Abstract
New influenza vaccines have been designed based on the fact that the extracellular domain of M2 protein (M2e) is nearly invariant in all influenza A strains. To clarify which exact region of M2e could induce antibodies with inhibitory activities against influenza virus replication, four overlapping peptides covering M2e were synthesized and then coupled to the carrier protein bovine serum albumin through the cysteine of the peptides. After a vaccination course, all these four peptide vaccines could induce high levels of rabbit antibodies with predefined peptide specificity (antibody dilution: 1:6400–1:25 600). Besides, the anti-N-terminal antibodies (AS2) reacted strongly with M2e, and reacted weakly with the middle part and C-terminus of M2e. The MDCK assay for cytopathic effect proved that antibodies recognizing the N-terminus of M2e could obviously inhibit replication of influenza A virus (A/wuhan/359/95) and influenza B virus (B/wuhan/321/99) in vitro in a dose-dependent manner, while antibodies recognizing the middle part and the C-terminus of M2e did not show such significant inhibitory activities. Sequence analysis indicates that the first nine N-terminal amino acid residues of M2e are extremely conservative. Just this region containing the first nine amino acid residues could induce antibodies with inhibitory activity against influenza A and influenza B virus replication, suggesting that the N-terminus of M2e may contain an epitope that could induce inhibitory antibodies against influenza virus replication in vitro.
Keywords: Influenza virus, M2 protein, N-terminus, Inhibitory activity
1. Introduction
Broad-spectrum and effective vaccines against influenza virus infection are still a subject of research of many investigators. The trivalent inactivated vaccines, which contain the hemagglutinin (HA) of influenza A (H1N1), influenza A (H3N2) and influenza B, are the only licensed commercial vaccines [1]. Because of the high mutation rate of HA, we should be immunized against influenza virus infection with these vaccines annually or biannually, as suggested by the World Health Organization [2]. Like HA and neuraminidase, M2 protein is also an essential integral membrane protein of influenza virus. However, M2 protein is only 97 amino acids long with 24 amino acids at the N-terminus exposed outside the membrane surface, 54 amino acids at the C-terminus located on the cytoplasmic side of the membrane and 19 amino acids spanning the lipid bilayer [3]. Pinto et al. [4] and Bron and co-workers [5] have found that the function of M2 protein is to form one highly selective and pH-regulated proton channel. Additionally, Zebedee and Lamb [6] and a few years later Ito et al. [7] proved that the extracellular domain of M2 protein (M2e) was remarkably conserved. Based on this characteristic, some new types of vaccines have been designed. Neirynck et al. [8] designed a universal influenza A vaccine, which provided mice with 90–100% protection against mouse-adapted X47 influenza A virus; Slepushkin and co-workers [9] found that vaccination of mice with baculovirus-expressed M2 protein could protect them from death following a lethal challenge with A/Ann Arbor/6/60 virus and heterologous A/Hong Kong/68 virus. Furthermore, some other experiments proved that the antibodies against M2e were really efficient against influenza A virus infection. Via passive immunization, Neirynck et al. [8] proved that antibodies induced by M2e mediated the protection; Treanor et al. [10] demonstrated that antibodies to M2 protein reduced the replication level of influenza A virus in the lungs of mice. Sesma et al. [11] produced a bispecific antibody (3F12) binding M2e and T cell receptor, and this bispecific antibody could redirect activated T cells to kill cells infected with influenza virus and inhibit virus replication in vitro. However, information on the immunogenicity of M2e is still scarce. To broaden this knowledge and to examine which exact region of M2e could induce antibodies with inhibitory activities, we designed four overlapping peptide sequences covering the extracellular domain of the M2 protein (Fig. 1 ). After a vaccination course, we identified their immunogenic specificity and the crossed immune reactions. Finally, we evaluated these antibodies’ inhibitory activities against influenza A and influenza B virus replication in vitro by the MDCK (Madin–Darby canine kidney) cytopathic effect (CPE) inhibitory assay.
Fig. 1.
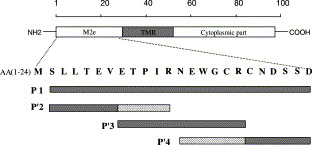
Four overlapping peptides covering the extracellular domain of M2 protein of influenza A virus. AA: amino acid residue; TMR: transmembrane region. P1: N-KSLLTEVETPIRNEWGCRCNDSSD; P2: KSLLTEVETPIR-G-SLLTEVETPIR (two repeats of P′2); P3: KETPIRNEWGCR-G-ETPIRNEWGCR (two repeats of P′3); P4: KNEWGCRCNDSSD-G-NEWGCRCNDSSD (two repeats of P′4).
2. Materials and methods
2.1. Cells and viruses
MDCK cells were obtained from the American Type Culture Collection (ATCC), and maintained in Dulbecco’s minimum essential medium (DMEM; Pierce, Rockford, IL, USA) supplemented with 100 U ml−1 of penicillin, 100 μg ml−1 of streptomycin and 10% fetal bovine serum (FBS; Pierce) at 37°C in a humidified atmosphere with 5% CO2. One human influenza A virus (A/wuhan/359/95) and another serologically identified human influenza B virus (B/wuhan/321/99) were multicycle replicated in 10-day-old embryonic eggs at 37°C for 48 h and plaque-forming units of the virus from the harvested allantoic fluid were obtained by plaque assay [12].
2.2. Peptides and antibodies
Four overlapping peptides (P1, P2, P3 and P4) covering the extracellular part of M2 protein were commercially synthesized at Genemed Synthesis (San Francisco, CA, USA). P1: N-KSLLTEVETPIRNEWGCRCNDSSD (aa 2–24); P2: KSLLTEVETPIR-G-SLLTEVETPIR (aa 2–12); P3: KETPIRNEWGCR-G-ETPIRNEWGCR (aa 8–18); P4: KNEWGCRCNDSSD-G-NEWGCRCNDSSD (aa 13–24) (Fig. 1). Peroxidase-conjugated goat anti-rabbit immunoglobulins were obtained from Dako (Denmark) and goat anti-human IgG (H+L) was obtained from Pierce. Peptides were chemically linked to the carrier protein bovine serum albumin (BSA; Sigma) by 3-maleimidobenzoic acid N-hydroxysuccinimide ester (Sigma). Rabbits were immunized subcutaneously with 50 μg peptide per rabbit in complete Freund’s adjuvant (1:1 ratio) at a final volume of 300 μl. Boosters were given in incomplete Freund’s adjuvant on days 14 and 28. Sera were separated 7 days after the last boosting immunization. Pre-immunized sera were collected before immunization and mixed as pooled normal serum (NS). The rabbit antiserum (AS0) against influenza A virus was induced by influenza virus A/wuhan/359/95 (50 μg virus per injection).
2.3. Detection of predefined peptide-specific antibodies in ELISA assay
The peptide-specific antibodies in rabbit antisera were detected in an enzyme-linked immunosorbent assay (ELISA). The peptides (5 μg ml−1) were coated overnight in a microtiter plate at 4°C. Non-specific binding was blocked for more than 2 h by incubation with 0.25% gelatin on phosphate-buffered saline (PBS). After washing twice with PBS–Tween 20 (0.5% Tween 20), rabbit sera at different dilutions were added and incubated for 1 h at room temperature. After washing twice, peroxidase-conjugated goat anti-rabbit immunoglobulins were added. After an additional five washes, freshly prepared o-phenylenediamine dihydrochloride peroxide solution (Sigma) was added and the optical density (OD) was measured with a microtiter plate reader at 450 nm (Bio-Rad).
2.4. Analysis of the immunogenicity of M2e through natural infection
The M2e-specific antibody titer in 66 serum samples from patients positive for influenza virus and 43 normal serum samples from individuals negative for influenza virus were tested by ELISA assay. The data obtained from 1:400 diluted influenza virus-positive sera and negative sera were analyzed to get the means. We performed a t-test to estimate the significance of the difference between the two means.
The standard errors of the difference:
| Sxc−xe=SSc+SSenc−ne−2×1nc+1ne, so: t=(Xc−Xe)−(μc−μe)Sxc−xe=0.490 |
Choosing significance level α=0.05 (two-tailed), t 0.05=1.98. Thus, t<t 0.05, which indicates that the difference of the means between the normal group and the patient group is not significant.
2.5. CPE assays
The CPE assays were performed as described by Schmidtke et al. [13]. MDCK cells were seeded at 2×104 per well in 96-well flat-bottom microtiter plate (Costar 3599) and cultured at 37°C in a humidified atmosphere with 5% CO2. The antiviral evaluation of the test sera was carried out in 2-day-old confluent MDCK cell monolayers grown in the internal 60 wells and determined by scoring the CPE microscopically. The sera diluted in 100 μl DMEM medium (1:40, 1:80, 1:160, 1:320 and 1:640 dilutions) were added to another sterile 96-well flat-bottom microtiter plate in two replicates per dilution. Immediately, a constant multiplicity of infection (MOI) of virus in a volume of 100 μl DMEM medium per well was added to that microtiter plate, and then the mixture was incubated at 37°C for 1 h. Afterwards the microtiter plate with confluent MDCK cell monolayers was washed twice with 200 μl DMEM medium per well before the incubated mixture was added. In addition, six wells of cell monolayers with 200 μl DMEM medium added per well served as positive controls, and another six wells of cell monolayers, with one MOI of virus in a volume of 200 μl DMEM medium added per well, served as negative controls. Then the following process was applied after the cell monolayers had been incubated at 37°C in a humidified atmosphere with 5% CO2 for 48 h.
Initially, the supernatant was removed and the cell monolayers were washed three times with 300 μl physiological sodium chloride solution to remove the dead cells. Secondly, the cell monolayers were fixed and stained in one step with 50 μl of 0.2% crystal violet (w/v) in 20% methanol (v/v) for 30 min. After six further manual washes with 300 μl of water, the stained cell monolayers were treated for 20 min with 100 μl of lysis buffer (0.9 g of sodium citrate and 1.25 ml of 1 N HCl in 98 ml 47.5% ethanol) to elute the crystal violet. Finally, the OD of individual wells was quantified spectrophotometrically with a microtiter plate reader at 630 nm (Bio-Rad). The validity of each test serum was evaluated as the protective rate calculated according to Pauwels et al. [14] using the following equation from three assays with two parallels each time: protective rate=[(mean value of OD of two test parallels minus mean value of OD of six negative controls) divided by (mean value of OD of six positive controls minus mean value of OD of six negative controls)]×100%.
3. Results and discussion
To clarify which region of M2e could induce inhibitory activity against influenza virus replication, four overlapping peptides covering M2e were synthesized (Fig. 1). After a vaccination course, all these four peptide vaccines could induce high levels of antibodies recognizing these four peptides (antibody dilution: 1:6400–1:25 600), while normal rabbit serum (pre-immune serum, NS) did not bind to any peptides (Fig. 2A ). In addition, the anti-P1 antibodies (AS1) reacted strongly with M2e (P1), and weakly with the other three peptides (P2, P3 and P4). The other three antibodies, anti-P2 antibodies (AS2), anti-P3 antibodies (AS3) and anti-P4 antibodies (AS4), could react intensely or very intensely with these three peptides (P2, P3 and P4), respectively, and these three antibodies with predefined peptide specificity all strongly bound to M2e (P1) (Fig. 2B). The peptides P2, P3 and P4 contain two repeated epitopes (Fig. 1). The high levels of antibodies to P2, P3 and P4 proved that these epitope peptides obviously enhanced the immunogenicity of these three epitopes located in the N-terminus, middle region and C-terminus of M2e (Fig. 2A), and the weak cross immune reactions proved that the antibodies induced by all these peptide vaccines were of high specificity (Fig. 2B). These results enable the epitope analysis of M2e in the following experiments.
Fig. 2.
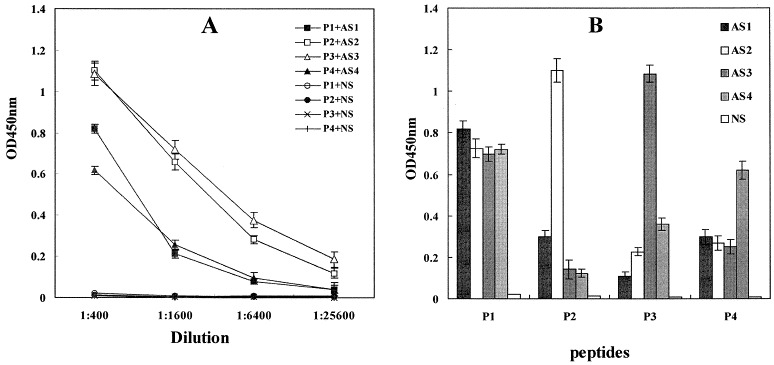
Titers of antibodies in AS1, AS2, AS3 and AS4 by ELISA assay. A: Titers of predefined peptide-specific antibodies. B: Possible cross immune reactions (antisera were diluted to 1:400). AS1, antiserum induced by P1-BSA; AS2, antiserum induced by P2-BSA; AS3, antiserum induced by P3-BSA; AS4, antiserum induced by P4-BSA; NS, pooled pre-immune normal serum (control). Data from all four experiments are shown as mean values.
We also examined the inhibitory activities of the four predefined peptide-specific antibodies against influenza virus replication in the MDCK CPE assay. Two kinds of influenza virus, A/wuhan/359/95 and B/wuhan/321/99, were used to evaluate the effectiveness of all these antibodies. M2e as a whole could induce antibodies with inhibitory activities against A/wuhan/359/95 and B/wuhan/321/99 replication in vitro in a dose-dependent manner (Fig. 3, Fig. 4 ), which is consistent with the results of other groups [8], [10], [11]. Zebedee and Lamb [15] demonstrated by observation of the macroscopic changes of the plaques that the M2 protein-specific antibodies were not able to inhibit virus adsorption or penetration of virions, but were able to reduce the rate of plaque growth and the size of plaque. Here, the antiviral evaluations of those antibodies were carried out by scoring the CPE spectrophotometrically with a microtiter plate reader at 630 nm (Bio-Rad), which was therefore more objective [13]. In the following experiments with the other three antibodies against P2, P3 and P4, respectively, we found that antibodies against P2 could obviously inhibit the replication of influenza A virus with the protective rate reaching 60% even at a dilution rate of 1:160 (Fig. 3, Fig. 4). When using one influenza B virus, B/wuhan/321/99, the same result was also obtained with the protective rate reaching 56% at a dilution rate of 1:80 by antibodies against P2, and the protective rate by NS (1:80 dilution) was less than 30% (Fig. 3, Fig. 4). However, antibodies against P3 and P4 did not show such significant inhibitory activities against influenza A and influenza B virus replication as did antibodies against P2 (Fig. 3, Fig. 4).
Fig. 3.
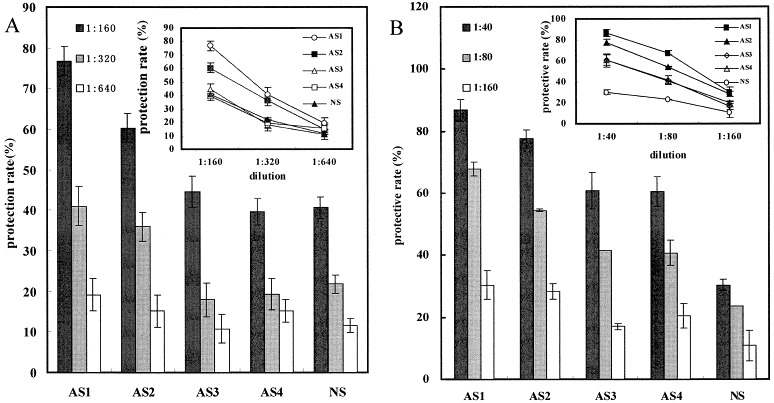
Evaluation of the inhibitory activities of the predefined peptide-specific antibodies against influenza virus replication in the MDCK CPE assay. A: Results of the experiments using A/wuhan/359/95. B: Results of the experiments using B/wuhan/321/99. AS1, antiserum induced by P1-BSA; AS2, antiserum induced by P2-BSA; AS3, antiserum induced by P3-BSA; AS4, antiserum induced by P4-BSA; NS, pooled pre-immune normal serum (control). Data from all four experiments are shown as mean values.
Fig. 4.
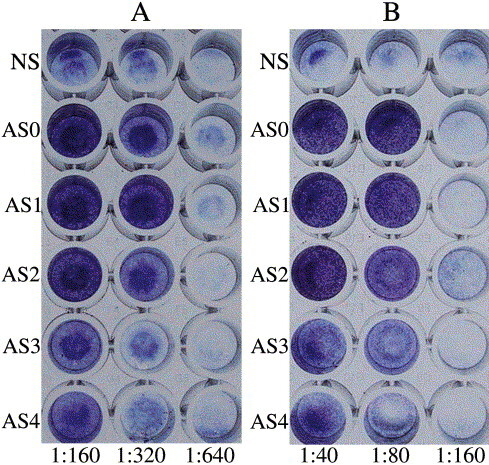
Antibodies against M2e (P1) and the N-terminus of M2e (P2) can inhibit the MDCK CPE. MDCK cell monolayers were infected with (A) influenza A virus, A/wuhan/359/95, or (B) influenza B virus, B/wuhan/321/99, using 1 unit of MOI after the viruses were incubated with different dilutions of NS and five kinds of antisera, AS0, AS1, AS2, AS3 and AS4.
Zebedee and Lamb [15] have already indicated that isoleucine at residue 11 and glutamic acid at residue 14 are important for antibody binding to the M2 protein. Our experiment further indicates that the N-terminus, which includes the first 12 amino acids of M2 protein, may contain one epitope that can induce antibodies with inhibitory activities against influenza virus A virus and influenza B virus replication in vitro, while the other parts of M2e do not seem to have the ability to induce such kind of antibodies. Further experiments are in progress to clarify whether the sequence of the first 12 N-terminal amino acids of M2 protein has similar ability in vivo in the mouse model. We also aligned and analyzed the amino acid sequences of M2e based on all 188 influenza A virus strains with available M2 protein amino acid sequences [16]. Surprisingly, no amino acid changes were found among the first 9 N-terminal amino acids of M2e (Fig. 5 ). Furthermore, proteins M1 and M2 share N-terminal residues 1–9 [17], and Latham and Galarza [18] proved that matrix protein played an important role in the virus assembly and release processes. To determine the antibody level against M2e of influenza virus in humans, 66 serum samples from influenza virus-positive patients and 43 normal serum samples from influenza virus-negative individuals were tested by ELISA. We found that the antibody levels of patients and the normal group showed no significant difference, which suggested that the inhibitory antibody titer induced by M2e against influenza virus replication in patients might be as low as that in healthy individuals (Fig. 6 ). Considering the obvious inhibitory activity of the antibodies induced by the very conservative N-terminus of M2e (Fig. 3, Fig. 4), this approach might hold a promise for creating novel vaccines for influenza virus in the future.
Fig. 5.
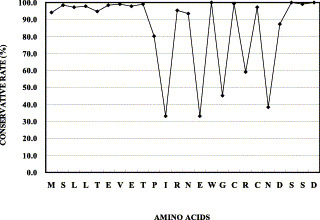
Alignment and analysis of the amino acid sequences of M2e based on all 188 influenza A virus strains with available M2 protein amino acid sequences. The conservation rate of every amino acid is calculated according to the chance that it appears among all 188 influenza A virus strains.
Fig. 6.
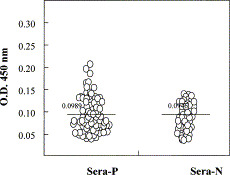
Analysis of the immunogenicity of M2e in humans through natural infection. Sixty-six serum samples from patients positive for influenza virus (Sera-P) and 43 normal sera from individuals negative for influenza virus (Sera-N) were tested by ELISA at dilution 1:400. The OD of each sample is represented by one circle. The two lines mark the means of all samples in the patient group and in the normal group. The t-test is taken to evaluate the difference between the two means from the two group. Significance level α=0.05 (two-tailed), t=0.490<t0.05=1.98.
Acknowledgements
This work was supported by the 973 Program (No. G1999054107), the National Science Foundation for Outstanding Young Scientist of China (No. 30025038), the fund of Tsinghua University and the Foundation for Advanced Visiting Scholars of the Ministry of Education.
References
- 1.Cox N.J, Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson, K.G., Webster, R.G. and Hay, A.J. (1998) Textbook of Influenza. Blackwell Science, Oxford.
- 3.Lamb R.A, Zebedee S.L, Richardson C.D. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 4.Pinto L.H, Holsinger L.J, Lamber R.A. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 5.Bron R, Kendal A.P, Klenk H.D, Wilschut J. Role of the M2 protein in influenza virus membrane fusion: effects of amatadine and monensin on fusion kinetics. Virology. 1993;195:808–811. doi: 10.1006/viro.1993.1435. [DOI] [PubMed] [Google Scholar]
- 6.Zebedee S.L, Lamb R.A. Nucleotide sequences of influenza A virus RNA segment 7: a comparison of five isolates. Nucleic Acids Res. 1980;17:2870. doi: 10.1093/nar/17.7.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito T, Gorman O.T, Kawaoka Y, Bean W.J, Webster R.G. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J. Virol. 1991;65:5491–5498. doi: 10.1128/jvi.65.10.5491-5498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou W.M, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 9.Slepushkin V.A, Katz J.M, Black R.A, Gamble W.C, Rota P.A, Cox N.J. Protection of mice against influenza A virus challenge by vaccination with baculovirus-expressed M2 protein. Vaccine. 1995;13:1339–1342. doi: 10.1016/0264-410x(95)92777-y. [DOI] [PubMed] [Google Scholar]
- 10.Treanor J.J, Tierney E.L, Zebedee S.L, Lamb R.A, Murphy B.R. Passively transferred monoclonal antibody to the M2 protein inhibit influenza A virus replication in mice. J. Virol. 1990;64:1357–1377. doi: 10.1128/jvi.64.3.1375-1377.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sesma A.F, Schulman J.L, Moran T.M. A bispecific antibody recognizing influenza A virus M2 protein restricts effector cells to inhibit virus replication in vitro. J. Virol. 1996;70:4800–4804. doi: 10.1128/jvi.70.7.4800-4804.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett, T. and Inglis, S.C. (1985) Growth purification and titration influenza viruses. In: Virology: A Practical Approach (Mahy, W.J., Ed.), pp. 119–151. IRL Press, Washington, DC.
- 13.Schmidtke M, Schnittler U, Jahn B, Dahse H.M, Stelzner A. A rapid assay for evaluation of antiviral activity against coxsachie virus B3, influenza virus A, and herpes simplex virus type 1. J. Virol. Methods. 2001;95:133–143. doi: 10.1016/s0166-0934(01)00305-6. [DOI] [PubMed] [Google Scholar]
- 14.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewjin P, Desmyter J, De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 15.Zebedee S.L, Lamb R.A. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J. Virol. 1988;62:2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macken, C., Lu, H., Goodman, J. and Boykin, L. (2001) The value of a database in surveillance and vaccine selection. In: Options for the Control of Influenza IV (Osterhaus, A.D.M.E., Cox, N. and Hampson, A.W., Eds.), pp. 103–106. Elsevier Science, Amsterdam.
- 17.Lamb R.A, Lai C.-J, Choppin P.W. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: collinear and interrupted mRNAs code for overlapping proteins. Proc. Natl. Acad. Sci. USA. 1981;78:4170–4174. doi: 10.1073/pnas.78.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latham T, Galarza J.M. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J. Virol. 2001;75:6154–6165. doi: 10.1128/JVI.75.13.6154-6165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


