Abstract
Cognitive training has shown promise for improving cognition in older adults. Age-related neuroanatomical changes may affect cognitive training outcomes. White matter hyperintensities are one common brain change in aging reflecting decreased white matter integrity. The current study assessed (1) proximal cognitive training performance following a 3-month randomized control trial and (2) the contribution of baseline whole-brain white matter hyperintensity load, or total lesion volume (TLV), on pre-post proximal training change. Sixty-two healthy older adults were randomized to either adaptive cognitive training or educational training control interventions. Repeated-measures analysis of covariance revealed two-way group × time interactions such that those assigned cognitive training demonstrated greater improvement on proximal composite (total training composite) and sub-composite (processing speed training composite, working memory training composite) measures compared to education training counterparts. Multiple linear regression showed higher baseline TLV associated with lower pre-post change on processing speed training sub-composite (β = -0.19, p = 0.04), but not other composite measures. These findings demonstrate the utility of cognitive training for improving post-intervention proximal performance in older adults. Additionally, pre-post proximal processing speed training change appears to be particularly sensitive to white matter hyperintensity load versus working memory training change. These data suggest that TLV may serve as an important factor for consideration when planning processing speed-based cognitive training interventions for remediation of cognitive decline in older adults.
Keywords: White matter hyperintensities, Total lesion volume, Cognitive training, Cognitive aging, Processing speed
Introduction
Cognitive decline in aging is a growing public health concern. Even minor declines in cognitive abilities affect older adults’ daily independent functioning and quality of life. As the proportion of older adults in the world population continues to increase, interventions that ameliorate cognitive changes in aging must be developed. Growing evidence demonstrates cognitive training as a promising technique for improving cognitive function in older adults [1–4]. Studies evaluating the effectiveness of cognitive training have demonstrated that direct assessment of performance on tasks for which subjects had trained (i.e., proximal outcome measures) was the most reliable and robust method for assessing cognitive training efficacy and produced the largest effect sizes [5]. Effectiveness may also be evaluated through near transfer (abilities trained) and far transfer (abilities not trained) measures. For example, the advanced cognitive training for independent and vital elderly (ACTIVE) study demonstrated proximal cognitive training improvement (i.e., improvement on tasks trained) lasting up to 10 years following reasoning and speed of processing training. The ACTIVE study also showed transfer beyond the task trained in that cognitive training groups of reasoning, speed of processing, and memory reported fewer self-reported difficulties in instrumental activities of daily living compared to control conditions [4, 6].
Several factors influence cognitive training outcomes and transfer, including the training type (e.g., single-component vs. multicomponent; process-based vs. strategy based), training features (e.g., duration, intensity, adaptivity, domain, setting), and target population [7]. In aging, processing speed and working memory are cognitive processes particularly vulnerable to decline and have garnered significant attention as intervention targets. Processing speed training studies reported immediate and long-term improvements in processing speed abilities [3, 6], and studies employing working memory training demonstrated improvements on the trained task and closely related memory measures [8–12]. A multicomponent approach may further increase proximal outcomes and transfer [13]. However, more research is needed to assess factors that might influence individual differences in older adults’ response to cognitive training. Biological characteristics including age and baseline cognitive status may negatively influence cognitive training outcomes, but results have been mixed between studies [14, 15]. An emerging area of research includes combining basic demographic, psychometric, and behavioral measures with magnetic resonance imaging (MRI)-based measures of brain structure and function to help resolve inconsistent findings.
Evidence suggests that baseline brain characteristics are a promising predictor of cognitive training outcomes [7]. Generally, neuroanatomical differences relate to cognitive difference in aging [16–19]. White matter hyperintensities (WMH) are one common age-related brain change evidenced by T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI. WMH are a proxy for localized white matter damage and suggestive of cerebral small vessel disease, reducing the threshold for the clinical expression of cognitive impairment and dementia [20]. Automated segmentation software allows for reliable quantification of WMH, characterized as whole-brain total lesion volume (TLV). Even in healthy aging, higher TLV and regional WMH load consistently associate with poorer cognitive performance in processing speed, memory, and executive functioning [21–25]. While relationships between WMH and executive function domains including set-shifting and fluency have been described, associations for working memory performance are inconsistent [25–27]. Evidence suggests working memory may be more susceptible to regional WMH load (e.g., periventricular and deep WMH in temporal and frontal regions) rather than whole-brain measurements [26]. However, the impact of TLV on cognitive training outcomes in older adults has yet to be investigated.
The present study hypothesized that those receiving cognitive training would perform significantly better on the post-intervention proximal composite (total training composite) and sub-composite (processing speed training composite, working memory training composite) measures, compared to education training control counterparts following a 3-month intervention. We further hypothesized that higher baseline TLV across the entire sample would associate with lower pre-post change on proximal composite and sub-composite measures. Given consistent relationships between WMH load and speed of processing and inconsistent associations for working memory, we specifically hypothesized that higher TLV would result in lower pre-post change on processing speed training composite but not working memory training composite.
Materials and methods
Participants
Eighty-seven healthy older adults ranging from 65 to 84 years old (mean age = 71.0 ± 4.6; 42 females; mean years of education = 16.3 ± 2.3) took part in the current study. Participants were recruited at the University of Florida (n = 52) and the University of Arizona (n = 35) for the augmenting cognitive training in older adults (ACT; R01AG054077) study [28]. Recruitment efforts are detailed in prior publications [28] and included newspaper, direct mail, radio and television advertisements, local events, and research contact registries. Participants were included if they were right-handed, without contra-indication for MRI, and demonstrated evidence of age-related cognitive decline on a cognitive training assessment (performance below the 80th percentile). Participants with prior history of major psychiatric illness, head injury resulting in loss of consciousness > 20 min, or formal diagnosis or evidence of mild cognitive impairment (MCI), dementia, or neurological brain disease were excluded from the study. The study was approved by and performed according to the policies of the Institutional Review Boards at the University of Florida (UF) and the University of Arizona (UA). Participants engaged in the informed consent process prior to the initiation of study activities. The data in the current study were collected at the screening, baseline, and three-month assessment visits. A total of 25 participants were excluded from final analyses due to missing or unreliable MRI data (n = 13), voluntary participant withdrawal (n = 8), and/or non-adherence to the intervention assignment (n = 4). Intervention adherence was defined as at least 80% completion of intervention activities. Therefore, those achieving less than 720 of 1232 maximum cognitive training levels (n = 3) or completing less than 48 of 60 maximum education training questionnaire items (n = 1) were considered non-adherent for their respective intervention conditions. Analyses included 62 participants (see Table 1 for sample demographics).
Table 1.
Sample demographics
| Demographics | M/SD |
|---|---|
| Age | 72.1 ± 4.4 |
| Years of education | 16.2 ± 2.3 |
| Sex | N (%) |
| Male | 34 (55%) |
| Female | 28 (45%) |
| Race | N (%) |
| White | 52 (83.9%) |
| African American or Black | 3 (4.8%) |
| American Indian/Alaska Native | 3 (4.8%) |
| Asian/Asian American | 1 (1.6%) |
| Native Hawaiian or other Pacific Islander | 1 (1.6%) |
| More than one race | 2 (3.2%) |
| Ethnicity | N (%) |
| Hispanic or Latino | 5 (8.1%) |
| Not Hispanic/Latino | 57 (91.9%) |
| Intervention assignment | N (%) |
| Cognitive training | 31 (50%) |
| Education training | 31 (45%) |
| Intervention adherence | M/SD |
| Cognitive training levels completed | 912.84 ± 89.07 |
| Education training questions completed | 58.77 ± 1.48 |
M mean, SD standard deviation, N number
Procedures
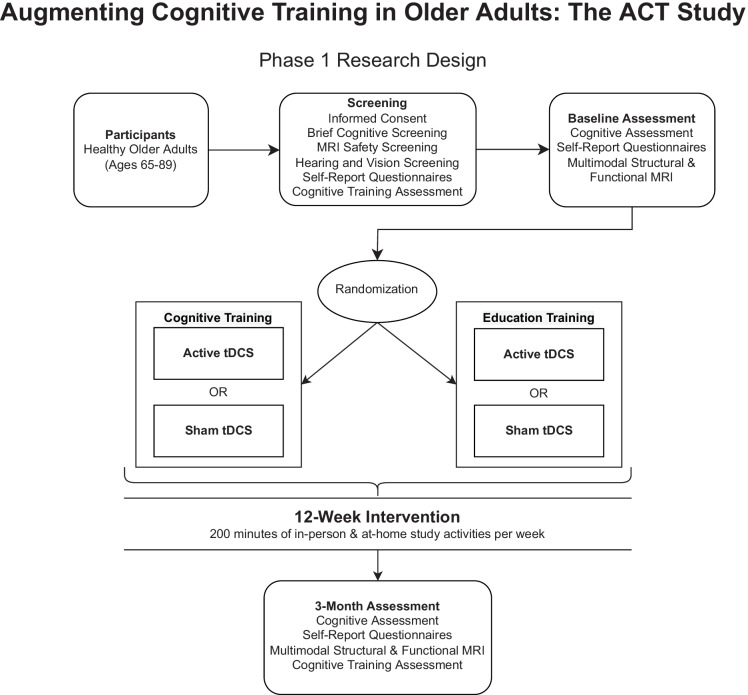
Data were collected as part of a larger multi-site phase III triple-blind randomized control trial funded by the National Institute on Aging (PI: Adam J. Woods, PhD, R01AG054077) with a target enrollment of 360 (180 female) healthy older adults. The trial is registered at clinicaltrials.gov as NCT02851511. The parent study employs a two-phase design. An initial cohort of 80 participants, collected across two study sites, was assigned to one of four intervention conditions. The primary aim of phase 1 was to investigate whether cognitive training is significantly better than a well-matched education training control in eliciting post-intervention improvement on the proximal composite measure. The sample was randomized to either an adaptive multidomain cognitive training intervention or education training control. All participants underwent sham or active transcranial direct current stimulation (tDCS) as an adjunctive intervention, which was not a variable of interest in phase 1. An interim analysis was performed to subsequently remove the education training condition in phase 2 and assess the relative impact of tDCS on cognitive training improvement in 280 healthy older adults. Phase 2 of the parent trial is still ongoing, and therefore, the randomization status of the tDCS intervention remains blinded to the study investigators. Only data pertinent to phase 1 study aims are currently available for analysis. Therefore, a dummy-coded variable was provided by the data management and quality control (DMAQC) team to control for tDCS status during analyses. Thus, tDCS status is not a variable of interest in the current study. A description of phase 1 study procedures follows.
Screening visits began with participants reviewing the informed consent with a trained researcher. Participants then completed a series of screening measures including a brief cognitive assessment, MRI safety screening, medical history and mood questionnaires, hearing and vision assessments, and the Posit Science cognitive training assessment (www.positscience.com). After determining whether participants met inclusion criteria, a baseline visit was scheduled. At the baseline visit, participants completed a battery of cognitive assessments, questionnaires, and a multimodal MRI scan. Following their baseline visit, participants were assigned to a 12-week intervention wherein they received either adaptive multidomain cognitive training (n = 34) or education training (n = 32), paired with either active or sham tDCS (Table 1). Participants attended ten daily in-person sessions over the first 2 weeks of the study, followed by weekly in-person sessions for 10 weeks. All participants were instructed to complete forty minutes of study activity at home for the remaining 4 days per week, on a 4G LTE-enabled laptop (Dell e5570) with a 15.5 in (diagonal) screen provided by study investigators. The intensity and duration of study activities for cognitive training and education training were identical; all participants were assigned 200 min of study activity per week (Fig. 1). Complete study materials are described in detail elsewhere [28]. In brief, participants were provided an optical mouse and comfortable headphones with audio level adjustment dial. All laptops were locked into a custom kiosk mode such that participants only accessed education training and cognitive training study portals and powered down by closing the laptop lid to facilitate ease of use by older adult participants. Participants underwent basic computer training and orientation with study investigators. A 24/7 telephone line was provided to participants to troubleshoot technological issues.
Fig. 1.
ACT study phase 1 procedures and conceptual model. Intensity and duration of study activities were identical between intervention assignments. Phase 2 of study is ongoing; therefore, tDCS condition remains blinded to investigators
Treatment conditions
Cognitive training
An eight-component, multidomain, cognitive training intervention was delivered via Posit Science’s BrainHQ suite (www.positscience.com); Posit Science’s BrainHQ tasks are commercially available. Detailed descriptions of these tasks are available through the Posit Science manuals/protocols. Over the course of the 12-week intervention, participants were assigned 20 h of training in 4 tasks targeting attention/processing speed (divided attention, target tracker, double decision, and hawk-eye tasks), and 20 h of training in four tasks targeting working memory (to-do list training, memory grid, card shark, and auditory aces). The cognitive training portal was programmed such that participants only accessed the eight chosen BrainHQ tasks. Participants completed four tasks per day for 10 min per task. A built-in timer progressed participants to the next task after 10 min of training. Training tasks were counterbalanced such that participants did not receive four of the same tasks each day but received timed training on all tasks over the 3-month intervention period [28].
Education training
The education training condition served as a control for the cognitive training condition. The education training group received the same materials (laptop, mouse, headphones, etc.), except for the contents accessed on the laptops. As with cognitive training, the education training participants were assigned 40 min of study activities 5 days per week for 12 weeks. The duration and frequency of study visits for education training were identical to that of cognitive training. The education training participants viewed educational videos produced by the National Geographic Channel, covering topics including history, nature, and wildlife. The education training portal was programmed such that participants navigated directly to daily videos. Participants followed a link to view the 40-min video assigned for each day. To ensure active engagement and attention, participants were asked to answer 4–6 questions regarding the content of each day’s videos. Questionnaires were collected during in-person visits to provide feedback on training adherence [28].
Proximal cognitive training assessment
The Posit Science BrainHQ cognitive training assessment was administered at the screening and 3-month time point and included all eight selected cognitive training tasks, set to a medium difficulty level. The goal was to measure proximal performance on the cognitive training tasks central to the cognitive training condition [28]. The assessment provided z-transformed scores for each of the eight cognitive training tasks and the proximal composite. Two additional sub-composite measures were created by combining the z-transformed scores from the four attention/processing speed cognitive training tasks and the four working memory cognitive training tasks mentioned above (see “Cognitive training”) [29]. This allowed for analyses of proximal change across all training tasks and their specific cognitive domains. Thus, there were three proximal assessment outcomes of interest: total training composite, processing speed training composite, and working memory training composite.
MRI acquisition and processing
The MRI acquisition and processing procedures have been described in detail in prior publications [20, 27]. Briefly, an MRI was collected using either a 3-Tesla Siemens Magnetom Prisma with a 64-channel head coil or a 3-Tesla Siemens Magnetom Skyra scanner with a 32-channel head coil at UF and UA, respectively. A high resolution T1-weighted 3D magnetization prepared rapid gradient echo (MPRAGE; repetition time [TR] = 1,800 ms; echo time [TE] = 2.26 ms; 1.0 × 1.0 × 1.0 mm3 voxels; 176 slices; field of view [FOV] = 256 × 256 mm; flip angle [FA] = 8; time = 3 min and 3 s) and T2-weighted fluid-attenuated inversion recovery (FLAIR; TR = 7,000 ms; TE = 101 ms; 1.0 × 1.0 × 2.5 mm3 voxels; 55 slices; FOV = 256 × 256 mm; FA = 120; time = 3 min and 9 s) were collected as part of the MRI session. The sequence parameters and scanning protocol were identical at the two study sites. Earplugs and foam padding were provided to the participant to minimize noise and head motion inside the scanner.
FLAIR and MPRAGE scans were acquired during the baseline visit. The images were processed using two freely available software applications, the lesion segmentation tool (LST) for SPM12 (www.statistical-modeling.de/lst.html), and the FreeSurfer 6.0 imaging analysis suite (http://surfer.nmr.mgh.harvard.edu/), as previously described [20, 27]. The FLAIR and MPRAGE were input to the lesion prediction algorithm of LST [30] (LPA; chapter 6.1). LPA is an accurate and reliable automated processing method for detecting WMH in healthy and neurodegenerative disease populations [31–33]. The full details of LST’s procedures are documented and available online (https://statistical-modelling.de/LST_documentation.pdf). The procedures include segmentation of brain tissue, registration of FLAIR to MPRAGE, and calculation of lesion maps. The LPA uses a spatial covariate that accounts for voxel-specific changes in lesion probability [30]. We used a lesion threshold of 0.30 to calculate TLV, consistent with previous research [27, 34]. All participants’ lesion segmentation results were visually reviewed, and no participants were removed for poor lesion classification. A log transformation was applied to TLV to normalize the distribution of white matter hyperintensities.
The MPRAGE was input to FreeSurfer’s default processing stream. FreeSurfer’s technical procedures are well-documented in previous publications [35–38] and thus are not described in detail here. In brief, FreeSurfer processing involves Talairach transformation, correction of magnetic field inhomogeneities via intensity normalization, and removal of non-brain tissues. Resultant FreeSurfer surface models were assessed visually and manually edited as appropriate. We applied FreeSurfer-generated estimated intracranial volume (eTIV) as a covariate in linear models to account for individual differences in participant head size.
Data analyses
Analyses were performed in the Statistical Package for the Social Sciences (SPSS; IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp). Primary analyses included Bonferroni-corrected repeated-measures analysis of covariance (RM-ANCOVA) and were conducted to examine pre-post differences in proximal composite and sub-composite measures between education training and cognitive training, while accounting for age, sex, education, and blinded tDCS intervention condition. Follow-up analyses examined the effect of several predictors, including TLV, on proximal post-intervention composite and sub-composite measures. Three baseline-adjusted linear regression models assessed pre-post change on the proximal composite and sub-composite measures. Baseline-adjusted linear regression was chosen because (1) its’ power compared to change scores in randomized studies [39–41] and (2) the ability to partial out baseline cognitive training performance — a potentially relevant predictor of cognitive training outcomes with mixed influence in previous literature [7]. Predictors included baseline performance, cognitive training group, blinded tDCS group, age, sex, years of education, scanner type, eTIV, log-adjusted whole-brain TLV, and binarized cardiovascular disease (CVD) risk. High CVD risk (n = 28, 45.2%) was defined by self-report of two or more of the following: prior or current smoker status, history of angina, atrial fibrillation, cardiac arrest, coronary artery disease, hypertension, high cholesterol, heart attack, heart failure, transient ischemic attacks, and diabetes [42].
Results
Change in proximal composite and sub-composites by intervention group
Preliminary analyses aimed to determine group differences (cognitive versus education) in proximal composite performance. RM-ANCOVA demonstrated a significant two-way interaction for group × time (Total Training Composite: p < 0.001, η2p = 0.56; Fig. 2). The intervention groups did not differ in their baseline performance (occasion 1 mean difference = 0.08, p = 0.49). Both intervention groups demonstrated improvement at their post-intervention assessment (education training mean difference = 0.23, p < 0.01; cognitive training mean difference = 1.16, p < 0.001). However, pairwise comparisons revealed cognitive training demonstrated much larger mean differences between occasions and performed significantly higher on the proximal composite at the post-intervention assessment (occasion 2 mean difference = 1.00, p < 0.001). These findings suggest that both groups improved on the proximal composite, but those who received cognitive training performed significantly better following intervention compared to those assigned the education training control condition.
Fig. 2.
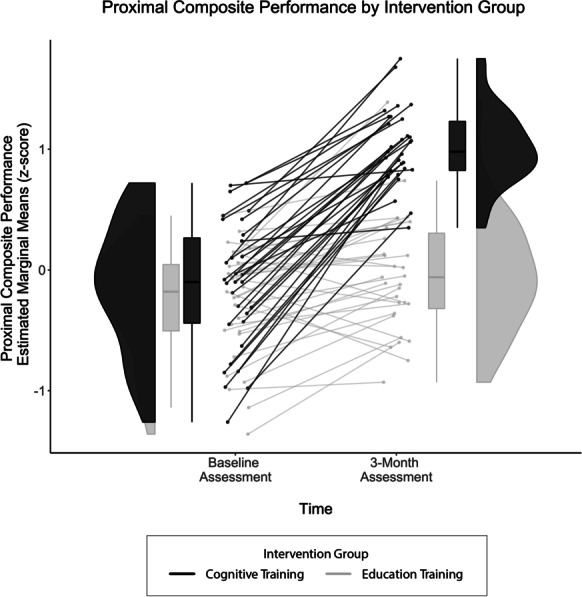
Three-month proximal composite performance by intervention group. Between-group differences on proximal composite as evidenced by Bonferonni-adjusted RM-ANCOVA. Covariates appearing in the model were evaluated at the following values: age = 71.11, sex = 0.45, years of education = 16.19, tDCS group = 0.50. Findings demonstrated that intervention groups did not differ at baseline, both groups improved significantly from baseline to 3-month assessments, and the cognitive training group (black) improved significantly more compared to the education training group (gray)
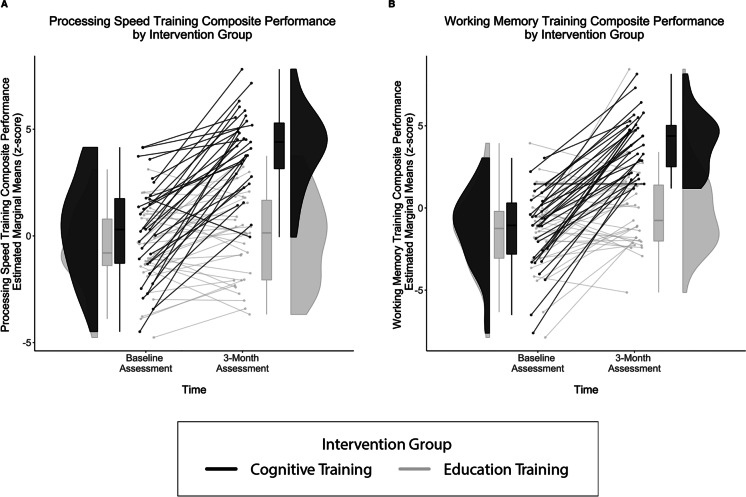
Sub-composite analyses revealed a similar pattern of results. RM-ANCOVA demonstrated a significant two-way interaction for group × time for each sub-composite (processing speed training composite: p < 0.001, η2p = 0.44; working memory training composite: p < 0.001, η2p = 0.43; Fig. 3). Intervention groups did not differ in their baseline performance for either sub-composite (processing speed training composite occasion 1 mean difference = 0.69, p = 0.17, working memory training composite occasion 1 mean difference = 0.08, p = 0.89). On the processing speed training composite, only the cognitive training group demonstrated significant improvement between time points (processing speed training composite: education training mean difference = 0.53, p = 0.15; cognitive training mean difference = 3.90, p < 0.001). In contrast, both the education training and cognitive training groups demonstrated significant improvement between time points on the working memory training composite (education training mean difference = 1.30, p < 0.01; cognitive training mean difference = 5.34, p < 0.001). Across both sub-composites, the cognitive training group evidenced larger mean differences between occasions and performed significantly better at the post-intervention assessment (processing speed training composite occasion 2 mean difference = 4.06, p < 0.001, working memory training composite occasion 2 mean difference = 3.96, p < 0.001). These findings further suggest that those who received cognitive training performed significantly better following intervention compared to those assigned the education training control condition.
Fig. 3.
Three-month sub-composite training performance by intervention group. Between-group differences in the A processing speed training composite, and B working memory training composite as evidenced by Bonferonni-adjusted RM-ANCOVA. Covariates appearing in the model are evaluated at the following values: age = 71.11, sex = 0.45, years of education = 16.19, tDCS group = 0.50. Findings demonstrate that intervention groups did not differ at baseline, both groups improved significantly from baseline to 3 months on working memory training composite, while only the cognitive training group improved significantly on processing speed training composite. The cognitive training group (black) improved significantly compared to the education training group (gray) across both sub-composites
Predictors of pre-post proximal composite and sub-composite change
Proximal composite
Baseline-adjusted linear regression explained 78.8% of the variance in the 3-month proximal composite, which was significantly greater than zero (F[10, 51] = 18.98, p < 0.001). Significant predictors of pre-post change on proximal composite performance included intervention assignment and baseline performance. Assignment to the cognitive training group (β = 0.73, p < 0.001) and higher baseline performance (β = 0.29, p < 0.001) were associated with higher pre-post change on the proximal composite. There was a potentially suggestive trend for a relationship between higher baseline TLV and lower change on 3-month proximal composite (β = − 0.14, p = 0.06; Fig. 4).
Fig. 4.
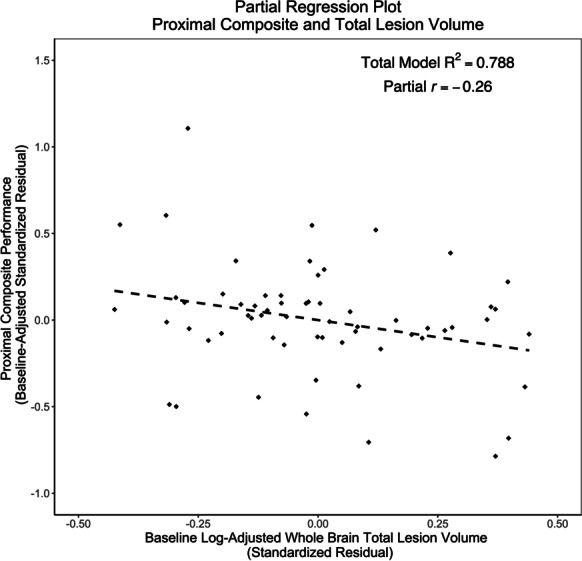
Partial regression plot for baseline TLV and 3-month proximal composite. Partial regression plot demonstrating a potentially suggestive trend between baseline log-adjusted TLV and post-intervention proximal composite performance (p = 0.06), while controlling for baseline performance, cognitive training group, tDCS group, age, sex, years of education, scanner type, estimated total intracranial volume, log-adjusted whole-brain TLV, and binarized cardiovascular disease (CVD) risk
Processing speed training composite
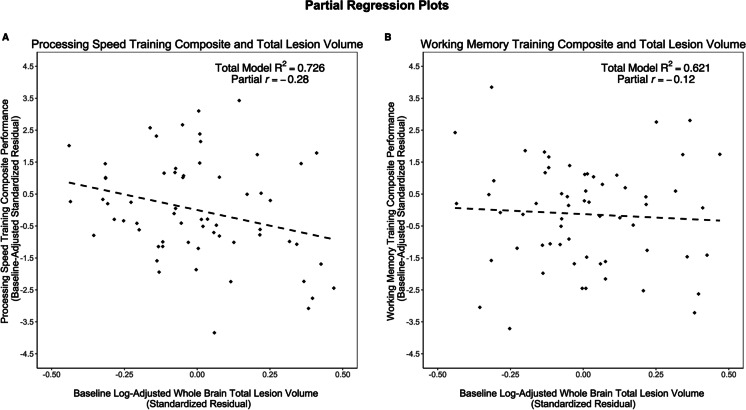
The overall model explained 72.6% of the variance in pre-post change on processing speed training composite performance, which was significantly greater than zero (F[10, 51] = 13.49, p < 0.001). Significant predictors included intervention assignment, baseline performance, education, and log-adjusted whole-brain TLV. Assignment to the cognitive training group (β = 0.66, p < 0.001), higher baseline performance (β = 0.27, p < 0.01), and higher education (β = 0.21, p = 0.02) associated with higher pre-post change on processing speed training composite performance. In contrast, higher baseline TLV is associated with lower change on processing speed training composite performance (β = − 0.18, p = 0.046) (Fig. 5A).
Fig. 5.
Partial regression plot for baseline TLV and 3-month sub-composite. A partial regression plot demonstrating the relationships between baseline log-adjusted TLV and A processing speed training composite (p = 0.046) and B working memory training composite (p = 0.38), while controlling for baseline performance, cognitive training group, tDCS group, age, sex, years of education, scanner type, estimated total intracranial volume, log-adjusted whole-brain TLV, and binarized cardiovascular disease (CVD) risk. Baseline TLV was a significant predictor for processing speed training composite but not the working memory training composite
Working memory training composite
The overall model explained 62.1% of the variance in pre-post change on working memory training composite performance, which was significantly greater than zero (F[10, 51] = 8.37, p < 0.001). Significant predictors of included intervention assignment and baseline performance. Assignment to the cognitive training group (β = 0.67, p < 0.001) and higher baseline (β = 0.29, p < 0.01) associated with higher pre-post change on working memory training composite performance. Post-intervention performance on the working memory training composite did not associate with baseline log-adjusted whole-brain TLV (β = − 0.09, p = 0.38) (Fig. 5B). The remaining predictors (age p = 0.63; sex p = 0.29; years of education p = 0.97; scanner type p = 0.89; eTIV p = 0.46; and CVD risk p = 0.61) were not significant in this model.
Discussion
Our aim was to investigate (1) the effect of a multidomain cognitive training intervention on improving proximal cognitive training outcomes compared to an education training control condition and (2) the impact of baseline whole-brain TLV, a proxy for WMH load, on pre-post proximal training outcomes following a 3-month intervention. Results from these analyses in older adults without manifest neurodegenerative disease supported our hypotheses that cognitive training results are in significantly greater improvement on proximal composite (total training composite) and sub-composite (processing speed training composite, and working memory training composite) measures compared to education training intervention. These findings emerged above and beyond the relevant predictors of age, sex, and years of education. Additionally, higher baseline TLV was related to lower pre-post change on processing speed training composite measure in both cognitive training and education training groups. We found a potentially suggestive trend for higher baseline TLV and lower pre-post change on the proximal composite, and no relationship between TLV and pre-post change working memory training composite performance. Findings related to TLV emerged above and beyond relevant predictors age and CVD risk, which are highly associated with future risk of stroke or conversion to dementia [14, 20]. The identification of baseline brain characteristics affecting pre-post cognitive training outcomes has gained considerable scientific interest in recent years [7, 43].
To our knowledge, we are the first study exploring baseline TLV as a predictor for proximal cognitive training outcomes. Total lesion volume (TLV) represents white matter hyperintensity burden in a single brain. WMH reflect age-related chronic macrostructural brain damage. Identified by T2-weighted MRI, WMH are characteristic of tissue alterations in the brain including small-vessel vascular occlusion, demyelination, increased water content, and loss of glial cells [44, 45]. The presence of WMH may reflect disruption of long-range axonal connections that indirectly mediate relationships between age-related cortical changes and cognitive decline [46]. Higher global and regional WMH load are related to poorer cognitive performance in processing speed, memory, and executive function [23, 24, 27, 34, 47–52]. The present study extends prior findings by demonstrating relationships between higher baseline TLV and lower pre-post change on proximal processing speed cognitive training measures.
The present study demonstrates that pre-post assessment of proximal cognitive training gains is sensitive to higher global WMH load. This finding was significant across the whole sample, that is, in both cognitive training and education training groups. Proximal outcome measures are considered robust and reliable for demonstrating training improvement. A recent systematic review and meta-analysis suggest proximal training measures that are not significantly influenced by age, education, or cognitive status [5]. The presented findings demonstrate that baseline brain characteristics, specifically global WMH load, may negatively impact pre-post change on proximal cognitive training measures. Future studies should consider TLV, and other baseline brain characteristics, when evaluating proximal cognitive training outcomes.
In our study, higher baseline TLV related to lower post-intervention performance for processing speed training composite, but not working memory training composite. These findings are in line with previous research demonstrating small but robust relationships between higher WMH load and decrements in domain-specific cognitive performance [20, 53]. Specifically, our findings are consistent with previous research in older adults without cognitive impairment demonstrating that larger WMH volumes associate with lower levels of perceptual speed but not working memory [27, 54]. While poorer processing speed is consistently related to higher whole-brain and regional WMHs, relationships between working memory ability and whole-brain WMH load are inconsistent and may vary by brain region [26, 55]. Our findings are consistent with these prior cognitive findings and suggest that processing speed-based cognitive training outcomes may be particularly sensitive to the size of the WMH load.
Proximal measures are considered the most reliable and robust method for assessing cognitive training efficacy [5]. However, we are not aware of any prior study examining baseline structural neuroimaging markers on proximal training outcomes following multidomain, adaptive, and cognitive training in healthy older adults. Prior studies have employed a wide array of training types (e.g., strategy videogames, mnemonic strategy, metamemory, and logical reasoning) and outcome measures (e.g., near and far transfer) in study populations that are not directly comparable to the methods employed in the current study. Nonetheless, evidence suggests larger regional volumes, lower cortical thickness, and higher structural integrity relate to improved training outcomes, faster learning rates, and higher cognitive performance on standardized neuropsychological assessments in older adults [14, 56–59]. The incorporation of proximal training outcomes provides a robust and appropriate measure for assessing effects of altered white matter integrity on training-specific gains.
Strengths and limitations
Strengths of the current study include the randomized control study design, allowing for comparison between intervention types, and incorporating an education training control group demonstrating the utility of an adaptive multicomponent cognitive training intervention to facilitate proximal cognitive training improvement. The present study identified a baseline neuroimaging marker that relates to pre-post change on proximal cognitive training measures. The examination of TLV in a sample of older adults absent of neurodegenerative disease is clinically relevant, as WMH in healthy older adults are less widespread but overlap when compared to neurodegenerative disease populations. Moreover, the presence and extent of WMH have been associated with lowered threshold for clinical expression of dementia [20] and are therefore an important factor to consider when employing interventions to remediate cognitive declines in aging. The present study examined proximal performance outcomes (i.e., the eight cognitive tasks trained) and did not examine neuropsychological measures capturing near or far transfer. Future studies should consider exploring the relationship between factors affecting proximal improvement, as well as near or far transfer. Understanding the role of TLV in proximal cognitive training measures would provide potential utility for personalized cognitive training paradigms in healthy older adult, mildly cognitively impaired, and dementia populations. Further, the results remained above and beyond CVD risk, which is associated with age-related brain changes and presence of WMH.
The present study only used the MPRAGE and FLAIR acquired at baseline to characterize TLV. This methodology provides a whole-brain measure of lesion load and does not allow for differentiation between the location of WMH (i.e., periventricular, deep, frontal, etc.). While findings here are consistent with prior literature, the spread and extent of WMHs have been shown to differentially affect cognitive function. Future studies should incorporate regional and periventricular analyses to further understand these relationships. Additionally, this methodology did not allow for the assessment of white matter bundles (i.e., diffusion-weighted imaging), or any changes to TLV that may have occurred during study participation. It would be valuable to combine measures of WMH with measures of microstructural white matter integrity/connectivity and further characterize how white matter changes over time affect cognitive training outcomes. The present study employed a freely available automated segmentation tool for efficient and reliable quantification of white matter hyperintensities, which matched those of previously published data in older adults [34]. Importantly, automated segmentation methods may vary depending on MRI scanner characteristics, acquisition parameters, and study populations. Ideal lesion segmentation may require differing processing pipelines or software depending on target sample or research/clinical settings.
Finally, the sample was largely Caucasian (83.3%) and non-Hispanic (90.9%) and had years of education consistent with a bachelor’s degree. The findings from the current study are not readily generalizable to other racial/ethnic groups such as Black/African Americans, Hispanic, or Asian/Asian Americans, where patterns of white matter hyperintensities, cardiovascular disease risk, and other relevant factors may vary. For example, systemic racism and marginalization are just two of many chronic stressors that can have a significant impact on health status and result in higher rates of CVD risk in minority populations [60]. Other social determinants of health, including lower socioeconomic status in childhood and adults, are related to greater white matter lesion burden [61]. Future studies should work toward equitable research practices, including attending to recruitment strategies and considering the impact of systemic racism to comprehensively evaluate the relationships presented in the current study.
Conclusion
Findings from this study contribute to the growing body of literature demonstrating the utility of cognitive training interventions in older adults. Adaptive, multidomain, cognitive training resulted in higher post-intervention proximal composite and sub-composite performance compared to the education training control group. Additionally, the present study provides a baseline brain characteristic (TLV) that may represent a challenge for evaluating the efficacy of single-and-multi-domain cognitive training interventions for remediating age-related cognitive declines. Higher age and greater vascular disease risks (e.g., hypertension, smoking, diabetes mellitus, atrial fibrillation) are major risk factors for increased white matter hyperintensity burden, higher TLV, and ultimately clinical expression of dementia. WMH may be modifiable, and early intervention on lifestyle factors (e.g., reducing cardiovascular disease risk, better control of hypertension, etc.) may facilitate greater response to cognitive interventions for an increasingly aging population. Regardless, our study demonstrates that pre-post assessment of processing speed proximal training gains is sensitive to TLV. The results demonstrate important considerations for future research aiming to intervene on age-related cognitive decline.
Acknowledgements
This manuscript would not be possible without our valued research participants at the McKnight Brain Institutes at the University of Florida and University of Arizona. We thank you for your commitment to research, your generosity, and hard work. We thank our research assistants for their hard work and instrumental role in making this manuscript possible.
Funding
We would like to acknowledge support by the National Institute of Aging/National Institutes of Health (R01AG054077, T32AG020499, P30AG019610); the National Heart, Lung, and Blood Institute (T32HL134621); the National Science Foundation (NSF1842473); the University of Florida Center for Cognitive Aging and Memory Clinical Translational Research; the state of Arizona and Arizona Department of Health Services; and the McKnight Brain Research Foundation.
Data availability
The datasets presented in this article are not readily available because, the data are managed under the data-sharing agreement established with NIA and the parent R01 clinical trial Data Safety and Monitoring Board in the context of an ongoing phase III clinical trial (ACT study, R01AG054077). All trial data will be made publicly available 2 years after completion of the parent clinical trial, per NIA and DSMB agreement. Requests for baseline data can be submitted to the ACT Publication and Presentation (P&P) Committee and will require submission of data use, authorship, and analytic plan for review by the P&P Committee. Requests to access the datasets should be directed to ajwoods@phhp.ufl.edu.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment Effects on Adult Cognitive Development: Can the Functional Capacity of Older Adults Be Preserved and Enhanced? Psychol Sci Public Interest. 2008;9(1):1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 2.Vance DE, Keltner NL, McGuinness T, Umlauf MG, Yuan Y-Y. The future of cognitive remediation training in older adults. J Neurosci Nurs. 2010;42(5):255–264. doi: 10.1097/JNN.0b013e3181ecb003. [DOI] [PubMed] [Google Scholar]
- 3.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mewborn CM, Lindbergh CA, Stephen Miller L. Cognitive interventions for cognitively healthy, mildly impaired, and mixed samples of older adults: A systematic review and meta-analysis of randomized-controlled trials. Neuropsychol Rev. 2017;27(4):403-39. 10.1007/s11065-017-9350-8 [DOI] [PubMed]
- 6.Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62:16–24. 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed]
- 7.Baykara E, Könen T, Unger K, Karbach J. MRI predictors of cognitive training outcomes. J Cogn Enhanc. 2021;5(2):245–258. doi: 10.1007/s41465-020-00188-y. [DOI] [Google Scholar]
- 8.Buschkuehl M, Jaeggi SM, Hutchison S, Perrig-Chiello P, Däpp C, Müller M, et al. Impact of working memory training on memory performance in old-old adults. Psychol Aging. 2008;23(4):743–753. doi: 10.1037/a0014342. [DOI] [PubMed] [Google Scholar]
- 9.Li S-C, Schmiedek Fl, Huxhold, Röcke C, Smith J, Lindenberger U. Working memory plasticity in old age: practice gain, transfer, and maintenance. Psychol Aging. 2008;23(4):731–742. doi: 10.1037/a0014343. [DOI] [PubMed] [Google Scholar]
- 10.Morrison AB, Chein JM. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychon Bull Rev. 2011;18(1):46-60. 10.3758/s13423-010-0034-0. [DOI] [PubMed]
- 11.Klingberg T. Training and plasticity of working memory. Trends Cogn Sci. 2010;14(7):317-24. 10.1016/j.tics.2010.05.002. [DOI] [PubMed]
- 12.McAvinue LP, Golemme M, Castorina M, Tatti E, Pigni FM, Salomone S, et al. An evaluation of a working memory training scheme in older adults. Front Aging Neurosci. 2013;5:20. 10.3389/fnagi.2013.00020. [DOI] [PMC free article] [PubMed]
- 13.Basak C, Qin S, O'Connell MA. Differential effects of cognitive training modules in healthy aging and mild cognitive impairment: A comprehensive meta-analysis of randomized controlled trials. Psychol Aging. 2020;35(2):220–249. doi: 10.1037/pag0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peter J, Schumacher LV, Landerer V, Abdulkadir A, Kaller CP, Lahr J, et al. Biological factors contributing to the response to cognitive training in mild cognitive impairment. J Alzheimers Dis. 2017;61(1):333–345. doi: 10.3233/JAD-170580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belleville S, Gilbert B, Fontaine F, Gagnon L, Ménard É, Gauthier S. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: Evidence from a cognitive intervention program. Dement Geriatr Cogn Disord. 2006;22(5-6):486–499. doi: 10.1159/000096316. [DOI] [PubMed] [Google Scholar]
- 16.Kraft JN, O’Shea A, Albizu A, Evangelista ND, Hausman HK, Boutzoukas E, et al. Structural neural correlates of double decision performance in older adults. Front. Aging Neurosci. 2020:278. Available from: 10.3389/fnagi.2020.00278. [DOI] [PMC free article] [PubMed]
- 17.Evangelista ND, O’Shea A, Kraft JN, Hausman HK, Boutzoukas EM, Nissim NR, et al. Independent contributions of dorsolateral prefrontal structure and function to working memory in healthy older adults. Cereb Cortex. 2021;31:1732–43. Available from: 10.1093/cercor/bhaa322. [DOI] [PMC free article] [PubMed]
- 18.Hardcastle C, O’Shea A, Kraft JN, Albizu A, Evangelista ND, Hausman HK, et al. Contributions of hippocampal volume to cognition in healthy older adults. Front Aging Neurosci. 2020:365. Available from: 10.3389/fnagi.2020.593833. [DOI] [PMC free article] [PubMed]
- 19.Hausman HK, O’Shea A, Kraft JN, Boutzoukas EM, Evangelista ND, Van Etten EJ, et al. The role of resting-state network functional connectivity in cognitive aging. Front Aging Neurosci. 2020:177. Available from: 10.3389/fnagi.2020.00177. [DOI] [PMC free article] [PubMed]
- 20.Alber J, Alladi S, Bae HJ, Barton DA, Beckett LA, Bell JM, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement Transl Res Clin Interv. 2019;5:107-17. 10.1016/j.trci.2019.02.001. [DOI] [PMC free article] [PubMed]
- 21.Bolandzadeh N, Davis JC, Tam R, Handy TC, Liu-Ambrose T. The association between cognitive function and white matter lesion location in older adults: A systematic review. BMC Neurol. 2012;12(1):1-0. 10.1186/1471-2377-12-126. [DOI] [PMC free article] [PubMed]
- 22.van Rooden S, van den Berg-Huysmans AA, Croll PH, Labadie G, Hayes JM, Viviano R, et al. Subjective cognitive decline is associated with greater white matter hyperintensity volume. J Alzheimers Dis. 2018;66(3):1283–1294. doi: 10.3233/JAD-180285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakefield DB, Moscufo N, Guttmann CR, Kuchel GA, Kaplan RF, Pearlson G, et al. White matter hyperintensities predict functional decline in voiding, mobility, and cognition in older adults. J Am Geriatr Soc. 2010;58(2):275–81. 10.1111/j.1532-5415.2009.02699.x. [DOI] [PMC free article] [PubMed]
- 24.Lampe L, Kharabian-Masouleh S, Kynast J, Arelin K, Steele CJ, Löffler M, et al. Lesion location matters: The relationships between white matter hyperintensities on cognition in the healthy elderly. J Cereb Blood Flow Metab. 2019;39(1):36-43. 10.1177/0271678X17740501. [DOI] [PMC free article] [PubMed]
- 25.Oosterman JM, Sergeant JA, Weinstein HC, Scherder EJA. Timed executive functions and white matter in aging with and without cardiovascular risk factors. Rev Neurosci. 2004;15(6):439-62. 10.1515/REVNEURO.2004.15.6.439. [DOI] [PubMed]
- 26.Brugulat-Serrat A, Salvadó G, Sudre CH, Grau-Rivera O, Suárez-Calvet M, Falcon C, et al. Patterns of white matter hyperintensities associated with cognition in middle-aged cognitively healthy individuals. Brain Imaging Behav. 2020;14(5):2012–23. 10.1007/s11682-019-00151-2. [DOI] [PMC free article] [PubMed]
- 27.Boutzoukas EM, O’Shea A, Albizu A, Evangelista ND, Hausman HK, Kraft JN, et al. Frontal white matter hyperintensities and executive functioning performance in older adults. Front Aging Neurosci. 2021:338. Available from: 10.3389/fnagi.2021.672535. [DOI] [PMC free article] [PubMed]
- 28.Woods AJ, Cohen R, Marsiske M, Alexander GE, Czaja SJ, Wu S. Augmenting cognitive training in older adults (The ACT Study): Design and methods of a phase III tDCS and cognitive training trial. Contemp Clin Trials. 2018;65:19–32. doi: 10.1016/j.cct.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song MK, Lin FC, Ward SE, Fine JP. Composite variables: When and how. Nurs Res. 2013;62(1):45–9. 10.1097/NNR.0b013e3182741948. [DOI] [PMC free article] [PubMed]
- 30.Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage. 2012;59(4):3774-83. 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed]
- 31.de Sitter A, Steenwijk MD, Ruet A, Versteeg A, Liu Y, van Schijndel RA, et al. Performance of five research-domain automated WM lesion segmentation methods in a multi-center MS study. Neuroimage. 2017;163:106-14. 10.1016/j.neuroimage.2017.09.011. [DOI] [PubMed]
- 32.Egger C, Opfer R, Wang C, Kepp T, Sormani MP, Spies L, et al. MRI FLAIR lesion segmentation in multiple sclerosis: Does automated segmentation hold up with manual annotation? NeuroImage Clin. 2017;13:264-70. 10.1016/j.nicl.2016.11.020. [DOI] [PMC free article] [PubMed]
- 33.Ribaldi F, Altomare D, Jovicich J, Ferrari C, Picco A, Pizzini FB, et al. Accuracy and reproducibility of automated white matter hyperintensities segmentation with lesion segmentation tool: A European multi-site 3T study. Magn Reson Imaging. 2021;76:108-15. 10.1016/j.mri.2020.11.008. [DOI] [PubMed]
- 34.Birdsill AC, Koscik RL, Jonaitis EM, Johnson SC, Okonkwo OC, Hermann BP, et al. Regional white matter hyperintensities: Aging, Alzheimer’s disease risk, and cognitive function. Neurobiol Aging. 2014;35(4)769-76. 10.1016/j.neurobiolaging.2013.10.072. [DOI] [PMC free article] [PubMed]
- 35.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179-94. https://doi.org/10.1006/nimg.1998.0395. [DOI] [PubMed]
- 36.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195-207. 10.1006/nimg.1998.0396. [DOI] [PubMed]
- 37.Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petscher Y, Schatschneider C. A simulation study on the performance of the simple difference and covariance-adjusted scores in randomized experimental designs. J Educ Meas. 2011;48(1):31–43. Available from: 10.1111/j.1745-3984.2010.00129.x. [DOI] [PMC free article] [PubMed]
- 40.Senn S. Change from baseline and analysis of covariance revisited. Stat Med. 2006;25(24):4344-44. 10.1002/sim.2682. [DOI] [PubMed]
- 41.Van Breukelen GJP. ANCOVA versus change from baseline had more power in randomized studies and more bias in nonrandomized studies. J Clin Epidemiol. 2006;59(9):920–925. doi: 10.1016/j.jclinepi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Bangen KJ, Nation DA, Clark LR, Harmell AL, Wierenga CE, Dev SI, et al. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front Aging Neurosci. 2014;6:159. 10.3389/fnagi.2014.00159. [DOI] [PMC free article] [PubMed]
- 43.Ansado J, Chasen C, Bouchard S, Northoff G. How brain imaging provides predictive biomarkers for therapeutic success in the context of virtual reality cognitive training. Neurosci Biobehav Rev. 2021;120:583-94. 10.1016/j.neubiorev.2020.05.018. [DOI] [PubMed]
- 44.Zhuang FJ, Chen Y, He WB, Cai ZY. Prevalence of white matter hyperintensities increases with age. Neural Regen Res. 2018;13(12):2141. 10.4103/1673-5374.241465. [DOI] [PMC free article] [PubMed]
- 45.Bauer CE, Zachariou V, Seago E, Gold BT. White Matter Hyperintensity Volume and Location: Associations With WM Microstructure, Brain Iron, and Cerebral Perfusion. Front Aging Neurosci. 2021:341. 10.3389/fnagi.2021.617947. [DOI] [PMC free article] [PubMed]
- 46.Tuladhar AM, Reid AT, Shumskaya E, De Laat KF, Van Norden AGW, Van Dijk EJ, et al. Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke. 2015;46(2):425–432. doi: 10.1161/STROKEAHA.114.007146. [DOI] [PubMed] [Google Scholar]
- 47.Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, et al. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiol Aging. 2011;32(9):1588-98. 10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed]
- 48.Smith EE, Salat DH, Jeng J, McCreary CR, Fischl B, Schmahmann JD, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76(17):1492-9. 10.1212/WNL.0b013e318217e7c8. [DOI] [PMC free article] [PubMed]
- 49.Meier IB, Manly JJ, Provenzano FA, Louie KS, Wasserman BT, Griffith EY, et al. White matter predictors of cognitive functioning in older adults. J Int Neuropsychol Soc. 2012;18(03):414–427. doi: 10.1017/S1355617712000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirsiger S, Koppelmans V, Mérillat S, Erdin C, Narkhede A, Brickman AM, et al. Executive functions in healthy older adults are differentially related to macro- and microstructural white matter characteristics of the cerebral lobes. Front Aging Neurosci. 2017;9:373. 10.3389/fnagi.2017.00373. [DOI] [PMC free article] [PubMed]
- 51.Dhamoon MS, Cheung YK, Moon Y, DeRosa J, Sacco R, Elkind MSV, et al. Cerebral white matter disease and functional decline in older adults from the Northern Manhattan Study: A longitudinal cohort study. PLoS Med. 2018;15(3):e1002529. doi: 10.1371/journal.pmed.1002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bangen KJ, Thomas KR, Weigand AJ, Sanchez DL, Delano-Wood L, Edmonds EC, Carmichael OT, Schwarz CG, Brickman AM, Bondi MW, Alzheimer's Disease Neuroimaging Initiative. Pattern of regional white matter hyperintensity volume in mild cognitive impairment subtypes and associations with decline in daily functioning. Neurobiology of aging. 2020;86:134–42. 10.1016/j.neurobiolaging.2019.10.016. [DOI] [PMC free article] [PubMed]
- 53.Kloppenborg RP, Nederkoorn PJ, Geerlings MI, Van Den Berg E. Presence and progression of white matter hyperintensities and cognition: A meta-analysis. Neurology. 2014;82(23):2127–2138. doi: 10.1212/WNL.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 54.Arvanitakis Z, Fleischman DA, Arfanakis K, Leurgans SE, Barnes LL, Bennett DA. Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct Funct. 2016;221(4):2135–46. 10.1007/s00429-015-1034-7. [DOI] [PMC free article] [PubMed]
- 55.Oosterman JM, Van Harten B, Weinstein HC, Scheltens P, Sergeant JA, Scherder EJA. White matter hyperintensities and working memory: An explorative study. Aging, Neuropsychol Cogn. 2008;15(3):384–99. 10.1080/13825580701879998. [DOI] [PubMed]
- 56.Basak C, Voss MW, Erickson KI, Boot WR, Kramer AF. Regional differences in brain volume predict the acquisition of skill in a complex real-time strategy videogame. Brain Cogn. 2011;76(3):407–414. doi: 10.1016/j.bandc.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Engvig A, Fjell AM, Westlye LT, Skaane NV, Sundseth Ø, Walhovd KB. Hippocampal subfield volumes correlate with memory training benefit in subjective memory impairment. Neuroimage. 2012;61(1):188–194. doi: 10.1016/j.neuroimage.2012.02.072. [DOI] [PubMed] [Google Scholar]
- 58.Park S, Ryu SH, Yoo Y, Yang JJ, Kwon H, Youn JH, et al. Neural predictors of cognitive improvement by multi-strategic memory training based on metamemory in older adults with subjective memory complaints. Sci Rep. 2018;8(1):1–1. 10.1038/s41598-018-19390-2. [DOI] [PMC free article] [PubMed]
- 59.Ray NR, O’Connell MA, Nashiro K, Smith ET, Qin S, Basak C. Evaluating the relationship between white matter integrity, cognition, and varieties of video game learning. Restor Neurol Neurosci. 2017;35(5):437–56. 10.3233/RNN-160716. [DOI] [PubMed]
- 60.Brewer LC, Cooper LA. State of the art and science: race, discrimination, and cardiovascular disease. The virtual mentor: VM. 2014;16(6):455–60. [PMC free article] [PubMed]
- 61.Shaked D, Leibel DK, Katzel LI, Davatzikos C, Gullapalli RP, Seliger SL, et al. Disparities in diffuse cortical white matter integrity between socioeconomic groups. Front Hum Neurosci. 2019;13:198. 10.3389/fnhum.2019.00198. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this article are not readily available because, the data are managed under the data-sharing agreement established with NIA and the parent R01 clinical trial Data Safety and Monitoring Board in the context of an ongoing phase III clinical trial (ACT study, R01AG054077). All trial data will be made publicly available 2 years after completion of the parent clinical trial, per NIA and DSMB agreement. Requests for baseline data can be submitted to the ACT Publication and Presentation (P&P) Committee and will require submission of data use, authorship, and analytic plan for review by the P&P Committee. Requests to access the datasets should be directed to ajwoods@phhp.ufl.edu.