Abstract
Introduction
Although diabetes mellitus (DM) increases the risk of proteinuria, the relationship between prediabetes and proteinuria remains not fully understood. Further, whether the change in glucose is associated with the risk for proteinuria is unknown.
Methods
This was a retrospective cohort study that included 1,849,074 participants (median age, 45 years; 59.3% men). No participants were taking glucose-lowering medications, and none had positive proteinuria at the initial health check-up. Each participant was categorized into three groups: normal (hemoglobin A1c [HbA1c] of <5.7%, n = 1,563,121), prediabetes (HbA1c of 5.7–6.4%, n = 253,490), and DM (HbA1c of ≥6.5%, n = 32,463) groups. We investigated the association between each HbA1c category and incident proteinuria using Cox proportional hazards models. We analyzed the association between the annual change in HbA1c and the risk for proteinuria.
Results
A total of 65,954 participants developed proteinuria during the observation period. Not only DM (hazard ratio [HR]: 2.15, 95% confidence interval [CI]: 2.07–2.24) but also prediabetes (HR: 1.14, 95% CI: 1.12–1.17) was associated with a greater risk for proteinuria. The relative risk reduction for proteinuria that was associated with prediabetes and DM was 12.3% and 53.5%, respectively. An annual increase in HbA1c was associated with a greater risk for proteinuria. This association was more pronounced in participants having prediabetes.
Conclusion
Not only DM but also prediabetes increased the risk for proteinuria. The influence of change in HbA1c on incident proteinuria was pronounced in people with prediabetes. Optimizing glucose would provide more benefit to individuals having prediabetes for proteinuria prevention.
Keywords: Prediabetes, Diabetes mellitus, Proteinuria, Epidemiology
Introduction
Proteinuria accelerates worsening kidney function and leads to the development of cardiovascular disease (CVD) events, thereby increasing the risk for all-cause mortality [1, 2, 3, 4, 5, 6]. Thus, proteinuria is considered a predisposing factor of kidney dysfunction, CVD, and life-threatening events. Diabetes mellitus (DM) is a major risk factor for proteinuria mainly via diabetic kidney disease [7, 8]. Moreover, given that up to one-third of patients newly diagnosed with DM already have kidney damage [9, 10, 11, 12], the risk of chronic kidney disease (CKD) could increase even in people with prediabetic hyperglycemia. However, whether prediabetes increases the risk for proteinuria development remains controversial [13, 14, 15, 16, 17, 18, 19, 20, 21]. For example, the SPRINT trial has showed that prediabetes was not associated with the development of albuminuria [18]. Although the relationship between prediabetes and the development of proteinuria needs to be elucidated in more detail, there are certain limitations in estimating the association between glycemia and the development of proteinuria because many studies do not completely exclude people receiving treatment with glucose-lowering medications that affect renal function and various classifications for glycemic status (based on hemoglobin A1c [HbA1c] or fasting plasma glucose [FPG]) were used in their analyses [13, 14, 15, 16, 17, 18, 19, 20, 21].
Further, although previous studies have examined a relationship between prediabetes (or DM) and an elevated risk for CKD [13, 14, 15, 16, 17, 18, 19, 20, 21], the specific CKD risk that is attributable to prediabetes (or DM) has not been studied. In particular, little is known regarding the relationship between changes in glucose levels and the risk of proteinuria development; therefore, whether normalizing glucose level can be a therapeutic goal for the prevention of proteinuria in people with elevated glucose levels remains unknown.
Using a nationwide population-based database, we studied individuals not taking glucose-lowering medications and examined at what medication-naïve HbA1c level is associated with an increased risk for proteinuria. Additionally, we analyzed the relationship between changes in HbA1c level and the risk for proteinuria to examine whether normalizing HbA1c levels could influence the incidence of future proteinuria events.
Materials and Methods
Study Design and Data Source
This was a retrospective cohort study using the health check-up and claims database in Japan (JMDC Claims Database) [22, 23, 24]. Claims data combined with health check-up data were included in this dataset. For the current study, we selected the records of individuals who had HbA1c levels available and were negative for proteinuria at their initial health check-up between January 2005 and April 2020 (n = 1,992,501). We excluded individuals aged <20 years (n = 11,380), those with a previous history of CVD or dialysis (n = 46,897), those with missing data regarding the use of glucose-lowering medications (n = 1,689), those taking glucose-lowering medications (n = 44,083), those with missing data on taking blood pressure-lowering or lipid-lowering medications (n = 76), current cigarette smokers (n = 2,255), and alcohol consumption (n = 37,047). Finally, 1,849,074 individuals were included in this study (online suppl. Fig. 1; see www.karger.com/doi/10.1159/000522280 for all online suppl. material).
Ethics
We performed this study based on the ethical guidelines of the University of Tokyo. This study was approved by the Ethics Committee of the University of Tokyo (No. 2018-10862) and was performed in accordance with the principles of the Declaration of Helsinki. Since data in the JMDC Claims Database are de-identified, the informed consent requirement was waived.
HbA1c Category and Other Measurements
Health check-up data, including body mass index, blood pressure, prior medical history, and fasting laboratory values, were obtained using standardized methods. HbA1c levels were described using the National Glycohemoglobin Standardization Program scale [25]. According to the HbA1c values at the initial health check-up, we classified the study population into the normal group (HbA1c level of <5.7%), prediabetes group (HbA1c level of ≥5.7% and ≤6.4%), and DM group (HbA1c level of ≥6.5%) [26, 27]. We defined overweight/obesity as a body mass index ≥25 kg/m2. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or receiving treatment with blood pressure-lowering medications. We defined dyslipidemia as low-density lipoprotein cholesterol level ≥140 mg/dL, high-density lipoprotein cholesterol level <40 mg/dL, triglycerides ≥150 mg/dL, or receiving treatment with lipid-lowering medications. We obtained information on cigarette smoking (current or noncurrent) and alcohol consumption (daily or occasional/none) using self-reported questionnaires.
Urine Dipstick Test
Urine dipstick tests were conducted using fresh, midstream urine samples. Protein concentrations were graded as negative (<10 mg/dL), trace (10–20 mg/dL), or positive (≥30 mg/dL). The sensitivity and specificity of the positive proteinuria on the urine dipstick test for detecting albumin-to-creatinine ratio ≥30 mg/g are 47.0% and 96.2% [28]. Furthermore, the sensitivity and specificity of the trace or positive proteinuria for detecting 150–499 mg/g or higher in the protein-to-creatinine ratio are 66.2% and 95.6% [29].
Outcomes
The primary outcome of this study is positive proteinuria (≥1+) assessed by the urine dipstick test. Study participants were followed up from the initial health check-up until the outcome occurrence date or date of last health check-up in our dataset.
Statistical Analysis
We described data as median (Q1–Q3) or number (percentage). Continuous and categorical variables were compared using one-way analyses of variance and the χ2 test among the three HbA1c categories. We performed Cox proportional hazards regression analyses to examine the relationship between the three HbA1c categories and the risk for proteinuria. We calculated hazard ratios (HRs) in an unadjusted model (model 1), age- and sex-adjusted model (model 2), and after adjusting for potential confounders, including age, sex, overweight/obesity, hypertension, dyslipidemia, cigarette smoking, and alcohol consumption (Model 3).
We analyzed the association between HbA1c levels as a continuous variable and incident proteinuria. To allow for a flexible interpretation of the association between HbA1c values and proteinuria development, continuous changes in HbA1c were evaluated using restricted cubic spline (RCS) regression models with five knots [30]. We set an HbA1c level of 5.7% as the reference. HRs were calculated after adjusting for covariates including age, sex, overweight/obesity, hypertension, dyslipidemia, cigarette smoking, and alcohol consumption.
We included 1,049,386 people who had HbA1c data available, were negative for proteinuria, and were not receiving treatment with glucose-lowering medications 1 year after the initial health check-up. We analyzed the relationship between changes in HbA1c levels and incident proteinuria. We calculated the HR of an increase in HbA1c level ≥0.5% after adjusting for potential confounders, including age, sex, overweight/obesity, hypertension, dyslipidemia, cigarette smoking, alcohol consumption, and HbA1c category at the initial health check-up. We calculated the relative risk reduction (RRR) based on HR of each HbA1c category. We also calculated the RRR of an increase in HbA1c level ≥0.5% for the risk of proteinuria.
We conducted seven sensitivity analyses. First, we performed multiple imputations for missing variables. Using the chained equation method described by Aloisio et al. [31], we imputed missing variables under the missing at random assumption. HRs and standard errors were calculated using Rubin's rules. Second, we defined proteinuria as ≥ trace on the urine dipstick test. Third, we excluded 322,711 people who had hypertension. Fourth, we conducted subgroup analyses stratified by age or sex. Fifth, we included 709,817 participants with data on serum creatinine level at the initial health check-up and conducted subgroup analyses stratified by estimated glomerular filtration rate (eGFR) (≥60 mL/min/1.73 m2 vs. <60 mL/min/1.73 m2). We calculated eGFR using the following GFR equation designed for the Japanese population: eGFR = 194 × (serum creatinine)−1.094 × (age)−0.287 (× 0.739, if women) [32]. Sixth, we analyzed 1,536,874 people with FPG data at their initial health check-up and categorized the study population into 4 groups: normal FPG group (FPG level of <100 mg/dL), normal-high FPG group (FPG level of ≥100 mg/dL and ≤109 mg/dL), impaired fasting glucose (IFG) group (FPG level of ≥110 mg/dL and ≤125 mg/dL), and DM group (FPG level of ≥126 mg/dL) [24]. Furthermore, we analyzed the relationship between FPG levels and incident proteinuria using RCS regression models, for which we set an FPG of 100 mg/dL as the reference. Seventh, we included 806,603 individuals who also had FPG data 1 year after the initial health check-up available. We estimated the HR and RRR of an increase in FPG level ≥5 mg/dL for the risk of proteinuria. Statistical significance was set at p < 0.05. Statistical analyses were performed using STATA v.17 (StataCorp LLC, College Station, TX, USA).
Results
Clinical Characteristics of Study Population
The median age of the study population was 45 (39–52) years, and 1,096,561 participants (59.3%) were men. According to HbA1c values, study participants were categorized into the normal (n = 1,563,121), prediabetes (n = 253,490), or DM (n = 32,463) group. Participants with prediabetes or DM were older than those with normal HbA1c levels. The proportion of men increased as the HbA1c-level category increased. The prevalence of overweight/obesity, hypertension, dyslipidemia, and cigarette smoking also increased with higher HbA1c categories (Table 1).
Table 1.
Clinical characteristics
| Normal (n = 1,563,121) | Prediabetes (n = 253,490) | DM (n = 32,463) | p value | |
|---|---|---|---|---|
| HbAlc | 5.3 (5.2, 5.5) | 5.9 (5.8, 6.0) | 6.9 (6.6, 7.6) | <0.001 |
| FPG, mg/dL | 90 (85, 96) | 99 (93, 107) | 130 (116, 150) | <0.001 |
| Age, years | 44 (38, 51) | 52 (45, 58) | 53 (46, 59) | <0.001 |
| Men | 917,759 (58.7) | 153,903 (60.7) | 24,899 (76.7) | <0.001 |
| Body mass index, kg/m2 | 22.0 (20.1, 24.3) | 23.8 (21.4, 26.5) | 26.1 (23.5, 29.2) | <0.001 |
| Overweight/obesity | 309,440 (19.8) | 96,678 (38.1) | 19,850 (61.1) | <0.001 |
| Hypertension | 233,493 (14.9) | 74,342 (29.3) | 14,876 (45.8) | <0.001 |
| Systolic blood pressure, mm Hg | 117 (107, 127) | 122 (112, 133) | 129 (120, 140) | <0.001 |
| Diastolic blood pressure, mm Hg | 72 (65, 80) | 76 (68, 84) | 81 (74, 88) | <0.001 |
| Dyslipidemia | 554,036 (35.4) | 149,505 (59.0) | 23,926 (73.7) | <0.001 |
| Low-density lipoprotein cholesterol, mg/dL | 116 (97, 138) | 130 (110, 151) | 134 (113, 157) | <0.001 |
| High-density lipoprotein cholesterol, mg/dL | 63 (53, 75) | 58 (48, 70) | 51 (44, 60) | <0.001 |
| Triglycerides, mg/dL | 79 (56, 118) | 103 (71, 153) | 136 (94, 201) | <0.001 |
| Cigarette smoking | 405,747 (26.0) | 68,351 (27.0) | 11,518 (35.5) | <0.001 |
| Alcohol drinking | 381,615 (24.4) | 56,355 (22.2) | 7,913 (24.4) | <0.001 |
Data are expressed as median (interquartile range) or number (percentage). Participants were categorized based on HbAlc category as normal (HbAlc level of <5.7%), prediabetes (HbAlc level of 5.7–6.4%), and DM (HbAlc level of ≥6.5%). HbAlc, hemoglobin Alc; DM, diabetes mellitus. p values were calculated using the analysis of variance for continuous variables and χ2 tests for categorical variables.
HbA1c Category and Proteinuria
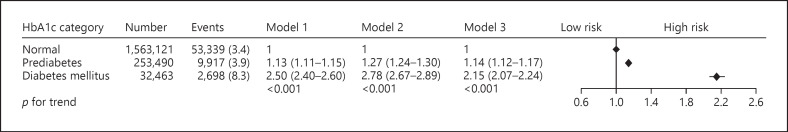
Figure 1 presents the association between HbA1c category and the risk for developing proteinuria. During the observational period, 65,954 participants developed proteinuria. Unadjusted regression analyses showed that prediabetes (HR: 1.13, 95% confidence interval [CI]: 1.11–1.15) and DM (HR: 2.50, 95% CI: 2.40–2.60) were associated with a higher incidence of proteinuria than normal. A stepwise increase in the risk for proteinuria with HbA1c category did not change even after adjusting for age and sex. Multivariable regression analysis (Model 3) demonstrated that prediabetes (HR: 1.14, 95% CI: 1.12–1.17) and DM (HR: 2.15, 95% CI: 2.07–2.24) were still associated with a greater risk for proteinuria than normal.
Fig. 1.
HbA1c category and proteinuria. The association of HbA1c category with the risk for the development of proteinuria. During the observational period, 65,954 participants developed proteinuria. Multivariable regression analyses demonstrated that prediabetes (HR: 1.14, 95% CI: 1.12–1.17) and DM (HR: 2.15, 95% CI: 2.07–2.24) were associated with a greater risk for the development of proteinuria than normal HbA1c category.
Restricted Cubic Spline
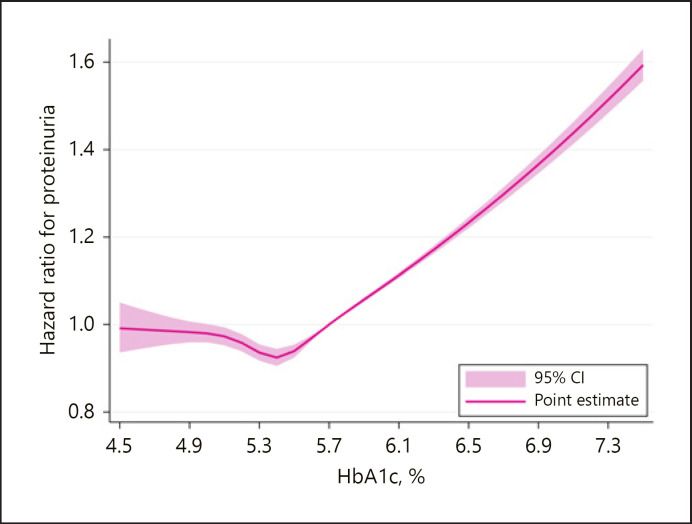
The dose-response association between medication-naïve HbA1c levels and the development of proteinuria is shown in Figure 2. The relationship between HbA1c levels and the subsequent risk for proteinuria development was modeled using multivariable-adjusted RCS regression models with a reference point set at an HbA1c of 5.7%. The risk for proteinuria development monotonically increased with HbA1c level after it exceeded approximately 5.4%.
Fig. 2.
RCS showed the linear dose-response relationship between medication-naïve HbA1c and the risk for proteinuria development. The reference point was set at HbA1c of 5.7%. The association between HbA1c and proteinuria development was adjusted for age, sex, overweight/obesity, hypertension, dyslipidemia, cigarette smoking, and alcohol consumption.
Change in HbA1c and Incident Proteinuria
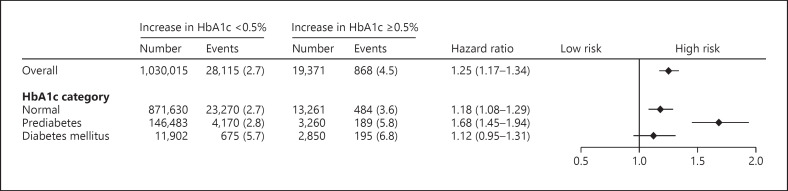
The median (Q1–Q3) change in HbA1c 1 year after the initial health check-up was 0.0 (−8.3 to +0.1)%. We defined an increase in HbA1c ≥0.5% as a significant metabolic response with reference to the National Institute for Health and Clinical Excellence clinical guideline [33]. The multivariable Cox regression analysis, which included an increase in HbA1c ≥0.5%, age, sex, overweight/obesity, hypertension, dyslipidemia, cigarette smoking, alcohol consumption, and HbA1c category showed that an increase in HbA1c ≥0.5% was associated with a greater risk for proteinuria development (HR: 1.25, 95% CI: 1.17–1.34). We also conducted a subgroup analysis stratified by HbA1c category. An increase in HbA1c level ≥0.5% was associated with a greater risk for proteinuria development in participants with normal levels (HR: 1.18, 95% CI: 1.08–1.29) and prediabetes (HR: 1.68, 95% CI: 1.45–1.94). An increase in HbA1c level ≥0.5% was not associated with incident proteinuria in participants with DM (HR: 1.12, 95% CI: 0.95–1.31) (Fig. 3).
Fig. 3.
Change in HbA1c and proteinuria. Multivariable regression analysis including increase in HbA1c ≥0.5%, age, sex, overweight/obesity, hypertension, dyslipidemia, cigarette smoking, alcohol consumption, and HbA1c category at the initial health check-up showed that increase in HbA1c ≥0.5% was associated with a greater risk for proteinuria development (HR: 1.25, 95% CI: 1.17–1.34). We conducted a subgroup analysis stratified by HbA1c category. Increase in HbA1c ≥0.5% was associated with a greater risk for proteinuria development in participants with normal (HR: 1.18, 95% CI: 1.08–1.29) and prediabetes (HR: 1.68, 95% CI: 1.45–1.94). Increase in HbA1c ≥0.5% was not associated with incident proteinuria in participants with DM (HR: 1.12, 95% CI: 0.95–1.31).
Relative Risk Reduction
The RRR for proteinuria development associated with prediabetes and DM was 12.3% (95% CI: 10.3–14.3%) and 53.5% (95% CI: 51.6–55.3%), respectively. Overall, the RRR for proteinuria development associated with an increase in HbA1c level ≥0.5% was 20.2% (95% CI: 14.4–25.5%). The RRR for proteinuria development associated with an increase in HbA1c level ≥0.5% in participants with normal levels and prediabetes was 15.1% (95% CI: 7.1–22.4%) and 40.4% (95% CI: 31.0–48.6%), respectively. The RRR for proteinuria development associated with an increase in HbA1c level ≥0.5% in participants with DM was 10.5% (95% CI: −5.3 to +23.9%).
Sensitivity Analyses
First, we conducted multiple imputations for missing data on blood pressure-lowering medications (n = 25), lipid-lowering medications (n = 51), cigarette smoking (n = 2,255), and alcohol consumption (n = 37,047). Totally, we included 1,888,452 participants in this model. The multivariable Cox proportional hazards regression analyses demonstrated that prediabetes and DM were associated with a greater risk for proteinuria than normal levels (online suppl. Table 1). Second, even when we defined proteinuria as ≥ trace on the urine dipstick test, prediabetes and DM were associated with an increased incidence for proteinuria compared with normal levels (online suppl. Table 2). Third, the relationship between HbA1c category and the stepwise increase in the risk for proteinuria did not change after excluding people with hypertension (online suppl. Table 3). Fourth, the association between HbA1c category and subsequent risk for proteinuria was observed in all subgroups stratified by age and sex (online suppl. Table 4). Fifth, we analyzed 709,817 participants with serum creatinine values. Median eGFR (Q1–Q3) was 81.3 (72.1–91.5) mL/min/1.73 m2. Among those participants with eGFR ≥60 mL/min/1.73 m2, prediabetes and DM were associated with a higher incidence of proteinuria in the multivariable-adjusted model. The relationship between prediabetes and proteinuria did not reach statistical significance in people with eGFR <60 mL/min/1.73 m2 (online suppl. Table 5). Sixth, we studied 1,536,874 people with available FPG data at their initial health check-up. Median FPG (Q1–Q3) was 91 (86–98) mg/dL. Compared with those with normal FPG levels, those with normal-high FPG levels, IFG levels, and DM were associated with an elevated risk for proteinuria (online suppl. Fig. 2). The RCS regression model of FPG levels for the risk for proteinuria with a reference point set at an FPG of 100 mg/dL showed that the risk for proteinuria development also increased with the FPG value (online suppl. Fig. 3). Seventh, we studied 806,603 individuals who also had FPG data 1 year after the initial health check-up available. The median (Q1–Q3) change in FPG 1 year after the initial health check-up was 0 (−4 to +5) mg/dL. An increase in FPG level ≥5 mg/dL was associated with a higher risk for proteinuria (HR: 1.11, 95% CI: 1.08–1.15). Subgroup analysis showed that an increase in FPG level ≥5 mg/dL was associated with a greater risk for proteinuria in participants with normal FPG levels, normal-high FPG levels, and IFG levels. Significant association between an increase in FPG level ≥5 mg/dL and proteinuria in participants with DM was not detected (online suppl. Fig. 4). Furthermore, the RRR for proteinuria development associated with normal-high FPG levels, IFG levels, and DM was 5.7% (95% CI: 3.3–8.1%), 24.1% (95% CI: 21.2–26.8%), and 49.8% (95% CI: 47.3–52.1%). The RRR for proteinuria development in relation to increasing in FPG level ≥5 mg/dL was 10.2% (95% CI: 7.4–12.8%). We estimated the RRR for proteinuria development with an increase in FPG level ≥5 mg/dL in participants with normal FPG levels, normal-high FPG levels, and IFG levels was 7.1% (95% CI: 3.9–10.2%), 16.2% (95% CI: 8.6–23.2%), and 31.0% (95% CI: 22.2–38.8%), respectively. The RRR for proteinuria development in relation to increasing in FPG level ≥5 mg/dL in participants with DM was 15.8% (95% CI: −0.5 to +29.6%).
Discussion
Using a nationwide population-based database that included the general population with negative proteinuria, we found that not only DM but also prediabetes was associated with a greater risk for proteinuria development than normal HbA1c levels. The risk of proteinuria started to increase from a prediabetes and further increased with a DM. The RCS showed a linear dose-dependent association between medication-naïve HbA1c or FPG levels and future risk for proteinuria. Lastly, we found that the annual change in HbA1c level was associated with a subsequent risk for proteinuria. This association was more pronounced in individuals having prediabetes. These findings support the importance of interventions for those not only with diabetes-range hyperglycemia but also those with prediabetes.
Although previous studies examined a relationship between prediabetes or DM and risk for proteinuria, results of these studies were not consistent [13, 14, 15, 16, 17, 18, 19, 20, 21]. In this study, we analyzed approximately two million individuals with negative proteinuria and found an association between prediabetic hyperglycemia and an elevated incidence of proteinuria. To the best of our knowledge, this study includes the largest study population in this field. Moreover, through various sensitivity analyses, we confirmed the robustness of our results. The relationship between HbA1c category and incident proteinuria did not change when we defined proteinuria as ≥ trace on the urine dipstick test, after excluding people with hypertension, which is a major risk factor for proteinuria. Further, the stepwise increase in the risk of proteinuria with HbA1c category was seen in all subgroups stratified by age or sex. This relationship was observed in people with preserved eGFR but not in those with reduced eGFR. Due to the limited sample size and number of proteinuria events in people with reduced eGFR, the statistical power was not high enough to reach a solid conclusion. However, given the analysis of the Chronic Renal Insufficiency Cohort showing the relationship between prediabetes and a greater risk for CKD progression [17], we should not underestimate the risks associated with prediabetes. Furthermore, our results did not change when we defined prediabetes, including normal-high FPG and IFG, and diabetes based on the FPG level. Compared with people with normal FPG (FPG level of <100 mg/dL), the risk of proteinuria increased in those with normal-high FPG (FPG level of 100–109 mg/dL), IFG (FPG level of 110–125 mg/dL), and DM (FPG level of ≥126 mg/dL). Since prediabetes may increase proteinuria, there is a possibility of kidney disease, which is known to be associated with hyperglycemia, starting to present before the onset of diabetes. Although the HbA1c threshold for diagnosing diabetes is a retinopathy-derived HbA1c threshold [34], considering that typical diabetic kidney disease is said to require a longer duration of diabetes than other complications, and the prevalence of kidney disease is not as high as that of neuropathy [12], proteinuria may develop differently from typical diabetic kidney disease. Other factors (i.e., genetic factors) may also have an impact.
This study is distinct from previous studies in several regards. By calculating the RRR, we aimed to show the potential benefits of normalizing HbA1c levels in people with hyperglycemia. Given the RRR of DM, half of the proteinuria events could be reduced if HbA1c levels were normalized in people with DM, and approximately 12.3% of proteinuria development could be prevented if those with prediabetes could normalize their glucose levels. We also found a linear relationship between medication-naïve HbA1c levels and an increased risk for proteinuria using the RCS analyses, suggesting that the positive association between higher levels of HbA1c and increased risk for proteinuria would be present irrespective of the cut-off value for the HbA1c level. Lastly, by demonstrating the relationship between the 1-year change in HbA1c level and future risk of proteinuria, we identified that decreasing HbA1c levels had the potential benefit of preventing proteinuria development. An increase in HbA1c ≥0.5% was significantly associated with incident proteinuria events in people with normal and prediabetes, and this association was more pronounced in people with prediabetes, suggesting that the impact of a temporal change in HbA1c on incident proteinuria would be greater in people with prediabetic hyperglycemia. Results of RRR showed that in people with prediabetes, the substantial proportion of proteinuria events might be prevented by normalizing HbA1c levels. These results could motivate efforts to optimize glucose levels in people with prediabetes.
These findings could have several possible explanations. People with prediabetes may meet the diagnostic requirements for DM if we performed an oral glucose tolerance test [35], and therefore, part of our results might simply be explained by diabetic nephropathy. Various studies have reported that the risk of adverse events increases in prediabetic hyperglycemia. We reported that the incidence of CVD events started to increase at normal-high FPG or IFG levels even in young adults [24]. We also reported that, compared with normal FPG levels, the risk of colorectal cancer was higher in not only those with DM but also in those with normal-high FPG and IFG levels [36]. Therefore, even before meeting the diagnostic criteria for DM, hyperglycemia could cause various harmful pathological effects. From a pathophysiological point of view, insulin resistance in people with prediabetes may be linked with a greater risk for proteinuria [37]. Further investigations are required to clarify the underlying mechanisms of our results.
This study has some clinical implications. Since proteinuria is known to precede the subsequent decline in renal function and development of CVD events, early detection and appropriate treatment measures for proteinuria are important. In this regard, the results of the present study suggest that HbA1c and FPG values obtained at health check-ups can be used to identify people who are at high risk for proteinuria development. Additionally, our results show that lowering glucose levels could potentially prevent proteinuria development. Particularly, our results showed that the influence that a change in HbA1c and FPG levels can have on incident proteinuria may be more pronounced in people with prediabetes. In the future, we need to clarify whether the risk for proteinuria could be prevented by lowering or normalizing glucose levels, particularly in people with prediabetes.
The strengths of the current study include the use of a large-scale population-based cohort with high levels of untreated HbA1c and negative urine dipstick proteinuria results and the documentation of proteinuria development over time. Our study is distinguishable from previous studies in that we analyzed a large-scale dataset and demonstrated that the annual changes in HbA1c was strongly associated with the risk of subsequent proteinuria development, particularly in people having prediabetes. However, this study also has several limitations. The possibility and potential influence of unmeasured confounders and residual bias could not be eliminated. For example, socio-economic status could influence both HbA1c levels and risk of proteinuria. However, data on socio-economic status were not available. People included in this database were mainly an employed working-age population, and therefore, we need to consider the possibility of selection bias. We also used urine dipstick test results, which are a semiquantitative assessment of proteinuria. Therefore, false-positive proteinuria could occur with specific conditions (e.g., concentrated urine, alkaline urine). Furthermore, although HbA1c was consistently associated with the risk of proteinuria in our analyses with different outcome definitions (i.e., positive or trace proteinuria), urinary protein is known to be an indicator with relatively large intra-individual variability [38]. Future studies should also elucidate the association of prediabetes or HbA1c with other indicators related to reduced renal function.
In conclusion, not only DM but also prediabetes was associated with a greater risk for proteinuria development. Furthermore, the annual change in HbA1c level was associated with the subsequent risk for proteinuria development, and this relationship was more pronounced in people having prediabetes. Our results suggest that detecting hyperglycemia may contribute to identifying people at a higher risk for proteinuria development and allow for appropriate preventive measures to be taken.
Statement of Ethics
This study was approval by the Ethics Committee of the University of Tokyo (No. 2018-10862) and was performed in accordance with the principles of the Declaration of Helsinki. Since data in the JMDC Claims Database are de-identified, the informed consent requirement was waived.
Conflict of Interest Statement
Research funding and scholarship funds (Hidehiro Kaneko and Katsuhito Fujiu) from Medtronic Japan Co., Ltd., Abbott Medical Japan Co., Ltd., Boston Scientific Japan Co., Ltd., and Fukuda Denshi, Central Tokyo Co., Ltd. Akira Okada and Satoko Yamaguchi are members of the Department of Prevention of Diabetes and Lifestyle-related Diseases, which is a cooperative program between The University of Tokyo and Asahi Mutual Life Insurance Company. Other authors have nothing to disclose. No potential conflicts of interest relevant to this study were reported.
Funding Sources
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (19AA2007 and H30-Policy-Designated-004), and the Ministry of Education, Culture, Sports, Science and Technology, Japan (17H04141). Hidehiro Kaneko and Katsuhito Fujiu received research funding and scholarship funds from Medtronic Japan Co., Ltd.; Biotronik Japan; SIMPLEX QUANTUM Co., Ltd.; Boston Scientific Japan Co., Ltd.; and Fukuda Denshi, Central Tokyo Co., Ltd.
Author Contributions
H.K., A.O., and H.M. conceived the study design. Y.S., A.O., H.I., and K.M. analyzed the data. K.M., K.F., N.M., T.J., N.T., H.M., S.Y., K.K., J.A., and K.N. reviewed the manuscript. K.N., T.Y., M.N., H.Y., and I.K. supervised the study. H.K. and I.K. are the guarantors of this work and, as such, had full access to all the data used in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability Statement
Hidehiro Kaneko, as the corresponding author, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This database is available for anyone who purchases it from the JMDC Inc (https://www.jmdc.co.jp/en/index).
Supplementary Material
Supplementary data
Supplementary data
Yuta Suzuki, Hidehiro Kaneko and Akira Okada contributed equally to this work, and shared first authorship.
References
- 1.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011 Jul;80((1)):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonelli M, Klarenbach SW, Lloyd AM, James MT, Bello AK, Manns BJ, et al. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int. 2011 Dec;80((12)):1306–14. doi: 10.1038/ki.2011.280. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009 Aug;54((2)):205–26. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Pinto-Sietsma SJ, Janssen WMT, Hillege HL, Navis G, Zeeuw D, Jong PE. Urinary albumin excretion is associated with renal functional abnormalities in a nondiabetic population. J Am Soc Nephrol. 2000 Oct;11((10)):1882–8. doi: 10.1681/ASN.V11101882. [DOI] [PubMed] [Google Scholar]
- 5.Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375((9731)):2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata M, Ninomiya T, Kiyohara Y, Murakami Y, Irie F, Sairenchi T, et al. Prediction of cardiovascular disease mortality by proteinuria and reduced kidney function: pooled analysis of 39,000 individuals from 7 cohort studies in Japan. Am J Epidemiol. 2013 Jul 1;178((1)):1–11. doi: 10.1093/aje/kws447. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012 Jan 14;379((9811)):165–80. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 8.Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, et al. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020 Jan;75((1 Suppl 1)):A6–7. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Olivarius Nde F, Andreasen AH, Keiding N, Mogensen CE. Epidemiology of renal involvement in newly-diagnosed middle-aged and elderly diabetic patients. Cross-sectional data from the population-based study “Diabetes Care in General Practiceˮ, Denmark. Diabetologia. 1993 Oct;36((10)):1007–16. doi: 10.1007/BF02374492. [DOI] [PubMed] [Google Scholar]
- 10.Kohler KA, McClellan WM, Ziemer DC, Kleinbaum DG, Boring JR. Risk factors for microalbuminuria in black americans with newly diagnosed type 2 diabetes. Am J Kidney Dis. 2000 Nov;36((5)):903–13. doi: 10.1053/ajkd.2000.19080. [DOI] [PubMed] [Google Scholar]
- 11.Davis TM, Stratton IM, Fox CJ, Holman RR, Turner RCUK. Prospective Diabetes Study 22. Effect of age at diagnosis on diabetic tissue damage during the first 6 years of NIDDM. Diabetes Care. 1997 Sep;20((9)):1435–41. doi: 10.2337/diacare.20.9.1435. [DOI] [PubMed] [Google Scholar]
- 12.Spijkerman AM, Dekker JM, Nijpels G, Adriaanse MC, Kostense PJ, Ruwaard D, et al. Microvascular complications at time of diagnosis of type 2 diabetes are similar among diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the hoorn screening study. Diabetes Care. 2003 Sep;26((9)):2604–8. doi: 10.2337/diacare.26.9.2604. [DOI] [PubMed] [Google Scholar]
- 13.Melsom T, Schei J, Stefansson VT, Solbu MD, Jenssen TG, Mathisen UD, et al. Prediabetes and risk of glomerular hyperfiltration and albuminuria in the general nondiabetic population: a Prospective Cohort Study. Am J Kidney Dis. 2016 Jun;67((6)):841–50. doi: 10.1053/j.ajkd.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa M, Onoue T, Kato K, Wada T, Shinohara Y, Kinoshita F, et al. Prediabetes is associated with proteinuria development but not with glomerular filtration rate decline: a longitudinal observational study. Diabet Med. 2021 Aug;38((8)):e14607. doi: 10.1111/dme.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markus MRP, Ittermann T, Baumeister SE, Huth C, Thorand B, Herder C, et al. Prediabetes is associated with microalbuminuria, reduced kidney function and chronic kidney disease in the general population: The KORA (Cooperative Health Research in the Augsburg Region) F4-Study. Nutr Metab Cardiovasc Dis. 2018 Mar;28((3)):234–42. doi: 10.1016/j.numecd.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Echouffo-Tcheugui JB, Narayan KM, Weisman D, Golden SH, Jaar BG. Association between prediabetes and risk of chronic kidney disease: a systematic review and meta-analysis. Diabet Med. 2016 Dec;33((12)):1615–24. doi: 10.1111/dme.13113. [DOI] [PubMed] [Google Scholar]
- 17.Neves JS, Correa S, Baeta Baptista R, Bigotte Vieira M, Waikar SS, Mc Causland FR. Association of prediabetes with CKD progression and adverse cardiovascular outcomes: an analysis of the CRIC Study. J Clin Endocrinol Metab. 2020 Apr 1;105((4)):e1772–80. doi: 10.1210/clinem/dgaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira MB, Neves JS, Leitão L, Baptista RB, Magriço R, Dias CV, et al. Impaired fasting glucose and chronic kidney disease, albuminuria, or worsening kidney function: a secondary analysis of the SPRINT. J Clin Endocrinol Metab. 2019 May 7;104((9)):4024–32. doi: 10.1210/jc.2019-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox CS, Larson MG, Leip EP, Meigs JB, Wilson PW, Levy D. Glycemic status and development of kidney disease: the Framingham Heart Study. Diabetes Care. 2005 Oct;28((10)):2436–40. doi: 10.2337/diacare.28.10.2436. [DOI] [PubMed] [Google Scholar]
- 20.Selvin E, Ning Y, Steffes MW, Bash LD, Klein R, Wong TY, et al. Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes. 2011 Jan;60((1)):298–305. doi: 10.2337/db10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing FY, Neeland IJ, Gore MO, Ayers CR, Paixao AR, Turer AT, et al. Association of prediabetes by fasting glucose and/or haemoglobin A1c levels with subclinical atherosclerosis and impaired renal function: observations from the Dallas Heart Study. Diab Vasc Dis Res. 2014 Jan;11((1)):11–8. doi: 10.1177/1479164113514239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20((5)):413–9. doi: 10.2188/jea.JE20090066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaneko H, Yano Y, Itoh H, Morita K, Kiriyama H, Kamon T, et al. Association of blood pressure classification using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline with risk of heart failure and atrial fibrillation. Circulation. 2021 Jun 8;143((23)):2244–53. doi: 10.1161/CIRCULATIONAHA.120.052624. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko H, Itoh H, Kiriyama H, Kamon T, Fujiu K, Morita K, et al. Fasting plasma glucose and subsequent cardiovascular disease among young adults: analysis of a nationwide epidemiological database. Atherosclerosis. 2021 Feb;319:35–41. doi: 10.1016/j.atherosclerosis.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Consensus Committee Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007 Sep;30((9)):2399–400. doi: 10.2337/dc07-9925. [DOI] [PubMed] [Google Scholar]
- 26.Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, et al. Japanese Clinical Practice Guideline for Diabetes 2016. Diabetol Int. 2018 Mar 27;9((1)):1–45. doi: 10.1007/s13340-018-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Diabetes Association 2. classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021 Jan;44((Suppl 1)):S15–33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 28.Sumida K, Nadkarni GN, Grams ME, Sang Y, Ballew SH, Coresh J, et al. Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: an individual participant-based meta-analysis. Ann Intern Med. 2020 Sep 15;173((6)):426–35. doi: 10.7326/M20-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Usui T, Yoshida Y, Nishi H, Yanagimoto S, Matsuyama Y, Nangaku M. Diagnostic accuracy of urine dipstick for proteinuria category in Japanese workers. Clin Exp Nephrol. 2020 Feb;24((2)):151–6. doi: 10.1007/s10157-019-01809-3. [DOI] [PubMed] [Google Scholar]
- 30.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989 May;8((5)):551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 31.Aloisio KM, Swanson SA, Micali N, Field A, Horton NJ. Analysis of partially observed clustered data using generalized estimating equations and multiple imputation. Stata J. 2014 Oct 1;14((4)):863–83. [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009 Jun;53((6)):982–92. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 33.National Institute for Health and Clinical Excellence . Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes. London: National Institute for Health and Clinical Excellence; 2009. (accessed December 27, 2021) [PubMed] [Google Scholar]
- 34.Atkin SL, Butler AE, Hunt SC, Kilpatrick ES. The retinopathy-derived HbA1c threshold of 6.5% for type 2 diabetes also captures the risk of diabetic nephropathy in NHANES. Diabetes Obes Metab. 2021 Sep;23((9)):2109–15. doi: 10.1111/dom.14449. [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association 2. classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020 Jan;43((Suppl 1)):S14–31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 36.Itoh H, Kaneko H, Okada A, Yano Y, Morita K, Seki H, et al. Fasting plasma glucose and incident colorectal cancer: analysis of a nationwide epidemiological database. J Clin Endocrinol Metab. 2021:dgab466. doi: 10.1210/clinem/dgab466. [DOI] [PubMed] [Google Scholar]
- 37.Ritz E, Koleganova N, Piecha G. Is there an obesity-metabolic syndrome related glomerulopathy? Curr Opin Nephrol Hypertens. 2011 Jan;20((1)):44–9. doi: 10.1097/MNH.0b013e3283414ca1. [DOI] [PubMed] [Google Scholar]
- 38.Waikar SS, Rebholz CM, Zheng Z, Hurwitz S, Hsu CY, Feldman HI, et al. Biological variability of estimated GFR and albuminuria in CKD. Am J Kidney Dis. 2018 Oct;72((4)):538–46. doi: 10.1053/j.ajkd.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
Hidehiro Kaneko, as the corresponding author, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This database is available for anyone who purchases it from the JMDC Inc (https://www.jmdc.co.jp/en/index).