Abstract
Background
Women are nearly twice as likely as men to suffer from major depressive disorder. Yet, there is a dearth of studies comparing the clinical outcomes of women and men with treatment-resistant depression (TRD) treated with similar augmentation strategies. We aimed to evaluate the effects of the augmentation strategies in women and men at the McGill University Health Center.
Methods
We reviewed health records of 76 patients (42 women, 34 men) with TRD, treated with augmentation strategies including antidepressants (AD) with mood stabilizers (AD+MS), antipsychotics (AD+AP), or in combination (AD+AP+MS). Clinical outcomes were determined by comparing changes on the 17-item Hamilton Depression Rating Scale (HAMD-17), Montgomery-Åsberg Depression Rating Scale (MADRS), Quick Inventory of Depressive Symptomatology (QIDS-C16), and Clinical Global Impression rating scale (CGI-S) at the beginning and after 3 months of an unchanged treatment. Changes in individual items of the HAMD-17 were also compared between the groups.
Results
Women and men improved from beginning to 3 months on all scales (P < .001, η p2 ≥ 0.68). There was also a significant sex × time interaction for all scales (P < .05, η p2 ≥ 0.06), reflecting a greater improvement in women compared with men. Specifically, women exhibited greater improvement in early (P = .03, η p2 = 0.08) and middle-of-the-night insomnia (P = .01, η p2 = 0.09) as well as psychomotor retardation (P < .001 η p2 = 0.16) and psychic (P = .02, η p2 = 0.07) and somatic anxiety (P = .01, η p2 = 0.10).
Conclusions
The combination of AD+AP/MS generates a significantly greater clinical response in women compared with men with TRD, supporting the existence of distinct pharmacological profiles between sexes in our sample. Moreover, they emphasize the benefit of augmentation strategies in women, underscoring the benefit of addressing symptoms such as insomnia and anxiety with AP and MS.
Keywords: Antidepressants, antipsychotics, mood stabilizers, major depressive disorder, treatment-resistant depression
Significance Statement.
Women are nearly twice as likely as men to suffer from major depressive disorder. Yet, there is a dearth of studies comparing the clinical outcomes of women and men with treatment-resistant depression treated with similar medication. We compared the improvement of women and men treated with similar combinations of medication (antidepressants with mood stabilizers and/or antipsychotics) at the McGill University Health Center. We found that the depressive symptoms of women and men improved significantly over 3 months. We also found that women improved more than men this period. Specifically, the use of mood stabilizers and/or antipsychotics in women improved insomnia, anxiety, and psychomotor retardation more than in men. Our results support the existence of distinct pharmacological profiles between sexes. Moreover, they emphasize the benefit of augmentation strategies in women, underscoring the benefit of addressing symptoms such as insomnia and anxiety with antipsychotics and mood stabilizers.
Introduction
Women are nearly twice as likely as men to suffer from major depressive disorder (MDD), and this sex difference is among the most robust of findings in psychopathology research (Weissman et al. 1996; Kessler et al. 2003; Wilhelm et al. 2008; Parker and Brotchie 2010; Salk et al. 2017). Despite the greater prevalence of depression among women, only a few studies have investigated the issue of sex differences and psychopharmacological response in MDD, particularly treatment-resistant depression (TRD) (LeGates et al. 2019; Rubinow and Schmidt 2019; Bartova et al. 2021).
Antidepressants (AD) are the first-line treatment for MDD (NICE 2010; Bauer et al. 2015; Cleare et al. 2015; Kennedy et al. 2016), yet more than 30% of patients show an inadequate response to initial pharmacological treatments (Rush et al. 2006; Berlim and Turecki 2007). International guidelines and clinical studies suggest that MDD non-responding to 2 adequate trials with AD, also called TRD, should be treated with a combination of different classes of AD, or augmentation strategies with antipsychotics (AP) and/or lithium and valproic acid (mood stabilizers [MS]) as well as other treatment modalities (including brain stimulation techniques) (Lam et al. 2009; Ghabrash et al. 2016; Kennedy et al. 2016; Gobbi et al. 2018).
Early evidence showed sex differences in the clinical outcome of augmentation strategies in MDD. For instance, T3 (L-triiodothyronine) was observed to be more effective in the augmentation of AD treatment in women than in men (Altshuler et al. 2001). Additional work in current and novel augmentation strategies may be useful in identifying personalized approaches to optimize treatment in both women and men (LeGates et al. 2019). To the best of our knowledge, clinical response rates to AD and a combination of augmentation strategies with either AP or MS has not been explored comprehensively between women and men. The present naturalistic study conducted at the specialized mood disorder clinic of McGill University primarily aimed to evaluate the use of pharmacological combinations of AD+AP, AD+MS, and AD+AP+MS in male compared with female TRD patients. The secondary objective is to investigate possible differences in sociodemographic, clinical, and treatment patterns between male and female TRD patients.
METHODS
This retrospective study was approved by the Institutional Review Board of McGill University (IRB no. 2020-6323) and was conducted from 2015 to 2020 in accordance with the Declaration of Helsinki and ICH Good Clinical Practice. Data were retrieved from a research database containing information systematically collected on patients followed at the Mood Disorders Clinic of the McGill University Health Center for ≥2 years (mean, 7.5 years). Written informed consent was not required because data were obtained by chart review. Diagnoses of MDD and comorbidities were confirmed by the Structured Clinical Interview for DSM-IV as well as thorough clinical interviews by experienced mood disorder specialists and research coordinators. Patients with a mixed episode or with a neurological/developmental disorder and/or a mood disorder secondary to a medical condition were excluded. The Maudsley Staging Method was used to establish the severity of the TRD patients (Fekadu et al. 2009). Some of the patients had been included in previous studies (Ghabrash et al. 2016; Nuñez et al. 2018).
Patients
Charts of 206 patients meeting DSM-IV criteria for a major depressive episode for ≥2 months were reviewed (American Psychiatric Association, 2000). A total 76 patients met the criteria for TRD by failing ≥2 pharmacological trials with different AD in mono or combination therapy at an adequate dose and for ≥3 weeks (Lam et al. 2009). All patients had at least a mild to severe major depressive episode, suggested by a score of ≥13 on the Hamilton-Rating Scale for Depression (HAMD-17) and a score of ≥20 on the Montgomery–Åsberg Depression Rating Scale (MADRS) based on cut-off values proposed by Zimmerman et al. (2013). Patients were treated with augmentation strategies, including ADs with MS (AD + MS), AP (AD + AP), or both (AD + AP + MS).
Clinical Evaluation
Chart analysis was performed by 2 authors (N.A.N. and G.G.) and evaluated at baseline, before the beginning (T0), and after at least 3 months of an unchanged pharmacological treatment (T3). At T0 and T3, patients were assessed on the following behavioral scales: HAMD-17 (Hamilton 1986), MADRS (Montgomery and Åsberg 1979), the Quick Inventory of Depressive Symptomatology (Rush et al. 2003) (QIDS-C16), and the Clinical Global Impression-Severity of Illness (Rush et al. 2003) (CGI-S). The response was defined as a ≥50% reduction from the pre-treatment in the HAMD-17 score. Remission was defined as a score <7 of the HAMD-17 at T3.
Reliability and Inter-Rater Agreement for Psychometric Scales
The internal consistency was previously assessed utilizing Cronbach’s alpha, and an acceptable reliability was found for all scales (HAMD-17: α = 0.82; QIDS-C16: α = 0.77) (Nuñez et al. 2018). Inter-rater reliability was previously assessed using Cohen’s kappa (Cohen 1968)27 on a sample of 140 patients (Nuñez et al. 2018) with a moderate to good agreement for all behavioral scales (HAMD-17: κ = 0.58; QIDS-C16: κ = 0.61; CGI-S: κ = 0.72).
Statistical Analyses
Group comparisons on patients’ demographics were computed through the Pearson’s chi-square (χ²) test or by Fisher’s exact test (if n ≤5 in each subgroup). Changes in scales were analyzed using repeated-measures ANOVA with sex as between-subject factor and time as a within-subject factor, followed by Tukey post-hoc analyses. Effect sizes are reported for t tests (Cohen’s d) and ANOVA (partial eta-squared, η p2). Small, medium, and large effect sizes were respectively 0.2, 0.5, and 0.8 for “d”, and 0.01, 0.06, and 0.14 for η p2 (Cohen 1968). Analyses were performed using R Statistical Software (R Core Team, 2020). Significance was set at P < .05. Data are presented as mean ± SD, except when otherwise specified.
RESULTS
Demographics
A total 76 patients were included in the study (age: 47.71 ± 12.50 years; Table 1). Women and men showed a moderate level of resistance based on the Maudsley Staging Method (women: 9.92 ± 1.89, men: 9.47 ± 1.67). Women previously had tried an average 5.2 (±3.2) medications and men an average 4.4 (±2.0) medications. Pharmacotherapies are described in Tables 2 and 3. At T0, women and men had a moderate/severe depression (HAMD-17: 24.98 ± 5.91 and 22.47 ± 5.99, respectively). Further characteristics of this sample can be found in our previous work (Nuñez et al. 2018; Moderie et al. 2022).
Table 1.
Socio-Demographic and Clinical Characteristics of Patients (Baseline)a
| Women | Men | Statistics | |
|---|---|---|---|
| No. of patients | 42 | 34 | |
| Age (y) (mean ± SD) | 47.95 ± 12.24 | 47.41 ± 12.99 | t = 0.18, P = .85, d = 0.04 |
| Duration of illness (y) (mean ± SD) | 12.4 ± 12.6 | 16.2 ± 12.3 | t = 1.72, P = .09, d = 0.38 |
| Place of birth | |||
| Africa | 2 (5%) | 4 (12%) | |
| North America | 28 (65%) | 21 (62%) | |
| Central or South America | 2 (5%) | 1 (3%) | Χ² = 20.32, P = .31 |
| Asia | 4 (10%) | 3 (9%) | |
| Europe | 6 (15%) | 5 (15%) | |
| No. of past suicide attempts (mean ± SD) | 0.67 ± 1.28 | 0.18 ± 0.46 | t = 2.30, P = .03, d = 0.50 |
| No. of past hospitalizations (mean ± SD) | 1.76 ± 1.45 | 1.23 ± 0.93 | t = 4.20, P = .18, d = 0.50 |
| No. of past medications (mean ± SD) | 5.21 ± 3.19 | 4.41 ± 2.01 | t = 1.28, P = .21, d = 0.30 |
| MSM (mean ± SD) | 9.92 ± 1.89 | 9.47 ± 1.67 | t = 1.33, P = .19, d = 0.25 |
| Depression severity (mean ± SD) | |||
| HAMD-17 | 24.98 ± 5.91 | 22.47 ± 5.99 | t = 1.82, P = .07, d = 0.42 |
| MADRS | 33.19 ± 9.00 | 29.79 ± 7.84 | t = 1.75, P = .08, d = 0.40 |
| QIDS-C16 | 15.71 ± 3.36 | 14.21 ± 3.81 | t = 1.80, P = .07, d = 0.42 |
| CGI-S | 5.38 ± 1.08 | 4.88 ± 1.15 | t = 1.93, P = .06, d = 0.45 |
| Comorbidities | |||
| Patients with anxiety disorders | 26 (62%) | 21 (62%) | Χ² = 0, P = 1.00 |
| Patients with substance-use disorders | 7 (17%) | 7 (21%) | Χ² = 0.2, P = .89 |
| Pharmacological strategy | |||
| AD+AP | 20 (48%) | 15 (44%) | Χ² = 0.09, P = .95 |
| AD+MS | 9 (21%) | 8 (24%) | |
| AD+AP+MS | 13 (31%) | 11 (46%) | |
| Psychotherapy | 21 (50%) | 11 (46%) | Χ² = 1.73, P = .18 |
a Abbreviations: AD, antidepressants; AP, antipsychotics; CGI-S, Clinical Global Impression rating scale; HAMD-17, 17-item Hamilton Depression Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; MS, mood stabilizers; MSM, Maudsley Staging Method; QIDS-C16, Quick Inventory of Depressive Symptomatology (Clinician-Rated); TRD, treatment-resistant depression.
Table 2.
Number of Patients Receiving Different Antidepressants and Respective Doses (mean ± SD)
| Women | Men | |||
|---|---|---|---|---|
| Antidepressants | n | Mean ± SD | n | Mean ± SD |
| Citalopram (mg) | 8 | 33.75 ± 25.04 | 8 | 32.50 ± 11.65 |
| Escitalopram (mg) | 9 | 15.56 ± 5.27 | 3 | 10.00 |
| Fluoxetine (mg) | 2 | 20.00 | 1 | 20.00 |
| Fluvoxetine (mg) | 1 | 50.00 | — | |
| Paroxetine (mg) | 1 | 40.00 | — | |
| Sertraline (mg) | 3 | 141.67 ± 62.92 | 4 | 100.00 ± 40.82 |
| Duloxetine (mg) | 1 | 60.00 | 5 | 54.00 ± 13.42 |
| Venlafaxine (mg) | 12 | 137.50 ± 68.47 | 12 | 146.88 ± 95.22 |
| Desvenlafaxine (mg) | — | 1 | 50.00 | |
| Bupropion (mg) | 12 | 175.00 ± 58.39 | 7 | 278.57 ± 134.96 |
| Amitriptyline (mg) | — | 3 | 58.33 ± 57.74 | |
| Clomipramine (mg) | 1 | 25.00 | 1 | 25.00 |
| Mirtazapine (mg) | 9 | 22.50 ± 13.52 | 5 | 34.50 ± 22.25 |
| Trazodone (mg) | 4 | 81.25 ± 12.50 | 2 | 50.00 |
Table 3.
Number of Patients Receiving Different Antipsychotics, Mood Stabilizers and Their Combination and Respective Doses (mean ± SD)
| Women | Men | |||
|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | |
| Antipsychotic | ||||
| Aripiprazole (mg) | 3 | 6.7 ± 7.2 | 3 | 1.4 ± 0.8 |
| Olanzapine (mg) | 3 | 9.2 ± 3.8 | — | — |
| Quetiapine (mg) | 11 | 127.5 ± 191.3 | 10 | 107.5 ± 84.2 |
| Risperidone (mg) | 3 | 1.8 ± 1.3 | 1 | 2 |
| Quetiapine (mg)/risperidone (mg) | — | — | 1 | 400/1 |
| Mood stabilizer | ||||
| Lamotrigine (mg) | 5 | 47.5 ± 28.5 | 1 | 300 |
| Lithium (mg) | 2 | 450 ± 212.1 | 1 | 600 |
| Topiramate (mg) | 1 | 25 | — | — |
| Valproic acid (mg) | 1 | 125 | 5 | 415.0 ± 213.3 |
| Lamotrigine (mg)/valproic acid (mg) | — | — | 1 | 150/1250 |
| Antipsychotic + mood stabilizer | ||||
| Aripiprazole/lithium (mg) | 1 | 5/300 | — | — |
| Aripiprazole/lamotrigine (mg) | 1 | 12.5/300 | — | — |
| Aripiprazole/valproic acid (mg) | — | — | 3 | 2/ 375 ± 216.5 |
| Olanzapine (mg)/lamotrigine (mg)/lithium (mg) | — | — | 1 | 5/ 50/300 |
| Olanzapine (mg)/quetiapine (mg)/lithium (mg) | — | — | 1 | 5/ 300/300 |
| Olanzapine (mg)/gabapentin (mg) | — | — | 1 | 15/600 |
| Olanzapine (mg)/valproic acid (mg) | 2 | 5/250 | — | — |
| Quetiapine (mg)/gabapentin (mg) | — | — | 1 | 50/600 |
| Quetiapine (mg)/topiramate (mg) | — | — | 1 | 50/25 |
| Quetiapine (mg)/gabapentin (mg) | 1 | 50/300 | 1 | 600/900 |
| Quetiapine(mg)/lamotrigine (mg) | 2 | 50/ 122.5 ± 123.7 | — | — |
| Quetiapine (mg)/valproic acid (mg) | 2 | 50/ 625 ± 530.3 | 2 | 125 ± 35.4/250 |
| Quetiapine (mg)/gabapentin (mg)/valproic acid (mg) | 1 | 300/300/125 | — | — |
| Quetiapine (mg)/topiramate (mg)/valproic acid (mg) | 1 | 125/25/125 | — | — |
| Risperidone (mg)/valproic acid (mg) | 1 | 0.5/25 | — | — |
Therapeutic range: Aripiprazole [2–15 mg]; Olanzapine [5–20 mg]; Quetiapine [50–300 mg]; Risperidone [0.25–3 mg]; Lamotrigine [25–200 mg]; Lithium [600–1200 mg, based on therapeutic serum levels]; Topiramate [N/A]; Valproic Acid [N/A, based on therapeutic serum levels].
Response and Remission
Response and remission rates of women and men did not differ significantly between the 2 groups (P ≥ .12; Table 4). Of note, no suicide attempt or suicidal behavior occurred during the 3-month follow-up of the patients.
Table 4.
Response and Remission Rates of Women (n = 42) and Men (n = 34) and Percentages
| Women | Men | Fisher/ X2 | ||
|---|---|---|---|---|
| HAMD-17 | Response Remission | 14 (33%) 4 (10%) |
6 (18%) 0 (0%) |
Χ² = 1.64, P = .20 P = .12 |
| MADRS | Response Remission | 27 (64%) 5 (12%) |
16 (47%) 2 (6%) |
Χ² = 1.62, P = .20 P = .16 |
Abbreviations: HAMD-17, 17-item Hamilton Depression Rating Scale; MADRS, Montgomery-Asberg Depression Rating Scale.
Clinical Outcomes in Women vs Men
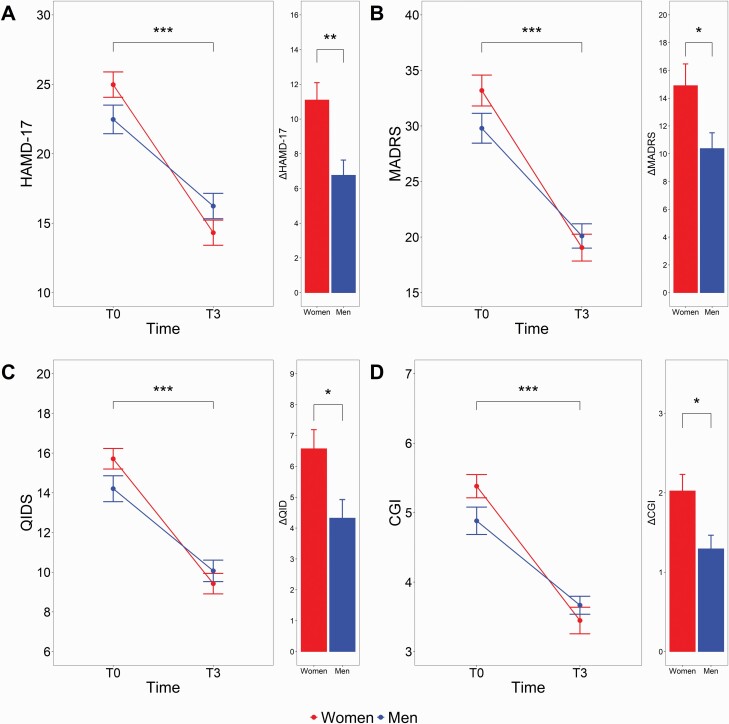
For the HAMD-17, 2-way repeated-measures ANOVA indicated a significant sex × time interaction (F1,67 = 8.55, P = .005, η p2 = 0.11; Figure 1) as well as significant main effect of time (F1,67 = 167.5, P < .001, η p2 = 0.71). There was no main effect of sex (F1,71 = 0.21, P = .64, η p2 = 0.01). For the MADRS, 2-way repeated-measures ANOVA indicated a significant sex × time interaction (F1,67 = 3.93, P = .05, η p2 = 0.06) as well as significant main effect of time (F1,67 = 144.97, P < .001, η p2 = 0.68). There was no main effect of sex (F1,71 = 0.80, P = .37, η p2 = 0.01). For the QIDS-C16, there were significant sex × time interactions (F1,65 = 5.40, P = .02, η p2 = 0.08) as well as a significant main effect of time (F1,65 = 171.42, P < .001, η p2 = 0.73). There was no main effect of sex (F1,71 = 0.65, P = .42, η p2 = 0.01). For the CGI-S, there were significant sex × time interactions (F1,69 = 5.47, P = .02, η p2 = 0.07) as well as significant main effect of time (F1,69 = 132.48, P < .001, η p2 = 0.68). There was no main effect of sex (F1,71 = 0.68, P = .41, η p2 = 0.01). For all scales, Tukey post-host analyses on the sex × time interactions revealed no between-group differences at T0 or T3.
Figure 1.
Changes in scales in women (n = 42) vs men (n = 34) with treatment-resistant depression (TRD). Two-way ANOVAs with sex as between-subject factor and time as a within-subject factor, followed by Tukey post-hoc analyses. Δ scores are reported for women and men (mean within-group change from T0 to T3). CGI, Clinical Global Impression rating scale; MADRS, Montgomery-Åsberg Depression Rating Scale; QIDS, Quick Inventory of Depressive Symptomatology (Clinician-Rated). *P < .05, **P < .01, ***P < .001.
Improvement in Individual Items of the HAMD-17 in Women vs Men
In Table 5, we compared 3-month changes in individual items of the HAMD-17 in women and men. Women exhibited greater improvement in both early (P = .03, η p2 = 0.08) and middle-of-the-night insomnia (P = .01, η p2 = 0.09) as well as retardation (P < .001 η p2 = 0.16), psychic (P = .02, η p2 = 0.07), and somatic anxiety (P = .01, η p2 = 0.10). There was a marginal finding for a greater improvement in general somatic symptoms in women vs men (P = .07, η p2 = 0.04). No significant findings were seen in other clinical scales (P ≥ .15).
Table 5.
Changes in Individual Items of the HAMD-17 in Women (n = 42) Vs Men (n = 34) with TRDa
| Women | Men | Sex effect | Time effect | Sex × Time effect | |
|---|---|---|---|---|---|
| Depressed mood | 1.10 ± 0.13 | 0.83 ± 0.14 | F1,75 = 1.50, P = .22, η p2 = 0.02 | ***F1,70 = 101.27, P < .001, η p2 = 0.59 | F1,70 = 2.02, P = .16, η p2 = 0.02 |
| Feelings of guilt | 0.78 ± 0.12 | 0.56 ± 0.14 | F1,75 =1.03, P = .31, η p2 = 0.01 | ***F1,70 = 53.07, P < .001, η p2 = 0.44 | F1,70 = 1.49, P = .23, η p2 = 0.02 |
| Suicide | 0.92 ± 0.14 | 0.82 ± 0.16 | F1,75 = 3.29, P = .07, η p2 = 0.04 | ***F1,70 = 64.06, P < .001, η p2 = 0.48 | F1,70 = 0.22, P = .64, η p2 = 0.00 |
| Insomnia: early in the night | 0.71 ± 0.09 | 0.41 ± 0.10 | F1,75 = 0.86, P = .36, η p2 = 0.01 | ***F1,70 = 71.79, P < .001, η p2 = 0.52 | *F1,70 = 5.26, P = .03, η p2 = 0.08 |
| Insomnia: middle of the night | 0.76 ± 0.10 | 0.37 ± 0.11 | F1,75 = 2.58, P = .11, η p2 = 0.04 | ***F1,70 = 58.49, P < .001, η p2 = 0.47 | **F1,70 = 6.92, P = .01, η p2 = 0.09 |
| Insomnia: early hours of the morning | 0.43 ± 0.09 | 0.23 ± 0.10 | F1,75 = 0, P = .97, η p2 = 0.00 | *** F1,70 = 23.67, P < .001, η p2 = 0.25 | F1,70 = 2.14, P = .15, η p2 = 0.03 |
| Work and activities | 0.99 ± 0.16 | 0.74 ± 0.18 | F1,75 = 0.05, P = .82, η p2 = 0.00 | *** F1,70 = 50.69, P < .001, η p2 = 0.42 | F1,70 = 1.06, P = .31, η p2 = 0.02 |
| Retardation | 0.68 ± 0.08 | 0.22 ± 0.09 | F1,75 = 0.68, P = .41, η p2 = 0.01 | *** F1,70 = 54.34, P < .001, η p2 = 0.43 | ***F1,70 = 12.69. P < .001, η p2 = 0.16 |
| Agitation | 0.41 ± 0.09 | 0.24 ± 0.10 | F1,75 = 3.41, P = .07, η p2 = 0.05 | *** F1,70 = 25.72, P < .001, η p2 = 0.28 | F1,70 = 1.75, P = .19, η p2 = 0.03 |
| Anxiety psychic | 0.91 ± 0.12 | 0.50 ± 0.14 | F1,75 = 1.16, P = .29, η p2 = 0.02 | *** F1,70 = 59.67, P < .001, η p2 = 0.47 | *F1,70 = 5.21, P = .02, η p2 = 0.07 |
| Anxiety somatic | 0.84 ± 0.11 | 0.40 ± 0.12 | F1,75 = 0.10, P = .76, η p2 = 0.00 | *** F1,70 = 56.49, P < .001, η p2 = 0.47 | **F1,70 = 6.92, P = .01, η p2 = 0.10 |
| Somatic symptoms gastro-intestinal | 0.40 ± 0.10 | 0.25 ± 0.12 | F1,75 = 0.69, P = .41, η p2 = 0.00 | *** F1,70 = 16.84, P < .001, η p2 = 0.19 | F1,70 = 0.98, P = .32, η p2 = 0.01 |
| General somatic symptoms | 0.49 ± 0.11 | 0.18 ± 0.12 | F1,75 = 0.37, P = .55, η p2 = 0.01 | *** F1,70 = 14.95, P < .001, η p2 = 0.19 | F1,70 = 3.33, P = .07, η p2 = 0.04 |
| Genital symptoms | 0.09 ± 0.08 | 0.14 ± 0.09 | * F1,75 = 5.22, P = .03, η p2 = 0.07 | F1,70 = 3.49, P = .07, η p2 = 0.05 | F1,70 = 0.12, P = .72, η p2 = 0.00 |
| Hypochondriasis | 0.50 ± 0.11 | 0.41 ± 0.12 | F1,75 = 0.07, P = .80, η p2 = 0.00 | *** F1,70 = 30.66, P < .001, η p2 = 0.31 | F1,70 = 0.29, P = .58, η p2 = 0.00 |
| Loss of weight | 0.48 ± 0.12 | 0.28 ± 0.13 | F1,75 = 0.41, P = .53, η p2 = 0.00 | *** F1,70 = 19.3, P < .001, η p2 = 0.22 | F1,70 = 1.39, P = .24, η p2 = 0.02 |
| Insight | 0.15 ± 0.07 | 0.16 ± 0.07 | F1,75 = 0.37, P = .55, η p2 = 0.00 | *** F1,70 = 9.90, P < .001, η p2 = 0.13 | F1,70 = 0.02, P = .88, η p2 = 0.00 |
| Total change | 10.70 ± 0.90 | 6.74 ± 1.00 | F1,75 = 0.21, P = .64, η p2 = 0.00 | *** F1,70 = 167.5, P < .001, η p2 = 0.71 | **F1,67 = 8.55, P = .005, η p2 = 0.11 |
a Two-way ANOVAs with sex as between-subject factor and time as a within-subject factors, followed by Tukey post-hoc analyses. Delta scores (changes from T0 to T3) are reported. *P < .05, **P < .01, ***P < .001
Abbreviations: HAMD-17, 17-item Hamilton Depression Rating Scale; TRD, treatment-resistant depression.
DISCUSSION
This is the first study, to our knowledge, comparing the clinical trajectory of women and men with TRD treated with similar augmentation strategies (AD and/or MS). One of our main findings is the greater reduction of depressive symptoms in women compared with men (medium effect size) treated with an AD and augmented with an AP and/or MS. Yet, a recent analysis by the Group for the Study of Resistant Depression showed a trend towards a more frequent administration of add-on treatments in men than in women (Bartova et al. 2021). Our results emphasize the importance of augmentation strategies in women with TRD. The synergistic effect of AD+AP is well studied in unipolar depression (Dold and Kasper 2017), and preclinical studies have underscored that augmentation with an AP allows for targeting multiple receptors and neurotransmitters systems (Blier and Blondeau 2011). There is also evidence undermining the importance of augmentation strategies including MS for TRD patients (Blier and Blondeau 2011; Dold and Kasper 2017; Gobbi et al. 2018). Historically, women were prescribed more tranquilizing and hypnotic drugs than men, but recently, AP appears to replace the use of those medications (Seifert et al. 2021a,b). Augmentation of AD with evidence-based pharmacotherapies rather than tranquilizing and hypnotic drugs will benefit women with TRD (Kennedy et al. 2016; Seifert et al. 2021b). Sex differences for the prescription of AD and MS among patients with MDD are largely unavailable and remain to be clarified (Seifert et al. 2021b). Our study design did not allow us to systematically address this question, but no significant differences were noted in terms of augmentation strategy. Most patients were augmented with an AP, and quetiapine was the agent most prescribed in both men and women. It is also the medication with the best evidence, as emphasized in a recent Cochrane Review for the management of TRD (Davies et al. 2019). In our study, MS was used less often than AP, without any sex differences. Others have reported a less common administration of MS in women compared with men with MDD (Bartova et al. 2021), a difference likely driven by the contraindication of valproic acid and lithium in women of childbearing age due to their potential teratogenic effects (Gentile 2010; Dold et al. 2016; Munk-Olsen et al. 2018).
The differential treatment outcomes observed between men and women could be explained by a myriad of factors. It has been suggested that women may be more likely to respond to selective serotonin reuptake inhibitors (SSRI) than a tricyclic AD, whereas men may be more likely to respond to tricyclic AD than an SSRI (Frank et al. 1988; Haykal and Akiskal 1999; Kornstein et al. 2000; Berlanga and Flores-Ramos 2006; Young et al. 2009). The occurrence of certain drug side effects (i.e., weight gain or sexual dysfunction) may also contribute to the differential AD efficacy and tolerability between sexes (Seifert et al. 2021a). Because most patients were treated with SSRIs in our study, this could contribute to explaining the greater improvement in specific symptoms in women compared with men. Nonetheless, there is no definite consensus on whether sex differences in AD efficacy actually exist (Keers and Aitchison 2010; Sramek et al. 2016; LeGates et al. 2019), and the National Institute for Health and Care Excellence explicitly states that little evidence supports prescribing AD according to sex (NICE 2010).
Besides, the pharmacokinetics of augmenting agents might exhibit sex differences with hypothesized differences in drug transporters (Benet et al. 1999), metabolizing enzymes (Harris et al. 1995; Cheung et al. 2006), and resulting plasma levels of medication (Ronfeld et al. 1997; Keers and Aitchison 2010). Pharmacodynamic properties of the augmenting agents may, furthermore, differ in men and women, with distinct effects on neurotransmitter synthesis in men and women (Keers and Aitchison 2010). More studies are needed to elucidate distinct effects of augmenting agents in relation to sex.
The groups in our study were comparable in terms of age, duration of illness, number of past hospitalizations and medications, and comorbidities with substance-use disorders (SUD) and anxiety disorders. Unlike our findings, in patients with MDD (non-TRD), alcohol and drug abuse is more common in men than in women (Marcus et al. 2008). Although the sample size is limited, our results may indicate that the prevalence of SUD in women with TRD might be higher compared with women with MDD, in line with increased risk for SUD among patients with TRD compared with other depressed patients (Brenner et al. 2019). Notably, most studies in TRD excluded individuals with SUD (Bennabi et al. 2015; De Carlo et al. 2016). Although the European Group for the Study of Resistant Depression did not identify SUD as a risk factor for TRD (Souery et al. 2007), sex differences were not investigated.
Women with MDD (non-TRD) present comorbid anxiety disorders more frequently than men and are more likely to suffer from anxiety prior to the development of depression (Breslau et al. 1995; Yonkers et al. 1996; Howell et al. 2001; Marcus et al. 2005; Grigoriadis and Erlick Robinson 2007; Bukh et al. 2010). Some data even suggest that the increase in anxiety–depression comorbidity may explain the greater lifetime prevalence of depression in women (Breslau et al. 1995). Interestingly, the European Group for the Study of Resistant Depression found no difference in comorbid anxiety disorders when comparing women and men with TRD, which might suggest an attenuation of this sex difference in TRD (Bartova et al. 2021). Likewise, we found no differences in comorbid anxiety disorders in women compared with men. However, as shown in the Zurich Cohort Study, women also have higher rates of sub-threshold co-morbid anxiety, which could contribute to the treatment resistance in MDD (Angst and Merikangas 2001; Souery et al. 2007). Our data also align with the findings from the DEPRES I and II studies reporting higher prevalence of insomnia and anxiety symptoms in women compared with men (Angst et al. 2002). Such evidence emphasizes the need to address anxiety and insomnia, particularly in women. AD, particularly SSRIs, may not sufficiently alleviate those symptoms in women (LeGates et al. 2019) and might contribute to the higher number of tranquilizers and hypnotics prescribed for women than for men (Boyd et al. 2015; Seifert et al. 2021b). In the current study, augmentation with AP and/or MS helped to significantly reduce (moderate effect size) both insomnia and anxiety in women more than in men.
Another important finding is the larger improvement noted in reported early and middle-of-the-night insomnia in women compared with men. Women with MDD generally report more insomnia symptoms than men (Silverstein 1999; Marcus et al. 2005). Insomnia is an established and modifiable risk factor for depression, the treatment of which offers the critical opportunity to prevent major depressive episodes (Plante 2021). The differential improvement in insomnia in women compared with men was accompanied by large-effect size difference in psychomotor retardation. Although no causality can be drawn, our results suggest that improving sleep with augmenting agents in women could decrease psychomotor retardation.
While we found a distinct clinical improvement on the severity of depression according to the different pharmacotherapy strategies, we did not observe an overall difference in their response or remission rates. Such outcomes should be viewed, considering the long period required to achieve remission or euthymic states in depression (Goodwin et al. 2016). The observed low rates of remission indeed reflect the refractory nature of patients included in this study. However, no suicide or suicide attempts were reported during the study follow-up, underscoring that even if the pharmacological combinations did not lead to remission within 3 months, they may be significant in certain depressive domains such as preventing suicidal behaviors. There was no sex difference observed in the suicidality item of the HAMD17. As in multiple studies, the number of suicide attempts was higher in women than in men, which could reflect the higher completion rate in men (Kessler et al. 1993; Oquendo et al. 2001). Larger studies are needed to elucidate preferential pharmacotherapy to prevent suicide. The absence of suicide in our cohort can also be linked to follow-up in a tertiary/quaternary clinic with staff fully trained in suicidal prevention and with 24/7 access to psychiatrists and/or psychiatry emergency.
Limitations
Several limitations should be considered while interpreting these findings. First, the external validity may be limited by data derived from a university hospital mood-specialized center. Second, we did not match the sample of women and men patients according to single pharmacological agents or dosages as well as to depressive severity. Third, we did not control for the menstrual status/phase of women, which can contribute to the severity of symptoms (Hartlage et al. 2004; Haley et al. 2013; Davari-Tanha et al. 2016; Salk et al. 2017) and AD response. Fourth, the non-blinded retrospective outcome assessments should be considered as well as the limitations of a naturalistic design study. Side effects and adverse events were not systematically documented. Nevertheless, the findings may reflect real-world interactions of clinically selected pharmacotherapies, as clinical treatment was individualized and adjusted to tolerability to favor patients’ preference and positive clinical outcomes (Kennedy et al. 2016; Dold and Kasper 2017). The long follow-up of patients at the clinic also prevents the inclusion of undiagnosed bipolar patients in the sample of TRD (Perlis et al. 2011).
CONCLUSION
In our naturalist study in patients with TRD, augmentation strategies generate a significantly greater clinical improvement in women compared with men, supporting the existence of distinct pharmacological profiles between sexes. Moreover, they emphasize the benefit of augmentation strategies in women and highlight the benefit of addressing insomnia and anxiety with AP and MS in this specific population. Further studies linking specific medication and symptoms outcomes in larger sample sizes should provide more insight into these clinical questions to provide personalized management of care of patients suffering from depression. This study paves the way for the investigation of sex differences in TRD, and the data reported here can be used to determine needed sample size in larger trials.
Acknowledgments
To all the patients and personnel of the Allan Memorial, McGill University Health Center, for their contribution to this research program. We want to thank Drs Marie Saint-Laurent, Nancy Low, and Pablo Cervantes for their support.
This study was supported by the Quebec Network on Suicide, Mood Disorders and Related Disorders and the Practice Plan fund of the Department of Psychiatry, McGill University Health Center.
Contributor Information
Christophe Moderie, Department of Psychiatry, McGill University, Montreal, Canada.
Nicolas Nuñez, Department of Psychiatry, McGill University, Montreal, Canada; Department of Psychiatry and Psychology, Mayo Clinic, Rochester, MN, USA.
Allan Fielding, Department of Psychiatry, McGill University, Montreal, Canada; McGill University Health Center, Montreal, Canada.
Stefano Comai, Department of Psychiatry, McGill University, Montreal, Canada; Department of Pharmaceutical and Pharmacological Sciences and Department of Biomedical Sciences, Padova, Italy; University of Padova, Padova, Italy.
Gabriella Gobbi, Department of Psychiatry, McGill University, Montreal, Canada; McGill University Health Center, Montreal, Canada.
Interest Statement
None.
References
- Altshuler LL, Bauer M, Frye MA, Gitlin MJ, Mintz J, Szuba MP, Leight KL, Whybrow PC (2001) Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 158:1617–1622. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: American Psychiatric Pub. [Google Scholar]
- Angst J, Gamma A, Gastpar M, Lépine JP, Mendlewicz J, Tylee A; Depression Research in European Society Study (2002) Gender differences in depression. Epidemiological findings from the European DEPRES I and II studies. Eur Arch Psychiatry Clin Neurosci 252:201–209. [DOI] [PubMed] [Google Scholar]
- Angst J, Merikangas KR (2001) Multi-dimensional criteria for the diagnosis of depression. J Affect Disord 62:7–15. [DOI] [PubMed] [Google Scholar]
- Bartova L, Dold M, Fugger G, Kautzky A, Mitschek MMM, Weidenauer A, Hienert MG, Frey R, Mandelli L, Zohar J, Mendlewicz J, Souery D, Montgomery S, Fabbri C, Serretti A, Kasper S (2021) Sex-related effects in major depressive disorder: results of the European Group for the Study of Resistant Depression. Depress Anxiety 38:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Severus E, Köhler S, Whybrow PC, Angst J, Möller HJ; Wfsbp Task Force on Treatment Guidelines for Unipolar Depressive Disorders (2015) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. part 2: maintenance treatment of major depressive disorder-update 2015. World J Biol Psychiatry 16:76–95. [DOI] [PubMed] [Google Scholar]
- Benet LZ, Izumi T, Zhang Y, Silverman JA, Wacher VJ (1999) Intestinal MDR transport proteins and P-450 enzymes as barriers to oral drug delivery. J Control Release 62:25–31. [DOI] [PubMed] [Google Scholar]
- Bennabi D, et al. (2015) Risk factors for treatment resistance in unipolar depression: a systematic review. J Affect Disord 171:137–141. [DOI] [PubMed] [Google Scholar]
- Berlanga C, Flores-Ramos M (2006) Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J Affect Disord 95:119–123. [DOI] [PubMed] [Google Scholar]
- Berlim MT, Turecki G (2007) Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry 52:46–54. [DOI] [PubMed] [Google Scholar]
- Blier P, Blondeau C (2011) Neurobiological bases and clinical aspects of the use of aripiprazole in treatment-resistant major depressive disorder. J Affect Disord 128 Suppl 1:S3–10. [DOI] [PubMed] [Google Scholar]
- Boyd A, Van de Velde S, Pivette M, Ten Have M, Florescu S, O’Neill S, Caldas-de-Almeida JM, Vilagut G, Haro JM, Alonso J, Kovess-Masféty V; EU-WMH investigators (2015) Gender differences in psychotropic use across Europe: results from a large cross-sectional, population-based study. Eur Psychiatry 30:778–788. [DOI] [PubMed] [Google Scholar]
- Brenner P, Brandt L, Li G, DiBernardo A, Bodén R., Reutfors J (2019) Treatment-resistant depression as risk factor for substance use disorders—a nation-wide register-based cohort study. Addiction 114:1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Schultz L, Peterson E (1995) Sex differences in depression: a role for preexisting anxiety. Psychiatry Res 58:1–12. [DOI] [PubMed] [Google Scholar]
- Bukh JD, Bock C, Vinberg M, Gether U, Kessing LV (2010) Gender differences among patients with a single depressive episode. Psychopathology 43:159–169. [DOI] [PubMed] [Google Scholar]
- Cheung C, Yu AM, Chen CS, Krausz KW, Byrd LG, Feigenbaum L, Edwards RJ, Waxman DJ, Gonzalez FJ (2006) Growth hormone determines sexual dimorphism of hepatic cytochrome P450 3A4 expression in transgenic mice. J Pharmacol Exp Ther 316:1328–1334. [DOI] [PubMed] [Google Scholar]
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, Dickens C, Ferrier IN, Geddes J, Gilbody S, Haddad PM, Katona C, Lewis G, Malizia A, McAllister-Williams RH, Ramchandani P, Scott J, Taylor D, Uher R; Members of the Consensus Meeting (2015) Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol 29:459–525. [DOI] [PubMed] [Google Scholar]
- Cohen J (1968) Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull 70:213–220. [DOI] [PubMed] [Google Scholar]
- Davari-Tanha F, Soleymani-Farsani M, Asadi M, Shariat M, Shirazi M, Hadizadeh H (2016) Comparison of citalopram and venlafaxine’s role in treating sleep disturbances in menopausal women, a randomized, double-blind, placebo-controlled trial. Arch Gynecol Obstet 293:1007–1013. [DOI] [PubMed] [Google Scholar]
- Davies P, Ijaz S, Williams CJ, Kessler D, Lewis G, Wiles N (2019) Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev 12:CD010557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carlo V, Calati R, Serretti A (2016) Socio-demographic and clinical predictors of non-response/non-remission in treatment resistant depressed patients: a systematic review. Psychiatry Res 240:421–430. [DOI] [PubMed] [Google Scholar]
- Dold M, Kasper S (2017) Evidence-based pharmacotherapy of treatment-resistant unipolar depression. Int J Psychiatry Clin Pract 21:13–23. [DOI] [PubMed] [Google Scholar]
- Dold M, Kautzky A, Bartova L, Rabl U, Souery D, Mendlewicz J, Porcelli S, Serretti A, Zohar J, Montgomery S, Kasper S (2016) Pharmacological treatment strategies in unipolar depression in European tertiary psychiatric treatment centers - a pharmacoepidemiological cross-sectional multicenter study. Eur Neuropsychopharmacol 26:1960–1971. [DOI] [PubMed] [Google Scholar]
- Fekadu A, Wooderson S, Donaldson C, Markopoulou K, Masterson B, Poon L, Cleare AJ (2009) A multidimensional tool to quantify treatment resistance in depression: the Maudsley staging method. J Clin Psychiatry 70:177–184. [DOI] [PubMed] [Google Scholar]
- Frank E, Carpenter LL, Kupfer DJ (1988) Possible role of antidepressants in precipitating mania and hypomania in recurrent depression. Am J Psychiatry 145:804–808. [DOI] [PubMed] [Google Scholar]
- Gentile S (2010) Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety 27:675–686. [DOI] [PubMed] [Google Scholar]
- Ghabrash MF, Comai S, Tabaka J, Saint-Laurent M, Booij L, Gobbi G (2016) Valproate augmentation in a subgroup of patients with treatment-resistant unipolar depression. World J Biol Psychiatry 17:165–170. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Ghabrash MF, Nuñez N, Tabaka J, Di Sante J, Saint-Laurent M, Vida S, Kolivakis T, Low N, Cervantes P, Booij L, Comai S (2018) Antidepressant combination versus antidepressants plus second-generation antipsychotic augmentation in treatment-resistant unipolar depression. Int Clin Psychopharmacol 33:34–43. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, et al. (2016) Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol 30:495–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis S, Robinson GE (2007) Gender issues in depression. Ann Clin Psychiatry 19:247–255. [DOI] [PubMed] [Google Scholar]
- Haley CL, Sung SC, Rush AJ, Trivedi MH, Wisniewski SR, Luther JF, Kornstein SG (2013) The clinical relevance of self-reported premenstrual worsening of depressive symptoms in the management of depressed outpatients: a STAR*D report. J Womens Health 22:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1986) The Hamilton Rating Scale for Depression. In: Assessment of depression, pp 143–152. Berlin, Heidelberg: Springer. [Google Scholar]
- Harris RZ, Benet LZ, Schwartz JB (1995) Gender effects in pharmacokinetics and pharmacodynamics. Drugs 50:222–239. [DOI] [PubMed] [Google Scholar]
- Hartlage SA, Brandenburg DL, Kravitz HM (2004) Premenstrual exacerbation of depressive disorders in a community-based sample in the United States. Psychosom Med 66:698–706. [DOI] [PubMed] [Google Scholar]
- Haykal RF, Akiskal HS (1999) The long-term outcome of dysthymia in private practice: clinical features, temperament, and the art of management. J Clin Psychiatry 60:508–518. [DOI] [PubMed] [Google Scholar]
- Howell HB, Brawman-Mintzer O, Monnier J, Yonkers KA (2001) Generalized anxiety disorder in women. Psychiatr Clin North Am 24:165–178. [DOI] [PubMed] [Google Scholar]
- Keers R, Aitchison KJ (2010) Gender differences in antidepressant drug response. Int Rev Psychiatry 22:485–500. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Müller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R; CANMAT Depression Work Group (2016) Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. pharmacological treatments. Can J Psychiatry 61:540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB (1993) Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord 29:85–96. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS; National Comorbidity Survey Replication (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095–3105. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB (2000) Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry 157:1445–1452. [DOI] [PubMed] [Google Scholar]
- Lam RW, Kennedy SH, Grigoriadis S, McIntyre RS, Milev R, Ramasubbu R, Parikh SV, Patten SB, Ravindran AV; Canadian Network for Mood and Anxiety Treatments (CANMAT) (2009) Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy. J Affect Disord 117 Suppl 1:S26–S43. [DOI] [PubMed] [Google Scholar]
- LeGates TA, Kvarta MD, Thompson SM (2019) Sex differences in antidepressant efficacy. Neuropsychopharmacology 44:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, Wisniewski SR, Balasubramani GK, Trivedi MH, Rush AJ (2005) Gender differences in depression: findings from the STAR*D study. J Affect Disord 87:141–150. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Kerber KB, Rush AJ, Wisniewski SR, Nierenberg A, Balasubramani GK, Ritz L, Kornstein S, Young EA, Trivedi MH (2008) Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression study. Compr Psychiatry 49:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moderie C, Nuñez N, Comai S, Saint-Laurent M, Fielding A, Low N, Gobbi G (2022) Distinct effects of antidepressants in association with mood stabilizers and/or antipsychotics in unipolar and bipolar depression. J Clin Psychopharmacol. doi: 10.1097/JCP.0000000000001500. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Munk-Olsen T, Liu X, Viktorin A, Brown HK, Di Florio A, D’Onofrio BM, Gomes T, Howard LM, Khalifeh H, Krohn H, Larsson H, Lichtenstein P, Taylor CL, Van Kamp I, Wesseloo R, Meltzer-Brody S, Vigod SN, Bergink V (2018) Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry 5:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE National Institute for Health and Clinical Excellence: Guidance (2010). Depression: the treatment and management of depression in adults (updated edition). Leicester, UK: British Psychological Society. [PubMed] [Google Scholar]
- Nuñez NA, Comai S, Dumitrescu E, Ghabrash MF, Tabaka J, Saint-Laurent M, Vida S, Kolivakis T, Fielding A, Low N, Cervantes P, Booij L, Gobbi G (2018) Psychopathological and sociodemographic features in treatment-resistant unipolar depression versus bipolar depression: a comparative study. BMC Psychiatry 18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Ellis SP, Greenwald S, Malone KM, Weissman MM, Mann JJ (2001) Ethnic and sex differences in suicide rates relative to major depression in the United States. Am J Psychiatry 158:1652–1658. [DOI] [PubMed] [Google Scholar]
- Parker G, Brotchie H (2010) Gender differences in depression. Int Rev Psychiatry 22:429–436. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Uher R, Ostacher M, Goldberg JF, Trivedi MH, Rush AJ, Fava M (2011) Association between bipolar spectrum features and treatment outcomes in outpatients with major depressive disorder. Arch Gen Psychiatry 68:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante DT (2021) The evolving nexus of sleep and depression. Am J Psychiatry 178:896–902. [DOI] [PubMed] [Google Scholar]
- R Core Team (2020) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ronfeld RA, Tremaine LM, Wilner KD (1997) Pharmacokinetics of sertraline and its N-demethyl metabolite in elderly and young male and female volunteers. Clin Pharmacokinet 32 Suppl 1:22–30. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ (2019) Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 44:111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB (2003) The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54:573–583. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Salk RH, Hyde JS, Abramson LY (2017) Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull 143:783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert J, Engel RR, Bernegger X, Führmann F, Bleich S, Stübner S, Sieberer M, Greil W, Toto S, Grohmann R (2021a) Time trends in pharmacological treatment of major depressive disorder: results from the AMSP Pharmacovigilance Program from 2001-2017. J Affect Disord 281:547–556. [DOI] [PubMed] [Google Scholar]
- Seifert J, Führmann F, Reinhard MA, Engel RR, Bernegger X, Bleich S, Stübner S, Rüther E, Toto S, Grohmann R, Sieberer M, Greil W (2021b) Sex differences in pharmacological treatment of major depressive disorder: results from the AMSP pharmacovigilance program from 2001 to 2017. J Neural Transm 128:827–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein B (1999) Gender difference in the prevalence of clinical depression: the role played by depression associated with somatic symptoms. Am J Psychiatry 156:480–482. [DOI] [PubMed] [Google Scholar]
- Souery D, Oswald P, Massat I, Bailer U, Bollen J, Demyttenaere K, Kasper S, Lecrubier Y, Montgomery S, Serretti A, Zohar J, Mendlewicz J; Group for the Study of Resistant Depression (2007) Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J Clin Psychiatry 68:1062–1070. [DOI] [PubMed] [Google Scholar]
- Sramek JJ, Murphy MF, Cutler NR (2016) Sex differences in the psychopharmacological treatment of depression. Dialogues Clin Neurosci 18:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lépine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK (1996) Cross-national epidemiology of major depression and bipolar disorder. JAMA 276:293–299. [PubMed] [Google Scholar]
- Wilhelm K, Parker G, Geerligs L, Wedgwood L (2008) Women and depression: a 30-year learning curve. Aust N Z J Psychiatry 42:3–12. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Warshaw MG, Massion AO, Keller MB (1996) Phenomenology and course of generalised anxiety disorder. Br J Psychiatry 168:308–313. [DOI] [PubMed] [Google Scholar]
- Young EA, Kornstein SG, Marcus SM, Harvey AT, Warden D, Wisniewski SR, Balasubramani GK, Fava M, Trivedi MH, John Rush A (2009) Sex differences in response to citalopram: a STAR*D report. J Psychiatr Res 43:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K (2013) Severity classification on the Hamilton Depression Rating Scale. J Affect Disord 150:384–388. [DOI] [PubMed] [Google Scholar]