Abstract
Background
After the acute phase of SARS-CoV-2 infection, children can develop long COVID symptoms. We aimed to investigate the prevalence of long-lasting symptoms, the duration and intensity of symptoms, quality of life, number of sick days and absences from daycare or school, and psychological and social outcomes in children aged 0–14 years who had been infected with SARS-CoV-2 relative to controls with no history of SARS-CoV-2 infection.
Methods
A nationwide cross-sectional study was conducted including children with a confirmed SARS-CoV-2-positive PCR test (cases) and matched controls from Danish national registers. A survey was sent to mothers (proxy reporting) of children aged 0–14 years who had had a positive SARS-CoV-2 test between Jan 1, 2020, and July 12, 2021, and a control group matched (1:4) by age and sex. The survey included the Pediatric Quality of Life Inventory (PedsQL) and the Children's Somatic Symptoms Inventory-24 (CSSI-24) to capture current overall health and wellbeing, and ancillary questions about the 23 most common long COVID symptoms. Descriptive statistics and logistic regression analysis were used. Clinically relevant differences were defined as those with a Hedges' g score greater than 0·2. This study is registered at ClinicalTrials.gov (NCT04786353).
Findings
Responses to the survey were received from 10 997 (28·8%) of 38 152 cases and 33 016 (22·4%) of 147 212 controls between July 20, 2021, and Sept 15, 2021. Median age was 10·2 years (IQR 6·6–12·8) in cases and 10·6 years (6·9–12·9) in controls. 5267 (48·2%) cases and 15 777 (48·3%) controls were female, and 5658 (51·8%) cases and 16 870 (51·7%) controls were male. Cases had higher odds of reporting at least one symptom lasting more than 2 months than did controls in the 0–3 years age group (478 [40·0%] of 1194 vs 1049 [27·2%] of 3855; OR 1·78 [95% CI 1·55–2·04], p<0·0001), 4–11 years age group (1912 [38·1%] of 5023 vs 6189 [33·7%] of 18 372; 1·23 [1·15–1·31], p<0·0001), and 12–14 years age group (1313 [46·0%] of 2857 vs 4454 [41·3%] of 10 789; 1·21 [1·11–1·32], p<0·0001). Differences in CSSI-24 symptom scores between cases and controls were statistically significant but not clinically relevant. Small clinically relevant differences in PedsQL quality-of-life scores related to emotional functioning were found in favour of cases in the children aged 4–11 years (median score 80·0 [IQR 65·0–95·0]) in cases vs 75·0 [60·0–85·0] in controls; p<0·0001) and 12–14 years (90·0 [70·0–100·0] vs (85·0 [65·0–95·0], p<0·0001). PedsQL social functioning scores were also higher in cases (100·0 [90·0–100·0] than controls (95·0 [80·0–100·0]) in the 12–14 years age group (p<0·0001; Hedges g>0·2).
Interpretation
Compared with controls, children aged 0–14 years who had a SARS-CoV-2 infection had more prevalent long-lasting symptoms. There was a tendency towards better quality-of-life scores related to emotional and social functioning in cases than in controls in older children. The burden of symptoms among children in the control group requires attention. Long COVID must be recognised and multi-disciplinary long COVID clinics for children might be beneficial.
Funding
A P Møller and Chastine Mc-Kinney Møller Foundation.
Introduction
Children worldwide are at risk of SARS-CoV-2 infection1 because of a lack of approved vaccines for children aged 0–4 years, few countries recommending vaccination for children aged 5–11 years, low vaccine uptake among children overall, difficulties practising physical distancing and, in particular, low vaccine effectiveness against the omicron variant of SARS-CoV-2.2 Thus, a considerable number of children have been infected with SARS-CoV-2,3 including in Denmark, where 58% of all children have had laboratory-confirmed infection during the period from Dec 15, 2021, to Feb 15, 2022.4 This situation leaves a considerable number of children at risk of long-term sequelae following SARS-CoV-2 infection (generally referred to as long COVID, post-COVID-19 condition, or post-COVID-19 syndrome). Different definitions of this condition exist, but WHO has defined post-COVID-19 condition among adults as persistent or fluctuating symptoms with an influence on daily functioning following SARS-CoV-2 infection for at least 2 months that cannot be explained by an alternative diagnosis.5 A duration of more than 12 weeks for symptoms has also been suggested.6 Only a few studies have investigated long COVID in children.7, 8, 9, 10, 11, 12, 13 These studies had substantial limitations regarding size,7, 8, 9, 13 lack of requirement for PCR confirmation of SARS-CoV-2 cases,7, 8, 13 heterogeneity in definitions of long COVID and follow-up periods,7, 8, 9, 10, 11, 12, 13 or the lack of a control group.7, 13 Furthermore, infants and toddlers are rarely represented.8, 10, 11 Five of the seven published studies reporting on children found persistent COVID-19-related symptoms to be more prevalent in children following COVID-19 (cases) than in controls who had not had COVID-19.8, 10, 11, 12, 13 The CLoCk study found symptoms after 12 weeks in 2038 (66%) of 3065 cases versus 1993 (53%) of 3739 controls among children aged 11–17 years in England.12 The most prevalent long COVID symptoms reported in children are headache (3–80%), fatigue (3–87%), sleep disturbance (2–63%), concentration difficulties (2–81%), and abdominal pain (1–76%).1
Research in context.
Evidence before this study
Before designing this study, we searched PubMed in Jan 4, 2021, to identify studies investigating long COVID in children. The search terms used were “child” OR “children” AND “COVID-19” AND “symptoms” OR “long COVID”. We found one paper from Sweden, including a case description of five children. We searched Google Scholar and found one preprint study from Italy that included 75 post-COVID-19 children with no control group and a website (longcovidkids.org) reporting a survey of child long COVID symptoms. Furthermore, we searched ClinicalTrials.gov for ongoing studies in the COVID-19 database. The first papers reporting on long COVID in children found high prevalence of long-lasting symptoms but were small, did not include the youngest children, and lacked laboratory-confirmed SARS-CoV-2 test results and a control group. Few recent studies including controls have reported symptoms to also be highly prevalent among controls. As of October, 2021, a new case definition from WHO defines long COVID to be symptoms lasting 8 weeks or longer, which is seldom reported in earlier studies, in which 4 weeks was often used as a cutoff.
Added value of this study
We investigated long COVID according to the WHO case definition in children aged 0–14 years. We reported on the duration of each symptom and the intensity of the symptom, as well as including findings on psychological wellbeing, social wellbeing, and daycare and school absence during the pandemic. The present study supports the findings from the literature, and contributes a large data set including a control group.
Implications of all the available evidence
Results from this LongCOVIDKidsDK study—combined with findings from other studies that included a control group—have shown persistent symptoms to be more prevalent in children after SARS-CoV-2 infection; however, the clinical differences on a population level seem to be small. Knowledge of long COVID in children is important to guide clinical recognition and management of COVID-19. Furthermore, the findings on the symptom burden among children in the control group require attention, as pandemic symptoms due to lockdown and social distancing have been suggested. Looking at the available evidence, long COVID must be recognised and multi-professional long COVID clinics for children might be beneficial. Long school closures and social distancing might compromise children's wellbeing and quality of life during a pandemic.
In the LongCOVIDKidsDK study, adolescents aged 15–18 years with a SARS-CoV-2 positive test had higher odds of experiencing at least one symptom lasting at least 2 months compared with controls (62% vs 57%; odds ratio [OR] 1·22 [95% CI 1·15–1·30], p<0·0001).14
The presence of long-lasting symptoms after a viral infection is no new phenomenon. In children, long-term symptoms following a respiratory viral infection can include persistent coughing and wheezing, as seen, for example, after respiratory syncytial virus infection in early childhood.15 Additionally, persistent fatigue, headaches, and abdominal pain can be present following Epstein-Barr virus infection.16
Knowledge of the long-term symptom burden in children who have tested positive for SARS-CoV-2 is essential to guide clinical recognition, parental caregiving, and societal decisions about isolation, lockdown, and vaccination strategies. On this basis, we conducted a national study of children aged 0–14 years who had tested positive for SARS-CoV-2 (cases) with a matched control group who had never had a positive PCR test for SARS-CoV-2. The objectives were to investigate: the prevalence of symptoms lasting more than 2 months in cases compared with matched controls; the duration and intensity of symptoms; quality of life; number of sick days and absence from daycare or school; and the psychological and social outcomes in cases versus controls. We hypothesised that, relative to controls, SARS-CoV-2-infected children (0–14 years) would have a higher prevalence of long-lasting symptoms with negative psychological and social effects, and more daycare or school absences and sick leave.
Methods
Study design and participants
The LongCOVIDKidsDK survey is a national cross-sectional study among children and adolescents with a history of a SARS-CoV-2-positive test and matched controls. In children aged 0–14 years, the survey was conducted by parent proxy report and was sent to mothers, or to a father or legal guardian if no mother existed,17 of all children who tested positive for SARS-CoV-2 in Denmark and of matched controls. From 15 years of age, adolescents can legally receive mail from public institutions; therefore, adolescents aged 15–18 years responded to the survey themselves, and are reported in a separate publication.14 Questionnaires, including an invitation and two reminders, were sent by mail via e-Boks (a secure digital post-box) and answered in REDCap (a web application for online surveys) between July 20 and Sept 15, 2021.18
All Danish children and adolescents aged 0–14 years with a positive SARS-CoV-2 test registered between Jan 1, 2020, and July 12, 2021, were included and sent invitations. Participants were identified from the Danish COVID-19 database, which covers all Danes with a positive SARS-CoV-2 test. As of July 12, the database included 4·2% of Danes aged 0–14 years. At the same time, controls who had never had a positive SARS-CoV-2 test were identified from the Danish Civil Registration System and included using exposure density matching by sex and age in a 1:4 ratio at the time of the cases' positive tests. All participants were alive at inclusion. Notably, open public testing free of charge was available for all by August, 2020, whereas testing was only available for symptomatic cases before that date. Thus, the control group might include children who had had COVID-19 and who were initially asymptomatic and therefore not tested.
In the survey, controls were asked whether they suspected that they had been infected with SARS-CoV-2 even though they did not have a positive test. Controls who reported suspicion of having been infected by SARS-CoV-2 but who had not been tested were excluded from the analyses. Controls who were infected in the time between register extraction of the population (July 12, 2021) and invitation to survey were instructed not to reply.
The study was permitted by the data protection agency (P-2021-195) and registered at ClinicalTrials.gov (NCT04786353). Register data access was granted by The Danish Health Data Authority (FSEID 00005625 and 00005757). Ethics committee approval is not required for surveys in Denmark.
Variables
Survey data were collected using ancillary questions regarding long-lasting COVID-19-related symptoms (appendix p 28), as well as the validated questionnaires Children's Somatic Symptoms Inventory (CSSI-24)19 and Pediatric Quality of Life Inventory (PedsQL), which captured current overall health and wellbeing.20 Ancillary questions about long COVID symptoms included the 23 most common symptoms identified from the Long COVID Kids Rapid Survey January 2021.21 The symptoms were stomach ache, chest pain, headache, fatigue, pain in muscles or joints, sore throat, dizziness, rashes, mood swings, nausea, fever, loss of appetite, trouble breathing, dark circles under the eyes, palpitations, trouble remembering or concentrating, cold hands or feet, cough, chapped lips, dizziness when standing, light sensitivity, discoloured fingers or toes, and extreme paleness. The following symptoms were not asked about in the 0–2 years age group: chest pain, headache, sore throat, dizziness, trouble remembering or concentrating, dizziness when standing, and light sensitivity.
The CSSI-24 is a 24-item generic questionnaire identifying the presence of various somatic symptoms in children. The items are scored on a five-point Likert scale from zero (not at all) to four (a whole lot) in the past 2 weeks. A higher sum score indicates more somatic distress.19 The CSSI-24 was not included in the survey for the 0–2 years age group as the symptoms would be too difficult to assess for that age group.
The 23-item generic questionnaire PedsQL consists of four dimensions of health-related quality of life in children aged 0–14 years. The questionnaire is available in different versions with items appropriate for different age groups. The dimensions include physical, emotional, social, and daycare or school functioning over the previous month.20 The scores range from 0–100, with higher scores indicating better health.
Questions were included about sick leave and absence from daycare or school within the past year and height and weight. The WHO classification of weight status in children and adolescents was used.22
Parents of children with a SARS-CoV-2 positive test were asked subjectively to assess the perceived acute symptom burden within the first 4 weeks following the positive test as either no symptoms, mild symptoms, or severe symptoms. These categories were not further defined.
Data sources and management
Cases were asked questions about the 23 common long COVID symptoms for the period since the SARS-CoV-2 infection was diagnosed, including questions about symptom intensity (never, almost never, sometimes, often, or almost always) and duration (eg, 1 week, 1 month, 2 months), up to a duration of 12 months or longer. The control group was asked about the same symptoms and was divided into four random groups of equal size with 3, 6, 9, or 12 months recall times to match the varying recall times in the case group.
We defined long-lasting symptoms as those lasting 2 months or longer, in line with the WHO definition of long COVID.5 In these analyses, participants were only included if they had a recall period long enough to have potentially had a symptom for 2 months. Participants with a recall period less than 2 months were excluded. We also did a sensitivity analysis in which we defined long COVID as symptoms lasting for more than 12 weeks, because other guidelines define the presence of symptoms lasting for 3 months or longer as long COVID.6
A nested population was established consisting of participants from the case group who had at least one new symptom starting after the positive SARS-CoV-2 test and present 8 weeks after the test (long COVID group).
Amounts of sick days and school absences within the past year were each categorised into two groups: 0–15 days and 16 days or more.
For the PedsQL questionnaire, results were presented for the 1–12 months, 13–24 months, 2–3 years, 4–11 years, and 12–14 years age groups, as these groups were asked different questions appropriate for their age. In other analyses, the population was divided into three age groups (0–3 years, 4–11 years, and 12–14 years) to allow for possible differences between age groups to be reflected and to avoid groups being too small.
In Denmark, daycare facilities and schools were closed for various periods between March 12, 2020, and May 21, 2021 (appendix p 29). Data were collected during a period when Denmark had reopened and had an epidemic of respiratory syncytial virus following the COVID-19 lockdown.
Statistical analysis
Numbers and percentages are presented for categorical variables, and means (with SDs) and medians (with IQRs) for continuous variables, as appropriate. ORs and corresponding 95% CIs were calculated by logistic regression. Analyses were adjusted for age and sex.
Differences between cases and controls were tested with a two-sided χ2 test for categorical variables (proportions) or t test for normally distributed continuous variables (means), or with an independent non-parametric Wilcoxon signed rank test for continuous variables that were not normally distributed. We also assessed differences in the proportions of participants with 2-month and 6-month symptom durations between the case and control groups.
Standardised differences between means were assessed using Hedges' g for continuous variables, with effect sizes categorised as small (g=0·2), medium (g=0·5), or large (g=0·8).23 We considered g>0·2 to indicate a clinically relevant difference.
The Bonferroni correction method was used to adjust for multiple testing. The significance level was set at <0·05 and 57 tests were conducted; therefore, a p value of 0·0009 or lower was considered statistically significant.
For results with fewer than five individuals per cell, numbers are presented as 1–4 and percentages are masked, in accordance with data protection rules set by the Danish Health Data Authorities.
All analyses were conducted using SAS 9.4M5.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
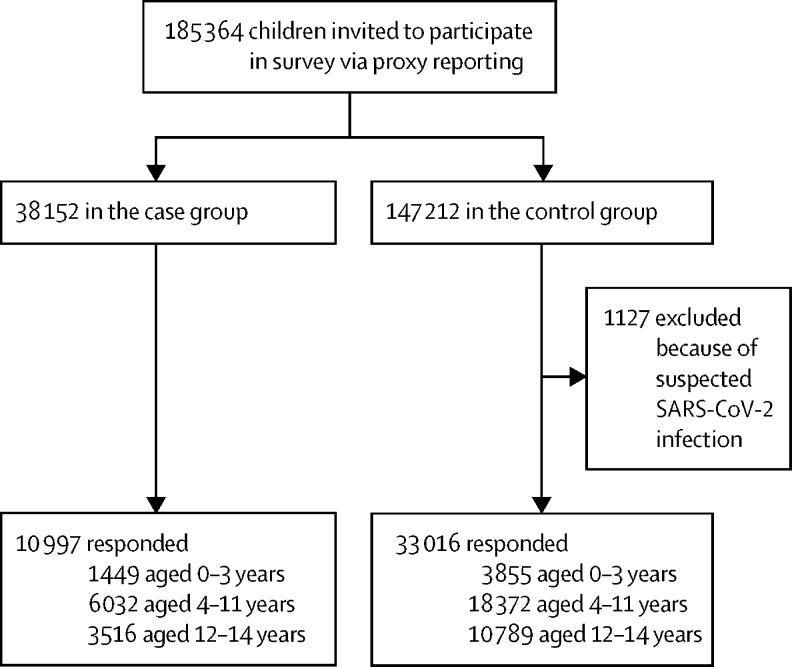
Between July 20, 2021, and Sept 15, 2021, 38 152 cases and 147 212 controls were invited to participate, of whom 10 997 (28·8%) cases and 33 016 (22·4%) controls responded to the survey (figure 1 ). Cases and controls were similar in age, sex, and prevalence of pre-existing comorbidity across age groups when responding to the questionnaire (table 1 ).
Figure 1.
Study profile
Table 1.
Demographic and clinical profile
|
0–3 years |
4–11 years |
12–14 years |
||||
|---|---|---|---|---|---|---|
| Cases (n=1449) | Controls (n=3855) | Cases (n=6032) | Controls (n=18 372) | Cases (n=3516) | Controls (n=10 789) | |
| Age, years | ||||||
| Median (IQR) | 2·4 (1·7–3·2) | 2·4 (1·7–3·3) | 9·1 (6·9–10·7) | 9·3 (7·0–10·9) | 13·6 (12·9–14·4) | 13·7 (13·0–14·4) |
| Range | 0·2–5·0 | 0·05–5·0 | 4·0–13·0 | 4·0–13·0 | 12·0–15·9 | 12·0–15·9 |
| Missing | 18 (1·2%) | 49 (1·3%) | 30 (0·5%) | 128 (0·7%) | 21 (0·6%) | 148 (1·4%) |
| Sex | ||||||
| Female | 684 (47·2%) | 1783 (46·3%) | 2857 (47·4%) | 8818 (48·0%) | 1727 (49·1%) | 5176 (48·0%) |
| Male | 747 (51·6%) | 2022 (52·5%) | 3145 (52·1%) | 9383 (51·1%) | 1768 (50·3%) | 5465 (50·7%) |
| Missing | 18 (1·2%) | 50 (1·3%) | 30 (0·5%) | 171 (0·9%) | 21 (0·6%) | 148 (1·4%) |
| Weight status* | ||||||
| Underweight | 69 (7·5%) | 178 (7·3%) | 463 (7·7%) | 1374 (7·5%) | 197 (5·6%) | 639 (5·9%) |
| Normal weight | 735 (80·2%) | 2015 (82·4%) | 4254 (70·5%) | 13 326 (72·5%) | 2605 (74·1%) | 7970 (73·9%) |
| Overweight | 53 (5·8%) | 132 (5·4%) | 973 (16·1%) | 2657 (14·5%) | 518 (14·7%) | 1473 (13·7%) |
| Obesity | 29 (3·2%) | 46 (1·9%) | 317 (5·3%) | 944 (5·1%) | 128 (3·6%) | 364 (3·4%) |
| Missing | 31 (3·4%) | 74 (3·0%) | 25 (0·4%) | 71 (0·4%) | 68 (1·9%) | 343 (3·2%) |
| Pre-existing comorbidity† | ||||||
| Asthma | 80 (5·5%) | 193 (5·0%) | 287 (4·8%) | 816 (4·4%) | 203 (5·8%) | 564 (5·2%) |
| Allergy | 46 (3·2%) | 163 (4·2%) | 543 (9·0%) | 2147 (11·7%) | 548 (15·6%) | 1887 (17·5%) |
| Eczema | 166 (11·5%) | 494 (12·8%) | 443 (7·3%) | 1403 (7·6%) | 227 (6·5%) | 676 (6·3%) |
| Tics | 1–4 | 1–4 | 68 (1·1%) | 349 (1·9%) | 58 (1·6%) | 246 (2·3%) |
| Colic | 24 (1·7%) | 27 (0·7%) | 13 (0·2%) | 9 (<0·1%) | 0 | 0 |
| Borrelia | NA | NA | 8 (0·1%) | 14 (0·1%) | 1–4 | 8 (0·1%) |
| ADHD/ADS | NA | NA | 90 (1·5%) | 455 (2·5%) | 114 (3·2%) | 440 (4·1%) |
| Epstein Barr virus infection | NA | NA | 11 (0·2%) | 14 (0·1%) | 15 (0·4%) | 14 (0·1%) |
| Arthritis | NA | NA | 13 (0·2%) | 31 (0·2%) | 13 (0·4%) | 31 (0·3%) |
| ME/CFS | NA | NA | 1–4 | 1–4 | 1–4 | 7 (0·1%) |
| Autism | NA | NA | 70 (1·2%) | 360 (2·0%) | 88 (2·5%) | 352 (3·3%) |
| Gout | NA | NA | 0 | 1–4 | 0 | 1–4 |
| Dyspraxia | NA | NA | 5 (0·1%) | 33 (0·2%) | 7 (0·2%) | 13 (0·1%) |
| Hypermobility | NA | NA | 69 (1·1%) | 358 (2·0%) | 75 (2·1%) | 231 (2·1%) |
| HPV infection | NA | NA | 1–4 | 1–4 | 1–4 | 1–4 |
| OCD, anxiety, or depression | NA | NA | 36 (0·6%) | 285 (1·6%) | 69 (2·0%) | 352 (3·3%) |
| Hyperthyroidism | NA | NA | 1–4 | 6 (<0·1%) | 0 | 0 |
| Hypertonia | NA | NA | 1–4 | 14 (0·1%) | 0 | 0 |
| Other | 59 (4·1%) | 170 (4·4%) | 200 (3·3%) | 894 (4·9%) | 12 (0·3%) | 593 (5·5%) |
| Time since positive COVID-19 test, months | ||||||
| <1 | 81 (5·6%) | NA | 348 (5·8%) | NA | 200 (5·7%) | NA |
| 1–3 | 214 (14·8%) | NA | 891 (14·8%) | NA | 574 (16·3%) | NA |
| 4–6 | 283 (19·5%) | NA | 1350 (22·4%) | NA | 473 (13·5%) | NA |
| 7–9 | 712 (49·1%) | NA | 2842 (47·1%) | NA | 1799 (51·2%) | NA |
| 10–12 | 95 (6·6%) | NA | 427 (7·1%) | NA | 394 (11·2%) | NA |
| ≥12+ | 64 (4·4%) | NA | 174 (2·9%) | NA | 76 (2·2%) | NA |
| Acute COVID-19 course‡ | ||||||
| No symptoms | 746 (51·5%) | NA | 3623 (60·1%) | NA | 1590 (45·2%) | NA |
| Mild symptoms | 653 (45·1%) | NA | 2317 (38·4%) | NA | 1838 (52·3%) | NA |
| Severe symptoms | 50 (3·5%) | NA | 92 (1·5%) | NA | 88 (2·5%) | NA |
Data are median (IQR), range, or n (%). For results with fewer than five individuals per cell, numbers are presented as 1–4 and percentages are masked according to data protection rules set by the Danish Health Data Authorities. ADHD/ADS=attention-deficit hyperactivity disorder (attention-deficit syndrome). HPV=human papillomavirus. ME/CFS=myalgic encephalomyelitis (chronic fatigue syndrome). NA=not applicable. OCD=obsessive-compulsive disorder.
WHO classification;22 not reported for children in the 0–2 years age group (cases n=532, controls n=1410).
Cases were asked about comorbidities before COVID-19 infection; controls were asked about present comorbidities; children aged 0–3 years were asked only about pre-existing comorbidities, including asthma, allergy, eczema, tics, and colic.
Self-reported burden of symptoms.
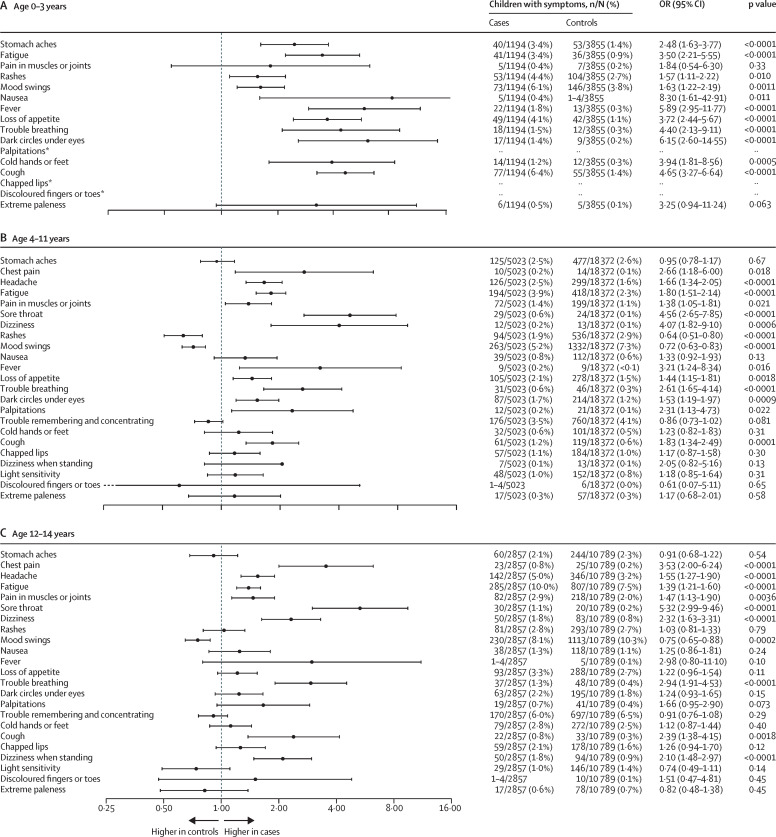
Cases had higher odds of reporting at least one symptom lasting more than 2 months than did controls in the 0–3 years age group (478 [40·0%] of 1194 vs 1049 [27·2%] of 3855; OR 1·78 [95% CI 1·55–2·04], p<0·0001), 4–11 years age group (1912 [38·1%] of 5023 vs 6189 [33·7%] of 18 372; 1·23 [1·15–1·31], p<0·0001), and 12–14 years age group (1313 [46·0%] of 2857 vs 4454 [41·3%] of 10 789; 1·21 [1·11–1·32], p<0·0001). The same applied when considering reporting at least one symptom lasting more than 3 months in children aged 0–3 years (435 [36·4%] of 1194 vs 872 [22·6%] of 3855; 1·94 [1·68–2·23], p<0·0001), 4–11 years (1710 [34·0%] of 5023 vs 5356 [29·2%] of 18 372; 1·28 [1·19–1·37], p<0·0001), and 12–14 years (1204 [42·1%] of 2857 vs 3966 [36·8%] of 10 789; 1·26 [1·11–1·32], p<0·0001). Small differences were seen across age groups when considering individual symptoms. For example, among the 0–3 years age group, cases had higher odds of reporting stomach aches than did controls, whereas no difference between cases and controls was observed for this symptom in the older age groups. Similarly, cases had higher odds of reporting mood swings in the 0–3 years age group, whereas controls had higher odds of reporting this symptom in the older age groups. Odds ratios for all symptoms are presented in figure 2 .
Figure 2.
Forest plot of symptoms lasting at least 2 months in cases and controls
OR=odds ratio. *For the 0–3-years age group, estimates are not shown for palpitations, chapped lips, and discoloured fingers or toes because the groups were too small.
The most common symptoms reported among children aged 0–3 years were mood swings, rashes, stomach aches, cough, and loss of appetite. Among those aged 4–11 years, mood swings, trouble remembering or concentrating, and rashes were most common; and among those aged 12–14 years, fatigue, mood swings, and trouble remembering or concentrating were most common. The prevalence of symptoms lasting at least 2, 3, 6, 9, and 12 months are presented in the appendix (pp 3–13). With increasing duration of symptoms, the proportion of children with those symptoms tended to decrease.
In cases aged 12–14 years, more girls than boys had at least one symptom lasting more than 2 months (732 [52·7%] of 1390 vs 573 [39·6%] of 1448; OR 1·70 [95% CI 1·47–1·97], p<0·0001), and a similar pattern was seen in the control group (2388 [46·1%] of 5176 vs 2010 [36·8%] of 5465; 1·47 [1·36–1·59], p<0·0001). In the younger age groups, sex differences were only found for controls aged 4–11 years (3054 [34·6%] of 8818 vs 3079 [32·8%] of 9383; 1·15 [1·08–1·23], p<0·0001; appendix p 14).
For CSSI-24 symptom scores, we found differences between cases and controls that were statistically significant but not clinically relevant (Hedges' g≤0·2) (table 2 ).
Table 2.
Symptom burden and health-related quality of life in COVID-19 cases and controls
|
1–12 months |
13–24 months |
2–3 years* |
4–11 years |
12–14 years |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n=105) | Controls (n=348) | Cases (n=427) | Controls (n=1062) | Cases (n=917) | Controls (n=2445) | Cases (n=6032) | Controls (n=18 372) | Cases (n=3516) | Controls (n=10 789) | ||
| CSSI-24 | |||||||||||
| Mean (SD) | NA | NA | NA | NA | 2·5 (4·8) | 2·5 (4·2) | 3·0 (5·1)† | 3·4 (4·8)† | 4·3 (7·0)† | 4·7 (6·2)† | |
| Median (IQR) | NA | NA | NA | NA | 1·0 (0·0–3·0) | 1·0 (0·0–3·0) | 1·0 (0·0–4·0) | 2· 0 (0·0–5·0) | 1·0 (0·0–5·0) | 3·0 (1·0–5·0) | |
| PedsQL | |||||||||||
| Physical functioning | |||||||||||
| Mean (SD) | 93·7 (11·2)† | 87·8 (12·2)† | 94·2 (9·1)† | 87·3 (12·0)† | 94·8 (10·2) | 94·8 (8·2) | 94·7 (11·4)† | 92·9 (11·8)† | 93·0 (13·0)† | 91·2 (13·3)† | |
| Median (IQR) | 100·0 (91·7–100·0) | 91·7 (79·2–100·0) | 100·0 (91·7–100·0) | 88·9 (80·6–97·2) | 100·0 (93·8–100·0) | 100·0 (90·6–100·0) | 100·0 (93·8–100·0) | 96·9 (90·6–100·0) | 100·0 (90·6–100·0) | 96·9 (87·5–100·0) | |
| Physical symptoms | |||||||||||
| Mean (SD) | 84·4 (13·0) | 85·1 (80·0–92·5) | 84·9 (12·9)†‡ | 89·1 (9·7)†‡ | NA | NA | NA | NA | NA | NA | |
| Median (IQR) | 85·0 (75·0–95·0) | 87·5 (80·0–92·5) | 87·5 (77·5–95·0) | 90·0 (82·5–97·5) | NA | NA | NA | NA | NA | NA | |
| Emotional functioning | |||||||||||
| Mean (SD) | 75·5 (16·9) | 75·8 (13·7) | 73·6 (16·2)†‡ | 77·0 (12·8)†‡ | 75·5 (18·1) | 73·5 (15·4) | 78·2 (19·1)†‡ | 73·3 (18·0)†‡ | 83·2 (19·5)†‡ | 79·2 (19·2)†‡ | |
| Median (IQR) | 75·0 (64·6–89·6) | 79·2 (68·8–85·4) | 75·0 (62·0–85·4) | 77·1 (68·8–87·5) | 75·0 (65·0–90·0) | 75·0 (65·0–85·0) | 80·0 (65·0–95·0) | 75·0 (60·0–85·0) | 90·0 (70·0–100·0) | 85·0 (65·0–95·0) | |
| Social functioning | |||||||||||
| Mean (SD) | 94·7 (9·3) | 93·0 (11·4) | 93·3 (11·0) | 93·0 (9·9) | 93·8 (10·8)† | 93·0 (10·8)† | 92·3 (13·3)† | 89·6 (15·0)† | 91·4 (15·4)†‡ | 87·9 (17·5)†‡ | |
| Median (IQR) | 100·0 (93·8–100·0) | 100·0 (87·5–100·0) | 100·0 (90·0–100·0) | 95·0 (90·0–100·0) | 100·0 (90·0–100·0) | 100·0 (90·0–100·0) | 100·0 (90·0–100·0) | 95·0 (85·0–100·0) | 100·0 (90·0–100·0) | 95·0 (80·0–100·0) | |
| School functioning | |||||||||||
| Mean (SD) | NA | NA | NA | NA | 92·9 (12·1) | 93·0 (11·3) | 86·8 (15·3)† | 84·2 (15·4)† | 83·7 (18·0)† | 80·9 (17·8)† | |
| Median (IQR) | NA | NA | NA | NA | 100·0 (90·0–100·0) | 100·0 (91·7–100·0) | 90·0 (80·0–100·0) | 90·0 (75·0–95·0) | 90·0 (75·0–100·0) | 85·0 (70·0–95·0) | |
| Cognitive functioning | |||||||||||
| Mean (SD) | 87·7 (17·4) | 88·6 (16·0) | 87·3 (16·5)† | 84·7 (16·3)† | NA | NA | NA | NA | NA | NA | |
| Median (IQR) | 100·0 (75·0–100·0) | 100·0 (75·0–100·0) | 94·4 (77·8–100·0) | 88·9 (75·0–100·0) | NA | NA | NA | NA | NA | NA | |
CSSI-24=Children's Somatic Symptoms Inventory. PedsQL=Pediatric Quality of Life Inventory.
Missing school functioning scores: 27 cases, 77 controls.
Statistically significant (p<0·0009), based on Wilcoxon signed rank test.
Hedges' g>0·2.
For PedsQL scores, small but clinically relevant differences (reflected by Hedges' g scores >0·2) were found for children aged 13–24 months on the physical symptoms scale, with lower scores, indicating worse health-related quality of life, reported in cases (mean 84·9 [SD 12·9]) than in controls (89·1 [9·7]; p<0·0001 [Wilcoxon signed rank test]), and on the emotional functioning scale, with lower scores reported in cases (73·6 [16·2]) than in controls (77·0 [12·8]; p<0·0001). However, in older age groups (ages 4–14 years), cases had higher health-related quality of life scores than did controls on some scales of the PedsQL. Among children aged 4–11 years, a small difference was found in emotional functioning scores, with higher scores reported in cases (78·2 [19·1]) than in controls (73·3 [18·0]; p<0·0001). Among children aged 12–14 years, small differences were found in emotional functioning scores, with higher scores in cases (83·2 [19·5]) than in controls (79·2 [19·2]; p<0·0001). Clinically relevant differences in social functioning scores were also found between cases (91·4 [15·4]) and controls (87·9 [17·5]; p<0·0001) in this age group (table 2).
Across all age groups, cases reported more sick leave and more absence from school or daycare within the past year than did controls (appendix p 15). Among children aged 13 months to 3 years, the proportion reporting having at least 16 days of sick leave was higher in cases (382 [28·4%] of 1344) than in controls (647 [18·4%] of 3507, p<0·0001), as was the proportion reporting at least 16 days of absence from daycare or school (321 [23·9%] of 1344 vs 494 [14·1%] of 3507, p<0·0001). The same applied for the 4–11 years age group (424 [7·0%] of 6032 vs 699 [3·8%] of 18 372, p<0·0001 for ≥16 days sick leave; and 269 [6·1%] of 4404 vs 450 [3·3%] of 13 508, p<0·0001 for ≥16 days of daycare or school absences) and the 12–14 years age group (317 [9·0%] of 3516 vs 565 [5·2%] of 10 789, p<0·0001; and 229 [6·5%] of 3516 vs 542 [5·0%] of 10 789, p<0·0001).
For all outcomes related to psychological and social symptoms, cases reported less frequent problems than did controls (appendix p 17). For example, in children aged 4–11 years and 12–14 years, the proportion who often felt scared was lower in cases than in controls (312 [5·2%] of 6032 cases vs 1027 [5·6%] of 18 372 controls, p<0·0001 in the 4–11 years age group; and 117 [3·3%] of 3516 cases vs 383 [3·5%] of 10 789 controls, p<0·0001 in the 12–14 years age group), as was the proportion who often had trouble sleeping (476 [7·9%] of 6032 cases vs 1642 [8·9%] of 18 372 controls, p<0·0001; and 275 [7·8%] of 3516 cases vs 911 [8·4%] of 10 789 controls, p<0·0001) and who felt worried about what would happen to them (305 [5·1%] of 6032 cases vs 1206 [6·6%] of 18 372 controls, p<0·0001; and 209 [5·9%] of 3516 cases vs 883 [8·2%] of 10 789 controls, p<0·0001).
Long COVID symptoms were defined as new symptoms that presented after SARS-CoV-2 infection and were present for 8 weeks after the positive SARS-CoV-2 test. Demographic and clinical profiles for children with long COVID symptoms are presented in the appendix (p 19). Long COVID was present in 427 (31·2%) of 1368 children aged 0–3 years, 1505 (26·5%) of 5684 children aged 4–11 years, and 1077 (32·5%) of 3316 children aged 12–14 years (appendix p 22). The prevalence and duration of each long COVID symptom and the amount of sick leave for the children with long COVID symptoms are presented in the appendix (pp 23–27).
Discussion
In this study of Danish children with a history of SARS-CoV-2 infection and age-sex-matched controls with no history of SARS-CoV-2 infection, we found that cases in all age groups had higher odds of having long-lasting symptoms, but cases in the oldest age groups (4–11 and 12–14 years) had better quality-of-life scores than did controls. Additionally, cases had more sick days and more daycare or school absences than did controls within the past year.
An important strength of this study is the use of Danish national registers that included all PCR test results from all public and private test providers, and allowed matched controls to be identified. Moreover, all citizens were reached through a national secure digital post box, which all Danish citizen are obliged to have, thereby ensuring complete coverage. The number of different symptoms suggested to be related to COVID-19 increased during the pandemic;1 thus, we cannot be sure that all relevant symptoms were included in the study. However, the use of validated health questionnaires was a strength of the study.19, 20
Parent proxy response rates of 28·8% for cases and 22·4% for controls in this study were slightly lower than in other studies of young adolescents in general (33%),24 but higher than in most studies investigating COVID-19 symptoms in children and adolescents (<14%).1, 12, 13 The low response rates and the difference in response rate between cases and controls might have caused some selection bias and sampling bias, and results should, therefore, be interpreted with caution. Furthermore, mothers of children with more symptoms or a more severe symptom burden might have been keener to respond in both groups; thus, results might represent the most affected children. As questionnaires were sent to mothers, age and sex differences between responders and non-responders are not expected.
Parental reporting is a valid proxy for child self-reported health-related quality of life25 and is generally found to have good agreement with children's reporting, although more so in physical domains than in social and psychological domains, which could have affected the results. Because no validated questionnaires about long COVID have been developed for children of various ages to respond to, we chose to use parent proxy reporting; therefore, this study reflects parents' perspectives. The validated questionnaires used to investigate quality of life and generic symptoms have proven reliable and valid when using parent proxy reporting up to the age of 16 years.26
Recall bias might have occurred in this study as some participants were asked to recall symptoms over a period of more than 12 months. However, inclusion of children infected near the beginning of the pandemic was necessary to explore whether symptoms for some could last 12 months or longer. Additionally, the CSSI-24 and PedsQL had 2–4 weeks' recall, meaning that some cases went a long time since infection and could be free of symptoms related to COVID-19 at the time of completing these questionnaires. CSSI-24 and PedsQL were included in the survey as indicators of current health and quality of life among cases and controls, whereas the 23 ancillary questions about long-term symptoms were included to explore the presence of symptoms for 12 months or longer following SARS-CoV-2 infection. Thus, the instruments serve different purposes, justifying the different recall periods.
The survey data did not include information regarding hospitalisation; therefore, we were not able to assess symptom burden in relation to hospital admission. By Sept 15, 2021, when data collection was completed, 242 children aged 0–14 years in Denmark had been hospitalised with SARS-CoV-2 infection as the primary or secondary diagnosis.27
Compared with controls, cases had a higher prevalence of longer-lasting symptoms, which could represent long COVID, as supported by the fact that a third of cases (the long COVID group) indicated that symptoms were not present before having SARS-CoV-2 infection. Because long COVID symptoms are the same as some ailments that are common in children, possible differences between groups could be masked by competing diseases and vaccine side-effects. Five of the seven previous studies of long COVID in children that included a control group found a difference in the prevalence of persistent symptoms,8, 11, 12, 13, 14 with more symptoms in the case group, whereas two studies found no difference.7, 9 Prevalence of long-lasting symptoms was 4–66%,1 depending on factors such as the number and type of symptoms included. Over time, we found that the prevalence and intensity of long COVID symptoms decreased, which was also found in adolescents aged 15–18 years in the LongCOVIDKidsDK study.14
In the present study, we used WHO's definition of long COVID as the presence of symptoms lasting at least 2 months. Other guidelines define the presence of symptoms lasting for 3 months or longer as long COVID.6 At present, there is still no consensus definition for paediatric long COVID. In our study, irrespective of symptom duration, cases had higher odds of reporting at least one symptom lasting either 8 or 12 weeks.
In the youngest group in our study (children aged 0–3 years), both cases and controls had worse quality-of-life scores on the PedsQL physical symptoms scale compared with a healthy sample of children aged 2 years,26 but better scores on the emotional function scale. Among children aged 4–11 years, both cases and controls had worse emotional health scores than the representative scores for a normal healthy population.26
Our analyses of quality of life showed several differences between cases and controls that were statistically significant. However, when evaluating those scores using Hedges' g,23 only the differences in emotional and social outcomes in favour of cases among the oldest groups were clinically relevant. This finding is also consistent with findings in adolescents.14 Possible explanations for the higher scores in cases versus controls could include increasing awareness about the pandemic (which might scare children who have not yet had the infection), the importance of contact with peers versus family, and the difficulties of lockdown and social restrictions in the oldest groups, which might affect quality of life in the overall population. The short-lasting symptom scores on the CSSI-24 were generally lower in the case group and highest for the oldest group.
Notably, controls generally had worse scores in the validated generic questionnaires for psychological outcomes. The demographic and clinical profiles are not completely identical between the groups, which could be one possible explanation for the difference. It could also be that the controls experienced fear of the unknown disease and had a more restricted everyday life because of protecting themselves from catching the virus. Pandemic symptoms in children have been suggested1 and might be caused by poor thriving from lockdown and social restrictions. A systematic review including 6743 participants revealed no or mild long-term anxiety and depression during the first waves of the COVID-19 pandemics. The authors concluded that the global increase in mental health sequelae was more likely due to psychosocial factors related to lockdown, quarantine, and interrupted everyday life than to long-term effects of the virus.28
Among children aged 0–3 and cases aged 4–11 years, no sex differences in the prevalence of long-lasting symptoms were found. Among those aged 12–14 years, in both cases and controls, girls had more symptoms than did boys. Adolescent girls generally report a larger burden of complaints and diseases and have more symptoms of any kind than do adolescent boys.29 Sex difference in perceived health and burden of disease is a known phenomenon, which manifests in puberty and might be related to hormonal balance.30 Sex differences in the presence of long-lasting symptoms were also found in adolescents aged 15–18 years in the LongCOVIDKidsDK study.14
Cases in our study had more sick days and school absences, reflecting, to some degree, the recommended quarantine period. We included these data in our study to examine if the absences in cases were substantially longer than what could be expected due to quarantine and acute symptoms. The data in cases reflect burden to the family, socioeconomic burden, and access to education.
Data in this study were obtained for the period in which the alpha and delta variants of SARS-CoV-2 were dominant, and it is therefore unknown whether the results are generalisable to children infected with a more recent variant or any future variants of the virus.
In conclusion, children who had a history of SARS-CoV-2 infection in all age groups from 0 to 14 years reported a higher prevalence of long-lasting symptoms compared with age-sex-matched controls, and, among the oldest respondents, more females than males had long-lasting symptoms. Many long-lasting symptoms were also found in the control group. There was a tendency towards better quality-of-life scores in cases than in controls in the oldest groups. Further research should study post-COVID-19 diagnoses, prescribed drugs, and health-care use to better understand symptom clusters and long-term consequences of COVID-19 and the pandemic in children. These data are to be reported by the LongCOVIDKidsDK study.
Data sharing
Data will not be made available for others according to Danish data protection legislation.
Declaration of interests
SDN declares a research grant from Novo Nordic Foundation, a travel grant from Gilead, and that she is on the Advisory board for Gilead, GlaxoSmithKline, and MSD. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The study was funded by the A P Møller and Chastine Mc-Kinney Møller Foundation (2021-00661). The research presented was investigator initiated. We are grateful to the parents and children who participated in the survey. The Danish Department of Clinical Microbiology and the Statens Serum Institut conducted the laboratory analysis, registration, and provided the national SARS-CoV-2 surveillance data for this study.
Contributors
SKB conceptualised the study with input from PP, SDN, UN, HB, and AVC. SKB and AVC directly assessed and verified the underlying data. AVC, MNSP, SR, and ABT conducted the statistical analysis. SKB wrote the first draft. All the authors provided critical scholarly feedback on the manuscript, had access to the study data, approved the final version of the manuscript, and were responsible for the decision to submit the manuscript.
Supplementary Material
References
- 1.Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. 2021;40:e482–e487. doi: 10.1097/INF.0000000000003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen CH, Schelde AB, Moustsen-Helm IR, et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. medRxiv. 2021 doi: 10.1101/2021.12.20.21267966. published online Dec 23. (preprint, version 3). [DOI] [Google Scholar]
- 3.Centers for Disease Controls and Prevention Omicron variant: what you need to know. 2022. https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html
- 4.Schønning K, Dessau RB, Jensen TG, et al. Electronic reporting of diagnostic laboratory test results from all healthcare sectors is a cornerstone of national preparedness and control of COVID-19 in Denmark. APMIS. 2021;129:438–451. doi: 10.1111/apm.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO A clinical case definition of post COVID-19 condition by a Delphi consensus. 6 October 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [DOI] [PMC free article] [PubMed]
- 6.National Institute for Health and Care Excellence. Scottish Intercollegiate Guidelines Network. Royal College of General Practitioners COVID-19 rapid guideline: managing the long-term effects of COVID-19. March 1, 2022. https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742
- 7.Blankenburg J, Wekenborg MK, Reichert J, et al. Mental health of adolescents in the pandemic: long-COVID-19 or long-pandemic syndrome? SSRN. 2021 doi: 10.2139/ssrn.3844826. published online May 12. (preprint). [DOI] [Google Scholar]
- 8.Miller F, Nguyen V, Navaratnam AMD, et al. Prevalence of persistent symptoms in children during the COVID-19 pandemic: evidence from a household cohort study in England and Wales. medRxiv. 2021 doi: 10.1101/2021.05.28.21257602. published online June 2. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA. 2021;326:869–871. doi: 10.1001/jama.2021.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children—a nationwide cohort study. Eur J Pediatr. 2022;181:1597–1607. doi: 10.1007/s00431-021-04345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roessler M, Tesch F, Batram M, et al. Post COVID-19 in children, adolescents, and adults: results of a matched cohort study including more than 150,000 individuals with COVID-19. medRxiv. 2021 doi: 10.1101/2021.10.21.21265133. published online Oct 22. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephenson T, Pinto Pereira SM, Shafran R, et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Health. 2022;6:230–239. doi: 10.1016/S2352-4642(22)00022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5:708–718. doi: 10.1016/S2352-4642(21)00198-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kikkenborg Berg S, Nielsen SD, Nygaard U, et al. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. 2022;6:240–248. doi: 10.1016/S2352-4642(22)00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fauroux B, Simões EAF, Checchia PA, et al. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther. 2017;6:173–197. doi: 10.1007/s40121-017-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawley E. Pediatric chronic fatigue syndrome: current perspectives. Pediatric Health Med Ther. 2018;9:27–33. doi: 10.2147/PHMT.S126253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen HAR, Ekholm O, Davidsen M, Christensen AI. The Danish health and morbidity surveys: study design and participant characteristics. BMC Med Res Methodol. 2019;19:91. doi: 10.1186/s12874-019-0733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen AI, Ekholm O, Kristensen PL, et al. The effect of multiple reminders on response patterns in a Danish health survey. Eur J Public Health. 2015;25:156–161. doi: 10.1093/eurpub/cku057. [DOI] [PubMed] [Google Scholar]
- 19.Walker LS, Beck JE, Garber J, Lambert W. Children's Somatization Inventory: psychometric properties of the revised form (CSI-24) J Pediatr Psychol. 2009;34:430–440. doi: 10.1093/jpepsy/jsn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varni JW, Burwinkle TM, Seid M. The PedsQL as a pediatric patient-reported outcome: reliability and validity of the PedsQL Measurement Model in 25,000 children. Expert Rev Pharmacoecon Outcomes Res. 2005;5:705–719. doi: 10.1586/14737167.5.6.705. [DOI] [PubMed] [Google Scholar]
- 21.Long Covid Kids Symptom gallery. https://www.longcovidkids.org/long-covid-kids-symptom-gallery
- 22.De Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450(suppl 450):76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 23.Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Stat. 1981;6:107–128. [Google Scholar]
- 24.Richards J, Wiese C, Katon W, et al. Surveying adolescents enrolled in a regional health care delivery organization: mail and phone follow-up—what works at what cost? J Am Board Fam Med. 2010;23:534–541. doi: 10.3122/jabfm.2010.04.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lifland BE, Mangione-Smith R, Palermo TM, Rabbitts JA. Agreement between parent proxy report and child self-report of pain intensity and health-related quality of life after surgery. Acad Pediatr. 2018;18:376–383. doi: 10.1016/j.acap.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children's health-related quality of life: an analysis of 13,878 parents' reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:2. doi: 10.1186/1477-7525-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Statens Serum Institut Monitoring data for COVID-19 in Denmark and Europe. 2022. https://covid19.ssi.dk/overvagningsdata (in Danish).
- 28.Bourmistrova NW, Solomon T, Braude P, Strawbridge R, Carter B. Long-term effects of COVID-19 on mental health: a systematic review. J Affect Disord. 2022;299:118–125. doi: 10.1016/j.jad.2021.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel G, Bisegger C, Fuhr DC, Abel T. Age and gender differences in health-related quality of life of children and adolescents in Europe: a multilevel analysis. Qual Life Res. 2009;18:1147–1157. doi: 10.1007/s11136-009-9538-3. [DOI] [PubMed] [Google Scholar]
- 30.Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369:1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will not be made available for others according to Danish data protection legislation.