Abstract
The composition of the commensal microbiota has recently emerged as a key element influencing the efficacy of cancer treatments. It has become apparent that the interplay between the microbiome and immune system within the host influences the response to immunotherapy, particularly immune checkpoint inhibitor therapy. Identifying the key components of the gut microbiota that influence this response is paramount for designing therapeutic interventions to enhance the response to cancer therapy. This review will discuss strategies being considered to modulate the gut microbiota, including fecal microbiota transplantation, administration of defined bacterial isolates as well as bacterial consortia, supplementation with probiotics, and lifestyle modifications such as dietary changes. Understanding the influence of the complex variables of the human microbiota on the effectiveness of cancer therapy will help drive the clinical design of microbial-based interventions in the field of oncology.
Key words: immunotherapy, biomarkers, resistance, microbiome
Highlights
-
•
Manipulation of the gut microbiome can influence the therapeutic efficacy of immune checkpoint inhibitors.
-
•
Clinical studies are currently exploring therapeutic methods to modulate commensal bacteria.
-
•
Strategies to modulate the microbiome include fecal microbiota transplantation, defined bacterial isolates and dietary manipulation.
Introduction
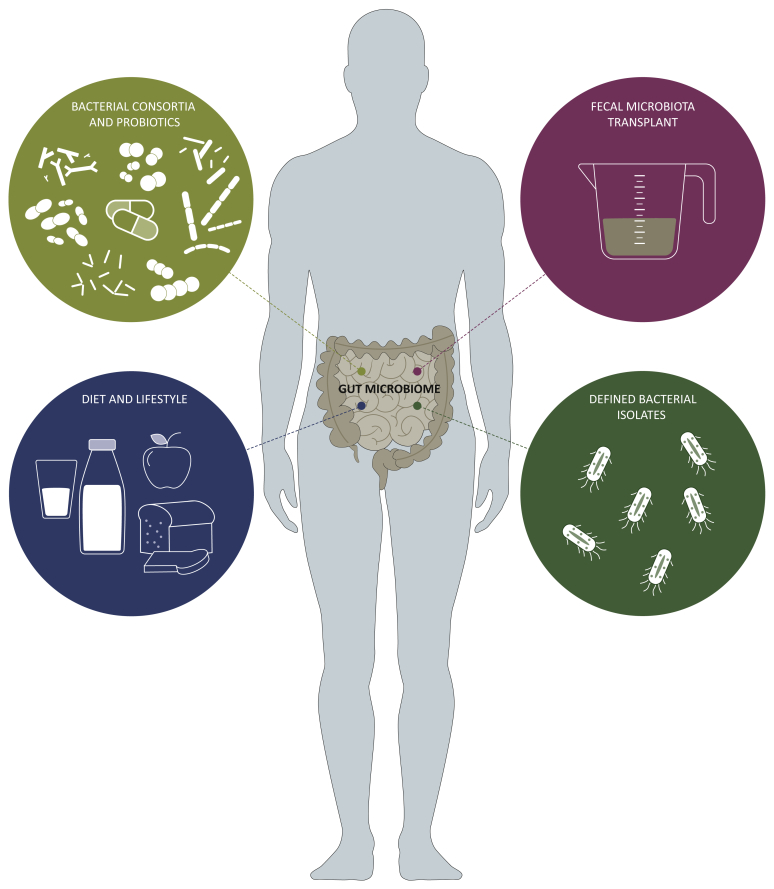
Host immunity typically involves a coordinated response of the innate and adaptive immune systems to ward off threats from both external (e.g. pathogens) and internal (e.g. cancer) sources. Factors frequently considered to impact on quantitative and qualitative aspects of immune responses include germline polymorphisms in immunoregulatory genes, history of prior antigen or pathogen exposures, and whether acquired immunosuppression (either pharmacologic or pathogenic) is present. However, recent work has indicated an additional host-associated factor that has a major regulatory influence on immune responses – the commensal microbiota. Originally acquired after birth, the intestinal microbiota exceeds 3 × 1013 bacterial cells and plays a crucial role in modulating innate and adaptive immunity.1, 2, 3 The composition of the gut microbiota has been demonstrated to influence immune responses in a variety of disease model systems, including autoimmune processes,4, 5, 6, 7, 8 viral infection,9, 10, 11 solid organ transplantation,12,13 allogeneic bone marrow transplantation14,15 and cancer.16, 17, 18, 19, 20 Modulation of antitumor immunity by the intestinal microbiota has prompted investigation into the potential for specific gut bacteria to potentiate the efficacy of immunotherapy with anti-CTLA-4 antibody or PD-1/PD-L1 blockade in mouse models.18,19,21, 22, 23 These preclinical studies have shown that manipulation of the microbiome can improve therapeutic efficacy over immune checkpoint blockade therapy alone. These exciting early results have motivated clinical exploration of microbiota-based interventions as a mechanism to optimize the clinical response to cancer immunotherapies in patients. This review will discuss various strategies being explored to enhance the therapeutic efficacy of immune checkpoint inhibitors: fecal microbiota transplantation (FMT), probiotics (isolated bacteria as well as bacterial consortia) and dietary modifications. These various approaches are illustrated in Figure 1.
Figure 1.
Microbiome-associated therapeutic strategies for modulating cancer treatment.
Interventional approaches targeting the gut microbiome to engineer a more desirable landscape for improving the outcome of cancer immunotherapeutics. Strategies to shape the gut microbiome include using specific bacterial consortia or probiotic supplementation, transfer of fecal microbiota, addition of specific bacterial organisms, and implementing a defined diet. Created using BioRender.com.
Therapeutic strategies
Fecal microbiota transplantation
One strategy for altering the composition of the intestinal microbiota is through FMT. FMT is the process of transferring fecal material from a donor into a recipient via a nasogastric tube, nasojejunal tube, upper tract endoscopy, colonoscopy, enema or as a prepared capsule. Transfer of fecal material from healthy donors has been shown to be effective in treating recurrent Clostridium difficile infection, and has been incorporated into the C. difficile infection management guidelines from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America.24, 25, 26 FMT has also been investigated in clinical trials for the treatment of ulcerative colitis, irritable bowel syndrome and other gastrointestinal conditions.27, 28, 29, 30, 31, 32, 33
The therapeutic potential of FMT in cancer immunotherapy has been supported by preclinical models in which patient-derived microbiota was transferred by gavage into germ-free mice (GFM). GFM colonized with FMT from responder patients exhibited slower tumor growth compared with those colonized with FMT from non-responder patients.22 In addition, reconstitution of GFM with responder-derived FMT resulted in greater efficacy of anti-PD-L1 therapy, while it was ineffective in mice with non-responder-derived FMT. In another cohort of patients with epithelial tumors, the therapeutic response to anti-PD-1 therapy was abrogated in mice that received FMT from non-responders or were treated with broad-spectrum antibiotics.21 Response to anti-PD-1 therapy could then be restored with FMT transfer from either responder-derived FMT or non-responder-derived FMT enriched with Akkermansia muciniphila. Similar correlation of improved antitumor response was conferred to mice that were administered FMT with Faecalibacterium spp.23 These findings suggest a functional association between the gut microbiome and the efficacy of anti-PD-1 immunotherapy, and the potential for therapeutic modulation.
Several independent research groups are currently investigating the effectiveness of FMT in enhancing the clinical response to anti-PD-1 therapy (Table 1). At the University of Pittsburgh Hillman Cancer Center, a clinical trial is exploring the delivery of FMT in combination with pembrolizumab in patients with melanoma refractory to anti-PD1 treatment (NCT03341143). This phase II feasibility study is exploring whether FMT from donor patients who responded to PD-1 blockade is able to improve the therapeutic outcome in patients with anti-PD-1 refractory melanoma. Functional and phenotypic changes in the innate and adaptive immune systems will be studied in parallel. Early data were presented by Giorgio Trinchieri at the American Association for Cancer Research 2019 annual meeting, where he reported stable disease or tumor regression in two of three patients with melanoma after undergoing FMT and immune checkpoint therapy.34 The team at Sheba Medical Center in Israel is conducting a phase I trial evaluating FMT from responder patients being transferred via colonoscopy followed by administration of stool-microbiota capsules and anti-PD-1 retreatment in patients with metastatic melanoma resistant to PD-1 inhibitors (NCT03353402).35 Preliminary results for the first three patients were presented, and revealed one patient with disease regression at the time of first imaging but with disease progression on subsequent scans, and with another patient showing a 45% decrease in disease burden with ongoing survival after 8 months. Tumor biopsies obtained from these patients showed a post-treatment increase in intratumoral CD68+ and CD8+ cells, suggesting a shift in the tumor microenvironment. These data have to be interpreted with caution as the sample sizes remain very small, and heterogeneity among the investigated patients (e.g. refractory to immune checkpoint inhibitor therapy versus relapse after initial response) may impact interpretation of the therapeutic effect of microbiome modifications. The designs of clinical trials will need to account for these variabilities among patient characteristics, as some patients who recur after prior anti-PD-1 treatment can respond to subsequent anti-PD-1 therapy even without any microbiome-based intervention. Nonetheless, thus far, these early results support the safety and feasibility of FMT in combination with anti-PD-1 therapy.
Table 1.
Cancer clinical trials investigating fecal microbiota transplantation (FMT) therapy
| Trial no. | Phase | Cancer type | Intervention | Sponsor/investigator |
|---|---|---|---|---|
| NCT03341143 | II | Melanoma | FMT (via colonoscopy) + immune checkpoint inhibitors | UPMC Hillman Cancer Center |
| NCT03353402 | I | Melanoma | FMT (via colonoscopy and stool capsules) + immune checkpoint inhibitors | Sheba Medical Center |
| NCT03772899 | I | Melanoma | FMT + immune checkpoint inhibitors | Lawson Health Research Institute |
| NCT04577729 | – | Melanoma | Autologous or allogeneic FMT + immune checkpoint inhibitors | Medical University of Graz |
| NCT04521075 | I/II | Melanoma NSCLC |
FMT (stool capsules) + immune checkpoint inhibitors | Sheba Medical Center |
| NCT04056026 | I | Mesothelioma | FMT (via colonoscopy) + immune checkpoint inhibitors | ProgenaBiome |
| NCT04116775 | II | Prostate | FMT (via endoscopy) + immune checkpoint inhibitors + enzalutamide | VA Portland Health Care System |
| NCT04130763 | I | Gastrointestinal | FMT (stool capsules) + immune checkpoint inhibitors | Peking University |
NSCLC, non-small cell lung cancer.
Although FMT is one potential strategy to modulate the gut microbiome with the goal of enhancing clinical responses to immunotherapy, there are some risks and uncertainties with the use of this modality. The first major issue is safety; asymptomatic donors can harbor unrecognized pathogens, including parasites and pathogenic bacteria. In addition, they may be colonized with antibiotic-resistant bacteria (multi-drug-resistant organisms). The US Food and Drug Administration issued a safety communication in June 2019 warning healthcare professionals of the potential risk of life-threatening infections following investigational FMT.36 This safety alert emerged as a result of two patients who developed serious bacterial infections after receiving FMT, resulting in one death. One of these individuals was enrolled on a clinical trial of FMT for the treatment of hepatic encephalopathy, and the other was receiving an allogeneic stem cell transplant for myelodysplastic syndrome.37 Clearly these were complex cases with multiple concomitant medical conditions, but nevertheless, these occurrences introduce an element of caution for FMT in patients with advanced cancer. FMT involves the transfer of not only commensal bacteria, but also viruses, protozoa, archaea and fungi, which are often not accounted for due to inadequate methods to characterize these organisms fully.38,39 There is growing evidence that, collectively, these microbial communities impact the development of cardiovascular disease,40,41 neurological disorders,42 metabolic disorders,43, 44, 45, 46 psychiatric conditions47 and lung disorders.48 Theoretically, the transfer of fecal microbiota may lead to unrecognized transmission and predisposition to these chronic health disorders, and thus may warrant more judicious selection of FMT donors as well as additional studies to better understand the long-term health impact of FMT.49,50 A recent detailed analysis of stool donors as candidates for FMT protocols, who underwent intensive screening for medical illnesses, subclinical presence of infectious pathogens in the specimen, or detection of antibiotic-resistant bacteria, revealed that only 3% of donors would qualify based on rigid criteria.51 Thus, FMT remains investigational, and it is anticipated that strict donor screening will be expected in future studies.
Defined bacterial isolates
Another microbiome-based intervention being explored for its potential to improve the efficacy of cancer immunotherapy is the administration of defined bacteria with immunomodulatory properties. In 2015, two independent research groups reported the identification of single bacterial species that could potentiate the response to checkpoint blockade (e.g. anti-CTLA-4 and anti-PD-1/PD-L1 antibodies) in preclinical models.18,19 In a study by Zitvogel and her team, mice with established sarcomas that were treated with antibiotics or housed in germ-free conditions showed tumor progression following treatment with CTLA-4 blockade.19 The anticancer efficacy of CTLA-4 blockade was restored when the mice were recolonized with specific Bacteroides spp. (i.e. B. fragilis and B. thetaiotaomicron), but not with other bacterial isolates, suggesting that Bacteroides spp. may play a key role in driving immune responses to anti-CTLA-4 therapy. It should be noted, however, that while reconstitution with live bacterial species was shown to restore the efficacy of anti-CTLA-4 therapy, intestinal epithelial cells, when exposed to microbial products (in the form of Toll-like receptor agonists), induced a response from intraepithelial lymphocytes in mice treated with anti-CTLA-4, suggesting the need for bacterial components alone to induce an inflammatory response from intestinal lymphocytes. This form of molecular mimicry may result in a beneficial or detrimental patient outcome (e.g. autoimmune reaction).52 Similarly, modulation of the intestinal microbiota with oral administration of Bifidobacterium spp. was shown to augment the response to PD-L1 blockade in a murine melanoma model.18 The therapeutic effect of Bifidobacterium spp. was associated with increased intratumoral and circulating tumor-antigen-specific CD8+ T cells, which could be attributed to increased ‘poising’ of dendritic cells throughout the animals.18
A similar increased abundance of Bifidobacterium spp. was observed in a cohort of patients with metastatic melanoma with improved responsiveness to anti-PD-1 therapy.22 Among multiple species of bacteria showing differential abundance in the stool of responders and non-responders was Bifidobacterium longum, which correlated with enhanced T-cell infiltration in the tumor microenvironment. The lack of detectable bifidobacterium sequences in the majority of patients with metastatic melanoma is in keeping with previous studies indicating loss of bifidobacterium colonization with aging,53,54 and also preferential loss in a cohort of patients with colorectal cancer compared with control individuals with non-cancerous intestinal illnesses.55 In a second study, analysis of the composition of the gut microbiota of patients with non-small cell lung carcinoma (NSCLC) and renal cell carcinoma similarly exhibited over-representation of distinct bacterial genera that were associated with improved clinical response.21 In that particular patient population, A. muciniphila was abundant in checkpoint inhibitor responders, and mice treated with antibiotics recolonized with A. muciniphila (alone or in combination with Enterococcus hirae) were shown to have a restored response to PD-1 blockade, emphasizing the influence that specific bacterial taxa can have on modulating the response to immunotherapy. Collectively, these studies provide further insight into the impact and immunostimulatory effect of isolated bacteria on regulating the response to immune checkpoint inhibitors. However, the most critical species and strains of bacteria for potentiation of antitumor T-cell responses are still unclear, and predicting the clinical outcome of patients from mice reconstituted in controlled environments with non-physiological levels of specific bacterial species has yet to be determined.
Despite these limitations, clinical trials aiming to improve the effectiveness of immunotherapy with defined bacterial strains have been initiated (Table 2). Evelo Biosciences is enrolling patients with various cancers in clinical trials investigating the oral administration of a bifidobacterial strain. Supplementation with Bifidobacterium animalis lactis (EDP1503) in the form of a capsule is administered for a 2-week period, followed by continued administration in combination with pembrolizumab (NCT03595683, NCT03775850). Preliminary data reported at the 2020 European Society for Medical Oncology World Congress on Gastrointestinal Cancer Virtual Meeting demonstrated safety and tolerability across various solid tumors, including colorectal cancer, triple-negative breast cancer and NSCLC.56 Notably, the overall response rate in the cohort with triple-negative breast cancer was 25%, which is encouraging given that the response with single-agent anti-PD1 therapy in this patient group ranges between 5% and 10%. Similarly, in a phase 1 study, the National Cancer Institute in collaboration with the City of Hope Medical Center are investigating the addition of Clostridium butyricum (CBM 588) to patients with advanced renal cell carcinoma undergoing combination therapy with nivolumab and ipilimumab (NCT03829111). Overall, single bacterial interventions as a tool to enhance the immunotherapeutic response are in the early stages of development, and questions remain regarding which precise bacterial isolate would best improve antitumor immunity, which assay is ideal to identify the relative abundance and functional status of specific bacteria (e.g. 16S sequencing, metagenomics, metatranscriptomics, culturomics, metabolomics), and which animal model is optimized to represent the interaction among human commensals and to detect single bacterial species with immune-potentiating effects.
Table 2.
Clinical trials investigating bacterial isolates
| Trial no. | Phase | Cancer type | Intervention | Sponsor/investigator |
|---|---|---|---|---|
| NCT03775850 | I/II | Solid tumors | EDP1503 (bifidobacterium capsule) + pembrolizumab | Evelo Biosciences |
| NCT03595683 | II | Melanoma | EDP1503 (bifidobacterium capsule) + pembrolizumab | Evelo Biosciences |
| NCT03829111 | I | Renal cell carcinoma | CBM588 (Clostridium butyricum probiotic) + nivolumab and ipilimumab | City of Hope Medical Center |
| NCT03637803 | I/II | Solid tumors | MRx0518 (Enterococcus gallinarum capsule) + immune checkpoint inhibitors | 4D Pharma |
Bacterial consortia and other probiotics
Administration of a consortium of commensal bacteria strains has emerged as an attractive method for manipulating the host microbiome to affect the therapeutic response to cancer immunotherapy (Table 3). Rather than transplanting the complete gut microbiome from human donors (e.g. FMT), which could produce unwanted side-effects from pathogenic strains, transfer of specific strains identified empirically as critical to the enhancement of immunotherapeutic intervention could allow for an ‘off-the-shelf’ approach for transferring multiple bacterial strains concurrently. To this end, work by Honda and colleagues identified 11 bacterial strains (seven Bacteroidales spp. and four non-Bacteroidales spp.) from healthy human donors that promoted the generation of interferon-γ-producing CD8+ T cells in mouse intestine.57 When this bacterial consortium was recolonized in a mouse syngeneic tumor model, enhancement of the anti-PD-1 therapeutic response was observed in conjunction with an increase in tumor-infiltrating CD8+ T cells. Interestingly, when the 11-strain consortium was analysed further, it was shown that the seven Bacteroidales spp. did not induce a CD8+ T-cell response whereas the four non-Bacteroidales spp. did, albeit at a much lower rate than the complete set of 11 species, pointing to a true consortium effect as well as highlighting the incomplete understanding of the interplay between multiple members of a bacterial community. Based on these data, a clinical trial is planned using this bacterial consortium in combination with anti-PD-1 therapy.58
Table 3.
Clinical trials investigating bacterial consortia and probiotics
| Trial no. | Phase | Cancer type | Intervention | Sponsor/investigator |
|---|---|---|---|---|
| NCT03686202 | I | Solid tumors | MET-4 (defined bacterial consortia) + immune checkpoint inhibitors | University Health Network, Toronto |
| NCT03817125 | Ib | Melanoma | SER-401 (defined bacterial consortia) + immune checkpoint inhibitors | Parker Institute for Cancer Immunotherapy |
| NCT01895530 | – | Colorectal cancer | Supplement with probiotic Saccharomyces boulardii | Federal University of Minas Gerais |
| NCT03072641 | – | Colorectal cancer | Supplement with ProBion Clinica (Bifidobacterium lactis, Lactobacillus acidophilus, inulin) | Vastra Gotaland Region |
| NCT03782428 | – | Colorectal cancer | Probiotic with six viable micro-organisms of Lactobacillus spp. and Bifidobacterium spp. | National University of Malaysia |
| NCT03358511 | – | Breast cancer | Over-the-counter probiotic with 13 bacterial species | Mayo Clinic |
While the application of a bacterial consortium to enhance cancer immunotherapy is relatively new, augmenting the antitumor response using scientifically designed clinical-grade probiotics or over-the-counter probiotics is currently under evaluation. A number of studies in colorectal cancer have shown changes in bacterial composition within the tumor, as well as differing cytokine profiles, after probiotic administration, but these results need to be investigated further regarding their impact on antitumor immunity (NCT01895530, NCT03072641, NCT03782428).59,60 A phase I trial is currently underway investigating the safety and engraftment of a defined mixture of bacterial species (MET-4) administered orally in combination with immune checkpoint inhibitors (NCT03686202). In another phase I study, the tolerability and preliminary efficacy of SER-401, a microbial cocktail correlated previously with response to immunotherapy in patients with melanoma, is being assessed (NCT03817125). In contrast, early retrospective data have indicated that over-the-counter probiotics are associated with decreased response to immune checkpoint inhibitors based on data collected from lifestyle surveys in patients with melanoma.61 Clinical trials assessing microbial manipulation with over-the-counter probiotics have been initiated (NCT03358511); however, particular attention is required when promoting their off-protocol use for patients with cancer until safety profiles of probiotic formulations are better understood. Together, these studies should help to identify microbial consortia that favor the responsiveness of immunotherapy, and will guide the design of next-generation microbiota-based therapeutics.
There is some question regarding the level of engraftment afforded by probiotics in healthy individuals and the resulting effect on the host.62 A recent study exploring the effect of an 11-strain probiotic regimen revealed differential colonization patterns (i.e. permissive versus non-permissive) within volunteers influenced by strain and gastrointestinal location.63 Gastrointestinal transcriptome analysis of permissive individuals revealed a rise in pathways involved in antigen presentation, humoral response and other immune-related functions, indicating probiotic-specific functional modification of the host.
A key component of consortia intervention will be selection of the optimal bacterial strains. To date, the signature of favorable bacterial strains based on 16S or metagenomic shotgun sequencing has not been consistent across patient studies, which could be a reflection of the tumor type being studied, the diet and geographic location of the patients, prior antibiotic history, concomitant medications and other factors. It is conceivable that different strains might be indicated for subsets of patients, and that a degree of individualization will ultimately be required. Analysing the gut microbiome prior to initiation of anticancer therapy could be a useful screening tool to identify the subset of patients who would benefit from gut microbiome modulation. One such strategy could employ GFM colonized with a patient's fecal material to serve as a surrogate system for identification of the ideal strategy (e.g. FMT, antibiotics, probiotics) to enhance the commensal microbiome in non-responders to immune checkpoint inhibitors. As with other novel forms of personalized therapy, the cost and time associated with developing these mouse models will have to be considered.
Diet and lifestyle factors
The commensal microbiota that resides in the gastrointestinal tract plays a vital role in food digestion, nutrient absorption, function of the intestinal innate and adaptive immune systems, and maintenance of the intestinal mucosal barrier. As such, human hosts have developed a mutually beneficial relationship with diverse bacterial species inhabiting the gut, and the composition of the gut commensals can be impacted by diet. Alterations to the complex commensal–host immune interaction through nutrition-based methods could consequently provide another approach to modulate the composition of the microbiome.64,65 Dietary interventions such as prebiotic supplementation, carbohydrate restriction, reducing fat intake or increasing fiber consumption can have major effects on the composition, and therefore the function, of the gut microbiota. Therefore, identifying dietary patterns that target and expand immunoregulatory bacteria, or conversely eliminate unfavorable bacteria, could theoretically enhance the potency of immunotherapy. Data presented by Spencer and colleagues from the MD Anderson Cancer Center suggest that diet may influence the gut microbiome and, in turn, affect anticancer immune responsiveness.61 Analysis of dietary surveys completed by a subset of 46 patients with melanoma who received anti-PD-1 therapy revealed that patients who consumed a high-fiber diet were more likely to have a favorable response to anti-PD-1 therapy than patients who consumed a low-fiber diet. Among patients who consumed diets rich in processed meats and sugars, their gut microbiome was not enriched with bacteria that have been correlated with an improved immunotherapeutic response. Additional studies on the role of dietary patterns (e.g. Mediterranean, Japanese, vegetarian diet) on alterations to the composition of the microbiota and their effects on the function of the immune system are being evaluated.66, 67, 68 Preliminary evidence further supports a link between diets low in fiber, high in animal protein and high in saturated fat with an unfavorable bacterial signature and lower amounts of beneficial bacteria, such as Bifidobacterium spp.
Further studies are needed to support a causal role linking specific dietary interventions to immunotherapy-related clinical outcomes. Several groups are actively assessing the dietary impact on patients with cancer undergoing combination treatment with immunotherapy and dietary interventions. A phase II study in Belgium is examining the effects of diet supplementation with curcumin in addition to an immunomodulatory cocktail (vitamin D, aspirin, cyclophosphamide and lansoprazole) followed by pembrolizumab in patients with cervical, endometrial and uterine sarcoma (Table 4). Similarly, patients with metastatic NSCLC receiving chemo-immunotherapy enrolled in a clinical trial at Indiana University have been randomized to a dietary modification arm (consuming a fasting-mimicking diet) or a placebo arm (consuming a regular diet) to determine clinical efficacy via response rate and progression-free survival (Table 4). Importantly, accurate collection of the dietary data of patients needs to be standardized to enable accurate comparison of treatments and outcomes. Additional limitations of dietary interventions include difficulty regulating patient compliance and interpretation of subjective information obtained through patient-reported surveys. Furthermore, clinical trials with sequential and multistage interventions will help simplify confounding variables, and allow better understanding of the relationship between food and commensal bacteria. Finally, nutrition-based approaches are not selective for modulating specific bacteria, and have the potential to result in divergent effects on varying patient populations (e.g. geographical or genetic differences altering the baseline gut microbiome and thus the response to dietary modifications).
Table 4.
Clinical trials investigating immunotherapy in combination with dietary modifications
| Trial no. | Phase | Cancer type | Intervention | Sponsor/investigator |
|---|---|---|---|---|
| NCT03192059 | II | Cervical/uterine | Pembrolizumab + RT + immunomodulatory cocktail + dietary supplement (curcumin) | University Hospital, Ghent |
| NCT03700437 | – | NSCLC | Carboplatin/pemetrexed + pembrolizumab, fasting-mimicking diet | Indiana University |
RT, radiation therapy; NSCLC, non-small cell lung cancer.
Conclusions and future directions
With this new treatment paradigm, many questions have yet to be answered, one of the foremost being how to match microbial-based interventions effectively with the appropriate patient population. For those who fail a cancer immunotherapy regimen (such as anti-PD-1 therapy), the reason for lack of efficacy may be due solely to suboptimal microbiota in a subset of patients. For other patients, poor tumor antigenicity, tumor-cell-intrinsic oncogenic events that are immune evasive, or germline genetic variants that bestow a high threshold for immune system activation may be causal, and consequently may not require any modification of the microbiome.69 Therefore, selection of patients who might benefit from microbial manipulation has yet to be defined. In addition, even if suboptimal microbiota is indeed causal, there are likely to be multiple mechanisms by which gut microbes impact systemic antitumor immunity. Some individuals may possess an abundance of immunoregulatory bacteria, whereas others may lack immune-potentiating bacteria. As such, selection of the most suitable microbiome donor is equally important, and clear demarcation of how these donors are identified should be provided in clinical trials as they may introduce additional variables that could impact clinical outcome. For example, FMT donors identified as ‘responders’ can be classified as achieving a response ranging from stable disease to partial or complete response within a defined time period. As one might imagine, such interpatient variability and differences across studies on how these patients are classified could influence the perceived conclusions. Until the deep biological mechanisms are elucidated, it will be challenging to identify the proper intervention that will optimally complement the existing microbial community in a given patient to improve immunotherapeutic efficacy.
While still in its infancy, manipulation of the microbiome has already shown great promise in modulating the therapeutic effect of cancer immunotherapy pre-clinically. Beyond the interventions discussed above, additional strategies under evaluation include depletion of pathogenic bacteria through the use of antibiotics, and selective elimination of targeted bacterial species with strain-specific bacteriophages. Critical to the advancement of these treatment options will be understanding the underlying mechanisms of how bacteria regulate antitumor immunity, in the presence and absence of specific immunotherapeutic interventions. As these mechanisms become more defined, and metabolites and/or specific bacteria-derived molecules are identified, new drug-based treatments could form the next generation of microbiome-informed therapies. This would allow for personalization of existing immune checkpoint inhibitor regimens; that is, patients presently on therapy whose response is unknown would either start therapy with a high probability of responding (e.g. based on proxy models) or receive microbiome modulation specific to their microbial composition in order to enhance their response. As with any new drug class, emphasis must also be placed on determining the safety parameters associated with delivering therapy. Overcoming these challenges will pave the way towards precise mapping of ‘ideal’ immunotherapy-potentiating microbiome interventions.
Acknowledgments
Funding
University of Chicago Basic Medical Research Training in Oncology (T32CA009566) (C.S.C.) and the Melanoma Research Alliance.
Disclosure
The University of Chicago has licensed IP to Evelo. In addition, Dr. Gajewski has a patent with the University of Chicago that is pending.
References
- 1.Sender R., Fuchs S., Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen D.S., Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature. 2017;541:321. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 3.de Vos W.M., de Vos E.A. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev. 2012;70(suppl 1):S45–S56. doi: 10.1111/j.1753-4887.2012.00505.x. [DOI] [PubMed] [Google Scholar]
- 4.Vieira S., Pagovich O., Kriegel M. Diet, microbiota and autoimmune diseases. Lupus. 2014;23(6):518–526. doi: 10.1177/0961203313501401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colpitts S.L., Kasper L.H. Influence of the gut microbiome on autoimmunity in the central nervous system. J Immunol. 2017;198(2):596–604. doi: 10.4049/jimmunol.1601438. [DOI] [PubMed] [Google Scholar]
- 6.Vatanen T., Franzosa E.A., Schwager R., et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562(7728):589–594. doi: 10.1038/s41586-018-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Zhang D., Jia H., et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 8.Jangi S., Gandhi R., Cox L.M., et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7(1):12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sui Y., Dzutsev A., Venzon D., et al. Influence of gut microbiome on mucosal immune activation and SHIV viral transmission in naive macaques. Mucosal Immunol. 2018;11(4):1219–1229. doi: 10.1038/s41385-018-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zilberman-Schapira G., Zmora N., Itav S., Bashiardes S., Elinav H., Elinav E. The gut microbiome in human immunodeficiency virus infection. BMC Med. 2016;14(1):83. doi: 10.1186/s12916-016-0625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosshart S.P., Vassallo B.G., Angeletti D., et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell. 2017;171(5):1015–1028.e13. doi: 10.1016/j.cell.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei Y.M., Chen L., Wang Y., et al. The composition of the microbiota modulates allograft rejection. J Clin Invest. 2016;126(7):2736–2744. doi: 10.1172/JCI85295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nellore A., Fishman J.A. The microbiome, systemic immune function, and allotransplantation. Clin Microbiol Rev. 2016;29(1):191–199. doi: 10.1128/CMR.00063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Köhler N., Zeiser R. Intestinal microbiota influence immune tolerance post allogeneic hematopoietic cell transplantation and intestinal GVHD. Front Immunol. 2019;9:3179. doi: 10.3389/fimmu.2018.03179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shono Y., van den Brink M.R.M. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer. 2018;18(5):283–295. doi: 10.1038/nrc.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrett W.S. Cancer and the microbiota. Science. 2015;348(6230):80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iida N., Dzutsev A., Stewart C.A., et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sivan A., Corrales L., Hubert N., et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vetizou M., Pitt J.M., Daillere R., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viaud S., Saccheri F., Mignot G., et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Routy B., Le Chatelier E., Derosa L., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 22.Matson V., Fessler J., Bao R., et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gopalakrishnan V., Spencer C.N., Nezi L., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leffler D.A., Lamont J.T. Clostridium difficile Infection. N Engl J Med. 2015;372(16):1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 25.van Nood E., Vrieze A., Nieuwdorp M., et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 26.McDonald L.C., Gerding D.N., Johnson S., et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66(7):e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian Y., Zhou Y., Huang S., et al. Fecal microbiota transplantation for ulcerative colitis: a prospective clinical study. BMC Gastroenterol. 2019;19(1):116. doi: 10.1186/s12876-019-1010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costello S.P., Hughes P.A., Waters O., et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321(2):156. doi: 10.1001/jama.2018.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cammarota G., Ianiro G. FMT for ulcerative colitis: closer to the turning point. Nat Rev Gastroenterol Hepatol. 2019;16(5):266–268. doi: 10.1038/s41575-019-0131-0. [DOI] [PubMed] [Google Scholar]
- 30.Xu D., Chen V.L., Steiner C.A., et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114(7):1043–1050. doi: 10.14309/ajg.0000000000000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aroniadis O.C., Brandt L.J., Oneto C., et al. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: a double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2019;4(9):675–685. doi: 10.1016/S2468-1253(19)30198-0. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Wiesnoski D.H., Helmink B.A., et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24(12):1804–1808. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohlke F., Stollman N. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Therap Adv Gastroenterol. 2012;5(6):403–420. doi: 10.1177/1756283X12453637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trinchieri G. The microbiome in cancer therapy. AACR Annual Meeting 2019; April 1, 2019; Atlanta, Georgia. Available at: https://www.abstractsonline.com/pp8/#!/6812/presentation/7757. Accessed March 30, 2020.
- 35.Baruch E.N., Youngster I., Ortenberg R., et al. Abstract CT042: Fecal microbiota transplantation (FMT) and re-induction of anti-PD-1 therapy in refractory metastatic melanoma patients – preliminary results from a phase I clinical trial ( NCT03353402) Cancer Res. 2019;79(13 suppl):CT042. [Google Scholar]
- 36.Food and Drug Administration. Important Safety Alert Regarding Use of Fecal Microbiota for Transplantation and Risk of Serious Adverse Reactions Due to Transmission of Multi-Drug Resistant Organisms. FDA. Published online June 18, 2019. Available at: http://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse. Accessed September 18, 2019.
- 37.DeFilipp Z., Bloom P.P., Torres Soto M., et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 38.Cho I., Blaser M.J. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson J., Garges S., Giovanni M., et al. The NIH human microbiome project. Genome Res. 2009;19(12):2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazemian N., Mahmoudi M., Halperin F., Wu J.C., Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome. 2020;8:36. doi: 10.1186/s40168-020-00821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Rodriguez E., Egea-Zorrilla A., Plaza-Díaz J., et al. The gut microbiota and its implication in the development of atherosclerosis and related cardiovascular diseases. Nutrients. 2020;12(3):605. doi: 10.3390/nu12030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vendrik K.E.W., Ooijevaar R.E., de Jong P.R.C., et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98. doi: 10.3389/fcimb.2020.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu E.W., Gao L., Stastka P., et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020;17(3):e1003051. doi: 10.1371/journal.pmed.1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin J., Li Y., Cai Z., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 45.Le Chatelier E., Nielsen T., Qin J., et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 46.Ridaura V.K., Faith J.J., Rey F.E., et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsiao E.Y., McBride S.W., Hsien S., et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arrieta M.-C., Stiemsma L.T., Dimitriu P.A., et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 49.Alang N., Kelly C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2(1):ofv004. doi: 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Clercq N.C., Frissen M.N., Davids M., Groen A.K., Nieuwdorp M. Weight gain after fecal microbiota transplantation in a patient with recurrent underweight following clinical recovery from anorexia nervosa. Psychother Psychosom. 2019;88(1):58–60. doi: 10.1159/000495044. [DOI] [PubMed] [Google Scholar]
- 51.Kassam Z., Dubois N., Ramakrishna B., et al. Donor screening for fecal microbiota transplantation. N Engl J Med. 2019;381(21):2070–2072. doi: 10.1056/NEJMc1913670. [DOI] [PubMed] [Google Scholar]
- 52.Gil-Cruz C., Perez-Shibayama C., Martin A.D., et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science. 2019;366(6467):881–886. doi: 10.1126/science.aav3487. [DOI] [PubMed] [Google Scholar]
- 53.Voreades N., Kozil A., Weir T.L. Diet and the development of the human intestinal microbiome. Front Microbiol. 2014;5:494. doi: 10.3389/fmicb.2014.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arboleya S., Watkins C., Stanton C., Ross R.P. Gut bifidobacteria populations in human health and aging. Front Microbiol. 2016;7:1204. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W., Liu F., Ling Z., Tong X., Xiang C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS One. 2012;7(6):e39743. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Francisco-Anderson L, Shariffudin S, Gardner H. EDP1503 induces antitumor responses via gut-mediated activation of both innate and adaptive immunity. Presented at: ESMO World Congress on Gastrointestinal Cancer 2020; July 1, 2020; Virtual. Abstract P-325. Available at: https://www.onclive.com/view/edp1503-has-anticancer-activity-across-histologies-with-or-without-pembrolizumab. Accessed November 9, 2020.
- 57.Tanoue T., Morita S., Plichta D.R., et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(7741):600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 58.Bristol-Myers Squibb and Vedanta Biosciences Announce a New Clinical Collaboration to Evaluate OPDIVO® (nivolumab) and VE800 in Patients with Advanced or Metastatic Cancers. Vedanta Biosciences, Inc. Available at: https://www.vedantabio.com/news-media/press-releases/detail/2492. Accessed March 8, 2020.
- 59.Hibberd A.A., Lyra A., Ouwehand A.C., et al. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017;4(1):e000145. doi: 10.1136/bmjgast-2017-000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Consoli M.L.D., da Silva R.S., Nicoli J.R., et al. Randomized clinical trial: impact of oral administration of Saccharomyces boulardii on gene expression of intestinal cytokines in patients undergoing colon resection. J Parenter Enteral Nutr. 2016;40(8):1114–1121. doi: 10.1177/0148607115584387. [DOI] [PubMed] [Google Scholar]
- 61.Spencer C.N., Gopalakrishnan V., McQuade J., et al. Tumor Biology. American Association for Cancer Research; 2019. Abstract 2838: The gut microbiome (GM) and immunotherapy response are influenced by host lifestyle factors; p. 2838. [Google Scholar]
- 62.Kristensen N.B., Bryrup T., Allin K.H., Nielsen T., Hansen T.H., Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8(1):52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zmora N., Zilberman-Schapira G., Suez J., et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–1405.e21. doi: 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 64.Singh R.K., Chang H.-W., Yan D., et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.David L.A., Maurice C.F., Carmody R.N., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown K., DeCoffe D., Molcan E., Gibson D.L. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4(8):1095–1119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maslowski K.M., Mackay C.R. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12(1):5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 68.Soldati L., Di Renzo L., Jirillo E., Ascierto P.A., Marincola F.M., De Lorenzo A. The influence of diet on anti-cancer immune responsiveness. J Transl Med. 2018;16(1):75. doi: 10.1186/s12967-018-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spranger S., Gajewski T.F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer. 2018;18(3):139–147. doi: 10.1038/nrc.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]