Abstract
Chronic diseases are the leading cause of death worldwide with increasing prevalence in all age groups, genders, and ethnicities. Most chronic disease deaths occur in middle-to low-income countries but are also a significant health problem in developed nations. Multiple chronic diseases now affect children and adolescents as well as adults. Being physically inactive is associated with increased chronic disease risk. Global societies are being negatively impacted by the increasing prevalence of chronic disease which is directly related to rising healthcare expenditures, workforce complications regarding attendance and productivity, military personnel recruitment, and academic success. However, increased physical activity (PA) and exercise are associated with reduced chronic disease risk. Most physiologic systems in the body benefit positively from PA and exercise by primary disease prevention and secondary disease prevention/treatment. The purpose of this brief review is to describe the significant global problem of chronic diseases for adults and children, and how PA and exercise can provide a non-invasive means for added prevention and treatment.
Keywords: Chronic disease, Noncommunicable diseases, Obesity, Diabetes, Cardiovascular disease, Cancer, Physical activity, Exercise
Introduction
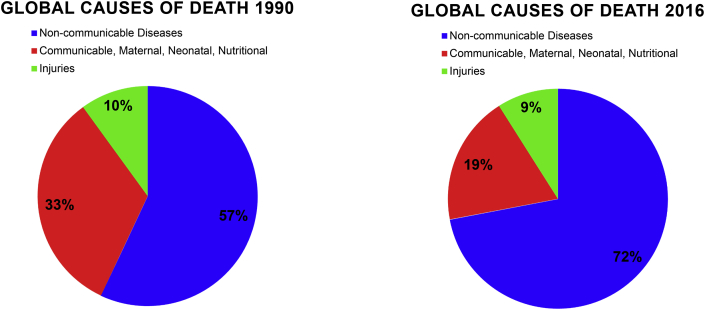
A chronic disease is an illness that is not contagious, usually of long duration, progresses slowly, and is typically a result of genetics, environment, or poor lifestyle.1 In 1990, more than 28 million (57%) of all global deaths were caused by chronic disease.2 This number increased to 36 million (63%) of all global deaths in 20083 and 39 million (72%) of all global deaths in 20164 (Fig. 1). Even though life expectancy estimates have consistently risen for the last two centuries,5,6 current estimations support a potential decline in life expectancy for future generations due to an increase in various chronic diseases such as lower respiratory disease, obesity, cancer, cardiovascular disease (CVD), diabetes, and stroke.7 Presently, the literature supports that the incorporation of daily physical activity (PA) and exercise into one's lifestyle will reduce risk for chronic diseases and mortality while providing a means for primary disease prevention.8 Furthermore, once a chronic illness is diagnosed, treatment is better managed when PA and exercise are part of the disease medical management plan. In either case of disease prevention or treatment, PA and regular exercise provide a higher quality of life and perhaps increased longevity.9
Fig. 1.
Global Causes of Death.
Global causes of death have shifted from communicable to non-communicable diseases. As communicable disease prevention and treatment has improved, deaths caused by non-communicable disease have increased and continues to rise.
The information for developing this figure was taken from Murray and Lopez. The Lancet. 1997 and Naghavi et al., The Lancet. 2017.
Although altering disease risk factors reduces overall chronic disease risk, modifiable risk factors such as sedentary behavior are associated with an increased risk for chronic disease.10 Non-modifiable risk factors are traits that cannot be changed such as age, ethnicity, and genetics. However even though not altered directly, genes are strongly influenced by the environment and lifestyle affecting gene expression.11 Modifiable risk factors are positively influenced by lifestyle such as daily PA, regular exercise, healthy diet, social engagement, spirituality, and stress management.12 However, other modifiable risk factors exist that are not directly related to lifestyle but negatively influence chronic disease risk such as education level, socioeconomic status, and employment. PA and regularly practiced exercise positively influence risk factors for chronic diseases such as CVD, type 2 diabetes, obesity, and cancer.13, 14, 15, 16, 17, 18, 19 Thus, the purpose of this brief review is to describe the global chronic disease problem for adults and children, describe the social-economic impact of chronic disease, and how PA and exercise can provide a non-invasive role for added chronic disease prevention and treatment. To achieve this purpose, a literature search was conducted using Pubmed, Medline, and Google Scholar databases. Search terms used were physical activity, exercise, multiple chronic diseases, chronic disease prevalence measures, and healthcare economy. In addition, the reference lists from systematic reviews incorporating PA and exercise and chronic disease were also reviewed.
The Rise of Chronic Disease
Diseases have always plagued humans. Infectious diseases remain a primary focus for prevention and treatment.20 Over time, the incidence and mortality rates for infectious diseases have dropped with advancements of vaccinations, antibiotics, sanitation, and the development of procedures for general infectious disease prevention.21 The reduction of infectious diseases is associated with decreased morbidity and mortality resulting in an increase in life expectancy.22 However, unanticipated consequences followed the gain in life years including a shift in the world's health burden from infectious to non-communicable diseases (NCDs) such as CVD.4
Many developing countries now experience the burden of both infectious and chronic disease rather than seeing a replacement of infectious disease with chronic disease. For example, India has the highest number of diabetic cases in the world23 while also having the highest number of certain infectious diseases such as tuberculosis every year.24 In this same regard, infectious diseases in South Africa account for almost the same number of deaths as chronic disease.25
In the past, chronic diseases were usually considered a problem only in developed countries.26 However, 80% of deaths in low- and middle-income countries are now caused by chronic diseases.26, 27, 28 Low- and middle-income countries are shown to have four times the mortality rate from NCDs than high-income countries.22 Ischemic heart disease and stroke represented 85% of CVD deaths and 28% of all-cause mortality in developing nations.26 Diabetes prevalence is expected to increase from about 400 million to 600 million globally by the year 2035 with most of this increase occurring in low-to middle-income countries.29 Cancer prevalence crosses all global economic gaps with 57% of the reported cancer cases occurring in low-to middle-income countries and 43% in developed nations.30 In China for example, cancer is the leading cause of death and is accounts for 25% of all deaths.31,32
Another global trend, especially in low-to middle-income countries, is the rate at which obesity prevalence has increased in recent decades. Currently, about 2 billion adults globally (approximately 25–33% of the world's population) are overweight and another 33% are obese. Obesity is associated with negative health implications and is a well-established risk factor for chronic diseases including CVD, type 2 diabetes, and certain cancers.33 In the United States, obesity prevalence (BMI ≥30) has more than doubled in the last two decades as has severe obesity (BMI ≥40). These trends lead to future predictions of a 33% increase in obesity and a 9% increase in severe obesity by 2030.34 Similar trends are seen across the globe. For instance, obesity prevalence in North and South America more than doubled between 1980 and 2015 with a 64% prevalence of overweight.35 In Europe, approximately 60% of the population are considered overweight and 23% are obese.35
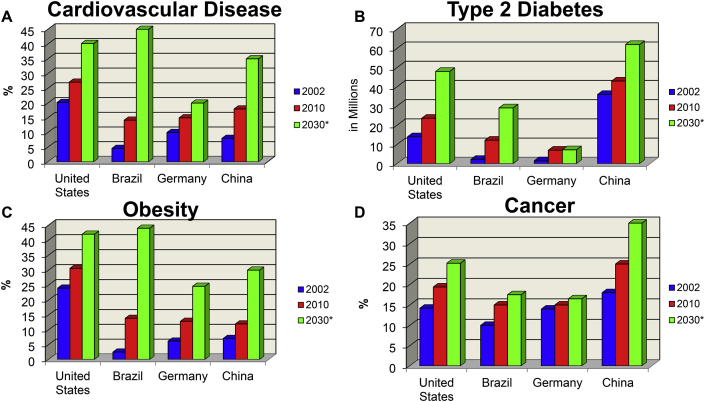
Countries once unaffected by increasing obesity now struggle with elevated obesity rates. For example, China has reported a significant increase in the number of obesity cases.36 While the proportion of the Chinese population classified as obese is relatively low (5%), China currently is ranked as second in the world with 250 million adults classified as overweight and another 40 million obese.36 Furthermore, the prevalence of overweight and obese children in China has more than doubled in the last 20 years.37 India has 135 million people affected by obesity with prevalence rates varying between states from 12 to 31%.38 These rates are expected to increase especially in middle-to low-income countries.39 Other obesity-related NCD rates such as CVD, type 2 diabetes, and cancer will also continue to rise and present a significant global health problem10,40, 41, 42 (Fig. 2).
Fig. 2.
Chronic Disease Trends.
Chronic disease trends from 2002 to 2010 and an estimation for 2030 including A) Cardiovascular disease, B) type 2 diabetes, C) obesity, and D) cancer in the United States, Brazil, Germany, and China.
Figure taken from Durstine, Gordon, Wang, Luo. J Sport Health Sci. 2013. (Permission obtained).
Socio-Economic Impact of Chronic Disease
Chronic diseases are an increasing worldwide concern that has tremendous social implications. In 2013, global healthcare costs for CVD, stroke, type 2 diabetes, breast cancer, and colon cancer were an estimated $54 billion in international currency (INT).43 Globally, approximately $21 billion INT in productivity losses are due to physical inactivity and chronic disease. Physical inactivity cost alone is associated with chronic disease and early death and is estimated at $145 billion INT.43
Increased occurrence of chronic diseases also has had a significant impact on the workforce. Individuals having chronic diseases are either not employed or work fewer hours and are less productive when compared to healthy workers.44 As chronic disease incidence rates increase, the likelihood of experiencing productivity losses and increases in welfare expenditures continue to rise.45
Global military workforces are dramatically affected. Militaries from around the world are experiencing a major decline in the ability to recruit, and obesity is the primary challenge for this recruiting dilemma. In North America, Asia, Europe, and Australia, approximately 50% of all military-aged adults (18–29 years) are overweight or obese.46 Young adults are reported to have low aerobic fitness that is associated with obesity. As a result, young adults wanting to enter the military will have difficulty meeting required physical fitness tests.46 In addition, young adults show less interest in enlisting as a result of an inactive lifestyle.46
A decline in academic performance is associated with the rise in chronic disease in children, adolescents, and young adults. Higher course grades and academic success are associated with regular classroom attendance.47 However, present data supports an inverse relationship between school attendance and standard test scores with being overweight and obese. More research is needed to determine whether these findings are due to social and behavioral factors related to obesity or the condition itself.48 Diabetes is also linked to lower academic test scores as well as a lower ability to focus.48 Lower test scores in grade school, high school, and college are associated with a decline in college degree attainment. In college and university settings, an association was reported between lower graduation rates with overweight, obesity, and/or other chronic diseases.49
Because chronic diseases are related to reduced academic success, global society is affected. For example, college graduates are less likely to utilize welfare programs,50 likely to vote at all levels of government,51 and likely to volunteer and donate to charities.52 As the global prevalence of chronic disease continues to rise, healthcare expenditures will also rise, productivity losses will become commonplace, national defenses will have greater difficulty in recruiting, academic success will likely be reduced, and global society will suffer.
Youth and Chronic Disease
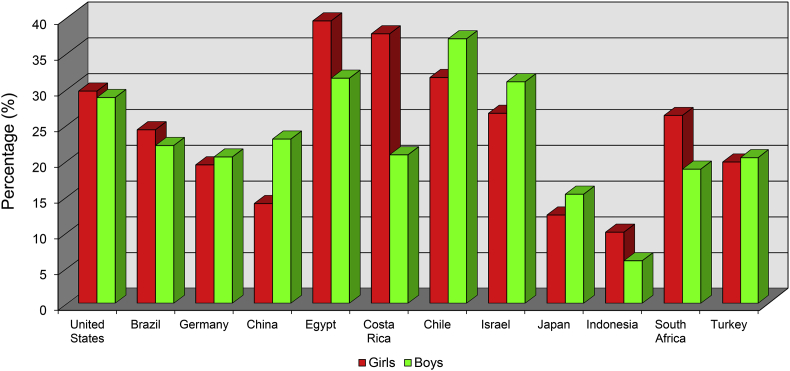
Middle-aged and older adults have historically been associated with a greater risk for chronic disease when considering that advanced age is a well-established risk factor.9 Nevertheless, a rise in chronic diseases are found in younger populations.53 Presently, a greater research focus is placed on the obesity rise in children and adolescents. In the United States, the prevalence of obese children (≥95th percentile) aged 2–19 years has dramatically increased from 5% in the 1960s, to 11% in 1994, to 15% in 2000,54 to 17% in 2014,55 and to 19% in 2016.56 Globally, similar trends are seen for overweight and obesity prevalence in children.53 Gender differences are also noted; young girls frequently have higher obesity prevalence than young boys. However, overweight, obesity, and gender differences do vary by country and culture, and is a global concern53 (Fig. 3).
Fig. 3.
Prevalence of Overweight and Obesity in Children - 2013.
Overweight and obesity prevalence varies between countries and cultures of the world. In 2013, Egypt and Costa Rica had the highest prevalence of young obese girls while Chile and Israel have the highest prevalence of young obese boys.
The information for developing this figure was taken from Ng et al., The Lancet. 2014.
Overweight and obesity in youth are associated with an increased risk for other chronic health problems. Children and adolescents are at an increased risk for hypertension when body weight is elevated, especially if body weight exceeds the 95th percentile.57 The prevalence of high blood pressure in adolescents varies between countries but is more prevalent in middle-to low-income nations.58 In Portugal, the prevalence of high blood pressure and borderline high blood pressure is about 8% in preschool aged children (3–6 years old) who are overweight/obese.59 Globally, hypertension prevalence in youth aged 8–17 years is approximately 11%.55,58 Overweight and obesity in youth is also associated increased risk for abnormal blood lipid levels including elevated total cholesterol and low-density lipoprotein (LDL)-cholesterol, and low levels of high-density lipoprotein (HDL)-cholesterol. The prevalence of abnormal blood lipid levels in adolescents between 12 and 19 years is 20% among overweight and obese youth.60
Metabolic syndrome (MetS) is the clustering of chronic conditions that relate to one another and is associated with an increased CVD risk. The conditions associated with the diagnosis of MetS include high blood pressure, hyperglycemia, central obesity, elevated LDL-cholesterol, elevated triglycerides, and low HDL-cholesterol. Present findings support non-alcoholic fatty liver disease as having a role in insulin resistance in obese adolescents.61 Describing, diagnosing, and treating MetS is very different for children than for adults, and implications for care are not completely understood.62 However, MetS in children and adolescents is a serious health problem. Because the mechanisms for MetS in children is not completely understood, prevalence and incidence estimations for youth are difficult to determine. Nevertheless, attempts to develop prevalence estimations have ranged in obese youth from 19 to 35%,55,60 and these values are increasing worldwide61 requiring immediate attention to provide proper treatment in children having cardiometabolic risk factors.62
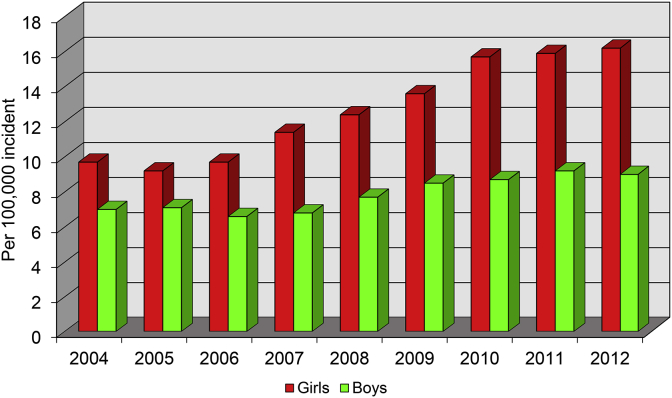
Prediabetes and type 2 diabetes have become an increasing concern among young people, especially among overweight and obese youth.63,64 In the United States for 2008, approximately 25% of adolescents between the ages of 12–19 years had prediabetes.55,60 Between 2004 and 2012, the United States incidence rate for type 2 diabetes in children and youth between the ages of 10–19 years increased by almost 5% each year with greater incidence rates in young girls65 (Fig. 4). Most children diagnosed with diabetes have poor glycemic control and have higher treatment failure rates.66 Similar trends for pediatric type 2 diabetes are seen throughout the world. The United Kingdom had an incidence increase of 6 children per 100,000 each year between 1994 and 1998 to 33 children per 100,000 each year between 2009 and 2013.63 Type 2 diabetes in Asia overtook type 1 diabetes as the predominant form of diabetes in children from Hong Kong and Taiwan.67
Fig. 4.
Youth Type 2 Diabetes Incidence in the United States.
The increase in Type 2 diabetes incidence in youth.
The information for developing this figure was taken from Mayer-Davis et al. NEJM. 2017.
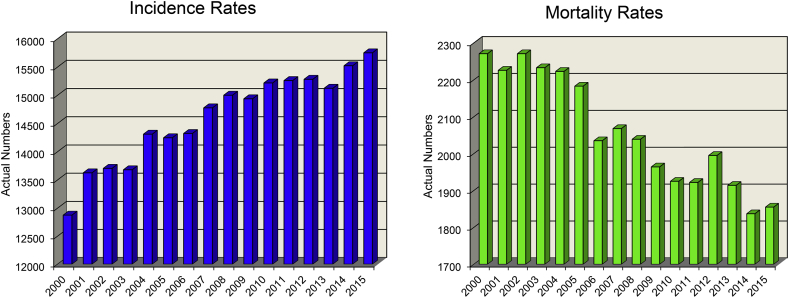
While childhood cancer mortality rates in the United States have fortunately declined, incidence rates unfortunately continue to rise (Fig. 5). The incidence rates of pediatric cancers rose from an annual increase of 0.6% in 1975 to 1.2% in 2010.68 Even though evidence supports a link between obesity and multiple types of cancer in adults,40 the same link in children is not well established. Preliminary data does suggest that certain childhood cancers may indeed be related to obesity,69 but further research in this area is needed. Although, evidence does support that children are at an increased risk of mortality if they are obese at diagnosis and during treatment for cancer.70,71 Childhood cancer survivors have a greater risk for adult obesity and other chronic diseases including cancer relapse within 10 years.71 As both childhood cancer rates and childhood obesity rates rise, mortality rates and the risk for adult obesity also increase, as does the risk for relapse and/or other future chronic diseases.
Fig. 5.
Childhood Cancer Incidence and Mortality Rates in the United States.
Incidence and Mortality rates in childhood cancer in the US between 2000 and 2015, including all types of cancer between the ages of 0–19 years, both male and female patients of all races and ethnicities.
The data provided for developing this figure was taken from The United States Cancer Statistics [1999–2015] at the Center for Disease Control and Prevention.
Physical inactivity
Physical inactivity is associated with increased chronic disease risk.72, 73, 74, 75 Moreover, the literature supports that lower morbidity and mortality rates are associated with maintaining moderate levels of PA and physical fitness.17 Support data come from epidemiologic and longitudinal studies reporting reduced disease risk following lifestyles incorporating daily PA and having higher cardiorespiratory fitness.76, 77, 78
Many countries and organizations such as the American College of Sports Medicine and the World Health Organization (WHO) have released PA guidelines to provide science-based recommendations for PA and exercise. These guidelines are for young children, adolescents, adults, elderly, and for individuals with chronic diseases. These guidelines consider different PA dimensions (mode, frequency, duration, and intensity) and domains (leisure time, transportation, occupation, and domestic activity) to allow for individualization.55,60 The different PA domains impact health and should be considered separately. For instance, increasing one PA domain (such as occupation activity) tends to cause another domain (such as leisure time activity) to decline55,60 and could cause an overall increase in sedentary time. Increasing sedentary time and sleep is inversely related to poor health and premature mortality.79,80
Physical inactivity prevalence is the percentage of individuals who do not perform sufficient daily PA to meet the PA and exercise guidelines of at least 150 min of moderate-intensity aerobic PA per week or at least 75 min of vigorous PA per week for adults81 and at least 60 min of moderate-to vigorous-intensity daily PA for children aged 5 to 17.82 Recent findings show an increase in physical inactivity globally.78 This increase in physical inactivity is associated with technology advances including increased use of television, computer, mobile devices, and video games.83 In the United States, only 42% of children between the ages of 6–11 years meet the WHO PA guidelines. Approximately 14% of adolescents report being regularly physically inactive while only 8% of 12- to 19-year-olds meet recommended PA levels.55,60 In this same regard, 30% of adults did not engage in enough PA during leisure time. Inactivity prevalence does increase with age: 25% of young adults (18–44 years), 33% of middle-aged adults (45–64 years), 36% of older adults (65–74 years), and 53% of the elderly (≥75 years) are reported inactive.60,84
Low PA levels result in harmful and even detrimental consequences. For example, if type 2 diabetic patients increase their sedentary time by just 60 min/day, mortality risk could increase by 13%.72 Additional problems arise with a physically inactive lifestyle including impaired circulation, osteoporosis, arthritis and/or other skeletal disabilities, diminished self-concept, greater dependence on others for daily living, reduced opportunity and ability for normal social interactions, and overall diminished quality of life.85
Health benefits of PA and exercise
Chronic disease prevention, rehabilitation/treatment, and other health benefits of daily PA and exercise are continually being investigated with new information being found and reported.3,74,86,87 A question often asked is how PA and exercise provide for chronic disease prevention and treatment. Because PA and exercise impact many health concerns in diverse ways, the answer to this question is dependent upon each condition and the severity of the disease. One way to understand how PA and exercise can improve health is best realized when comparing the effects of PA to medication use on heart rate at rest and during exercise. For example, beta-blockers are typically used to treat different CVDs and will lower resting, submaximal, and maximal exercise heart rates. Daily PA and exercise also result in lower resting and submaximal heart rates but not maximal heart rate. The use of beta blockers and PA and exercise participation both have the same effect on heart rate except for maximal heart rate. While conventional medications treat symptoms or alter physiologic functioning in a synthetic or non-physiologic fashion, PA and exercise cause the physiologic systems of the body to function optimally. Thus, daily PA and exercise act as a natural treatment for many diseases. For example, PA and exercise participation improves myocardial function88,89 by increasing myocardial strength and oxygen delivery90 while decreasing myocardial oxygen demand.91 Other cardiovascular improvements associated with daily PA and exercise include lower systolic blood pressure and lower blood catecholamine levels at rest and at all submaximal exercise levels, hence aiding in prevention and treatment of CVD risk factors. These adaptations cause improved systemic functioning and overall individual health without the potential side effects of traditional medicines. PA and exercise participation function both in prevention and treatment for chronic disease.92 The cardiovascular system is not the only physiologic system to benefit from PA and exercise. The scientific literature supports that most if not all physiologic systems are positively altered by PA and exercise.17,87,93,94 Thus, exercise can be viewed as a medicine.
Current research literature supports the concept of a dose-response curve for PA and exercise with high person-to-person variability.17,95,96 The concept of an exercise dose-response curve for health benefits was first introduced for PA and exercise in two landmark epidemiological studies.97,98 These studies demonstrated that increases in PA and physical fitness are associated with decreased all-cause mortality. Other clinical investigations also successfully depicted the dose-response curve.99 For example, older men and women have significantly lower mortality risk when moderate cardiorespiratory fitness levels are maintained.100
Other means for optimizing bodily functioning by PA and exercise are found in the literature. Quality of life is increased when PA and exercise are included as part of the medical management plan for individuals living with chronic disease.101, 102, 103 Improved functional capacity and muscular strength, reduced inflammation, increased HDL-cholesterol, and body weight reductions are a result of PA and exercise in children and adults.104 The implementation of daily PA and exercise prevention interventions support an 80% reduction in CVD risk,15 90% reduction in type 2 diabetes risk,18 33% reduction in cancer risk,17 and in some cases reductions in all-cause mortality.13 Results from exercise-based cardiac rehabilitation programming found no effect on all-cause mortality but a greatly reduced cardiac mortality.19,103,105 Also reported is that an exercise-based cardiac rehabilitation program, when compared to a usual care control group, reduced the need for percutaneous transluminal coronary angioplasty by 19%, reductions in nonfatal myocardial infarctions by 21%, and cardiac mortality reductions by 26%.106 A review of 63 studies incorporating exercise programming as part of cardiac rehabilitation demonstrate reduced cardiovascular mortality by 8–10% and hospital re-admission by 26–31%.19 Furthermore, myocardial infarction patients enrolled in 3–6 months of cardiac rehabilitation programming experienced an 11–36% increase in aerobic functional capacity, improved quality of life, and decreased risk for subsequent cardiac events.15,16
Health improvements seen with PA and exercise are not limited to the cardiovascular system. After becoming physically active, type 2 diabetics improve their overall insulin sensitivity and positively altered skeletal muscle proteins and enzymes associated with glucose metabolism and insulin signaling.107 As a result, structured exercise programming is an important part of a diabetic's medical management plan.104,108 Another health benefit example is the inverse relationship found between cancer mortality and PA and exercise. Cancer mortality rates are reduced by 7–17% with increased PA.14 Depression and anxiety symptoms are also improved with daily PA and exercise.109
Additional health benefits exist for preventing disease complications and improved quality of life. Daily PA and exercise enhances bone health by increasing bone mineral density. These interventions are recommended in the prevention and treatment of osteoporosis and to decrease the risk of future bone fractures.110,111 Also, PA and exercise improve the immune system enabling the body to fight infectious diseases resulting in less overall susceptibility to sicknesses.112 As part of this immune adaptation, lymphatic function is enhanced and inflammation is reduced by decreasing inflammatory cell accumulation.113
In conjunction with a proper nutritional diet, PA and exercise exert synergistic effects to improve infertility which is often associated with obesity. Infertility linked to hypogonadism in obese men is improved with daily PA and exercise.114 Ovulation and pregnancy rates are increased by PA and exercise in overweight and obese women who struggle with infertility.115 PA and exercise have a positive influence during pregnancy for both the mother and the baby. Present literature supports daily PA and exercise as safe and improves maternal and fetal well-being.116,117 Women who exercise while pregnant avoid excessive weight gain116 and improve fetus nervous system.118 PA and exercise during pregnancy can also decrease time spent in labor119 and reduce the risk of having a large (≥4 kg body weight) newborn.120
PA and exercise induce molecular adaptations in multiple brain regions, improving functional and structural neural properties, allows for enhanced learning and skill acquisition,121, 122, 123 and improves cognition in healthy adults124 and in neurologically disabled adults.123,125 An inverse relationship exists between the amount of PA and exercise with risk for developing dementia (including Alzheimer's) and Parkinson's disease.122 PA and exercise is proposed to delay the onset of those conditions and is recommended as a preventative measure for cognitive decline and as part of the treatment/management plan.122,126
The portions of the brain most adaptable to change (i.e., memory/learning, emotion, etc.) are the first enhanced by PA and exercise.123 Clearly, neurological deficits in addition to mental health conditions improve with PA and exercise127 which in turn prevents or reduces other health conditions associated with poor stress management,128 depression, and anxiety.129,130
Conclusion
Chronic diseases are the leading cause of death worldwide as disease incidence rates continue to rise. Most chronic disease deaths occur in middle-to low-income societies as well as in developed countries. As the prevalence of these diseases rise in adults, chronic diseases such as type 2 diabetes and obesity rise in youth and adolescents. Including PA and exercise into daily lifestyle activities provides multiple health benefits, promote societal growth, and provide long-term chronic disease prevention and treatment while improving overall global health. Thus, PA and exercise provide a non-invasive means for added chronic disease prevention and treatment. Though additional physiologic, biochemical, and molecular information regarding PA and exercise health benefits are useful, important areas for future research include how to get more people to overcome PA and exercise barriers, better understand the interaction of medications with PA and regular exercise, determine the mechanisms for MetS in children, design research projects that yield better MetS prevalence and incidence estimations for youth, and determine the mechanisms for certain childhood cancers and their relationships to other diseases such as obesity.
Conflicts of interest
The authors have no conflicts of interest to report.
Each authors’ contributions
Each author contributed equally to the drafting of this manuscript. JLD provided an initial draft for the Introduction, ― The Rise of Chronic Disease, ― Health Benefits of Exercise, created Fig. 2, and was responsible for the overall editorial development. EA expanded on the initial drafts for the previously mentioned sections and wrote the sections on ― Youth and Chronic Disease, ― Physical Inactivity, ― Socio-Economic Impact of Chronic Disease, and the Conclusion. EA created Figs. 1, Fig. 3, Fig. 4, and Fig. 5.
Submission statement
The manuscript has not been published and is not under consideration for publication elsewhere.
References
- 1.Noncommunicable Diseases World health organization. 2018. http://www.who.int/en/news-room/fact-sheets/detail/noncommunicable-diseases
- 2.Murray C.J., Lopez A.D. Alternative projections of mortality and disability by cause 1990-2020: global burden of disease study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 3.Alwan A., Armstrong T., Bettcher D., et al. World Health Organization; 2011. Global Status Report on Noncommunicable Diseases 2010. [Google Scholar]
- 4.Naghavi M., Abajobir T., Bettcher D., et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. The Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olshansky S.J., Passaro D.J., Hershow R.C., et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 6.GBD Mortality, Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy S.L., Xu J., Kochanek K.D., et al. Deaths: final data for 2015. Natl Vital Stat Rep. 2017;66(6) [PubMed] [Google Scholar]
- 8.Lear S.A., Hu W., Rangarajan S., et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390(10113):2643–2654. doi: 10.1016/S0140-6736(17)31634-3. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen B.K., Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sport. 2015;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 10.Katzmarzyk P.T., Lear S.A. Physical activity for obese individuals: a systematic review of effects on chronic disease risk factors. Obes Rev. 2012;13(2):95–105. doi: 10.1111/j.1467-789X.2011.00933.x. [DOI] [PubMed] [Google Scholar]
- 11.Booth F.W., Lees S.J. Fundamental questions about genes, inactivity, and chronic diseases. Physiol Genom. 2007;28(2):146–157. doi: 10.1152/physiolgenomics.00174.2006. [DOI] [PubMed] [Google Scholar]
- 12.Danaei G., Ding E.L., Mozaffarian D., et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4) doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogelholm M. Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors. A systematic review. Obes Rev. 2010;11(3):202–221. doi: 10.1111/j.1467-789X.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- 14.Li T., Wei S., Shi Y., et al. The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 2016;50(6):339–345. doi: 10.1136/bjsports-2015-094927. [DOI] [PubMed] [Google Scholar]
- 15.Ades P., Balady G., Berra K. Transforming exercise-based cardiac rehabilitation programs into secondary prevention centers: a national imperative. J Cardiopulm Rehabil Prev. 2001;21(5):263–272. doi: 10.1097/00008483-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Wenger N.K., Froelicher E.S., Smith L.K., et al. Cardiac rehabilitation as secondary prevention. Agency for health care policy and research and national heart, lung, and blood institute. Clin Pract Guidel Quick Ref Guide Clin. 1995;17:1–23. [PubMed] [Google Scholar]
- 17.Warburton D.E., Nicol C.W., Bredin S.S. Health benefits of physical activity: the evidence. CMAJ (Can Med Assoc J) 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamman R.F., Wing R.R., Edelstein S.L., et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson L., Oldridge N., Thompson D.R., et al. Exercise-based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67(1):1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 20.Fauci A.S. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32(5):675–685. doi: 10.1086/319235. [DOI] [PubMed] [Google Scholar]
- 21.Yach D., Hawkes C., Gould C.L., et al. The global burden of chronic diseases: overcoming impediments to prevention and control. J Am Med Assoc. 2004;291(21):2616–2622. doi: 10.1001/jama.291.21.2616. [DOI] [PubMed] [Google Scholar]
- 22.Piot P., Caldwell A., Lamptey P., et al. Addressing the growing burden of non-communicable disease by leveraging lessons from infectious disease management. J Glob Health. 2016;6(1) doi: 10.7189/jogh.06.010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization Country and regional data on diabetes. 2019. https://www.who.int/diabetes/facts/world_figures/en/index5.html
- 24.World Health Organization . World Health Organization; 2018. Global Tuberculosis Report. Report: WHO/CDS/TB/2018.25. [Google Scholar]
- 25.Kahn K., Garenne M.L., Collinson M.A., et al. Mortality trends in a new South Africa: hard to make a fresh start. Scand J Public Health Suppl. 2007;69:26–34. doi: 10.1080/14034950701355668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barquera S., Pedroza-Tobias A., Medina C., et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46(5):328–338. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Alwan A., MacLean D., Riley L., et al. Monitoring and surveillance of chronic non-communicable diseases: progress and capacity in high-burden countries. The Lancet. 2010;376(9755):1861–1868. doi: 10.1016/S0140-6736(10)61853-3. [DOI] [PubMed] [Google Scholar]
- 28.Bollyky T.J., Templin T., Cohen M., et al. Lower-income countries that face the most rapid shift in noncommunicable disease burden are also the least prepared. Health Aff (Millwood) 2017;36(11):1866–1875. doi: 10.1377/hlthaff.2017.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guariguata L., Whiting D.R., Hambleton I., et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA A Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 31.Wang J.B., Jiang Y., Wei W.Q., et al. Estimation of cancer incidence and mortality attributable to smoking in China. Cancer Causes Control. 2010;21(6):959–965. doi: 10.1007/s10552-010-9523-8. [DOI] [PubMed] [Google Scholar]
- 32.Chen W., Zheng R., Baade P.D., et al. Cancer statistics in China, 2015. CA A Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 33.Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 34.Finkelstein E.A., Khavjou O.A., Thompson H., et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Chooi Y.C., Ding C., Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Huang T.T. Watching China's weight. Obesity (Silver Spring) 2016;24(7):1407. doi: 10.1002/oby.21545. [DOI] [PubMed] [Google Scholar]
- 37.Wang H., Xue H., Du S., et al. Time trends and factors in body mass index and obesity among children in China: 1997-2011. Int J Obes (Lond) 2017;41(6):964–970. doi: 10.1038/ijo.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrivastava U., Misra A., Mohan V., et al. Obesity, diabetes and cardiovascular diseases in India: public health challenges. Curr Diabetes Rev. 2017;13(1):65–80. doi: 10.2174/1573399812666160805153328. [DOI] [PubMed] [Google Scholar]
- 39.Seidell J.C., Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66(Suppl 2):7–12. doi: 10.1159/000375143. [DOI] [PubMed] [Google Scholar]
- 40.Font-Burgada J., Sun B., Karin M. Obesity and cancer: the oil that feeds the flame. Cell Metabol. 2016;23(1):48–62. doi: 10.1016/j.cmet.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Ortega F.B., Lavie C.J., Blair S.N. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 42.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 43.Ding D., Lawson K.D., Kolbe-Alexander T.L., et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016;388(10051):1311–1324. doi: 10.1016/S0140-6736(16)30383-X. [DOI] [PubMed] [Google Scholar]
- 44.Zwieten M., Vromme E., Mol M., et al. 2014. National Working Conditions Survey 2013. Methodology and Global Results. [Google Scholar]
- 45.Besen E., Jetha A., Gaines B. Examining the likelihood of experiencing productivity loss and receiving social security disability income following the onset of chronic disease. J Occup Environ Med. 2018;60(1):48–54. doi: 10.1097/JOM.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 46.Denis T.S. Future soldiers: “the few...” military personnel trends in the developed world. CMJ. 2015;15(4):12–21. [Google Scholar]
- 47.Aucejo E.M., Romano T. Assessing the effect of school days and absences on test score performance. Econ Educ Rev. 2016;55:70–87. doi: 10.1016/j.econedurev.2016.08.007. [DOI] [Google Scholar]
- 48.Michael S.L., Merlo C.L., Basch C.E., et al. Critical connections: health and academics. J Sch Health. 2015;85(11):740–758. doi: 10.1111/josh.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenbaum J.E. Disabilities and degrees: identifying health impairments that predict lower chances of college enrollment and graduation in a nationally representative sample. Community Coll Rev. 2018;46(2):145–175. doi: 10.1177/0091552118762630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster A.C., Hawk W.R. vol 2. US Bureau of Labor Statistics); 2013. (Spending Patterns of Families Receiving Means-Tested Government Assistance. Beyond the Numbers: Prices and Spending). (26) [Google Scholar]
- 51.Hillygus D.S. The missing link: exploring the relationship between higher education and political engagement. Political Behav. 2005;27(1):25–47. doi: 10.1007/s11109-005-3075-8. [DOI] [Google Scholar]
- 52.Trostel P.A. The Benefits of College Education to Individuals and to Society; 2015. It's Not Just the Money. [Google Scholar]
- 53.Ng M., Fleming T., Robinson M., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogden C.L., Flegal K.M., Carroll M.D., et al. Prevalence and trends in overweight among US children and adolescents, 1999-2000. J Am Med Assoc. 2002;288(14):1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 55.Benjamin E.J., Virani S.S., Callaway C.W., et al. Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 56.Hales C.M., Fryar C.D., Carroll M.D., et al. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. J Am Med Assoc. 2018;319(16):1723–1725. doi: 10.1001/jama.2018.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lo J.C., Chandra M., Sinaiko A., et al. Severe obesity in children: prevalence, persistence and relation to hypertension. Int J Pediatr Endocrinol. 2014;2014(1):3. doi: 10.1186/1687-9856-2014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Moraes A.C., Lacerda M.B., Moreno L.A., et al. Prevalence of high blood pressure in 122,053 adolescents: a systematic review and meta-regression. Medicine (Baltim) 2014;93(27):e232. doi: 10.1097/MD.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vale S., Trost S.G., Rego C., et al. Physical activity, obesity status, and blood pressure in preschool children. J Pediatr. 2015;167(1):98–102. doi: 10.1016/j.jpeds.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 60.Benjamin E.J., Blaha M.J., Chiuve S.E., et al. Heart disease and stroke statistics-2017 update: a report from the American heart association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poyrazoglu S., Bas F., Darendeliler F. Metabolic syndrome in young people. Curr Opin Endocrinol Diabetes Obes. 2014;21(1):56–63. doi: 10.1097/01.med.0000436414.90240.2c. [DOI] [PubMed] [Google Scholar]
- 62.Magge S.N., Goodman E., Armstrong S.C., et al. The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. 2017;140(2) doi: 10.1542/peds.2017-1603. [DOI] [PubMed] [Google Scholar]
- 63.Abbasi A., Juszczyk D., van Jaarsveld C.H.M., Gulliford M.C. Body mass index and incident type 1 and type 2 diabetes in children and young adults: a retrospective cohort study. J Endocr Soc. 2017;1(5):524–537. doi: 10.1210/js.2017-00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhupathiraju S.N., Hu F.B. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118(11):1723–1735. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mayer-Davis E.J., Lawrence J.M., Dabelea D., et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017;376(15):1419–1429. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Today Study Group, Zeitler P., Hirst K., et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma R.C., Lin X., Jia W. Causes of type 2 diabetes in China. Lancet Diabetes Endocrinol. 2014;2(12):980–991. doi: 10.1016/S2213-8587(14)70145-7. [DOI] [PubMed] [Google Scholar]
- 68.Ward E., DeSantis C., Robbins A., et al. Childhood and adolescent cancer statistics, 2014. CA A Cancer J Clin. 2014;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 69.Ghosh T., Richardson M., Ryder J., et al. Paper Presented at: AACR Annual Meeting. 2019. Obesity as a risk factor for pediatric acute lymphoblastic leukemia: a report from the Children's Oncology Group. Atlanta, GA. [DOI] [Google Scholar]
- 70.Rogers P.C., Meacham L.R., Oeffinger K.C., et al. Obesity in pediatric oncology. Pediatr Blood Cancer. 2005;45(7):881–891. doi: 10.1002/pbc.20451. [DOI] [PubMed] [Google Scholar]
- 71.Saenz A.M., Stapleton S., Hernandez R.G., et al. Body mass index at pediatric leukemia diagnosis and the risks of relapse and mortality: findings from a single institution and meta-analysis. J Obes. 2018;2018:7048078. doi: 10.1155/2018/7048078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riegel B., Moser D.K., Buck H.G., et al. Self-care for the prevention and management of cardiovascular disease and stroke: a scientific statement for healthcare professionals from the American heart association. J Am Heart Assoc. 2017;6(9) doi: 10.1161/JAHA.117.006997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kesaniemi A., Riddoch C.J., Reeder B., et al. Advancing the future of physical activity guidelines in Canada: an independent expert panel interpretation of the evidence. Int J Behav Nutr Phys Act. 2010;7:41. doi: 10.1186/1479-5868-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendis S., Armstrong T., Bettcher D., et al. World Health Organization; 2014. Global Status Report on Noncommunicable Diseases 2014. [Google Scholar]
- 75.Brawner C.A., Churilla J.R., Keteyian S.J. Prevalence of physical activity is lower among individuals with chronic disease. Med Sci Sport Exerc. 2016;48(6):1062–1067. doi: 10.1249/MSS.0000000000000861. [DOI] [PubMed] [Google Scholar]
- 76.Pate R.R., Pratt M., Blair S.N., et al. Physical activity and public health. A recommendation from the centers for disease control and prevention and the American college of Sports medicine. J Am Med Assoc. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 77.King A.C., Sallis J.F. Why and how to improve physical activity promotion: lessons from behavioral science and related fields. Prev Med. 2009;49(4):286–288. doi: 10.1016/j.ypmed.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guthold R., Stevens G.A., Riley L.M., et al. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018;6(10):e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 79.Bouchard C., Blair S.N., Katzmarzyk P.T. Less sitting, more physical activity, or higher fitness? Mayo Clin Proc. 2015;90(11):1533–1540. doi: 10.1016/j.mayocp.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez K., Fuentes J., Marquez J.L. Physical inactivity, sedentary behavior and chronic diseases. Korean J Fam Med. 2017;38(3):111–115. doi: 10.4082/kjfm.2017.38.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.World Health Organization Physical activity and adults. 2019. https://www.who.int/dietphysicalactivity/factsheet_adults/en/
- 82.World Health Organization Global recommendations on physical activity for health. 2011. https://www.who.int/dietphysicalactivity/publications/physical-activity-recommendations-5-17years.pdf?ua=1 [PubMed]
- 83.Delfino L.D., Dos Santos Silva D.A., Tebar W.R., et al. Screen time by different devices in adolescents: association with physical inactivity domains and eating habits. J Sports Med Phys Fitness. 2018;58(3):318–325. doi: 10.23736/S0022-4707.17.06980-8. [DOI] [PubMed] [Google Scholar]
- 84.Spittaels H., Van Cauwenberghe E., Verbestel V., et al. Objectively measured sedentary time and physical activity time across the lifespan: a cross-sectional study in four age groups. Int J Behav Nutr Phys Act. 2012;9:149. doi: 10.1186/1479-5868-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Durstine J.L., Painter P., Franklin B.A., et al. Physical activity for the chronically ill and disabled. Sport Med. 2000;30(3):207–219. doi: 10.2165/00007256-200030030-00005. [DOI] [PubMed] [Google Scholar]
- 86.Bullard T., Ji M., An R., et al. A systematic review and meta-analysis of adherence to physical activity interventions among three chronic conditions: cancer, cardiovascular disease, and diabetes. BMC Public Health. 2019;19(1):636. doi: 10.1186/s12889-019-6877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neufer P.D., Bamman M.M., Muoio D.M., et al. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metabol. 2015;22(1):4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 88.Farinatti P., Monteiro W., Oliveira R., et al. Cardiorespiratory responses and myocardial function within incremental exercise in healthy unmedicated older vs. young men and women. Aging Clin Exp Res. 2018;30(4):341–349. doi: 10.1007/s40520-017-0776-x. [DOI] [PubMed] [Google Scholar]
- 89.Billman G.E. Aerobic exercise conditioning: a nonpharmacological antiarrhythmic intervention. J Appl Physiol. 1985;92(2):446–454. doi: 10.1152/japplphysiol.00874.2001. 2002. [DOI] [PubMed] [Google Scholar]
- 90.Halle M., Schoenberg M.H. Physical activity in the prevention and treatment of colorectal carcinoma. Dtsch Arztebl Int. 2009;106(44):722–727. doi: 10.3238/arztebl.2009.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beck D.T., Martin J.S., Casey D.P., et al. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am J Hypertens. 2013;26(9):1093–1102. doi: 10.1093/ajh/hpt080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nunan D., Mahtani K.R., Roberts N., et al. Physical activity for the prevention and treatment of major chronic disease: an overview of systematic reviews. Syst Rev. 2013;2:56. doi: 10.1186/2046-4053-2-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Silvestri L. Benefits of physical activity. Percept Mot Skills. 1997;84(3 Pt 1):890. doi: 10.2466/pms.1997.84.3.890. [DOI] [PubMed] [Google Scholar]
- 94.Ruegsegger G.N., Booth F.W. Health benefits of exercise. Cold Spring Harb Perspect Med. 2018;8(7) doi: 10.1101/cshperspect.a029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haskell W.L. J.B. Wolffe Memorial Lecture. Health consequences of physical activity: understanding and challenges regarding dose-response. Med Sci Sport Exerc. 1994;26(6):649–660. doi: 10.1249/00005768-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 96.Simon H.B. Exercise and health: dose and response, considering both ends of the curve. Am J Med. 2015;128(11):1171–1177. doi: 10.1016/j.amjmed.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 97.Paffenbarger R.S., Jr., Hyde R.T., Wing A.L., et al. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 98.Blair S.N., Kohl H.W., 3rd, Paffenbarger R.S., Jr., et al. Physical fitness and all-cause mortality. A prospective study of healthy men and women. J Am Med Assoc. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 99.Warburton D.E.R., Bredin S.S.D. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32(5):541–556. doi: 10.1097/HCO.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 100.Sui X., LaMonte M.J., Blair S.N. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165(12):1413–1423. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buffart L.M., Kalter J., Sweegers M.G., et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 102.Baptista L.C., Machado-Rodrigues A.M., Martins R.A. Exercise but not metformin improves health-related quality of life and mood states in older adults with type 2 diabetes. Eur J Sport Sci. 2017;17(6):794–804. doi: 10.1080/17461391.2017.1310933. [DOI] [PubMed] [Google Scholar]
- 103.Sagar V.A., Davies E.J., Briscoe S., et al. Exercise-based rehabilitation for heart failure: systematic review and meta-analysis. Open Heart. 2015;2(1) doi: 10.1136/openhrt-2014-000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Durstine J.L., Gordon B., Wang Z., et al. Chronic disease and the link to physical activity. J Sport Health Sci. 2013;2(1):3–11. doi: 10.1016/j.jshs.2012.07.009. [DOI] [Google Scholar]
- 105.Powell R., McGregor G., Ennis S., et al. Is exercise-based cardiac rehabilitation effective? A systematic review and meta-analysis to re-examine the evidence. BMJ Open. 2018;8(3) doi: 10.1136/bmjopen-2017-019656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leon A.S., Franklin B.A., Costa F., et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American heart association scientific statement from the council on clinical cardiology (subcommittee on exercise, cardiac rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity), in collaboration with the American association of cardiovascular and pulmonary rehabilitation. Circulation. 2005;111(3):369–376. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- 107.Kirwan J.P., Sacks J., Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Clevel Clin J Med. 2017;84(7 Suppl 1):S15–S21. doi: 10.3949/ccjm.84.s1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aune D., Norat T., Leitzmann M., et al. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015;30(7):529–542. doi: 10.1007/s10654-015-0056-z. [DOI] [PubMed] [Google Scholar]
- 109.Dudgeon W.D., Phillips K.D., Bopp C.M., et al. Physiological and psychological effects of exercise interventions in HIV disease. AIDS Patient Care STDS. 2004;18(2):81–98. doi: 10.1089/108729104322802515. [DOI] [PubMed] [Google Scholar]
- 110.Xu J., Lombardi G., Jiao W., et al. Effects of exercise on bone status in female subjects, from young girls to postmenopausal women: an overview of systematic reviews and meta-analyses. Sport Med. 2016;46(8):1165–1182. doi: 10.1007/s40279-016-0494-0. [DOI] [PubMed] [Google Scholar]
- 111.Daly R.M. Exercise and nutritional approaches to prevent frail bones, falls and fractures: an update. Climacteric. 2017;20(2):119–124. doi: 10.1080/13697137.2017.1286890. [DOI] [PubMed] [Google Scholar]
- 112.Simpson R.J., Kunz H., Agha N., et al. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci. 2015;135:355–380. doi: 10.1016/bs.pmbts.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 113.Hespe G.E., Kataru R.P., Savetsky I.L., et al. Exercise training improves obesity-related lymphatic dysfunction. J Physiol. 2016;594(15):4267–4282. doi: 10.1113/JP271757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chambers T.J., Richard R.A. The impact of obesity on male fertility. Hormones (Basel) 2015;14(4):563–568. doi: 10.14310/horm.2002.1621. [DOI] [PubMed] [Google Scholar]
- 115.Best D., Avenell A., Bhattacharya S. How effective are weight-loss interventions for improving fertility in women and men who are overweight or obese? A systematic review and meta-analysis of the evidence. Hum Reprod Update. 2017;23(6):681–705. doi: 10.1093/humupd/dmx027. [DOI] [PubMed] [Google Scholar]
- 116.Barakat R., Perales M., Bacchi M., et al. A program of exercise throughout pregnancy. Is it safe to mother and newborn? Am J Health Promot. 2014;29(1):2–8. doi: 10.4278/ajhp.130131-QUAN-56. [DOI] [PubMed] [Google Scholar]
- 117.Davies G.A.L., Wolfe L.A., Mottola M.F., et al. No. 129-Exercise in pregnancy and the postpartum period. J Obstet Gynaecol Can. 2018;40(2):e58–e65. doi: 10.1016/j.jogc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 118.Labonte-Lemoyne E., Curnier D., Ellemberg D. Exercise during pregnancy enhances cerebral maturation in the newborn: a randomized controlled trial. J Clin Exp Neuropsychol. 2017;39(4):347–354. doi: 10.1080/13803395.2016.1227427. [DOI] [PubMed] [Google Scholar]
- 119.Perales M., Calabria I., Lopez C., et al. Regular exercise throughout pregnancy is associated with a shorter first stage of labor. Am J Health Promot. 2016;30(3):149–154. doi: 10.4278/ajhp.140221-QUAN-79. [DOI] [PubMed] [Google Scholar]
- 120.Wiebe H.W., Boule N.G., Chari R., et al. The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstet Gynecol. 2015;125(5):1185–1194. doi: 10.1097/AOG.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 121.Morgan J.A., Corrigan F., Baune B.T. Effects of physical exercise on central nervous system functions: a review of brain region specific adaptations. J Mol Psychiatry. 2015;3(1):3. doi: 10.1186/s40303-015-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paillard T., Rolland Y., de Souto Barreto P. Protective effects of physical exercise in alzheimer's disease and Parkinson's disease: a narrative review. J Clin Neurol. 2015;11(3):212–219. doi: 10.3988/jcn.2015.11.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hotting K., Roder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. 2013;37(9 Pt B):2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 124.Northey J.M., Cherbuin N., Pumpa K.L., et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52(3):154–160. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 125.Karssemeijer E.G.A., Aaronson J.A., Bossers W.J., et al. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: a meta-analysis. Ageing Res Rev. 2017;40:75–83. doi: 10.1016/j.arr.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 126.Samieri C., Perier M.C., Gaye B., et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. J Am Med Assoc. 2018;320(7):657–664. doi: 10.1001/jama.2018.11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bland H., Melton B., Bigham L., et al. Quantifying the impact of physical activity on stress tolerance in college students. Coll Stud J. 2014;48(4):559–568. [Google Scholar]
- 128.Dhabhar F.S. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58(2-3):193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 129.Rebar A.L., Stanton R., Geard D., et al. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. 2015;9(3):366–378. doi: 10.1080/17437199.2015.1022901. [DOI] [PubMed] [Google Scholar]
- 130.Mikkelsen K., Stojanovska L., Polenakovic M., et al. Exercise and mental health. Maturitas. 2017;106:48–56. doi: 10.1016/j.maturitas.2017.09.003. [DOI] [PubMed] [Google Scholar]