Abstract
Although there is growing evidence that cellular senescence influences wound healing, a clear understanding of how senescence can be beneficial and/or detrimental to wound healing is unknown. Wound healing may also be influenced by the baseline tissue senescence, which is elevated in aging and chronic wounds, both of which have significant healing delays. To study the effects of skin senescence on wound healing, we developed an elevated skin senescence model based on the subcutaneous transfer of irradiated fibroblasts into young 8-week-old wild-type C57BL/6 male mice. This senescent cell transfer significantly increased skin senescence levels compared to control transfers of non-irradiated fibroblasts. There was an increased presence of SA-β-Gal- and p21-positive senescent cells throughout the skin. Furthermore, the entire skin showed significantly elevated gene expression of senescence (p16, p21) and SASP markers (IL-6, MCP-1, MMP-3, MMP-9, and TGF-β). Subsequent wound healing in the skin with elevated senescence was markedly delayed and had similar kinetics to naturally aged 2-year-old mice. After the wounds had healed, the skin developed persistently elevated senescence. Our results demonstrate that states of elevated skin senescence can delay wound healing and result in sustained senescence after healing. Therefore, the accumulation of senescent cells in aged skin or chronic wounds may be a driver of delayed healing and can be considered a potential target to improve healing.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00551-1.
Keywords: Mouse, Cell, Senescence, Aging, Wound healing
Introduction
Senescent cells accumulate in multiple tissues during natural aging and have the potential to disrupt normal tissue homeostasis and repair processes [1, 2]. Therefore, cellular senescence is believed to be a key biological process that contributes to age-related dysfunction and chronic diseases, particularly in response to illness or injury [3–8]. Numerous studies have established and reviewed the importance of damage-induced senescence in wound healing [3, 9]. The emerging role of cellular senescence in wound healing biology and tissue repair includes the regulation of fibrosis and the stimulation of reprogramming [9–12]. The accumulation of senescent cells in the skin of aged mice and humans has been associated with DNA damage, upregulation of DNA damage response pathways, and increased production of reactive oxygen species (ROS) without causing apoptosis, as well as an inflammatory state that causes both local and systemic inflammation and tissue damage via a secretory phenotype associated with senescence called SASP [13–17]. This may include mitochondrial dysfunction, which has been shown to induce senescence and impact wound healing [18]. The accumulation of pathologic senescent cells has been linked to a variety of diseases and age-related morbidity in various organ systems [19]. Interventions that extend healthspan and lifespan delay the accumulation of cellular senescence markers; removing senescent cells extends healthspan in progeroid mouse models, and transplantation of a relatively small number of senescent cells into previously healthy animals results in multisystem dysfunction similar to that seen in aged animals [20–22]. Furthermore, it was discovered that the link between senescent cell accumulation and disease extends to humans [23]. On the other hand, the effect of increased skin senescence on wound healing is unknown. Our study aimed to develop a model of increased skin senescence in young mice to determine how levels of skin senescence impact subsequent wound healing.
Methods
Animals and cell culture
All procedures were carried out under the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the Boston University Subcommittee on Research and Animal Care (202000006). CAG-luciferase transgenic C57BL/6 young male mice (2 months) (Stock No: 000664) were obtained from The Jackson Laboratory. Ear tissue from CAG-luciferase transgenic C57BL/6 young male mice was used to isolate primary fibroblasts. In brief, the ear samples were washed three times with serum-free media, finely cut, and incubated for 2–3 h at 37°C in collagenase A. The digested tissue was then transferred to a 70-µm cell strainer and the cells were forced out of the strainer using a syringe plunger. The cell suspension was centrifuged for 7 min at 580 g and 4°C. The cell pellet was resuspended in DMEM, seeded, and expanded. After two passages, cells were gamma-irradiated with 10 Gy. Irradiated cells were maintained in culture for 10 days prior to cell transfer.
Bioluminescence imaging
Bioluminescence activity of senescent and non-senescent fibroblast cells was determined in vitro and in vivo using the Xenogen In Vivo Imaging System. Initially, senescent and non-senescent fibroblast cells in 6-well plates were treated with d-luciferin, and bioluminescent imaging was performed. Furthermore, young C57BL/6 mice were subcutaneously transplanted with 1 × 106 cells/500 µl volume of senescent and non-senescent fibroblast cells, followed by an intraperitoneal injection of 150 µl of RediJect d-luciferin Ultra Bioluminescent Substrate (PerkinElmer) (Part Number: 770505). The Living Image® 4.5.1 Software was used to perform bioluminescence imaging under isoflurane anesthesia on day 0 and day 7 after senescent cell transfer.
Animal surgery
Seven days after subcutaneous cell transfers, a 1 cm2 full-thickness excisional wound was created on the dorsal skin of mice that had previously received irradiated or non-irradiated fibroblast cells under isoflurane anesthesia. 3M™ Tegaderm™ Transparent Film Dressing was used to cover the wounds. For analgesia, mice were given 0.03 mg/kg buprenorphine subcutaneously and were housed with food and water ad libitum. Postoperatively, mice wounds were digitally imaged. ImageJ software was used to calculate the wound area (version 1.53 k).
Senescence-associated beta-galactosidase (SA-β-gal) staining
SA-β-gal staining was performed in vitro according to the manufacturer’s protocol for the Senescence β-Galactosidase Staining Kit (Cell Signaling Technology, cat #9860) on irradiated or non-irradiated fibroblast cells 10 days after irradiation. Briefly, media was removed; 1X PBS was used to rinse the plate. Cells were fixed for 15 min at room temperature using Fixative Solution. In a dry non-CO2 incubator, β-galactosidase staining solution was added and incubated overnight at 37°C (no CO2).
The tissues were isolated 7 days after transfers of senescent and non-senescent fibroblast cells, as well as after 19 days from mice with healed wounds. The tissues were first fixed with neutralized buffer formalin for 4–6 h before being incubated overnight at 4°C in a 30% sucrose solution. To detect SA-β-gal in skin tissue, the CellEvent™ Senescence Green Detection Kit (Invitrogen, cat. #C10851) was used. Frozen 5-µm-thick sections were washed with buffer and then with 1% BSA. Sections were treated with a prewarmed working solution and placed in a humidified chamber to prevent moisture loss before being incubated overnight at 37°C without CO2 and protected from light. After washing in buffer, sections were mounted with VECTASHIELD® Antifade Mounting Medium with DAPI (H-1200–10). A Nikon Eclipse E400 fluorescent microscope at 10X magnification was used to capture microscopic images. The SA-β-gal-positive cells were counted using Fiji-ImageJ (version 1.53c).
Real-time PCR
The gentleMACS dissociator (Miltenyl Biotec) was used to homogenize the tissue and the TRIzol™ Plus RNA Purification Kit (catalog number: 12183555) was used to extract RNA from skin tissue isolated from mice 7 days after transfer of senescent and non-senescent fibroblast cells, as well as at 19 days from mice with healed wounds. RNA was reverse transcribed to cDNA using the Verso cDNA synthesis kit (Thermo Scientific, ref. #AB-1453/B), after NanoDrop quantification. For quantitative real-time PCR, Maxima SYBR Green/ROX qPCR master mix (Thermo Fisher Scientific, ref. #K0222) (Applied Biosystems) was used. The primers purchased from Thermo Scientific are listed in Table S1. To calculate the relative mRNA levels of the senescence and SASP markers, β-actin was used as a control.
Histological assessment
After fixation in 4% paraformaldehyde in PBS for 2 h at 4°C with subsequent overnight sucrose treatment at 4°C, the 5-μm-thick cryosections were washed in PBS. Antigen retrieval was performed in EnVision FLEX Target Retrieval Solution Low pH (Dako, ref. #K8005) using Dako PT Link antigen retrieval machine. The sections were washed with PBS and traced with a hydrophobic pen. The sections were incubated in BLOXALL® blocking solution (SP-6000) for 10 min for the quenching of endogenous peroxidase activity. Blocking was carried out for 20 min at room temperature with 2.5% horse serum. The sections were incubated with rabbit anti-p21 primary antibodies (ab188224) overnight at 4°C (1:100) and washed with PBS, followed by incubation with ImmPRESS polymer reagent provided in the ImmPRESS® horse anti-rabbit IgG Polymer Kit (Vector Laboratories: Cat. No.: MP-7401) for 30 min. After aspiration of secondary antibodies and extensive washing to remove unbound antibodies, sections were incubated with peroxidase (HRP) detection system (ImmPACT® DAB Peroxidase Substrate, Vector Laboratories: Cat. No.: SK-4105) for 30 s to 1 min or until desired stain intensity develops. The sections were rinsed in tap water and counterstained with Hematoxylin, mounted with VECTASHIELD® Antifade Mounting Medium (H-1000–10), and coverslipped. Brightfield microscopy images were captured using a Nikon Eclipse E400 fluorescent microscope at 10X SA-β-gal and anti-p21 staining, respectively. Percentages of SA-β-gal and p21-positive cells were calculated using ImageJ software.
For hematoxylin and eosin staining, deparaffinized sections were rehydrated with distilled water. Mayer’s hematoxylin staining was performed for 1 min, washed with distilled water, and then counterstained with alcoholic eosin for 1 min before dehydrating with ethanol. Stained slides were mounted and coverslipped. A Nikon Eclipse E400 fluorescent microscope was used to capture brightfield microscopy images at a magnification of 10X .
Statistical analysis
For comparisons between transferred animals with irradiated and non-irradiated cells, an unpaired t-test was used, and for comparisons between different time points, ANOVA with post hoc Tukey tests was used. The results are presented as mean ± SEM, with p < 0.05 considered statistically significant.
Results and discussion
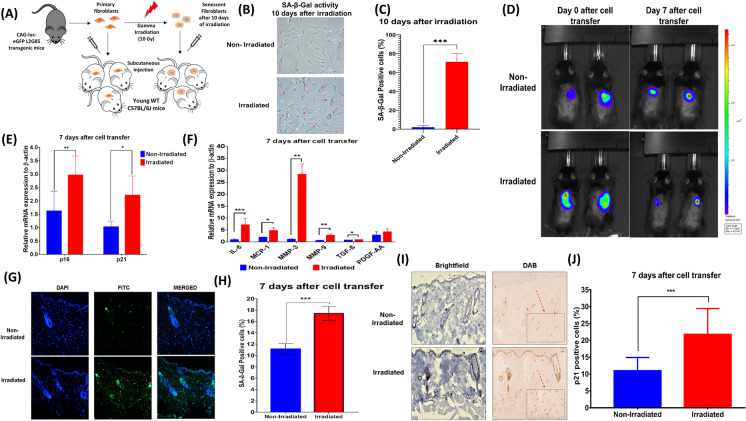
Primary ear fibroblasts were isolated and cultured from CAG-luciferase transgenic C57BL/6 mice. To induce senescence, cultured fibroblasts were treated in vitro with 10-Gy gamma irradiation. Ten days after irradiation, we observed senescent changes including altered cellular morphology and more than 70% of irradiated cells demonstrated SA-β-gal positivity compared to 2% of non-irradiated cells (Fig. 1B and D). We isolated cells 10 days after irradiation for subcutaneous cell transfer of 1 × 106 cells/500 µl of irradiated or non-irradiated control fibroblasts to young C57BL/6 male mice (2 months, n = 40) using 18-G needles for subcutaneous delivery under the dorsal skin. On days 0 and 7 after cell transfer, transplanted cells were visualized using in vivo bioluminescence imaging (Fig. 1C) confirming their presence and viability. Seven days after initial cell transplantation, the skin was harvested and analyzed for gene expression of senescence and SASP markers. There were statistically significant increases in the expression of senescent markers (p16, p21) and SASP factors (IL-6, MCP-1, MMP-3, MMP-9, and TGF-β) in mice transferred with irradiated senescent fibroblasts compared to mice transferred with non-irradiated fibroblasts (Fig. 1E and F). We hypothesize that subcutaneous transplantation of senescent cells likely increased skin senescence due to bystander effects (i.e., SASP effects) as we did not see any evidence of cell migration of the transplanted cells out of the subcutaneous plane (Supplementary Figure S1).
Fig. 1.
Increasing levels of skin senescence through subcutaneous irradiated fibroblast transfer. A Study design: primary mouse-ear fibroblasts isolated from CAG-Luc-eGFP L2G85 transgenic mice. 10-Gy irradiated (or) non-irradiated fibroblast cells were transplanted subcutaneously into wild-type C57BL/6 J mice (8 weeks old). B, C SA-β-gal cells in non-irradiated and irradiated fibroblast cultures 10 days after irradiation. D Xenogen IVIS Imaging System of transplanted cells in young mice on day 0 and after 7 days after transplantation. E, F The relative expression of senescence and SASP markers in the skin 7 days after cell transfer. G, H SA-β-gal staining and quantification of positive cells 7 days after cell transfer, nuclei (DAPI), 10 × magnification. I, J p21 staining and quantification of positive cells 7 days after cell transfer, 10 × magnification. t-test; ***p < 0.001, **p < 0.01, *p < 0.05
We next examined how increasing levels of baseline skin senescence in our model affected subsequent wound healing. Seven days after cell transfers, additional young mice with irradiated (N = 12) and non-irradiated (N = 12) fibroblast transfers underwent a 1 cm2 surgical full-thickness dorsal skin wound. Wounds were assessed daily with digital imaging and wound healing was significantly delayed in young mice pretreated with irradiated cell transfers versus non-irradiated cell transfers. This delayed wound healing remarkably had kinetics more similar to naturally aged 2-year-old mice (Fig. 2A, B) which also have increased baseline skin senescence [24, 25]. After healing was complete in both control and irradiated mice, the healed wound and skin were evaluated for residual senescence and SASP. Mice that had received non-irradiated fibroblasts had a return to baseline senescence and SASP factors after healing was completed, whereas mice that received irradiated fibroblasts maintained elevated levels of p16, p21, MCP-1, MMP-9, and TGF-β. This suggests persistent senescence occurs possibly due to dysregulation of normal senescence clearance mechanisms. Prior studies have shown transient wound-induced senescence during wound healing that normally resolves by the end of healing [26]. We also specifically evaluated PDGF-AA expression, which was identified as a critical growth factor secreted by wound senescent cells [26], at two time points: (1) 7 days after cell transfer and (2) after wounds had healed. We found that both non-irradiated and irradiated cell transfers demonstrated equivalent skin PDGF-AA expression (Fig. 1F). However, after wounds had healed in both groups, PDGF-AA levels were much lower in the irradiated group (Fig. 2E), suggesting that increased skin senescence may alter the ability for sustained PDGF-AA signaling required for optimal wound healing. This has been seen in delayed aging [26] and diabetic wounds [27], where there is a decreased presence of PDGF signaling.
Fig. 2.
Transplantation of senescent cells causes significant delays in wound healing and persistent senescence. A Study design: induction of full-thickness excision wound on C57BL/6 J young mice 7 days after cell transplantation. B Representative images of wound healing in young mice after senescence cell transplantation compared with aged C57BL/6J mice (24 months old). C Wound contraction assessment. D, E Relative expressions of senescence and SASP markers in the skin after completion of wound healing. F, G SA-β-gal staining and quantification of positive cells after wound healing, nuclei (DAPI), 10 × magnification. H, I p21 staining and quantification of positive cells after wound healing, 10 × magnification. t-test; ***p < 0.001, **p < 0.01, *p < 0.05
Cellular senescence appears to play an intricate and context-dependent role in wound repair [28, 29]. Senescent cells can cause surrounding cells to become senescent. The accumulation of senescent cells with age can result in a loss of tissue homeostasis, which may impair the normal and timely healing of wounds and other injuries [24, 30]. The DNA damage response and cellular senescence of neighboring cells are also induced by senescent cells through processes involving reactive oxygen species (ROS) and cell–cell contact, which are mediated by gap junctions. This promotes senescent cell accumulation in multiple tissues via a signaling network involving complex interactions between ROS and SASP factors, impairing wound healing [31, 32].
While a transient senescent response to wounding is beneficial in young mice [26], the effects of chronic senescence associated with aging are thought to be detrimental to wound healing. We used subcutaneous cell transfer to develop a model of increasing skin senescence in young mice. These transferred cells are persistent and mimic chronic skin senescence; however, our model accelerates skin senescence to baseline levels greater than those associated with natural aging. This, elevated skin senescence was found to be detrimental to wound healing, resulting in delays in young mice compared to those observed in aged mice. We hypothesize that young mice with elevated skin senescence from cell transfer lost the ability to regulate an acute and transient wound-induced senescence response that is needed in normal wound healing [26]. Although the mechanisms of senescent clearance have not been fully elucidated, this may involve normal immune-mediated clearance mechanisms that are either overwhelmed or become dysfunctional in states of chronic senescence. An additional mechanism of delayed wound healing due to elevated skin senescence likely involves dysregulation of matrix metalloproteinases, as we found increased expression of matrix metalloproteinases MMP-3 and MMP-9, in mice with irradiated cell transfers, which can promote growth factor degradation and delay in wound healing [33–35]. It will be important to determine the mechanisms by which increased senescence disrupts the normal cellular and molecular balance of wound healing events. However, it is becoming more evident that chronic senescence contributes to delayed wound healing, and strategies for reversing chronic senescence states can likely improve delayed wound healing [36].
Conclusion
The extraordinary potency of senescent cells has been shown in multiple studies demonstrating that the transfer of a relatively small population of these cells into healthy young organisms can cause significant morbidity and age-related disease, and alter the trajectory of normal aging [8, 37, 38]. Our data add to the growing evidence that senescent cells alter normal physiological responses by demonstrating that a single subcutaneous transfer of senescent cells causes elevated skin senescence and significantly alters normal wound healing and tissue repair. We believe that our delayed healing wound healing model will be useful in studying chronic wounds in mice and will help us to understand the consequences of chronic senescent cell accumulation in tissue repair. Identifying the mechanisms by which senescent cells contribute to pathologic wound healing may lead to the development of senescence-modifying strategies to alleviate the burden of chronic wounds [20, 21, 31, 39, 40]. Senescent cell targeting may be an advantageous strategy for delaying, preventing, or treating chronic wounds.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
DR designed and supervised the study and acquired funding. RJRST wrote the original manuscript draft, analyzed wound imaging and immunostaining data, and performed the statistical analysis. MS performed qPCR experiments and analyzed the qPCR results. RJRST and MS performed and analyzed IVIS imaging and wound healing experiments. RJRST, MS, GHS, and JC performed in vitro experiments. All authors reviewed, edited, and approved the final version of the manuscript.
Funding
This work was supported by a grant from the National Institute on Aging [R03AG067983]; Laszlo N. Tauber Professorship in Surgery; Boston Claude D. Pepper Older Americans Independence Center (NIA) Research Education Core Award 5P30AG031679-10: Sub award 115900.
Data availability
Data supporting the findings of this study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rex Jeya Rajkumar Samdavid Thanapaul, Email: haiiamrex@gmail.com.
Maria Shvedova, Email: mariashv@bu.edu.
Grace Haeun Shin, Email: gshin98@bu.edu.
Jack Crouch, Email: jcrouch@bu.edu.
Daniel S. Roh, Email: droh@bu.edu
References
- 1.Chia CW, Sherman-Baust CA, Larson SA, Pandey R, Withers R, Karikkineth AC, et al. Age-associated expression of p21and p53 during human wound healing. Aging cell. 2021;20. [DOI] [PMC free article] [PubMed]
- 2.Wang Z, Shi C. Cellular senescence is a promising target for chronic wounds: a comprehensive review. Burns Trauma. 2020;8:21. doi: 10.1093/burnst/tkaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thanapaul RJRS, Shvedova M, Shin GH, Roh DS. An insight into aging, senescence, and their impacts on wound healing. Adv Geriatr Med Res. 2021;3. [DOI] [PMC free article] [PubMed]
- 4.Antelo-Iglesias L, Picallos-Rabina P, Estévez-Souto V, da Silva-Álvarez S, Collado M. The role of cellular senescence in tissue repair and regeneration. Mech Ageing Dev. 2021;198. [DOI] [PubMed]
- 5.Ding X, Kakanj P, Leptin M, Eming SA. Regulation of the wound healing response during aging. J Invest Dermatol. 2021;141:1063–1070. doi: 10.1016/j.jid.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 6.di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22:75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song S, Lam EWF, Tchkonia T, Kirkland JL, Sun Y. Senescent cells: emerging targets for human aging and age-related diseases. Trends Biochem Sci. 2020;45:578–592. doi: 10.1016/j.tibs.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naylor RM, Baker DJ, van Deursen JM. Senescent cells: a novel therapeutic target for aging and age-related diseases. Clin Pharmacol Ther. 2013;93:105. doi: 10.1038/clpt.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson HN, Hardman MJ. Senescence in wound repair: emerging strategies to target chronic healing wounds. Front Cell Dev Biol. 2020;8:773. doi: 10.3389/fcell.2020.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosteiro L, Pantoja C, Alcazar N, Marión RM, Chondronasiou D, Rovira M, et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science (New York, NY). 2016;354. [DOI] [PubMed]
- 11.Resnik SR, Egger A, AbdoAbujamra B, Jozic I. Clinical implications of cellular senescence on wound healing. Curr Dermatol Rep. 2020;9:286–297. doi: 10.1007/s13671-020-00320-3. [DOI] [Google Scholar]
- 12.Wilkinson HN, Clowes C, Banyard KL, Matteuci P, Mace KA, Hardman MJ. Elevated local senescence in diabetic wound healing is linked to pathological repair via CXCR2. J Invest Dermatol. 2019;139:1171–1181.e6. doi: 10.1016/j.jid.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Wang AS, Dreesen O. Biomarkers of cellular senescence and skin aging. Front Genet. 2018;9 AUG:247. [DOI] [PMC free article] [PubMed]
- 14.Demaria M, Desprez PY, Campisi J, Velarde MC. Cell autonomous and non-autonomous effects of senescent cells in the skin. J Investig Dermatol. 2015;135:1722–1726. doi: 10.1038/jid.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewitt G, Jurk D, Marques FDM, Correia-Melo C, Hardy T, Gackowska A, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3. [DOI] [PMC free article] [PubMed]
- 16.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 18.Velarde MC, Demaria M, Melov S, Campisi J. Pleiotropic age-dependent effects of mitochondrial dysfunction on epidermal stem cells. Proc Natl Acad Sci USA. 2015;112:10407–10412. doi: 10.1073/pnas.1505675112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasek NS, Kuchel GA, Kirkland JL, Xu M. Strategies for targeting senescent cells in human disease. Nat Aging 2021 1:10. 2021;1:870–9. [DOI] [PMC free article] [PubMed]
- 20.Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J Gerontol A Biol Sci Med Sci. 2017;72:780. doi: 10.1093/gerona/glw186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker DJ, Wijshake T, Tchkonia T, Lebrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Justice JN, Gregory H, Tchkonia T, Lebrasseur NK, Kirkland JL, Kritchevsky SB, et al. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci Med Sci. 2018;73:939–945. doi: 10.1093/gerona/glx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karin O, Agrawal A, Porat Z, Krizhanovsky V, Alon U. Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nat Commun. 2019;10:1–9. doi: 10.1038/s41467-019-13192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agren MS, Steenfos HH, Dabelsteen S, Hansen JB, Dabelsteen E. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent. J Investig Dermatol. 1999;112:463–469. doi: 10.1046/j.1523-1747.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 28.Kumari R, Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021;9:485. doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratsinis H, Mavrogonatou E, Kletsas D. Scarless wound healing: from development to senescence. Adv Drug Deliv Rev. 2019;146:325–343. doi: 10.1016/j.addr.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife. 2015;4. [DOI] [PMC free article] [PubMed]
- 32.da Silva PFL, Ogrodnik M, Kucheryavenko O, Glibert J, Miwa S, Cameron K, et al. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell. 2019;18. [DOI] [PMC free article] [PubMed]
- 33.Caley MP, Martins VLC, O’Toole EA. Metalloproteinases and wound healing. Adv Wound Care. 2015;4:225. doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen TT, Mobashery S, Chang M. Roles of matrix metalloproteinases in cutaneous wound healing. Wound healing - new insights into ancient challenges. 2016 doi: 10.5772/64611. [DOI] [Google Scholar]
- 35.Liu Y, Min D, Bolton T, Nub́e V, Twigg SM, Yue DK, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care. 2009;32:117–9. [DOI] [PMC free article] [PubMed]
- 36.Wilkinson HN, Hardman MJ. Wound senescence: a functional link between diabetes and ageing? Exp Dermatol. 2021;30:68–73. doi: 10.1111/exd.14082. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X, Chen Z, Shen W, Huang G, Sedivy JM, Wang H, et al. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct Target Ther. 2021;6:1–29. doi: 10.1038/s41392-020-00451-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Docherty MH, Baird DP, Hughes J, Ferenbach DA. Cellular senescence and senotherapies in the kidney: current evidence and future directions. Front Pharmacol. 2020;11:755. doi: 10.3389/fphar.2020.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang B, Liu Z, Chen VP, Wang L, Inman CL, Zhou Y, et al. Transplanting cells from old but not young donors causes physical dysfunction in older recipients. Aging cell. 2020;19. [DOI] [PMC free article] [PubMed]
- 40.Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author on reasonable request.