Abstract
We aimed to identify brain structural changes in cortical and subcortical regions linked to recent suicidal behavior. We performed secondary analyses of structural MRI data of two independent studies, namely the Establishing Moderators/Biosignatures of Antidepressant Response - Clinical Care (EMBARC) study and a Little Rock study on acute suicidal behavior. Study 1 (EMBARC, N = 187), compared individuals with remote suicide attempts (Remote-SA), individuals with lifetime suicide ideation but no attempts (Lifetime-SI only), and depressed individuals without lifetime suicide ideation or attempts (non-suicidal depressed). Study 2 (Little Rock data, N = 34) included patients recently hospitalized for suicide ideation or attempt constituted by: patients who recently attempted suicide (Recent-SA), individuals with remote suicide attempts (Remote-SA), and Lifetime-SI only. Study 3 combined the EMBARC and Little Rock datasets including Recent-SA, Remote-SA, Lifetime-SI only and non-suicidal depressed individuals. In Study 1 and Study 2, no significant differences were observed between groups. In Study 3, significantly lower middle temporal gyrus thickness, insular surface area, and thalamic volume and higher volume in the nucleus accumbens were observed in Recent-SA. This pattern of structural abnormalities may underlie pain and emotion dysregulation, which have been linked to the transition from suicidal thoughts to action.
1. Introduction
Suicide rates have continued to rise for the past several decades (Dyer, 2018). Over 1.4 million adults attempt suicide annually in the United States (Administration, 2019); this is equivalent to two suicide attempts per minute. A better understanding of the neurobiology underlying the progression of suicidal thoughts to suicidal behavior can advance identification of biological markers and interventions for effective suicide prevention.
Structural and functional abnormalities in the frontal, parietal, and temporal lobes have been linked to suicidal behavior (Hwang et al., 2010; Mann, 2003; Schmaal et al., 2020). Reduced frontal and temporal lobe volumes have been observed in depressed patients with a history of suicide attempts (Gosnell et al., 2016). Decreased gray matter volume in the orbitofrontal cortex (OFC) has been described in several populations with a history of suicide attempts, including patients with major depressive disorder (MDD) (Ding et al., 2015; Hwang et al., 2010; Monkul et al., 2007), bipolar disorder (Benedetti et al., 2011; Johnston et al., 2017), and schizophrenia (Aguilar et al., 2008). Additionally, a history of suicide attempts has been linked to reduced cortical thickness in the dorsolateral PFC (dlPFC), the ventrolateral PFC (vlPFC), and the anterior cingulate cortex (ACC) in patients with MDD (Wagner et al., 2012). Decreased resting-state functional connectivity between the rostral ACC, the OFC, and the right middle temporal pole was described in depressed patients with suicidal ideation (Du et al., 2017). In addition, functional hypoconnectivity was observed in the frontoparietal network of depressed individuals with a history of suicide attempts (Hwang et al., 2018; Kaiser et al., 2015).
The frontal, temporal, and parietal lobes are highly interconnected and are involved in cognitive, emotional, and attentional processes. Structural alterations in the frontal regions, including the dorsal and ventral PFC, OFC, and inferior frontal gyrus (IFG), are associated with impairments in impulse control (Cáceda et al., 2014), control inhibition (Richard-Devantoy et al., 2016), emotional reactivity (Pan et al., 2013), and anhedonia (Downar et al., 2014), which are implicated in suicidal behavior (Jollant et al., 2011). Moreover, subcortical abnormalities have been observed in patients with depression and suicidal behavior. A large meta-analysis reported lower hippocampal volume in patients with MDD (Schmaal et al., 2015). In two studies of depressed suicide attempters, lower hippocampal volume was observed as compared to depressed non-attempters (Colle et al., 2015) and to healthy controls (Gosnell et al., 2016). Structural abnormalities have been described in patients with MDD with a history of suicide attempts including larger amygdala volume (Monkul et al., 2007), smaller caudate and globus pallidus volume (Vang et al., 2010), and smaller putamen gray matter volume (Dombrovski et al., 2012). However, other studies have observed no differences in subcortical volume measures between depressed patients with and without suicidal behavior (Gifuni et al., 2016; Rentería et al., 2017). The key difference between these studies may be attributed to the recency of the suicide attempt.
A recent elegant review by Schmaal and colleagues described a set of brain regions associated with suicidal behavior clustered in two circuits, one associated with the dlPFC and another with the vlPFC (Schmaal et al., 2020). These regions are associated with impaired internal states and emotional dysregulation associated with suicide. Additionally, subcortical structures may also be involved in cognitive and behavioral vulnerabilities underlying the transition from suicidal ideation to behavior. Thus, we chose a priori regions including the dorsal (caudal) ACC, IFG, inferior temporal, middle temporal and superior temporal gyri, lateral and medial OFC, insula, superior frontal gyrus and superior parietal cortex, caudate, hippocampus, putamen, thalamus and nucleus accumbens. We hypothesized that a history of suicide attempts would be associated with structural alterations including decreased cortical thickness and surface area in our a priori cortical regions and volume in subcortical regions. Furthermore, we anticipate that these anomalies would be more pronounced in patients with a recent suicide attempt as compared to individuals with remote suicide attempts.
2. Study 1 methods (EMBARC dataset)
2.1. Participants
Study 1 presents an analysis of depressed individuals (N = 187) with data shared from the National Institute of Health (NIMH) Data Archive “Establishing Moderators/Biosignatures of Antidepressant Response - Clinical Care (EMBARC) MDD Treatment and Controls” collection. The study design and participant enrollment criteria have been previously published (Trivedi et al., 2016), and the study abided by the principles of the Declaration of Helsinki. The data included adults of both sexes, ages 18–65 years. Participants who met criteria for nonpsychotic MDD (Diagnostic and Statistical Manual of Mental Disorders ([DSM])-IV-TR using the Structural Clinical Interview for DSM Diagnoses [SCID]) (Dsm-Iv-tr, 2000) were enrolled in a placebo-controlled randomized clinical trial of sertraline. Additional inclusion criteria included early onset MDD (before age 30) and chronic (episode duration of more than two years) or recurrent MDD (two or more recurrences including current episode) as described previously (Bartlett et al., 2018; Trivedi et al., 2016). The Institutional Review Board (IRB) at each imaging study site approved the research procedures. Written informed consents were obtained from all the study participants. In our analysis, three groups were compared: remote suicide attempters (Remote-SA; n = 21) included currently depressed patients with at least one lifetime suicide attempt but not within the last six months; lifetime suicide ideators (Lifetime-SI only; n = 72) included currently depressed patients with lifetime suicidal ideation but no lifetime history of suicide attempts; and non-suicidal depressed patients (n = 94) included currently depressed patients with no lifetime suicidal ideation or suicide attempts.
2.2. Study 1 procedure
Recruitment and study design have been previously published (Bartlett et al., 2018; Trivedi et al., 2016). Briefly, after obtaining a signed informed consent, psychiatric diagnoses were established with the SCID, and the Quick Inventory of Depressive symptoms (Rush et al., 2003) was administered. The 17-item Hamilton Depression Rating Scale (HDRS-17) (Hamilton, 1960) was used to determine depression severity.
2.3. MRI acquisition
The MRI acquisition parameters of the EMBARC dataset have been previously described (Bartlett et al., 2018; Trivedi et al., 2016). The EMBARC MRI sites and scanners were the following: University of Michigan (UM—Philips Ingenia, 15-channel), Massachusetts General Hospital (MGH—Siemens TrioTim, 12-channel), University of Texas Southwestern Medical Center (TX—Philips Achieva, 8-channel head-coil), and Columbia University Medical Center (CU—GE Signa HDx, 8-channel). MPRAGE sequences were acquired from the former two sites, a 3D turbo field echo (TFE) sequence was acquired at TX, and an inversion recovery-fast spoiled gradient-echo (IR-FSPGR) sequence was acquired at CU. Sequence parameters were as follows: TR/TE = 5.9–8.2/2.4–4.6 ms, 8–12° flip angle, 1 mm slice thickness, 4.4–5.5 min acquisition, and 1 mm isotropic voxel dimensions. OurThe current study was based on the baseline data.
2.4. Image processing
The EMBARC processed images from a previous report (Bartlett et al., 2018) were downloaded from the controlled access datasets maintained by the NIMH-supported National Database for Clinical Trials (NDCT) (Trivedi et al., 2016). The dataset identifier is #2199, titled “Establishing Moderators/Biosignatures of Antidepressant Response – Clinical Care (EMBARC) MDD Treatment and Controls”. FreeSurfer 5.3.0 (http://surfer.nmr.mgh.harvard.edu/) was run on the raw structural images to calculate cortical thickness and surface area, as well as subcortical volume, using the Desikan-Killiany (DK) atlas (Desikan et al., 2006) and FreeSurfer’s subcortical atlas (Filipek et al., 1994; Makris et al., 2008). A standardized quality control procedure was performed that was originally validated using EMBARC data (Iscan et al., 2015). Briefly, raw T1-weighted images were first examined for imaging artifacts including ghosting, blurring, and ringing. Then, coronal and axial sections of FreeSurfer’s pial and white surfaces were visually assessed for accuracy and subsequently approved or disapproved (Iscan et al., 2015). Regions of interest were selected a priori based on previous reports of suicidal patients: caudal ACC, IFG (pars opercularis, pars triangularis, pars orbitalis), lateral and medial OFC, inferior temporal, middle temporal and superior temporal gyri, insula, and superior frontal gyrus and superior parietal cortex in addition to subcortical volumes: nucleus accumbens, caudate, hippocampus, putamen and thalamus (Ding et al., 2015; Gifuni et al., 2016; Gosnell et al., 2016; Jollant et al., 2011; Rentería et al., 2017; Schmaal et al., 2020, 2015). We have included both cortical thickness and surface area in our analyses as both contribute to cortical volume, but may be reflected differently with increasing age (Storsve et al., 2014; Winkler et al., 2012). In order to reduce variability associated with the use of different imaging scanners at multiple sites, the ComBat technique was used to harmonize the imaging data within EMBARC; this method has been validated and applied in previous studies leveraging EMBARC data (Bartlett et al., 2018; Fortin et al., 2018).
2.5. Statistical analyses
Descriptive statistics were calculated. Univariate linear regressions were performed for a priori ROI cortical thickness, surface area, and subcortical volume. Mean differences between Remote-SA, Lifetime-SI only, and non-suicidal depressed groups were compared with analysis of covariance (ANCOVA). Age, depression severity (HDRS-17), education, and imaging sites were factored in as covariates. A linear regression analysis was used with the number of suicide attempts as the dependent variable and age and depression severity as independent variables. Spearman correlation analyses were performed between the ROI and number of suicide attempts and months since last suicide attempt. The false discovery rate was adjusted (p-FDR ≤ 0.10) using the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995). All analyses were conducted using SPSS 26 (SPSS Inc., Chicago, IL, USA).
3. Study 2 methods (Little rock dataset)
3.1. Participants
Study 2 included participants recruited from the psychiatric inpatient units and outpatient clinics of the University of Arkansas for Medical Sciences (UAMS) and the Little Rock community between March 2014 and June 2016. Three groups were analyzed: currently depressed patients with a recent (within the previous three days) suicide attempt (Recent-SA; n = 11), currently depressed patients with at least one lifetime suicide attempt but not within the last six months (Remote-SA; n = 11), and currently depressed patients with lifetime suicidal ideation but no lifetime history of suicide attempts (Lifetime-SI only; n = 12).
The Recent-SA group included only hospitalized patients in the psychiatric inpatients. They were hospitalized for a recent suicide attempt (within 3 days) of moderate–high intent and lethality as defined by a score of ≥2 in the actual lethality/medical damage subscale of the Columbia Suicide Severity Rating Scale (C-SSRS) (Posner et al., 2011). The Remote-SA and Lifetime-SI only groups included a combination of hospitalized patients and patients from outpatient clinics.
All subjects fulfilled DSM-IV-TR criteria for major depressive episode and either MDD, bipolar disorder, or depression not otherwise specified. Duration of disease or number of recurrences were not inclusion criteria for this study. Exclusion criteria were the following: inability to read, write and speak English; inability to provide informed consent; history of dementia, neurovascular or neurodegenerative conditions; current pain of any kind; opioid use within the last month; history of non-suicidal self-harm; undergoing alcohol, benzodiazepine, opioid or barbiturate withdrawal; non-removable ferromagnetic objects; history of claustrophobia; positive pregnancy test; and involuntary hospitalization. Our group and others have previously used this empirical six-month cut-off for remote suicidal behavior in order to identify cognitive and physiological changes associated with recent suicidal behavior (Caceda et al., 2017; Carbajal et al., 2017; Cáceda et al., 2014; Gibbs et al., 2016; van Heeringen et al., 2017). The UAMS IRB approved all procedures, and written informed consents were obtained from all the study participants.
3.2. Study 2 procedure
Recruitment and study procedures were described in detail previously (Caceda et al., 2018). After written informed consent, participants underwent an interview to obtain demographic data, behavioral ratings, and psychiatric and medical histories. Psychiatric diagnoses were established with the SCID. The C-SSRS and Beck Depression Inventory (BDI-II) (Beck et al., 1996) were used to characterize suicidal ideation and behavior and depression severity, respectively.
3.3. MRI acquisition and analysis
Imaging data were acquired using a Philips 3T Achieva X-series MRI scanner (Philips Healthcare, Eindhoven, The Netherlands). Anatomic images were acquired with 3D TFE sequence (matrix = 256 × 256, 220 sagittal slices, TR/TE/FA = shortest/shortest/8°, final resolution =0.94 × 0.94 × 1 mm3 resolution). The raw structural images were processed with FreeSurfer 5.3.0 (http://surfer.nmr.mgh.harvard.edu/) to extract cortical thickness, surface area, and subcortical gray matter volume from the Desikan-Killiany atlas (Desikan et al., 2006) (same as Study 1). The same ROIs from Study 1 were investigated.
3.4. Statistical analyses
Univariate linear regressions were performed for a priori ROI cortical thickness and surface area, and subcortical volume. Mean differences between Recent-SA, Remote SA and Lifetime-SI only were compared with ANCOVA. For the univariate linear regressions, age, depression severity, and education were factored in as covariates. The false discovery rate was adjusted (p-FDR ≤ 0.10) using the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995). All analyses were conducted using SPSS 26 (SPSS Inc., Chicago, IL, USA).
4. Study 3 methods (EMBARC + little rock datasets)
4.1. Participants
Study 3 presents two analyses of pooled data from two studies (total N = 223) and included adults of both sexes, ages 18–65 years. In the first analysis within Study 3, four groups were compared: Recent-SA (n = 11); Remote-SA (n = 32); Lifetime-SI only (n = 84); and non-suicidal depressed (n = 96). The Recent-SA group included only patients from the Little Rock dataset (Study 2), whereas the Remote-SA, Lifetime-SI only and non-suicidal depressed groups pooled participants from the EMBARC dataset in Study 1 and from the Little Rock dataset in Study 2. The Remote-SA group included currently depressed patients with at least one lifetime suicide attempt but not within the last six months. Because the Recent-SA group solely contained participants from the Little Rock dataset, in the second analysis within Study 3, a Lifetime-SA group was created that pooled the Remote-SA and Recent-SA groups to allow for representation from both datasets within in all groups (Lifetime-SA, Lifetime-SI only, and non-suicidal depressed). The ComBat technique was then run on this rearranged dataset as it is unlikely that it could have completely harmonized the effect of all participants in one group originating from a sole site. After regrouping the two SA groups, the ComBat technique was performed.
4.2. Statistical analyses
Descriptive statistics were calculated. Univariate linear regressions were performed for the a priori ROI cortical thickness, surface area, and subcortical volume. Mean differences between the four groups: Recent-SA, Remote-SA, Lifetime-SI only and non-suicidal depressed were compared with ANCOVA. We used univariate linear regressions with age, depression severity, education, and imaging sites factored in as covariates, followed by pairwise comparisons and adjusted for multiple comparisons using the Bonferroni correction. Depression severity was classified as mild, moderate and severe. For the HAM-D, a score of 9–16 was categorized as mild, 17–23 as moderate, and 24+ as severe. For the BDI-II, a score of 14–19 was categorized as mild, 20–28 as moderate, and 29+ as severe. Since two depression scales (HAM-D for the EMBARC dataset and BDI-II for the Arkansas dataset) were used, a non-parametric test was run for the 3 different depression severity ranks using Kruskal-Wallis H method. The false discovery rate was adjusted (p-FDR ≤ 0.10) using the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995). A second ANCOVA was run with the same covariates as the first ANCOVA to compare mean differences between three groups: Lifetime-SA (Recent SA + Remote SA), Lifetime-SI only and non-suicidal depressed after the data was harmonized with the ComBat technique. All analyses were conducted using SPSS 26 (SPSS Inc., Chicago, IL, USA).
5. Study 1 results
Significantly higher depression severity (HDRS-17) was observed in the Remote-SA group as compared to the Lifetime-SI only group (t = 3.520; p=.001) and to the non-suicidal depressed group (t = 3.594; p<.001) (Table 1). No differences in cortical thickness, surface area, or subcortical gray volume matter were observed between the three groups (Table 4). No significant correlations were observed between the number of suicide attempts and months since last suicide attempt and structural changes in the ROIs.
Table 1.
Demographic and clinical characteristics of the EMBARC study sample analyzed in Study 1.
| EMBARC dataset N = 187 | Remote-SA (n = 21) Mean ± SD | Lifetime-SI only (n = 72) Mean ± SD | Depressed (n = 94) Mean ± SD | p-value |
|---|---|---|---|---|
| Age | 39.19 ± 12.54 | 33.71 ± 12.04 | 38.19 ± 13.44 | p=.053 |
| Gender (female%) | 14 (66.7%) | 51 (70.8%) | 55 (58.5%) | p=.252 |
| Education (years) | 15.17 ± 2.61 | 15.06 ± 2.30 | 14.96 ± 2.96 | p=.937 |
| Number of suicide attempts | 1.58 ± 0.96 | N/A | N/A | |
| Ethnicity (Caucasian%) | 16 (76.2%) | 50 (69.4%) | 60 (63.8%) | p=.822 |
| Depression HAM-D | 22.10 ± 3.97 | 18.43 ± 4.32 | 18.62 ± 3.87 | p=.001 |
| HAM-D score | p=.001 | |||
| Remote-SA vs. Lifetime-SI only | ||||
| HAM-D score | p=.001 | |||
| Remote-SA vs. Depressed | ||||
| HAM-D score | p=.770 | |||
| Lifetime-SI only vs. Depressed | ||||
| Lifetime Diagnosis | ||||
| Anxiety Disorder | 0 (0%) | 0 (0%) | 0 (0%) | |
| Bipolar Disorder | 0 (0%) | 0 (0%) | 0 (0%) | |
| Panic Disorder | 4 (19.0%) | 18 (25.4%) | 19 (20.4%) | p=.721 |
| Posttraumatic Stress Disorder | 8 (38.1%) | 16 (22.2%) | 26 (27.7%) | p=.337 |
| Substance Abuse | 7 (33.3%) | 16 (22.2%) | 28 (29.8%) | p=.446 |
HAM-D: Hamilton Depression Inventory; SA: suicide attempters; SI: suicide ideators.
Table 4.
Comparison of brain structures between Remote-SA, Lifetime-SI only, and non-suicidal depressed analyzed in Study 1 with ComBat harmonization.
| Region of Interest | Remote-SA (n = 21) Mean ± SD | Lifetime-SI only (n = 72) Mean ± SD | Depressed (n = 94) Mean ± SD | p-value adjusted |
|---|---|---|---|---|
| Cortical thickness | ||||
| Caudal ACC | 4.96 ± 0.45 | 5.22 ± 0.39 | 5.10 ± 0.37 | p=.163 |
| IFG | 15.39 ± 0.73 | 15.84 ± 0.90 | 15.67 ± 0.88 | p=.499 |
| Inferior temporal | 5.53 ± 0.29 | 5.67 ± 0.38 | 5.63 ± 0.26 | p=.465 |
| Insula | 5.96 ± 0.29 | 6.05 ± 0.36 | 6.02 ± 0.32 | p=.721 |
| Lateral OFC | 5.22 ± 0.32 | 5.39 ± 0.33 | 5.34 ± 0.29 | p=.575 |
| Medial OFC | 4.74 ± 0.37 | 4.90 ± 0.35 | 4.87 ± 0.29 | p=.385 |
| Middle temporal | 5.68 ± 0.24 | 5.81 ± 0.35 | 5.82 ± 0.32 | p=.118 |
| Superior frontal | 5.32 ± 0.30 | 5.51 ± 0.32 | 5.46 ± 0.36 | p=.180 |
| Superior parietal | 4.16 ± 0.23 | 4.34 ± 0.25 | 4.28 ± 0.26 | p=.098 |
| Superior temporal | 5.45 ± 0.28 | 5.68 ± 0.35 | 5.61 ± 0.34 | p=.161 |
| Surface area | ||||
| Caudal ACC | 1361.02 ± 232.94 | 1385.68 ± 267.82 | 1390.49 ± 228.09 | p=.805 |
| IFG | 7043.28 ± 608.77 | 7049.91 ± 858.15 | 7122.42 ± 850.38 | p=.461 |
| Inferior temporal | 6042.10 ± 701.65 | 6200.07 ± 823.18 | 6114.38 ± 852.41 | p=.691 |
| Insula | 4296.49 ± 431.42 | 4300.67 ± 467.82 | 4388.45 ± 481.65 | p=.344 |
| Lateral OFC | 4969.45 ± 406.97 | 4967.83 ± 552.57 | 5050.15 ± 640.72 | p=.312 |
| Medial OFC | 3590.52 ± 438.23 | 3481.19 ± 420.08 | 3567.48 ± 439.26 | p=.395 |
| Middle temporal | 6270.01 ± 716.28 | 6160.69 ± 783.14 | 6266.21 ± 788.10 | p=.416 |
| Superior frontal | 13,937.37 ± 1238.26 | 13,640.99 ± 1526.56 | 13,857.16 ± 1549.99 | p=.399 |
| Superior parietal | 10,745.24 ± 1127.78 | 10,389.64 ± 1220.73 | 10,504.54 ± 1141.43 | p=.356 |
| Superior temporal | 7276.52 ± 761.19 | 7122.71 ± 782.95 | 7173.04 ± 786.20 | p=.794 |
| Subcortical volume | ||||
| Caudate | 6929.61 ± 679.74 | 7253.30 ± 1076.68 | 7069.78 ± 1020.22 | p=.281 |
| Hippocampus | 8709.81 ± 616.48 | 8558.47 ± 883.98 | 8624.69 ± 796.66 | p=.741 |
| Putamen | 10,194.39 ± 1542.83 | 10,897.33 ± 1420.78 | 10,545.93 ± 1434.16 | p=.412 |
| Thalamus | 15,604.80 ± 1298.98 | 16,087.51 ± 1759.06 | 16,060.78 ± 1958.85 | p=.414 |
| Nucleus Accumbens | 1103.83 ± 189.46 | 1114.61 ± 192.53 | 1085.16 ± 215.35 | p=.921 |
ACC: anterior cingulate cortex; IFG: inferior frontal gyrus; OFC = orbitofrontal cortex.
Cortical thickness is expressed in millimeters; Surface area is expressed in millimeters2; Volume is expressed in millimeters3.
6. Study 2 results
There were no significant differences in depression severity (BDI-II) between the three patient groups (p=.069) (Table 2). The Remote-SA group had higher depression severity as compared to Lifetime-SI only group (t = 2.668; p=.014). No differences in cortical thickness, surface area, or subcortical gray volume matter were observed between the three groups (Table 5). There were no significant correlations between the number of suicide attempts and months since last suicide attempt and the ROI structural changes.
Table 2.
Demographic and clinical characteristics of the Little Rock study sample analyzed in Study 2.
| Little Rock dataset N = 34 | Recent-SA (n = 11) Mean ± SD | Remote-SA (n = 11) Mean ± SD | Lifetime-SI only (n = 12) Mean ± SD | p-value |
|---|---|---|---|---|
| Age | 34.09 ± 10.39 | 36.00 ± 11.34 | 37.83 ± 14.12 | p=.763 |
| Gender (female%) | 9 (81.8%) | 7 (63.6%) | 7 (58.3%) | p=.277 |
| Education (years) | 12.82 ± 1.25 | 13.32 ± 2.17 | 13.88 ± 1.93 | p=.394 |
| Number of suicide attempts | 2.73 ± 2.15 | 1.40 ± 0.70 | N/A | p=.078 |
| Ethnicity (Caucasian%) | 9 (81.8%) | 8 (80%) | 10 (83.3%) | p=.980 |
| Antidepressant (yes%) | 100% | 100% | 100% | |
| Depression BDI-II | 36.18 ± 11.91 | 39.09 ± 7.79 | 29.75 ± 8.90 | p=.074 |
| Depression score | Recent-SA vs. Remote-SA | p=.505 | ||
| BDI-II | ||||
| Depression score | Recent-SA vs. Lifetime-SI only | p=.155 | ||
| BDI-II | ||||
| Depression score | Remote-SA vs. Lifetime-SI only | p=.014 | ||
| BDI-II | ||||
| Lifetime Diagnosis | ||||
| Anxiety Disorder | 1 (0.09%) | 1 (9.1%) | 1 (9.1%) | p=.616 |
| Bipolar Disorder | 2 (22.2%) | 1 (9.1%) | 2 (18.2%) | p=.710 |
| Panic Disorder | 0 (0%) | 2 (18.2%) | 1 (9.1%) | p=.391 |
| Posttraumatic Stress Disorder | 4 (44.4%) | 2 (18.2%) | 4 (36.4%) | p=.429 |
| Substance Abuse | 2 (22.2%) | 0 (0%) | 1 (9.1%) | p=.246 |
BDI-II: Beck Depression Inventory; SA: suicide attempters; SI: suicide ideators.
Table 5.
Comparison of brain structures between Recent-SA, Remote-SA, and Lifetime-SI only analyzed in Study 2.
| Region of Interest | Recent-SA (n = 11) Mean ± SD | Remote-SA (n = 11) Mean ± SD | Lifetime-SI (n = 12) Mean ± SD | p-value adjusted |
|---|---|---|---|---|
| Cortical thickness | ||||
| Caudal ACC | 5.40 ± 0.23 | 5.40 ± 0.47 | 5.29 ± 0.56 | p=.818 |
| IFG | 15.00 ± 0.51 | 14.94 ± 1.25 | 14.52 ± 1.08 | p=.822 |
| Inferior temporal | 5.06 ± 0.25 | 5.15 ± 0.39 | 5.02 ± 0.39 | p=.760 |
| Insula | 6.00 ± 0.24 | 5.99 ± 0.44 | 5.86 ± 0.37 | p=.943 |
| Lateral OFC | 5.02 ± 0.24 | 5.11 ± 0.42 | 4.96 ± 0.32 | p=.736 |
| Medial OFC | 4.62 ± 0.23 | 4.81 ± 0.46 | 4.66 ± 0.30 | p=.345 |
| Middle temporal | 5.38 ± 0.28 | 5.49 ± 0.39 | 5.17 ± 0.36 | p=.364 |
| Superior frontal | 5.42 ± 0.24 | 5.31 ± 0.42 | 5.18 ± 0.36 | p=.419 |
| Superior parietal | 4.32 ± 0.19 | 4.35 ± 0.32 | 4.01 ± 0.31 | p=.024 |
| Superior temporal | 5.39 ± 0.25 | 5.45 ± 0.38 | 5.24 ± 0.28 | p=.592 |
| Surface area | ||||
| Caudal ACC | 1359.36 ± 209.15 | 1530.45 ± 337.42 | 1417.67 ± 396.03 | p=.430 |
| IFG | 6266.36 ± 671.27 | 7062.18 ± 1188.93 | 7056.17 ± 933.93 | p=.141 |
| Inferior temporal | 5878.91 ± 639.32 | 6323.64 ± 1250.79 | 6070.75 ± 914.19 | p=.411 |
| Insula | 3747.09 ± 370.48 | 4021.91 ± 577.48 | 4290.58 ± 629.89 | p=.260 |
| Lateral OFC | 4682.55 ± 463.46 | 4900.55 ± 872.49 | 4988.33 ± 688.79 | p=.806 |
| Medial OFC | 3307.73 ± 390.57 | 3488.18 ± 653.03 | 3620.25 ± 462.98 | p=.566 |
| Middle temporal | 5845.55 ± 573.91 | 6262.09 ± 1230.60 | 6197.25 ± 875.65 | p=.589 |
| Superior frontal | 12,914.64 ± 1174.78 | 13,530.45 ± 2093.53 | 14,231.25 ± 1654.05 | p=.458 |
| Superior parietal | 9787.27 ± 1280.46 | 10,564.73 ± 1155.82 | 10,661.00 ± 1338.10 | p=.322 |
| Superior temporal | 6611.27 ± 381.47 | 7048.45 ± 1146.12 | 7254.33 ± 878.86 | p=.367 |
| Subcortical volume | ||||
| Caudate | 7257.12 ± 599.28 | 7188.78 ± 1207.95 | 7426.52 ± 1138.37 | p=.995 |
| Hippocampus | 7604.11 ± 578.20 | 7723.02 ± 1149.66 | 8049.52 ± 958.42 | p=.779 |
| Putamen | 11,098.31 ± 802.74 | 11,931.23 ± 1749.28 | 11,637.27 ± 1600.05 | p=.212 |
| Thalamus | 12,944.33 ± 864.88 | 13,390.44 ± 2022.57 | 13,941.46 ± 2344.56 | p=.498 |
| Nucleus Accumbens | 1411.95 ± 206.48 | 1406.87 ± 283.40 | 1420.84 ± 266.07 | p=.967 |
ACC: anterior cingulate cortex; IFG: inferior frontal gyrus; OFC = orbitofrontal cortex.
Cortical thickness is expressed in millimeters; Surface area is expressed in millimeters2; Volume is expressed in millimeters3.
7. Study 3 results
Data was analyzed for a total of 223 currently depressed patients. Forty-three patients had a lifetime history of suicide attempts, of which, 11 attempted suicide within the last three days, and 32 had remote lifetime suicide attempts. There were 84 patients with lifetime suicidal ideation, but no lifetime suicide attempts, and 96 depressed patients with no lifetime history of suicidal ideation or attempts. Table 3 presents the demographic and clinical characteristics of the study samples. The EMBARC baseline scan data from medication-free patients was analyzed. All the patients in the Little Rock study were taking antidepressants. The results showed that there was a statistically significant difference in depression severity between the 4 groups, χ2(2) = 11.511, p=.0009. The mean rank depression severity score was the highest for the Remote-SA group (141.33), then the Recent-SA group (123.59), followed by the Lifetime-SI only group (107.43), and finally the non-suicidal depression group (104.90).
Table 3.
Demographic and clinical characteristics of both study samples analyzed in Study 3.
| Combined EMBARC and Little Rock study samples N = 223 | Recent-SA (n = 11) Mean ± SD | Remote-SA (n = 32) Mean ± SD | Lifetime-SI (n = 84) Mean ± SD | Depressed (n = 96) Mean ± SD | p-value |
|---|---|---|---|---|---|
| Age | 34.09 ± 10.39 | 38.09 ± 12.06 | 34.30 ± 12.35 | 38.61 ± 13.62 | p=.118 |
| Gender (female %) | 9 (81.8%) | 21 (65.6%) | 58 (69.0%) | 55 (57.3%) | p=.225 |
| Education (years) | 12.82 ± 1.25 | 14.53 ± 2.59 | 14.89 ± 2.28 | 14.96 ± 2.94 | p=.070 |
| Number of suicide attempts | 2.73 ± 2.15 | 1.52 ± 0.87 | N/A | N/A | p=.097 |
| Ethnicity (Caucasian%) | 9 (81.8%) | 24 (77.4%) | 60 (71.4%) | 60 (64.2%) | p=.899 |
| Antidepressant-only Little Rock sample (yes%) | 100% | 34.4% | 14.3% | 2.1% | p<.001 |
| Depression severity | 123.59 | 141.33 | 107.43 | 104.90 | p=.009* |
Kruskal-Wallis H test was used for depression severity ranking (BDI-II and HAM-D).
The FDR multiple comparison correction method was used (n = 25 ROI), so all p-values are reported after adjusting for multiple comparison correction (threshold p<.02). Significantly lower cortical thickness middle temporal gyri (F = 5.19; p=.002) (Fig. 1A) and a trend towards significance in the inferior temporal (F = 3.27; p=.022) were observed in the Recent-SA group compared to all of the other groups (see Table 6). Also, significantly smaller surface area of the insula (F = 4.74; p=.003) (Fig. 1B) was observed in the Recent-SA group (Table 6) relative to the other groups. Significantly smaller volume of the thalamus (F = 4.86; p=.003) (Fig. 1C) and larger volume of the nucleus accumbens (F = 3.61; p=.014) (Fig. 1D) were observed in the Recent-SA group as compared to the other groups (See Table 6). There were no significant correlations between cortical thickness, surface area, or volume with the number of lifetime suicide attempts or months since last attempt. Combining the Remote-SA and Recent-SA groups into a Lifetime-SA group did not yield any significant results when compared to Lifetime-SI only and non-suicidal depression groups after ComBat harmonization (Table 7).
Fig. 1.
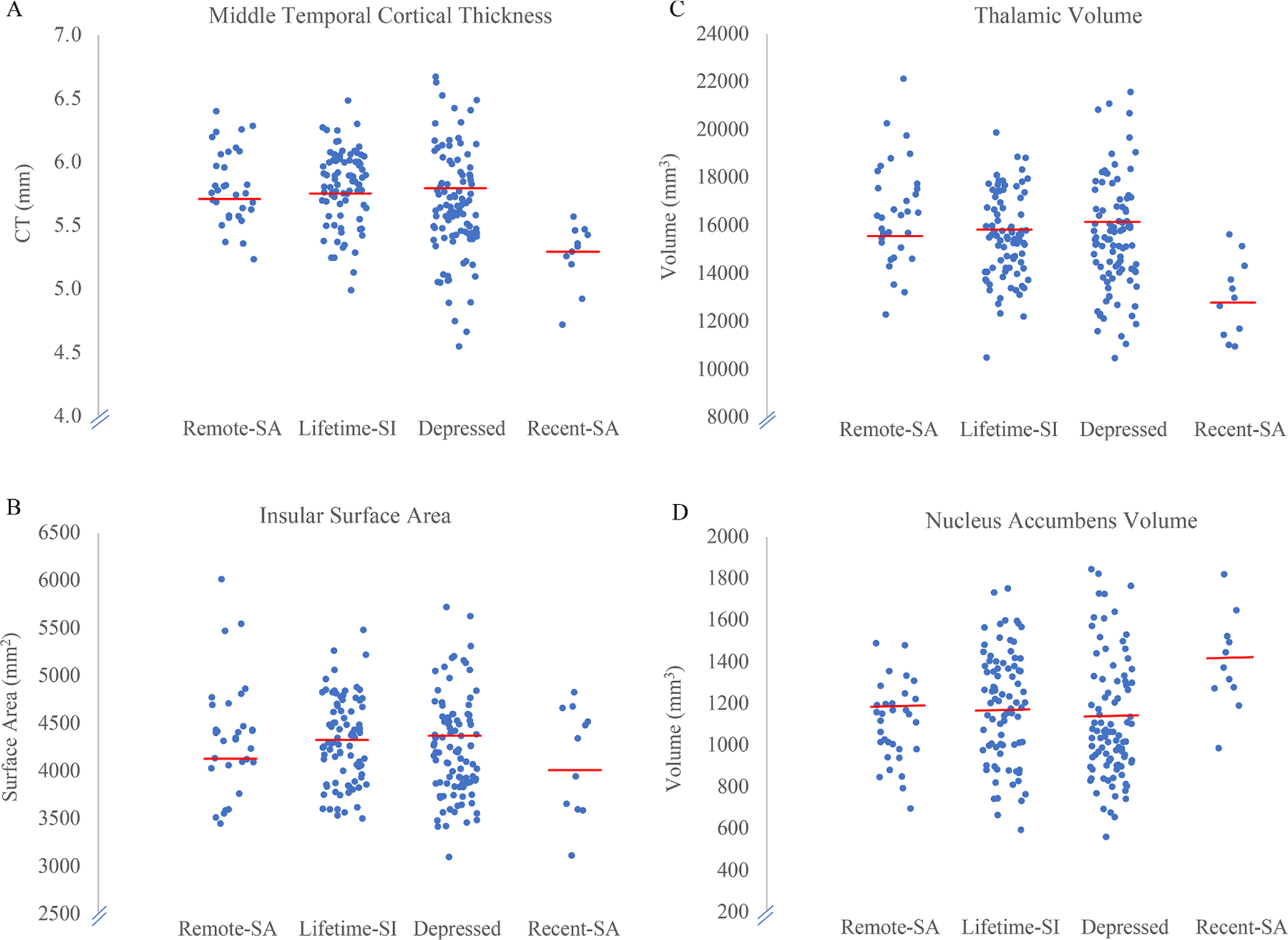
Scatterplots of cortical thickness, surface area, and volume in the 4 groups. A) Lower middle temporal cortical thickness was observed in the Recent-SA and Lifetime-SI only groups as compared to the non-suicidal depressed group. B) Lower insular surface area was observed in the Recent-SA group as compared to the Lifetime-SI only and non-suicidal depressed groups. C) Lower thalamic volume was observed in the Recent-SA group as compared to the Lifetime-SI only and non-suicidal depressed groups. D) Higher volume in the nucleus accumbens was observed in the Recent-SA group as compared to the Lifetime-SI only and non-suicidal depressed groups.
Table 6.
Comparison of brain structures between Recent-SA, Remote-SA, Lifetime-SI only, and non-suicidal depressed analyzed in Study 3 (EMBARC + Little Rock combined).
| Region of Interest | Recent-SA (n = 11) Mean ± SD | Remote-SA (n = 32) Mean ± SD | Lifetime-SI (n = 84) Mean ± SD | Depressed (n = 96) Mean ± SD | p-value adjusted |
|---|---|---|---|---|---|
| Cortical Thickness | |||||
| Caudal ACC | 5.40 ± 0.23 | 5.10 ± 0.52 | 5.23 ± 0.44 | 5.11 ± 0.35 | p=.224 |
| IFG | 15.00 ± 0.51 | 15.22 ± 0.92 | 15.61 ± 1.04 | 15.62 ± 0.95 | p=.048 |
| Inferior temporal | 5.06 ± 0.25b | 5.39 ± 0.39 | 5.59 ± 0.45 | 5.61 ± 0.28b | p=.022 # |
| Insula | 6.00 ± 0.24 | 5.96 ± 0.36 | 6.03 ± 0.38 | 6.02± 0.33 | p=.822 |
| Lateral OFC | 5.02 ± 0.24 | 5.17 ± 0.35 | 5.32 ± 0.37 | 5.32 ± 0.35 | p=.374 |
| Medial OFC | 4.62 ± 0.23 | 4.75 ± 0.43 | 4.86 ± 0.35 | 4.86 ± 0.34 | p=.749 |
| Middle temporal | 5.38 ± 0.28b | 5.61 ± 0.31 | 5.72 ± 0.4d | 5.80 ± 0.33b,d | p=.002 * |
| Superior frontal | 5.42 ± 0.24 | 5.30 ± 0.34 | 5.46 ± 0.34 | 5.45 ± 0.36 | p=.218 |
| Superior parietal | 4.32± 0.19 | 4.22 ± 0.29 | 4.30 ± 0.29 | 4.28 ± 0.26 | p=.617 |
| Superior temporal | 5.39 ± 0.25 | 5.44 ± 0.33 | 5.62 ± 0.38 | 5.60 ± 0.35 | p=.247 |
| Surface area | |||||
| Caudal ACC | 1359.36 ± 209.15 | 1416.56 ± 293.22 | 1391.63 ± 286.14 | 1390.08 ± 235.99 | p=.914 |
| IFG | 6266.36 ± 671.27 | 7027.56 ± 862.52 | 7059.37 ± 885.63 | 7048.59 ± 862.42 | p=.088 |
| Inferior temporal | 5844.55 ± 638.42 | 6135.34 ± 944.01 | 6184.64 ± 830.98 | 6124.66 ± 897.57 | p=.494 |
| Insula | 3682.82 ± 289.84a,b | 4192.50 ± 497.88 | 4295.49 ± 512.30a | 4373.02 ± 480.52b | p=.003 * |
| Lateral OFC | 4626.91 ± 469.19 | 4927.97 ± 611.31 | 4975.86 ± 579.66 | 5047.33 ± 636.58 | p=.246 |
| Medial OFC | 3252.18 ± 404.17 | 3543.59 ± 508.88 | 3507.33 ± 436.77 | 3563.06 ± 432.95 | p=.277 |
| Middle temporal | 5773.45 ± 575.01 | 6246.44 ± 945.15 | 6180.11 ± 798.68 | 6263.04 ± 783.26 | p=.314 |
| Superior frontal | 12,837.00 ± 1180.85 | 13,752.19 ± 1555.43 | 13,742.50 ± 1608.75 | 13,836.16 ± 1536.50 | p=.541 |
| Superior parietal | 9522.36 ± 1178.00 | 10,661.29 ± 1182.44 | 10,433.12 ± 1238.30 | 10,523.22 ± 1137.02 | p=.092 |
| Superior temporal | 6544.45 ± 350.20 | 7174.16 ± 900.24 | 7150.95 ± 814.10 | 7168.88 ± 752.90 | p=.172 |
| Subcortical volume | |||||
| Caudate | 7257.12 ± 599.28 | 7007.24 ± 966.71 | 7304.42 ± 1136.52 | 7073.77 ± 1067.36 | p=.499 |
| Hippocampus | 7604.11 ± 578.20 | 8351.74 ± 929.84 | 8493.43 ± 940.24 | 8600.43 ± 817.52 | p=.192 |
| Putamen | 11,098.31 ± 802.74 | 10,778.74 ± 1890.76 | 11,045.56 ± 1622.42 | 10,556.02 ± 1601.43 | p=.898 |
| Thalamus | 12,944.33 ± 864.88a,b | 14,805.66 ± 1906.41 | 15,740.29 ± 2144.77a | 15,951.06 ± 2060.73b | p=.003 * |
| Nucleus Accumbens | 1411.95 ± 206.48a,b | 1205.84 ± 302.87 | 1163.60 ± 261.04a | 1088.16 ± 247.88b | p=.014 * |
ACC: anterior cingulate cortex; IFG: inferior frontal gyrus; OFC = orbitofrontal cortex.
Cortical thickness is expressed in millimeters; Surface area is expressed in millimeters2. Volume is expressed in millimeters3.
Statistically significant.
Trending towards significance.
Statistically significant between Recent-SA and Lifetime-SI.
Statistically significant between Recent-SA and Depressed.
Statistically significant between Remote-SA and Depressed.
Statistically significant between Lifetime-SI and Depressed.
p-FDR ≤ 0.10.
Table 7.
Comparison of brain structures between Lifetime-SA, Lifetime-SI only, and non-suicidal depressed analyzed in Study 3 (EMBARC + Little Rock combined) with ComBat harmonization.
| Region of Interest | Remote-SA (n = 43) Mean ± SD | Lifetime-SI only (n = 84) Mean ± SD | Depressed (n = 96) Mean ± SD | p-value adjusted |
|---|---|---|---|---|
| Cortical thickness | ||||
| Caudal ACC | 5.09 ± 0.40 | 5.23 ± 0.41 | 5.13 ± 0.37 | p=.506 |
| IFG | 15.43 ± 0.82 | 15.63 ± 0.93 | 15.52 ± 0.90 | p=.574 |
| Inferior temporal | 5.51 ± 0.31 | 5.58 ± 0.38 | 5.54 ± 0.26 | p=.675 |
| Insula | 6.01 ± 0.31 | 6.03 ± 0.37 | 6.00 ± 0.33 | p=.696 |
| Lateral OFC | 5.23 ± 0.31 | 5.32 ± 0.33 | 5.28 ± 0.30 | p=.764 |
| Medial OFC | 4.77 ± 0.35 | 4.86 ± 0.34 | 4.84 ± 0.30 | p=.542 |
| Middle temporal | 5.70 ± 0.29 | 5.72 ± 0.36 | 5.74 ± 0.33 | p=.113 |
| Superior frontal | 5.38 ± 0.32 | 5.46 ± 0.33 | 5.43 ± 0.36 | p=.251 |
| Superior parietal | 4.25 ± 0.25 | 4.30 ± 0.28 | 4.27 ± 0.26 | p=.921 |
| Superior temporal | 5.51 ± 0.30 | 5.61 ± 0.35 | 5.57 ± 0.35 | p=.495 |
| Surface area | ||||
| Caudal ACC | 1371.28 ± 251.01 | 1162.88 ± 203.17 | 1125.57 ± 217.07 | p=.841 |
| IFG | 6927.52 ± 817.05 | 7038.82 ± 861.42 | 7087.43 ± 843.53 | p=.320 |
| Inferior temporal | 6075.48 ± 829.22 | 6178.43 ± 829.15 | 6119.39 ± 854.77 | p=.797 |
| Insula | 4152.77 ± 459.01 | 4289.09 ± 493.14 | 4348.21 ± 476.62 | p=.070 |
| Lateral OFC | 4913.73 ± 554.84 | 4965.78 ± 566.72 | 5024.73 ± 633.27 | p=.277 |
| Medial OFC | 3507.06 ± 483.29 | 3495.02 ± 424.21 | 3556.73 ± 436.55 | p=.340 |
| Middle temporal | 6172.69 ± 823.95 | 6159.76 ± 779.49 | 6255.30 ± 785.23 | p=.383 |
| Superior frontal | 13,580.99 ± 1477.36 | 13,710.54 ± 1533.50 | 13,831.17 ± 1536.39 | p=.395 |
| Superior parietal | 10,428.76 ± 1212.38 | 10,412.48 ± 1223.01 | 10,507.64 ± 1134.17 | p=.621 |
| Superior temporal | 7118.20 ± 788.14 | 7130.85 ± 805.46 | 7140.55 ± 784.77 | p=.688 |
| Subcortical volume | ||||
| Caudate | 7086.65 ± 791.64 | 7275.51 ± 1070.00 | 7076.90 ± 1025.47 | p=354 |
| Hippocampus | 8440.46 ± 748.83 | 8475.14 ± 884.62 | 8504.50 ± 793.66 | p=.741 |
| Putamen | 10,517.63 ± 1147.51 | 11,018.29 ± 1412.51 | 10,690.82 ± 1433.49 | p=.348 |
| Thalamus | 15,176.89 ± 1386.73 | 1571.61 ± 1825.32 | 15,657.08 ± 1946.97 | p=.122 |
| Nucleus Accumbens | 7043.28 ± 608.77 | 7049.91 ± 858.15 | 7122.42 ± 850.38 | p=.461 |
ACC: anterior cingulate cortex; IFG: inferior frontal gyrus; OFC = orbitofrontal cortex.
Cortical thickness is expressed in millimeters; Surface area is expressed in millimeters2; Volume is expressed in millimeters3.
8. Discussion
The goal of the present study was to examine brain structures to gain insight into the neurobiological mechanisms underlying remote and recent suicidal behavior. Study 1, based on currently depressed patients from the EMBARC study, did not find differences in brain structure between Remote-SA, Lifetime-SI only and non-suicidal depressed groups. In Study 2, we aimed to examine brain structural changes in acute suicide risk between Recent-SA, Remote-SA, and Lifetime-SI only and also did not observe any differences. In Study 3, we combined both samples and found decreased cortical thickness in the middle temporal gyrus, decreased insular surface area, as well as smaller thalamic volume and larger volume in the nucleus accumbens in depressed patients after a recent suicide attempt (Recent-SA) as compared to all the other groups.
Reduced thicknesses in the middle temporal and inferior temporal gyri were observed in the Recent-SA group. The middle and inferior temporal gyri are associated with language processing, semantic memory (Chao et al., 1999), and visual perception (Ishai et al., 1999), respectively. The inferior and superior temporal gyri underlie sensory integration and visceral reactions to emotional stimuli (Drevets et al., 2008). A predisposition for suicidal behavior may arise when these impaired processes result in a negative information bias, leading to worsening suicidal thoughts including mental imagery (Crane et al., 2012; Holmes et al., 2007) and suicidal planning (van Heeringen et al., 2014). Abnormal resting state brain activity in the middle temporal and superior temporal gyri has been associated with impulsivity in young adults with a history of suicide attempts (Cao et al., 2016). We previously described increased transient impulsivity in patients hospitalized for recent suicide attempts (Cáceda et al., 2014). Noteworthy, Jollant and collaborators found no structural differences between patients with a history of suicide attempts but described reduced volumes of temporal and dorsolateral prefrontal cortices and putamen in first-degree biological relatives of depressed individuals who died from suicide (Jollant et al., 2018). Altogether, these findings illustrate that structural brain alterations may be associated with a profile of impaired cognitive and behavioral tendencies that may drive susceptibilities for suicidal behavior.
Surface area of the insula was reduced in the Recent-SA group. While we did not observe structural alterations in the dACC, a proposed key region for mediating roles between suicidal thoughts to behavior, the IFG is a key region that interacts with the insula and dACC and may facilitate suicidal behavior (Schmaal et al., 2020). As such, impairment in these structures, which belong to the limbic system, are linked to impaired cognitive and decision-making processes associated with the transition from suicidal ideation to suicidal action.
We observed an increase in nucleus accumbens volume in the Recent-SA group. The nucleus accumbens is central for reward (Mogenson et al., 1980; Nestler and Carlezon, 2006) and has feedback projections to the frontal and temporal regions (Floresco, 2015). A recent meta-analysis found no differences in nucleus accumbens volume in patients with MDD as well as no associations between depression severity and volume (Schmaal et al., 2016). Gifuni and colleagues did not find an association between nucleus accumbens volume and suicidal behavior, however, they reported a negative correlation between nucleus accumbens volume and suicide attempt lethality (Gifuni et al., 2016). Of note, Gifuni and colleagues recruited euthymic participants with HDRS scores below 7 (Gifuni et al., 2016). In contrast, our sample from both datasets included patients with at least moderate depression severity. In our study, no relationship was found between nucleus accumbens volume and the highest rated suicide attempt lethality, however, the lack of lethality scores from both datasets limited our results. The nucleus accumbens is a key region for action selection guided by frontal-cognitive and temporal-emotional inputs (Floresco, 2015) and may modulate the decision and lethality for the suicide attempt.
Thalamic volume was smaller in the Recent-SA group. The thalamus is highly interconnected and plays a critical role in relaying sensory input from subcortical regions. It is at the center of cognitive, emotional, and motivational processes that guide goal-directed behaviors (Haber and Calzavara, 2009), memory, and attention (Aggleton et al., 2010; de Bourbon-Teles et al., 2014; Tekin and Cummings, 2002). Smaller thalamic volume has also been observed in patients with current depression (Nugent et al., 2013). On the other hand, increased thalamic volume was observed in depressed Veterans with mild traumatic brain injury and a history of suicidal behavior (Lopez-Larson et al., 2013). In a postmortem study, more neurons and larger thalamic volume were observed in patients who died by suicide (Young et al., 2008). However, others did not find a significant association between suicidal behavior and thalamic volume in depressed patients (Gifuni et al., 2016; Spoletini et al., 2011). The discrepancy in thalamic volume between our findings and others may be explained by the heterogeneity in patient populations, comorbidity, and focus on suicide attempts versus suicides.
Our results reveal a pattern of structural changes in brain regions belonging to the limbic system (i.e. middle temporal gyrus, nucleus accumbens and insula), as well as the thalamus in recent suicide attempters. Structural and functional connectivity between these brain regions have been previously described (Cauda et al., 2011; Craig and Zhang, 2006; Wiech et al., 2014; Xu et al., 2015). As mentioned above, similar alterations have been found in patients with a history of suicide attempts (Gosnell et al., 2016; Nugent et al., 2013; Schmaal et al., 2020). Structural abnormalities in the limbic system in recent suicide attempters compared to remote suicide attempters may point to the limbic system as a crucial element in the progression to suicidal behavior by disrupting cognitive and behavioral processes found in acutely suicidal patients, such as affect and pain regulation, reward, and self-reference (Conejero et al., 2018; DeVille et al., 2020; Downar et al., 2014; Ducasse et al., 2018; Jollant et al., 2011; Nock et al., 2010; Pan et al., 2013). The structural changes seen within two months of suicidal behavior (Colle et al., 2015; Gosnell et al., 2016), the changes in hippocampal volume after 4 weeks of electroconvulsive therapy (Abbott et al., 2014), and particularly, the changes in cortical thickness reported within a week of initiation of antidepressant treatment (Bartlett et al., 2018) support the case for brain structural changes in a short time span, as seen in recent suicide attempters.
Investigating neurobiological mechanisms of acute suicide risk remains a pressing need despite the difficulties associated with the inherent variability in suicide research. In a large meta-analysis, there were no subcortical volume differences in a subset analyses of MDD patients with suicidal behavior that included suicidal ideation, planning, or attempts (Rentería et al., 2017). However, out of their total sample of MDD patients, only 33% had suicidal planning, and 3% had a lifetime suicide attempt (Rentería et al., 2017). These findings illustrate the need to investigate characteristics associated with recent suicidal behavior in order to understand acute suicide risk. Although speculative, the dissimilarities in the findings may be due to a combination of differences including medication usage, depression severity, and active suicidal ideation. The elevated depression severity and suicidal behavior in the Little Rock patient sample necessitated psychiatric hospitalization. Although some patients with recent suicide attempts had high depression severity, some did not have active suicidal ideation at the time of the study. Some participants in the EMBARC study had mild active suicidal ideation. Previous reports show that after a suicide attempt, there is an improvement in depressive symptoms and suicidality (Jallade et al., 2005; Sarfati et al., 2003), in addition to reductions in risky behaviors such as impulsivity (Cáceda et al., 2014). Recently, our group and others have demonstrated the use of complex mathematical algorithms based on structural imaging and resting-state connectivity to identify risk for suicidal behavior (Caceda et al., 2018; Gosnell et al., 2019). Functional brain imaging or white matter imaging may provide promising indicators of brain plasticity attributable to acute suicide risk (Fields, 2010).
The main limitations of the study are that all the subjects of the Recent-SA group were medicated and recruited at one scanning site (Little Rock), and the sample size was small (n = 11). We anticipate that the interpretation of our findings regarding recent suicide attempters may be confounded by the study design (all recent attempters recruited in one site). As such, we controlled for multiple scan sites and for multiple ROIs. We performed ComBat harmonization as an additional statistical analysis for 3 groups (Lifetime-SA, Lifetime-SI only, and non-suicidal depressed groups). Nevertheless, after adjusting for FDR correction, the findings indicate structural alterations in the inferior and middle temporal gyri, insula, nucleus accumbens and thalamus. These structural findings in recent suicide attempters suggest that specific key regions may show considerable plasticity associated with acute suicide risk. Moreover, the patients in the EMBARC study were medication-free during their baseline imaging scans, while the patients in the Little Rock study were mostly medicated. Given the critical conditions for the hospitalized Little Rock patients, treatment could not have been with-held. Of note, the medicated patients were all acutely suicidal. Lastly, the three-day window for suicide attempts may be too short to observe structural changes in cortical thickness despite the structural changes seen within one week of initiation of antidepressant therapy (Bartlett et al., 2018). However, smaller frontal and temporal lobe volumes have been observed in depressed patients who had attempted suicide within the previous two months as compared to those with no suicide attempt history (Gosnell et al., 2016). Studies that replicate with larger acutely suicidal patients are warranted in order to evaluate high suicide risks whereby reducing the critical evaluation time between onset of suicidal behavior and post-suicidal behavior assessments.
Most previous studies have been performed in symptom-free patients, often months or years after engaging in suicidal behavior or experiencing suicidal thoughts. In this current study, we sought to examine the structural differences between individuals with recent and remote suicidal behavior and thoughts. As such, a perceived strength of this study was the inclusion of depressed patients within a few days of a suicide attempt (three days) to examine the underlying neurobiology of acute suicide risk. Additionally, we included a depressed control group of similar depression severity and compared them to suicide attempters and patients with suicide ideation, who presented at least moderately severe depression. Thus, the structural differences observed in suicide attempters seem to be specific to suicidal behavior rather than to depression. Future studies that include the characterization of recent suicidal behavior may reveal more accurately the underlying neurobiology of acute suicide risk and further advance our understanding of high-risk states.
Acknowledgements
This work was partially funded by the Clinician Scientist Program of the University of Arkansas for Medical Sciences, TRI NCATS UL1TR000039 and by NIGMS P30 GM110702. The EMBARC study data acquisition was supported by the National Institute of Mental Health of the National Institutes of Health under Award numbers U01MH092221 and U01MH092250. The funding sources had no role in this study design, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Declarations of Competing Interest
Ricardo Cáceda, MD, PhD received research funding from Neuronetics and Janssen Pharmaceuticals; Clint Kilts, PhD served as a member of a scientific advisory meeting for Allergan Pharmaceuticals and as a member of the national advisory board for Skyland Trail. He is also a co-holder of US Patent No. 6373, 990 (Method and device for the transdermal delivery of lithium). All the other authors declare no conflicts of interest.
References
- Abbott CC, Jones T, Lemke NT, Gallegos P, McClintock SM, Mayer AR, Bustillo J, Calhoun VD, 2014. Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry 4, e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Administration, S.A.a.M.H.S., 2019. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19–5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 19. [Google Scholar]
- Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT, 2010. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci 31, 2292–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar EJ, Garcia-Marti G, Marti-Bonmati L, Lull JJ, Moratal D, Escarti MJ, Robles M, Gonzalez JC, Guillamon MI, Sanjuan J, 2008. Left orbitofrontal and superior temporal gyrus structural changes associated to suicidal behavior in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 32, 1673–1676. [DOI] [PubMed] [Google Scholar]
- Bartlett EA, DeLorenzo C, Sharma P, Yang J, Zhang M, Petkova E, Weissman M, McGrath PJ, Fava M, Ogden RT, Kurian BT, Malchow A, Cooper CM, Trombello JM, McInnis M, Adams P, Oquendo MA, Pizzagalli DA, Trivedi M, Parsey RV, 2018. Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacology 43, 2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W, 1996. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 67, 588–597. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Radaelli D, Poletti S, Locatelli C, Falini A, Colombo C, Smeraldi E, 2011. Opposite effects of suicidality and lithium on gray matter volumes in bipolar depression. J Affect Disord 135, 139–147. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal statistical society: series B (Methodological) 57, 289–300. [Google Scholar]
- Caceda R, Bush K, James GA, Stowe ZN, Kilts CD, 2018. Modes of Resting Functional Brain Organization Differentiate Suicidal Thoughts and Actions: a Preliminary Study. J Clin Psychiatry 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceda R, Kordsmeier NC, Golden E, Gibbs HM, Delgado PL, 2017. Differential Processing of Physical and Psychological Pain during Acute Suicidality. Psychother Psychosom 86, 116–118. [DOI] [PubMed] [Google Scholar]
- Cao J, Chen X, Chen J, Ai M, Gan Y, Wang W, Lv Z, Zhang S, Wang S, Kuang L, Fang W, 2016. Resting-state functional MRI of abnormal baseline brain activity in young depressed patients with and without suicidal behavior. J Affect Disord 205, 252–263. [DOI] [PubMed] [Google Scholar]
- Carbajal JM, Gamboa JL, Moore J, Smith F, Eads L, Clothier J, Caceda R, 2017. Response to unfairness across the suicide risk spectrum. Psych Res 258, 365–373. [DOI] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A, 2011. Functional connectivity of the insula in the resting brain. Neuroimage 55, 8–23. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A, 1999. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci 2, 913–919. [DOI] [PubMed] [Google Scholar]
- Colle R, Chupin M, Cury C, Vandendrie C, Gressier F, Hardy P, Falissard B, Colliot O, Ducreux D, Corruble E, 2015. Depressed suicide attempters have smaller hippocampus than depressed patients without suicide attempts. J Psychiatr Res 61, 13–18. [DOI] [PubMed] [Google Scholar]
- Conejero I, Olíe E, Calati R, Ducasse D, Courtet P, 2018. Psychological Pain, Depression, and Suicide: recent Evidences and Future Directions. Curr Psychiatry Rep 20, 33. [DOI] [PubMed] [Google Scholar]
- Craig AD, Zhang ET, 2006. Retrograde analyses of spinothalamic projections in the macaque monkey: input to posterolateral thalamus. J Comp Neurol 499, 953–964. [DOI] [PubMed] [Google Scholar]
- Crane C, Shah D, Barnhofer T, Holmes EA, 2012. Suicidal imagery in a previously depressed community sample. Clin Psychol Psychother 19, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceda R, Durand D, Cortes E, Prendes S, Moskovciak TN, Harvey PD, Nemeroff CB, 2014. Impulsive choice and psychological pain in acutely suicidal depressed patients. Psychosom Med 76, 445–451. [DOI] [PubMed] [Google Scholar]
- de Bourbon-Teles J, Bentley P, Koshino S, Shah K, Dutta A, Malhotra P, Egner T, Husain M, Soto D, 2014. Thalamic control of human attention driven by memory and learning. Curr Biol 24, 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Śegonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- DeVille DC, Kuplicki R, Stewart JL, Tulsa I, Aupperle RL, Bodurka J, Cha YH, Feinstein J, Savitz JB, Victor TA, Paulus MP, Khalsa SS, 2020. Diminished responses to bodily threat and blunted interoception in suicide attempters. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Lawrence N, Olie E, Cyprien F, le Bars E, Bonafe A, Phillips ML, Courtet P, Jollant F, 2015. Prefrontal cortex markers of suicidal vulnerability in mood disorders: a model-based structural neuroimaging study with a translational perspective. Transl Psychiatry 5, e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H, 2012. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychol Med 42, 1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, Bakker N, Blumberger DM, Daskalakis ZJ, Kennedy SH, Flint AJ, Giacobbe P, 2014. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry 76, 176–185. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML, 2008. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213, 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dsm-Iv-tr A, 2000. Diagnostic and Statistical Manual of Mental disorders, Text Revision. American Psychiatric Association, Washington, DC. [Google Scholar]
- Du L, Zeng JK, Liu H, Tang DJ, Meng HQ, Li YM, Fu YX, 2017. Fronto-limbic disconnection in depressed patients with suicidal ideation: a resting-state functional connectivity study. J Affect Disord 215, 213–217. [DOI] [PubMed] [Google Scholar]
- Ducasse D, Holden RR, Boyer L, Artéro S, Calati R, Guillaume S, Courtet P, Olíe E, 2018. Psychological Pain in Suicidality: a Meta-Analysis. J Clin Psychiatry 79. [DOI] [PubMed] [Google Scholar]
- Dyer O, 2018. US suicide rate is climbing steadily with highest prevalence in sparsely populated western states. BMJ 361, k2586. [DOI] [PubMed] [Google Scholar]
- Fields RD, 2010. Neuroscience. change in the brain’s white matter. Science 330, 768–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS Jr, 1994. The young adult human brain: an MRI-based morphometric analysis. Cerebral cortex 4, 344–360. [DOI] [PubMed] [Google Scholar]
- Floresco SB, 2015. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol 66, 25–52. [DOI] [PubMed] [Google Scholar]
- Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, Adams P, Cooper C, Fava M, McGrath PJ, McInnis M, Phillips ML, Trivedi MH, Weissman MM, Shinohara RT, 2018. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 167, 104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs HM, Davis L, Han X, Clothier J, Eads LA, Caceda R, 2016. Association between C-reactive protein and suicidal behavior in an adult inpatient population. J Psychiatr Res 79, 28–33. [DOI] [PubMed] [Google Scholar]
- Gifuni AJ, Ding Y, Olíe E, Lawrence N, Cyprien F, Le Bars E, Bonafé A, Phillips ML, Courtet P, Jollant F, 2016. Subcortical nuclei volumes in suicidal behavior: nucleus accumbens may modulate the lethality of acts. Brain Imaging Behav 10, 96–104. [DOI] [PubMed] [Google Scholar]
- Gosnell SN, Fowler JC, Salas R, 2019. Classifying suicidal behavior with resting-state functional connectivity and structural neuroimaging. Acta Psychiatr Scand 140, 20–29. [DOI] [PubMed] [Google Scholar]
- Gosnell SN, Velasquez KM, Molfese DL, Molfese PJ, Madan A, Fowler JC, Christopher Frueh B, Baldwin PR, Salas R, 2016. Prefrontal cortex, temporal cortex, and hippocampus volume are affected in suicidal psychiatric patients. Psychiatry Res Neuroimaging 256, 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Calzavara R, 2009. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull 78, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J Neurol Neurosurg Psych 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes EA, Crane C, Fennell MJ, Williams JM, 2007. Imagery about suicide in depression–”Flash-forwards”? J Behav Ther Exp Psychiatry 38, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Legarreta M, Bueler CE, DiMuzio J, McGlade E, Lyoo IK, Yurgelun-Todd D, 2018. Increased efficiency of brain connectivity networks in veterans with suicide attempts. Neuroimage Clin 20, 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JP, Lee TW, Tsai SJ, Chen TJ, Yang CH, Lirng JF, Tsai CF, 2010. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J Geriatr Psychiatry Neurol 23, 171–184. [DOI] [PubMed] [Google Scholar]
- Iscan Z, Jin TB, Kendrick A, Szeglin B, Lu H, Trivedi M, Fava M, McGrath PJ, Weissman M, Kurian BT, Adams P, Weyandt S, Toups M, Carmody T, McInnis M, Cusin C, Cooper C, Oquendo MA, Parsey RV, DeLorenzo C, 2015. Test-retest reliability of freesurfer measurements within and between sites: effects of visual approval process. Hum Brain Mapp 36, 3472–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV, 1999. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci U S A 96, 9379–9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallade C, Sarfati Y, Hardy-Bayĺe M-C, 2005. Clinical evolution after self-induced or accidental traumatism: a controlled study of the extent and the specificity of suicidal catharsis. J Affect Disord 85, 283–292. [DOI] [PubMed] [Google Scholar]
- Johnston JAY, Wang F, Liu J, Blond BN, Wallace A, Liu J, Spencer L, Cox Lippard ET, Purves KL, Landeros-Weisenberger A, Hermes E, Pittman B, Zhang S, King R, Martin A, Oquendo MA, Blumberg HP, 2017. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am J Psychiatry 174, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant F, Lawrence NL, Olié E, Guillaume S, Courtet P, 2011. The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World J Biol Psychiatry 12, 319–339. [DOI] [PubMed] [Google Scholar]
- Jollant F, Wagner G, Richard-Devantoy S, Kohler S, Bar KJ, Turecki G, Pereira F, 2018. Neuroimaging-informed phenotypes of suicidal behavior: a family history of suicide and the use of a violent suicidal means. Transl Psychiatry 8, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA, 2015. Large-Scale network dysfunction in major depressive disorder: a Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 72, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson M, King JB, McGlade E, Bueler E, Stoeckel A, Epstein DJ, Yurgelun-Todd D, 2013. Enlarged thalamic volumes and increased fractional anisotropy in the thalamic radiations in veterans with suicide behaviors. Front Psychiatry 4, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ, 2008. Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry 64, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, 2003. Neurobiology of suicidal behaviour. Nat Rev Neurosci 4, 819–828. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY, 1980. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14, 69–97. [DOI] [PubMed] [Google Scholar]
- Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, Sassi RB, Mallinger AG, Keshavan MS, Soares JC, 2007. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry 12, 360–366. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA Jr., 2006. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Nock MK, Park JM, Finn CT, Deliberto TL, Dour HJ, Banaji MR, 2010. Measuring the suicidal mind: implicit cognition predicts suicidal behavior. Psychol Sci 21, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Davis RM, Zarate CA, Drevets WC, 2013. Reduced thalamic volumes in major depressive disorder. Psychiatry Res 213, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan LA, Hassel S, Segreti AM, Nau SA, Brent DA, Phillips ML, 2013. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychol Med 43, 2129–2142. [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ, 2011. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentería ME, Schmaal L, Hibar DP, Couvy-Duchesne B, Strike LT, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Gillespie NA, Hatton SN, Lagopoulos J, Veltman DJ, van der Wee N, van Erp TGM, Wittfeld K, Grabe HJ, Block A, Hegenscheid K, Völzke H, Veer IM, Walter H, Schnell K, Schramm E, Normann C, Schoepf D, Konrad C, Zurowski B, Godlewska BR, Cowen PJ, Penninx BWJH, Jahanshad N, Thompson PM, Wright MJ, Martin NG, Christensen H, Hickie IB, 2017. Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl Psychiatry 7, e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard-Devantoy S, Ding Y, Lepage M, Turecki G, Jollant F, 2016. Cognitive inhibition in depression and suicidal behavior: a neuroimaging study. Psychol Med 46, 933–944. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB, 2003. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Sarfati Y, Bouchaud B, Hardy-Bayĺe M−C, 2003. Cathartic effect of suicide attempts not limited to depression: a short-term prospective study after deliberate self-poisoning. Crisis: The Journal of Crisis Intervention and Suicide Prevention 24, 73. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Hibar D, Sämann P, Hall G, Baune B, Jahanshad N, Cheung J, van Erp T, Bos D, Ikram M, 2016. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, van Harmelen AL, Chatzi V, Lippard ETC, Toenders YJ, Averill LA, Mazure CM, Blumberg HP, 2020. Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Mol Psychiatry 25, 408–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TG, Sämann PG, Frodl T, Jahanshad N, Loehrer E, Tiemeier H, Hofman A, Niessen WJ, Vernooij MW, Ikram MA, Wittfeld K, Grabe HJ, Block A, Hegenscheid K, Völzke H, Hoehn D, Czisch M, Lagopoulos J, Hatton SN, Hickie IB, Goya-Maldonado R, Krämer B, Gruber O, Couvy-Duchesne B, Rentería ME, Strike LT, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Wright MJ, Hall GB, MacQueen GM, Frey EM, Carballedo A, van Velzen LS, van Tol MJ, van der Wee NJ, Veer IM, Walter H, Schnell K, Schramm E, Normann C, Schoepf D, Konrad C, Zurowski B, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Sussmann JE, Godlewska BR, Cowen PJ, Fischer FH, Rose M, Penninx BW, Thompson PM, Hibar DP, 2015. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoletini I, Piras F, Fagioli S, Rubino IA, Martinotti G, Siracusano A, Caltagirone C, Spalletta G, 2011. Suicidal attempts and increased right amygdala volume in schizophrenia. Schizophr Res 125, 30–40. [DOI] [PubMed] [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, Walhovd KB, 2014. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J Neuroscience 34, 8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin S, Cummings JL, 2002. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res 53, 647–654. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, Oquendo MA, Bruder G, Pizzagalli D, Toups M, Cooper C, Adams P, Weyandt S, Morris DW, Grannemann BD, Ogden RT, Buckner R, McInnis M, Kraemer HC, Petkova E, Carmody TJ, Weissman MM, 2016a. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): rationale and design. J Psychiatr Res 78, 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, McGrath PJ, Weissman M, Parsey R, Fava M, 2016. Establishing moderators and biosignatures of antidepressant response for clinical care of depression (EMBARC). [DOI] [PMC free article] [PubMed]
- van Heeringen K, Bijttebier S, Desmyter S, Vervaet M, Baeken C, 2014. Is there a neuroanatomical basis of the vulnerability to suicidal behavior? A coordinate-based meta-analysis of structural and functional MRI studies. Front Hum Neurosci 8, 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heeringen K, Wu GR, Vervaet M, Vanderhasselt MA, Baeken C, 2017. Decreased resting state metabolic activity in frontopolar and parietal brain regions is associated with suicide plans in depressed individuals. J Psychiatr Res 84, 243–248. [DOI] [PubMed] [Google Scholar]
- Vang FJ, Ryding E, Traskman-Bendz L, van Westen D, Lindstrom MB, 2010. Size of basal ganglia in suicide attempters, and its association with temperament and serotonin transporter density. Psychiatry Res 183, 177–179. [DOI] [PubMed] [Google Scholar]
- Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlosser RG, 2012. Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J Psychiatr Res 46, 1449–1455. [DOI] [PubMed] [Google Scholar]
- Wiech K, Jbabdi S, Lin CS, Andersson J, Tracey I, 2014. Differential structural and resting state connectivity between insular subdivisions and other pain-related brain regions. Pain 155, 2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Sabuncu MR, Yeo BT, Fischl B, Greve DN, Kochunov P, Nichols TE, Blangero J, Glahn DC, 2012. Measuring and comparing brain cortical surface area and other areal quantities. Neuroimage 61, 1428–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang J, Fan L, Li H, Zhang W, Hu Q, Jiang T, 2015. Tractography-based parcellation of the human middle temporal gyrus. Sci Rep 5, 18883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Bonkale WL, Holcomb LA, Hicks PB, German DC, 2008. Major depression, 5HTTLPR genotype, suicide and antidepressant influences on thalamic volume. Br J Psychiatry 192, 285–289. [DOI] [PubMed] [Google Scholar]


