ABSTRACT
Medication non-adherence (MNA) is a major issue in kidney transplantation and it is associated with increased risk of rejection, allograft loss, patients’ death and higher healthcare costs. Despite its crucial importance, it is still unclear what are the best strategies to diagnose, prevent and treat MNA. MNA can be intentional (deliberate refusal to take the medication as prescribed) or unintentional (non-deliberate missing the prescribed medication). Its diagnosis may rely on direct methods, aiming at measuring drug ingestions, or indirect methods that analyse the habits of patients to adhere to correct drug dose (taking adherence) and interval (time adherence). Identifying individual risk factors for MNA may provide the basis for a personalized approach to the treatment of MNA. Randomized control trials performed so far have tested a combination of strategies, such as enhancing medication adherence through the commitment of healthcare personnel involved in drug distribution, the use of electronic reminders, therapy simplification or various multidisciplinary approaches to maximize the correction of individual risk factors. Although most of these approaches reduced MNA in the short-term, the long-term effects on MNA and, more importantly, on clinical outcomes remain unclear. In this review, we provide a critical appraisal of traditional and newer methods for detecting, preventing and treating non-adherence to immunosuppression after kidney transplantation from the perspective of the practising physician.
Keywords: behaviour therapy, drug monitoring, graft rejection, immunosuppressive agents, medication adherence, organ transplantation, patient education, risk factors
INTRODUCTION
Kidney transplantation is the treatment of choice for end-stage kidney disease. However, despite advances in short-term outcomes, long-term renal allograft loss remains a significant issue [1–3]. One of the most important and often underestimated modifiable factors that strongly affects graft fate is medication non-adherence (MNA) [4]. It has been reported that MNA is responsible for nearly 20% of antibody-mediated rejections [5] and 16% of early graft losses [6]. This is a matter of concern, as rejection-induced graft loss is associated with an increased risk of sensitization, which reduces the chances of being re-transplanted [7]. Finally, rejection and the associated increased immunosuppression burden increase hospitalization rates, healthcare costs [8], and the risk of dying from cardiovascular disease and cancer [9, 10].
This phenomenon is extremely common, as up to one-third of kidney transplant recipients (KTRs) may be non-adherent to immunosuppressive medications. The rate of non-adherence may also increase with time post-transplantation. Two studies reported that every 5 years after transplant, cases of MNA increase by approximately 20% [11, 12]. Despite the crucial importance of a correct intake of immunosuppressive medications, there is little guidance on how to identify MNA and promote therapy adherence [13].
Addressing MNA in routine clinical practice is challenging because MNA is often ill-defined, the diagnosis is difficult, treatment strategies are not widely available and their efficacy on clinical outcomes is not always proven.
This review aims at critically assessing the currently available evidence on MNA diagnosis, risk factors and treatment, with particular focus on those aspects that may be useful for the practising physician (Box 1).
Box 1.
Take home messages on MNA
| MNA is associated with increased risk of rejection, allograft loss, patients’ death and higher healthcare costs. |
| The degree of MNA, which can influence the clinical outcomes and that requires a specific treatment strategy, is not defined |
| MNA risk factors are associated with patients, therapy, disease characteristics, healthcare organization, and socioeconomic and cultural characteristics. Some of these factors can be modifiable and represent the corner of treatment strategies. |
| Because MNA can appear/worsen during extremely stressful moments or anytime, it must be constantly monitored. Since risk factors can vary at any moment, different strategies may need to be adopted in the same patient. |
| Intentional MNA, which is characterized by a—usually unrecognized—deliberate refusal to comply with the prescribed therapy, is hard to diagnose and treat. These patients are hardly included in clinical trials. Constant motivational-behavioural interventions may represent the only viable resource to prevent and treat intentional MNA. |
| Unintentional MNA is characterized by non-deliberate reduced adherence to the prescribed therapy. It is easier to diagnose and to treat. Unintentional MNA diagnostic tools might occasionally be oversensitive. |
| Strategies that have been assessed for the prevention and treatment of MNA include: - the commitment of healthcare personnel involved in drug distribution (i.e. pharmacist, nurses) - the use of electronic reminders (i.e. alarmed drug container, phone alarms and Apps) - therapy simplification - multidisciplinary approaches (i.e. nurses, psychologists, medical doctors and trained therapy coaches) to maximize the correction of individual risk factors. |
| Overall, they were shown to improve MNA, but the effect generally vanished thereafter. Moreover, no trial published so far has shown any improvement in clinical outcomes. Lack of benefit may be related to failure to include MNA patients because of the ‘streetlight effect’ |
Defining MNA
Adherence implies that the medication is taken at the prescribed dose and time [14]. MNA can be quantitatively assessed with the percentage of medication intake (taking adherence) or the percentage of correct inter-dose intervals (timing adherence). However, strict adherence to prescribed medications should not necessarily be regarded as an absolute requirement for every patient [15]. Many patients often practice various forms of non-adherent behaviours, not all of them carrying the risk of jeopardizing clinical outcomes [15, 16]. Most transplant physicians would agree that minor deviations from a prescribed treatment schedule (e.g. occasionally taking tacrolimus (Tac) 1–3 later than the prescribed time) are acceptable [15]. In contrast, establishing the degree of non-adherence that impairs clinical outcomes is not an easy task [15, 17].
A useful distinction is the one between intentional and non-intentional MNA [18]. Intentional MNA represents a deliberate refusal to take the recommended medications properly. This attitude seems to involve almost 14% of the KTRs [18]. It may take place shortly after transplantation, or later over the course of follow-up [19]. Late-onset intentional MNA may follow stressful events. Intentional MNA is almost universally mis-diagnosed and does not usually respond to the standard treatment strategies.
Non-intentional MNA, which refers to a non-deliberate attitude to missing the prescribed drugs, can involve up to 62% of KTRs [18]. Among unintentional MNA, we can distinguish two further subgroups: the unintentional non-adherent patients who seek medical advice after having realized they have missed the dose. They are usually prone to follow healthcare suggestions to improve MNA. This attitude, which can be enhanced by various factors such as hectic lifestyle, low health literacy, immigration/ethnical background, is the least dangerous and the one that may benefit the most from medication reminder interventions. The other type of unintentional MNA is represented by initially unintentionally non-adherent patients whom, however, hide their mistakes. Upon not-experiencing any evident immediate adverse consequences, they eventually become intentional non-adherent patients. These patients suffer more commonly from timing rather than dose adherence. A typical setting is represented by the so-called ‘drug holidays’ [20, 21], an interval of time when a chronically medicated patient temporarily stops taking the medication. This may happen during weekends, vacations or at any unpredictable time [22]. This category of patients may be easier to treat at earlier stages, but eventually presents similar problems in identification and treatment as the genuine intentional MNA patients. One relatively common manifestation of the development of this condition, which should alert the transplant team, is the frequent missing of outpatient clinic visits (Box 2).
Box 2.
Patients’ sentences suggestive of intentional and non-intentional MNA
| Intentional MNA | Non-intentional MNA |
|---|---|
| After missing clinic visit: ‘Sorry, I forgot to come to the clinic, but I am very busy for the moment.’ | ‘Sorry, I realize that yesterday I forgot my medication, what should I do?’ |
| ‘I feel intoxicated with all these drugs.’ | ‘I wrongly took twice my medication and now I am worried.’ |
| ‘What?? Are you asking me if I am properly taking my medication? You are offending me!’ | ‘My wife is out for the weekend and I am not sure about my medication!’ |
| ‘I read that vitamins can counteract the toxic effect of immunosuppressive medication, can I take them?’ | ‘Sorry, it's a hard time, I realize that I started forgetting my pills, can you help me?’ |
Measuring MNA
Every strategy for measuring MNA has its own pros and cons and no approach can be regarded as a gold standard. Herein, we summarize the most common strategies that can be distinguished between direct and indirect strategies (Table 1).
Table 1.
Diagnostic methods to measure MNA; definition, advantages and disadvantages of direct and indirect methods for diagnosis of MNA
| Methods | Definition | Advantages | Disadvantages |
|---|---|---|---|
| Direct | |||
| Directly observed therapy | Sightly supervised drug administration by healthcare personnel or caregiver | High reliability | Expensive Time-consuming Loss of independence |
| Wireless observed therapy [23] | Ingestible sensor system embedded in pills. | High reliability | Expensive Gastrointestinal discomfort Skin reaction to ingestion detector |
| Therapeutic drug monitoring [15, 27, 24–35] | Investigate the discrepancies between expected and observed drug blood levels. | Easily available at every transplant centre | Not available for every drug Reflect a short interval of time |
| Indirect | |||
| Pill counts [36] | Healthcare personnel, caregivers and pharmacists can count pill and monitor drug refills | Inexpensive | Patients can hide pills Requires a single distribution system Time-consuming |
| Electronic monitoring [37–42] | Use of microprocessors embedded in the medication container | Do not assure drug ingestion Uncomfortable device Expensive |
|
| Self-reported questionnaire [14, 43, 44] | Questions to determine whether and how often the patients did not correctly take the prescribed medication | Easy, inexpensive and can be done during routine visits | Can underestimate intentional MNA |
Direct methods
Direct methods are aimed at directly measuring patient drug ingestion. Ideally, such methods should be easy, cheap, non-time-consuming and should not represent an excessive burden for the patients. Unfortunately, none of the available strategies fulfils all these requirements. Moreover, despite being the most efficacious strategies to identify MNA, they may not always be effective in strongly intentionally non-adherent patients.
Direct observed therapy consists of a sight view supervised drug administration by a healthcare personnel or a caregiver. This strategy, which is cost- and time-consuming, has never been tested via clinical trials. Moreover, most patients, particularly the most obstinate intentional non-adherent ones, would hardly be willing to accept such a close direct supervision.
Recently, wireless observed therapy (WOT) has been proposed to diagnose MNA. WOT is based on an ingestible sensor system, which is embedded in pills or capsules. Upon encountering gastric fluid, a signal is released that is recorded by an adhesive personal monitor (APM). This theoretically allows 100% certainty to be achieved concerning the actual number and timing of drug intake [23]. A pilot study on 20 stable adult KTRs used ingestible event marker-enteric coated mycophenolate sodium (IEM-ECMPS) [23]. This study showed that the detection rate was 99%. After 9 weeks of mean follow-up, patients did not experience any serious adverse event or acute rejection. However, eight patients prematurely discontinued treatment due to IEM-ECMPS gastrointestinal symptoms (n = 2), skin intolerance to APM (n = 2) or insufficient system usability (n = 4). Moreover, rash or erythema due to APM was reported in seven (37%) patients during the first month of use. Some patients have also reported feeling anxious with this type of constant surveillance [23]. An additional limitation of WOT is represented by its high cost. Therefore, despite the potential benefit, the applications of WOT may be limited to specific settings.
The most common method used to directly assess drug intake deploys the fact that, over the post-transplant follow-up, every solid organ transplant recipient undergoes regular therapeutic drug monitoring (TDM) of calcineurin inhibitors (cyclosporine and Tac) and/or of mammalian target of rapamycin (mTOR)-inhibitors (sirolimus and everolimus). The largest experience with TDM for assessing MNA comes from Tac. The presence of MNA is diagnosed based on the discrepancy between expected and observed Tac blood drug levels. The two main approaches are based on measuring the variability of Tac trough levels [intra-patient variability (IPV)], most commonly measured as medication level variability index (MLVI) or standard deviation (Tac SD) [24–26], coefficient of variation (CV) and on calculating the Tac dose to concentration ratio [27–29] (Table 2).
Table 2.
Methods to measure MNA using TDM of tacrolimus
| Method | Definition | Time interval | Tac dose | Normal range | Values correlated with MNA | Limitations | Other determinants besides MNA |
|---|---|---|---|---|---|---|---|
| MLVIa [24, 25] | Tac SD of at least 3 Tac trough levels | >1 year | Changed or unchanged | <2 | >2 | Better used as a threshold rather than a continuous number | - Dietary preferences - High metabolizers - Drug-to-drug interactions (steroids) - Drug–food interactions - Different laboratory assays - Different Tac formulations - Clinical conditions that may increase CV independently from MNA (diarrhoea, infections, hypotension, abdominal surgery) |
| CV [26–28] | Tac SD/Tac mean ×100 | >4–6 months (during stable phases) | Unchanged | 15–30% | >50% | It is reliable only if the dose remains unchanged | |
| Concentration/dose ratio [26] | Tac concentration (ng/mL)/Tac daily dose (mg/day) measured at steady state | Changed or unchanged | Stable within the same patients (ranging from 0.5 to 5.0 ng/mL per mg/day) | Lower than expected for each patient | Less standardized |
aPaediatrics liver transplant studies.
MNA, medication non-adherence; TDM, therapeutic drug monitoring; Tac, tacrolimus; SD, standard deviation.
IPV is related to clinical outcomes. Among 356 Canadian KTRs, there was a significant 27% increase in the adjusted hazard ratio of the composite endpoint of late allograft rejection, transplant glomerulopathy or total graft loss (including death). For every 1-unit increase in Tac SD, the hazard ratio increased by approximately 30% [30]. A Tac SD > 2 has been associated with late acute rejection within 190 days in 379 adolescent liver transplant recipients (70% sensitivity and specificity) [25]. In another study on 297 KTRs, 71% (24/34) of the patients developing graft failure had high IPV during the first year post-transplantation [31].
Despite the fact that intentionally non-adherent patients may increase drug intake (through ‘pulses’) selectively at times of laboratory testing and clinic visits, they are not generally able to guess the right timing and dosage to maintain unaltered the drug trough levels; for this reason IPV can be a useful tool to uncover intentional MNA, especially in the context of unplanned or shortly planned visits [25, 32]. Unfortunately, TDM cannot be used for all immunosuppressive medications, like steroids or azathioprine, and it is challenging to use with mycophenolate (because of the limitation of TDM) and mTOR-inhibitors (because of the long half-life).
Tac fluctuations can also be observed in the context of drug-to-drug [33] and drug–food interactions and acute clinical conditions. However, in settings in which there is no alternative explanation for high IPV, especially in the context of a concomitant risky behaviour (as judged by clinical assessment, self-reporting or missed outpatient visit), then high IPV can be regarded as a valuable surrogate of MNA [15, 25, 34].
Indirect methods
Indirect methods include pill count, electronic monitoring, self-reporting questionnaire and healthcare-provided inquires. All these measures, when used individually, have poor sensitivity. However, in combination with drug monitoring, these approaches can reach a high sensitivity, although they can be cost- and time-consuming [35].
In the case of suboptimal drug levels, pill counts can help the clinician to diagnose MNA [36]. However, in cases of intentional MNA, this method can be misleading, because the patients deliberately hide the missed pills from the caregivers.
Electronic monitoring (EM) is based on the use of expensive microprocessors, which are embedded in the medication container or blister, that record the time and date of medication intake [37, 38]. In theory, EM is a highly accurate recorder of patterns of medication intake. However, the event of opening the vial does not ensure that the patient ingests the medication, especially in the case of intentional MNA. Some devices can also be uncomfortable, therefore they may lead to non-usage and to falsely categorizing patients as non-adherent [14]. EM-assessed MNA has been used in clinical trials to objectively measure the response to specific treatment [39–42].
An inexpensive measure of MNA is self-reporting questionnaires. The Basel Assessment Adherence to Immunosuppressive (BAASIS©) Medication scale, which is the most used questionnaire, includes questions to determine if and how often in the last month, the patient (1a) missed a dose immunosuppressive medication, (1b) missed more than two consecutive doses, (2) took their medication more than 2 h after the recommended dosing time and (3) changed their dose without their doctor’s instruction [43]. Such measures of MNA have been associated with the rate of viral rebound in HIV patients [44]. Although self-reporting can underestimate MNA, it is helpful as an initial screening and helps identify patients worth more careful discussion regarding all medication-taking practices [14, 44].
Donor-specific antibody (DSA) formation has been linked to MNA [45]. However, this late finding gives fewer opportunities to invert the immune process. Of note, MNA can induce the appearance of non-DSA anti-HLA antibodies well before the development of full-blown anti-HLA DSA and chronic-active antibody-mediated rejection [46, 47].
Individual risk factors for MNA
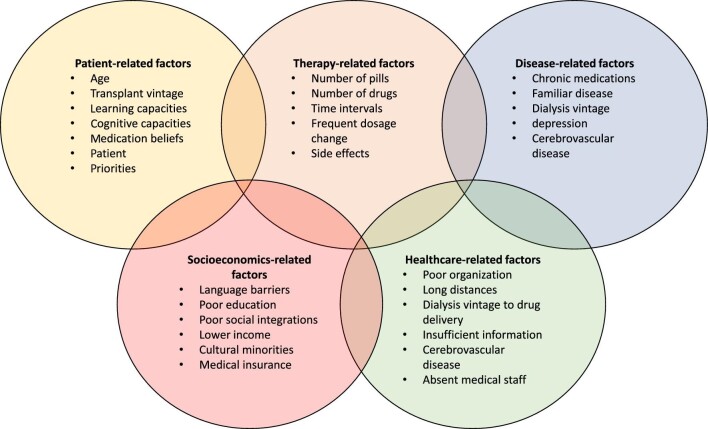
The relevance of identifying risk factors in clinical practice is that they help in preventing MNA. The World Health Organization has defined five main risk factor domains: patient-related, therapy-related, disease-related, and healthcare organizational and socioeconomic factors (Figure 1).
FIGURE 1:
Interplay between the five different domains concerning individual risk factors for medical non adherence.
Risk factors for non-adherence can also be divided into modifiable and non-modifiable ones [48]. Patients’ physical characteristics and disease factors are generally considered unmodifiable, whereas therapy complexities and organization issues can be modified by interventions. Patients’ beliefs and psychological factors can be modified as well, but this generally requires a multicomponent approach. Other risk factors include time post-transplant, health literacy, sociocultural barriers related to immigration status and ethnicities, learning and cognitive capacities, medication beliefs, overall patient lifestyle, and competing priorities. They may also be corrected, provided that ad hoc interventions are put in place.
Risk factors for MNA can coexist and change over time, therefore it is crucially important to continuously monitor these factors to address them as soon as they arise.
Patient-related factors
MNA in elderly patients is usually unintentional and related to factors such as forgetfulness, complex medication regimen, side effects and need for caregivers. In contrast, MNA in adolescents and young adults is related to lifestyle disruptions and progressive empowerment over caregivers to assuming responsibility for self-management [49, 50]. Overall, it has been estimated that the prevalence of MNA in adolescents and young adults is about 32% and that it accounts for 44% of all graft losses and 23% of late acute rejection episodes in this setting [43, 51, 52].
Therapy-related factors
Therapy-related factors include the number and complexity of daily medications [53], the frequent changes in dosages and drug-related side effects [54]. Identifying specific lifestyle factors that cause MNA may sometimes provide an easy way to improve medical adherence; for instance, by tailoring the timing of drug administration to the patient's working hours or to the timing of leisure activities.
Disease-related factors
Disease-related factors can be related to the history of chronic medications and the dialysis vintage. For instance, depression or cognitive impairment due to cerebrovascular disease have been linked to poor adherence in KTRs [12].
Healthcare organizational factors
The poor healthcare organization, non-private medical insurance in the USA, distance and time factors can affect MNA. Long distances from the place where patients get medications can greatly affect drug adherence. Limited time allotted by the healthcare personnel to provide patients with adequate information at the time of hospital discharge, or absence of medical staff in the outpatient clinic for consultation after forgetting drug intake, all negatively impact MNA [55].
Socioeconomic and cultural factors
Socioeconomic and cultural determinants of MNA are factors such as belonging to cultural minorities with poor social integration, low socioeconomic status and lack of insurance coverage [56–58]. Constantiner et al., who analysed the adherence of 312 KTRs in New York City through a self-reporting questionnaire, found that younger age and lower income were significantly associated with reported MNA [56]. A recent European study found that, compared with EU-born KTRs, non-EU-born KTRs had a hazard ratio of graft failure beyond 1 year of 1.36, probably related to barriers to adherence related to recent immigration background [58].
General interventions for MNA prevention and treatment
Despite the critical impact of MNA on graft survival, there are still limited interventions that comprehensively address MNA in KTRs and that have been tested in clinical trials.
Unfortunately, the randomized control trials (RCTs) performed so far on intervention strategies showed some efficacy in reducing measured MNA, but none of them was designed or be able to show any benefit on clinical outcomes [16, 59–63]. One of the additional explanations of this lack of effect on clinical outcomes could be the ‘streetlight effect’ in which biases in population and endpoint selection could drive misleading conclusions [63]. In fact, since non-adherent patients are less likely to accept being enrolled in monitoring and intervention trials, most of these studies probably included adherent or partially adherent patients. This might have prevented the trials from detecting the true effect of the intervention on MNA patients. Moreover, because the MNA measurements are often considered as primary endpoints, many trials have concluded that the intervention was effective, despite the lack of relevant improvement in clinical outcomes [63].
Clinical pharmacist care
Clinical pharmacists may be involved in MNA monitoring by overseeing the direct medication distribution and providing counselling [64–66]. One RCT reported that pharmacist care strategies increased measured medication adherence (mean compliance rate 95 versus 82% for intervention and controls, respectively, P < 0.001), but this had no impact on self-reported medication adherence and on graft outcomes [65]. Another RCT on 128 KTRs found no difference in Tac CV (31.4 versus 32.5%) or in questionnaire-based adherence rate (27% versus 25%). The main limitation of implementing pharmacist care is represented by the inaccuracy of the estimated discrepancy (i.e. the measure of MNA) between the medication collection and the actual intake. Moreover, not all patients are centralized to a single pharmacist facility. Therefore, it may be logistically challenging to track prescription refill for all KTRs [15].
Medication reminder interventions
These interventions aim at reminding the unintentionally non-adherent patients to assume their medication at the correct dose and timing, using electronic medication dispensers, freely available smartphone settings and Apps. Reese et al. randomized 120 KTRs to EM with customized reminders, EM with customized reminders plus provider notification or EM alone (control). Despite a significantly increased customized-EM-based adherence at 180-day assessment (78, 88 and 55%, respectively), no difference was detected in mean Tac levels [67]. Another RCT, which randomized 80 KTRs to EM monitoring plus electronic and healthcare reminder versus standard of care, found that the intervention group had a high EM-based compliance rate (98%). However, the compliance rate in controls was not reported. Moreover, the study found no difference in mean Tac level between the intervention and control group (approximately 7ng/L in both groups). Of note, 6 of the 40 participants in the intervention group withdrew from the study prematurely, mainly due to excessive stress or feeling of being controlled. One participant died 6 months after inclusion because of a serious infection [68], this serious adverse event being apparently not related to the intervention.
Electronic reminders have been largely replaced by phone alarms and various Apps on patients’ smartphones [69]. A single-centre RCT investigated the effect of using a free mobile application on Tac IPV. The authors found a marginally, albeit statistically significant, lower Tac CV at 1 month (28 versus 37%). However, the difference vanished at the 3-month assessment [70].
Remote monitoring and telemedicine
One RCT analysed the impact of telemedicine versus standard of care in 46 living-donor KTRs. The intervention arm included both chronic management process and acute-care situation support. The authors found a significantly lower questionnaire-based adherence rate (17% in the telemedicine monitoring group versus 56% in the control group). The effect persisted for up to 12 months after the end of the intervention. The authors also reported a lower incidence of hospital re-admission and shorter length of stay (median re-admission 0 versus 2; median length of stay 6 versus 13 days). It is unclear whether this resulted from the intervention itself or rather the fact that patients in the intervention groups received more intensive and close follow-up compared with the control group [60]. The practice of telemedicine has received a substantial boost from the coronavirus disease 2019 (COVID-19) epidemic [71]. Moreover, it has been reported that the COVID-19 epidemic and the related logistical problems have increased the rejection rates [72]. One recent trial including paediatric lung transplant recipients found a reduction in the Tac IPV at 6 months in 10 patients undergoing 3-weekly phone calls and regular follow-up calls (Tac SD −1.84; 95% CI: −2.95, −0.74; P = 0.004) compared with 7 controls undergoing only regular follow-up calls (Tac SD 0.59; 95% CI: −1.42, 2.60; P = 0.46) [73]. Telemedicine may be integrated with home-based dried blood spots (DBS) sampling of Tac for the purpose of therapeutic drug monitoring [74, 75]. The use of remote monitoring and telemedicine can improve patient quality of life and independence [60], limiting the patients’ psychological distress, but its role in everyday clinical practice needs further validation. In our own experience during the COVID-19 pandemic, there are some patients who strongly prefer undergoing regular outpatient clinical visits rather than relying on telemedicine visits.
Therapy simplification
Studies consistently showed that medication complexity is an obstacle to medication adherence [76, 77]. Thus, regimens should be kept as simple as possible and they should be adapted to the patient’s habits and lifestyle [76]. Over the last decade, several strategies to improve MNA have been focussed on therapy simplification.
Four single-arm cross over pre–post comparison studies and one RCT showed higher adherence after pill burden reduction, through switch to once-daily Tac alone [53, 78] or in combination with a full once-daily therapy [79]. Overall, these cross-over studies showed a net improvement in 10–20% of the patients. However, all these studies lack a control group. The only published RCT analysed the effect of once-daily Tac switching, based on electronic-monitored MNA. 219 KTRs were included and randomized 2:1 to once-daily (n = 145) and twice-daily Tac (n = 74) and then followed for 6 months after randomization. Medication adherence increased by 10% in the once-daily group compared with the twice-daily group (88% versus 79%) [80]. The relatively small effect of once-daily Tac in improving MNA is not unexpected, because this strategy also has its own pitfalls [81]. For instance, while young patients with an active life can benefit from a once-daily regimen, patients doing day and night shifts or elderly patients living a drug-paced life could be more comfortable with twice-daily regimens. Moreover, adherence becomes even more critical in once-daily regimens, when missing one dose has more serious consequences as opposed to cases of regular formulations [82]. In the study from Wu et al., the highest coefficient of variation before switching was associated with a higher risk of reduced Tac levels after conversion (area under the receiver operating characteristic curve 0.84, sensitivity 68.3%, specificity 92%) [53].
Therapy simplification has largely experimented with Tac monotherapy. In fact, some drugs such as mycophenolate and everolimus require b.i.d. administration. Even in the case of calcineurin-free regimens, such as those based on the costimulatory drug blocker belatacept, which requires i.v. monthly administration, patients receive concomitant administration of b.i.d. mycophenolate or everolimus [83]. However, due to its long half-life, everolimus could be theoretically administered once daily, as suggested by a small recent RCT [84].
Patients should be aware that medication regimens can be personalized to meet their needs, but they should also be aware of the pros and cons of each option. Tailored therapies seem to be particularly helpful for the empowerment of patients, to reduce the feeling of overmedicalization and to lower the risk of MNA, whereas they seem to be less impactful and even harmful in the case of intentional MNA. We recommend that individual habits and lifestyle hurdles to medical adherence should be discussed with the patients and drug treatment schedules should be the result of a shared decision-making process.
Educational-behavioural intervention
The information-motivation-behavioural skills model (‘IMB skills model’) is a validated theoretical framework that includes three essential factors to engage and maintain a health behaviour: information, motivation and behavioural skills. The interventions provide psychoeducation, address barriers, foster motivation and discuss cultural messages on adherence behaviour. They also include electronic reminders and meetings with ‘coaches’. These approaches have been effective in promoting medication adherence in other chronic diseases, such as HIV infection [39–42], and may help preventing intentional MNA in transplant recipients.
Most studies performed in transplanted patients used an RCT design and examined multicomponent interventions [85] delivered by healthcare professionals across multiple face-to-face and/or telephone sessions [59, 62, 85–90]. Four studies randomized the whole population of KTRs [59, 85, 88, 90], while four other RCTs included only non-adherent KTRs on the basis of an EM survey [62, 86, 87, 89] (Table 3).
Table 3.
Clinical trial investigation to prevent or treat MNA
| Strategy | First author | -Publication year -Time frame -Location |
Design | -Inclusion criteria -Exclusion criteria |
Patients’ characteristics | -Intervention (n) -Comparator (n) -Duration |
Outcome(s) | Results | Patient survival | Death-censored graft survival | Graft function | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical pharmacist care | ||||||||||||
| Chisholm [64] | - 2001 - 1997–1999 - Augusta (USA) |
RCT | - All single KTRs, aged 18–60 years, receiving the same immunosuppressive drugs for 1 year, followed at the hospital clinic >1 year, receiving the therapy from the hospital pharmacy - Dual or combined transplant recipients, change in immunosuppressive medications within the 1st-year post-transplant, not followed at hospital clinic, receiving drugs from other pharmacies |
N = 24 Males 75% Caucasians 58%, African-Americans 38%, and Hispanic 4% Living-donor KTRs 33% Age 49.2 ± 10.2 CNI use: cyclosporine 88%, Tac 12% |
- Pharmacist counselling and instruction (n = 12) - Standard of care (n = 12) 12 months |
- Mean compliance rate (CR) for each month - Mean time of patients’ compliance (CR > 80%) - % of patients reaching therapeutic target drug levels Compliance rate was calculated as dose refill/dose prescribed × 100 |
96.1 ± 4.7% versus 81.6 ± 11.5% (P < 0.001). 11 months (95% CI of 10 – 12) versus 9 (95% CI of 7 – 11) 64% versus 48% (P < 0.05) |
NA | NA | NA | NA | |
| Joost [65] | - 2014 - 2008–2010 - Erlangen (Germany) |
Case–control study | - All adult German-speaking KTRs, independent of others for medication management or questionnaire completion and followed at visit to the outpatient clinic, on MMF/MPA therapy and willing to use electronic-monitored (EM) bottle for MMF/MPA |
N = 67 Male 69% Living-donor KTRs 23% Age 53 (12.6) First transplant 91% CNI use: cyclosporine 82%, Tac 18% |
- Pharmacist counselling and adherence support (n = 32) - Standard of care (n = 35) 12 months |
- % of days with the correct dosing of MMF/MPA through EM during the 1st year post-transplant - Taking adherence (% of bottle opening/total number of doses prescribed) - Timing adherence (% of doses taken within a 6-h interval around patients’ standard intake time) - Adherence rates (pill counts) -n of drug holidays (no MMF/MPA intake for >48 h). |
(91%, CI 90.52– 91.94) versus (75%, CI 74.57–76.09) P = 0.014 82% versus 95% P = 0.006 94% versus 95% P = 0.142 90% versus 97% P = 0.008 19% versus 67% P = 0.001 |
NA | NA | eGFR at 12 months 46 ± 15.4 mL/min versus 49 ± 14.3 mL/min P = 0.446 |
NA | |
| Bessa [66] | - 2016 – 2014 - Sao Paulo (Brazil) |
RCT | - Adult KTRs who received immunosuppressive regimens consisting of Tac, prednisone and mycophenolate sodium or azathioprine - KTRs receiving concomitant medications known to interfere with TAC pharmacokinetics |
N = 128 Age 45.7 ± 11.6 versus 43.1 ± 12.5 Male 59 versus 66% Living donor 23% versus 20% |
- Pharmacist counselling and adherence support (n = 64) - Standard of care (n = 64) 90 days |
- % coefficient variation of Tac - Patient adherence through the BAASIS Scale at Day 28 - Patient adherence through the BAASIS at Day 90 |
31.4% ± 12.3% versus 32.5% ± 16.1% P = 0.673 17% versus 26% P = 0.135 27% versus 25%, P = 0.457 |
NA | NA | NA | NA | |
| Medication reminder interventions | ||||||||||||
| Reese [67] | - 2016 - 2021–2014 - Philadelphia (USA) |
RCT | - Adults KTRs on twice daily Tac medication - Patients with inability to manage medications, poor English comprehension, HIV-positive serostatus, living more than 120 miles from the centre and/or discharge to an acute-care facility |
N = 117 age 50 ± 11 years male 60% African-American 40% Prior transplant 12% |
- Arm 1: reminders group (n = 40) - Arm 2: reminders-plus-notification group (n = 39) - Arm 3: control group. (n = 38) - 6 months |
- Adherence at 90 days - Adherence at 14 days - CV for Tac level - Patient adherence through the BAASIS scale at Day 90 |
78% versus 88% versus 55% 82% versus 88% versus 58% 0.25 ± 0.14 versus 0.26 ± 0.11 versus 0.26 ± 0.13 P = 0.05 78% versus 74% versus 72% P = 0.58 |
1 death in arm 1 | 1 graft failure in the control arm | NA | No | |
| Henriksson [68] |
- 2016 - 2011–2013 - Stockholm (Sweden) |
RCT | - all consecutive KTRs |
N = 80 Age 44.65 (2–69) years Male 65% Living donor 45% |
- Using electronic medication dispenser (EMD) (n = 40) - Not using EMD (n = 40) - 12 months |
- Medication non- adherence rate - Patients with diagnosis of rejection |
2% versus nonadherence 10% versus 33% (P = 0.054) |
- 1 death for infection in the intervention group | NA | NA | - 3 patients felt being monitored. - stroke (n = 1) - 1 participant experienced extremely stressed by EMD use |
|
| Torabi [70] | - 2017 - NA - New York City (USA) |
RCT | - All KTRs or SPKTRs |
N = 67 Age 53.7 ± 14.3 versus 51.6 ± 14.3 years Living-donor KTRs 28% versus 83% |
- Use of Transplant Hero mobile App (n = 18) - Non-users (n = 18) - 3 months |
- Tac CV at 1 month - Tac CV at 3 months |
28% versus 37% (P = 0.014) 34% versus 35% (P = 0.63) |
NA | NA | s-Creatinine reported to be not statistically different at 1 (P = 0.65) and 3 (P = 0.83) months | NA | |
| Remote monitoring and telemedicine | ||||||||||||
| Schmid [60] | - 2017 -2011–2013 - Freiburg im Breisgau (Germany) |
RCT | - living-donor KTRs |
N = 46 Age 46 (18–59) versus 51 (19–66) Male 61 versus 48% Living relate donor 39 versus 52% ABO-incompatible KT 30 versus 26% |
- telemedically supported case management (n = 23) - Standard of care (n = 23) - 12 months |
- Median unplanned hospital admission at 12 months - Median hospitalization days at 12 months questionnaire-based MNA rate |
0 [(IQR) = 1] versus 1 [(IQR) = 2] U = 132.5, P = 0.002, r = 0.44 0 days (IQR = 6) versus 13 days (IQR = 23) U = 141.0, P = 0.005, r = 0.41 56.5% versus 17.4% (P = 0.013) |
NA | 0 versus 2 (1 rejection, 1 haemorrhage) | NA | ||
| Therapy simplification | ||||||||||||
| van Boekel [79] | - 2013 - 2006–2010 - Nijmegen (The Netherlands) |
Cross-over study with no control group | - Adult KTRs with stable renal function on once daily Tac, with unchanged Tac dose in the previous 3 months, on potential full once daily regimen, Dutch speaking - Patients on mycophenolate regimen |
N = 75 Age 49.6 ± 12.1 Male 61% Time after transplant 3.1 ± 3.3 years Living-donor KTR 75% Concomitant IS: prednisone 92%, azathioprine 7%, both 1% Working in shifts 24% |
- After switching to fully once daily therapy (n = 75) - Same patients before switching to fully once daily therapy - 6 months |
- Treatment convenience score at 3 weeks - Daily number of medications at 2 weeks - Daily number of tablets at 2 weeks - Self-reported adherence at 3 weeks Measured by Treatment Satisfaction Questionnaire for Medication version II |
66.0 ± 14.5 versus 78.5 ± 14.5 (P < 0.001) 2.4 ± 0.7 versus 1.6 ± 0.7 (P < 0.001) 12.4 ± 3.3 versus 9.1 ± 2.6 (P < 0.001) 78% versus 95% |
NA | NA | NA | Not registered at 6 months | |
| Cassuto [78] | - 2016 - NA - Multicentric (France) |
Cross-over study with no control group | - Adult kidney and/or liver transplant recipients, on initial twice-daily Tac regimen - Enrolled in clinical trials |
N = 1106 Age 52.4 ± 13.2 years Male 62% Self-reported adherence assessment at baseline: good adherence (GA) 21%, minor non-adherence (mNA) 72%, non-adherence 7% Mean general acceptance score 78% |
- After switching from twice to once-daily Tac regimen (n = 1106) - Same patients before switching to once daily Tac 6 months |
- Adherence rate at 3 months compared with baseline - Adherence rate at 6 months compared with baseline |
28 versus 21% GA, 68 versus 72% nMA, 4.4 versus 7.1% non-adherence (P < 0.001) 26 versus 21% GA, 68 versus 72% nMA, 6.5 versus 7.1% non-adherence (P < 0.001) |
NA | NA | NA | NA | |
| Wu [53] | - 2011 - 2010 - Multicentric (Taiwan) |
Cross-over study with no control group | - Adult KTRs, on twice daily Tac-based therapy for 3 months, with stable health status |
N = 129 Age 51 ± 12 years Living donor 5% |
- Switch to once daily Tac (n = 129) - Same patients before switching to once daily Tac - 6 months |
- % CV of Tac | 8.5 ± 5 versus 14 ± 7.5 (P < 0.05) |
|||||
| Fellstrom [81] | - 2018 - 2012–2015 - multicentric (Sweden) |
Cross-over study with no control group | - Adult KTRs with stable renal function, on twice daily Tac regimen |
N = 233 Age 50 (19–82) versus 53.5 (20–77) years Male 65% versus 76% Prior transplant 18% versus 19% MNA assessed by BAASIS questionnaire at baseline 54% versus 66% |
- Switch to once daily Tac (n = 175) - Remain twice-daily Tac (n = 58) - 12 months |
- Increase in adherence assessed by BAASIS questionnaire at 12 months - Reduction in through Tac levels |
+2.6% versus 3.9% −0.6 ± 2.7 versus −0.2 ± 1.7 ng/mL |
1 due to spleen haemorrhage in the intervention group and 1 for cardiac surgery complications in the control group | NA | No difference in eGFR at 0–12 months | 8 patients in the once daily Tac group experienced AE (tumors, gastrointestinal problem, skin reaction, angina pectoris and diabetes None in the control group |
|
| Kuypers [80] | - 2013 - 2008–2009 - multicentric (Belgium) |
RCT | - Adult KTRs, with transplant vintage 6 months–6 years, on twice daily Tac-based therapy, with stable health status |
N = 219 Male 57% versus 62% Prior transplant 11% versus 11% Transplant vintage 3.1 ± 2.0 versus 2.9 ± 2.1 years |
After 3 months of EM-based MNA assessment: - Switch to once daily Tac (n = 145) - Remain twice-daily Tac (n = 74) - 6 months |
- MNA measured as % of patients who remain engaged with the same regimen at 6 months - Day-by-day % of patients with correct dosing - Difference in pre–post randomization MNA - % patients missing daily dose at 6 months |
81.5 versus 71.9% (P = 0.08) 88.2 versus 78.8% (P = 0.001) +6% versus −0.7% (P < 0.001) 62% versus 40% |
NA | NA | NA | Gastrointestinal 2.8% in the intervention group | |
| Educational-behavioural intervention | ||||||||||||
| De Geest [86] | - 2006 - NA - Basel (Switzerland) |
RCT | - Adult KTRs, previously assessed as non-adherent through EM for 3 months, transplant vintage >1 year, French or German speaking - Lack of mental clarity, blindness, without a phone |
N = 18 NA |
- One home visit at the inclusion and behavioural interventions, individualized education and social support through monthly phone call for 3 months (n = 6) - Standard of care (n = 12) - 6 months |
- EM-based adherence at 6 months | 84% versus 81% P = 0.58 |
NA | NA | NA | NA | |
| Russell [87] | - 2011 - NA - Columbia (USA) |
RCT | - Adult KTRs, previously assessed as non-adherent through EM for 3 months, one twice daily immunosuppressive drug, English speaking, able to open EM cap, independent in medication administration, access to a telephone - No cognitive impairment, or other diagnosis who shorten lifespan |
N = 15 Age 51.5 ± 7.2 years Male 47% Caucasian 80% Less than high school education 60% Living donor 27% Prior transplant 47% |
- Continuous self-improvement intervention through monthly specialist nurse support (1 home visits +5 phone calls) for 6 months (n = 8) - Standard of care (n = 7) - 6 months |
- EM-based MNA at 6 months | 84 versus 81% P = 0.039 |
NA | NA | NA | NA | |
| Garcia [88] | - 2015 - 2011–2012 - Sao Paulo (Brazil) |
RCT | - Adult KTRs |
N = 108 Age 46 ± 14.1 versus 49.3 ± 12.1 years Male 56% versus 63% Living-donor KTRs 38% versus 18% (P = 0.017) Duration of dialysis 25 ± 18 versus 42 ± 31 months (P = 0.007) |
- Personalized counselling by a transplant nurse through 30 consultation once a week for 3 months (n = 55) - Standard of care (n = 56) - 12 months |
- % adherence assessed by Immunosuppressant Therapy Adherence Scale (ITAS) questionnaire at 3 months - Mean drug trough levels |
86 versus 54% (P = 0.001) 8.7 ± 1.6 versus 8.7 ± 1.8 ng/mL |
NA | NA | eGFR at 12 months 61 ± 21 versus 55 ± 24 mL/min/1.73 m2 (P = 0.46) |
NA | |
| Breu-Dejean [85] | - 2016 - 2002–2003 - Toulouse (France) |
RCT | - Adult stable KTRS, KT within 5 years - Cognitive or psychiatric disorders |
N = 110 Age 48 ± 12 years Male 57% Related- living-donor KTs 3.6% Prior transplant 10% |
- 2-h psychoeducational intervention in group of 10 persons, every week for 2 months, conducted by a multidisciplinary team (physician, psychologist, nurses, kinesiotherapist, dietitian and social worker) (n = 55) - Standard of care (n = 55) - 10 years |
- Questionnaire-based adherence at 3 months |
75% versus 47% |
Death 12.7% versus 9.1% (P = 0.35) Death with functioning graft 8.2 versus 3.6% (P = 0.13) A log rank test not significant difference (P = 0.06) |
Death-censored graft survival 69% versus 87% (P = 0.02) Duration with a functioning graft 3481 ± 894 versus 3562± 717 days (P = 0.97) |
NA | NA | |
| Cukor [89] | - 2017 - 2011 - New York City (USA) |
RCT | - KTRs on Tac regimen, aged >25 years, <98% adherence to medication prescription determined by 3 baseline pill counts and Tac trough levels - Lack of a telephone, non-English speaker |
N = 33 Age 52 ± 12 years Male 40% African-American 88% Transplant vintage 37.6 ± 13.4 months |
- 2 sessions of 2-h cognitive behavioural therapy in 2 weeks (n = 15) - Standard of care (n = 18) - 6 weeks |
- Increased in adherence based on pill counts - Grouped Tac trough levels SD |
+6% versus 0% (P = 0.04) 1.8 versus 3.5 (P < 0.05) |
NA | NA | No difference | NA | |
| Foster [59] | - 2018 - 2012–2016 - Multicentric (Canada and USA) |
RCT | - KTRs, aged 11-24 years, transplant vintage >3 months, stable graft function - Impending graft failure, severe neurocognitive disabilities, lack of electronic pill box connectivity, use of liquid immunosuppressive medication, having a sibling participating in the study, participating in other adherence study, English or French speaking |
N = 169 Age 15 (13.2–17.4) versus 16 13.3 –17.5 years Male 57 versus 61% African-American 11 versus 13% Prior transplant 9 versus 8% Living donor 46 versus 58% |
- EM reminder + receive text message, email, and/or visual cue dose reminders and met with a coach at 3-month intervals (n = 81) - Standard of care (n = 88) - 12 months |
- Taking adherence At 6 months - Timing adherence |
78 versus 68% 73% versus 61% |
NA | NA | NA | Higher number of CMV infection 0.59 versus 0% patient/month | |
| Low [90] | - 2019 - 2014–2015 - multicentric (Australia) |
RCT | - Adult KTRs, self-manage medication, English speaking - Visually impaired patients, or unable to answer the telephone unassisted |
N = 71 Age 51 ± 11 years Male 58% Living donor 20% Transplant vintage 28 (20–41) days |
- Face-to-face meeting and telephone health coaching every 2 weeks for 3 months (n = 35) - Standard of care (n = 36) - 12 months |
- Difference in EM-based taking adherence from 3 to 12 months - Timing adherence - SD of Tac trough levels for each patient |
- 17.0 versus - 2.3% - 6.9 versus 14.0% 2.1 versus 2.3 |
NA | NA | NA | NA | |
| Russell [62] | - 2020 - 2015–2017 - Multicentric (USA) |
RCT | - Adult EM- based non-adherent KTRs, self-administered therapy, at least one twice daily immunosuppressive medication, English speaking - No access to the telephone, unable to open an EM cap, mini-mental score < 4 |
N = 89 Age 52 ± 10 years Male 58% African-American 61% Prior transplant 15% Living donor KT 28% |
- 6 months multicomponent adherence‐promoting interventions (n = 45) - Standard of care (n = 44) - 6 months |
- Average EM-base adherence at 6 months - Average EM-base adherence at 12 months |
91 versus 67% (P < 0.001) 77 versus 60% (P = 0.004) |
NA | NA | - S-Creatinine at 12 months 1.3 versus 2.1 mg/dL - BUN at 12 months 21 versus 28 mg/dL |
No |
RCT, randomized controlled trial; CNI, calcineurin inhibitor; BAASIS, Basel Assessment of Adherence to Immunosuppressive Medication Scale; EM, electronic monitoring; CI, confidential interval; NA, not applicable; MMF, mycophenolate mofetil; MPA, mycophenolic acid; eGFR, estimated glomerular filtration rate; SPKTR, simultaneous pancreas kidney transplant recipient; IQR, interquartile range; IS, immunosuppression; AE, adverse event; CMV, cytomegalovirus; BUN, blood urea nitrogen.
Two small RCTs found no difference in EM-based adherence between educational-behavioural intervention and control group [86, 90], while the other six RCTs proved that intervention significantly reduces MNA, but this effect generally vanished thereafter [59, 62, 85, 87–89]. Among these six RCTs, four of them investigated the clinical outcomes [59, 62, 85, 88], with two studies finding no difference in graft survival between groups [59, 88], one study finding a negative impact on 10-year death-censored graft survival in the intervention group [85] and the last RCT (SystemCHANGE [62]) showing a positive numerical trend, despite not statistically significant, on graft function at 12 months [62]. Interestingly, there was also a numerical trend toward an increased infection risk in the intervention groups, which needs to be further addressed in future RCT and meta-analyses. A possible explanation for this outcome is that increased adherence to antirejection drugs may result in a higher risk of overimmunosuppression.
In summary, educational-behavioural interventions are effective strategies in improving MNA, at least in the short term. Unfortunately, the extent of the long-term benefit is uncertain. Moreover, they are expensive, time-consuming and their widespread implementation may be hard to achieve in several clinical settings.
CONCLUSIONS
MNA is one of the leading causes of patient and graft loss after kidney transplantation. Unfortunately, there is no evidence to date that any single strategy for treating MNA improves the two major clinical outcomes, namely, patient death and death-censored graft failure. Therefore, every effort should be made to identify individual risk factors for MNA and to discuss with patients what are the major hurdles to adherence to the prescribed treatment schedule. Then, the plan to improve medical adherence should be personalized to the peculiar issues raised in the individual patient. While unintentional non-adherent patients can benefit from various personalized and multi-disciplinary interventions such as electronic reminders and phone Apps and therapy simplification, intentional MNA remains an Achille's heel of any transplant centre. To preventing KTRs from becoming intentional MNA patients, constant monitoring via motivational-behavioural interventions may represent the only viable resource.
Contributor Information
Ilaria Gandolfini, Department of Medicine and Surgery, University of Parma, Parma, Italy; Nephrology Unit, University Hospital of Parma, Parma, Italy.
Alessandra Palmisano, Nephrology Unit, University Hospital of Parma, Parma, Italy.
Enrico Fiaccadori, Department of Medicine and Surgery, University of Parma, Parma, Italy; Nephrology Unit, University Hospital of Parma, Parma, Italy.
Paolo Cravedi, Department of Medicine, Division of Nephrology and Translational Transplant Research Center, Recanati Miller Transplant Institute, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Umberto Maggiore, Department of Medicine and Surgery, University of Parma, Parma, Italy; Nephrology Unit, University Hospital of Parma, Parma, Italy.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Gondos A, Dohler B, Brenner Het al. Kidney graft survival in Europe and the United States: strikingly different long-term outcomes. Transplantation 2013; 95: 267–274 [DOI] [PubMed] [Google Scholar]
- 2. Gonzalez-Molina M, Burgos D, Cabello Met al. Impact of immunosuppression treatment on the improvement in graft survival after deceased donor renal transplantation: a long-term cohort study. Nefrologia 2014; 34: 570–578 [DOI] [PubMed] [Google Scholar]
- 3. Wang JH, Skeans MA, Israni AK. Current status of kidney transplant outcomes: dying to survive. Adv Chronic Kidney Dis 2016; 23: 281–286 [DOI] [PubMed] [Google Scholar]
- 4. Vlaminck H, Maes B, Evers Get al. Prospective study on late consequences of subclinical non-compliance with immunosuppressive therapy in renal transplant patients. Am J Transplant 2004; 4: 1509–1513 [DOI] [PubMed] [Google Scholar]
- 5. Denhaerynck K, Burkhalter F, Schafer-Keller Pet al. Clinical consequences of non adherence to immunosuppressive medication in kidney transplant patients. Transpl Int 2009; 22: 441–446 [DOI] [PubMed] [Google Scholar]
- 6. Butler JA, Roderick P, Mullee Met al. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation 2004; 77: 769–776 [DOI] [PubMed] [Google Scholar]
- 7. Neuberger JM, Bechstein WO, Kuypers DRet al. Practical Recommendations for Long-term Management of Modifiable Risks in Kidney and Liver Transplant Recipients: A Guidance Report and Clinical Checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation 2017; 101: S1–S56 [DOI] [PubMed] [Google Scholar]
- 8. Pinsky BW, Takemoto SK, Lentine KLet al. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant 2009; 9: 2597–2606 [DOI] [PubMed] [Google Scholar]
- 9. Kabani R, Quinn RR, Palmer Set al. Risk of death following kidney allograft failure: a systematic review and meta-analysis of cohort studies. Nephrol Dial Transplant 2014; 29: 1778–1786 [DOI] [PubMed] [Google Scholar]
- 10. Clayton PA, McDonald SP, Russ GRet al. Long-term outcomes after acute rejection in kidney transplant recipients: an ANZDATA analysis. J Am Soc Nephrol 2019; 30: 1697–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burkhalter H, Wirz-Justice A, Cajochen Cet al. Daytime sleepiness in renal transplant recipients is associated with immunosuppressive non-adherence: a cross-sectional, multi-center study. Clin Transplant 2014; 28: 58–66 [DOI] [PubMed] [Google Scholar]
- 12. Jindal RM, Neff RT, Abbott KCet al. Association between depression and nonadherence in recipients of kidney transplants: analysis of the United States renal data system. Transplant Proc 2009; 41: 3662–3666 [DOI] [PubMed] [Google Scholar]
- 13. Ranahan M, Von Visger J, Kayler LK. Describing barriers and facilitators for medication adherence and self-management among kidney transplant recipients using the information-motivation-behavioral skills model. Clin Transplant 2020; 34: e13862. [DOI] [PubMed] [Google Scholar]
- 14. Nevins TE, Nickerson PW, Dew MA. Understanding medication nonadherence after kidney transplant. J Am Soc Nephrol 2017; 28: 2290–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lieber SR, Helcer J, Shemesh E. Monitoring drug adherence. Transplant Rev (Orlando) 2015; 29: 73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shemesh E, Shneider BL, Mazariegos GV. Weekend versus weekday adherence: do we, or do we not, thank God it's Friday? Am J Transplant 2020; 20: 7–9 [DOI] [PubMed] [Google Scholar]
- 17. Shemesh E. Measuring adherence to medications: are complex methods superior to simple ones? Pediatr Transplant 2012; 16: 315–317 [DOI] [PubMed] [Google Scholar]
- 18. Griva K, Davenport A, Harrison Met al. Non-adherence to immunosuppressive medications in kidney transplantation: intent vs. forgetfulness and clinical markers of medication intake. Ann Behav Med 2012; 44: 85–93 [DOI] [PubMed] [Google Scholar]
- 19. Nevins TE, Thomas W. Quantitative patterns of azathioprine adherence after renal transplantation. Transplantation 2009; 87: 711–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denhaerynck K, Steiger J, Bock A. et al. Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant 2007; 7: 108–116 [DOI] [PubMed] [Google Scholar]
- 21. Prendergast MB, Gaston RS. Optimizing medication adherence: an ongoing opportunity to improve outcomes after kidney transplantation. Clin J Am Soc Nephrol 2010; 5: 1305–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boucquemont J, Pai ALH, Dharnidharka VRet al. Association between day of the week and medication adherence among adolescent and young adult kidney transplant recipients. Am J Transplant 2020; 20: 274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eisenberger U, Wuthrich RP, Bock Aet al. Medication adherence assessment: high accuracy of the new Ingestible Sensor System in kidney transplants. Transplantation 2013; 96: 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shemesh E, Mitchell J, Neighbors Ket al. Recruiting a representative sample in adherence research-The MALT multisite prospective cohort study experience. Pediatr Transplant 2017; 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shemesh E, Bucuvalas JC, Anand Ret al. The Medication Level Variability Index (MLVI) predicts poor liver transplant outcomes: a prospective multi-site study. Am J Transplant 2017; 17: 2668–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shemesh E, Lurie S, Stuber MLet al. A pilot study of posttraumatic stress and nonadherence in pediatric liver transplant recipients. Pediatrics 2000; 105: E29. [DOI] [PubMed] [Google Scholar]
- 27. Shuker N, van Gelder T, Hesselink DA. Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev (Orlando) 2015; 29: 78–84 [DOI] [PubMed] [Google Scholar]
- 28. Whalen HR, Glen JA, Harkins Vet al. High intrapatient tacrolimus variability is associated with worse outcomes in renal transplantation using a low-dose tacrolimus immunosuppressive regime. Transplantation 2017; 101: 430–436 [DOI] [PubMed] [Google Scholar]
- 29. Leino AD, King EC, Jiang Wet al. Assessment of tacrolimus intrapatient variability in stable adherent transplant recipients: establishing baseline values. Am J Transplant 2019; 19: 1410–1420 [DOI] [PubMed] [Google Scholar]
- 30. Sapir-Pichhadze R, Wang Y, Famure Oet al. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int 2014; 85: 1404–1411 [DOI] [PubMed] [Google Scholar]
- 31. Borra LC, Roodnat JI, Kal JAet al. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant 2010; 25: 2757–2763 [DOI] [PubMed] [Google Scholar]
- 32. Modi AC, Ingerski LM, Rausch JRet al. White coat adherence over the first year of therapy in pediatric epilepsy. J Pediatr 2012; 161: 695–699.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anglicheau D, Flamant M, Schlageter MHet al. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant 2003; 18: 2409–2414 [DOI] [PubMed] [Google Scholar]
- 34. van Gelder T. Within-patient variability in immunosuppressive drug exposure as a predictor for poor outcome after transplantation. Kidney Int 2014; 85: 1267–1268 [DOI] [PubMed] [Google Scholar]
- 35. Burnier M, Wuerzner G, Struijker-Boudier Het al. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension 2013; 62: 218–225 [DOI] [PubMed] [Google Scholar]
- 36. Fine RN, Becker Y, De Geest Set al. Nonadherence consensus conference summary report. Am J Transplant 2009; 9: 35–41 [DOI] [PubMed] [Google Scholar]
- 37. Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int 2015; 2015: 217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park LG, Howie-Esquivel J, Dracup K. Electronic measurement of medication adherence. West J Nurs Res 2015; 37: 28–49 [DOI] [PubMed] [Google Scholar]
- 39. Fisher JD, Fisher WA, Bryan ADet al. Information-motivation-behavioral skills model-based HIV risk behavior change intervention for inner-city high school youth. Health Psychol 2002; 21: 177–186 [PubMed] [Google Scholar]
- 40. Fisher JD, Amico KR, Fisher WAet al. Computer-based intervention in HIV clinical care setting improves antiretroviral adherence: the LifeWindows Project. AIDS Behav 2011; 15: 1635–1646 [DOI] [PubMed] [Google Scholar]
- 41. Kalichman SC, Cherry J, Cain D. Nurse-delivered antiretroviral treatment adherence intervention for people with low literacy skills and living with HIV/AIDS. J Assoc Nurses AIDS Care 2005; 16: 3–15 [DOI] [PubMed] [Google Scholar]
- 42. Mannheimer SB, Morse E, Matts JPet al. Sustained benefit from a long-term antiretroviral adherence intervention. Results of a large randomized clinical trial. J Acquir Immune Defic Syndr 2006; 43Suppl 1: S41–S47 [DOI] [PubMed] [Google Scholar]
- 43. Dobbels F, Ruppar T, De Geest Set al. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant 2010; 14: 603–613 [DOI] [PubMed] [Google Scholar]
- 44. Glass TR, De Geest S, Hirschel Bet al. Self-reported non-adherence to antiretroviral therapy repeatedly assessed by two questions predicts treatment failure in virologically suppressed patients. Antivir Ther 2008; 13: 77–85 [PubMed] [Google Scholar]
- 45. Yamazaki F, Kitajima T, Nukada Tet al. Synthesis of an appropriately protected core glycotetraoside, a key intermediate for the synthesis of “bisected” complex-type glycans of a glycoprotein. Carbohydr Res 1990; 201: 15–30 [DOI] [PubMed] [Google Scholar]
- 46. Susal C, Wettstein D, Dohler Bet al. Association of kidney graft loss with de novo produced donor-specific and non-donor-specific HLA antibodies detected by single antigen testing. Transplantation 2015; 99: 1976–1980 [DOI] [PubMed] [Google Scholar]
- 47. Hourmant M, Cesbron-Gautier A, Terasaki PIet al. Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol 2005; 16: 2804–2812 [DOI] [PubMed] [Google Scholar]
- 48. Chisholm-Burns MA, Kwong WJ, Mulloy LLet al. Nonmodifiable characteristics associated with nonadherence to immunosuppressant therapy in renal transplant recipients. Am J Health Syst Pharm 2008; 65: 1242–1247 [DOI] [PubMed] [Google Scholar]
- 49. Tong A, Morton R, Howard Ket al. Adolescent experiences following organ transplantation: a systematic review of qualitative studies. J Pediatr 2009; 155: 542–549 [DOI] [PubMed] [Google Scholar]
- 50. Foster BJ, Dahhou M, Zhang Xet al. Association between age and graft failure rates in young kidney transplant recipients. Transplantation 2011; 92: 1237–1243 [DOI] [PubMed] [Google Scholar]
- 51. Simons LE, McCormick ML, Mee LLet al. Parent and patient perspectives on barriers to medication adherence in adolescent transplant recipients. Pediatr Transplant 2009; 13: 338–347 [DOI] [PubMed] [Google Scholar]
- 52. Zelikovsky N, Schast AP, Palmer Jet al. Perceived barriers to adherence among adolescent renal transplant candidates. Pediatr Transplant 2008; 12: 300–308 [DOI] [PubMed] [Google Scholar]
- 53. Wu MJ, Cheng CY, Chen CHet al. Lower variability of tacrolimus trough concentration after conversion from prograf to advagraf in stable kidney transplant recipients. Transplantation 2011; 92: 648–652 [DOI] [PubMed] [Google Scholar]
- 54. Morrissey PE, Flynn ML, Lin S. Medication noncompliance and its implications in transplant recipients. Drugs 2007; 67: 1463–1481 [DOI] [PubMed] [Google Scholar]
- 55. Kobayashi S, Tsutsui J, Okabe Set al. Medication nonadherence after kidney transplantation: an internet-based survey in Japan. Psychol Health Med 2020; 25: 91–101 [DOI] [PubMed] [Google Scholar]
- 56. Constantiner M, Rosenthal-Asher D, Tedla Fet al. Differences in attitudes toward immunosuppressant therapy in a multi-ethnic sample of kidney transplant recipients. J Clin Psychol Med Settings 2018; 25: 11–19 [DOI] [PubMed] [Google Scholar]
- 57. Pagani FD. Insurance coverage and heart transplant outcomes. Circ Cardiovasc Qual Outcomes 2016; 9: 501–503 [DOI] [PubMed] [Google Scholar]
- 58. Grossi AA, Maggiore U, Puoti Fet al. Association of immigration background with kidney graft function in a publicly funded health system: a nationwide retrospective cohort study in Italy. Transpl Int 2020; 33: 1405–1416 [DOI] [PubMed] [Google Scholar]
- 59. Foster BJ, Pai ALH, Zelikovsky Net al. A randomized trial of a multicomponent intervention to promote medication adherence: the Teen Adherence in Kidney Transplant Effectiveness of Intervention Trial (TAKE-IT). Am J Kidney Dis 2018; 72: 30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schmid A, Hils S, Kramer-Zucker Aet al. Telemedically supported case management of living-donor renal transplant recipients to optimize routine evidence-based aftercare: a single-center randomized controlled trial. Am J Transplant 2017; 17: 1594–1605 [DOI] [PubMed] [Google Scholar]
- 61. Kaier K, Hils S, Fetzer Set al. Results of a randomized controlled trial analyzing telemedically supported case management in the first year after living donor kidney transplantation - a budget impact analysis from the healthcare perspective. Health Econ Rev 2017; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Russell CL, Hathaway D, Remy LMet al. Improving medication adherence and outcomes in adult kidney transplant patients using a personal systems approach: SystemCHANGE results of the MAGIC randomized clinical trial. Am J Transplant 2020; 20: 125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Duncan S, Annunziato RA, Dunphy Cet al. A systematic review of immunosuppressant adherence interventions in transplant recipients: decoding the streetlight effect. Pediatr Transplant 2018; 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chisholm MA, Mulloy LL, Jagadeesan Met al. Impact of clinical pharmacy services on renal transplant patients' compliance with immunosuppressive medications. Clin Transplant 2001; 15: 330–336 [DOI] [PubMed] [Google Scholar]
- 65. Joost R, Dorje F, Schwitulla Jet al. Intensified pharmaceutical care is improving immunosuppressive medication adherence in kidney transplant recipients during the first post-transplant year: a quasi-experimental study. Nephrol Dial Transplant 2014; 29: 1597–1607 [DOI] [PubMed] [Google Scholar]
- 66. Bessa AB, Felipe CR, Hannun Pet al. Prospective randomized trial investigating the influence of pharmaceutical care on the intra-individual variability of tacrolimus concentrations early after kidney transplant. Ther Drug Monit 2016; 38: 447–455 [DOI] [PubMed] [Google Scholar]
- 67. Reese PP, Bloom RD, Trofe-Clark Jet al. Automated reminders and physician notification to promote immunosuppression adherence among kidney transplant recipients: A randomized trial. Am J Kidney Dis 2017; 69: 400–409 [DOI] [PubMed] [Google Scholar]
- 68. Henriksson J, Tyden G, Hoijer Jet al. A prospective randomized trial on the effect of using an electronic monitoring drug dispensing device to improve adherence and compliance. Transplantation 2016; 100: 203–209 [DOI] [PubMed] [Google Scholar]
- 69. Duettmann W, Naik MG, Zukunft Bet al. eHealth in transplantation. Transpl Int 2021; 34: 16–26 [DOI] [PubMed] [Google Scholar]
- 70. Torabi J, Choinski K, Courson Aet al. Letter to the Editor: Mobile technology can improve adherence and lessen tacrolimus variability in patients receiving kidney transplants. Ochsner J 2017; 17: 218–219 [PMC free article] [PubMed] [Google Scholar]
- 71. El Aoufy K, Melis MR, Bellando Randone Set al. The positive side of the coin: Sars-Cov-2 pandemic has taught us how much Telemedicine is useful as standard of care procedure in real life. Clin Rheumatol 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Aziz F, Muth B, Parajuli Set al. Unusually high rates of acute rejection during the COVID-19 pandemic: cause for concern? Kidney Int 2020; 98: 513–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Duncan-Park S, Dunphy C, Becker Jet al. Remote intervention engagement and outcomes in the Clinical Trials in Organ Transplantation in Children consortium multisite trial. Am J Transplant 2021; 21: 3112–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zijp TR, Izzah Z, Aberg Cet al. Clinical value of emerging bioanalytical methods for drug measurements: a scoping review of their applicability for medication adherence and therapeutic drug monitoring. Drugs 2021; 81: 1983–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Martial LC, Hoogtanders KEJ, Schreuder MFet al. Dried blood spot sampling for tacrolimus and mycophenolic acid in children: analytical and clinical validation. Ther Drug Monit 2017; 39: 412–421 [DOI] [PubMed] [Google Scholar]
- 76. Martin LR, Feig C, Maksoudian CRet al. A perspective on nonadherence to drug therapy: psychological barriers and strategies to overcome nonadherence. Patient Prefer Adherence 2018; 12: 1527–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fogarty L, Roter D, Larson Set al. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns 2002; 46: 93–108 [DOI] [PubMed] [Google Scholar]
- 78. Cassuto E, Pageaux GP, Cantarovich Det al. Adherence to and acceptance of once-daily tacrolimus after kidney and liver transplant: results from OSIRIS, a French observational study. Transplantation 2016; 100: 2099–2106 [DOI] [PubMed] [Google Scholar]
- 79. van Boekel GA, Kerkhofs CH, Hilbrands LB. Treatment satisfaction in renal transplant patients taking tacrolimus once daily. Clin Ther 2013; 35: 1821–1829e1 [DOI] [PubMed] [Google Scholar]
- 80. Kuypers DR, Peeters PC, Sennesael JJet al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation 2013; 95: 333–340 [DOI] [PubMed] [Google Scholar]
- 81. Fellstrom B, Holmdahl J, Sundvall Net al. Adherence of renal transplant recipients to once-daily, prolonged-release and twice-daily, immediate-release tacrolimus-based regimens in a real-life setting in Sweden. Transplant Proc 2018; 50: 3275–3282 [DOI] [PubMed] [Google Scholar]
- 82. Oberbauer R, Bestard O, Furian Let al. Optimization of tacrolimus in kidney transplantation: New pharmacokinetic perspectives. Transplant Rev (Orlando) 2020; 34: 100531. [DOI] [PubMed] [Google Scholar]
- 83. Kirk AD, Adams AB, Durrbach Aet al. Optimization of de novo belatacept-based immunosuppression administered to renal transplant recipients. Am J Transplant 2021; 21: 1691–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Taber DJ, Posadas-Salas A, Su Zet al. Preliminary assessment of safety and adherence to a once-daily immunosuppression regimen in kidney transplantation: results of a randomized controlled pilot study. Clin Transplant 2020; 34: e13844. [DOI] [PubMed] [Google Scholar]
- 85. Breu-Dejean N, Driot D, Dupouy Jet al. Efficacy of psychoeducational intervention on allograft function in kidney transplant patients: 10-year results of a prospective randomized study. Exp Clin Transplant 2016; 14: 38–44 [PubMed] [Google Scholar]
- 86. De Geest S, Schafer-Keller P, Denhaerynck Ket al. Supporting medication adherence in renal transplantation (SMART): a pilot RCT to improve adherence to immunosuppressive regimens. Clin Transplant 2006; 20: 359–368 [DOI] [PubMed] [Google Scholar]
- 87. Russell C, Conn V, Ashbaugh Cet al. Taking immunosuppressive medications effectively (TIMELink): a pilot randomized controlled trial in adult kidney transplant recipients. Clin Transplant 2011; 25: 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Garcia MF, Bravin AM, Garcia PDet al. Behavioral measures to reduce non-adherence in renal transplant recipients: a prospective randomized controlled trial. Int Urol Nephrol 2015; 47: 1899–1905 [DOI] [PubMed] [Google Scholar]
- 89. Cukor D, Ver Halen N, Pencille Met al. A pilot randomized controlled trial to promote immunosuppressant adherence in adult kidney transplant recipients. Nephron 2017; 135: 6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Low JK, Manias E, Crawford Ket al. Improving medication adherence in adult kidney transplantation (IMAKT): A pilot randomised controlled trial. Sci Rep 2019; 9: 7734. [DOI] [PMC free article] [PubMed] [Google Scholar]