Abstract
RNA binding proteins mediate posttranscriptional RNA metabolism and play regulatory roles in many developmental processes in eukaryotes. Despite their known effects on the floral transition from vegetative to reproductive growth in plants, the underlying mechanisms remain largely obscure. Here, we show that a hitherto unknown RNA binding protein, hnRNP R-LIKE PROTEIN (HRLP), inhibits cotranscriptional splicing of a key floral repressor gene FLOWERING LOCUS C (FLC). This, in turn, facilitates R-loop formation near FLC intron I to repress its transcription, thereby promoting the floral transition in Arabidopsis thaliana. HRLP, together with the splicing factor ARGININE/SERINE-RICH 45, forms phase-separated nuclear condensates with liquid-like properties, which is essential for HRLP function in regulating FLC splicing, R-loop formation, and RNA Polymerase II recruitment. Our findings reveal that inhibition of cotranscriptional splicing of FLC by nuclear HRLP condensates constitutes the molecular basis for down-regulation of FLC transcript levels to ensure the reproductive success of Arabidopsis.
Phase separation of HRLP promotes flowering through inhibiting cotranscriptional splicing of a key flowering repressor FLC.
INTRODUCTION
Posttranscriptional regulation is an indispensable mechanism for controlling gene expression in eukaryotes. A nascent pre-mRNA transcript undergoes several tightly regulated RNA processing steps during or after transcription, including capping, splicing, modification, and polyadenylation, to form the mature mRNA before being exported to the cytoplasm for translation (1). All RNA processing events are mediated by the associated RNA binding proteins (RBPs), including the heterogeneous nuclear ribonucleoproteins (hnRNPs) representing a large family of RBPs that profoundly affect nearly every aspect of mRNA metabolism from transcription to RNA decay (2). The binding specificity and functional diversity of RBPs mostly rely on various combinations of RNA binding domains (RBDs) (3, 4), such as the RNA recognition motif (RRM) (5), hnRNP K homology (KH) domain (6), and zinc finger domain (7), among which the RRM containing the two short conserved sequences, RNP1 and RNP2, is the most commonly found RBD (4, 8). Although 196 RRM-containing RBPs have been annotated in the model plant Arabidopsis thaliana (9), most of them are yet to be functionally characterized.
RBPs play central regulatory roles in a multitude of diverse developmental and cellular processes in eukaryotes (10–12). In Arabidopsis, RBPs have been shown to affect the floral transition, a key developmental switch from vegetative to reproductive growth that determines the plant reproductive success (10, 13, 14). This transition is controlled by a complex network of genetic pathways in response to environmental and endogenous flowering signals (13). Several RBPs have been implicated to function in the autonomous pathway that monitors endogenous cues to affect the expression of FLOWERING LOCUS C (FLC), which encodes a potent floral repressor directly inhibiting the expression of two floral pathway integrators, FLOWERING LOCUS T (FT) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) (15–17). In particular, both the sense FLC transcript and alternative polyadenylation of the antisense COOLAIR transcripts from the FLC locus are modulated by two RBPs, FLOWERING CONTROL LOCUS A (FCA) and FPA, together with 3′ processing factors including FY, CLEAVAGE STIMULATING FACTOR 64 (CstF64), and CstF77 (18, 19). Several other RBPs, such as RZ-1B, RZ-1C, SC35, and SC35-LIKE (SCL) proteins, have been shown to modulate splicing and expression of the sense FLC transcripts to regulate flowering (20, 21). Single-molecule RNA fluorescence in situ hybridization (FISH) assay has revealed the colocalization of the nonspliced FLC RNA with FLC DNA FISH signals (22), implying that FLC pre-mRNA undergoes cotranscriptional splicing. Despite the above progress in understanding the effects of RBPs on FLC expression, the concrete mechanisms underlying cotranscriptional splicing at the FLC locus remain largely elusive.
Increasing evidence suggests that multiple RNA processing events occur within RBP-rich condensates, which are formed through phase separation driven by multivalent interactions between RNA and RBPs or intrinsically disordered regions (IDRs) of RBPs (23–26). Formation of protein condensates, including RBP-rich ones, have been shown to mediate multiple biological processes in plants, such as phytohormone signaling (27), flowering (28), intrachloroplast cargo sorting (29), thermosensory response (30), plant immune response (31), and microRNA processing (32). Notably, liquid-liquid phase separation (LLPS) of FCA promoted by FLX-LIKE 2 (FLL2) is crucial for FCA function in regulating the alternative polyadenylation of antisense FLC transcripts in flowering time control (28).
In this study, we reveal that a hitherto uncharacterized RBP, hnRNP R-LIKE PROTEIN (HRLP), plays a key role in suppressing cotranscriptional splicing of FLC, which enhances R-loop formation near FLC intron I, resulting in reduced recruitment of RNA Polymerase II (Pol II) and a consequential low FLC expression. HRLP, together with the splicing factor ARGININE/SERINE-RICH 45 (SR45), undergoes LLPS both in vitro and in vivo to form phase-separated nuclear condensates, which is required for inhibiting the cotranscriptional splicing process of FLC. In contrast, loss of HRLP or failure in forming HRLP nuclear bodies facilitates the cotranscriptional splicing and transcription of FLC, thus preventing the floral transition. Our study uncovers that inhibition of cotranscriptional splicing of FLC by nuclear HRLP condensates is an integral mechanism underlying posttranscriptional regulation of FLC to determine the flowering time in Arabidopsis.
RESULTS
HRLP promotes flowering in Arabidopsis
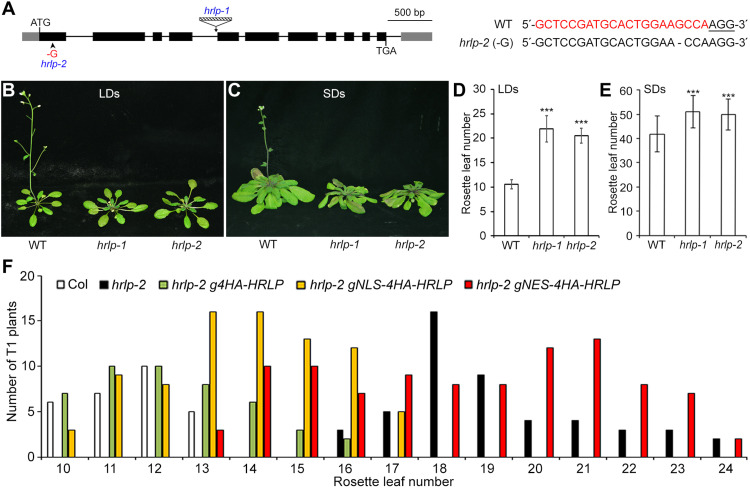
HRLP (AT2G44710) is one of the Arabidopsis orthologs of the human hnRNP R (fig. S1) and shares high sequence similarity with its closest homologs in other plant species (fig. S2A). HRLP contains one coiled-coil domain, three RRMs, and multiple low-complexity regions (LCRs) as predicted by the Simple Modular Architecture Research Tool (SMART; fig. S2B). To study the biological function of HRLP, we obtained a mutant, hrlp-1 (Salk_124411), harboring a T-DNA insertion in the fourth intron in the Columbia (Col-0) background from the Arabidopsis Biological Resource Center (Fig. 1A). This mutant, in which HRLP transcripts spanning the T-DNA insertion site were undetectable (fig. S3, A and B), displayed significantly late flowering compared with wild-type plants under both long days (LDs) and short days (SDs; Fig. 1, B to E). To confirm the role of HRLP in affecting flowering, we further generated another mutant by the CRISPR-Cas9–mediated gene editing using a single-guide RNA (sgRNA) that targeted a region within the first exon of HRLP (Fig. 1A). We subsequently identified one homozygous mutant without the CRISPR-Cas9 transgene, designated hrlp-2, that contained 1 base pair (bp) of guanine (G) deletion at 3 bp upstream of the protospacer adjacent motif (Fig. 1A). Similarly, hrlp-2 showed a late-flowering phenotype comparable to hrlp-1 under both LDs and SDs (Fig. 1, B to E). These results suggest that HRLP promotes flowering in Arabidopsis.
Fig. 1. HRLP regulates flowering time in Arabidopsis.
(A) Schematic diagram shows the T-DNA insertion site in hrlp-1 (Salk_124411) and the 1-bp G deletion site in hrlp-2. Exons in the coding region and untranslated regions (UTRs) are shown by black and gray boxes, respectively, and introns are indicated by black lines. The right panel shows the alignment of genomic DNA sequences of wild-type (WT) and hrlp-2 containing the CRISPR-Cas9 target site. The 21-bp sgRNA sequence is shown in red color and the 3-bp protospacer adjacent motif is underlined. (B and C) hrlp mutants exhibit late flowering under LDs (B) and SDs (C). (D and E) Flowering time of hrlp mutants under LDs (D) and SDs (E). Error bars, means ± SD; n = 20. Asterisks indicate statistically significant differences between WT and hrlp plants (two-tailed paired Student’s t test, ***P < 0.001). (F) Flowering time distribution of T1 transgenic plants of hrlp-2 g4HA-HRLP, hrlp-2 gNLS-4HA-HRLP, and hrlp-2 gNES-4HA-HRLP.
To verify that the flowering defect of hrlp mutants was caused by loss of HRLP function, we transformed hrlp-2 mutants with a 5.0-kb HRLP genomic construct (g4HA-HRLP) including the 0.8-kb 5′ upstream region, the 3.7-kb coding sequence fused in frame with a 4HA tag immediately after ATG plus introns, and the 0.5-kb 3′ untranslated region (fig. S3A). Most of the hrlp-2 g4HA-HRLP T1 transformants exhibited comparable flowering time to wild-type plants (Fig. 1F), demonstrating that loss of HRLP is responsible for the late-flowering phenotype of hrlp-2. We then selected one representative hrlp-2 g4HA-HRLP line that may contain the transgene at a single locus based on a 3:1 Mendelian segregation ratio for further investigation.
HRLP is highly expressed in developing seedlings
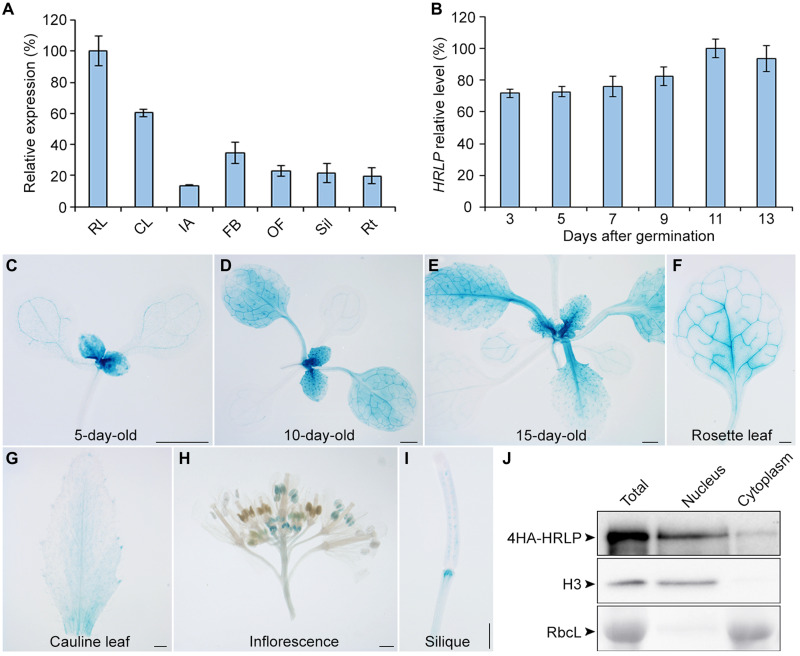
Quantitative real-time polymerase chain reaction (qRT-PCR) revealed that HRLP mRNA was expressed in all tissues examined with the highest expression in rosette leaves (Fig. 2A). Its expression was gradually increased in developing seedlings during the floral transition occurring at 9 to 13 days after gemination under our growth conditions (Fig. 2B), which is consistent with HRLP function as a flowering promoter. To examine the expression pattern of HRLP protein, we generated the gHRLP-GUS reporter lines, in which the same genomic fragment used in g4HA-HRLP was translationally fused to the β-glucuronidase (GUS) reporter gene (fig. S3A). Most of the gHRLP-GUS transgenic lines exhibited similar staining patterns. HRLP-GUS signals were detectable intensively in shoot apices and young rosette leaves, but weakly in old rosette leaves in developing seedlings in the course of the floral transition (Fig. 2, C to F). GUS signals were also detected in cauline leaves, stamens of young floral buds, and siliques (Fig. 2, G to I). In general, the expression of HRLP mRNA and HRLP-GUS protein was stronger in vegetative organs than reproductive organs.
Fig. 2. Expression pattern of HRLP.
(A) qRT-PCR analysis of HRLP expression in various tissues of WT Col plants. RL, rosette leaves; CL, cauline leaves; IA, inflorescence apices; FB, floral buds; OF, open flowers; Sil, siliques; Rt, roots. Levels of gene expression normalized to TUB2 expression are shown relative to the maximal expression level set at 100%. Error bars, means ± SD; n = 3. (B) qRT-PCR analysis shows the temporal expression pattern of HRLP in WT seedlings from 3 to 13 days after germination under LDs. Levels of gene expression normalized to TUB2 expression are shown relative to the maximal expression level set at 100%. Error bars, means ± SD; n = 3. (C to I) GUS staining of a representative gHRLP-GUS transgenic line reveals the HRLP expression pattern in a 5-day-old seedling (C), 10-day-old seedling (D), 15-day-old seedling (E), a rosette leaf (F), a cauline leaf (G), an inflorescence (H), and a silique (I). Scale bars, 1 mm. (J) HRLP is present in both nucleus and cytoplasm. Total, nuclear, and cytoplasmic proteins were extracted from 9-day-old hrlp-2 g4HA-HRLP seedlings and detected using anti-HA antibody. Histone 3 (H3) examined by anti-H3 antibody and the Rubisco large subunit (RbcL) stained with Ponceau S served as the internal controls for nuclear and cytosol fractions, respectively.
To understand how HRLP affects flowering in response to various flowering signals, we analyzed HRLP expression in various flowering mutants and under different environmental conditions. HRLP expression levels were comparable in wild-type plants and mutants of the autonomous pathway, including fca-2, fpa-7, flk-2, ld-1, and fld-3, fve-4 (fig. S3C), as well as the mutants of the photoperiod pathway, gi-1, co-9, and ft-10 (fig. S3, D and E), indicating that HRLP expression is not regulated by the autonomous and photoperiod pathways. In addition, hrlp-2 responded normally to the changes in ambient temperature (fig. S3F), implying that HRLP is not involved in the thermosensory pathway. HRLP expression was not obviously altered in the gibberellin (GA)–deficient mutant ga1-3 compared with wild-type plants grown under SDs (fig. S3G). Moreover, GA treatment of ga1-3 did not notably alter HRLP expression (fig. S3H), indicating that HRLP expression is not affected by the GA pathway. In contrast, although vernalization treatment of wild-type Col plants barely affected HRLP expression, vernalization treatment of FRI FLC significantly up-regulated HRLP, which was associated with the marked down-regulation of FLC (fig. S3, I and J). This observation implies that vernalization influences HRLP expression particularly when FLC expression levels are high.
To investigate the subcellular localization of HRLP, we examined HRLP protein levels in the nuclear and cytosolic fractions extracted from 9-day-old hrlp-2 g4HA-HRLP seedlings and found that 4HA-HRLP was present predominantly in the nucleus and weakly in the cytoplasm (Fig. 2J). To further explore which subcellular fraction of HRLP confers a promotive effect on flowering, we transformed hrlp-2 with gNLS-4HA-HRLP and gNES-4HA-HRLP constructs, in which the nuclear localization signal (NLS) and the nuclear export signal (NES) were fused translationally with the start codon in the g4HA-HRLP construct, respectively (fig. S3A). Most of the hrlp-2 gNLS-4HA-HRLP lines showed earlier flowering than hrlp-2, whereas most of the hrlp-2 gNES-4HA-HRLP lines exhibited late flowering like hrlp-2 (Fig. 1F). Further immunoblot analysis revealed that NLS-4HA-HRLP and NES-4HA-HRLP proteins were exclusively localized in the nucleus and cytoplasm, respectively (fig. S3K). These results suggest that the nuclear HRLP plays a major role in promoting flowering.
HRLP down-regulates FLC transcript levels
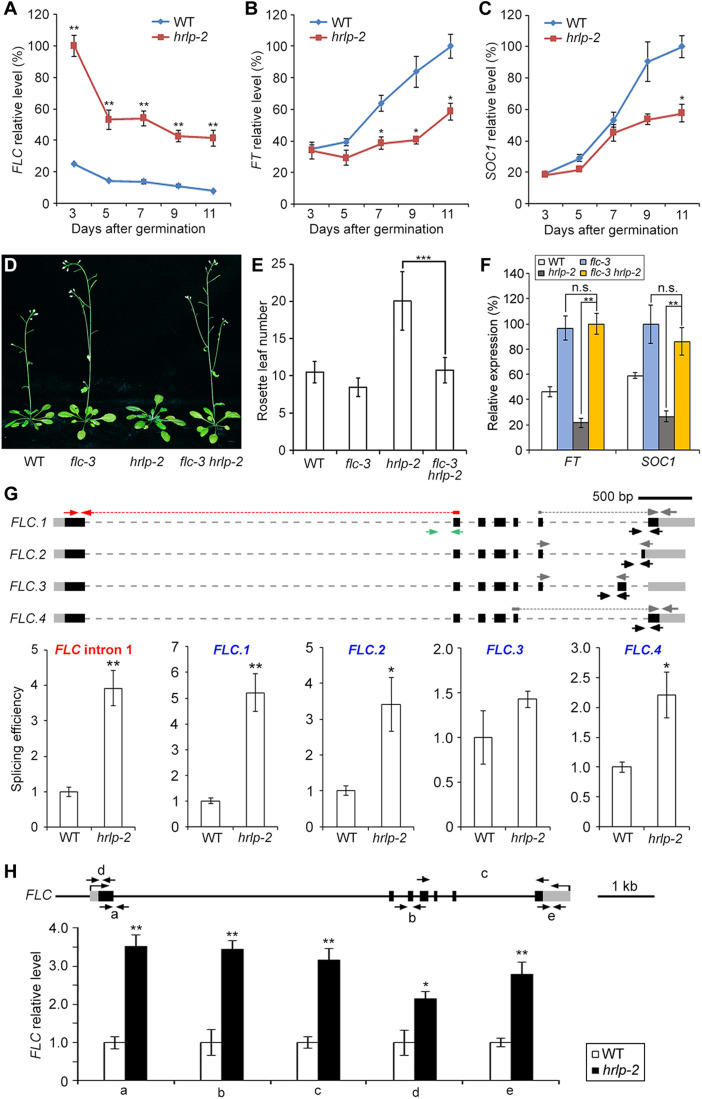
To explore the potential downstream targets of HRLP in regulating flowering time, we performed high-throughput sequencing of RNA extracted from 9-day-old wild-type and hrlp-2 seedlings grown under LDs. Among these differentially expressed genes (fold change >2.0, P < 0.05) in hrlp-2, the expression levels of the key floral repressor FLC were evidently up-regulated (fig. S4A), which was in line with the late-flowering phenotype of hrlp-2. To verify the RNA sequencing (RNA-seq) result, we examined FLC expression in developing seedlings during the floral transition and again observed considerably up-regulated FLC transcript levels in hrlp-2 versus wild-type plants (Fig. 3A). As FLC directly represses FT and SOC1 expression (33), the expression of these two genes was consequently down-regulated in hrlp-2 (Fig. 3, B and C). In contrast, the expression of the closest homologs of FLC, MADS AFFECTING FLOWERING 1-5 (MAF1-5), and the FLC partner gene SHORT VEGETATIVE PHASE (SVP) was only slightly altered in hrlp-2 during the floral transition (fig. S4, B to G). The above results were also corroborated by gene expression analyses in 9-day-old wild-type and hrlp-1 seedlings (fig. S4H). Meanwhile, we also examined the expression of the FLC antisense transcript COOLAIR in hrlp-2 mutants. Expression of the proximally polyadenylated class I COOLAIR remained unchanged, whereas expression of the distally polyadenylated class II COOLAIR was slightly increased in hrlp-2 (fig. S4I). The latter could be associated with the increased FLC expression (18, 34) rather than unaltered splicing of COOLAIR in hrlp-2 (fig. S4J). In addition, the expression of several autonomous pathway genes acting upstream of FLC, including FVE, FCA, FLD, FPA, LD, FLK, and FY, were not affected by HRLP (fig. S4K). Together, these results imply that HRLP may directly modulate the FLC transcript levels to control flowering.
Fig. 3. HRLP affects FLC transcripts levels.
(A to C) Expression of FLC (A), FT (B), and SOC1 (C) in WT and hrlp-2 seedlings under LDs. Gene expression levels in (A), (B), (C), and (F) normalized to TUB2 expression are shown relative to the maximal level set at 100%. (D) Flowering phenotype of flc-3, hrlp-2, and flc-3 hrlp-2. (E) Flowering time of various plants under LDs. Error bars, means ± SD; n = 20. (F) Expression of FT and SOC1 in various seedlings. (G) Splicing efficiency of FLC introns in 9-day-old WT and hrlp-2 seedlings. The top panel shows schematic diagrams of FLC isoforms and primers for determining splicing efficiency. Black boxes, exons; gray boxes, UTRs; gray dashed lines, introns. Red arrows with dashed lines and green arrows indicate primers for exon I and intron I, respectively, while gray arrows with or without dashed lines and black arrows represent primers that determine other featured exons and the corresponding introns of each isoform, respectively. Splicing efficiency (bottom) was determined by normalizing expression levels of the featured exon against the corresponding unspliced alternative intron. The levels in WT plants are set as 1.0. (H) Nascent FLC levels in 9-day-old WT and hrlp-2 seedlings. FLC genomic structure and primers for determining nascent FLC levels were shown above. Black lines, introns and upstream sequences; black boxes, exons; gray boxes, UTRs. Gene expression levels normalized to EF1A are shown relative to WT levels set as 1.0. Error bars (A, B, C, F, G, and H), means ± SD; n = 3. Asterisks indicate statistically significant differences between WT and hrlp-2 plants (A, B, C, G, and H) or between indicated genotypes (E and F) (two-tailed paired Student’s t test, *P < 0.05, **P < 0.01, and ***P < 0.001), whereas n.s. indicates no statistically significant differences (P > 0.05) (F).
Furthermore, flc-3 largely suppressed the late-flowering phenotype of hrlp-1 and hrlp-2 (Fig. 3, D and E, and fig. S4L), suggesting that the flowering defect of hrlp is mainly attributed to increased FLC levels. As expected, low expression levels of SOC1 and FT in hrlp-2 were restored in flc-3 hrlp-2 to the extents similar to those in flc-3 (Fig. 3F). These results suggest that HRLP promotes flowering mainly through modulating FLC transcript levels. The late-flowering phenotype of hrlp-2 was still suppressed by vernalization treatment (fig. S4M), implying that the effect of vernalization on FLC expression is at least partially independent of HRLP.
HRLP regulates splicing and transcription of FLC
Because RBPs affect almost all aspects of mRNA metabolism, including transcriptional and posttranscriptional processes (1, 2), we proceeded to explore how HRLP affects FLC transcript levels during the floral transition. At the posttranscriptional level, we first examined the splicing efficiency of FLC by calculating the levels of each featured exons against those of their respective featured introns in FLC sense variants, FLC.1-4 (Fig. 3G). The splicing efficiency of all featured introns of the four FLC isoforms except intron 6 of FLC.3 was significantly increased in hrlp-2, especially for the major isoform FLC.1 (Fig. 3G). Moreover, the splicing efficiency of FLC intron I was also significantly higher in hrlp-2 than wild-type plants (Fig. 3G). These results indicate that HRLP negatively regulates the intron splicing of FLC. In contrast, HRLP did not affect the transcript stability or the nuclear-cytoplasmic transport of FLC mRNA (fig. S5, A and B). Next, we analyzed the abundance of nascent FLC transcripts to explore whether HRLP also regulates FLC at the transcriptional level. Nascent FLC RNA levels were significantly increased in hrlp-2 compared with wild-type plants (Fig. 3H), indicating that HRLP also inhibits FLC transcription. Together, these results suggest that HRLP represses both splicing and transcription of FLC.
HRLP is directly associated with FLC RNA
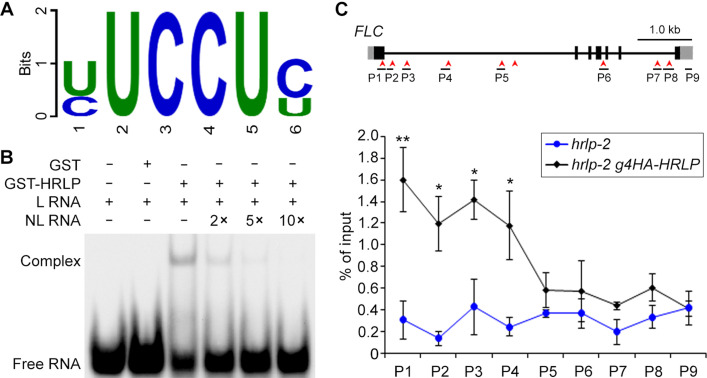
The nature of HRLP as an RBP with triple RRMs and its effects on suppressing splicing and transcription of FLC prompted us to examine whether HRLP is directly associated with FLC mRNA. In vitro RNA pull-down assay using the FLC mRNA probe showed that only glutathione S-transferase (GST)–HRLP(RRM) containing the triple RRMs of HRLP bound to the FLC probe, but not a GFP probe (fig. S5C), indicating that FLC mRNA bears the HRLP binding site(s). To determine the sequence preference for HRLP binding, we performed the systematic evolution of ligands by exponential enrichment (SELEX) experiment through screening sequences highly enriched with GST-HRLP versus GST (fig. S5, D and E) and identified the YUCCUY (Y=U/C) motif as a potential binding site for HRLP (Fig. 4A). We then carried out electrophoretic mobility shift assay (EMSA) using biotin-labeled RNA probes containing either four repeats of 5′-YUCCUY-3′ or four repeats of 5′-RAGGAR-3′ as a control. Only migration of the 5′-YUCCUY-3′ probe was retarded by GST-HRLP (Fig. 4B and fig. S5F), and this migration was weakened and even abolished by the addition of an increasing amount of the unlabeled probe (Fig. 4B). In addition, deletion of YUCCUY from FLC mRNA abolished its binding with GST-HRLP(RRM) (fig. S5, G and H). These observations strongly suggest that HRLP binds to the YUCCUY motif.
Fig. 4. HRLP is directly associated with the FLC pre-mRNA.
(A) The sequence logo shows the mostly enriched motif associated with GST-HRLP in SELEX experiments. The size of the letter indicates the information content (measured in bits). (B) EMSA shows the in vitro binding of GST-HRLP to the YUCCUY motif. The RNA oligo containing four repeats of 5′-YUCCUY-3′ was used as the RNA probe. L RNA, labeled RNA probe; NL RNA, unlabeled RNA competitor. (C) RIP analysis using anti-HA antibody reveals the binding of HRLP to the FLC pre-mRNA in 9-day-old hrlp-2 g4HA-HRLP versus hrlp-2 seedlings. The structure of FLC pre-mRNA and positions of 9 pairs of primers used in RIP assay are shown in the top panel. Red arrowheads indicate the positions of YUCCUY motifs. Exons, UTRs, and introns are indicated by black boxes, gray boxes, and black lines, respectively. Error bars, means ± SD; n = 3. Asterisks indicate statistically significant differences in HRLP association with FLC between hrlp-2 g4HA-HRLP and hrlp-2 seedlings (two-tailed paired Student’s t test, *P < 0.05, **P < 0.01).
As there are nine YUCCUY motifs in the FLC pre-mRNA (Fig. 4C), we next investigated whether HRLP directly binds to FLC transcripts in vivo by RNA immunoprecipitation followed by qRT-PCR (RIP-qPCR) using the primers designed to cover the regions containing these YUCCUY motifs in the FLC pre-mRNA (Fig. 4C). RIP-qPCR performed on 9-day-old hrlp-2 g4HA-HRLP and hrlp-2 seedlings revealed significant enrichment of HRLP on FLC pre-mRNA in hrlp-2 g4HA-HRLP, especially in the first exon and the first intron (Fig. 4C), suggesting a direct association of HRLP with FLC pre-mRNA in vivo.
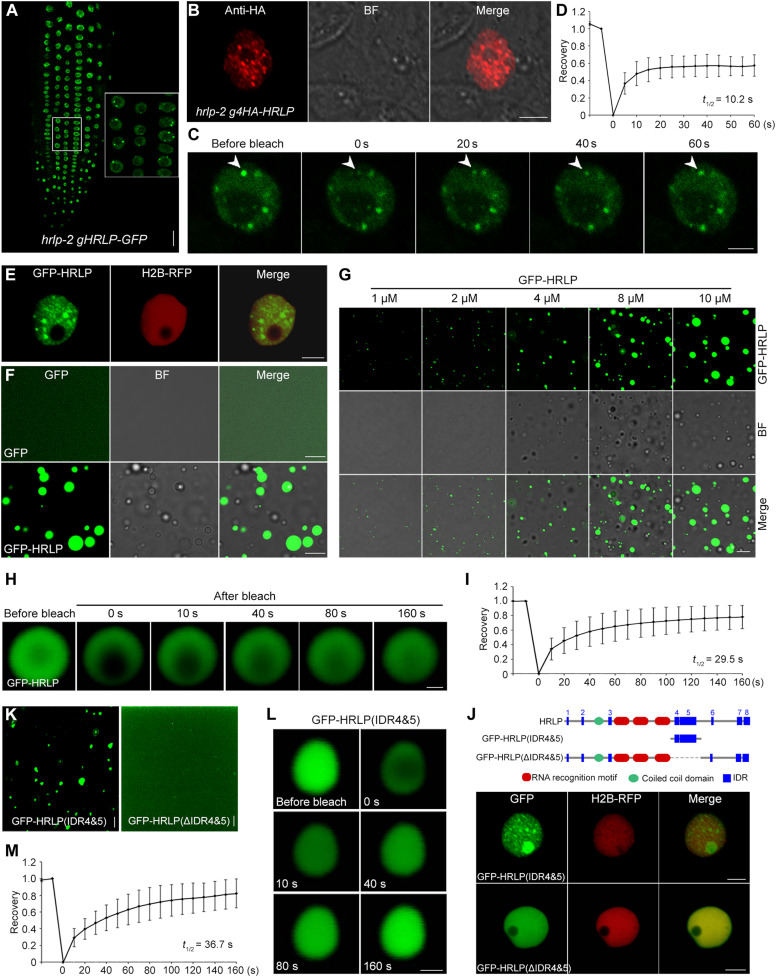
HRLP forms liquid-like nuclear condensate in vivo
To further elucidate the molecular mechanism by which HRLP modulates FLC splicing and transcription, we examined the distribution pattern of HRLP in the nucleus where HRLP functions in promoting flowering (Fig. 1F). HRLP–green fluorescent protein (GFP) was localized in multiple nuclear bodies in root tip cells of a functional hrlp-2 gHRLP-GFP line (Fig. 5A), in which the transgene bearing the HRLP genomic fragment translationally fused to the GFP reporter rescued the late-flowering phenotype of hrlp-2 (fig. S6, A and B). Consistently, 4HA-HRLP was also localized in nuclear bodies in both leaf protoplasts (Fig. 5B) and root tip cells (fig. S6C) of hrlp-2 g4HA-HRLP. To test whether HRLP nuclear condensates display liquid-like characteristics, we performed the fluorescence recovery after photobleaching (FRAP) assay on HRLP-GFP nuclear bodies in hrlp-2 gHRLP-GFP. HRLP-GFP was rapidly redistributed from the unbleached area to the bleached area (Fig. 5, C and D), indicating a liquid-like state of HRLP condensates. Moreover, in Nicotiana benthamiana leaf epidermal cells transiently expressing 35S:GFP-HRLP, GFP-HRLP was also localized in multiple nuclear bodies (Fig. 5E) and exhibited a similar liquid-like property under FRAP analysis (fig. S6, D and E). Together, these observations indicate that HRLP protein forms nuclear bodies with liquid-like properties via LLPS in vivo.
Fig. 5. HRLP undergoes LLPS both in vitro and in vivo.
(A) HRLP-GFP localization in root tip cells of 5-day-old hrlp-2 gHRLP-GFP seedlings. Inset shows a magnified view. Scale bar, 20 μm. (B) Immunolocalization of 4HA-HRLP in an hrlp-2 g4HA-HRLP mesophyll protoplast. BF, bright field. Scale bar, 5 μm. (C) FRAP assay of an HRLP-GFP nuclear body (arrowheads) in hrlp-2 gHRLP-GFP. Time in (C), (H), and (L) indicates the duration after the photobleaching pulse. Scale bar, 5 μm. (D) FRAP recovery plot of HRLP-GFP nuclear bodies in hrlp-2 gHRLP-GFP. The value at the beginning of photobleaching in (D), (I), and (M) was set as 0. The half-time of recovery (t1/2) in (D), (I), and (M) was calculated from the logarithmic curve and the best-fit values were generated by GraphPad. Error bars in (D), (I), and (M), means ± SD; n = 15. (E) Subcellular localization of GFP-HRLP in N. benthamiana leaf epidermal cells. H2B-RFP, RFP fluorescence of the nuclear reporter (core histone 2B fused to red fluorescent protein). Scale bar, 5 μm. (F) In vitro phase separation of GFP and GFP-HRLP proteins with the addition of PEG-8000. Scale bars, 10 μm. (G) Formation of droplets at the indicated concentrations of GFP-HRLP in the presence of PEG-8000 in vitro. Scale bar, 10 μm. (H) FRAP assay showing the recovery of GFP signals in a GFP-HRLP droplet. Scale bar, 1 μm. (I) FRAP recovery plot of GFP-HRLP droplets. (J) Subcellular localization of GFP-HRLP(IDR4&5) and GFP-HRLP(ΔIDR4&5) in N. benthamiana leaf epidermal cells. Scale bars, 5 μm. (K) In vitro phase separation of GFP-HRLP(IDR4&5) and GFP-HRLP(ΔIDR4&5) proteins with the addition of PEG-8000. Scale bars, 5 μm. (L) FRAP assay of a GFP-HRLP(IDR4&5) droplet. Scale bar, 1 μm. (M) FRAP recovery plot of GFP-HRLP(IDR4&5) droplets.
To investigate whether HRLP itself undergoes phase separation in vitro, we produced the recombinant GST-GFP-HRLP protein, from which the GST-tag was cleaved to generate GFP-HRLP. In the presence of PEG-8000 (polyethylene glycol, molecular weight 8000) as a crowding agent, GFP-HRLP, but not GFP, formed spherical droplets whose size gradually increased with increasing concentrations of GFP-HRLP proteins in a dosage-dependent manner (Fig. 5, F and G). FRAP assay further revealed that GFP-HRLP signals recovered shortly after photobleaching (Fig. 5, H and I), demonstrating that GFP-HRLP diffuses freely within the droplets. These results suggest that HRLP protein undergoes phase separation in vitro.
RBP condensate formation is often mediated by the IDRs containing high prevalence of glycine (G), arginine (R), lysine (K), or serine (S) residues (23, 25, 35–37). As predicted by SMART (fig. S6F), HRLP contains eight IDRs (IDR1 to IDR8), also called the LCRs, and the IDR(7&8) in the C-terminal region was recognized as a prion-like domain by PLACC (38). To identify the region(s) of HRLP conferring its phase separation feature, we first separated HRLP into two parts, the C-terminal part (HRLP-C) containing IDR(7&8) and the rest of the N-terminal part (HRLP-N; fig. S6G). Only GFP-HRLP-N formed nuclear bodies in N. benthamiana leaf epidermal cells (fig. S6H), indicating that the N-terminal part of HRLP is required for the phase separation of HRLP. We then divided HRLP-N into three regions, including HRLP(1-213) containing IDR (1-3), HRLP(214-473) containing the triple RRMs, and HRLP(474-727) containing IDR (4-6), among which only GFP-HRLP(474-727) was localized in nuclear bodies (fig. S6, G and H). Consistently, only GFP-HRLP(474-727) formed droplets in the presence of PEG-8000 in vitro (fig. S6I). We further dissected HRLP(474-727) and found that only GFP-HRLP(474-601) containing IDR(4&5) formed nuclear bodies, whereas neither IDR4 nor IDR5 itself was sufficient to drive nuclear body formation (fig. S6, G and H). These observations indicate that IDR(4&5) is required for phase separation of HRLP. As expected, the truncated HRLP without these two IDRs, GFP-HRLP(ΔIDR4&5), was expressed uniformly in the nucleus (Fig. 5J). Moreover, only the recombinant protein GFP-IDR(4&5), but not GFP-HRLP(ΔIDR4&5), formed droplets in vitro in the presence of PEG-8000 (Fig. 5K). FRAP assay subsequently detected a quick redistribution of GFP-IDR(4&5) from the unbleached area to the beached area after photobleaching (Fig. 5, L and M), suggesting that IDR(4&5) is sufficient for mediating phase separation. Together, these results demonstrate that IDR(4&5) is essential for the phase separation property of HRLP.
Phase separation of HRLP promotes flowering
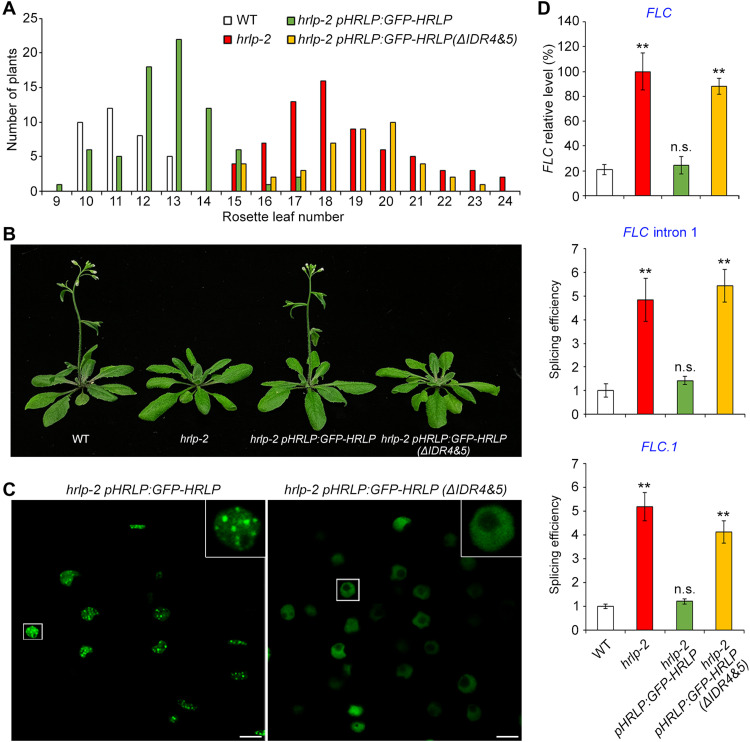
To assess the biological consequence of the phase separation of HRLP, we transformed hrlp-2 with GFP-HRLP or GFP-HRLP(ΔIDR4&5) cDNA driven by the HRLP promoter. In the T1 generation, most of the hrlp-2 pHRLP:GFP-HRLP lines exhibited comparable flowering time to wild-type plants, whereas all hrlp-2 pHRLP:GFP-HRLP(ΔIDR4&5) lines exhibited late flowering like hrlp-2 (Fig. 6A). This demonstrates that deletion of IDR(4&5) compromises HRLP’s role in promoting flowering. We then selected representative pHRLP:GFP-HRLP and pHRLP:GFP-HRLP(ΔIDR4&5) lines that expressed GFP-HRLP and GFP-HRLP(ΔIDR4&5) mRNAs and proteins at similar levels (Fig. 6B and fig. S7, A and B) to observe their subcellular localization. In agreement with their localization in N. benthamiana leaf epidermal cells (Fig. 5, E and J), GFP-HRLP was localized in pHRLP:GFP-HRLP nuclear bodies, whereas GFP-HRLP(ΔIDR4&5) was distributed uniformly in pHRLP:GFP-HRLP(ΔIDR4)&5) nuclei (Fig. 6C). Moreover, overexpression of GFP-HRLP or GFP-HRLP(ΔIDR4)&5) cDNA in hrlp-2 also revealed a similar link between phase separation of HRLP and its role in rescuing late flowering of hrlp-2 (fig. S7, C and D). These observations suggest that phase separation of HRLP conferred by IDR(4&5) is critical for HRLP function in promoting flowering. Next, we examined whether phase separation of HRLP regulates FLC transcript levels and splicing efficiency of FLC introns. In line with the flowering behavior, both FLC expression and splicing were restored to the wild-type level in hrlp-2 pHRLP:GFP-HRLP, but not in hrlp-2 pHRLP:GFP-HRLP(ΔIDR4&5) (Fig. 6D). Single-molecule RNA FISH (smFISH) assay using smFISH probes against the FLC intron I sequence in hrlp-2 gHRLP-GFP also revealed that HRLP condensates were colocalized with FLC nascent transcripts (fig. S7E). These observations indicate that phase separation of HRLP is essential for its function in regulating FLC splicing and transcript levels. Overexpression of GFP-HRLP(ΔIDR4&5) in wild-type plants caused a late flowering phenotype (fig. S7F), indicating that overexpressed HRLP(ΔIDR4&5) could dominantly compete against wild-type HRLP for binding to FLC, thus possibly failing to suppress FLC expression.
Fig. 6. Phase separation of HRLP promotes flowering in Arabidopsis.
(A) Flowering time distribution of T1 transgenic plants of hrlp-2 pHRLP:GFP-HRLP and hrlp-2 pHRLP:GFP-HRLP(ΔIDR4&5). (B) pHRLP:GFP-HRLP(ΔIDR4&5) is unable to rescue the late-flowering phenotype of hrlp-2. (C) Subcellular localization of GFP-HRLP and GFP-HRLP(ΔIDR4&5) in root tip cells of 5-day-old respective transgenic lines. The insets show enlarged images of the indicated nuclei in the white boxes. Scale bar, 10 μm. (D) FLC expression (top) and splicing efficiency of FLC intron I (middle) and intron 6 of FLC.1 (bottom) in 9-day-old seedlings in different genetic backgrounds. FLC expression levels normalized to TUB2 expression are shown relative to the maximal expression level set at 100%. The splicing efficiency was determined by normalizing the expression levels of the featured exon against the corresponding unspliced alternative intron of each isoform. The levels in WT plants are set as 1.0. Error bars, means ± SD; n = 3. Asterisks or n.s. indicate significant or no significant differences, respectively, between WT seedlings and the other genotypes (two-tailed paired Student’s t test, **P < 0.01; n.s., P > 0.05).
HRLP interacts with SR45 to modulate splicing and transcription of FLC
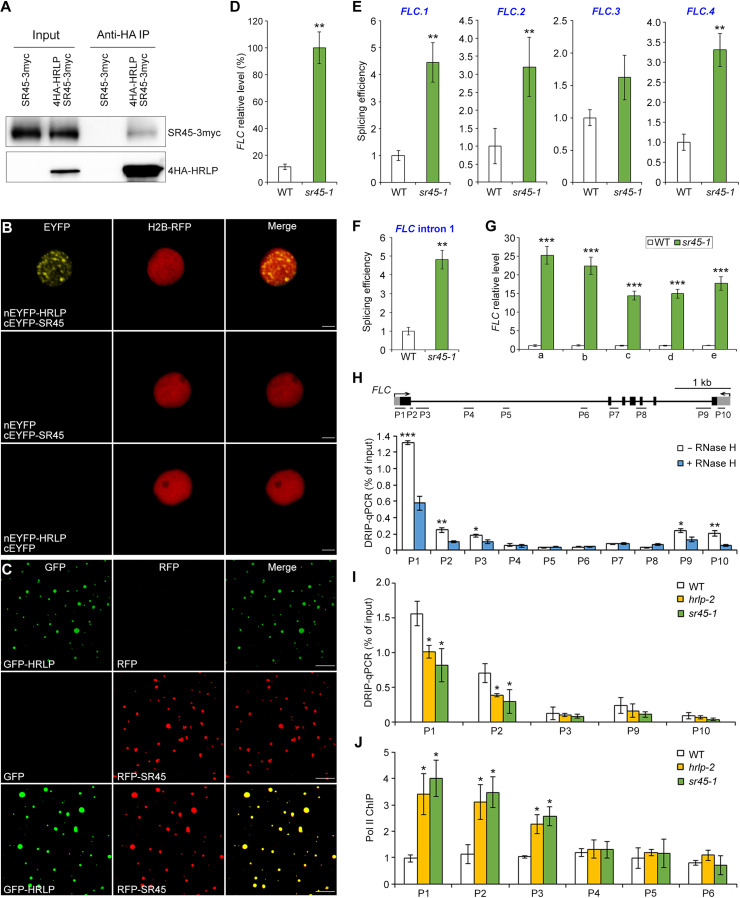
To uncover potential interacting partners in HRLP condensates, we performed coimmunoprecipitation (CoIP) assay followed by liquid chromatography–tandem mass spectrometry (LC-MS/MS) with hrlp-2 g4HA-HRLP. Notably, HRLP condensates were enriched with splicing factors, including SR45 (table S1), which is a plant-specific splicing factor involved in regulating FLC expression (39). We found that GFP-HRLP and SR45–red fluorescent protein (RFP) were colocalized in nuclear bodies in both Arabidopsis protoplasts and N. benthamiana leaf epidermal cells (fig. S8, A and B). CoIP assays in N. benthamiana confirmed the protein interaction between HRLP1 and SR45 in vivo (fig. S8C). We generated sr45-1 gSR45-3myc transgenic plants, in which gSR45-3myc fully rescued the late-flowering phenotype of sr45-1 (fig. S8D), and confirmed the interaction between HRLP and SR45 in Arabidopsis F1 seedlings from crosses between sr45-1 gSR45-3myc and hrlp-2 g4HA-HRLP (Fig. 7A). Bimolecular fluorescence complementation (BiFC) assays further revealed a direct interaction between HRLP and SR45 mainly in nuclear bodies (Fig. 7B). In addition, we found that SR45-RFP was incorporated into GFP-HRLP droplets (Fig. 7C and fig. S8, E and F), indicating that they may function together via phase separation. These results suggest that HRLP interacts with SR45 in nuclear bodies.
Fig. 7. HRLP, together with SR45, regulates R-loop levels of FLC.
(A) CoIP shows the interaction between HRLP and SR45 in F1 seedlings from crosses between sr45-1 gSR45-3myc and hrlp-2 g4HA-HRLP. Proteins were detected by anti-myc (top) or anti-HA (bottom) antibodies. (B) BiFC analysis of the interaction between HRLP and SR45. Scale bars, 10 μm. (C) In vitro phase separation of 10 μM GFP-HRLP and RFP-SR45 proteins with the addition of PEG-8000. Scale bars, 10 μm. (D) Expression of FLC normalized to TUB2 in 9-day-old WT and sr45-1 seedlings. (E and F) Splicing efficiency of intron 6 of FLC.1, FLC.2 and FLC.3, and intron 5 of FLC.4 (E) and FLC intron I (F) in 9-day-old WT and sr45-1 seedlings. Primer positions are indicated in Fig. 3G. (G) Measurement of nascent FLC levels in 9-day-old WT and sr45-1 seedlings. Levels of gene expression normalized to EF1A expression are shown relative to WT expression levels set as 1.0. The positions of primers are shown in Fig. 3H. (H) DRIP analysis of R-loop formation at the FLC locus in 9-day-old WT seedlings with (+) and without (−) RNase H treatment. The gene structure of FLC and positions of primers used are shown in the top panel. (I) DRIP analysis of R-loop at the FLC locus in 9-day-old WT, hrlp-2 and sr45-1 seedlings. (J) Chromatin immunoprecipitation (ChIP) analysis of RNA Pol II enrichment at the FLC locus in 9-day-old WT, hrlp-2, and sr45-1 seedlings. Error bars (D to J), means ± SD; n = 3. Asterisks (D to J) indicate significant differences between WT and mutants or between untreated and treated seedlings (two-tailed paired Student’s t test, *P < 0.05, **P < 0.01, and ***P < 0.001).
Similar to hrlp mutants, sr45-1 exhibited a late-flowering phenotype (fig. S8, D and G) as previously reported (39). In sr45-1 mutants, FLC transcript levels were up-regulated during the floral transition, whereas the expression of FT and SOC1 was down-regulated (Fig. 7D and fig. S8H). We also found that the splicing efficiency of FLC intron I and the featured introns of the three FLC isoforms (FLC.1,2,4) was significantly higher in sr45-1 than wild-type plants (Fig. 7, E and F). Moreover, nascent FLC levels were also considerably increased in sr45-1 compared with wild-type plants (Fig. 7G). These effects of SR45 on FLC are mostly similar to those displayed by HRLP (Fig. 3), supporting the idea that HRLP interacts with SR45 to regulate FLC splicing and transcription. We further generated sr45-1 hrlp-2 double mutants and found that sr45-1 hrlp-2 double mutants flowered only slightly later than their single mutants (fig. S8I), supporting the notion that HRLP and SR45 function in a protein complex in regulating flowering.
Phase separation of HRLP affects R-loop formation and RNA Pol II recruitment at FLC
Because the formation of DNA:RNA hybrids (R-loops) has been reported to affect gene expression, including the transcription at the FLC locus (40–43), we further tested whether the effects of HRLP and SR45 on FLC are relevant to cotranscriptional R-loop formation. DNA-RNA hybrid immunoprecipitation (DRIP) assay on 9-day-old seedlings revealed potential R-loop formation in the region spanning the first exon and the first intron (P1 to P3) as well as the region near the transcription termination site (P9 and P10) (Fig. 7H), which largely overlap with the regions reported in previous studies (42–44). Further R-loop foot-printing assay confirmed the R-loop formation in the first exon and intron of FLC (fig. S8J). Notably, high levels of R-loops in the region near intron I (P1 and P2) were significantly reduced in both hrlp-2 and sr45-1 mutants (Fig. 7I), but not in FRI FLC (fig. S8K) where FLC is highly expressed, but its splicing efficiency is not altered (45). To test whether this reduction of R-loops is correlated to RNA Pol II function as implicated in a previous study (41), we compared Pol II occupation at the FLC locus in hrlp-2 and sr45-1 mutants versus wild-type plants through chromatin immunoprecipitation (ChIP) assay using an antibody recognizing the C-terminal domain (CTD) of Pol II (anti-Pol II CTD). RNA Pol II enrichment at the region near intron I (P1 to P3) was increased in both hrlp-2 and sr45-1 versus wild-type plants, indicating that recruitment of RNA Pol II at the FLC locus is enhanced in hrlp-2 and sr45-1. Together, these results suggest that HRLP and SR45 inhibit splicing and transcription of FLC, which is associated with R-loop formation and impediment of RNA Pol II recruitment in the region near intron I.
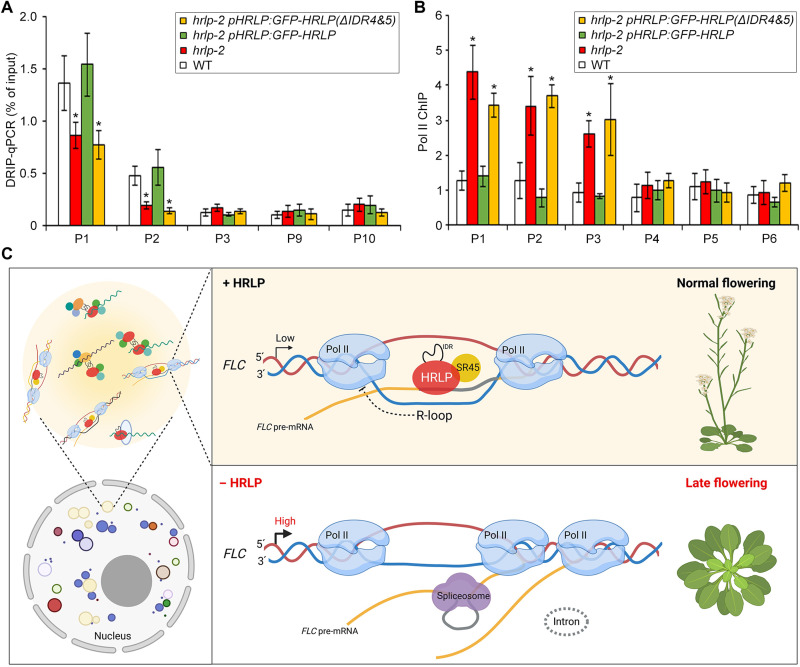
To assess whether phase separation of HRLP affects R-loop formation and RNA Pol II recruitment at the FLC locus, we examined R-loop formation at FLC in 9-day-old hrlp-2 pHRLP:GFP-HRLP and hrlp-2 pHRLP:GFP-HRLP(ΔIDR4&5) seedlings. DRIP assay showed high levels of R-loops in the region near intron I (P1 and P2) in both wild-type and hrlp-2 pHRLP:GFP-HRLP seedlings, whereas R-loop formation in the same region was significantly reduced in hrlp-2 and hrlp-2 pHRLP:GFP-HRLP(ΔIDR4&5) (Fig. 8A). ChIP assay further revealed that RNA Pol II enrichment in the region near intron I (P1 to P3) was increased in hrlp-2 and hrlp-2 pHRLP:GFP-HRLP(ΔIDR4&5) compared with wild-type and hrlp-2 pHRLP:GFP-HRLP seedlings (Fig. 8B). These findings, together with the observations on the effect of phase separation of HRLP on FLC splicing and transcript levels (Fig. 6D), suggest that phase separation of HRLP mediated by IDR(4&5) is crucial for its regulation of FLC mRNA levels.
Fig. 8. Phase separation of HRLP affects R-loop formation and RNA Pol II recruitment at the FLC locus.
(A) DRIP analysis of R-loop at the FLC locus in 9-day-old seedlings in various genetic backgrounds. (B) ChIP analysis of the enrichment of RNA Pol II over the genomic region of FLC in 9-day-old seedlings in various genetic backgrounds. In (A) and (B), error bars, means ± SD; n = 3. Asterisks indicate significant differences between WT plants and other specified genotypes (two-tailed paired Student’s t test, *P < 0.05). (C) A model depicting regulation of the cotranscriptional splicing of FLC by HRLP via phase separation. HRLP, together with SR45, is recruited to the region near intron I of the nascent FLC RNA by forming nuclear bodies to inhibit the splicing process, which enhances R-loop formation and compromises Pol II recruitment near intron I, thus suppressing FLC transcription. Loss of HRLP or failure in forming HRLP nuclear bodies enables cotranscriptional splicing of FLC introns. This compromises R-loop formation and facilitates recruitment of Pol II near intron I of FLC, resulting in higher FLC mRNA levels and the late-flowering phenotype. Created with BioRender.com.
DISCUSSION
Throughout the life cycle of mRNA molecules, they are associated and modulated by diverse RBPs that influence all aspects of RNA metabolism, including RNA synthesis, splicing, modification, transport, translation, and degradation (1, 46). Recent studies have suggested that a complex and complicated transcriptome control involving a large number of RBPs and their associated proteins possibly facilitates plants to evolve with more flexible and resilient strategies to cope with an ever-changing environment (10, 14, 47). Although, by now, more than 800 RBPs have been identified in Arabidopsis (14), their biological functions are largely unknown. In this study, we have shown that the plant hnRNP R-like RBP, HRLP, acts with the splicing factor SR45 to promote flowering through inhibiting the cotranscriptional splicing of the key flowering repressor FLC, which is associated with increased R-loop formation near its intron I to repress its transcription (Fig. 8C). This promotive effect of HRLP on flowering requires a liquid-like property conferred by its IDRs. Thus, HRLP condensate–mediated regulation of FLC splicing and transcription constitutes a hitherto unknown regulatory module that down-regulates FLC transcript levels to facilitate flowering under favorable conditions.
Our findings establish HRLP as a previously uncharacterized flowering promoter in modulating FLC mRNA levels. First, hrlp mutants exhibit a daylength-insensitive late-flowering phenotype under both LDs and SDs. HRLP expression is not obviously affected by most flowering genetic pathways tested, but positively regulated by vernalization in the presence of high FLC levels. Second, FLC transcripts are greatly increased in hrlp mutants, while flc-3 almost completely suppresses the late-flowering phenotype of hrlp, suggesting that FLC is the major target of HRLP during the floral transition. Third, HRLP significantly reduces the splicing efficiency and transcription of FLC pre-mRNA via direct binding to its exon I and intron I. Moreover, HRLP interacts with the splicing factor SR45, which also down-regulates the splicing efficiency and transcription of FLC to promote flowering. In addition, because there are two other HRLP homolog proteins in the Arabidopsis genome, it would be interesting to investigate whether HRLP homologs also function in modulating FLC transcript levels in flowering time control.
Mounting evidence suggests that the processes of transcription and splicing are tightly coupled and mutually influence each other (48, 49). For example, transcription elongation rate regulates alternative splicing, which, in turn, can also affect transcription (50–54). FLC pre-mRNA undergoes cotranscriptional splicing, as evident by the single molecular FISH analysis showing the colocalization of nonspliced FLC pre-mRNA with FLC DNA (22). Loss of HRLP or SR45 leads to enhanced splicing efficiency and transcription of FLC pre-mRNA, which is coupled with reduced R-loop formation near FLC intron I, suggesting that HRLP-mediated inhibition of cotranscriptional splicing of FLC may alter the local chromatin context, thereby regulating FLC transcription. We have observed increased Pol II occupancy in hrlp or sr45 mutants, particularly in the region near intron I where R-loop formation is reduced. Two ALBA family members, ALBA4 and ALBA5, whose homologs are R-loop readers acting to maintain genome stability (55), have been identified as the HRLP-associated proteins (table S1), implying that HRLP may coordinate molecular events pertaining to R-loop formation and its subsequent biological interpretation. Thus, our findings suggest a previously unidentified regulatory paradigm in which low splicing efficiency of FLC is associated with the formation of R-loop involving introns, thus impeding Pol II recruitment to repress FLC expression. Given the widespread cotranscriptionally splicing and R-loop structures in Arabidopsis (44, 56, 57), it would be intriguing to examine whether this regulatory paradigm represents a general mechanism in the whole transcriptome.
Notably, phase separation of HRLP is required for its function in suppressing splicing and transcription of FLC and the associated R-loop formation and impediment of RNA Pol II recruitment. This exemplifies that phase separation-mediated formation of nonmembrane cellular compartments could facilitate functional coupling of transcription and RNA processing (58). In agreement with the observations that the phase separation of RBPs is often driven by IDRs capable of weak multivalent interactions (25, 35, 36), our results demonstrate that HRLP undergoes phase separation to form nuclear condensates in vitro and in vivo through its IDR(4&5). Removal of IDR(4&5) compromises the formation of HRLP condensates, resulting in successive molecular and phenotypic changes as those displayed by hrlp loss-of-function mutants, including increased splicing efficiency, reduced R-loop formation, enhanced RNA Pol II recruitment, and, consequently, late flowering. These causal links suggest that phase separation of HRLP is fundamental for its cellular function in suppressing FLC expression. HRLP condensates may serve as biological scaffolds that accommodate other regulatory components, including the splicing factor SR45 to enable efficient coupling of transcription and splicing of their downstream targets, such as the FLC pre-mRNA, and possibly other interacting partners to control various RNA processing steps in addition to splicing (fig. S8, L and M). While our study correlates the IDR-driven phase separation of HRLP with its function in mediating cotranscriptional splicing of FLC, further characterization of RNA binding proteome (47, 59), including those associated with HRLP and FLC, will provide important insights into the mechanisms underlying the response of cellular RNA interactome to environmental and developmental signals.
MATERIALS AND METHODS
Plant materials and growth conditions
Arabidopsis plants were grown on Murashige and Skoog (MS) plates or soil under LDs (16-hour light/8-hour dark) or SDs (8-hour light/16-hour dark) at 23° ± 2°C, 16° ± 1°C, or 28° ± 2°C. The mutant seeds of hrlp-1 (SALK_124411) and sr45-1 (SALK_004132) in the Col background were obtained from the Arabidopsis Biological Resource Center (ABRC), and hrlp-2 was generated by CRISPR-Cas9. flc-3, ft-10, co-9, gi-1, fca-2, fpa-7, ld-1, fve-4, flk-2, and fld-3 mutants are in the Col background. Double mutants of flc-3 hrlp-1, flc-3 hrlp-2, and sr45-1 hrlp-2 were generated by genetic crossing.
Plasmid construction
To construct g4HA-HRLP, gHRLP-GFP, and gHRLP-GUS, the 5.0-kb HRLP genomic sequence was amplified and cloned into pENTR/D-TOPO (Invitrogen, catalog no. K240020) to generate gHRLP. The Spe I restriction site was introduced to gHRLP immediately after the start codon or before the stop codon by the QuikChange Site-Directed Mutagenesis Kit (Stratagene) to generate g(SpeI)HRLP or gHRLP(SpeI), respectively. On the basis of these constructs, g4HA-HRLP, gNLS-4HA-HRLP, gNES-4HA-HRLP, gHRLP-GFP, and gHRLP-GUS were generated via cloning the corresponding sequences into the Spe I site. To construct 35S:GFP-HRLP and 35S:GFP-HRLP (truncated forms), the full-length coding sequence and various truncated sequences of HRLP were amplified and cloned into pGreen 0299 35S-GFP (60). To construct GST-HRLP or GST-HRLP (truncated forms), the full-length coding sequence and various truncated sequences of HRLP were amplified and cloned into pGEX-6p-2. To construct 35S:SR45-RFP, the coding sequence of SR45 was amplified and cloned into pGreen 0229-35S-RFP. The coding sequence of SR45 was amplified and cloned into pENTR vector (61) harboring 9myc to generate 35S:SR45-9myc. To construct gSR45-3myc, the 4.3-kb SR45 genomic sequence including 1.5-kb upstream region and 2.8-kb coding region plus introns was amplified and cloned into pENTR vector harboring 3myc. The primers used for plasmid construction are listed in table S2.
Plant transformation
Transgenic plants were generated through the floral dipping method (62) using the Agrobacterium strain GV3101 harboring the desired constructs. g4HA-HRLP, gNLS-4HA-HRLP, gNES-4HA-HRLP, gHRLP-GFP, pHRLP:GFP-HRLP, pHRLP:GFP-HRLP(ΔIDR4&5), 35S:GFP-HRLP, and 35S:GFP-HRLP(ΔIDR4&5) were transformed into hrlp-2 mutants, while gHRLP-GUS was transformed into wild-type (Col) plants. gSR45-3myc was transformed into sr45-1 mutants. These transgenic plants were all selected by Basta.
Expression analysis
Total RNA was extracted from approximately 15 seedlings for each sample using the FavorPrep Plant Total RNA Mini Kit (Favorgen) and reverse-transcribed using the M-MLV Reverse Transcriptase System (Promega). qRT-PCR was performed with SYBR Green PCR Master Mix (Applied Biosystems, catalog no. A25742) on the 7900HT Fast Real-Time PCR system (Applied Biosystems). Gene expression was calculated as previously described (61). Primers used for gene expression analysis are listed in table S2. GUS staining was performed as previously described (63).
In vitro RNA pull-down assay
The coding sequence of the triple RRM domain of HRLP was cloned into pGEX-6p-2 (Pharmacia). GST and GST-HRLP-RRM were expressed in Escherichia coli Rosetta (DE3) cells by induction with isopropyl-β-d-1-thiogalactopyranoside at 16°C overnight and purified with Glutathione Sepharose (GE Healthcare, catalog no. GE17-0756-05). The biotin-labeled FLC RNA probe was in vitro transcribed using T7 RNA polymerase (Roche, catalog no. 10881767001) and Biotin RNA Labelling Mix (Roche, catalog no. 11685597910) and prefolded in RNA structure buffer [10 mM MgCl2, 0.1 M KCl, and 10 mM tris-HCl (pH 7.0)]. The purified GST or GST-HRLP-RRM protein was incubated with the biotin-labeled FLC RNA probe for 1 hour, followed by incubation with Streptavidin Magnetic Beads (Roche, catalog no. 11641786001) overnight. The beads were extensively washed four times with 1× phosphate-buffered saline (PBS) buffer and boiled for 10 min, and the eluate was analyzed by Western blot using anti-GST antibody [Santa Cruz Biotechnology, sc-138 horseradish peroxidase (HRP), RRID:AB_627677].
Systematic evolution of ligands by exponential enrichment
SELEX was performed as previously described with some modifications (21, 64). The resulting matrix was in vitro transcribed into RNA population with T3 RNA polymerase (Promega, catalog no. P208C). After precipitation with ethanol, the RNA was dissolved in the binding buffer [20 mM Hepes (pH 7.9), 150 mM KCl, 1 mM dithiothreitol (DTT), tRNA (500 ng/ml), and 0.1% Triton X-100] and heated at 80°C for 5 min, followed by reducing the temperature to 22°C to allow the formation of the secondary structure. The resulting RNA and Recombinant RNasin Ribonuclease Inhibitor (Promega, catalog no. N2515) were incubated with GST or GST-HRLP bound to GST beads. The beads were then washed and treated with Proteinase K (Thermo Fisher Scientific, catalog no. 25530049) to release the bound RNA for further purification. The purified RNA was reverse-transcribed and amplified by PCR. The products from different amplification cycles were examined, and those from the minimum cycle showing a visible band were purified for subsequent selections. After seven rounds of selection, the final PCR products were ligated into pGEM-T vector (Promega, catalog no. A1360) and sequenced. Sequences were analyzed by MEME (http:/meme.nbcr.net/meme/). The primers used in SELEX are listed in table S2.
Electrophoretic mobility shift assay
Biotin-labeled RNA (5′-Bio-YUCCUYCAYUCCUYCAYUCCUYCAYUCCUYCA-3′) were incubated with GST or GST-HRLP protein in the binding buffer [10 mM Hepes (pH 7.3), 1 mM MgCl2, 20 mM KCl, and 1 mM DTT] for 1 hour. For competition groups, the binding reaction occurred in the presence of different concentrations of unlabeled RNA probes. The mixture was separated on a 5% native polyacrylamide gel and transferred to the nylon membrane (GE Healthcare). After ultraviolet cross-linking, the membrane was detected with HRP-conjugated streptavidin (Thermo Fisher Scientific, catalog no. N100).
Measurement of splicing efficiency
Total RNA extracted from seedlings were reverse-transcribed with a mixture of specified primers to obtain different forms of spliced and unspliced FLC isoforms. The ratios of spliced/unspliced of each isoform were calculated by dividing the value of spliced levels against that of the corresponding unspliced levels. The primers used are listed in table S2.
Isolation of chromatin-bound RNA
Chromatin-bound RNA was isolated as previously described with some modifications (65). Around 300 individual seedlings were ground into powder and resuspended in Honda buffer [0.44 M sucrose, 10 mM MgCl2, 20 mM Hepes, 1.25% (w/v) Ficoll, 2.5% (w/v) Dextran, 0.5% Triton X-100, 5 mM DTT, 1× proteinase inhibitor, ribonuclease (RNase) inhibitor (20 U/ml), and tRNA (50 ng/μl)]. The mixture was filtered with two layers of Miracloth followed by centrifugation. The pellet was resuspended in the resuspension buffer [25 mM tris-HCl (pH 7.5), 50% glycerol, 100 mM NaCl, 1 mM DTT, and 0.5 mM EDTA] and washed twice with the UREA wash buffer [0.5 mM EDTA, 25 mM tris-HCl (pH 7.5), 1% Tween 20, 1 mM DTT, 300 mM NaCl, and 1 M urea]. After eliminating DNA by deoxyribonuclease (DNase) I treatment, the chromatin-bound RNA was further purified for reverse transcription. The primers used for detecting nascent FLC expression are listed in table S2.
RNA-seq and bioinformatics analysis
The aerial parts of 9-day-old wild-type and hrlp-2 seedlings grown under LDs were harvested for total RNA extraction using the RNeasy Plus Mini kit (Qiagen) according to the manufacturer’s instructions. The amount and quality of RNA were examined by gel electrophoresis and an Agilent Bioanalyzer 2100 system. rRNA was removed by a Ribo-Zero rRNA Removal kit (Eipcentre). The NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (NEB) was used to construct the library, and sequencing was performed on the Illumina Novaseq 6000 platform. RNA-seq data were analyzed using Partek Flow (Partek). Briefly, paired-end raw reads were trimmed by removing reads with PHRED scores below 20 or with length shorter than 25 nt. Trimmed data were aligned to the reference genome TAIR10 using STAR 2.7.3a with default parameters. Filtered gene counts were normalized to the counts per million values. Differential gene expression analysis was performed using the Partek Gene Specific Analysis algorithm.
RNA immunoprecipitation
RIP was carried out as previously described with some modifications (66). Around 300 individual seedlings were ground into powder and fixed with 1% formaldehyde for 10 min. The pellet was lysed in the lysis buffer [50 mM tris-HCl (pH 7.5), 150 mM NaCl, 4 mM MgCl2, 0.25% Igepal CA-630, 1% SDS, 0.25% sodium deoxycholate, and 5 mM DTT] supplemented with RNase Inhibitor and Complete EDTA-free Protease Inhibitor Cocktail (Roche, catalog no. 5056489001). After preclearing with Protein A/G Plus Agarose (Santa Cruz Biotechnology, catalog no. sc-2003), the cell lysate was treated with Turbo DNase (Thermo Fisher Scientific, catalog no. AM2239) and RNase T1 (Thermo Fisher Scientific, catalog no. EN0541) for 15 min followed by incubation with anti-hemagglutinin (HA) agarose conjugate (Sigma-Aldrich, catalog no. A2095, RRID:AB_257974) for 3 hours at 4°C. RNA in the input and associated with beads was extracted using the RNeasy Plus Mini Kit (Qiagen) and reverse-transcribed using random hexamers (Invitrogen, catalog no. 51709). TUB2 was included as an internal control. The primers used in RIP assay are listed in table S2.
Chromatin immunoprecipitation
ChIP assay was performed as previously described (67). The chromatin extracted from around 300 individual seedlings was fixed and sonicated to produce DNA fragment at ~500 bp, followed by detection with anti-HA agarose conjugate (Sigma-Aldrich) or anti-RNA Pol II CTD antibody (Abcam, ab26721, RRID:AB_777726) bound to Protein A/G Plus Agarose Beads (Santa Cruz Biotechnology, catalog no. sc-2003). Fold enrichment of each fragment was determined by qRT-PCR, and a genomic fragment of TUB2 was included as an internal control. The primers used in ChIP assay are listed in table S2.
DNA-RNA immunoprecipitation
The nuclei from around 300 seedlings were isolated with the Honda buffer and treated with proteinase K in the lysis buffer [50 mM tris-HCl (pH 8.0), 10 mM EDTA, and 1% SDS] overnight. After purification, the DNA was treated with proteinase K for another 2 hours, followed by extraction and precipitation. The DNA pellet was dissolved in the lysis buffer and sonicated into fragments of ~500 bp. Purified DNA was immunoprecipitated with the S9.6 antibody (Kerafast, catalog no. ENH001, RRID:AB_2687463) bound to Protein A/G Plus Agarose Beads (Santa Cruz Biotechnology, catalog no. sc-2003) at 4°C overnight. The beads were subsequently washed thrice with wash buffer I [75 mM KCl, 50 mM tris (pH 8.0), 1% Triton X-100, and 0.1% sodium deoxycholate], once with wash buffer II [300 mM KCl, 50 mM tris (pH 8), 1% Triton X-100, and 0.1% sodium deoxycholate], and twice with wash buffer III [10 mM tris (pH 8) and 1 mM EDTA (pH 8)]. The DNA-RNA hybrid was eluted and purified with the PCR Purification Kit (Qiagen). For RNase H–treated samples, the sonicated DNA sample was treated with RNase H (NEB, catalog no. M0297S) at 37°C for 3 hours.
R-loop foot-printing
R-loop foot-printing was performed as previously described (42). DNA was purified from around 300 9-day-old wild-type seedlings as described for the DRIP assay. After purification, 1.5 μg of DNA was treated with the bisulfite solution (EpiTect Bisulfite Kit, Qiagen) for at least 12 hours at 37°C. The modified DNA was amplified with primers Footp-C-F and Footp-R, and the resulting PCR products were ligated into the pGEM-T vector (Promega, catalog no. A1360). Around 15 individual clones were sequenced, and the sequencing data were aligned to the FLC genomic sequence. The primers are listed in table S2.
CoIP coupled with LC-MS/MS analysis
Nuclear protein was isolated from seedlings using the nuclear isolation buffer [20 mM KCl, 25% glycerol, 20 mM tris (pH 7.0), 30 mM β-mercaptoethanol, 2.5 mM MgCl2, 0.7% Triton X-100, 2 mM EDTA (pH 8.0), and 250 mM sucrose] with freshly added 1× protease inhibitor cocktail (Roche), and resuspended in IP buffer [50 mM Hepes (pH 7.5), 150 mM KCl, 10 μM ZnSO4, 0.05% SDS, 5 mM MgCl2, 1% Triton X-100, and 1× protease inhibitor cocktail], followed by incubation with anti-HA agarose conjugate (Sigma-Aldrich) for 4 hours at 4°C. After extensive washing, the immunoprecipitated proteins were eluted and analyzed by a TripleTOF5600 System (AB Sciex).
Immunoblotting
Various proteins were resolved by SDS–polyacrylamide gel electrophoresis and detected using specific antibodies, including anti-HA (Santa Cruz Biotechnology, catalog no. sc-7392 HRP, RRID:AB_2894930), anti-myc (Santa Cruz Biotechnology, catalog no. sc-40, RRID:AB_627268), anti-GFP antibody (Santa Cruz Biotechnology, catalog no. sc-9996 HRP, RRID:AB_627695), and anti-H3 (Abcam, catalog no. ab1791, RRID:AB_302613) antibodies.
In vitro phase separation assay
Various GST-tagged recombinant proteins, including GST-GFP, GST-GFP-HRLP, GST-GFP-HRLP(IDR4&5), GST-GFP-HRLP(ΔIDR4&5), and GST-RFP-SR45, were expressed in E. coli and purified with the Glutathione Sepharose beads (Amersham Bioscience). GST-free GFP fusion proteins were generated by PreScission Protease (GE Healthcare, catalog no. 27-0843-01) cleavage. Droplet assembly was performed with an addition of PEG-8000 (Sigma-Aldrich, catalog no. 25322-68-3) at a final concentration of 10% (w/v). The fluorescence was observed under an FV3000 Olympus confocal microscope.
FRAP assay
FRAP was performed using a 40× oil immersion objective of the FV3000 Olympus confocal microscope. After nuclear bodies were bleached using the 488-nm laser, recovery was recorded every 5 or 10 s. Recovery curves were analyzed by the FV31S-SW software.
Immunolocalization
Immunolocalization of 4HA-HRLP was performed as previously described (68, 69). Protoplasts were isolated from 2-week-old hrlp-2 g4HA-HRLP and hrlp-2 leaves, while roots were collected from 3-day-old hrlp-2 g4HA-HRLP and hrlp-2 seedlings. Anti-HA (Santa Cruz Biotechnology, catalog no. sc-7392, RRID:AB_627809) and CF555 goat anti-mouse immunoglobulin G (IgG; Biotium, Cat# 20231, RRID:AB_10854844) antibodies were used as primary and secondary antibodies, respectively. The samples were examined with an FV3000 Olympus confocal microscope.
Single-molecule RNA FISH
smFISH was performed as previously described (70) on root tips of 5-day-old hrlp-2 gHRLP-GFP seedlings using Quasar570-labeled probes against FLC intron I (71) (Biosearch Technologies). The samples were examined under an FV3000 Olympus confocal microscope.
Acknowledgments
We thank G. B. Chen for analyzing the RNA-seq data, and the Protein and Proteomics Centre (PPC) in the Department of Biological Sciences, National University of Singapore for the LC-MS/MS service.
Funding: This work was supported by the National Research Foundation Competitive Research Programme (NRF-CRP22-2019-0001), Singapore Food Story R&D Programme (SFS_RND_SUFP_001_04), and the intramural research support from Temasek Life Sciences Laboratory and National University of Singapore.
Author contributions: Y.Z., L.S., and H.Y. conceived this study and designed the experiments. Y.Z., S.F., C.H., Z.W.N.T., J.X.K., and L.S. performed the experiments. Y.Z., L.S., and H.Y. analyzed data and wrote the paper. All authors read and approved the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Sequencing reads are available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database (accession no. GSE200390). Request for plant materials should be submitted to H.Y. (dbsyuhao@nus.edu.sg).
Supplementary Materials
This PDF file includes:
Figs. S1 to S8
Tables S1 and S2
REFERENCES AND NOTES
- 1.Dreyfuss G., Kim V. N., Kataoka N., Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell Biol. 3, 195–205 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Geuens T., Bouhy D., Timmerman V., The hnRNP family: Insights into their role in health and disease. Hum. Genet. 135, 851–867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burd C. G., Dreyfuss G., Conserved structures and diversity of functions of RNA-binding proteins. Science 265, 615–621 (1994). [DOI] [PubMed] [Google Scholar]
- 4.Lunde B. M., Moore C., Varani G., RNA-binding proteins: Modular design for efficient function. Nat. Rev. Mol. Cell Biol. 8, 479–490 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clery A., Blatter M., Allain F. H., RNA recognition motifs: Boring? Not quite. Curr. Opin. Struct. Biol. 18, 290–298 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Valverde R., Edwards L., Regan L., Structure and function of KH domains. FEBS J. 275, 2712–2726 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Font J., Mackay J. P., Beyond DNA: Zinc finger domains as RNA-binding modules. Methods Mol. Biol. 649, 479–491 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Maris C., Dominguez C., Allain F. H., The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 272, 2118–2131 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Lorkovic Z. J., Barta A., Genome analysis: RNA recognition motif (RRM) and K homology (KH) domain RNA-binding proteins from the flowering plant Arabidopsis thaliana. Nucleic Acids Res. 30, 623–635 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prall W., Sharma B., Gregory B. D., Transcription is just the beginning of gene expression regulation: The functional significance of RNA-Binding proteins to post-transcriptional processes in plants. Plant Cell Physiol. 60, 1939–1952 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Glisovic T., Bachorik J. L., Yong J., Dreyfuss G., RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 582, 1977–1986 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Salas E., Lozano G., Fernandez-Chamorro J., Francisco-Velilla R., Galan A., Diaz R., RNA-binding proteins impacting on internal initiation of translation. Int. J. Mol. Sci. 14, 21705–21726 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao S., Hua C., Shen L., Yu H., New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 62, 118–131 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Cho H., Cho H. S., Hwang I., Emerging roles of RNA-binding proteins in plant development. Curr. Opin. Plant Biol. 51, 51–57 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Simpson G. G., Dean C., Arabidopsis, the Rosetta stone of flowering time? Science 296, 285–289 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Liu C., Thong Z., Yu H., Coming into bloom: The specification of floral meristems. Development 136, 3379–3391 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Whittaker C., Dean C., The FLC locus: A platform for discoveries in epigenetics and adaptation. Annu. Rev. Cell Dev. Biol. 33, 555–575 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Hornyik C., Terzi L. C., Simpson G. G., The spen family protein FPA controls alternative cleavage and polyadenylation of RNA. Dev. Cell 18, 203–213 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Liu F., Marquardt S., Lister C., Swiezewski S., Dean C., Targeted 3′ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science 327, 94–97 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Yan Q., Xia X., Sun Z., Fang Y., Depletion of Arabidopsis SC35 and SC35-like serine/arginine-rich proteins affects the transcription and splicing of a subset of genes. PLOS Genet. 13, e1006663 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z., Zhu D., Lin X., Miao J., Gu L., Deng X., Yang Q., Sun K., Zhu D., Cao X., Tsuge T., Dean C., Aoyama T., Gu H., Qu L. J., RNA binding proteins RZ-1B and RZ-1C play critical roles in regulating pre-mRNA splicing and gene expression during development in Arabidopsis. Plant Cell 28, 55–73 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosa S., Duncan S., Dean C., Mutually exclusive sense-antisense transcription at FLC facilitates environmentally induced gene repression. Nat. Commun. 7, 13031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiedner H. J., Giudice J., It’s not just a phase: Function and characteristics of RNA-binding proteins in phase separation. Nat. Struct. Mol. Biol. 28, 465–473 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao S. E., Regev O., Splicing at the phase-separated nuclear speckle interface: A model. Nucleic Acids Res. 49, 636–645 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y., Protter D. S., Rosen M. K., Parker R., Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberti S., Gladfelter A., Mittag T., Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176, 419–434 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powers S. K., Holehouse A. S., Korasick D. A., Schreiber K. H., Clark N. M., Jing H., Emenecker R., Han S., Tycksen E., Hwang I., Sozzani R., Jez J. M., Pappu R. V., Strader L. C., Nucleo-cytoplasmic partitioning of ARF proteins controls auxin responses in Arabidopsis thaliana. Mol. Cell 76, 177–190.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang X., Wang L., Ishikawa R., Li Y., Fiedler M., Liu F., Calder G., Rowan B., Weigel D., Li P., Dean C., Arabidopsis FLL2 promotes liquid-liquid phase separation of polyadenylation complexes. Nature 569, 265–269 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang M., Li X., Zhang J., Feng P., Pu H., Kong L., Bai Z., Rong L., Xu X., Chi W., Wang Q., Chen F., Lu C., Shen J., Zhang L., Liquid-liquid phase transition drives intra-chloroplast cargo sorting. Cell 180, 1144–1159.e20 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Jung J. H., Barbosa A. D., Hutin S., Kumita J. R., Gao M., Derwort D., Silva C. S., Lai X., Pierre E., Geng F., Kim S. B., Baek S., Zubieta C., Jaeger K. E., Wigge P. A., A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 585, 256–260 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Zavaliev R., Mohan R., Chen T., Dong X., Formation of NPR1 condensates promotes cell survival during the plant immune response. Cell 182, 1093–1108.e18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie D., Chen M., Niu J., Wang L., Li Y., Fang X., Li P., Qi Y., Phase separation of SERRATE drives dicing body assembly and promotes miRNA processing in Arabidopsis. Nat. Cell Biol. 23, 32–39 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Helliwell C. A., Wood C. C., Robertson M., James Peacock W., Dennis E. S., The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 46, 183–192 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Li P., Tao Z., Dean C., Phenotypic evolution through variation in splicing of the noncoding RNA COOLAIR. Genes Dev. 29, 696–701 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong P. A., Forman-Kay J. D., Liquid-liquid phase separation in cellular signaling systems. Curr. Opin. Struct. Biol. 41, 180–186 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Brangwynne C. P., Tompa P., Pappu R. V., Polymer physics of intracellular phase transitions. Nat. Phys. 11, 899–904 (2015). [Google Scholar]
- 37.Castello A., Fischer B., Eichelbaum K., Horos R., Beckmann B. M., Strein C., Davey N. E., Humphreys D. T., Preiss T., Steinmetz L. M., Krijgsveld J., Hentze M. W., Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Lancaster A. K., Nutter-Upham A., Lindquist S., King O. D., PLAAC: A web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics 30, 2501–2502 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali G. S., Palusa S. G., Golovkin M., Prasad J., Manley J. L., Reddy A. S., Regulation of plant developmental processes by a novel splicing factor. PLOS ONE 2, e471 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aguilera A., Garcia-Muse T., R loops: From transcription byproducts to threats to genome stability. Mol. Cell 46, 115–124 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Skourti-Stathaki K., Proudfoot N. J., A double-edged sword: R loops as threats to genome integrity and powerful regulators of gene expression. Genes Dev. 28, 1384–1396 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Q., Csorba T., Skourti-Stathaki K., Proudfoot N. J., Dean C., R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science 340, 619–621 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu C., Wu Z., Duan H. C., Fang X., Jia G., Dean C., R-loop resolution promotes co-transcriptional chromatin silencing. Nat. Commun. 12, 1790 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu W., Xu H., Li K., Fan Y., Liu Y., Yang X., Sun Q., The R-loop is a common chromatin feature of the Arabidopsis genome. Nat. Plants 3, 704–714 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Geraldo N., Baurle I., Kidou S., Hu X., Dean C., FRIGIDA delays flowering in Arabidopsis via a cotranscriptional mechanism involving direct interaction with the nuclear cap-binding complex. Plant Physiol. 150, 1611–1618 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dreyfuss G., Matunis M. J., Pinol-Roma S., Burd C. G., hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem. 62, 289–321 (1993). [DOI] [PubMed] [Google Scholar]
- 47.Koster T., Marondedze C., Meyer K., Staiger D., RNA-binding proteins revisited—The emerging Arabidopsis mRNA interactome. Trends Plant Sci. 22, 512–526 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Kornblihtt A. R., de la Mata M., Fededa J. P., Munoz M. J., Nogues G., Multiple links between transcription and splicing. RNA 10, 1489–1498 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bentley D. L., Coupling mRNA processing with transcription in time and space. Nat. Rev. Genet. 15, 163–175 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Ding Y., The simultaneous coupling of transcription and splicing in plants. Mol. Plant 13, 184–186 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Ji X., Zhou Y., Pandit S., Huang J., Li H., Lin C. Y., Xiao R., Burge C. B., Fu X. D., SR proteins collaborate with 7SK and promoter-associated nascent RNA to release paused polymerase. Cell 153, 855–868 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das R., Yu J., Zhang Z., Gygi M. P., Krainer A. R., Gygi S. P., Reed R., SR proteins function in coupling RNAP II transcription to pre-mRNA splicing. Mol. Cell 26, 867–881 (2007). [DOI] [PubMed] [Google Scholar]
- 53.de la Mata M., Kornblihtt A. R., RNA polymerase II C-terminal domain mediates regulation of alternative splicing by SRp20. Nat. Struct. Mol. Biol. 13, 973–980 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Monsalve M., Wu Z., Adelmant G., Puigserver P., Fan M., Spiegelman B. M., Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol. Cell 6, 307–316 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Yuan W., Zhou J., Tong J., Zhuo W., Wang L., Li Y., Sun Q., Qian W., ALBA protein complex reads genic R-loops to maintain genome stability in Arabidopsis. Sci. Adv. 5, eaav9040 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu D., Mao F., Tian Y., Lin X., Gu L., Gu H., Qu L. J., Wu Y., Wu Z., The features and regulation of co-transcriptional splicing in Arabidopsis. Mol. Plant 13, 278–294 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Li S., Wang Y., Zhao Y., Zhao X., Chen X., Gong Z., Global co-transcriptional splicing in Arabidopsis and the correlation with splicing regulation in mature RNAs. Mol. Plant 13, 266–277 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J., Cao Y., Ma L., Co-transcriptional RNA processing in plants: Exploring from the perspective of polyadenylation. Int. J. Mol. Sci. 22, 3300 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bach-Pages M., Homma F., Kourelis J., Kaschani F., Mohammed S., Kaiser M., van der Hoorn R. A. L., Castello A., Preston G. M., Discovering the RNA-binding proteome of plant leaves with an improved RNA interactome capture method. Biomolecules 10, 661 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen L., Liang Z., Gu X., Chen Y., Teo Z. W., Hou X., Cai W. M., Dedon P. C., Liu L., Yu H., N6-methyladenosine RNA modification regulates shoot stem cell fate in Arabidopsis. Dev. Cell 38, 186–200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y., Song S., Gan Y., Jiang L., Yu H., Shen L., SHAGGY-like kinase 12 regulates flowering through mediating CONSTANS stability in Arabidopsis. Sci. Adv. 6, eaaw0413 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clough S. J., Bent A. F., Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- 63.Shen L., Thong Z., Gong X., Shen Q., Gan Y., Yu H., The putative PRC1 RING-finger protein AtRING1A regulates flowering through repressing MADS AFFECTING FLOWERING genes in Arabidopsis. Development 141, 1303–1312 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Cavaloc Y., Bourgeois C. F., Kister L., Stévenin J., The splicing factors 9G8 and SRp20 transactivate splicing through different and specific enhancers. RNA 5, 468–483 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Z., Ietswaart R., Liu F., Yang H., Howard M., Dean C., Quantitative regulation of FLC via coordinated transcriptional initiation and elongation. Proc. Natl. Acad. Sci. U.S.A. 113, 218–223 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyer K., Koster T., Nolte C., Weinholdt C., Lewinski M., Grosse I., Staiger D., Adaptation of iCLIP to plants determines the binding landscape of the clock-regulated RNA-binding protein AtGRP7. Genome Biol. 18, 204 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen L., Kang Y. G., Liu L., Yu H., The J-domain protein J3 mediates the integration of flowering signals in Arabidopsis. Plant Cell 23, 499–514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee M. H., Lee Y., Hwang I., In vivo localization in Arabidopsis protoplasts and root tissue. Methods Mol. Biol. 1043, 113–120 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Shen L., Zhang Y., Sawettalake N., A molecular switch for FLOWERING LOCUS C activation determines flowering time in Arabidopsis. Plant Cell 34, 818–833 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duncan S., Olsson T. S. G., Hartley M., Dean C., Rosa S., Single molecule RNA FISH in Arabidopsis root cells. Bio. Protoc. 7, e2240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ietswaart R., Rosa S., Wu Z., Dean C., Howard M., Cell-size-dependent transcription of FLC and its antisense long non-coding RNA COOLAIR explain cell-to-cell expression variation. Cell Syst. 4, 622–635.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S8
Tables S1 and S2