Abstract
The process of dog domestication likely involved at least two functional stages. The initial stage occurred when subpopulations of wolves became synanthropes, benefiting from life nearby or in human environments. The second phase was characterized by the evolution of novel forms of interspecific cooperation and social relationships between humans and dogs. Here, we discuss possible roles of the oxytocin system across these functional stages of domestication. We hypothesize that in early domestication, oxytocin played important roles in attenuating fear and stress associated with human contact. In later domestication, we hypothesize that oxytocin's most critical functions were those associated with affiliative social behavior, social engagement, and cooperation with humans. We outline possible neurobiological changes associated with these processes and present a Siberian fox model of canid domestication in which these predictions can be tested. Lastly, we identify limitations of current studies on the neuroendocrinology of domestication and discuss challenges and opportunities for future research.
Keywords: Domestication, Oxytocin, Stress, Aggression, Affiliative behavior, Canid, Dog, Wolf, Fox
Highlights
-
•
We propose various roles for oxytocin across canid domestication.
-
•
In early domestication, oxytocin primarily regulated fear and anxiety toward humans.
-
•
In late domestication, oxytocin facilitated interspecific social bonds and cooperation.
-
•
Comparative neurobiology is critical for understanding oxytocin's roles in domestication.
-
•
Experimentally domesticated Siberian foxes provide a powerful model for these studies.
1. Introduction: Functional stages of domestication
The domestication of animals represents one of the largest cultural-evolutionary events in human history. Whereas modern humans have existed for at least two hundred thousand years [1], animal domestication began only in the last 15–40 thousand years, with most domestication events occurring much more recently [2]. Although there remain many open questions regarding how, when, and why various species have been domesticated, these evolutionary events transformed the biology of diverse species in ways that enabled novel forms of human-animal interactions.
The selective pressures involved in domestication have likely varied across both time and taxa, yet it is believed that, across many species, the earliest stages of animal domestication depended largely on evolutionary reductions in fear and aggression toward humans [3]. In some species, such as dogs (Canis familiaris), domestication favored not only social tolerance of humans, but also active social bonds and relationships rooted in attachment [4]. Our ability to form such interspecific relationships is based in part on empathy toward nonhuman animals, a defining characteristic of human psychology [[5], [6], [7]], but likely also on specific behavioral and neurobiological adaptations in the domesticates themselves [8].
The first domesticated taxa were dogs, who diverged from their principal ancestor – the gray wolf (Canis lupus) between ∼ 32,000 and ∼11,000–16,000 years ago [9,10]. How and why dog domestication began remains controversial, but likely involved several functionally distinct stages. Below we briefly describe two important transitions during dog domestication to provide context for our hypotheses regarding oxytocin's roles in these processes.
The first stage of dog domestication likely involved the opening of an anthropogenic niche that facilitated novel and sustained associations between some wolf and human populations. Some theories suggest that wolf populations were first attracted to refuse generated by humans, providing a novel feeding ecology that was most profitably exploited by wolves (Canis lupus) with the least fear of humans [11]. In contrast, other theories propose that human-wolf interactions began by humans capturing and rearing infant wolves [[12], [16]] or via mutualism stemming from the complementary hunting strategies of Paleolithic humans and wolves [13]. Regardless of the specific circumstances that favored these early human-wolf associations, wolf populations initially occupied these niches as synanthropes (non-domesticated species benefiting from an anthopogenic environment). It is unlikely that humans at this time foresaw or actively planned for future stages of domestication, as would subsequent generations of dog breeders.
In a subsequent phase of domestication, human-dog interactions began to take new forms, and dogs began to be selected for particular behavioral characteristics [14]. During this period it is likely that social bonds between individual dogs and people became increasingly important, possibly favoring dogs who were most biologically prepared to develop such interspecific relationships. Although wolves can develop attachment relationships with humans when hand-fed from a young age [15,16], domestication appears to have relaxed the conditions required for such relationships in dogs [11,17,18]. Thus, whereas the initial stages of dog domestication likely targeted reductions in fear and anxiety that were required for life in anthropogenetic environments, selection in later stages of domestication may have acted more specifically on socioemotional processes related to interspecific social bonding and cooperation.
Due to its historical nature, understanding the sequence of biological changes associated with dog domestication remains challenging. However, an experimental model of canid domestication – initiated with silver foxes (Vulpes vulpes) more than 60 years ago in the former USSR – provides important clues about these processes. Below, we briefly describe this remarkable and long running domestication experiment and propose a model in which both the initial and later physiological changes associated with domestication may have depended critically on the neuropeptide oxytocin.
2. A canid model of domestication: Siberian foxes
In 1959, Russian geneticist Dmitri Belyaev initiated a study designed to mimic the process of animal domestication. In doing so, he aimed not only to create a selective regime reflecting the conditions he envisioned as critical for animal domestication, but also to answer fundamental questions about the phenotypic changes in domestication and their biological bases [3]. Although the genetic changes associated with dog domestication were unknown at the time, Belyaev proposed that, fundamentally, domestication was based on selection for tamability.
To test this hypothesis, Belyaev employed a species he had worked with extensively at the Institute for Fur Breeding, the silver fox (Vulpes vulpes). Year after year, he and his team bred and systematically tested hundreds of foxes for “friendliness” towards humans [19]. In the population selected for tamability, only the most friendly and least aggressive foxes (∼10% of the population) were selected to be parents for the next generation (Fig. 1).
Fig. 1.
Experimentally-domesticated foxes with researchers at a farm near Novosibirsk, Russia (Photos: A. Fedorova, with permission).
Across 60 years, Belyaev and his colleagues in Novosibirsk documented phenotypic changes in these foxes that closely resembled those in dog domestication (e.g., increased docility, alterations to developmental timing, and morphological changes including an increased prevalence of depigmentation, floppy ears, and curly tails). In addition to characterizing rapid and remarkable morphological and behavioral changes in these foxes, Belyaev's team discovered a dramatic (two-fold) reduction of blood cortisol concentrations and decreased reactivity of the adrenal cortex in foxes selected for tamability, relative to wild-type controls [8,20]. These findings were the first to characterize neuroendocrine changes related to reduced stress reactivity in an experimental model of model of domestication [3,20]. Similar results have since been reported in domesticated rats [21,22] and guinea pigs [23]. Foxes selected for tamability also exhibited increased serotonin concentrations in diverse areas of the brain, which has been hypothesized as another important mechanism inhibiting aggressive behavior in this lineage [24].
3. Properties of oxytocin
Oxytocin is a neuropeptide synthesized in the mammalian hypothalamus, that is produced (with some minor molecular variations) in all vertebrates [25,26]. This molecule has important actions both in the central nervous system, where it functions as a neurotransmitter, and in the periphery, where it acts as a hormone. Peripherally, oxytocin is secreted into the bloodstream via the posterior pituitary where it contributes to diverse physiological processes including parturition, lactation, metabolism, cardiovascular function, skeletal homeostasis, muscle maintenance, and autonomic regulation [[27], [28], [29], [30], [31], [32], [33], [34]]. Many of oxytocin's actions in the periphery feed back to the central nervous system, for example, via pathways involving the vagus nerve [[35], [36], [37], [38], [39]]. It has also been shown that oxytocin is actively transported from peripheral blood into the brain via the receptor for advanced glycation end-products (RAGE), a process that can influence neural activity related to social behavior [40,41]. In the central nervous system, oxytocin acts as a non-canonical neurotransmitter or neuromodulator, regulating emotional states, social cognition, and behaviors ranging from fear, anxiety, and aggression to pair bonding [[42], [43], [44], [45]]. Oxytocin can be released from cell bodies and dendrites, diffusing to nearby receptors, but also by targeted long-range axonal projections [46].
In some species, oxytocin plays critical roles in the development of selective social bonds, for example, between monogamous dyads, or adults and infants [[137], [138]]. Outside of reproductive contexts, oxytocin can sometimes increase social trust [47,48], modulate social attention and aspects of social engagement, such as eye gaze [49,50] and promote behavioral synchrony [51,52].
Importantly, oxytocin can also have potent anxiolytic effects. By binding to oxytocin receptors in the amygdala and vagal nuclei, oxytocin can modulate the autonomic nervous system [[53], [54], [55]]. Through actions on hypothalamic nuclei, oxytocin can influence activity in the hypothalamic-pituitary-adrenal (HPA) axis [[56], [57], [58]]. These actions of oxytocin on the HPA axis have been shown to robustly attenuate release of glucocorticoids, specifically by modulating synaptic transmission at corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus [59].
Lastly, studies in primates and rodents suggest that oxytocin also influences serotonergic function by stimulating release of serotonin in limbic regions and increasing the availability of serotonin receptors [[60], [61], [62]]. Given the serotonergic changes observed in Belyaev's foxes [24], and known roles for serotonin in inhibiting aggressive behavior [63,64], it is possible that oxytocin-serotonin interactions also contributed to reductions in aggressive behavior across domestication.
As described above, dog domestication likely involved a sequence of phenotypic changes, first involving selection against fear and aggression towards humans, and later involving selection for affiliative socioemotional processes that enabled dogs to (more) easily form enduring social bonds with humans. We hypothesize that each of these changes depended, in part, on neuroendocrine processes involving oxytocin. Below, we expand on the hypothesized roles of oxytocin across these phases of domestication.
4. Oxytocin in early domestication: Effects on fear, stress, and selective sociality
We hypothesize that in early domestication, the most important functions of oxytocin were those associated with dampening stress reactivity and inhibiting aggressive behavior. Indeed, in most experimental models of domestication, reductions in stress and aggressive responses are among the first phenotypic changes observed. For example, when Norway rats that were selected for reduced aggressiveness towards humans were exposed to an emotional stressor, their resting plasma corticosterone levels and overall HPA axis activity were drastically lower compared to rat lines selected for increased aggression [22,65]. Preliminary evidence reveals oxytocinergic correlates of these changes to fear and aggressive behavior. For example, comparisons between laboratory and wildtype strains of rats indicate that, relative to wildtypes, laboratory-domesticated strains exhibit higher densities of oxytocin-immunoreactive cells in several regions of the hypothalamus [66]. In feral rats strains – which exhibit higher levels of offensive aggression than laboratory strains – both acute, and chronic oxytocin administration yield dose-dependent reductions in aggressive behavior, whereas antagonizing the oxytocin receptor cause increases in aggression [67,68].
The studies reviewed above suggest several mechanisms through which increases in oxytocin signaling may have contributed to reductions in fear, anxiety, and aggression toward humans during the early stages of domestication. Further, this work illustrates pathways through which oxytocin may have altered other neurohormonal systems, which to date, have been principal foci in the neuroendocrinology of domestication (i.e., glucocorticoids and serotonin). Nonetheless, it is unlikely that a simple upregulation of the oxytocin system could account for the behavioral and emotional changes in early domestication.
Indeed, the effects of oxytocin are known to be highly context dependent and moderated by a wide range of biological and social factors. Recent research has confirmed these phenomena in studies of domestic dogs. For example, during calm and affiliative forms of human-animal interaction, both humans and dogs exhibit increases in circulating oxytocin [[69], [70], [71], [72]], and exogenous administration of oxytocin can increase affiliative social behaviors, such as eye-gaze and contact seeking in these settings [73,74]. However, under other conditions, including threat, exogenous oxytocin administration has been shown to decrease dogs’ friendly behavior toward humans [75]. Similar “antisocial” effects of oxytocin have been observed in several other mammals, including both domesticated and non-domesticated species. For example, intranasal oxytocin administered to newborn piglets resulted in increased aggression to other piglets under stressful conditions [76]. Central oxytocin infusion promoted aggression in dominant male squirrel monkeys [77] and dominant male rats, which exhibited increased oxytocin receptor binding in socially relevant brain regions [78], and Gray rats became more aggressive toward conspecifics in the resident-intruder test after prolonged oxytocin administration [79].
The results of such studies can be challenging to interpret, especially because (at high concentrations) oxytocin can also bind to vasopressin receptors, sometimes yielding opposite effects to those when oxytocin acts at its own receptor [46,[80], [81], [82]]. Nonetheless, a substantial literature confirms that the effects of oxytocin are not always prosocial, particularly under conditions of threat or stress [[83], [84], [85]].
Several hypotheses, relevant to domestication, have been proposed to account for these varying effects of oxytocin. The social salience hypothesis [86] posits that via interactions with the dopaminergic system, oxytocin enhances the salience of social cues, regardless of their valence. Thus, in social contexts without stress or fear, oxytocin may promote prosocial behaviors by enhancing the salience of (positively-valanced) social cues among interacting partners. This effect could account for responses to oxytocin such as increased eye gaze, proximity seeking, and enhanced responses to human gestural communication in companion dogs interacting with familiar partners [73,74,87]. However, this hypothesis can also account for why dogs receiving exogenous oxytocin exhibit reduced friendly behavior toward their owners when approached in a threatening manner [75]. Related hypotheses emphasize differential roles of oxytocin in social interactions between in-group versus out-group members, such that oxytocin serves primarily prosocial functions when individuals interact with in-group members, but can decrease cooperative or affiliative behaviors toward out-group members [88,89]. Although the latter hypothesis was formulated largely based on studies of human behavior, and tested in contexts involving cultural identities, is bears relevance to domestication given that at some point, the core social group for early dogs shifted from being one consisting of conspecifics to one composed (predominantly) of humans.
It is unlikely that initial wolf-human interactions took place in the favorable, non-stressful situations where many prosocial effects of oxytocin have been demonstrated in dogs. Thus, it is important to consider the possible roles of oxytocin under the likely stressful conditions present in early stages of domestication.
Wolf packs occupy a home territory, that is delineated by scent marking and vocalizations, and which is defended from neighboring packs [90,91]. Although intergroup encounters are rare, when they do occur, they frequently involve aggression leading to serious injuries or fatalities [90]. Thus, wolf societies are characterized by highly selective sociality [45], with strong social bonds and high levels of affiliative behavior within a pack, but predominantly agonistic inter-group dynamics. Presumably, the initial stages of domestication required conditions in which some wolf and human populations maintained regular physical proximity. During this period, which predates agriculture, humans lived highly mobile hunter-gatherer lifestyles, and it is likely that humans exploited many of the same territories as wolves. Although we know little about how human presence would have affected wolf populations at the time, many believe that wolves have long been fearful of humans [16]; but see Ref. [92]. Recent studies illustrate that wolf packs adjust activity within a territory to avoid human encroachment [93]. These conditions may have created chronic stressors for some wolf populations, who were required to either reduce their territorial usage, to find new territories, or to adopt behavioral strategies that allowed them to coexist in areas trafficked by humans.
Studies using animal models suggest that oxytocin can facilitate adaptative fear by promoting rapid detection of threats and strengthening memories associated with fear [94]. But at the same time, in stressogenic territories, oxytocin tends to reduce anxiety, allowing greater expression of exploratory behavior in novel areas of the environment [58]. Thus, it is possible that these seemingly paradoxical roles of oxytocin contributed to divergent strategies in wolf populations living near humans. In general, oxytocin may have contributed to sustained fear and avoidance learning in most wolves. However, among wolves who chose, or were forced to live nearby humans, oxytocin may have reduced (chronic) maladaptive anxiety, enabling these animals to approach and explore areas associated with human activity. Across generations such wolves may have been more likely to form new territories in proximity to humans [95], rearing offspring who exhibited further reductions in fear and anxiety due to developmental exposure to anthropogenic environments. Similar processes have recently been demonstrated in coyotes. Across reproductive bouts, parents become habituated to anthropogenic environments, and in turn, successive litters exhibit further reductions in fear of humans as a function of parental behavior [96].
Although speculative, the scenario described above outlines conditions in which a novel anthropogenic niche may have favored divergent adaptations among wolves, which depended in part on the roles of oxytocin in fear and anxiety. Once established, the novel anthropogenic niche would have continually favored animals with the least fear and anxiety toward humans, creating persistent selection pressures on canid socioemotional systems. We hypothesize that physiological responses to these conditions aligned closely with those observed in Belaev's foxes, including general reductions in HPA activity, and increases in serotonergic activity, effects that as described above, may have been coordinated via the actions of oxytocin.
Relative to wolves, feral dogs occupy less rigidly defined territories (which often overlap substantially between packs), and though these territories are defended, and intergroup encounters are frequent, territorial conflict rarely results in lethal aggression. Feral dogs also exhibit less cooperation in activities such as hunting and breeding (where paternal care is rare, relative to wolves), and their mating system has been characterized as promiscuous [97,98]. One possibility is that the anthropogenic niche described above favored a transition from the highly selective sociality observed in wolves – in which packs hunt cooperatively, social units are based around a single monogamous breeding pair, and intergroup encounters are highly agonistic – to a less structured social system, in which selective sociality (including monogamous breeding) became less critical, perhaps in response to a shift from cooperative hunting to scavenging. Returning to Belyaev's foxes, changes in social selectivity with reproductive partners presented a major challenge in early captive fox breeding, and initially male foxes refused to copulate with unfamiliar females, with only a few males from the wild colony exhibiting the ability to breed polygamously [99].
This distinction between general gregariousness and highly selective sociality is important here, as both sets of processes depend on an integrative system involving both oxytocin and the evolutionarily-related neuropeptide, arginine vasopressin [100,101]. Vasopressin has shown to modulate selective aggression and partner preference in monogamous species [102] and may be particularly important for more selective forms of sociality. The possibility that dogs became less selectively social during domestication is supported by recent work linking “hypersociality” in dogs to genetic variants associated with Williams-Beuren syndrome [103], a neurodevelopmental disorder characterized by extreme gregariousness and attraction to strangers, and in which both oxytocin and vasopressin appear to be dysregulated [104]. Intriguingly, recent comparative genomic studies with wildtype and domesticated mammals suggest evolutionary changes in domestication involving all three subtypes of vasopressin's receptor [105]. Relative to oxytocin, there has been comparatively little focus on the roles of vasopressin in domestication, but we hypothesize that functional changes involving vasopressin have also been important for changes to stress physiology, selective sociality, and aggression across the course of domestication. Notably, in initial studies on neuropeptides and aggression in dogs, aggressive behavior has been linked to circulating vasopressin, but not oxytocin concentrations [136].
5. Oxytocin in late domestication: Social affiliation with humans
As described above, we hypothesize that the central neuropeptide-dependent processes in early domestication were those associated with downregulation of stress responses, inhibition of fear, and relaxation to the social structure and highly selective sociality of wolves. Whereas these adaptations may have allowed canids to become synanthropic, the development of strong interspecific emotional bonds and cooperative behaviors likely depended on further adaptations involving prosocial functions of oxytocin. Relative to wolves, dogs easily form social bonds with humans, requiring minimal exposure to people during early development in order to be successfully socialized [17,106]. Social bonds between dogs and humans have been characterized using attachment theory (reviewed in Ref. [107], and assessed using modifications of Ainsworth's Strange Situation Test [108], which was first developed to assess attachment between children and their caregivers. These and related studies reveal that social bonds between dogs and their caregivers exhibit many similarities to those between children and parents [109], including secure-base effects, and aspects of “social engagement” [110] such as sustained eye contact and immobility without fear [71,111]. Although it was initially reported that socialized wolves could not form such attachment relationships with humans [112], these findings have been challenged in recent years [15,113]. From a functional perspective, however, what matters is not a categorical distinction of whether such relationships are possible, but rather the relative ease with which they develop. In this regard, the evidence that dogs have evolved predispositions to seek and maintain social bonds with humans is abundant.
Importantly, many aspects of these social behaviors are known to involve oxytocin, and there is a rapidly growing literature demonstrating ways in which oxytocin facilitates and responds to these forms of interspecific social interaction. For example, several studies have shown that social touch, vocal cues, and mutual eye gaze increase peripheral oxytocin concentrations in both humans and dogs [69,72,114]. Similarly, under conditions of psychological safety [39] intranasal administration of oxytocin to dogs can increases affiliative behaviors toward humans, including proximity seeking and eye gaze [73,74]; but see Ref. [115] for breed differences in these effects), effects that have not been observed in wolves [73]; but see Refs. [116,117].
6. Predictions and future research opportunities
Although the number of scientific studies investigating possible roles of oxytocin in domestication is increasing rapidly, we still lack fundamental knowledge about core features of the oxytocinergic system in domesticated animals, and their wild counterparts. In the remainder of this article, we address possible neurobiological changes associated with the scenarios described above, as well as future data and experiments that will be required to powerfully test hypotheses about oxytocin's roles in domestication.
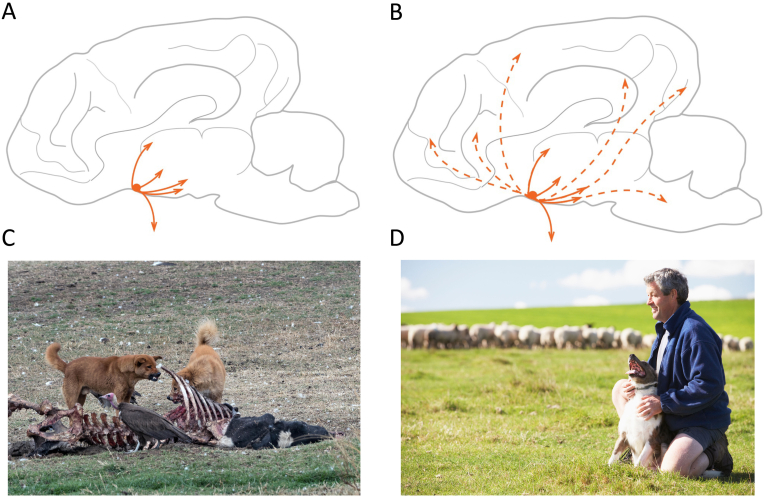
In early domestication, we propose that oxytocin's most important roles related to regulating stress and fear, effected by actions HPA axis, autonomic nervous system, and serotonergic systems. It is also possible that modifications to oxytocin signaling, in conjunction with arginine vasopressin, contributed to a relaxation of the social structure and reproductive monogamy of gray wolf ancestors. These aspects of oxytocin signaling may have relied primarily on evolutionarily ancient modes of oxytocin release (Fig. 2), involving diffusion from neuronal cell bodies and dendrites as well as limited axonal projections to structures in the midbrain and limbic regions [46,66]. Similarly, these target regions may have become enriched for oxytocin receptors, increasing sensitivity to oxytocin's release.
Fig. 2.
Hypothesized changes to the central oxytocin system across domestication. A: In early domestication, oxytocin is predominantly released in the hypothalamus from somatodendritic compartments of OT neurons, locally suppressing activity of the HPA axis and reducing aggression. Medial surface of the dog brain is from Ref. [118]. B: Visual depiction of hypothesized changes in later domestication. There is an elaboration of neurons projecting beyond the hypothalamus (dashed lines) allowing targeted release of OT in diverse socially-relevant forebrain regions that orchestrate complex forms of affiliative behaviors with humans. C: Feral dogs in Ethiopia exploiting an anthropogenic niche, a process that was likely critical in early domestication D: Herding dogs as human assistants and companions, an example of novel forms of human-animal interaction associated with late domestication. The images in panels C and D are subject to copyright and were licensed for use by the authors.
In later domestication, we propose that oxytocin began to facilitate the interspecific forms of social engagement and attachment that characterize many modern-day relationships between humans and dogs. These processes may have required elaboration of the neural pathways described above, but perhaps also axonal projections to more distal sites, including those in the forebrain, which are evolutionarily more recent, and implicated in complex aspects of social behavior (Fig. 2; [25,119]. However, the extent to which behavioral and socioemotional changes in late domestication would have required elaboration to the neural targets of oxytocin signaling remains unknown. Given that prior to domestication, wolves (and silver foxes) already exhibited selective social bonds and highly coordinated forms of social behavior, it is possible that the neurobiological effects of late domestication consisted primarily of changes that allowed novel interspecific social partners to stimulate already existing oxytocinergic pathways involved in social bonding. On this model, existing oxytocin pathways that functioned in intraspecific sociality may have been evolutionarily co-opted for novel forms of interspecific sociality, without the need for substantial modifications to existing oxytocin pathways.
Testing hypotheses about the roles of oxytocin in canid domestication will require novel comparative studies. Current research has addressed these questions largely through genetic studies of the oxytocin receptor gene [120] or biomarkers from peripheral matrices such as urine [73,[121], [122], [123]]. Though assessments of peripheral oxytocin concentrations hold value, it remains unclear to what extent these measurements reflect central oxytocinergic activity [124] and this approach cannot address questions about the neural architecture of oxytocin signaling in the brain. Thus, an important priority for future research will involve the comparative study of fine organization of oxytocin neurons, their synaptic apparatus and central axonal projections as well as mapping of oxytocin receptor expressing regions in the brains of domesticated and wild-type species. Although beyond the scope of this article, similar work investigating arginine vasopressin and its receptors will be crucial for understanding the neuroendocrine pathways of domestication [125]. Many of the neurobiological consequences of domestication – especially those related to selective sociality and stress responses – may involve coordinated actions of both oxytocin and vasopressin, and thus their study as an integrative system will be critical [126]. In addition to this comparative neurobiological work, it will also be important to better understand to the consequences of exogenous exposure to oxytocin (or vasopressin) in both domesticated and non-domesticated species, at different points in development, and across contexts [127,128].
The use of comparative studies, drawing on diverse species along a domestication continuum will be critical for our ability to understand and differentiate general processes involved in domestication from those which may be lineage specific. For example, although oxytocin's roles in pair bonding were first demonstrated in studies with voles [129,130] specific signatures of oxytocinergic evolution in voles do not necessarily generalize to other pair-bonded taxa [[131], [132], [133]]. In this regard, it will be important to embrace a broadly comparative perspective for questions about the roles of oxytocin in domestication. Within the canid lineage, comparisons of dogs and wolves will be essential, but studies with other (partially) domesticated taxa such as dingoes [134], or urban coyotes [135] will also provide important comparative data.
Uniquely situated within this comparative landscape are the Siberian fox populations, from which so many other insights about domestication have emerged. These populations afford valuable opportunities for investigating the full range of questions delineated above, under the rigorous experimental conditions that have been sustained for more than 60 years. Thus, we expect that future studies with Siberian foxes will contribute significantly to the next phases of research on possible roles of oxytocin in animal domestication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by RSF-DFG grant to YEH (21-44-04405) and to VG (GR 3619/15–1). We gratefully acknowledge Sue Carter for thoughtful comments on an earlier draft of the manuscript.
Contributor Information
Yury E. Herbeck, Email: herbek@bionet.nsc.ru.
Marina Eliava, Email: marina.eliava@zi-mannheim.de.
Valery Grinevich, Email: valery.grinevich@zi-mannheim.de.
Evan L. MacLean, Email: evanmaclean@arizona.edu.
References
- 1.Hublin Jean-Jacques, Ben-Ncer Abdelouahed, Bailey Shara E., Freidline Sarah E., Neubauer Simon, Skinner Matthew M., Bergmann Inga, Le Cabec Adeline, Benazzi Stefano, Harvati Katerina. New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens. Nature. 2017;546(7657):289–292. doi: 10.1038/nature22336. [DOI] [PubMed] [Google Scholar]
- 2.Larson Greger, Fuller Dorian Q. The evolution of animal domestication. Annu. Rev. Ecol. Evol. Syst. 2014;45:115–136. [Google Scholar]
- 3.Belyaev Dmitry K. Destabilizing selection as a factor in domestication. J. Hered. 1979;70(5):301–308. doi: 10.1093/oxfordjournals.jhered.a109263. [DOI] [PubMed] [Google Scholar]
- 4.Previde Emanuela Prato, Valsecchi Paola. 2014. "The Immaterial Cord: the Dog–Human Attachment bond." the Social Dog; pp. 165–189. [Google Scholar]
- 5.Angantyr Malin, Eklund Jakob, Hansen Eric M. A comparison of empathy for humans and empathy for animals. Anthrozoös. 2011;24(4):369–377. [Google Scholar]
- 6.Panksepp Jaak, Panksepp Jules B. Toward a cross-species understanding of empathy. Trends Neurosci. 2013;36(8):489–496. doi: 10.1016/j.tins.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez-Leal Raquel, Ana Costa, Megías-Robles Alberto, Fernández-Berrocal Pablo, Faria Luísa. Relationship between emotional intelligence and empathy towards humans and animals. PeerJ. 2021;9 doi: 10.7717/peerj.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbeck Yury E., Gulevich Rimma G., Eliava Marina, Shepeleva Darya V., Trut Lyudmila N., Grinevich Valery. 2018. Domestication: Neuroendocrine Mechanisms of Canidae‐human Bonds." Model Animals in Neuroendocrinology: from Worm to Mouse to Man; pp. 313–334. [Google Scholar]
- 9.Freedman Adam H., Gronau Ilan, Schweizer Rena M., Ortega-Del Vecchyo Diego, Han Eunjung, Silva Pedro M., Galaverni Marco, Fan Zhenxin, Marx Peter, Lorente-Galdos Belen. Genome sequencing highlights the dynamic early history of dogs. PLoS Genet. 2014;10(1) doi: 10.1371/journal.pgen.1004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Guo-Dong, Zhai Weiwei, Yang He-Chuan, Wang Lu, Zhong Li, Liu Yan-Hu, Fan Ruo-Xi, Yin Ting-Ting, Zhu Chun-Ling, Poyarkov Andrei D. Out of southern East Asia: the natural history of domestic dogs across the world. Cell Res. 2016;26(1):21–33. doi: 10.1038/cr.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppinger Raymond, Coppinger Lorna. University of Chicago Press; 2002. Dogs: a New Understanding of Canine Origin, Behavior and Evolution. [Google Scholar]
- 12.Serpell James A. Commensalism or cross-species adoption? A critical review of theories of wolf domestication. Frontiers in Veterinary Science. 2021;8 doi: 10.3389/fvets.2021.662370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shipman Pat. How do you kill 86 mammoths? Taphonomic investigations of mammoth megasites. Quat. Int. 2015;359:38–46. [Google Scholar]
- 14.Vigne Jean-Denis. The origins of animal domestication and husbandry: a major change in the history of humanity and the biosphere. Comptes Rendus Biol. 2011;334(3):171–181. doi: 10.1016/j.crvi.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Wheat Christina Hansen, Larsson Linn, Berner Patricia, Temrin Hans. Hand-reared wolves show attachment comparable to dogs and use human caregiver as a social buffer in the Strange Situation Test. bioRxiv. 2020 2020.02. 17.952663. [Google Scholar]
- 16.Mech L David, Janssens Luc AA. 2021. An Assessment of Current Wolf Canis lupus Domestication Hypotheses Based on Wolf Ecology and Behaviour. Mammal Review. [Google Scholar]
- 17.Serpell James, Duffy Deborah L., Jagoe J Andrew. vol. 2. 2016. pp. 93–117. ("Becoming a Dog: Early Experience and the Development of behavior." the Domestic Dog: its Evolution, Behavior and Interactions with People). [Google Scholar]
- 18.Price Edward O. Cabi; 2002. Animal Domestication and Behavior. [Google Scholar]
- 19.Trut L.N. Early Canid Domestication: the Farm-Fox Experiment: foxes bred for tamability in a 40-year experiment exhibit remarkable transformations that suggest an interplay between behavioral genetics and development. Am. Sci. 1999;87(2):160–169. [Google Scholar]
- 20.Trut Lyudmila, Oskina Irina, Kharlamova Anastasiya. Animal evolution during domestication: the domesticated fox as a model. Bioessays. 2009;31(3):349–360. doi: 10.1002/bies.200800070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plyusnina Irene, Oskina I. Behavioral and adrenocortical responses to open-field test in rats selected for reduced aggressiveness toward humans. Physiol. Behav. 1997;61(3):381–385. doi: 10.1016/s0031-9384(96)00445-3. [DOI] [PubMed] [Google Scholar]
- 22.Plyusnina Irina Z., Oskina Irina N., Tibeikina Marina A., Popova Nina K. Cross-fostering effects on weight, exploratory activity, acoustic startle reflex and corticosterone stress response in Norway gray rats selected for elimination and for enhancement of aggressiveness towards human. Behav. Genet. 2009;39(2):202–212. doi: 10.1007/s10519-008-9248-6. [DOI] [PubMed] [Google Scholar]
- 23.Künzl Christine, Sachser Norbert. The behavioral endocrinology of domestication: a comparison between the domestic Guinea pig (Cavia apereaf. porcellus) and its wild ancestor, the cavy (Cavia aperea) Horm. Behav. 1999;35(1):28–37. doi: 10.1006/hbeh.1998.1493. [DOI] [PubMed] [Google Scholar]
- 24.Popova N.K., Voitenko N.N., Kulikov A.V., Avgustinovich D.F. Evidence for the involvement of central serotonin in mechanism of domestication of silver foxes. Pharmacol. Biochem. Behav. 1991;40(4):751–756. doi: 10.1016/0091-3057(91)90080-l. [DOI] [PubMed] [Google Scholar]
- 25.Knobloch H Sophie, Grinevich Valery. Evolution of oxytocin pathways in the brain of vertebrates. Front. Behav. Neurosci. 2014;8:31. doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee P., Joy K.P., Chaube R. Structural and functional diversity of nonapeptide hormones from an evolutionary perspective: a review. Gen. Comp. Endocrinol. 2017;241:4–23. doi: 10.1016/j.ygcen.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Neumann I., Russell J.A., Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993;53(1):65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- 28.Gutkowska J., Jankowski M. Oxytocin revisited: its role in cardiovascular regulation. J. Neuroendocrinol. 2012;24(4):599–608. doi: 10.1111/j.1365-2826.2011.02235.x. [DOI] [PubMed] [Google Scholar]
- 29.Chaves, Ernestânia Valéria, Tilelli Cristiane Queixa, Almeida Brito Nilton, Nascimento Brito Márcia. Role of oxytocin in energy metabolism. Peptides. 2013;45:9–14. doi: 10.1016/j.peptides.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Kasahara Yoshiyuki, Sato Keisuke, Takayanagi Yuki, Mizukami Hiroaki, Ozawa Keiya, Hidema Shizu, So Kyoung-Ha, Kawada Teruo, Inoue Nao, Ikeda Ikuo. Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology. 2013;154(11):4305–4315. doi: 10.1210/en.2012-2206. [DOI] [PubMed] [Google Scholar]
- 31.Elabd Christian, Cousin Wendy, Pavan Upadhyayula, Chen Robert Y., Chooljian Marc S., Ju Li, Kung Sunny, Jiang Kevin P., Conboy Irina M. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat. Commun. 2014;5(1):1–11. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurlemann Rene, Grinevich Valery. Springer; 2018. Behavioral Pharmacology of Neuropeptides: Oxytocin. [Google Scholar]
- 33.Sun Li, Lizneva Daria, Ji Yaoting, Colaianni Graziana, Hadelia Elina, Gumerova Anisa, Ievleva Kseniia, Kuo Tan-Chun, Korkmaz Funda, Ryu Vitaly. Oxytocin regulates body composition. Proc. Natl. Acad. Sci. Unit. States Am. 2019;116(52):26808–26815. doi: 10.1073/pnas.1913611116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwasaki Yusaku, Maejima Yuko, Suyama Shigetomo, Yoshida Masashi, Arai Takeshi, Katsurada Kenichi, Kumari Parmila, Nakabayashi Hajime, Kakei Masafumi, Yada Toshihiko. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308(5):R360–R369. doi: 10.1152/ajpregu.00344.2014. [DOI] [PubMed] [Google Scholar]
- 35.Moscovice Liza R., Ziegler Toni E. Peripheral oxytocin in female baboons relates to estrous state and maintenance of sexual consortships. Horm. Behav. 2012;62(5):592–597. doi: 10.1016/j.yhbeh.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cushing Bruce S., Carter C Sue. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm. Behav. 2000;37(1):49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- 37.Quintana Daniel S., Kemp Andrew H., Gail A Alvares, Guastella Adam J. A role for autonomic cardiac control in the effects of oxytocin on social behavior and psychiatric illness. Front. Neurosci. 2013;7:48. doi: 10.3389/fnins.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porges Stephen W. The polyvagal theory: phylogenetic contributions to social behavior. Physiol. Behav. 2003;79(3):503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 39.Porges S.W. The polyvagal perspective. Biol. Psychol. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto Yasuhiko, Liang Mingkun, Munesue Seiichi, Deguchi Kisaburo, Ai Harashima, Furuhara Kazumi, Yuhi Teruko, Zhong Jing, Akther Shirin, Goto Hisanori. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Communications biology. 2019;2(1):76. doi: 10.1038/s42003-019-0325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto Yasuhiko, Higashida Haruhiro. RAGE regulates oxytocin transport into the brain. Communications biology. 2020;3(1):1–4. doi: 10.1038/s42003-020-0799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacDonald Kai, Feifel David. Oxytocin׳ s role in anxiety: a critical appraisal. Brain Res. 2014;1580:22–56. doi: 10.1016/j.brainres.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 43.Grinevich Valery, Stoop Ron. Interplay between oxytocin and sensory systems in the orchestration of socio-emotional behaviors. Neuron. 2018;99(5):887–904. doi: 10.1016/j.neuron.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 44.de Jong, Trynke R., Neumann Inga D. 2017. "Oxytocin and aggression." Behavioral Pharmacology of Neuropeptides: Oxytocin; pp. 175–192. [DOI] [PubMed] [Google Scholar]
- 45.Carter C Sue, Cushing Bruce S. The Origins and Nature of Sociality. Routledge; 2017. Proximate mechanisms regulating sociality and social monogamy, in the context of evolution; pp. 99–121. [Google Scholar]
- 46.Grinevich Valery, Knobloch-Bollmann H Sophie, Eliava Marina, Busnelli Marta, Chini Bice. Assembling the puzzle: pathways of oxytocin signaling in the brain. Biol. Psychiatr. 2016;79(3):155–164. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Nave Gideon, Camerer Colin, McCullough Michael. Does oxytocin increase trust in humans? A critical review of research. Perspect. Psychol. Sci. 2015;10(6):772–789. doi: 10.1177/1745691615600138. [DOI] [PubMed] [Google Scholar]
- 48.Kosfeld Michael, Heinrichs Markus, Paul J Zak, Fischbacher Urs, Fehr Ernst. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 49.Guastella, Adam J., Mitchell Philip B., Dadds Mark R. Oxytocin increases gaze to the eye region of human faces. Biol. Psychiatr. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 50.Domes Gregor, Steiner Angela, Porges Stephen W., Heinrichs Markus. Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology. 2013;38(7):1198–1202. doi: 10.1016/j.psyneuen.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Mu Yan, Guo Chunyan, Han Shihui. Oxytocin enhances inter-brain synchrony during social coordination in male adults. Soc. Cognit. Affect Neurosci. 2016;11(12):1882–1893. doi: 10.1093/scan/nsw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feldman Ruth. Sensitive periods in human social development: new insights from research on oxytocin, synchrony, and high-risk parenting. Dev. Psychopathol. 2015;27(2):369–395. doi: 10.1017/S0954579415000048. [DOI] [PubMed] [Google Scholar]
- 53.Huber Daniel, Veinante Pierre, Stoop Ron. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 54.Grippo Angela J., Trahanas Diane M., Zimmerman Robert R., II, Porges Stephen W., Carter C Sue. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34(10):1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porges Stephen W. Love: an emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology. 1998;23(8):837–861. doi: 10.1016/s0306-4530(98)00057-2. [DOI] [PubMed] [Google Scholar]
- 56.Neumann Inga D. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog. Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- 57.Petersson Maria, Hulting Anna-Lena, Uvnäs-Moberg Kerstin. Oxytocin causes a sustained decrease in plasma levels of corticosterone in rats. Neurosci. Lett. 1999;264(1):41–44. doi: 10.1016/s0304-3940(99)00159-7. [DOI] [PubMed] [Google Scholar]
- 58.Windle R.J., Shanks N., Lightman Stafford L., Ingram Colin D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138(7):2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 59.Jamieson B.B., Nair B.B., Iremonger K.J. Regulation of hypothalamic corticotropin‐releasing hormone neurone excitability by oxytocin. J. Neuroendocrinol. 2017;29(11) doi: 10.1111/jne.12532. [DOI] [PubMed] [Google Scholar]
- 60.Lefevre Arthur, Richard Nathalie, Jazayeri Mina, Beuriat Pierre-Aurélien, Fieux Sylvain, Zimmer Luc, Duhamel Jean-René, Sirigu Angela. Oxytocin and serotonin brain mechanisms in the nonhuman primate. J. Neurosci. 2017;37(28):6741–6750. doi: 10.1523/JNEUROSCI.0659-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida Masahide, Takayanagi Yuki, Inoue Kiyoshi, Kimura Tadashi, Young Larry J., Onaka Tatsushi, Nishimori Katsuhiko. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. 2009;29(7):2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pagani Jerome H., Avram SK Williams, Cui Zhenzhong, Song June, Mezey Éva, Senerth Julia M., Baumann Michael H., Scott Young W. Raphe serotonin neuron‐specific oxytocin receptor knockout reduces aggression without affecting anxiety‐like behavior in male mice only. Gene Brain Behav. 2015;14(2):167–176. doi: 10.1111/gbb.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Audero Enrica, Mlinar Boris, Baccini Gilda, Skachokova Zhiva K., Corradetti Renato, Gross Cornelius. Suppression of serotonin neuron firing increases aggression in mice. J. Neurosci. 2013;33(20):8678–8688. doi: 10.1523/JNEUROSCI.2067-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi Aki, Miczek Klaus A. Neurogenetics of aggressive behavior: studies in rodents. Neuroscience of aggression. 2013:3–44. doi: 10.1007/7854_2013_263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naumenko E.V., Popova N.K., Nikulina Ella M., Dygalo N.N., Shishkina G.T., Borodin P.M., Markel A.L. Behavior, adrenocortical activity, and brain monoamines in Norway rats selected for reduced aggressiveness towards man. Pharmacol., Biochem. Behav. 1989;33(1):85–91. doi: 10.1016/0091-3057(89)90434-6. [DOI] [PubMed] [Google Scholar]
- 66.Ruan Chao, Zhang Zhibin. Laboratory domestication changed the expression patterns of oxytocin and vasopressin in brains of rats and mice. Anat. Sci. Int. 2016;91(4):358–370. doi: 10.1007/s12565-015-0311-0. [DOI] [PubMed] [Google Scholar]
- 67.Calcagnoli Federica, de Boer Sietse F., Althaus Monika, Den Boer Johan A., Koolhaas Jaap M. Antiaggressive activity of central oxytocin in male rats. Psychopharmacology. 2013;229(4):639–651. doi: 10.1007/s00213-013-3124-7. [DOI] [PubMed] [Google Scholar]
- 68.Calcagnoli Federica, Meyer Neele, F de Boer Sietse, Althaus Monika, Koolhaas Jaap M. Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats. Horm. Behav. 2014;65(4):427–433. doi: 10.1016/j.yhbeh.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 69.MacLean Evan L., Gesquiere Laurence R., Gee Nancy R., Levy Kerinne, Martin W Lance, Carter C Sue. Effects of affiliative human–animal interaction on dog salivary and plasma oxytocin and vasopressin. Front. Psychol. 2017;8:1606. doi: 10.3389/fpsyg.2017.01606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Handlin Linda, Hydbring-Sandberg Eva, Nilsson Anne, Ejdeback Mikael, Jansson Anna, Uvnas-Moberg Kerstin. Short-Term interaction between dogs and their owners: effects on oxytocin, cortisol, insulin and heart RateAn exploratory study. Anthrozoös. 2011;24(3):301–315. [Google Scholar]
- 71.Nagasawa M., Kikusui T., Onaka T., Ohta M. Dog's gaze at its owner increases owner's urinary oxytocin during social interaction. Horm. Behav. 2009;55(3):434–441. doi: 10.1016/j.yhbeh.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 72.Odendaal J.S.J., Meintjes R.A. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet. J. 2003;165(3):296–301. doi: 10.1016/s1090-0233(02)00237-x. [DOI] [PubMed] [Google Scholar]
- 73.Nagasawa Miho, Mitsui Shouhei, En Shiori, Ohtani Nobuyo, Ohta Mitsuaki, Sakuma Yasuo, Onaka Tatsushi, Mogi Kazutaka, Kikusui Takefumi. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science. 2015;348(6232):333–336. doi: 10.1126/science.1261022. [DOI] [PubMed] [Google Scholar]
- 74.Romero Teresa, Nagasawa Miho, Mogi Kazutaka, Hasegawa Toshikazu, Kikusui Takefumi. Proceedings of the National Academy of Sciences; 2014. Oxytocin Promotes Social Bonding in Dogs; p. 201322868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hernádi Anna, Kis Anna, Kanizsár Orsolya, Tóth Katinka, Miklósi Bernadett, Topál József. Intranasally administered oxytocin affects how dogs (Canis familiaris) react to the threatening approach of their owner and an unfamiliar experimenter. Behav. Process. 2015;119:1–5. doi: 10.1016/j.beproc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Rault Jean-Loup, Carter C Sue, Garner Joseph P., Marchant-Forde Jeremy N., Richert Brian T., Lay Donald C., Jr. Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiol. Behav. 2013;112:40–48. doi: 10.1016/j.physbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 77.Winslow James T., Insel Thomas R. Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J. Neurosci. 1991;11(7):2032–2038. doi: 10.1523/JNEUROSCI.11-07-02032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee Won, Hiura Lisa C., Yang Eilene, Broekman Katherine A., Ophir Alexander G., Curley James P. Social status in mouse social hierarchies is associated with variation in oxytocin and vasopressin 1a receptor densities. Horm. Behav. 2019;114:104551. doi: 10.1016/j.yhbeh.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 79.Gulevich Rimma, Kozhemyakina Rimma, Shikhevich Svetlana, Konoshenko Maria, Herbeck Yury. Aggressive behavior and stress response after oxytocin administration in male Norway rats selected for different attitudes to humans. Physiol. Behav. 2019;199:210–218. doi: 10.1016/j.physbeh.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 80.Jurek Benjamin, Neumann Inga D. The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev. 2018;98(3):1805–1908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- 81.Carter C Sue, Kenkel William M., MacLean Evan L., Wilson Steven R., Perkeybile Allison M., Yee Jason R., Ferris Craig F., Nazarloo Hossein P., Porges Stephen W., Davis John M. Is oxytocin “nature's medicine”. Pharmacol. Rev. 2020;72(4):829–861. doi: 10.1124/pr.120.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song Zhimin, Borland Johnathan M., Larkin Tony E., O'Malley Maureen, Elliott Albers H. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology. 2016;74:164–172. doi: 10.1016/j.psyneuen.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crespi, Bernard J. Oxytocin, testosterone, and human social cognition. Biol. Rev. 2016;91(2):390–408. doi: 10.1111/brv.12175. [DOI] [PubMed] [Google Scholar]
- 84.Ne'eman, Ronnie N Perach-Barzilay, Fischer-Shofty Meytal, Atias A., Shamay-Tsoory S.G. Intranasal administration of oxytocin increases human aggressive behavior. Horm. Behav. 2016;80:125–131. doi: 10.1016/j.yhbeh.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 85.Egito Julia H., Nevat Michael, Shamay-Tsoory Simone G., C Osório Ana Alexandra. Oxytocin increases the social salience of the outgroup in potential threat contexts. Horm. Behav. 2020;122:104733. doi: 10.1016/j.yhbeh.2020.104733. [DOI] [PubMed] [Google Scholar]
- 86.Shamay-Tsoory, Simone G., Ahmad Abu-Akel. The social salience hypothesis of oxytocin. Biol. Psychiatr. 2016;79(3):194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 87.Oliva Jessica Lee, Rault J.-L., Appleton Belinda, Lill Alan. Oxytocin enhances the appropriate use of human social cues by the domestic dog (Canis familiaris) in an object choice task. Anim. Cognit. 2015;18(3):767–775. doi: 10.1007/s10071-015-0843-7. [DOI] [PubMed] [Google Scholar]
- 88.De Dreu, Carsten K.W., Kret Mariska E. Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biol. Psychiatr. 2016;79(3):165–173. doi: 10.1016/j.biopsych.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 89.De Dreu, Carsten K.W., Greer Lindred L., Van Kleef Gerben A., Shalvi Shaul, Handgraaf Michel JJ. Oxytocin promotes human ethnocentrism. Proc. Natl. Acad. Sci. Unit. States Am. 2011;108(4):1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mech L David. U of Minnesota Press; 2003. The Wolves of Denali. [Google Scholar]
- 91.Mech L David. Alpha status, dominance, and division of labor in wolf packs. Can. J. Zool. 1999;77(8):1196–1203. [Google Scholar]
- 92.Fritts Steven H., Stephenson Robert O., Hayes Robert D., Boitani Luigi. Wolves. University of Chicago Press; 2010. 12. Wolves and humans; pp. 289–316. [Google Scholar]
- 93.Anton Colby B., Smith Douglas W., Suraci Justin P., Stahler Daniel R., Duane Timothy P., Wilmers Christopher C. Gray wolf habitat use in response to visitor activity along roadways in Yellowstone National Park. Ecosphere. 2020;11(6) [Google Scholar]
- 94.Janeček Michael, Dabrowska Joanna. Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies—potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST) Cell Tissue Res. 2019;375(1):143–172. doi: 10.1007/s00441-018-2889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zepeda Emily, Payne Eric, Wurth Ashley, Sih Andrew, Gehrt Stanley. Early life experience influences dispersal in coyotes (Canis latrans) Behav. Ecol. 2021;32(4):728–737. doi: 10.1093/beheco/arab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schell Christopher J., Young Julie K., Lonsdorf Elizabeth V., Santymire Rachel M., Mateo Jill M. Parental habituation to human disturbance over time reduces fear of humans in coyote offspring. Ecology and evolution. 2018;8(24):12965–12980. doi: 10.1002/ece3.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pal S.K., Ghosh B., Roy S. Inter-and intra-sexual behaviour of free-ranging dogs (Canis familiaris) Appl. Anim. Behav. Sci. 1999;62(2–3):267–278. [Google Scholar]
- 98.Bonanni Roberto, Cafazzo Simona. The Social Dog. Elsevier; 2014. The social organisation of a population of free-ranging dogs in a suburban area of Rome: a reassessment of the effects of domestication on dogs' behaviour; pp. 65–104. [Google Scholar]
- 99.Petryaev P.A. Gostorga RSFSR; 1929. Razvedeniye Serebristo-Chernykh Lisits [Breeding of the Silver-Black Foxes] [Google Scholar]
- 100.Carter C Sue. The role of oxytocin and vasopressin in attachment. Psychodyn. Psychiatry. 2017;45(4):499–517. doi: 10.1521/pdps.2017.45.4.499. [DOI] [PubMed] [Google Scholar]
- 101.Cho Mary M., DeVries A Courtney, Williams Jessie R., Carter C Sue. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav. Neurosci. 1999;113(5):1071. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 102.Winslow J.T., Hastings N., Carter C.S., Harbaugh C.R., Insel T.R. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 103.vonHoldt Bridgett M., Shuldiner Emily, Koch Ilana Janowitz, Kartzinel Rebecca Y., Hogan Andrew, Brubaker Lauren, Wanser Shelby, Stahler Daniel, Wynne Clive DL., Ostrander Elaine A., Sinsheimer Janet S., Udell Monique AR. Structural variants in genes associated with human Williams-Beuren syndrome underlie stereotypical hypersociability in domestic dogs. Science advances. 2017;3(7) doi: 10.1126/sciadv.1700398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dai Li, Carter C Sue, Ying Jian, Bellugi Ursula, Pournajafi-Nazarloo Hossein, Korenberg Julie R. Oxytocin and vasopressin are dysregulated in Williams syndrome, a genetic disorder affecting social behavior. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fam Bibiana SO., Paré Pamela, Felkl Aline B., Vargas-Pinilla Pedro, Paixão-Côrtes Vanessa R., Viscardi Lucas Henriques, Cátira Bortolini Maria. Oxytocin and arginine vasopressin systems in the domestication process. Genet. Mol. Biol. 2018;41:235–242. doi: 10.1590/1678-4685-GMB-2017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scott J.P., Fuller J.L. University of Chicago Press; Chicago: 1965. Genetics and the Social Behavior of the Dog. [Google Scholar]
- 107.Bretherton Inge. The origins of attachment theory: John Bowlby and mary Ainsworth. Dev. Psychol. 1992;28(5):759. [Google Scholar]
- 108.Topál József, Miklósi Ádám, Csányi Vilmos, Antal Dóka. Attachment behavior in dogs (Canis familiaris): a new application of Ainsworth's (1969) Strange Situation Test. J. Comp. Psychol. 1998;112(3):219. doi: 10.1037/0735-7036.112.3.219. [DOI] [PubMed] [Google Scholar]
- 109.MacLean Evan L., Hare Brian. Dogs hijack the human bonding pathway. Science. 2015;348(6232):280–281. doi: 10.1126/science.aab1200. [DOI] [PubMed] [Google Scholar]
- 110.Porges S.W. The role of social engagement in attachment and bonding. Attachment and bonding. 2005;3:33–54. [Google Scholar]
- 111.Miklósi Ádám, Kubinyi E., Topál J., Gácsi M., Virányi Z., Csányi V. A simple reason for a big difference: wolves do not look back at humans, but dogs do. Curr. Biol. 2003;13(9):763–766. doi: 10.1016/s0960-9822(03)00263-x. [DOI] [PubMed] [Google Scholar]
- 112.Topál József, Gácsi Márta, Miklósi Ádám, Virányi Zsófia, Kubinyi Enikő, Csányi Vilmos. Attachment to humans: a comparative study on hand-reared wolves and differently socialized dog puppies. Anim. Behav. 2005;70(6):1367–1375. [Google Scholar]
- 113.Lenkei Rita, Újváry Dóra, Bakos Viktória, Faragó Tamás. Adult, intensively socialized wolves show features of attachment behaviour to their handler. Sci. Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-74325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rehn Therese, Handlin Linda, Uvnäs-Moberg Kerstin, Keeling Linda J. Dogs' endocrine and behavioural responses at reunion are affected by how the human initiates contact. Physiol. Behav. 2014;124:45–53. [PubMed] [Google Scholar]
- 115.Kovács Krisztina, Kis Anna, Pogány Ákos, Koller Dóra, Topál József. Differential effects of oxytocin on social sensitivity in two distinct breeds of dogs (Canis familiaris) Psychoneuroendocrinology. 2016;74:212–220. doi: 10.1016/j.psyneuen.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 116.Fiset Sylvain, Plourde Vickie. Commentary: oxytocin-gaze positive loop and the coevolution of human-dog bonds. Front. Psychol. 2015;6:1845. doi: 10.3389/fpsyg.2015.01845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kekecs Zoltan, Szollosi Aba, Palfi Bence, Szaszi Barnabas, Kovacs Krisztina J., Dienes Zoltan, Aczel Balazs. Commentary: oxytocin-gaze positive loop and the coevolution of human–dog bonds. Front. Neurosci. 2016;10:155. doi: 10.3389/fnins.2016.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lim Robert Kho-Seng, Chan-Nao Liu, Moffitt Robert L. Charles C. Thomas, Springfield, IL; 1960. A Stereotaxic Atlas of the Dog’s Brain. [Google Scholar]
- 119.Lefevre Arthur, Benusiglio Diego, Tang Yan, Krabichler Quirin, Charlet Alexandre, Grinevich Valery. Oxytocinergic feedback circuitries: an anatomical Basis for neuromodulation of social behaviors. Front. Neural Circ. 2021;15 doi: 10.3389/fncir.2021.688234. https://www.frontiersin.org/articles/10.3389/fncir.2021.688234/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bence M., Marx P., Szantai E., Kubinyi E., Ronai Z., Banlaki Z. Lessons from the canine Oxtr gene: populations, variants and functional aspects. Gene Brain Behav. 2017;16:427–438. doi: 10.1111/gbb.12356. [DOI] [PubMed] [Google Scholar]
- 121.Marshall-Pescini Sarah, Schaebs Franka S., Gaugg Alina, Meinert Anne, Tobias Deschner, Range Friederike. The role of oxytocin in the dog–owner relationship. Animals. 2019;9(10):792. doi: 10.3390/ani9100792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wirobski Gwendolyn, Range Friederike, Schaebs Franka S., Palme Rupert, Tobias Deschner, Marshall-Pescini Sarah. Life experience rather than domestication accounts for dogs' increased oxytocin release during social contact with humans. Sci. Rep. 2021;11(1):1–12. doi: 10.1038/s41598-021-93922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wirobski G F Range, Schaebs F.S., Palme R., Deschner T., Marshall-Pescini S. Endocrine changes related to dog domestication: comparing urinary cortisol and oxytocin in hand-raised, pack-living dogs and wolves. Horm. Behav. 2021;128:104901. doi: 10.1016/j.yhbeh.2020.104901. [DOI] [PubMed] [Google Scholar]
- 124.MacLean Evan L., Wilson Steven Ray, Martin W Lance, Davis John M., Nazarloo Hossein P., Carter C Sue. Challenges for measuring oxytocin: the blind men and the elephant? Psychoneuroendocrinology. 2019;107:225–231. doi: 10.1016/j.psyneuen.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Freeman Sara M., Young Larry J. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J. Neuroendocrinol. 2016;28(4) doi: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carter C.S. Oxytocin pathways and the evolution of human behavior. Annu. Rev. Psychol. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- 127.Bales Karen L., A van Westerhuyzen Julie, Lewis-Reese Antoniah D., Grotte Nathaniel D., Lanter Jalene A., Carter C Sue. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm. Behav. 2007;52(2):274–279. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carter C Sue. Developmental consequences of oxytocin. Physiol. Behav. 2003;79(3):383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 129.Insel Thomas R., Shapiro Lawrence E. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. Unit. States Am. 1992;89(13):5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Williams Jessie R., Insel Thomas R., Harbaugh Carroll R., Carter C Sue. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J. Neuroendocrinol. 1994;6(3):247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 131.Grebe Nicholas M., Sharma Annika, Freeman Sara M., Palumbo Michelle C., Patisaul Heather B., Bales Karen L., Drea Christine M. Neural correlates of mating system diversity: oxytocin and vasopressin receptor distributions in monogamous and non-monogamous Eulemur. Sci. Rep. 2021;11(1):1–13. doi: 10.1038/s41598-021-83342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Freeman Sara M., Walum Hasse, Inoue Kiyoshi, Smith Aaron L., Goodman Mark M., Bales Karen L., Young Larry J. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carter C Sue, Perkeybile Allison M. The monogamy paradox: what do love and sex have to do with it? Frontiers in ecology and evolution. 2018;6:202. doi: 10.3389/fevo.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johnston Angie M., Turrin Courtney, Watson Lyn, Arre Alyssa M., Santos Laurie R. Uncovering the origins of dog–human eye contact: dingoes establish eye contact more than wolves, but less than dogs. Anim. Behav. 2017;133:123–129. [Google Scholar]
- 135.Brooks James, Kays Roland, Hare Brian. Coyotes living near cities are bolder: implications for dog evolution and human-wildlife conflict. Behaviour. 2020;157(3–4):289–313. [Google Scholar]
- 136.MacLean Evan L., Gesquiere Laurence R., Gruen Margaret E., Sherman Barbara L., Martin W Lance, Carter C Sue. Endogenous oxytocin, vasopressin, and aggression in domestic dogs. Front. Psychol. 2017;8:1613. doi: 10.3389/fpsyg.2017.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kenkel W.M., Paredes J., Yee J.R., Pournajafi‐Nazarloo H., Bales K.L., Carter C.S. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J. Neuroendocrinol. 2012;24(6):874–886. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- 138.Feldman R. Oxytocin and social affiliation in humans. Horm. Behav. 2012;61(3):380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]