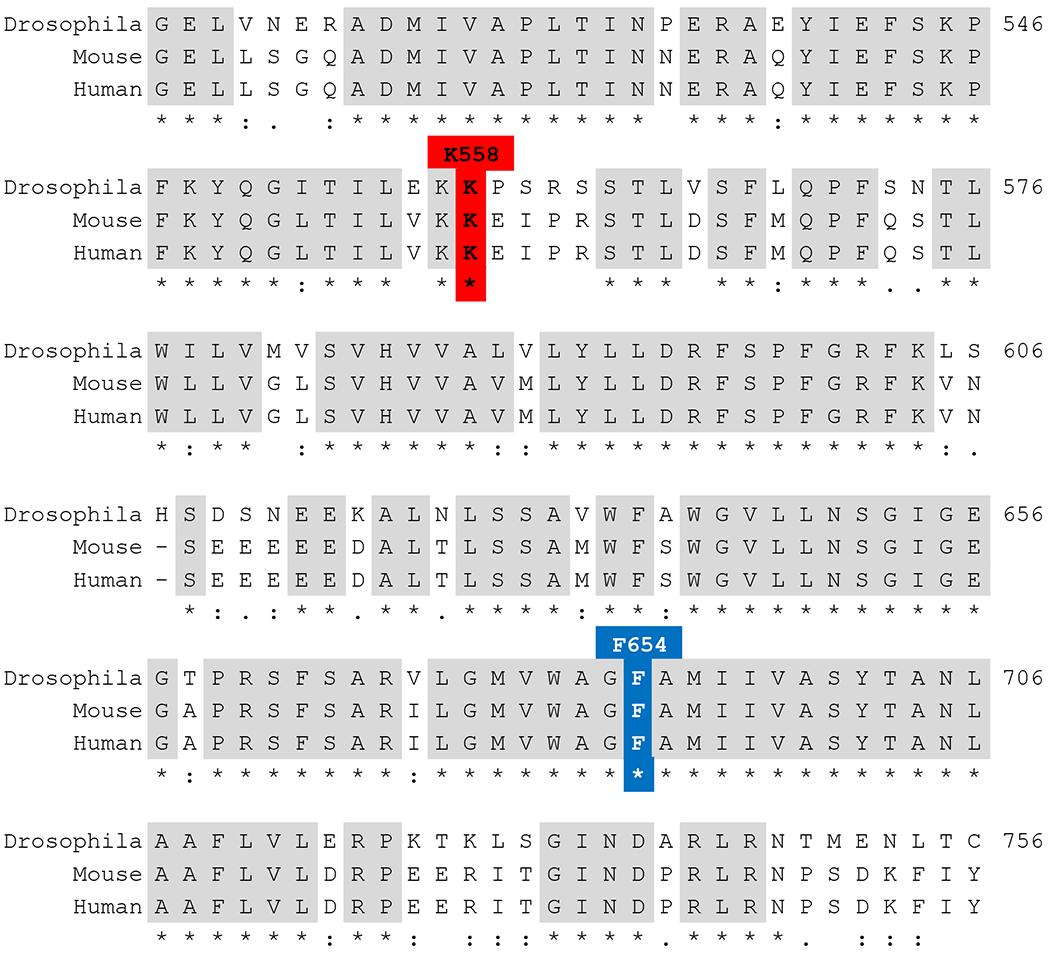
Alignment of NMDAR1 protein sequences. Alignment of the NMDAR1 genes from Drosophila melanogaster, Mus musculus, and Homo sapiens generated in Tcoffee (Notredame et al., 2000). Only the portion containing the residues studied here is included. “*” denotes identical residues, “:” denotes conservative change, “.” denotes less conservative change.
Figure 2.
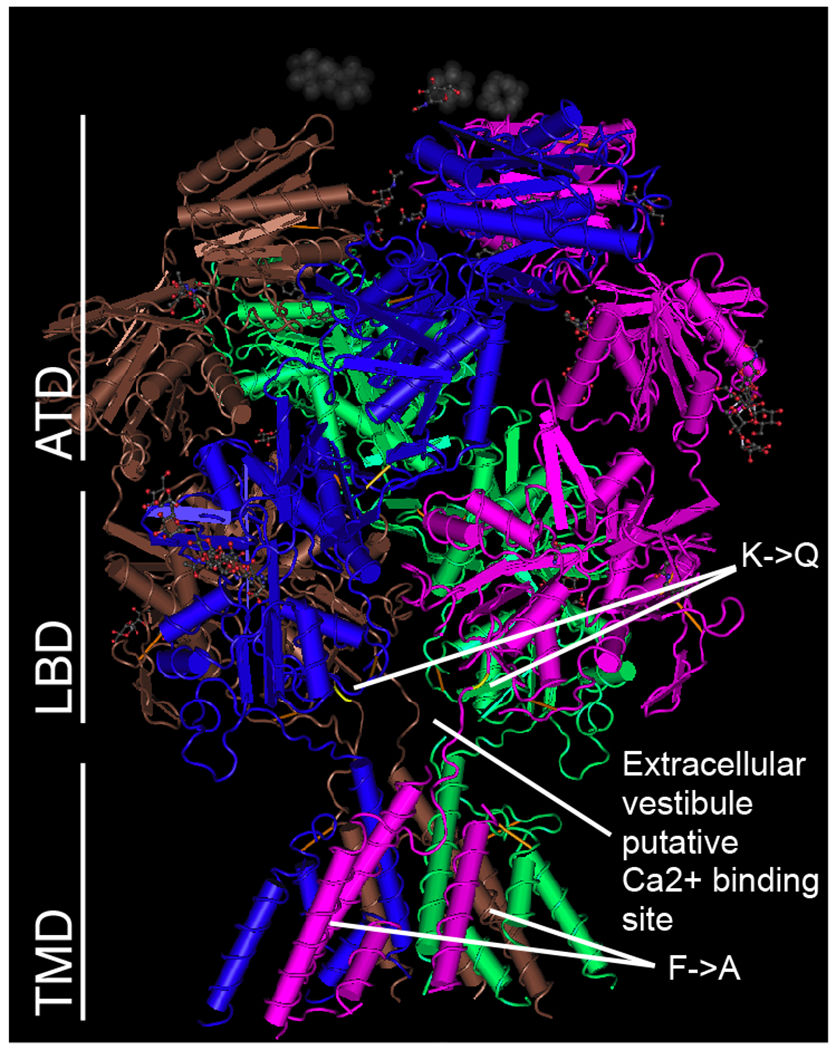
Position of amino acid substitutions mapped onto the crystal structure of a GluN1-GluN2B Rattus norvegicus heterotetrameric channel as described by (Karakas and Furukawa, 2014). X-ray diffraction 3.96 Å model downloaded from NCBI (https://www.ncbi.nlm.nih.gov/Structure/pdb/4PE5). Channel is organized into three domains: ATD (amino-terminal domain); LBD (ligand-binding domain), and TMD (transmembrane domain). The K->Q substitution maps to the LBD near the putative Ca2+ binding site at the extracellular vestibule while the F->A substitution maps to the TMD.
The mutant allele that carries both K558Q and F654A mutations will be referred to as NMDAR1DBL. This allele is homozygous viable and produces subtle behavioral effects. The effect of NMDAR1DBL on ethanol sensitivity was measured in an ethanol-sedation assay and a recovery-from-ethanol-sedation assay. In the sedation assay, age-matched females are exposed to ethanol vapor and monitored as they gradually become intoxicated and are sedated (Pohl et al., 2013). In this assay, NMDAR1DBL flies sedate slightly more slowly than wild type (Fig.3A, p < 0.04). That is, the NMDAR1DBL mutant reduced ethanol sensitivity. In the recovery-from-sedation assay, flies were exposed to ethanol vapor until all were sedated, and then the rate of recovery was measured (Cowmeadow et al., 2005; Krishnan et al., 2012). In this second assay, the NMDAR1DBL flies were found to be much less sensitive to ethanol than wild type (Fig.3B, p < 0.0001). We then tested NMDAR1DBL flies for the ability to acquire ethanol tolerance. In the tolerance test, half the animals are sedated on day one, while the other half are mock sedated. On day two, both groups are sedated simultaneously and the recovery rate measured. If the second-ethanol-exposure group recovers faster than the first-exposure group, the stock is denoted as being capable of acquiring tolerance. The presence of the NMDAR1DBL mutation does not block the ability to acquire tolerance (Fig.3C, p < 0.05).
Figure 3.
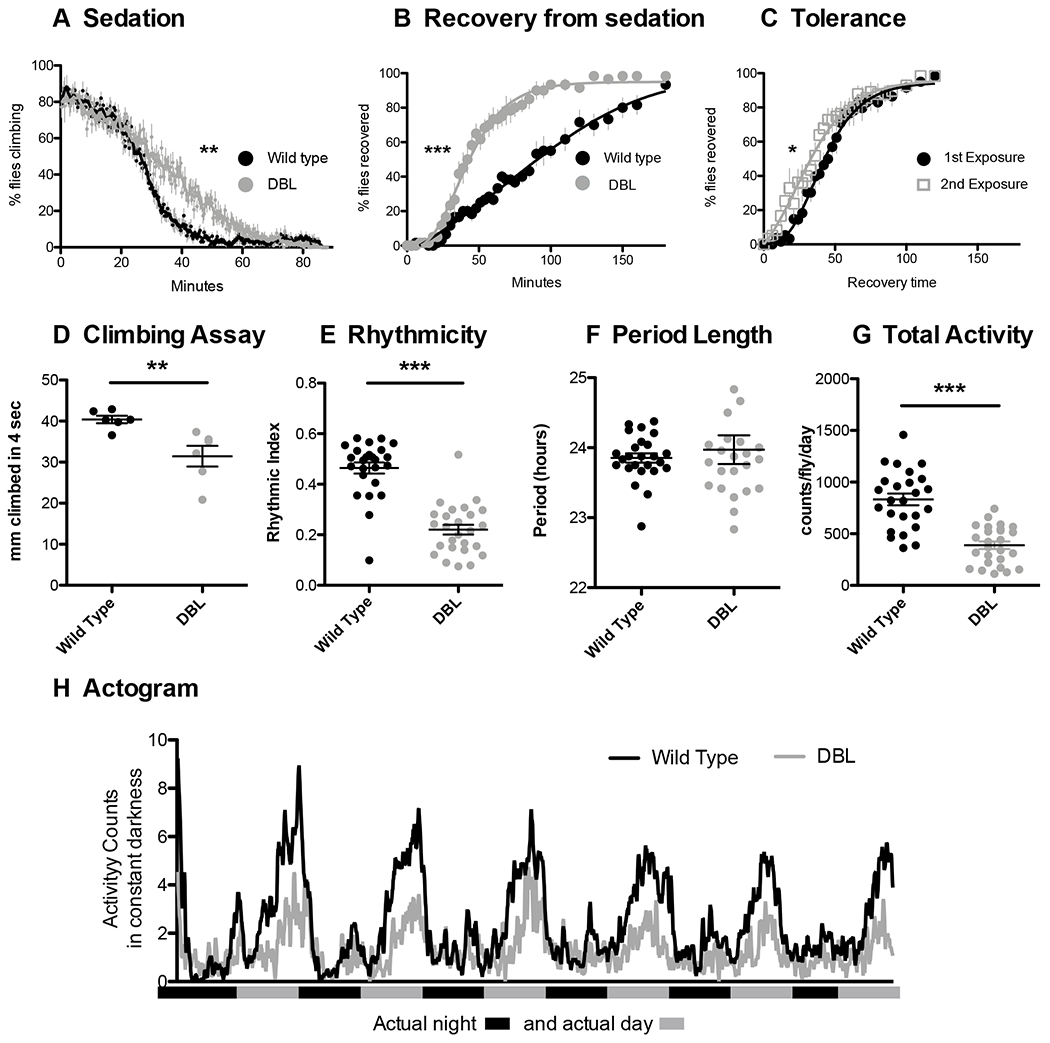
NMDAR1DBL (NMDAR1K558Q;F654A) flies show reductions in ethanol sensitivity, circadian rhythmicity, climbing speed, and activity level. A) Age-matched females were monitored as they become intoxicated and sedate. In this assay animals homozygous for NMDAR1DBL take longer to sedate than wild type controls (p < 0.04). B) Recovery-from-sedation assay where flies are placed in vials and exposed to ethanol vapor until sedated, and then animals are allowed to recover in a humidified air stream. 0 minutes denotes the beginning of the recovery period. NMDAR1DBL flies recover faster than wild type controls (p < 0.0001). C) Twenty-four hour tolerance assay. Half of the animals were sedated with ethanol vapor 24 h prior to the test, while the other half were mock-sedated. Tolerance is seen as a left-shift in the sedation curve of animals experiencing their second sedation (p < 0.05). D) In the RING assay of climbing ability, NMDAR1DBL flies climb slower than wild type (p < 0.008). In a circadian rhythm assay, flies show E) a reduced rhythmic index (p < 0.0001), F) normal period length (p>0.05), and G) reduced activity level (p < 0.0001). H) Actograms showing activity levels of wild type and NMDAR1DBL flies over six days in constant darkness. All circadian rhythmicity measures were conducted in constant darkness starting with newly eclosed animals that had been entrained for 3 days in a 12:12 light cycle. The gray and black bars on the x-axis represent the actual day and night cycle during the six days of imposed darkness.
The NMDAR1DBL flies also have a visibly altered gait: they appear to stagger slightly as they walk. As a measure of coordination, we performed the RING (rapid iterative negative geotaxis) assay to measure climbing ability. This assay takes advantage of Drosophila’s natural negative geotaxis behavior—flies knocked to the bottom of a vial rapidly climb back toward the top of the vial (Gargano et al., 2005). In this assay, the NMDAR1DBL flies climbed more slowly than the wild type (Fig.3D, p < 0.008).
NMDAR1DBL animals were also tested for circadian rhythmicity under free-running conditions. The rationale for this was the reported link between NMDAR1 and Drosophila circadian rhythm (Tomita et al., 2015) and that mutations that disrupt circadian rhythms also affect ethanol behaviors (Ceriani et al., 2002; Cowmeadow et al., 2005; Pohl et al., 2013). NMDAR1DBL flies were able to maintain circadian rhythmicity and a 24 h period (Fig.3E and 3F). Although the mutant has a deflated rhythmic index (Fig.3E, p < 0.001), it appears that this deflation is a reflection of the flies’ decreased overall activity level (p < 0.0001, Fig.3G).
To determine which amino acid change in NMDAR1DBL caused the reduction in ethanol sensitivity, we generated each mutation individually in separate alleles using the CRISPR/Cas9 system. NMDAR1F654A homozygotes and NMDAR1K558Q homozygotes were subjected to a battery of behavioral tests. An NMDAR1EP331 mutant was included in many tests to evaluate whether the new alleles resembled a simple loss-of-function mutation. NMDAR1EP331 is a hypomorphic allele caused by a P-element transposon insertion in NMDAR1 (Miyashita et al., 2012; Xia et al., 2005).
To evaluate the effect of NMDAR1 mutations on ethanol sensitivity, we used both the sedation assay and the recovery-from-sedation assay described above. It has been reported that the EP331 allele does not affect alcohol sensitivity in Drosophila (Maiya et al., 2012). However, in the sedation assay and the recovery-from-sedation assay, we observed that NMDAR1EP331 homozygotes displayed increased ethanol sensitivity compared to wild-type animals (Fig.4A, p < 0.0001 and Fig.4D, p < 0.0001, respectively). Similarly, the NMDAR1F654A allele increased ethanol sensitivity in the sedation assay and the recovery assay (Fig.4B, p < 0.02 and Fig.4E, p < 0.0001, respectively).
Figure 4.
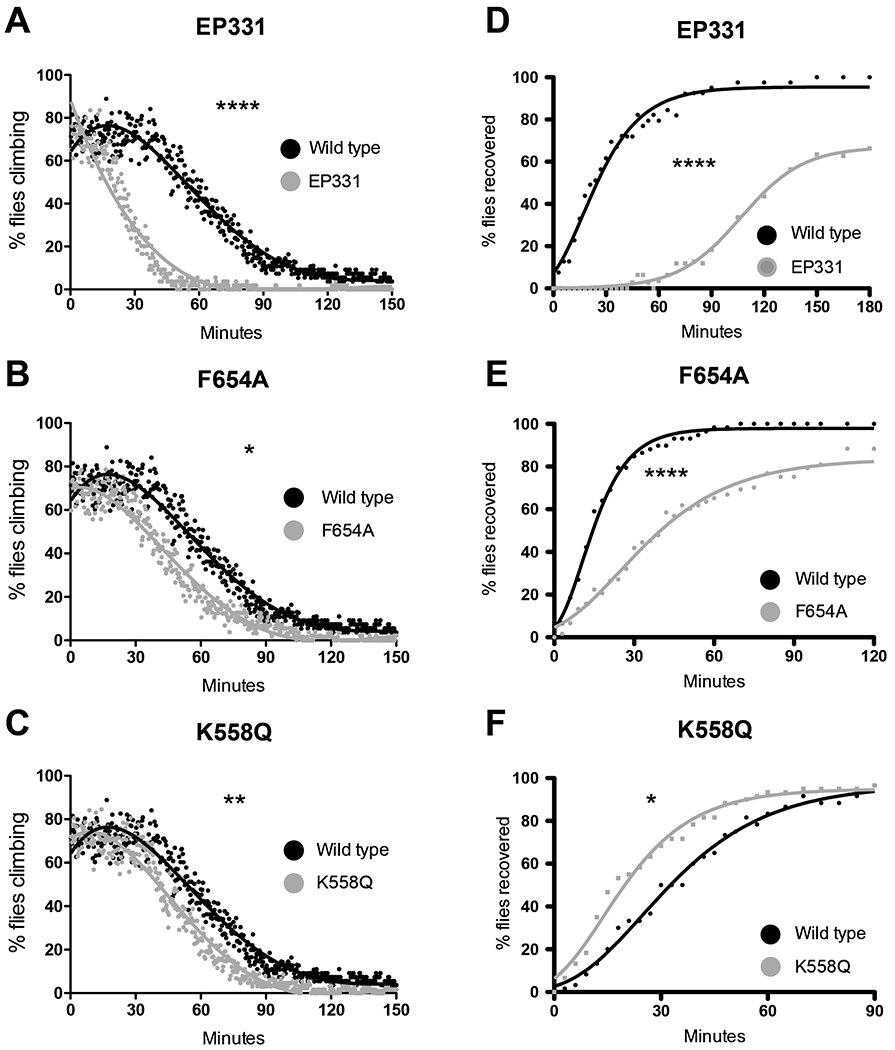
Three alleles of NMDAR1 affect ethanol sensitivity in a sedation assay and in a recovery from sedation assay. To account for day-to-day variation in the rate of sedation, all are compared to the same wild type stock which were tested in parallel with the mutant line. Panels A through C are an ethanol vapor sedation assay. A) Animals homozygous for NMDAR1EP331 are more sensitive than wild type controls (p < 0.0001) in the ethanol sedation assay. B) Animals homozygous for NMDAR1F654A are more sensitive than wild type controls (p < 0.02) in the ethanol sedation assay. C) Animals homozygous for NMDAR1K558Q are more sensitive than wild type controls (p < 0.008) in the ethanol sedation assay, although some independent repeats showed no difference from the wild type (not shown). Panels D through F are a recovery from ethanol sedation assay. Zero minutes denotes the beginning of the recovery period. D) NMDAR1EP331 homozygotes recover from ethanol sedation more slowly (are more sensitive) than wild type controls (p < 0.0001). E) NMDAR1F654A homozygotes recover from ethanol sedation more slowly (are more sensitive) than wild type controls (p < 0.0001). F) NMDAR1K558Q homozygous recover from ethanol sedation more rapidly (are less sensitive) than wild type controls (p < 0.02).
The NMDAR1K558Q mutant allele, however, differentially influenced sensitivity to ethanol sedation and the rate of recovery from ethanol sedation. NMDAR1K558Q homozygotes sedated more quickly than wild-type controls (Fig.4C, p < 0.008). In some repeats of this sedation assay, there was no difference (p > 0.05) between the rate of sedation of the mutant and wild-type animals, suggesting that ethanol sensitivity was influenced by a subtle unknown environmental variable. However, in the recovery-from-sedation assay, the NMDAR1K558Q mutant animals reliably recovered from sedation more quickly than wild-type flies (Fig.4F, p < 0.02). That is, the NMDAR1K558Q mutants were less sensitive to sedation with ethanol.
The ability to acquire tolerance was measured using the recovery-from-sedation assay. None of the NMDAR1 mutant alleles eliminated the capacity to acquire tolerance (Fig.5 A–C). We also performed tolerance tests using the sedation assay and observed the same result—none of the mutant alleles blocked the ability to acquire 24 h tolerance (Fig.5D–F).
Figure 5.

NMDAR1 mutant alleles do not block the acquisition of tolerance. Half of the animals were sedated with ethanol vapor 24h prior to the test, while the other half were mock-sedated. Tolerance is seen as a right-shift in the sedation curve of animals experiencing their second sedation. A through C are a recovery-from-sedation tolerance assays and D through F are sedation-tolerance assays. A) Animals homozygous for NMDAR1EP331 acquire tolerance (p < 0.0007). B) Animals homozygous for NMDAR1F654A acquire tolerance (p < 0.002). C) Animals homozygous for NMDAR1K558Q acquire tolerance (p < 0.0003). D) Animals homozygous for NMDAR1EP331 acquire tolerance (p < 0.04). E) Animals homozygous for NMDAR1F654A acquire tolerance (p < 0.003). F) Animals homozygous for NMDAR1K558Q acquire tolerance (p < 0.002).
To determine if the NMDAR1 mutations alter the preference for ethanol food, we used the feeding-preference method described by Park et al. (2018). Neither the EP331, F654A, or K558Q allele shifts the ethanol-preference profile (Fig.6A). However, all three mutant alleles increase relative total feeding in the preference chamber (Fig.6B).
Figure 6.
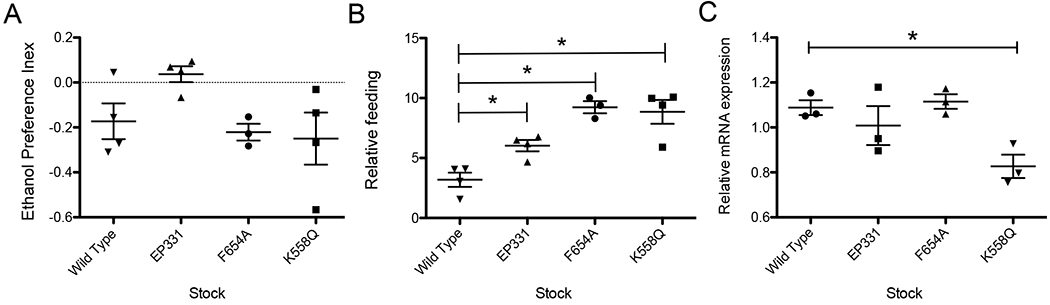
Ethanol preference of males was determined in a chamber in which flies are free to feed on standard solid fly food or on the same food supplemented with 5% ethanol by volume Males are known to show no preference or slight aversion for 5% ethanol. A) None of the alleles produce significant differences from the wild type stock (One-way ANOVA and Dunnett’s Multiple Comparison Test). B) All three of the NMDA mutants significantly increase the level of relative level of feeding when compared to the wild type stock (same data as panel A, One-way ANOVA and Dunnett’s Multiple Comparison Test p < 0.05 level). C) There is no correlation between any of the behaviors tested and the relative level of expression of the NMDAR1 gene in wild type or mutants (Relative mRNA expression is NMDAR1 normalized to cyclophilin mRNA, that is 2−ΔCT NMDAR1/Cyp).
To evaluate coordination and climbing ability, we tested the animals in the RING climbing assay. The F654A allele degraded the capacity to climb. In four seconds, F654A flies reached 27.97 mm ± 1.597, while wild type flies were able to climb 39.31 mm ± 3.857 (Fig.7, p < 0.03). The EP331 and K558Q alleles, however, did not differ from wild type in the rate of climbing (p > 0.5 and p > 0.1, respectively). The staggering-gait phenotype seen in the DBL flies appeared only in flies carrying the F654A substitution.
Figure 7.

RING assay shows that only the NMDAR1F654A allele affects the capacity to climb. Age-matched female flies are knocked to the bottom of a vial, and naturally climb toward the top of the container. This is repeated five times and the height climbed by each fly is measured. The EP331 (Panel A; n.s. p > 0.5) and K558Q (Panel C; n.s. p > 0.1) alleles do not affect climbing (p > 0.05), while the F654A allele (Panel B; p < 0.03) of the NMDAR1 gene reduces climbing ability (p < 0.03).
The ability to maintain behavioral circadian rhythmicity was assessed for each mutant. A previous study reported that knockdown of NMDAR1 in neurons resulted in higher total activity (Tomita et al., 2015). In our assay, EP331 homozygotes were normal with respect to overall activity, period length, and rhythmic index (Fig 8A–D). The NMDAR1F654A flies showed reduced activity (Fig.8A) and almost no circadian rhythmicity (RI of 0.05281 for F654A compared to 0.5085 for wild type, Fig.8B and D, p < 0.0001). Because the NMDAR1F654A mutants are almost completely arrhythmic, interpretation of the period length is not appropriate. NMDAR1K558Q flies showed lower activity (Fig.8A, p < 0.0001) and reduced rhythmic index (Fig.8B and F) but normal period length (Fig.8C and F) compared to wild type.
Figure 8.

Circadian Rhythms are affected by mutations in NMDAR1. A) The rhythmic index of NMDAR1EP331 hypomorphs is normal compared to wild type. Flies homozygous for NMDAR1F654A and NMDAR1K558Q have severely reduced rhythmic index (p < 0.0001 and p < 0.01, respectively). B) The period length is unaffected in NMDAR1EP331 and NMDAR1K558Q animals. The period of NMDAR1F654A is calculated to be longer than wild type, but these flies are arrhythmic and this value is spurious, therefore interpretation of period for this allele is not meaningful. C) NMDAR1EP331 have normal activity levels, but NMDAR1F654A and NMDAR1K558Q are both less active (p < 0.0001). D-F) Actograms showing activity levels of wild type, NMDAR1EP331, NMDAR1F654A, and NMDAR1K558Q over six days in constant darkness. All circadian rhythmicity measures were conducted in constant darkness starting with newly eclosed animals that had been entrained for 3 days in a 12:12 light cycle. The gray and black bars on the x-axis represent the actual day and night cycle during the six days of imposed darkness.
NMDAR1 mutations can affect learning (Chen et al., 2012; Wu et al., 2007; Xia et al., 2005). To assess whether the new alleles affected learning, we used two learning assays. The first was the courtship-suppression learning assay (Ejima and Griffith, 2007; Siegel and Hall, 1979), which takes advantage of the fact that males previously rejected by a mated female reduce their courtship effort when paired with a second, unmated female. Reduced ability to learn or form memories reduces the courtship-suppression response. Courtship effort is quantified as a courtship index: the percentage of time spent courting in a 10 minute window. In this courtship-suppression assay, naïve males are compared to males who had experienced one hour of rejection by a mated female immediately before the test. Wild-type rejected males show a reduced courtship index (Fig.9, p < 0.05). The hypomorphic EP331 allele did not reduce courtship effort after training, indicating that this allele strongly compromised learning (Fig.9, p > 0.6). This is in concordance with reports that NMDAR1EP3511 flies have reduced performance in an olfactory learning test (Xia et al., 2005).
Figure 9.
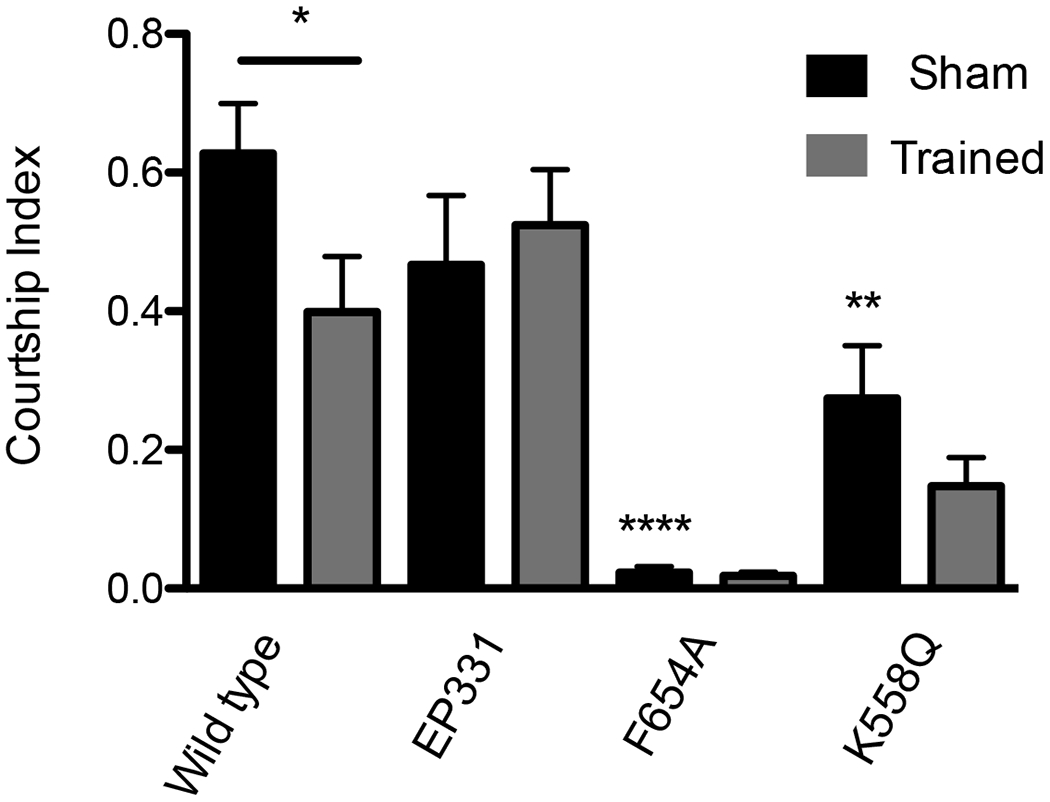
NMDA mutants affect courtship and/or learning in a courtship-suppression assay. Males kept in isolation since eclosion were placed in a courtship chamber in the presence of a freeze-killed virgin female and the courtship index measured. Solid black bars are the courtship index of sexually naive males courting a virgin female. Gray bars are represent the male courtship index of a virgin female after repetitive rejection training by a previously mated, non-receptive female. Male courtship suppression is a learned response. Wild type males reduce courtship after training (p < 0.05). The NMDAR1EP331 males do not learn or remember their rejection training– training does not affect courtship levels (p > 0.6). NMDAR1F654A males court at a very low level (p < 0.0001 compared to WT Sham). There no statistically significant difference between the sham and trained NMDAR1F654A males. NMDAR1K558Q animals also court less than wild type (p < 0.01). For NMDAR1K558Q flies the reduction in courtship after training is not significant (p > 0.1).
No evidence of learning was observed with the NMDAR1F654A males (p > 0.6 trained vs. sham). However, this result is compromised because the NMDAR1F654A males show extremely low courtship in the sham-trained animals (Fig.9, p < 0.0001 vs. wild-type sham). Therefore, with this assay, we cannot say whether NMDAR1F654A males can or cannot learn. However, the reduction of courtship in NMDAR1K558Q mutants was not as severe as that in NMDAR1F654A. The NMDAR1K558Q mutation only reduced the basal courtship index from a wild-type index of 0.63 to 0.27 (Fig.9, p < 0.01). This mutant showed a clear inability to learn in this assay (Fig.9, p > 0.1).
We also used a learned-suppression-of-phototaxis assay (Le Bourg and Buecher, 2002) to test whether the new alleles interfere with the capacity to learn. In this assay, flies choose an illuminated arm (leading to an illuminated vial) or dark arm (leading to a darkened vial). Untrained animals almost always choose the lit arm. However, after only two trials with negative quinine reinforcement (0.1M quinine exposure after the flies enter the lit vial), most wild-type animals choose to enter the darkened arm. Wild-type and mutant lines show indistinguishable baseline phototaxis (Fig.10A). After seven training trials, all animals were tested for recall in the absence of quinine (Fig.10 B–F shaded regions). The wild-type animals (Fig.10B, p < 0.001) and F654A mutant animals (Fig.10C, p < 0.001) show strong learning, and the magnitude of learning between the wild type and the F654A mutant is not distinguishable (Figure 10.E, p = 1.0000). However, the K558Q mutant does not show learning as assayed in the recall phase (Fig.6D, p > 0.2).
Figure 10.
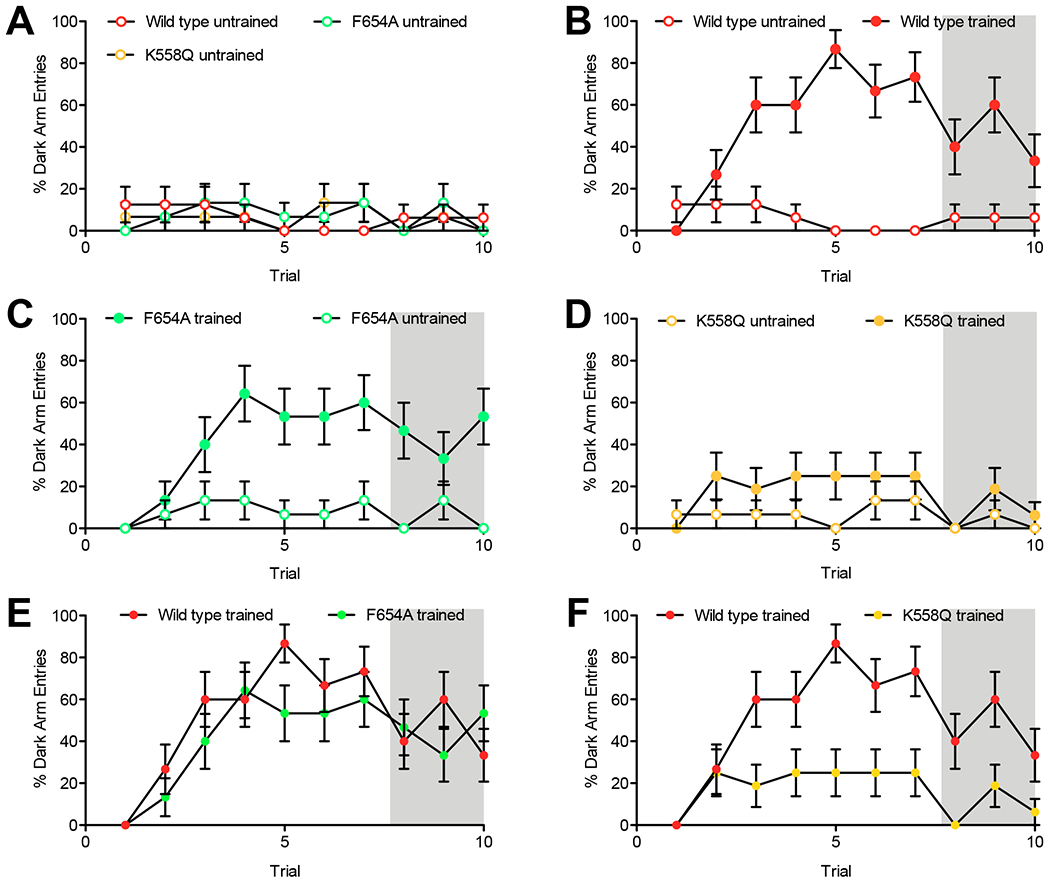
The K558Q mutation but not the F654A mutation interferes with learning in the Learned-Suppression-of-Phototaxis Assay. Males kept in isolation prior to eclosion were entrained to avoid white light illumination that has been repeatedly paired with quinine exposure. Flies are placed in a choice chamber in which they can choose a light or dark arm. The illuminated arm is coated with quinine negative reinforcer for the first 7 trials. Values plotted are percentage of trials that the fly chooses the dark arm. Shaded area shows trials 8-10 in which quinine was not added to the illuminated arm and which reflects the capacity for recall. Trained refers to animals in which the quinine negative reinforcer was paired with light. Untrained refers to animals in which neither illuminated nor dark chamber include quinine. A) When given the choice of an illuminated chamber or a dark chamber untrained wild type, K558Q, or F654A mutants most often choose the illuminated chamber and do not differ from each other (Canton S/F654A p > 0.9, Canton S/K558Q p > 0.6, and F654A /K558Q p > 0.8). B) Wild type Canton S flies rapidly learn to avoid the illuminated arm when it is paired with quinine and recall this training in the absence of the negative reinforcer (trials 8-10). C) F654A mutants also show robust learning and capacity to recall this learning. D) K558Q mutants did not demonstrate learning during the recall phase. For A through F a mixed model ANOVA shows a main effect of genotype p < 0.0001 and a main effect of training trial p < 0.0001. For B through D, N = 15 or 16, Canton S trained versus untrained, p < 0.0003; F654A trained versus untrained, p < 0.0004; and K558Q trained versus untrained, p > 0.2. E) The degree of learning displayed by wild type (Canton S) and F654A were similar. F) Wild type greatly outperformed the K558Q mutant in this assay). For E and F, N = 15 or 16, Canton S paired versus F654A paired, p = 1.0000; and Canton S paired versus K558Q paired p < 0.001.
Discussion
The scientific utility of evolutionary conservation is that observations made in one organism can be used to infer function in another. Most often, the motivation for such demonstrations is to gather evidence that the gene product has a similar function in humans. Functional conservation between distantly related model organisms is a strong indicator that such conservation will extend to humans. Here we demonstrate that the NMDAR1 shows a conserved role in ethanol responsivity. Both flies and mammals have a single NMDAR1 gene, and in mice, the F639A change reduces ethanol sensitivity of the mammalian NMDAR channels, reduces behavioral ethanol sensitivity in some but not all rodent behavioral assays, and alters ethanol drinking in some but not all drinking assays (den Hartog et al., 2013). After a false start in which we inadvertently created a novel double mutant (NMDARDBL), we successfully created F654A, a Drosophila analog of the mouse F639A mutation. The phenotype of the DBL mutation (less sensitive to sedation by ethanol vapor) seemed to indicate that, as in mammals, the F654A change would reduce ethanol sensitivity in flies. However, when NMDAR1F654A was tested, we observed fundamental phenotypic differences between the mammalian and Drosophila versions of the mutation (NMDAR1F639A in mice and NMDAR1F654A in Drosophila). These cover all of the salient behavioral attributes of NMDAR1F639A mutant mice—recessive lethality of the allele (by P21), reduced ethanol sensitivity in some behavioral paradigms, and reduced ethanol preference (den Hartog et al., 2013). In contrast, in flies, the F654A allele is homozygous viable with no obvious health effect, causes increased ethanol sensitivity in both a sedation and a recovery assay, and does not alter ethanol preference in a feeding assay.
The Drosophila K558 position in the NMDAR1 subunit is orthologous to K544 in the human NMDAR1 subunit. Wafford et al. (1995) described the K544Q allele as producing a five-fold reduction in the affinity of the receptor for glycine in a Xenopus expression system. Regalado et al. (2001), in turn, showed that K544Q alters intersubunit cooperativity between the NMDAR1 glycine-binding subunit and the glutamate-binding NMDAR2 subunit. More recently, a crystal structure showed that the position of the K544 residue lies near the junction of the ligand-binding domain and the transmembrane domain of the NMDAR1 subunit and is almost coincident with the proposed extracellular-vestibule Ca2+-binding site (Figure 2 and Karakas and Furukawa, 2014). The change from a positively charged to a negatively charged amino acid residue near the mouth of the pore should have a substantial effect on the ion flux through the NMDAR. An interesting tangential observation is that the K558Q mutation appears to be dominant when in cis with the F564A allele in that the behavioral effects of the NMDA1DBL allele most closely resembles the NMDA1K558Q allele. Both result in reduced ethanol sensitivity in a recovery from sedation assay and result in similar circadian actograms and both differ strongly from the effects of F654A in these measures—in some manner K558Q suppresses the effects of F654A.
In flies it was the K558Q allele that reliably reduced ethanol sensitivity in a recovery-from-sedation assay. Albeit in a rate-of-sedation assay, this allele produced a partially penetrant increase in ethanol sensitivity. This difference between the sensitivity profile in the assays might be a product of the ethanol dose—higher in the recovery assay but lower in the sedation assay. Dose-dependent effects on ethanol sensitivity were observed for the mouse F639A mutation—in a rotarod test, mutant mice did not differ from wild type at 2.0 mg/kg of ethanol but showed decreased sensitivity at 2.5 mg/kg ethanol (den Hartog et al., 2013). While we observed reduced NMDAR1 gene expression in the K558Q line (Fig.6c), it appears unlikely that the difference between sensitivity in the sedation assay and in the recovery assay is merely a consequence of decreased expression of the K558Q allele, because while EP331 is hypomorphic, it reliably shows increased ethanol sensitivity in both assays. Instead, in flies, in place of F654A generating reducing ethanol sensitivity, it appears that it is the K558Q mutation that reduces ethanol sensitivity, perhaps because of the effects of K558Q on glycine modulation of the channel (described only for mammalian channels Wafford et al., 1995; Regalado et al., 2001; Karakas and Furukawa, 2014).
Even though the EP331, F654A, and K55Q mutations affect ethanol sensitivity, none of them prevent the acquisition of tolerance. Although counterintuitive, it is becoming abundantly clear that reduced innate ethanol sensitivity and inducible ethanol tolerance (ethanol-induced reduction in ethanol sensitivity), two behaviors that seem so similar, have distinct molecular origins (Berger et al., 2008; Kong et al., 2010; van der Linde and Lyons, 2011; Pohl et al., 2013; Morozova et al., 2015; Troutwine et al., 2016). From the observation that NMDAR1 mutants do not suppress tolerance, we draw the conclusion that, in flies, ethanol-modulated changes in NMDAR activity are unlikely to be an initiating factor in the production of ethanol tolerance. Furthermore, neither the EP331, F654A, or K558Q mutations altered the preference of males for ethanol food. However, we did observe that all three NMDAR1 mutants substantially increase the overall interest in food.
We also examined a number of other behaviors not directly related to the response to alcohol. We saw that NMDAR1F654A homozygotes perform poorly in walking, climbing, circadian rhythm, and male courtship assays. Despite the unusual gait of NMDAR1F654A animals, we do not think that poor performance in all of these categories is simply a consequence of reduced locomotor activity, because NMDAR1K558Q also reduces overall activity (Fig.8f) but these animals have near normal climbing ability, rhythmicity index, and a greatly superior capacity for male courtship. Furthermore, the observation that the previously described hypomorphic EP331 mutation does not affect circadian rhythmicity or courtship capacity suggests that the F654A and K558Q alleles do not compromise these behaviors merely by reducing NMDAR activity but instead do so by altering NMDA activity in another manner.
Because NMDARs play an important role in learning in both flies and mammals, we expected that the new NMDAR1 alleles would compromise learning. We were therefore surprised that F654A, which had major effects on locomotion, circadian rhythm, and male courtship, did not reduce associative learning in the light-aversion-learning assay (learning by F654A could not be evaluated in the courtship-suppression assay because the mutation had a major effect on baseline courtship). However, the K558Q mutation, which in other assays seemed to have less severe effects, suppressed associative learning in the courtship-suppression assay and in the light-aversion-learning assay. The only mutation that affects the expression level from the NMDAR1 gene was K558Q, which generates a drop in the mean normalized expression (2−ΔCT for WT is 1.1, for K558Q is 0.83; Fig.6C)—albeit the EP331 allele is thought to be a functional hypomorphic allele (Miyashita et al., 2012; Xia et al., 2005). Therefore, there does not appear to be a simple correlation of behavioral phenotype with level of NMDAR expression.
To determine whether any of the mutations produced channels with altered ethanol sensitivity, we attempted to express the channel in Xenopus oocytes as described by Xia et al. (2005). Unfortunately, despite repeated attempts, we were unable to express functional channels from the wild-type NMDAR1 mRNA by itself or in combination with the NMDAR2 subunit. In the absence of direct testing of the effect of ethanol on the mutant channels we recognize that the reduced behavioral sensitivity to ethanol caused by K558Q could result from changed brain expression or localization of the channel. Evaluating all such secondary effects would be a substantial and perhaps open-ended undertaking. Instead, it is hoped that this work will stimulate evaluation of this residue (K544Q in mammals) for an effect on the sensitivity of the channel to ethanol. This residue has not been previously studied for its effect on ethanol responses.
Although we were unable to determine if the K->Q mutation directly affects ethanol sensitivity of the channel, this work highlights fly-to-mammal similarities and differences with regards to the interaction between ethanol and the NMDAR1 receptor. As in mammals, in flies a single amino acid change in the NMDAR1 receptor can also reduce the behavioral sensitivity of animals to ethanol. However, unlike mammals, it is not a mutation affecting the transmembrane domain (F639A mammals, F654A flies) but a mutation affecting the ligand-binding domain (K544Q mammal, K558Q flies) that produces this effect (Fig.2). Differences in wiring or abundance of glutamatergic synapses between these animals could help account for the differences in the effects of mutations on ethanol sensitivity in flies and mammals. Thus, while the absolute phenotype produced by each amino acid substitution in the NMDAR channel may not be conserved over evolutionary time, the role of the NMDAR channel in modulating ethanol sensitivity is clearly conserved.
Methods
Behavioral experiments were performed on 5-7 day old age-matched flies unless otherwise stated. Mutant alleles were backcrossed into our wild type Canton S stock six times, using allele-specific PCR to track the mutations, yielding flies approximately 98% identical to the wild type.
Generation of NMDAR1DBL by homologous recombination
We attempted to generate the F654A mutation by ends-out homologous recombination (Gong and Golic, 2003). Four kb homology arms were cloned into pW25 and mutated by PCR. This construct was injected into w1118 flies and founders screened for white expression. Genomic sequencing showed that the knock-in mutant had two mutations F654A and K558Q. The allele is referred to as NMDAR1DBL.
Generation of NMDAR1 F654A and K558Q mutations using the CRISPR/Cas9 system
These mutations were created as described in Port et al. (2014) and detailed at www.crisprflydesign.org. Target sites were chosen using the DRSC CRISPR tool version 2 (www.flyrnai.org/crispr2). DNA oligomers were from Integrated DNA Technologies (Coralville, IA; http://idtdna.com). Briefly, plasmid encoding the gRNA and single stranded donor DNA carrying the desired mutations were co-injected into vas-Cas9-expressing embryos (BSC# 52669) by Rainbow Transgenic Flies (Camarillo, California).
For the F654A mutation, a target with a cut site eight base pairs from the mutation site was identified. The sequences of the guide RNA targeting oligos were sense 5’GTCGTGCTGGGCATGGTCTGGGC3’, and antisense 5’AAACGCCCAGACCATGCCCAGCA3’. These have overhangs that base pair with the digested plasmid. Oligos were annealed and ligated (New England Biolabs, Ipswich, MA) into BbsI-cut pCFD3 plasmid (Addgene #49410). Donor DNA was an 120 nucleotide single-stranded DNA oligomer. To prevent the gRNA from recognizing the donor, it contained a silent mutation that had a codon usage bias closest to the wild-type codon, to prevent re-cutting after insertion. The sequence of the donor was: 5’GCTCCAGCACGAGGAAGGCGGCCAGGTTGGCGGTGTACGAGGCAACGATGATCATAGCAgcTCCGGCCCAaACCATGCCCAGCACCCGGGCGGAGAAGCTCCGGGGCGTGCCCTCGCCGA3’. The lower case nucleotides are residues mutated to avoid recutting.
The protocol for generating the K558Q mutation used different oligomers but was otherwise essentially identical to that above. The guide RNA oligos were : sense 5’GTCGACGAGAGTACTCGATCGTGA3’ and antisense 5’AAACTCACGATCGAGTACTCTCGT3’. Single-stranded donor was: 5’GCCGAGTACATTGAATTCTCGAAGCCATTCAAATACCAAGGCATTACAATACTCGAGAAGcAACCGTCtCGATCcAGTACTCTCGTGTCTTTCTTGCAGCCGTTTAGTAACACGCTATGGA3’. The underlined lower case nucleotides are residues mutated to avoid recutting.
The mutations were screened for by allele-specific PCR. The primer pair for F654A was 5’ATGGTtTGGGCCGGAgc3’ and 5’ACATGCGATGCTACGCTCATTTA3’. The primer pair for K558Q was 5’GTACTgGATCGaGACGGTTg3’ and 5’GATCAACGACAGCGAGATCAT3’. Mutant alleles were confirmed in the F2 lines by sequencing a genomic PCR product directly surrounding the mutation and then re-verified by sequencing of cDNA from the mutants.
Recovery from Ethanol Sedation Assay and Ethanol Tolerance Assay
Experiments were performed as described in Krishnan et al., (2012) and Cowmeadow et al., (2005). Groups of ten 5-7 day old females were exposed to a stream of ethanol vapor until all flies were sedated (15-18 minutes). Flies were allowed to recover in a humidified air stream and the recovery time at which they regain postural control recorded. n = 4-6 vials for each group. All experiments were performed between 11:00 and 16:00 (zeitgeber time 3-8). Statistical significance between wild type and mutants was determined using the log rank test in GraphPad Prism 6 (La Jolla, CA, USA).
For the sedation tolerance assay, on day one, half of the flies were treated in the ethanol air stream until sedated, while the control group received a mock treatment. Sedated animals recovered in a humidified air stream until they regained postural control, and then both groups were allowed to recover overnight in food vials. Twenty-four hours later both groups received the same ethanol treatment and recovery from sedation was monitored. Statistical significance determined as described for the Recovery from Ethanol Sedation Assay above. The recovery-from-sedation tolerance assay, was conducted in a similar manner with the following changes. On day 1, the experimental group is exposed for 1 hour to vapor from 1 ml of a 75% EtOH solution that was pipetted onto a Flug in the bottom of the vial. A second group is mock exposed at the same time (day 1) using a Flug containing 1 ml of water (0% EtOH control). Both groups are returned to fresh fly food for 24 hours. On day 2, both the groups are exposed to vapor from a Flug that contains 1 ml of 35% EtOH. A camera takes pictures every 20 seconds during the gradual sedation that occurs during the day 2 exposure.
Ethanol Sedation Assay
The rate of ethanol sedation and the magnitude of ethanol tolerance was measured essentially as described in Pohl et al., (2013). Briefly, groups of ten females were placed in plastic tubes with a platform made from a Kim Wipe and a plastic ring about halfway up the tube. One ml of 35% ethanol/water solution was placed on one third of a cotton Flug (Genesee Scientific, San Diego, CA) at the bottom of the vial and flies were exposed to the vapor from an evaporating ethanol solution.
Ethanol Preference Assay
Ethanol preference was measured as described by Park et al., (2018) using the photographic observational method. Briefly, 50 flies were housed in a rectangular chamber with a food tray that provided 24 food cells (6 x 4 grid). Each food cell was 1.5 cm x 1.5 cm x 0.5 cm deep and was filled with molten standard cornmeal/molasses/agar media. Alternating food cells were supplemented with 5% ethanol (concentration stable for at least two days). A digital computer-controlled camera at the top of the chamber provides a photographic record of the behavior. Preference was determined by recording the number of flies on each food square every five minutes over two days. These values were used to generate cumulative averages over the entire period. Ethanol preference was calculated as follows: .
Circadian Rhythm Assay
Flies were raised in a 12:12 light:dark cycle for 3 days. Single males were loaded, without anesthesia, into 5 mm × 65 mm glass tubes with food at one end (5% sucrose 2% agar) and locomotor activity recorded using the DAM2 Drosophila Activity Monitor system (Trikinetics, Waltham, MA). Monitors were placed into a sound-isolated incubator at 24°C, and free-running rhythms measured in constant darkness in 5 minute bins for 14 days. Flies that did not move for any 24 hour period in the first ten days were excluded. Period and rhythm indices were generated by autocorrelation as described (Levine et al., 2002). Rhythmic animals were those with a rhythm index greater than 0.1 (Ng et al., 2011). Average daily activity was determined by summing and then averaging the number of DAM2 beam passes over 7 days.
Rapid iterative negative geotaxis (RING) Climbing Assay
In the rapid iterative negative geotaxis (RING) assay (Gargano et al., 2005) six mutant and six wild type vials of age-matched females (15 flies/vial) were interdigitated and secured in a custom-built rack on a vertical track. Flies were allowed to acclimate for one minute and then were dropped three times in rapid succession. The flies rapidly up the vial after being knocked down, and this was video recorded for analysis. Flies were given 30 seconds to recover between iterations, and the entire process was repeated five times. Four seconds after knock down the average height climbed was measured using ImageJ (https://imagej.nih.gov). Iterations were averaged to determine a single value for each vial. A Student’s t-test was used to determine statistical significance in Prism 6 (Graphpad, San Diego, CA).
Courtship Suppression Assay
The courtship suppression assay was performed as described by Siegel and Hall (1979), and Mehren et al., (2004). Males were collected within four hours of eclosion and stored individually in a two ml centrifuge tube with breathing holes on standard food for four days. Wild type virgin females were collected and stored in groups of 30 on standard food. Wild type age-matched males were also collected and stored in groups of 30 for mating to trainer females. Twenty four hours prior to the test, groups of 12 virgins were paired with 18 males and left to mate. These mated females were used as trainers.
On the day of the test, half of the virgin males were paired with one mated female for one hour in their individual 2ml tube. Sham males were left in isolation in their 2ml tube during this period. A virgin female that had been killed by freezing for 10 minutes was then placed in a circular observation chamber (14mm diameter x 5mm height), and then a single male was loaded. The behavior of the male was filmed, and courtship was manually scored. Ten minutes of video was examined for each male, and the courtship index was calculated as the percentage of time spent courting.
Learned-Suppression-of-Phototaxis Assay
Five day old singly housed males were tested for associative learning in a light dark choice chamber as described by Le Bourg and Buecher (2002) and Seugnet et al. (2009). Individual pupae were transferred into vials containing fly food and allowed to eclose. Flies were sexed after eclosion and aged for 5 days in isolation.
The learning chamber was built using 3 pieces of plexiglass. The middle piece has a 3 cm by 5 cm T shaped corridor maze cut into it. The top piece has holes to accommodate two fly vials on the ends of the T-maze. In this assay, flies walk down a corridor and at the T-junction choose to walk toward the illuminated or the darkened vial. Illumination was provided by a gooseneck light source, set at the lowest luminance, shining through a paper cowl set about 1 cm from the vial. The darkened vial was encased in black cardboard and the assay was performed in a dark room under dim red light illumination. Each fly vial also contained a piece of rolled filter paper (Fisher Brand Filter Paper P5). During training (first seven trials), the illuminated vial was paired with the negative reinforcer (~1.3 ml of 0.1M quinine monohydrochloride dihydrate, Sigma-Aldrich part number 145920, added to the paper in the vial). New vials are used for each trial and the orientation of the illuminated and dark arms is randomized between trials. All flies were tested between the hours of 10 AM - 3 PM. During the assay, individual males were allowed to choose between illuminated and dark arm, captured and serially retrained for 7 iterations. In each iteration, the light arm is paired with 0.1M quinine. When flies entered either of the arms during the training phase the experimenter would wait for 1 minute then take the fly out of the vial, replace the vials, then start the fly at the beginning of the maze. Iterations 8-10 do not include quinine punishment and are a recall test. During the recall phase flies were taken immediately out of the vials once they made a decision.
When evaluating the capacity of animals to recall their training we used a generalized-linear-mixed-effects model as implemented by lme4:glmer and afex in R (Bates et al., 2015; Singmann et al., 2019) in order to account for variability from two sources—across-animal variability (N =15 or 16 animals) and the within-animal variability (between repeated trials for each animal).
Xenopus oocyte electrophysiology
Drosophila NR1 and NR2 NMDAR subunit cDNAs, subcloned into the pBK-CMV vector, were used in this study. Xenopus laevis oocytes were harvested, isolated and injected with cDNAs as described in Pflanz et al. (2018). NR1 and NR2 cDNAs were combined in a 1:1 ratio and injected into oocytes at a final concentration of 1.5 ng/30 nl. Two electrode voltage-clamp electrophysiological recordings were performed on oocytes 3-5 days post-injection. Individual oocytes were placed in a 100 μL bath and perfused with a buffer consisting of 10 mM HEPES (pH 7.4), 115 mM NaCl, 2.5 mM KCl and 2 mM BaCl2, at a rate of 2 ml/min. The animal poles of oocytes were impaled with two 3M KCl-filled borosilicate glass electrodes, each with a resistance between 1 and 10MΩ. Oocytes were voltage clamped at −70mV using an OC-725 oocyte clamp (Warner Instruments, CT) and data was collected at a rate of 1 kHz using a PowerLab ML866 digitizer and LabChart software (ADInstruments, Australia). NMDAR expression was assayed using a solution of 10 mM glutamate + 10 mM glycine made up in the perfusion buffer and applied for 30 seconds.
Acknowledgements
This work was supported by NIH grant number 1F31AA021326-01 to BRT. and NIH grant number 2R01AA018037-06A1 to NSA. We thank Jane Kirschman for copy editing and other feedback on the manuscript.
References
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67:1–48. [Google Scholar]
- Berger KH, Kong EC, Dubnau J, Tully T, Moore MS, Heberlein U (2008) Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin Exp Res 32:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA (2002) Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci 22:9305–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Wu JK, Lin HW, Pai TP, Fu TF, Wu CL, Tully T, Chiang AS (2012) Visualizing long-term memory formation in two neurons of the Drosophila brain. Science 335:678–685. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Atkinson NS (2005) The slowpoke gene underlies rapid ethanol tolerance in Drosophila. Alcohol Clin Exp Res 29:1777–1786. [DOI] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP (2014) Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol 118:315–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN (2004) Role of distinct NMDA receptor subtypes at central synapses. Sci STKE 2004:re16. [DOI] [PubMed] [Google Scholar]
- den Hartog CR, Beckley JT, Smothers TC, Lench DH, Holseberg ZL, Fedarovich H, Gilstrap MJ, Homanics GE, Woodward JJ (2013) Alterations in ethanol-induced behaviors and consumption in knock-in mice expressing ethanol-resistant NMDA receptors. PLoS One 8:e80541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Zwarts L, Yamamoto A, Callaerts P, Mackay TF (2009) Mutations in many genes affect aggressive behavior in Drosophila melanogaster. BMC Biol 7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima A, Griffith LC (2007) Measurement of Courtship Behavior in Drosophila melanogaster. CSH Protoc 2007:pdb.prot4847. [DOI] [PubMed] [Google Scholar]
- French RL, Heberlein U (2009) Glycogen synthase kinase-3/Shaggy mediates ethanol-induced excitotoxic cell death of Drosophila olfactory neurons. Proc Natl Acad Sci U S A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargano JW, Martin I, Bhandari P, Grotewiel MS (2005) Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol 40:386–395. [DOI] [PubMed] [Google Scholar]
- Gong WJ, Golic KG (2003) Ends-out, or replacement, gene targeting in Drosophila. Proc Natl Acad Sci U S A 100:2556–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E, Furukawa H (2014) Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 344:992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong EC, Allouche L, Chapot PA, Vranizan K, Moore MS, Heberlein U, Wolf FW (2010) Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcohol Clin Exp Res 34:302–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HR, Al-Hasan YM, Pohl JB, Ghezzi A, Atkinson NS (2012) A role for dynamin in triggering ethanol tolerance. Alcohol Clin Exp Res 36:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin A, Karak S, Priya R, Das A, Ayyub C, Ito K, Rodrigues V, Ramaswami M (2010) Central synaptic mechanisms underlie short-term olfactory habituation in Drosophila larvae. Learn Mem 17:645–653. [DOI] [PubMed] [Google Scholar]
- Le Bourg E, Buecher C (2002) Learned suppression of photopositive tendencies in Drosophila melanogaster. Anim Learn Behav 30:330–341. [DOI] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC (2002) Signal analysis of behavioral and molecular cycles. BMC Neurosci 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiya R, Lee S, Berger KH, Kong EC, Slawson JB, Griffith LC, Takamiya K, Huganir RL, Margolis B, Heberlein U (2012) DlgS97/SAP97, a Neuronal Isoform of Discs Large, Regulates Ethanol Tolerance. PLoS One 7:e48967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehren JE, Ejima A, Griffith LC (2004) Unconventional sex: fresh approaches to courtship learning. Curr Opin Neurobiol 14:745–750. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Oda Y, Horiuchi J, Yin JC, Morimoto T, Saitoe M (2012) Mg(2+) block of Drosophila NMDA receptors is required for long-term memory formation and CREB-dependent gene expression. Neuron 74:887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova TV, Huang W, Pray VA, Whitham T, Anholt RR, Mackay TF (2015) Polymorphisms in early neurodevelopmental genes affect natural variation in alcohol sensitivity in adult Drosophila. BMC Genomics 16:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng FS, Tangredi MM, Jackson FR (2011) Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol 21:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, Tran T, Atkinson NS (2018) Monitoring food preference in Drosophila by oligonucleotide tagging. Proc Natl Acad Sci U S A 115:9020–9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanz NC, Daszkowski AW, Cornelison GL, Trudell JR, Mihic SJ (2018) An intersubunit electrostatic interaction in the GABAA receptor facilitates its responses to benzodiazepines. J Biol Chem 293:8264–8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl JB, Ghezzi A, Lew L, Robles RB, Cormack L, Atkinson NS (2013) Circadian genes differentially affect tolerance to ethanol in Drosophila. Alcohol Clin Exp Res 37:1862–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F, Chen HM, Lee T, Bullock SL (2014) Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc Natl Acad Sci U S A 111:E2967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regalado MP, Villarroel A, Lerma J (2001) Intersubunit cooperativity in the NMDA receptor. Neuron 32:1085–1096. [DOI] [PubMed] [Google Scholar]
- Ronald KM, Mirshahi T, Woodward JJ (2001) Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site-directed mutagenesis of a transmembrane domain phenylalanine residue. J Biol Chem 276:44729–44735. [DOI] [PubMed] [Google Scholar]
- Sambandan D, Yamamoto A, Fanara JJ, Mackay TF, Anholt RR (2006) Dynamic genetic interactions determine odor-guided behavior in Drosophila melanogaster. Genetics 174:1349–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Suzuki Y, Stidd R, Shaw PJ (2009) Aversive phototaxic suppression: evaluation of a short-term memory assay in Drosophila melanogaster. Genes Brain Behav 8:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RW, Hall JC (1979) Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci U S A 76:3430–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singmann H, Bolker B, Westfall J, Aust F (2019) afex: Analysis of Factorial Experiments. R package version 0.23-0. [Google Scholar]
- Smothers CT, Clayton R, Blevins T, Woodward JJ (2001) Ethanol sensitivity of recombinant human N-methyl-D-aspartate receptors. Neurochem Int 38:333–340. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ (2006) Effects of amino acid substitutions in transmembrane domains of the NR1 subunit on the ethanol inhibition of recombinant N-methyl-D-aspartate receptors. Alcohol Clin Exp Res 30:523–530. [DOI] [PubMed] [Google Scholar]
- Tomita J, Ueno T, Mitsuyoshi M, Kume S, Kume K (2015) The NMDA Receptor Promotes Sleep in the Fruit Fly, Drosophila melanogaster. PLoS One 10:e0128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutwine BR, Ghezzi A, Pietrzykowski AZ, Atkinson NS (2016) Alcohol resistance in Drosophila is modulated by the Toll innate immune pathway. Genes Brain Behav 15:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi A, Caldwell JC, Tracey WD (2012) Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr Biol 22:2124–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linde K, Lyons LC (2011) Circadian modulation of acute alcohol sensitivity but not acute tolerance in Drosophila. Chronobiol Int 28:397–406. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Kathoria M, Bain CJ, Marshall G, Le Bourdellès B, Kemp JA, Whiting PJ (1995) Identification of amino acids in the N-methyl-D-aspartate receptor NR1 subunit that contribute to the glycine binding site. Mol Pharmacol 47:374–380. [PubMed] [Google Scholar]
- Wu CL, Xia S, Fu TF, Wang H, Chen YH, Leong D, Chiang AS, Tully T (2007) Specific requirement of NMDA receptors for long-term memory consolidation in Drosophila ellipsoid body. Nat Neurosci 10:1578–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Miyashita T, Fu TF, Lin WY, Wu CL, Pyzocha L, Lin IR, Saitoe M, Tully T, Chiang AS (2005) NMDA receptors mediate olfactory learning and memory in Drosophila. Curr Biol 15:603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Lei Z, Li H, Yi W, Zhang Z, Guo A (2010) NMDA receptors-dependent plasticity in the phototaxis preference behavior induced by visual deprivation in young and adult flies. Genes Brain Behav 9:325–334. [DOI] [PubMed] [Google Scholar]


