Abstract
The cytoskeleton plays an integral role in maintaining the integrity of epithelial cells. Epithelial cells primarily employ cytokeratin in their cytoskeleton, whereas mesenchymal cells use vimentin. During the epithelial-mesenchymal transition (EMT), cytokeratin-positive epithelial cells begin to express vimentin. EMT induces stem cell properties and drives metastasis, chemoresistance, and tumor relapse. Most studies of the functions of cytokeratin and vimentin have relied on the use of either epithelial or mesenchymal cell types. However, it is important to understand how these two cytoskeleton intermediate filaments function when co-expressed in cells undergoing EMT. Here, we discuss the individual and shared functions of cytokeratin and vimentin that coalesce during EMT and how alterations in intermediate filament expression influence carcinoma progression.
Keywords: Intermediate filaments, cytokeratin, vimentin, epithelial-mesenchymal transition, cancer stem cells
1. Introduction
Cellular integrity is important at the level of individual cells and in the architectures of tissues and organs. The cytoskeleton is necessary for the coherence of cells and is critical for functions of both normal and cancerous cells including cell division, cellular shape, endocytosis, exocytosis, migration, invasion, and intravasation 1–4. The cytoskeleton is composed of intermediate filaments, microfilaments (primarily actin), and microtubules (primarily tubulin). Of these three components, intermediate filaments are the most diverse. Unlike the ubiquitous microfilaments and microtubules, different tissues have different intermediate filament types 5.
Some intermediate filaments are found in nearly all cell types. For example, lamins provide nuclear support in all cells 6. Other intermediate filaments are tissue specialized with unique functions. The most striking examples of tissue-specific intermediate filaments are those composed of either cytokeratin or vimentin 7. Cytokeratin is expressed in epithelial cells, where it provides cell-cell adhesion support 8. Vimentin is expressed in mesenchymal cells that usually lack both cell-cell adhesion and polarity. In these cells, vimentin provides resistance to migration-related stress 9.
Carcinomas hijack the epithelial-to-mesenchymal (EMT) program, which is important during embryonic development and wound healing, to drive invasion and metastasis 10. While EMT is further classified into type 1 (embryogenesis), type 2 (wound healing and fibrosis), and type 3 (carcinoma progression and metastasis) 11, we will refer to EMT in the context of this review as the gaining of mesenchymal properties by epithelial cells. Typically, the profile of intermediate filaments in epithelial cells is static, but during EMT cells begin to express vimentin. Recent findings indicate that cancer cells need not fully proceed through EMT to become invasive or obtain stem cell-like properties. Rather than a binary epithelial-mesenchymal state, cells progress through EMT on a spectrum that continues until it reaches the mesenchymal state or stalls at a particular stage within the EMT spectrum 12. These intermediate cells referred to as hybrid E/M cells, express both epithelial and mesenchymal markers and are more tumorigenic than cells with either a highly epithelial or a highly mesenchymal phenotype 13,14. Patients with tumors that co-express both cytokeratin and vimentin have an increased likelihood of metastasis and worse prognosis than those with tumors that express only cytokeratin 15–18. Due to the importance of EMT in carcinoma progression, this review will discuss how individual and shared functions of cytokeratin and vimentin coalesce during EMT. We also discuss therapeutic strategies to target this unique alteration in intermediate filament expression to block cancer progression.
2. Cytokeratin structure and function in epithelial cells
Most intermediate filaments are constructed similarly through a stepwise assembly process from monomers to the final 10-nm filaments. Cytokeratin deviates from other intermediate filament proteins as cytokeratin strictly forms heterodimers between the two classes of cytokeratins: type I (acidic) and type II (basic) 19,20. The demarcation of type I and type II cytokeratins was initially used due to the separation in 2D-PAGE with type I cytokeratins being smaller (40–56.5 kDa) and separating in the acidic gradient of the gel (isoelectric point, pI 4.5–6.0), while the type II cytokeratins a generally larger (50–70 kDa) and separate in the neutral-basic gradient (pI 6.5–8.5) 8,20,21. This terminology was initially temporary; however, dividing the cytokeratins based on this rule is reliable 20,21. The general segregation based on acid and basic cytokeratins into type I and type II, respectively, was still utilized following refinement of the nomenclature of cytokeratins 19. Collectively there are 54 human cytokeratins with 28 genes encode type I cytokeratins and 26 genes that encode type II cytokeratins (Table 1) 7,8,19. Both types of cytokeratins share an α-helical central rod domain of ~310 amino acids flanked by variable domains at the non-helical head and tail 8,19,21 (Figure 1A).
Table 1. Characteristics of cytokeratin and vimentin intermediate filament types.
Individual types of intermediate filaments are categorized by primary method of assembly, tissue location, isoelectric point (pI), molecular weight, and expressed proteins in a given category.
| Category | Primary assembly method | Tissue expression | pI | MW (kDa) | Protein(s) |
|---|---|---|---|---|---|
| Type I Cytokeratins | Heteropolymerize with Type II | Epithelial cells | Acidic (4.5 – 6.0) | 40 – 56.5 | K9, 10, 12–20, 23–28, 31–40 |
| Type II Cytokeratins | Heteropolymerize with Type I | Epithelial cells | Basic (6.5 – 8.5) | 50 – 70 | K1–8, 71–86 |
| Vimentin | Homopolymerize | Mesenchymal cells | Acidic (5.6) | 57 | Vimentin |
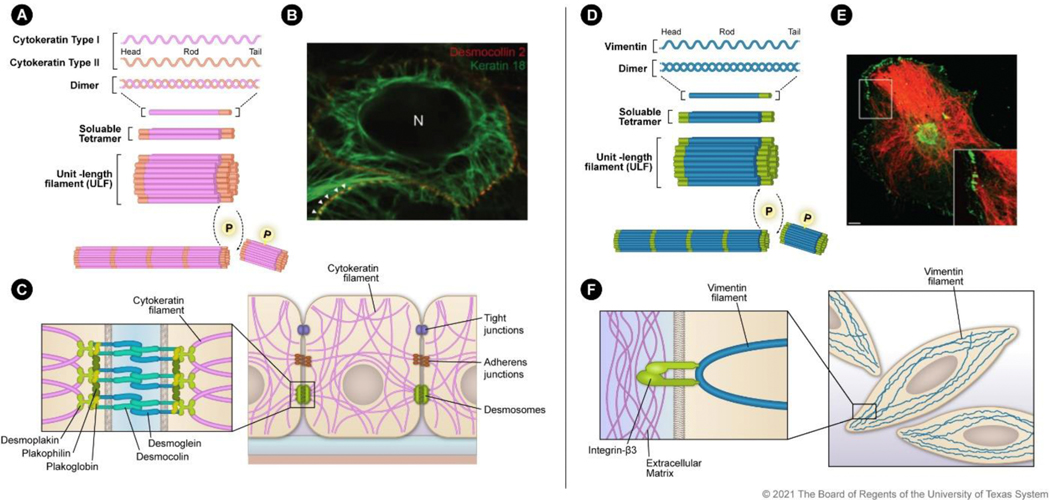
Figure 1. Filamentous assembly and localization of cytokeratin and vimentin:
A) Cytokeratin assembly initiates with type I and type II heterodimer formation. The heterodimers combine to form soluble tetramers that interact to form ULFs made up of eight monomers. ULFs are then assembled in a phosphorylation-dependent manner into 10-nm filaments. B) Survey fluorescence micrograph of MDCK cells that express YFP-labeled desmocollin (red) and mRFP-labeled human cytokeratin 18 (green). Image reprinted with permission from Quinlan et al., J. Cell Sci., 130, 3437–3445 (2017). C) Schematic of cytokeratin distribution in an epithelial cell with cell-cell adhesion processes. Zoomed inset shows the protein-protein interactions between cytokeratin filaments and desmosomes. D) Vimentin assembles as homodimers that combine to form soluble tetramers and then ULFs made up of eight monomers. ULFs are then assembled in a phosphorylation-dependent manner into filaments. E) Transformed human bone marrow endothelial cells stained for integrin β3 (green) and vimentin (red). Image reprinted with permission from Bhattacharya et al., J. Cell Sci., 122, 1390–1400 (2009). F) Schematic of vimentin distribution in a mesenchymal cell. Zoomed inset indicates the vimentin filament interaction with β3 integrin that anchors the filament to the extracellular matrix.
Coiled-coil heterodimers occur initially between type I and type II cytokeratins 8. The heterodimer then forms a heterotetramer between two heterodimers. Finally, the heterotetramer forms unit-length filaments (ULFs) by lateral association (Figure 1A), a process identified as self-assembly in vitro 21,22. Improper dimerization between cytokeratin filaments from the same type (i.e., type I + type I) results in rapid degradation, showing a preference for the association between type I and type II filaments 23. ULFs assemble longitudinally in a phosphorylation-directed manner into 10-nm filaments. The 10-nm filamentous network of cytokeratins spans from the nucleus to the cell periphery and provides structural support (Figure 1B, C). Phosphorylation is a crucial regulator of controlling assembly and organization for intermediate filaments 1. Multiple phosphorylation sites on cytokeratin allow rapid disassembly into soluble ULFs for distribution and reassembly into the insoluble 10-nm filaments. This plasticity of assembly and disassembly is critical for intermediate filament functions such as organelle arrangement, stress resistance, and cell division.
2.1. Active role of cytokeratin in cell adhesion
The filamentous network of cytokeratin is necessary for cell-cell adhesion in sheets of epithelial cells. Cytokeratin filaments associate with desmosomes at the cell periphery. The binding of cytokeratin filaments with desmoplakin supports the formation of adhesion plaques that resist stress at the tissue level. The interaction between cytokeratin filaments with desmosomes maintains cell shape and facilitates cell-to-cell contacts 24,25. Cytokeratin also ensures proper localization of E-cadherin, an adhesion molecule that functions to maintain cell-cell contacts. Via interaction with cytokeratin, E-cadherin is localized to the basolateral portion of the cell membrane to form adherens junctions that serve in both cell-cell adhesion and signaling 26,27. E-cadherin expression is a critical factor of epithelial identity. During EMT, E-cadherin function is reduced through downregulation or delocalization from the cell membrane. When cytokeratin production is disrupted, E-cadherin is mislocalized to the basolateral membrane, and cells lose polarity 27,28. Epithelial cellular polarity is a defining feature of a differentiated cell state, and the loss of cellular polarity is an early stage of EMT. The adhesion-promoting property of cytokeratin is a major driver of epithelial morphology, and the downregulation of this process occurs during EMT 29. Thus, cytokeratin is not just a passive bystander of the epithelial identity but proactively reinforces it.
2.2. Cytokeratin allows epithelial cells to withstand mechanical stress
The cytokeratin network spans the cell, forming a support mesh from the nucleus to the cell membrane providing integral stress resistance (Fig 1B, C) 30. At the nuclear membrane, cytokeratin binds to nesprin-3. At the cell membrane, cytokeratins attach to desmosomes. When all cytokeratin-encoding genes are knocked out, cells become severely susceptible to stress, such as force applied by magnetic tweezers, demonstrating the importance of cytokeratins in cellular stress mitigation 31. The filamentous network throughout the cytoplasm also functions in mechanosensing as it transmits mechanical stress into signals allowing for directional migration 32,33. This action is essential for the collective migration of epithelial cells that normally occurs during embryogenesis and tissue regeneration; however, this process also drives the migration of tumor cells during metastasis 34,35. Collectively migrating cells do not need to undergo EMT fully; instead, the sheet of carcinoma cells has a spectrum of epithelial and mesenchymal properties. The type of cytokeratin expressed is critical, as reductions in levels of only cytokeratin-8 are sufficient to disrupt epithelial sheet formation 36.
Mechanical stress mitigation is only the initial function of cytokeratins in the stress response. In skin wounding, cytokeratins 6, 16, and 17 are upregulated in keratinocytes through MAPK signaling to provide additional stability 37. While keratinocytes deficient in cytokeratin 6 have enhanced migratory capacity, mice that lack cytokeratin 6 have defects in wound repair 38. This is due to the resulting fragility of keratinocytes. Keratinocytes without cytokeratin 6 can migrate in response to a wound, but stress at the wound edge leads to lysis 38. Mutations in cytokeratins lead to severe epithelial tissue damage and fragility in humans 39. For example, epidermolysis bullosa simplex is a disease caused by mutations in cytokeratins 5 and 14 that impacts skin integrity and leads to severe blistering.
In summary, cytokeratin networks function at the tissue level to maintain epithelial cellular architecture, and loss of certain cytokeratins leads to severe disease. However, when levels of all cytokeratins are reduced in keratinocytes, the cytoskeleton is not completely disrupted 31. This suggests that even though resistance to mechanical stress is profoundly impaired in the absence of cytokeratins, the overall function of the cytoskeleton is maintained. This robustness to cytokeratin loss is an important characteristic as cells transition to the mesenchymal phenotype during EMT. However, during EMT progression, the downregulation of cytokeratin does not occur in a vacuum. Vimentin is upregulated as cytokeratin is downregulated, and vimentin takes over some of the cellular responsibilities of cytokeratin while contributing mesenchymal-reinforcing properties. The similarities of cytokeratin and vimentin are essential for a smooth transition from epithelial to mesenchymal phenotype.
3. Vimentin structure and its role in maintaining mesenchymal properties
Vimentin resembles cytokeratins structurally and functionally; however, some key differences result in unique properties. Much like cytokeratins, vimentin has a head, rod, and tail domain (Figure 1D). Vimentin is similar in size and pI to type I cytokeratins with an acidic pI of 5.6 and a MW of 57 kDa (Table 1) 40. At the initial steps of assembly, vimentin differs from cytokeratins as it forms homotetramers rather than heterotetramers; however, the dimers are assembled in a similar coiled-coil process(Figure 1D) 7. Vimentin assembles much like cytokeratins into ULFs and 10-nm filaments. Phosphorylation events mediate vimentin assembly, and phosphorylation sites are clustered in the head domain of the protein 41,42. Like the cytokeratin network, the vimentin cytoplasmic network extends from the cell membrane to the nuclear membrane, where it interacts with nesprin-3 (Figure 1E, F) 43. A crucial difference between cytokeratins and vimentin is that vimentin is recruited to the cellular membrane by directly binding to integrin β3 rather than desmosomes (Figure 1F) 44–46. Disruption of integrin β3 is sufficient for the collapse of vimentin filaments around the nucleus, whereas overexpression of integrin β3 establishes the vimentin filament mesh from the nucleus to the cytoplasmic membrane 47. At the cytoplasmic membrane, vimentin is recruited to the focal adhesions that function in mechanical sensing and the interaction of the cell with the extracellular matrix.
3.1. Contribution of vimentin to elastic resilience
Vimentin primarily provides resistance to stress and compression at the single-cell level, unlike cytokeratin networks, which mediate tissue-level resistance to mechanical stress and compression 4,48. Vimentin forms a hyper-elastic network that maintains cell viability by enhancing cell stretchability, strength, resilience, and toughness by dispersing local mechanical stress to other regions of the cell; this is essential when cells are migratory. The act of migration introduces unique stressors on a cell relative to the stress experienced by a non-motile cell. A migrating cancer cell must adapt to compression and stretching during invasion of tissue. Shear stress is also applied after intravasation as the cell travels through the bloodstream. Based on these factors, cancer cells expressing vimentin have an advantage during migration and invasion 49.
The importance of vimentin in resilience was demonstrated by stretching mouse embryonic fibroblasts embedded in a hydrogel to induce mechanical stress, which is comparable to the stress experienced during motility and invasion in vivo 50. Vimentin-depleted cells were less viable than cells that expressed normal levels of vimentin when force was applied to the hydrogel 50. However, organisms deficient in vimentin have much milder phenotypes than organisms that lack cytokeratins. Vimentin knockout was initially reported to have no detrimental effects in mice 51. The mild defects of vimentin knockout relative to depletion of cytokeratin are likely due to the impacted tissue types rather than vimentin’s overall importance. Epithelial cells present in the skin function as the physical barrier of the body. Because of this, the loss of cytokeratins is readily observed as diseases such as epidermolysis bullosa simplex. Further studies revealed that loss of vimentin results in abnormal mammary gland development, vascular stiffness in arteries, and impairment of wound healing 52–55. These observations collectively support the crucial functions of vimentin at both the cellular and organismal level 56
3.2. Motility mediated by vimentin
Vimentin is important for directed cell migration, which requires interplay with cytoskeleton components actin and tubulin. Directional migration relies on the polarization of the cell based on the reaction to extracellular signals. Migration in the direction of an extracellular signal is important for the efficient dissemination of tumor cells toward blood vessels 57. During this process, actin first assembles at the leading edge of the cell to form pseudopodia for an extension. In the second step, the disassembly of the actin fibers at the rear of the cells leads to contraction 58. Vimentin interacts with actin in the stress fiber complex to facilitate the disassembly process 59. In cells depleted of vimentin, actin stress fiber assembly and contractility are increased, a property that is reversed upon re-expression of vimentin 59. Vimentin regulates actin assembly and contraction by downregulating the phosphorylation of GEF-H1, a component of the Rho A pathway involved in actin reorganization 59. Vimentin also regulates the activity of the small GTPase Rac1, which is involved in actin polymerization and migration 60,61. The molecular mechanisms involved in the vimentin-mediated regulation of actin, GEF-H1, and Rac1 are unknown. It has been speculated that vimentin acts as a “phosphorylation sink.” As vimentin is heavily phosphorylated by multiple kinases, it may function to reduce the net activity of these kinases on other substrates such as GEF-H1 59,61. The vimentin sink could also interfere with the phosphorylation of actin, leading to increased instability of the actin filament complex.
In migratory cells, tubulin microtubule function is also influenced by vimentin. Tubulin mediates cell motility by enhancing polarity leading to directed migration 62. Microtubules provide this directionality, but they have a short grow-shrink cycle of 3 to 5 minutes, introducing instability in the directionality of migration. In contrast, vimentin filaments have a grow-shrink cycle of more than 10 minutes. The regular grow-shrink cycle of vimentin enhances the memory of tubulin filamentous patterns by providing a memory-like template for tubulin reassembly 63. This template is lost upon vimentin depletion resulting in a reduction in directed tubulin assembly.
Vimentin’s role in migration is regulated by phosphorylation. Phosphorylation of vimentin at Ser39 catalyzed by protein kinase B (ATK1) mediates assembly and disassembly of vimentin filaments 64. Ablation of Ser39 phosphorylation with an alanine substitution is sufficient to reduce the migratory and invasive capacity of soft tissue sarcoma cells. In contrast, a phosphomimetic mutation at residue 39 results in increased migration and invasiveness 64. These findings suggest that invasive cancers likely have a high amount of phosphorylation at this site during migration.
During cancer progression, the expansive growth of cancer cells results in crowded conditions that can provide additional stress on cells. However, enhanced cellular stiffness enables cancer cells to tolerate these crowded conditions 65. Vimentin functions to stiffen cells and thus may facilitate cancer cell survival. An increase in cell stiffness also relieves the stress of migration in clustered cells but not in sparse or single cells. Due to the importance of cluster migration and the potential for crowding, vimentin is likely to bolster the resilience of carcinomas. Collectively, the increase in vimentin levels that occur during the EMT mediates multiple facets of cellular motility and resistance to the stress encountered during carcinoma progression. During EMT, vimentin competes with and eventually overthrows the bias toward immobility that results from cytokeratin expression in favor of motility and invasion. However, the extent to which vimentin overwrites cytokeratin function depends on the shared characteristics between the filaments.
4. Shared characteristics of cytokeratin and vimentin filaments
The networks of both vimentin and cytokeratin intermediate filaments can interact with identical linker proteins in some scenarios. Plectin and nesprin-3 anchor to both vimentin and cytokeratin filaments to the nuclear envelope 30. Desmosomal interactions with both types of filaments occur through desmoplakin, although the functional outcomes differ, with cytokeratin leading to cell-cell adhesion and vimentin promoting tension and contraction responses 44,45. Additionally, both filaments have similar roles in the organization of organelles such as the Golgi apparatus, vesicles, nucleus, and autophagosomes 4,66–71. Understanding the shared functions between the filaments in normal cells is essential for mapping the interplay that occurs in the carcinogenic hybrid E/M cells, as some functions may be shared or delegated to one filament depending on the stage of the EMT process.
4.1. Vimentin and cytokeratin participate in cell division
Although the filamentous mesh of the cytoskeleton is useful in non-dividing cells, the web of filaments must be organized during cell division to clear the metaphase plate and allow the two daughter cells to pull apart. During cell division, a clear metaphase plate ensures that the filament networks do not interfere with the function of kinetochore microtubules as they connect to and retract the chromosomes. The clearing of the intermediate filament network can occur either by complete disassembly or by redistribution (Figure 2A, B). Whether the network is disassembled or redistributed depends on cell type and the expression of other intermediate filaments. For vimentin filaments, progression to either redistribution or disassembly is determined by the expression of the type VI filament nestin. Nestin forms heteropolymers with vimentin; disassembly of these filaments is regulated during mitosis by cell-cycle kinases 72,73.
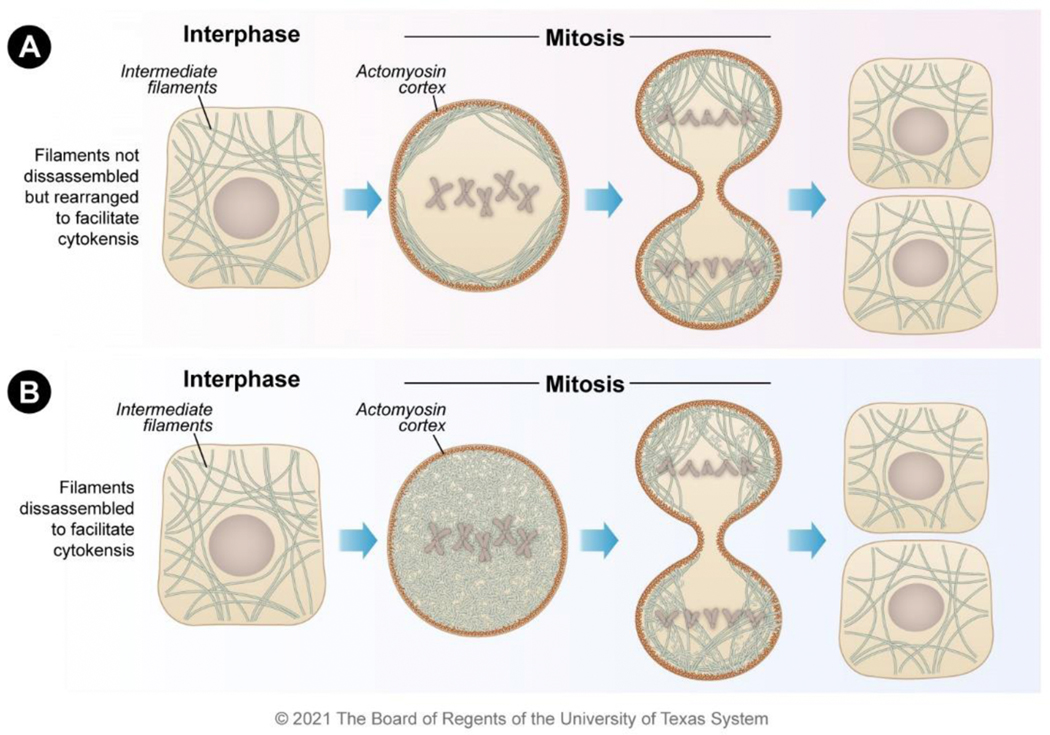
Figure 2. Localization mechanisms for vimentin and cytokeratin during mitosis.
Both vimentin and cytokeratin filaments exist as filamentous forms during interphase. During mitosis, the filaments either A) remain filamentous but are organized to the actin cortex to clear the mitotic plate or B) become disassembled into unit-length filaments to clear the mitotic plate. Both mechanisms enable the efficient completion of cytokinesis resulting in daughter cells.
During prometaphase, CDK1-cyclin B phosphorylates vimentin at Ser56, which primes vimentin for phosphorylation at Ser83 by PLK1 in metaphase 74,75. Subsequently, during anaphase and telophase, Aurora-B phosphorylates vimentin located in the cleavage furrow at Ser72 and Rho K phosphorylates it at Ser71 76. Phosphorylation decreases the stability of vimentin-nestin heterotetramers. Nestin has a shorter N-terminal domain than vimentin, and the N-terminal region of vimentin has many phosphorylation sites involved in intermediate filament assembly. As a result, vimentin-nestin heterotetramers are likely to form less stable filaments during mitosis when vimentin phosphorylation is increased 72,73.
Vimentin filaments are not disassembled during mitosis in cells that lack nestin. The vimentin network is redistributed in cells lacking nestin during cytokinesis rather than disassembling (Figure 2A) 72. This occurs through the interaction of the vimentin tail (amino acids 412–466) with the actomyosin cortex formed during mitosis at the cell periphery 77. Vimentin interacts with actomyosin arcs during interphase, but the interaction of vimentin and the actomyosin cortex dramatically increases during mitosis 77,78. This is likely due to the mitotic-specific kinases that are upregulated during mitosis and that phosphorylate vimentin. The vimentin-actomyosin interaction is overridden during nestin expression resulting in vimentin filament collapse due to the decreased stability of the filaments that contain nestin.
The cytokeratin filamentous network is also reorganized during cell division in a phosphorylation-dependent manner 79. Phosphorylation occurs primarily in the N-terminal region of cytokeratin and is catalyzed by the same kinases that act on vimentin, such as CDK1, Aurora-B, and Rho 80. For cytokeratin filaments, the outcome of phosphorylation during mitosis is dependent on cellular context. An intact cytokeratin network is maintained and relocalized (Figure 2A), whereas in others, cytokeratin is solubilized in foci of concentrated cytokeratin granules (Figure 2B) 81. The outcome may be determined by kinase expression or by the types of cytokeratins expressed in the cell, much like how nestin determines whether vimentin filaments disassemble or relocalize. For example, the type II filament cytokeratin 5 contains unique phosphorylation sites relative to its heterodimer partner cytokeratin 14 80. Given the similarities between the cytokeratin and vimentin breakdown during cell division, it is likely that cytokeratin also interacts with the actin cortex in cells in which the filamentous structure is maintained for the proper division of cytokeratin. However, the C-terminal vimentin tail domain necessary for the actomyosin cortex interaction (amino acids 412–466) does not have significant homology to cytokeratin, suggesting that the molecular mechanism differs.
4.2. Phospho-malleability is essential for the proper functioning of both filaments
Phospho-malleability is the balance between effective phosphorylation by kinase and efficient resolution of phosphorylation by phosphatase. During mitosis, both vimentin and cytokeratin go through a series of phosphorylation and dephosphorylation events. Phosphorylation by mitosis-related kinases (e.g., PLK1, CDK1, Aurora B) induces reorganization or disassembly of the intermediate filaments, and these sites are subsequently dephosphorylated after mitosis by PP1 and PP2A phosphatases 82. Phospho-malleability can be disrupted through mutations that prevent proper phosphorylation (i.e., alanine substitutions), altered levels of kinases or phosphatases, or small-molecule compounds that alter the action of kinases or phosphatases.
In the cases of vimentin and cytokeratin, disrupting phospho-malleability has catastrophic effects on proper mitosis. Alanine substitutions or reduced phosphorylation of mitotic sites on vimentin (i.e., Ser39, Ser56, Ser72, or Ser83) result in cells that form intermediate filament bridges between daughter cells that prevent cytokinesis and segregation of nuclei, ultimately causing polyploidy (Figure 3A) 3,83. Alanine substitutions in vimentin also have impacts in vivo, as observed in genetically engineered mice expressing vimentin with alanine substitutions at multiple phosphorylation sites. These mice showed age-related phenotypes associated with increased senescence, specifically in tissues with high levels of vimentin expression attributed to the presence of polyploidy and binucleated cells 84,85. Polyploidy is associated with senescence and aging, as well as chromosomal instability and cancer 86,87.
Figure 3. Mutations in intermediate filament proteins result in mitotic defects in cell division.
A) Preventing phosphorylation through alanine mutations results in intermediate filaments that are unable to be disassembled during mitosis and cytokinesis. These filaments form an intermediate filament (IF) bridge between the daughter cells that can result in polyploidy. B) Phosphomimetic mutations or hyperphosphorylation of the intermediate filaments alters mitotic dynamics through tubulin localization and also leads to polyploidy.
Similar effects are observed upon inhibition of PLK1, a kinase involved in centrosome maturation and mitosis. PLK1 phosphorylates vimentin at Ser83, resulting in the collapse of vimentin filaments during mitosis and blocking the phosphorylation of vimentin with the PLK1 inhibitor volasertib results in polyploidy, much like the alanine mutations 88,89. Inhibiting phospho-malleability by mimicking or stabilizing the phosphorylation of these residues can be just as detrimental as preventing phosphorylation. The small molecule FiVe1, which binds directly to vimentin, stabilizes phosphorylation at Ser38, Ser56, and Ser83 and causes polyploidy 90.
Unlike the alanine substitutions that disrupt mitosis because vimentin remains filamentous, the stabilization of phosphorylation causes erroneous vimentin filamentous collapse leading to mitotic catastrophe and polyploidy (Figure 3B). This vimentin collapse results in tubulin mislocalization during mitosis, impairing tubulin function in the formation of the mitotic spindle. This suggests that vimentin aids in tubulin filament assembly during mitosis, much as it does during migration 63. However, vimentin’s role in the mitotic function of tubulin needs to be further investigated.
As it is for vimentin, cytokeratin phospho-malleability is also critical for proper cell division. If phosphorylations of cytokeratin by mitosis-related kinases CDK1 and Aurora-B are prevented by alanine substitutions, the intermediate filament-bridge formation that leads to polyploidy is observed (Figure 3A) 80. Although more work needs to be done to identify the context that regulates the choice between disassembly and localization of cytokeratin, it appears that the processes that cytokeratin and vimentin filaments undergo during mitosis are similar and similarly regulated.
5. Coexpression of vimentin and cytokeratin during EMT
Coexpression of epithelial-based cytokeratin and mesenchymal-related vimentin in cells is rare. Typically, cells adopt either a mesenchymal or epithelial differentiation state and express the corresponding filament protein. However, hybrid E/M cells co-express both cytokeratin and vimentin. These hybrid E/M cells are observed during embryonic development, wound healing, and carcinomas 91. As a result of the transient nature and rarity of hybrid E/M cells, there is ambiguity regarding the functions of vimentin and cytokeratin when natively expressed in the same cell. Epithelial cells engineered to express vimentin or microinjected with vimentin have been studied; however, the gradual induction of vimentin during the early stages of EMT may differ from these non-physiological increases of vimentin 92. Thus, it will be important to study the interplay between cytokeratin and vimentin during the early stages of EMT.
5.1. Cytokeratin is crucial in early EMT stages
To study the function of cytokeratin during EMT, epithelial cells are exposed to TGF-β1 in vitro, which induces the expression of several EMT factors such as Snail and Zeb1 in a Smad2/3-dependent manner 93–95. One would expect that cytokeratin would hinder EMT progression; however, it has EMT-promoting roles during the early stages of TGF-β1-induced EMT. Cytokeratin 18 promotes nuclear translocation and activation of Smad2/3, and cytokeratin 18-deficient cells lose epithelial markers and gain mesenchymal markers at a reduced rate relative to cells with endogenous levels of cytokeratin 18 96. The reason cytokeratin-18 contributes to EMT rather than hindering it is unknown, but cells deficient in cytokeratin-18 have decreased Smad2/3 phosphorylation. Cytokeratin-18 mediated translocation of Smad2/3 to the cell membrane may enhance its phosphorylation by the TGF-β1 receptor kinase. This suggests that cytokeratins are crucial during the early and potentially most important stages of EMT, functioning as a priming step for EMT induction in epithelial cells. More work needs to be performed to determine if this is a cytokeratin-18-specific effect or a shared property of cytokeratins. Interestingly, the cytoskeleton network is suggested to provide a pattern for the newly gained vimentin during EMT, further supporting the role of cytokeratin in the transition during EMT 97.
5.2. Vimentin reinforces EMT
Once EMT has initiated, vimentin plays an active role in enforcing the mesenchymal state. Vimentin is involved in signaling transduction that promotes and stabilizes pro-EMT pathways. Loss of vimentin is sufficient to override Slug- and Ras-driven EMT induction, characterized based on decreased migration and mesenchymal cellular phenotype, suggesting that vimentin functions as a signaling integrator in EMT 98. In addition, loss of vimentin results in a decrease of Axl expression; Axl is a receptor tyrosine kinase involved in metastasis, EMT, and stemness 98. Moreover, vimentin serves as a scaffold to recruit and localize proteins to mediate signal transduction. For example, vimentin interacts with both activated ERK and Slug, enhancing ERK-mediated phosphorylation of Slug at Ser87 99. This phosphorylation event activates Slug resulting in EMT induction and increased Axl expression 99. Recruitment of phosphorylated ERK to the vimentin filament network in vitro results in longer-lasting phosphorylation by shielding ERK from alkaline phosphatase 99.
How vimentin functions in cells of a hybrid E/M state are not understood as most previous studies have focused on the vimentin-positive cells with an extreme mesenchymal phenotype. The presence of vimentin in filamentous networks in hybrid E/M cells suggests that the mesenchymal-like enforcing functions of vimentin observed during EMT are active 13. Thus, in E/M hybrid cells, vimentin likely functions in EMT-associated signaling by acting as a signaling scaffold. Analysis of the effect of the vimentin filaments on signal transduction pathways and functional interactions of vimentin with EMT-related signaling proteins will help understand how cells progress through the spectrum of EMT.
5.3. Cellular dominance of vimentin over cytokeratin
Introducing vimentin through ectopic expression or microinjection in epithelial cells leads to the adoption of a mesenchymal-like morphology with a loss of desmosome contacts and increased cell motility 92. Furthermore, when mesenchymal cells are depleted of vimentin, migration is decreased, and cells adopt an epithelial-like morphology 100. These data support the idea that vimentin has a dominant function in cells that co-express vimentin and cytokeratins and that vimentin dictates the morphology and interactions of these cells. However, further studies will be needed to determine how exogenous cytokeratin functions in mesenchymal cells. Analyses revealed the locations of vimentin and cytokeratin in hybrid E/M cells further support the idea that vimentin filaments dominate cytokeratin 13,101,102. Hybrid E/M cells can be isolated using a combination of the basal epithelial marker CD104+ (also known as Integrin β4), which is related to stemness, and the CD44hi adhesion marker associated with tumorigenic breast cancer cells 13,101,102. The hybrid E/M cells isolated with these markers express cytokeratin and vimentin and have increased tumorigenicity compared to epithelial and extreme mesenchymal cells isolated from the same parental population 13.
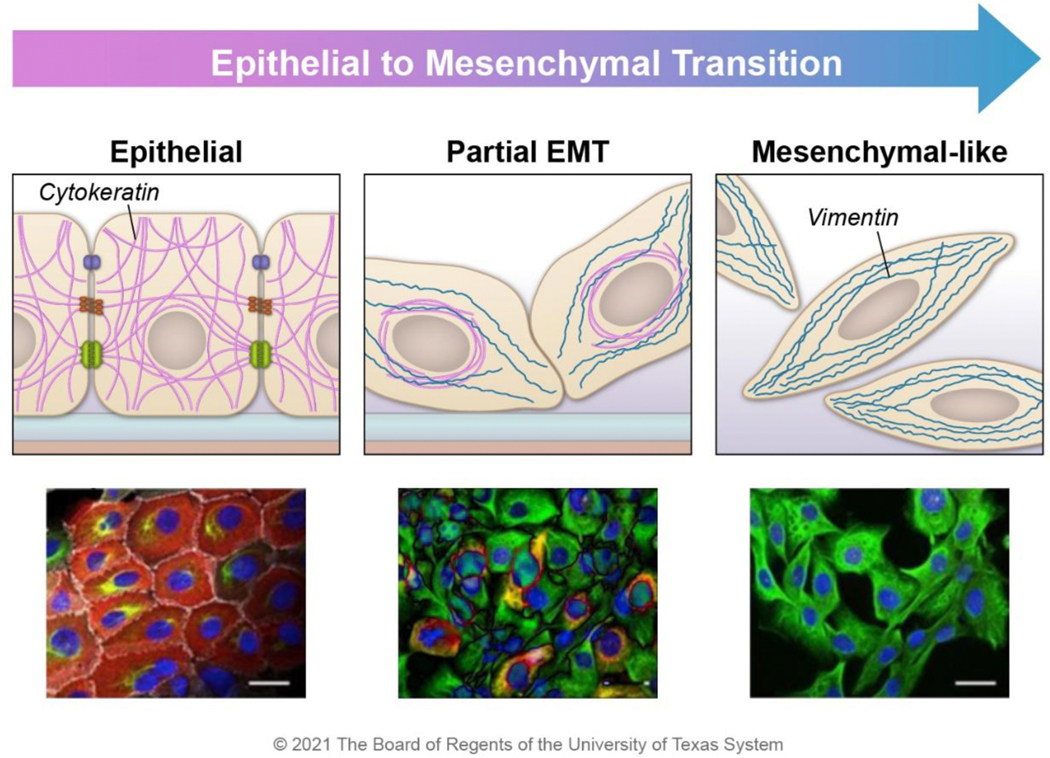
Interestingly, in CD104+ CD44hi cells that express cytokeratin and vimentin, cytokeratin is collapsed around the nucleus instead of its normal cytoplasm spanning meshwork (Figure 4) 13. In contrast, vimentin forms a filamentous network that reaches from the nucleus to the cell periphery and resembles the network observed in mesenchymal-like cells (Figure 4) 13. Because of these cells’ robust filamentous vimentin network, vimentin may perform most intermediate filament functions in stress resistance, cell stability, organelle positioning, and signaling processes.
Figure 4. Intermediate filament expression changes during EMT.
Epithelial-like cancer cells express cytokeratin (red), which aids in stress resistance and supports cell-cell adhesion; these cells do not express vimentin. As cells progress through EMT, mesenchymal markers such as vimentin (green) begin to be expressed. In E/M hybrid cells, cytokeratin is perinuclearly localized, and the vimentin filament network stretches from the nucleus to the periphery of the cell. At the far end of the EMT spectrum, epithelial markers are lost. The cartoons were constructed based on the observed morphology of HMLER cells during the transition from epithelial (CD104+ CD44low) to hybrid E/M (CD104+ CD44hi) to mesenchymal (CD104− CD44hi) populations 13. Images are reprinted with permission from Kröger, C. et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc. Natl. Acad. Sci. 116, 7353–7362 (2019).
The mechanism that results in vimentin’s dominance at the cell periphery and cytokeratin’s perinuclear localization is unknown. The reduction in desmosomes in hybrid E/M cells may decrease the recruitment of cytokeratin to the cell membrane to form filamentous networks, leading to perinuclear collapse. Concurrently, various integrins in the hybrid E/M cell may recruit vimentin to the membrane forming a filamentous network spanning the cytoplasm. Alternatively, kinases and signaling pathways altered in hybrid E/M cells may specifically increase cytokeratin phosphorylation leading to more perinuclear localization. Regardless of the reason, it is unlikely that cytokeratin is non-functional in the hybrid E/M cells. Instead, the interplay between the two filaments in this unique population likely provides some beneficial properties to the cancer cells during dissemination and invasion.
6. Coexpression of vimentin and cytokeratin in carcinomas benefits cancer
The cooperation between cytokeratin and vimentin is likely to enhance carcinoma progression since cells with both epithelial and mesenchymal traits possess higher levels of tumorigenesis than those that are phenotypically epithelial or mesenchymal 13. Collective cell migration is proposed to be a major driver of invasion and metastasis in carcinomas 35,103,104. To disseminate, tumor cell clusters first adhere to and then invade the tissue surrounding the primary tumor. A major contributor to maintaining cell-cell contacts during collective cell migration is the adhesion molecule E-cadherin. E-cadherin is important for epithelial cells, and it also has pro-metastatic roles, suggesting that retaining some epithelial properties is beneficial in carcinoma progression 105,106. The invasive leader cells express basal epithelial genes in collective migration, specifically the basal cytokeratin 14 107. These leader cells also express EMT transcription factors such as Snail, suggesting they could be hybrid E/M cells 108. Although these pro-metastatic processes rely on retaining epithelial and mesenchymal properties in the invasive cells, the extent to which the coexpression of vimentin and cytokeratin is important in these cells is unknown.
Direct interactions between cytokeratin and vimentin have been described in non-cancerous cell types such as human epidermal keratinocytes 109. In these cells, vimentin and the type, I cytokeratin 14 interact depending on the vimentin amino acid sequence YRKLLEGEE. Interestingly, this vimentin amino acid sequence is found in cytokeratin 4 and is required for cytokeratin-cytokeratin interaction between cytokeratins 14 and 4 110. Disruption of this sequence in vimentin results in reduced vimentin-cytokeratin interaction as shown by immunoprecipitation and decreased keratinocyte migration, suggesting a functional role for the interaction between vimentin and cytokeratin. It may be that this interaction also occurs between vimentin and another type I cytokeratin in hybrid E/M cells to facilitate migration. Suppose this type of interaction enhances the migratory properties of carcinoma cells or enhances the ability of cell clusters to withstand migratory stress. In that case, it should be considered as a putative target for drug development. More work needs to be done to characterize the cytokeratin-vimentin interactions in carcinomas and to determine their functional effects.
6.1. Novel roles for vimentin identified using lineage tracing of EMT
To characterize the role of EMT during carcinoma progression, lineage tracing has been performed using the mesenchymal marker fsp-1 to mark cells that have undergone EMT 111. Although this study suggested that the gain of mesenchymal properties may not be critical for metastasis, it did show that the cells marked for late EMT lineage by fsp-1 promoter activity functioned to drive local invasion out of the region of the primary tumor 111. This study was able to mark cells that had fully undergone EMT, but there are shortcomings with the lineage tracing system regarding EMT. The ability of fsp-1 to accurately mark cells that have undergone EMT is uncertain as cells with EMT properties were falsely identified as EMT-null and unlabeled 112. Because of this experimental issue, there are likely contributions of cells in the hybrid E/M state to metastasis not observed in lineage tracing studies. Regardless, the lineage tracing analysis suggests different functional roles of cells based on their progression on the EMT transition path, with cells that are more mesenchymal-like functioning in the local invasion.
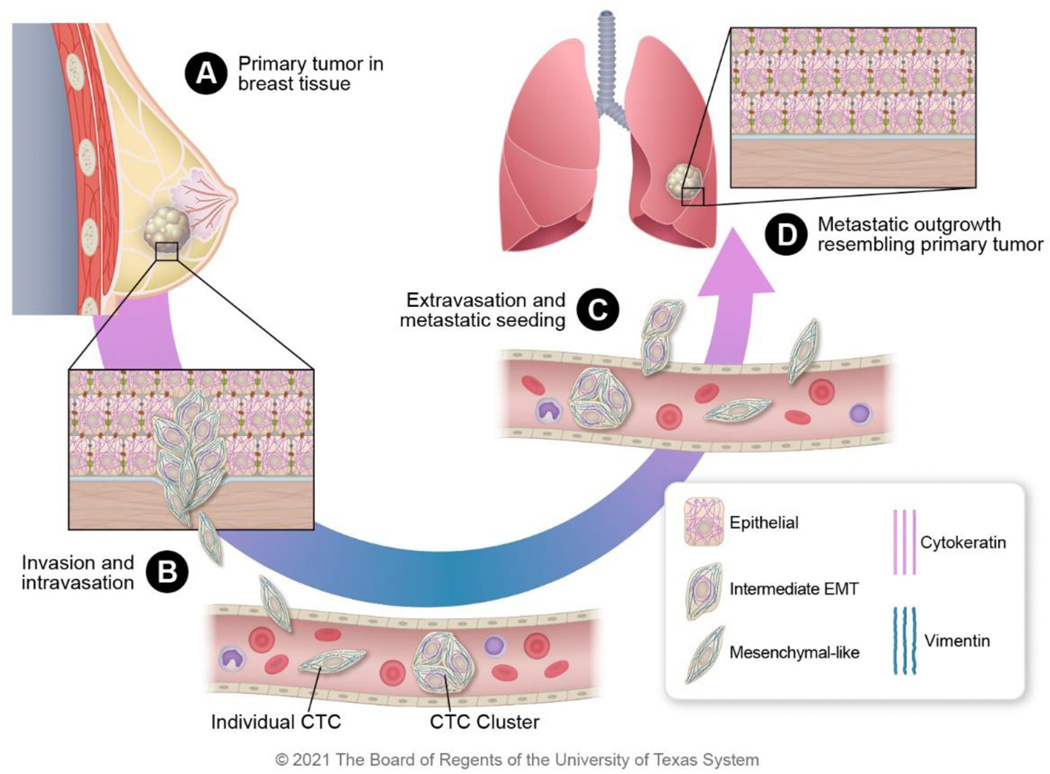
Our current understanding of the intermediate filament’s role during carcinoma progression from the primary tumor to metastasis is summarized in Figure 5. Initial invasion is spearheaded by leader cells with more mesenchymal properties than the bulk of the collectively migrating cells. The initial invasion is followed by intravasation into the bloodstream. Individual cancer cells and clusters of circulating tumor cells with the hybrid E/M phenotype can survive better in the bloodstream in part due to shear stress resistance imparted by vimentin expression 113. The circulating tumor cells then extravasate into the metastatic site by exiting the blood vessel. During extravasation, cells experience other types of shear stress; thus, both vimentin and cytokeratin expression may be beneficial. Eventually, cells that seed the metastatic site proliferate, and these cells resemble the morphology of the primary tumor with dominant cytokeratin expression and a more differentiated phenotype.
Figure 5: Intermediate filament expression changes during cancer progression.
A) During primary tumor growth in the breast, some cells begin to undergo EMT. B) The cells with mesenchymal-like phenotypes function as leader cells during invasion and intravasation into the bloodstream. Individual circulating tumor cells (CTCs) and clusters of CTCs have both been observed in circulation. C) CTCs extravagate to metastatic sites to seed the tissues and remain dormant (in lungs in this example). D) At the metastatic site, the dormant carcinoma cells resume proliferation and ultimately resemble the primary tumor with largely epithelial morphology as a result of the mesenchymal to epithelial transition.
7. Future perspective: targeting intermediate filaments in cancer
Metastasis is a major contributor to mortality in cancer patients 114. Identifying the unique functions of metastatic cancer cells should lead to the development of therapeutics to treat and eliminate this disease. EMT imparts properties of highly malignant tumors to carcinoma cells and is tightly associated with metastasis. The unique pattern of vimentin upregulation that occurs during EMT in cancer cells suggests that interfering with vimentin function is a potential therapeutic strategy to block metastatic disease progression. A benefit of targeting vimentin to block EMT is that disruption of vimentin expression or phosphorylation has marginal side effects in mice 51–53,84,85. This supports the hypothesis that targeting vimentin to inhibit EMT would not have serious detrimental side effects on the non-diseased cell population and would limit cancer progression to metastatic disease.
The area of targeting vimentin intermediate filaments that are gained during EMT in carcinomas is promising 115. Small molecules have been identified that inhibit vimentin filament formation by altering vimentin phosphorylation at assembly residues, demonstrating anti-cancer effects due to vimentin’s importance on cell division without impacting vimentin-negative cells (Figure 6A) 90,116,117. The disruption of vimentin filaments leads to mitotic catastrophe and polyploidy due to mislocalization of vimentin and tubulin during mitosis 90. Mislocalization of vimentin is a common effect of targeting the intermediate filament with a small molecule, and this observation has been used to identify vimentin targeting small molecules 118.
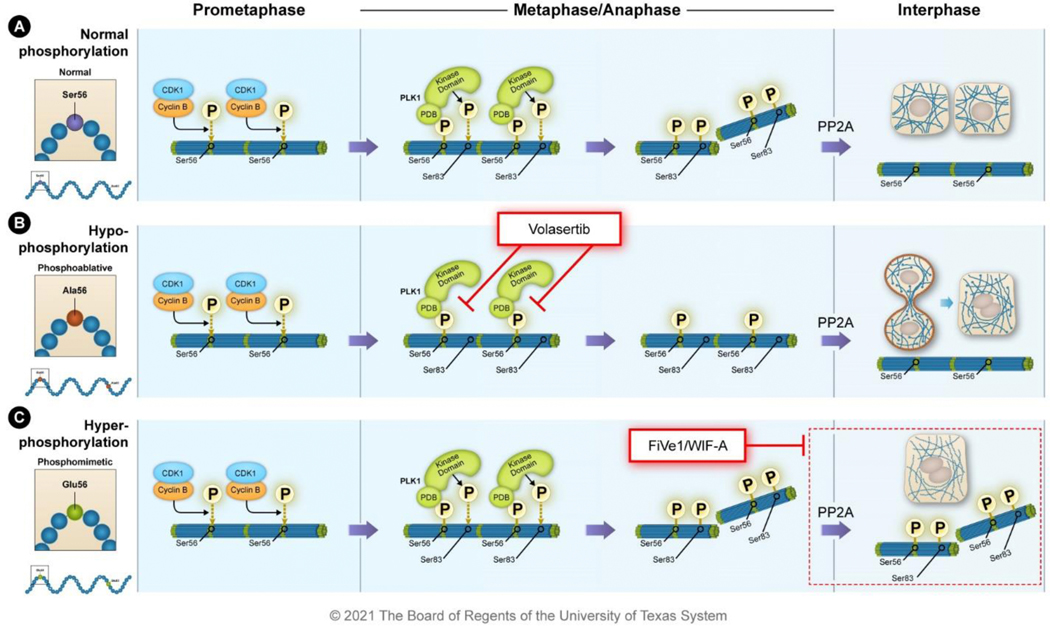
Figure 6. Small-molecule inhibitors have been identified that disrupt vimentin phospho-malleability.
A) During prometaphase, vimentin is phosphorylated at Ser56 by CDK1-cyclin B; this primes subsequent phosphorylation events. The polo-box domain (PDB) of PLK1 binds to the primed phosphorylation site at Ser56 and the kinase domain of PLK1 phosphorylates vimentin Ser83. This leads to the disassembly of the 10-nm filaments into ULFs during metaphase and anaphase. The phosphatase PP2A dephosphorylates vimentin stabilizing the filaments after cytokinesis. B) The PLK1 inhibitor volasertib binds to the ATP pocket of PLK1 preventing phosphorylation of substrates like vimentin. In the presence of volasertib, vimentin remains filamentous during metaphase causing issues during mitosis and cytokinesis that culminate in intermediate filament bridges and polyploidy. C) The small molecules FiVe1 and Withaferin-A (WIF-A) stabilize vimentin phosphorylation causing improper destabilization of vimentin filaments resulting in mitotic catastrophe and polyploidy.
The PLK1 inhibitor, volasertib, inhibits the phospho-malleability of vimentin by reducing the phosphorylation at Ser83 (Figure 6B). Volasertib binds the ATP-binding pocket of PLK1, reducing kinase activity to decrease phosphorylation of downstream targets such as vimentin 119,120. In cell-based assays, volasertib treatment decreases vimentin phosphorylation at Ser83 and polyploidy, much like phosphoablative mutations at this key residue 74,88,89. In phase I clinical trials, volasertib showed low toxicity, long terminal half-life, a good pharmacokinetic profile, and efficacy in patients with solid tumors and leukemia 121–123. Volasertib was shown to have a good safety profile in a phase II trial but lacked efficacy as a monotherapy in patients with urothelial cancer who had progressed following platinum-based chemotherapies 124. However, when volasertib was combined with the anti-metabolite cytarabine to treat patients with acute myeloid leukemia, the response rate was higher in the combination therapy group versus cytarabine alone 125. This suggests potential synergistic roles of volasertib that should be further investigated for anti-tumoral effects in both solid tumors and leukemia. These clinical trials did not evaluate the impact of volasertib on vimentin function but instead focused on its general PLK1 inhibitory action due to the known roles of PLK1 during mitosis. Thus, preclinical trials will need to be done to assess the impact of volasertib inhibition on vimentin, the hybrid E/M cell phenotype, and tumor progression and drug resistance.
Another class of phospho-malleability-inhibiting small molecules are known to increase vimentin phosphorylation. The small molecule FiVe1 directly binds to the rod domain of vimentin stabilizing vimentin phosphorylation at Ser56, leading to multinucleation of mesenchymal-like breast cancer cells (Figure 6C) 90. This leads to perinuclear vimentin aggregation as well as tubulin mislocalization during mitosis. FiVe1 also decreases the migratory and stem cell properties of cancer cells in culture. FiVe1 is currently being evaluated in preclinical studies, and more research is needed to determine its efficacy and safety. The action of FiVe1 is similar to that of Withaferin-A (WIF-A), which also leads to an increase of vimentin phosphorylation at Ser56 and inhibition of invasion and metastasis of breast cancer cells (Figure 6C) 90,126. WIF-A’s covalent binding to vimentin results in vimentin aggregation, aneuploidy, and apoptosis 127. However, WIF-A has several targets other than vimentin such as GFAP, β-tubulin, NF-κB, and Sp1 so treatment with this compound may have undesirable side effects 116,128,129. WIF-A has been evaluated in phase I clinical trials for various diseases, including osteosarcomas, and it was well tolerated. However, the formulation for oral delivery needs to be improved before antitumor effects are tested clinically 130,131.
The mechanism that results in the stabilization of vimentin Ser56 phosphorylation by FiVe1 and WIF-A is unknown. Based on the direct binding to vimentin by FiVe1, these small molecules likely bind to vimentin and competitively prevent the interaction of vimentin with the phosphatase PP2A 82. It is more likely that a small molecule prevents a normal interaction, such as dephosphorylation, from occurring than that it induces a gain of interaction such as promoting the interaction between vimentin and a kinase. However, the molecular mechanisms by which FiVe1 or WIF-A results in increased phosphorylation needs to be elucidated.
The statin simvastatin also targets vimentin leading to decreased proliferation and apoptosis 118. Simvastatin treatment results in vimentin perinuclear reorganization. Unlike the previously discussed compounds, simvastatin does not appear to function through altering the major vimentin phospho-sites (Ser39, Ser56, or Ser83); however, it did increase the negative charge of vimentin suggesting some type of posttranslational modification 118. More work will be needed to identify the targeting mechanism of simvastatin towards vimentin and the functional outcomes.
8. Conclusion
The functions of cytokeratin and vimentin in epithelial and mesenchymal cells, respectively, are well understood; however, the interplay that occurs upon vimentin induction in epithelial cells during EMT is not. Cytokeratin and vimentin intermediate filaments have similar functions: these filaments impart stress resistance, role in signaling pathways, and support the cytoskeleton and protein complexes at the cell periphery. However, distinctions that derive from different interacting partners and structures lead to their various functions. This results in critical differences in epithelial- and mesenchymal-like cells during EMT and in hybrid E/M cells, which have been implicated in driving cancer progression. Due to the significance of these cancer cells with epithelial and mesenchymal features in carcinoma progression and metastasis, understanding the properties of intermediate filaments in the rare cells that co-express vimentin and cytokeratin is critical for developing novel therapeutic options. There are promising therapeutics under development to target vulnerabilities in the hybrid E/M cells that play an important role in carcinoma progression; however, more work needs to be performed, and more needs to be learned before these therapies can benefit patients.
Acknowledgments
Funding information:
This research was supported by CPRIT (RP160710/RP170172), NSF (15-597-1605817), NIH/NCI (R01CA200970, 2R01CA155243), and the Bowes Foundation, (to S.A.M.) and NIH T32 (5T32CA186892) (to N.A.K).
Abbreviations
- CK
cytokeratin
- EMT
epithelial-mesenchymal transition
- Hybrid E/M
Hybrid cells with epithelial and mesenchymal properties
- Ser
Serine
- ULFs
unit length filaments
- CSCs
cancer stem-like cells
- FSP1
fibroblast-specific protein 1
- ERK
extracellular-signal-regulated kinase
- PLK1
polo-like kinase 1
- ATK1
protein kinase B
- TGF-β1
Transforming growth factor-beta 1
- CDK1
cyclin-dependent kinase 1
- GEF
guanine nucleotide exchange factor
- WIF-A
withaferin A
- FiVe1
FOXC2-inhibiting Vimentin effector 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snider NT & Omary MB Post-translational modifications of intermediate filament proteins: Mechanisms and functions. Nat. Rev. Mol. Cell Biol 15, 163–177 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivaska J. et al. PKCε-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 24, 3834–3845 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura Y, Kasahara K. & Inagaki M. Intermediate filaments and IF-associated proteins: from cell architecture to cell proliferation. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci 95, 479–493 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo M. et al. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys. J 105, 1562–1568 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szeverenyi I. et al. The human intermediate filament database: Comprehensive information on a gene family involved in many human diseases. Hum. Mutat 29, 351–360 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Gruenbaum Y. & Foisner R. Lamins: Nuclear Intermediate Filament Proteins with Fundamental Functions in Nuclear Mechanics and Genome Regulation. Annu. Rev. Biochem 84, 131–164 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Sharma P, Alsharif S, Fallatah A. & Chung BM Intermediate Filaments as Effectors of Cancer Development and Metastasis: A Focus on Keratins, Vimentin, and Nestin. Cells 1–21 (2019) doi: 10.3390/cells8050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob JT, Coulombe PA, Kwan R. & Omary MB Types I and II keratin intermediate filaments. Cold Spring Harb. Perspect. Biol 10, 1–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokkinos MI et al. Vimentin and epithelial-mesenchymal transition in human breast cancer - Observations in vitro and in vivo. Cells Tissues Organs 185, 191–203 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Dongre A. & Weinberg RA New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol 20, 69–84 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R. & Weinberg RA The basics of epithelial-mesenchymal transition. J. Clin. Invest 119, 1420–1428 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFaline-Figueroa JL et al. A pooled single-cell genetic screen identifies regulatory checkpoints in the continuum of the epithelial-to-mesenchymal transition. Nat. Genet 51, 1389–1398 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kröger C. et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc. Natl. Acad. Sci 116, 7353–7362 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jolly MK et al. Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol. Ther 194, 161–184 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Hendrix MJC, Seftor EA, Seftor REB & Trevor KT Experimental coexpression of vimentin and keratin intermediate filaments in human breast cancer cells results in phenotypic interconversion and increased invasive behavior. Am. J. Pathol 150, 483–495 (1997). [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas PA et al. Association between keratin and vimentin expression, malignant phenotype, and survival in postmenopausal breast cancer patients. Clin. Cancer Res 5, 2698–2703 (1999). [PubMed] [Google Scholar]
- 17.Hendrix MJC et al. Coexpression of vimentin and keratins by human melanoma tumor cells: Correlation with invasive and metastatic potential. J. Natl. Cancer Inst 84, 165–174 (1992). [DOI] [PubMed] [Google Scholar]
- 18.Armstrong AJ et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res 9, 997–1007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweizer J. et al. New consensus nomenclature for mammalian keratins. J. Cell Biol 174, 169–174 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moll R, Franke WW, Schiller DL, Geiger B. & Krepler R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 31, 11–24 (1982). [DOI] [PubMed] [Google Scholar]
- 21.Moll R, Divo M. & Langbein L. The human keratins: Biology and pathology. Histochem. Cell Biol 129, 705–733 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinert PM, Idler WW & Zimmerman SB Self-assembly of bovine epidermal keratin filaments in vitro. J. Mol. Biol 108, 547–567 (1976). [DOI] [PubMed] [Google Scholar]
- 23.Lu X. & Lane EB Retrovirus-mediated transgenic keratin expression in cultured fibroblasts: Specific domain functions in keratin stabilization and filament formation. Cell 62, 681–696 (1990). [DOI] [PubMed] [Google Scholar]
- 24.Stappenbeck TS et al. Functional analysis of desmoplakin domains: Specification of the interaction with keratin versus vimentin intermediate filament networks. J. Cell Biol 123, 691–705 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowery J, Kuczmarski ER, Herrmann H. & Goldma RD Intermediate filaments play a pivotal role in regulating cell architecture and function. J. Biol. Chem 290, 17145–17153 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shafraz O. et al. E-cadherin binds to desmoglein to facilitate desmosome assembly. Elife 7, 1–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanada S. et al. Keratin-containing inclusions affect cell morphology and distribution of cytosolic cellular components. Exp. Cell Res 304, 471–482 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Ameen NA, Figueroa Y. & Salas PJI Anomalous apical plasma membrane phenotype in CK8-deficient mice indicates a novel role for intermediate filaments in the polarization of simple epithelia. J. Cell Sci 114, 563–575 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Palacios F, Tushir JS, Fujita Y. & D’Souza-Schorey C. Lysosomal Targeting of E-Cadherin: a Unique Mechanism for the Down-Regulation of Cell-Cell Adhesion during Epithelial to Mesenchymal Transitions. Mol. Cell. Biol 25, 389–402 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilhelmsen K. et al. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J. Cell Biol 171, 799–810 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramms L. et al. Keratins as the main component for the mechanical integrity of keratinocytes. Proc. Natl. Acad. Sci. U. S. A 110, 18513–18518 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber GF, Bjerke MA & DeSimone DW A Mechanoresponsive Cadherin-Keratin Complex Directs Polarized Protrusive Behavior and Collective Cell Migration. Dev. Cell 22, 104–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinlan RA et al. A rim-and-spoke hypothesis to explain the biomechanical roles for cytoplasmic intermediate filament networks. J. Cell Sci 130, 3437–3445 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedl P. & Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol 10, 445–457 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Aceto N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long HA, Boczonadi V, McInroy L, Goldberg M. & Määttä A. Periplakin-dependent reorganisation of keratin cytoskeleton and loss of collective migration in keratin-8-downregulated epithelial sheets. J. Cell Sci 119, 5147–5159 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Yin M. & Zhang L. Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 8, 807 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong P. & Coulombe PA Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J. Cell Biol 163, 327–337 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamcheu JC et al. Keratin gene mutations in disorders of human skin and its appendages. Arch. Biochem. Biophys 508, 123–137 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang P, Sharpe CR, Mohun TJ & Wylie CC Vimentin expression in oocytes, eggs and early embryos of Xenopus laevis. Development 103, 279–287 (1988). [DOI] [PubMed] [Google Scholar]
- 41.Eriksson JE et al. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J. Cell Sci 117, 919–932 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Sihag RK, Inagaki M, Yamaguchi T, Shea TB & Pant HC Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp. Cell Res 313, 2098–2109 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmisano MG et al. Skeletal muscle intermediate filaments form a stress-transmitting and stress-signaling network. J. Cell Sci 128, 219–224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kartenbeck J, Franke WW, Moser JG & Stoffels U. Specific attachment of desmin filaments to desmosomal plaques in cardiac myocytes. EMBO J. 2, 735–742 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kartenbeck J, Schwechheimer K, Moll R. & Franke WW Attachment of vimentin filaments to desmosomal plaques in human meningiomal cells and arachnoidal tissue. J. Cell Biol 98, 1072–1081 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J. et al. Vimentin filaments regulate integrin-ligand interactions by binding to the cytoplasmic tail of integrin β3. J. Cell Sci 129, 2030–2042 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Bhattacharya R. et al. Recruitment of vimentin to the cell surface by β3 integrin and plectin mediates adhesion strength. J. Cell Sci 122, 1390–1400 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendez MG, Restle D. & Janmey PA Vimentin enhances cell elastic behavior and protects against compressive stress. Biophys. J 107, 314–323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kidd ME, Shumaker DK & Ridge KM The role of Vimentin intermediate filaments in the progression of lung cancer. Am. J. Respir. Cell Mol. Biol 50, 1–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu J. et al. High stretchability, strength, and toughness of living cells enabled by hyperelastic vimentin intermediate filaments. Proc. Natl. Acad. Sci 201903890 (2019) doi: 10.1073/pnas.1903890116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colucci-Guyon E. et al. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 79, 679–694 (1994). [DOI] [PubMed] [Google Scholar]
- 52.Peuhu E, Virtakoivu R, Mai A, Wärri A. & Ivaska J. Epithelial vimentin plays a functional role in mammary gland development. Dev. 144, 4103–4113 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Langlois B. et al. Vimentin knockout results in increased expression of sub-endothelial basement membrane components and carotid stiffness in mice. Sci. Rep 7, 1–15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Battaglia RA, Delic S, Herrmann H. & Snider NT Vimentin on the move: New developments in cell migration. F1000Research 7, 1–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng F. et al. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc. Natl. Acad. Sci. U. S. A 113, E4320–E4327 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ivaska J, Pallari HM, Nevo J. & Eriksson JE Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res 313, 2050–2062 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Wyckoff JB et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67, 2649–2656 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Caswell PT & Zech T. Actin-Based Cell Protrusion in a 3D Matrix. Trends Cell Biol. 28, 823–834 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiu Y. et al. Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA. J. Cell Sci 130, 892–902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, He L, Wu YI, Hahn KM & Montell DJ Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat. Cell Biol 12, 591–597 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colburn ZT & Jones JCR Complexes of α6β4 integrin and vimentin act as signaling hubs to regulate epithelial cell migration. J. Cell Sci 131, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Etienne-Manneville S. Microtubules in Cell Migration. Annu. Rev. Cell Dev. Biol 29, 471–499 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Gan Z. et al. Vimentin Intermediate Filaments Template Microtubule Networks to Enhance Persistence in Cell Polarity and Directed Migration. Cell Syst. 3, 252–263.e8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu QS et al. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene 30, 457–470 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Messica Y. et al. The role of Vimentin in Regulating Cell Invasive Migration in Dense Cultures of Breast Carcinoma Cells. Nano Lett. 17, 6941–6948 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Kumemura H. et al. Aggregation and Loss of Cytokeratin Filament Networks Inhibit Golgi Organization in Liver-Derived Epithelial Cell Lines. Cell Motil. Cytoskeleton 57, 37–52 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Toivola DM, Tao GZ, Habtezion A, Liao J. & Omary MB Cellular integrity plus: Organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 15, 608–617 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Gao YS & Sztul E. A novel interaction of the Golgi complex with the vimentin intermediate filament cytoskeleton. J. Cell Biol 152, 877–893 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biskou O. et al. The type III intermediate filament vimentin regulates organelle distribution and modulates autophagy. PLoS One 14, 1–20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang HL et al. Vimentin supports mitochondrial morphology and organization. Biochem. J 410, 141–146 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Li Y. et al. Moving Cell Boundaries Drive Nuclear Shaping during Cell Spreading. Biophys. J 109, 670–686 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chou YH, S K, Herrmann H. & Goldman RD. Nestin Promotes the Phosphorylation-dependent Disassembly of Vimentin Intermediate Filaments During Mitosis. Mol. Biol. Cell 14, 1468–1478 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steinert PM et al. A high molecular weight intermediate filament-associated protein in BHK- 21 cells is nestin, a type VI intermediate filament protein: Limited co- assembly in vitro to form heteropolymers with type III vimentin and type IV α-internexin. J. Biol. Chem 274, 9881–9890 (1999). [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi T. et al. Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis. J. Cell Biol 171, 431–436 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsujimura K. et al. Visualization and function of vimentin phosphorylation by cdc2 kinase during mitosis. J. Biol. Chem 269, 31097–31106 (1994). [PubMed] [Google Scholar]
- 76.Goto H. et al. Aurora-B regulates the cleavage furrow-specific vimentin phosphorylation in the cytokinetic process. J. Biol. Chem 278, 8526–8530 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Duarte S. et al. Vimentin filaments interact with the actin cortex in mitosis allowing normal cell division. Nat. Commun 10, 4200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiu Y. et al. Bidirectional Interplay between Vimentin Intermediate Filaments and Contractile Actin Stress Fibers. Cell Rep. 11, 1511–1518 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Sawant MS & Leube RE Consequences of Keratin Phosphorylation for Cytoskeletal Organization and Epithelial Functions. International Review of Cell and Molecular Biology vol. 330 (Elsevier Inc., 2017). [DOI] [PubMed] [Google Scholar]
- 80.Inaba H. et al. Regulation of keratin 5/14 intermediate filaments by CDK1, Aurora-B, and Rho-kinase. Biochem. Biophys. Res. Commun 498, 544–550 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Lane EB, Goodman SL & Trejdosiewicz LK Disruption of the keratin filament network during epithelial cell division. EMBO J. 1, 1365–1372 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turowski P, Myles T, Hemmings B, Fernandez A. & Lamb NJ Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol. Biol. Cell 10, 1997–2015 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yasui Y. et al. Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene 20, 2868–2876 (2001). [DOI] [PubMed] [Google Scholar]
- 84.Matsuyama M. et al. Defect of mitotic vimentin phosphorylation causes microophthalmia and cataract via aneuploidy and senescence in lens epithelial cells. J. Biol. Chem 288, 35626–35635 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka H. et al. Cytokinetic failure-induced tetraploidy develops into aneuploidy, triggering skin aging in phosphovimentin-deficient mice. J. Biol. Chem 290, 12984–12998 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erenpreisa J. & Cragg MS Three steps to the immortality of cancer cells: Senescence, polyploidy and self-renewal. Cancer Cell Int. 13, 1–12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sliwinska MA et al. Induction of senescence with doxorubicin leads to increased genomic instability of HCT116 cells. Mech. Ageing Dev 130, 24–32 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Sanhaji M. et al. p53 is not directly relevant to the response of Polo-like kinase 1 inhibitors. Cell Cycle 11, 543–553 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Singh R. et al. Non- canonical cMet regulation by vimentin mediates Plk1 inhibitor–induced apoptosis. EMBO Mol. Med 11, 1–20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bollong MJ et al. A vimentin binding small molecule leads to mitotic disruption in mesenchymal cancers. Proc. Natl. Acad. Sci 114, E9903–E9912 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sha Y. et al. Intermediate cell states in epithelial-to-mesenchymal transition. Phys. Biol 16, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mendez MG, Kojima SI & Goldman RD Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 24, 1838–1851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Derynck R. & Zhang YE Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature vol. 425 577–584 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Miettinen PJ, Ebner R, Lopez AR & Derynck R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: Involvement of type I receptors. J. Cell Biol 127, 2021–2036 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi Y. & Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Jung H, Kim B, Moon BI & Oh ES Cytokeratin 18 is necessary for initiation of TGF-β1-induced epithelial–mesenchymal transition in breast epithelial cells. Mol. Cell. Biochem 423, 21–28 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Pagan R, Martín I, Alonso A, Llobera M. & Vilaró S. Vimentin filaments follow the preexisting cytokeratin network during epithelial-mesenchymal transition of cultured neonatal rat hepatocytes. Exp. Cell Res 222, 333–344 (1996). [DOI] [PubMed] [Google Scholar]
- 98.Vuoriluoto K. et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 30, 1436–1448 (2011). [DOI] [PubMed] [Google Scholar]
- 99.Virtakoivu R. et al. Vimentin-ERK signaling uncouples slug gene regulatory function. Cancer Res. 75, 2349–2362 (2015). [DOI] [PubMed] [Google Scholar]
- 100.Liu C, Lin H, Tang M. & Wang Y. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. 6, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bierie B. et al. Integrin-β4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc. Natl. Acad. Sci. U. S. A 114, E2337–E2346 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ & Clarke MF Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A 100, 3983–3988 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheung KJ et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl. Acad. Sci. U. S. A 113, E854–E863 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bronsert P. et al. Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer-host interface. J. Pathol 234, 410–422 (2014). [DOI] [PubMed] [Google Scholar]
- 105.Shamir ER et al. Twist1-induced dissemination preserves epithelial identity and requires E-cadherin. J. Cell Biol 204, 839–856 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shamir ER & Ewald AJ Adhesion in mammary development: Novel roles for E-cadherin in individual and collective cell migration. Current Topics in Developmental Biology vol. 112 (Elsevier Inc., 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheung KJ, Gabrielson E, Werb Z. & Ewald AJ Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155, 1639–1651 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ye X. et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525, 256–260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Velez-Delvalle C, Marsch-Moreno M, Castro-Muñozledo F, Galván-Mendoza IJ & Kuri-Harcuch W. Epithelial cell migration requires the interaction between the vimentin and keratin intermediate filaments. Sci. Rep 6, 1–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herrmann H. et al. The intermediate filament Protein Consensus motif of helix 2B: Its atomic structure and contribution to assembly. J. Mol. Biol 298, 817–832 (2000). [DOI] [PubMed] [Google Scholar]
- 111.Lourenco AR et al. Differential contributions of pre- and post- EMT tumor cells in breast cancer metastasis. Cancer Res. (2019) doi: 10.1158/0008-5472.CAN-19-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bornes L. et al. Fsp1-Mediated Lineage Tracing Fails to Detect the Majority of Disseminating Cells Undergoing EMT. Cell Rep. 29, 2565–2569.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jolly MK et al. Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front. Oncol 5, 1–19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steeg PS Targeting metastasis. Nat. Rev. Cancer 16, 201–218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Strouhalova K. et al. Vimentin intermediate filaments as potential target for cancer treatment. Cancers (Basel). 12, 1–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bargagna-Mohan P. et al. Withaferin A targets intermediate filaments glial fibrillary acidic protein and vimentin in a model of retinal gliosis. J. Biol. Chem 285, 7657–7669 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thaiparambil JT et al. Withaferin A inhibits breast cancer invasion and metastasis at subcytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer 129, 2744–2755 (2011). [DOI] [PubMed] [Google Scholar]
- 118.Trogden KP et al. An image-based small-molecule screen identifies vimentin as a pharmacologically relevant target of simvastatin in cancer cells. FASEB J. 32, 2841–2854 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rudolph D. et al. Efficacy and mechanism of action of volasertib, a potent and selective inhibitor of polo-like kinases, in preclinical models of acute myeloid leukemia. J. Pharmacol. Exp. Ther 352, 579–589 (2015). [DOI] [PubMed] [Google Scholar]
- 120.Rudolph D. et al. BI 6727, a polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin. Cancer Res 15, 3094–3102 (2009). [DOI] [PubMed] [Google Scholar]
- 121.Lin CC et al. A phase i study of two dosing schedules of volasertib (BI 6727), an intravenous polo-like kinase inhibitor, in patients with advanced solid malignancies. Br. J. Cancer 110, 2434–2440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Awada A. et al. Phase I trial of volasertib, a Polo-like kinase inhibitor, plus platinum agents in solid tumors: Safety, pharmacokinetics and activity. Invest. New Drugs 33, 611–620 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kobayashi Y. et al. Phase I trial of volasertib, a Polo-like kinase inhibitor, in Japanese patients with acute myeloid leukemia. Cancer Sci. 106, 1590–1595 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stadler WM et al. An open-label, single-arm, phase 2 trial of the polo-like kinase inhibitor volasertib (BI 6727) in patients with locally advanced or metastatic urothelial cancer. Cancer 120, 976–982 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Michael L. et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood 124, 1426–1434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thaiparambil JT et al. Withaferin A inhibits breast cancer invasion and metastasis at subcytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer 129, 2744–2755 (2011). [DOI] [PubMed] [Google Scholar]
- 127.Bargagna-mohan P. et al. The Tumor Inhibitor and Antiangiogenic Agent Withaferin A Targets the Intermediate Filament Protein Vimentin. 14, 623–634 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Antony ML et al. Growth arrest by the antitumor steroidal lactone withaferin a in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of β-tubulin. J. Biol. Chem 289, 1852–1865 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heyninck K, Lahtela-Kakkonen M, Van der Veken P, Haegeman G. & Vanden Berghe W. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKβ. Biochem. Pharmacol 91, 501–509 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Dutta R, Khalil R, Green R, Mohapatra SS & Mohapatra S. Withania somnifera (Ashwagandha) and withaferin a: Potential in integrative oncology. Int. J. Mol. Sci 20, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pires N. et al. Safety and pharmacokinetics of Withaferin-A in advanced stage high grade osteosarcoma: A phase I trial. J. Ayurveda Integr. Med 11, 68–72 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]