Abstract
CD47 is a “don’t eat me” signal to phagocytes that is overexpressed on many tumor cells as a potential mechanism for immune surveillance evasion. CD47 and its interaction with signal-regulating protein alpha (SIRPα) on phagocytes is therefore a promising cancer target. Therapeutic antibodies and fusion proteins that block CD47 or SIRPα have been developed and have shown activity in preclinical models of hematologic and solid tumors. Anemia is a common adverse event associated with anti-CD47 treatment, but mitigation strategies—including use of a low ‘priming’ dose—have substantially reduced this risk in clinical studies. While efficacy in single-agent clinical studies is lacking, findings from studies of CD47–SIRPα blockade in combination with agents that increase ‘eat me’ signals or with antitumor antibodies are promising. Magrolimab, an anti-CD47 antibody, is the furthest along in clinical development among agents in this class. Magrolimab combination therapy in phase Ib/II studies has been well tolerated with encouraging response rates in hematologic and solid malignancies. Similar combination therapy studies with other anti-CD47–SIRPα agents are beginning to report. Based on these early clinical successes, many trials have been initiated in hematologic and solid tumors testing combinations of CD47–SIRPα blockade with standard therapies. The results of these studies will help determine the role of this novel approach in clinical practice and are eagerly awaited.
Key words: CD47, SIRPα, magrolimab, macrophage, phagocytosis, innate immunotherapy
Highlights
-
•
CD47 is a “don’t eat me” signal overexpressed on cancer cells.
-
•
Blockade of the CD47–SIRPα signaling pathway leads to phagocytosis of tumor cells.
-
•
CD47–SIRPα blockade plus standard treatment shows promising clinical efficacy.
-
•
Clinically, CD47–SIRPα blockade plus standard treatment is well tolerated.
-
•
Clinical trials targeting CD47–SIRPα in hematologic and solid tumors are ongoing.
Introduction
Immune surveillance between normal cells, defective cells, and foreign pathogens is regulated by cell-surface receptors, which mediate interactions between immune cells and their targets. These include markers of ‘self’ or “don’t eat me” signals that interact with proteins expressed on the surface of phagocytes to inhibit phagocytosis, such as the tumor cell major histocompatibility complex type 1 component, β2-microglobulin, interaction with macrophage leukocyte immunoglobulin (Ig)-like receptor B1 (LILRB1)1; tumor cell programmed death-ligand 1 (PD-L1) interaction with programmed cell death protein 1 (PD-1) on tumor-associated macrophages2; tumor cell CD24 interaction with sialic-acid-binding Ig-like lectin 10 (Siglec-10) on tumor-associated macrophages3; and CD47, a cell-surface protein that has ubiquitous expression and an array of cellular functions with multiple binding partners.4, 5, 6, 7 CD47 inhibits phagocytosis through an interaction with signal-regulating protein alpha (SIRPα) on the surface of phagocytic cells (Figure 1).8,9 Interaction with CD47 promotes localization of SIRPα to the phagocytic synapse, which activates Src homology region 2 domain-containing phosphatase-1 (SHP-1) phosphatase, and ultimately inhibits non-muscle myosin IIA accumulation at the cell membrane, preventing engulfment.10,11 Blockade of CD47–SIRPα signaling has recently been investigated as a means of activating phagocytic cells, particularly macrophages, for therapeutic purposes.
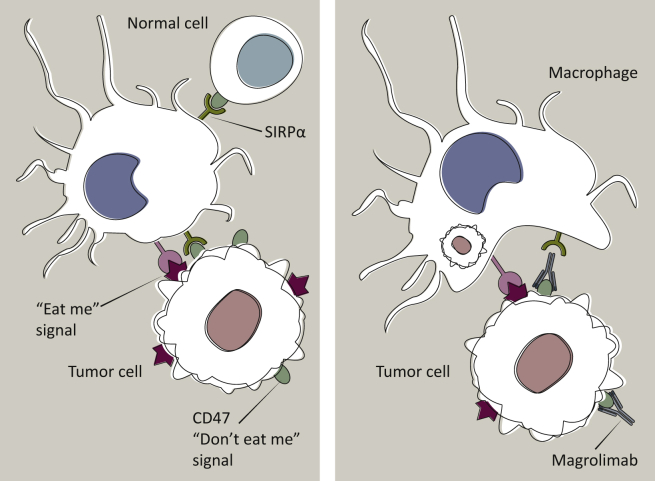
Figure 1.
Prevention of phagocytosis by CD47–SIRPα interactions and mechanism of action of CD47–SIRPα therapeutic blockade with magrolimab.
CD47 is a “don’t eat me” signal expressed on the cell surface. Interaction of CD47 with SIRPα on phagocytes prevents phagocytic elimination of healthy cells. CD47 is overexpressed on cancer cells to overcome the expression of ‘eat me’ signals and help tumor cells evade macrophage immune surveillance. Blockade of the CD47–SIRPα interaction, as shown with the anti-CD47 antibody (magrolimab) on the right, unmasks the ‘eat me’ signals and promotes phagocytic elimination of tumor cells. Most healthy cells do not express ‘eat me’ signals, and therefore are spared from phagocytosis under CD47–SIRPα blockade. SIRPα, signal-regulating protein alpha. Adapted from Chao et al.111
Basis for targeting CD47 in cancer
Evasion of immune system surveillance is a fundamental step in tumorigenesis.12 Malignant cells from multiple tumor types express higher levels of CD47 than do normal cells,13, 14, 15, 16, 17, 18 suggesting that using CD47 overexpression to masquerade as ‘self’ is a common mechanism for cancer cells to escape immune surveillance. Several mechanisms may lead to advantageous overexpression of CD47. CD47 transcription is induced by MYC,19 a potent oncogene and driver of many malignancies.20 In a hypoxic tumor microenvironment, CD47 is up-regulated by direct binding of hypoxia-inducible factor 1 (HIF-1) to the CD47 promoter.21 CD47 transcription is also regulated by tumor-specific enhancers and super enhancers, which can be activated by pro-inflammatory pathways.22 CD47 overexpression may counteract the overexpression of prophagocytic ‘eat me’ signals that are up-regulated in response to cell stress or because these prophagocytic signals provide a tumorigenic advantage.23
Therapeutic blockade of CD47–SIRPα interactions: preclinical evidence
Genetic knockdown of CD47 expression renders cells vulnerable to phagocytosis by macrophages in vitro and in vivo, and indirect reduction of CD47 expression by knockdown of transcription-inducing pathways also increases phagocytosis of tumor cells.8,13,21,22,24,25 Monoclonal antibodies to CD47 and SIRPα, and SIRPα fusion proteins, have been developed to block the interaction between tumor cell CD47 and macrophage SIRPα, providing several ways to kill tumor cells, depending on the specific agent used (Figure 2): blocking CD47 or SIRPα removes the “don’t eat me” signal, permitting phagocytosis by macrophages; antibodies may activate Fc-dependent mechanisms, including antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC); antibodies may induce apoptosis directly; and phagocytes may present tumor antigens for CD8+ T-cell activation.26 CDC activation by antibodies to CD47 has not been reported; however, other therapeutic antibodies (e.g. rituximab, daratumumab, and ofatumumab) are known to activate CDC, and this appears to depend on several factors including antibody isotype, binding strength, valence, and epitope; receptor cluster formation; and expression of complement regulatory proteins on tumor cells.27, 28, 29, 30 Direct induction of apoptosis occurs with some anti-CD47 antibodies but not others, and with differing potency.14,31, 32, 33, 34, 35 This process is caspase-independent,31 and mechanisms may involve ligation and activation of the CD47 ligand, thrombospondin,32 cytoskeletal reorganization,31 and up-regulation of HIF-1α.34
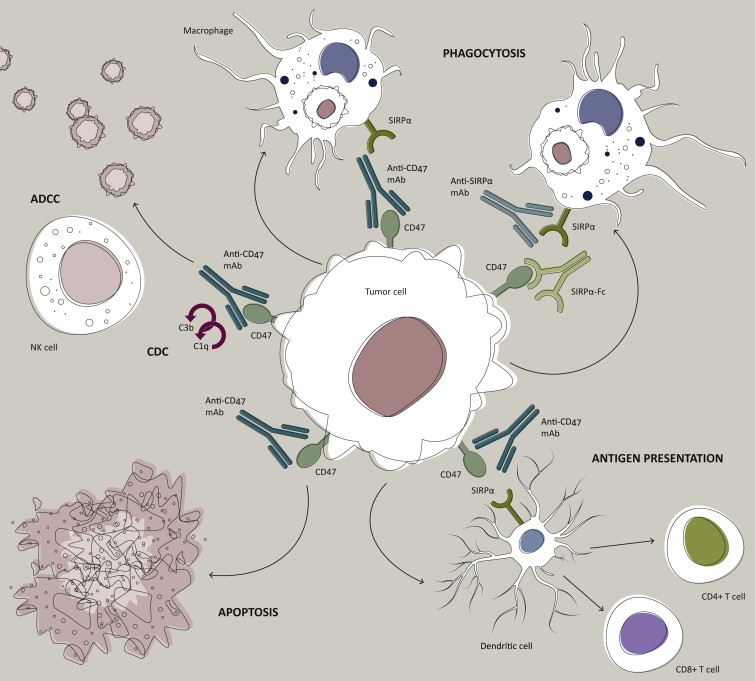
Figure 2.
Mechanisms of targeting the CD47–SIRPα pathway in cancer.
Therapeutic targeting of the CD47–SIRPα pathway can cause elimination of cancer cells through multiple mechanisms. Firstly, inhibition of the CD47–SIRPα interaction with a blocking anti-CD47 antibody, a blocking anti-SIRPα antibody, or a recombinant SIRPα protein (depicted here as a bivalent Fc-fusion protein) leads to phagocytic uptake of tumor cells by macrophages. Secondly, an anti-CD47 antibody can eliminate tumor cells through traditional antibody Fc-dependent mechanisms including natural killer cell-mediated ADCC and CDC. Thirdly, anti-CD47 antibody may directly stimulate apoptosis of tumor cells through a caspase-independent mechanism. Fourthly, anti-CD47 antibody may enable phagocytic uptake of tumor cells by dendritic cells and subsequent antigen presentation to CD4 and CD8 T cells, thereby stimulating an antitumor adaptive immune response.
ADCC, antibody-dependent cellular cytotoxicity; CDC, complement-dependent cytotoxicity; mAb, monoclonal antibody; NK, natural killer; SIRPα, signal-regulating protein alpha. Reprinted from Chao et al.,26 with permission from Elsevier.
Blockade of the CD47–SIRPα interaction with anti-CD47 antibodies has anticancer effects in preclinical models.14,16, 17, 18,35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 Magrolimab (formerly Hu5F9-G4) is a humanized anti-CD47 antibody that binds CD47 with low nanomolar affinity and is based on an Ig G4 scaffold to minimize Fc-mediated effector toxicity for non-tumor cells expressing CD47.35 Magrolimab binding is sufficient to induce phagocytosis of cancer cells by macrophages in vitro, but it does not activate ADCC or CDC, or directly induce apoptosis.35 In immunodeficient [NOD/SCID/IL-2Rgnull (NSG)] models, magrolimab shows strong monotherapy activity against human hematological malignancies35; in ovarian cancer cell lines and patient-derived xenograft models, including taxane-resistant tumors; and in orthotopic xenografts of five aggressive pediatric brain tumor types, without evidence of harm to other central nervous system cells.44,46 CC-90002 elicited phagocytosis of hematologic and solid tumor cells in vitro and showed significant dose-dependent antitumor effects in multiple myeloma (MM) cell line xenograft models in vivo. Significant tumor regression was also observed in cell line- and patient-derived triple-negative breast cancer (TNBC) and acute myeloid leukemia (AML) HL-60 xenograft models.47 Lemzoparlimab (TJC4) monotherapy completely eradicated Raji cell tumors in a xenograft model and extended survival in AML xenografted mice.39 SRF231 induced phagocytosis of human hematopoietic tumor cell lines by human macrophages; interaction of SRF231 Fc domain with FcγR induced apoptosis and ADCP; and significant antitumor activity with sustained tumor regression was observed across several xenograft models of hematologic malignancies.42 AO-176 led to apoptosis selectively in tumor cells, not normal cells including activated T cells, and promoted dose-dependent phagocytosis of several hematologic and solid tumor cell types. Tumor growth inhibition was observed in xenograft lymphoma, TNBC, ovarian and gastric carcinoma models.40 In one MM xenograft model, AO-176 induced complete remission lasting up to 120 days in all treated mice.48 Interestingly, most anti-SIRPα antibodies and non-Fc CD47-targeting agents have not shown single-agent activity in the preclinical setting,49, 50, 51, 52 suggesting that engaging Fc receptors contribute a key component of efficacy in preclinical models.
Activity in rational therapeutic combinations
Targeted antibodies to tumor cell markers are a mainstay of cancer treatment and are thought to act through Fc effector mechanisms including ADCC, ADCP, CDC, and induction of apoptosis.27,53 Co-treatment with CD47–SIRPα-blocking agents may synergize with tumor-targeting antibodies by enhancing the potential of phagocytes, particularly macrophages, to execute these effector functions (Figure 3). Indeed, CD47–SIRPα blockade combined with tumor-targeting antibodies rituximab, cetuximab, panitumumab, trastuzumab, daratumumab, and dinutuximab has had additive or synergistic efficacy in preclinical models, including cancer lines selected to be resistant to ADCC.12,35,39,46,54, 55, 56, 57, 58, 59, 60, 61, 62, 63 Individual tumor-targeting antibodies have been shown to activate multiple Fc effector functions,30,64, 65, 66 broadening the therapeutic potential of these combinations.
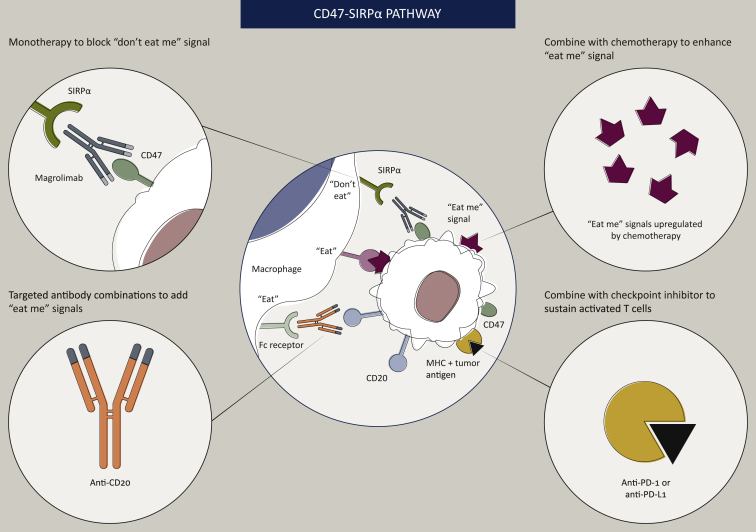
Figure 3.
Potential synergistic combinations with anti-CD47 treatment.
CD47–SIRPα pathway blockade in combination with therapies that increase the ‘eat me’ signals on tumor cells has the potential for synergistic clinical efficacy. Some types of chemotherapy and other cytotoxic agents increase the expression of ‘eat me’ signals on tumor cells. Similarly, tumor-targeted antibodies present Fc regions to the Fc receptors on phagocytes, triggering ADCP. Phagocytosis of tumor cells by macrophages or dendritic cells can lead to tumor cell antigen presentation to T cells, activating antitumor T-cell responses; therefore, combination of CD47–SIRPα pathway blockade with T-cell checkpoint inhibitors may also produce synergistic efficacy.
ADCP, antibody-dependent cellular phagocytosis; MHC, major histocompatibility complex; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; SIRPα, signal-regulating protein alpha.
Calreticulin (CRT), an ‘eat me’ signal, is up-regulated on tumor cell surfaces by cytotoxic stimuli, including anthracyclines and inhibitors of protein phosphatase 1/GADD34 (tautomycin, calyculin A, and salubrinal), suggesting potential synergy between cytotoxic agents and CD47–SIRPα blockade (Figure 3).67 Azacitidine has also increased the expression of CRT on the surface of AML cells.68,69 Because tumor cells express relatively high levels of CRT as well as CD47, and most normal cells express low levels of CD47 without CRT, combining CD47–SIRPα blockade and cytotoxic agents can selectively target tumor cells.23 Magrolimab combined with azacitidine increased phagocytosis of HL60 cells by macrophages in vitro significantly beyond the level observed with either agent alone.68 Growth of HL60 cells engrafted into NSG mice was also inhibited with magrolimab + azacitidine treatment as early as day 10, and growth elimination with 100% survival was maintained up to study termination 255 days post-engraftment.68 Another anti-CD47-blocking antibody increased the sensitivity of high CD47-expressing hepatocellular carcinoma (HCC) cells to doxorubicin or cisplatin in vitro, and the combination was synergistic in patient-derived HCC xenografts.70
Combinations of anti-CD47–SIRPα with T-cell checkpoint inhibitors can independently activate both innate and adaptive immune responses, and, based on the ability of phagocytes to cross-present antigens to T cells, have the potential for a synergistic antitumor immune response (Figure 3).71,72 In fact, evidence suggests that the effects of CD47 blockade in some cancer models require cross-priming of T cells.71 Preclinically, anti-mouse CD47 nanobody treatment (lacking single-agent activity) significantly enhanced response to anti-PD-L1 in mouse melanoma cells induced to express PD-L1; and in a syngeneic mouse melanoma model, the combination significantly delayed tumor growth and prolonged survival.73 Similar tumor-specific effects of anti-CD47 and anti-PD-L1 antibodies in mouse pancreatic cancer models suggest that the efficacy of a dual anti-CD47-SIRPα/PD-L1 approach will depend on tumor type and microenvironment.74,75 In combination with anti-cytotoxic T-lymphocyte associated protein 4 (CTLA4) antibodies, anti-CD47 antibodies significantly suppressed tumor growth, extended survival, and increased CD8+ T-cell proliferation in tumor-draining lymph nodes in a mouse model of pancreatic cancer, suggesting another potential avenue for clinical development.76
Collectively, preclinical studies of CD47–SIRPα blockade across hematologic and solid tumor models suggest potential for clinical efficacy, particularly in combination regimens. Hematologic tumors may be especially susceptible, because macrophage clearance of those cells is a natural part of their life cycle. Questions remain, including the role of prophagocytic signals such as CRT expressed on tumor cells versus macrophage-secreted CRT, which binds to asialoglycans on target cells and CD91 on macrophages and helps mediate phagocytosis.77,78 Tumor and other cells also express sialylglycoproteins on their cell surfaces; hypersialylation could be another means of immune surveillance escape.79 Regulation of tumor cell expression of CRT and sialylglycoproteins may offer additional therapeutic avenues.
Safety of targeting the CD47–SIRPα axis
The ubiquitous expression of CD47 is unique among current immunotherapy targets, sparking concerns regarding on-target toxicity resulting from phagocytosis of normal CD47-expressing cells after treatment with either CD47- or SIRPα-blocking drugs.72 In particular, red blood cells (RBCs) express CD47 as a regulator of their lifespan by macrophages and are therefore vulnerable to anti-CD47 antibodies late in their lives.8 Other blood cells and many other cell types also express CD47, which broadens the potential range of on-target toxicities. In addition, the Fc region of some anti-CD47 and anti-SIRPα antibodies has the potential to activate ADCC and CDC, which may be toxic to normal cells.4,5,28,80 IgG1 and IgG3 are the most potent activators of ADCC and CDC, but IgG4 is not associated with either28; therefore, isotype scaffold may define the toxicity profile of antibody-based therapies.
Evaluation of potential toxicities in mouse models is limited by lack of cross-reactivity between most humanized or fully human antibodies and fusion proteins with mouse proteins on sensitive cell types.35,38,40 Nonhuman primates (NHPs) closely recapitulate human physiology and protein sequences.81 In particular, cynomolgous monkey CD47 differs from human CD47 in only three amino acids, which are outside of the SIRPα-binding domain, so this NHP has been used routinely in pharmacokinetic and toxicology studies involving CD47.35,37, 38, 39, 40,45 These studies have confirmed that RBCs are quite sensitive to CD47–SIRPα blockade; while others, including white blood cells, are surprisingly resistant against any measurable toxic effects. Other than RBCs, normal cells do not express prophagocytic signals, which likely protects them from phagocytosis under CD47–SIRPα blockade.23 Multiple in vitro studies have confirmed that, in contrast to tumor cells and RBCs, normal cells are not eliminated in the presence of an anti-CD47 antibody.14,23
RBC depletion
Aging RBCs are naturally cleared from circulation by macrophages.82 CD47 on RBCs inhibits phagocytic clearance and, conversely, RBCs that lack CD47 are rapidly cleared from the circulation.8 As RBCs age, they gradually lose CD47 expression and increase expression of prophagocytic signals, which increases their susceptibility to phagocytosis.25,83 On-target dose-dependent anemia, with a parallel increase in reticulocytosis, was observed in NHPs 5-7 days after magrolimab dosing, and hemoglobin spontaneously returned to baseline levels ∼2 weeks after dosing.35 Despite high CD47 expression, white blood cells and platelets were unaffected, and there was no evidence of intravascular hemolysis. Although anemia occurred in all NHPs who received magrolimab, the degree of anemia varied among individuals at the same dose. Similar findings in NHPs have been reported for other anti-CD47 antibodies.84 While not fully understood, this variance may be driven by differences in expression of pro-phagocytic signals by RBCs. Further study of this phenomenon could help to identify clinical biomarkers that may be associated with more severe anemia in patients.
Safety and mitigation strategies for RBC depletion with monotherapy
Recognition of potential on-target anemia with CD47–SIRPα blockade has led to a broad variety of efforts to develop therapeutic strategies that avoid anemia. The antibody Fc scaffold used may also influence the clinical toxicity profile of anti-CD47–SIRPα agents. Data on NHPs are available for some agents; clinical data are preliminary and based on conference presentations for all but magrolimab.
Achieving magrolimab serum levels in NHPs associated with efficacy in xenograft models required doses of 10-30 mg/kg to saturate the ‘antigen sink’ of CD47 on non-tumor cells; therefore, a low priming dose was tested based on the hypothesis that it could serve to remove the most vulnerable RBCs and activate reticulocytosis before initiating a higher maintenance dose. With the use of a priming dose, there was no further decrease in hemoglobin with maintenance doses and hemoglobin levels returned to the normal range.35 This provided rationale for the clinical priming/maintenance dosing strategy to mitigate anemia in magrolimab trials. Clinically, anemia with increased need for RBC transfusion was observed in a dose-escalation trial of magrolimab in relapsed/refractory (R/R) AML.85 In contrast, with a magrolimab priming/maintenance dosing schedule, mild transient anemia in ∼25%-57% of patients with compensatory reticulocytosis was observed in subsequent clinical trials.58,69,86, 87, 88 In these trials, hemoglobin decreased by a mean of 0.4-0.9 g/dl (maximum 2.5 g/dl) after the first dose, and then returned to baseline; therapy continued to improve hemoglobin levels and reduce the need for RBC transfusion.69,87 Evidence from ex vivo studies of bone marrow and peripheral blood samples from patients with solid tumors (NCT02216409) or AML (NCT02678338) shows that CD47 protein is ‘pruned’ from RBCs but not white blood cells or AML blasts by magrolimab treatment which, in addition to replacement of RBCs with a younger cell population by the priming dose, leads to lack of further IgG4-driven elimination of RBCs, given the remaining RBCs are CD47 negative and not bound by magrolimab.89 These findings further support the use of a priming/maintenance dose to mitigate on-target anemia with magrolimab use. No data have been published regarding this phenomenon for other CD47 agents, but the degree or kinetics of pruning may differ between them. If so, this may underlie some observed differences in the specific toxicity profiles of different drugs targeting this pathway.
Trillium (TTI)-621 consists of a fusion of the N-terminal V domain of human SIRPα with the IgG1 Fc domain. This ‘decoy receptor’ was designed to bind to CD47 on tumor cells and activate phagocytosis and Fc effector functions for maximum efficacy. TTI-621 binds to CD47 on a variety of hematologic cells and causes anemia in NHPs but exhibits minimal binding to human RBCs, purportedly because it binds to clustered CD47 in the cell membrane but not distributed CD47 associated with the spectrin cytoskeleton in human RBCs.37 During the dose-escalation period of a phase I study of IgG1-based TTI-621, thrombocytopenia was observed in 30% (22% grade ≥3) of 214 patients with R/R non-Hodgkin’s lymphoma (NHL).90 Results from 25 patients in a dose-finding trial of the related TTI-622 in R/R lymphoma suggest a similar safety profile, with thrombocytopenia in 16% (grade 4 in 1) and neutropenia in 12% (all grade ≥3).91
ALX148 (ALX Oncology, South San Francisco, CA) is a combination of the N-terminal D1 CD47-binding domain of SIRPα engineered to provide picomolar affinity, fused to a modified human IgG1 which is ‘Fc dead’ to prevent ADCC, CDC, and targeting normal cells for ADCP. ALX148 had no reported effects on hematologic parameters in NHP studies.45 A bispecific antibody, TG-1801 (TG Therapeutics, New York, NY; formerly NI-1701 from Novimmune), has also been developed on an IgG1 Fc scaffold; it binds weakly to CD47 and with a higher affinity to CD19, which is widely expressed on malignant B cells.38 This targets antibody binding preferentially to B cells, avoiding RBCs and other CD47+ cells. No clinical monotherapy data have been presented on either compound.
AO-176 (Arch Oncology, Brisbane, CA) is an anti-CD47 antibody with an IgG2 Fc domain that binds selectively to CD47 on tumor cells but not on other cells, and does not activate ADCC but does have a direct cell-killing effect; the mechanisms for selective tumor cell binding and direct killing effect are unknown. AO-176 had minimal effect on hematologic parameters in NHPs, and transient anemia was not seen40; however, interim data from a phase I/II trial of AO-176 showed thrombocytopenia in 33% (grade 3 brief thrombocytopenia in 22%) and anemia in 22%.92
Like magrolimab, anti-CD47 antibodies lemzoparlimab, IBI188, SRF231, CC-90002, and AK117 are based on an IgG4 scaffold.42,59,93, 94, 95, 96 Lemzoparlimab (I-Mab Biopharma, Shanghai, China) binds in such a way that glycosylation near the binding epitopes on RBC CD47 ‘shields’ the RBC from lemzoparlimab binding. Lemzoparlimab had minimal and transient effects on RBCs in NHPs.39 However, despite this characterized mechanism of binding, anemia was observed in 30% of patients treated with lemzoparlimab in a phase I study, with an average transient decrease in hemoglobin of 1.5 g/dl, similar to magrolimab.95 Interim phase I data in patients with R/R solid tumors or lymphoma treated with IBI188 (using a priming and maintenance dosing strategy) show anemia in 15% (3/20) of patients and 1 patient having a grade 4 platelet count decrease.93 SRF231 was associated with fatigue (43%), headache (35%), and pyrexia (30%); anemia was not reported in the abstract.94 In the CC-90002 monotherapy trial in patients with R/R AML or high-risk myelodysplastic syndromes (MDS), the expected anemia was observed in 32% of patients; however, thrombocytopenia also occurred in 39% of patients, suggesting an unknown mechanism independent of ADCC and CDC activation.97 Among the first 15 patients treated with AK117 in a phase I study, grade 2 anemia and grade 1 thrombocytopenia occurred in 1 patient.96 These spectrum of adverse events (AEs) highlight the possibility that even anti-CD47 agents with a similar IgG4 scaffold may have distinct safety profiles.
Antibodies to SIRPα, which is not expressed on RBCs, have also been developed—including ADU-1805 (Aduro Biotech, Berkeley, CA) based on IgG2 and BI 765063 (OSE-172; Boehringer Ingelheim, Ingelheim am Rhein, Germany) based on IgG4—to antagonize this pathway while avoiding the RBC binding of anti-CD47 drugs.50,98 The most common AEs in patients with advanced solid tumors who completed dose escalation with BI 765063 (n = 50) were infusion-related reactions, fatigue, headache, arthralgia, and diarrhea; as expected, anemia was not observed.99
These monotherapy studies provide more questions than answers regarding the role of IgG isotype scaffold and specific mechanisms of target engagement in the safety profiles of CD47–SIRPα-blocking therapies. Importantly, AEs associated with T-cell immune checkpoint inhibitors (colitis, pneumonitis, and hypothyroidism) have not been observed with these macrophage checkpoint inhibitors.100
Clinical safety in combination therapy studies
Combinations of CD47–SIRPα-blocking therapies or tumor-opsonizing antibodies with other treatments that increase expression of ‘eat me’ signals have shown promising results in early-stage clinical trials and were well tolerated. A phase Ib/II trial of magrolimab + rituximab in 115 patients with R/R B-cell lymphoma showed the expected mild, transient anemia following the priming dose that resolved on maintenance dose therapy. Most AEs occurred in the first month, only 7% of patients discontinued for AEs, and no late-emerging safety signals were seen up to 24 months.58,101 In a phase Ib trial of magrolimab + azacitidine in patients with untreated AML ineligible for intensive chemotherapy or untreated higher-risk MDS, the combination was well tolerated, with no significant immune-related AEs or increases in infections or cytopenia observed, 0% (MDS) and 4.7% (AML) of patients discontinuing for treatment-related AEs, and improvement in cytopenias on therapy.69,87 Long-term treatment with magrolimab (up to 25 months) in patients with AML has not shown any late-emerging toxicities.69
A small phase Ib study of magrolimab with the PD-L1 inhibitor avelumab in patients with platinum-R/R ovarian cancer or advanced solid tumors (n = 34) showed a safety profile consistent with PD-L1 therapy and magrolimab-related anemia, and discontinuation of either drug because of AEs in 15% of patients.88
Clinical efficacy of CD47–SIRPα blockade
Clinical efficacy in monotherapy studies
Monotherapy phase I clinical trials of anti-CD47 antibodies have generally yielded limited signs of efficacy compared to combination strategies. Magrolimab monotherapy was evaluated in 62 heavily pretreated patients with solid tumors or lymphoma, with objective partial responses (PRs) observed in 2 patients with ovarian cancer and a mixed response in 1 patient with diffuse large B-cell lymphoma (DLBCL).86 CC-90002 monotherapy in AML and high-risk MDS was terminated during the dose-escalation stage because of an insufficiently promising clinical profile; best response was stable disease (SD) in two patients, and anti-drug antibodies were observed in most patients regardless of dose.97,102 Interim data from dose escalation of TTI-621 in R/R NHL (n = 214) showed objective responses in 20% of 71 patients.90 Similarly, interim results of intra-lesional administration of TTI-621 in cutaneous T-cell lymphoma showed Composite Assessment of Index Lesion Severity response (decrease of ≥50%) in 34% of 29 patients.103 Interim results on SRF231 in 37 patients with R/R solid tumors indicated no complete response (CR) or PR, although prolonged SD was reported.94 AO-176 monotherapy produced 1 PR and 7 SD responses in an interim analysis of 27 patients with advanced solid tumors expressing high levels of CD47.92 Finally, monotherapy with BI 765063, which binds to SIRPα, led to clinical benefit in 45% of patients with advanced solid tumors, including one PR, during dose escalation.99 These studies highlight the limitations of preclinical cancer models for predicting efficacy in human malignancies.
Clinical efficacy in combination therapy studies
Rational combinations of CD47–SIRPα blockade with treatments that increase the presence of prophagocytic markers have produced encouraging preliminary data, and many additional studies are underway (Table 1). Magrolimab + rituximab has shown benefit in R/R NHL to at least two prior lines of therapy in a phase Ib study [n = 22, 15 with DLBCL, and 7 with follicular lymphoma (FL)]; overall response rates (ORRs) and CR rates, respectively, in DLBCL were 40% and 33%, and in FL were 71% and 43%.58 More mature data from the phase Ib/II expansion (n = 115, 70 with DLBCL and 45 with indolent lymphoma), including 85% who were rituximab-refractory, show ORR and CR rate, respectively, of 36% and 15% in patients with DLBCL, and 61% and 24% in patients with indolent lymphoma, with a median time to response of 1.8 months and duration of response (DOR) not reached. Similar responses were observed across multiple DLBCL subtypes and primary refractory patients, irrespective of prior lines of therapy.101 Early results from clinical studies in NHL with other anti-CD47-SIRPα agents also suggest efficacy, although patient numbers were small and populations differed. CC-90002 + rituximab in 24 patients with R/R NHL showed an ORR of 13% with 1 durable CR and 2 patients with PR. DOR was 3.9 months.59 In the subset of phase I study, patients with B-cell NHL who received TTI-621 + rituximab (n = 35), ORR was 23% (9% CR, 14% PR).61 Preliminary analysis of data on ALX148 + rituximab yielded a 40.9% ORR (4 CR, 5 PR, 6 SD) in 22 patients on 10 mg/kg, and 63.6% ORR (3 CR, 4 PR, 1 SD, n = 11 total) in 11 patients on 15 mg/kg.60
Table 1.
Ongoing and recruiting trials of anti-CD47 and anti-SIRPα agents (by estimated study completion date, grouped by agent)
| Agent | Company | Regimen | Population | Estimated enrollment, n | Estimated study completion | NCT identifier | Phase | Status |
|---|---|---|---|---|---|---|---|---|
| AO-176 (anti-CD47 IgG2 mAb) | Arch Oncology | AO-176 OR AO-176 + paclitaxel OR AO-176 + pembrolizumab | Advanced solid tumors | 183 | March 2023 | NCT03834948 | I/II | Recruiting |
| AO-176 OR AO-176 + either dexamethasone OR dexamethasone + bortezomib | R/R MM | 102 | March 2023 | NCT04445701 | I/II | Recruiting | ||
| HX009 (anti-CD47/PD-1 bifunctional antibody) | Waterstone Hanxbio Pty Ltd | HX009 monotherapy | R/R advanced malignant tumors | 21 | September 2021 | NCT04097769 | I | Active, not recruiting |
| HX009 monotherapy | Unresectable locally advanced/metastatic solid tumors | 210 | February 2023 | NCT04886271 | II | Recruiting | ||
| TTI-621 (SIRPaFc) | Trillium Therapeutics Inc. | TTI-621 OR TTI-621 + either rituximab OR nivolumab | R/R hematological malignancies and selected solid tumors (PTCL, CTCL) | 260 | December 2021 | NCT02663518 | I | Recruiting |
| TTI-622 (SIRPaFc) | TTI-622 OR TTI-622 + either azacitidine OR azactidine + venetoclax OR carfilzomib + dexamethasone | R/R lymphoma or MM | 150 | December 2022 | NCT03530683 | I | Recruiting | |
| IBI188 (anti-CD47 mAb) IBI188 (anti-CD47 mAb) |
Innovent Biologics (Suzhou) Co. Ltd. | IBI188 OR IBI188 + rituximab | Solid tumors and lymphomas | 49 | January 2022 | NCT03717103 | I | Active, not recruiting |
| IBI188 + azacitidine | Newly diagnosed high-risk MDS | 12 | February 2022 | NCT04485065 | I | Recruiting | ||
| IBI188 + azacitidine | AML | 126 | May 2022 | NCT04485052 | I/II | Recruiting | ||
| IBI188 monotherapy | Advanced malignant tumors and lymphomas | 42 | August 2022 | NCT03763149 | I | Active, not recruiting | ||
| IBI188 + GM-CSF + cisplatin/carboplatin + bevacizumab + sintilimab + pemetrexed | Advanced malignancies | 120 | October 2022 | NCT04861948 | I | Recruiting | ||
| BI-765063/OSE172 (anti-SIRPα Mab) | Boehringer Ingelheim | BI-765063 OR BI-765063 + BI-754091 (a PD-1 receptor antagonist) |
Japanese adults w/ advanced solid tumors | 36 | June 2022 | NCT04653142 | I | Recruiting |
| BI-765063 OR BI-765063 + BI-754091 (a PD-1 receptor antagonist) |
Advanced solid tumors (NSCLC, TNBC, pancreatic cancer, melanoma, HNSCC, RCC, UC, SCL, gastric cancer, CRC, and OC) | 116 | December 2022 | NCT03990233 | I | Recruiting | ||
| SL-172154 | Shattuck Labs, Inc. | SL-172154 monotherapy | Unresectable, locally advanced/metastatic ovarian, primary peritoneal, or fallopian tube cancer | 40 | July 2022 | NCT04406623 | I | Recruiting |
| SL-172154 monotherapy | Cutaneous SCC or HNSCC | 18 | July 2022 | NCT04502888 | I | Recruiting | ||
| ALX148 (CD47 antagonist) | ALX Oncology, Inc. | ALX148 OR ALX148 + either pembrolizumab OR trastuzumab OR rituximab OR pembrolizumab + 5-FU + cisplatin OR trastuzumab + ramucirumab + paclitaxel | Advanced/metastatic solid tumor malignancy; or R/R NHL | 174 | December 2022 | NCT03013218 | I | Active, not recruiting |
| ALX148 + azacitidine | Previously untreated or R/R higher-risk MDS | 173 | December 2023 | NCT04417517 | I/II | Recruiting | ||
| ALX148 + venetoclax and azacitidine | Newly diagnosed or R/R AML | 97 | December 2023 | NCT04755244 | I/II | Recruiting | ||
| ALX148 + pembrolizumab + cisplatin/carboplatin + 5-FU | Advanced HNSCC | 112 | October 2024 | NCT04675333 | II | Recruiting | ||
| ALX148 + pembrolizumab | Advanced HNSCC | 111 | October 2024 | NCT04675294 | II | Recruiting | ||
| IBI322 (recombinant anti-human CD47/PD-L1 bispecific antibody) | Innovent Biologics (Suzhou) Co. Ltd. | IBI322 monotherapy | Advanced malignant tumors | 45 | December 2022 | NCT04338659 | I | Not yet recruiting |
| IBI322 monotherapy | Hematologic malignancy that failed standard treatment | 230 | November 2023 | NCT04795128 | I | Recruiting | ||
| IBI322 monotherapy | Locally advanced, unresectable or metastatic tumors | 218 | December 2023 | NCT04328831 | Ia/Ib | Recruiting | ||
| IMC-002 (IgG4 CD47 mAb) | ImuneOncia Therapeutics Inc. | IMC-002 monotherapy | Metastatic or locally advanced solid tumors and R/R lymphomas | 24 | December 2022 | NCT04306224 | I | Recruiting |
| TQB2928 (blocks CD47 and SIRPa) | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | TQB2928 monotherapy | Locally advanced unresectable/metastatic solid tumors, hematological malignancies, or lymphoma | 20 | December 2022 | NCT04854681 | I | Not yet recruiting |
| TG-1801 (NI-1701) (CD47 and CD19 antibody) |
TG Therapeutics, Inc. | TG-1801 OR TG-1801 + ublituximab | B-cell lymphoma | 16 | December 2022 | NCT03804996 | I | Recruiting |
| TG-1801 OR TG-1801 + ublituximab | B-cell lymphoma or CLL | 60 | December 2023 | NCT04806035 | Ib | Recruiting | ||
| AK117 (anti-CD47 mAb) | Akeso | AK117 monotherapy | R/R advanced solid tumor, NHL (including R/R transformed lymphoma) | 162 | January 2023 | NCT04728334 | I | Recruiting |
| AK117 monotherapy | NHL | 159 | September 2023 | NCT04349969 | I | Not yet recruiting | ||
| GS-189 (FSI-189) (anti-SIRPα mAb) | Gilead Sciences/Forty Seven Inc. | FSI-189 OR FSI-189 + rituximab | R/R NHL | 75 | August 2023 | NCT04502706 | I | Recruiting |
| Magrolimab (anti-CD47 mAb) Magrolimab (anti-CD47 mAb) |
Gilead Sciences Gilead Sciences |
Magrolimab + daratumumab + pomalidomide + dexamethasone + bortezomib | R/R MM | 153 | September 2023 | NCT04892446 | II | Not yet recruiting |
| Magrolimab + azacitidine + venetoclax OR magrolimab + mitoxantrone + etoposide + cytarabine OR magrolimab + CC-486 | Myeloid malignancies | 164 | March 2024 | NCT04778410 | II | Not yet recruiting | ||
| Magrolimab OR magrolimab + azacitidine | R/R AML, MDS (monotherapy); untreated or R/R AML, MDS (with azacitidine) | 287 | February 2025 | NCT03248479 | I | Recruiting | ||
| Magrolimab + azacitidine OR venetoclax + azacididine OR 7+3 | TP53-mutant AML | 346 | November 2024 | NCT04778397 | III | Recruiting | ||
| Magrolimab + pembrolizumab + 5-FU + platinum OR magrolimab + docetaxel | HNSCC | 233 | December 2024 | NCT04854499 | II | Recruiting | ||
| Magrolimab + azacitidine | Treatment-naïve higher-risk MDS | 520 | February 2025 | NCT04313881 | III | Recruiting | ||
| Magrolimab + docetaxel | Solid tumors (mNSCLC, mSCLC) | 116 | March 2025 | NCT04827576 | II | Recruiting | ||
| Magrolimab + rituximab OR rituximab + gemcitabine + oxaliplatin | R/R B-cell NHL | 422 | August 2026 | NCT02953509 | I/II | Recruiting | ||
| Stanford University, Merck Sharp & Dohme Corp. | Magrolimab + pembrolizumab | Hodgkin’s lymphoma, or R/R Hodgkin’s lymphoma | 24 | May 2026 | NCT04788043 | II | Not yet recruiting | |
| ZL-1201 (anti-CD47 mAb) | Zai Lab (Shanghai) Co., Ltd. | ZL-1201 monotherapy | Locally advanced unresectable or metastatic solid tumors and lymphomas | 66 | January 2024 | NCT04257617 | I | Recruiting |
| PF-07257876 (CD47/PD-L1 bispecific antibody) | Pfizer | PF-07257876 monotherapy | NSCLC, HNSCC, ovarian cancer | 90 | July 2024 | NCT04881045 | I | Not yet recruiting |
| CPO107 (JMT601) (CD20-CD47 bispecific fusion protein) | Conjupro Biotherapeutics, Inc. | CPO107 monotherapy | CD20-positive NHL | 75 | December 2024 | NCT04853329 | I/II | Not yet recruiting |
| CC-95251 (anti-SIRPα mAb) | Celgene | CC-95251 OR CC-95251 + rituximab OR cetuximab | Advanced solid and hematologic cancers/neoplasms | 230 | November 2024 | NCT03783403 | I | Recruiting |
| TJC4 (TJ01133, lemzoparlimab) | AbbVie | TJ01133 + dexamethasone, carfilzomib, pomalidomide, daratumumab | R/R MM | 163 | July 2025 | NCT04895410 | I | Not yet recruiting |
5-FU, 5-fluorouracil; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CRC, colorectal cancer; CTCL, cutaneous T-cell lymphoma; GM-CSF, granulocyte–macrophage colony-stimulating factor; HNSCC, head and neck squamous cell carcinoma; Ig, immunoglobulin; mAb, monoclonal antibody; MDS, myelodysplastic syndrome; MM, multiple myeloma; mNSCLC, metastatic non-small-cell lung cancer; mSCLC, metastatic small-cell lung cancer; NHL, non-Hodgkin’s lymphoma; NSCLC, non-small-cell lung cancer; OC, ovarian cancer; PD-1, programmed cell death protein 1; PD-L1, PD-1 ligand; PTCL, peripheral T-cell lymphoma; R/R, relapsed/refractory; RCC, renal cell carcinoma; SCC, squamous cell carcinoma; SCL, small-cell lung cancer; SIRPα, signal-regulating protein alpha; TNBC, triple-negative breast cancer; TTI, trillium; UC, urothelial carcinoma.
Source: ClinicalTrials.gov (updated as of 30 August 2021).
Data from phase Ib clinical trials of magrolimab + azacitidine in patients with untreated higher-risk MDS and untreated AML who were ineligible for intensive chemotherapy have been reported. In 33 assessable patients with MDS (of 39 total, 64% high or very high risk); ORR was 91% and CR was 42%, which increased to 56% after 6 months on therapy. In four patients with TP53 mutations, ORR was 75% and CR was 50%. Median time to response was 1.9 months and median DOR was not reached. Twenty percent of responders were minimal residual disease (MRD) negative after a median of 5.8 months of follow-up.87 The AML trial (n = 64) included 70% with poor cytogenetic risk and 73% with TP53 mutations. In patients assessable for efficacy (n = 43), ORR was 63% and CR was 42% (69% and 45%, respectively, in patients with TP53 mutations). MRD negativity was achieved in 35% and 29% of the overall and TP53-mutant populations. Median time to response was 1.95 months, median DOR 9.6 months, and median overall survival (OS) 18.9 months in patients with TP53-wild-type and 12.9 months in patients with TP53-mutant AML.69 Several other anti-CD47 agents have also begun clinical testing in combination with azacitidine, but no results have been published.
Early clinical data are also emerging on combinations of anti-CD47-SIRPα agents with targeted antibodies and T-cell checkpoint inhibitors in solid tumors. Magrolimab was combined with cetuximab in patients with advanced colorectal cancer in a phase Ib/II study, with PR achieved in 6% and SD in 44% of patients with KRAS-wild-type disease, and SD in 38% of those with KRAS-mutant disease. The investigators noted low tumor epidermal growth factor receptor expression at baseline, which may have limited synergy with CD47 blockade.104 Similarly, a phase Ib study of magrolimab with avelumab in ovarian cancer (n = 24 assessable for efficacy) found SD in 42%; the only patient with documented tumor cell PD-L1 expression had an unconfirmed PR.88 Investigators from both studies suggested that alternative strategies to enhance prophagocytic signals are needed in the respective tumor types. In a phase I study of ALX148 with pembrolizumab [with or without 5-fluorouracil (5-FU) + platinum] in head and neck squamous cell carcinoma (HNSCC) or trastuzumab (with or without ramucirumab + paclitaxel) in human epidermal growth factor receptor 2-positive (HER2+) gastric/gastroesophageal cancer (GC), checkpoint inhibitor-naïve patients with HNSCC had an ORR of 40% with a median progression-free survival (PFS) of 4.6 months and OS not reached (n = 10), and those with GC had an ORR of 20% with a median PFS of 2.2 months and median OS of 8.1 months.105
Near-term opportunities for CD47–SIRPα therapeutics
Many clinical trials of anti-CD47–SIRPα agents have been initiated, primarily in combinations with standard therapy (Table 1), including magrolimab, which is currently being tested in multiple trials, some with registrational potential.58,69,86,87 While data from trials in solid tumors are eagerly awaited, CD47–SIRPα-targeted therapy has the potential to combine with novel treatment to make a near-term impact in hematological malignancies. For example, in AML, phase III trials of venetoclax combined with azacitidine or low-dose cytarabine in previously untreated AML ineligible for intensive chemotherapy were recently published.106,107 Venetoclax is a B Cell Lymphoma 2 (BCL2) inhibitor that induces apoptosis,108 which increases and redistributes ‘eat me’ signals leading to phagocytic clearance.109 In experimental studies, the loss of BCL2 in neutrophils signals their disappearance from the blood and tissues, and in mice with enforced expression of neutrophil BCL2 they do not undergo apoptosis; yet at the time normal neutrophils would undergo apoptosis, the BCL2 neutrophils bind CRT and are removed by macrophages.110 CD47–SIRPα blockade thus has potential to synergize with venetoclax. AML clinical trials of IBI188 with azacitidine and ALX148 with venetoclax and azacitidine have been initiated, as have several trials of magrolimab: with venetoclax and azacitidine in newly diagnosed unfit AML; with mitoxantrone, etoposide, and cytarabine in R/R AML; with oral azacitidine as maintenance therapy for patients in complete remission; and a phase III trial of magrolimab + venetoclax versus venetoclax + azacitidine versus 7 + 3 in TP53-mutant AML (Table 1). For high-risk MDS patients, azacitidine and decitabine are the only approved single-agent therapeutics; as responses are generally limited, combination therapies are under investigation. Magrolimab, IBI188, and ALX148 are being combined with azacitidine in treatment-naïve, higher-risk MDS; the ALX148 study will also enroll R/R MDS (Table 1).
Conclusions
Evidence supporting the CD47–SIRPα interaction as a therapeutic target in cancer is accumulating rapidly, and clinical trials have so far supported key aspects of the preclinical findings. Clinical data, particularly from the hematologic malignancy trials of magrolimab, indicate that CD47–SIRPα blockade in combination with standard treatment is highly efficacious and well tolerated, representing a meaningful advance in patient care. Future studies will determine the ultimate role of anti-CD47 therapy in many cancer indications.
Acknowledgments
Funding
Medical writing support was provided by Impact Communication Partners, Inc., and funded by Gilead Sciences, Inc.
Disclosure
RM reports employment by Gilead Sciences, Inc., at the time of the study and holds the US20160340397A1 patent and has the US20210115140A1 and US20210147568A1 patents pending. JX reports employment by and stock in Gilead Sciences, Inc. at the time of the study. ILW reports receiving a percentage of the royalties paid to his institution by Gilead Sciences, Inc.
References
- 1.Barkal A.A., Weiskopf K., Kao K.S., et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19:76–84. doi: 10.1038/s41590-017-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon S.R., Maute R.L., Dulken B.W., et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkal A.A., Brewer R.E., Markovic M., et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572:392–396. doi: 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown E., Hooper L., Ho T., Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;111:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mawby W.J., Holmes C.H., Anstee D.J., Spring F.A., Tanner M.J. Isolation and characterization of CD47 glycoprotein: a multispanning membrane protein which is the same as integrin-associated protein (IAP) and the ovarian tumour marker OA3. Biochem J. 1994;304:525–530. doi: 10.1042/bj3040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindberg F.P., Gresham H.D., Schwarz E., Brown E.J. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sick E., Jeanne A., Schneider C., Dedieu S., Takeda K., Martiny L. CD47 update: a multifaceted actor in the tumour microenvironment of potential therapeutic interest. Br J Pharmacol. 2012;167:1415–1430. doi: 10.1111/j.1476-5381.2012.02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldenborg P.A., Zheleznyak A., Fang Y.F., Lagenaur C.F., Gresham H.D., Lindberg F.P. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 9.Vernon-Wilson E.F., Kee W.J., Willis A.C., Barclay A.N., Simmons D.L., Brown M.H. CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPalpha 1. Eur J Immunol. 2000;30:2130–2137. doi: 10.1002/1521-4141(2000)30:8<2130::AID-IMMU2130>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Veillette A., Thibaudeau E., Latour S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J Biol Chem. 1998;273:22719–22728. doi: 10.1074/jbc.273.35.22719. [DOI] [PubMed] [Google Scholar]
- 11.Tsai R.K., Discher D.E. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaiswal S., Chao M.P., Majeti R., Weissman I.L. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31:212–219. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiswal S., Jamieson C.H., Pang W.W., et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majeti R., Chao M.P., Alizadeh A.A., et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao M.P., Alizadeh A.A., Tang C., et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374–1384. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao M.P., Alizadeh A.A., Tang C., et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao Z., Chung H., Banan B., et al. Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett. 2015;360:302–309. doi: 10.1016/j.canlet.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willingham S.B., Volkmer J.P., Gentles A.J., et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casey S.C., Tong L., Li Y., et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabay M., Li Y., Felsher D.W. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H., Lu H., Xiang L., et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A. 2015;112:E6215–E6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betancur P.A., Abraham B.J., Yiu Y.Y., et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun. 2017;8:14802. doi: 10.1038/ncomms14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao M.P., Jaiswal S., Weissman-Tsukamoto R., et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsson M., Bruhns P., Frazier W.A., Ravetch J.V., Oldenborg P.A. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood. 2005;105:3577–3582. doi: 10.1182/blood-2004-08-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsson M., Nilsson A., Oldenborg P.A. Dose-dependent inhibitory effect of CD47 in macrophage uptake of IgG-opsonized murine erythrocytes. Biochem Biophys Res Commun. 2007;352:193–197. doi: 10.1016/j.bbrc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Chao M.P., Weissman I.L., Majeti R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225–232. doi: 10.1016/j.coi.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glennie M.J., French R.R., Cragg M.S., Taylor R.P. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 28.van Erp E.A., Luytjes W., Ferwerda G., van Kasteren P.B. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol. 2019;10:548. doi: 10.3389/fimmu.2019.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bondza S., Marosan A., Kara S., et al. Complement-dependent activity of CD20-specific IgG correlates with bivalent antigen binding and C1q binding strength. Front Immunol. 2021;11:609941. doi: 10.3389/fimmu.2020.609941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evers M., Rösner T., Dünkel A., et al. The selection of variable regions affects effector mechanisms of IgA antibodies against CD20. Blood Adv. 2021;5:3807–3820. doi: 10.1182/bloodadvances.2021004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mateo V., Brown E.J., Biron G., et al. Mechanisms of CD47-induced caspase-independent cell death in normal and leukemic cells: link between phosphatidylserine exposure and cytoskeleton organization. Blood. 2002;100:2882–2890. doi: 10.1182/blood-2001-12-0217. [DOI] [PubMed] [Google Scholar]
- 32.Manna P.P., Frazier W.A. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64:1026–1036. doi: 10.1158/0008-5472.can-03-1708. [DOI] [PubMed] [Google Scholar]
- 33.Uno S., Kinoshita Y., Azuma Y., et al. Antitumor activity of a monoclonal antibody against CD47 in xenograft models of human leukemia. Oncol Rep. 2007;17:1189–1194. [PubMed] [Google Scholar]
- 34.Sagawa M., Shimizu T., Fukushima N., et al. A new disulfide-linked dimer of a single-chain antibody fragment against human CD47 induces apoptosis in lymphoid malignant cells via the hypoxia inducible factor-1α pathway. Cancer Sci. 2011;102:1208–1215. doi: 10.1111/j.1349-7006.2011.01925.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu J., Wang L., Zhao F., et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D., Wang J., Willingham S.B., Martin R., Wernig G., Weissman I.L. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538–2545. doi: 10.1038/leu.2012.141. [DOI] [PubMed] [Google Scholar]
- 37.Petrova P.S., Viller N.N., Wong M., et al. TTI-621 (SIRPαFc): a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res. 2017;23:1068–1079. doi: 10.1158/1078-0432.CCR-16-1700. [DOI] [PubMed] [Google Scholar]
- 38.Buatois V., Johnson Z., Salgado-Pires S., et al. Preclinical development of a bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B-cell lymphoma and leukemia. Mol Cancer Ther. 2018;17:1739–1751. doi: 10.1158/1535-7163.MCT-17-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng Z., Wang Z., Guo B., Cao W., Shen H. TJC4, a differentiated anti-CD47 antibody with novel epitope and RBC sparing properties. Blood. 2019;134:4063. [Google Scholar]
- 40.Puro R.J., Bouchlaka M.N., Hiebsch R.R., et al. Development of AO-176, an ext-generation humanized anti-CD47 antibody with novel anticancer properties and negligible red blood cell binding. Mol Cancer Ther. 2020;19:835–846. doi: 10.1158/1535-7163.MCT-19-1079. [DOI] [PubMed] [Google Scholar]
- 41.Ribeiro M.L., Normant E., Garau D.R., et al. PS1310 the novel bispecific CD47-CD19 antibody TG-1801 potentiates the activity of UBLITUXIMAB-UMBRALISIB (U2) drug combination in preclinical models of B-NHL. HemaSphere. 2019;3:598. [Google Scholar]
- 42.Peluso M.O., Adam A., Armet C.M., et al. The fully human anti-CD47 antibody SRF231 exerts dual-mechanism antitumor activity via engagement of the activating receptor CD32a. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L., Zhang L., Yang L., et al. Anti-CD47 antibody as a targeted therapeutic agent for human lung cancer and cancer stem cells. Front Immunol. 2017;8:404. doi: 10.3389/fimmu.2017.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gholamin S., Esparza R., Cheshier S., Mitra S. TRTH-32. Combination immunotherapy to activate the innate immune microenvironment against pediatric brain tumors. Neuro Oncol. 2017;19:iv58–iv59. [Google Scholar]
- 45.Kauder S.E., Kuo T.C., Harrabi O., et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kathawala R., Mundy D., Duran G., et al. Abstract 4001: The anti-CD47 antibody Hu5F9-G4 activates macrophages and inhibits ovarian cancer xenografts, alone and in combination with chemotherapy or immunotherapy. Cancer Res. 2016;76:4001. [Google Scholar]
- 47.Narla R., Modi H., Wong L., et al. Abstract 4694: The humanized anti-CD47 monoclonal antibody, CC-90002, has antitumor activity in vitro and in vivo. Cancer Res. 2017;77 [Google Scholar]
- 48.Wilson C., Richards J., Puro R., et al. AO-176, a highly differentiated clinical stage anti-CD47 antibody, exerts potent anti-tumor activity in preclinical models of multiple myeloma as a single agent and in combination with approved therapeutics. Blood. 2020;136:3–4. [Google Scholar]
- 49.Murata Y., Tanaka D., Hazama D., et al. Anti-human SIRPalpha antibody is a new tool for cancer immunotherapy. Cancer Sci. 2018;109:1300–1308. doi: 10.1111/cas.13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voets E., Parade M., Lutje Hulsik D., et al. Functional characterization of the selective pan-allele anti-SIRPα antibody ADU-1805 that blocks the SIRPα-CD47 innate immune checkpoint. J Immunother Cancer. 2019;7:340. doi: 10.1186/s40425-019-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J., Xavy S., Mihardja S., et al. Targeting macrophage checkpoint inhibitor SIRPα for anticancer therapy. JCI Insight. 2020;5 doi: 10.1172/jci.insight.134728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiskopf K., Ring A.M., Ho C.C., et al. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nimmerjahn F., Ravetch J.V. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 54.Ring N.G., Herndler-Brandstetter D., Weiskopf K., et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A. 2017;114:E10578–E10585. doi: 10.1073/pnas.1710877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chao M, McKenna KM, Cha A, et al. Abstract PR13: the anti-CD47 antibody HU5F9-G4 is a novel immune checkpoint inhibitor with synergistic efficacy in combination with clinically active cancer targeting antibodies. Presented at the 2016 American Association for Cancer Research. December 6-10, 2016; San Antonio, TX.
- 56.Zhao X.W., van Beek E.M., Schornagel K., et al. CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci U S A. 2011;108:18342–18347. doi: 10.1073/pnas.1106550108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Storti P., Vescovini R., Costa F., et al. CD14(+) CD16(+) monocytes are involved in daratumumab-mediated myeloma cells killing and in anti-CD47 therapeutic strategy. Br J Haematol. 2020;190:430–436. doi: 10.1111/bjh.16548. [DOI] [PubMed] [Google Scholar]
- 58.Advani R., Flinn I., Popplewell L., et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med. 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abrisqueta P., Sancho J.-M., Cordoba R., et al. Anti-CD47 antibody, CC-90002, in combination with rituximab in subjects with relapsed and/or refractory non-Hodgkin lymphoma (R/R NHL) Blood. 2019;134:4089. [Google Scholar]
- 60.Kim T, Lakhani N, Gainor J, et al. 3016 ALX148, a CD47 blocker, in combination with rituximab in patients with non-Hodgkin lymphoma. Presented at the 62nd ASH Annual Meeting and Exposition; December 5-8, 2020.
- 61.Ansell S.M., Maris M.B., Lesokhin A.M., et al. Phase I study of the CD47 blocker TTI-621 in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2021;27:2190–2199. doi: 10.1158/1078-0432.CCR-20-3706. [DOI] [PubMed] [Google Scholar]
- 62.Theruvath J., Menard M., Smith B., et al. Anti-GD2 antibody disrupts GD2:Siglec-7 interactions and synergizes with CD47 blockade to mediate tumor eradication. BioRxiv. March 21, 2021 doi: 10.1101/2021.03.19.436221. [DOI] [Google Scholar]
- 63.Upton R., Banuelos A., Feng D., et al. Combining CD47 blockade with trastuzumab eliminates HER2-positive breast cancer cells and overcomes trastuzumab tolerance. Proc Natl Acad Sci U S A. 2021:118. doi: 10.1073/pnas.2026849118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Weers M., Tai Y.-T., van der Veer M.S., et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 65.Overdijk M.B., Verploegen S., Bögels M., et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7:311–321. doi: 10.1080/19420862.2015.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herter S., Herting F., Mundigl O., et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12:2031–2042. doi: 10.1158/1535-7163.MCT-12-1182. [DOI] [PubMed] [Google Scholar]
- 67.Obeid M., Tesniere A., Ghiringhelli F., et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 68.Feng D., Glip P., McKenna K., et al. Combination treatment with 5F9 and azacitidine enhances phagocytic elimination of acute myeloid leukemia. Blood. 2018;132:2729. [Google Scholar]
- 69.Sallman D, Asch A, Kambhampati S, et al. The first in class anti CD47 antibody magrolimab combined with azacitidine is well tolerated and effective in AML patients: phase 1b results. Presented at the 2020 American Society of Hematology Annual Meeting & Exposition; December 5-8, 2020; Atlanta, GA.
- 70.Lo J., Lau E.Y., So F.T., et al. Anti-CD47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma. Liver Int. 2016;36:737–745. doi: 10.1111/liv.12963. [DOI] [PubMed] [Google Scholar]
- 71.Tseng D., Volkmer J.P., Willingham S.B., et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110:11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X., Pu Y., Cron K., et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sockolosky J.T., Dougan M., Ingram J.R., et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A. 2016;113:E2646–E2654. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pan Y., Lu F., Fei Q., et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J Hematol Oncol. 2019;12:124. doi: 10.1186/s13045-019-0822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lian S., Xie R., Ye Y., et al. Dual blockage of both PD-L1 and CD47 enhances immunotherapy against circulating tumor cells. Sci Rep. 2019;9:4532. doi: 10.1038/s41598-019-40241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song X., Lu Z., Xu J. Targeting cluster of differentiation 47 improves the efficacy of anti-cytotoxic T-lymphocyte associated protein 4 treatment via antigen presentation enhancement in pancreatic ductal adenocarcinoma. Exp Ther Med. 2020;20:3301–3309. doi: 10.3892/etm.2020.9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng M., Chen J., Weissman-Tsukamoto R., et al. Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. Proc Natl Acad Sci U S A. 2015;112:2145–2150. doi: 10.1073/pnas.1424907112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feng M., Marjon K.D., Zhu F., et al. Programmed cell removal by calreticulin in tissue homeostasis and cancer. Nat Commun. 2018;9:3194. doi: 10.1038/s41467-018-05211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodrigues E., Macauley M.S. Hypersialylation in cancer: modulation of Inflammation and therapeutic opportunities. Cancers (Basel) 2018;10:207. doi: 10.3390/cancers10060207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reinhold M.I., Lindberg F.P., Plas D., Reynolds S., Peters M.G., Brown E.J. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47) J Cell Sci. 1995;108:3419–3425. doi: 10.1242/jcs.108.11.3419. [DOI] [PubMed] [Google Scholar]
- 81.Cauvin A., Peters C., Brennan F. In: The Nonhuman Primate in Nonclinical Drug Development and Safety Assessment. Blumei J., Korte S., Schenck E., Weinbauer G., editors. Elsevier Inc.; Amsterdam, Netherlands: 2015. Advantages and limitations of commonly used nonhuman primate species in research and development of biopharmaceuticals; pp. 379–395. [Google Scholar]
- 82.Bennett G.D., Kay M.M. Homeostatic removal of senescent murine erythrocytes by splenic macrophages. Exp Hematol. 1981;9:297–307. [PubMed] [Google Scholar]
- 83.Khandelwal S., van Rooijen N., Saxena R.K. Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation. Transfusion. 2007;47:1725–1732. doi: 10.1111/j.1537-2995.2007.01348.x. [DOI] [PubMed] [Google Scholar]
- 84.Pietsch E.C., Dong J., Cardoso R., et al. Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J. 2017;7:e536. doi: 10.1038/bcj.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brierley C.K., Staves J., Roberts C., et al. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion. 2019;59:2248–2254. doi: 10.1111/trf.15397. [DOI] [PubMed] [Google Scholar]
- 86.Sikic B.I., Lakhani N., Patnaik A., et al. First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol. 2019;37:946–953. doi: 10.1200/JCO.18.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sallman D, Asch A, Al-Malki M, et al. The first in class anti CD47 antibody magrolimab combined with azacitidine is well tolerated and effective in MDS patients: phase 1b results. Presented at the 2020 European Hematology Association; November 13, 2020; Virtual.
- 88.Lakhani N.J., Patnaik A., Liao J.B., et al. A phase Ib study of the anti-CD47 antibody magrolimab with the PD-L1 inhibitor avelumab (A) in solid tumor (ST) and ovarian cancer (OC) patients. J Clin Oncol. 2020;38:18. [Google Scholar]
- 89.Chen J., McKenna K., Choi T., et al. RBC-specific CD47 pruning confers protection and underlies the transient anemia in patients treated with anti-CD47 antibody 5F9. Blood. 2018;132:2327. [Google Scholar]
- 90.Horwitz S., Foran J., Maris M., et al. Updates from ongoing, first-in-human phase 1 dose escalation and expansion study of TTI-621, a novel biologic targeting CD47, in patients with relapsed or refractory hematologic malignancies. Blood. 2020;136:41–43. [Google Scholar]
- 91.Patel K., Ramchandren R., Maris M., et al. Investigational CD47-blocker TTI-622 shows single-agent activity in patients with advanced relapsed or refractory lymphoma: update from the ongoing first-in-human dose escalation study. Blood. 2020;136:46–47. [Google Scholar]
- 92.Burris H., III, Spira A., Taylor M., et al. A first-in-human study of AO-176, a highly differentiated anti-CD47 antibody, in patients with advanced solid tumors. J Clin Oncol. 2021;39:2516. [Google Scholar]
- 93.Lakhani N., Orloff M., Fu S., et al. First-in-human phase I trial of IBI188, an antiCD47 targeting monoclonal antibody, in patients with advanced solid tumors and lymphomas. J Immunother Cancer. 2020;8:A180. [Google Scholar]
- 94.Patnaik A., Spreafico A., Paterson A., et al. Results of a first-in-human phase I study of SRF231, a fully human, high-affinity anti-CD47 antibody. J Clin Oncol. 2020;38:3064. [Google Scholar]
- 95.Berlin J., Harb W., Adjei A., et al. A first-in-human study of lemzoparlimab, a differentiated anti-CD47 antibody, in subjects with relapsed/refractory malignancy: initial monotherapy results. J Immunother Cancer. 2020;8:A233–A234. [Google Scholar]
- 96.Gan H., Coward J., Mislang A., et al. Safety of AK117, an anti-CD47 monoclonal antibody, in patients with advanced or metastatic solid tumors in a phase I study. J Clin Oncol. 2021;39:2630. [Google Scholar]
- 97.Zeidan A., DeAngelo D., Palmer J., et al. A phase I study of CC-90002, a monoclonal antibody targeting CD47, in patients with relapsed and/or refractory (R/R) acute myeloid leukemia (AML) and high-risk myelodysplastic syndromes (MDS): final results. Blood. 2019;134:1320. [Google Scholar]
- 98.Delord J.-P., Kotecki N., Marabelle A., et al. A phase 1 study evaluating BI 765063, a first in class selective myeloid SIRPa inhibitor, as stand-alone and in combination with BI 754091, a programmed death-1 (PD-1) inhibitor, in patients with advanced solid tumours. Blood. 2019;134:1040. [Google Scholar]
- 99.Champiat S., Cassier P., Kotecki N., Korakis I., Vinceneux A., Jungels C. Safety, pharmacokinetics, efficacy, and preliminary biomarker data of first-in-class BI 765063, a selective SIRPα inhibitor: results of monotherapy dose escalation in phase 1 study in patients with advanced solid tumors. J Clin Oncol. 2021;39:2623. [Google Scholar]
- 100.Magee D.E., Hird A.E., Klaassen Z., et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: a systematic review and meta-analysis of randomized clinical trials. Ann Oncol. 2020;31:50–60. doi: 10.1016/j.annonc.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 101.Advani R, Bartlett N, Smith S, et al. Activity of the first in class anti CD47 antibody Hu5F9 G4 with rituximab in relapsed/refractory non-Hodgkin’s lymphoma: interim phase 1b/2 results. Presented at the 15th International Conference on Malignant Lymphoma (ICML); June 18-22, 2019; Lugano, Switzerland.
- 102.A study of CC-90002 in subjects with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS). https://clinicaltrials.gov/ct2/show/NCT02641002 ClinicalTrials.gov identifier: NCT02641002. Available at.
- 103.Querfeld C., Thompson J.A., Taylor M.H., et al. Intralesional TTI-621, a novel biologic targeting the innate immune checkpoint CD47, in patients with relapsed or refractory mycosis fungoides or Sézary syndrome: a multicentre, phase 1 study. Lancet Haematol. 2021;8:e808–e817. doi: 10.1016/S2352-3026(21)00271-4. [DOI] [PubMed] [Google Scholar]
- 104.Fisher G., Jr., Lakhani N., Eng C., et al. A phase 1b/2 study of the anti-CD47 antibody magrolimab with cetuximab in solid tumor and colorectal cancer patients. J Clin Oncol. 2020;38:114. [Google Scholar]
- 105.Chow L.Q.M., Gainor J.F., Lakhani N.J., et al. A phase I study of ALX148, a CD47 blocker, in combination with standard anticancer antibodies and chemotherapy regimens in patients with advanced malignancy. J Clin Oncol. 2020;38:3056. [Google Scholar]
- 106.DiNardo C.D., Jonas B.A., Pullarkat V., et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 107.Wei A.H., Montesinos P., Ivanov V., et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135:2137–2145. doi: 10.1182/blood.2020004856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Venclexta® (venetoclax) [Package Insert] Genentech USA, Inc; South San Francisco, CA: 2020. [Google Scholar]
- 109.Gardai S.J., McPhillips K.A., Frasch S.C., et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 110.Lagasse E., Weissman I.L. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179:1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chao M.P., Takimoto C.H., Feng D.D., et al. Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front Oncol. 2020;9:1380. doi: 10.3389/fonc.2019.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]