Background.
Humoral responses to coronavirus disease 2019 (COVID-19) vaccines are attenuated in solid organ transplant recipients (SOTRs), necessitating additional booster vaccinations. The Omicron variant demonstrates substantial immune evasion, and it is unknown whether additional vaccine doses increase neutralizing capacity versus this variant of concern (VOC) among SOTRs.
Methods.
Within an observational cohort, 25 SOTRs with low seroresponse underwent anti–severe acute respiratory syndrome coronavirus 2 spike and receptor-binding domain immunoglobulin (Ig)G testing using a commercially available multiplex ELISA before and after a fourth COVID-19 vaccine dose (D4). Surrogate neutralization (percent angiotensin-converting enzyme 2 inhibition [%ACE2i], range 0%–100% with >20% correlating with live virus neutralization) was measured against full-length spike proteins of the vaccine strain and 5 VOCs including Delta and Omicron. Changes in IgG level and %ACE2i were compared using the paired Wilcoxon signed-rank test.
Results.
Anti–receptor-binding domain and anti-spike seropositivity increased post-D4 from 56% to 84% and 68% to 88%, respectively. Median (interquartile range) anti-spike antibody significantly increased post-D4 from 42.3 (4.9–134.2) to 228.9 (1115.4–655.8) World Health Organization binding antibody units. %ACE2i (median [interquartile range]) also significantly increased against the vaccine strain (5.8% [0%–16.8%] to 20.6% [5.8%–45.9%]) and the Delta variant (9.1% [4.9%–12.8%] to 17.1% [10.3%–31.7%]), yet neutralization versus Omicron was poor, did not increase post-D4 (4.1% [0%–6.9%] to 0.5% [0%–5.7%]), and was significantly lower than boosted healthy controls.
Conclusions.
Although a fourth vaccine dose increases anti-spike IgG and neutralizing capacity against many VOCs, some SOTRs may remain at high risk for Omicron infection despite boosting. Thus, additional protective interventions or alternative vaccination strategies should be urgently explored.
INTRODUCTION
Solid organ transplant recipients (SOTRs) have blunted responses to coronavirus disease 2019 (COVID-19) vaccines.1,2 Thus, the Centers for Disease Control and Prevention has recommended that all SOTRs should receive a third primary dose (D3) of mRNA-based vaccine and consider a fourth booster dose as well.3 Although a fourth dose (D4) of vaccine seems to increase both severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike-specific (anti-spike antibody [anti-S]) antibody response and T cell responses in a subset of SOTRs, it is unclear whether this additional vaccine dose elicits antibodies capable of neutralizing variants of concern (VOCs), including the now dominant Omicron variant that demonstrates significant immune evasion.4,5 To evaluate whether D4 of the COVID-19 vaccine improves the neutralizing capacity of plasma from SOTRs, we measured total anti-S, antireceptor binding domain (anti-RBD), and angiotensin-converting enzyme 2 (ACE2) neutralization to VOCs in a sample of SOTRs pre- and post-D4 of the COVID-19 vaccine.
MATERIALS AND METHODS
Cohorts
SOTRs were enrolled in a national prospective observational study of immunocompromised COVID-19 vaccine recipients as previously described.1,2,4,6 All participants gave written or oral consent as approved by the Johns Hopkins Institutional Review Board (IRB00248540). Within the larger cohort, participants independently obtained D4 of the vaccine in the community between April and December 2021 and submitted blood samples pre- and post-D4. Participants with available demographic and immunological data on pre- and post-D4 of vaccine were included in the analysis. Healthy control (HC) participants were enrolled under Johns Hopkins IRB00027183 and provided samples at a median of 14 d after third (booster) mRNA vaccination. Blood was collected in acid citrate dextrose tubes, and plasma was isolated by Ficoll centrifugation and stored at –80 °C.
IgG Measurement
Antinucleocapsid antibody (anti-N), anti-RBD, and anti-S immunoglobulin (Ig)G were measured in thawed participant plasma in duplicate using the multiplex chemiluminescent Meso Scale Diagnostics (MSD, Rockville, MD) V-PLEX COVID-19 Respiratory Panel 3 Kit according to the manufactures’ directions at a dilution of 1:5000. IgG was expressed in World Health Organization binding antibody units, and seropositivity was defined in accordance with manufacturer recommendations.
ACE2 Neutralizing Antibody Measurement
The MSD ACE2 inhibition assay measures the ability of participant plasma to inhibit ACE2 binding to full-length spike protein (a surrogate for neutralizing activity). Plasma was thawed, and ACE2 blocking was measured using the ACE2 MSD V-PLEX SARS-CoV-2 ACE2 Panel 23 kit according to the manufacturer’s protocol at a dilution of 1:100 as previously described.2 Results were reported as percent ACE2 inhibition based on the equation provided by the manufacturer ([1 – average sample electrochemiluminescence/average electrochemiluminescence signal of blank well] × 100). Adequate ACE2 inhibition was defined as >20%, based upon correlation with live virus-neutralizing antibody in SOTRs.2
Statistical Analysis
A paired Wilcoxon signed-rank test was used to compare the median of anti-N, anti-S, anti-RBD, and percent ACE2 inhibition pre- and post-D4 of the vaccine among the cohort. An unpaired Wilcoxon-Mann-Whitney test was used to compare median percent ACE2 inhibition between post-D4 SOTR plasma and post-D3 HC plasma.
RESULTS
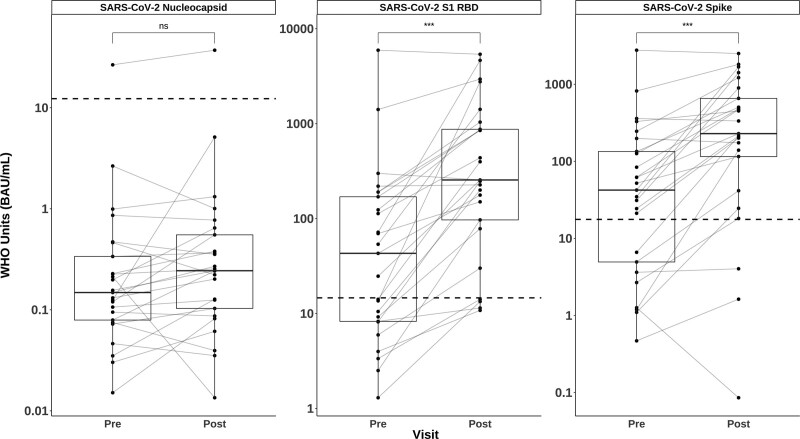
Twenty-five SOTRs provided pre- and post-D4 samples and were included in the study. Demographic and baseline clinical data for the cohort are included in Table 1. The median age (interquartile range [IQR]) was 59 y (45–66), 52% were male, 92% were White, and 64% were primarily kidney transplant recipients (16/25). All received 2 mRNA-based vaccines as initial series, followed by mRNA vaccine (17/25; 68%) or Ad26.COV2.S vaccine (8/25; 32%) as a D3. All, however, received an mRNA-based D4 (15/25 [60%] mRNA-1273, 10/25 [40%] BNT162b2) at a median (IQR) of 93 d (28–134) post-D3. Most participants were taking immunosuppressive regimens containing an antimetabolite (21/24; 84%), 1 had previously received belatacept, and 2 of 25 (8%) had a history of acute rejection. Pre-D4 samples were obtained at a median (IQR) of 8 d (1–19) before vaccination, and post-D4 samples were collected at a median (IQR) of 29 d (17–38) after vaccination. No participants reported a clinical diagnosis of COVID-19, although 1 participant did have a positive anti-N antibody before receiving a D4, suggesting prior infection (Figure 1). The median (IQR) anti-N IgG value did not significantly differ post-D4 (0.15 [0.1–0.33] BAU/mL versus 0.24 [0.1–0.55] BAU/mL, P = 0.2). Anti-RBD and anti-S seropositivity increased from 56% (14/25) to 84% (21/25) and from 68% (17/25) to 88% (22/25), respectively. Corresponding median (IQR) values for anti-RBD and anti-S also significantly increased from 43.1 (8.3–115.4) BAU/mL to 255.3 (96.9–873.2) BAU/mL and from 42.3 (4.9–134.2) BAU/mL to 228.9 (115.4–655.8) BAU/mL, respectively (P < 0.001 for both assays; Figure 1).
TABLE 1.
Cohort demographics and transplant factors
| Factor | Value |
|---|---|
| N = 25 | |
| Age at vaccination (y) | 59 (45–66) |
| Years since transplant | 4.3 (2.7–8.8) |
| Female | 14 (56) |
| Organ | |
| Kidney | 16 (64) |
| Liver | 3 (12) |
| Liver-kidney | 1 (4) |
| Pancreas | 1 (4) |
| Kidney-pancreas | 1 (4) |
| Heart | 1 (4) |
| Lung | 2 (8) |
| Immunosuppressives | |
| Prednisone | 18 (72) |
| Calcineurin inhibitor | 24 (96) |
| Mycophenolate | 21 (84) |
| mTOR inhibitor | 2 (8) |
| Belatacept | 1 (4) |
| Triplea | 17 (68) |
| Days from D3 to D4 | 93 (28–134) |
| Days from D4 to antibody measurement | 29 (17–38) |
| Vaccine brand (D4) | |
| Moderna | 15 (60) |
| Pfizer | 10 (40) |
Data for continuous variables are reported as median (Q1–Q3). Data for categorical variables are reported as N (%).
aPrednisone, antimetabolite, and calcineurin inhibitor or mTOR inhibitor. Includes 1 participant also taking belatacept as a fourth medication.
D3, third primary dose; D4, fourth dose; mTOR, mammalian target of rapamycin.
FIGURE 1.
Changes in SARS-CoV-2–specific IgG after a fourth dose of COVID-19 vaccine. Total IgG in WHO BAUs against SARS-CoV-2 nucleocapsid, S1 RBD, and full-length spike before and after a fourth dose of the vaccine among SOTRs. The box plots represent the IQR. The median is represented by a solid horizontal line in the box. The lower and upper whiskers represent 1.5 times the IQR beyond the quartiles. Each dot represents an individual sample. Gray lines between dots indicate change after a fourth dose. Dashed horizontal lines represent seropositivity cutoffs as determined by the test manufacturer based on convalescent and prepandemic samples. Statistical differences between measurements were determined by the paired Wilcoxon signed-rank test. P values of <0.05 were considered significant. (*** indicates P < 0.001, ns indicates P > 0.05). BAU, binding antibody unit; COVID-19, coronavirus disease 2019; IgG, immunoglobulin G; IQR, interquartile range; RBD, receptor-binding domain antibody; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOTR, solid organ transplant recipient; WHO, World Health Organization.
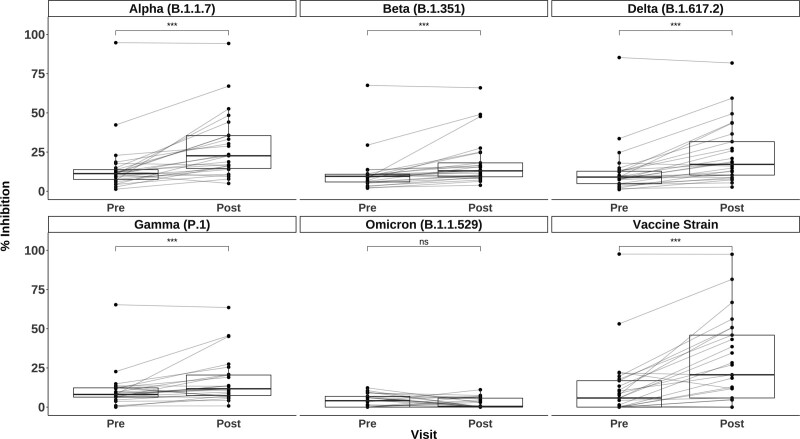
Similarly, median (IQR) plasma neutralization by percent ACE2 inhibition significantly increased post-D4 versus the vaccine strain: 5.8% (0%–16.8%) to 20.6% (5.8%–45.9%); the Alpha variant: 11.3% (7.5%–13.8%) to 22.6% (14.6%–35.5%); the Beta variant: 9.5% (5.9%–10.9%) to 13.0% (9.2%–18.1%); the Gamma variant: 8.1% (6.3%–12.2%) to 11.7% (7.4%–20.5%); and the Delta variant: 9.1% (4.9%–12.8%) to 17.1% (10.3%–31.7%; all P values <0.001). However, plasma neutralization of Omicron variant spike protein for all participants was low, below expected neutralizing antibody threshold, and did not increase post-D4: median (IQR) inhibition from 4.1% (0%–5.7%) to 0.5% (0%–6.9%) (P = 0.06; Figure 2).2 Notably, the participant with the highest inhibition of all other VOCs demonstrated minimal inhibition of Omicron post-D4 (6.1%). Furthermore, the participant with possible prior infection per positive anti-N exhibited no ACE2 binding to Omicron spike (0% pre- and post-D4). SOTR ACE2 inhibition against all VOCs post-D4 was significantly lower than that of HCs post-D3 of mRNA-based COVID-19 vaccine (n = 24; Supplemental Table S1, SDC, http://links.lww.com/TP/C403). This was particularly notable for the Omicron variant, where median (IQR) inhibition for the HCs was 30.2% (19.7% - 53.4%) post-D3 compared with 0.5% (0%–6.9%) for the SOTRs post-D4 (Supplemental Figure S1, SDC, http://links.lww.com/TP/C403).
FIGURE 2.
Changes plasma surrogate neutralizing capacity (percent ACE2 inhibition) after a fourth dose of the COVID-19 vaccine. Inhibition of full-length SARS-CoV-2 spike variants (indicated in the top header of each panel) before and after a fourth dose of the vaccine among SOTRs. The box plots represent the IQR. The median is represented by a solid horizontal line in the box. The lower and upper whiskers represent 1.5 times the IQR beyond the quartiles. Each dot represents an individual sample. Gray lines between dots indicate change after a fourth dose. Statistical differences between measurements were determined by the paired Wilcoxon signed-rank test. P values of <0.05 were considered significant (*** indicates P < 0.001, and ns indicates P > 0.05). COVID-19, coronavirus disease 2019; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOTR, solid organ transplant recipient.
DISCUSSION
In this observational series, SOTRs, with poor prior seroresponse demonstrated increased binding titers and improved neutralizing ability against many VOCs on receiving D4 of an mRNA-based COVID-19 vaccine. Yet, D4 failed to induce significant neutralization of the now dominant Omicron variant in this cohort. Although anti-S IgG in BAU was comparable with previously published studies of D4, the profound discrepancy with Omicron ACE2 binding is consistent with early in vitro series in immunocompetent persons indicating 10- to 20-fold higher titers required to neutralize this heavily mutated variant in vitro.5,7-11 Although Omicron ACE2 inhibition in HCs post-D3 was also lower than for other VOCs, the majority of participants achieved levels associated with live virus neutralization.2 These findings raise concern that, in contrast to the general population, in which boosters connote much reduced risk of infection, some SOTRs may have ongoing high vulnerability to infection despite additional doses and boosting.11
Notable features of this cohort include high frequency of kidney transplant recipients and use of antimetabolites in combination with other immunosuppressants, a phenotype associated with reduced seroresponse to 2- and 3-dose series.1,2,12 Additionally, approximately one third of recipients had received heterologous vaccines as part of their primary series, which might be associated with differential seroresponse, although all received an mRNA-based D4. Thus, although this cohort may not be representative of all vaccinated SOTRs, it does reflect a common and concerning subgroup of persons who seem to be at high risk for SARS-CoV-2 Omicron variant infection despite receiving 4 vaccine doses. Importantly, at least 1 participant had evidence of prior infection, which in combination with 3-dose vaccination connotes potent “hybrid immunity” in the immunocompetent population,13 yet did not generate Omicron neutralization.
Limitations include a small, observational convenience sample of persons pursuing D4 in the community and may not represent the greater SOTR population. Furthermore, we were unable to measure cellular responses, which may provide some antibody-independent protection, although studies in immunocompetent people suggest that cross-reactive T cells do not necessarily correlate with neutralizing antibodies.10 Although we did not use live virus to measure neutralization, we and others previously demonstrated a good correlation between ACE2 inhibition assays and live virus neutralization.2,14
These findings suggest that additional and booster dosing of the original vaccines to select SOTRs may not generate robust protection against infection in the form of neutralizing antibodies against the Omicron variant or future variants evolved from Omicron. Therefore, additional strategies such as modulation of immunosuppressant regimens before additional vaccine doses, vaccines with alternative antigen sequences, or broadly neutralizing passive immunity products, such as monoclonal antibody cocktails or high-titer convalescent plasma, may be necessary to provide protection in highly immunosuppressed SOTRs.
ACKNOWLEDGMENTS
The authors thank Teresa P. Y. Chiang for her assistance with the statistical analysis.
Supplementary Material
Footnotes
A.H.K. and W.A.W. conceived of the study, designed the experiments, performed the analysis, and wrote the original article. T.S.J. and T.Y.A. performed the assays and contributed to data visualization. O.A., Y.E., and J.E.R. collected the samples and prepared them for study. A.T.A. and J.L.A. collected and curated the clinical and demographic data. J.N.B., A.L.C., J.R.B., S.L.K., A.P., D.L.S., and A.A.R.T. secured funding, provided supervision, and contributed to interpretation of the results. All authors contributed to editing the article.
D.L.S. has received consulting and speaking honoraria from Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. A.H.K. has received consulting fees from Roche. The other authors declare no conflicts of interest.
This work was supported by the Ben-Dov family, the National Cancer Institute U54CA260491 (A.L.C. and S.L.K.), grant T32DK007713 (J.L.A.) from the National Institute of Diabetes and Digestive and Kidney Diseases, and grants K24AI144954 (D.L.S.), K08AI156021 (A.H.K.), K23AI157893 (W.A.W.), U01AI138897 (W.A.W. and D.L.S.), and R01AI120938S1 (A.A.R.T.) from the National Institute of Allergy and Infectious Diseases.
Reasonable requests to the corresponding author for deidentified data will be granted.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karaba AH, Zhu X, Liang T, et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2022;22:1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the United States. Available at https://www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf. Accessed February 4, 2022.
- 4.Alejo JL, Mitchell J, Chiang TP, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105:e280–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamar N, Abravanel F, Marion O, et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA-based vaccine in recipients of a solid organ transplant. JAMA Netw Open. 2021;4:e2136030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimeglio C, Herin F, Martin-Blondel G, et al. Antibody titers and protection against a SARS-CoV-2 infection. J Infect. 2022;84:248–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng S, Phillips DJ, White T, et al. ; Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GeurtsvanKessel CH, Geers D, Schmitz KS, et al. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol. 2022;7:eabo2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med. 2022;386:1088–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reischig T, Kacer M, Vlas T, et al. Insufficient response to mRNA SARS-CoV-2 vaccine and high incidence of severe COVID-19 in kidney transplant recipients during pandemic. Am J Transplant. 2022;22:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates TA, McBride SK, Leier HC, et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol. 2022;7:eabn8014. doi:10.1126/sciimmunol.abn8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skelly DT, Harding AC, Gilbert-Jaramillo J, et al. ; Medawar Laboratory Team; OPTIC (Oxford Protective T cell Immunology for COVID-19) Clinical Group; PITCH (Protective Immunity T cells in Health Care Worker) Study Group; C-MORE/PHOSP-C Group. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat Commun. 2021;12:5061. [DOI] [PMC free article] [PubMed] [Google Scholar]