Abstract
Successful immunity to infection, malignancy, and tissue damage requires the coordinated recruitment of numerous immune cell subsets to target tissues. Once within the target tissue, effector T cells rely on local chemotactic cues and structural cues from the tissue matrix to navigate the tissue, interact with antigen-presenting cells, and release effector cytokines. This highly dynamic process has been “caught on camera” in situ by intravital multiphoton imaging. Initial studies revealed a surprising randomness to the pattern of T cell migration through inflamed tissues, behavior thought to facilitate chance encounters with rare antigen-bearing cells. Subsequent tissue-wide visualization has uncovered a high degree of spatial preference when it comes to T cell activation. Here, we discuss the basic tenants of a successful effector T cell activation niche, taking cues from the dynamics of Tfh positioning in the lymph node germinal center. In peripheral tissues, steady-state microanatomical organization may direct the location of “pop-up” de novo activation niches, often observed as perivascular clusters, that support early effector T cell activation. These perivascular activation niches appear to be regulated by site-specific chemokines that coordinate the recruitment of dendritic cells and other innate cells for local T cell activation, survival, and optimized effector function.
Keywords: cell trafficking, chemokines, cytokines, infectious disease, inflammation, T cells
1 ∣. INTRODUCTION
Cells of the immune system have a remarkable ability to mobilize, moving within and between tissues for their development, for activation, and for the precise delivery of anti-microbial effector function necessary for pathogen clearance. The ability to locate to specific sites within a tissue is regulated by microanatomical display of chemotactic and adhesive cues. Zonal organization of distinct stromal and immune cell types within lymphoid tissues is critical for immune surveillance and rapid response to infection.1-3 The discovery of compartmentalized chemokine expression that defined the major T and B cell zones of the lymph node (LN) back in the 1990s established a paradigm for immune cell positioning that remains central to our understanding of location-dependent immune function in lymphoid and non-lymphoid peripheral tissues. With recent advances in intravital imaging and tissue-wide high-definition histocytometry, the simple spatial map of T cell and B cell zones in the LN has been redrawn with a network of new microanatomical zones, or niches, that position immune cells for specialized function. These niches poise immune cells for initial rapid detection and response to immune challenge, shape Th differentiation, and coordinate delivery of anti-microbial function by effector T cells. Common features of these niches include a distinct physical microenvironment established through cooperative signaling between LN stroma and innate immune cells, and an ecosystem of cells clustered together that serve as producers and consumers of chemotactic, growth, and survival factors. Chemokines and lipid attractants act as molecular location pins for the recruitment and retention of naive and/or effector T cells. In turn, local T cell recruitment and activation leads to secretion of cytokines that amplify the niche through additional recruitment, immune activation, and survival. These activation clusters in the LN increase the chances that rare antigen-specific T cells will be optimally activated by cognate antigen-bearing antigen-presenting cells (APC).
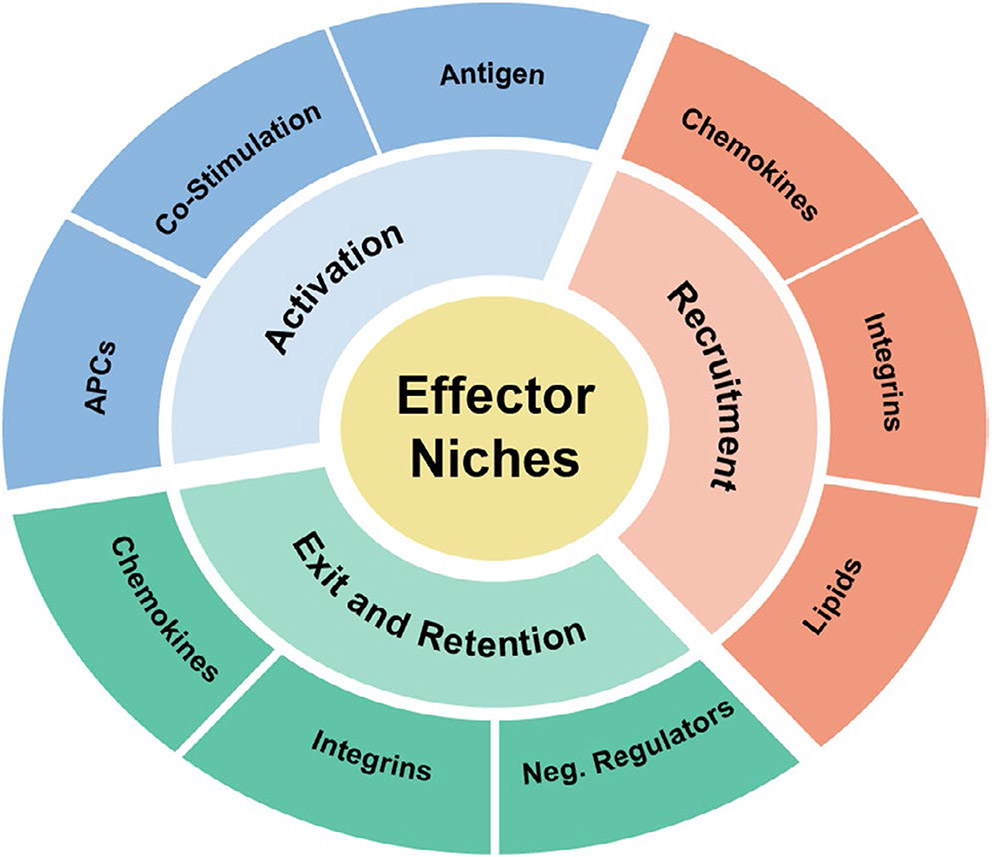
How effector T cells re-group once recruited to inflamed, infected, or malignant peripheral tissues, in contrast to lymphoid tissues, is less well understood. Nonetheless, the challenges for effector T cell activation in peripheral tissues remain similar to, if not heightened from, those in the LN. Despite clonal expansion in the LN, effector T cells of distinct specificities remain at low frequency among the pool of effector T cells entering an inflamed site. Moreover, the location and frequency of specific antigen-bearing cells may be random and rare, often in poorly organized tissues seemingly lacking discrete anatomic compartmentalization. Extra challenges to productive activation are faced by effector T cells at infected sites in which pathogens actively manipulate local microenvironments to evade immune attack and at tumors sites where immunosuppressive microenvironments are often established. Emerging studies in a variety of tissues, including the skin, brain, liver, and lung, as well as tumors, suggest that T cell activation niches can be induced in inflamed tissues under the control of localized chemokine signals emanating from tissue resident or recruited innate immune cells. These activation niches are context and tissue specific but serve the same purpose, to amplify the recruitment (and possible retention) of effector T cells to microanatomical sites enriched for antigen presentation and activation.4-8 Positional cues that facilitate niche development and function include chemokines and other chemotactic or chemo-repulsive factors, adhesive signals from integrins and their ligands, and antigen and costimulatory molecules (Figure 1). Restricting effector activation events to a physically defined microenvironment in tissues may help to separate potentially damaging immune responses from vital functions of the surrounding tissue.
FIGURE 1.
Effector niche components
2 ∣. SPATIAL PREFERENCE FOR ACTIVATION IN THE LN
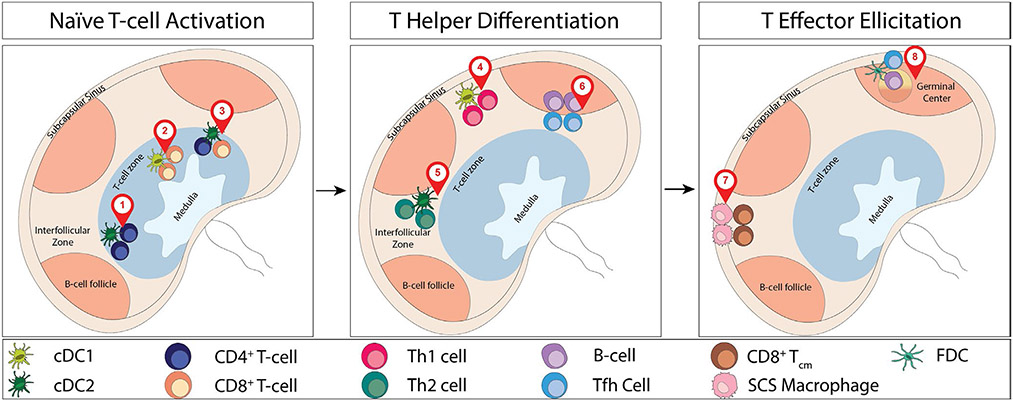
The LN is poised to rapidly respond to immune challenge through the strategic positioning of LN stroma and resident innate immune cells, located to best capture incoming lymph-borne particulate material.9-11 In the steady state, macrophages line the subcapsular sinus (SCS) floor and trap antigen, immune complexes, and viral particles and can deliver these antigens to follicular B cells.12-14 Similarly, DCs (of the cDC2 subset) line the lymphatic sinuses for rapid particulate antigen capture,15,16 while other DCs position along the LN conduit network for soluble antigen uptake in the paracortex.17-19 Intranodal repositioning of lymph node-resident DCs to these sites, and non-random distribution of incoming monocytes, may also potentiate the activation niches.20,21 With antigen capture optimized, local niche-specific production of chemokines by stroma or APCs actively creates a road-map for T cell recruitment to these areas of productive antigen presentation3 (Figure 2).
FIGURE 2.
Lymph node positioning for activation, differentiation, and elicitation. Numbered locator pins denote 1. CD4+ T cell activation (Baptista et al, 2019; Gerner et al, 2015; Gerner et al, 2017); 2. CD8+ T-cell activation (Bajenoff et al, 2003); 3. CD4+ T cell help for CD8+ T cells (Castellino et al, 2006); 4. Th1 differentiation (Groom et al, 2012); 5. Th2 differentiation (Leon et al, 2012); 6. Tfh differentiation (Kerfoot et al, 2011); 7. CD8+ Tcm activation (Kastenmuller et al, 2013); 8. Tfh help for B-cell maturation (Gitlin et al, 2014)
2.1 ∣. Initiating T cell activation
CD4+ T cells and CD8+ T cells, and respective stimulatory DC subsets, cDC2 and cDCl, have been shown to be asymmetrically distributed in the LN prior to antigen challenge,15,16,22-24 leading to spatially distinct initial activation events (Figure 2A). XCR1+ cDCl cells specialized in cross-presentation for CD8+ T cell priming were found more centrally in the T cell zone paracortex, while cDC2 cells associated with CD4+ T cell priming were observed toward the periphery of the paracortex adjacent to the SCS and the interfollicular area. The molecular signals that determine DC subset positioning in the LN are incompletely understood, but likely result from cross-talk with spatially distinct LN stromal cells.11 The zonal activation of CD4+ T cells and CD8+ T cells is also regulated by the nature of the activating event (virally infected versus particulate-uptake) and timing (early versus late during infection).19,23 It is becoming clear that chemokine production by these spatially distinct APCs is key to promoting initial T cell activation by reducing the need for extensive search for antigen by rare antigen-specific naive T cells. Indeed, prior to antigen challenge, there appears to be a steady-state spatial preference for naive CD4+ T cells at the periphery of the lymph node paracortex, matching the position of the cDC2s.24 The position of these CD4+ T cells is regulated by their higher expression of the GPCR Ebi2, compared to naive CD8+ T cells.24 Loss of Ebi2, or one of its main oxysterol ligands, 7α,25-dihydroxycholesterol (7α,25-OHC), disrupted the naive CD4+ T cell spatial preference. Changing the steady-state positioning of naive CD4+ T cells delayed antigen recognition, proliferation, and numerous effector functions. This study highlighted the functional significance of these LN pre-positioned T:DC activation niches in optimizing initial antigen-specific CD4+ T cell activation and expansion. A central function for CD4+ T cells in the LN is the provision of local help to naive CD8+ T cells, thought to depend on CD4+ and CD8+ T cell activation by the same DC, or at least activation in close proximity.25,26 Such a need for spatial coordination of the two T cell types appeared at odds with the asymmetry in naive CD4+ and CD8+ T cell distributions in the steady state. Once again, the production of chemokines by antigen-specific CD4+ T cell:DC clusters appears to provide a localization cue that increases the chances of CD8+ T cells encountering areas of productive antigen presentation. Inflammatory signals, prior to antigen encounter, led to CD8+ T cell upregulation of the chemokine receptor CCR5, enabling CD8+ T cells to localize to active CD4+ T cell:DC clusters producing the CCR5 ligands CCL3 and CCL4.27 Disrupting this cognate antigen positioning mechanism reduced the ability of CD4+ T cells to promote CD8+ T cell memory.
2.2 ∣. Th differentiation
Much of our knowledge of Th differentiation has been driven by in vitro studies of the cytokine and costimulatory signals that imprint the distinct effector programs.28-30 When and where these differentiation signals are encountered in vivo is less well understood but the process can also be aided by chemokine-guided, non-random localization of differentiating T cells from initial LN activation sites to LN niches that reinforce distinct differentiation programs. The emergence of the CXCR5+ T follicular helper (Tfh) CD4+ T cell subset,31 functionally defined based specifically on their ability to position to B cell follicles, established a precedent for zonal regulation of Th differentiation (Figure 2B). Distinct cellular sources and spatial expression of the chemokines CXCL9 and CXCL10 (ligands for CXCR3) are used to choregraph the CXCR3-dependent differentiation of CD4+ T cells into Th1 effector cells.32 Initial activation following immunization occurred via CXCL10-producing DCs in the T cell zone, while further differentiation was dependent on intranodal migration of Th1 cells to interfollicular regions enriched for CXCL9 and CXCL10 produced by local stroma, DCs, and macrophages in response to pathogen challenge. As discussed, the interfollicular region specializes in antigen capture but it is also likely to provide important spatially restricted differentiation signals, as yet ill-defined, that are induced, or preferentially collect, at the SCS from infected tissues.
In an analogous fashion, Th2 differentiation during infection with the nematode H polygyrus is optimized by upregulation of CXCR5 on both DCs and CD4+ T cells facilitating their repositioning to the CXCL13-rich perifollicular regions of the LN. While the Th2-differentiating signals proffered within the perifollicular region have not been characterized, this Th2 differentiation niche was dependent on the upregulation of CXCL13 expression by lymphotoxin-expressing B cells.33 In the spleen, a similar chemokine-based zonal segregation instructs CD8+ T cell effector versus memory fate decisions following viral or bacterial infection. In the absence of CXCR3, CD8 T cells failed to localize to the inflammatory cytokine-rich splenic marginal zone to receive signals for effector differentiation, resulting in preferential development of memory cells instead.34-36
These examples highlight the role of chemokines in directing T cells to distinct differentiation niches within the LN. However, more work needs to be done to understand the immune and stromal cell types and local signals in those niches that drive specific differentiation programs. Also, as yet unclear is the molecular imprinting of CD4+ T cells for effector function that occurs at each microanatomical location, a multi-step process that could see iterative or distinct epigenetic modifications in each location.
2.3 ∣. Effector function
Delivery of effector function demands spatial precision to rapidly target areas of pathogen infection and to avoid collateral damage to surrounding healthy tissues. As with the differential pre-positioning of naive CD4+ and CD8+ T cells, differences in naive and memory CD8+ T cell pre-positioning suggests CD8+ central memory T cells (Tcm) are poised in the interfollicular regions for prompt encounter with lymph-borne pathogens37 (Figure 2C). Local, virally induced, chemokine expression facilitates additional rapid intranodal trafficking of Tcm to the subcapsular and interfollicular sites of viral entry.37,38 In the case of LCMV, CXCR3 ligands CXCL9 and CXCL10 were locally induced by distinct arms of the immune response and synergized to accelerate Tcm repositioning for rapid viral clearance.38 Viral infection of macrophages in the SCS triggered type 1 interferon-dependent induction of CXCL10, while subsequent recruitment and cognate activation of CXCR3+ Tcm amplified local chemokine production through IFNγ-mediated upregulation of CXCL9. As will become apparent in our subsequent discussion of activation niches in infected peripheral tissues, this coordinated response between stromal and innate cells and the T cells themselves optimize and accelerate rapid repositioning and activation of effector T cells for microanatomical pathogen containment.
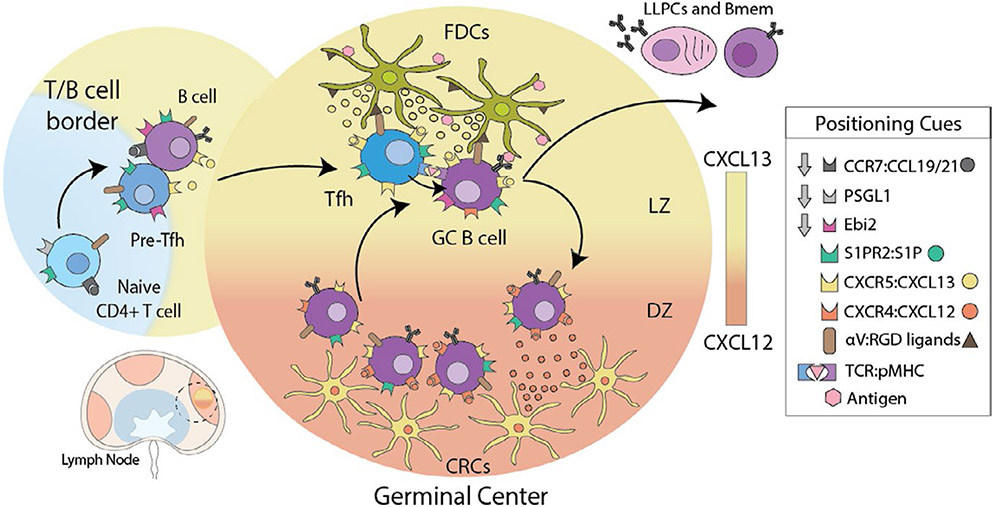
3 ∣. THE GERMINAL CENTER: A BLUEPRINT FOR EFFECTOR T CELL ACTIVATION NICHES
While knowledge of zonal activation for effector function in peripheral tissues is an emerging field, the LN germinal center (GC) has been extensively studied and is arguably the best characterized microanatomical activation niche, serving to position antigen and immune cell subsets for optimal communication and effector function. The GC is formed de novo within the LN B cell follicle following immunization or infection and coalesce B cells and Tfh with antigen-bearing follicular dendritic cells (FDC). Within this niche, B cells undergo repeated rounds of somatic hypermutation, with high-affinity B cells being selected to survive based on strength of antigen binding and competition for limited T cell help from Tfh. Close communication is critical for the development of high-affinity protective antibody responses. Zones within the GC control specific events, with GC B cell interactions with FDC and Tfh optimized in the light zone and B cell proliferation and somatic hypermutation occurring in the dark zone39 (Figure 3). The intravital study of GC dynamics has also become a rich field for innovation with respect to discovery of chemoattractant and repulsive receptor:ligand interactions that promote local cellular confinement. Recent reviews by experts in the field provide a comprehensive account of the organization and functional consequences of activation in the GC.31,40-44 Here, we use examples from the GC literature to highlight the core principles for a successful effector T cell activation niche: antigen presentation, recruitment, and optimized function (Figure 1).
FIGURE 3.
Germinal Center guidance signals. Dendritic cell-activated CD4 T cells downregulate receptors for T cell zone localization cues (PSGL-1 and CCR7), while increasing the expression of receptors that bind follicular cues (Ebi2 and CXCR5) enabling pre-Tfh to re-locate to the T-B border and interfollicular zone to interact with B cells. Stable interactions with B cells presenting cognate antigen result in the reinforcement of the Tfh program and migration into GCs, directed by expression of S1PR2 and repression of EBI2 chemotactic receptors. GC localization of Tfh is further refined by physical interactions with B cells, controlled by surface receptors that can either have an attractant or retention function. Expression of integrin αVβ3 enhances Tfh localization to GC through interactions with FDC coated with RGD ligands. After B cells receive T cell help in the GC they upregulate CXCR4 and move toward a gradient of CXCL12 expressed by dark zone (DZ) CXCL12-expressing reticular cells (CRC). Within the DZ, B cells proliferate before downregulating CXCR4 and becoming responsive to CXCL13. They return to the light zone (LZ) by following a follicular dendritic cell (FDC)-generated CXCL13 gradient for further rounds of T cell help and somatic hypermutation. B cells that survive this rigorous process of mutation and selection in the GC differentiate into either long-lived plasma cells (LLPCs) or memory B cells (Bmem)
3.1 ∣. Antigen capture and delivery
As previously discussed, the strategic positioning of specialized macrophages at the LN SCS optimizes particulate antigen capture from the lymphatics draining the site of infection. The position of the B cell follicles underneath the SCS allows for B cell sampling of antigen from SCS macrophage projections.12-14 The FDC are embedded in the B cell follicle, close to the LN sinus, and are also highly effective at capturing and displaying immune complexes in the form of antigens opsonized by antibody or complement, mediated by FDC expression of Fc and complement receptors.45 FDC can directly acquire antigen transported via the LN conduit system,46 or can be “handed” antigen by follicular B cells that have acquired antigen from the SCS macrophages. FDC form an extensive dendritic cell network that provides a large surface area for antigen display, and are non-phagocytic enabling them to retain antigen complexes on their cell surface for weeks (even months). Antigen display is coupled with chemokine production (CXCL13) to attract CXCR5+ GC B cells. B cells were found to migrate along the FDC network and capture aggregated antigen along with membrane fragments from the FDC surface.47 It is thought that GC B cells with high affinity for antigen can better “extract” opsonized antigen from the FDC. Those GC B cells are the primary MHC-II+ APC in the GC, where they internalize, process, and present antigen to compete for limiting Tfh help.
3.2 ∣. Recruitment and retention cues that colocalize immune effectors
The initiation of the GC reaction requires positioning of both Tfh and cognate antigen-presenting B cells at the T:B border, followed by follicular entry and retention in the GC itself. The study of the GC response has revealed local confinement to this niche requires a complex balance of multiple positive and negative cues that recruit to the GC and exclude from other regions of the LN (Figure 3). These confinement signals include chemokine and lipid chemoattractants that recruit cells to a region, and an emerging set of receptors that signal to stop migration and restrain movement to or within a region. The control of this positioning is regulated by temporal regulation of chemotactic receptors, and the spatial availability of ligand gradients, the production and processing of which is controlled by micro-anatomically distinct stromal cell types.48
The positioning of activated T and B cells at the T:B border is dependent on antigen-driven upregulation of CXCR5 and Ebi2 receptors. CXCL13, the CXCR5 ligand, is locally produced by the GC FDC and marginal reticular cells (MRC).49 Similarly, generation of the oxysterol lipid ligand for Ebi2, 7α,25-OHC, from cholesterol, is dependent on the enzyme Ch25h that is more highly expressed by stromal cells positioned in the outer region of the B cell follicle.50 This intranodal migration to the T:B border is also facilitated by the downregulation of CCR7 and PSGL-151 to reduce responsiveness to chemokine ligands CCL19 and CCL21 produced by fibroblastic reticular cells (FRC) in the T cell zone.
The GC forms centrally within the B cell follicle coincident with the FDC network,52 and requires movement of B cells and Tfh from the T:B border into the follicle and then to the GC. This relocation requires both the downregulation of receptors that facilitated T:B border positioning and the upregulation of receptors that actively maintain GC confinement. Downregulation of Ebi2 enables CXCR5-dependent migration toward the FDC and away from the Ebi2-Iigand enriched in the outer B cell follicle. Both GC B cells and Tfh upregulate S1PR2, a receptor that exerts an inhibitory migration signal to its ligand the lipid sphingosine-1-phosphate (S1P). S1P is abundant in lymph and blood and is required for lymphocyte exit from the LN.53 Interestingly, S1P seems to be particularly absent within germinal centers. Therefore, enhanced S1PR2 expression by GC lymphocytes prevents premature exit from the GC and LN.54 Recent studies in human have found an additional inhibitory migration receptor that promotes GC retention, P2PY8.55,56 While the ligand for P2PY8 (S-geranylgeranyl-L-glutathione, Ggg) is expressed broadly across the LN, it can be metabolized to an inactive form by glutamyltransferase enzymes. In a fascinating addition to the GC retention story, FDC were shown to express one of these enzymes (Ggt5), actively playing a role in creating a gradient of P2PY8-ligand from the GC (low expression) to the outer follicle (high expression) to constrain GB B cells to the GC.57,58 Additional positional control for GC B cells comes with movement from the light zone to the dark zone. This repositioning within the GC is once again regulated by expression of a chemokine receptor, CXCR4, by the B cells that is responsive to CXCL12 enriched in the dark zone and made by a specific subset of stromal cells, termed CXCL12-expressing reticular cells (CRCs).59
In addition to secreted chemotactic and chemo-repulsive gradients, a number of cell:cell, contact-dependent interactions also direct and maintain GC positioning. Interactions between GC B cells and Tfh are promoted and stabilized by a variety of membrane receptor:ligand interactions.40 Reciprocally, long-lasting signaling by Tfh:B cell conjugates at the T:B cell border is critical for progression into the follicle and GC development. For example, disruption of the adaptor signaling lymphocytic activation molecule (SLAM)-associated protein (SAP) in T cells reduces the stability of Tfh:B cell interactions and leads to a defect in localization to the GC.60,61 Similarly, expression of the adhesion guidance receptor semaphorin 4C on the surface of Tfh facilitates the interaction with plexin B2 presented on GC B cells.62 Semaphorin 4C sensing of plexin B2 by Tfh biased their movement into the GC, and the absence of semaphorin 4C on Tfh led to mis-localization to the edge of the GC. Additional integrin-based adhesive interactions between GC B cells, Tfh, and the FDC may also help retain cells in the GC. FDC display distinct extracellular matrix components, such as vitronectin, on their cell surface that may serve as adhesive ligands for matrix-binding integrins on GC B cells and Tfh for GC retention.63,64 Indeed, loss of the integrin αv on Tfh cells led to mis-localization outside of the GC and impaired GC-dependent long-lived plasma cell (LLPC) generation.64
3.3 ∣. Functional maturation
The interactions between T and B cells at the T:B border and within the GC play important roles in stabilizing Tfh differentiation, and in shaping the functional maturation of the GC B cells into LLPC or memory B cells.44,65 Tfh differentiation is a complex and incompletely understood process, which requires both initial activation by DCs followed by secondary interactions with antigen-bearing B cells.31,42 Tfh differentiation begins with induction of the master transcriptional regulator BCL6, which has been shown to be both necessary and sufficient for phenotypic and functional Tfh.66-68 BCL6 is a transcriptional repressor of BLIMP-1 and directly or indirectly regulates the expression of many other target genes.69 Interactions with DC are thought to induce BCL6 through synergy of TCR, ICOS, IL-6, and IL-12 signaling. This pre-Tfh program is sufficient for a fraction of the primed CD4 T cells to acquire CXCR5 expression; however, this program is lost rapidly in the absence of cognate B cell interactions.70,71 Pre-Tfh form both cognate and non-cognate interactions with B cells at the border of the follicle and within the interfollicular zone. Non-cognate interactions with B cells enhance pre-Tfh motility at the T-B boarder effectively enhancing the probability of interaction with cognate B cells.72 Once cognate pairs of T-B cells form, ICOSL expression by antigen-specific B cells is critical to maintaining and stabilizing the Tfh program prior to entry into the germinal center.73 This interaction stabilizes BCL6 expression, downregulates EBI2, and upregulates S1PR2 for migration into and retention within the GC. Tfh deliver their effector function within the GC in the form of T cell help for B cells. Tfh help is a summation of cognate signals between Tfh and GC B cells. Key signals include CD40L-CD40 engagement74-77 and the supply of the cytokines IL-21 and IL-4.78,79 A number of factors enhance these “help” signals including ICOS-ICOSL,80 and more recently, the level of BCL6 itself expressed in Tfh that appears to control the degree of CD40L “delivered” to GC B cells.81 Thus, the GC niche both stabilizes the Tfh differentiation program and supports Tfh effector function.82
4 ∣. T CELL ACTIVATION NICHES IN PERIPHERAL TISSUES
In contrast to the highly organized and functionally distinctzones present in the LN and spleen for optimized T cell encounter with antigen presentation, inflamed peripheral tissues are often disorganized and have been extensively remodeled by inflammatory cytokines, infection, or malignancy. Ex vivo phenotyping of immune infiltrates and inflammatory mediators has provided context-specific profiles of infection sites, but how these immune cell types are positioned within the tissue to optimize re-activation of effector T cells and targeted delivery of effector function has been difficult to dissect. Immune cell clusters have been observed in static slices from infected tissue for decades, supporting the idea that lymphocytes have the capacity to self-organize once recruited to the inflamed tissue. However, basic organizational information at inflamed sites remains elusive, including: the availability and distribution of cognate antigen-bearing APC; the source and three-dimensional patterning of chemokine signals; the search capacity and range of incoming effector T cells and the local manipulation of effective immune positioning by the pathogens themselves. The ability to observe immunity in action, in situ, with the use of intravital multiphoton imaging has provided an opportunity to study the tissue-specific dynamics of cell movement and cell communication in a variety of disease states.83 Peripheral activation clusters are often self-organized and require context-dependent sequential signals from resident stromal/innate cells (macrophages, DC) and early attraction of additional innate cell subsets (monocytes, neutrophils, and moDCs) followed by effector T cell recruitment.84-88 Observations of such dynamic behavior, combined with whole tissue histocytometry, have shaped key mechanistic questions that will continue to lead to new insight into the molecular basis of coordinated immunity in infected, inflamed, or malignant tissues. Parallels to the LN are now being seen with both strategic tissue-specific pre-positioning of innate immune cells for rapid response to pathogens, and induced hubs where chemokines orchestrate the repositioning of innate cells, APCs, and effector T cells into peripheral activation niches (Table 1, Figure 4).
TABLE 1.
Effector T cell activation niches in inflamed, infected, and malignant tissues
| Innate cells |
Key effector molecules |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T cell | Context | Tissue | Mac | Mono | DC | MoDC | Neu | ILC | Chemokines | Cytokines | Ref |
| Effector CD4 | Immunization | Skin (PV) | ✓ | CXCR3-CXCL10 | IFNγ | 4 | |||||
| Contact Hypersensitivity | Skin (PV) | ✓ | ✓ | CXCR2-CXCL2 | IL-1R-IL-1α, IFNγ | 5 | |||||
| Skin (HF) | ✓ | ✓ | CCR2-CCL2, CXCL1, CXCL2 | IFNγ, TNFα, MIF | 135 | ||||||
| Viral | Skin (D) | IFNγ | 136 | ||||||||
| Bacterial | Liver (G) | ✓ | ✓ | TNFα, IFNγ | 84,134 | ||||||
| Autoimmune | CNS (PV) | ✓ | CXCR3-CXCL10/9/11, CCL5 | IFNγ | 138,139 | ||||||
| Parasite | Skin (PV) | ✓ | CXCR3-CXCL10 | IFNγ | 4 | ||||||
| Lung (AC) | ✓ | IL-33, IL-5 | 103 | ||||||||
| Tumor | Colon | ✓ | ✓ | ✓ | CXCL10/11, CXCL13 | IFNγ | 8 | ||||
| Effector CD8 | Contact Hypersensitivity | Skin (HF, PV) | ✓ | ✓ | ✓ | CXCR2-CXCL2, CCR2-CCL2, CXCL1 | IL-1R-IL-1α, IFNγ, TNFα, MIF | 5,135 | |||
| Viral | Skin (HF, E-D) | ✓ | CXCR3-CXCL10/9 | IFNγ | 136,140-142 | ||||||
| Liver | ✓ | ✓ | ✓ | CCR2 | 143 | ||||||
| CNS (PV, AC, MN) | ✓ | CCR7-CCL19/21 | IFNγ, TNFα | 86,141 | |||||||
| Lung (PV) | ✓ | ✓ | ✓ | 122,145 | |||||||
| Lymph Node | ✓ | CXCR3-CXCL10, CCR7 | 146 | ||||||||
| Bacterial | Spleen | ✓ | ✓ | ✓ | 147 | ||||||
| Tumor | Skin (PV) | ✓ | ✓ | CXCL16-CXCR6 | IL15-IL-15rα | 6,7 | |||||
| Colon | ✓ | ✓ | ✓ | CXCL10/11, CXCL13 | IFNγ | 8 | |||||
| Memory CD4 | Viral | Skin (HF) | ✓ | CCL5 | IFNγ | 87 | |||||
| Vaginal mucosa | ✓ | ✓ | CCL5 | IFNγ | 88 | ||||||
| Parasite | Lung (AC) | ✓ | IL-33, IL-5 | 103 | |||||||
| Steady state | CNS (DS) | ✓ | ✓ | CXCR4-CXCL12 | 111 | ||||||
| Memory CD8 | Viral | Skin (HF) | ✓ | IFNγ | 87 | ||||||
| Vaginal mucosa | ✓ | ✓ | CCL5, CXCL9 | IFNγ | 88 | ||||||
| Autoimmune | CNS (PV) | ✓ | CXCR6-CXCL16, CCL5 | 139 | |||||||
| Skin (HF) | ✓ | ✓ | CXCR6-CXCL16 | 148 | |||||||
| Steady state | CNS (DS) | ✓ | ✓ | CXCR4-CXCL12 | 111 | ||||||
| Regulatory CD4 | Autoimmune | CNS (PV) | ✓ | CXCR3-CXCL10/9/11 | IFNγ | 138 | |||||
| Parasite | Lung (AC) | ✓ | IL-33, IL-5 | 103 | |||||||
Abbreviations: AC, adventitial cuff; D, dermis; DC, dendritic cell; DS, dural sinus; E-D, epidermal-dermal border; G, granuloma; HF, hair follicle; ILC, innate-like lymphocyte. Tissue: PV, perivascular; Innate cells: Mac, macrophage; MN, microglial nodules; MoDC, monocyte-derived DC; Mono, monocyte; Neu, neutrophil.
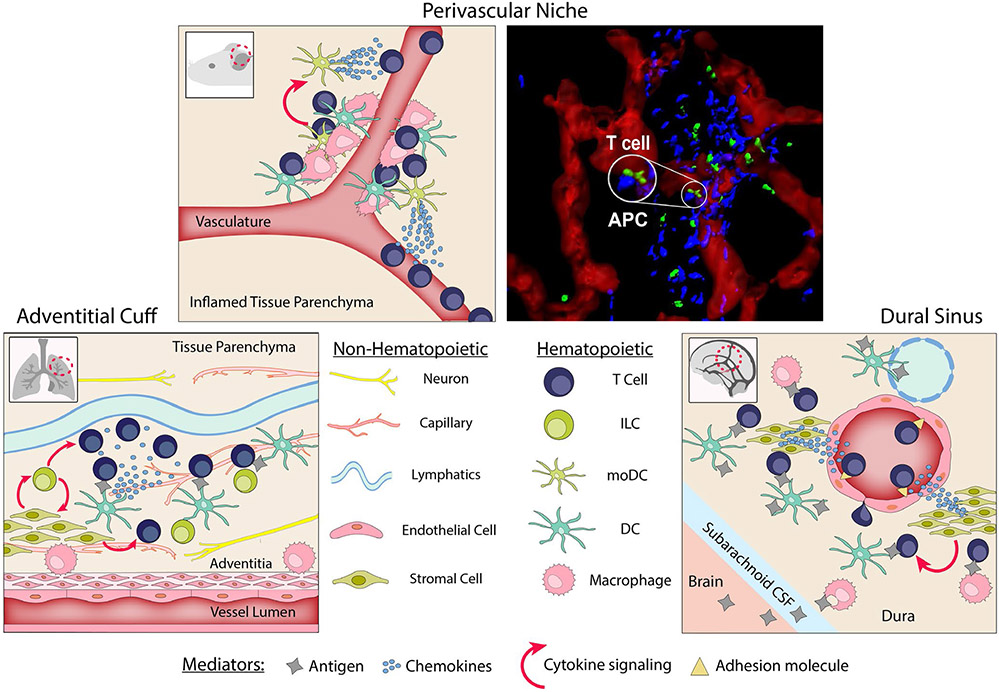
FIGURE 4.
Peripheral activation niches. The Perivascular Niche. Induced following foreign antigen challenge, perivascular macrophages recruit APCs (DCs and moDCs) for de novo assembly of the perivascular niche. APCs secrete chemokines that locally induce effector T cell extravasation. Within the perivascular niche, local antigen presentation reactivates effector T cells to secrete effector cytokines. Secretion of cytokines by effector T cells further upregulates chemokine secretion by the moDCs to enhance the niche and attract and activate additional effector T cells. Right panel, rendered image of the inflamed dermis obtained by intravital multiphoton microscopy: red, CD31 vessels; green, effector T cells (Th1); blue, chemokine-producing APC (CXCL10+) (Prizant et al, 2021). The Adventitial Cuff. Pre-positioned prior to foreign antigen challenge at the confluence of blood vessels, lymphatics, nerves, and niche-specific stromal cells. Adventitial stromal cells support a population of ILCs which modulate stromal secretions, and together support resident immune cells. During inflammation, ILCs stimulate local DCs to secrete a T cell chemoattractant. Resident macrophages act as alternative APCs. The Dural Sinus. Pre-positioned prior to foreign antigen challenge at the confluence of blood vessels, lymphatics, and niche-specific endothelium and stroma. Homeostatic patrolling by T cells is mediated through the expression of adhesins and chemokines by vascular endothelia and dural stroma, respectively. Once in the dural sinus, T cells rely on DC/macrophage antigen presentation for activation and retention cues. Resident Tregs rely in part on stromal-derived cytokines for long term survival
4.1 ∣. Innate pre-positioned niches
It has long been appreciated that tissue-resident innate cells are positioned for rapid response to pathogen challenge, with macrophages and dendritic cells poised beneath or between epithelial cell surfaces of the skin, lung, and gut. More recently, a series of studies focused on innate lymphoid cells (ILCs)89 have revealed distinct innate activation niches in the steady state, at barrier tissues. Innate activation in these niches appears to be facilitated by the juxtaposition of tissue stroma, peripheral nerves, dendritic cells or macrophages and the ILCs.90-93 ILC3s are found in the small intestines and colon in cryptopatches, clustering among dendritic cells and fibroblastic stromal cells. The stromally produced oxysterol 7α,25-OHC acts as a positioning cue for Ebi2+ ILC3 recruitment into the niche and is critical for tissue homeostasis and pathogen control.94,95 For ILC2s, cytokines such as IL-33, IL-25, and TSLP released from epithelium or tissue stroma expand and activate the ILCs, while neural sensing regulates this process. Mucosal tissues are heavily innervated and ILC2s express receptors for neurotransmitters and neuropeptides. ILC2s were found positioned in close contact with neurons in the intestinal mucosa.96,97 In support of active cross-talk between ILCs and neurons, parasite and allergen challenge led to the rapid production of the neuropeptide NMU, which was shown to amplify ILC numbers and function.96-98 Alternative neural signals have been identified as negative regulators of ILC2, with enhanced ILC2 function in response to the helminth Nippostrongyrus brasiliensis in the absence of β2 adrenergic receptors.99 It is likely that the outcome of neuroimmune sensing will differ depending on the tissue location and nature of the damage signal.
Here, we highlight two vascular-associated anatomical structures, the adventitial cuff and the dural sinus, that appear to be particularly poised to facilitate microbial sensing and antigen uptake by innate immune cells, and that may serve as important tissue “outposts” for optimized presentation of antigens to newly recruited effector T cells. These spatially distinct niches mark microenvironments primed for APC accumulation, immune cell infiltration, and efficient T cell reactivation.100,101 Innervation of the surrounding tissue positions these immune cells to be directly receptive to nervous system triggering in response to environmental stimuli during an inflammatory response.102 The colocalization of draining lymphatics makes many of these perivascular niches also ideal antigen-rich environments for effector T cell activation.
4.1.1 ∣. The adventitial cuff
The adventitial cuff forms an interesting microanatomical site where tissue-derived signals (antigens, TLR ligands, and cytokines) carried in the draining interstitial fluid meet specialized stroma and resident DCs and ILCs poised to respond.92 Adventitial cuffs are the outer layer of ECM-rich extravascular space surrounding medium to large-sized blood vessels, a vascular area responsible for supplying oxygen to the muscular walls of the vessel and for the removal of tissue fluids. The anatomical niche is enriched for lymphatics and peripheral nerves in addition to innate immune cells, and therefore serves as an important steady-state surveillance site (Figure 4). Specialized “adventitial” stromal cells (ASCs) in this niche respond to a variety of environmental stimuli and secrete chemokines and cytokines to modulate local immune cells such as macrophages and DCs. A recent study using tissue clearing and 3D imaging has identified the adventitial cuff as a preferred site for ILC2s in the lung and other tissues including the brain and adipose tissue.103 The ILC2s were found in close proximity to ASCs, the ASCs being responsible for supporting resident ILC2 via IL-33 and TSLP secretion. Subsequent ILC2 production of IL-13 reciprocally regulated ASCs, enhancing IL-33 production. The ILC2 population in the cuff may also be responsive to regulation by the nerves in this niche, similar to the studies previously described.96-98 Interestingly, regulatory T cells (Tregs) were also enriched in the adventitial cuff and may help to maintain tissue homeostasis and limit excessive immunity.
Important in the context of this review, the adventitial cuff was also a preferred site of effector Th2 cell expansion following Nippostrongyrus brasiliensis infection.103 The lymphatic drainage of antigen through these cuffs ensures rapid and efficient presentation by the resident APC populations and local chemokine production from ASCs and ILCs promote recruitment of T cells to these active sites of antigen presentation. Indeed, deletion of ASCs reduced both the number of ILC2s and Th2 cells in the infected lung.103 ASCs express a number of chemokines and the enzymes needed for the generation of oxysterols to help guide effector T cell positioning. ASCs are able to secrete CCL8 to assist in Th2 homing in allergically inflamed skin as part of an atopic dermatitis model.104 In addition, ILC2-derived IL-13 can stimulate DCs to produce the Th2 chemoattractant CCL17.105 Resident macrophage populations, which expand during inflammation, can act as alternative APCs. B cells have also been observed infiltrating the adventitia and accumulating in nodules in a CXCL13-dependent manner, with CXCL13 being secreted by ASCs after IFN or IL-6 stimulation.106,107 Thus, the innate activation niche of the adventitial cuff in the steady state also provides a preferred niche for re-activation of effectors cells following infection. At this stage, the route of lymphocyte trafficking to the adventitial cuff is not known. It is unlikely that cells exit the bloodstream from these large vessels, so they may migrate from the parenchymal of the tissue to this site in response to local chemokines.
What makes the adventitial cuff especially interesting is the potential to generate a “memory niche,” reshaping the landscape for better effector function later on. ASCs are able to develop a type of trained memory with IL-8 expression being modulated by prior IFN exposure, VCAM-1 surface expression remaining elevated long after removal of stimuli, and IL-33 expression remaining elevated months after helminth infection.92,108 Resident ILC2s act as drivers for the accumulation of tissue-resident memory (TRM) Th2s to contribute to the memory pool.103 In addition, the local lymphatics expand along with the influx of immune cells at the adventitial cuff and persist long after the clearance of the infection.109 Lymphatic cells can express IL-7 and contribute to the survival of TRM Th2 cells following allergic challenge in the airways.110
Overall, the evidence points to these adventitial cuffs, home to ASCs as well as innate and adaptive immune cells, being potent modulators of immune surveillance and regional tissue immunity. Their ability to monitor tissue antigen drainage through the locally expanded lymphatic network, supporting ILCs, and maintaining TRM T cells makes them spatial hotspots for immune response initiation, effector function, and memory.100
4.1.2 ∣. The dural sinus
The dural sinuses, spread throughout the dural meninges of the brain, colocalize draining Ag with potent APCs in perivascular regions to allow for optimized presentation to resident and patrolling T cells (Figure 4). The sinuses collect blood from the cerebrum before emptying into the jugular vein and gather central nervous system (CNS) derived antigens from the cerebral-spinal fluid (CSF)101 T cells, MHC-II+ macrophages, and DCs are found to be highly localized to the dural sinus and in close proximity to both blood vessels and lymphatics.111-113 Analogous to the adventitial cuff, these niches rely on signals from resident stromal and endothelial cells to enhance antigen presentation and recruit effector T cells. The recent generation of a cellular and molecular atlas of the dural sinus revealed distinct region-specific signatures of the endothelium and dural stroma.111 The dural sinus endothelium lacked tight junctions and preferentially expressed adhesion molecules VCAM-1 and ICAM-1 to facilitate homeostatic T cell leukocyte trafficking through the dural sinuses. The sinusoidal stroma homeostatically expressed a range of chemokines including CXCL12 and CXCL16, which recruited CXCR4- and CXCR6-expressing T cells, respectively. Basal levels of CX3CL1 expression were also observed in the stroma, a chemokine known to attract CX3CR1+ macrophages. Once recruited to the dural sinus, elegant studies tracing CSF solutes confirmed that brain-enriched proteins can be captured by dural macrophages and DCs and presented to the colocalized T cells.111
Acting as one of the main means of immune surveillance in the immune-privileged CNS, the meninges and dural sinuses must maintain a balance of inflammation to prevent local swelling and damage.114 Tregs have been shown to be key immunomodulators in the CNS, using IL-10 secretion to control TNF and IFNγ expression by local immune cells.115 A population of Tregs is maintained in the sinuses and may be sustained by the expression of IGF1 by the dural stroma.111,116 After dural APC presentation of Ag to patrolling T cells, some cells are polarized into Tregs to bolster this population and can last for weeks as resident long-lived effectors.
Thus, the dural sinuses serve as regional hubs of immune surveillance in the CNS. The colocalization of vascular and lymphatic drainage makes this an ideal location for monitoring of the CNS inflammatory environment, with resident stroma and APC optimizing immune cell recruitment, activation, and regulation.
4.2 ∣. Induced activation niches
While the recent study of the adventitial cuff suggests this pre-positioned innate activation niche can also serve as a site for subsequent effector T cell activation,103 it remains unclear whether the induced clustering of effector T cells with APCs seen in many inflamed tissues (Table 1) arises at predetermined locations within the tissue parenchyma. A growing body of evidence points to the de novo assembly of microanatomical activation hubs located perivascularly that are induced following immune challenge and that under-pin T cell immunity within inflamed tissues.4,5,7,8,86,117-119
4.2.1 ∣. Perivascular activation niches
The perivascular activation niche has emerged as a preferred site of initial effector T cell activation in a variety of inflamed, infected, and malignant tissues. The niche is induced following immune challenge and has been identified by the accumulation of myeloid cells with antigen presentation capacity close to post-capillary venules and the colocalization of effector T cells. The specific immune cells recruited to this niche will likely differ depending on the tissue and the type of pathogen; however, common themes are emerging. The induction of innate signals, such as type 1 interferons, initiate the activation and recruitment of myeloid subsets to the perivascular space, where local niche-specific chemokine production and antigen presentation optimizes encounter with incoming effector T cells.4,5 It is not yet known if the induced perivascular niche arises from any strategic positioning that might optimize enrichment of antigen, as seen in the SCS of the LN or the adventitial cuff. In the skin, following complete Freund's adjuvant (CFA)-protein immunization, perivascular activation niches “popped up” throughout the ear pinna, often thousands of microns away from the antigen depot, suggesting they may be broadly induced to maximize opportunities for antigen encounter.4 Interestingly, in a contact hypersensitivity (CSH) model, the induced perivascular activation niche occurred alongside focal areas of edema, which may locally enhance interstitial flow and antigen accumulation.5
Sequential recruitment of leukocyte subsets to the perivascular niche is coordinated by a cascade of chemokine signals. Macrophages appear to play a critical role in perivascular APCs clustering. IL-1α signaling promotes macrophage CXCL2 release that triggers dermal DC perivascular clustering,5 an essential prerequisite for highly localized activation of effector T cells and IFNγ release. CXCR3 expression by effector T cells was implicated in positioning of T cells in the inflamed dermis through engagement with induced tissue expression of CXCL10.120,121
In a recent study, the use of a CXCL9/CXCL10 dual reporter mouse (REX3)32 and intravital multiphoton microscopy enabled dissection of the cellular dynamics within the perivascular niche of the inflamed dermis.4 Dermal inflammation induced the formation of de novo perivascular CXCL10+ cellular clusters, enriched for chemokine-producing CD11b+ CD11c+ MHC-IIhigh monocyte-derived DCs (moDCs). Perivascular expression of CXCL10 marked “hotspots” for CXCR3+ Th1 effector T cell extravasation into the tissue. Upon entry, Th1 cell motility was confined within the perivascular niche and Th1 cells made prolonged contact with the CXCL10+ cells in the clusters. Chemokine signaling acted as a positioning signal to locate to the niche, while MHC-II-dependent antigen presentation provided a critical retention cue. Failure to enter the perivascular niche led to a marked reduction in Th1 activation and IFNγ production. Thus, the CXCL10-rich perivascular niche serves to link effector T cell tissue entry with early tissue activation for Th1 effector function. Moreover, early Th1 cell activation in the perivascular cluster amplified the CXCL9/10+ niche via an IFNγ-dependent feedback loop4 and may encourage addition effector T cell recruitment. Single-cell analysis of colorectal cancers in humans8 also identified a CXCL10+ perivascular niche associated with an accumulation of IFNγ+effector T cells (see discussion below).
Activation in these perivascular niches appears to be an important first step in T cell activation in inflamed peripheral tissues. In a study following effector CD8+ T cells through the course of influenza infection, CD8+ T cells were associated with prolonged contacts with perivascular DC clusters during the early clearance phase of infection.122 In later stages, CD8+ T cells shifted in behavior to rapid movement throughout the tissue likely allowing them to survey and locate residual infected cells. A similar phenomenon was observed in a model of fetal cerebral malaria in which T cells were initially associated with perivascular APCs but were found to be moving rapidly throughout the tissue in later stages of the disease.119 This points to a two-step process in peripheral tissues wherein antigen-specific T cells become reactivated at perivascular niches during the early response and then, as functionally poised effectors, remain in the tissue to deliver effector cytokines that contribute to pathogen control or immunopathology.
The advantage to spatial compartmentalization of effector T cell activation in the periphery is perhaps best illustrated by recent reports describing perivascular niches in tumors. High-resolution spatial imaging using histocytometry revealed perivascular niches containing DCs, MHC-II+ macrophages, and CD8+ T cells in two murine tumor models.7 These niches resided in the outer vascularized region, away from the deeper tumor nest and increased in abundance in response to bispecific T cell antibody therapy. Interestingly, the perivascular niche was populated by TCF+ PD1+ T cells (non-exhausted, stem-like) while the deeper tumor nest was populated by TCF− PD1+ terminal or exhausted effector T cells. TCF+ PD1+ T cells are thought to be progenitors of the exhausted effector T cells123 and responsive to checkpoint blockade.124 Similarly, in a mouse model of melanoma, perivascular clusters of CXCL16-producing DCs positioned CXCR6+ effector CD8+ T cells in the activation niche and provided an IL-15-dependent survival signal.6 The expression of CXCR6 marked a transition from stem-like TCF+ PD1+ T cells to TCF− PD1+ effector T cells. Taken together,6-8 these studies indicate that perivascular niches may provide a protected activation microenvironment that feeds the pool of anti-tumor effectors. This component of the tumor microenvironment may be an effective target for expanding the pool of effector T cells for subsequent infiltration and control of the tumor mass.
Thus, inducible perivascular activation niches can be found in a variety of tissues and assemble in response to a host of immune challenges. The microanatomical location of these “pop-up” activation hubs is poorly understood, but may be governed by an as yet unidentified stromal cell type that is strategically poised to initiate activation of resident innate cells such as the perivascular macrophages. The fate of effector T cells activated in this niche is unclear, but emerging data suggest early activation upon tissue entry may provide important signals that strengthen or maintain the effector T cell program for better anti-microbial or anti-tumor immune responses. Whether these sites resolve with the general resolution of the immune response remains to be determined. Drawing parallels with the adventitial cuff and the dural sinus, it is tempting to speculate that stromal/innate cells within the perivascular niche may retain some trained memory for efficient activation at that particular spot in response to subsequent immune challenge, and/or provide a safe harbor for TRM.
5 ∣. THE NICHE ADVANTAGE
In the fields of marketing and economics, a competitive niche advantage seeks to target and expand a specific segment of the market. The advantage is that a company can focus on quality engagement, customize offerings (or product/service), hone expertise, reduce competition, and increase visibility. Using these “cues” from the economic world, we speculate on the functional advantage of activation of effector T cells in spatially distinct activation niches in inflamed tissues.
5.1 ∣. Quality engagements, better visibility
Efficiency of immune activation is enhanced when trial and error is reduced. The random, non-directional, patterns of T cell interstitial movement observed by intravital microscopy83 led to a series of studies aimed at determining cell intrinsic and extrinsic mechanisms that might optimize the random search for APCs at inflamed sites.125-127 Lack of information on the distribution of cognate antigen-bearing APC and an inability to visualize the chemotactic landscape has limited investigations. The identification of induced perivascular activation niches in inflamed tissues suggests a model where local chemokine production is linked to enriched hubs of antigen presentation. By effector T cells exiting the circulation directly into these activation niches,4 the chance of rapidly encountering antigen is increased and the need for protracted search reduced.
Crosstalk between resident and recruited innate effectors and incoming effector T cells likely enhances the quality of engagement signals in the niche. Th1 cells in the CXCL10-enriched perivascular niche had more prolonged contacts with CXCL10+ cells in the niche than CXCL10+ cells outside of the niche suggesting the niche supports sustained T:APC interactions.4 APCs in the perivascular niche were enriched for high MHC-II expression and were more likely to co-express chemokines that have been implicated in stabilizing the T:APC synapse and providing costimulatory signals.128,129 Further analysis is needed to determine niche-specific properties of the APC in these perivascular activation hubs.
There may also be a kinetic advantage to rapid TCR engagement on entry into the inflamed milieu. The 3-signal model of CD4+ T cell activation (antigen, costimulation, and cytokines) results in optimal activation and expansion of antigen-specific CD4+ T cells, but if these signals are out of sequence a hyporesponsive state occurs in both naive and memory CD4+ T cells.130 Specifically, exposure to inflammatory cytokine signaling before antigen engagement impaired CD4+ T cell function. A protracted search for APC in the inflamed tissue may expose effector T cells to signal 3 cytokines in the absence of TCR triggering. Thus, initial effector CD4+ T cell re-activation in the inflamed tissue may benefit from provision of pMHC/TCR signals in concert with the inflammatory signals of the infected tissue. Indeed, for CD8+ T cells, provision of inflammatory signals coincident with pMHC/TCR was able to tune sensitivity to antigen, lowering the threshold requirements for antigen density to trigger effector function.131
Niche visibility appears to be enhanced by a T cell-mediated positive feedback loop, where cytokines secreted by T cells in the niche upregulate local chemokine expression.4 At early stages in the immune response to infection, antigen-specific effector T cells entering the infected tissue are at low frequency. These early T cell tissue pioneers may increase visibility of the niche for more efficient recruitment of effector T cells that follow later in the infection.
5.2 ∣. Reduce competition
Effector T cells are subject to functional competition from a variety of sources within the inflamed tissue. Competition can arise from antagonizing effector or regulatory immune cell types. Th2 cells can antagonize Th1 cell recruitment and activation, and myeloid suppressor cells and Tregs can locally block immune activation. Moreover, there is often active tampering with immune function by pathogens with strategies to evade immune detection. Insulating early effector T cell activation away from the tissue milieu, by initial activation in a supportive niche that provides positive activation signals, is likely to amplify the incoming antigen-specific pool and/or boost function. Indeed, in the dermal CXCL10+ perivascular niche enriched for Th1 cells, Th2 cells were excluded from the niche.4 In tumors, the chemokine-rich perivascular niche appears to provide a “safe haven” microenvironment that supports the survival or activation of new, not yet exhausted, cohorts of anti-tumor CD8+ T cell effectors.6 Of therapeutic interest, this niche appeared to expand following immunotherapy, suggesting cells within the perivascular microenvironment may be especially receptive to immune interventions aimed at boosting effector function.7 In contrast to the reduced competition in the induced perivascular niche that enhances immune stimulation, the pre-positioned innate niche appears poised for homeostatic regulation. The adventitial cuff and dural sinus are both associated with an enriched Treg population, that likely limits immune activation by outcompeting potentially harmful effector T cells that might respond to self-antigens.
5.3 ∣. Hone expertise
Initial peripheral activation in the inflamed tissue at these perivascular niches could hone effector T cell expertise at two levels: by enrichment of “useful” antigen-specificities and/or by enhancing functional gene programs. The entry of effector T cells into inflamed tissues occurs in an antigen non-specific fashion. Therefore, having an early sorting mechanism that expands those effectors with antigen-specificities that are relevant to the particular pathogen would have an immediate impact, or rapid return on investment, on the anti-microbial response. The coupling of targeted extravasation at perivascular niches enriched for antigen presentation capacity may help to quickly hone the specificity of the effector T cell pool.
Effector T cells within the perivascular niche appear to be better stimulated and have enhanced cytokine production compared to effector T cells outside the niche, but the functional consequence of activation in the niche is yet to be fully investigated. Th differentiation is inherently plastic28,29 and the inflammatory milieu of the infected tissue has the potential to enforce or reprogram effector potential through epigenetic changes to the effector transcriptome. Context-specific recruitment of innate effectors to the niche, such as inflammation-modified macrophage and moDC populations, could provide potent instructional cues for effector cytokine gene regulation. In this way, those innate cells recruited to the niche may act a market analysts, having surveyed the target tissue they are best placed to direct the most efficient functional strategy, the unmet need, which they deploy through optimizing the effector T cell product in the activation niche. This idea that the inflamed target tissue may ultimately be the site where stable effector programs are established is not new,132 but these perivascular activation niches may provide an optimized location for commitment to a particular effector program.
6 ∣. CONCLUSIONS AND FUTURE DIRECTIONS
The emergence of specialized niches that provide a platform for activation of effector T cells in inflamed peripheral (non-lymphoid) tissues raises numerous questions, but at the same time holds great promise for therapeutic targeting. The basic activation unit appears to include niche-specific stroma, enriched antigen and antigen presentation, elevated chemokine expression, access to the vasculature and targeted recruitment of functionally distinct effector T cells. The main outcome being a robust and finely tuned (specificity and function) effector T cell response for better pathogen or tumor clearance. The cellular players and the molecular positioning cues will likely differ depending on the inflammatory context and the target tissue. Current examples focus on the positive contributions that activation within a specialized niche can provide, to bolster effector T cell numbers and strengthen function in infected and malignant tissues. However, these niches in an autoimmune or chronic inflammatory setting may serve to exacerbate disease. So, what more do we need to know?
6.1 ∣. Position of activation niches?
Blood vessels appear to be key organizational hubs for peripheral activation niches that expand the effector T cell pool in infected and malignant tissues. These inducible niches are associated with post-capillary venules where leukocytes preferentially exit the circulation. It is unclear at present if these sites originate from an existing pre-positioned steady-state niche, akin to the advential cuff. At least in the skin,4 they are seen “scattered” across large areas of the inflamed tissue suggesting they may serve to cast a wide net for initial effector T cell recruitment. Alternatively, the distribution of the activation niches could be stochasitic and directed by the incoming T cells themselves. Initial tissue entry may be random, with the niche being assembled de novo at sites where incoming effector T cells happen to encounter cognate APCs in the perivascular space. The spatial relationship between these initial activation hubs and the infection foci itself is also not known, but it is likely that the initial activation event upon entry into the tissue will necessitate a subsequent relocation of that expanded effector T cell pool to the infection site for pathogen clearance, as previously predicted.119,122
6.2 ∣. Positional cues?
Recent studies have utilized spatially distinct hubs of chemokine production as markers of the perivascular niche, namely CXCL104,8 and CXCL16.6 Manipulating these chemokines or their receptivity disrupted effector T cell positioning to the activation niche, establishing that the chemokines play an active role in directing effector T cell location. Studies revealing the complex combinatorial control of cell positioning in the GC highlight that spatial confinement is controlled by balancing multiple positive and negative signals. These include cell- and location-specific generation and processing of chemoattractants and adhesins. The GC field is at the forefront of understanding such microanatomical confinement, and their continued discovery of new positioning cues suggests our current studies of spatially distinct chemokine hubs are just scratching the surface of a complex set of signals that position effector T cell for activation at infected sites.
6.3 ∣. Exiting and reusing the niche?
While the perivascular, adventitial, and dural niches specialize in antigen presentation in tissues, they are rarely the site of direct infection. As discussed, effector T cells activated in the niche need to exit and move to the infection foci to deliver anti-microbial activities. This requires desensitization to the attractants that enabled niche recruitment in the first place and possible upregulation of receptors that enable interstitial migration and the “search” for the pathogen itself. How this may be achieved is not well understood. Interestingly, terminating TCR-signaling via upregulation of negative regulators such as PD1 or CTLA-4 may be a sufficient trigger for the transition from arrest in the niche to resumption of migration capacity.133,134
Intravital imaging in combination with a photoactivatable fluorescent probe revealed dynamic exchange of Tfh between GCs in the LN.78 Tfh could move between neighboring GCs and participate in B cell selection in multiple GC throughout the LN. It is not yet known if effector T cells can similarly move between perivascular niches in the same tissue and what function that might serve. To that end, understanding of the dynamic nature of the niche itself would be informative: do the perivascular niches resolve following pathogen clearance or do they persist to be reused upon subsequent pathogen challenge. There is also the possibility the perivascular niche may transition into a T cell tissue-resident memory niche, as seen for the adventitial cuff.
6.4 ∣. Technological solutions
Continued development of high-resolution spatial transcriptomic and multiplexed histocytometry will fuel much discovery over the coming years. The ability to perform whole tissue imaging will help determine the spatial relationship between immune activation and immune function relative to pathogen or malignant foci. These tools will enable an unprecedented look into diseased states in human tissues and direct mechanistic studies to link position with function. Although powerful, this analysis will still represent a snap-shot in a disease process. Parallel development of optogenetic tools that enable cells to be marked in time and space, and novel ways to visualize antigen and chemokine presentation in situ, in real-time, will be essential to determine the sequential steps in orchestrating successful, or pathogenic, immunity.
The more we understand about the molecular cues that assemble and maintain these peripheral activation niches the better informed we will be to target these microanatomical sites to improved disease outcome.
ACKNOWLEDGEMENTS
We gratefully acknowledge support from the following agencies: National Institutes of Health Grants P01 AI02851, R01 AI072690, and R01 AI136536 to DJF.
Footnotes
This article is part of a series of reviews covering Insights into immune function from imaging appearing in Volume 306 of Immunological Reviews.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
REFERENCES
- 1.Rodda LB, Lu E, Bennett ML, et al. Single-Cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity. 2018;48(5):1014–1028.e6. doi: 10.1016/J.IMMUNI.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Förster R, Braun A, Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. 2012;33(6):271–280. doi: 10.1016/J.IT.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 3.Grant SM, Lou M, Yao L, Germain RN, Radtke AJ, Lennon-Duménil AM. The lymph node at a glance – how spatial organization optimizes the immune response. J Cell Sci. 2020;133(5):133. doi: 10.1242/JCS.241828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prizant H, Patil N, Negatu S, et al. CXCL10+ peripheral activation niches couple preferred sites of Th1 entry with optimal APC encounter. Cell Rep. 2021;36(6):109523. doi: 10.1016/J.CELREP.2021.109523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Natsuaki Y, Egawa G, Nakamizo S, et al. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat Immunol. 2014;15(11):1064–1069. doi: 10.1038/ni.2992 [DOI] [PubMed] [Google Scholar]
- 6.di Pilato M, Kfuri-Rubens R, Pruessmann JN, et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell. 2021;184(17):4512–4530.e22. doi: 10.1016/J.CELL.2021.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoltzfus CR, Sivakumar R, Kunz L, et al. Multi-parameter quantitative imaging of tumor microenvironments reveals perivascular immune niches associated with anti-tumor immunity. Front Immunol. 2021;12:3049. doi: 10.3389/FIMMU.2021.726492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelka K, Hofree M, Chen JH, et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell. 2021:184:4734–4752. doi: 10.1016/j.cell.2021.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poirot J, Medvedovic J, Trichot C, Soumelis V. Compartmentalized multicellular crosstalk in lymph nodes coordinates the generation of potent cellular and humoral immune responses. Eur J Immunol. 2021;1–15. doi: 10.1002/EJI.202048977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckert N, Permanyer M, Yu K, Werth K, Förster R. Chemokines and other mediators in the development and functional organization of lymph nodes. Immunol Rev. 2019;289(1):62–83. doi: 10.1111/IMR.12746 [DOI] [PubMed] [Google Scholar]
- 11.Krishnamurty AT, Turley SJ. Lymph node stromal cells: cartographers of the immune system. Nat Immunol. 2020;21(4):369–380. doi: 10.1038/s41590-020-0635-3 [DOI] [PubMed] [Google Scholar]
- 12.Carrasco YR, Batista FD. B Cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27(1):160–171. doi: 10.1016/J.IMMUNI.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 13.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nature Immunol. 2007;8(9):992–1000. doi: 10.1038/ni1494 [DOI] [PubMed] [Google Scholar]
- 14.Junt T, Moseman EA, Iannacone M, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450(7166):110–114. doi: 10.1038/nature06287 [DOI] [PubMed] [Google Scholar]
- 15.Gerner MY, Torabi-Parizi P, Germain RN. Strategically localized dendritic cells promote rapid T Cell Responses To Lymph-Borne Particulate Antigens. Immunity. 2015;42(1):172–185. doi: 10.1016/J.IMMUNI.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 16.Gerner MY, Casey KA, Kastenmuller W, Germain RN. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J Exp Med. 2017;214(10):3105–3122. doi: 10.1084/JEM.20170335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itano AA, McSorley SJ, Reinhardt RL, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19(1):47–57. doi: 10.1016/S1074-7613(03)00175-4 [DOI] [PubMed] [Google Scholar]
- 18.Sixt M, Kanazawa N, Selg M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22(1):19–29. doi: 10.1016/J.IMMUNI.2004.11.013 [DOI] [PubMed] [Google Scholar]
- 19.Reynoso GV, Weisberg AS, Shannon JP, et al. Lymph node conduits transport virions for rapid T cell activation. Nat Immunol. 2019;20(5):602–612. doi: 10.1038/s41590-019-0342-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodruff MC, Heesters BA, Herndon CN, et al. Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. J Exp Med. 2014;211(8):1611–1621. doi: 10.1084/JEM.20132327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leal JM, Huang JY, Kohli K, et al. Innate cell microenvironments in lymph nodes shape the generation of T cell responses during type I inflammation. Sci Immunol. 2021;6(56):9435. doi: 10.1126/SCIIMMUNOL.ABB9435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingulli E, Ulman DR, Lucido MM, Jenkins MK. In situ analysis reveals physical interactions between CD11b+ dendritic cells and antigen-specific cd4 t cells after subcutaneous injection of antigen. J Immunol. 2002;169(5):2247–2252. doi: 10.4049/JIMMUNOL.169.5.2247 [DOI] [PubMed] [Google Scholar]
- 23.Eickhoff S, Brewitz A, Gerner MY, et al. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell. 2015;162(6):1322–1337. doi: 10.1016/J.CELL.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baptista AP, Gola A, Huang Y, et al. The chemoattractant receptor Ebi2 drives intranodal naive CD4+ T cell peripheralization to promote effective adaptive immunity. Immunity. 2019;50(5):1188–1201.e6. doi: 10.1016/J.IMMUNI.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 25.Bedoui S, Heath WR, Mueller SN. CD4+ T-cell help amplifies innate signals for primary CD8+ T-cell immunity. Immunol Rev. 2016;272(1):52–64. doi: 10.1111/IMR.12426 [DOI] [PubMed] [Google Scholar]
- 26.Hor JL, Whitney PG, Zaid A, Brooks AG, Heath WR, Mueller SN. Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T cell activation to localized viral infection. Immunity. 2015;43(3):554–565. doi: 10.1016/J.IMMUNI.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 27.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440(7086):890–895. doi: 10.1038/nature04651 [DOI] [PubMed] [Google Scholar]
- 28.Zhou L, Chong MMW, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/J.IMMUNI.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 29.Nakayamada S, Takahashi H, Kanno Y, O’Shea JJ. Helper T cell diversity and plasticity. Curr Opin Immunol. 2012;24(3):297–302. doi: 10.1016/J.COI.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruterbusch M, Pruner KB, Shehata L, Pepper M. In vivo CD4+ T Cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev Immunol. 2020;38:705–725. doi: 10.1146/annurev-immunol-103019-085803 [DOI] [PubMed] [Google Scholar]
- 31.Crotty S. T Follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50(5):1132–1148. doi: 10.1016/J.IMMUNI.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groom JR, Richmond J, Murooka TT, et al. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity. 2012;37(6):1091–1103. doi: 10.1016/j.immuni.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.León B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of TH2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol. 2012;13(7):681–690. doi: 10.1038/ni.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu JK, Kagari T, Clingan JM, Matloubian M. Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proc Natl Acad Sci USA. 2011;108(21):E118–E127. doi: 10.1073/PNAS.1101881108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohlmeier JE, Reiley WW, Perona-Wright G, et al. Inflammatory chemokine receptors regulate CD8+ T cell contraction and memory generation following infection. J Exp Med. 2011;208(8):1621–1634. doi: 10.1084/JEM.20102110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurachi M, Kurachi J, Suenaga F, et al. Chemokine receptor CXCR3 facilitates CD8+ T cell differentiation into short-lived effector cells leading to memory degeneration. J Exp Med. 2011;208(8):1605–1620. doi: 10.1084/JEM.20102101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kastenmüller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T Cell responses in the lymph node. Immunity. 2013;38(3):502–513. doi: 10.1016/J.IMMUNI.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sung JH, Zhang H, Moseman EA, et al. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;150(6):1249–1263. doi: 10.1016/J.CELL.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Victora GD, Schwickert TA, Fooksman DR, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143(4):592–605. doi: 10.1016/J.CELL.2010.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi H. T follicular helper cells in space-time. Nat Rev Immunol. 2016;16(10):612–625. doi: 10.1038/nri.2016.94 [DOI] [PubMed] [Google Scholar]
- 41.Cyster JG, Allen CDC. B cell responses: cell interaction dynamics and decisions. Cell. 2019;177(3):524–540. doi: 10.1016/J.CELL.2019.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vinuesa CG, Linterman MA, Yu D, MacLennan ICM. Follicular helper T cells. Ann Rev Immunol. 2016;34(1):335–368. doi: 10.1146/annurev-immunol-041015-055605 [DOI] [PubMed] [Google Scholar]
- 43.Song W, Craft J. T follicular helper cell heterogeneity: time, space, and function. Immunol Rev. 2019;288(1):85–96. doi: 10.1111/IMR.12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mesin L, Ersching J, Victora GD. Germinal center B cell dynamics. Immunity. 2016;45(3):471–482. doi: 10.1016/J.IMMUNI.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heesters BA, Myers RC, Carroll MC. Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol. 2014;14(7):495–504. doi: 10.1038/nri3689 [DOI] [PubMed] [Google Scholar]
- 46.Roozendaal R, Mempel TR, Pitcher LA, et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30(2):264–276. doi: 10.1016/J.IMMUNI.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki K, Grigorova I, Phan TG, Kelly LM, Cyster JG. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J Exp Med. 2009;206(7):1485. doi: 10.1084/JEM.20090209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu E, Cyster JG. G-protein coupled receptors and ligands that organize humoral immune responses. Immunol Rev. 2019;289(1):158–172. doi: 10.1111/IMR.12743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katakai T. Marginal reticular cells: a stromal subset directly descended from the lymphoid tissue organizer. Front Immunol. 2012;3(JUL):200. doi: 10.3389/FIMMU.2012.00200/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi T, Wang X, Kelly LM, et al. Oxysterol gradient generation by lymphoid stromal cells guides activated b cell movement during humoral responses. Immunity. 2012;37(3):535–548. doi: 10.1016/J.IMMUNI.2012.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poholek AC, Hansen K, Hernandez SG, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185(1):313–326. doi: 10.4049/JIMMUNOL.0904023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avancena P, Song T, Yao Y, et al. The magnitude of germinal center reactions is restricted by a fixed number of preexisting niches. Proc Natl Acad Sci USA. 2021;118(30):e2100576118. doi: 10.1073/PNAS.2100576118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Ann Rev Immunol. 2012;30(1):69–94. doi: 10.1146/annurev-immunol-020711-075011 [DOI] [PubMed] [Google Scholar]
- 54.Green JA, Suzuki K, Cho B, et al. The sphingosine 1-phosphate receptor S1P2 maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunol. 2011;12(7):672–680. doi: 10.1038/ni.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muppidi JR, Schmitz R, Green JA, et al. Loss of signalling via Gα13 in germinal centre B-cell-derived lymphoma. Nature. 2014;516(7530):254–258. doi: 10.1038/nature13765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muppidi JR, Lu E, Cyster JG. The G protein–coupled receptor P2RY8 and follicular dendritic cells promote germinal center confinement of B cells, whereas S1PR3 can contribute to their dissemination. J Exp Med. 2015;212(13):2213–2222. doi: 10.1084/JEM.20151250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu E, Wolfreys FD, Muppidi JR, Xu Y, Cyster JG. S-Geranylgeranyl-l-glutathione is a ligand for human B cell-confinement receptor P2RY8. Nature. 2019;567(7747):244–248. doi: 10.1038/s41586-019-1003-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallman AE, Wolfreys FD, Nguyen DN, et al. Abcc1 and Ggt5 support lymphocyte guidance through export and catabolism of S-geranylgeranyl-l-glutathione. Sci Immunol. 2021;6(60):eabg1101. doi: 10.1126/SCIIMMUNOL.ABG1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodda LB, Bannard O, Ludewig B, Nagasawa T, Cyster JG. Phenotypic and morphological properties of germinal center dark zone Cxcl12-expressing reticular cells. J Immunol. 2015;195(10):4781–4791. doi: 10.4049/JIMMUNOL.1501191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T–B cell interactions underlie germinal centre formation. Nature. 2008;455(7214):764–769. doi: 10.1038/nature07345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cannons JL, Qi H, Lu KT, et al. Optimal germinal center responses require a multistage T cell: B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32(2):253–265. doi: 10.1016/J.IMMUNI.2010.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan L, de Leur K, Hendriks RW, et al. T follicular helper cells as a new target for immunosuppressive therapies. Front Immunol. 2017;8:1510. 10.3389/fimmu.2017.01510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Rodda LB, Bannard O, Cyster JG. Integrin-mediated interactions between B cells and follicular dendritic cells influence germinal center B cell fitness. J Immunol. 2014;192(10):4601–4609. doi: 10.4049/JIMMUNOL.1400090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schrock DC, Leddon SA, Hughson A, Miller J, Lacy-Hulbert A, Fowell DJ. Pivotal role for α V integrins in sustained Tfh support of the germinal center response for long-lived plasma cell generation. Proc Natl Acad Sci USA. 2019;116(10):4462–4470. doi: 10.1073/pnas.1809329116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35(5):671–680. doi: 10.1016/J.IMMUNI.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 66.Johnston RJ, Poholek AC, DiToro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/SCIENCE.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nurieva RI, Chung Y, Martinez GJ, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/SCIENCE.1176676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu D, Rao S, Tsai LM, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31(3):457–468. doi: 10.1016/J.IMMUNI.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 69.Choi J, Crotty S. Bcl6-mediated transcriptional regulation of follicular helper T cells (TFH). Trends Immunol. 2021;42(4):336–349. doi: 10.1016/J.IT.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baumjohann D, Preite S, Reboldi A, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38(3):596–605. doi: 10.1016/J.IMMUNI.2012.11.020 [DOI] [PubMed] [Google Scholar]
- 71.Deenick EK, Chan A, Ma CS, et al. Follicular helper T Cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33(2):241–253. doi: 10.1016/J.IMMUNI.2010.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu H, Li X, Liu D, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496(7446):523–527. doi: 10.1038/nature12058 [DOI] [PubMed] [Google Scholar]
- 73.Choi YS, Kageyama R, Eto D, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34(6):932–946. doi: 10.1016/J.IMMUNI.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foy TM, Page DM, Waldschmidt TJ, et al. An essential role for gp39, the ligand for CD40, in thymic selection. J Exp Med. 1995;182(5):1377–1388. doi: 10.1084/JEM.182.5.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7–2 in established germinal centers. J Immunol. 1995;155(2):556–567. [PubMed] [Google Scholar]
- 76.Watanabe M, Fujihara C, Radtke AJ, et al. Co-stimulatory function in primary germinal center responses: CD40 and B7 are required on distinct antigen-presenting cells. J Exp Med. 2017;214(9):2795–2810. doi: 10.1084/JEM.20161955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ise W, Fujii K, Shiroguchi K, et al. T follicular helper cell-germinal center B cell interaction strength regulates entry into plasma cell or recycling germinal center cell fate. Immunity. 2018;48(4):702–715.e4. doi: 10.1016/J.IMMUNI.2018.03.027 [DOI] [PubMed] [Google Scholar]
- 78.Shulman Z, Gitlin AD, Weinstein JS, et al. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345(6200):1058–1062. doi: 10.1126/SCIENCE.1257861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weinstein JS, Herman EI, Lainez B, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17(10):1197–1205. doi: 10.1038/ni.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu D, Xu H, Shih C, et al. T–B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 2015;517(7533):214–218. doi: 10.1038/nature13803 [DOI] [PubMed] [Google Scholar]
- 81.Liu Z, Liu S, Zhang Y, et al. Distinct roles of ICOS and CD40L in human T-B cell adhesion and antibody production. Cell Immunol. 2021;368:104420. doi: 10.1016/J.CELLIMM.2021.104420 [DOI] [PubMed] [Google Scholar]
- 82.Mintz MA, Cyster JG. T follicular helper cells in germinal center B cell selection and lymphomagenesis. Immunol Rev. 2020;296(1):48–61. doi: 10.1111/IMR.12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fowell DJ, Kim M. The spatio-temporal control of effector T cell migration. Nat Rev Immunol. 2021;21(9):582–596. doi: 10.1038/S41577-021-00507-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T Cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28(2):271–284. doi: 10.1016/J.IMMUNI.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waite JC, Leiner I, Lauer P, et al. Dynamic imaging of the effector immune response to listeria infection in vivo. PLoS Pathog. 2011;7(3):e1001326. doi: 10.1371/JOURNAL.PPAT.1001326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cupovic J, Onder L, Gil-Cruz C, et al. Central nervous system stromal cells control local CD8+ T cell responses during virus-induced neuroinflammation. Immunity. 2016;44(3):622–633. doi: 10.1016/J.IMMUNI.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Collins N, Jiang X, Zaid A, et al. Skin CD4+ memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat Commun. 2016;7(1):1–13. doi: 10.1038/ncomms11514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iijima N, Iwasaki A. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346(6205):93–98. doi: 10.1126/science.1257530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klose CSN, Artis D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 2020;30(6):475–491. doi: 10.1038/s41422-020-0323-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McFarland AP, Colonna M. Sense and immuno-sensibility: innate lymphoid cell niches and circuits. Curr Opin Immunol. 2020;62:9–14. doi: 10.1016/J.COI.2019.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moriyama S, Artis D. Neuronal regulation of group 2 innate lymphoid cells and type 2 inflammation. Adv Immunol. 2019;143:1–9. doi: 10.1016/BS.AI.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 92.Dahlgren MW, Molofsky AB. Adventitial cuffs: regional hubs for tissue immunity. Trends Immunol. 2019;40(10):877–887. doi: 10.1016/j.it.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164(3):378–391. doi: 10.1016/J.CELL.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Emgård J, Kammoun H, García-Cassani B, et al. Oxysterol sensing through the receptor GPR183 promotes the lymphoid-tissue-inducing function of innate lymphoid cells and colonic inflammation. Immunity. 2018;48(1):120–132.e8. doi: 10.1016/J.IMMUNI.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chu C, Moriyama S, Li Z, et al. Anti-microbial functions of group 3 innate lymphoid cells in gut-associated lymphoid tissues are regulated by G-protein-coupled receptor 183. Cell Rep. 2018;23(13):3750–3758. doi: 10.1016/J.CELREP.2018.05.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klose CSN, Mahlakõiv T, Moeller JB, et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature. 2017;549(7671):282–286. doi: 10.1038/nature23676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cardoso V, Chesné J, Ribeiro H, et al. . Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 2017;549(7671):277–281. doi: 10.1038/nature23469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wallrapp A, Riesenfeld SJ, Burkett PR, et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature. 2017;549(7672):351–356. doi: 10.1038/nature24029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moriyama S, Brestoff JR, Flamar AL, et al. β2-adrenergic receptor–mediated negative regulation of group 2 innate lymphoid cell responses. Science. 2018;359(6379):1056–1061. doi: 10.1126/SCIENCE.AAN4829 [DOI] [PubMed] [Google Scholar]
- 100.Cautivo KM, Steer CA, Molofsky AB. Immune outposts in the adventitia: one foot in sea and one on shore. Curr Opin Immunol. 2020;64:34–41. doi: 10.1016/J.COI.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Croese T, Castellani G, Schwartz M. Immune cell compartmentalization for brain surveillance and protection. Nat Immunol. 2021;22(9):1083–1092. doi: 10.1038/s41590-021-00994-2 [DOI] [PubMed] [Google Scholar]
- 102.Chu C, Artis D, Chiu IM. Neuro-immune interactions in the tissues. Immunity. 2020;52(3):464–474. doi: 10.1016/J.IMMUNI.2020.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dahlgren MW, Jones SW, Cautivo KM, et al. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity. 2019;50(3):707–722.e6. doi: 10.1016/j.immuni.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Islam SA, Chang DS, Colvin RA, et al. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ TH2 cells. Nat Immunol. 2011;12(2):167–177. doi: 10.1038/ni.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Halim TYF, Hwang YY, Scanlon ST, et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2016;17(1):57–64. doi: 10.1038/ni.3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Denton AE, Innocentin S, Carr EJ, et al. Type I interferon induces CXCL13 to support ectopic germinal center formation. J Exp Med. 2019;216(3):621–637. doi: 10.1084/JEM.20181216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goya S, Matsuoka H, Mori M, et al. Sustained interleukin-6 signalling leads to the development of lymphoid organ-like structures in the lung. J Pathol. 2003;200(1):82–87. doi: 10.1002/PATH.1321 [DOI] [PubMed] [Google Scholar]
- 108.Crowley T, Buckley CD, Clark AR. Stroma: the forgotten cells of innate immune memory. Clin Exp Immunol. 2018;193(1):24–36. doi: 10.1111/CEI.13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baluk P, Adams A, Phillips K, et al. Preferential lymphatic growth in bronchus-associated lymphoid tissue in sustained lung inflammation. Am J Pathol. 2014;184(5):1577–1592. doi: 10.1016/J.AJPATH.2014.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shinoda K, Hirahara K, Iinuma T, et al. Thy1+IL-7+ lymphatic endothelial cells in iBALT provide a survival niche for memory T-helper cells in allergic airway inflammation. Proc Natl Acad Sci USA. 2016;113(20):E2842–E2851. doi: 10.1073/PNAS.1512600113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rustenhoven J, Drieu A, Mamuladze T, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184(4):1000–1016.e27. doi: 10.1016/J.CELL.2020.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anandasabapathy N, Victora GD, Meredith M, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208(8):1695–1705. doi: 10.1084/JEM.20102657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Quintana E, Fernández A, Velasco P, et al. DNGR-1+ dendritic cells are located in meningeal membrane and choroid plexus of the noninjured brain. Glia. 2015;63(12):2231–2248. doi: 10.1002/GLIA.22889 [DOI] [PubMed] [Google Scholar]
- 114.de Lima KA, Rustenhoven J, Kipnis J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Ann Rev Immunol. 2020;38(1):597–620. doi: 10.1146/annurev-immunol-102319-103410 [DOI] [PubMed] [Google Scholar]
- 115.Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15(2):192–199. doi: 10.1038/nm.1927 [DOI] [PubMed] [Google Scholar]
- 116.Bilbao D, Luciani L, Johannesson B, Piszczek A, Rosenthal N. Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease. EMBO Mol Med. 2014;6(11):1423–1435. doi: 10.15252/EMMM.201303376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goddery EN, Fain CE, Lipovsky CG, et al. Microglia and perivascular macrophages act as antigen presenting cells to promote CD8 T cell infiltration of the brain. Front Immunol. 2021;12:3428. doi: 10.3389/FIMMU.2021.726421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barron AMS, Mantero JC, Ho JD, et al. Perivascular adventitial fibroblast specialization accompanies T cell retention in the inflamed human dermis. J Immunol Author Choice. 2019;202(1):56–68. doi: 10.4049/JIMMUNOL.1801209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qin J, Lovelace MD, Mitchell AJ, de Koning-Ward T, Grau GE, Pai S. Perivascular macrophages create an intravascular niche for CD8+ T cell localisation prior to the onset of fatal experimental cerebral malaria. Clin Translat Immunol. 2021;10(4):e1273. doi: 10.1002/CTI2.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Suga H, Sugaya M, Miyagaki T, Ohmatsu H, Okochi H, Sato S. CXCR3 deficiency prolongs Th1-type contact hypersensitivity. J Immunol. 2013;190(12):6059–6070. doi: 10.4049/JIMMUNOL.1201606 [DOI] [PubMed] [Google Scholar]
- 121.Tokuriki A, Seo N, Ito T, Kumakiri M, Takigawa M, Tokura Y. Dominant expression of CXCR3 is associated with induced expression of IP-10 at hapten-challenged sites of murine contact hypersensitivity: a possible role for interferon-y-producing CD8+ T cells in IP-10 expression. J Dermatol Sci. 2002;28(3):234–241. doi: 10.1016/S0923-1811(01)00172-4 [DOI] [PubMed] [Google Scholar]
- 122.Matheu MP, Teijaro JR, Walsh KB, et al. Three phases of CD8 T cell response in the lung following H1N1 influenza infection and sphingosine 1 phosphate agonist therapy. PLoS One. 2013;8(3):e58033. doi: 10.1371/JOURNAL.PONE.0058033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jansen CS, Prokhnevska N, Master VA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576(7787):465. doi: 10.1038/S41586-019-1836-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. doi: 10.1038/nature19330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gérard A, Patino-Lopez G, Beemiller P, et al. Detection of rare antigen-presenting cells through T cell-intrinsic meandering motility, mediated by Myo1g. Cell. 2014;158(3):492–505. doi: 10.1016/J.CELL.2014.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harris TH, Banigan EJ, Christian DA, et al. Generalized Lévy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486(7404):545–548. doi: 10.1038/nature11098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Overstreet MG, Gaylo A, Angermann BR, et al. Inflammation-induced interstitial migration of effector CD4+ T cells is dependent on integrin αV. Nat Immunol. 2013;14(9):949–958. doi: 10.1038/ni.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Friedman RS, Jacobelli J, Krummel MF. Surface-bound chemokines capture and prime T cells for synapse formation. Nat Immunol. 2006;7(10):1101–1108. doi: 10.1038/ni1384 [DOI] [PubMed] [Google Scholar]
- 129.Molon B, Gri G, Bettella M, et al. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6(5):465–471. doi: 10.1038/ni1191 [DOI] [PubMed] [Google Scholar]
- 130.Sckisel GD, Bouchlaka MN, Monjazeb AM, et al. Out-of-sequence signal 3 paralyzes primary CD4+ T-cell-dependent immunity. Immunity. 2015;43(2):240–250. doi: 10.1016/J.IMMUNI.2015.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Richer MJ, Nolz JC, Harty JT. Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8+ T cells by enhancing T cell receptor signaling. Immunity. 2013;38(1):140–152. doi: 10.1016/J.IMMUNI.2012.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ley K. The second touch hypothesis: T cell activation, homing and polarization. F1000Research. 2014;3(May):1–15. doi: 10.12688/f1000research.3-37.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Honda T, Egawa G, Kabashima K. Antigen presentation and adaptive immune responses in skin. Int Immunol. 2019;31(7):423–429. doi: 10.1093/intimm/dxz005 [DOI] [PubMed] [Google Scholar]
- 134.Schneider H, Downey J, Smith A, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313(5795):1972–1975. doi: 10.1126/SCIENCE.1131078 [DOI] [PubMed] [Google Scholar]
- 135.Liu Z, Yang F, Zheng H, et al. Visualization of T cell-regulated monocyte clusters mediating keratinocyte death in acquired cutaneous immunity. J Investig Dermatol. 2018;138(6):1328–1337. doi: 10.1016/J.JID.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 136.Macleod BL, Bedoui S, Hor JL, et al. Distinct APC subtypes drive spatially segregated CD4+ and CD8+ T-cell effector activity during skin infection with HSV-1. PLoS Pathog. 2014;10(8):e1004303. doi: 10.1371/journal.ppat.1004303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, Germain RN. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity. 2011;34(5):807–819. doi: 10.1016/j.immuni.2011.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Müller M, Carter SL, Hofer MJ, et al. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol. 2007;179(5):2774–2786. doi: 10.4049/JIMMUNOL.179.5.2774 [DOI] [PubMed] [Google Scholar]
- 139.Steinbach K, Vincenti I, Egervari K, et al. Brain-resident memory T cells generated early in life predispose to autoimmune disease in mice. Sci Transl Med. 2019;11(498): doi: 10.1126/SCITRANSLMED.AAV5519 [DOI] [PubMed] [Google Scholar]
- 140.Hickman HD, Reynoso GV, Ngudiankama BF, et al. CXCR3 chemokine receptor enables local CD8+ T cell migration for the destruction of virus-infected cells. Immunity. 2015;42(3):524–537. doi: 10.1016/j.immuni.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hickman HD, Reynoso G, v., Ngudiankama BF, et al. Anatomically restricted synergistic antiviral activities of innate and adaptive immune cells in the skin. Cell Host Microbe. 2013;13(2):155–168. doi: 10.1016/j.chom.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zaid A, Mackay LK, Rahimpour A, et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc Natl Acad Sci USA. 2014;111(14):5307–5312. doi: 10.1073/PNAS.1322292111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang LR, Wohlleber D, Reisinger F, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8+ T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol. 2013;14(6):574–583. doi: 10.1038/ni.2573 [DOI] [PubMed] [Google Scholar]
- 144.Schwabenland M, Salié H, Tanevski J, et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity. 2021;54(7):1594–1610.e11. doi: 10.1016/J.IMMUNI.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dunbar PR, Cartwright EK, Wein AN, et al. Pulmonary monocytes interact with effector T cells in the lung tissue to drive TRM differentiation following viral infection. Mucosal Immunol. 2020;13(1):161–171. doi: 10.1038/s41385-019-0224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Duckworth BC, Lafouresse F, Wimmer VC, et al. Effector and stem-like memory cell fates are imprinted in distinct lymph node niches directed by CXCR3 ligands. Nat Immunol. 2021;22(4):434–448. doi: 10.1038/s41590-021-00878-5 [DOI] [PubMed] [Google Scholar]
- 147.Aoshi T, Zinselmeyer BH, Konjufca V, et al. Bacterial entry to the splenic white pulp initiates antigen presentation to CD8+ T cells. Immunity. 2008;29(3):476–486. doi: 10.1016/J.IMMUNI.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 148.Vella JL, Molodtsov A, Angeles CV, et al. Dendritic cells maintain anti-tumor immunity by positioning CD8 skin-resident memory T cells. Life Sci Alliance. 2021;4(10):e202101056. doi: 10.26508/LSA.202101056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bajénoff M, Granjeaud S, & Guerder S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J Exp Med. 2003;198(5):715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kerfoot SM, Yaari G, Patel JR, et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34(6):947–960. doi: 10.1016/j.immuni.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gitlin AD, Shulman Z, & Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509(7502):637–640. doi: 10.1038/nature13300 [DOI] [PMC free article] [PubMed] [Google Scholar]