Abstract
Background
The laryngeal mask airway (LMA) is a safe and effective modality to maintain the airway for general anaesthesia during surgical procedures. The LMA is removed at the end of surgery and anaesthesia, when the patient maintains an adequate respiratory rate and depth. This removal of the LMA can be done either when the patient is deep under anaesthesia (early removal) or only after the patient has regained consciousness (late removal). It is not clear which of these techniques is superior.
Objectives
The objective of this review was to compare the safety of LMA removal in the deep plane of anaesthesia (early removal) versus removal in the awake state (late removal) for participants undergoing general anaesthesia.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 8); MEDLINE (1966 to August 2014); EMBASE (1980 to August 2014); LILACS (1982 to August 2014); CINAHL (WebSPIRS; 1984 to August 2014); and ISI Web of Science (1984 to August 2014). We searched for ongoing trials through various trial registration websites. In addition, we searched conference proceedings and reference lists of relevant articles.
Selection criteria
We included randomized controlled trials (RCTs) on adults and children undergoing elective general anaesthesia using the LMA, that compared early removal of the LMA (defined as removal of the LMA in the deep plane of anaesthesia) versus late removal of the LMA (defined as removal of the LMA after the patient is awake).
Data collection and analysis
Two authors independently assessed trial quality and extracted data. We contacted study authors for additional information. We used a random‐effects model to generate forest plots from the data.
Main results
We identified a total of 9188 citations and included 15 RCTs conducted on 2242 participants in this review. All trials used the LMA Classic in American Society of Anesthesiologists (ASA) physical status I or II for patients undergoing elective general anaesthesia. Children were enrolled in 11 trials and adults in five trials. None of the trials were of high methodological quality. Eight of the 15 studies had adequate generation of random sequence, whereas only one trial had adequate concealment of random sequence. Three trials had blinded the outcome assessor. Thus, the majority of the studies appeared to have a high risk of bias in the study design.
Using the GRADE approach, we found low quality evidence that the risk of laryngospasm was similar with early removal of the LMA (3.3%) versus late removal (2.7%): risk ratio (RR) 1.23, 95% confidence interval (CI) 0.74 to 2.03; 11 trials, 1615 participants. The quality of evidence was very low that the risk of coughing was less after early removal (13.9%) than late removal (19.4%): RR 0.52, 95% CI 0.29 to 0.94; 11 trials, 1430 participants. The quality of evidence for the risk of desaturation was also very low; there was no difference between early removal (7.9%) and late removal (10.1%): RR 0.68, 95% CI 0.4 to 1.16; 13 trials, 2037 participants. We found low quality evidence that the risk of airway obstruction was higher with early removal (15.6%) compared to late removal of the LMA (4.6%): RR 2.69, 95% CI 1.32 to 5.5; eight trials, 1313 participants.
Authors' conclusions
This systematic review suggests that current best evidence comparing early versus late removal of the LMA in participants undergoing general anaesthesia does not demonstrate superiority of either intervention. However, the quality of evidence available is either low or very low. There is a paucity of well designed RCTs and a need for large scale RCTs to demonstrate whether early removal or late removal of the LMA is better after general anaesthesia.
Keywords: Adult; Child; Humans; Anesthesia, General; Device Removal; Device Removal/adverse effects; Laryngeal Masks; Airway Obstruction; Airway Obstruction/etiology; Anesthesia Recovery Period; Cough; Cough/etiology; Laryngismus; Laryngismus/etiology; Oxygen; Oxygen/blood; Randomized Controlled Trials as Topic; Time Factors
Plain language summary
Removal of the laryngeal mask airway after surgery while under general anaesthesia (early removal) or after regaining consciousness (late removal)
Review question
We undertook this Cochrane review to compare the safety of early removal of the laryngeal mask airway (LMA) versus late removal, in people undergoing general anaesthesia.
Background
The LMA is an airway device used to keep the airway open during general anaesthesia in adults and children. The LMA is removed at the end of the surgical procedure either while the person remains anaesthetized (referred to as early removal) or after the person is fully awake (referred to as late removal). At present it is unclear which of these approaches (early removal versus late removal) is better in terms of the safety of the patient.
Study characteristics
The evidence is current to August 2014. We found 15 randomized controlled trials on 2242 participants addressing this question. All the trials were performed in individuals who were not seriously ill under elective general anaesthesia. A LMA Classic was used for all studies. Children were enrolled in 11 studies and adults in five studies. None of the trials were of high methodological quality.
Key results
The risks of complications such as laryngospasm (tight closure of the windpipe preventing effective breathing), and lowering of oxygen content in the blood (desaturation), were similar with early removal and late removal of the LMA. Coughing was less frequent after early removal of the LMA, with a risk of 13.9% as compared to the risk of 19.4% after late removal of the LMA. However, airway obstruction was more likely after early removal, with a risk of 15.6%, as compared to a risk of 4.6% after late removal of the LMA. No data were available on length of stay in the recovery room or hospital, or patient satisfaction. Thus, overall, this systematic review suggests that with the current available evidence, early and late removal of the LMA are comparable in persons undergoing general anaesthesia, and neither is superior in terms of safety.
Quality of evidence
The quality of the evidence that is available is either low or very low for all the outcomes described. This was mainly due to poorly conducted studies, the small number of people that they recruited, and to a lesser extent, some variation in the study results.
Summary of findings
Summary of findings for the main comparison. Early removal of the LMA versus late removal of the LMA after general anaesthesia.
| Early removal of the LMA versus late removal of the LMA after general anaesthesia | ||||||
| Patient or population: patients with general anaesthesia Settings: operating room Intervention: early removal of the LMA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Early removal of the LMA | |||||
| Laryngospasm (immediate) | Study population1 | RR 1.23 (0.74 to 2.03) | 1615 (11 studies) | ⊕⊕⊝⊝ low2,3 | ||
| 27 per 1000 | 33 per 1000 (20 to 55) | |||||
| Low1 | ||||||
| 6 per 1000 | 8 per 1000 (5 to 13) | |||||
| High1 | ||||||
| 85 per 1000 | 105 per 1000 (63 to 173) | |||||
| Desaturation (immediate) | Study population4 | RR 0.68 (0.40 to 1.16) | 2037 (13 studies) | ⊕⊝⊝⊝ very low2,3,5 | ||
| 101 per 1000 | 69 per 1000 (40 to 117) | |||||
| Low4 | ||||||
| 9 per 1000 | 6 per 1000 (4 to 11) | |||||
| High4 | ||||||
| 280 per 1000 | 190 per 1000 (112 to 325) | |||||
| Airway obstruction (immediate) | Study population6 | RR 2.69 (1.32 to 5.50) | 1313 (8 studies) | ⊕⊕⊝⊝ low2,5 | ||
| 46 per 1000 | 122 per 1000 (60 to 250) | |||||
| Low6 | ||||||
| 11 per 1000 | 30 per 1000 (15 to 61) | |||||
| High6 | ||||||
| 80 per 1000 | 215 per 1000 (106 to 440) | |||||
| Coughing (immediate) | Study population6 | RR 0.52 (0.29 to 0.94) | 1430 (11 studies) | ⊕⊝⊝⊝ very low2,7 | ||
| 194 per 1000 | 101 per 1000 (56 to 182) | |||||
| Low6 | ||||||
| 76 per 1000 | 40 per 1000 (22 to 71) | |||||
| High6 | ||||||
| 293 per 1000 | 152 per 1000 (85 to 275) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The assumed risk for laryngospasm is derived from the included studies, Splinter 1997 and Sinha 2006, who reported second lowest and second highest control group risks, respectively, for laryngospasm. 2 Quality of evidence downgraded by one level due to lack of allocation concealment and blinding. 3 Quality of evidence was downgraded by one level as the pooled estimate of effect includes both no effect and appreciable benefit/harm. 4 The assumed risk for desaturation is derived from the included studies, Thomas 2012 and Baird 1999, who reported second lowest and second highest control group risks, respectively, for both outcomes. 5 Quality of evidence downgraded by one level due to moderate unexplainable heterogeneity (I2 = 52%). 6 The assumed risk for airway obstruction and coughing are derived from the included studies, Varughese 1994 and Baird 1999, who reported second lowest and second highest control group risks, respectively, for this outcome. 7 Quality of evidence downgraded by two levels due to significant heterogeneity (I2 = 75%).
Background
During general anaesthesia there is a decrease in the tone of the pharyngeal and laryngeal muscles that maintain patency of the upper airways (Stone 1994). Therefore, artificial devices are introduced to maintain airway patency. A patent airway is crucial for the flow of oxygen and carbon dioxide between the lungs and the atmosphere. The primary hazard of an obstructed airway is hypoxaemia, leading to impaired oxygenation of tissues. If hypoxaemia persists, reversible hypoxic damage has the potential to worsen into irreversible cell death in vital organs and becomes life‐threatening.
Description of the condition
At the end of general anaesthesia, the device to maintain airway patency is removed as the patient emerges from unconsciousness. During emergence, the patient is susceptible to complications related to the airways because airway patency depends on the return of the natural tone of the pharyngeal and laryngeal muscles. This return of tone varies from individual to individual and is affected by the drugs used during anaesthesia. Airway‐related complications of emergence include pharyngeal and laryngeal obstruction (Jaffe 1972; Morikawa 1961; Ruben 1961) which may contribute to impaired tissue oxygenation. The risk of an adverse event is enhanced by any manipulation or intervention of the airways in light planes of anaesthesia.
Description of the intervention
The laryngeal mask airway (LMA) has been a safe and effective modality to maintain the airways during general anaesthesia for surgical procedures (Lopez‐Gill 1996). Like other devices the LMA has to be removed at the end of surgery, while the patient maintains an adequate respiratory rate and depth. This is done in one of two ways: either when the patient is still in the deep anaesthetized state (referred to as early removal), or when the patient regains consciousness and is in the awake state (referred to as late removal). Both methods have theoretical advantages and disadvantages.
How the intervention might work
The advantages of early removal might include reduced coughing and haemodynamic disturbance. The airway must be scrupulously maintained following removal, however, because airway obstruction, arterial oxygen desaturation, coughing, retching and vomiting are possible complications (Dolling 2003). On the other hand, the advantages of removing the LMA when the patient is awake might include a lower incidence of laryngospasm and upper airway obstruction but at the potential cost of increased coughing, restlessness and biting on the LMA (Mason 1990).
Why it is important to do this review
The appropriate time to remove the LMA has been studied by various investigators but with conflicting results and conclusions. Archie Brain, who pioneered the LMA, recommends that it can be left in place until the patient has regained consciousness (Brain 1993). Some investigators have indeed demonstrated a higher incidence of oxygen desaturation (Dolling 2003) and airway complications like upper airway obstruction (Nunez 1998) following early removal of the LMA. Kitching 1996 and Varughese 1994, however, found that early removal of the LMA was associated with fewer complications than late removal. Samarkandi 1998 found no difference in the incidence of complications between early versus late removal of the LMA. There are no systematic reviews available to determine whether the LMA should be removed early or late.
Objectives
The objective of this review was to compare the safety of LMA removal in the deep plane of anaesthesia (early removal) versus removal in the awake state (late removal) for participants undergoing general anaesthesia.
Methods
Criteria for considering studies for this review
Types of studies
We included prospective randomized controlled trials (RCTs) irrespective of blinding, publication status, or language.
Types of participants
We included trials on adults and children who underwent elective general anaesthesia using a laryngeal mask airway (LMA) irrespective of their American Society of Anesthesiologists (ASA) physical status.
We excluded trials on participants with a history of gastro‐oesophageal reflux and those at risk of pulmonary aspiration of gastric contents.
Types of interventions
We included studies that compared the early removal of the LMA to the late removal of the LMA in participants who had surgical procedures under general anaesthesia, with or without muscle relaxants. We defined early removal as removal of the LMA at the end of the surgical procedure, but before cessation of general anaesthesia, i.e. with the patient still deeply anaesthetized and without airway reflexes. We defined late removal as removal of the LMA after cessation of general anaesthesia, when the patient was awake. For this review, trials using any type of LMA were eligible for inclusion.
We excluded trials involving procedures where endotracheal intubation was planned after removal of the LMA and when the LMA was used for emergency access to the airways.
Types of outcome measures
Primary outcomes
The primary outcome measures after removal of the LMA were:
laryngospasm;
coughing;
desaturation; and
airway obstruction.
These outcomes were included in Table 1.
Secondary outcomes
Use of additional airway devices apart from manual maintenance of the upper airway.
Incidence of nausea, vomiting or retching.
Incidence of aspiration.
Biting on the LMA or LMA damage and biting on the bite block.
Length of stay in the recovery room.
Length of stay in the hospital.
Patient satisfaction.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 8), MEDLINE (via Ovid SP, 1966 to August 2014), EMBASE (via Ovid SP, 1980 to August 2014), LILACS (via BIREME, 1982 to August 2014), CINAHL (via EBSCOhost, 1984 to August 2014), and ISI Web of Science (1984 to August 2014).
We used a specific search strategy for each database. Please see Appendix 1 for CENTRAL, Appendix 2 for MEDLINE, Appendix 3 for EMBASE, Appendix 4 for CINAHL, Appendix 5 for ISI Web of Science and Appendix 6 for LILACS.
We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy phases one and two as contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We searched for relevant ongoing trials using the following websites (last searched in September 2014).
Controlled‐Trials.com.
ClinicalStudyResults.org.
NHS National Research Register.
Clinicaltrials.gov.
Searching other resources
We handsearched the bibliographies of retrieved articles to identify potentially relevant trials. We looked for additional trials by searching the abstracts of relevant conference proceedings. We did not use any language restriction in the search strategy.
Data collection and analysis
Selection of studies
We (PJM and JLM) independently examined the titles and abstracts of reports identified by both electronic and manual searching for potentially relevant studies. We retrieved the full‐text versions of all trials chosen by at least one author for evaluation. We (PJM and JLM) independently selected trials that met the inclusion criteria using a checklist designed in advance for that purpose. We resolved disagreements by mutual discussion.
Data extraction and management
We (PJM and JLM) independently extracted data using a standardized checklist. Information was recorded on the study design and setting, study inclusion and exclusion criteria, patient characteristics, details of anaesthetic technique, including the definitions of early and late removal of the LMA and the study outcomes. In addition, we extracted information on methods of randomization, allocation concealment, blinding, frequency and handling of missing data and selective reporting of outcomes. We contacted the corresponding authors of selected studies to seek further clarification on issues of reporting or to obtain additional outcome data. Data extractors were not blinded to study citations. We (PJM and JLM) entered data into Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
We (PJM and JLM) judged the quality of trials independently by assessing the risk of bias using the Cochrane 'Risk of bias' tool (Higgins 2011). We resolved differences in opinions by mutual discussion. The domains evaluated using this tool included:
random sequence generation;
allocation concealment;
blinding;
incomplete outcome data; and
selective reporting.
For each domain, we assigned a judgement regarding the risk of bias as 'high risk of bias’, ‘low risk of bias’, or ‘unclear risk of bias’ (Higgins 2011). We attempted to contact the trial corresponding author for clarification when insufficient detail was reported to assess the risk of bias. We constructed a ‘Risk of bias’ table in RevMan 2014 to present the results.
Measures of treatment effect
We expressed dichotomous outcomes in terms of risk ratios (RRs) and 95% confidence intervals (CIs). There were no continuous outcomes among reported variables. The pooled estimate was estimated using a random‐effects model as heterogeneity was anticipated.
Unit of analysis issues
Individual participants in each trial arm comprised the unit of analysis. All included trials had a parallel group design and thus no adjustment was necessary for crossover or clustering.
Dealing with missing data
We contacted the corresponding authors of selected trials to obtain missing data.
Assessment of heterogeneity
Statistical heterogeneity was evaluated from forest plots of the study estimates, and formally using the I2 statistic. For results showing moderate to significant heterogeneity as measured by I2 > 50% (Higgins 2002) we explored the possible sources of heterogeneity. We evaluated the clinical heterogeneity in studies by qualitative assessment of study differences in terms of study population, surgical setting and anaesthetic technique.
Assessment of reporting biases
We constructed a funnel plot (graphical display) of the treatment effect indicated by the RR for the primary outcomes, against trial precision indicated by standard error (SE) of log (RR) using RevMan 2014. We visually inspected the funnel plot for asymmetry.
Data synthesis
We (JLM and PJM) organized the data, conducted analyses, and reported summary statistics. We identified sufficient studies to perform meta‐analyses using RevMan 2014. The findings are presented in Table 1.
We employed the GRADE approach (Guyatt 2008) using GRADEproGDT 2015 to assess the overall quality of evidence for each primary outcome to create Table 1. We downgraded the evidence from 'high quality' by one level for serious, and by two levels for very serious study limitations or risk of bias, indirectness of evidence, inconsistency across the included studies, lack of precision of effect estimates or potential publication bias.
Subgroup analysis and investigation of heterogeneity
We expected clinical heterogeneity based on the age of the participants (children below 12 years versus adults) and the anaesthetic technique (with and without the use of muscle relaxants). Therefore, we used these two variables to perform subgroup analyses.
Sensitivity analysis
We did not identify any trial at low risk of bias and therefore did not perform sensitivity analysis.
Results
Description of studies
(See: Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification)
Results of the search
We identified 9188 citations from database searches, manual searches and citation review (Figure 1). After screening by title and then abstract, we identified 26 citations that were potentially eligible for inclusion in the review. The study published in the Turkish language (Iyilikci 1999) is awaiting translation (see Characteristics of studies awaiting classification). We evaluated the full texts of the remaining 26 citations in detail. Only the abstracts were available for two studies (Thomas 2012; Varughese 1994), as these were abstracts presented in conferences and thereafter published in journals as conference abstracts. We made attempts to contact the authors of Heidari 2005, Sinha 2006 and Splinter 1997 to seek further information of these trials, and obtained clarifications from Sinha 2006 alone. There was no contact information available for Thomas 2012 or Varughese 1994.
1.

Study flow diagram.
Included studies
We included 15 trials in the review. These trials included 2242 participants; individual trials' participant numbers ranged from 60 to 333 participants. Four trials were exclusively in adults (Cheong 1999; Gataure 1995; Heidari 2005; Nunez 1998) and one trial (Baird 1999) included adults and children, and reported the outcomes in adults and children separately. The remaining 10 trials (Dolling 2003; Kitching 1996; Laffon 1994; Pappas 2001; Park 2012; Samarkandi 1998; Sinha 2006; Splinter 1997; Thomas 2012; Varughese 1994) were done exclusively in children. All the trials were conducted using the LMA Classic in ASA I or II patients for elective general anaesthesia.
Two trials (Cheong 1999; Heidari 2005) used muscle relaxants as a component of general anaesthesia, whereas other trials maintained the patients on spontaneous respiration. Four trials (Baird 1999; Dolling 2003; Park 2012; Varughese 1994) included only day‐case procedures in their studies. The trial by Dolling 2003 was in children for dental surgery, whereas all other trials were in patients for peripheral, orthopaedic, urogenital or lower limb procedures. For full details of included trials, please see Characteristics of included studies.
The length of stay in recovery room, length of stay in hospital, use of additional airway devices after removal of the LMA, and patient satisfaction were not reported in any of the 15 studies. Biting or damage to the LMA or bite block was reported only by Gataure 1995. Only Cheong 1999 looked for the incidence of pulmonary aspiration after LMA removal. Five trials (Heidari 2005; Pappas 2001; Splinter 1997; Thomas 2012; Varughese 1994) defined desaturation as peripheral capillary oxygen saturation (SpO2) < 90% and seven trials (Cheong 1999; Dolling 2003; Kitching 1996; Nunez 1998; Park 2012; Samarkandi 1998; Sinha 2006) defined desaturation as SpO2 < 95%. Baird 1999 reported desaturation in detail: incidence of desaturation < 96%, < 94% and < 91%. The reported incidence of < 96% was utilized in the meta‐analysis.
Excluded studies
We excluded 10 studies for the reasons described in the Characteristics of excluded studies. We excluded two studies (Cameron 2001; Lerman 2010) as the study design was not randomized for early and late removal of the LMA. We excluded one citation as it was an editorial (Mencke 2010). We excluded the remaining seven studies (Flynn 2007; Goldmann 2011; Lee 2011; Lema 2010; McKay 2006; Sinha 2009; White 2009) as the interventions and outcomes studied were different from those of this systematic review.
Awaiting classification
One study is awaiting classification (Iyilikci 1999) (see Characteristics of studies awaiting classification).
Ongoing studies
There are no ongoing studies
Risk of bias in included studies
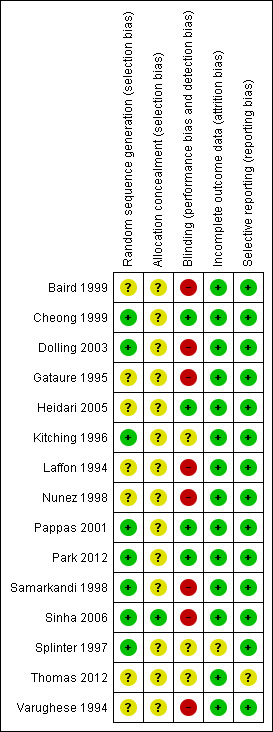
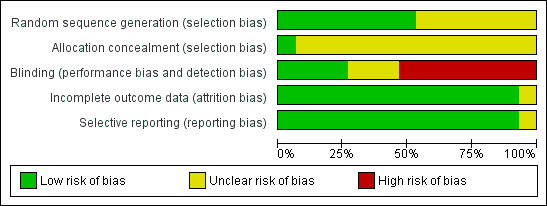
Eight of the 15 studies had adequate generation of random sequence either by computer generated random numbers or coin tossing (Cheong 1999; Dolling 2003; Kitching 1996; Pappas 2001; Park 2012; Samarkandi 1998; Sinha 2006; Splinter 1997), whereas only one trial had adequate concealment of random sequence (Sinha 2006). Three trials had blinded the outcome assessor (Cheong 1999; Heidari 2005; Pappas 2001) and one trial had blinded the data analyst (Park 2012). Therefore, the majority of the included studies seem to have a high risk of bias in the study design (Figure 2; Figure 3).
2.
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Of the 15 included studies, 14 had complete data reported (Figure 2; Figure 3). Fourteen trial reports appear to be free of selective reporting as the outcomes mentioned in the methodology have been reported in the results despite lack of explicit statement in the text. Five authors (Dolling 2003; Pappas 2001; Park 2012; Splinter 1997; Thomas 2012) have explicit statements to address the cases excluded after recruitment into the study and one author (Sinha 2006) reported cases excluded on enquiry (see Characteristics of included studies). The other nine trials did not have any exclusions or dropouts after randomization. Splinter 1997 reported the exclusion of 25 patients for whom the LMA was not used (from a total of 333) after enrolment. We presume that these exclusions were possibly before randomization (a request was sent to the authors to clarify the issue, however there was no response). Park 2012 reported excluding seven patients after recruitment but before randomization.
Patients were excluded after randomization in four trials: four in Pappas 2001, five in Sinha 2006, one in Dolling 2003 and four in Thomas 2012 totaling 14 exclusions from a total of 2209 patients randomized. All four authors performed available case analysis. This amounts to 0.63% of missing data in this meta‐analysis. The reasons for missing data mentioned in these four trials where available case analysis was performed appear to be “missing at random.” Considering these, the outcome data appears to be of low risk of bias. Therefore, we did not do the sensitivity analysis for the imputation of missing data using best and worst case scenarios.
We determined that the quality of evidence generated from these pooled studies suffers serious limitations due to risk of bias and, therefore, downgraded the quality of all primary outcomes evaluated by GRADE by one level.
Effects of interventions
See: Table 1
(See: Data and analyses and Table 1)
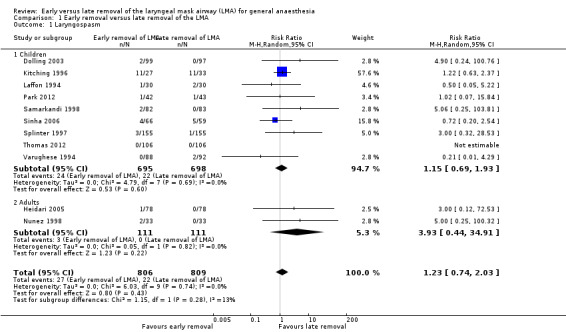
Laryngospasm (Comparison 1, Outcome 1) (Analysis 1.1, Analysis 1.2)
Eleven trials enrolling a total of 1615 patients reported this outcome, 806 (49.9%) of whom were enrolled in the early removal arm and 809 (50.1%) in the late removal arm. Twenty‐seven (3.3%) patients in the early arm experienced laryngospasm versus 22 (2.7%) in the late arm. This difference of 0.6% was not statistically significant, and the risk ratio (RR) of experiencing laryngospasm with the early removal compared to late removal was 1.23 (95% confidence interval (CI) 0.74 to 2.03, P = 0.43, I2 = 0%). We graded the quality of evidence as low for this outcome as the pooled estimate was imprecise in addition to there being a high risk of bias in the included studies.
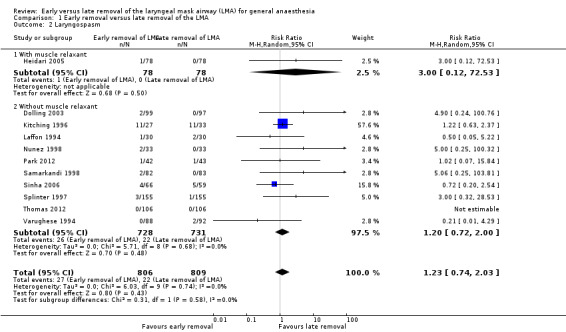
We performed two planned subgroup analyses based on clinical heterogeneity. Subgroup analysis by age (Analysis 1.1) did not suggest any statistically significant difference between early and late removal of the LMA in children (RR 1.15, 95% CI 0.69 to 1.93, P = 0.6, I2 = 0%; nine trials, 1393 participants) or adults (RR 3.93, 95% CI 0.44 to 34.91, P = 0.22, I2 = 0%; two trials, 222 participants). Subgroup analysis by whether muscle relaxants were used (RR 3.0, 95% CI 0.12 to 72.53; one trial, 156 participants) or not used (RR 1.20, 95% CI 0.72 to 2.00, P = 0.48, I2 = 0 %; 10 trials, 1459 participants) (Analysis 1.2) also did not reveal a significant difference based on timing of removal. The differences between the subgroup estimates were not statistically significant (P = 0.28, I2 = 13.3%).
1.1. Analysis.
Comparison 1 Early removal versus late removal of the LMA, Outcome 1 Laryngospasm.
1.2. Analysis.
Comparison 1 Early removal versus late removal of the LMA, Outcome 2 Laryngospasm.
The funnel plot of RR versus SE log (RR) for laryngospasm appears symmetrical (Figure 4), suggesting a potentially low risk of publication bias for this outcome.
4.
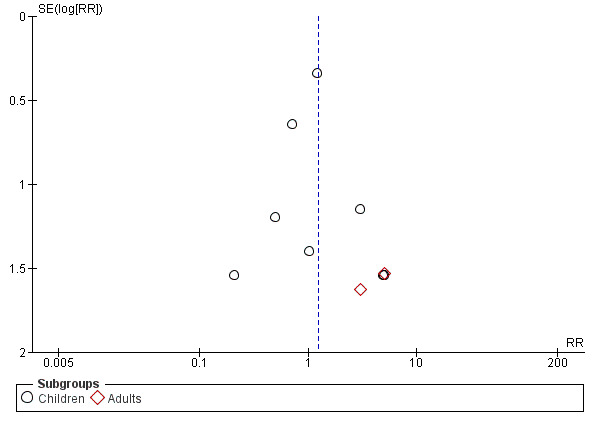
Funnel plot of comparison: 1 Early removal versus late removal, outcome: 1.1 Laryngospasm.
Coughing (Comparison 1, Outcome 2) (Analysis 1.3, Analysis 1.4)
Eleven studies on 1430 participants reported the incidence of coughing. Of these participants, 713 (49.9%) were enrolled in the early removal arm and 717 (50.1%) in the late removal arm. Ninety‐nine participants (13.9%) in the early arm had coughing events after LMA removal, whereas 139 participants (19.4%) coughed after late removal. This difference of 5.5% was statistically significant as the RR of coughing after early removal was 0.52 (95% CI 0.29 to 0.94, P = 0.03) as compared to late removal. Significant heterogeneity (I2 = 75%) was observed and the high risk of bias further downgraded this outcome to very low quality of evidence.
The subgroup analysis by age (Analysis 1.3) showed a similar risk of coughing between groups in children (RR 0.50, 95% CI 0.19 to 1.34, P = 0.17, I2 = 82%; 7 trials, 835 participants) and adults (RR 0.51, 95% CI 0.25 to 1.03, P = 0.06, I2 = 58%; five trials, 595 participants). The subgroup analysis on patients who were given muscle relaxants showed a statistically significantly lower risk of coughing after early removal compared to late removal of the LMA (RR 0.41, 95% CI 0.20 to 0.83, P = 0.01, I2 = 1%; two trials, 219 participants). In the subgroup of participants where muscle relaxants were not used, the risk of coughing was similar with early or late removal of the LMA (RR 0.46, 95% CI 0.21 to 1.04, P = 0.06, I2 = 79%; 9 trials, 1211 participants) Analysis 1.4. There appears to be no significant differences between the subgroups (P = 0.83, I2 = 0%).
1.3. Analysis.
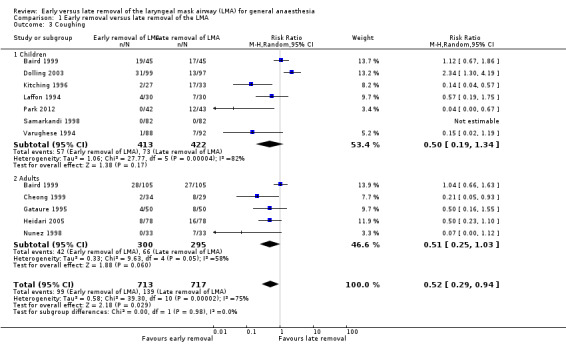
Comparison 1 Early removal versus late removal of the LMA, Outcome 3 Coughing.
1.4. Analysis.
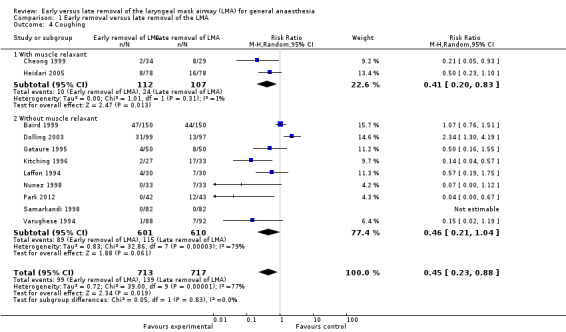
Comparison 1 Early removal versus late removal of the LMA, Outcome 4 Coughing.
On exploring reasons for heterogeneity, we identified that the study by Dolling 2003 was done in children scheduled for elective dental day‐case surgery and the rest of the studies were on children undergoing orthopaedic, urogenital, lower abdominal and peripheral surgical procedures. In fact, Splinter 1997 excluded participants scheduled for airway surgery or with conditions that may affect airway reflexes. Since dental procedures can lead to bleeding into the upper airway and thus stimulate airway reflexes, the variation in surgical setting may have contributed to the heterogeneity encountered in this result.
The funnel plot for coughing appeared symmetrical, suggesting a low risk of publication bias for this outcome.
Desaturation (Comparison 1, Outcome 3) (Analysis 1.5, Analysis 1.6, Analysis 1.7)
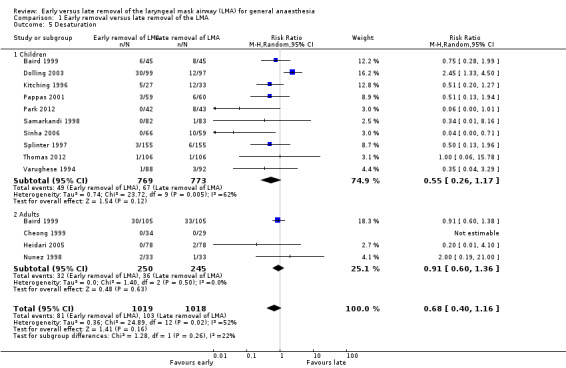
Thirteen trials on 2037 participants reported the outcome of desaturation. Among them, 1019 participants (50.0%) were randomized to early removal of the LMA and the other 1018 participants (50.0%) to late removal of the LMA. Eighty‐one (7.9%) participants had desaturation after early removal of the LMA, whereas 103 (10.1%) participants had desaturation after late removal. This difference of 2.2% was not statistically significant as the RR of desaturation is similar after early and late removal of the LMA (RR 0.68, 95% CI 0.40 to 1.16, P = 0.16, I2 = 52%; Figure 5). The overall quality of evidence for this outcome is very low due to high risk of bias, poor precision of the pooled estimate and inconsistency among the included studies.
5.
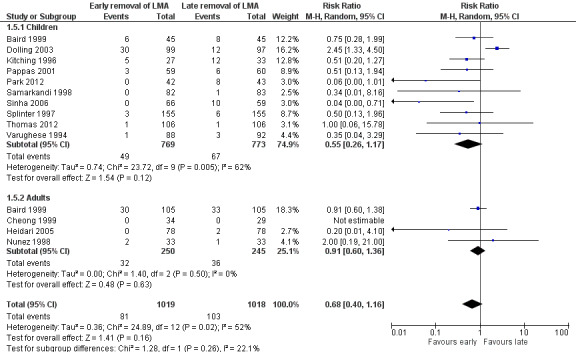
Forest plot of comparison: 1 Early removal versus late removal, outcome: 1.6 Desaturation in children and adults.
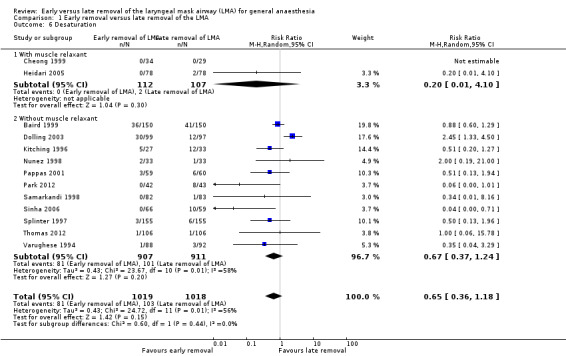
The subgroup analysis by age (Analysis 1.5) showed a similar risk of desaturation between groups in children (RR 0.55, 95% CI 0.26 to 1.17, P = 0.12, I2 = 62%; 10 trials, 1542 participants) and adults (RR 0.91, 95% CI 0.6 to 1.36, P = 0.63, I2 = 0%; 4 trials, 495 participants). The risk of desaturation was similar between groups following general anaesthesia with or without muscle relaxant (with muscle relaxant: RR 0.20, 95% CI 0.01 to 4.10, two trials, 219 participants, only a single trial had events that contributed to this analysis and reporting of heterogeneity was therefore not appropriate; without muscle relaxant: RR 0.67, 95% CI 0.37 to 1.24, P = 0.2, I2 = 58%; 11 trials, 1818 participants; Analysis 1.6). There appear to be no significant differences between the subgroups (P = 0.44, I2 = 0%).
1.5. Analysis.
Comparison 1 Early removal versus late removal of the LMA, Outcome 5 Desaturation.
1.6. Analysis.
Comparison 1 Early removal versus late removal of the LMA, Outcome 6 Desaturation.
The variations in the surgical setting in the study by Dolling 2003 and its effect on airway reflexes may have contributed to the heterogeneity observed for desaturation. In addition, the lack of a uniform cut‐off in the reading of pulse oximetry to define desaturation also may have contributed to this heterogeneity. We performed a subgroup analysis by grouping studies that used a cut‐off of < 90% on pulse oximetry to define desaturation and those that used a value higher than 90% to define desaturation (Analysis 1.7). Heterogeneity was negligible in the subgroup where the cut‐off was < 90% and moderate heterogeneity was observed in the trials where the cut‐offs were > 90% as the cut‐off varied from 94% to 96%.
1.7. Analysis.
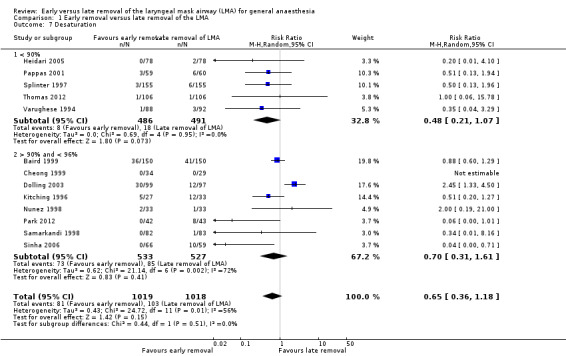
Comparison 1 Early removal versus late removal of the LMA, Outcome 7 Desaturation.
The funnel plot for desaturation (Figure 6) appeared symmetrical indicating a low risk of publication bias for this outcome.
6.
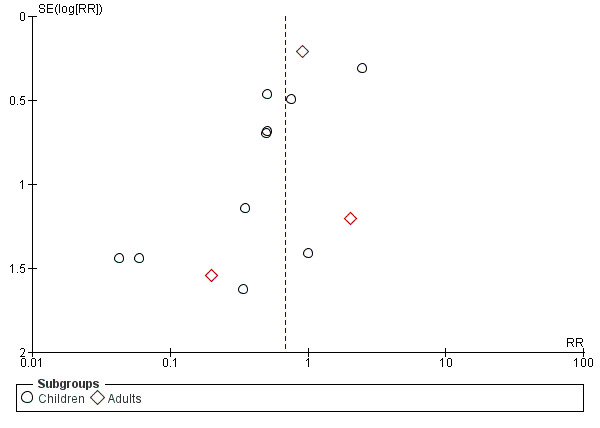
Funnel plot of comparison: 1 Early removal versus late removal, outcome: 1.5 Desaturation.
Airway obstruction (Comparison 1, Outcome 4) (Analysis 1.8)
Six authors reported the incidence of airway obstruction in addition to laryngospasm as an outcome. Another two authors reported airway obstruction, but not laryngospasm. There was no specific definition for airway obstruction used in any of these studies. Since laryngospasm is one of the reasons for airway obstruction in the immediate recovery period, we considered airway obstruction as a separate outcome for the purpose of this review.
Eight trials on 1313 participants reported this outcome. Of these participants, 654 (49.8%) were randomized to early removal and 659 (50.2%) to late removal of the LMA. One hundred and two participants (15.6%) experienced airway obstruction after early removal whereas 30 participants (4.6%) experienced airway obstruction after late removal of the LMA. This difference of 11% was statistically significant with an increased risk of airway obstruction after early removal of the LMA (RR 2.69, 95% CI 1.32 to 5.50, P = 0.007, I2 = 52%; Figure 7). The meta‐analysis showed moderate heterogeneity (I2 = 52%) which may be due to the lack of a uniform criterion to define airway obstruction reported in various studies. This inconsistency along with the high risk of bias downgrades the evidence to low quality for this outcome.
7.
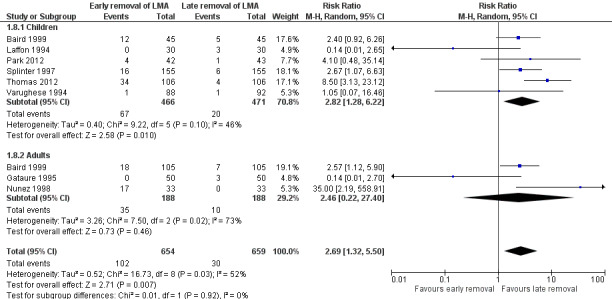
Forest plot of comparison: 1 Early removal versus late removal, outcome: 1.8 Airway obstruction.
The subgroup analysis by age (Analysis 1.8) showed a similarly higher risk of airway obstruction in children after early removal of the LMA compared to late removal (RR 2.82, 95% CI 1.28 to 6.22, P = 0.01, I2 = 46%; six trials, 937 participants) whereas in the adults, the difference in risk was not significant (RR 2.46, 95% CI 0.22 to 27.40, P = 0.46, I2 = 73%; three trials, 376 participants). There appears to be no significant difference between the subgroups (P = 0.92, I2 = 0%). We did not perform the planned subgroup analysis for use of muscle relaxants as none of the trials that studied this outcome used muscle relaxants.
1.8. Analysis.
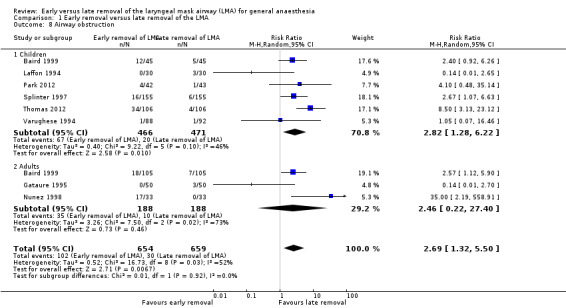
Comparison 1 Early removal versus late removal of the LMA, Outcome 8 Airway obstruction.
The funnel plot for airway obstruction (Figure 8) appeared symmetrical suggesting a low risk of publication bias for this outcome.
8.
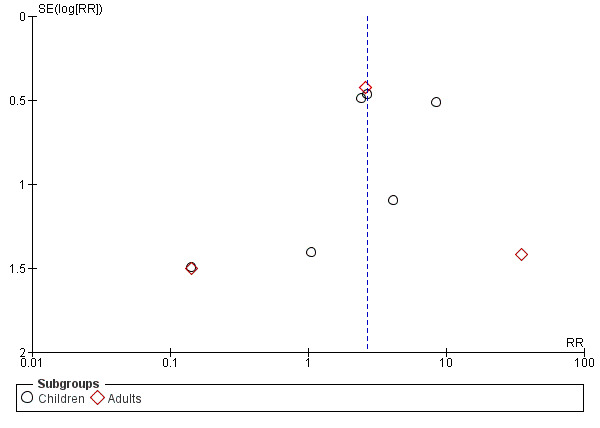
Funnel plot of comparison: 1 Early removal versus late removal, outcome: 1.8 Airway obstruction.
Secondary outcome measures
Use of additional airway devices apart from manual maintenance of the upper airway (Comparison 1, Outcome 4)
Only one trial (Varughese 1994) reported on the use of an additional airway device where it was not required either after early or late removal of the LMA. However, seven trials (Cheong 1999; Gataure 1995; Heidari 2005; Kitching 1996; Pappas 2001; Samarkandi 1998; Sinha 2006) used oropharyngeal airway after early removal of the LMA as part of the study design. This could be the reason that use of additional airway devices was not reported as a separate outcome in these seven trials.
Incidence of nausea, vomiting or retching (Comparison 1, Outcome 5) (Analysis 1.9)
Seven trials on 1192 participants reported the incidence of nausea, vomiting or retching following removal of the LMA. Of them, 594 (49.8%) were randomized to the early removal arm and 598 (50.2%) to the late removal arm. Three participants in the early arm (0.5%) and 12 participants in the late arm (2.0%) reported an incidence of nausea, vomiting or retching following LMA removal. This difference of 1.5% was not statistically significant as the RR was 0.41 (95% CI 0.15 to 1.13, P = 0.08, I2 = 0%).
The subgroup analysis by age showed a similar risk of nausea, vomiting or retching between groups in children (RR 0.45, 95% CI 0.13 to 1.51, P = 0.19, I2 = 0%; five trials, 936 participants) and adults (RR 0.33, 95% CI 0.05 to 2.08, P = 0.24, I2 = 0%; two trials, 256 participants). There appears to be no significant difference between the subgroups (P = 0.79, I2 = 0%).
Incidence of aspiration (Comparison 1, Outcome 6)
Only one trial (Cheong 1999) reported the incidence of aspiration during or after LMA removal which was none. The other trials did not report this outcome.
Biting on the LMA or LMA damage and biting on the bite block (Comparison 1, Outcome 7) (Analysis 1.10)
Two trials on 185 participants reported on the incidence of damage to the LMA during removal; the LMA was removed early in 92 (49.7%) and late in 93 participants (50.3%). Five participants in the early removal group (5.4%) had bitten/damaged the LMA during emergence whereas 25 participants (26.9%) did so in the late removal group. The difference in risk of damage to the LMA was statistically significant and 21.5% less when the LMA was removed early as compared to late (RR 0.20, 95% CI 0.08 to 0.51, P = 0.0007, I2 = 0%). We did not perform the planned subgroup analysis as there were only two trials reporting this outcome.
Length of stay in the recovery room (Comparison 1, Outcome 8)
None of the trials reported on the length of stay in the recovery room.
Length of stay in the hospital (Comparison 1, Outcome 9)
No trial studied the length of stay in the hospital.
Patient satisfaction (Comparison 1, Outcome 10)
Patient satisfaction was not studied in any of the trials.
Discussion
Summary of main results
We have considered several clinically relevant outcomes that have a bearing on immediate and subsequent patient management. The primary outcomes of laryngospasm, coughing, oxygen desaturation and airway obstruction, individually or together, have a direct bearing on the immediate management and postoperative course and outcome of patients following general anaesthesia.
Meta‐analysis suggests that the risks of laryngospasm and desaturation are similar whether the LMA is removed early or late. There is a significantly lower risk of coughing if the LMA is removed early whereas the risk of airway obstruction is higher if the LMA is removed early. However, the risk of airway obstruction does not increase the risk of events like laryngospasm or desaturation. This may be because of the use of an oropharyngeal airway after removal of the LMA as part of the study design in seven out of 15 included trials. The incidences of other side‐effects including nausea and vomiting were similar with early and late removal of the LMA. The risk of biting on the LMA/damage to the LMA is significantly higher if the LMA is removed late. None of the included studies evaluated patient‐centred outcomes like duration of hospital stay or patient satisfaction.
Overall completeness and applicability of evidence
The outcomes of laryngospasm and desaturation are clinically more relevant than the other outcomes studied in this meta‐analysis as both are potentially life‐threatening, critical events. Prevention of these critical incidents is vital to improve the safety of anaesthesia practice. Therefore, it is important to differentiate the risk of laryngospasm and desaturation involved with early and late removal of the LMA after general anaesthesia and choose the technique that produces less risk.
Although the results suggest similar risks of laryngospasm and desaturation with either technique, it is not possible to draw conclusions, as the pooled data is insufficient to categorically indicate that there is evidence of similar risks with early or late removal of the LMA. This comparison can only be properly addressed through additional well conducted randomized controlled trials (RCTs) with adequate sample size.
Airway obstruction is a preventable outcome and therefore it is important to consider during emergence. This outcome, which was found to occur significantly more frequently after early removal of the LMA, was not studied in seven out of 15 included studies. This was probably because, in six of these seven trials, use of Guedel's airway after early removal of the LMA was part of the study design, indicating that the authors were anticipating airway obstruction, and therefore took preventive measures to avoid it. This incomplete reporting confounds the inferences that we can draw about the risk of airway obstruction from this meta‐analysis.
Ten of the 15 included trials in this meta‐analysis were conducted exclusively in children, which may affect the applicability of the review findings to adolescents and adults. Despite the majority of trials enrolling children, the analyses remain underpowered to draw firm conclusions on the outcomes in either children or adults individually.
Quality of the evidence
Overall, the quality of evidence detected in this systematic review on early versus late removal of the LMA after general anaesthesia ranks from low to very low for the studied outcomes. The main limiting factor, decreasing the quality of all outcomes, was the high risk of bias in study design and conduct of included trials. None of the 15 trials fulfilled all five criteria for a low risk of bias. All but one trial (Sinha 2006) had inappropriate or unclear generation of allocation sequence and concealment of allocation, suggesting high risk of selection bias. The outcomes of interest for the review, such as desaturation and laryngospasm occur immediately after the intervention, i.e. removal of the LMA. Therefore, these are to be observed immediately following the intervention, making blinding difficult. Nevertheless, three trials (Cheong 1999; Heidari 2005; Pappas 2001) achieved blinding of outcome assessor and Park 2012 blinded the data analyst. All the other trials have the possibility of observer bias weakening the quality of evidence generated. Most trials were able to report complete data for all selected outcomes in all the included participants, thereby they possess a low risk of attrition bias.
The second limiting factor, decreasing the quality of evidence, is the small number of subjects studied, as the outcomes of interest are primarily complications with low but significant incidence rates. The meta‐analysis shows that the baseline risk of laryngospasm is 2.7% with late removal of the LMA. The optimal information sample size required to demonstrate a relative risk increase of 25% for this outcome after early removal of the LMA with a type I error of 0.05 and type II error of 0.2 is estimated to be 20,110 and the same to demonstrate a relative risk increase of 50% would be 5640. Similarly, the optimal information sample size to demonstrate a relative risk reduction of 25% from the baseline risk of desaturation of 10.1% after late removal of the LMA is 3984 participants. Thus, the current meta‐analysis with pooled data of 1615 participants on the outcome of laryngospasm and 2037 participants on the outcome of desaturation is underpowered to detect relative risk reduction/increase of 25% in the incidence of either outcomes.
The third factor that contributed to lower the quality of evidence is the inconsistency of results across the included studies, probably due to variations in inclusion criteria and diagnostic criteria for coughing, desaturation and airway obstruction. Selection bias and observer bias are significant in the included trials of this systematic review and, therefore, the results of the meta‐analyses need to be interpreted with caution.
Potential biases in the review process
We found low risk of publication bias as assessed by funnel plots. Two of the included studies (Thomas 2012; Varughese 1994) were published as conference abstracts with no information for the contact author in the publication. This has limited the availability of information and data that have been included in the review from these studies. The data from the Turkish study (Iyilikci 1999) is not included in the analysis for want of translation.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the first systematic review and meta‐analysis examining the benefits and harms of early versus late removal of the LMA after general anaesthesia.
Authors' conclusions
Implications for practice.
At the current state of evidence, we have insufficient information to decide whether early removal or late removal of the LMA is associated with less risk of laryngospasm and desaturation. However, early removal carries a higher risk of airway obstruction as compared with late removal. This has implications for clinical practice, as the clinician must anticipate this risk and take precautionary measures to maintain a clear airway when choosing to remove the LMA early.
Implications for research.
The evidence presented in this review does not enable us to determine the superiority of either technique. Given the low incidence of complications and laryngospasm, the sample sizes may be too small to determine the size of any treatment effect reliably. Considering that the subgroup analysis by age was underpowered in spite of the large proportion of studies in children, more studies are required in both adults and children. Only one of the 15 included studies had taken precautions to eliminate selection and observer bias in the study design. These limitations need to be addressed in future research.
We suggest that future researchers who take up this clinical question formulate well designed RCTs where generation of allocation sequence and allocation concealment are done appropriately to prevent selection bias of participants to interventions. Observer bias needs to be addressed by proper blinding, although this would require specific effort by the investigators to introduce blinded observers into the setting immediately after the intervention of early or late removal of the LMA. The fact that three of the included trials in this systematic review used blinded outcome assessors suggests that it is possible in the study design.
We also suggest that the future RCTs dealing with this issue be of a large scale to randomize at least 4000 participants in order to generate evidence that will yield information to aid clinical decision‐making. This is primarily because the incidence of important complications of the interventions studied are in the range of 2.7% to 10%.
Notes
We would like to thank Dr Mathew Zacharias for his help and advice during the preparation of the protocol (Mathew 2008) for this systematic review.
Acknowledgements
We would like to thank Mike Bennett (content editor), Cathal Walsh (statistical editor), Maurizio Solca, Ozan Akca (peer reviewers) and Janet Wale (consumer editor) for their help and editorial advice during the preparation of this systematic review.
We also thank Dr Georgia Salanti for the time and support she gave to the assessment of the quality of trials during the review process.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Laryngeal Masks
#2 (LMA or Proseal):ti,ab
#3 removal near LMA
#4 (extubation near (early or late or deep or awake or LMA):ti,ab
#5 (mask* near airway*):ti,ab
#6 (Laryngeal near Mask*):ti, ab
#7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. exp Laryngeal Masks/ or (Laryngeal adj3 Mask*).ti,ab. or (Mask* adj3 Airway*).ti,ab. or LMA.mp. or Proseal.mp. or (removal adj3 LMA).mp. or (extubation adj3 (early or late or deep or awake or LMA)).mp. 2. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) and humans.sh. 3. 1 and 2
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. exp Laryngeal Masks/ or (Laryngeal adj3 Mask*).ti,ab. or (Mask* adj3 Airway*).ti,ab. or LMA.mp. or Proseal.mp. or (removal adj3 LMA).mp. or (extubation adj3 (early or late or deep or awake or LMA)).mp. 2. (RANDOMIZED‐CONTROLLED‐TRIAL/ or RANDOMIZATION/ or CONTROLLED‐STUDY/ or MULTICENTER‐STUDY/ or PHASE‐3‐CLINICAL‐TRIAL/ or PHASE‐4‐CLINICAL‐TRIAL/ or DOUBLE‐BLIND‐PROCEDURE/ or SINGLE‐BLIND‐PROCEDURE/ or (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER* or ((SINGL* or DOUBL* or TREBL* or TRIPL*) adj3 (BLIND* or MASK*))).ti,ab.) and human*.ec,hw,fs. 3. 1 and 2
Appendix 4. Search strategy for CINAHL (EBSCOhost)
S1 MJ Laryngeal‐Masks
S2 TX Laryngeal and Mask*
S3 TX Mask* and Airway*
S4 TX LMA or Proseal
S5 TX removal and LMA
S6 TX extubation and (early or late or deep or awake or LMA)
S7 S6 or S5 or S4 or S3 or S2 or S1
Appendix 5. Search strategy for ISI Web of Science
#1 TS=(Laryngeal Mask*) or TS=(Mask* SAME Airway*) or TS=LMA or TS=Proseal or TS=(removal near LMA) or TS=(extubation SAME (early or late or deep or awake or LMA))
#2 TS=random* or TS=placebo or TS="DOUBLE BLIND" or TS="SINGLE BLIND" or TS="MULTICENTER STUDY" or TS="CONTROLLED STUDY" or TS=((SINGL* or DOUBL* or TREBL* or TRIPL*) SAME (BLIND* or MASK*))
#3 #2 AND #1
Field Tags: TS=Topic
Appendix 6. Search strategy for LILACS (BIREME interface)
"LARYNGEAL MASKS" or "LARYNGEAL MASKS/" or "Laryngeal Mask$" or "Mask$ Airway$" or "Proseal" or "LMA removal" or "early extubation" or "LMA extubation" or "awake extubation" or "deep extubation" or "late extubation"
Data and analyses
Comparison 1. Early removal versus late removal of the LMA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Laryngospasm | 11 | 1615 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.74, 2.03] |
| 1.1 Children | 9 | 1393 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.69, 1.93] |
| 1.2 Adults | 2 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 3.93 [0.44, 34.91] |
| 2 Laryngospasm | 11 | 1615 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.74, 2.03] |
| 2.1 With muscle relaxant | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.53] |
| 2.2 Without muscle relaxant | 10 | 1459 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.72, 2.00] |
| 3 Coughing | 11 | 1430 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.29, 0.94] |
| 3.1 Children | 7 | 835 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.19, 1.34] |
| 3.2 Adults | 5 | 595 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.25, 1.03] |
| 4 Coughing | 11 | 1430 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.23, 0.88] |
| 4.1 With muscle relaxant | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.20, 0.83] |
| 4.2 Without muscle relaxant | 9 | 1211 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.21, 1.04] |
| 5 Desaturation | 13 | 2037 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.40, 1.16] |
| 5.1 Children | 10 | 1542 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.26, 1.17] |
| 5.2 Adults | 4 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.60, 1.36] |
| 6 Desaturation | 13 | 2037 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.36, 1.18] |
| 6.1 With muscle relaxant | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.10] |
| 6.2 Without muscle relaxant | 11 | 1818 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.37, 1.24] |
| 7 Desaturation | 13 | 2037 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.36, 1.18] |
| 7.1 < 90% | 5 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.21, 1.07] |
| 7.2 > 90% and < 96% | 8 | 1060 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.31, 1.61] |
| 8 Airway obstruction | 8 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 2.69 [1.32, 5.50] |
| 8.1 Children | 6 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [1.28, 6.22] |
| 8.2 Adults | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 2.46 [0.22, 27.40] |
| 9 Incidence of nausea, vomiting or retching | 7 | 1192 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.15, 1.13] |
| 9.1 Children | 5 | 936 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.13, 1.51] |
| 9.2 Adults | 2 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.05, 2.08] |
| 10 Incidence of biting on the LMA/LMA damage | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.08, 0.51] |
1.9. Analysis.
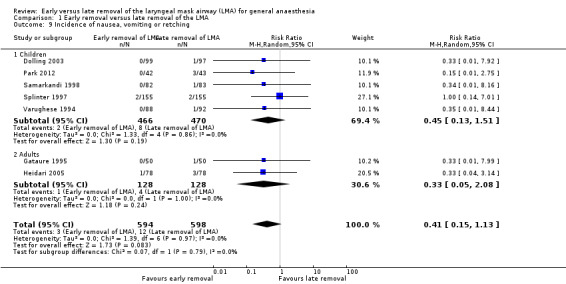
Comparison 1 Early removal versus late removal of the LMA, Outcome 9 Incidence of nausea, vomiting or retching.
1.10. Analysis.
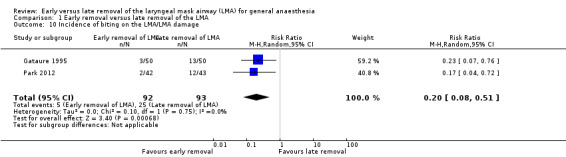
Comparison 1 Early removal versus late removal of the LMA, Outcome 10 Incidence of biting on the LMA/LMA damage.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baird 1999.
| Methods | Prospective, randomized, single‐centre trial | |
| Participants | N = 300, age 1.5 to 81 years, scheduled for elective day‐case surgery | |
| Interventions | Type of LMA: LMA Classic Early removal ‐ The LMA removed by anaesthetist in operating theatre and replaced by appropriately sized Guedel airway and oxygen delivered by Hudson mask Late removal ‐ The LMA left in situ with oxygen delivered through modified T piece ‐ removed by awake patients themselves or nurse under supervision in PACU. Patient was considered awake if he or she could open eyes and mouth |
|
| Outcomes | Coughing Desaturation defined as oxygen saturation < 96% Airway obstruction |
|
| Notes | No premedication Anaesthesia induced with propofol and fentanyl, maintained on N2O + O2 + Isoflurane Patients breathed spontaneously during the procedure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients assigned to one of two groups by random allocation. Randomization was carried out by "envelope randomization in the operating theatre" COMMENT ‐ There is no description of method used for generation of random sequence such as shuffling of envelope or coin tossing |
| Allocation concealment (selection bias) | Unclear risk | "No patient was aware of their randomization status, and envelopes were opened after induction of anaesthesia and towards the end of surgery" COMMENT ‐ It is not clear whether the sequence was concealed from the investigators as the authors do not mention that the envelopes were opaque |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | High risk | "It was not possible to double‐blind the study, as the observers needed to be able to see patients' airway in order to record the incidence of complications" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ Though no explicit statement by authors, the outcomes are reported for 300 patients who were randomized |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
Cheong 1999.
| Methods | Prospective, randomized clinical trial | |
| Participants | N = 63, adult patients scheduled for orthopaedic surgery of lower extremity under general anaesthesia. Patients with anticipated airway difficulty, history of gastro‐oesophageal reflux, hiatal hernia, previous gastric surgery or morbid obesity, and those who were receiving medications affecting gastric pH (H2 blockers) were excluded from the study | |
| Interventions | Type of LMA: LMA Classic Early removal ‐ The LMA was removed when signs of rejection like swallowing, struggling and restlessness appeared Late removal ‐ The LMA was removed when the patient could open his or her mouth on command |
|
| Outcomes | Coughing Desaturation <95% Bucking and straining Pulmonary aspiration |
|
| Notes | Induction ‐ thiopental + succinylcholine Maintenance ‐ N2O + O2 + enflurane+ vecuronium |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "At the end of the operation (to avoid the anaesthesiologists' bias during the period of anaesthetic induction and maintenance), patients were allocated randomly by coin toss to one of the two groups" |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ It is not clear if the anaesthesiologist observing the outcomes was aware or unaware of the allocation |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | Low risk | "Physical events during the arousal phase ‐ bucking, straining and coughing ‐ were recorded by an independent observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The situations to consider eliminating the subject from data analysis did not arise" |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported for all patients |
Dolling 2003.
| Methods | Prospective, randomized controlled trial | |
| Participants | N= 205, children admitted for elective dental day‐case surgery. Exclusion criteria ‐ ASA III‐V, history of gastro‐oesophageal reflux | |
| Interventions | Type of LMA: LMA Classic Early removal ‐ The LMA was removed whilst deeply anaesthetized, immediately after the end of the procedure Late removal ‐ The LMA was removed when patient was able to open eyes and mouth on command |
|
| Outcomes | Laryngospasm Coughing Desaturation Use of additional airway devices Postoperative nausea or vomiting |
|
| Notes | Induction ‐ propofol Maintenance ‐ N2O + O2 + sevoflurane Spontaneous breathing |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned to one of the two groups by toss of a coin" |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ There is no mention about how the sequence was concealed from the investigators |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | High risk | Neither the patient nor the guardian was aware of the group to which the patient had been assigned. The anaesthetist was informed of the assigned group before surgery was completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Out of 205 patients, nine patients were excluded from the analysis after recruitment; an LMA was deemed unnecessary by the anaesthetist in six patients having only one or two front teeth removed; two were obese and were withdrawn from the study by the anaesthetist. The ninth patient was excluded from analysis because his LMA was accidentally removed deep when he had been randomly assigned to the awake group" COMMENT ‐ Available case analysis (on 196 patients) was performed by authors. Of the nine excluded, eight exclusions occurred before randomization |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
Gataure 1995.
| Methods | Prospective, randomized clinical trial | |
| Participants | N = 100, spontaneously breathing patients undergoing urological surgery. Patients < 18 years of age and those at risk of regurgitation were excluded | |
| Interventions | Type of LMA: LMA Classic Early removal ‐ The LMA was removed by anaesthetist in OR while deeply anaesthetized ‐ N2O and enflurane were not turned off until after removal of laryngeal mask Late removal ‐ The LMA was removed by recovery nurse when patient responded to commands |
|
| Outcomes | Coughing Desaturation (cut‐off not defined) Biting Retching Vomiting Excessive salivation Airway obstruction Regurgitation before and after removal of the LMA |
|
| Notes | Induction ‐ alfentanil + propofol Maintenance ‐ N2O + O2 + enflurane, Bain's circuit and spontaneous breathing |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients randomly allocated" COMMENT ‐ No information on the method of generation of allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ No information on the method of allocation concealment |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | High risk | Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ Outcomes reported for all 100 patients recruited |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
Heidari 2005.
| Methods | Prospective, randomized double‐blind study in 4 groups | |
| Participants | N = 156, ASA I‐II, age 18 to 65 years Short time elective surgery under general anaesthesia |
|
| Interventions | Type of LMA: LMA Classic 4 groups ‐ Group 1: halothane anaesthesia and early removal of the LMA; Group 2: halothane anaesthesia and late removal of the LMA; Group 3: propofol anaesthesia and early removal of the LMA; Group 4: propofol anaesthesia and late removal of the LMA Early removal ‐ The LMA was removed at the end of surgery when patient was still receiving anaesthetic drug, N2O was discontinued and after inhalation of 100% O2 for at least 5 minutes, while the patient was still anaesthetized. Oral airway was inserted and finally anaesthetic drug was discontinued Late removal ‐ The LMA was removed in the presence of spontaneous eye opening, purposeful movement of extremities without any physical stimulation, and responding to verbal commands |
|
| Outcomes | Laryngospasm Cough and straining Desaturation (SpO2 < 90%) Postoperative nausea and vomiting Bronchospasm Breath‐holding |
|
| Notes | Induction ‐ fentanyl + lidocaine + thiopental Muscle relaxant ‐ atracurium Maintenance ‐ N2O + O2+ halothane/propofol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients randomly assigned in halothane and propofol group, using computerized generated random list. Then in each group, patients assigned randomly in early and late LMA removal groups" COMMENT ‐ The generation of allocation sequence for early and late LMA removal groups not mentioned (investigators could not be contacted at the correspondence address provided) |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ No information on the method of allocation concealment |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | Low risk | In PACU, an anaesthesiologist who was blind to the type of anaesthetic drug and timing of LMA removal recorded study variables |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ Outcomes reported for all 156 patients randomized |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
Kitching 1996.
| Methods | Randomized, prospective single‐blind study | |
| Participants | N = 60, age 12 months to 8 years, elective surgery (urogenital or plastic) general anaesthesia combined with regional anaesthesia Exclusion criteria: anaesthesia within preceding 3 months, current or chronic upper airway disease, asthma and congenital heart disease |
|
| Interventions | Type of LMA: LMA Classic Early removal ‐ The LMA was removed at twice MAC of halothane (adjusted for age) with N2O Late removal ‐ The LMA was removed in recovery room when the child was awake and could swallow |
|
| Outcomes | Laryngospasm Coughing Desaturation (< 95%) Excessive salivation requiring suction |
|
| Notes | Premedication ‐ oral diazepam 0.3 mg/kg and oral atropine 30 mcg/kg Induction ‐ halothane, oxygen and nitrous oxide, fentanyl 1 mcg/kg followed by insertion of the LMA Maintenance ‐ N2O + O2 + halothane, morphine 0.1 mg/kg intramuscular if required Anaesthesia deepened 5 minutes before anticipated end of surgery by giving twice the alveolar MAC of halothane adjusted for age with N2O in the early removal group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocated randomly, by tossing a coin, to have the LMA removed or left in situ" |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ No information on the method of allocation concealment |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | Unclear risk | "single blinded" COMMENT ‐ It is not clear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ Outcomes reported for all 60 patients randomized |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
Laffon 1994.
| Methods | Prospective, randomized study | |
| Participants | N = 60, ASA I‐II, age 4 months to 12 years, scheduled for minor urologic or upper abdominal surgery | |
| Interventions | Type of LMA: LMA Classic Early removal ‐ patients received halothane at twice MAC and oxygen for 5 minutes before the LMA was removed Late removal ‐ The LMA was removed after the patients opened their eyes or mouths, demonstrated satisfactory volume, respiratory rate or facial grimace |
|
| Outcomes | Laryngospasm Coughing SpO2 before removal of the LMA and lowest SpO2 after removal of the LMA Airway obstruction Apnoea Bronchospasm Arrhythmias |
|
| Notes | Induction & maintenance ‐ N2O + O2 + halothane and spontaneous respiration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ No information on the method of allocation concealment |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | High risk | Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ 60 patients were randomized and their outcomes reported |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
Nunez 1998.
| Methods | Prospective, randomized, single‐centre trial | |
| Participants | N = 66, ASA I‐II, scheduled for elective urological, orthopaedic and minor breast surgery Exclusion criteria ‐ age < 18 and > 80 years Pathology of neck, upper respiratory or upper alimentary tract pathology, those at risk of pulmonary aspiration of gastric contents |
|
| Interventions | Early removal ‐ The LMA was removed while the patient was still deeply anaesthetized at the end of the operation, immediately after discontinuing the volatile agent and nitrous oxide Late removal ‐ The LMA was removed once the patient regained consciousness spontaneously and was able to respond to the verbal command to open the mouth |
|
| Outcomes | Laryngospasm Coughing Desaturation Airway obstruction |
|
| Notes | Induction ‐ propofol 2.5 to 3.0 mg/kg + alfentanil 10 to 20 mcg/kg Maintenance ‐ isoflurane 1% to 2% in nitrous oxide and O2 on spontaneous respiration LMA size 4 for females and size 5 for males |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Allocated randomly to one of two groups by blocked randomization (in blocks of 10)" |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ No information on the method of allocation concealment |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | High risk | Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ Outcomes reported for all 66 patients randomized |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
Pappas 2001.
| Methods | Randomized observer‐blinded study | |
| Participants | N = 123, age 4 months to 7 years, ASA I‐II, scheduled for infra‐umbilical surgery 4 groups ‐ Group 1: isoflurane anaesthesia and early removal of the LMA; Group 2: isoflurane anaesthesia and late removal of the LMA; Group 3: sevoflurane anaesthesia and early removal of the LMA; Group 4: sevoflurane anaesthesia and late removal of the LMA |
|
| Interventions | Type of LMA: LMA Classic Early removal ‐ The LMA was removed at twice the age‐adjusted MAC end‐tidal concentration of isoflurane/ sevoflurane Late removal ‐ The LMA was removed when patients were awake i.e. in the presence of spontaneous eye opening, grimacing, coughing, and purposeful movement of extremities without any physical stimulation |
|
| Outcomes | Desaturation Airway reactivity Vomiting Bronchospasm Overall airway adverse event |
|
| Notes | Induction & maintenance ‐ according to study groups Spontaneous breathing Supplemental regional analgesia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Assigned to one of the four groups using computer‐generated random sequence" |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ No description of method of allocation concealment |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | Low risk | "A research nurse, blinded to the anaesthetic drug and technique, recorded all study variables" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ 123 patients randomized and four excluded after randomization (3.3% dropout). Authors have done available case analysis |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
Park 2012.
| Methods | Randomized, prospective, parallel‐group study | |
| Participants | Enrolled = 92, Randomized = 85 Excluded after enrolment and before randomization = 7 Age: 2 to 6 years, ASA I‐II, elective inguinal hernia repair or hydrocelectomy as outpatients |
|
| Interventions | Type of LMA: LMA Classic Early removal ‐ The LMA was removed during anaesthesia with 2.2% sevoflurane Late removal ‐ The LMA was removed when patients met the recovery criteria, including facial grimace, spontaneous eye opening, and purposeful arm movement |
|
| Outcomes | Laryngospasm defined as respiratory effort without airflow despite chin lift and jaw thrust, thus requiring assisted positive pressure ventilation Coughing Desaturation (SpO2 < 95%) Breath holding defined as apnoea longer than 5 seconds Excessive secretions requiring pharyngeal suction after LMA removal LMA biting (no removal of LMA due to biting, even when the patient was fully awake) Vomiting Upper airway obstruction requiring use of airway adjuncts or airway support for both chin lift and jaw thrust |
|
| Notes | No premedication Induction & maintenance ‐ sevoflurane, spontaneous respiration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned to either anaesthesia group or the awake group using a computer‐generated random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ No description of method of allocation concealment |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | Low risk | "One investigator was aware of group allocation and was responsible for anaesthesia emergence and LMA removal. The other investigators observed the recovery process and recorded data. The data analyst was blinded to study group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ All the 85 patients randomized completed the study |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ All the outcomes mentioned in methods have been reported in results |
Samarkandi 1998.
| Methods | Prospective, randomized trial | |
| Participants | N = 165, ASA I, infants and children of both sexes aged 2 months to 13 years, scheduled for elective lower limb or perineal surgery Exclusion criteria ‐ current or chronic upper airway disease, asthma, congenital heart disease, major abdominal/thoracic/vascular surgery, at risk of aspiration |
|
| Interventions | Type of LMA: LMA Classic Early removal ‐ The LMA was removed and replaced with a Guedel airway Late removal ‐ The LMA was left in situ until patients demonstrated recovery of airway reflexes and had opened their eyes or mouths |
|
| Outcomes | Laryngeal spasm Coughing Desaturation Retching Bronchospasm Postop nausea and vomiting Excessive salivation |
|
| Notes | Premedication ‐ trimeprazine Induction ‐ halothane, N2O + O2 Maintenance ‐ isoflurane 1‐2% and N2O 50% in O2 followed by the LMA, then caudal with 0.125% bupivacaine End of operation ‐ Patient was turned to one side breathing 100% oxygen 4 L/min |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Assigned randomly according to random number generated by computer" |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ No description of method of allocation concealment |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | High risk | Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ Outcomes have been reported for all 165 patients mentioned in methodology section |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
Sinha 2006.
| Methods | Prospective randomized single‐blind study | |
| Participants | N = 130 children (as confirmed by the corresponding author on query, but 120 children reported in the publication), ASA I‐II, 1‐8 years for herniotomy/orchidopexy/plastic surgery of lower limb Excluded: BMI > 30, symptoms of respiratory tract infection, asthma, risk of aspiration, anticipated difficult airway, hearing defect, neurological disorder, taking medication known to affect electroencephalography (EEG) |
|
| Interventions | Early removal ‐ The LMA was removed in the deep plane of anaesthesia when airway reflexes were still depressed Late removal ‐ The LMA was removed in the awake plane after return of airway reflexes, spontaneous activity and eye opening |
|
| Outcomes | Laryngospasm Coughing Desaturation Clenching of teeth Biting of the LMA Breath holding Vomiting Excessive salivation |
|
| Notes | Premedication ‐ triclofos 50 mg/kg Induction ‐ O2 + N2O + sevoflurane Maintenance ‐ O2 + N2O + sevoflurane + fentanyl and caudal with 0.125% bupivacaine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly allocated to one of the two groups using computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | "Sealed opaque envelopes ‐ the anaesthesiologist who was to insert the LMA opened these envelopes just prior to surgery" |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | High risk | "Single blinding" COMMENT ‐ Patients were blinded to achieve single blinding ‐ communicated by the authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ No explicit statement; 120 patients mentioned in 'Methods' and outcomes reported for 125 patients in 'Results.' On enquiry, trial authors stated that 130 patients were enrolled and 125 patients completed the study (3.9% dropout). Available case analysis performed |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
Splinter 1997.
| Methods | Prospective, randomized, single‐blind study | |
| Participants | N = 333, ASA I‐II, age 1.5 to 15 years | |
| Interventions | Early removal ‐ The LMA was removed before return of airway reflexes. Late removal ‐ The LMA was removed after patient had awakened and had intact airway reflexes, exhibiting purposeful movements including swallowing as an indication of protective reflexes |
|
| Outcomes | Laryngospasm Desaturation (< 90%) Postop nausea or vomiting Stridor Breath holding Excessive salivation |
|
| Notes | Induction ‐ N2O + O2+ halothane/propofol Maintenance ‐ N2O + halothane, isoflurane in older children |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned via a computer generated random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ No mention of method of allocation concealment |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | Unclear risk | "single blinding" COMMENT ‐ It is not clear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | COMMENT ‐ 333 patients were enrolled but 25 did not undergo intervention (the LMA was not used in 17 and surgery was cancelled in 8). However, the results are reported in 310 patients (no response to correspondence seeking clarification on this discrepancy) |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
Thomas 2012.
| Methods | Randomized controlled trial | |
| Participants | N = 216, children aged 1 to 16 years, ASA I‐II, undergoing non‐airway surgery. | |
| Interventions | Four groups for LMA removal ‐ Group 1: early removal in lateral position; Group 2: late removal in lateral position; Group 3: early removal in supine position; Group 4: late removal in supine position Early removal ‐ The LMA was removed in deep plane of anaesthesia Late removal ‐ The LMA was removed when the patients were awake after anaesthesia |
|
| Outcomes | Laryngospasm Desaturation Upper airway obstruction Vomiting Excessive secretions Biting the stem of the LMA |
|
| Notes | This trial is published as an abstract. There is no contact information for the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | COMMENT ‐ The details of randomization process are not specified |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ Allocation concealment not specified |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | Unclear risk | COMMENT ‐There is no mention of the blinding of the observer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ 216 patients were enrolled, 4 patients were excluded for anaesthetic reasons and outcome data are reported in 212 patients |
| Selective reporting (reporting bias) | Unclear risk | COMMENT ‐ 6 outcome variables are mentioned in methodology while three of them are not reported in the results. It is unclear if the variable "Number of complications" includes all of them |
Varughese 1994.
| Methods | Prospective, randomized trial | |
| Participants | N = 180 children, age 3 to 12 years, weight < 30 kg, ASA I‐II for minor day surgery | |
| Interventions | Early removal ‐ The LMA was removed at the end of surgery when 2% halothane in oxygen 100% was administered for 5 minutes Late removal ‐ The LMA was removed upon return of protective airway reflexes and consciousness |
|
| Outcomes | Laryngospasm Coughing Desaturation Postop nausea or vomiting Obstruction Excess salivation Clenching of teeth Use of continuous positive airway pressure (CPAP) after LMA removal Use of oropharyngeal airway, altered head position, jaw lift, suctioning |
|
| Notes | No premedication Induction ‐ propofol Maintenance ‐ N2O + O2+ halothane followed by spontaneous breathing |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated to one of two groups" |
| Allocation concealment (selection bias) | Unclear risk | COMMENT ‐ No mention of allocation concealment |
| Blinding (performance bias and detection bias) Of outcome assessor to all outcomes | High risk | Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT ‐ Outcome data reported for all 180 patients included |
| Selective reporting (reporting bias) | Low risk | COMMENT ‐ Though no explicit statement, the outcomes mentioned in methodology have been reported |
ASA = American Society of Anesthesiologists (physical classification scale) CPAP = continuous positive airway pressure EEG = electroencephalography ITT = intention‐to‐treat LMA = laryngeal mask airway MAC = minimal alveolar concentration OR = operating room PACU = postanaesthesia care unit SpO2 = peripheral capillary oxygen saturation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cameron 2001 | The patients were allocated to the intervention groups in a non‐randomized fashion |
| Flynn 2007 | All the LMAs were removed after the children regained consciousness. There was no intervention group of early removal of the LMA |
| Goldmann 2011 | The interventions compared were awake removal of the LMA in the operating room versus recovery room. There was no group receiving early removal of the LMA |
| Lee 2011 | Early removal of the LMA was compared against early and late removal of endotracheal tube and was not compared against late removal of the LMA |
| Lema 2010 | The interventions studied in this trial were two different anaesthetic agents (desflurane and sevoflurane) delivered through the LMA and the outcomes assessed were complications like cough and laryngeal spasm during and after anaesthesia. Both the intervention and outcomes studied were not relevant to the current systematic review |
| Lerman 2010 | The interventions randomised in this trial were two different anaesthetic agents (desflurane and isoflurane) for maintenance of anaesthesia. The early or late removal of the LMA was at the discretion of the attending anaesthesiologist and therefore, not a randomised intervention |
| McKay 2006 | The interventions in this trial were two anaesthetic agents (desflurane and sevoflurane) delivered through the LMA to patients who smoke to assess the incidence of complications like cough, laryngospasm and desaturation. Both the intervention and outcomes studied were not relevant to the current systematic review |
| Mencke 2010 | It is an editorial |
| Sinha 2009 | In this trial, the intervention groups differed by the type of ventilation instituted through the LMA during anaesthesia ‐ either spontaneous ventilation or pressure controlled ventilation. All the LMAs were removed at the onset of swallowing in these patients. Thus, the intervention studied was different from the review protocol |
| White 2009 | The trial studied early and late recovery characteristics of patients maintained on either desflurane anaesthesia or sevoflurane anaesthesia through the LMA which was removed after the patient woke up from anaesthesia in both groups. Thus the intervention studied did not meet the inclusion criteria of the review |
LMA = laryngeal mask airway
Characteristics of studies awaiting assessment [ordered by study ID]
Iyilikci 1999.
| Methods | Not yet assessed |
| Participants | Thirty urological patients of ASA I‐II risk were randomly divided into two groups |
| Interventions | Early removal of the LMA ‐ patients were subjected to removal of the LMA under deep anaesthesia Late removal of the LMA ‐ removal of the LMA was planned when gag reflex was present and the patient was awake |
| Outcomes | Complications and haemodynamic changes after LMA removal |
| Notes | Induction ‐ propofol + alfentanil Maintenance ‐ O2 + N2O + propofol + alfentanil This study has been published in Turkish and the data extraction as well as risk of bias assessment are pending translation |
ASA = American Society of Anesthesiologists (physical classification scale) LMA = laryngeal mask airway
Differences between protocol and review
One of the co‐authors (Georgia Salanti) of the protocol (Mathew 2008) withdrew from the review process.
It was mentioned in the protocol that blinding in individual trials would not be considered to assess methodological quality. However, we used the Cochrane 'Risk of bias' tool to assess quality. Therefore, blinding was included as one of the criteria.
The primary outcomes decided in the protocol stage were laryngospasm, coughing and desaturation. The outcome of airway obstruction was not included either as a primary or a secondary outcome. During the process of data extraction, we encountered nine included trials that reported airway obstruction which is also important during emergence from anaesthesia. Therefore, we added airway obstruction as a primary outcome for this systematic review.
Contributions of authors
Conceiving the review: Preethy J Mathew (PJM) Designing the review: PJM and Joseph L Mathew (JLM) Co‐ordinating the review: PJM
Undertaking manual searches: PJM Screening search results: PJM and JLM Organizing retrieval of papers: PJM Screening retrieved papers against inclusion criteria: PJM and JLM Appraising quality of papers: PJM and JLM Abstracting data from papers: PJM and JLM Writing to authors of papers for additional information: PJM Providing additional data about papers: PJM Obtaining and screening data on unpublished studies: PJM Data management for the review: PJM and JLM Entering data into Review Manager (RevMan 2014): PJM and JLM Analysis of data: PJM and JLM Interpretation of data: PJM and JLM Writing the review: PJM and JLM Guarantor of the review: PJM Statistical analysis: PJM and JLM
Sources of support
Internal sources
Postgraduate Institute of Medical Education and Research, Chandigarh, India.
External sources
-
South Asian Cochrane Network Centre, India.
Workshop to complete the review
Declarations of interest
Preethy J Mathew: none known Joseph L Mathew: none known
New
References
References to studies included in this review
Baird 1999 {published data only}
- Baird MB, Mayor AH, Goodwin APL. Removal of the laryngeal mask airway: Factors affecting the incidence of post‐operative adverse respiratory events in 300 patients. European Journal of Anaesthesiology 1999;16:251‐6. [PUBMED: 10234495] [DOI] [PubMed] [Google Scholar]
Cheong 1999 {published data only}
- Cheong YP, Park SK, Son Y, Lee KC, Song YK, Yoon JS, et al. Comparison of incidence of gastroesophageal reflux and regurgitation associated with timing of removal of the laryngeal mask airway: On appearance of signs of rejection versus after recovery of consciousness. Journal of Clinical Anesthesia 1999;11:657‐62. [PUBMED: 10680108] [DOI] [PubMed] [Google Scholar]
Dolling 2003 {published data only}
- Dolling S, Anders NRK, Rolfe SE. A comparison of deep vs. awake removal of the laryngeal mask airway in paediatric dental daycase surgery. A randomised controlled trial. Anaesthesia 2003;58:1224‐8. [PUBMED: 14705688] [DOI] [PubMed] [Google Scholar]
Gataure 1995 {published data only}
- Gataure PS, Latto IP. Complications associated with removal of the laryngeal mask airway: A comparison of removal in deeply anaesthetised versus awake patients. Canadian Journal of Anaesthesia 1995;42:1113‐6. [PUBMED: 8595687] [DOI] [PubMed] [Google Scholar]
Heidari 2005 {published data only}
- Heidari SM, Abbasi S, Rahimi M. Removal of Laryngeal Mask Airway: Awake or deep anesthesia?. Journal of Research in Medical Sciences 2005;10:59‐62. [Google Scholar]
Kitching 1996 {published data only}
- Kitching AJ, Walpole AR, Blogg CE. Removal of the laryngeal mask airway in children: Anaesthetized compared with awake. British Journal of Anaesthesia 1996;76:874‐6. [PUBMED: 8679367] [DOI] [PubMed] [Google Scholar]
Laffon 1994 {published data only}
- Brain AIJ. Removal of laryngeal mask airway: airway complications in children, anaesthetized versus awake. Paediatric Anaesthesia 1994;4:271. [Google Scholar]
- Laffon M, Plaud B, Dubousset AM, Ben Haj'hmida R, Ecoffey C. Removal of laryngeal mask airway: airway complications in children, anaesthetized versus awake. Paediatric Anaesthesia 1994;4:35‐7. [Google Scholar]
Nunez 1998 {published data only}
- Nunez J, Hughes J, Wareham K, Asai T. Timing of removal of the laryngeal mask airway. Anaesthesia 1998;53:126‐30. [PUBMED: 9534633] [DOI] [PubMed] [Google Scholar]
Pappas 2001 {published data only}
- Pappas AL, Sukhani R, Lurie J, Pawlowski J, Sawicki K, Corsino A. Severity of airway hyperreactivity associated with laryngeal mask airway removal: Correlation with volatile anesthetic choice and depth of anaesthesia. Journal of Clinical Anesthesia 2001;13:498‐503. [PUBMED: 11704447] [DOI] [PubMed] [Google Scholar]
Park 2012 {published data only}
- Park JS, Kim KJ, Choi EK, Lee JR. A randomized controlled trial comparing Laryngeal Mask Airway removal during adequate anesthesia and after awakening in children aged 2 to 6 years. Journal of Clinical Anesthesia 2012;24:537‐41. [PUBMED: 22999984] [DOI] [PubMed] [Google Scholar]
Samarkandi 1998 {published data only}
- Samarkandi AH. Awake removal of the laryngeal mask airway is safe in paediatric patients. Canadian Journal of Anaesthesia 1998;45:150‐2. [PUBMED: 9512850] [DOI] [PubMed] [Google Scholar]
Sinha 2006 {published data only (unpublished sought but not used)}
- Sinha A, Sood J. Safe removal of LMA in children ‐ At what BIS?. Pediatric Anesthesia 2006;16:1144‐7. [PUBMED: 17040303] [DOI] [PubMed] [Google Scholar]
Splinter 1997 {published data only}
- Splinter WM, Reid CW. Removal of the laryngeal mask airway in children: Deep anesthesia versus awake. Journal of Clinical Anesthesia 1997;9:4‐7. [PUBMED: 9051538] [DOI] [PubMed] [Google Scholar]
Thomas 2012 {published data only}
- Kasisomayajula A, Thomas‐Kattappurathu G, Hageman AD, Short J. Best position and depth of anaesthesia for laryngeal mask airway removal in children ‐ A randomized controlled trial. Paediatric Anaesthesia 2012;22:909‐10. [DOI] [PubMed] [Google Scholar]
- Thomas‐Kattappurathu G, Kasisomayajula A, Hageman AD, Short J. Best position and depth of anaesthesia for LMA removal in children ‐ A randomised controlled trial. Paediatric Anaesthesia 2012;22:923. [DOI] [PubMed] [Google Scholar]
Varughese 1994 {published data only}
- Varughese A, McCulloch D, Lewis M, Stokes M. Removal of laryngeal mask airway (LMA) in children: Awake or deep?. Anesthesiology 1994;81:3A. [Google Scholar]
References to studies excluded from this review
Cameron 2001 {published data only}
- Cameron AJD, Sellers WFS. Early vs late LMA removal; risk to patients and damage to equipment. Anaesthesia and Intensive Care 2001;29:80‐1. [PUBMED: 11261922] [PubMed] [Google Scholar]
Flynn 2007 {published data only}
- Flynn P, Ahmed FB, Mitchell V, Patel A, Clarke S. A randomised comparison of the single use LMA FlexibleTM with reusable LMA FlexibleTM in paediatric dental day‐case patients. Anaesthesia 2007;62:1281‐4. [PUBMED: 17991266] [DOI] [PubMed] [Google Scholar]
Goldmann 2011 {published data only}
- Goldmann K, Kuhlmann S, Gerlach M, Borntrager C. Removal of the laryngeal mask airway in the post‐anesthesia care unit. A means of process optimization?. Anaesthesist 2011;60:1002‐8. [PUBMED: 21881929] [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee YC, Kim JM, Ko HB, Lee SR. Use of laryngeal mask airway and its removal in a deeply anaesthetized state reduces emergence agitation after sevoflurane anaesthesia in children. The Journal of International Medical Research 2011;39:2385‐92. [PUBMED: 22289558] [DOI] [PubMed] [Google Scholar]
Lema 2010 {published data only (unpublished sought but not used)}
- Lema FE, Tafur LA, Giraldo C, Delgado MA. Incidence of cough after desflurane and sevoflurane administration through a laryngeal mask: a controlled clinical trial. Revista Española de Anestesiología y Reanimación 2010;57:141‐6. [PUBMED: 20422846] [DOI] [PubMed] [Google Scholar]
Lerman 2010 {published data only}
- Lerman J, Hammer GB, Verghese S, Ehlers M, Khalil SN, Betts E, et al. Airway responses to desflurane during maintenance of anesthesia and recovery in children with laryngeal mask airways. Pediatric Anesthesia 2010;20:495‐505. [PUBMED: 20456065] [DOI] [PubMed] [Google Scholar]
McKay 2006 {published data only}
- McKay RE, Bostrom A, Balea MC, McKay WR. Airway responses during desflurane versus sevoflurane administration via a laryngeal mask airway in smokers. Anesthesia and Analgesia 2006;103:1147‐54. [PUBMED: 17056947] [DOI] [PubMed] [Google Scholar]
Mencke 2010 {published data only}
- Mencke T, Schomburg GN. Laryngeal morbidity after use of laryngeal mask airway. Acta Anaesthesiologica Scandinavica 2010;54:127‐8. [DOI: 10.1111/j.1399-6576.2009.02159.x.] [DOI] [PubMed] [Google Scholar]
Sinha 2009 {published data only}
- Sinha A, Sharma B, Sood J. Proseal laryngeal mask airway in infants and toddlers with upper respiratory tract infections: A randomised control trial of spontaneous versus pressure control ventilation. Middle Eastern Journal of Anaesthesia 2009;20:437‐43. [PubMed] [Google Scholar]
White 2009 {published data only}
- White PF, Tang J, Wender RH, Yumul R, Stokes OJ, Sloninsky A, et al. Desflurane versus sevoflurane for maintenance of outpatient anesthesia: The effect on early versus late recovery and perioperative coughing. Anesthesia and Analgesia 2009;109:387‐93. [PUBMED: 19608808] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Iyilikci 1999 {published data only}
- Iyilikci L, Tezcan G, Gokel E. Comparison of complications and hemodynamic changes during and after removal of laryngeal mask airway in deep anesthesia versus awake patients. Turk‐Anesteziyoloji‐ve‐Reanimasyon 1999;27:423‐6. [Google Scholar]
Additional references
Brain 1993
- Brain AIJ. The Intavent Laryngeal Mask: Instruction Manual. 2nd Edition. Henley‐on‐Thames, England: Intavent International, 1993. [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc). GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Version [17 Sept 2014]. Hamilton: McMaster University (developed by Evidence Prime, Inc), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;15(21):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jaffe 1972
- Jaffe BF. Postoperative hoarseness. American Journal of Surgery 1972;123:432. [DOI] [PubMed] [Google Scholar]
Lopez‐Gill 1996
- Lopez‐Gill M, Brimacombe J, Alvarez M. Safety and efficacy of the laryngeal mask airway. A prospective survey of 1400 children. Anaesthesia 1996;51:969‐72. [DOI] [PubMed] [Google Scholar]
Mason 1990
- Mason DG, Bingham RM. The laryngeal mask airway in children. Anaesthesia 1990;45:760‐3. [DOI] [PubMed] [Google Scholar]
Morikawa 1961
- Morikawa S, Safer P, Decarlo J. Influence of the head‐jaw position upon upper airway patency. Anesthesiology 1961;22:265. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruben 1961
- Ruben HM, Elam JO, Ruben AM, Greene DG. Investigation of upper airway problems in resuscitation. Anesthesiology 1961;22:271‐9. [DOI] [PubMed] [Google Scholar]
Stone 1994
- Stone DJ, Gal TJ. Airway management. In: Miller RD editor(s). Anesthesia. Churchill Livingstone, New York, 1994. [Google Scholar]
References to other published versions of this review
Mathew 2008
- Mathew PJ, Mathew JL, Salanti G. Early versus late removal of laryngeal mask airway (LMA) for general anaesthesia. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD007082] [DOI] [PMC free article] [PubMed] [Google Scholar]


