Abstract
The gasotransmitter hydrogen sulfide (H2S) produced by the transsulfuration pathway (TSP) is an important biological mediator, involved in many physiological and pathological processes in multiple higher organisms, including humans. Cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) enzymes play a central role in H2S production and metabolism. Here, we investigated the role of H2S in learning and memory processes by exploring several Drosophila melanogaster strains with single and double deletions of CBS and CSE developed by the CRISPR/Cas9 technique. We monitored the learning and memory parameters of these strains using the mating rejection courtship paradigm and demonstrated that the deletion of the CBS gene, which is expressed predominantly in the central nervous system, and double deletions completely block short- and long-term memory formation in fruit flies. On the other hand, the flies with CSE deletion preserve short- and long-term memory but fail to exhibit long-term memory retention. Transcriptome profiling of the heads of the males from the strains with deletions in Gene Ontology terms revealed a strong down-regulation of many genes involved in learning and memory, reproductive behavior, cognition, and the oxidation–reduction process in all strains with CBS deletion, indicating an important role of the hydrogen sulfide production in these vital processes.
Keywords: Drosophila melanogaster, CBS, CSE, H2S, learning, memory, courtship rejection paradigm, transcriptome
1. Introduction
Various roles of the third described endogenous gasotransmitter (H2S) under normal conditions and in various human diseases and pathologies were described in several excellent reviews [1,2,3]. Similarly, the transsulfuration pathway (TSP), which results in the production of hydrogen sulfide (H2S) and includes the conversion of homocysteine to cysteine following the breakdown of methionine, was described in detail by several authors including a brilliant analysis performed by Kimura [4,5,6,7]. Briefly, the accumulated data demonstrate that, in Drosophila and other eukaryotes, H2S is produced by cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (MST) genes that have different functions and may be expressed in different organs and tissues [8]. It becomes clear that H2S plays an important role in brain functions as a neuroprotector and neuromodulator in different organisms, including humans. There are several lines of evidence in favor of this conclusion. First, the level of endogenous H2S is significantly decreased throughout aging and in the case of many neurodegenerative diseases including Alzheimer’s Disease (AD) [9,10]. Second, the application of various H2S donors often exhibited a strong neuroprotective effect, and such treatment may ameliorate memory impairment and restore cognitive functions in various model studies and the case of several human neurodegenerative diseases [11,12,13,14]. Finally, numerous missing mutations that disrupt the structure of CBS in humans result in classical homocystinuria due to cystathionine β-synthase (CBS) deficiency [15,16]. Individuals with homocystinuria, the most frequent disorder of sulfur metabolism, have many developmental and cognitive difficulties, with a significant number of cases having a learning disability, atherosclerosis, or thromboembolic disease [15,17]. A decrease in endogenous H2S generation contributes to homocysteine (Hcy)-induced deficit in learning and memory in rats [18]. In contrast, exogenous H2S ameliorated Hcy-induced cognitive dysfunction [19]. Interestingly, the vision phenotype of CBS knock-down flies is consistent with severe myopia observed in homocystinuria patients [20].
However, to the best of our knowledge, there are only scattered data implicating the synthesis of endogenous H2S in the memory process and mating behavior under normal conditions in higher organisms [21,22]. Drosophila melanogaster strains obtained in our laboratory [23] containing single and double deletions of the three major genes implicated in H2S production represent a convenient model to study the role of H2S production in learning and memory processes in higher organisms.
We hypothesized that the disturbance of endogenous H2S generation and metabolism in the brain may affect cognitive functions in Drosophila melanogaster, a model organism often used in the investigations of molecular mechanisms underlying learning, memory, and aging in higher eukaryotes [24,25,26,27].
In the experiments described herein, we made use of several D. melanogaster strains obtained using the CRISPR/Cas9 technique. These strains with deletions of the three genes (CBS, CSE, and MST) were used and described in detail in our previous studies [23,28]. Transcriptomic studies demonstrated that the deletions of both CBS and CSE (CBS-/-; CSE-/-) have a cumulative effect and drastically alter genome expression, with a more pronounced impact exerted by the deletion of the CBS gene. The previous analysis demonstrated that the obtained deletions of CBS and CSE affected numerous genes involved in various biological pathways including oxidation-reduction process, glutathione metabolic process, stress-response genes, housekeeping genes, and genes participating in olfactory and reproduction, while the deletion of MST affected a comparatively small number of genes [23]. Based on the above-mentioned facts and considerations, we decided to monitor the learning and memory parameters of these strains (CBS-/- and CSE-/-), as well as strains with deletions of both CBS and CSE genes (CBS-/-; CSE-/-) using the mating rejection courtship paradigm. Our studies demonstrated that the deletion of the CBS gene and double deletions completely block short- and long-term memory formation in D. melanogaster males, while the flies with CSE deletion preserve short- and long-term memory but failed to exhibit long-term memory retention. Transcriptomic studies revealed a pronounced down-regulation in the expression of multiple pertinent genes and signal pathways in the heads of the male flies from the studied strains with deletions of major H2S-producing genes.
2. Materials and Methods
2.1. Fly Strains and Maintenance
All flies were reared on a standard sugar–yeast–agar medium at 25 °C, 60% humidity, and a light–dark cycle of 12:12 h. As a control, we used strain 58492 with genotype (y1 M{Act5C-Cas9.P. RFP-}ZH-2A w1118 DNAlig4169) obtained from the Bloomington Drosophila Stock Center. Additionally, we used transgenic CBS-/-(5) and CBS-/-(8), CSE-/-, (CBS-/-; CSE-/-(1)) and (CBS-/-; CSE-/-(2)) strains developed in our laboratory [23].
2.2. Test for Learning and Memory of Flies in Conditioned Courtship Suppression Paradigm
To evaluate memory formation in drosophila males, we used the conditioned courtship suppression paradigm (CCSP) [29]. Drosophila melanogaster males drastically reduce courtship behavior after mating failure. Under laboratory conditions, such conditioned courtship suppression serves as a complex learning and memory assay. Interestingly, variations in the courtship conditioning assay can establish different types of memory [27,30,31]. CCSP is used widely for learning ability and memory retention in Drosophila [32,33,34]. All procedures were performed exactly as described in our previous paper [35].
Briefly, the resulting courtship index [36], i.e., percentage of time spent in courtship over a 300-s period) was calculated for each male [36]. The CI was used to calculate the learning index (LI) as follows: LI = ({CIna − CItr}/CIna) × 100 = (1 − CItr/CIna) × 100 [37], where CIna and CItr are the mean courtship indices for independent samples of naïve and trained males, respectively.
Statistical comparisons of behavioral data were performed using a two-sided randomization test [38] by directly computing the probability of rejection of the null hypothesis αR. The sampled randomization test with 10,000 permutations was used. The null hypothesis was rejected at αR < 0.05. We compared all experimental groups with each other.
2.3. Olfactory Behavior Assay
Olfactory behavior assays were performed in a temperature-controlled darkroom at +25 °C. For experiments, we used a classical T-maze [39]. Two airflows were supplied to tubes of T-maze. The first airflow carried the odorant (4-methyl cyclohexanol, Fluka, Sigma-Aldrich, Germany) dissolved in mineral oil (Vecton, Russia) with 1:100 dilution, another one carried fresh air passed through mineral oil without odorant. The concentration of odorant was chosen based on the previous investigation [40] to ensure strong repulsive behavior in the control flies. About fifteen 5-day-old naïve males were transferred into a T-maze central camera for 2 min for adaptation without any airflow, and then the test was performed for 3 min for a choice between airflows with or without the odorant. Thereafter, flies in the odorant-contained tube were counted (Nodor) and the percentage (V) of flies that were attracted (non-repulsed) by the odorant was counted:
The experiment included 31 replicates and about 400 flies participated in the assay for each genotype. Statistical analysis was performed using KyPlot 5.0 software (KyensLab Inc., Tokyo, Japan, software 5.0). All samples were tested for normality with the Shapiro–Wilk test which suggested that samples have non-normal distribution, so the Mann–Whitney test was applied to compare each experimental sample with the control one.
2.4. RNA Extraction from Fly Heads for Library Preparations
Three biological replicates of 5-day-old naïve males were collected for each transcriptome analysis. For each replicate, total RNA was isolated from approximately 100 heads of naïve males collected by decapitation of drosophila males on an ice-cooled surface. The extraction was made using RNAzol RT (Molecular Research Center, Cincinnati, OH, USA) according to the company’s protocol. The concentration and quality of RNA were determined via Qubit Fluorometer (Invitrogen) and an Agilent BioAnalyzer 2100, respectively, using RNA 6000 nano kit (Agilent technologies, Santa-Clara, CA, USA). For each of the three biological replicates taken for libraries, 100 heads were collected. For libraries RNA with Integrity Number (RIN), no less than 8 were taken.
2.5. cDNA Library Preparation and Data Analysis
Illumina NEB Next Ultra II Directional RNA Library Prep Kit (NEB, Ipswich, MA, USA) was used for mRNA libraries preparation. The sequencing was performed on the Illumina NextSeq 500 platform. For each library sequencing provided around 15–20 million reads. For analysis, the PPLine script [41] was used: the reads were mapped to the D. melanogaster genome (Dm6) with STAR [42] following adapter, length, and quality trimming by Trimmomatic [43]. The edgeR package [44] was used for differential gene expression analysis. Differential expression parameters for each gene were estimated using TMM read normalization method, with the fitting quasi-likelihood negative binomial generalized log-linear model. Raw p-values were corrected with the FDR method. The topGO (v.2.36.0) and clusterProfiler Bioconductor packages [45] were used to perform Gene Ontology and KEGG enrichment analyses. Visualization of the gene set enrichment analysis (GSEA) was performed using custom scripts written in Python and R. Sequence data were deposited in the NCBI GEO database under the number—GSE200397. RNA sequencing and further differential expression estimation were performed using the equipment of the Engelhardt Institute of Molecular Biology RAS “Genome” center (http://www.eimb.ru/rus/ckp/ccu_genome_c.php, accessed on 22 February 2022).
2.6. Quantitative Real-Time PCR
For real PCR, RNA was isolated from three biological replicates (fly heads). One microgram of total RNA was used for cDNA synthesis with an MMLV RT kit (Evrogen, Moscow, Russia, cat# SK021). All qRT-PCR reactions were conducted using the SYBR Green fluorescent dye (Evrogen, Moscow, Russia, cat.# PK156S) in an ABI PRISM VR 7500 device (Applied Biosystems). The relative expression of the studied genes was calculated based on the ΔΔCt method [46]. Quantifications were normalized to the housekeeping gene rp49 [47]. Experiments were performed with three replicates and three experimental replicates. The primers sequences are listed in Table S1.
2.7. Amino Acid Quantitative Analysis
Intracellular metabolites from total flies were extracted using cold 80% (v/v) aqueous methanol [48]. The profile of amino acids was analyzed during separation in a lithium buffer system. Amino acids were quantified according to [49] using an L-8800 amino acid analyzer (Hitachi Ltd., Tokyo, Japan). For high-performance liquid chromatography, the 2622SC-PF ion-exchange column (Hitachi Ltd., P/N 855–4507, 4.6 mm × 60 mm, Tokyo, Japan) was eluted at a rate of 0.35 mL/min by step gradients of Li–citrate buffers and temperature (in the range 30–70 °C).
3. Results
3.1. Learning and Memory of Flies in Conditioned Courtship Suppression Paradigm
Courtship behavior is an important aspect of mating success, and many animals have intricate behavioral rituals that they use to attract mates. Thus, males of D. melanogaster have a highly stereotyped courtship routine that involves orientation towards the female, vibrating an outstretched wing to produce a courting song, licking, and attempted copulation. Courtship conditioning involves a training period, where learning and memory are induced, followed by a testing period where the behavioral effects of training are observed over time [30]. Courtship conditioning can be used to induce different temporal forms of memory, such as short- and long-term memory (STM and LTM).
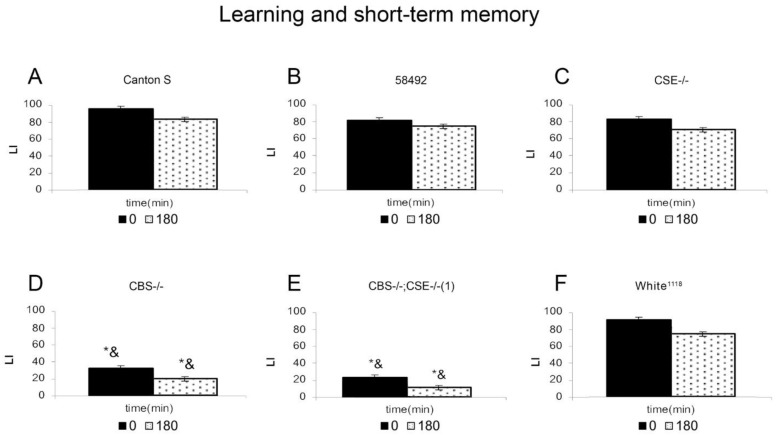
Figure 1 shows the results of the short-term memory analysis. To check the influence of differences in the genetic background on memory formation in the mating rejection paradigm, we used three strains as controls. These are the wild-type Canton-S (CS) strain, strain 58492, which, like the transformants, carries mutations in the yellow and white genes, and the white-eyed strain white1118. Males from wild-type CS, 58492, white1118 and CSE-/- are capable of learning and forming short-term memory. In contrast, males from strains CBS-/- and (CBS-/-; CSE-/-) (double deletion) exhibited very low ability to learn and, therefore, to form short-term memory. These mutant males showed learning ability that was four-fold lower than that of CS flies. This fact demonstrates the involvement of sulfur metabolism genes in the learning process and the formation of short-term memory. It is noteworthy, that in the case of single deletions, we observe different results. Thus, CSE-/- males showed excellent learning and short memory formation and did not differ in this respect from males of all control strains. Characteristically, CBS-/- males are characterized by an inability to learn and form short-term memory, like double deletion flies. This indicates the involvement of cystathionine β-synthase expression in learning and memory processes.
Figure 1.
Dynamics of learning acquisition and short-term memory retention of conditioned courtship suppression in mutant males. Males from Canton-S (A), 58492 (B), CSE-/- (C), CBS-/- (D), CBS-/-; CSE-/- (1) (E), and white1118 (F) strains were tested. Abscissa: time after training (min); ordinate: LI—learning index, standard units. The sample size for each time point was 20 males. *—LI significantly lower than that 58492 strain under similar conditions (two-sided randomization test, αR < 0.05); &—LI significantly lower than that of wild type Canton-S strain under similar conditions (two-sided randomization test, αR < 0.05).
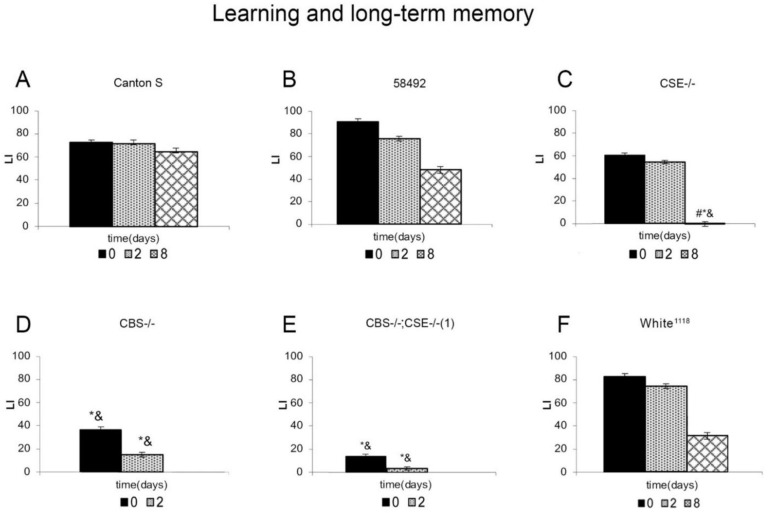
In the next stage, we performed an analysis of learning acquisition and long-term memory retention in the studied strains (Figure 2). As expected, males from all control strains exhibited normal learning and long-term memory ability as the duration of learning increased, which is consistent with previous results [35,50]. However, in the case of males from white-eyed strains 58492 and white1118, a slight decrease in memory retention was observed. The results obtained in the analysis of learning acquisition and long-term memory retention in the strains with double deletion CBS-/-; CSE-/- are consistent with those of short-term memory; these males are incapable of learning and long-term memory formation. Thus, the presence of a double deletion leads to a disturbance of the ability to learn and form both short- and long-term memory.
Figure 2.
Dynamics of learning acquisition and long-term memory retention of conditioned courtship suppression in mutant males. Males from Canton-S (A), 58492 (B), CSE-/- (C), CBS-/- (D), CBS-/-; CSE-/- (1) (E), and white1118 (F) strains were tested. Abscissa: time after training (days); ordinate: LI—learning index, standard units. The sample size for each time point was 20 males. *—LI significantly lower than that 58492 strain under similar conditions (two-sided randomization test, αR < 0.05); &—LI significantly lower than that of wild type Canton-S strain under similar conditions (two-sided randomization test, αR < 0.05); and #—LI in the delayed test significantly lower than in test immediately following training (two-sided randomization test, αR < 0.05).
Flies with CSE gene deletion exhibit a very peculiar behavioral pattern in our studies. In this case, learning indices immediately and after 2 days after training remain at a high level and do not significantly differ from those of the control strains, which indicates a normal implementation of learning processes and formation of long-term memory. However, after eight days, the learning index in this strain dropped catastrophically. Therefore, the absence of the cystathionine γ-lyase gene (CSE) does not affect the ability to learn and form short- and long-term memory but disrupts the retention of the latter, which in the wild type males lasts for up to 9 days. On the other hand, the CBS mutants, like the flies carrying the double deletion, turned out to be incapable of learning and long-term memory formation. Therefore, the ability to learn, regardless of the duration of the training, and the formation of short- and long-term memory, is severely impaired both in the absence of both genes (CBS-/-; CSE-/-) and in the case when only CBS gene is deleted.
All behavioral experiments investigating the mating rejection paradigm described above are sensible only if the flies being tested have normal olfactory abilities. To be sure that the males with deletions retain normal olfactory abilities, we performed a special series of experiments described below.
3.2. The Monitoring of Olfactory Abilities of the Strains Used in the Study
Our previous work dealing with transcriptome analysis of whole flies with the deletions of the three sulfur metabolism genes (CBS, CSE, and MST) revealed a decreased expression of several genes associated with response to pheromone [23]. Therefore, we decided to investigate the olfactory behavior of our strains with deletions as described in the Materials and Methods section. Our results demonstrated that all the experimental strains have the same repulsive behavior in response to high odorant concentration as the control ones (Figure S1).
3.3. Transcriptome Analysis of the Strains with Deletions
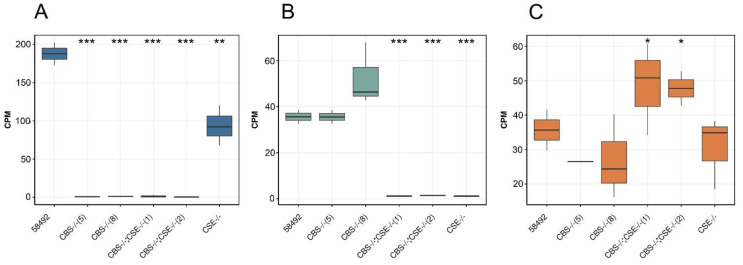
The performed analysis of the transcriptomic libraries obtained from the heads of the flies with deletions and control flies demonstrated that the expression of CBS in the heads of the control males from strain 58492 is 4–5 fold higher than the expression of CSE or MST (Figure 3). Surprisingly, in the strains with CBS deletion, we did not observe a compensatory increase in MST gene expression, which also produces hydrogen sulfide in the brain [51]. (Figure 3). The absence of a significant compensatory increase in the expression level of the MST gene in the strains with CBS deletion was confirmed by real-time PCR (Figure S2).
Figure 3.
The expression of H2S producing genes in the heads of the males from the studied strains. (A) Box plot, demonstrating the expression levels of CBS in the heads of the flies from the studied strains. (B) Box plot, demonstrating the expression levels of CSE in the heads of the flies from the studied strains. (C) Boxplot, demonstrating the expression levels of MST in the heads of the flies from the studied strains. (* represents p < 0.05, ** represents p < 0.005, and *** denotes FDR < 0.001).
It is of note, that a performed amino acid quantitative analysis (see Materials and Methods) demonstrated (Figure S3) that, as expected, the deletion of the CBS gene resulted in the accumulation of homocysteine (Hcy) which behaves as a strong excitotoxic neurotransmitter that causes cognitive impairments, vascular dementia, and many other abnormalities in humans and mice [15,17,52]. The level of methionine/homocysteine mixture increases about 8-fold in CBS-/- strain and 7-fold in CBS-/-; CSE-/- strain. At the same time, the cystathionine level in the CSE-/- strain increases 170-fold. However, cystathionine accumulation detected in the case of CSE deletion (Figure S3) should have no deleterious effect because cystathioninemia is considered a biochemical abnormality without visible clinical symptoms [53].
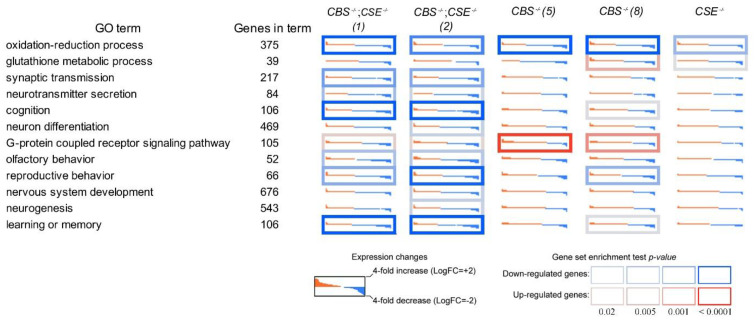
The performed transcriptome analysis of libraries obtained from drosophila male heads of the strains with the deletions and a control 58492 strain enabled us to determine major cellular processes affected by the deletion of the two major genes involved in the transsulfuration pathway and H2S production. Thus, transcriptome profiling of the heads from the strains with deletions in Gene Ontology terms revealed significant changes in various cellular processes, including learning and memory, cognition, synaptic transmission, neurotransmitter transport, and secretion. Importantly, the performed analysis revealed a significant decrease in many genes involved in oxidation—reduction process, memory formation, and consolidation in the strains with the deletions. Characteristically, the most pronounced changes were revealed in the strains with double deletions (CBS-/-; CSE-/-) and the least in the strain comprising a deletion of the CSE gene (Figure 4).
Figure 4.
Gene ontology (GO) and pathway analysis of differentially expressed genes in the heads of naïve males. Expression level changes induced in the strains with single CBS-/-(5) and CBS-/-(8), CSE-/- and double deletions CBS-/-; CSE-/-(1), and CBS-/-; CSE-/-(2) in the male heads. Each cell represents the sorted binary logarithms of expression value fold changes (LogFC) in the mutant strains versus control species for genes participating in a specific GO pathway. LogFC (vertical axis) ranges from -2 to +2, i.e., -2 from a 4-fold decrease (blue) to a 4-fold increase (red). Cell borders demonstrate the statistical significance of gene set enrichment analysis (Fisher test p-value): blue (enriched with downregulated genes) and red (enriched with overexpressed ones). (Color figure online). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.).
The performed KEGG analysis of transcriptomic data from Drosophila heads (Figure S4) is discussed in the Supplementary Material.
For quantitative analysis of our transcriptomic data (to minimize unique single strain differences) at the first stage we picked up all genes that exhibited a similar pattern of expression changes: (1) in both strains with CBS deletions, (2) in both strains with double deletion (CBS-/-; CSE-/-) (Table 1).
Table 1.
The number of genes with altered expression in the strains with deletions after pairwise comparison to control strain 548492 (FDR < 0.05).
| Category | Number of Genes | Category | Number of Genes |
|---|---|---|---|
| CBS-/- genes down-regulated | 295 | CBS-/- genes up-regulated | 72 |
| CBS-/-; CSE-/- genes down-regulated | 190 | CBS-/-; CSE-/- genes up-regulated | 61 |
| CSE-/- genes down-regulated | 53 | CSE-/- genes up- regulated | 20 |
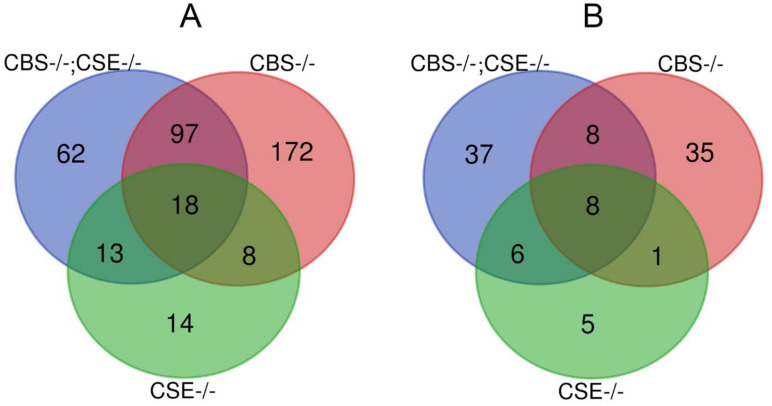
Based on these data, Venn diagrams were made (Figure 5). It is evident from Figure 5 and Table 1 that the maximal number of genes that significantly changed their expression was revealed in the strains comprising the deletion of the CBS gene. Surprisingly, the strains with double deletion (CBS-/-; CSE-/-) exhibited a significantly smaller number of genes with expression changes in comparison with CBS strains (Table 1), while the strain with CSE deletion is characterized by a minimal number of genes with the altered expression.
Figure 5.
Venn diagram showing common and unique sets of differentially expressed genes between investigated strains (CBS-/-; CSE-/-: blue, CBS-/-: red, and CSE-/-: green) after pairwise comparison to control strain 548492 (FDR < 0.05); (A) Down-regulated genes. (B) Up-regulated genes.
It is necessary to emphasize that, in most cases, the genes with altered expression exhibited down-regulation. The group of down-regulated genes predominantly consists of the genes found in the strains with CBS deletion and the strains with double deletion (CBS-/-; CSE-/-) (Figure 5). Characteristically, most (70–75%) of the genes with altered expression in CSE-/- strain exhibited a similar pattern of expression changes in CBS-/- or (CBS-/-; CSE-/-) strains (Figure 5). In Table S2 (genes down-regulated) and Table S3 (genes up-regulated), we provide a list of all the genes with altered expression quantified in (Figure 5) with FDR < 0.05.
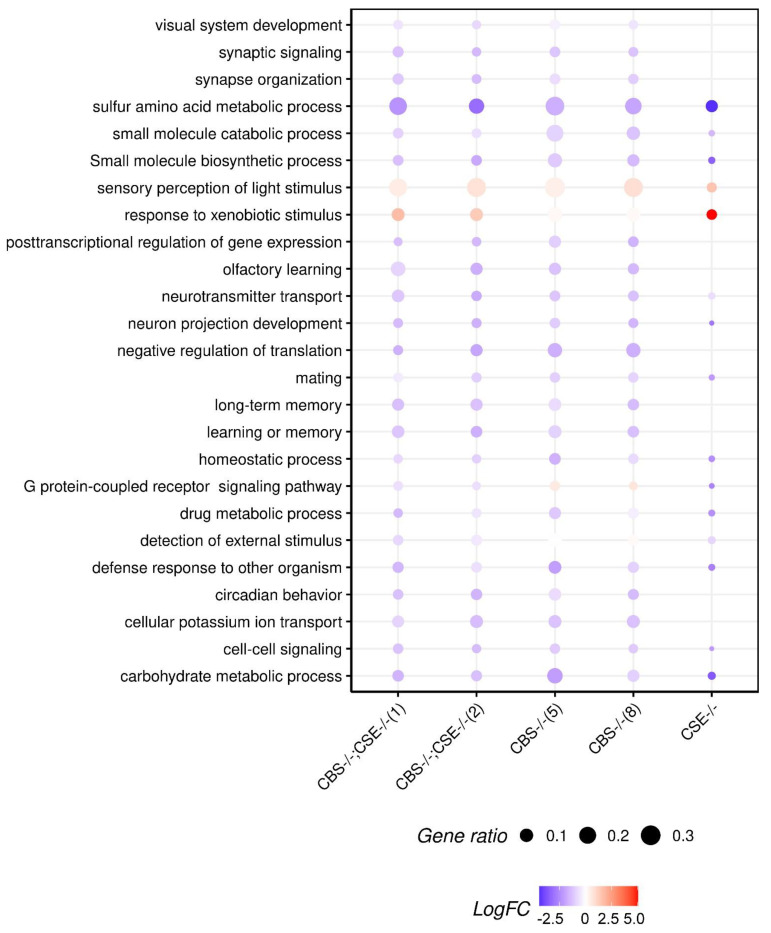
Additionally, we have performed a GSEA analysis of differentially expressed genes to reveal the gene categories that exhibited significant changes in their expression in the strains with deletions (Figure 6).
Figure 6.
GSEA analysis of differentially expressed genes: X-axis represents strains, Y-axis represents, enriched Gene Ontology terms. Point colors represent averaged LogFC value between all genes (FDR < 0.05) appearing in the corresponding Gene Ontology group on the Y axis, point size—the proportion of genes with altered expression to the total number of genes in such group.
It is clear that GO terms related to the functioning of the nervous system and memory formation are of the greatest interest to our analysis. Figure 6 illustrates drastic changes in several major processes related to memory formation and consolidation observed in the strains with CBS deletion. These GO terms include learning and memory, short-term memory, long-term memory, cognition, synaptic signaling, synapse organization, neuron projection development, neurotransmitter transport, olfactory learning, cell–cell signaling, regulation of trans-synaptic signaling, synaptic vesicle localization, and vesicle-mediated transport in the synapse. It is of note that, in the strain with CSE deletion, significant changes (FDR < 0.05) in the expression of genes belonging to these groups are either absent or very small.
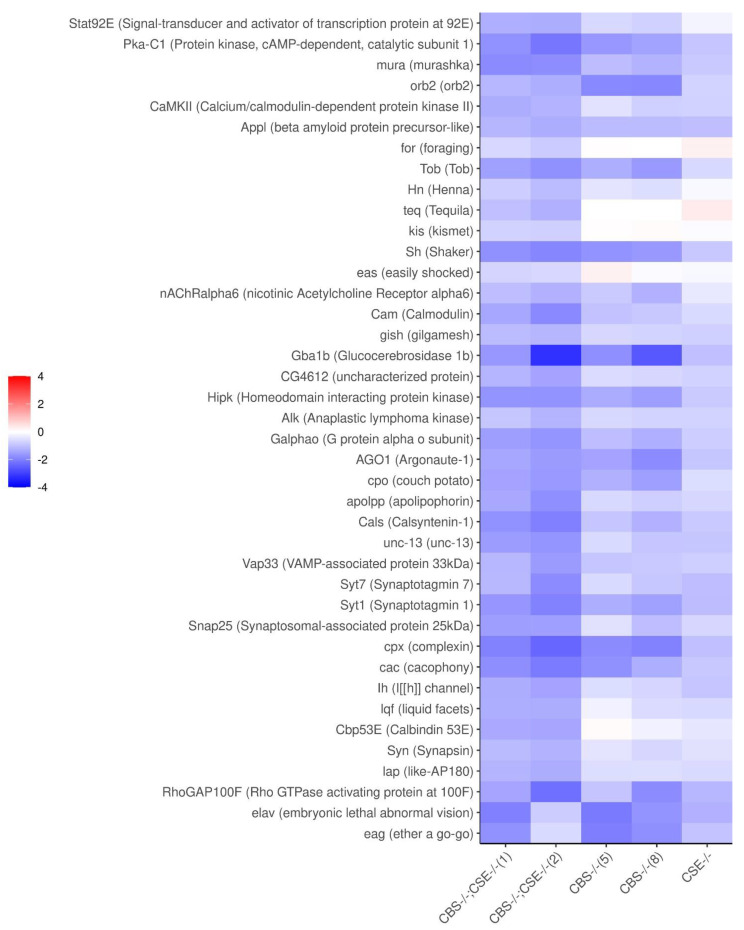
To better illustrate the changes in pertinent gene expression in the studied strains we provided a heat map of the genes involved in learning and memory, cognition, synapse organization, and signaling (Figure 7). The heat map demonstrated that the CSE strain exhibited a slight, statistically insignificant decrease in the expression of almost all genes that belong to the down-regulation group in strains with CBS deletion. Importantly, the most drastic decrease in the expression of these genes is observed in both strains with double deletion.
Figure 7.
Heat map for down-regulated genes involved in learning and memory, cognition, synapse organization, and signaling in the strains with deletions. (Genes with FDR < 0.05 in at least one comparison were considered significant). All comparisons were with the control strain (58492).
Several genes with altered expression revealed by transcriptome analysis (i.e., glucosidase beta acid (GBA1) gene, Tequila (Teq) gene, and Argonaute-1 (Ago1)) were studied using quantitative real-time PCR. It is evident that the most pronounced decrease in the level of expression in all strains with CBS gene deletion is observed for the GBA1b gene. Previously, using the courtship test, it was shown that Drosophila GBA1b mutants lack long-term memory [54]. The expression of the Tequila (Teq) gene, which encodes a serine protease (an orthologue of the human neurotrophin gene), is also necessary for long-term memory formation [55]. Ago1 plays a role in miRNA-mediated translational control of neurons during LTM [56]. It shows a slight decrease in the expression level in all strains with CBS gene deletion. The expression of the Teq gene is downregulated only in (CBS-/-; CSE-/-) strains. Interestingly, Rutabaga (rut) gene, which encodes Ca [2+]/calmodulin-activated adenylyl cyclase that is responsible for synthesis of cAMP and needed in learning and memory [57], did not change its expression in our strains with deletions. The results of quantitative real-time PCR are depicted in Figure S5.
To better illustrate the relationship between genes with reduced expression levels and GO terms related to memory formation processes, a network was created for the (CBS-/-; CSE-/-(1)) strain. The picture demonstrates the complex relationships between such genes and the involvement of the same genes in different processes responsible for memory acquisition and consolidation (Figure S6).
4. Discussion
The physiological role of hydrogen sulfide was described in detail for the first time in the classical studies of Kimura in 1996 [4]. It has been also demonstrated in several investigations that H2S functions as a signaling molecule in the central nervous system (CNS), being involved in the regulation of ion channels, neurotransmitter functions, and various signaling molecules such as tyrosine kinases [4,58,59]. It has been established that CBS is the predominant H2S synthetase in CNS in all higher organisms [4,58,59,60]. Thus, neuronal localization of CBS protein was demonstrated in all major areas of the brain [59], in radial glia/astrocyte lineage of developing mouse brain [61,62], and in reactive astrocytes [63]. In contrast, the level of the other major H2S synthetase (CSE) and its activity is comparatively low in the mammalian brain [64].
In our studies presented here, we made use of several D. melanogaster strains developed in our laboratory exploring the CRISPR/Cas9 technique [23]. These strains containing single and double deletions of major genes responsible for H2S production represent a unique tool to investigate the role of endogenous H2S in learning and memory.
Previous numerous experiments demonstrated a strong protective role of exogenous H2S treatment in various pathologies, including neurodegenerative diseases and aging [65,66]. In addition, human mutations of CBS genes result in homocystinuria accompanied by strong cognitive impairments and many other abnormalities [16,18,19]. These data led us to hypothesize that the obtained deletions of CBS and CSE genes in Drosophila may be a perfect tool to reveal the role of individual H2S-producing genes in learning and memory processes.
Experiments have shown that strains with CBS deletions are characterized by a complete absence of short- and long-term memory, as shown in the courtship suppression paradigm (Figure 1 and Figure 2). Moreover, transcriptome studies using RNA isolated from the male heads demonstrated that all strains with CBS deletion exhibited a dramatic decrease in the expression of many genes involved in memory formation processes (Figure 6 and Figure 7). We are well aware that, in all strains containing a deletion of the CBS gene, the accumulation of toxic neurotransmitter homocysteine may influence the functioning of the nervous system and be at least partially responsible for the observed learning and memory impairments. However, here we demonstrated that while strains with double deletions and strains comprising only CBS deletion are characterized by a very similar level of Hcy (Figure S2), the most drastic drop of most pertinent gene expression was detected in the strains with double deletion (Figure 7). Furthermore, we revealed a highly significant loss of LTM memory retention in the strain with CSE deletion, which also suggests an important role of endogenous H2S in the cognitive processes. It is clear that there is a complex interaction between two major genes (CBS and CSE) playing a central role in cysteine metabolism and H2S production. Analysis of the number of genes that change the level of expression in strains with a single deletion of the CBS gene and strains with a double deletion revealed an interesting phenomenon: the number of genes with reduced expression level decreases almost three-fold in the strain with the double deletion as compared to the CBS-/- strain (Figure 5). This phenomenon should have a positive effect on various physiological processes. Indeed, previously, we demonstrated [67] to our surprise that the strains with double deletions exhibited significantly better stress tolerance and higher longevity than strains with a single CBS deletion.
It is likely the learning and memory failure observed in the strains with CBS deletion stems from a cumulative effect of simultaneous down-regulation of several pertinent genes involved in the cognitive processes in Drosophila males and the deleterious effect of Hcy accumulation (Figure 7).
It is well-known that molecular mechanisms underlying learning, memory formation, and long-term memory retention are quite different [68]. Thus, learning and short-term memory do not require the synthesis of new proteins and are formed by modifications of existing target proteins in the neurons responsible for cell membrane conductance and neuronal excitation [69,70].
In the case of learning and STM formation, the main processes include adenylate cyclase activation, cAMP production, and protein kinase A (PKA) activation. These processes include PKA-dependent phosphorylation of various proteins, including potassium and calcium channel subunits, resulting in the strengthening of pre-existing synaptic connections [67]. Therefore, the initial level of expression of both PKA itself and the genes related to potassium and calcium channel functioning may affect the formation of short-term memory. Consistent with this, our transcriptome analysis revealed a decrease in the expression of the following genes that may be responsible for the observed STM and learning disorders in strains with CBS deletions, and especially in the case of double deletions: pka-c1, eag, Sh, and cac. The ether a go-go gene (eag) participates in voltage-gated potassium channel activity and is involved in learning [71]. Gene Shaker (Sh), encoding the structural alpha subunit of a voltage-gated potassium channel, plays a key role in maintaining electrical excitability in neurons regulating neurotransmitter release at the synapses [72]. The cacophony (cac) gene is of special interest for the understanding of our results [73,74]. Like Sh, it encodes the structural subunit of the voltage-gated calcium channel located in presynaptic active zones and is involved in the release of neurotransmitters. The expression of this gene is essential for a wide range of neurophysiological processes; in particular, it contributes to male courtship behavior. Interestingly, D. melanogaster strains with deletions of 4–6 copies of the hsp70 gene are also characterized by impaired memory formation processes, and exhibited a decrease in the expression level of the above mentioned genes [35].
In contrast to STM, long-term memory requires a synthesis of new proteins and the formation of new synapses [67,75]. During the formation of LTM, there is a persistent increase in the level of cAMP, phosphorylation of the transcription factor CREB (CRE-binding protein), and induction of CREB-induced gene transcription [68,76]. Surprisingly, strains with CBS and CSE deletions showed no changes in the expression level of the creb gene. It is known that LTM formation and long-term potentiation (LTP) are associated with enhanced neurotransmitter release and strengthening of synaptic connections [77,78]. A postsynaptic increase in calcium concentration is observed in most synapses that support LTP. Increased calcium levels lead to activation of Calcium/calmodulin-dependent protein kinase II (CaMKII) which is regulated by the Ca2+/calmodulin complex and autophosphorylation process [62]. Therefore, CaMKII is a key protein kinase involved in neural plasticity and memory formation [79,80,81,82]. Experimental blocking of CaMKII inhibits synaptic transmission [83]. Accordingly, in our studies, we observed a decrease in the expression level of the CaMKII gene predominantly in the strains with double deletions and strains with CBS deletion. This observation is consistent with the evidence demonstrating that, in mammals, H2S promotes long-term potentiation and regulates intracellular calcium concentration in brain cells. It has also been shown that H2S enhances LTP in synapses, and Ca2+ waves are induced in the surrounding astrocytes [84,85]. These facts corroborated the conclusion postulating that H2S may act as a neuromodulator and/or intracellular messenger and play an important role in synapse remodeling [85].
It is also well-established, that the regulation of RNA transport and the regulation of translation of localized mRNA play a very important role in synaptic remodeling [86]. Characteristically, in our studies, we observed down-regulation of several genes (appl, ago1, orb2, elav, and cam) belonging to this category in all strains with CBS deletion. In addition, the genes controlling the neuronal signaling and neurotransmission, as well as genes involved in neurotransmitter transport cpx, Syt1, Syt7, unc-13, cac, Vap33, and Syn, are down-regulated in the strains with deletion of CBS (Figure 7).
Analysis of genes, down-regulated in all strains with deletions, revealed genes with serine hydrolase activity or serine endopeptidase activity (Tables S1 and S2). Serine hydrolases have been shown to regulate proteolysis at the synapses and alter neuronal plasticity [87]. These genes also participate in posttranslational modification of key brain signaling proteins [88,89] and may affect the metabolism of a wide range of chemical messengers, including neurotransmitters [90,91,92]. It is possible that the altered expression level of genes involved in proteolysis may also contribute to the observed lack of long-term memory consolidation observed in the CSE-/- strain. We hypothesize that complete memory loss in flies with CBS gene deletion was caused by the cumulative effect of homocysteine accumulation and the lack of neuroprotective and neuromodulatory effects of H2S.
5. Concluding Remarks
In the experiments performed in this study, we used several D. melanogaster strains developed by the CRISPR/Cas9 technique containing double and single deletions of two major genes (CBS and CSE) responsible for H2S production. These strains with deletions have been investigated in experiments controlling learning and memory formation in flies using the mating rejection paradigm. The performed experiments demonstrated that all strains containing a single or double deletion of CBS were characterized by the complete block of long- (LTM) and short-term (STM) memory formation. Characteristically, flies with CSE deletion exhibited normal STM and LTM, but failed to exhibit LTM retention in our behavioral experiments. Transcriptomic studies have shown that deletions of the CBS and CSE genes significantly alter genome expression in male fly heads to varying degrees, with the CBS gene deletion having a more pronounced effect. Importantly, the genes with altered expression predominantly belong to the down-regulated group and include genes involved in learning and memory, reproductive behavior, cognition, and the oxidation-reduction process. Strains with double deletions exhibited the maximal degree of downregulation of the above-mentioned genes, while in the flies with the deletion of CSE only, we observed a very slight, hardly detectable decrease in the expression of the same groups of genes. The accumulated data enables us to conclude that neurotransmitter H2S plays an important role in learning and memory processes in the fruit flies by interacting with definite groups of genes and signal systems.
Acknowledgments
We thank Svetlana Sorokina for technical support. We also grateful to Mark Evgenev, from the Notre Dame University USA for careful reading the manuscript and language correction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom12060751/s1, Figure S1. Olfactory behavior assay results; Figure S2. Validation of MST level by quantitative RT-PCR in the heads of 5-day-old naïve males. Figure S3. Quantitative analysis of methionine/homocysteine and cystathionine amino acid levels in control strain, CBS-/-, CSE-/-, and double deletion strain CBS-/-; CSE-/-. Figure S4. KEGG pathways; Figure S5. Validation of GBA1b, Teq, Ago1, and rut gene expression levels by quantitative RT-PCR in the heads of 5-day-old naïve males. Figure S6. Graphical illustration of interrelations between differentially expressed genes (blue) and corresponding GeneOntology terms, involved in memory formation (purple) in a network of (CBS-/-; CSE-/-(1)) strain. Table S1. Primers for Q RT-PCR. Table S2. Representation of differentially expressed genes (down-regulated) from Figure 5A.; Table S3. Representation of differentially expressed genes (up-regulated) from Figure 5A.
Author Contributions
Conceptualization, M.B.E., O.G.Z. and E.A.N.; methodology, S.V.S., L.N.C., N.V.S., V.Y.S., A.L.K. and A.P.R.; software, A.P.R. and L.N.C. formal analysis, O.G.Z., M.B.E. and S.V.S.; investigation, O.G.Z., V.Y.S., E.A.N., L.A.B., A.S.Z. and A.L.K.; data curation, A.P.R. and L.N.C.; writing—original draft preparation, O.G.Z., M.B.E. and E.A.N.; writing—review and editing, M.B.E., O.G.Z. and E.A.N.; supervision: M.B.E. and O.G.Z. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Sequence data were deposited in the NCBI GEO database under the number—GSE200397.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The research was funded by Russian Science Foundation Grant 17-74-30030.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013;63:492–497. doi: 10.1016/j.neuint.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Kimura H. Hydrogen sulfide and polysulfides as signaling molecules. Proc. Jpn. Acad. Ser. B. 2015;91:131–159. doi: 10.2183/pjab.91.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul B.D., Snyder S.H. Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem. Pharmacol. 2018;149:101–109. doi: 10.1016/j.bcp.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura H. Hydrogen Sulfide and Polysulfides as Biological Mediators. Molecules. 2014;19:16146–16157. doi: 10.3390/molecules191016146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filipovic M.R. Persulfidation (S-sulfhydration) and H2S. Handb. Exp. Pharmacol. 2015;230:29–59. doi: 10.1007/978-3-319-18144-8_2. [DOI] [PubMed] [Google Scholar]
- 7.Kabil O., Banerjee R. Enzymology of H2S Biogenesis, Decay and Signaling. Antioxid. Redox Signal. 2014;20:770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015;14:329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 9.Giovinazzo D., Bursac B., Sbodio J.I., Nalluru S., Vignane T., Snowman A.M., Albacarys L.M., Sedlak T.W., Torregrossa R., Whiteman M., et al. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA. 2021;118:e2017225118. doi: 10.1073/pnas.2017225118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng S.-Y., Wu X., Lu T., Cui G., Chen G. Research progress of hydrogen sulfide in Alzheimer′s disease from laboratory to hospital: A narrative review. Med. Gas Res. 2020;10:125–129. doi: 10.4103/2045-9912.296043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kshirsagar V., Thingore C., Gursahani M., Gawali N., Juvekar A. Hydrogen Sulfide Ameliorates Lipopolysaccharide-Induced Memory Impairment in Mice by Reducing Apoptosis, Oxidative, and Inflammatory Effects. Neurotox. Res. 2021;39:1310–1322. doi: 10.1007/s12640-021-00374-6. [DOI] [PubMed] [Google Scholar]
- 12.Gao S., Tang Y.-Y., Jiang L., Lan F., Li X., Zhang P., Zou W., Chen Y.-J., Tang X.-Q. H2S Attenuates Sleep Deprivation-Induced Cognitive Impairment by Reducing Excessive Autophagy via Hippocampal Sirt-1 in WISTAR RATS. Neurochem. Res. 2021;46:1941–1952. doi: 10.1007/s11064-021-03314-0. [DOI] [PubMed] [Google Scholar]
- 13.Vandini E., Ottani A., Zaffe D., Calevro A., Canalini F., Cavallini G.M., Rossi R., Guarini S., Giuliani D. Mechanisms of Hydrogen Sulfide against the Progression of Severe Alzheimer’s Disease in Transgenic Mice at Different Ages. Pharmacology. 2018;103:50–60. doi: 10.1159/000494113. [DOI] [PubMed] [Google Scholar]
- 14.Gao R., Chen G., Zhang J.-Y., Ding Y.-P., Wang Z., Kong Y. Hydrogen sulfide therapy in brain diseases: From bench to bedside. Med Gas Res. 2017;7:113–119. doi: 10.4103/2045-9912.208517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Bashir H., Dekair L., Mahmoud Y., Ben-Omran T. Neurodevelopmental and Cognitive Outcomes of Classical Homocystinuria: Experience from Qatar. JIMD Rep. 2014;21:89–95. doi: 10.1007/8904_2014_394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoss G.R.W., Poloni S., Blom H.J., Schwartz I.V.D. Three Main Causes of Homocystinuria: CBS, cblC and MTHFR Deficiency. What do they Have in Common? J. Inborn Errors Metab. Screen. 2019;7:e20190007. doi: 10.1590/2326-4594-jiems-2019-0007. [DOI] [Google Scholar]
- 17.Keating A.K., Freehauf C., Jiang H., Brodsky G.L., Stabler S.P., Allen R.H., Graham D.K., Thomas J.A., Van Hove J.L., Maclean K.N. Constitutive induction of pro-inflammatory and chemotactic cytokines in cystathionine beta-synthase deficient homocystinuria. Mol. Genet. Metab. 2011;103:330–337. doi: 10.1016/j.ymgme.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M.-H., Tang J.-P., Zhang P., Li X., Wang C.-Y., Wei H.-J., Yang X.-F., Zou W., Tang X.-Q. Disturbance of endogenous hydrogen sulfide generation and endoplasmic reticulum stress in hippocampus are involved in homocysteine-induced defect in learning and memory of rats. Behav. Brain Res. 2014;262:35–41. doi: 10.1016/j.bbr.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Li M., Zhang P., Wei H.-J., Li M.-H., Zou W., Li X., Gu H.-F., Tang X.-Q. Hydrogen Sulfide Ameliorates Homocysteine-Induced Cognitive Dysfunction by Inhibition of Reactive Aldehydes Involving Upregulation of ALDH2. Int. J. Neuropsychopharmacol. 2016;20:305–315. doi: 10.1093/ijnp/pyw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flores-Flores M., Moreno-García L., Castro-Martínez F., Nahmad M. Cystathionine β-synthase Deficiency Impairs Vision in the Fruit Fly, Drosophila melanogaster. Curr. Eye Res. 2020;46:600–605. doi: 10.1080/02713683.2020.1818262. [DOI] [PubMed] [Google Scholar]
- 21.Kuntz S., Poeck B., Strauss R. Visual Working Memory Requires Permissive and Instructive NO/cGMP Signaling at Presynapses in the Drosophila Central Brain. Curr. Biol. 2017;27:613–623. doi: 10.1016/j.cub.2016.12.056. [DOI] [PubMed] [Google Scholar]
- 22.Brown E.B., Layne J.E., Elchert A.R., Rollmann S.M. Behavioral and Transcriptional Response to Selection for Olfactory Behavior in Drosophila. G3 Genes Genomes Genet. 2020;10:1283–1296. doi: 10.1534/g3.120.401117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zatsepina O., Karpov D., Chuvakova L., Rezvykh A., Funikov S., Sorokina S., Zakluta A., Garbuz D., Shilova V., Evgen’Ev M. Genome-wide transcriptional effects of deletions of sulphur metabolism genes in Drosophila melanogaster. Redox Biol. 2020;36:101654. doi: 10.1016/j.redox.2020.101654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tully T., Boynton S., Brandes C., Dura J., Mihalek R., Preat T., Villella A. Genetic Dissection of Memory Formation in Drosophila melanogaster. Cold Spring Harb. Symp. Quant. Biol. 1990;55:203–211. doi: 10.1101/SQB.1990.055.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Akalal D.-B.G., Yu D., Davis R.L. A Late-Phase, Long-Term Memory Trace Forms in the Neurons of Drosophila Mushroom Bodies after Olfactory Classical Conditioning. J. Neurosci. 2010;30:16699–16708. doi: 10.1523/JNEUROSCI.1882-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mery F., Belay A.T., So A.K.-C., Sokolowski M.B., Kawecki T.J. Natural polymorphism affecting learning and memory in Drosophila. Proc. Natl. Acad. Sci. USA. 2007;104:13051–13055. doi: 10.1073/pnas.0702923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhuravlev A.V., Nikitina E., Savvateeva-Popova E.V. Learning and memory in Drosophila: Physiologic and genetic bases. Usp Fiziol Nauk. 2015;46:76–92. [PubMed] [Google Scholar]
- 28.Shaposhnikov M.V., Zakluta A.S., Zemskaya N.V., Guvatova Z.G., Shilova V.Y., Yakovleva D.V., Gorbunova A.A., Koval L.A., Ulyasheva N.S., Evgen’Ev M.B., et al. Deletions of the cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) genes, involved in the control of hydrogen sulfide biosynthesis, significantly affect lifespan and fitness components of Drosophila melanogaster. Mech. Ageing Dev. 2022;203:111656. doi: 10.1016/j.mad.2022.111656. [DOI] [PubMed] [Google Scholar]
- 29.Kamyshev N.G., Iliadi K.G., Bragina J.V. Drosophila Conditioned Courtship: Two Ways of Testing Memory. Learn. Mem. 1999;6:1–20. doi: 10.1101/lm.6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raun N., Jones S., Kramer J.M. Conditioned courtship suppression in Drosophila melanogaster. J. Neurogenet. 2021;35:154–167. doi: 10.1080/01677063.2021.1873323. [DOI] [PubMed] [Google Scholar]
- 31.Keleman K., Vrontou E., Krüttner S., Yu J.Y., Kurtovic-Kozaric A., Dickson B.J. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature. 2012;489:145–149. doi: 10.1038/nature11345. [DOI] [PubMed] [Google Scholar]
- 32.Kuzin B.A., Nikitina E.A., Cherezov R.O., Vorontsova J.E., Slezinger M.S., Zatsepina O.G., Simonova O.B., Enikolopov G.N., Savvateeva-Popova E.V. Combination of Hypomorphic Mutations of the Drosophila Homologues of Aryl Hydrocarbon Receptor and Nucleosome Assembly Protein Family Genes Disrupts Morphogenesis, Memory and Detoxification. PLoS ONE. 2014;9:e94975. doi: 10.1371/journal.pone.0094975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savvateeva-Popova E., Popov A., Grossman A., Nikitina E., Medvedeva A., Peresleni A., Korochkin L., Moe J.G., Davidowitz E., Pyatkov K., et al. Pathogenic chaperone-like RNA induces congophilic aggregates and facilitates neurodegeneration in Drosophila. Cell Stress Chaperon. 2007;12:9–19. doi: 10.1379/CSC-222R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savvateeva-Popova E., Popov A., Grossman A., Nikitina E., Medvedeva A., Molotkov D., Kamyshev N., Pyatkov K., Zatsepina O., Schostak N., et al. Non-coding RNA as a trigger of neuropathologic disorder phenotypes in transgenic Drosophila. J. Neural Transm. 2008;115:1629–1642. doi: 10.1007/s00702-008-0078-8. [DOI] [PubMed] [Google Scholar]
- 35.Zatsepina O.G., Nikitina E.A., Shilova V.Y., Chuvakova L.N., Sorokina S., Vorontsova J.E., Tokmacheva E.V., Funikov S.Y., Rezvykh A.P., Evgen’Ev M.B. Hsp70 affects memory formation and behaviorally relevant gene expression in Drosophila melanogaster. Cell Stress Chaperon. 2021;26:575–594. doi: 10.1007/s12192-021-01203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koemans T.S., Oppitz C., Donders R.A.T., van Bokhoven H., Schenck A., Keleman K., Kramer J.M. Drosophila Courtship Conditioning as a Measure of Learning and Memory. J. Vis. Exp. 2017:e55808. doi: 10.3791/55808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallagher M., Rapp P.R. The use of animal models to study the effects of aging on cognition. Annu. Rev. Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- 38.Rohlf F.J., Sokal R.R. Biometry: The Principles and Practice of Statistics in Biological Research. Freeman; New York, NY, USA: 1981. [Google Scholar]
- 39.Tully T., Quinn W.G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X., Yuan C., Guo A. Drosophila Olfactory Response Rhythms Require Clock Genes but Not Pigment Dispersing Factor or Lateral Neurons. J. Biol. Rhythm. 2005;20:237–244. doi: 10.1177/0748730405274451. [DOI] [PubMed] [Google Scholar]
- 41.Krasnov G.S., Dmitriev A.A., Kudryavtseva A.V., Shargunov A.V., Karpov D.S., Uroshlev L.A., Melnikova N.V., Blinov V.M., Poverennaya E.V., Archakov A.I., et al. PPLine: An Automated Pipeline for SNP, SAP, and Splice Variant Detection in the Context of Proteogenomics. J. Proteome Res. 2015;14:3729–3737. doi: 10.1021/acs.jproteome.5b00490. [DOI] [PubMed] [Google Scholar]
- 42.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson M.D., McCarthy D.J., Smyth G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu G., Wang L.-G., Han Y., He Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schefe J.H., Lehmann K.E., Buschmann I.R., Unger T., Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s C T difference” formula. Klin. Wochenschr. 2006;84:901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 47.Ponton F., Chapuis M.-P., Pernice M., Sword G.A., Simpson S.J. Evaluation of potential reference genes for reverse transcription-qPCR studies of physiological responses in Drosophila melanogaster. J. Insect Physiol. 2011;57:840–850. doi: 10.1016/j.jinsphys.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Parkhitko A.A., Binari R., Zhang N., Asara J.M., Demontis F., Perrimon N. Tissue-specific down-regulation of S-adenosyl-homocysteine via suppression of dAhcyL1/dAhcyL2 extends health span and life span in Drosophila. Genes Dev. 2016;30:1409–1422. doi: 10.1101/gad.282277.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ksenofontov A.L., Boyko A.I., Mkrtchyan G.V., Tashlitsky V.N., Timofeeva A.V., Graf A.V., Bunik V.I., Baratova L.A. Analysis of free amino acids in mammalian brain extracts. Biochemistry. 2017;82:1183–1192. doi: 10.1134/S000629791710011X. [DOI] [PubMed] [Google Scholar]
- 50.Kaminskaya A.N., Nikitina E.A., Medvedeva A.V., Gerasimenko M.S., Chernikova D.A., Savateeva-Popova E.V. Influence of limk1 Gene Polymorphism on Learning Acquisition and Memory Formation with pCREB Distribution and Aggregate Formation in Neuromuscular Junctions in Drosophila melanogaster. Genetika. 2015;51:685–693. doi: 10.1134/S1022795415060071. [DOI] [PubMed] [Google Scholar]
- 51.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K., Kimura H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 52.Kumar M., Tyagi N., Moshal K.S., Sen U., Pushpakumar S., Vacek T., Lominadze D., Tyagi S.C. GABAA receptor agonist mitigates homocysteine-induced cerebrovascular remodeling in knockout mice. Brain Res. 2008;1221:147–153. doi: 10.1016/j.brainres.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mudd S. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill Professiona; New York, NY, USA: 2001. Disorders of transsulfuration. [DOI] [Google Scholar]
- 54.Davis M.Y., Trinh K., Thomas R.E., Yu S., Germanos A.A., Whitley B.N., Sardi S.P., Montine T.J., Pallanck L.J. Glucocerebrosidase Deficiency in Drosophila Results in α-Synuclein-Independent Protein Aggregation and Neurodegeneration. PLoS Genet. 2016;12:e1005944. doi: 10.1371/journal.pgen.1005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Didelot G., Molinari F., Tchénio P., Comas D., Milhiet E., Munnich A., Colleaux L., Preat T. Tequila, a Neurotrypsin Ortholog, Regulates Long-Term Memory Formation in Drosophila. Science. 2006;313:851–853. doi: 10.1126/science.1127215. [DOI] [PubMed] [Google Scholar]
- 56.Sudhakaran I.P., Hillebrand J., Dervan A., Das S., Holohan E.E., Hülsmeier J., Sarov M., Parker R., VijayRaghavan K., Ramaswami M. FMRP and Ataxin-2 function together in long-term olfactory habituation and neuronal translational control. Proc. Natl. Acad. Sci. USA. 2013;111:E99–E108. doi: 10.1073/pnas.1309543111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levin L., Han P.-L., Hwang P.M., Feinstein P.G., Davis R., Reed R.R. The Drosophila learning and memory gene rutabaga encodes a Ca2+calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-F. [DOI] [PubMed] [Google Scholar]
- 58.Singh S., Padovani D., Leslie R.A., Chiku T., Banerjee R. Relative Contributions of Cystathionine β-Synthase and γ-Cystathionase to H2S Biogenesis via Alternative Trans-sulfuration Reactions. J. Biol. Chem. 2009;284:22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robert K., Vialard F., Thiery E., Toyama K., Sinet P.-M., Janel N., London J. Expression of the Cystathionine β Synthase (CBS) Gene During Mouse Development and Immunolocalization in Adult Brain. J. Histochem. Cytochem. 2003;51:363–371. doi: 10.1177/002215540305100311. [DOI] [PubMed] [Google Scholar]
- 60.Hu L.-F., Lu M., Hon Wong P.T., Bian J.-S. Hydrogen Sulfide: Neurophysiology and Neuropathology. Antioxid. Redox Signal. 2011;15:405–419. doi: 10.1089/ars.2010.3517. [DOI] [PubMed] [Google Scholar]
- 61.Enokido Y., Suzuki E., Iwasawa K., Namekata K., Okazawa H., Kimura H. Cystathionine β-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J. 2005;19:1854–1856. doi: 10.1096/fj.05-3724fje. [DOI] [PubMed] [Google Scholar]
- 62.Lee M., Schwab C., Yu S., McGeer E., McGeer P.L. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol. Aging. 2009;30:1523–1534. doi: 10.1016/j.neurobiolaging.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Kimura Y., Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 64.Ishii I., Akahoshi N., Yu X.-N., Kobayashi Y., Namekata K., Komaki G., Kimura H. Murine cystathionine γ-lyase: Complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem. J. 2004;381:113–123. doi: 10.1042/BJ20040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeong N.Y., Jung J., Tabassum R. Therapeutic importance of hydrogen sulfide in age-associated neurodegenerative diseases. Neural Regen. Res. 2020;15:653–662. doi: 10.4103/1673-5374.266911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagpure B.V., Bian J.-S. Brain, Learning, and Memory: Role of H2S in Neurodegenerative Diseases. Handb. Exp. Pharmacol. 2015;230:193–215. doi: 10.1007/978-3-319-18144-8_10. [DOI] [PubMed] [Google Scholar]
- 67.Kandel E.R. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kandel E.R. The Molecular Biology of Memory Storage: A Dialogue Between Genes and Synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 69.Bailey C.H., Bartsch D., Kandel E.R. Toward a molecular definition of long-term memory storage. Proc. Natl. Acad. Sci. USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tully T. Discovery of genes involved with learning and memory: An experimental synthesis of Hirschian and Benzerian perspectives. Proc. Natl. Acad. Sci. USA. 1996;93:13460–13467. doi: 10.1073/pnas.93.24.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cowan T.M., Siegel R.W. Drosophila Mutations that Alter Ionic Conduction Disrupt Acquisition and Retention of a Conditioned Odor Avoidance Response. J. Neurogenet. 1986;3:187–201. doi: 10.3109/01677068609106849. [DOI] [PubMed] [Google Scholar]
- 72.Lichtinghagen R., Stocker M., Wittka R., Boheim G., Stühmer W., Ferrus A., Pongs O. Molecular basis of altered excitability in Shaker mutants of Drosophila melanogaster. EMBO J. 1990;9:4399–4407. doi: 10.1002/j.1460-2075.1990.tb07890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawasaki F., Zou B., Xu X., Ordway R.W. Active Zone Localization of Presynaptic Calcium Channels Encoded by the cacophony Locus of Drosophila. J. Neurosci. 2004;24:282–285. doi: 10.1523/JNEUROSCI.3553-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith L.A., Peixoto A.A., Kramer E.M., Villella A., Hall J.C. Courtship and Visual Defects of cacophony Mutants Reveal Functional Complexity of a Calcium-Channel α1 Subunit in Drosophila. Genetics. 1998;149:1407–1426. doi: 10.1093/genetics/149.3.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mayford M., Siegelbaum S.A., Kandel E.R. Synapses and Memory Storage. Cold Spring Harb. Perspect. Biol. 2012;4:a005751. doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flexner J.B., Flexner L.B., Stellar E. Memory in Mice as Affected by Intracerebral Puromycin. Science. 1963;141:57–59. doi: 10.1126/science.141.3575.57. [DOI] [PubMed] [Google Scholar]
- 77.Bliss T.V.P., Collingridge G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 78.Lynch M.A. Long-Term Potentiation and Memory. Physiol. Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 79.Elgersma Y., Fedorov N.B., Ikonen S., Choi E.S., Elgersma M., Carvalho O., Giese K.P., Silva A.J. Inhibitory Autophosphorylation of CaMKII Controls PSD Association, Plasticity, and Learning. Neuron. 2002;36:493–505. doi: 10.1016/S0896-6273(02)01007-3. [DOI] [PubMed] [Google Scholar]
- 80.Silva A.J., Paylor R., Wehner J.M., Tonegawa S. Impaired Spatial Learning in α-Calcium-Calmodulin Kinase II Mutant Mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 81.Lisman J., Yasuda R., Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang J.-Y., Nakahata Y., Hayano Y., Yasuda R. Mechanisms of Ca2+/calmodulin-dependent kinase II activation in single dendritic spines. Nat. Commun. 2019;10:1–12. doi: 10.1038/s41467-019-10694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tao W., Lee J., Chen X., Díaz-Alonso J., Zhou J., Pleasure S., Nicoll R.A. Synaptic memory requires CaMKII. eLife. 2021;10:e60360. doi: 10.7554/eLife.60360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagai Y., Tsugane M., Oka J.-I., Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J. 2004;18:557–559. doi: 10.1096/fj.03-1052fje. [DOI] [PubMed] [Google Scholar]
- 85.Kamat P.K., Kalani A., Tyagi N. Role of Hydrogen Sulfide in Brain Synaptic Remodeling. Methods Enzymol. 2015;555:207–229. doi: 10.1016/bs.mie.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernandez-Moya S.M., Bauer K.E., Kiebler M.A. Meet the players: Local translation at the synapse. Front. Mol. Neurosci. 2014;7:84. doi: 10.3389/fnmol.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Melchor J.P., Strickland S. Tissue plasminogen activator in central nervous system physiology and pathology. Thromb. Haemost. 2005;93:655–660. doi: 10.1160/TH04-12-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sontag E., Nunbhakdi-Craig V., Sontag J.-M., Diaz-Arrastia R., Ogris E., Dayal S., Lentz S., Arning E., Bottiglieri T. Protein Phosphatase 2A Methyltransferase Links Homocysteine Metabolism with Tau and Amyloid Precursor Protein Regulation. J. Neurosci. 2007;27:2751–2759. doi: 10.1523/JNEUROSCI.3316-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siegel G., Obernosterer G., Fiore R., Oehmen M., Bicker S., Christensen M., Khudayberdiev S., Leuschner P.F., Busch C.J.L., Kane C., et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat. Cell Biol. 2009;11:705–716. doi: 10.1038/ncb1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phillis J.W. Acetylcholine Release from the Central Nervous System: A 50-Year Retrospective. Crit. Rev. Neurobiol. 2005;17:161–217. doi: 10.1615/CritRevNeurobiol.v17.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 91.Blankman J.L., Cravatt B.F. Chemical Probes of Endocannabinoid Metabolism. Pharmacol. Rev. 2013;65:849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Viader A., Ogasawara D., Joslyn C.M., Sanchez-Alavez M., Mori S., Nguyen W., Conti B., Cravatt B.F. A chemical proteomic atlas of brain serine hydrolases identifies cell type-specific pathways regulating neuroinflammation. eLife. 2016;5:e12345. doi: 10.7554/eLife.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data were deposited in the NCBI GEO database under the number—GSE200397.