Abstract
Simple Summary
Honey bees are key pollinators in agricultural crops. Today, honey bee colonies in decline are a global concern as a result of various stressors, including pesticides, pathogens, honey bee health, and parasites. A healthy honey bee colony refers to colonies that are not exposed to biotic and abiotic stressors. In this study, we examine how thiamethoxam (pesticide) and deformed wing virus type A (DWV-A) interact in effects on honey bee health. The results revealed that the honey bees were infected with DWV-A and were additionally exposed to thiamethoxam, showing effects that increased the mortality rate, and crippled wings in newly emerged adult honey bees. Moreover, the exposure to thiamethoxam and DWV-A injection resulted in induced expression of immune genes (hymenoptaecin gene) while downregulation of two apoptosis genes (caspase8-like, caspase9-like genes). The impact interaction of pesticide and DWV-A have on the expression of apoptosis genes can directly affect viral susceptibility in the honey bee host.
Abstract
Honey bees are economically important insects for crop pollination. They play a significant role as pollinators of wild plants and agricultural crops and produce economical products, such as honey, royal jelly, wax, pollen, propolis, and venom. Despite their ecological and economical importance, the global honey bee population is in decline due to factors including pathogens, parasites, intensive agriculture, and pesticides. Moreover, these factors may be interlinked and exacerbate the loss of honey bees. This study aimed to investigate the interaction between a pesticide, thiamethoxam, and deformed wing virus type A (DWV-A) to honey bees and the effects on survival rate, wing characteristics, and expression of immune and apoptosis genes in Apis mellifera. We described the potential interaction between thiamethoxam and DWV-A on honey bee wing characteristics, DWV-A loads, and the expressions of immune (defensin, abaecin, and hymenoptaecin) and apoptosis genes (buffy, apaf1, caspase3-like, caspase8-like, and caspase9-like). Honey bee larvae were fed with three different thiamethoxam doses (0.001, 1.4, and 14.3 ng/µL of the diet). Then, thiamethoxam-treated white-eyed pupae were injected with 107 copy numbers/honey bee of the DWV-A genome. The interaction between thiamethoxam and DWV-A caused a high mortality rate, crippled wings in newly emerged adult honey bees (100%), and resulted in induced expression of hymenoptaecin gene compared to the control group, while downregulation of caspase8-like, caspase9-like genes compared to the DWV injection group. Therefore, the potential interaction between thiamethoxam and DWV-A might have a deleterious effect on honey bee lifespan. The results from this study could be used as a tool to combat DWV-A infection and mitigate pesticide usage to alleviate the decrease in the honey bee population.
Keywords: apoptosis, DWV-A, immune, pathogens, pesticides, thiamethoxam
1. Introduction
The western honey bee, Apis mellifera, is the main pollinator of wild plants and agricultural crops. They also produce honey and other hive products such as royal jelly, wax, pollen, propolis, and venom [1,2,3]. The global and rapid loss of honey bee colonies has been associated with various factors, including pesticides, pathogens, honey bee health, and parasites [4]. However, these losses are thought to be largely attributed to the pesticide as well as emergent pathogens, including viruses [5]. Furthermore, viruses and pesticides can be concurrent threats to honey bee colonies, as honey bees infected with different pathogens encounter pesticides when collecting pollen and nectar [6,7].
When honey bees forage pollen, pesticide residue in crops up to 10 km away can pollute collected pollen and nectar and consequently cause pesticide contamination in colonies. Thiamethoxam (nitro-substituted neonicotinoid) is now the most commonly used insecticide in crops worldwide for seed coatings or directly sprayed on crops [8,9]. Previous studies have shown that thiamethoxam had negative effects on honey bees in the larval stage [10,11,12,13], pupal stage [14], and adult stage [15,16,17,18]. Moreover, neonicotinoid insecticides are likely to cause changes in honey bee physiology, such as hypopharyngeal gland development [19,20], honey bee behavior [21,22,23], colony development [24], foraging [25,26], and memory and learning [27,28,29].
Among honey bee pathogens, viruses have been one of the main culprits associated with honey bees’ colony decline [30,31]. To date, about 26 honey bee viruses have been described, most of which are single-stranded RNA viruses, primarily belonging to the Dicistroviridae and Iflaviridae families [30]. The most common honey bee viruses include acute bee paralysis virus (ABPV), black queen cell virus (BQCV), chronic bee paralysis virus (CBPV), deformed wing virus (DWV), Israeli acute bee paralysis virus (IABPV), Kashmir bee virus (KBV), and sacbrood virus (SBV) were detected in honey bee colonies [5,32]. DWV is widespread and dominant in A. mellifera, positively correlated with varroa mites and tropilaelaps mites infestation [32,33,34,35]. DWV causes crippled wings and reduced body size in adult honey bees [36]. Several studies have documented that DWV has been linked to colony losses [32,37,38]. Three master variants of DWV (DWV-A, DWV-B, and DWV-C) have been discovered, with DWV-A being the most widespread variant [39,40,41]
Effects of co-exposure between pesticides and honey bee viruses have already been reported, resulting in an increase in DWV loads [42], BQCV loads [43], and CBPV loads [44] in honey bees. The effect of these factors has also been found to cause higher mortality rates in honey bee larvae [45]. Moreover, the change in gene expression pattern has been observed in immune and detoxification genes in honey bees [44]. Although the co-exposure of honey bees to DWV and thiamethoxam were investigated in previous studies [45], information on the relationship between crippled wings honey bees and gene pattern is still scarce. In this study, we described the effects of DWV-A infection and different concentrations of thiamethoxam treatment on the survival, viral loads, wing characteristics, and expressions of immune and apoptosis genes in newly emerged adult honey bees.
2. Materials and Methods
2.1. Honey Bee Samples
Seven Apis mellifera colonies maintained at Bee Protection Laboratory (BeeP) apiary, Chiang Mai University, Thailand (18°48′14.3″ N 98°57′22.2″ E) during 2018–2019 were used in this study. The crippled honey bees were collected from four colonies kept without ectoparasitic mites treatment. Three honey bee colonies, no visible clinical symptoms, and low/no ectoparasitic mites infestation were used for in vitro larval rearing.
2.2. Preparation of Deformed Wing Virus Type A (DWV-A) Lysate
The crippled adult honey bees were collected from A. mellifera colonies to prepare a DWV-A lysate. Five crippled adult honey bees were frozen in liquid nitrogen and crushed with a mortar. The ground crippled adult honey bees were suspended in 5 mL of phosphate buffer solution (pH 7.4) and then centrifuged at 6440× g for 10 min at 4 °C (K3 Series, Centurion Scientific Ltd., London, UK). The supernatant was collected after centrifugation and filtered through a 0.2-micron filter (Millipore, Merck, Darmstadt, Germany) to eliminate bacteria, fungi, and Nosema. The absence of six common honey bee viruses (acute bee paralysis virus (ABPV) [46], black queen cell virus (BQCV) [46], chronic bee paralysis virus (CBPV) [46], Israeli acute bee paralysis virus (IABPV) [47], Kashmir bee virus (KBV) [46], and sacbrood virus (SBV) [46]) in the lysate was confirmed by quantitative real-time PCR (qRT-PCR). The sequence of primers used is shown in Table S1. Lysate without all six common honey bee viruses was used for this study. The lysate was kept at −80 °C until use [48]. The level of DWV genome equivalents in the lysate was measured using the same qRT-PCR technique described later in the Materials and Methods (Section 2.6 and Section 2.7).
2.3. Diet and Larval Feeding
The first instar larval stage of A. mellifera was grafted onto an artificial diet plate. The levels of different sugar and yeast extract concentrations in food were provided for each developmental larval stage to meet the nutritional requirements. The artificial diet consisting of 50% w/w of royal jelly and 50% w/w of distilled water that contained either diet A (12% w/v glucose, 12% w/v fructose, and 2% w/v yeast extract), or diet B (15% w/v glucose, 15% w/v fructose, and 3% w/v yeast extract), or diet C (18% w/v glucose, 18% w/v fructose, and 4% w/v yeast extract) was refreshed every day. On the first and second days of in vitro rearing, each larva was fed with diet A, and then diet B was fed on the third day. Finally, diet C was fed on the fourth, fifth, and sixth days of the larvae developmental stage. Plates of larvae were incubated at 34 ± 1 °C and 96% RH [49].
2.4. Exposure to Thiamethoxam
Thiamethoxam was mixed in with diet C at three concentrations, including 0.001 (LT group), 1.4 (MT group), and 14.3 (HT group) ng/µL of the diet (note that the concentration of 0.001 ng/µL was the equivalent level of residues found in nectar, pollen, and beebread) [13]. The medium and high concentrations of thiamethoxam were selected according to a previous study [12], which were the lethal and sub-lethal concentrations of thiamethoxam to honey bee larvae reared in vitro. Diet C with no thiamethoxam was used in the control group (C group). The experimental groups were fed with diet C at different concentrations on the 4th day after grafting. After that, larvae received only food without the insecticide on the 5th and 6th days. On the 4th, 5th, and 6th days, each larva was fed 30, 40, and 50 ng/µL of diet C, respectively [12]. Overall, 105 honey bee larvae were subject to each treatment. Larval mortality was checked individually by observation under a stereomicroscope (Olympus, Tokyo, Japan) until they developed into the white-eyed pupae stage.
2.5. Injection of DWV-A to Honey Bee White-Eyed Pupae
The white-eyed pupae were collected and divided into 9 groups. Thiamethoxam-treated white-eyed pupae were injected laterally between the second and third tergite of the abdomen with 2 µL per honey bee of PBS containing 107 copy numbers/honey bee of DWV-A genome. Thiamethoxam-treated white-eyed pupae were divided into six groups: LT/V- (treated with 0.001 ng/µL thiamethoxam); LT/NC (treated with 0.001 ng/µL thiamethoxam with PBS injection); LT/V+ (treated 0.001 ng/µL thiamethoxam with DWV-A injection); MT/V- (treated 1.4 ng/µL thiamethoxam); MT/NC (treated 1.4 ng/µL thiamethoxam with PBS injection); MT/V+ (treated 1.4 ng/µL thiamethoxam with DWV-A injection). Thiamethoxam-untreated white-eyed pupae were injected with 2 µL per honey bee of 107 copy numbers/honey bee of DWV-A genome as a positive DWV-A control group (PC group). White-eyed pupae that were not treated with thiamethoxam and PBS injected were used as a negative control group (NC group). The PBS injection treatments were used as a control for the injection [45,50]. White-eyed pupae that were not treated with thiamethoxam and not injected were used as a handling control group (C group). All white-eyed pupae were incubated at 34 ± 1 °C and 70% RH until developing into newly emerged adult honey bees [51,52]. The honey bee survival rate was monitored during development.
2.6. RNA Extraction and cDNA Synthesis
Total RNA of adult honey bees was individually extracted by using TRIzol® (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. RNA concentration and quantity were determined using a BioDrop Duo spectrophotometer (BioDrop Ltd., Cambridge, UK). Reverse transcription was performed from 1 μg RNA to cDNA using the Tetro cDNA synthesis kit (Bioline, Alexandria, NSW, Australia) following the manufacturer’s protocol.
2.7. Quantitative Real-Time PCR Parameters
The number of DWV-A genome copies was determined by the absolute quantification method. The standard curve was established by plotting seven 10-fold dilutions of DWV-A insert in TOPO ®TA Cloning® plasmid (Invitrogen, Carlsbad, CA, USA). The qRT-PCR was performed on BioRad iQTM 5 (Bio-Rad Crop., Hercules, CA, USA), using SensiFAST SYBRR® No-ROX Kit master mix (Bioline, Alexandria, NSW, Australia). The amplification was performed in a 20 μL reaction volume using SensiFAST SYBR® No-ROX Mix, consisting of 10 µL of 2x SensiFAST SYBR® No-ROX Mix, 0.8 µL of each 10 µM primer, 1 μL of 10-fold diluted cDNA and nuclease-free water to adjust the volume to 20 μL. For amplification step with the following profile was used: 50 °C for 30 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s, 55 °C for 1 min, and 72 °C for 30 s. The melting curve was generated from 55 °C to 95 °C in 0.5 °C/s increments. The sequence of DWV-A [53], and housekeeping genes [54,55] primers is described in Table S1.
Relative quantification in real-time PCR was determined in antimicrobial peptides (AMPs), and apoptosis-related genes [50,56]. Ribosomal protein subunit 5 (RPS5) and β-actin were used as housekeeping genes for all primers shown in Table S1. qRT-PCR was performed as described above. All reactions were carried out using a thermal program of 95 °C for 30 s followed by 40 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min. The final qRT-PCR amplification was confirmed by the analysis of the melting curve generated from 55 °C to 95 °C in 0.5 °C/s increments. Each experiment was performed in triplicate, and negative controls (no template) were included in each reaction. Gene expression was calculated as 2−ΔΔCT [57].
2.8. Statistical Analysis
The survival of white-eyed pupae and newly emerged adult honey bees were established using Kaplan–Meier survival statistics with the log-rank test. Log-transformed DWV-A loads and gene transcripts were analyzed using one-way ANOVA (Welch ANOVA in cases of unequal variance) followed by the Games-Howell post-hoc t-test. The data were analyzed using generalized linear models (GLMs) to evaluate significant variations among treatments and genes, with treatments and genes as fixed factors, and the interaction was included. p-values less than 0.05 were noted as significant. All statistical analyses were tested using the SPSS v 25 program (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Effects of Thiamethoxam on Survival of Larvae to White-Eyed Pupae
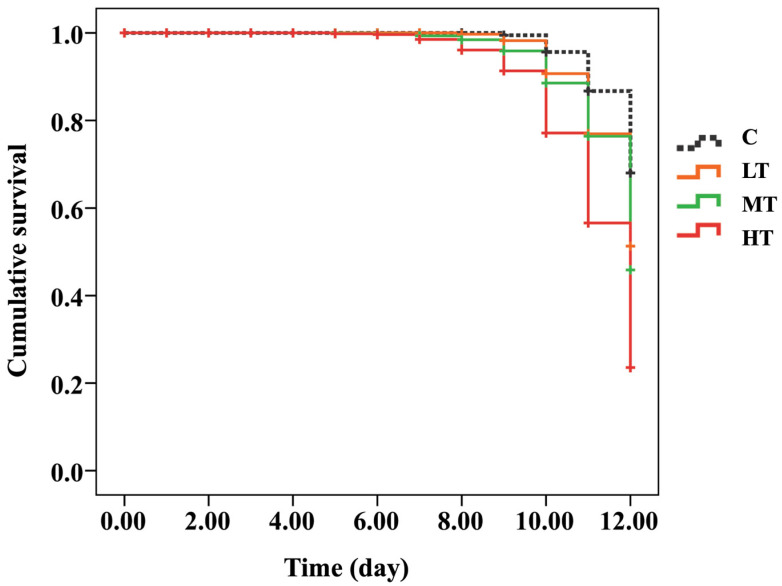
The cumulative survival curves of A. mellifera white-eyed pupae were significantly different between the C group (thiamethoxam-untreated) and thiamethoxam-treated groups after 12 days post feed (Kaplan–Meier log-rank test, x2 = 170.826, p < 0.0001; Figure 1 and Figure S1). The survival rate of the C group (90%) was not significantly different compared to the LT group (70%) (log-rank test, p = 0.059). In addition, the survival rates between MT (39%) and HT (22%) groups were not significantly different (log-rank test, p = 0.128; Figure 1 and Figure S1 and Table S2). Honey bees fed with the highest thiamethoxam dose (14.3 ng/µL; HT group) showed a significantly lower survival rate than the C group (log-rank test, p < 0.0001), and honey bees fed with 0.001 (LT group), 1.4 (MT group) ng/µL of thiamethoxam (log-rank test, p = 0.013 and 0.028, respectively) (Figure 1 and Table S2).
Figure 1.
Kaplan–Meier survival curve of white-eyed pupae that were treated with three concentrations of thiamethoxam (0.001, 1.4, and 14.3 ng/µL) and control (untreated thiamethoxam) in the larval stage.
3.2. Effects of Co-Exposure of Thiamethoxam and DWV-A on the Survival of White-Eyed Pupae to Newly Emerged Adult Honey Bees
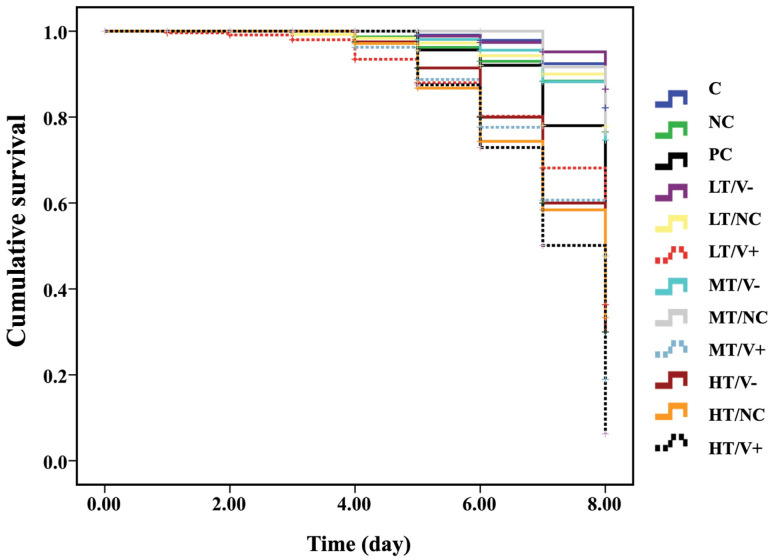
The cumulative survival curves of A. mellifera newly emerged adult honey bees were significantly different between the control and treated groups 8 days post injection (Kaplan–Meier log-rank test, x2 = 131.182, p < 0.0001; Figure 2). There was no significant difference in cumulative survival rates among the C group (thiamethoxam-untreated with no DWV-A injection) (89%), NC (thiamethoxam-untreated with PBS injection) (87%), LT/V- (91%), LT/NC (86%), MT/V- (85%), and MT/NC (83%) (log-rank test, p > 0.05). However, these treatment groups showed higher cumulative survival rates when compared to groups injected with DWV-A. The injection with DWV-A-untreated thiamethoxam group (PC group) and LT/V+, MT/V+, and HT/V+ groups showed survival rates of 61%, 47%, 50%, and 13%, respectively. The HT/V- and HT/NC groups resulted in survival rates of 13% and 14%, respectively (at p < 0.05, Figure 2 and Figure S2, and Table S3). Moreover, PC groups showed higher cumulative survival rate than LT/V+, MT/V+, and HT/V+ groups at p-value = 0.012, <0.0001, and <0.0001, respectively (Figure 2). A significant effect of interaction between thiamethoxam and DWV-A on mortality was found in all co-exposure groups when compared with DWV-A alone or thiamethoxam alone, except for the high dose thiamethoxam groups (HT/V- and HT/NC).
Figure 2.
Kaplan–Meier survival curve of newly emerged adult honey bees treated with three concentrations of thiamethoxam (0.001, 1.4, and 14.3 ng/µL) in the larval stage that were injected with DWV-A, PBS, and control (not treated with thiamethoxam and uninfected group) in the white-eyed pupal stage.
3.3. Effects of Co-Exposure of Thiamethoxam and DWV-A on Wing Characteristics of Newly Emerged Adult Honey Bees
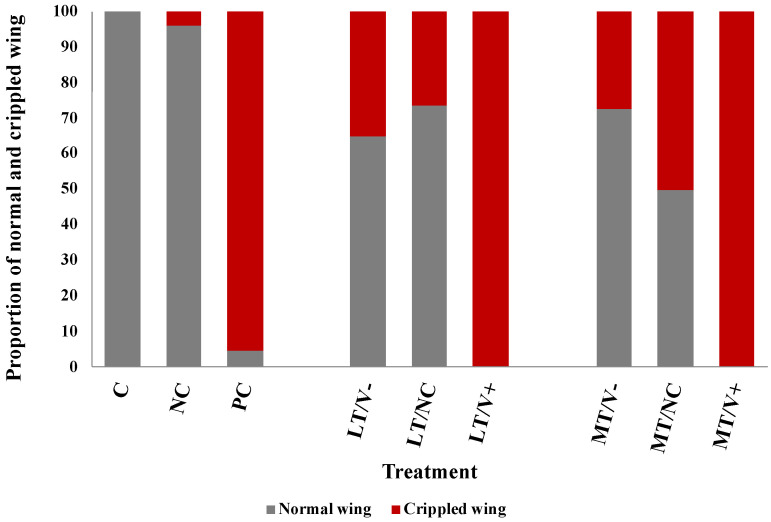
All newly emerged adult honey bees showed normal wings in the C group (100%) and the NC group (96%). Newly emerged adult honey bees that were not treated with thiamethoxam and injected with DWV-A (PC group) showed both normal and deformed wings at 5% and 95%, respectively. All thiamethoxam-treated groups were investigated for the crippled wings. The groups that were subject to 0.001 ng/µL of thiamethoxam (LT/V- group) and 0.001 ng/µL of thiamethoxam with PBS (LT/NC group) showed normal wing at 65% and 74%, respectively. The groups that were subject to 1.4 ng/µL of thiamethoxam (MT/V- group) and 1.4 ng/µL of thiamethoxam with PBS (MT/NC group) showed normal wing at 73% and 50%, respectively. The results showed that all concentrations of thiamethoxam treatments that were injected with DWV-A resulted in crippled wings in newly emerged adult honey bees (100%) (Figure 3). The survival rate of the HT/V+, HT/V-, and HT/NC groups was very low, and, therefore, the wing characteristic analysis was not performed.
Figure 3.
Percentages of the normal and crippled wings of newly emerged adult honey bees after being treated with thiamethoxam at 0.001 and 1.4 ng/µL in the larval stage and injected with DWV-A and PBS in the white-eyed pupal stage. The untreated and uninjected larvae were used as controls.
3.4. DWV-A Loads in Newly Emerged Adult Honey Bees
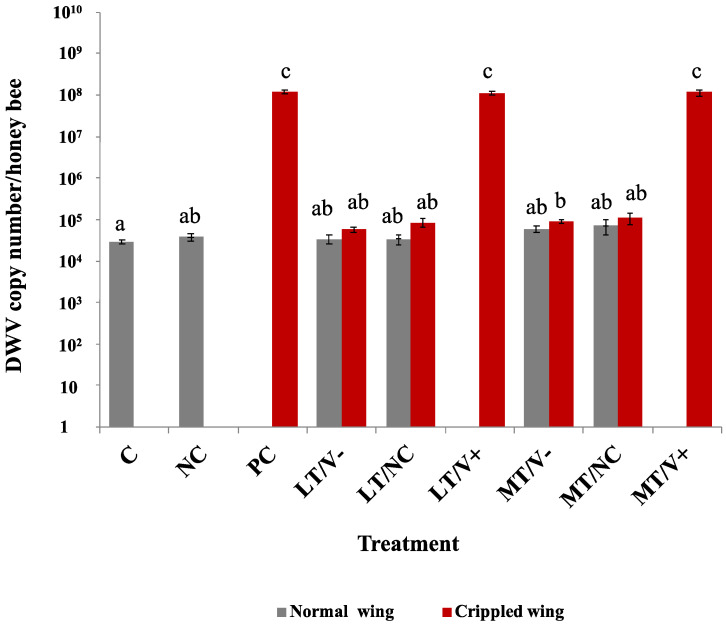
Low DWV-A loads were detected in C and NC groups in newly emerged adult honey bees (2.9 × 104 ± 8.0 × 103 and 3.8 × 104 ± 1.6 × 104 copy numbers/honey bee, respectively). The DWV-A levels of the MT/V- group showed a statistically significant difference in DWV-A levels compared to the C group (p = 0.031). The PC groups had higher DWV-A levels compared to the C group and all treatment groups at a p-value less than 0.05, except LT/V+ and MT/V+ groups (Figure 4 and Table S4). Crippled wings honey bees in the PC, LT/V+, and MT/V+ groups showed DWV-A loads of 1.2 × 108 ± 1.4 × 107, 1.1 × 108 ± 1.1 × 107, and 1.2 × 108 ± 1.9 × 107 copy numbers/honey bee, respectively, and there was no statistically significant difference in DWV-A loads between each other (p > 0.05). The interaction between thiamethoxam and DWV-A did not result in a significant modulation of DWV-A loads.
Figure 4.
DWV-A loads in newly emerged adult honey bees treated with thiamethoxam at 0.001 and 1.4 ng/µL in the larval stage and injected with DWV-A and PBS in the white-eyed pupal stage. The control group was not treated with thiamethoxam and not injected. Vertical bars represent means ± SEM. One-way ANOVA with Games–Howell post-hoc test was used. The lowercase letters indicate significant differences at p-values less than 0.05.
3.5. Immune- and Apoptosis-Related Gene Expression in Newly Emerged Adult Honey Bees
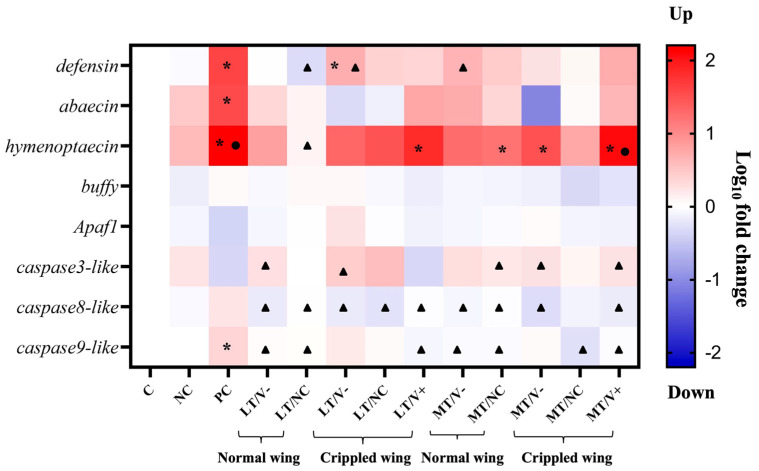
White-eyed honey bee pupae treated with 0.001 and 1.4 ng/µL thiamethoxam and control (thiamethoxam-untreated) in the larval stage were injected with PBS and DWV-A. Three immune (defensin, abaecin, and hymenoptaecin) and five apoptosis-related genes (buffy, apaf1, caspase3-like, caspase8-like, and caspase9-like) were investigated at the newly emerged adult honey bee stage. The results showed no significant differences in the expressions of buffy and apaf1 (Welch ANOVA, p = 0.062 and 0.095, respectively) in all experimental groups. In contrast, there were statistically significant differences in the expressions of six genes, including defensin, abaecin, hymenoptaecin, caspase3-like, caspase8-like, and caspase9-like (Welch ANOVA, all genes, p < 0.01). PC group showed an upregulation in three immune genes, including defensin, abaecin, and hymenoptaecin, compared to the C group (Games-Howell, p = 0.003, 0.034, and 0.011, respectively) (Figure 5 and Tables S5–S7). The expressions of two immune genes (defensin and abaecin) were lower in all groups treated with thiamethoxam and DWV-A injection than in the PC group, though not significantly different. Only the hymenoptaecin gene showed slightly higher upregulation in LT/V+ and MT/V+ groups than in the C group (Games–Howell, p = 0.005 and 0.006, respectively) (Figure 5 and Table S7). Honey bees that were treated with 1.4 ng/µL thiamethoxam and with DWV-A injection (MT/V+ group) showed an upregulation of the caspase3-like gene (Games-Howell, p = 0.008) compared to the PC group (Figure 5 and Table S8). The caspase8-like and caspase9-like genes showed the highest upregulation in the PC group, but only caspase9-like was significantly different compared to the control at p = 0.018 (Figure 5 and Tables S9 and S10). Moreover, the mRNA levels of the two genes were significantly suppressed in LT/V+ and MT/V+ groups compared to the PC group (Games-Howell, caspase8-like p = 0.046 and 0.031, respectively, and caspase9-like p = 0.014 and 0.026, respectively).
Figure 5.
Heatmap of immune and apoptosis genes expression levels in newly emerged adult honey bees. A black asterisk (*) indicates a significant difference between the treatment compared to the control group. A black circle (●) indicates a significant difference between the treatment compared to the PBS group. A black triangle (▲) indicates a significant difference between the treatment compared to the DWV-A group (p < 0.05; Welch ANOVA and Games-Howell).
Gene expression was significantly influenced by treatments and genes, and the interaction was also significant (GLMs: p < 0.001 for treatments; p < 0.001 for genes; and p < 0.001 for the interaction).
4. Discussion
Our study provides an insight into the effects on survival, DWV-A loads, wing characteristic, and expression of immune and apoptosis genes of Apis mellifera after exposure to different doses of thiamethoxam and DWV-A infection in newly emerged adult honey bees. Our results are consistent with previous reports, as we found that honey bees exposed to thiamethoxam in the larval stage had a significantly reduced survival rate in the white-eyed pupal stage [13,39]. Moreover, the combined effect of thiamethoxam and DWV-A further decreased the survival rate of newly emerged adult honey bees. Coulon et al. [45] also reported that a high dosage of thiamethoxam decreased the survival rate of honey bees after being injected with DWV. In this study, we showed that treatment with a low concentration of thiamethoxam (environmental dose in the colony) induces increased crippled wings in newly emerged adult honey bees. Nevertheless, there are limitations in this study that could be addressed in future research. The study used two high concentrations (1.4 and 14.3 ng/µL) that are not environmentally relevant. These concentrations not only induced high mortality but also resulted in an uneven number of individual tested.
Previous studies have shown that thiamethoxam caused changes in honey bee physiology [19,20]. Honey bees exposed to pesticides in the larval stage developed deformed physical characteristics in the adult stage, such as wing malformation, stunted bodies, and crippled legs [58]. Our study demonstrated that the effects of treatment with only thiamethoxam induce increased wing deformity in newly emerged adult honey bees. Moreover, numerous studies have also demonstrated that DWV is also the cause of crippled wings in honey bees [56,59]. Our result showed that honey bees exposed to thiamethoxam and DWV-A stressors had a high percentage of crippled wings as newly emerged adult honey bees. As a consequence, exposure to pesticides and DWV-A in honey bee colonies may impact the ability of adult honey bees to perform duties and forage effectively, leading to a decreased rate of colony survival.
In the present study, DWV-A levels of honey bees co-exposed to DWV-A and thiamethoxam were significantly higher than in treated groups, except for the PC group. Thus, the combination of neonicotinoid insecticides and DWV infection induced significantly higher DWV viral loads in honey bees [42]. These results coincide with the low survival rate of honey bees co-exposed to DWV-A and thiamethoxam, suggesting the effect between thiamethoxam and DWV-A infection on honey bee survival.
The immune-related gene expressions of honey bees co-exposed to thiamethoxam and DWV-A were upregulated in newly emerged adult honey bees. However, only the hymenoptaecin gene was significantly upregulated compared to the control group. The hymenoptaecin gene is one of the antimicrobial peptides that have been identified in honey bees to be active against microorganisms [60]. AMPs play a crucial role in the insect immune system and contribute to individual and social immunity [50,61,62]. Previous studies have indicated that the expressions of AMP genes in honey bees were upregulated after the invasion of pathogens, including microsporidian Nosema [63], Paenibacillus larvae [64], viruses [56], and ectoparasitic mites [65]. Viral infection within the host via viral entry, replication, and spreading can induce the antiviral innate immune responses [66]. Upregulation of several AMP genes, including abaecin, hymenoptaecin, and defensin, was also shown in other studies where honey bees were infected with DWV-A [56]. Treatment with thiamethoxam led to the downregulation of abaecin and defensin genes in crippled wings adult honey bees. Interestingly, honey bees exposed to thiamethoxam and DWV-A injection were also found to downregulate abaecin and defensin genes, implying immunological toxicity.
We found that co-exposure to thiamethoxam and DWV-A decreased the expression of apoptosis-related genes and significantly down-regulated caspases8-like and caspases9-like genes. The caspases gene is known to be related to programmed cell death and is associated with the final proteases in apoptosis [67]. Apoptosis is an important component of various processes, including normal cell development, embryonic development, function of the immune system, hormone-dependent atrophy, and chemical-induced cell death [68,69]. Evidence from previous studies suggested that virus infection induced apoptosis in insects and that the infection was mitigated by the elimination of the infected cells [70,71,72]. Honey bees injected with DWV had suppressed the expression of caspases in the pupal stage, which likely promoted the virus survival in hosts [56]. Honey bee co-exposure groups showed a strong alteration of immune gene expressions and downregulation of two apoptosis genes. Further studies are needed to investigate in greater detail the mechanisms for the viruses and pesticides that destroy immune pathways and the ability of viral replication in honey bee hosts.
5. Conclusions
This study showed the combined effect of DWV-A and thiamethoxam on A. mellifera, resulting in an increased mortality rate, crippled wings, and increased DWV-A loads. Our finding showed that honey bees exposed to thiamethoxam and DWV-A could intensify DWV-A infection, which could result in long-term physical deformity and decreased honey bees’ life span. Data from our investigation revealed that gene expression patterns changed in each treatment group. The effect of both thiamethoxam and DWV-A results in the transcriptome imbalance, which may also have an effect on stress recovery and, subsequently, on honey bees’ survival rate. Therefore, the results of our study could be explained by a negative interaction between thiamethoxam and DWV-A on honey bee lifespan in laboratory conditions. Future studies should be undertaken to examine the effects of pesticide exposure and viral infection occurring under field conditions.
Acknowledgments
P.C. and P.P. are supported by Post-Doctoral Fellowship 2022, Office of Research Administration, Chiang Mai University, Thailand. We thank Terd Disayathanoowat for editing this paper, providing many valuable comments, and financial support. This research was supported by Chiang Mai University. The Department of Biology, Faculty of Science, Chiang Mai University, Thailand is also acknowledged.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13060515/s1, Table S1: Primers used for qRT-PCR amplification in this study, Table S2: Statistic of survival in white-eyed pupae honey bees, Table S3: Statistic of survival in newly emerged adult honey bees, Table S4: Statistic of DWV-A load in newly emerged adult honey bees, Table S5: Statistic of defensin in newly emerged adult honey bees, Table S6: Statistic of abaecin in newly emerged adult honey bees, Table S7: Statistic of hymenoptaecin in newly emerged adult honey bees, Table S8: Statistic of caspase3-like in newly emerged adult honey bees, Table S9: Statistic of caspase8-like in newly emerged adult honey bees, Table S10: Statistic of caspase9-like in newly emerged adult honey bees, Figure S1: The percentage of survival rate of white-eyed pupae honey bees at 12 days post feed, Figure S2: The percentage of survival rates in newly emerged adult honey bees.
Author Contributions
Conceptualization, P.C. and P.P.; methodology, P.C.; software, S.K.; validation, P.P.; formal analysis, P.P., W.M., S.K. and C.S.; investigation, P.P.; resources, P.C.; data curation, P.P.; writing—original draft preparation, P.P., W.M., S.K., C.S. and P.C.; writing—review and editing, P.P., W.M., S.K., C.S. and P.C.; visualization, P.C. and P.P.; supervision, P.C.; project administration, P.C.; funding acquisition, P.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary material.
Conflicts of Interest
The authors declared that there are no competing interest.
Funding Statement
This research work was partially supported by Chiang Mai University Fund to P.C., and P.P. Open Access funding was covered by Mekong-Lancang Cooperation Special Fund.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gallai N., Salles J.-M., Settele J., Vaissière B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009;68:810–821. doi: 10.1016/j.ecolecon.2008.06.014. [DOI] [Google Scholar]
- 2.Liu Z., Chen C., Niu Q., Qi W., Yuan C., Su S., Liu S., Zhang Y., Zhang X., Ji T. Survey results of honey bee (Apis mellifera) colony losses in China (2010–2013) J. Apic. Res. 2016;55:29–37. doi: 10.1080/00218839.2016.1193375. [DOI] [Google Scholar]
- 3.Crane E. The past and present importance of bee products to man. In: Avshalom M., Yaacov L., editors. Bee Products: Properties, Applications, and Apitherapy. Springer Science & Business Media; Berlin, Germany: 2013. pp. 1–14. [Google Scholar]
- 4.Potts S.G., Biesmeijer J.C., Kremen C., Neumann P., Schweiger O., Kunin W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Cox-Foster D.L., Conlan S., Holmes E.C., Palacios G., Evans J.D., Moran N.A., Quan P.-L., Briese T., Hornig M., Geiser D.M., et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science. 2007;318:283–287. doi: 10.1126/science.1146498. [DOI] [PubMed] [Google Scholar]
- 6.Neumann P., Carreck N.L. Honey bee colony losses. J. Apic. Res. 2010;49:1–6. doi: 10.3896/IBRA.1.49.1.01. [DOI] [Google Scholar]
- 7.van der Sluijs J.P., Simon-Delso N., Goulson D., Maxim L., Bonmatin J.-M., Belzunces L.P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013;5:293–305. doi: 10.1016/j.cosust.2013.05.007. [DOI] [Google Scholar]
- 8.Nauen R., Ebbinghaus-Kintscher U., Salgado V.L., Kaussmann M. Thiamethoxam is a neonicotinoid precursor converted to clothianidin in insects and plants. Pestic. Biochem. Phys. 2003;76:55–69. doi: 10.1016/S0048-3575(03)00065-8. [DOI] [Google Scholar]
- 9.van Sluijs L., Pijlman G.P., Kammenga J.E. Why do Individuals differ in viral susceptibility? A story told by model organisms. Viruses. 2017;9:284. doi: 10.3390/v9100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friol P.S., Catae A.F., Tavares D.A., Malaspina O., Roat T.C. Can the exposure of Apis mellifera (Hymenoptera, Apiadae) larvae to a field concentration of thiamethoxam affect newly emerged bees? Chemosphere. 2017;185:56–66. doi: 10.1016/j.chemosphere.2017.06.113. [DOI] [PubMed] [Google Scholar]
- 11.Grillone G., Laurino D., Manino A., Porporato M. Toxicity of thiametoxam on in vitro reared honey bee brood. Apidologie. 2017;48:635–643. doi: 10.1007/s13592-017-0506-6. [DOI] [Google Scholar]
- 12.Tavares D.A., Roat T.C., Carvalho S.M., Silva-Zacarin E.C.M., Malaspina O. In vitro effects of thiamethoxam on larvae of Africanized honey bee Apis mellifera (Hymenoptera: Apidae) Chemosphere. 2015;135:370–378. doi: 10.1016/j.chemosphere.2015.04.090. [DOI] [PubMed] [Google Scholar]
- 13.Tavares D.A., Dussaubat C., Kretzschmar A., Carvalho S.M., Silva-Zacarin E.C.M., Malaspina O., Bérail G., Brunet J.-L., Belzunces L.P. Exposure of larvae to thiamethoxam affects the survival and physiology of the honey bee at post-embryonic stages. Environ. Pollut. 2017;229:386–393. doi: 10.1016/j.envpol.2017.05.092. [DOI] [PubMed] [Google Scholar]
- 14.Tesovnik T., Cizelj I., Zorc M., Čitar M., Božič J., Glavan G., Narat M. Immune related gene expression in worker honey bee (Apis mellifera carnica) pupae exposed to neonicotinoid thiamethoxam and Varroa mites (Varroa destructor) PLoS ONE. 2017;12:e0187079. doi: 10.1371/journal.pone.0187079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badiou-Bénéteau A., Carvalho S.M., Brunet J.-L., Carvalho G.A., Buleté A., Giroud B., Belzunces L.P. Development of biomarkers of exposure to xenobiotics in the honey bee Apis mellifera: Application to the systemic insecticide thiamethoxam. Ecotoxicol. Environ. Saf. 2012;82:22–31. doi: 10.1016/j.ecoenv.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Coulon M., Schurr F., Martel A.C., Cougoule N., Bégaud A., Mangoni P., Dalmon A., Alaux C., Le Conte Y., Thiéry R., et al. Metabolisation of thiamethoxam (a neonicotinoid pesticide) and interaction with the Chronic bee paralysis virus in honeybees. Pestic. Biochem. Phys. 2018;144:10–18. doi: 10.1016/j.pestbp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Henry M., Béguin M., Requier F., Rollin O., Odoux J.-F., Aupinel P., Aptel J., Tchamitchian S., Decourtye A. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science. 2012;336:348–350. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- 18.Tosi S., Burgio G., Nieh J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 2017;7:1201. doi: 10.1038/s41598-017-01361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatjina F., Papaefthimiou C., Charistos L., Dogaroglu T., Bouga M., Emmanouil C., Arnold G. Sublethal doses of imidacloprid decreased size of hypopharyngeal glands and respiratory rhythm of honeybees in vivo. Apidologie. 2013;44:467–480. doi: 10.1007/s13592-013-0199-4. [DOI] [Google Scholar]
- 20.Renzi M.T., Rodríguez-Gasol N., Medrzycki P., Porrini C., Martini A., Burgio G., Maini S., Sgolastra F. Combined effect of pollen quality and thiamethoxam on hypopharyngeal gland development and protein content in Apis mellifera. Apidologie. 2016;47:779–788. doi: 10.1007/s13592-016-0435-9. [DOI] [Google Scholar]
- 21.Suchail S., Guez D., Belzunces L.P. Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ. Toxicol. Chem. 2001;20:2482–2486. doi: 10.1002/etc.5620201113. [DOI] [PubMed] [Google Scholar]
- 22.Williamson S., Moffat C., Gomersall M., Saranzewa N., Connolly C., Wright G. Exposure to Acetylcholinesterase Inhibitors Alters the Physiology and Motor Function of Honeybees. Front. Physiol. 2013;4:13. doi: 10.3389/fphys.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright G.A., Softley S., Earnshaw H. Low doses of neonicotinoid pesticides in food rewards impair short-term olfactory memory in foraging-age honeybees. Sci. Rep. 2015;5:15322. doi: 10.1038/srep15322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J.Y., Anelli C.M., Sheppard W.S. Sub-Lethal Effects of Pesticide Residues in Brood Comb on Worker Honey Bee (Apis mellifera) Development and Longevity. PLoS ONE. 2011;6:e14720. doi: 10.1371/journal.pone.0014720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider C.W., Tautz J., Grünewald B., Fuchs S. RFID Tracking of Sublethal Effects of Two Neonicotinoid Insecticides on the Foraging Behavior of Apis mellifera. PLoS ONE. 2012;7:e30023. doi: 10.1371/journal.pone.0030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang E.C., Chuang Y.C., Chen Y.L., Chang L.H. Abnormal Foraging Behavior Induced by Sublethal Dosage of Imidacloprid in the Honey Bee (Hymenoptera: Apidae) J. Econ. Entomol. 2008;101:1743–1748. doi: 10.1603/0022-0493-101.6.1743. [DOI] [PubMed] [Google Scholar]
- 27.Frost E.H., Shutler D., Hillier N.K. Effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival. J. Exp. Biol. 2013;216:2931–2938. doi: 10.1242/jeb.086538. [DOI] [PubMed] [Google Scholar]
- 28.Desneux N., Decourtye A., Delpuech J.-M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007;52:81–106. doi: 10.1146/annurev.ento.52.110405.091440. [DOI] [PubMed] [Google Scholar]
- 29.Williamson S.M., Wright G.A. Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J. Exp. Biol. 2013;216:1799–1807. doi: 10.1242/jeb.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMenamin A.J., Genersch E. Honey bee colony losses and associated viruses. Curr. Opin. Insect Sci. 2015;8:121–129. doi: 10.1016/j.cois.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 31.McMahon D.P., Natsopoulou M.E., Doublet V., Fürst M., Weging S., Brown M.J., Gogol-Döring A., Paxton R.J. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. R. Soc. Lond. B Biol. Sci. 2016;283:20160811. doi: 10.1098/rspb.2016.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin S.J., Highfield A.C., Brettell L., Villalobos E.M., Budge G.E., Powell M., Nikaido S., Schroeder D.C. Global honey bee viral landscape altered by a parasitic mite. Science. 2012;336:1304–1306. doi: 10.1126/science.1220941. [DOI] [PubMed] [Google Scholar]
- 33.Bowen-Walker P.L., Martin S.J., Gunn A. The transmission of deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. J. Invertebr. Pathol. 1999;73:101–106. doi: 10.1006/jipa.1998.4807. [DOI] [PubMed] [Google Scholar]
- 34.Highfield A.C., El Nagar A., Mackinder L.C.M., Noël L.M.L.J., Hall M.J., Martin S.J., Schroeder D.C. Deformed wing virus implicated in overwintering honeybee colony losses. Appl. Environ. Microbiol. 2009;75:7212–7220. doi: 10.1128/AEM.02227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordström S. Distribution of deformed wing virus within honey bee (Apis mellifera) brood cells infested with the ectoparasitic mite Varroa destructor. Exp. Appl. Acarol. 2003;29:293–302. doi: 10.1023/A:1025853731214. [DOI] [PubMed] [Google Scholar]
- 36.Yue C., Genersch E. RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor) J. Gen. Virol. 2005;86:3419–3424. doi: 10.1099/vir.0.81401-0. [DOI] [PubMed] [Google Scholar]
- 37.Dainat B., Vanengelsdorp D., Neumann P. Colony collapse disorder in Europe. Environ. Microbiol. Rep. 2012;4:123–125. doi: 10.1111/j.1758-2229.2011.00312.x. [DOI] [PubMed] [Google Scholar]
- 38.Martin S. A population model for the ectoparasitic mite Varroa jacobsoni in honey bee (Apis mellifera) colonies. Ecol. Model. 1998;109:267–281. doi: 10.1016/S0304-3800(98)00059-3. [DOI] [Google Scholar]
- 39.Lanzi G., de Miranda J.R., Boniotti M.B., Cameron C.E., Lavazza A., Capucci L., Camazine S.M., Rossi C. Molecular and biological characterization of deformed wing virus of honeybees (Apis mellifera L.) Virol. J. 2006;80:4998–5009. doi: 10.1128/JVI.80.10.4998-5009.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mordecai G.J., Wilfert L., Martin S.J., Jones I.M., Schroeder D.C. Diversity in a honey bee pathogen: First report of a third master variant of the Deformed Wing Virus quasispecies. ISME J. 2016;10:1264–1273. doi: 10.1038/ismej.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ongus J.R., Peters D., Bonmatin J.M., Bengsch E., Vlak J.M., van Oers M.M. Complete sequence of a picorna-like virus of the genus Iflavirus replicating in the mite Varroa destructor. J. Gen. Virol. 2004;85:3747–3755. doi: 10.1099/vir.0.80470-0. [DOI] [PubMed] [Google Scholar]
- 42.Di Prisco G., Cavaliere V., Annoscia D., Varricchio P., Caprio E., Nazzi F., Gargiulo G., Pennacchio F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA. 2013;110:18466. doi: 10.1073/pnas.1314923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doublet V., Labarussias M., de Miranda J.R., Moritz R.F.A., Paxton R.J. Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 2015;17:969–983. doi: 10.1111/1462-2920.12426. [DOI] [PubMed] [Google Scholar]
- 44.Coulon M., Schurr F., Martel A.-C., Cougoule N., Bégaud A., Mangoni P., Di Prisco G., Dalmon A., Alaux C., Ribière-Chabert M., et al. Influence of chronic exposure to thiamethoxam and chronic bee paralysis virus on winter honey bees. PLoS ONE. 2019;14:e0220703. doi: 10.1371/journal.pone.0220703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coulon M., Dalmon A., Di Prisco G., Prado A., Arban F., Dubois E., Ribière-Chabert M., Alaux C., Thiéry R., Le Conte Y. Interactions Between Thiamethoxam and Deformed Wing Virus Can Drastically Impair Flight Behavior of Honey Bees. Front. Microbiol. 2020;11:766. doi: 10.3389/fmicb.2020.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locke B., Forsgren E., Fries I., de Miranda J.R. Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control. Appl. Environ. Microbiol. 2012;78:227–235. doi: 10.1128/AEM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Miranda J.R., Cordoni G., Budge G. The acute bee paralysis virus–kashmir bee virus–israeli acute paralysis virus complex. J. Invertebr. Pathol. 2010;103:S30–S47. doi: 10.1016/j.jip.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Khongphinitbunjong K., de Guzman L.I., Rinderer T.E., Tarver M.R., Frake A.M., Chen Y., Chantawannakul P. Responses of varroa-resistant honey bees (Apis mellifera L.) to deformed wing virus. J. Asia-Pac. Entomol. 2016;19:921–927. doi: 10.1016/j.aspen.2016.08.008. [DOI] [Google Scholar]
- 49.Aupinel P., Fortin D., Dufour H., Tasei J.-N., Michaud B., Odoux J., Pham-Delègue M.-H. Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae. Bull. Insectol. 2005;58:107–111. [Google Scholar]
- 50.Evans J.D., Aronstein K., Chen Y.P., Hetru C., Imler J.L., Jiang H., Kanost M., Thompson G.J., Zou Z., Hultmark D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006;15:645–656. doi: 10.1111/j.1365-2583.2006.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gätschenberger H., Azzami K., Tautz J., Beier H. Antibacterial immune competence of honey bees (Apis mellifera) is adapted to different life stages and environmental risks. PLoS ONE. 2013;8:e66415. doi: 10.1371/journal.pone.0066415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azzami K., Ritter W., Tautz J., Beier H. Infection of honey bees with acute bee paralysis virus does not trigger humoral or cellular immune responses. Arch. Virol. 2012;157:689–702. doi: 10.1007/s00705-012-1223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeGrandi-Hoffman G., Chen Y., Huang E., Huang M.H. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.) J. Insect Physiol. 2010;56:1184–1191. doi: 10.1016/j.jinsphys.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Evans J.D., Pettis J.S. Colony-level impacts of immune responsiveness in honey bees, Apis mellifera. EVO. 2005;59:2270–2274. doi: 10.1111/j.0014-3820.2005.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 55.Simone M., Evans J.D., Spivak M. Resin collection and social immunity in honey bees. EVO. 2009;63:3016–3022. doi: 10.1111/j.1558-5646.2009.00772.x. [DOI] [PubMed] [Google Scholar]
- 56.Mookhploy W., Krongdang S., Chantawannakul P. Effects of Deformed Wing Virus Infection on Expressions of Immune- and Apoptosis-Related Genes in Western Honeybees (Apis mellifera) Insects. 2021;12:82. doi: 10.3390/insects12010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 58.Atkins E.L., Kellum D. Comparative Morphogenic and toxicity Studies on the Effect of Pesticides on Honeybee Brood. J. Apic. Res. 1986;25:242–255. doi: 10.1080/00218839.1986.11100725. [DOI] [Google Scholar]
- 59.de Miranda J.R., Genersch E. Deformed wing virus. J. Invertebr. Pathol. 2010;103((Suppl. 1)):S48–S61. doi: 10.1016/j.jip.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 60.Casteels P., Ampe C., Jacobs F., Tempst P. Functional and chemical characterization of hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee (Apis mellifera) J. Biol. Chem. 1993;268:7044–7054. doi: 10.1016/S0021-9258(18)53143-4. [DOI] [PubMed] [Google Scholar]
- 61.Evans J.D. Transcriptional immune responses by honey bee larvae during invasion by the bacterial pathogen, Paenibacillus larvae. J. Invertebr. Pathol. 2004;85:105–111. doi: 10.1016/j.jip.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Ilyasov R., Gaifullina L., Saltykova E., Poskryakov A., Nikolenko A. Review of the expression of antimicrobial peptide defensin in honey bees Apis mellifera L. J. Apic. Sci. 2012;56:115–124. doi: 10.2478/v10289-012-0013-y. [DOI] [Google Scholar]
- 63.Sinpoo C., Paxton R.J., Disayathanoowat T., Krongdang S., Chantawannakul P. Impact of Nosema ceranae and Nosema apis on individual worker bees of the two host species (Apis cerana and Apis mellifera) and regulation of host immune response. J. Insect Physiol. 2018;105:1–8. doi: 10.1016/j.jinsphys.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Krongdang S., Evans J.D., Chen Y., Mookhploy W., Chantawannakul P. Comparative susceptibility and immune responses of Asian and European honey bees to the American foulbrood pathogen, Paenibacillus larvae. Insect Sci. 2018;26:831–842. doi: 10.1111/1744-7917.12593. [DOI] [PubMed] [Google Scholar]
- 65.Khongphinitbunjong K., de Guzman L.I., Tarver M.R., Rinderer T.E., Chantawannakul P. Interactions of Tropilaelaps mercedesae, honey bee viruses and immune response in Apis mellifera. J. Apic. Res. 2015;54:40–47. doi: 10.1080/00218839.2015.1041311. [DOI] [Google Scholar]
- 66.Mueller S.N., Rouse B.T. Clinical Immunology. 3rd ed. Mosby; Maryland Heights, MO, USA: 2008. Immune responses to viruses; pp. 421–431. [DOI] [Google Scholar]
- 67.McIlwain D.R., Berger T., Mak T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Esch H., Esch I., Kerr W.E. Sound: An element common to communication of stingless bees and to dances of the honey bee. Science. 1965;149:320–321. doi: 10.1126/science.149.3681.320. [DOI] [PubMed] [Google Scholar]
- 71.Settles E.W., Friesen P.D. Flock House Virus Induces Apoptosis by Depletion of Drosophila Inhibitor-of-Apoptosis Protein DIAP1. Virol. J. 2008;82:1378–1388. doi: 10.1128/JVI.01941-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marques J.T., Imler J.-L. The diversity of insect antiviral immunity: Insights from viruses. Curr. Opin. 2016;32:71–76. doi: 10.1016/j.mib.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and supplementary material.