Abstract
Psoriasis is a chronic, inflammatory skin disease that affects 2‒3% of the global population. Besides skin manifestations, patients with psoriasis have increased susceptibility to a number of comorbidities, including psoriatic arthritis, cardiovascular disease, and inflammatory bowel disease. To understand the systemic component of psoriasis pathogenesis, we performed a pilot study to examine the fecal metagenome, host colonic transcriptome, and host peripheral blood immune profiles of patients with psoriasis and healthy controls. Our study showed increased functional diversity in the gut microbiome of patients with psoriasis. In addition, we identified microbial species that preferentially associate with patients with psoriasis and which have been previously found to associate with other autoimmune diseases. Intriguingly, our data revealed three psoriasis subgroups that have distinct microbial and host features. Integrating these features revealed host‒microbe associations that are specific to psoriasis or particular psoriasis subgroups. Our findings provide insight into the factors that may affect the development of comorbidities in patients with psoriasis and may hold diagnostic potential for early identification of patients with psoriasis at risk for these comorbidities.
Abbreviations: IBD, inflammatory bowel disease; RNA-seq, RNA sequencing
Introduction
Psoriasis is a prevalent immune-mediated disease characterized by inflamed skin lesions and epidermal hyperproliferation. A total of 2‒3% of the global population is affected by psoriasis, and the disease is known to be heterogeneous and multifactorial. It has been well established that psoriasis has different subtypes on the basis of distinct disease characteristics. Plaque psoriasis (psoriasis vulgaris) is the most prevalent psoriasis subtype, with others being guttate, inverse, erythrodermic, and pustular. Psoriasis pathogenesis is multifactorial with a strong genetic component (Elder et al., 2010). Recent studies have suggested that the microbiome, diet, and other environmental factors may also have a role in psoriasis pathogenesis (Afifi et al., 2017; Benhadou et al., 2018; Chang et al., 2018; Codoñer et al., 2018; Fahlén et al., 2012; Fyhrquist et al., 2019; Gao et al., 2008; Loesche et al., 2018; Takemoto et al., 2015; Tett et al., 2017; Yan et al., 2017). At the molecular level, aberrant activation of IL-17 signaling pathway is one of the major contributors to the disease (Brembilla et al., 2018). Therapeutically blocking components in IL-17 signaling pathway usually controls skin inflammation. However, the effectiveness of psoriasis treatments varies across patients, further highlighting the heterogeneous nature of psoriasis.
In addition to the skin manifestations, patients with psoriasis are at higher risk of developing a number of other comorbidities. Psoriatic arthritis is one of the most prevalent comorbidities among patients with psoriasis because up to one third of patients with psoriasis transition into psoriatic arthritis (Ritchlin et al., 2017). Patients with psoriasis also have a 2.5-fold higher risk of developing Crohn’s disease and a 1.7-fold higher risk of developing ulcerative colitis, linking psoriasis to inflammatory bowel disease (IBD) (Fu et al., 2018). Other common psoriasis comorbidities include cardiovascular disease (Prodanovich et al., 2009) and type 2 diabetes (Wan et al., 2018). Although the association between psoriasis and its comorbidities is firmly established, the underlying cause and triggers are yet to be elucidated. Developing these comorbidities in patients with psoriasis not only increases the disease burden for the patients but also complicates strategies in treatment and diagnosis. The ability to risk stratify patients with psoriasis for the development of certain comorbidities would greatly enhance strategies toward prevention, early detection, and treatment.
Although the skin microbiome has been the focus for cutaneous autoimmune disease, dysbiosis in the gut microbiome has been observed in psoriasis (Codoñer et al., 2018; Eppinga et al., 2016; Hidalgo-Cantabrana et al., 2019; Scher et al., 2015; Tan et al., 2018). Moreover, gut microbiome dysbiosis has been implicated in many psoriasis comorbidities such as psoriatic arthritis (Scher et al., 2015), IBD (Gevers et al., 2014; Knights et al., 2014; Lloyd-Price et al., 2019; Morgan et al., 2012), and type 2 diabetes (Zhou et al., 2019). Together, these findings suggest that the gut microbiome might be an important contributing factor for psoriasis pathogenesis and the emergence of psoriasis comorbidities. To better understand the systemic component of pathogenesis associated with psoriasis, we performed a detailed multiomics analysis with a cohort of patients with psoriasis and healthy individuals. We utilized shotgun metagenomic sequencing to profile both the taxonomic composition and functional capacity of the gut microbiome. In addition, we carried out RNA sequencing (RNA-seq) to profile the host intestinal transcriptome. Host peripheral blood was also collected to measure both systemic immune populations and their cytokine-producing capacity. Our study revealed several microbial features associated with psoriasis. Next, using clustering analysis, we identified three psoriasis subgroups, each with distinct microbial features that may predispose to certain psoriasis comorbidities. Finally, we performed multiomics analysis by integrating all our omics data and revealed disease and subgroup-specific host‒microbe associations. Our work highlights the heterogeneity of psoriasis and the potential role of microbial and host‒microbe associations in psoriasis pathogenesis and comorbidities. Moreover, this pilot study provides an analytical framework that can be applied to study host‒microbe association in other diseases.
Results
Study design and cohort summary
We recruited a cohort of 33 subjects with psoriasis not on systemic therapy and 15 age- and sex-matched healthy individuals (Table 1) to study the gut microbial features associated with psoriasis and their potential contribution to psoriasis pathogenesis. All patients with psoriasis were clinically diagnosed with psoriasis at the University of California San Francisco Psoriasis and Skin Treatment Center (San Francisco, CA) and had a mean PASI of 14.2, representing moderate-to-severe disease. To characterize microbial composition, we collected stool samples and subjected each sample to shotgun metagenomic sequencing that provides both taxonomic composition and functional capacity. For the subsequent analyses, we focused on bacterial species, microbial UniRef90 gene families, and microbial MetaCyc pathways. To link the changes of microbial features in psoriasis gut to the host response, we collected biopsies from the sigmoid colon and subjected these samples to RNA-seq. We also isolated PBMCs from blood samples to measure immune cell profiles and cytokine production capacity. Together, our study design (Figure 1) provided a comprehensive survey on both host biology and microbiome capacity in a cohort of patients with psoriasis in comparison with those in the healthy controls.
Table 1.
Demographic Information of Metagenomics Cohort
| Characteristic | Healthy | Psoriasis | P-Value |
|---|---|---|---|
| Sample size | 15 | 33 | NA |
| Sex (% female) | 47% | 52% | 1 |
| Age (mean age), y | 45.8 ± 13.9 | 43.2 ± 14.6 | 0.5654 |
| Mean PASI score | NA | 14.2 ± 13.3 | NA |
| Median PASI score | NA | 10.1 | NA |
Abbreviation: NA, not applicable.
Figure 1.
Multiomic study design. In this study, we collected six different datasets for multiomic analysis: shotgun metagenomic sequencing from stool samples generated profiles of (i) microbial species, (ii) microbial gene families, and (iii) microbial gene pathways. RNA-seq from sigmoid colon biopsies generated (iv) host colonic transcriptome data. Flow cytometry analyses from PBMCs generated (v) immune population profiles and (vi) cytokine production profiles. The datasets measuring microbial features are in the green box, and the datasets measuring host features are in the yellow box. Stool samples were collected from 15 healthy subjects and 33 patients with psoriasis. Sigmoid colon biopsies and PBMCs were collected from 16 healthy subjects and 26 patients with psoriasis. A total of 14 healthy subjects and 26 patients with psoriasis had six fully complete datasets. RNA-seq, RNA sequencing.
Microbial diversity and community structure between psoriasis and healthy gut microbiome
Gut microbiome dysbiosis has been previously associated with decreased microbial diversity. Low microbial diversity has been observed in several human diseases, including IBD, obesity, and autism (Gevers et al., 2014; Hsiao et al., 2013; Turnbaugh et al., 2009). It has been hypothesized that losing microbial diversity rise from a missing group of beneficial microbes in the gut microbiome, which can lead to many detrimental consequences such as loss of control in the growth of opportunistic pathogens and lack of production of beneficial microbial-derived compounds. Alpha diversities assess the microbial diversity within a community by calculating richness (numbers of species) and evenness (even distribution of each species within a community) (Lozupone and Knight, 2005). To compare the microbial diversity in patients with psoriasis with that in healthy subjects, we calculated different alpha diversity indices to estimate the overall diversity (Shannon), evenness (Simpson), and richness (chao1) of each community. We observed higher evenness of microbial functional diversities associated with patients with psoriasis, whereas similar taxonomical diversity was observed between patients with psoriasis and healthy controls (Figure 2a and Supplementary Figure S1a and b). The overall microbial community structures were similar between psoriasis samples and healthy samples because no distinct clusters were observed in principal coordinate analysis plots for taxonomic and functional profiles (Supplementary Figure S1c and d). All patients with psoriasis in our cohort had a normal-appearing lower gastrointestinal endoscopic examination, so drastic differences in diversity and community structure in the psoriasis microbiome as observed with other gastrointestinal diseases might not be expected.
Figure 2.
Microbial features associated with PSO and healthy subjects. Boxplots compare alpha diversities of gut microbiome in patients with PSO and those in the healthy subjects. Alpha diversity was measured by Shannon index, Simpson diversity index, and chao1 estimation for (a) microbial Uniref90 gene families. Statistical significance was determined by Wilcoxon test. (b) Dot plot summary of DA microbial species identified by DEseq2. Each dot represents a DA microbial species with dot size‒present adjusted P-value, and x-axis represents log2 fold change. (c) Boxplots of select DA microbial species. Blue boxes represent PSO sample, and red boxes represent healthy samples. ∗∗P < 0.01. adj, adjusted; DA, differential abundant; n.s., not significant; PSO, psoriasis.
Supplementary Figure S1.
Microbial diversity metrics. (a) Boxplot comparing the alpha diversity of gut microbiome in patients with PSO and healthy subjects. Alpha diversity was measured by Shannon index, Simpson diversity index, and chao1 estimation for microbial species. PCoA of the microbial community structures based on Bray‒Curtis distance matrix for (b) microbial species and (c) microbial gene families. (d) PCA of host transcriptome by top 5,000 most variable genes. In all plots, red denotes healthy samples, and blue denotes PSO samples. n.s., not significant; PC, principal component; PCA, principal component analysis; PCoA, principal coordinate analysis; PSO, psoriasis; RNA-seq, RNA sequencing.
Identification of the microbial features associated with the psoriasis gut microbiome
We hypothesized that even though the gut microbiome from patients with psoriasis has seemingly normal overall microbial community structure and diversity, the differences between psoriasis and healthy microbiome may be in specific microbial features. To identify the microbial features that are differentially abundant between gut microbiome from patients with psoriasis and healthy individuals, we performed differentially abundance analysis using DEseq2 (Love et al., 2014), which is designed for RNA-seq analysis but is widely adapted for microbiome data (McMurdie and Holmes, 2014). We estimated differential abundant features using a negative binomial model after controlling for known confounding factors for gut microbiome such as sex, age, and experimental batch. Our analysis revealed bacterial species and microbial gene families and pathways that are differentially abundant between microbiome in patients with psoriasis and healthy individuals (Figure 2b and Supplementary Table S1, Supplementary Table S2, Supplementary Table S3). Among bacterial species that are differentially abundant between psoriasis and healthy gut microbiome, we found an increase of Bacteroides vulgatus and Parasutterella excrementihominis and a decrease of Phascolarctobacterium succinatutens (Figure 2c).
Microbial gene family analysis reveals three psoriasis subgroups with distinct microbial and host features
We then performed a hierarchical clustering analysis on the microbial gene families differentially abundant between patients with psoriasis and healthy controls and identified three distinct groups in our cohort (Figure 3a). To confirm that these subgroups were not observed by chance, we performed bootstrapped gap statistics on both the Euclidean distance and Bray‒Curtis distance, which confirmed the presence of the three distinct groups in the cohort (Supplementary Figure S2a and b). Among the three groups identified by clustering, group 1 consists of a mixture of healthy and psoriasis samples (14 healthy samples and 15 psoriasis samples), group 2 consists of all patients with psoriasis (nine psoriasis samples), and group 3 consists of almost all patients with psoriasis (one healthy sample and nine psoriasis samples) (Figure 3b). For the subsequent investigations of these psoriasis subgroups, we termed the subjects with psoriasis from these subgroups PSO1, PSO2, and PSO3. The clustering was not confounded by body mass index, age, and sex or diet (Supplementary Figure S3a‒c and g and Supplementary Table S4). All psoriasis subgroups had similar disease severity, disease duration, and age of disease onset (Supplementary Figure S3d‒f). However, we found that each psoriasis subgroup had a number of distinct microbial and host features (Figure 3c). Some of the interesting features associated with each psoriasis subgroup are highlighted below.
Figure 3.
PSO subgroups identified by a differential abundance of microbial gene families. (a) The hierarchical cluster dendrogram shows the membership of all samples in this cohort. The colored boxes represent the grouping of each sample into three distinct groups. The red box depicts group 1, the blue box depicts group 2, and the green box depicts group 3. The red dotted line represents where the tree is cut to derive the three subgroups. The AU bootstrap confidence scores and BP values are represented in red and green, respectively, at the major branches. (b) The stacked bar plot represents the distribution of disease status of the three groups identified by cluster analysis. The height of each bar represents the size of each group, and the color represents the disease status, with red for healthy subjects and blue for PSO samples. (c) Heatmap of microbial and host features associated with each PSO subgroup. Columns represent PSO subgroups, and rows represents microbial features identified from shotgun metagenomics or host features from colonic RNA-seq. Differential abundance microbial features with nonzero counts for at least 10 samples were plotted on the heatmap to exclude features with high dropout rates. The color of each cell represents the average abundance and is scaled by means of the features. The data type of each feature is indicated in the side bar: pink represents microbial species, blue represents microbial pathway, and yellow represents host GEx. AU, arbitrary unit; BP, bootstrap probability; GEx, gene expression; PSO, psoriasis; RNA-seq, RNA sequencing.
Supplementary Figure S2.
Bray-Curtis analysis. (a) Gap statistics calculated using Bray‒Curtis and Euclidean dissimilar matrix of the DA UniRef90 gene families with bootstrapping for 1,000 times. (b) PCoA plot shows the Bray‒Curtis dissimilarity matrix of the DA UniRef90 gene families grouped the cohort into three groups that are similar to the grouping defined by hierarchical clustering (as represented by the color of each point). The shapes represent the disease status (round circles depict healthy samples, and triangles depict PSO samples). DA, differentially abundant; DE, differentially expressed; maxSE, maximum numeric vector of function values; PCoA, principal coordinate analysis; PSO, psoriasis.
Supplementary Figure S3.
Comparisons of metadata and microbial diversity in the three subgroups identified in the cohort. Only subjects with a complete dataset from the three measurement types are included. (a‒c) Comparisons of BMI, age, and gender in the three groups. (d‒f) Comparisons of PASI, disease onset, and disease duration in each PSO subgroup. (g, h) Comparison of diet scores and self-reported joint pain or swelling in the three groups. Comparisons of observed microbial, Shannon index, and Simpson diversity index in the three groups for (i) microbial species, (j) UniRef90 gene families, and (k) MetaCyc pathway. BMI, body mass index; Dz, disease; F, female; M, male; n.s., not significant; PSO, psoriasis; y, year.
Both PSO2 and PSO3 are dominated by subjects with psoriasis, suggesting that these are two distinct psoriasis-specific subgroups. We identified several common microbial features shared by these psoriasis subgroups as well as some microbial features that are distinct to each subgroup (Figure 3c and Supplementary Table S5, Supplementary Table S6, Supplementary Table S7). Although psoriasis samples have a lower abundance in P. succinatutens than healthy controls (Figure 2c), the reduced abundance of P. succinatutens is specific to PSO2 and PSO3 but not to PSO1 (Figure 3c). In addition, samples in PSO2 and PSO3 are less abundant with Turicibacter sanguinis and unclassified Turicibacter species (Figure 3c). Samples in PSO2 are more abundant with Bacteroides xylanisolvens and less abundant with Prevotella copri, Streptococcus thermophilus, and Coprococcus sp ART55 1 (Figure 3c). On the contrary, samples in PSO3 have a lower abundance in Ruminococcaceae bacterium D16 and Lachnospiraceae bacterium 1 1 57FAA (Figure 3c). In addition to distinct taxonomic features, PSO2 has a distinct profile in microbial functions, especially in microbial pathways. Both abundant and depleted pathways were found in PSO2 relative to those in other psoriasis subgroups, suggesting a shift of microbial functions in PSO2 (Figure 3c). Microbial communities in PSO2 have lower abundance in the arginine and polyamine biosynthesis superfamily, suggesting a lower capacity for polyamine production (Figure 3c). In contrast to PSO2, we did not identify microbial pathways that are uniquely associated with PSO1 and PSO3.
From the perspective of host response, PSO2 and PSO3 patients have distinct intestinal transcriptomic signatures (Figure 3c and Supplementary Table S8). Gut biopsies from PSO2 patients have increased expression in ATP13A5 and PDE9A and reduced expression in sulfotransferases, SULT1C2 and SULT1C3. In contrast, most of the transcriptomic signatures associated with PSO3 have increased expression compared with those associated with other psoriasis samples (Figure 3c). To gain more insight into the host intestinal immune response, we deconvoluted the immune cell composition in sigmoid colon bulk RNA-seq using the digital cytometry framework CIBERSORTx (Newman et al., 2019). As part of the CIBERSORTx framework, we first defined gene signatures of various immune cell populations in the sigmoid colon using single-cell RNA-seq generated from healthy sigmoid colon (James et al., 2020). We then used CIBERSORTx to apply the gene signature matrix to the bulk RNA-seq to infer the composition of cell populations. Sigmoid colon from patients in PSO3 has more abundant CD8+ T cells and less abundant NK cells and activated CD4+ T cells than that from other psoriasis subgroups (Figure 4a and Supplementary Table S9).
Figure 4.
PSO subgroup‒specific microbial and host features. (a) Boxplots show differences in sigmoid colon immune cell compositions deconvoluted by CIBERSORTx between different PSO subgroups. (b) Boxplots showing circulating host immune responses measured by flow cytometry. The statistical significance in boxplots was determined by Wilcox test on pairwise comparison of each group of interest. P-values were depicted by symbols: ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05. Comparisons with P > 0.05 are not shown. PSO, psoriasis; Teff, effector T cell.
In addition to colonic transcriptomic signatures, flow cytometry analysis revealed distinct immune features in circulatory blood between the two psoriasis-dominant groups. Patients in PSO3 have higher activated memory CD4+ effector T cells than those in PSO2, whereas the frequency of memory CD4+ effector T cells and total CD4+ effector T cells are comparable between these two psoriasis subgroups (Figure 4b). Similarly, memory CD8+ T cells have a more activated population in PSO3 patients (Figure 4b). Despite lower T-cell activation, patients in PSO2 have a higher capacity to produce IL-22 by circulating CD8+ T cells and TNF-α by circulating γδ T cells (Figure 4b) than healthy controls or those in PSO1. Together, our data reveal differential underlying circulatory immune responses associated with PSO2 and PSO3. PSO2 patients have a higher capacity for proinflammatory cytokine production, whereas PSO3 patients have higher baseline T-cell activation.
Distinct correlations between psoriasis severity with microbial features in each psoriasis subgroups
To further understand the relationship between the psoriasis subgroups and psoriasis heterogeneity, we tested whether microbial features in these psoriasis subgroups are significantly correlated with psoriasis parameters. In PSO1 patients, disease severity is positively correlated with the microbial pathways involved in purine degradation (Figure 5). Disease severity in PSO2 patients is positively correlated with several microbial pathways, including pentose phosphate biosynthesis and L-arginine biosynthesis (Figure 5). The microbial L-rhamnose degradation pathway is positively correlated with disease duration in PSO2 (Figure 5). The microbial L-isoleucine biosynthesis pathway is correlated with disease duration positively in PSO1 patients and negatively in PSO2 patients (Figure 5). No significant correlations between microbial features and disease information were observed in PSO3.
Figure 5.
Correlations between microbial features and psoriasis-related clinical features. Dot plot summarizes the correlation between microbial features and disease parameters of PASI scores and disease duration. Each dot represents a significant correlation between a disease parameter and a microbial feature. The direction and strength of the correlation are presented by dot color (red represents positive correlation, and blue represents negative correlation). The dot size represents false discovery rate‒adjusted P-values. adj, adjusted; corr., correlation value; Dz, disease.
Multiomic analysis in patients with psoriasis and subgroups reveals distinct host‒microbe associations
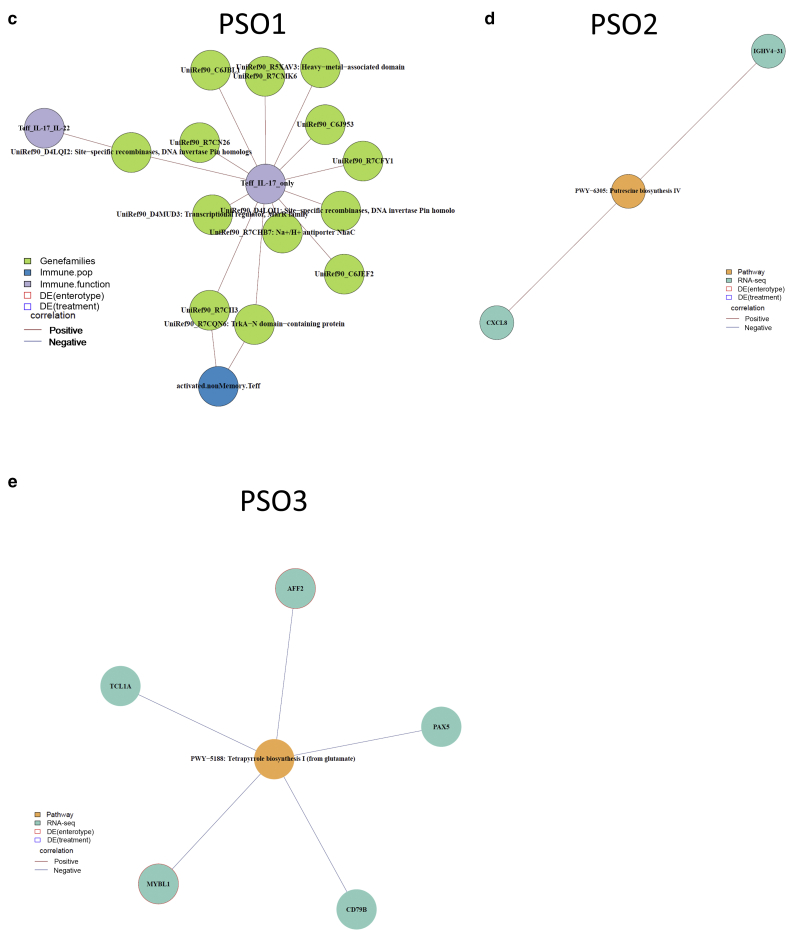
To gain a comprehensive view of the crosstalk between the gut microbiome and host biology, we constructed multiomic correlation networks by integrating microbial and host features across different measurements (Supplementary Table S10). A subset of 40 subjects from our cohort with complete measurements from shotgun metagenomic sequencing, gut RNA-seq, and circulating immune profiling were included in the multiomic analysis. This multiomic cohort consists of 26 subjects with psoriasis and 14 age- and sex-matched healthy subjects (Table 2). Multiomic networks were constructed within each disease status and within each psoriasis subgroup to reveal disease-specific and subgroup-specific host‒microbe relationships. The resulting multiomic networks consist of nodes that represent host or microbial features and edges that represent significant correlations between nodes. The network in patients with psoriasis is denser and consists of more edges than the multiomic network in healthy subjects (Figure 6a). The final multiomic network consists of 73 significant correlations within healthy subjects and 97 significant correlations within patients with psoriasis (Table 3). Intriguingly, the multiomic network in each psoriasis subgroup displayed a distinct network structure. Multiomic networks in PSO1 and PSO2 were more interconnected than the network in PSO3 (Figure 6a). It is interesting that PSO3 had fewer edges and nodes than other psoriasis subgroups and all psoriasis samples combined (Table 3). Overall, our data revealed distinct multiomic network structures in patients with psoriasis and healthy controls as well as among different psoriasis subgroups.
Table 2.
Demographic Information of Subjects with Full Set of Multiomics Data
| Characteristic | Healthy | Psoriasis | P-Value |
|---|---|---|---|
| Sample size | 14 | 26 | NA |
| Sex (% female) | 43% | 58% | 0.5096 |
| Age (mean age), y | 44.8 ± 13.8 | 44.7 ± 15.5 | 0.9789 |
| Mean PASI score | NA | 11 ±8.9 | NA |
| Median PASI score | NA | 7.7 | NA |
Abbreviation: NA, not applicable.
Figure 6.
Multiomic networks associated with PSO and PSO-specific subgroups. (a) Overview of multiomic networks within healthy subjects, patients with PSO, and each PSO subgroup. Each node represents a microbial or host feature, and each edge represents a significant association between the two nodes. The color of nodes represents the measurement type of the node. Host‒microbe modules are identified using greedy optimization of modularity. (b) PSO-specific modules associate microbial features with circulating IL-17 production. (c‒e) PSO subgroup‒specific modules are also identified in each PSO-specific subgroup. For each module, the color of nodes represents the measurement type of the node, and the color of edges indicates the direction of the correlation (red edges represent positive associations, and blue edges represent negative associations). DE, differentially expressed; PSO, psoriasis; RNA-seq, RNA sequencing.
Table 3.
Summary of Multiomic Networks
| Characteristic | Healthy | Psoriasis | PSO1 | PSO2 | PSO3 |
|---|---|---|---|---|---|
| Number of subjects | 14 | 26 | 12 | 8 | 6 |
| Number of edges | 73 | 97 | 124 | 126 | 53 |
| Number of features | 99 | 110 | 143 | 160 | 74 |
| Total number of modules | 30 | 35 | 28 | 55 | 26 |
| Number of modules with at least three features | 16 | 15 | 15 | 21 | 10 |
| Number of edges with host immune features | 22 | 13 | 95 | 12 | 0 |
| Number of edges with host transcriptome | 51 | 84 | 27 | 109 | 53 |
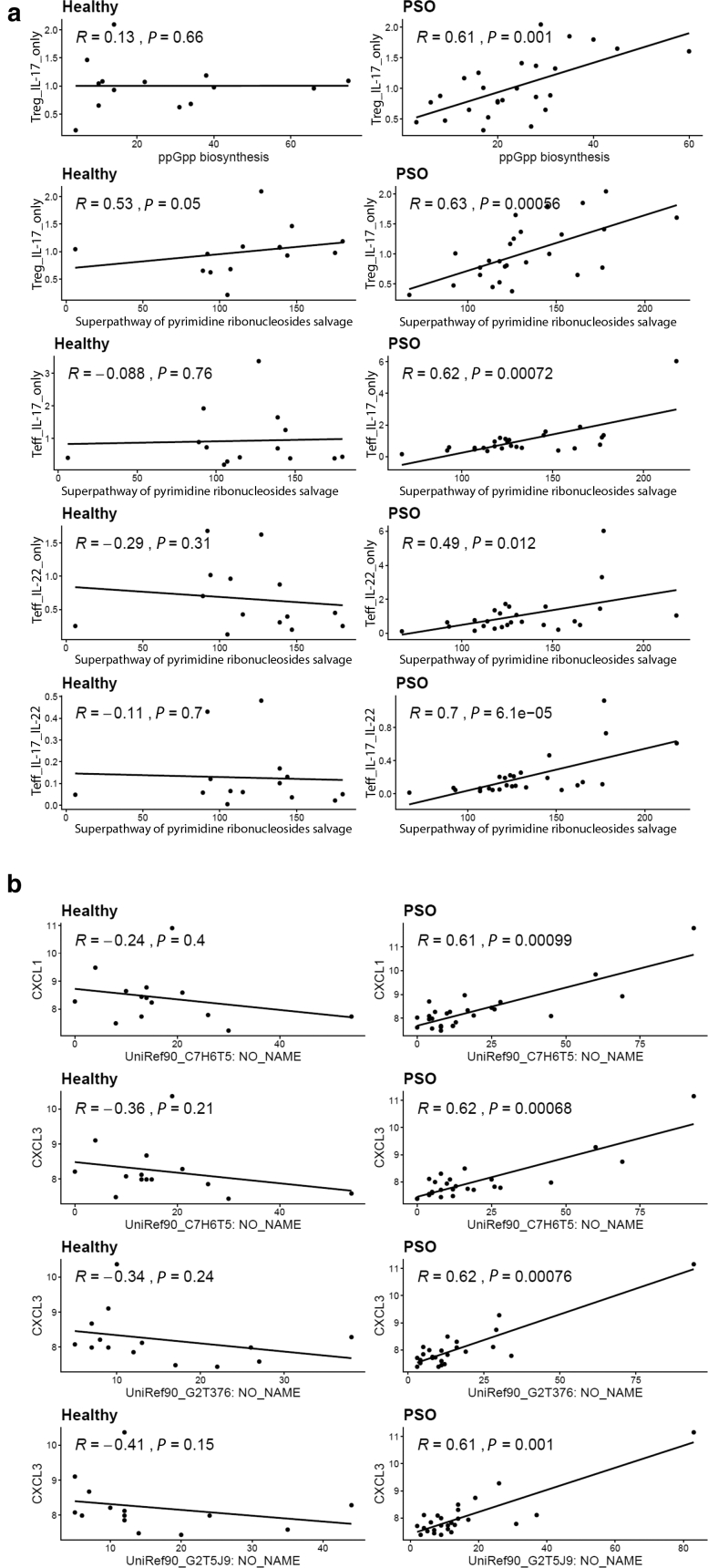
The different multiomic networks might reflect the different host‒microbe associations in subjects with psoriasis and healthy subjects. One of the modules in psoriasis-associated multiomic network positively links microbial pathways involved in microbial ppGpp biosynthesis and pyrimidine ribonucleosides salvage pathways with circulating IL-17 production in both CD4+ effector T cells and regulatory T cells (Figure 6b). The associations between these microbial pathways and IL-17 production were only observed in psoriasis samples despite that these microbial pathways are also being abundant in healthy samples (Supplementary Figure S4a).
Supplementary Figure S4.
PSO-specific correlations between microbial functions and proinflammatory host response. (a) Scatter plots show positive correlations between microbial MetaCyc pathways and IL-17 production in psoriatic PBMCs but not in healthy PBMCs. (b) Scatter plots show a positive correlation between microbial UniRef90 gene families with the colonic expression of CXCL1 and CXCL3 in patients with PSO but not in healthy control. The correlations were calculated by Spearman’s rank-order correlation. PSO, psoriasis; Teff, effector T cell; Treg, regulatory T cell.
Besides psoriasis-specific host‒microbe associations, our analysis also identified host‒microbe associations that are specific in psoriasis subgroups. A multiomic network identified in PSO1 consists of a module that links microbial gene families with activated nonmemory CD4+ T cells and IL-17 production capacity in CD4+ effector T cells (Figure 6c), suggesting potential microbial controls in T-cell activation and effector function in this psoriasis subgroup. Indeed, 95 of 124 host‒microbe associations in PSO1 link the features of host circulating immunity to microbial features, which is higher than the networks in PSO2 and PSO3 patients (Table 3). In addition, we also identified some subgroup-specific associations in psoriasis-dominant PSO2 and PSO3. In PSO2, the expression of proinflammatory chemokine CXCL8 is positively correlated with microbial putrescine biosynthesis pathway in PSO2 patients (Figure 6d). We identified a module in PSO3 network that negatively correlates microbial tetrapyrrole biosynthesis pathway with several genes in B-cell biology, including CD79B, PAX5, TCL1A, and MYBL1 (Figure 6e). Tetrapyrroles are metal-binding compounds that serve as different cofactors, such as heme, cobalamin (vitamin B12), and coenzyme F430, and have crucial roles in regulating diverse cell functions.
Discussion
The pathogenesis of psoriasis is highly heterogeneous, which poses challenges in diagnosis and disease control. Accumulating evidence from recent studies suggests that the heterogeneity of psoriasis pathogenesis may be the result of the interplay between microbiome and host immune response. Our study focused on studying the heterogeneity of psoriasis pathogenesis through the lens of multiomic datasets assessing both host and microbial features. In this study, we show that psoriasis gut microbiome has increased B. vulgatus and P. excrementihominis and reduced P. succinatutens compared with the ones in healthy controls. These microbial features have also been associated with intestinal inflammation. Increased intestinal colonization of B. vulgatus and elevated B. vulgatus reactive serum antibodies have been reported in patients with ulcerative colitis (Matsuda et al., 2000). It is intriguing that the gut microbiome of patients with psoriasis in our cohort shares some microbial features associated with IBD or irritable bowel syndrome despite no intestinal symptoms reported in our patients.
Enterotypes defined by different microbiome compositions have been previously described in healthy subjects (Arumugam et al., 2011). Our study revealed three psoriasis subgroups in our cohort as defined by the differential abundance of microbial gene families. Each psoriasis subgroup has distinct microbial and host features (Table 4). The reduced Turicibacter species in PSO2 and PSO3 are reminiscent of the gut microbiome associated with pediatric Crohn’s disease (El Mouzan et al., 2018; Wang et al., 2016). PSO2 has the most distinct microbial functional profile (Figure 3c) from that of other psoriasis subgroups. The gut microbiome in PSO2 shows a higher biosynthetic capacity of several important immune regulators, including pyridoxal 5-phosphate (vitamin B6), L-ornithine, and flavin. We also found lower capacity in arginine and polyamine biosynthesis in PSO2. Some of these molecules have been linked to immune-mediated intestinal inflammation. For example, vitamin B6 plays a crucial role in lymphocyte trafficking into the intestines (Yoshii et al., 2019). Vitamin B6 deficiency has been linked to several immune-mediated diseases, including rheumatoid arthritis and IBD (Selhub et al., 2013; Yoshii et al., 2019). Having increased capacity of vitamin B6 biosynthesis may have a protective role for subjects in PSO2 from autoimmune diseases. On the contrary, polyamines play a crucial role in intestinal mucosa maintenance and resident immune cell development (Yoshii et al., 2019). Interestingly, it has been shown that spermine, a class of polyamine, reduces the secretion of proinflammatory IL-18 cytokine by inhibiting NLRP6 inflammasome activation (Levy et al., 2015). Our data suggest that gut microbial communities in PSO2 have increased vitamin B6 biosynthesis and reduced polyamine production, but the clinical implication requires further study.
Table 4.
Summary of Host and Microbial Features of Psoriasis Subgroups
| Characteristic | PSO2 and PSO3 Common Features | PSO2-Specific Features | PSO3-Specific Features |
|---|---|---|---|
| Metagenomics (stool) |
Turicibacter spp. ↓ Phascolarctobacterium succinatutens ↓ |
Bacteroides xylanisolvans ↑ Prevetella copri ↓ Streptococcus thermophilus ↓ Coprococcus sp ART55 1 arginine and polyamine biosynthesis ↓ pyridoxal 5-phosphate biosynthesis ↑ |
Megamonas spp. ↑ Ruminococcaceae bacterium D16 ↓ Lachnospiraceae bacterium 1 1 57FAA ↓ |
| Transcriptomics (sigmoid colon) | NA | SULT1C2 and SULT1C3 ↓ FOHL1 ↓ PDE9A ↑ |
FOHL1 ↑ UCP2 ↑ HTR3A ↑ |
| Gut immune cell population (sigmoid colon digital cytometry) | NA | NA | CD8 T cells ↑ Activated CD4 T cells ↓ NK cells ↓ |
| Immune population (PBMC flow cytometry) | NA | Reduced activated memory CD4 and CD8 | NA |
| Cytokine production (PBMC flow cytometry) | NA | Higher IL-22 production in CD8 and higher TNF-α production in γδ T cells | NA |
Abbreviation: NA, not applicable.
Although both PSO2 and PSO3 are psoriasis-enriched subgroups, we have observed some differences in host circulatory and intestinal immune profiles between the two subgroups. PSO2 has increased memory CD8+ T-cell population in peripheral blood and higher IL-22 production capacity from CD8+ T cells (Figure 4 and Supplementary Table S9). Although PSO3 has no obvious immune signatures in peripheral blood, PSO3 has higher CD8+ T cells and lower active CD4+ T cells in sigmoidal gut as identified by in silico immunoprofiling. Conventionally, CD8+ T cells are known for their cytotoxic activities and are important for clearing infected cells or cancerous cells. Recent studies have been focusing on cytokine-producing CD8+ T cells because they were found in psoriatic skin to be an important source of proinflammatory IL-17 and IL-22 (Hijnen et al., 2013; Liu et al., 2021). Interestingly, expanded proinflammatory cytokine-producing CD8+ T cells have been found in PBMCs of patients with psoriatic arthritis (Diani et al., 2019). Our findings confirm the presence of cytokine-producing CD8+ T cells in patients with psoriasis and implicate differential roles of CD8+ T cells in different psoriasis subgroups.
Many of the host transcriptomic signatures found in PSO3 are similar to the ones reported in patients with IBD or IBD animal models, including elevated expression of folate hydrolase (FOLH1) (Noble et al., 2010; Rais et al., 2016), one of the serotonin receptors (HTR3A) (Shajib et al., 2019), and mitochondrial UCP2 (Jin et al., 2017; Yu et al., 2009). Elevated FOLH1 expression has been reported in intestinal biopsies of patients with IBD, and inhibiting FOLH1 activity ameliorates IBD-associated abnormalities in mouse models (Noble et al., 2010; Rais et al., 2016). UCP2 encodes for mitochondrial UCP2 and has been implicated in several autoimmune diseases (Yu et al., 2009). Expression of UCP2 is elevated in a dextran sodium sulfate‒induced mouse model of IBD, and the severity of IBD can be ameliorated by knocking down UCP2 expression through an expression by small interfering RNA (Jin et al., 2017). In addition to the IBD-related signatures, patients in PSO3 also have a reduced abundance of Ruminococcaceae and Lachnospiraceae, which is also observed in the gut microbiome of psoriatic arthritis (Scher et al., 2015). It is worth noting that all patients in PSO3 reported having joint pain or swelling, whereas only a fraction of patients in PSO1 or PSO2 did (Supplementary Figure S3h). Both IBD and psoriatic arthritis are common psoriatic comorbidities, and our study suggests an intriguing possibility that these psoriasis subgroups might represent psoriasis populations with differential risk for developing comorbidities such as IBD and psoriatic arthritis.
Our multiomic analysis revealed interesting associations between microbial pathways with circulating IL-17A production and expression of proinflammatory chemokines, CXCL1 and CXCL3, in the colon (Figure 6b). These host‒microbe associations are only observed in patients with psoriasis but not in healthy subjects (Supplementary Figure S4a and b), suggesting that psoriasis-specific host‒microbe associations might be crucial drivers of the proinflammatory changes in patients with psoriasis. In addition to psoriasis-specific host‒microbe associations, our analysis also revealed specific host‒microbe associations in each psoriasis subgroup in our cohort. Together, our data suggest that the host‒microbe interaction can be context dependent, whereby the same microbial species or function may have a different effect on the basis of the disease state or disease subgroup.
We are aware of several limitations of our study that could be improved by future work. Although multidimensional, our analysis is limited by a modest cohort size, so it would be important to validate our findings in a larger independent psoriasis cohort. In addition to validation, a larger cohort will allow for the application of machine learning approaches to better characterize psoriasis subgroups. Owing to the scope of the study, we focused our efforts on cross-sectional observations of our cohort. Host‒microbe interaction can be relatively dynamic over time, and some of the features or associations identified may be relatively transient. Collecting longitudinal data may assist in the identification of stable associations compared with transient associations. In addition, a longitudinal dataset would allow for better tracking of host‒microbe associations associated with the development of comorbidities. In this study, we have identified several microbial species that are differentially abundant in psoriasis gut compared with those in healthy controls. However, their roles in psoriasis and inflammation are not previously well understood. Testing the microbial species identified in this study for their roles in eliciting local and system inflammation can shed some insights into their roles in modulating psoriasis. Even with these limitations, the findings of this study provide valuable insights into the complexity of psoriasis biology that can help us to better identify patients with psoriasis with a higher risk of comorbidities and unique biological pathways. To date, most of the disease-related microbiome studies are still focusing on signatures identification aimed to identify specific microbes or microbial functions that contribute to the pathogenesis of the disease. Our results suggest that pathogenesis of psoriasis and its comorbidities is highly individualized and that both host and microbiome should be considered when assessing disease risks and strategizing disease management in both clinical and scientific settings. The findings of this study are relevant because they may have diagnostic potential for psoriasis comorbidities and may provide insights in developing personalized disease management strategies for patients with psoriasis.
Materials and Methods
Cohort recruitment and sample collection
Adult patients with psoriasis and healthy volunteers recruited from the San Francisco Bay area were enrolled in the study after providing written informed consent. All procedures performed on human subjects were reviewed and approved by Institutional Review Board at the University of California San Francisco (Institutional Review Board protocols 10-02830 and 10-01218). Individuals with abnormal coagulation parameters, positive HIV screening test, and history of bleeding disorders, abdominal surgery, gastrointestinal cancer, IBD, AIDS, immunodeficiency or immunosuppressive medications, or concurrent inflammatory skin condition were excluded. All patients with psoriasis had a diagnosis of psoriasis from a physician for at least 6 months before study enrollment, which was verified by study staff. To assess the psoriatic microbiome in an untreated state, subjects were excluded if they had received systemic biologic therapy in the last 6 months, nonbiologic systemic medications (methotrexate, cyclosporine, corticosteroids, cyclophosphamide, retinoids, and photochemotherapy) or antibiotics in the last month, or phototherapy or topical therapy in the last 2 weeks before the clinical visit. Healthy volunteers had no personal or family history of psoriasis. Gut biopsies, stool samples, and blood samples were collected on the same day. Gut biopsies were taken from the sigmoid colon of each subject. All patients with psoriasis in our cohort had a normal-appearing lower gastrointestinal endoscopic examination. Two biopsies were preserved in RNAlater solution (Ambion, Austin, TX) and kept at 4 °C overnight before storage at ‒80 °C. Stool samples were collected before gut biopsies and stored at ‒80 °C. Blood samples were taken on the same day as and preceded the gut biopsies. PBMCs were isolated from whole blood and stored in liquid nitrogen. We collected a total of 48 stool samples (15 from healthy subjects and 33 from patients with psoriasis) for shotgun metagenomics, and a total of 42 gut biopsies and blood samples (16 from healthy subjects and 26 from patients with psoriasis) were collected for host transcriptomics and immune profiling.
Diet habit analysis
We assessed dietary habits of healthy controls and patients with psoriasis by an in-person distribution of the 2009‒2010 National Health and Nutrition Examination Survey dietary screener questionnaire. The questionnaire consists of 25 questions about nutrient intake frequencies of different food groups. The differences in intake frequencies of each food group were compared between disease status and psoriasis subgroups with Fisher’s exact test, and results with P < 0.05 were considered significant. To further assess the quality of dietary habits, we summarized diet survey by calculating a Mediterranean diet score that would quantify the overall quality of diet. The traditional Mediterranean diet has been shown to reduce the risk of developing heart disease, metabolic syndrome, diabetes, and depression. The foundation for the Mediterranean diet consists of an abundance of fresh fruits, vegetables, whole grains, legumes, and omega-3 fatty acids and limited food such as milk, cheese, red meats, and sweets with added sugars. We adopted Mediterranean score calculation from a previous study (Panagiotakos et al., 2006). Briefly, we used six components of the Mediterranean diet (nonrefined cereals, fruit, vegetables, potatoes, whole grains, and legumes). For the consumption of food groups that aligned with the Mediterranean diet, we assigned scores 0, 1, 2, 3, 4, and 5 when a participant reported no consumption, rare (one time per month), frequent (2‒3 times per /month), very frequent (one time per week), weekly (2‒6 times per week), and daily (1‒2 times per day), respectively. For non-Mediterranean diet food components, we assigned scores on a reverse scale with scores 0, 1, 2, 3, 4, and 5 when a participant reported daily (1‒2 times per day), weekly (2‒6 times per week), very frequent (one time per week), rare (one time per month), and no consumption, respectively. All scores were then combined to produce a total score that ranged from 0 to 50, where the higher the score, the better the diet. To test for statistical significance between both participant groups, we utilized the Wilcox test to compare the means and P-values of our data.
Shotgun metagenomics
DNA was extracted from fecal samples with the modified cetyl trimethylammonium bromide method. The quality of the extracted DNA was assessed by gel electrophoresis and Quant-iT PicoGreen dsDNA Assay (Life Technologies, Carlsbad, CA). The extracted DNA was submitted to Vincent J. Coates Genomic Sequencing Laboratory at the California Institute of Technology for Quantitative Biosciences (San Francisco, CA) (www.qb3.berkeley.edu/gsl) for metagenomic library construction and sequencing. The metagenomic libraries were constructed using PrepX DNA library kit (Takara Bio, Kusatsu, Japan) and sequenced on HiSeq4000 (Illumina, San Diego, CA) for pair-end 150 base pair sequencing. An average of 22 million pair-end total reads were generated per sample. Reads were first mapped to human genome (GRCh38, GENCODE release 25) by bowtie2 (version 2.2.8) to identify human DNA. The nonhuman reads were extracted and sorted by using samtool (version 0.1.19). This data processing workflow results in an average of 16 million nonhuman reads per sample. The resulting nonhuman reads were used for the subsequent taxonomic and functional profiling.
Taxonomic profiles were generated using MetaPhlAn2 (version 2.6.0), which uses a library of ∼1 million unique clade-specific marker genes for taxonomic profiling (Franzosa et al., 2018). The abundance table of bacterial species was extracted for the subsequent analysis. Functional profiles were generated by HUMAnN2 (version 0.11.1), which is an assemble-free method to infer the functional capacities for each microbiome (Franzosa et al., 2018). For each sample, HUMAnN2 maps read the pangenomes of species identified in the sample and performed additional translated searches on unclassified reads. The resulting dataset is the abundance table of microbial gene families (UniRef90), which is summarized into the higher-level MetaCyc gene pathways. Our analysis identified a total of 339 bacterial species, 409 MetaCyc pathways, and 650,048 UniRef90 gene families in our dataset.
Subsequent metagenomic analyses were performed using the R package phyloseq (McMurdie and Holmes, 2013). The abundance of microbial species, UniRef90 gene families, and MetaCyc pathway was normalized to relative abundance to account for different library sizes and input as phyloseq objects and used for calculating microbial community diversities. Owing to the high dimensionality and high dropout rate of the microbial gene families table, we focus our analysis on the microbial gene families with at least three counts for at least 80% of the total samples, which results in 3,356 UniRef90 gene families. Alpha diversity was calculated as four indices: observed (number of observed microbial features), chao1, Shannon, and Simpson index, which summarize both richness and evenness of the community. The alpha diversities were compared by disease status and psoriasis subgroups with two-sided Wilcox test. Dissimilarities between each microbiome isolated are represented by Bray‒Curtis dissimilarity matrix and visualized on a principal coordinate analysis plot.
Unfiltered counts per million tables were used for differential abundance analysis. Differential abundant microbial features between psoriasis samples and healthy samples were identified using DESeq2 (version 1.18.1) (Love et al., 2014) with the design ⋍sex+age.bin+batch+Status. Microbial features with adjusted P < 0.05 and absolute log2 fold change > 0.6 are considered statistically significant. Differential abundant microbial features associated with each subgroup identified in this cohort were identified by pairwise comparison of each subgroup using DESeq2 (version 1.18.1) (Love et al., 2014). Microbial features with adjusted P < 0.1 and absolute log2 fold change > 0.6 are considered statistically significant. Differential abundance microbial features with none zero counts for at least 10 samples were plotted on the heatmap to exclude features with high dropout rates.
Clustering analysis to identify subgroups in the cohort
The subgroups in this cohort were identified by complete linkage hierarchical clustering on the differentially abundant microbial UniRef90 gene families identified as described earlier. The clustering was done using the R package pheatmap (version 1.0.12), which implements hclust for hierarchical clustering, or the gapstat_ord function in phyloseq (version 1.22.3). Gap statistics on differentially abundant UniRef90 gene families estimated three clusters present in our dataset. Gap statistics were done using the clusGap function from the cluster package (version 2.0.7-1) with bootstrapping 1,000 times (B = 1,000). The optimal number of clusters was determined as the smallest k that is within one standard error from the local maximal as suggested in Tibshirani et. al. (2001). The hierarchical dendrogram was cut by cutree function for three clusters (k = 3) in stats package (version 3.4.2) to assign membership of clusters for each sample.
Host RNA-seq
RNA was extracted from sigmoidal colon biopsies by RNeasy Plus Universal Kits (Qiagen, Hilden, Germany). Total RNA integrity was assessed using Agilent 2100 Nano and Pico RNA kit, and all samples have RNA integrity number score >7. cDNA libraries construction and sequencing were done by Beijing Genomics Institute (Shenzhen, China). In brief, ribosomal RNA was removed from the total RNA by Ribo-Zero Gold Kit (Illumina), and library was constructed by TruSeq Stranded Total RNA Library Prep kit (Illumina). cDNA libraries were sequenced on HiSeq4000 (Illumina) for paired-end 100 base pair sequencing. The average sequencing depth is 66 million per sample. Sequencing reads were mapped to the human genome (GRCh38, GENCODE release 25) using STAR (version 2.4.2a). The number of counts mapped to each transcript is summarized by HTSeq-count (version 0.6.0) and used as input for differential analysis in DESeq2 (version 1.18.1) (Love et al., 2014). Raw read counts were normalized by median of ratios method using DESeq2. Differentially expressed genes between psoriasis samples and healthy samples were determined by DESeq2 with the model design ∼sex+age+batch+status. Genes with adjusted P < 0.05 and absolute log2 fold change > 0.6 are considered significantly different between psoriasis samples and healthy samples. A similar analysis was performed to identify differentially expressed genes between different subgroups (genecluster.group) in this cohort with the DESeq2 model design ∼sex+age+batch+genecluster.group. Results of pairwise comparisons were extracted, and significant differentially expressed genes were determined with the same significant criteria. The expression of the top 5,000 most variable genes was transformed by variance stabilizing transformation for principal component analysis.
In silico cytometry methods
The colon immune cell composition was imputed from the sigmoid colon bulk RNA-seq data by CIBERSORTx (https://cibersortx.stanford.edu/) (Newman et al., 2019). In brief, single-cell RNA-seq dataset associated with sigmoid colon was extracted from colon immune atlas of Gut Cell Atlas project by Seurat (James et al., 2020). The first part of the CIBERSORTx workflow computed the signature matrix of immune cell populations in the sigmoid colon using the sigmoid colon single-cell RNA-seq dataset. The second part of the CIBERSORTx workflow imputed cell fractions in the sigmoid colon by comparing bulk sigmoid colon RNA-seq with sigmoid colon immune cell signature matrix with the following parameters: relative run mode, 100 permutations, disabled quantile normalization, and S-mode batch correction, which was implemented to account for cross-platform deconvolution. The cell compositions were compared by disease status and psoriasis subgroups with two-sided Wilcoxon test or Kruskal‒Wallis test, and results with P < 0.05 were considered significant. Statistical analyses were performed with R.
Multiomic analysis
We integrated six different datasets collected from three different measurement types: shotgun metagenomic sequencing, flow cytometry, and host RNA-seq from 14 healthy subjects and 26 patients with psoriasis. We included the following datasets for data integration: (i) bacterial species from shotgun metagenomics with nonzero count in at least 10 samples, (ii) microbial MetaCyc pathway from shotgun metagenomics with nonzero count in at least 10 samples, (iii) microbial UniRef90 gene families from shotgun metagenomics with at least three counts for 80% of the total samples with metagenomic data, (iv) cell population from flow cytometry, (v) cytokine production capacity from flow cytometry, and (vi) top 500 most variable genes plus all differentially expressed genes identified by DEseq2 from host RNA-seq. More information about datasets included in the multiomic analysis is summarized in Supplementary Table S10. Pairwise datasets from different measurement types were integrated by Spearman’s rank-order correlation. We constructed interaction networks with only strong and robust correlations that fit the following criteria: correlations need to have a false discovery rate‒adjusted P < 0.1 and absolute correlation coefficient > 0.6. We required microbial or host features with a nonzero count for >70% of samples within the group of interest. Multiomic analyses were done within all subjects (both healthy subjects and subjects with psoriasis), within healthy subjects alone, within subjects with psoriasis alone, and within each psoriasis subgroups. The significant host‒microbe associations identified in our analyses are listed in Supplementary Table S11. The multiomic networks were visualized using the R package, igraph (version 1.2.4.1) The community modules within each network were identified using fast greedy modularity optimization algorithm (Clauset et al., 20041).
Host flow cytometry
Flow cytometry for cell population
Frozen PBMCs were thawed and counted, and one million cells were plated in a 96-well V-bottom plate. The cells were centrifuged for 5 minutes at 400g and surfaced stained with a predetermined antibody staining cocktail for 15 minutes at room temperature. The cells were washed with sort buffer, centrifuged, resuspended in BD Perm/Wash Buffer (BD Biosciences, Franklin Lakes, NJ), and centrifuged for 5 minutes at 700g. The supernatant was aspirated, and the cells were stained for FoxP3 by adding diluted fluorophore-labeled anti-human FoxP3 antibody in BD Perm/Wash Buffer for 30 minutes at room temperature. Cells were centrifuged for 5 minutes at 700g, the supernatant was aspirated, and the wash was repeated with BD Perm/Wash buffer. The cells were resuspended a final time in 50 ul of PBS and held at 4 ºC until analyzed on a BD LSR-II instrument (BD Biosciences). Brilliant Violet Buffer was added to each staining step. Fluorescent-minus-one controls were used for setting gates. A minimum of 300,000 events were analyzed for each sample. The gating strategy is provided in Supplementary Figure S5, and the antibodies used are listed in Table 5.
Supplementary Figure S5.
Representative flow cytometry gating for identifying various cell populations. (a) Cell populations (CD4+ Teff, CD4+ Treg, CD8+ T cell, γδ T cell, and innate lymphoid cells) and (b) their memory and activation state. APC, allophycocyanin; FSC-A, forward scatter area; FSC-H, forward scatter height; K, thousand; PE, phycoerythrin; Q, quadrant; SSC-A, side scatter area; Teff, effector T cell; Treg, regulatory T cell.
Table 5.
List of the Antibodies Used for the Flow Cytometry
| Marker | Antibody | Cell Type |
|---|---|---|
| Phenotype panel | ||
| AARD | AARD | Viability |
| CD45 | CD45-APCH7 | Lymphocytes |
| CD3 | CD3-PerCpCy55 | T cells |
| CD4 | CD4-APC | CD4+ T cells |
| CD8 | CD8-BV650 | CD8+ T cells |
| FoxP3 | FoxP3-A488 | Treg |
| CD25 | CD25-PECF594 | Treg |
| TCRgd | TCRgd-BV711 | γδ T cells |
| HLADR | HLADR-BV421 | Activation |
| CD38 | CD38-PE | Activation |
| CD117 | CD117-BV786 | ILCs |
| NKp44 | NKp44-BV605 | ILC3 |
| CD45RO | CD45RO-PECy7 | Memory |
| Functional panel | ||
| AARD | AARD | Viability |
| CD45 | CD45-APC-Cy7 | Lymphocytes |
| CD3 | CD3-PerCpCy55 | T cells |
| CD4 | CD4-APC | CD4+ T cells |
| CD8 | CD8-BV650 | CD8+ T cells |
| FoxP3 | FoxP3-A488 | Treg |
| CD127 | CD127-PE-Dazzle594 | Low in Tregs |
| TCRVS2 | TCRVS2-BV711 | Vd2 T cells |
| NKp44 | NKp44-BV605 | ILC3 |
| CD117 | CD117-BV786 | ILC3 |
| TNF-α | TNFa-A700 | Cytokine |
| IFNγ | IFNg-PE-Cy7 | Cytokine |
| IL-17 | IL17-BV421 | Cytokine |
| IL-22 | IL22-PE | Cytokine |
Abbreviations: APC, allophycocyanin; PE, phycoerythrin; Treg, regulatory T cell.
Flow cytometry for cytokine production
Frozen PBMCs were thawed and counted and one million cells were plated in a 96-well U-bottom plate. PBMCs were left unstimulated in complete culture media (RPMI + 10% heat-inactivated fetal bovine serum supplemented with penicillin/streptomycin/L-glutamine) or stimulated with 5 ng/ml of phorbol myristate acetate and 0.5 ug/ml of ionomycin for 4 hours in the presence of brefeldin A (10 ug/ml). After 4 hours of stimulation, the cells were centrifuged and stained with an antibody staining cocktail listed for 15 minutes at room temperature. The cells were washed with sort buffer, centrifuged, and resuspended in BD Perm/Wash Buffer and centrifuged for 5 minutes at 700g. The supernatant was aspirated, and the cells were stained for intracellular antigens by adding diluted fluorophore-labeled antibodies in BD Perm/Wash Buffer for 30 minutes at room temperature. Cells were centrifuged for 5 minutes at 700g, the supernatant was aspirated, and the wash was repeated with BD Perm/Wash buffer. The cells were resuspended a final time in 50 ul of PBS and held at 4 ºC until analyzed on a BD LSR-II instrument. Brilliant Violet Buffer was added to each staining step. Fluorescent-minus-one controls were used for setting gates. A minimum of 300,000 events were analyzed for each sample. Reported data represent the stimulated cytokine expression minus the unstimulated cytokine expression referred to as background corrected cytokine expression. fcs files were analyzed by FlowJo (version 10; Tree Star, Ashland, OR), and the immune profiles were exported as txt files, which are imported into R for statistical analysis. Wilcox test was used to assess the significance of the differences between disease status or disease subgroups. The gating strategy is provided in Supplementary Figure S6, and the antibodies used are listed in Table 5.
Supplementary Figure S6.
Representative flow cytometry gating for measuring cytokine production in various cell populations after 4 hours of PMA and ionomycin stimulation. (a) Gating strategies for identification of Teff, Treg, CD8+ T cells, and γδ T cells. (b) Gating strategies for measuring the production of IL-17A, IL-22, TNF-α, and IFNγ. APC, allophycocyanin; FSC-A, forward scatter area; FSC-H, forward scatter height; K, thousand; PMA, phorbol myristate acetate; Teff, effector T cell; Treg, regulatory T cell; vs. versus.
Data availability statement
Expression data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus and is accessible through Gene Expression Omnibus Series accession number GSE150851. Metagenomics sequence data are accessible through Sequence Read Archive BioProject PRJNA634145. All clinical and experimental data available are shown in Supplementary Table S12.
ORCIDs
Hsin-Wen Chang: http://orcid.org/0000-0003-2881-245X
Di Yan: http://orcid.org/0000-0003-1338-9847
Rasnik Singh: http://orcid.org/0000-0002-7372-6692
Audrey Bui: http://orcid.org/0000-0003-3055-463X
Kristina Lee: http://orcid.org/0000-0002-5879-817X
Alexa Truong: http://orcid.org/0000-0002-4748-5581
Jeffrey M. Milush: http://orcid.org/0000-0002-0773-6411
Ma Somsouk: http://orcid.org/0000-0001-6116-3645
Wilson Liao: http://orcid.org/0000-0001-7883-6439
Author Contributions
Conceptualization: WL, HWC; Methodology (Colonic Biopsies Collection): MS; Formal Analysis (Diet): AT; Flow Cytometry Data Analysis: JMM, HWC; Methodology (Flow Cytometry): JMM; Methodology (In Silico Cytometry): AB; Methodology: WL, HWC, JMM, MS; Methodology (Patient Recruitment): DY, RS, KL; Supervision: WL; Formal Analysis (Bioinformatics): HWC; Writing - Original Draft Preparation: WL, HWC
Acknowledgments
Metagenomic sequencing was performed by the Vincent J. Coates Genomics Sequencing Laboratory at the University of California Berkeley (Berkeley, CA), supported by the National Institutes of Health S10 OD018174 Instrumentation Grant. Flow cytometry was performed by the San Francisco General Hospital Flow Core Facility, supported by a grant from the National Institutes of Health, University of California San Francisco-Gladstone Institute of Virology and Immunology Center for AIDS Research (P30 AI027763). This work was performed at the University of California San Francisco (San Francisco, CA). This study was supported in part by a National Psoriasis Foundation Translational Research Award and National Institutes of Health grants to WL (R01AR065174 and U01AI119125) and a National Psoriasis Foundation Medical Research Fellowship to DY.
Conflict of Interest
WL has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. The remaining authors state no conflict of interest.
accepted manuscript published online 10 March 2022; corrected proof published online 12 May 2022
Footnotes
Cite this article as: JID Innovations 2022;X:100115
Clauset A, Newman MEJ, Moore C. Finding community structure in very large networks. arXiv 2004.
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.xjidi.2022.100115.
Supplementary Materials
Supplementary Table S1.
List of Microbial Species that Are Differentially Abundant between Psoriasis and Healthy Samples
| Microbial Species | Base Mean | Log2 Fold Change | P-Value | Padj |
|---|---|---|---|---|
| Prevotella_disiens | 12.48 | 26.94 | 5.07382E–16 | 2.08027E–14 |
| Clostridium_sp_KLE_1755 | 95.27 | 26.20 | 3.98903E–15 | 9.08612E–14 |
| Bacteroides_coprocola | 1,787.37 | 22.57 | 1.30691E–11 | 1.33958E–10 |
| Erysipelotrichaceae_bacterium_5_2_54FAA | 4.22 | 21.86 | 5.56657E–11 | 5.18704E–10 |
| Dorea_unclassified | 2.09 | 20.75 | 4.90663E–10 | 3.59236E–09 |
| Prevotella_buccae | 2.70 | 18.20 | 4.78393E–08 | 3.26902E–07 |
| Brachyspira_unclassified | 0.73 | 16.74 | 5.16488E–07 | 3.30875E–06 |
| Haemophilus_pittmaniae | 4.37 | 16.29 | 9.98499E–07 | 6.02036E–06 |
| Gemella_sanguinis | 3.32 | 15.09 | 5.99289E–06 | 3.41262E–05 |
| Roseburia_unclassified | 1,416.55 | 14.32 | 1.53925E–05 | 8.09092E–05 |
| Bacteroides_oleiciplenus | 12.19 | 13.46 | 5.31623E–05 | 0.000272457 |
| Burkholderiales_bacterium_1_1_47 | 579.13 | 12.17 | 3.16928E–08 | 2.24035E–07 |
| Weissella_unclassified | 1.08 | 11.51 | 0.000557326 | 0.002393849 |
| Lachnospiraceae_bacterium_9_1_43BFAA | 56.00 | 10.31 | 0.001862232 | 0.007485442 |
| Clostridium_hylemonae | 3.72 | 10.17 | 0.002285859 | 0.008841529 |
| Anaerotruncus_unclassified | 7.27 | 9.86 | 0.003047001 | 0.011567319 |
| Megasphaera_unclassified | 3,017.86 | 9.60 | 0.001021066 | 0.004186369 |
| Collinsella_stercoris | 11.98 | 9.54 | 0.004187753 | 0.015061219 |
| Enterococcus_faecium | 291.35 | 9.24 | 0.003988014 | 0.014598981 |
| Dialister_invisus | 11,846.88 | 9.16 | 0.00038163 | 0.001738535 |
| Eubacterium_dolichum | 126.53 | 8.43 | 0.000771769 | 0.003228827 |
| Parabacteroides_goldsteinii | 179.97 | 6.59 | 0.010261261 | 0.035346823 |
| Parasutterella_excrementihominis | 232.32 | 4.89 | 0.006925784 | 0.024479064 |
| Sutterella_wadsworthensis | 2,783.26 | 4.82 | 0.011048633 | 0.037130653 |
| Bacteroides_vulgatus | 16,890.23 | 2.08 | 0.003156479 | 0.01176506 |
| Clostridiaceae_bacterium_JC118 | 68.59 | ‒5.10 | 0.010345412 | 0.035346823 |
| Phascolarctobacterium_succinatutens | 16,877.87 | ‒7.49 | 0.012182033 | 0.040279303 |
| Bifidobacterium_pseudocatenulatum | 843.21 | ‒8.15 | 0.000560511 | 0.002393849 |
| Peptostreptococcaceae_noname_unclassified | 407.41 | ‒8.64 | 6.40326E–05 | 0.00031254 |
| Streptococcus_mitis_oralis_pneumoniae | 31.93 | ‒9.77 | 1.50858E–05 | 8.09092E–05 |
| Atopobium_parvulum | 1.79 | ‒10.15 | 0.002104515 | 0.008296648 |
| Coprococcus_sp_ART55_1 | 17,348.22 | ‒11.50 | 0.000206357 | 0.000961438 |
| Lactobacillus_delbrueckii | 5.36 | ‒11.56 | 0.000500643 | 0.002231124 |
| Mitsuokella_multacida | 247.94 | ‒12.11 | 9.84218E–05 | 0.00046922 |
| Fusobacterium_ulcerans | 188.80 | ‒13.32 | 6.19636E–05 | 0.000309818 |
| Mitsuokella_unclassified | 1,185.04 | ‒14.76 | 1.95683E–06 | 1.14614E–05 |
| Leuconostoc_lactis | 12.28 | ‒14.80 | 8.64274E–06 | 4.78854E–05 |
| Gemella_unclassified | 1.35 | ‒16.59 | 6.05617E–07 | 3.76217E–06 |
| Desulfovibrio_desulfuricans | 43.84 | ‒17.53 | 1.0359E–07 | 6.85032E–07 |
| Proteus_penneri | 0.22 | ‒20.93 | 3.3238E–10 | 2.52363E–09 |
| Citrobacter_freundii | 1.54 | ‒21.36 | 1.38522E–10 | 1.09219E–09 |
| Leuconostoc_gelidum | 0.28 | ‒21.40 | 1.32504E–10 | 1.08653E–09 |
| Proteus_unclassified | 0.29 | ‒21.44 | 1.22431E–10 | 1.04577E–09 |
| Streptococcus_anginosus | 6.60 | ‒21.57 | 9.26092E–11 | 8.2543E–10 |
| Porphyromonas_somerae | 4.71 | ‒21.89 | 5.18674E–11 | 5.06325E–10 |
| Lactobacillus_curvatus | 13.67 | ‒22.92 | 5.84E–12 | 6.30105E–11 |
| Alloscardovia_omnicolens | 2.16 | ‒23.22 | 3.21802E–12 | 3.66497E–11 |
| Lactobacillus_sakei | 1.23 | ‒23.34 | 2.45901E–12 | 2.96528E–11 |
| Streptococcus_sp_BS35b | 2.20 | ‒23.89 | 7.47425E–13 | 9.57638E–12 |
| Enterococcus_avium | 2.11 | ‒24.12 | 4.44676E–13 | 6.07724E–12 |
| Bacteroides_gallinarum | 16.02 | ‒24.34 | 2.59664E–13 | 3.80222E–12 |
| Carnobacterium_maltaromaticum | 12.49 | ‒24.74 | 1.15388E–13 | 1.81958E–12 |
| candidate_division_TM7_single_cell_isolate_TM7c | 0.18 | ‒25.12 | 4.69133E–14 | 8.01436E–13 |
| Weissella_cibaria | 6.65 | ‒25.57 | 1.65562E–14 | 3.08547E–13 |
| Subdoligranulum_variabile | 28.34 | ‒25.57 | 1.27799E–14 | 2.61988E–13 |
| Bacteroides_sp_1_1_6 | 133.01 | ‒26.24 | 3.4666E–15 | 8.88316E–14 |
| Citrobacter_unclassified | 4.40 | ‒26.39 | 1.9069E–15 | 5.58448E–14 |
| Butyricicoccus_pullicaecorum | 12.00 | ‒26.47 | 1.86029E–15 | 5.58448E–14 |
| Bacteroides_sp_2_1_22 | 425.75 | ‒29.27 | 1.42946E–18 | 7.326E–17 |
| Corynebacterium_glutamicum | 1.95 | ‒29.98 | 2.24401E–19 | 1.54839E–17 |
| Ruminococcus_champanellensis | 1,472.34 | ‒30.00 | 2.26594E–19 | 1.54839E–17 |
| Campylobacter_hominis | 1.51 | ‒30.00 | 2.09163E–19 | 1.54839E–17 |
Abbreviation: adj, adjusted.
Supplementary Table S2.
List of Microbial Gene Families that Are Differentially Abundant between Psoriasis and Healthy Samples
| UniRef90_Genefamilies | Base Mean | Log2 Fold Change | P-Value | Padj |
|---|---|---|---|---|
| UniRef90_D4JKB5: NO_NAME | 20.44971008 | 8.184774568 | 3.76E–07 | 3.33E–05 |
| UniRef90_D4JKB4: NO_NAME | 9.737750875 | 7.318193992 | 2.7E–06 | 0.000218 |
| UniRef90_D4JKB3: NO_NAME | 5.604615949 | 5.972567491 | 2.98E–06 | 0.000239 |
| UniRef90_C6JHT6: NO_NAME | 11.59795247 | 3.503976143 | 3.74E–06 | 0.000297 |
| UniRef90_V8CCN6: NO_NAME | 7.911723223 | ‒8.466033916 | 2.02E–05 | 0.001506 |
| UniRef90_R5HHX1: NO_NAME | 3.291298903 | 5.617246745 | 2.23E–05 | 0.001652 |
| UniRef90_B0MUZ9: NO_NAME | 17.2243469 | 7.779663835 | 2.63E–05 | 0.001914 |
| UniRef90_B0NKZ9: NO_NAME | 4.866770601 | 4.68406674 | 0.000104 | 0.007173 |
| UniRef90_E1YTP4: NO_NAME | 9.980238914 | 4.364093512 | 0.000124 | 0.008475 |
| UniRef90_C0EYV3: NO_NAME | 5.843250501 | ‒1.156773775 | 0.00013 | 0.008856 |
| UniRef90_K9DSD1: NO_NAME | 20.70420605 | 7.4409631 | 0.000134 | 0.009178 |
| UniRef90_D4V9S6: Toxin-antitoxin system, antitoxin component family protein | 3.433982731 | 3.624603291 | 0.000137 | 0.009363 |
| UniRef90_T4Z7D4: NO_NAME | 12.26511689 | ‒1.374370748 | 0.00014 | 0.009561 |
| UniRef90_A7A642: NO_NAME | 9.61390528 | ‒3.999917984 | 0.00018 | 0.012157 |
| UniRef90_C4Z8L3: NO_NAME | 8.953334146 | 4.14025387 | 0.000198 | 0.013365 |
| UniRef90_D4L5Q7: Dockerin type I repeat | 3.709257355 | 4.458714365 | 0.000214 | 0.014395 |
| UniRef90_C4ZDT0: NO_NAME | 11.02944175 | 2.61803876 | 0.000221 | 0.014875 |
| UniRef90_Q89YV2: NO_NAME | 3.960842604 | 2.459102717 | 0.000231 | 0.015496 |
| UniRef90_R5ENA9: NO_NAME | 7.31968454 | 2.809734777 | 0.000273 | 0.018301 |
| UniRef90_R5VN00: NO_NAME | 6.66944193 | 2.726824008 | 0.000283 | 0.018902 |
| UniRef90_C0FYT5: NO_NAME | 5.608127022 | 5.494622505 | 0.000289 | 0.019302 |
| UniRef90_U2DKA0: Toxin-antitoxin system, antitoxin component, Xre family | 2.41752419 | ‒2.07552754 | 0.000315 | 0.021031 |
| UniRef90_U2NXE6: NO_NAME | 0.823846701 | 4.956194737 | 0.000317 | 0.0212 |
| UniRef90_R5F076: NO_NAME | 6.570912784 | 2.6,53219048 | 0.000329 | 0.021951 |
| UniRef90_I7ASX7: Aminoglycoside 6-adenylyltransferase | 5.981679914 | ‒1.229156672 | 0.000339 | 0.022617 |
| UniRef90_D6E2K8: NO_NAME | 6.220313047 | 7.882367351 | 0.00035 | 0.023302 |
| UniRef90_C4ZCN8: NO_NAME | 4.739971235 | 4.531804983 | 0.000367 | 0.02438 |
| UniRef90_A8SDV6: NO_NAME | 3.111716292 | ‒6.463421902 | 0.00037 | 0.024535 |
| UniRef90_A7B7Y7: NO_NAME | 3.835647149 | ‒4.216283036 | 0.000381 | 0.02528 |
| UniRef90_R5DHM8: 50S ribosomal protein L14 | 8.05231442 | ‒1.091553729 | 0.000387 | 0.025643 |
| UniRef90_F7JRF6: NO_NAME | 2.10652993 | ‒2.258144862 | 0.000397 | 0.026326 |
| UniRef90_R5ENA5: NO_NAME | 6.145444386 | 2.76594925 | 0.000404 | 0.026733 |
| UniRef90_C4ZB30: NO_NAME | 13.98634953 | 6.26094269 | 0.000416 | 0.027527 |
| UniRef90_C4ZHW5: NO_NAME | 7.601796234 | 2.21832247 | 0.000424 | 0.028048 |
| UniRef90_A6NXS5: NO_NAME | 3.085119571 | ‒1.949411921 | 0.000431 | 0.028513 |
| UniRef90_R5ES70: NO_NAME | 6.142418982 | 2.70370766 | 0.000447 | 0.029252 |
| UniRef90_R5ENC3: NO_NAME | 6.187279035 | 2.701041839 | 0.00045 | 0.029276 |
| UniRef90_R5VTV0: NO_NAME | 11.84385896 | ‒8.859754987 | 0.000484 | 0.031431 |
| UniRef90_R5ES82: NO_NAME | 6.218044543 | 2.631291787 | 0.000495 | 0.03205 |
| UniRef90_A8SDV1: PTS system, lactose/cellobiose family IIC component | 1.577472175 | ‒5.394020156 | 0.000498 | 0.032135 |
| UniRef90_R5F071: NO_NAME | 5.795869956 | 2.671859836 | 0.0005 | 0.032226 |
| UniRef90_R5EN96: NO_NAME | 6.316661771 | 2.597760365 | 0.000522 | 0.033606 |
| UniRef90_D4LVD7: Bacterial mobilisation protein (MobC) | 2.997706936 | ‒3.203584633 | 0.000527 | 0.033927 |
| UniRef90_R5EP09: NO_NAME | 6.497763827 | 2.664311777 | 0.000545 | 0.035039 |
| UniRef90_A8SDV2: PTS system, Lactose/Cellobiose specific IIB subunit | 1.67041892 | ‒5.420005822 | 0.000571 | 0.036645 |
| UniRef90_R5ENY1: NO_NAME | 6.055002091 | 2.605312597 | 0.000597 | 0.038285 |
| UniRef90_R5EXU5: NO_NAME | 6.640770231 | 2.590729061 | 0.000608 | 0.039021 |
| UniRef90_R5B346: Peptidyl-prolyl cis-trans isomerase | 1.333716976 | ‒2.172687536 | 0.000617 | 0.039563 |
| UniRef90_R5F1R6: NO_NAME | 6.195974482 | 2.670392787 | 0.00062 | 0.039746 |
| UniRef90_R5ENZ3: NO_NAME | 5.847885346 | 2.536486311 | 0.000661 | 0.042259 |
| UniRef90_R5F1S4: NO_NAME | 6.301820127 | 2.594059152 | 0.000673 | 0.043049 |
| UniRef90_F9Z9M1: Putative transposase | 3.863722566 | 2.56148742 | 0.000689 | 0.044002 |
| UniRef90_R5F5V1: NO_NAME | 6.214726081 | 2.736286892 | 0.000691 | 0.044147 |
| UniRef90_C0B519: NO_NAME | 2.42564728 | ‒2.370035967 | 0.000713 | 0.045513 |
| UniRef90_J9GFK7: Transposase | 10.34428213 | 3.257865985 | 0.000716 | 0.045681 |
| UniRef90_R5F1T5: NO_NAME | 7.6286111 | 2.573141893 | 0.000747 | 0.047598 |
| UniRef90_R5ES58: NO_NAME | 6.279915456 | 2.537809692 | 0.000753 | 0.047953 |
| UniRef90_R5END3: NO_NAME | 6.459389346 | 2.620179025 | 0.000759 | 0.048362 |
| UniRef90_C4ZAI7: Putative regulatory components of sensory transduction system | 8.358269459 | 1.883595829 | 0.000763 | 0.04859 |
| UniRef90_R5ENB7: NO_NAME | 6.194816868 | 2.563624339 | 0.000778 | 0.049511 |
Abbreviation: adj, adjusted.
Supplementary Table S3.
List of Microbial Metacyc Pathways that Are Differentially Abundant between Psoriasis and Healthy Samples
| MetaCyc_Pathway | Base Mean | Log2 Fold Change | P-Value | Padj |
|---|---|---|---|---|
| P163-PWY: L-lysine fermentation to acetate and butanoate | 0.174625879 | ‒17.84752508 | 8.47E–08 | 1.63E–05 |
| PWY-5304: superpathway of sulfur oxidation (Acidianus ambivalens) | 0.135640038 | ‒18.83320162 | 1.60E–08 | 6.16E–06 |
| PWY-7200: superpathway of pyrimidine deoxyribonucleoside salvage | 0.627023649 | 12.74062955 | 6.12E–05 | 0.007856 |
Abbreviation: adj, adjusted.
Supplementary Table S4.
Summary of Statistics of Diet Survey
| Diet | P-Value (Disease Status) | P-Value (Enterotype) |
|---|---|---|
| Milk | 0.76 | 0.88 |
| Fruit | 0.66 | 0.16 |
| Cereal | 0.12 | 0.93 |
| Soda | 0.51 | 0.52 |
| Pure fruit juices | 0.87 | 0.86 |
| Sweetened tea or coffee | 0.25 | 0.49 |
| Sweetened fruit drinks | 0.6 | 0.87 |
| Salad | 0.44 | 0.48 |
| Fried potatoes | 0.1 | 0.07 |
| Other kinds of potatoes | 0.0564 | 0.09 |
| Beans | 0.29 | 0.18 |
| Brown rice | 0.64 | 0.67 |
| Other veggies | 0.48 | 0.28 |
| Salsa | 0.11 | 0.0958 |
| Pizza | 0.38 | 0.69 |
| Tomato sauces | 0.68 | 0.12 |
| Cheese | 0.47 | 0.46 |
| Red meat | 0.65 | 0.65 |
| Processed meat | 0.13 | 0.28 |
| Whole grain bread | 0.74 | 0.1 |
| Chocolate/candy | 0.84 | 0.62 |
| Doughnuts | 0.46 | 0.33 |
| Pastries | 0.5 | 0.07 |
| Frozen desserts | 0.79 | 0.23 |
| Popcorn | 0.52 | 0.75 |
Supplementary Table S5.
List of Microbial Species that Are Differentially Abundant between Different Subgroup in the Cohort
| Microbial Species | Base Mean | Log2 Fold Change | P-Value | Padj | Comparison Pair |
|---|---|---|---|---|---|
| Turicibacter unclassified | 148.1650263 | ‒25.09944303 | 1.48E–25 | 2.77E–23 | c2c1 |
| Coprococcus sp ART55 1 | 17,348.22439 | ‒30 | 4.07E–22 | 3.80E–20 | c2c1 |
| Phascolarctobacterium succinatutens | 16,877.86806 | ‒30 | 5.75E–20 | 3.58E–18 | c2c1 |
| Turicibacter sanguinis | 30.89947547 | ‒23.71828011 | 1.25E–19 | 5.84E–18 | c2c1 |
| Coprococcus sp ART55 1 | 17,348.22439 | ‒32.10303282 | 4.81E–18 | 9.82E–16 | c2c3 |
| P. succinatutens | 16,877.86806 | ‒27.87316104 | 1.19E–12 | 8.08E–11 | c2c3 |
| Turicibacter unclassified | 148.1650263 | ‒20.02159204 | 2.65E–12 | 1.35E–10 | c2c3 |
| Megamonas unclassified | 13,738.87404 | ‒10.70556321 | 1.26E–06 | 1.03E–05 | c2c1 |
| Megamonas unclassified | 13,738.87404 | ‒12.64875264 | 1.93E–06 | 2.81E–05 | c2c3 |
| T. sanguinis | 30.89947547 | ‒15.01564462 | 2.39E–06 | 3.25E–05 | c2c3 |
| Ruminococcaceae bacterium D16 | 318.9654536 | ‒8.1375798 | 0.000312498 | 0.003363951 | c3c1 |
| T. sanguinis | 30.89947547 | ‒8.702635493 | 0.000532915 | 0.005417965 | c3c1 |
| Lachnospiraceae bacterium 1 1 57FAA | 2,253.140867 | ‒6.756348377 | 0.000705118 | 0.006791396 | c3c1 |
| Streptococcus thermophilus | 2,905.078536 | ‒4.197322843 | 0.002619562 | 0.020410751 | c2c1 |
| Prevotella copri | 69,851.06368 | ‒4.796452699 | 0.003805748 | 0.028466996 | c2c1 |
| S. thermophilus | 2,905.078536 | ‒5.017689601 | 0.002810256 | 0.035830762 | c2c3 |
| Bacteroides xylanisolvens | 1,773.658642 | 3.277724558 | 0.005561142 | 0.039997442 | c2c1 |
Abbreviation: adj, adjusted.
Supplementary Table S6.
List of Microbial Gene Families that Are Differentially Abundant between Different Subgroups in the Cohort
| UniRef90_Genefamilies | Base Mean | Log2 Fold Change | P-Value | Padj | Comparison Pair |
|---|---|---|---|---|---|
| UniRef90_R5F5T8: NO_NAME | 6.13241864 | 3.855214514 | 8.38E–09 | 9.43E–06 | c2c3 |
| UniRef90_R5F5U3: NO_NAME | 7.146133182 | 3.742380578 | 6.57E–09 | 7.87E–06 | c2c3 |
| UniRef90_R5F5U7: NO_NAME | 5.872476462 | 3.736858351 | 3.22E–09 | 5.40E–06 | c2c3 |
| UniRef90_R5F5V1: NO_NAME | 6.214726081 | 3.729014981 | 1.70E–07 | 8.76E–05 | c2c3 |
| UniRef90_R5F5V6: NO_NAME | 6.843317692 | 3.810172235 | 7.23E–08 | 4.24E–05 | c2c3 |
| UniRef90_R5F6I7: NO_NAME | 5.66902435 | 3.508892329 | 1.28E–08 | 1.30E–05 | c2c3 |
| UniRef90_R5F7E6: NO_NAME | 7.654869731 | 3.781212969 | 8.36E–08 | 4.80E–05 | c2c3 |
| UniRef90_R5F7E9: NO_NAME | 6.014946714 | 3.700272305 | 1.07E–07 | 5.95E–05 | c2c3 |
| UniRef90_R5F7F4: NO_NAME | 6.252090198 | 3.70429003 | 2.70E–09 | 4.97E–06 | c2c3 |
| UniRef90_R5F7F6: NO_NAME | 7.03037544 | 3.73575232 | 6.86E–08 | 4.14E–05 | c2c3 |
| UniRef90_R5F826: NO_NAME | 6.25853359 | 3.553654489 | 5.32E–06 | 0.002125 | c2c3 |
| UniRef90_R5F830: NO_NAME | 4.020772359 | 3.303446928 | 0.000126 | 0.041501 | c2c3 |
| UniRef90_R6BXA1: Pyridoxal biosynthesis lyase PdxS | 18.20827345 | –4.738434723 | 0.000139 | 0.025007 | c2c1 |
| UniRef90_R6EYN1: Phosphoenolpyruvate carboxykinase [ATP] | 12.14532041 | ‒5.300791062 | 0.000284 | 0.045255 | c2c1 |
| UniRef90_R5UHP6: NO_NAME | 7.404456209 | –2.502048554 | 0.000151 | 0.048523 | c2c3 |
| UniRef90_R5V1S5: NO_NAME | 6.469822898 | 3.674332424 | 3.90E–08 | 2.69E–05 | c2c3 |
| UniRef90_R5VEH7: NO_NAME | 5.638179874 | 3.783296451 | 3.44E–08 | 2.46E–05 | c2c3 |
| UniRef90_R5VN00: NO_NAME | 6.66944193 | 3.804730154 | 9.58E–10 | 2.11E–06 | c2c3 |
| UniRef90_R6FSV7: dTDP-4-dehydrorhamnose 3 5-epimerase | 3.836852934 | ‒4.712602303 | 0.00015 | 0.026547 | c2c1 |
| UniRef90_UPI00046F6872: NO_NAME | 3.841073774 | ‒3.288653597 | 7.37E–05 | 0.025081 | c2c3 |
| UniRef90_R6YLJ5: dTDP-glucose 4 6-dehydratase | 25.36463194 | ‒5.501203549 | 4.07E–05 | 0.008208 | c2c1 |
| UniRef90_R6YTB4: Virulence protein | 6.475252167 | 3.598839048 | 1.42E–11 | 5.13E–08 | c3c1 |
| UniRef90_R7ERI0: Protein phosphatase 2C | 3.917056058 | 2.488315558 | 0.000229 | 0.03792 | c2c1 |
| UniRef90_W1I1K8: Uncultured bacterium extrachromosomal DNA RGI00018 | 44.18955643 | ‒8.421153783 | 7.98E–05 | 0.027004 | c2c3 |
| UniRef90_W1I4A6: Uncultured bacterium extrachromosomal DNA RGI00018 | 19.44468222 | ‒5.622500646 | 0.000293 | 0.046458 | c2c1 |
Abbreviation: adj, adjusted.
Supplementary Table S7.
List of Microbial Metacyc Pathways that Are Differentially Abundant between Different Subgroups in the Cohort
| MetaCyc_Pathway | Base Mean | Log2 Fold Change | P-Value | Padj | Comparison Pair |
|---|---|---|---|---|---|
| NAD-BIOSYNTHESIS-II: NAD salvage pathway II | 6.885975523 | ‒2.032470385 | 0.004596 | 0.081003 | c2c1 |
| POLYAMINSYN3-PWY: super pathway of polyamine biosynthesis II | 16.58305195 | ‒2.61298583 | 0.002204 | 0.069068 | c2c1 |
| PWY-5121: superpathway of geranylgeranyl diphosphate biosynthesis II (via MEP) | 39.92534701 | ‒1.094952419 | 0.003983 | 0.081003 | c2c1 |
| PWY-5384: sucrose degradation IV (sucrose phosphorylase) | 6.824519225 | ‒3.287493629 | 0.0009 | 0.061079 | c2c1 |
| PWY-6147: 6-hydroxymethyl-dihydropterin diphosphate biosynthesis I | 54.32244565 | ‒0.893067591 | 0.002654 | 0.074673 | c2c1 |
| PWY-6167: flavin biosynthesis II (archaea) | 5.972757342 | ‒5.596005317 | 0.005971 | 0.098575 | c2c1 |
| PWY-6270: isoprene biosynthesis I | 88.15679759 | ‒1.066870021 | 0.006642 | 0.098575 | c2c1 |
| PWY-6859: all-trans-farnesol biosynthesis | 4.012058217 | ‒3.717715695 | 0.001083 | 0.061079 | c2c1 |
| PWY-7392: taxadiene biosynthesis (engineered) | 9.386270018 | ‒3.60306079 | 0.000283 | 0.042061 | c2c1 |
| PWY-7560: methylerythritol phosphate pathway II | 79.44418324 | ‒1.069297573 | 0.007032 | 0.099156 | c2c1 |
| ANAEROFRUCAT-PWY: homolactic fermentation | 143.8794075 | 0.779635572 | 0.0046 | 0.097618 | c2c3 |
| COLANSYN-PWY: colanic acid building blocks biosynthesis | 35.8384929 | 1.550221291 | 0.0044 | 0.097618 | c2c3 |
| HEXITOLDEGSUPER-PWY: superpathway of hexitol degradation (bacteria) | 54.54908312 | 0.909153433 | 0.002063 | 0.066483 | c2c3 |
| OANTIGEN-PWY: O-antigen building blocks biosynthesis (E. coli) | 99.70191306 | ‒1.121948089 | 0.006771 | 0.097618 | c2c3 |
| PHOSLIPSYN-PWY: superpathway of phospholipid biosynthesis I (bacteria) | 70.46008483 | 1.331355977 | 0.001855 | 0.066483 | c2c3 |
| PWY4FS-7: phosphatidylglycerol biosynthesis I (plastidic) | 51.5490991 | 1.391404667 | 0.006811 | 0.097618 | c2c3 |
| PWY-5101: L-isoleucine biosynthesis II | 66.49625492 | 1.214578922 | 0.005292 | 0.097618 | c2c3 |
| PWY-6168: flavin biosynthesis III (fungi) | 172.2879499 | 0.689038903 | 0.005629 | 0.097618 | c2c3 |
| PWY-7323: superpathway of GDP-mannose-derived O-antigen building blocks biosynthesis | 25.89416668 | 1.771497516 | 0.002522 | 0.067784 | c2c3 |
| UDPNAGSYN-PWY: UDP-N-acetyl-D-glucosamine biosynthesis I | 67.18613175 | ‒1.506925835 | 0.001515 | 0.066483 | c2c3 |
| ARG+POLYAMINE-SYN: super pathway of arginine and polyamine biosynthesis | 69.58601257 | ‒0.960789029 | 0.000298 | 0.042061 | c2c1 |
| ARG+POLYAMINE-SYN: super pathway of arginine and polyamine biosynthesis | 69.58601257 | ‒1.05230553 | 0.000918 | 0.066483 | c2c3 |
| ARGININE-SYN4-PWY: L-ornithine de novo biosynthesis | 91.08538996 | 1.466876718 | 0.001004 | 0.066483 | c2c3 |
| ARGININE-SYN4-PWY: L-ornithine de novo biosynthesis | 91.08538996 | 1.144773909 | 0.001917 | 0.069068 | c2c1 |
| PWY-6471: peptidoglycan biosynthesis IV (Enterococcus faecium) | 10.68397596 | ‒2.509515632 | 0.004524 | 0.081003 | c2c1 |
| PWY-6471: peptidoglycan biosynthesis IV (Enterococcus faecium) | 10.68397596 | ‒3.293509459 | 0.001507 | 0.066483 | c2c3 |
| PYRIDOXSYN-PWY: pyridoxal 5-phosphate biosynthesis I | 83.95260245 | 1.49487838 | 0.002165 | 0.066483 | c2c3 |
| PYRIDOXSYN-PWY: pyridoxal 5-phosphate biosynthesis I | 83.95260245 | 1.201030452 | 0.002913 | 0.074673 | c2c1 |
Abbreviation: adj, adjusted.
Supplementary Table S8.
List of Differentially Expressed Genes that Are Differentially Abundant between Different Subgroups in the Cohort
| Ensembl_Gene_ID | Base Mean | Log2 Fold Change | Padj | Gene Name | Comparison Pair |
|---|---|---|---|---|---|
| ENSG00000155966 | 70.519 | 3.74964 | 0.000392968 | AFF2 | c3c1 |
| ENSG00000233680 | 35.444 | 3.02891 | 0.001437268 | HNRNPA1P27 | c2c1 |
| ENSG00000187527 | 19.131 | 2.03977 | 0.049354713 | ATP13A5 | c2c3 |
| ENSG00000214671 | 84.817 | 0.83427 | 0.022303138 | RPL6P12 | c2c1 |
| ENSG00000214595 | 713.756 | 1.01421 | 0.018629526 | EML6 | c3c1 |
| ENSG00000173040 | 124.774 | ‒0.84891 | 0.038601379 | EVC2 | c2c3 |
| ENSG00000238025 | 58.599 | ‒1.37982 | 0.039362413 | ZDHHC4P1 | c2c1 |
| ENSG00000162897 | 190.900 | 1.35991 | 0.02346219 | FCAMR | c3c1 |
| ENSG00000086205 | 10.244 | ‒2.40497 | 0.000829552 | FOLH1 | c2c3 |
| ENSG00000181867 | 0.673 | ‒20.54710 | 0.000897703 | FTMT | c2c1 |
| ENSG00000158089 | 21.123 | 2.26847 | 0.013980259 | GALNT14 | c3c1 |
| ENSG00000274618 | 372.111 | 1.52264 | 0.008495718 | HIST1H4F | c3c1 |
| ENSG00000166736 | 16.410 | ‒3.99495 | 0.032119336 | HTR3A | c2c3 |
| ENSG00000166736 | 16.410 | 3.54715 | 0.013195954 | HTR3A | c3c1 |
| ENSG00000241244 | 319.624 | ‒1.36024 | 0.020133239 | IGKV1D-16 | c2c3 |
| ENSG00000129451 | 40.860 | ‒4.35734 | 0.035837072 | KLK10 | c2c3 |
| ENSG00000129451 | 40.860 | 3.74074 | 0.025102134 | KLK10 | c3c1 |
| ENSG00000225128 | 0.677 | 23.60138 | 0.005620613 | LINC00972 | c2c3 |
| ENSG00000225128 | 0.677 | ‒17.31295 | 0.038958367 | LINC00972 | c3c1 |
| ENSG00000182333 | 0.399 | 12.17337 | 0.003881171 | LIPF | c2c3 |
| ENSG00000264251 | 0.669 | 10.28858 | 0.024886441 | RN7SL819P | c2c3 |
| ENSG00000182333 | 0.399 | ‒12.36307 | 5.74E–05 | LIPF | c3c1 |
| ENSG00000249333 | 0.470 | ‒14.41122 | 1.34E–06 | LL22NC03-23C6.12 | c2c1 |
| ENSG00000234031 | 27.687 | 1.34310 | 0.039573133 | RPS3AP44 | c2c3 |
| ENSG00000249333 | 0.470 | ‒16.86785 | 1.46E–05 | LL22NC03-23C6.12 | c2c3 |
| ENSG00000271765 | 63.342 | 0.91097 | 0.038601379 | RN7SKP299 | c2c3 |
| ENSG00000118308 | 1,060.651 | 1.25761 | 0.049743284 | LRMP | c3c1 |
| ENSG00000266276 | 0.285 | ‒17.22075 | 0.004989858 | MIR4743 | c3c1 |
| ENSG00000240666 | 3.840 | ‒5.06250 | 0.015579404 | MME-AS1 | c2c3 |
| ENSG00000228232 | 975.118 | ‒1.27782 | 0.014609163 | GAPDHP1 | c2c3 |
| ENSG00000215182 | 291.333 | ‒4.57590 | 0.005872574 | MUC5AC | c2c3 |
| ENSG00000215182 | 291.333 | 4.07199 | 0.002093636 | MUC5AC | c3c1 |
| ENSG00000185697 | 288.454 | 1.51200 | 0.012506363 | MYBL1 | c3c1 |
| ENSG00000160505 | 11.579 | ‒5.48847 | 0.008074731 | NLRP4 | c2c3 |
| ENSG00000265018 | 49.486 | ‒2.47429 | 0.009651198 | AGAP12P | c2c3 |
| ENSG00000160505 | 11.579 | 4.54221 | 0.006739737 | NLRP4 | c3c1 |
| ENSG00000163545 | 1,334.424 | ‒0.72970 | 0.030687143 | NUAK2 | c2c3 |
| ENSG00000278561 | 12.597 | ‒3.56800 | 0.024920457 | PTPN20CP | c2c3 |
| ENSG00000189233 | 376.826 | ‒1.37169 | 0.015794615 | NUGGC | c2c3 |
| ENSG00000160191 | 3,535.762 | 1.00276 | 0.026512132 | PDE9A | c2c1 |
| ENSG00000236717 | 0.463 | ‒23.22885 | 6.47E–16 | RP11-100G15.10 | c2c1 |
| ENSG00000236717 | 0.463 | ‒23.12038 | 1.84E–08 | RP11-100G15.10 | c2c3 |
| ENSG00000231090 | 0.555 | ‒13.99578 | 7.79E–05 | RP11-101C11.1 | c2c1 |
| ENSG00000231090 | 0.555 | ‒17.07458 | 0.000194189 | RP11-101C11.1 | c2c3 |
| ENSG00000257513 | 3.422 | ‒4.72468 | 0.008074731 | NPIPB1P | c2c3 |
| ENSG00000272988 | 0.267 | ‒15.44717 | 0.023939688 | RP11-150D20.5 | c3c1 |
| ENSG00000259772 | 165.237 | ‒0.83726 | 0.011698659 | RP11-16E12.2 | c2c3 |
| ENSG00000282950 | 0.907 | ‒26.60203 | 6.09E–05 | RP11-174O3.6 | c2c3 |
| ENSG00000260303 | 23.670 | ‒4.86844 | 0.01227057 | RP11-203B7.2 | c2c3 |
| ENSG00000260303 | 23.670 | 4.12079 | 0.010443355 | RP11-203B7.2 | c3c1 |
| ENSG00000258675 | 0.457 | ‒12.87191 | 0.006683019 | RP11-299L17.3 | c3c1 |
| ENSG00000275314 | 0.741 | 10.03950 | 0.024849813 | RP11-32A1.2 | c2c3 |
| ENSG00000275314 | 0.741 | ‒10.90604 | 0.000262234 | RP11-32A1.2 | c3c1 |
| ENSG00000259476 | 1.221 | 13.39380 | 0.001571351 | RP11-50C13.2 | c2c3 |
| ENSG00000259476 | 1.221 | ‒13.63577 | 2.56E–05 | RP11-50C13.2 | c3c1 |
| ENSG00000206532 | 8.420 | ‒3.15479 | 0.04914993 | RP11-553A10.1 | c2c3 |
| ENSG00000249877 | 3.764 | ‒13.38306 | 0.010980955 | RP11-706F1.2 | c3c1 |
| ENSG00000280022 | 44.512 | ‒5.02298 | 0.001165293 | RP11-707O23.1 | c2c1 |
| ENSG00000258962 | 113.659 | 2.06291 | 0.004479337 | RP11-747H7.1 | c2c1 |
| ENSG00000260711 | 1,768.668 | 1.06849 | 0.039573133 | RP11-747H7.3 | c2c3 |
| ENSG00000203620 | 0.321 | 13.96751 | 0.032593228 | RP11-84A19.2 | c2c3 |
| ENSG00000203620 | 0.321 | ‒13.23645 | 0.008565686 | RP11-84A19.2 | c3c1 |
| ENSG00000231654 | 8.334 | ‒2.42875 | 0.024773164 | RPS6KA2-AS1 | c2c1 |
| ENSG00000170054 | 54.004 | 6.27792 | 0.001444378 | SERPINA9 | c3c1 |
| ENSG00000142583 | 152.205 | 1.50167 | 0.008107758 | SLC2A5 | c3c1 |
| ENSG00000243770 | 14.943 | ‒1.76051 | 0.02152624 | RN7SL65P | c3c1 |
| ENSG00000198203 | 1,020.018 | ‒1.26205 | 0.000897703 | SULT1C2 | c2c1 |
| ENSG00000198203 | 1,020.018 | ‒1.24560 | 0.049945372 | SULT1C2 | c2c3 |
| ENSG00000196228 | 130.871 | ‒1.37044 | 0.006397675 | SULT1C3 | c2c1 |
| ENSG00000157303 | 105.695 | 1.10998 | 0.013195954 | SUSD3 | c3c1 |
| ENSG00000187621 | 53.221 | 2.55063 | 0.013195954 | TCL6 | c3c1 |
| ENSG00000088992 | 54.739 | 1.29575 | 0.013195954 | TESC | c3c1 |
| ENSG00000160182 | 2,794.990 | 1.52164 | 0.038058354 | TFF1 | c3c1 |
| ENSG00000160181 | 62.719 | 1.80337 | 0.021301346 | TFF2 | c3c1 |
| ENSG00000169903 | 40.103 | 3.38885 | 0.012506363 | TM4SF4 | c3c1 |
| ENSG00000275154 | 16.556 | 2.73116 | 0.024773164 | U3 | c2c1 |
| ENSG00000175567 | 592.509 | ‒0.78874 | 0.006712427 | UCP2 | c2c3 |
| ENSG00000112303 | 146.742 | ‒1.84313 | 0.027895978 | VNN2 | c2c3 |
| ENSG00000112303 | 146.742 | 1.50589 | 0.040547819 | VNN2 | c3c1 |
| ENSG00000101842 | 47.686 | ‒2.79232 | 0.049611364 | VSIG1 | c2c3 |
| ENSG00000101842 | 47.686 | 2.42312 | 0.034021561 | VSIG1 | c3c1 |
| ENSG00000158023 | 52.914 | 1.39125 | 0.012506363 | WDR66 | c3c1 |
Abbreviations: adj, adjusted; ID, identification.
Supplementary Table S9.
Comparison of Immune Profiles from Flow Cytometry and Cibersort among Different Psoriasis Subgroups
| Data Source | Immune Population | AVG ± SD |
Significance (Wilcox Test) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy | PSO1 | PSO2 | PSO3 | Healthy Versus PSO1 | Healthy Versus PSO2 | Healthy Versus PSO3 | PSO1 Versus PSO2 | PSO1 Versus PSO3 | PSO2 Versus PSO3 | ||
| Immunephenotype | Teff (CD3+ CD4+ CD25‒ FoxP3‒) | 80.49 ± 5.98% | 83.33 ± 3.15% | 83.49 ± 3.71% | 80.8 ± 3.29% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Active Teff (CD3+ CD4+ CD25+ FoxP3‒ CD38+ HLADR+) | 0.13 ± 0.09% | 0.27 ± 0.26% | 0.09 ± 0.03% | 0.36 ± 0.25% | n.s. | n.s. | 0.035 | n.s. | n.s. | 0.024 | |
| Memory Teff (CD3+ CD4+ CD25‒ FoxP3‒ CD45RO+) | 33.72 ± 10.14% | 34.55 ± 13.64% | 35.81 ± 12.12% | 36.3 ± 10.2% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Active memory Teff (CD3+ CD4+ CD25‒ FoxP3‒ CD45RO+ CD38+ HLADR+) | 0.31 ± 0.34% | 0.55 ± 0.55% | 0.18 ± 0.08% | 0.67 ± 0.56% | n.s. | n.s. | n.s. | 0.018 | n.s. | 0.02 | |
| Tregs (CD3+ CD4+ CD25+ FoxP3+) | 6.46 ± 2.66% | 5.81 ± 1.03% | 6.13 ± 1.22% | 6.53 ± 1.26% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Activated Tregs (CD3+ CD4+ CD25+ FoxP3+, CD45RO+) | 70.65 ± 11.6% | 67.99 ± 9.84% | 66.94 ± 10.33% | 69.95 ± 6.3% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Cytokine production | Teff (CD3+ CD4+ CD127+) | 58.51 ± 15.43% | 52.79 ± 18.71% | 58.85 ± 10.83% | 39.48 ± 18.33% | n.s. | n.s. | 0.02 | n.s. | n.s. | 0.04 |
| Teff IL17+ only | 0.93 ± 0.87% | 1.1 ± 1.56% | 0.78 ± 0.53% | 1.11 ± 0.5% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Teff IL22+ only | 0.66 ± 0.51% | 0.68 ± 0.39% | 1.75 ± 2% | 0.89 ± 0.58% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Teff IL17+ IL22+ | 0.13 ± 0.15% | 0.15 ± 0.16% | 0.32 ± 0.41% | 0.15 ± 0.06% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Teff TNF-α+ only | 0.69 ± 0.77% | 0.84 ± 0.87% | 1.28 ± 1.37% | 0.6 ± 0.21% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Teff IFNγ+ only | 10.73 ± 3.92% | 11 ± 4.07% | 12.02 ± 7.43% | 11.81 ± 4.17% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Teff TNF-α+ IFNγ+ | 0.63 ± 0.71% | 0.44 ± 0.3% | 1 ± 1.64% | 0.44 ± 0.23% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| CIBERSORTx | Th1 | 0.02 ± 0.02% | 0.01 ± 0.01% | 0.03 ± 0.02% | 0.02 ± 0.02% | n.s. | n.s. | n.s. | 0.012 | n.s. | n.s. |
| Th17 | 0.08 ± 0.02% | 0.08 ± 0.02% | 0.09 ± 0.02% | 0.09 ± 0.03% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Treg | 0.05 ± 0% | 0.05 ± 0% | 0.05 ± 0.01% | 0.06 ± 0.01% | n.s. | n.s. | n.s. | n.s. | n.s. | 0.02 | |
| Tcm | 0.02 ± 0% | 0.02 ± 0% | 0.02 ± 0% | 0.02 ± 0% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Tfh | 0.01 ± 0% | 0.01 ± 0% | 0.01 ± 0% | 0.01 ± 0% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Activated CD4 T | 0.14 ± 0.03% | 0.14 ± 0.02% | 0.14 ± 0.02% | 0.11 ± 0.01% | n.s. | n.s. | 0.014 | n.s. | 0.024 | 0.02 | |
| Immunephenotype | CD8(CD3+ CD8+) | 30.19 ± 10.33% | 32.09 ± 12.33% | 24.66 ± 9.14% | 31 ± 12.52% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Active CD8 (CD3+ CD8+ CD38+ HLADR+) | 0.52 ± 0.28% | 1.3 ± 1.65% | 0.44 ± 0.25% | 1.09 ± 0.81% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Memory CD8 (CD3+ CD8+ CD45RO+) | 15.36 ± 6.22% | 15.57 ± 8.13% | 25.34 ± 6.52% | 16.45 ± 6.41% | n.s. | 0.002 | n.s. | 0.02 | n.s. | 0.029 | |
| Activated memory CD8 (CD3+ CD8+ CD45RO+ CD38+ HLADR+) | 2.04 ± 1.61% | 3.5 ± 4.8% | 1.18 ± 1.01% | 3.09 ± 1.81% | n.s. | n.s. | n.s. | n.s. | n.s. | 0.04 | |
| Cytokine production | CD8 (CD3+ CD8+) | 27.21 ± 10.1% | 30.32 ± 12.55% | 23.69 ± 9.09% | 27.08 ± 13.52% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| CD8+ IL17+ only | 0.12 ± 0.08% | 0.15 ± 0.08% | 0.28 ± 0.36% | 0.17 ± 0.08% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| CD8+ IL22+ only | 0.14 ± 0.19% | 0.07 ± 0.06% | 0.47 ± 0.64% | 0.08 ± 0.04% | n.s. | n.s. | n.s. | 0.023 | n.s. | n.s. | |
| CD8+ IL17+ IL22+ | 0.02 ± 0.01% | 0.02 ± 0.01% | 0.15 ± 0.2% | 0.02 ± 0.03% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| CD8+ TNF-α+ only | 0.04 ± 0.07% | 0.09 ± 0.22% | 0.14 ± 0.18% | 0.06 ± 0.06% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| CD8+ IFNγ+ only | 16.47 ± 7.85% | 16.09 ± 9.52% | 20.17 ± 12.71% | 20.45 ± 8.55% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| CD8+ TNF-α+ IFNγ+ | 0.4 ± 0.29% | 0.35 ± 0.35% | 0.46 ± 0.65% | 0.66 ± 0.58% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| CIBERSORTx | CD8 T | 0.00375 ± 0.0065% | 0.00326 ± 0.00408% | 0.00053 ± 0.00133% | 0.01483 ± 0.01089% | n.s. | n.s. | 0.026 | 0.043 | 0.042 | 0.011 |
Abbreviations: AVG, average; n.s., not significant; Tcm, T central memory; Teff, effector T cell; tfh, T follicular helper; Th, T helper; Treg, regulatory T cell.
Supplementary Table S10.
Summary of Datasets Used in Multiomic Analysis
| Dataset | Measurement Type | Inclusion Criteria | No. of Features Included | Normalization Method |
|---|---|---|---|---|
| Microbial spp. (n = 40) | Shotgun metagenomics from stool samples | Nonzero counts in at least 10 samples | 142 | Counts per million |
| Microbial UniRef90 gene families (n = 40) | Shotgun metagenomics from stool samples | At least three counts for 80% of all metagenomics samples (total is 52 samples) | 2,488 | Counts per million |
| Microbial MetaCyc pathways (n = 40) | Shotgun metagenomics from stool samples | All metacyc pathways identified by humann2 | 334 | Counts per million |
| Flow cell population (n = 40) | Flow cytometry from PBMCs | Include all data collected | 25 | Percentage of parental gate |
| Flow cytokine production (n = 40) | Flow cytometry from PBMCs | Include all data collected | 29 | Percentage of parental gate |
| Host RNA-seq (n = 40) | RNA-seq from sigmoidal colon biopsies | Top 500 most variable genes + all DE genes identified by either disease comparison or PSO subgroup comparisons + inflammatory genes | 601 | vst transformation to ensure variance is constant across the data |
Abbreviations: DE, differentially expressed; No., number; PSO, psoriasis; RNA-seq, RNA sequencing; vst, variance stabilizing transformation.
Supplementary Table S11.
List of Significant Associations in Host Microbial Multiomics Network
| From | To | Cor | P-Value | Padj | Greedy Membership |
|---|---|---|---|---|---|
| Significant associations in health-associated network | |||||
| s__Catenibacterium_mitsuokai | CD8_TNFa_only | 0.80837 | 0.000118 | 0.047311 | 1 |
| PWY4FS-7: phosphatidylglycerol biosynthesis I (plastidic) | Teff_IFNg_Only | 0.643956 | 0.00019 | 0.095866 | |
| PWY4FS-8: phosphatidylglycerol biosynthesis II (non-plastidic) | Teff_IFNg_Only | 0.643956 | 0.00019 | 0.095866 | |
| PWY4FS-7: phosphatidylglycerol biosynthesis I (plastidic) | Teff_TNFa_INFg | 0.621978 | 1.49E–06 | 0.003106 | |
| PWY4FS-8: phosphatidylglycerol biosynthesis II (non-plastidic) | Teff_TNFa_INFg | 0.621978 | 1.49E–06 | 0.003106 | |
| CD8_TNFa_only | IL1R2 | ‒0.68647 | 2.35E–06 | 0.006511 | |
| s__Catenibacterium_mitsuokai | NOS2 | 0.805281 | 0.000223 | 0.096093 | |
| Teff_TNFa_INFg | NOS2 | 0.775824 | 0.000257 | 0.091006 | |
| CD8_TNFa_only | ST6GAL2 | ‒0.64466 | 3.23E–05 | 0.030873 | |
| s__Catenibacterium_mitsuokai | ST6GAL2 | ‒0.66447 | 4.78E–06 | 0.005155 | |
| PHOSLIPSYN-PWY: superpathway of phospholipid biosynthesis I (bacteria) | CD8_IFNg_only | 0.767033 | 0.000162 | 0.092121 | 2 |
| Treg_INFg_only | CCDC144A | 0.749451 | 0.000105 | 0.062263 | |
| Treg_INFg_only | RP11-219A15.1 | 0.796066 | 2.5E–05 | 0.02777 | |
| CD8_IFNg_only | RP11-336A10.5 | 0.818482 | 2.1E–05 | 0.02777 | |
| CD8_IL22_only | RP11-336A10.5 | 0.752476 | 0.000181 | 0.080494 | |
| Treg_INFg_only | RP11-336A10.5 | 0.673268 | 0.000104 | 0.062263 | |
| Treg_TNFa_IFNg | RP11-336A10.5 | 0.790289 | 0.000219 | 0.088193 | |
| Treg_INFg_only | USP32P1 | 0.783672 | 0.000112 | 0.06403 | |
| PWY-6125: superpathway of guanosine nucleotides de novo biosynthesis II | activated.memory.Teff | 0.686469 | 0.000357 | 0.021066 | 3 |
| PWY-7184: pyrimidine deoxyribonucleotides de novo biosynthesis I | activated.memory.Teff | 0.638767 | 0.000445 | 0.025005 | |
| PWY-7197: pyrimidine deoxyribonucleotide phosphorylation | activated.memory.Teff | 0.613862 | 0.001624 | 0.073648 | |
| s__Escherichia_coli | activated.memory.Teff | 0.60066 | 1.89E–05 | 0.003131 | |
| FUC-RHAMCAT-PWY: super pathway of fucose and rhamnose degradation | Active.Teff | 0.786207 | 0.001056 | 0.051489 | |
| PWY-6125: super pathway of guanosine nucleotides de novo biosynthesis II | Active.Teff | 0.63631 | 0.001602 | 0.073212 | |
| PWY-6628: superpathway of L-phenylalanine biosynthesis | Active.Teff | 0.728019 | 7.38E–06 | 0.0016 | |
| s__Escherichia_coli | Active.Teff | 0.660784 | 8.59E–06 | 0.001669 | |
| UniRef90_K6B179: NO_NAME | Active.Teff | 0.604715 | 1.58E–06 | 0.041485 | |
| s__Eubacterium_ventriosum | IGLV8-61 | ‒0.73511 | 0.000141 | 0.073261 | 4 |
| s__Ruminococcus_obeum | IGLV8-61 | ‒0.6 | 0.000147 | 0.0755 | |
| UniRef90_A5ZMH0: NO_NAME | IGLV8-61 | ‒0.81588 | 2.75E–06 | 0.065021 | |
| UniRef90_D4LLH7: ATPases involved in chromosome partitioning | IGLV8-61 | ‒0.76686 | 3.19E–06 | 0.070247 | |
| UniRef90_R6S2S2: Ketol-acid reductoisomerase | IGLV8-61 | ‒0.71356 | 5.13E–06 | 0.091049 | |
| GLYCOLYSIS: glycolysis I (from glucose 6-phosphate) | memory.CD8 | 0.78022 | 0.000181 | 0.011891 | 5 |
| PWY-5484: glycolysis II (from fructose 6-phosphate) | memory.CD8 | 0.789011 | 0.000203 | 0.013021 | |
| UniRef90_D4M0P1: ADP-ribose pyrophosphatase | memory.CD8 | 0.899252 | 1.03E–05 | 0.087323 | |
| NONOXIPENT-PWY: pentose phosphate pathway (non-oxidative branch) | RP11-716A19.3 | 0.61012 | 7.69E–06 | 0.031569 | 6 |
| UniRef90_R7JV77: NO_NAME | RP11-716A19.3 | 0.610793 | 4.62E–06 | 0.086393 | |
| NONOXIPENT-PWY: pentose phosphate pathway (non-oxidative branch) | RP4-672N11.1 | 0.61012 | 7.69E–06 | 0.031569 | |
| UniRef90_R7JV77: NO_NAME | RP4-672N11.1 | 0.610793 | 4.62E–06 | 0.086393 | |
| PPGPPMET-PWY: ppGpp biosynthesis | activated.memory.CD8 | 0.601323 | 0.001952 | 0.08521 | 7 |
| PWY-821: superpathway of sulfur amino acid biosynthesis (Saccharomyces cerevisiae) | activated.memory.CD8 | 0.686806 | 7.64E–05 | 0.006382 | |
| NONMEVIPP-PWY: methylerythritol phosphate pathway I | ADGRG7 | 0.868132 | 2.01E–05 | 0.062622 | 8 |
| s__Prevotella_copri | ADGRG7 | 0.871288 | 3.52E–05 | 0.0262 | |
| GLUCUROCAT-PWY: superpathway of β-D-glucuronide and D-glucuronate degradation | SULT1C2 | ‒0.92527 | 2.97E–05 | 0.078414 | 9 |
| UniRef90_D4K8E3: Predicted phosphatase homologous to the C-terminal domain of histone macroH2A1 | SULT1C2 | ‒0.9083 | 7.16E–07 | 0.027384 | |
| CD8_Immune.function | ZG16 | 0.732674 | 0.000189 | 0.080761 | 10 |
| CD8_Immune.Pop | ZG16 | 0.767033 | 3.68E–05 | 0.065578 | |
| UniRef90_C7H1R7: NO_NAME | MST1L | ‒0.90309 | 1.41E–06 | 0.040723 | 11 |
| UniRef90_C7H1R7: NO_NAME | MST1P2 | ‒0.89428 | 3.41E–07 | 0.020076 | |
| s__Eubacterium_hallii | AP000350.5 | 0.91339 | 9.22E–05 | 0.05574 | 12 |
| s__Eubacterium_hallii | AP000350.6 | 0.792502 | 0.000112 | 0.062974 | |
| UniRef90_D4K1Q8: Predicted integral membrane protein | HELLPAR | 0.719472 | 1.51E–06 | 0.04237 | 13 |
| UniRef90_E2ZN70: NO_NAME | HELLPAR | 0.803967 | 4.87E–06 | 0.089006 | |
| s__Bacteroides_dorei | LL22NC03-23C6.12 | 0.61662 | 7.52E–05 | 0.049355 | 14 |
| s__Bacteroides_dorei | RP11-174O3.6 | 0.604902 | 9.1E–05 | 0.055665 | |
| UniRef90_G2SYP7: NO_NAME | RP11-745A24.3 | 0.704199 | 2.9E–06 | 0.065432 | 15 |
| UniRef90_G2T527: Filamentation induced by cAMP protein Fic | RP11-745A24.3 | 0.72507 | 3.86E–06 | 0.07883 | |
| Teff_TNFa_only | IGHV6-1 | ‒0.80968 | 2.48E–05 | 0.02777 | 16 |
| Teff_TNFa_only | REG1A | 0.610445 | 7.99E–05 | 0.062263 | |
| PWY-7219: adenosine ribonucleotides de novo biosynthesis | ITGA2 | ‒0.91969 | 2.74E–05 | 0.076724 | 17 |
| s__Dorea_formicigenerans | Teff_Immune.Pop | ‒0.91209 | 0.000234 | 0.020021 | 18 |
| Treg_IL17_IL22 | IGLV2-18 | ‒0.76005 | 0.000208 | 0.086495 | 19 |
| CD8_TNFa_IFNg | TM4SF1 | ‒0.65714 | 0.000312 | 0.098059 | 20 |
| UniRef90_R6LGS5: NO_NAME | AC131056.3 | ‒0.89989 | 2.79E–06 | 0.065021 | 21 |
| ILC3 | IGHD | 0.748352 | 7.96E–06 | 0.018936 | 22 |
| UniRef90_R5TP61: 50S ribosomal protein L30 | IGHV3-33 | ‒0.70751 | 1.16E–06 | 0.036628 | 23 |
| UniRef90_R9HTU6: NO_NAME | FTMT | 0.612826 | 1.82E–07 | 0.012321 | 24 |
| PWY-5177: glutaryl-CoA degradation | CDA | 0.816282 | 1.43E–05 | 0.049172 | 25 |
| s__Ruminococcus_lactaris | EML6 | 0.76199 | 4.06E–05 | 0.029981 | 26 |
| PWY-7328: superpathway of UDP-glucose-derived O-antigen building blocks biosynthesis | IL17F | 0.780793 | 2.95E–05 | 0.078414 | 27 |
| UniRef90_D4KCX5: Rhamnulose-1-phosphate aldolase | CD177P1 | 0.963551 | 2.32E–06 | 0.056574 | 28 |
| PWY0-1061: superpathway of L-alanine biosynthesis | CD8_IL17_only | 0.760754 | 4.74E–06 | 0.006165 | 29 |
| s__Ruminococcus_sp_5_1_39BFAA | CD19 | 0.781079 | 0.000124 | 0.066737 | 30 |
| Significant associations in psoriasis-associated network | |||||
| 1CMET2-PWY: N10-formyl-tetrahydrofolate biosynthesis | GAPDHP1 | ‒0.6841 | 9.75E–05 | 0.064441 | 1 |
| ARGININE-SYN4-PWY: L-ornithine de novo biosynthesis | GAPDHP1 | ‒0.7126 | 2.17E–05 | 0.023794 | |
| PWY-3841: folate transformations II | GAPDHP1 | ‒0.65402 | 0.00017 | 0.092741 | |
| PWY-5101: L-isoleucine biosynthesis II | GAPDHP1 | ‒0.68194 | 0.000136 | 0.080946 | |
| PWY-6353: purine nucleotides degradation II (aerobic) | GAPDHP1 | 0.663131 | 0.000167 | 0.091415 | |
| PWY-7282: 4-amino-2-methyl-5-phosphomethylpyrimidine biosynthesis (yeast) | GAPDHP1 | ‒0.69345 | 2.78E–05 | 0.027634 | |
| PWY0-845: super pathway of pyridoxal 5-phosphate biosynthesis and salvage | GAPDHP1 | ‒0.71761 | 9.73E–06 | 0.013577 | |
| PYRIDOXSYN-PWY: pyridoxal 5-phosphate biosynthesis I | GAPDHP1 | ‒0.71319 | 1.28E–05 | 0.01645 | |
| UniRef90_A7V4G2: NO_NAME | GAPDHP1 | ‒0.75316 | 5.57E–06 | 0.058996 | |
| UniRef90_Q5LC85: Elongation factor 4 | GAPDHP1 | ‒0.75949 | 1.37E–06 | 0.02837 | |
| UniRef90_Q5LES4: Sulfate adenylyltransferase subunit 2 | GAPDHP1 | ‒0.78468 | 1.2E–06 | 0.027047 | |
| ARGININE-SYN4-PWY: L-ornithine de novo biosynthesis | MIR222HG | 0.677723 | 0.000185 | 0.098183 | |
| PWY-6703: preQ0 biosynthesis | MIR222HG | 0.635198 | 0.000171 | 0.092877 | |
| PWY0-845: super pathway of pyridoxal 5-phosphate biosynthesis and salvage | MIR222HG | 0.697094 | 0.000186 | 0.098528 | |
| s__Bacteroides_uniformis | MIR222HG | 0.718291 | 8.08E–05 | 0.030159 | |
| PWY-6353: purine nucleotides degradation II (aerobic) | MTND1P23 | ‒0.73465 | 9.63E–05 | 0.06407 | |
| s__Bacteroides_uniformis | NPIPB15 | ‒0.60205 | 0.000127 | 0.043035 | |
| PWY-6703: preQ0 biosynthesis | NUAK2 | ‒0.69266 | 0.000187 | 0.098672 | |
| ARGININE-SYN4-PWY: L-ornithine de novo biosynthesis | RN7SKP299 | 0.705078 | 0.000141 | 0.082044 | |
| PWY-5101: L-isoleucine biosynthesis II | RN7SKP299 | 0.773598 | 1.22E–05 | 0.01603 | |
| ARGININE-SYN4-PWY: L-ornithine de novo biosynthesis | RP11-16E12.2 | ‒0.71602 | 0.000189 | 0.098755 | |
| UniRef90_R5EN96: NO_NAME | AP001627.1 | 0.718549 | 7.47E–06 | 0.070498 | 2 |
| UniRef90_R5ENA5: NO_NAME | AP001627.1 | 0.705786 | 5.84E–06 | 0.060871 | |
| UniRef90_R5ENA9: NO_NAME | AP001627.1 | 0.651182 | 7.75E–06 | 0.072187 | |
| UniRef90_R5ENB7: NO_NAME | AP001627.1 | 0.676065 | 4.98E–06 | 0.05694 | |
| UniRef90_R5ENC3: NO_NAME | AP001627.1 | 0.695925 | 6.58E–06 | 0.065657 | |
| UniRef90_R5END3: NO_NAME | AP001627.1 | 0.660024 | 8.62E–06 | 0.075666 | |
| UniRef90_R5ENY1: NO_NAME | AP001627.1 | 0.678813 | 5.08E–06 | 0.057196 | |
| UniRef90_R5ENZ3: NO_NAME | AP001627.1 | 0.660032 | 9.77E–06 | 0.080475 | |
| UniRef90_R5EP09: NO_NAME | AP001627.1 | 0.607497 | 9.06E–06 | 0.077542 | |
| UniRef90_R5ES58: NO_NAME | AP001627.1 | 0.712702 | 7.09E–06 | 0.068494 | |
| UniRef90_R5ES70: NO_NAME | AP001627.1 | 0.698935 | 5.74E–06 | 0.06032 | |
| UniRef90_R5ES82: NO_NAME | AP001627.1 | 0.726586 | 7.68E–06 | 0.072187 | |
| UniRef90_R5EXU5: NO_NAME | AP001627.1 | 0.665197 | 7.19E–06 | 0.069054 | |
| UniRef90_R5F071: NO_NAME | AP001627.1 | 0.690192 | 6.4E–06 | 0.064816 | |
| UniRef90_R5F076: NO_NAME | AP001627.1 | 0.759732 | 4.82E–06 | 0.056148 | |
| UniRef90_R5F1R6: NO_NAME | AP001627.1 | 0.669014 | 9.65E–06 | 0.080053 | |
| UniRef90_R5F1S4: NO_NAME | AP001627.1 | 0.698402 | 5.46E–06 | 0.058301 | |
| UniRef90_R5F1T5: NO_NAME | AP001627.1 | 0.677638 | 3.89E–06 | 0.051875 | |
| UniRef90_R5VN00: NO_NAME | AP001627.1 | 0.707391 | 5.26E–06 | 0.057636 | |
| UniRef90_C7H6T5: NO_NAME | CXCL1 | 0.607639 | 1.68E–07 | 0.006928 | 3 |
| UniRef90_C7H6T5: NO_NAME | CXCL3 | 0.62271 | 3.3E–08 | 0.002159 | |
| UniRef90_G2T376: NO_NAME | CXCL3 | 0.61847 | 9.73E–10 | 0.000185 | |
| UniRef90_G2T5J9: NO_NAME | CXCL3 | 0.607058 | 2.91E–08 | 0.001971 | |
| PWY-7196: superpathway of pyrimidine ribonucleosides salvage | Teff_IL17_IL22 | 0.703267 | 0.00045 | 0.078835 | 4 |
| PWY-7196: superpathway of pyrimidine ribonucleosides salvage | Teff_IL17_only | 0.62053 | 0.000127 | 0.064567 | |
| PPGPPMET-PWY: ppGpp biosynthesis | Treg_IL17_only | 0.607528 | 0.000525 | 0.085532 | |
| PWY-7196: superpathway of pyrimidine ribonucleosides salvage | Treg_IL17_only | 0.630174 | 0.000704 | 0.095585 | |
| COLANSYN-PWY: colanic acid building blocks biosynthesis | IGHV3-30 | ‒0.6278 | 0.000129 | 0.077505 | 5 |
| PWY-7323: super pathway of GDP-mannose-derived O-antigen building blocks biosynthesis | IGHV3-30 | ‒0.62012 | 5.2E–05 | 0.043212 | |
| COLANSYN-PWY: colanic acid building blocks biosynthesis | RP11-16E12.2 | ‒0.61959 | 0.00017 | 0.092741 | |
| ANAGLYCOLYSIS-PWY: glycolysis III (from glucose) | IGHV2-70 | ‒0.69299 | 2.94E–05 | 0.028746 | 6 |
| CALVIN-PWY: Calvin-Benson-Bassham cycle | IGHV2-70 | ‒0.75205 | 9.6E–06 | 0.01349 | |
| NONOXIPENT-PWY: pentose phosphate pathway (non-oxidative branch) | IGHV2-70 | ‒0.75603 | 1.87E–05 | 0.021434 | |
| PWY-1042: glycolysis IV (plant cytosol) | IGHV2-70 | ‒0.73312 | 2.18E–06 | 0.00494 | |
| PWY-7242: D-fructuronate degradation | IGHV2-70 | ‒0.69802 | 8.1E–05 | 0.056949 | |
| COA-PWY: coenzyme A biosynthesis I | MTND2P28 | ‒0.61331 | 1.79E–05 | 0.020776 | 7 |
| FAO-PWY: fatty acid β-oxidation I | MTND2P28 | ‒0.64143 | 2.21E–05 | 0.023794 | |
| PWY-5100: pyruvate fermentation to acetate and lactate II | MTND2P28 | ‒0.66359 | 5.47E–05 | 0.044043 | |
| PWY-5136: fatty acid β-oxidation II (peroxisome) | MTND2P28 | ‒0.61916 | 3.21E–05 | 0.030777 | |
| PWY-1269: CMP-3-deoxy-D-manno-octulosonate biosynthesis I | Vd2 | 0.679672 | 0.000179 | 0.071033 | 8 |
| PWY-1269: CMP-3-deoxy-D-manno-octulosonate biosynthesis I | Vd2T | 0.661191 | 0.000251 | 0.022565 | |
| PWY-1269: CMP-3-deoxy-D-manno-octulosonate biosynthesis I | IGHV2-26 | ‒0.63313 | 0.000189 | 0.098755 | |
| HEME-BIOSYNTHESIS-II: heme biosynthesis I (aerobic) | IGKV1-17 | ‒0.74011 | 4.13E–05 | 0.036607 | 9 |
| PWY-5918: super pathway of heme biosynthesis from glutamate | IGKV1-17 | ‒0.71627 | 0.000159 | 0.087773 | |
| s__Streptococcus_parasanguinis | KRTAP13-2 | ‒0.6127 | 1.05E–05 | 0.006086 | 10 |
| s__Streptococcus_parasanguinis | RN7SL65P | ‒0.62108 | 5.04E–06 | 0.003209 | |
| s__Dorea_formicigenerans | CD8_TNFa_IFNg | 0.651556 | 0.000876 | 0.036379 | 11 |
| UniRef90_G1WU72: NO_NAME | CD8_TNFa_IFNg | 0.641326 | 6.26E–05 | 0.083128 | |
| PWY-5384: sucrose degradation IV (sucrose phosphorylase) | AGAP12P | 0.660269 | 2.54E–05 | 0.026031 | 12 |
| PWY-5384: sucrose degradation IV (sucrose phosphorylase) | NPIPB1P | 0.648085 | 0.000185 | 0.098183 | |
| UniRef90_C9YMF0: Sigma-24 (Feci) | IL1RN | 0.810404 | 8.15E–06 | 0.074295 | 13 |
| UniRef90_D4L1Z0: DNA-directed RNA polymerase specialized sigma subunit, sigma24 homolog | IL1RN | 0.807528 | 7.07E–06 | 0.068494 | |
| PWY-3001: super pathway of L-isoleucine biosynthesis I | Treg_TNFa_IFNg | ‒0.63367 | 0.000231 | 0.071033 | 14 |
| THRESYN-PWY: super pathway of L-threonine biosynthesis | Treg_TNFa_IFNg | ‒0.72483 | 7.24E–05 | 0.050646 | |
| P461-PWY: hexitol fermentation to lactate, formate, ethanol and acetate | SIGLEC12 | 0.722109 | 7.4E–05 | 0.053292 | 15 |
| UniRef90_R6SAP9: Orotate phosphoribosyltransferase | SIGLEC12 | 0.743519 | 2.73E–06 | 0.040374 | |
| FUC-RHAMCAT-PWY: super pathway of fucose and rhamnose degradation | RP11-693J15.5 | 0.622961 | 0.000178 | 0.095618 | 16 |
| UniRef90_R5DXN6: NO_NAME | TMEM72 | ‒0.6137 | 9.9E–06 | 0.081082 | 17 |
| PWY-5695: urate biosynthesis/inosine 5-phosphate degradation | IGHV3-53 | ‒0.7541 | 0.000161 | 0.088313 | 18 |
| UniRef90_R5J0A8: Transposase IS605 OrfB family central region | SLC6A14 | 0.727059 | 9.13E–06 | 0.077542 | 19 |
| UniRef90_R9HTU6: NO_NAME | RPL6P12 | 0.644934 | 1.12E–05 | 0.087267 | 20 |
| UniRef90_D4K3E4: Desulfoferrodoxin ferrous iron-binding domain | IGLV5-45 | 0.677288 | 6.46E–06 | 0.065139 | 21 |
| s__Odoribacter_splanchnicus | PCK1 | ‒0.67833 | 4.84E–05 | 0.020322 | 22 |
| UniRef90_C7H7L8: NO_NAME | LL22NC03-23C6.12 | 0.603595 | 6.12E–09 | 0.000802 | 23 |
| Vd2T_TNFa_IFNg | KLK10 | 0.719637 | 7.31E–09 | 2.1E–05 | 24 |
| UniRef90_C4ZEM9: NO_NAME | REG1A | 0.653404 | 3.24E–07 | 0.010793 | 25 |
| UniRef90_R5J281: NO_NAME | Treg_TNFa | 0.66736 | 2.24E–07 | 0.004274 | 26 |
| UniRef90_A8SCB2: NO_NAME | GSTA1 | ‒0.64042 | 4E–06 | 0.052695 | 27 |
| Treg_INFg_only | FOS | 0.640109 | 1.57E–05 | 0.015843 | 28 |
| PWY-5345: super pathway of L-methionine biosynthesis (by sulfhydrylation) | Teff_IFNg_Only | ‒0.67739 | 0.000424 | 0.078253 | 29 |
| P185-PWY: formaldehyde assimilation III (dihydroxyacetone cycle) | IGLV4-60 | ‒0.68048 | 9.42E–05 | 0.063081 | 30 |
| UniRef90_V8CHB5: NO_NAME | DSG3 | 0.644924 | 2.65E–06 | 0.039778 | 31 |
| PWY0-1296: purine ribonucleosides degradation | ALDH1L1 | 0.623461 | 0.000178 | 0.095618 | 32 |
| HEMESYN2-PWY: heme biosynthesis II (anaerobic) | Teff_Immune.Pop | ‒0.6012 | 0.001917 | 0.065199 | 33 |
| s__Alistipes_shahii | ADAMTS4 | 0.607468 | 0.000263 | 0.071968 | 34 |
| s__Bacteroidales_bacterium_ph8 | IL22 | 0.621457 | 1.92E–07 | 0.000212 | 35 |
| Significant associations in PSO1-associated network | |||||
| activated.memory.CD8 | RP11-299L17.3 | 0.650444 | 1.13E–06 | 0.00596 | 1 |
| activated.nonMemory.CD8 | RP11-299L17.3 | 0.650444 | 1.79E–07 | 0.001895 | |
| activeCD8 | RP11-299L17.3 | 0.650444 | 2.14E–06 | 0.007538 | |
| UniRef90_D4JPG6: NO_NAME | RP11-299L17.3 | 0.65273 | 1.51E–06 | 0.090737 | |
| UniRef90_U4D938: NO_NAME | RP11-299L17.3 | 0.651584 | 1.37E–07 | 0.030625 | |
| UniRef90_D4LM01: Site-specific recombinase XerD | activated.memory.CD8 | 0.804932 | 0.000156 | 0.095101 | |
| UniRef90_D4LWR3: RNA polymerase sigma factor, sigma-70 family | activated.memory.CD8 | 0.712285 | 1.54E–05 | 0.033677 | |
| UniRef90_D4MWC2: Replication initiator protein A (RepA) N-terminus | activated.memory.CD8 | 0.642109 | 3.2E–05 | 0.047927 | |
| UniRef90_U4D938: NO_NAME | activated.memory.CD8 | 0.612961 | 3.6E–05 | 0.047962 | |
| UniRef90_D4LWR3: RNA polymerase sigma factor, sigma-70 family | activated.memory.Teff | 0.648507 | 2.11E–05 | 0.038011 | |
| UniRef90_C4Z761: Citrate synthase | activeCD8 | 0.617548 | 0.000109 | 0.08439 | |
| UniRef90_G2T5D6: Glutathione synthase | activeCD8 | 0.624565 | 3.18E–05 | 0.047927 | |
| s__Flavonifractor_plautii | activated.nonMemory.Teff | 0.666676 | 6.31E–10 | 1.56E–07 | 2 |
| s__Ruminococcus_sp_5_1_39BFAA | activated.nonMemory.Teff | 0.616463 | 0.000705 | 0.069518 | |
| UniRef90_C6JCI4: NO_NAME | activated.nonMemory.Teff | 0.645614 | 0.000177 | 0.098154 | |
| UniRef90_D4LLE0: Aldehyde dehydrogenase, molybdenum-binding subunit apoprotein | activated.nonMemory.Teff | 0.660215 | 9.81E–05 | 0.082059 | |
| UniRef90_R5HR90: NO_NAME | activated.nonMemory.Teff | 0.635567 | 6.87E–05 | 0.069169 | |
| UniRef90_R5HVG1: NO_NAME | activated.nonMemory.Teff | 0.690686 | 1.74E–05 | 0.034534 | |
| UniRef90_R5I7T7: ATP-dependent 6-phosphofructokinase | activated.nonMemory.Teff | 0.695959 | 0.000192 | 0.098154 | |
| UniRef90_R5XT20: Rhamnulose-1-phosphate aldolase | activated.nonMemory.Teff | 0.706504 | 0.000132 | 0.092377 | |
| UniRef90_R6GH13: NO_NAME | activated.nonMemory.Teff | 0.731108 | 3.57E–05 | 0.047962 | |
| UniRef90_R6PH30: NO_NAME | activated.nonMemory.Teff | 0.682456 | 1.65E–05 | 0.034534 | |
| UniRef90_R6Q745: NO_NAME | activated.nonMemory.Teff | 0.742962 | 2.36E–05 | 0.040223 | |
| UniRef90_R7CD60: NO_NAME | activated.nonMemory.Teff | 0.695959 | 8.4E–05 | 0.076379 | |
| UniRef90_R7CD72: NO_NAME | activated.nonMemory.Teff | 0.695959 | 0.000145 | 0.092467 | |
| UniRef90_R7CDD0: NO_NAME | activated.nonMemory.Teff | 0.700709 | 0.000163 | 0.096324 | |
| UniRef90_R7CGR6: NO_NAME | activated.nonMemory.Teff | 0.685965 | 0.00013 | 0.092377 | |
| UniRef90_R7CGS0: NO_NAME | activated.nonMemory.Teff | 0.73592 | 0.000135 | 0.092377 | |
| UniRef90_R7CGX0: Nicotinate phosphoribosyltransferase | activated.nonMemory.Teff | 0.64437 | 0.000179 | 0.098154 | |
| UniRef90_R7CGX6: NO_NAME | activated.nonMemory.Teff | 0.607018 | 0.00012 | 0.09182 | |
| UniRef90_R7CH88: NO_NAME | activated.nonMemory.Teff | 0.697716 | 0.000174 | 0.098154 | |
| UniRef90_R7CIC0: NO_NAME | activated.nonMemory.Teff | 0.666676 | 0.000155 | 0.095101 | |
| UniRef90_R7CIF8: NO_NAME | activated.nonMemory.Teff | 0.64386 | 0.000128 | 0.092377 | |
| UniRef90_R7CK52: NO_NAME | activated.nonMemory.Teff | 0.741653 | 0.000141 | 0.092467 | |
| UniRef90_R7CLQ2: Cyclic pyranopterin monophosphate synthase accessory protein | activated.nonMemory.Teff | 0.674929 | 9.2E–05 | 0.080527 | |
| UniRef90_R7CLX2: NO_NAME | activated.nonMemory.Teff | 0.655537 | 0.000181 | 0.098154 | |
| UniRef90_R7CPD9: NO_NAME | activated.nonMemory.Teff | 0.652022 | 3.76E–05 | 0.048 | |
| UniRef90_R7CPY8: NO_NAME | activated.nonMemory.Teff | 0.721193 | 0.000124 | 0.092377 | |
| UniRef90_R7CR35: ATP synthase | activated.nonMemory.Teff | 0.698246 | 0.000102 | 0.082059 | |
| UniRef90_R7CRE2: Acetylglutamate kinase | activated.nonMemory.Teff | 0.700709 | 0.00019 | 0.098154 | |
| UniRef90_R7CSG7: NO_NAME | activated.nonMemory.Teff | 0.675439 | 8.46E–05 | 0.076379 | |
| UniRef90_R7CSS1: NO_NAME | activated.nonMemory.Teff | 0.734623 | 0.000134 | 0.092377 | |
| UniRef90_R7DCE8: NO_NAME | activated.nonMemory.Teff | 0.601055 | 5.27E–05 | 0.058851 | |
| UniRef90_R7NEY7: Acetyltransferases | activated.nonMemory.Teff | 0.625457 | 1.66E–06 | 0.008485 | |
| UniRef90_T5LV87: IS605 OrfB family transposase | activated.nonMemory.Teff | 0.624561 | 0.000135 | 0.092377 | |
| UniRef90_T5M0M6: NO_NAME | activated.nonMemory.Teff | 0.711034 | 6.03E–05 | 0.062774 | |
| UniRef90_E1YTP4: NO_NAME | activated.nonMemory.Teff | 0.790135 | 5.17E–07 | 0.003527 | 3 |
| UniRef90_R7CII3: NO_NAME | activated.nonMemory.Teff | 0.670823 | 0.000184 | 0.098154 | |
| UniRef90_R7CQN6: TrkA-N domain-containing protein | activated.nonMemory.Teff | 0.699664 | 0.000171 | 0.098154 | |
| UniRef90_D4LQI2: Site-specific recombinases, DNA invertase Pin homologs | Teff_IL17_IL22 | 0.652583 | 2.91E–06 | 0.015436 | |
| UniRef90_C6J953: NO_NAME | Teff_IL17_only | 0.671967 | 1.62E–06 | 0.011888 | |
| UniRef90_C6JBL1: NO_NAME | Teff_IL17_only | 0.610238 | 4.71E–05 | 0.065888 | |
| UniRef90_C6JEF2: NO_NAME | Teff_IL17_only | 0.62527 | 2.19E–05 | 0.047594 | |
| UniRef90_D4LQI1: Site-specific recombinases, DNA invertase Pin homologs | Teff_IL17_only | 0.698422 | 4.94E–05 | 0.066521 | |
| UniRef90_D4LQI2: Site-specific recombinases, DNA invertase Pin homologs | Teff_IL17_only | 0.627224 | 3.5E–06 | 0.016735 | |
| UniRef90_E1YTP4: NO_NAME | Teff_IL17_only | 0.62082 | 6.42E–06 | 0.022067 | |
| UniRef90_R5XAV3: Heavy-metal-associated domain | Teff_IL17_only | 0.62566 | 9.01E–07 | 0.008609 | |
| UniRef90_R7CFY1: NO_NAME | Teff_IL17_only | 0.669696 | 2.91E–05 | 0.054164 | |
| UniRef90_R7CHB7: Na+/H+ antiporter NhaC | Teff_IL17_only | 0.811625 | 1.33E–05 | 0.035418 | |
| UniRef90_R7CII3: NO_NAME | Teff_IL17_only | 0.610643 | 0.000114 | 0.091508 | |
| UniRef90_R7CMK6: NO_NAME | Teff_IL17_only | 0.637328 | 1.29E–07 | 0.002051 | |
| UniRef90_R7CN26: NO_NAME | Teff_IL17_only | 0.666082 | 3.98E–06 | 0.017267 | |
| UniRef90_R7CQN6: TrkA-N domain-containing protein | Teff_IL17_only | 0.61839 | 0.00011 | 0.090629 | |
| UniRef90_A5ZNM4: NO_NAME | Teff_IL17_IL22 | 0.734266 | 5.1E–06 | 0.019503 | 4 |
| UniRef90_A5ZYV3: NO_NAME | Teff_IL17_IL22 | 0.706294 | 3.54E–05 | 0.06035 | |
| UniRef90_A5ZYV6: NO_NAME | Teff_IL17_IL22 | 0.686516 | 2.57E–06 | 0.015322 | |
| UniRef90_C6JC21: NO_NAME | Teff_IL17_IL22 | 0.607808 | 6.47E–06 | 0.022067 | |
| UniRef90_D4JAB3: Glyoxalase/Bleomycin resistance protein/Dioxygenase superfamily | Teff_IL17_IL22 | 0.794387 | 0.000109 | 0.090629 | |
| UniRef90_D4LKT7: 50S ribosomal protein L7/L12 | Teff_IL17_IL22 | 0.684624 | 0.000117 | 0.091508 | |
| UniRef90_D4LRE4: Transcriptional regulator, BadM/Rrf2 family | Teff_IL17_IL22 | 0.666693 | 9.86E–05 | 0.086726 | |
| UniRef90_D4LVZ1: Predicted transcriptional regulators | Teff_IL17_IL22 | 0.766035 | 8.23E–07 | 0.008609 | |
| UniRef90_D4MTV2: Transcriptional regulator, MerR family | Teff_IL17_IL22 | 0.713542 | 5.75E–05 | 0.073251 | |
| UniRef90_D4MUD3: Transcriptional regulator, MarR family | Teff_IL17_IL22 | 0.729829 | 1.16E–06 | 0.009737 | |
| UniRef90_F7JEX2: NO_NAME | Teff_IL17_IL22 | 0.711285 | 3.92E–06 | 0.017267 | |
| UniRef90_R5Z6G3: NO_NAME | Teff_IL17_IL22 | 0.678483 | 2.85E–06 | 0.015436 | |
| UniRef90_R7CCT5: NO_NAME | Teff_IL17_IL22 | 0.678393 | 0.000148 | 0.099236 | |
| UniRef90_D4MUD3: Transcriptional regulator, MarR family | Teff_IL17_only | 0.75747 | 8.66E–08 | 0.002044 | |
| s__Bacteroides_thetaiotaomicron | CD300E | 0.640982 | 2.63E–05 | 0.02332 | 5 |
| UniRef90_G2T376: NO_NAME | CXCL2 | 0.735024 | 1.52E–06 | 0.090737 | |
| s__Bacteroides_thetaiotaomicron | CXCL3 | 0.704029 | 3.68E–06 | 0.007238 | |
| UniRef90_G2T376: NO_NAME | CXCL3 | 0.699686 | 6.32E–07 | 0.045297 | |
| UniRef90_G2T5J9: NO_NAME | CXCL3 | 0.727956 | 7.33E–07 | 0.0507 | |
| UniRef90_G2T376: NO_NAME | LRFN5 | 0.657281 | 4.08E–07 | 0.040956 | |
| s__Bacteroides_thetaiotaomicron | SOCS3 | 0.704029 | 8.85E–05 | 0.050575 | |
| UniRef90_D4LVH8: KaiC | activated.memory.CD8 | 0.78459 | 0.000144 | 0.092467 | 6 |
| UniRef90_R9JXY1: 50S ribosomal protein L33 | activated.nonMemory.Teff | 0.648507 | 0.000165 | 0.096324 | |
| UniRef90_A7B829: NO_NAME | Active.Teff | 0.620145 | 0.000188 | 0.098154 | |
| UniRef90_D4LVH8: KaiC | Active.Teff | 0.697716 | 0.000175 | 0.098154 | |
| UniRef90_G2T5D0: NO_NAME | Active.Teff | 0.651408 | 0.000155 | 0.095101 | |
| UniRef90_K1S8B5: Transcriptional regulator | Active.Teff | 0.739437 | 0.000158 | 0.09531 | |
| UniRef90_R9JXY1: 50S ribosomal protein L33 | Active.Teff | 0.669014 | 0.00014 | 0.092467 | |
| UniRef90_U2PAU4: NO_NAME | Active.Teff | 0.870181 | 5.14E–07 | 0.003527 | |
| s__Barnesiella_intestinihominis | LL22NC03-23C6.12 | 0.651584 | 9.5E–05 | 0.052803 | 7 |
| UniRef90_C7H7L9: ABC transporter, ATP-binding protein | LL22NC03-23C6.12 | 0.65273 | 2.34E–07 | 0.036053 | |
| s__Barnesiella_intestinihominis | SERPINA9 | 0.747806 | 3.18E–05 | 0.026686 | |
| s__Eubacterium_hallii | GSTA1 | 0.72028 | 0.000187 | 0.078443 | 8 |
| UniRef90_E2ZGA1: NO_NAME | GSTA1 | ‒0.82312 | 1.06E–06 | 0.071004 | |
| s__Eubacterium_ventriosum | Vd2T_TNFa_only | 0.797189 | 5.33E–05 | 0.006695 | 9 |
| UniRef90_B0NZF6: NO_NAME | Vd2T_TNFa_only | 0.64675 | 0.000105 | 0.090542 | |
| UniRef90_D4M5F3: dTDP-glucose pyrophosphorylase | memory.Teff | ‒0.88422 | 0.000101 | 0.082059 | 10 |
| UniRef90_D4M5F3: dTDP-glucose pyrophosphorylase | nonMemory.Teff | 0.880707 | 5.17E–05 | 0.058792 | |
| DZ_duration | CD8_IL17_IL22 | 0.654946 | 0.000478 | 0.040147 | 11 |
| PWY-5104: L-isoleucine biosynthesis IV | DZ_duration | 0.736492 | 0.000281 | 0.068952 | |
| UniRef90_B0G2Q7: NO_NAME | Treg_IL17_IL22 | 0.776803 | 7.01E–05 | 0.077728 | 12 |
| UniRef90_D4J5V6: Site-specific recombinase XerD | Treg_IL17_IL22 | 0.693832 | 9.86E–05 | 0.086726 | |
| UniRef90_A5ZSX2: NO_NAME | CD8_TNFa_IFNg | 0.907182 | 7.34E–05 | 0.077728 | 13 |
| UniRef90_D4JAA1: Phage integrase family | CD8_TNFa_IFNg | 0.795496 | 6.81E–05 | 0.077728 | |
| s__Parabacteroides_distasonis | IL17A | 0.649429 | 0.000219 | 0.085842 | 14 |
| s__Parabacteroides_distasonis | LINC00939 | 0.751742 | 2.07E–05 | 0.020128 | |
| s__Bacteroides_dorei | FOSB | 0.605649 | 4.51E–05 | 0.033931 | 15 |
| s__Bacteroides_dorei | Treg_IL22_only | 0.643114 | 3.18E–05 | 0.004358 | |
| Treg_INFg_only | RP11-301M17.1 | 0.650444 | 2.22E–06 | 0.009133 | 16 |
| UniRef90_G2SYP7: NO_NAME | Teff_TNFa_only | 0.704243 | 5.65E–06 | 0.020765 | 17 |
| UniRef90_C4Z763: GTP pyrophosphokinase | Teff_TNFa_INFg | 0.804196 | 0.000117 | 0.091508 | 18 |
| PWY0-1297: superpathway of purine deoxyribonucleosides degradation | PASI | 0.713137 | 1.85E–05 | 0.018036 | 19 |
| UniRef90_C0FPA9: BAAT/acyl-CoA thioester hydrolase C-terminal domain protein | nonMemory.CD8 | ‒0.87834 | 0.000123 | 0.092377 | 20 |
| s__Alistipes_putredinis | CD55 | 0.881119 | 0.000117 | 0.059771 | 21 |
| s__Dorea_formicigenerans | TRHDE | 0.895105 | 4.16E–05 | 0.032046 | 22 |
| s__Coprococcus_comes | CCDC85A | ‒0.63398 | 0.000222 | 0.086043 | 23 |
| s__Lachnospiraceae_bacterium_3_1_46FAA | UCA1 | 0.609458 | 0.000162 | 0.072164 | 24 |
| THRESYN-PWY: super pathway of L-threonine biosynthesis | Treg_TNFa_IFNg | ‒0.85365 | 7.05E–06 | 0.016049 | 25 |
| UniRef90_C7H2P0: NO_NAME | CD8_TNFa_only | 0.704029 | 8.19E–05 | 0.079819 | 26 |
| s__Roseburia_inulinivorans | CD177 | ‒0.83916 | 1.39E–06 | 0.003509 | 27 |
| PYRIDNUCSAL-PWY: NAD salvage pathway I | URAD | 0.882718 | 1.02E–07 | 0.005333 | 28 |
| Significant associations in PSO2-associated network | |||||
| BIOTIN-BIOSYNTHESIS-PWY: biotin biosynthesis I | CYP2B7P | 0.833333 | 2.98E–05 | 0.009035 | 1 |
| FASYN-ELONG-PWY: fatty acid elongation -- saturated | CYP2B7P | 0.833333 | 1.4E–05 | 0.005053 | |
| FASYN-INITIAL-PWY: super pathway of fatty acid biosynthesis initiation (E. coli) | CYP2B7P | 0.826362 | 2.64E–05 | 0.008035 | |
| GLUDEG-I-PWY: GABA shunt | CYP2B7P | 0.714286 | 0.000278 | 0.074586 | |
| PWY-5022: 4-aminobutanoate degradation V | CYP2B7P | 0.714286 | 0.000334 | 0.087365 | |
| PWY-5971: palmitate biosynthesis II (bacteria and plants) | CYP2B7P | 0.833333 | 2.8E–05 | 0.008503 | |
| PWY-6113: superpathway of mycolate biosynthesis | CYP2B7P | 0.833333 | 4.57E–05 | 0.013734 | |
| PWY-6282: palmitoleate biosynthesis I (from (5Z)-dodec-5-enoate) | CYP2B7P | 0.833333 | 7.21E–05 | 0.021414 | |
| PWY-6284: super pathway of unsaturated fatty acids biosynthesis (E. coli) | CYP2B7P | 0.833333 | 4.07E–05 | 0.012269 | |
| PWY-6519: 8-amino-7-oxononanoate biosynthesis I | CYP2B7P | 0.833333 | 3.09E–05 | 0.009339 | |
| PWY-7388: octanoyl-[acyl-carrier protein] biosynthesis (mitochondria, yeast) | CYP2B7P | 0.833333 | 1.83E–05 | 0.006357 | |
| PWY-7664: oleate biosynthesis IV (anaerobic) | CYP2B7P | 0.833333 | 1.18E–05 | 0.004438 | |
| PWY0-862: (5Z)-dodec-5-enoate biosynthesis | CYP2B7P | 0.833333 | 2.54E–05 | 0.007747 | |
| PWYG-321: mycolate biosynthesis | CYP2B7P | 0.833333 | 1.31E–05 | 0.004806 | |
| BIOTIN-BIOSYNTHESIS-PWY: biotin biosynthesis I | STC1 | –0.7619 | 0.00013 | 0.03689 | |
| FASYN-ELONG-PWY: fatty acid elongation -- saturated | STC1 | –0.7619 | 0.000173 | 0.047907 | |
| FASYN-INITIAL-PWY: super pathway of fatty acid biosynthesis initiation (E. coli) | STC1 | –0.7545 | 0.000271 | 0.073002 | |
| PWY-5971: palmitate biosynthesis II (bacteria and plants) | STC1 | ‒0.7619 | 0.000209 | 0.05735 | |
| PWY-6113: super pathway of mycolate biosynthesis | STC1 | ‒0.7619 | 0.000287 | 0.076805 | |
| PWY-6282: palmitoleate biosynthesis I (from (5Z)-dodec-5-enoate) | STC1 | ‒0.7619 | 0.00028 | 0.075069 | |
| PWY-6284: super pathway of unsaturated fatty acids biosynthesis (E. coli) | STC1 | ‒0.7619 | 0.000347 | 0.090509 | |
| PWY-6519: 8-amino-7-oxononanoate biosynthesis I | STC1 | ‒0.7619 | 0.0002 | 0.055236 | |
| PWY-7388: octanoyl-[acyl-carrier protein] biosynthesis (mitochondria, yeast) | STC1 | ‒0.7619 | 0.00016 | 0.044499 | |
| PWY-7664: oleate biosynthesis IV (anaerobic) | STC1 | ‒0.7619 | 0.000235 | 0.063921 | |
| PWY0-862: (5Z)-dodec-5-enoate biosynthesis | STC1 | ‒0.7619 | 0.000229 | 0.062421 | |
| PWYG-321: mycolate biosynthesis | STC1 | ‒0.7619 | 0.00012 | 0.0343 | |
| s__Bacteroides_ovatus | Teff_TNFa_only | 0.666667 | 0.000994 | 0.091836 | 2 |
| s__Bacteroides_ovatus | Vd2T_IL17_only | 0.761905 | 0.00021 | 0.043201 | |
| UniRef90_C0FN95: NO_NAME | Vd2T_IL17_only | 0.880952 | 5.48E–07 | 0.026184 | |
| UniRef90_D6E3G6: Site-specific recombinase XerD | Vd2T_IL17_only | 0.90368 | 1.25E–07 | 0.011927 | |
| ANAEROFRUCAT-PWY: homolactic fermentation | FOS | ‒0.97619 | 0.000119 | 0.034027 | 3 |
| GLYCOLYSIS: glycolysis I (from glucose 6-phosphate) | FOS | ‒0.95238 | 8.01E–05 | 0.023227 | |
| PWY-5484: glycolysis II (from fructose 6-phosphate) | FOS | ‒0.95238 | 5.05E–05 | 0.015132 | |
| ANAEROFRUCAT-PWY: homolactic fermentation | PDE4C | ‒0.90476 | 9.72E–05 | 0.028114 | |
| ARGSYN-PWY: L-arginine biosynthesis I (via L-ornithine) | PASI | 0.754505 | 0.001298 | 0.055236 | 4 |
| PENTOSE-P-PWY: pentose phosphate pathway | PASI | 0.833333 | 0.002607 | 0.055236 | |
| PWY-5154: L-arginine biosynthesis III (via N-acetyl-L-citrulline) | PASI | 0.785714 | 0.001279 | 0.055236 | |
| PWY-7400: L-arginine biosynthesis IV (archaebacteria) | PASI | 0.754505 | 0.001234 | 0.055236 | |
| ARGSYN-PWY: L-arginine biosynthesis I (via L-ornithine) | XIST | ‒0.64672 | 7.14E–06 | 0.00271 | |
| ARGSYNBSUB-PWY: L-arginine biosynthesis II (acetyl cycle) | XIST | ‒0.92857 | 4.15E–06 | 0.001618 | |
| GLUTORN-PWY: L-ornithine biosynthesis | XIST | ‒0.6627 | 2.01E–07 | 9.8E–05 | |
| PWY-5154: L-arginine biosynthesis III (via N-acetyl-L-citrulline) | XIST | ‒0.63474 | 1.07E–05 | 0.004031 | |
| PWY-7400: L-arginine biosynthesis IV (archaebacteria) | XIST | ‒0.62655 | 1.07E–05 | 0.004046 | |
| PWY-6121: 5-aminoimidazole ribonucleotide biosynthesis I | BMX | ‒0.95238 | 0.000205 | 0.056554 | 5 |
| PWY-6122: 5-aminoimidazole ribonucleotide biosynthesis II | BMX | ‒0.97619 | 0.000225 | 0.061454 | |
| PWY-6277: super pathway of 5-aminoimidazole ribonucleotide biosynthesis | BMX | ‒0.97619 | 0.000225 | 0.061454 | |
| PWY-6121: 5-aminoimidazole ribonucleotide biosynthesis I | CCDC129 | ‒0.88095 | 0.000105 | 0.030152 | |
| PWY-6122: 5-aminoimidazole ribonucleotide biosynthesis II | CCDC129 | ‒0.90476 | 0.000107 | 0.030556 | |
| PWY-6277: superpathway of 5-aminoimidazole ribonucleotide biosynthesis | CCDC129 | ‒0.90476 | 0.000107 | 0.030556 | |
| PWY-6121: 5-aminoimidazole ribonucleotide biosynthesis I | SH2D6 | ‒0.97619 | 2.49E–05 | 0.007595 | |
| PWY-6122: 5-aminoimidazole ribonucleotide biosynthesis II | SH2D6 | ‒0.95238 | 7.94E–05 | 0.023078 | |
| PWY-6277: superpathway of 5-aminoimidazole ribonucleotide biosynthesis | SH2D6 | ‒0.95238 | 7.94E–05 | 0.023078 | |
| PWY-6121: 5-aminoimidazole ribonucleotide biosynthesis I | SH2D7 | ‒0.95238 | 0.000325 | 0.085378 | |
| PWY-6122: 5-aminoimidazole ribonucleotide biosynthesis II | SH2D7 | ‒0.97619 | 0.000326 | 0.08554 | |
| PWY-6277: super pathway of 5-aminoimidazole ribonucleotide biosynthesis | SH2D7 | ‒0.97619 | 0.000326 | 0.08554 | |
| P461-PWY: hexitol fermentation to lactate, formate, ethanol and acetate | MT1X | ‒0.85714 | 7.8E–05 | 0.022737 | 6 |
| P461-PWY: hexitol fermentation to lactate, formate, ethanol, and acetate | MYBL1 | ‒0.90476 | 0.000309 | 0.081754 | |
| P461-PWY: hexitol fermentation to lactate, formate, ethanol, and acetate | NTRK2 | ‒0.95238 | 0.000321 | 0.084551 | |
| s__Clostridium_bartlettii | IL17F | 0.76835 | 0.000202 | 0.059482 | 7 |
| s__Veillonella_unclassified | IL17F | 0.76835 | 2.91E–05 | 0.011329 | |
| s__Clostridium_bartlettii | RN7SL301P | 0.76835 | 0.00012 | 0.038644 | |
| s__Veillonella_unclassified | RN7SL301P | 0.76835 | 7.23E–05 | 0.025955 | |
| s__Eggerthella_lenta | Vd2 | 0.89822 | 0.000353 | 0.054394 | 8 |
| s__Flavonifractor_plautii | Vd2 | 0.785714 | 0.000613 | 0.072663 | |
| s__Eggerthella_lenta | RP11-707O23.5 | 0.76835 | 1.96E–06 | 0.001075 | |
| s__Flavonifractor_plautii | RP11-707O23.5 | 0.763763 | 0.000134 | 0.04259 | |
| s__Eubacterium_siraeum | Vd2T_IL22_only | 0.814386 | 0.000264 | 0.048712 | 9 |
| s__Eubacterium_siraeum | IL17C | 0.76835 | 7.73E–06 | 0.003364 | |
| s__Subdoligranulum_unclassified | IRX2 | 0.754505 | 0.000291 | 0.083959 | 10 |
| s__Subdoligranulum_unclassified | PAX8-AS1 | ‒0.83333 | 0.000316 | 0.087851 | |
| s__Roseburia_hominis | C5orf17 | 0.904762 | 0.000139 | 0.043995 | 11 |
| s__Roseburia_hominis | RP11-726G1.1 | 0.904762 | 0.000122 | 0.038958 | |
| GALACTUROCAT-PWY: D-galacturonate degradation I | GHR | 0.928571 | 0.000275 | 0.073927 | 12 |
| PWY-6507: 4-deoxy-L-threo-hex-4-enopyranuronate degradation | GHR | 0.97619 | 5.83E–05 | 0.017377 | |
| CITRULBIO-PWY: L-citrulline biosynthesis | PCK1 | 0.904762 | 0.00033 | 0.086443 | 13 |
| PWY-4984: urea cycle | PCK1 | 0.904762 | 0.000355 | 0.092057 | |
| PWY-6305: putrescine biosynthesis IV | CXCL8 | 0.97619 | 0.000123 | 0.034844 | 14 |
| PWY-6305: putrescine biosynthesis IV | IGHV4-31 | 0.928571 | 0.000159 | 0.044272 | |
| FAO-PWY: fatty acid β-oxidation I | IGKV1D-16 | 0.946125 | 0.000305 | 0.080971 | 15 |
| PWY-5136: fatty acid β-oxidation II (peroxisome) | IGKV1D-16 | 0.952381 | 7.44E–05 | 0.022051 | |
| P42-PWY: incomplete reductive TCA cycle | IGLV7-46 | ‒0.97619 | 0.000353 | 0.091828 | 16 |
| PWY-5104: L-isoleucine biosynthesis IV | IGLV7-46 | ‒0.95238 | 0.000101 | 0.029049 | |
| s__Lachnospiraceae_bacterium_1_4_56FAA | CD8_IL22_only | 0.809524 | 0.000137 | 0.036152 | 17 |
| s__Lachnospiraceae_bacterium_1_4_56FAA | Teff_TNFa_INFg | 0.718576 | 6.25E–05 | 0.020997 | |
| PWY-5189: tetrapyrrole biosynthesis II (from glycine) | CA1 | ‒0.78571 | 0.000311 | 0.082075 | 18 |
| PWY-5189: tetrapyrrole biosynthesis II (from glycine) | CD274 | 0.928571 | 0.000299 | 0.079439 | |
| FOLSYN-PWY: super pathway of tetrahydrofolate biosynthesis and salvage | TMEM37 | 0.934148 | 3.07E–06 | 0.001317 | 19 |
| PWY-6612: superpathway of tetrahydrofolate biosynthesis | TMEM37 | 0.904762 | 4.47E–06 | 0.001739 | |
| Treg_IL17_only | FCRL1 | ‒0.97619 | 6.99E–05 | 0.041248 | 20 |
| Treg_IL17_only | FCRLA | ‒1 | 0.000158 | 0.087172 | |
| PWY-6969: TCA cycle V (2-oxoglutarate:ferredoxin oxidoreductase) | REG1A | ‒0.76376 | 0.000365 | 0.094544 | 21 |
| s__Bacteroides_xylanisolvens | REG1A | 0.736989 | 0.000162 | 0.048778 | |
| GLCMANNANAUT-PWY: super pathway of N-acetylglucosamine, N-acetylmannosamine, and N-acetylneuraminate degradation | C11orf86 | ‒0.83333 | 5.42E–06 | 0.002104 | 22 |
| GLUCONEO-PWY: gluconeogenesis I | IGLV3-10 | ‒0.7619 | 0.000271 | 0.073002 | 23 |
| s__Ruminococcus_bromii | IGHM | 0.904762 | 1.28E–05 | 0.005526 | 24 |
| PWY-621: sucrose degradation III (sucrose invertase) | CLCA4 | ‒0.97619 | 1.29E–05 | 0.00476 | 25 |
| PWY-2941: L-lysine biosynthesis II | NLGN4Y | 0.912328 | 0.000273 | 0.073524 | 26 |
| PHOSLIPSYN-PWY: super pathway of phospholipid biosynthesis I (bacteria) | IGLV5-37 | ‒0.90476 | 0.000222 | 0.060934 | 27 |
| UniRef90_B0GA46: NO_NAME | CD8_TNFa_IFNg | 0.8485 | 2.77E–06 | 0.088269 | 28 |
| TRNA-CHARGING-PWY: tRNA charging | CHIT1 | ‒0.92857 | 1.07E–05 | 0.004046 | 29 |
| RHAMCAT-PWY: L-rhamnose degradation I | DZ_duration | 0.886243 | 0.00443 | 0.078996 | 30 |
| s__Clostridium_bolteae | Active.Teff | ‒0.87427 | 0.000195 | 0.092483 | 31 |
| PWY0-1297: superpathway of purine deoxyribonucleosides degradation | MYEOV | 0.904762 | 3.78E–05 | 0.011407 | 32 |
| NONOXIPENT-PWY: pentose phosphate pathway (non-oxidative branch) | CLIC6 | 0.904762 | 0.000182 | 0.050256 | 33 |
| PWY-3001: super pathway of L-isoleucine biosynthesis I | CIDEC | ‒0.88095 | 0.000207 | 0.056999 | 34 |
| s__Bilophila_wadsworthia | NLRP2 | ‒0.95238 | 4.72E–05 | 0.018207 | 35 |
| PWY-5005: biotin biosynthesis II | KRT8P36 | 0.922172 | 0.000142 | 0.040129 | 36 |
| PWY0-1061: superpathway of L-alanine biosynthesis | RP11-575F12.2 | 0.970077 | 0.000254 | 0.068804 | 37 |
| PWY0-1296: purine ribonucleosides degradation | SULT1C2 | ‒0.97619 | 0.000106 | 0.030556 | 38 |
| MET-SAM-PWY: super pathway of S-adenosyl-L-methionine biosynthesis | FRMD1 | ‒0.80952 | 0.000175 | 0.048401 | 39 |
| LACTOSECAT-PWY: lactose and galactose degradation I | IGHV1-3 | 0.952381 | 0.000237 | 0.064542 | 40 |
| s__Bilophila_unclassified | RPS6KA2-AS1 | ‒1 | 1.82E–05 | 0.007734 | 41 |
| PWY-7383: anaerobic energy metabolism (invertebrates, cytosol) | IGLV4-60 | ‒0.92857 | 0.000302 | 0.080129 | 42 |
| HEME-BIOSYNTHESIS-II: heme biosynthesis I (aerobic) | RP11-958N24.2 | 0.788009 | 0.000292 | 0.077896 | 43 |
| PWY-7234: inosine-5-phosphate biosynthesis III | HERC2P4 | ‒0.92857 | 0.000167 | 0.0464 | 44 |
| PWY0-162: super pathway of pyrimidine ribonucleotides de novo biosynthesis | ARL14 | ‒0.97008 | 6.97E–05 | 0.020733 | 45 |
| PWY-7229: superpathway of adenosine nucleotides de novo biosynthesis I | CTAGE15 | 0.857143 | 0.000298 | 0.079314 | 46 |
| Vd2T_IFNg_only | IGLC7 | 0.880952 | 0.000167 | 0.08878 | 47 |
| PWY-6897: thiamin salvage II | MME-AS1 | 0.76835 | 0.000346 | 0.09024 | 48 |
| PWY-5030: L-histidine degradation III | AC131056.3 | 0.952381 | 0.000379 | 0.097971 | 49 |
| s__Eubacterium_eligens | RP11-259G18.3 | ‒0.95181 | 6.08E–05 | 0.022759 | 50 |
| Treg_IL17_IL22 | IGKV1D-43 | 0.730552 | 6.75E–05 | 0.041248 | 51 |
| s__Lachnospiraceae_bacterium_7_1_58FAA | ZBTB16 | 0.928571 | 0.000347 | 0.095663 | 52 |
| s__Coprococcus_catus | THNSL2 | 1 | 5.97E–05 | 0.02245 | 53 |
| s__Erysipelotrichaceae_bacterium_6_1_45 | PTGS2 | ‒0.83333 | 0.000359 | 0.098475 | 54 |
| s__Bifidobacterium_longum | Vd2T_TNFa_only | ‒0.91573 | 0.000601 | 0.072663 | 55 |
| List of significant associations in PSO3 associated network | |||||
| P122-PWY: heterolactic fermentation | EDDM3DP | 0.664211 | 1.49E–05 | 0.022213 | 1 |
| P122-PWY: heterolactic fermentation | RP11-100G15.10 | 0.664211 | 1.49E–05 | 0.022213 | |
| PWY-5083: NAD/NADH phosphorylation and dephosphorylation | EDDM3DP | 0.695608 | 4.42E–07 | 0.001001 | |
| PWY-5083: NAD/NADH phosphorylation and dephosphorylation | RP11-100G15.10 | 0.695608 | 4.42E–07 | 0.001001 | |
| PWY-821: super pathway of sulfur amino acid biosynthesis (Saccharomyces cerevisiae) | RP11-100G15.10 | 0.695608 | 7.39E–05 | 0.070103 | |
| PWY-821: super pathway of sulfur amino acid biosynthesis (Saccharomyces cerevisiae) | EDDM3DP | 0.695608 | 7.39E–05 | 0.070103 | |
| PWY0-1061: superpathway of L-alanine biosynthesis | RP11-100G15.10 | 0.654654 | 9.11E–05 | 0.081919 | |
| PWY0-1061: superpathway of L-alanine biosynthesis | EDDM3DP | 0.654654 | 9.11E–05 | 0.081919 | |
| s__Bacteroides_xylanisolvens | EDDM3DP | 0.654654 | 0.000129 | 0.063831 | |
| s__Bacteroides_xylanisolvens | RP11-100G15.10 | 0.654654 | 0.000129 | 0.063831 | |
| s__Haemophilus_parainfluenzae | EDDM3DP | 0.654654 | 3.59E–07 | 0.000407 | |
| s__Haemophilus_parainfluenzae | RP11-100G15.10 | 0.654654 | 3.59E–07 | 0.000407 | |
| PWY-5188: tetrapyrrole biosynthesis I (from glutamate) | MYBL1 | ‒1 | 1.56E–05 | 0.022439 | 2 |
| PWY-5188: tetrapyrrole biosynthesis I (from glutamate) | PAX5 | ‒1 | 4.29E–05 | 0.046051 | |
| PWY-5188: tetrapyrrole biosynthesis I (from glutamate) | AFF2 | ‒1 | 1.32E–05 | 0.020625 | |
| PWY-5188: tetrapyrrole biosynthesis I (from glutamate) | TCL1A | ‒0.88571 | 1.28E–05 | 0.020213 | |
| PWY-5188: tetrapyrrole biosynthesis I (from glutamate) | CD79B | ‒0.88571 | 3.53E–05 | 0.040084 | |
| PWY-5345: superpathway of L-methionine biosynthesis (by sulfhydrylation) | PWP2 | ‒0.94286 | 1.25E–05 | 0.019878 | 3 |
| PWY-5345: super pathway of L-methionine biosynthesis (by sulfhydrylation) | IGKV1-9 | ‒0.94286 | 8.57E–05 | 0.078976 | |
| SULFATE-CYS-PWY: super pathway of sulfate assimilation and cysteine biosynthesis | IGKV1-9 | ‒0.89865 | 8.22E–05 | 0.076628 | |
| COBALSYN-PWY: adenosylcobalamin salvage from cobinamide I | ATP13A5 | ‒1 | 8.76E–05 | 0.079978 | 4 |
| DTDPRHAMSYN-PWY: dTDP-L-rhamnose biosynthesis I | ATP13A5 | ‒1 | 3.25E–05 | 0.037635 | |
| DTDPRHAMSYN-PWY: dTDP-L-rhamnose biosynthesis I | RGS13 | ‒0.94286 | 6.46E–05 | 0.063204 | |
| s__Roseburia_intestinalis | IL36RN | 0.845154 | 7.21E–05 | 0.042737 | 5 |
| s__Roseburia_intestinalis | SLC44A5 | 0.771429 | 0.000123 | 0.061427 | |
| s__Roseburia_intestinalis | RP11-313J2.1 | ‒0.82857 | 6.98E–05 | 0.041714 | |
| s__Lachnospiraceae_bacterium_7_1_58FAA | RP11-462B18.1 | 0.654654 | 0.000174 | 0.083495 | 6 |
| s__Lachnospiraceae_bacterium_7_1_58FAA | TM4SF4 | 0.885714 | 3.85E–05 | 0.024936 | |
| s__Lachnospiraceae_bacterium_7_1_58FAA | IGHV3-66 | ‒1 | 6.57E–06 | 0.005182 | |
| GOLPDLCAT-PWY: superpathway of glycerol degradation to 1,3-propanediol | NARF | ‒1 | 0.000113 | 0.097624 | 7 |
| GOLPDLCAT-PWY: superpathway of glycerol degradation to 1,3-propanediol | PNLIPRP2 | ‒1 | 4.67E–05 | 0.04818 | |
| MET-SAM-PWY: superpathway of S-adenosyl-L-methionine biosynthesis | TMEM150B (DRAM-3 regulate autophagy) | 1 | 3.53E–05 | 0.040084 | 8 |
| PWY-5347: superpathway of L-methionine biosynthesis (transsulfuration) | TMEM150B | 1 | 8.06E–05 | 0.075717 | |
| FAO-PWY: fatty acid β-oxidation I | TEKT4P2 | ‒1 | 2.74E–05 | 0.035602 | 9 |
| PWY-5136: fatty acid β-oxidation II (peroxisome) | TEKT4P2 | ‒1 | 3.71E–05 | 0.040923 | |
| UniRef90_A7B4W0: NO_NAME | AQP5 | 0.970588 | 4.31E–07 | 0.015343 | 10 |
| UniRef90_D6DY27: ABC-type antimicrobial peptide transport system, ATPase component | AQP5 | 0.955882 | 1.67E–06 | 0.057497 | |
| UniRef90_C6JCY5: NO_NAME | IGLV4-60 | ‒0.98561 | 2.08E–06 | 0.070312 | 11 |
| NAD-BIOSYNTHESIS-II: NAD salvage pathway II | NLGN4Y | 0.941124 | 7.1E–05 | 0.068116 | 12 |
| CALVIN-PWY: Calvin-Benson-Bassham cycle | HBEGF | 1 | 7.11E–05 | 0.068116 | 13 |
| GLUCONEO-PWY: gluconeogenesis I | RP11-575F12.3 | 1 | 7.47E–06 | 0.013124 | 14 |
| PWY-5097: L-lysine biosynthesis VI | RP11-30P6.6 | ‒1 | 4.54E–05 | 0.047844 | 15 |
| PYRIDNUCSYN-PWY: NAD biosynthesis I (from aspartate) | RP11-747H7.3 | 1 | 5.69E–05 | 0.055963 | 16 |
| UniRef90_G1WWG9: NO_NAME | CD55 | 1 | 2.77E–06 | 0.090568 | 17 |
| PWY-5005: biotin biosynthesis II | TDRD1 | 0.941124 | 8.24E–05 | 0.076628 | 18 |
| OANTIGEN-PWY: O-antigen building blocks biosynthesis (E. coli) | ISG15 | 1 | 4.61E–05 | 0.048107 | 19 |
| UniRef90_D4L1Y1: Site-specific recombinases, DNA invertase Pin homologs | SERPINA9 | ‒1 | 1.8E–08 | 0.000665 | 20 |
| PWY-2941: L-lysine biosynthesis II | PI15 | 0.828571 | 4.83E–05 | 0.049266 | 21 |
| PWY4LZ-257: superpathway of fermentation (Chlamydomonas reinhardtii) | IGLC7 | ‒0.94286 | 8.84E–05 | 0.080307 | 22 |
| UniRef90_D4KBZ1: Ribonuclease H | HRCT1 | ‒1 | 2.95E–06 | 0.094916 | 23 |
| UniRef90_C7H3L3: NO_NAME | FOSB | ‒0.95618 | 1.65E–06 | 0.057497 | 24 |
| CRNFORCAT-PWY: creatinine degradation I | VWA5B1 | 1 | 5.56E–06 | 0.010772 | 25 |
| s__Anaerostipes_hadrus | RP11-424G14.1 | 0.845154 | 2.53E–07 | 0.000295 | 26 |
Abbreviations: adj, adjusted; Cor, correlation value.
Supplementary Table S12.
Available Clinical and Experimental Data
| PID | Status | Gender | Age | PASI | PSO onset | Disease Duration |
Metagenomics Data (Stool) |
Host Transcriptomic Data (Sigmoid) |
Flow Cytometry Data (PBMCs) |
PSO Subgroups Identified |
|---|---|---|---|---|---|---|---|---|---|---|
| 6532 | Healthy | M | 61 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7300 | PSO | F | 58 | 5.9 | 18 | 37 | Yes | Yes | Yes | PSO2 |
| 7301 | PSO | M | 37 | 27.8 | 24 | 10 | Yes | Yes | Yes | PSO2 |
| 7303 | PSO | F | 35 | NA | 18 | 14 | Yes | Yes | Yes | PSO2 |
| 7304 | PSO | F | 41 | 1.2 | 19 | 19 | Yes | Yes | Yes | PSO2 |
| 7305 | PSO | M | 58 | 19.4 | 33 | 22 | Yes | Yes | Yes | PSO1 |
| 7306 | PSO | M | 70 | 2.1 | 20 | 47 | Yes | Yes | Yes | PSO1 |
| 7307 | PSO | M | 58 | 15.6 | 50 | 5 | Yes | Yes | Yes | PSO3 |
| 7308 | PSO | F | 60 | 5.2 | 48 | 10 | Yes | Yes | Yes | PSO1 |
| 7309 | PSO | F | 29 | 0.2 | 10 | 16 | Yes | Yes | Yes | PSO3 |
| 7310 | PSO | M | 54 | 7.7 | 44 | 10 | Yes | Yes | Yes | PSO1 |
| 7311 | PSO | M | 58 | 5.6 | 20 | 47 | Yes | Yes | Yes | PSO2 |
| 7312 | PSO | M | 39 | 4.3 | 10 | 26 | Yes | Yes | Yes | PSO1 |
| 7313 | PSO | F | 76 | 7.5 | 60 | 14 | Yes | Yes | Yes | PSO2 |
| 7314 | PSO | M | 29 | 4 | 7 | 19 | Yes | Yes | Yes | PSO3 |
| 7315 | PSO | M | 55 | 3.9 | 42 | 10 | Yes | Yes | Yes | PSO1 |
| 7317 | PSO | F | 28 | 2.6 | 10 | 15 | Yes | Yes | Yes | PSO1 |
| 7320 | PSO | F | 60 | 13.2 | 29 | 32 | Yes | Yes | Yes | PSO3 |
| 7322 | PSO | F | 42 | 3.4 | 10 | 32 | Yes | Yes | Yes | PSO1 |
| 7323 | PSO | F | 53 | 31.1 | 22 | 31 | Yes | Yes | Yes | PSO3 |
| 7324 | PSO | M | 57 | 7.8 | 55 | 2 | Yes | Yes | Yes | PSO1 |
| 7325 | PSO | F | 22 | 27.8 | 15 | 7 | Yes | Yes | Yes | PSO1 |
| 7327 | PSO | F | 29 | 11.3 | 6 | 23 | Yes | Yes | Yes | PSO2 |
| 7328 | PSO | F | 28 | 13.2 | 8 | 20 | Yes | Yes | Yes | PSO2 |
| 7330 | PSO | F | 28 | 16.1 | 16 | 12 | Yes | Yes | Yes | PSO1 |
| 7331 | PSO | F | 29 | 18.7 | 12 | 17 | Yes | Yes | Yes | PSO3 |
| 7332 | PSO | M | 28 | 19.8 | 24 | 4 | Yes | Yes | Yes | PSO1 |
| 7352 | Healthy | F | 60 | NA | NA | NA | Yes | No | No | Healthy |
| 7353 | Healthy | M | 25 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7355 | Healthy | F | 23 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7358 | Healthy | F | 69 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7360 | Healthy | F | 38 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7364 | Healthy | M | 53 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7365 | Healthy | F | 57 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7368 | Healthy | F | 49 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7369 | Healthy | M | 38 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7371 | Healthy | M | 47 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7372 | Healthy | M | 59 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7374 | Healthy | M | 35 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7375 | Healthy | M | 36 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| 7376 | Healthy | F | 37 | NA | NA | NA | Yes | Yes | Yes | Healthy |
| N1 | PSO | M | 52 | 23.9 | 27 | 25 | Yes | No | No | PSO3 |
| N11 | PSO | F | 31 | 31.9 | 5 | 16 | Yes | No | No | PSO3 |
| N2 | PSO | M | 49 | 8.8 | 26 | 23 | Yes | No | No | PSO1 |
| N3 | PSO | M | 37 | 22.7 | 27 | 10 | Yes | No | No | PSO1 |
| N4 | PSO | M | 32 | 66.6 | 25 | 7 | Yes | No | No | PSO1 |
| N6 | PSO | M | 35 | 6.8 | 35 | 1 | Yes | No | No | PSO2 |
| N9 | PSO | F | 29 | 18.7 | 27 | 2 | Yes | No | No | PSO3 |
Abbreviations: F, female; M, male; NA, not available; PSO, psoriasis.
References
- Afifi L., Danesh M.J., Lee K.M., Beroukhim K., Farahnik B., Ahn R.S., et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National survey. Dermatol Ther (Heidelb) 2017;7:227–242. doi: 10.1007/s13555-017-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R., et al. Enterotypes of the human gut microbiome [published corrections appear in Nature 2014;506:516 and Nature 2011;474:666] Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhadou F., Mintoff D., Schnebert B., Thio H.B. Psoriasis and microbiota: a systematic review. Diseases. 2018;6:47. doi: 10.3390/diseases6020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembilla N.C., Senra L., Boehncke W.-H. The IL-17 Family of cytokines in psoriasis: IL-17A and beyond. Front Immunol. 2018;9:1682. doi: 10.3389/fimmu.2018.01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.W., Yan D., Singh R., Liu J., Lu X., Ucmak D., et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome. 2018;6:154. doi: 10.1186/s40168-018-0533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codoñer F.M., Ramírez-Bosca A., Climent E., Carrión-Gutierrez M., Guerrero M., Pérez-Orquín J.M., et al. Gut microbial composition in patients with psoriasis. Sci Rep. 2018;8:3812. doi: 10.1038/s41598-018-22125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diani M., Casciano F., Marongiu L., Longhi M., Altomare A., Pigatto P.D., et al. Increased frequency of activated CD8+ T cell effectors in patients with psoriatic arthritis. Sci Rep. 2019;9:10870. doi: 10.1038/s41598-019-47310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mouzan M.I., Winter H.S., Assiri A.A., Korolev K.S., Al Sarkhy A.A., Dowd S.E., et al. Microbiota profile in new-onset pediatric Crohn’s disease: data from a non-Western population. Gut Pathog. 2018;10:49. doi: 10.1186/s13099-018-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J.T., Bruce A.T., Gudjonsson J.E., Johnston A., Stuart P.E., Tejasvi T., et al. Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol. 2010;130:1213–1226. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- Eppinga H., Sperna Weiland C.J., Thio H.B., van der Woude C.J., Nijsten T.E.C., Peppelenbosch M.P., et al. Similar depletion of protective Faecalibacterium prausnitzii in psoriasis and inflammatory bowel disease, but not in hidradenitis suppurativa. J Crohns Colitis. 2016;10:1067–1075. doi: 10.1093/ecco-jcc/jjw070. [DOI] [PubMed] [Google Scholar]
- Fahlén A., Engstrand L., Baker B.S., Powles A., Fry L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15–22. doi: 10.1007/s00403-011-1189-x. [DOI] [PubMed] [Google Scholar]
- Franzosa E.A., McIver L.J., Rahnavard G., Thompson L.R., Schirmer M., Weingart G., et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods. 2018;15:962–968. doi: 10.1038/s41592-018-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Lee C.H., Chi C.C. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417–1423. doi: 10.1001/jamadermatol.2018.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhrquist N., Muirhead G., Prast-Nielsen S., Jeanmougin M., Olah P., Skoog T., et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat Commun. 2019;10:4703. doi: 10.1038/s41467-019-12253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Tseng C.H., Strober B.E., Pei Z., Blaser M.J. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One. 2008;3:e2719. doi: 10.1371/journal.pone.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers D., Kugathasan S., Denson L.A., Vázquez-Baeza Y., Van Treuren W., Ren B., et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Cantabrana C., Gómez J., Delgado S., Requena-López S., Queiro-Silva R., Margolles A., et al. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br J Dermatol. 2019;181:1287–1295. doi: 10.1111/bjd.17931. [DOI] [PubMed] [Google Scholar]
- Hijnen D., Knol E.F., Gent Y.Y., Giovannone B., Beijn S.J.P., Kupper T.S., et al. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J Invest Dermatol. 2013;133:973–979. doi: 10.1038/jid.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James K.R., Gomes T., Elmentaite R., Kumar N., Gulliver E.L., King H.W., et al. Distinct microbial and immune niches of the human colon. Nat Immunol. 2020;21:343–353. doi: 10.1038/s41590-020-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Chen D., Zheng R.H., Zhang H., Chen Y.P., Xiang Z. miRNA-133a-UCP2 pathway regulates inflammatory bowel disease progress by influencing inflammation, oxidative stress and energy metabolism. World J Gastroenterol. 2017;23:76–86. doi: 10.3748/wjg.v23.i1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knights D., Silverberg M.S., Weersma R.K., Gevers D., Dijkstra G., Huang H., et al. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med. 2014;6:107. doi: 10.1186/s13073-014-0107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M., Thaiss C.A., Zeevi D., Dohnalová L., Zilberman-Schapira G., Mahdi J.A., et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163:1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chang H.W., Huang Z.M., Nakamura M., Sekhon S., Ahn R., et al. Single-cell RNA sequencing of psoriatic skin identifies pathogenic Tc17 cell subsets and reveals distinctions between CD8+ T cells in autoimmunity and cancer. J Allergy Clin Immunol. 2021;147:2370–2380. doi: 10.1016/j.jaci.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Price J., Arze C., Ananthakrishnan A.N., Schirmer M., Avila-Pacheco J., Poon T.W., et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche M.A., Farahi K., Capone K., Fakharzadeh S., Blauvelt A., Duffin K.C., et al. Longitudinal study of the psoriasis-associated skin microbiome during therapy with ustekinumab in a randomized phase 3b clinical trial. J Invest Dermatol. 2018;138:1973–1981. doi: 10.1016/j.jid.2018.03.1501. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Fujiyama Y., Andoh A., Ushijima T., Kajinami T., Bamba T. Characterization of antibody responses against rectal mucosa-associated bacterial flora in patients with ulcerative colitis. J Gastroenterol Hepatol. 2000;15:61–68. doi: 10.1046/j.1440-1746.2000.02045.x. [DOI] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol. 2014;10 doi: 10.1371/journal.pcbi.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan X.C., Tickle T.L., Sokol H., Gevers D., Devaney K.L., Ward D.V., et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A.M., Steen C.B., Liu C.L., Gentles A.J., Chaudhuri A.A., Scherer F., et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 2019;37:773–782. doi: 10.1038/s41587-019-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble C.L., Abbas A.R., Lees C.W., Cornelius J., Toy K., Modrusan Z., et al. Characterization of intestinal gene expression profiles in Crohn’s disease by genome-wide microarray analysis. Inflamm Bowel Dis. 2010;16:1717–1728. doi: 10.1002/ibd.21263. [DOI] [PubMed] [Google Scholar]
- Panagiotakos D.B., Pitsavos C., Stefanadis C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis. 2006;16:559–568. doi: 10.1016/j.numecd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Prodanovich S., Kirsner R.S., Kravetz J.D., Ma F., Martinez L., Federman D.G. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–703. doi: 10.1001/archdermatol.2009.94. [DOI] [PubMed] [Google Scholar]
- Rais R., Jiang W., Zhai H., Wozniak K.M., Stathis M., Hollinger K.R., et al. FOLH1/GCPII is elevated in IBD patients, and its inhibition ameliorates murine IBD abnormalities. JCI Insight. 2016;1:e88634. doi: 10.1172/jci.insight.88634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchlin C.T., Colbert R.A., Gladman D.D. Psoriatic arthritis [published correction appears in N Engl J Med 2017;376:2097] N Engl J Med. 2017;376:957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- Scher J.U., Ubeda C., Artacho A., Attur M., Isaac S., Reddy S.M., et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128–139. doi: 10.1002/art.38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J., Byun A., Liu Z., Mason J.B., Bronson R.T., Crott J.W. Dietary vitamin B6 intake modulates colonic inflammation in the IL10−/− model of inflammatory bowel disease. J Nutr Biochem. 2013;24:2138–2143. doi: 10.1016/j.jnutbio.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajib M.S., Chauhan U., Adeeb S., Chetty Y., Armstrong D., Halder S.L.S., et al. Characterization of serotonin signaling components in patients with inflammatory bowel disease. J Can Assoc Gastroenterol. 2019;2:132–140. doi: 10.1093/jcag/gwy039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto A., Cho O., Morohoshi Y., Sugita T., Muto M. Molecular characterization of the skin fungal microbiome in patients with psoriasis. J Dermatol. 2015;42:166–170. doi: 10.1111/1346-8138.12739. [DOI] [PubMed] [Google Scholar]
- Tan L., Zhao S., Zhu W., Wu L., Li J., Shen M., et al. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp Dermatol. 2018;27:144–149. doi: 10.1111/exd.13463. [DOI] [PubMed] [Google Scholar]
- Tett A., Pasolli E., Farina S., Truong D.T., Asnicar F., Zolfo M., et al. Unexplored diversity and strain-level structure of the skin microbiome associated with psoriasis. NPJ Biofilms Microbiomes. 2017;3:14. doi: 10.1038/s41522-017-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R., Hastie T. Hastie. Estimating the number of clusters in a data set via the gap statistic. Royal Stat Soc. 2002;457 doi: 10.1111/1467-9868.00293. [DOI] [Google Scholar]
- Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M.T., Shin D.B., Hubbard R.A., Noe M.H., Mehta N.N., Gelfand J.M. Psoriasis and the risk of diabetes: a prospective population-based cohort study. J Am Acad Dermatol. 2018;78:315–322.e1. doi: 10.1016/j.jaad.2017.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Kaplan J.L., Gold B.D., Bhasin M.K., Ward N.L., Kellermayer R., et al. Detecting microbial dysbiosis associated with pediatric Crohn disease despite the high variability of the gut microbiota. Cell Rep. 2016;14:945–955. doi: 10.1016/j.celrep.2015.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Issa N., Afifi L., Jeon C., Chang H.W., Liao W. The role of the skin and gut microbiome in psoriatic disease. Curr Dermatol Rep. 2017;6:94–103. doi: 10.1007/s13671-017-0178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii K., Hosomi K., Sawane K., Kunisawa J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr. 2019;6:48. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Wieczorek S., Franke A., Yin H., Pierer M., Sina C., et al. Association of UCP2 -866 G/A polymorphism with chronic inflammatory diseases. Genes Immun. 2009;10:601–605. doi: 10.1038/gene.2009.29. [DOI] [PubMed] [Google Scholar]
- Zhou W., Sailani M.R., Contrepois K., Zhou Y., Ahadi S., Leopold S.R., et al. Longitudinal multi-omics of host–microbe dynamics in prediabetes. Nature. 2019;569:663–671. doi: 10.1038/s41586-019-1236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Expression data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus and is accessible through Gene Expression Omnibus Series accession number GSE150851. Metagenomics sequence data are accessible through Sequence Read Archive BioProject PRJNA634145. All clinical and experimental data available are shown in Supplementary Table S12.