Abstract
Senescent cells are in a cell cycle arrest state and accumulate with aging and obesity, contributing to a chronic inflammatory state. Treatment with senolytic drugs dasatinib and quercetin (D + Q) can reduce senescent cell burden in several tissues, increasing lifespan. Despite this, there are few reports about senescent cells accumulating in female reproductive tissues. Therefore, the aim of the study was to characterize the ovarian reserve and its relationship with cellular senescence in genetically obese mice (ob/ob). In experiment 1, ob/ob (n = 5) and wild-type (WT) mice (n = 5) at 12 months of age were evaluated. In experiment 2, 2-month-old female ob/ob mice were treated with senolytics (D + Q, n = 6) or placebo (n = 6) during the 4 months. Obese mice had more senescent cells in ovaries, indicated by increased p21 and p16 and lipofuscin staining and macrophage infiltration. Treatment with D + Q significantly reduced senescent cell burden in ovaries of obese mice. Neither obesity nor treatment with D + Q affected the number of ovarian follicles. In conclusion, our data indicate that obesity due to leptin deficiency increases the load of senescent cells in the ovary, which is reduced by treatment by senolytics. However, neither obesity nor D + Q treatment affected the ovarian reserve.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00573-9.
Keywords: Ovarian reserve, Obesity, Cell senescence, Dasatinib, Quercetin
Introduction
Aging is a physiological process of declining tissue function [1] due to cumulative damage resulting from genomic instability, telomere shortening, epigenetic changes, and loss of proteostasis [2]. As a response, compensatory mechanisms aimed at mitigating damage are initiated including the dysregulation of nutrient sensitivity, mitochondrial dysfunction, and cellular senescence [2]. In females, along with common somatic health-related complications of aging, there is a severe decline in fertility [3]. In this sense, ovarian aging refers to the reduction in the number of ovarian follicles with advancing age. The ovarian reserve is determined in utero and comprised primordial follicles that remain in a quiescent state until activated, at which point they are ovulated or undergo atresia [4]. When the ovarian reserve is severely reduced, menopause occurs in women [4]. In many strains of mice, menopause, or estropause, does not naturally occur, although the ovarian reserve does become severely reduced with age [5]. Therefore, the reproductive lifespan of a female depends on the initial size of the primordial follicle reserve, as well as its rate of depletion [6]. In addition to infertility, menopause considerably increases the risk for several metabolic diseases including cardiovascular disease, osteoporosis, hypertension, diabetes mellitus, and ovarian cancer [7, 8].
Interestingly, obesity can accelerate the development of age-related diseases [9]. Leptin-deficient mice (ob/ob) are widely studied as a model of human obesity [10]. The ob/ob mice are severely obese, insulin resistant, have impaired fertility, and display a shortened lifespan [11]. It is becoming well recognized that obesity reduces the primordial follicle ovarian reserve in young animals [12, 13]. Female ob/ob mice are infertile due to leptin signaling being required in the hypothalamus for gonadotropin release and ovulation to occur [14]. However, fertility is restored when ob/ob mice are supplemented with exogenous leptin [14] or receive adipose tissue transplants from lean littermates [15].
The accumulation of senescent cells with advancing age is also surmised to play a causal role in decreasing health span and lifespan [16]. Obesity is associated with increased senescent cell burden in various tissues, thereby contributing to increased systemic inflammation [2, 13, 17]. Senescence is a cellular defense mechanism, inhibiting cell cycle progression and tumorigenesis. However, cell cycle arrest also inhibits apoptosis thereby promoting the accumulation of senescent cells during aging [18–20]. Senescent cells often accumulate lipofuscin [21] which is accompanied by high expression of p16 and p21 [22]. In addition, senescent cells are characterized by the secretion of pro-inflammatory factors that attract macrophages [23]. The senescence-associated secretory phenotype (SASP) contributes to the propagation of pro-inflammatory signals in the tissue microenvironment, which is believed to promote the progression of age-related diseases [2, 24]. We previously reported that several markers of cellular senescence increase with age in the ovary of mice [25]. However, the emergence of senescent cells in the ovary is not well defined, and perhaps more importantly, the role that obesity plays in promoting the premature accumulation of senescent cells in the ovary is critically understudied.
Therapeutics known as senolytics are a relatively new class of drugs that can selectively eliminate senescent cells from the body. These treatments have been reported to decrease aging-related disease burden and increase lifespan [16]. One of the earliest senolytic cocktails to be developed and studied is the combination of dasatinib and quercetin (D + Q), which have been shown to be effective at killing senescent cells [26]. Quercetin is a bioflavonoid highly present in the human diet, whereas dasatinib is a tyrosine kinase inhibitor used in cancer therapy [27]. Despite other reports demonstrating that senolytic treatment can reduce senescent cell accumulation in genetically- and diet-induced obese mice [28, 29], it remains unclear if obesity increases senescence cell burden in the ovary, or if senolytic treatment can attenuate senescent cell accumulation in the ovary and/or improve follicular reserve. Thus, our study aimed to understand how obesity affects the ovarian reserve and accumulation of ovarian senescent cells and if senolytics can lessen/prevent this senescent cell burden within the ovary.
Methods
Mice and experimental design
Experiments were approved by the Animal Experimentation Ethics Committee from Universidade Federal de Pelotas (Numbers 3715–2014 and 24,915–2020). All mice (C57BL/6 background) were maintained in ventilated cages with ad libitum access to food and water unless otherwise specified. The vivarium is maintained at 20 ± 2 °C at 40–70% humidity on a 12-h light cycle. In experiment 1, 12-month-old ob/ob (n = 5) and C57Bl/6 wild-type (WT; n = 5) females were euthanized, and ovarian tissue was collected. In a second experiment, 2-month-old ob/ob female mice were divided into control (n = 6) and D + Q (n = 6) treatment groups. Treated females received the senolytic drugs dasatinib and quercetin (D + Q) for 4 months beginning at 2 months of age. Treatment consisted of dasatinib (5 mg/kg) and quercetin (50 mg/kg) dissolved in 60% phosal, 30% PEG400, and 10% ethyl alcohol [16] for 3 consecutive days every 2 weeks. Control mice received the vehicle solution, composed of 60% phosal, 30% PEG400, and 10% ethyl alcohol. Females from experiment 2 were euthanized at 6 months of age to collect ovarian tissue. For both experiments, one of the ovaries was stored in 10% formaldehyde for histological and immunofluorescence assays, whereas the other was stored at − 80 °C for RNA extraction.
Insulin tolerance test
An insulin tolerance test (ITT) was performed at 6 months of age in obese mice from experiment 2 receiving D + Q or vehicle. After 2-h fasting, basal glycemia was measured and, subsequently, human insulin (5 IU/kg body weight, Novolin R®, Novo Nordisk) was administered i.p. [30]. Glycemia was measured in a drop of blood from the tail at 5, 20, 35, and 60 min after insulin injections using a commercial glucometer (Accu-Chek Performa, ROCHE).
Follicle counting and classification
The ovaries were processed as previously described [25]. Nine ovarian sections from each mouse were used to count follicles at 40 × magnification and to calculate follicle density. Follicles were classified according previous descriptions [25, 31]. The number of follicles for each of the nine ovarian sections was then divided by the section area to calculate the follicle density (number of follicles/mm2) [25, 31]. The average density of the nine sections/mice was used for statistical analysis.
Lipofuscin staining by Sudan black
The lipofuscin staining was performed with the Sudan black dye on ovarian sections as an indicator of senescence. The protocol used was adjusted from a previous description [32]. The slides were dewaxed with xylol, washed in a gradient of ethyl alcohol until reaching 70% alcohol, and rehydrated with water. After diluting the Sudan black in 70% ethyl alcohol, avoiding its precipitation, a 10-ml syringe with disc filter was used to drip it onto a clean slide, and the slide containing the tissue sections was inverted over for approximately 2 min. After this procedure, the slide with the sections was separated and immediately washed with 50% ethyl alcohol and distilled water. The slides were assembled with glycerol and then observed under a light microscope at 4 and 10 × magnification. The area of lipofuscin staining in the images was calculated using the ImageJ software and expressed as the percentage of the total area of the section.
Immunofluorescence for macrophage, p21 and p16
Slides were deparaffinized and rehydrated with 3 washes of Safeclear (Fisher Scientific, Pittsburgh, PA. USA) for 10 min each, followed by 3 washes of each concentration in a graded series of ethanol (100%, 95%, 80%, 70%) for 5 min. After rinsing the slides 2 times for 5 min in distilled water, the slides were incubated in sodium citrate pH 6.0 during 40 min at 95 °C. Permeabilization was performed in 0.2% of Triton-X 100 in PBS for 1 h. Sections were blocked for 4 h in a blocking solution (2.52 mg/ml glycine, 10% goat serum, 3% BSA, 0.2% Triton-X in PBS-T) and then incubated with primary antibody (diluted in blocking solution) overnight at RT (CD68 #ab955 Abcam Plc, UK; P16 #10,883–1-AP; and P21 #10,355–1-AP, Proteintech, USA) [33–37]. After 2 washes of 10 min with PBST, the slides were incubated with secondary antibodies for 2 h at RT (goat anti-rabbit Cy3 for p16 and p21, Jackson Immuno Research Laboratories, West Grove PA, USA; and Alexa Fluor® 488 #ab150113, Abcam). The slides were rinsed twice with PBST for 5 min. DNA was counterstained by applying an antifade solution (Vector laboratories, Burligame, CA, USA) containing 0.1 µg/ml of DAPI (4′,6′-diamidino-2-phenylindole) (Sigma). Image acquisition was performed using a Zeiss AxioImager M2 microscope (Carl Zeiss AG, Oberkochen. Germany). Images were processed using Zen 2 (Carl Zeiss AG, Oberkochen. Germany). Digital images were analyzed for relative fluorescence intensity using Fiji (ImageJ, NIH). Positive and negative controls for p16 (Suppl. Fig. 1) and p21 (Suppl. Fig. 2) antibodies are provided. Macrophage number was evaluated as a total number of macrophages per ovary section in 3 random ovary sections/mice.
RNA extraction and gene expression
Total RNA from ovarian samples was extracted and processed as previously described [38]. The genes Gapdh (glyceraldehyde-3-phosphate dehydrogenase), β2m (beta-2 microglobulin), Actb (actin beta) and Ppia (peptidylprolyl isomerase A) were evaluated as endogenous controls. According to results from the geNorm software [39], the β2m gene was the most stable in both experiments. Relative gene expression was calculated using the comparative CT method [40]. Primer sequences used in these analyses are shown in Table 1.
Table 1.
Murine primer pairs (forward and reverse) used in the experiments
| Gene | Primers | Size | Accession |
|---|---|---|---|
| β-2-Microglobulin (β2m) |
F: AAGTATACTCACGCCACCCA R: CAGGCGTATGTATCAGTCTC |
217 | NM_00935.3 |
| Anti-Mullerian hormone (Amh) |
F:TCCTACATCTGGCTGAAGTGATATG R: CAGGTGGAGGCTCTTGGAACT |
66 | XM_006513119.3 |
| Growth/differentiation factor 15 (Gdf15) |
F: GAGCTACGGGGTCGCTTC R: GGGACCCCAATCTCACCT |
130 | NM_001330687.1 |
| Matrix metallopeptidase 12 (Mmp12) |
F: GAGTCCAGCCACCAACATTAC R: GCGAAGTGGGTCAAAGACAG |
232 | NM_001320076.1 |
| Chemokine ligand 2 (Ccl2) |
F: GAAGCCAGCTCTCTCTTCCTC R: TTGCTGGTGAATGAGTAGCAG |
150 | NM_011333.3 |
| Interleukin 1 alpha (Il1a) |
F: GAGTCGGCAAAGAAATCAAGATG R: CAATGGCAGAACTGTAGTCTTCGT |
96 | NM_010554.4 |
| Stanniocalcin-1(Stc1) |
F: CTACTTTCCAGAGGATGATCGC R: ACTTCAGTGATGGCTTCCGG |
100 | NM_009285.3 |
Statistical analysis
All the data are presented as mean ± SEM and a P < 0.05 was considered significantly different. All the statistical analyses were performed using student’s t-test in GraphPad Prism 5.0.
Results
Experiment 1: ovarian senescence is increased in obese mice
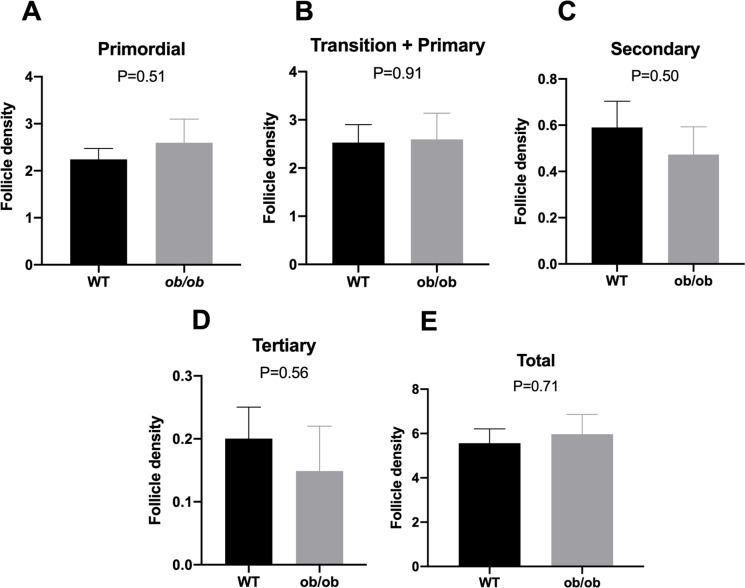
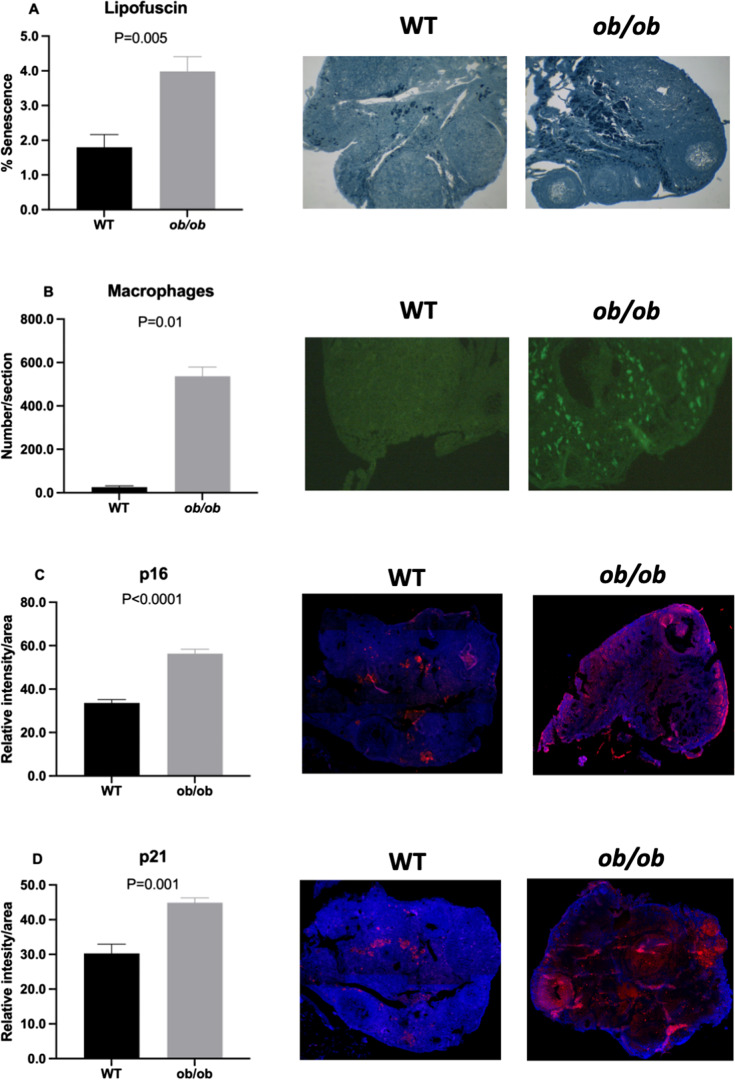
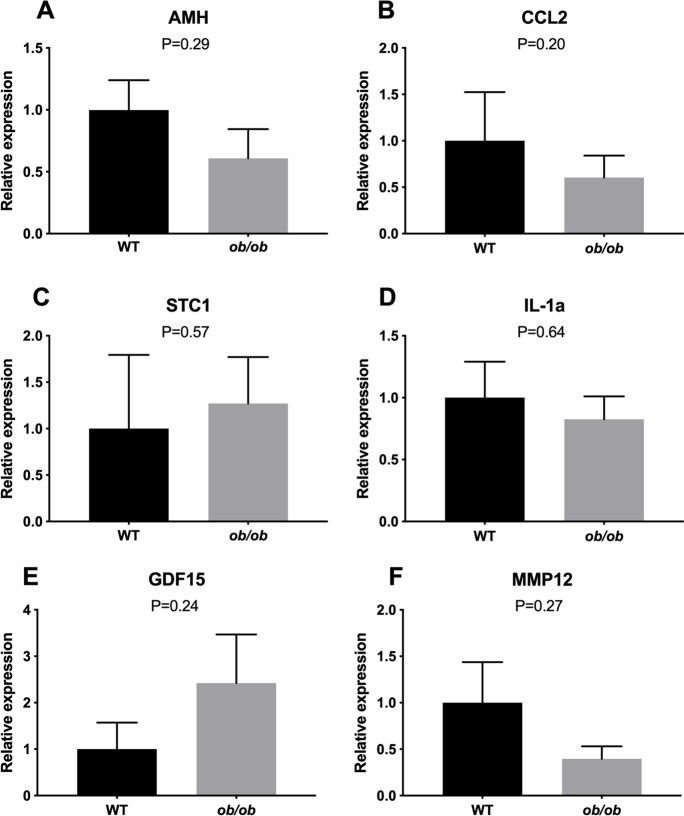
As expected, body weight (Suppl. Fig. 3) was two-fold higher in ob/ob animals when compared to lean WT animals. Conversely, to our surprise ob/ob mice had an ovarian follicular reserve similar to age-matched WT lean mice. No difference was observed in the number of primordial, primary, secondary, tertiary, or total follicles between ob/ob and WT mice (Fig. 1). Interestingly, the percentage of lipofuscin positivity, a marker of senescent cells, was greater (P = 0.005; Fig. 2A) in the ovary of ob/ob females when compared to lean WT females. Furthermore, the number of macrophages per ovarian section was higher in the ovary of ob/ob females when compared to lean WT females of the same age (P = 0.01; Fig. 2B). p16 (Fig. 2C) and p21 (Fig. 2D) positivity was also found to be greater in ob/ob females (P < 0.0001 and P = 0.001, respectively), suggesting that obesity does indeed promote a greater abundance of senescent cells in the ovary. There was no change in Amh gene expression (P = 0.29; Fig. 3A), an ovarian reserve indicator, which supports our follicular reserve findings. Other transcriptional markers of senescence including ovarian expression of Ccl2 (P = 0.20), Stc1 (P = 0.57), Il1-a (P = 0.64), Gdf-15 (P = 0.24), and Mmp12 (P = 0.27) were not different between ob/ob and WT mice (Fig. 3B–F).
Fig. 1.
Mean follicle density at different growth stages in obese (ob/ob, n = 5) or lean wild-type (WT, n = 5) mice at 12 months of age. Primordial (A), transition and primary (B), secondary (C), tertiary (D), and total number of follicles (E). Values of P < 0.05 were considered significant
Fig. 2.
Lipofuscin staining (A), macrophage infiltration (B), and p16 (C) and p21 (D) staining in obese (ob/ob, n = 5), and wild-type lean (WT, n = 5) females at 12 months of age. Values of P < 0.05were considered significant
Fig. 3.
Analysis of relative gene expression in ovaries from obese (ob/ob, n = 5) and wild-type lean (WT, n = 5) females at 12 months of age. A Anti-Mullerian hormone (Amh), (B) chemokine ligand 2 (CCL2), (C) Stanniocalcin-1 (Stc1), (D) Interleukin 1 alpha (IL1a), (E) growth/differentiation factor 15 (Gdf15), (F) matrix metallopeptidase 12 (Mmp12). Values of P < 0.05 were considered significant
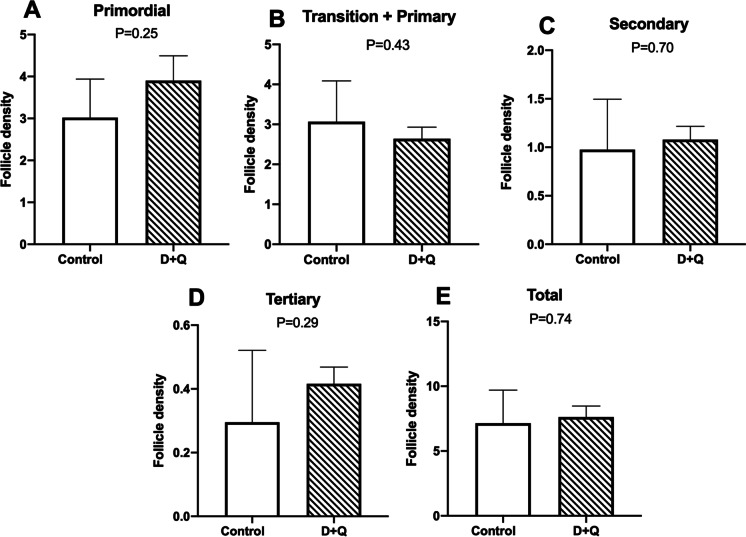
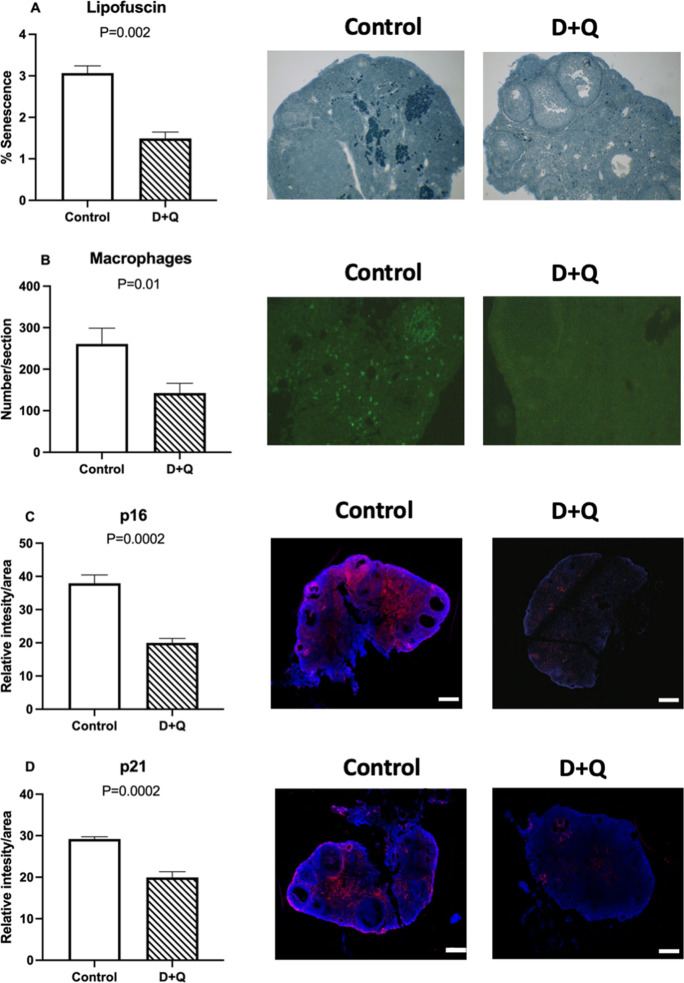
Experiment 2: senolytics can reduce ovarian senescence in obese mice
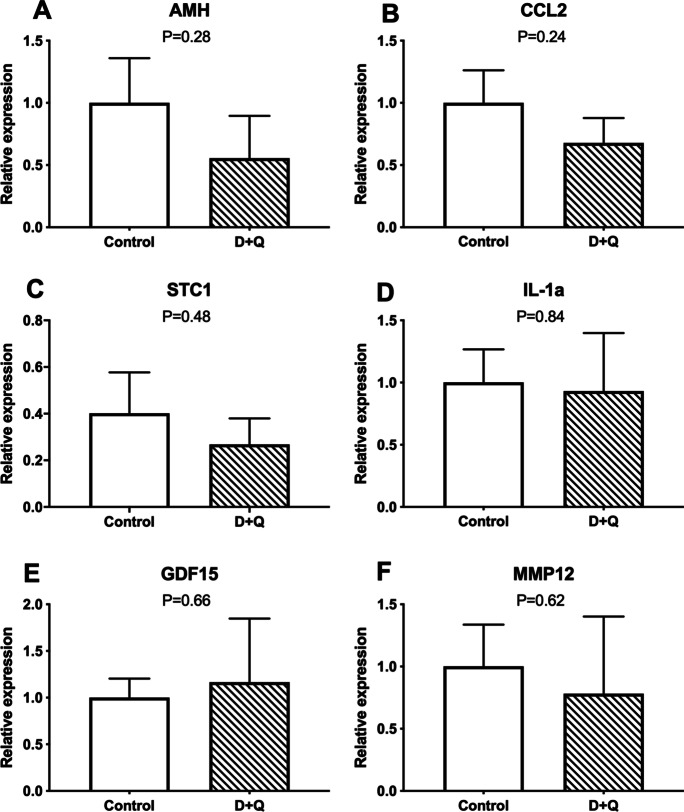
In experiment 2, treatment with D + Q in ob/ob mice did not affect body weight or insulin sensitivity after 4 months of treatment (Suppl. Fig. 4). No changes were observed in follicle numbers after treatment with D + Q. No difference was found for the number of primordial (Fig. 4A), transitional and primary (Fig. 4B), secondary (Fig. 4C), tertiary (Fig. 4D), and total (Fig. 4E) follicles between the control and D + Q-treated obese mice. Treatment with senolytics (D + Q), however, decreased the percentage of senescent cells in the ovaries of ob/ob females treated with D + Q compared to controls, as indicated by lipofuscin staining (P = 0.002; Fig. 5A). Similarly, the number of macrophages per ovarian section was lower in the ovary of D + Q-treated obese females (P = 0.01; Fig. 5B). In addition, p16 (Fig. 5C) and p21 positivity (Fig. 5D) were lower in D + Q-treated females (P = 0.0002 and P = 0.0002, respectively), further suggesting the ability of D + Q to reduce ovarian senescence. There was also no change in Amh gene expression (P = 0.28; Fig. 6A). Other markers of senescence, including Ccl2 (P = 0.24), Stc1(P = 0.48), Il1-a (P = 0.84), Gdf-15 (P = 0.66), and Mmp12 (P = 0.62) were not different between groups (Fig. 6B–F).
Fig. 4.
Analysis of the follicle density (number/mm2) at different growth stages in 6-month-old obese (ob/ob) females, control (n = 6) or treated with dasatinib plus quercetin (n = 6). P values < 0.05 were considered significant. Values were represented as mean ± standard error
Fig. 5.
Lipofuscin staining (A), macrophage infiltration (B), and p16 (C) and p21 (D) staining in 6-month-old obese (ob/ob) females, control (n = 6) or treated with dasatinib plus quercetin (n = 6). Values of P < 0.05 were considered significant
Fig. 6.
Analysis of relative gene expression in ovaries from 6-month-old obese (ob/ob) females, control (n = 6) or treated with dasatinib plus quercetin (n = 6). A Anti-Mullerian hormone (Amh), (B) chemokine ligand 2 (CCL2), (C) Stanniocalcin-1 (Stc1), (D) Interleukin 1 alpha (IL-1a), (E) growth/differentiation factor 15 (Gdf15), (F) matrix metallopeptidase 12 (Mmp12). Values of P < 0.05 were considered significant
Discussion
In this study, we characterized the ovarian reserve and accumulation of senescent cells in the ovaries of lean and obese mice. We also evaluated the effects of senolytic treatment on senescent cell burden in the ovary. We found that obese mice displayed a greater presence of senescent cells in the ovary when compared to lean littermates. This was demonstrated by greater lipofuscin accumulation and elevated p21 and p16 expression. To our knowledge, this is the first report demonstrating that obese mice accumulate more senescent cells within the ovary. We previously reported that lipofuscin accumulates in the ovary with advancing age, and that this occurs in conjunction with increased p21 and p16 expression [25]. Lipofuscin staining is a senescence marker highly correlated to β-galactosidase staining [21]. In addition, senescent cells have a high expression of p16 and p21, which result in cell cycle arrest [22]. It is well known that senescent cells are present in several tissues [41, 42]; however, data regarding the presence of senescent cells in female reproductive organs is scarce, especially in obese mice.
It is known that cellular senescence can be triggered by different types of stressors, such as oxidative stress, DNA damage, metabolic dysfunction, inflammation, and elevated glucose [43]. Interestingly, exposure of cells to high glucose concentration can induce senescence, observed by higher β-galactosidase staining [44]. As insulin resistance and increased circulating levels of glucose are a common phenotype of diet-induced or genetic obesity, they can be linked to the increased presence of senescent cells. High glucose levels also trigger oxidative stress and inflammation [45]; therefore, obesity can stress the organism similarly to aging. Obesity has been implicated with the accelerated development of aging diseases, remodeling of tissues, acquisition of a pro-inflammatory phenotype, [42], which are changes commonly associated to senescence. There is an abundance of senescent cells in adipose tissue that increases with age [46]. Obesity has also been shown to increase senescent cell burden in adipose tissues [28], thereby supporting the idea that obesity may represent a mild progeria syndrome [42, 47–49]. In this sense, it was showed that both aging and obesity lead to accumulation of cellular senescence and SASP in a similar fashion [16, 28]. In fact, treatment of obese mice with senolytics reduced senescent cell burden and decreased insulin resistance [28], which suggests that these two conditions are closely interconnected. In this context, we can infer that obesity can accelerate the aging phenotype, resulting in accumulation of senescent cells. Our current findings support this idea by demonstrating that obesity also increases senescent cell burden in the ovary. Furthermore, senolytic treatment decreased the proportion of senescent cells in the ovaries of obese mice as shown by declines in lipofuscin accumulation and the expression of p16 and p21. This is the first study to evaluate the effects of senolytic treatment on ovarian senescence, and findings are analogous to previous reports indicating that senolytics can effectively reduce senescent cell burden in the context of obesity [28].
Despite displaying a reduction in senescent cell burden, we did not observe reduced expression of several SASP factors in ovarian tissue following senolytic treatment. It remains unclear if ovarian cells establish a unique SASP following the conversion to senescence, which could explain our inability to detect changes in known SASP factors. This is supported by a recent report demonstrating that different mouse tissues develop unique SASP profiles [50], although the ovary was not included in these analyses. To our knowledge, there has never been a characterization of the ovarian SASP, or an analysis to determine which ovarian cells become senescent. Given that tissue remodeling and pro-inflammatory processes are part of a normal functioning ovary, further studies will be needed to firmly establish the best markers for ovarian senescence and the SASP. Despite the fact that no changes were observed in SASP factors in our studies, ovaries from obese females did display a substantial increase in macrophage infiltration when compared to lean mice, and this was prevented by senolytic treatment. This finding supports previous work indicating that senescent cells attract macrophages as a means of removing damaged cells [23]. These findings also do not appear to be isolated to mice, since a report found that senolytic treatment decreased senescent cell burden, p21 and p16 expression, and macrophage infiltration in adipose tissue of humans [51].
In the current study, the ovarian follicular reserve was not affected by obesity. It is well established that diet-induced obesity accelerates activation of primordial follicles leading to premature exhaustion of the follicular reserve [12]. Calorie restriction (CR) has the opposite effect and preserves ovarian reserve [38]. Previous studies have shown that 3-month-old ob/ob mice have fewer preantral follicles when compared to WT mice [13]. Similarly, fewer primordial and total follicles were found in 7-week-old ob/ob mice compared to lean WT mice [52]. Both of these studies used very young mice; therefore, it is possible that differences in follicle counts disappear with aging as the ovarian reserve becomes severely reduced. It remains unclear if this is the reason; we did not observe differences between 12-month-old WT and ob/ob mice in our study. It is possible that long-term leptin deficiency has beneficial effects on the ovarian reserve, as the follicular density we observed is similar to previous reports for normal mice using similar methods [53–55]. The ovarian follicular reserve in ob/ob mice was also not affected by treatment with senolytics (D + Q). This suggests that the reduction in ovarian senescent cell burden is uncoupled from the ovarian reserve, at least in leptin-deficient mice. Our previous study indicated that an age-related increase in senescent cell burden in the ovaries of WT mice does indeed correlate with ovarian reserve [25]; therefore, additional studies will be required to provide greatly clarity.
We observed that the D + Q treatment did not affect body weight. Others also found that the reduction in senescent cells did not affect body weight gain using leptin receptor-deficient mice (db/db) [28]. An additional study using dietary-induced obese-male mice treated with senolytics (D + Q) also failed modulate body mass following the elimination of senescent cells [29]. We also found that senolytic treatment did not affect insulin sensitivity, which is in contrast to previous reports [28]. Obese mice induced by a high-fat diet treated with D + Q also had an improvement in insulin sensitivity in a short period of time (3 treatment cycles), but these beneficial effects disappeared after 16 weeks of treatment [29]. These results suggest that chronic treatments with senolytics exert a transient effect on insulin sensitivity, which peaks after several weeks of treatment but decreases over time, as in the study mentioned before.
The present study has limitations, which should be acknowledged. First, leptin- deficient mice are anovulatory; therefore, we could not evaluate the impact of senescent cells and its clearance on ovulation rate and fertility. Future studies using diet-induced obesity should provide evidence if senescent cell clearance will positively impact fertility of obese mice. It is known that obesity negatively affects fertility of female mice; therefore, treating obese females with senolytics can elucidate if senescent cells are involved in this process. Using a diet-induced obesity model will also help to establish the role of leptin in the findings of the current study. Although previous studies had shown that both diet-induced obesity and leptin-receptor deficiency obesity resulted in increased senescent cell burden in adipose tissue [28], the same remains to be determined in the ovary. Another limitation of the current study is that despite demonstrating the presence of senescent cells in the ovary, we were not able to determine which type of ovarian cells become senescent. Further studies using single-cell RNA sequencing and spatial transcriptomics will help to uncover the type of ovarian cells that become senescent. This can have important implications for the use of senolytic treatments to recover fertility or prevent age and obesity-associated infertility. The type of senescent cell will also have an impact in the choice of senolytics. To date, other drugs, such as fisetin and navitoclax have been also shown as effective for removing senescent cells. Future studies should compare the effectiveness of different drugs to target and remove ovarian-senescent cells.
In conclusion, female ob/ob mice accumulate more senescent cells in the ovary, and treatment with D + Q senolytic drugs can reduce this burden. Despite this, no effect of obesity or D + Q treatment was observed on ovarian reserve size, suggesting that senescence has no direct effect on ovarian reserve in leptin-deficient obese mice. It must be noted that D + Q treatment is unable to kill all types of senescent cells; therefore, combination therapies including alternative senolytics and senomorphics must be tested to determine the efficacy of these treatments for ovarian health.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by CAPES, CNPq, and FAPERGS to A. S.; the National Institutes of Health (R01 AG069742 to M. B. S.; R56 AG061414, R15 AG059190, R03 AG059846, and R21 AG062985 to M. M. M.; and Eunice Kennedy Shriver National Institute of Child Health and Human Development (R00HD090289) and the Magee Auxiliary Research Scholar (MARS) endowment to M. A. B. E.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alonso-Fernández P, De la Fuente M. Role of the immune system in aging and longevity. Curr Aging Sci. 2011. 10.2174/1874609811104020078. [DOI] [PubMed]
- 2.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed TA, Ahmed SM, El-Gammal Z, Shouman S, Ahmed A, et al. Oocyte aging: the role of cellular and environmental factors and impact on female fertility. Adv Exp Med Biol. 2020 doi: 10.1007/5584_2019_456. [DOI] [PubMed] [Google Scholar]
- 4.Baker TG. A Quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 5.Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, et al. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006 doi: 10.1210/en.2005-1588. [DOI] [PubMed] [Google Scholar]
- 6.Richardson MC, Guo M, Fauser BC, Macklon NS. Environmental and developmental origins of ovarian reserve. Hum Reprod Update. 2014 doi: 10.1093/humupd/dmt057. [DOI] [PubMed] [Google Scholar]
- 7.Newson L. Menopause and cardiovascular disease. Post Reprod Health. 2018 doi: 10.1177/2053369117749675. [DOI] [PubMed] [Google Scholar]
- 8.Prestwood KM, Raisz LG. Prevention and treatment of osteoporosis. Clin Cornerstone. 2002 doi: 10.1016/s1098-3597(02)90034-7. [DOI] [PubMed] [Google Scholar]
- 9.Mancuso P, Bouchard B. The impact of aging on adipose function and adipokine synthesis. Front Endocrinol (Lausanne) 2019 doi: 10.3389/fendo.2019.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, et al. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006 doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- 11.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978 doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 12.Wang N, Luo LL, Xu JJ, Xu MY, Zhang XM, et al. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism. 2014 doi: 10.1016/j.metabol.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Serke H, Nowicki M, Kosacka J, Schroder T, Kloting N, et al. Leptin-deficient (ob/ob) mouse ovaries show fatty degeneration, enhanced apoptosis and decreased expression of steroidogenic acute regulatory enzyme. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2011.220. [DOI] [PubMed] [Google Scholar]
- 14.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996 doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 15.Barros CC, Almeida SS, Mori MA, Valero VB, Haro AS, et al. Efficient method for obtaining Lep(ob)/Lep(ob)-derived animal models using adipose tissue transplantations. Int J Obes (Lond) 2009 doi: 10.1038/ijo.2009.95. [DOI] [PubMed] [Google Scholar]
- 16.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018 doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafer MJ, Miller JD, LeBrasseur NK. Cellular senescence: implications for metabolic disease. Mol Cell Endocrinol. 2017 doi: 10.1016/j.mce.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Wu KKL, Jiang X, Xu A, Cheng KKY. The role of adipose tissue senescence in obesity- and ageing-related metabolic disorders. Clin Sci (Lond) 2020 doi: 10.1042/CS20190966. [DOI] [PubMed] [Google Scholar]
- 19.Passos JF, Simillion C, Hallinan J, Wipat A, von Zglinicki T. Cellular senescence: unravelling complexity. Age (Dordr) 2009 doi: 10.1007/s11357-009-9108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karin O, Alon U. Senescent cell accumulation mechanisms inferred from parabiosis. Geroscience. 2021 doi: 10.1007/s11357-020-00286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EA Georgakopoulou, K Tsimaratou, K Evangelou, PJ Fernandez Marcos, V Zoumpourlis, et al (2013) Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence A method applicable in cryo-preserved and archival tissues. Aging (Albany NY), 10.18632/aging.100527. [DOI] [PMC free article] [PubMed]
- 22.Rodier, F and J Campisi. Four faces of cellular senescence. J Cell Biol, 2011; 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed]
- 23.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013 doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anerillas C, Abdelmohsen K, Gorospe M. Regulation of senescence traits by MAPKs. Geroscience. 2020 doi: 10.1007/s11357-020-00183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ansere VA, Ali-Mondal S, Sathiaseelan R, Garcia DN, Isola JVV, et al. Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion. Mech Ageing Dev. 2021 doi: 10.1016/j.mad.2020.111425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu YL, Xu YQ, Yang J, Li J. One-stage reconstruction of Achilles tendon and skin defects by the sliding gastrocnemius musculocutaneous flap without anastomosis. J Trauma. 2009 doi: 10.1097/TA.0b013e31817dac20. [DOI] [PubMed] [Google Scholar]
- 27.Montero JC, Seoane S, Ocaña A, Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2616. [DOI] [PubMed] [Google Scholar]
- 28.Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019 doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sierra-Ramirez A, López-Aceituno JL, Costa-Machado LF, Plaza A, Barradas M et al (2020) Transient metabolic improvement in obese mice treated with navitoclax or dasatinib/quercetin. Aging (Albany NY). 10.18632/aging.103607 [DOI] [PMC free article] [PubMed]
- 30.Sreejayan N, Dong F, Kandadi MR, Yang X, Ren J. Chromium alleviates glucose intolerance, insulin resistance, and hepatic ER stress in obese mice. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.217. [DOI] [PubMed] [Google Scholar]
- 31.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004 doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- 32.Evangelou K, Lougiakis N, Rizou SV, Kotsinas A, Kletsas D, et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell. 2017 doi: 10.1111/acel.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chae JB, Jang H, Son C, Park CW, Choi H, et al. Targeting senescent retinal pigment epithelial cells facilitates retinal regeneration in mouse models of age-related macular degeneration. Geroscience. 2021 doi: 10.1007/s11357-021-00457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maciel-Baron LA, Morales-Rosales SL, Aquino-Cruz AA, Triana-Martinez F, Galvan-Arzate S, et al. Senescence associated secretory phenotype profile from primary lung mice fibroblasts depends on the senescence induction stimuli. Age (Dordr) 2016 doi: 10.1007/s11357-016-9886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu S, Wu W, Huang H, Huang R, Xie L, et al. The p53/miRNAs/Ccna2 pathway serves as a novel regulator of cellular senescence: complement of the canonical p53/p21 pathway. Aging Cell. 2019 doi: 10.1111/acel.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirzayans R, Andrais B, Scott A, Paterson MC, Murray D. Single-cell analysis of p16(INK4a) and p21(WAF1) expression suggests distinct mechanisms of senescence in normal human and Li-Fraumeni Syndrome fibroblasts. J Cell Physiol. 2010 doi: 10.1002/jcp.22002. [DOI] [PubMed] [Google Scholar]
- 37.Saccon TD, Rovani MT, Garcia DN, Mondadori RG, Cruz LAX, et al. Primordial follicle reserve, DNA damage and macrophage infiltration in the ovaries of the long-living Ames dwarf mice. Exp Gerontol. 2020 doi: 10.1016/j.exger.2020.110851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia DN, Saccon TD, Pradiee J, Rincon JAA, Andrade KRS, et al. Effect of caloric restriction and rapamycin on ovarian aging in mice. Geroscience. 2019 doi: 10.1007/s11357-019-00087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002 doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Höhn A, Weber D, Jung T, Ott C, Hugo M, et al. Happily (n)ever after: aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017 doi: 10.1016/j.redox.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010 doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumari R, Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021 doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong JG, Park JB, Lee D, Park EY. Effect of high glucose on stress-induced senescence of nucleus pulposus cells of adult rats. Asian Spine J. 2015 doi: 10.4184/asj.2015.9.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004 doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 46.Darcy J, Bartke A. Functionally enhanced brown adipose tissue in Ames dwarf mice. Adipocyte. 2017. 10.1080/21623945.2016.1274470. [DOI] [PMC free article] [PubMed]
- 47.Salvestrini V, Sell C, Lorenzini A. Obesity may accelerate the aging process. Front Endocrinol (Lausanne) 2019 doi: 10.3389/fendo.2019.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tzanetakou IP, Katsilambros NL, Benetos A, Mikhailidis DP, Perrea DN. Is obesity linked to aging?: adipose tissue and the role of telomeres. Ageing Res Rev. 2012 doi: 10.1016/j.arr.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda) 2017 doi: 10.1152/physiol.00012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudgins AD, Tazearslan C, Tare A, Zhu Y, Huffman D, et al. Age- and tissue-specific expression of senescence biomarkers in mice. Front Genet. 2018 doi: 10.3389/fgene.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hickson LJ, LanghiPrata LGP, Bobart SA, Evans TK, Giorgadze N, et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019 doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamm ML, Bhat GK, Thompson WE, Mann DR. Folliculogenesis is impaired and granulosa cell apoptosis is increased in leptin-deficient mice. Biol Reprod. 2004 doi: 10.1095/biolreprod.104.027292. [DOI] [PubMed] [Google Scholar]
- 53.Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012 doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Schneider A, Matkovich SJ, Victoria B, Spinel L, Bartke A, et al. Changes of ovarian microRNA profile in long-living ames dwarf mice during aging. PLoS One. 2017 doi: 10.1371/journal.pone.0169213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saccon TD, Moreira F, Cruz LA, Mondadori RG, Fang Y, et al. Ovarian aging and the activation of the primordial follicle reserve in the long-lived Ames dwarf and the short-lived bGH transgenic mice. Mol Cell Endocrinol. 2017 doi: 10.1016/j.mce.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.