Abstract
With age, long-lived proteins in the human body deteriorate, which can have consequences both for aging and disease. The aging process is often associated with the formation of covalently crosslinked proteins. Currently our knowledge of the mechanism of formation of these crosslinks is limited. In this study, proteomics was used to characterize sites of covalent protein-protein crosslinking and identify a novel mechanism of protein-protein crosslinking in the adult human lens. In this mechanism, Lys residues are crosslinked to C-terminal Asp residues that are formed by non-enzymatic protein truncation. Ten different crosslinks were identified in lens major proteins such as αA-crystallin, αB-crystallin and AQP0. Crosslinking in AQP0 increased significantly with age and also increased significantly in cataract lenses compared with normal lenses. Using model peptides, a mechanism of formation of the Lys-Asp crosslink was elucidated. The mechanism involves spontaneous peptide cleavage on the C-terminal side of Asp residues which can take place in the pH range 5-7.4. Cleavage appears to involve attack by the side chain carboxyl group on the adjacent peptide bond, resulting in the formation of a C-terminal Asp anhydride. This anhydride intermediate can then either react with water to form Asp, or with a nucleophile, such as a free amine group to form a crosslink. If an ε-amino group of Lys or an N-terminal amine group attacks the anhydride, a covalent protein-protein crosslink will be formed. This bi-phasic mechanism represents the first report to link two spontaneous events: protein cleavage and crosslinking that are characteristic of long-lived proteins.
Keywords: Long-lived proteins, Aging, Lens, Racemisation, Cataract
1. Introduction:
The human body contains numerous long-lived proteins (LLPs) [1-3]. With age, certain amino acids within these proteins; in particular Asn, Asp, Gln, Cys, Thr and Ser, undergo deterioration [4-6] and these spontaneous modifications can result in racemization, deamidation and protein cleavage [1]. Another common modification associated with aged LLPs is non-disulphide covalent crosslinking. Protein-protein crosslinking is increasingly observed with age in tissues such as the lens [7], brain[8], heart [9], arteries [10], cartilage [11] and has been associated with decreased function and disease [7, 12, 13]. Several crosslinking mechanisms have been proposed such as via advanced glycation end products [14, 15], transglutaminase activity [16], and through dehydroalanine (DHA) intermediates [7, 17]; however, identification of novel age-related protein-protein crosslinking processes is challenging due to the large number of potential crosslink combinations that could form coupled with technical difficulties in sequencing crosslinked peptides.
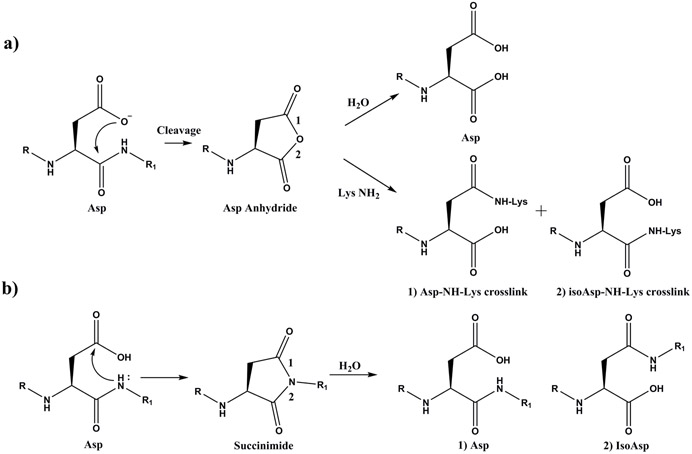
With advances in proteomics technology and data analysis methodology, identification of novel crosslinking sites formed in aged tissue become increasingly feasible [7]. Recently, one mechanism for crosslinking of aspartic acid and lysine residues in the human eye lens, involving the formation of a succinimide intermediate, was described [18]. This discovery provided the first link between the age-related processes of protein-protein crosslinking and protein racemisation/isomerisation. Racemisation and isomerisation of Asp and Asn are two of the most common modifications present in LLPs [19-21] and the reaction pathway is thought to involve the peptide bond NH attacking the Asn or Asp side chains to form a cyclic succinimide intermediate [22, 23]. Hydrolysis of this succinimide intermediate can result in the formation of four Asp isomers: L- Asp, D-Asp, L-iso Asp and D-isoAsp[24]. Whilst, formation of a succinimide is a major pathway of Asp degradation, a competing mechanism can take place where the side chain carboxylic acid attacks the adjacent peptide bond carbonyl resulting in peptide cleavage and the formation of a C-terminal succinic anhydride. This anhydride can then hydrolyse to yield a C-terminal Asp. Such cleavages, are typically observed under low pH conditions, for example, those employed for storage of protein pharmaceuticals such as monoclonal antibodies [25-27]. The mechanism of this peptide bond cleavage has been examined in detail, and it appears to involve attack of the ionized carboxyl side chain on the protonated carbonyl group of the peptide bond [28]. A separate computational study outlined a similar mechanism of peptide bond cleavage that involved the protonated side chain of Asp which also resulted in the formation of a C-terminal anhydride [29].
As part of a project to characterize the covalent crosslinking of LLPs and their mechanisms of formation, we used proteomic methods to examine proteins from adult lenses with the aim of detecting sites of novel crosslinks. When aged human lenses were examined for the presence of non- disulphide covalently crosslinked proteins, several sites were found that involved Lys residues crosslinked to C-terminal Asp residues. This paper describes the mechanism of formation of these crosslinks.
2. Materials and Methods:
Frozen human lenses were obtained from NDRI (Philadelphia, PA) or from the Kansas Eye Bank (Wichita, KS). All lenses were isolated from the donor no later than 8 hours post mortem and shipped on dry ice. Human lens work was conducted in compliance with the Declaration of Helsinki. The lenses were classified as normal or cataract lenses by the eye bank. All lenses received were stored at −80°C until use. All chemicals were purchased from Sigma (St. Louis, MO). All HPLC grade solvents were purchased from Thermo Fisher Scientific (Fair Lawn, NJ). Sequence-grade modified trypsin was obtained from Thermo Fisher Scientific (Rockford, IL).
2.1. Lens extraction
To identify and quantify crosslinked peptides as a function of age, lens region and cataract, twelve lenses, grouped into three were examined: a young lens group (18 y, 19 y, 21 y and 22 y), a middle-aged normal lens group (48 y, 53 y, 53 y and 56 y) and a middle-aged cataract lens group (50 y, 57 y, 58 y and 64 y). Each lens was dissected into three regions; Outer Cortex (OC), Outer Nucleus (ON) and Inner Nucleus (IN) as described previously [30]. Briefly, 30 μm thick equatorial cryostat sections (LEICA CM 3050S, Leica Microsystems Inc., Bannockburn, IL) were obtained and collected on parafilm. Three different lens regions were isolated by punching the section using 4.5 mm and 6 mm diameter trephines. Isolated lens tissue regions were separated from parafilm by five sequential washes with 100 μL of 25 mM Tris, 150 mM NaCl, 5 mM EDTA, pH 7.4. The samples were passed through a 25 G needle 5 times and centrifuged at 20,000 g for 30 min., The samples were then further processed to obtain water soluble fraction (WSF), urea soluble fraction (USF) and urea insoluble (UIF) as described previously [30]. Samples were reduced by DTT, alkylated by IAA and digested by trypsin as described previously [30]. After digestion, all samples were dried in a speedvac and proteins in each sample were reconstituted in 0.1% formic acid to make a final total protein concentration of 0.25 μg/μL for subsequent analysis.
To identify low abundant crosslinked peptides, the urea-insoluble fraction from the nucleus region of two additional cataract lenses aged 68 and 71 were processed as above and the tryptic peptides fractionated by strong cation exchange as described previously [7, 31]. Desalted tryptic peptides from a 68 year-old lens were step-eluted sequentially from SCX resins with 40%, 60% and 100% buffer B (5 mM potassium phosphate buffer containing 30% ACN, 350 mM KCl, pH 2.5) balanced with buffer A (5 mM potassium phosphate buffer containing 30% ACN, pH 2.5). The 60% buffer B eluate was analyzed by a three-step multidimensional protein identification technology (MudPIT) analysis as described previously [7]. Tryptic peptides from a 71 year-old lens were step-eluted sequentially from SCX resins by 30%, 40%, 60%, 80% and 100% buffer B. Peptides from 10 μg of total proteins were fractioned using 1 mg of SCX resins (Luna SCX, 5 μm, 100 Å media, Phenomenex, Torrance, CA). The amount of sample loaded on the column was based on a pre-run screen using a small aliquot of each fraction and the amount of peptides loaded for the final runs corresponds to 1 μg original digest for the 40% buffer B eluate and 2-3 μg original digest for the 60-100% buffer B eluates.
2.2. LC-MS/MS
Tryptic peptides were loaded on a C18 trap column (50 mm x 100 μm) packed with Phenomenex Jupiter resin (5 μm mean particle size, 300 Å pore size) and separated on a one-dimensional fused silica capillary column (250 mm x 100 μm) packed with Phenomenex Jupiter resin (3 μm mean particle size, 300 Å pore size) or analyzed by MudPIT as described previously [7]. For one-dimensional liquid chromatography, a 70-minute gradient was performed, consisting of the following: 0-60 min, 2-45% ACN (0.1% formic acid); 60-70 min, 45-95% ACN (0.1% formic acid) balanced with 0.1% formic acid. The eluate was directly infused into a Q Exactive instrument (Thermo Scientific, San Jose, CA) with a nanoelectrospray ionization source. A data-dependent acquisition method consisted of MS1 acquisition (R=70,000), using an MS AGC target value of 1e6, followed by up to 15 MS/MS scans (R=17,500) of the most abundant ions detected in the preceding MS scan. The MS2 AGC target value was set to 2e5, with a maximum ion time of 200 ms, a 5% underfill ratio, and an intensity threshold of 5e4. HCD collision energy was set to 27, dynamic exclusion was set to 5 s, and peptide match and isotope exclusion were enabled. For MudPIT analysis, the samples were run on a Orbitrap Velos mass spectrometer (Thermo Scientific, San Jose, CA). The instrument was operated in a 17-step data dependent mode with one precursor scan event (R=60,000) to identify the top 16 most abundant ions in each MS scan for fragmentation in the ion trap. Dynamic exclusion (repeat count 1, exclusion list size 500, and exclusion duration 15s) was enabled. +1 and +2 precursors were excluded from MS2.
2.3. Data Analysis
For crosslinked peptide identification, the raw data were processed in two steps. Firstly, a list of truncated peptides from major lens proteins (crystallins, AQP0, and cytoskeletal proteins) with C-terminal Asp residues that were detected in a 48 year-old normal lens and a 50 year-old cataract lens was generated from the results of nine LC-MS/MS analyses of WSF, USF and UIF of different regions of each lens. The nine raw data files were converted to mzML files and searched by TagRecon [32] against a custom human lens database as previously described [7]. The searching parameters include semi-constrained trypsin specificity, a maximum of two missed cleavage sites, a static modification of carbamidomethylation of cysteine, variable modification of oxidation of methionine and deamination of asparagines and glutamine. Manual analysis was then performed to verify each C-terminal Asp containing peptide. Each truncated peptide was then added as a separate entry to a major lens protein database (15 major lens proteins including crystallins, AQP0, BFSPs) to generate a protein database for searching crosslinked peptides. The second step involved analysing for crosslinked peptides using StavroX (version 3.6.6) [33] to generate candidate crosslinked peptides. The crosslinker setting was set to search for crosslinking of Lys and Asp residues with a water loss. Other parameters included trypsin-specific cleavage with a static modification of carbamidomethylation of cysteine, variable modification of oxidation of methionine, precursor mass deviation<5 ppm and fragment ions mass deviation<10 ppm. Manual analysis was then performed to identify crosslinked peptides from the list of candidate peptides generated from StavroX.
For quantification of the levels of crosslinking of the C-terminus of AQP0 in different lens regions, the selected ion chromatograms of crosslinked AQP0 peptides (AQP0 227-233 crosslinked to AQP0 239-243) and the linear peptide, AQP0 234-238 were extracted with a +/−10 ppm mass tolerance. The peak areas were calculated within Xcalibur software. The level of crosslinking was defined as the ratio of the peak area of crosslinked peptide to the peak area of peptide 234-238. To obtain the peak area of the crosslinked peptide, the peak areas of all isomers (Supplemental Figure 1) were summed.
The results are presented as mean ± standard deviation (SD) of 4 independent experiments from four different lenses in each group shown in Figure 2. Statistical analysis was done by one-way analysis of variance (ANOVA) at p<0.05 followed by Tukey’s multiple comparison test.
Figure 2:
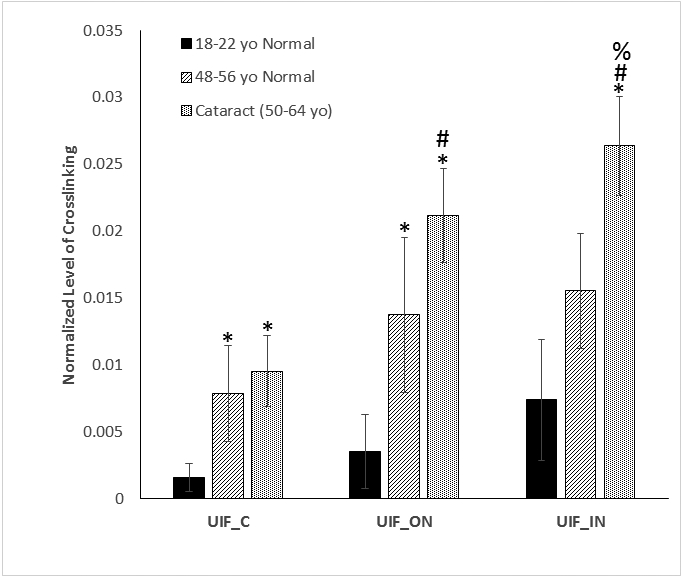
Levels of crosslinking between AQP0 227-233(LKSISER) and AQP0 239-243 (GAKPD) in different aged normal and cataract lenses
The average levels of the crosslinked peptide from four different lenses in similar age and disease condition were plotted. The error bars indicate the standard deviation from four analyses. An asterisk (*) indicates crosslinking increases significantly compared with the same region of the young lenses (p<0.05). A pound sign (#) Indicates significantly increases in crosslinking in the inner nucleus regions compared with cortex region in the same group of lenses (p< 0.05); A percent sign (%) showed significantly increased in crosslinking in cataract lenses compared with age-matched normal lenses (p<0.05). n=4 +/− SD (UIF = Urea insoluble fraction, C= cortex, ON=outer nucleus, IN= inner nucleus
2.4. Asp cleavage and stability experiments
All synthetic peptides were purchased from GME biochemicals (Shanghai, China) at 95% purity. The peptides Ac-PDVF, Ac-PDGF, Ac-YLDAF and Ac-YLDSF (1mg/mL) were dissolved in 50mM citric acid pH 5.0, 50mM sodium phosphate pH 6.7 or pH 7.4 and incubated at 60°C. Aliquots were taken at timed intervals and injected onto a C18 RP HPLC column (Aeris, 2.6μ, XB-C18, 100mm x 2.1m Phenomenex). A 50-minute gradient was performed at a flow rate of 0.2mL/min, consisting of the following: 0-5 min, 2% CH3CN; 5-15 min, 20% CH3CN, 15-20min 40% CH3CN, 25-35min 80% CH3CN, 35-50 2% CH3CN. Breakdown of the peptides were monitored at 216nm and 280nm with peaks collected, dried down and their identity confirmed by tandem mass spectrometry using a Thermo LTQ mass spectrometer (Thermo Fisher Scientific). The elution times of breakdown products were confirmed using synthetic peptides standards. The percentages of cleavage and racemised peptides were determined by the total peak area of Asp cleavage products by comparison to the original peptide. For peptides that contained Tyr, absorbance at 280nm was used and the amount of cleavage was determined by total peak area of Ac-YSD in comparison to the original peptide. Peptides without Tyr were monitored at 216nm.
2.5. Formation of C-terminal Asp crosslinks.
Ac-PDVF, Ac-YLDAF and Ac-YLDSF (1mg/mL) were incubated at 60°C with a 5-molar excess of phenylethylamine (PE) or N-Ac-K, at pH 5.0 (50mM citric acid), pH 6.7 (50mM sodium phosphate) and pH 7.4 (50mM sodium phosphate). Aliquots were separated as described in section 2.4. All HPLC peaks were collected and peaks containing the crosslinks were confirmed by MS/MS using a Thermo LTQ mass spectrometer. The crosslink was quantified as a percentage of total peak area of starting peptide. Asp crosslinks involving an internal succinimide were made as described [18].
3. Results:
3.1. Identification of protein-protein crosslinking in human lenses
Previously, DHA and DHB-mediated protein-protein crosslinking was identified as one of the mechanisms that contributes to irreversible protein-protein crosslinking in human lenses [7]. During our searching protocol a strong signal was detected in tryptic digest of the urea-insoluble fraction from the nucleus region of a 68 year old cataract lens corresponding to crosslinking between peptides AQP0 227-233 (LK*SISER) and AQP0 239-243 (GAKPD*). The crosslink appeared to involve an amide bond between the Lys side chain of one peptide, and the C-terminal Asp residue of another. This signal was repeatedly detected in majority of human lens urea-insoluble fractions. The measured parent mass was less than 0.8 ppm of the theoretical parent mass on a Q Exactive instrument. The assignment to this crosslinked peptide was confirmed by the tandem mass spectrum shown in Figure 1 that was acquired on the Q Exactive instrument where all fragments were measured with ≤5 ppm mass accuracy. This tandem mass spectrum was obtained in the tryptic digest of UIF from the nucleus region of a 22 year old normal lens. The presence of y1β+, b3β+, b2α+ and y5α+ ions clearly indicate the crosslink is through K228 and D243. Isopeptide bonds have been found to form spontaneously in bacterial coat proteins.[34]
Figure 1.
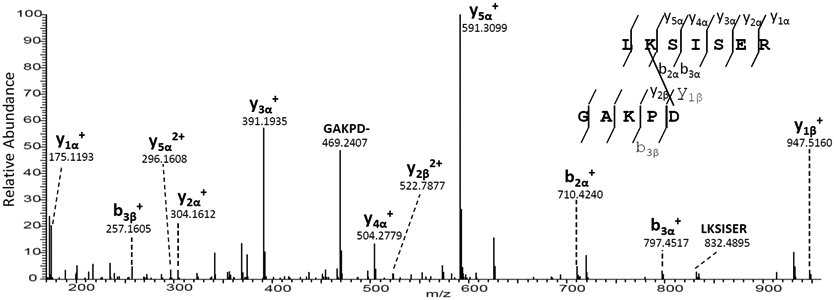
MS/MS spectrum of a crosslinked peptide that is detected in the tryptic digest of UIF from a 22 year old normal lens nucleus. The tandem mass spectrum shows the crosslink between Asp 243 (GAKPD*) and Lys228 (LK*SISER) both from AQP0.
3.2. Quantification of AQP0-AQP0 crosslinking in different lens regions
In order to determine if the amount of AQP0 crosslinking in the lens increased as a function of age or cataract, individual lenses were dissected into three regions [30]. Since the lens grows continuously throughout life the cortex, outer nucleus and inner nucleus correspond to regions and proteins that were synthesized at different times during life [35]. Each region was extracted initially with buffer and then with 8M urea prior to proteomic analysis. Crosslinking of AQP0 was found to occur early in life. The crosslinked AQP0 peptide LK*SISER-GAKPD* could be detected readily in young normal human lenses by 1D LC-MS/MS analysis. The selected ion chromatograms for this crosslinked peptide can be found in Supplemental Figure 1 and indicates the presence of multiple isomers. To characterize the effect of aging and disease on crosslinking of AQP0, the levels of crosslinking in the different regions of three groups of the lenses (young normal, middle-aged normal and cataract) were quantified by summing the peak areas of all isomers. The quantification results are shown in Figure 2.
For all three groups of lenses, the crosslinking increased in the outer nucleus and inner nucleus regions compared with cortex. This increase reached statistical significance for cataract lenses. Comparing the same lens regions between young lenses and middle-aged lenses, the crosslinking level also increased and the differences were statistically significant in the cortex and outer nucleus. The extent of crosslinking was found to be higher in cataract lenses compared with age-matched normal lenses and the difference reached statistically significance in the inner nucleus. In summary, in all three lens regions, the amount of crosslink increased with age and also with cataract.
3.3. Lys-Asp crosslinks in other lens proteins
Having characterised the AQP0-AQP0 crosslink, we searched for other sites in proteins where crosslinking involved a Lys residue and a C-terminal Asp residue. Nine other sites of such crosslinking in the lens were detected as shown in Table 1. Crosslinks were identified in the major structural proteins αA-crystallin, αB-crystallin, γC/D crystallin, and γS crystallin. Two other homologous crosslinks formed between the N-terminal amino group and a C-terminal Asp were also detected. The tandem mass spectra for the assignments shown in Table 1 are displayed in Supplemental Figures 2-10. There are additionally 4 peptides detected that match the predicted parent masses and their tandem mass spectra confirmed one peptide, but lack b- or y-ions from the other peptide; most likely because these peptides are too short to be confidently confirmed. These four peptides can be found in the Supplemental Table 1. There are an additional six peptides with addition of a single Asp residue to Lys residues within a peptide. It is conceivable that truncation at an Asp residue with a preceding Arg or Lys residue would result in a single Asp crosslinked to a Lys residue after trypsin digestion of lens proteins. Thus, these Asp modified peptides could arise from crosslinked proteins and are also listed in Supplemental Table 1.
Table 1:
Crosslinked Peptides Identified in Human Lenses.
| Peptide | [MH]+exp | [MH]+cal | Error (ppm) |
|---|---|---|---|
| AQP0 227-233: LK*SISER AQP0 239-243: GAKPD* |
1300.7210 | 1300.7219 | 0.74 |
| AQP0 1-5: *MWELR AQP0 239-243: GAKPD* |
1202.5967 | 1202.5987 | 0.43 |
| αB 124-129: IPADVD* αB 83-92: HFSPEELK*VK |
1823.9510 | 1823.9538 | 1.53 |
| αB 124-129: IPADVD* αB 91-103: VK*VLGDVIEVHGK |
2003.1169 | 2003.1172 | 0.17 |
| αA 146-151: IQTGLD* αB 91-103: VK*VLGDVIEVHGK |
2020.1403 | 2020.1437 | 1.68 |
| αA 146-151: IQTGLD* αB 150-157:K*QVSGPER |
1527.8096 | 1527.8125 | 1.89 |
| αB 124-129: IPADVD* αB 121-123: K*YR |
1076.5742 | 1076.5734 | 0.76 |
| AQP0 239-243: GAKPD* γS 8-19: ITFYEDK*NFQGR |
1985.9687 | 1985.9716 | 1.44 |
| αA 70-88:FVIFLDVK*HPSPEDLTVK αA55-58: TVLD* |
2562.3997 | 2562.3854 | 4.9 |
| αA 146-151: IQTGLD* γC/γD 2-3: *GK |
831.4572 | 831.4571 | 0.15 |
Asterisks indicate the residues that are involved in crosslinking. All masses listed are monoisotopic masses.
3.4. Spontaneous cleavage of peptides at Asp
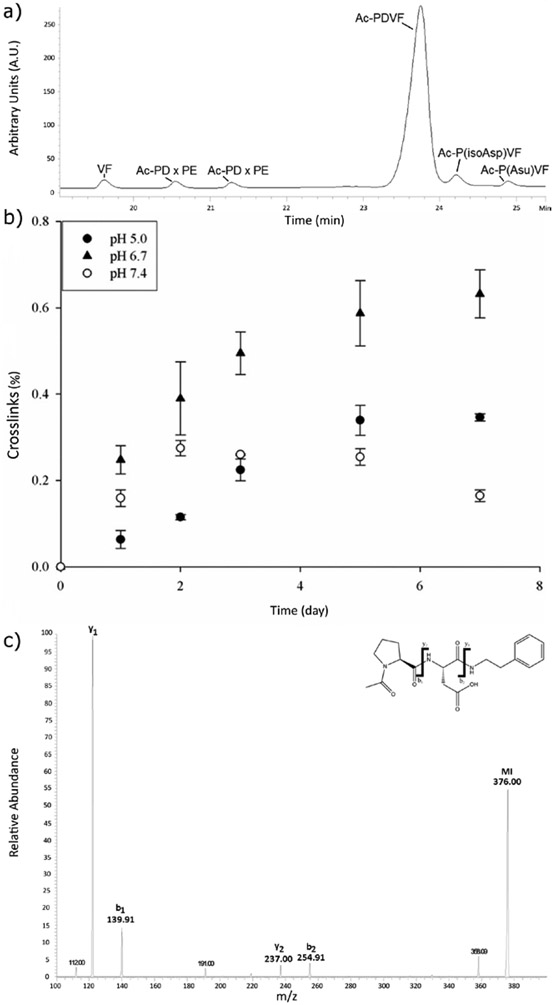
The crosslinked peptides characterised in the lens (Table 1) involved cleavage on the C-terminal side of Asp. To understand the cleavage/crosslinking mechanism, the tetrapeptide, Ac-PDVF, was examined since this incorporates a Pro Asp cleavage and crosslinking site detected in AQP0 (see Fig 1). Three physiologically relevant pHs: pH 5.0, representative of the pH within lysozomes [36]; pH 6.7, the pH in the centre of the lens [37] and pH 7.4, the pH of extracellular fluid, were employed. Peptide bond cleavage was studied initially and this was monitored by HPLC. During the incubation period, several HPLC peaks appeared which corresponded to the breakdown of Ac-PDVF. These included Ac-PD, VF, Ac-P(isoAsp)VF and the succinimide of Asp denoted as Ac-P(Asu)VF. The structures of these products were confirmed by co-elution of synthetic standards as well as by MS/MS of each HPLC peak.
Peptide bond cleavage on the C-terminal side of Asp was found to be measurable at each pH examined (Figure 3). The degree of scission was pH-dependent, with the greatest amount of cleavage detected at pH 5.0 and the least at pH 7.4. This finding of more peptide bond cleavage at acidic pHs is consistent with the reaction mechanism proposed for this process [38]
Figure 3.
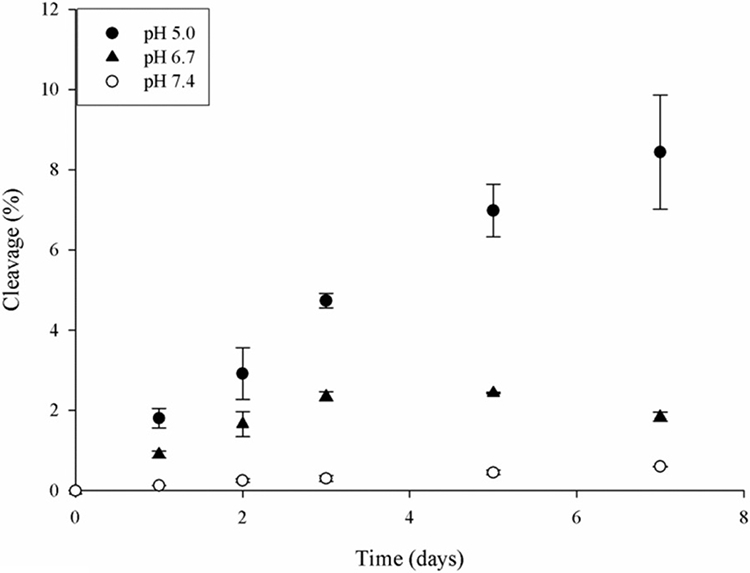
Time course of Asp cleavage in Ac-PDVF. Ac-PDVF was incubated at pH 5.0, 6.7 and 7.4. Cleavage of Ac-PDVF and generation of Ac-PD was determined by the HPLC peak area at 216nm as described in Materials and Methods. n=3 +/− SD.
When another two peptides, Ac-YLDAF and Ac-YLDSF, incorporating the Leu Asp sites of Asp cleavage and crosslinking in αA crystallin, (Table 1) were incubated under the same conditions similar results were obtained (Figure 4). Both Ac-YLDAF and Ac-YLDSF were found to cleave on the C-terminal side of the Asp residue. Again, there was an effect of pH on the time course of cleavage (Fig 4) with the greatest amount of cleavage detected at pH 5.0.
Figure 4.
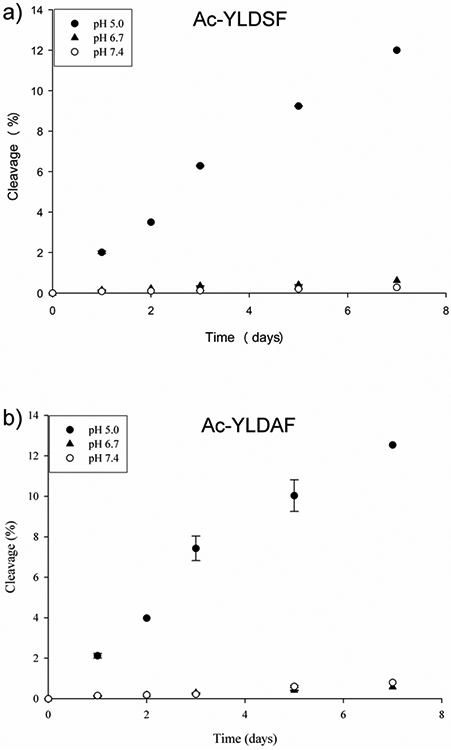
Time course of Asp cleavage in a) Ac-YLDSF and b) Ac-YLDAF at pH 5.0, 6.7 and 7.4.Cleavage at Asp and generation of Ac-YLD was determined by HPLC peak area at 280nm as described in Materials and Methods. n=3 +/− SD.
There was a noticeable effect of amino acid sequence on the overall reaction and this was evident from the fact that relatively less cleavage was noted at pH 6.7 and 7.4 for Ac-YLDAF and Ac-YLDSF compared to Ac-PDVF. In addition, there were differences in the ratios of cleaved to racemised/ isomerised products in the three peptides examined (Fig 5).
Figure 5.
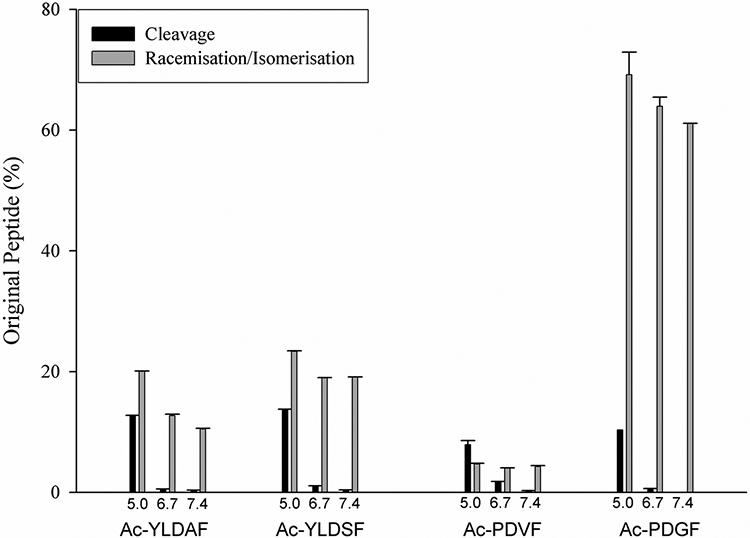
Cleavage at Asp compared with racemisation of Asp in Ac-YLDSF, Ac-YLDAF, Ac-PDVF and Ac-PDGF after 7 days at pH 5.0, pH 6.7 and pH 7.4. Cleavage was calculated by HPLC peak area of the C-terminal Asp peptides by comparison to the original peptide peak area as described in Materials and Methods. The amount of racemisation/isomerisation was calculated by the HPLC peak area of racemised/isomerised peptides plus the succinimide, in comparison to original peptide peak area. n=3 +/− SEM. See Scheme 1 for a summary of the pathways involved. Peptide identity was confirmed by elution time of synthetic peptides and mass spectrometry.
The bulkiness of the amino acid residue on the C-terminal side of Asp appears to have a significant effect on the overall degradation pathway. Thus, replacement of Val in Ac-PDVF by Gly resulted in a decrease in the extent of peptide scission at pH 5.0, compared to alanine and serine, with almost no cleavage of Ac-PDGF at pH 6.7 and 7.4 (Figure 5). It is probable that the reason for this can be traced to the fact that there are two competing pathways operating. These competing reaction pathways are outlined in Scheme 1. Small residues on the C-terminal side of Asp are known to facilitate succinimide formation, and may effectively reduce the amount of Asp available for other alternative reactions, such as cleavage.
Scheme 1. Asp residues in proteins can breakdown in two ways: cleavage or racemisation/isomerisation.
Pathway a) Cleavage. Asp residues can undergo spontaneous cyclisation forming a cyclic anhydride intermediate. Detailed analyses indicate that the mechanism probably involves attack of the ionized side chain carboxyl on a protonated carbonyl of the peptide bond [38]. The cyclic anhydride can hydrolyse, or can react with an amine group such as that of a Lys residue, forming an Asp-Lys crosslink. The ε-amino group of Lys can potentially attack either carbonyl of the anhydride leading to two isomeric Asp-Lys crosslinks as shown.
Pathway b) Racemisation/isomerisation. Formation of a succinimide can occur by attack of the adjacent peptide bond NH group on the side chain carboxyl group of Asp. The succinimide can isomerise and hydrolyse to yield four different Asp isomers: L-Asp, D- Asp, L-isoAsp, and D-isoAsp [22].
3.5. Crosslinking and cleavage at Asp
In order to discover if crosslinking could also be observed under the same conditions as used for cleavage, incubations of Ac-PDVF were repeated in the presence of a 5-fold excess of the lysine mimic, phenylethylamine (PE). In the presence of PE, two additional HPLC peaks were found eluting at 20.5 and 21.3 minutes (Figure 6a) and these were identified by MS/MS to correspond to Ac-PD crosslinked to PE (Figure 6c). The two peaks presumably correspond to Asp/isoAsp isomers of the crosslink (see Scheme 1a).
Figure 6.
a) HPLC profile of peptide PDVF with N-terminal acetylation (Ac- PDVF) incubated with a five-fold molar excess of phenylethylamine (PE) after 5 days at pH 5. The two peaks Ac-PD x PE corresponding to the isomerised formed of the peptide crosslinked to PE.
b) Time course of Ac-PD- PE crosslink formation. Ac-PDVF was incubated at three different buffer pHs (5.0, 6.7 and 7.4) in the presence of a five-fold molar excess of PE. Ac-PD- PE was determined by the HPLC peak area at 216nm as described in Materials and Methods. n=3 +/− SD.
c) MS/MS spectrum of Ac-PD crosslinked to PE. Ac-PDVF was incubated with PE at pH 5 for 72h and the HPLC peak collected for MS/MS. MI = molecular ion.
The time course shown in Figure 6b revealed that significant crosslinking was detected and the amount of crosslink increased with time. Interestingly the pH-dependence of crosslinking was not the same as that for cleavage (Figure 4), with the largest amount of crosslinking being detected at pH 6.7, the pH of the lens interior. The reason for this difference in cleavage and crosslinking could be due to the stability of the reactive anhydride intermediate toward competing reactions such as hydrolysis. These experiments were repeated using N-acetyl Lys in place of PE, and crosslinking with Ac- PD was also detected (data not shown).
Similar Ac-YLD- PE crosslinked peptides were obtained when Ac-YLDAF and Ac-YLDSF were incubated in the presence of PE. When the incubations of Ac-YLDAF and Ac-YLDSF were repeated in the presence of N-Ac-Lys, Ac-YLD crosslinked with N-Ac-Lys was also detected (e.g. see Supplementary Figure 11).
3.6. Crosslinking with Lys involves a succinic anhydride intermediate
Previous detailed mechanistic investigations of Asp cleavage [25, 29, 38] suggested that a likely intermediate product of the scission at Asp involves a C-terminal anhydride (see Scheme 1a). If this were indeed the case, and its lifetime in aqueous solution were sufficiently long, the anhydride could react readily with amine nucleophiles. This process would therefore provide an explanation for the crosslinking with Lys and N-terminal residues.
Aspects of this proposed mechanism were tested in the following ways. If the mechanism of crosslinking involved an anhydride intermediate, then anhydride synthesis, and incubation with PE under the same incubation conditions, should also yield a PE-crosslinked product. Ac-PAsp anhydride was therefore synthesized using a literature procedure [39]and its structure confirmed by MS before being mixed with excess PE at pH 5, 6.7 and pH 7.4. Ac-PAsp anhydride crosslinked with PE at each pH examined. The MS/MS spectrum of the product was the same as that from the incubation of Ac-PDVF with PE. In agreement with this anhydride mechanism, peptides with a C-terminal Asp, failed to form crosslinks when incubated with PE.
Overall these results show that spontaneous cleavage of proteins can take place at the peptide bond on the C-terminal side of Asp through the formation of a cyclic anhydride intermediate. Once this anhydride forms in biological tissues it can follow one of two pathways. If an amine nucleophile, such as the side chain of Lys or an α-amino group, is in close proximity then a protein-protein crosslink will form. Otherwise water will hydrolyse the anhydride producing a C-terminal Asp residue.
4. Discussion
Since LLPs undergo a range of modifications with time, degradation of LLPs is of major importance for human aging and age-related diseases. Some modifications, such as glycation [40] and oxidation [41] are the result of external reactive molecules. Many other modifications are spontaneous and occur due to the structures of the amino acid side chains and their local environment e.g. unstructured regions permit more allowable conformations. As we age, non-disulphide crosslinks involving proteins occur in many tissues in the human body [42, 43]; however, the mechanism for formation of these crosslinks is poorly understood. As part of a long-term project to define age-related modifications to human lens proteins, we have been characterizing the covalent crosslinks that occur in human LLPs with age. The lens was chosen since it contains abundant LLPs [44] and therefore serves as a model tissue to examine protein aging. Lens LLPs include cytosolic crystallin proteins, as well as cell membrane LLPs, the most abundant of which is the water channel protein, AQP0 [45].
Crystallin aggregation, crosslinking and insolubilization are characteristics of lens protein aging and are believed to form light scattering centers which may contribute to the development of cataract [46]. Protein-protein crosslinking is also thought to contribute to protein insolubilization [42], aging [43] and is implicated in a number of diseases of aging such as Alzheimer’s disease [8, 47]. Several mechanisms have been proposed to explain non-disulfide crosslinking such as glycation [14, 15], transglutaminase-mediated crosslinking [16] and DHA-mediated crosslinking [7]. Age-related truncation of lens proteins is a well-established phenomenon and many including αA crystallin [48], AQP0 [49], and connexin50 and connexin 46 [50], have been reported to undergo truncation at Asp residues., Truncated crystallins have been found to be more prone to aggregation [51]. It has also been hypothesized that the development of cataract is due, in part, to the aggregation of crystallin fragments generated by the breakdown of crystallin proteins [52] and peptides with C-terminal Asp are abundant in aged human lenses [53].
Proteomic analysis of human lenses revealed multiple sites where a Lys residue from one protein was crosslinked to an Asp from either the same, or another, polypeptide. The formerly internal Asp residues were found at the C-termini of the crosslinked peptides, indicating that they had been cleaved either before, or after, crosslinking by Lys. Of note, many of these same Asp sites are known to be racemised with age [21, 54, 55]. Several synthetic Asp-containing peptides were examined in an effort to elucidate the mechanism underpinning this novel crosslinking.
It was found that Asp-containing peptides can undergo cleavage to varying extents at pH 5.0, 6.7 and 7.4. When the experiments were repeated in the presence of PE, or N-acetyl Lys, a crosslinked product was detected. The most likely mechanism involves the formation of a reactive C-terminal Asp anhydride intermediate, and this mechanism was confirmed by synthesis of a C-terminal Asp anhydride. Since this mechanism was able to be replicated under biological pH conditions, it could therefore explain the novel crosslinks found in the lens.
Once formed, the anhydride would be subject to two competing reactions: hydrolysis, and attack by a nucleophilic group. Anhydrides are readily hydrolysed by water and this process is pH-dependent. Other studies have shown that the greatest anhydride stability is observed between pH 4 and 6 [56]. In the cell, the degree of crosslinking will depend on the availability and proximity of Lys residues or α–amino groups and the local environment. For example, it is likely that Lys in a membrane will be more reactive as hydrophobic conditions depress the pKa of the side chain NH2 [57] making it more likely to act as a nucleophile at neutral pH.
Our results under biological pH conditions are consistent with literature data on the changes that take place on storage of proteins, such as monoclonal antibodies (MAbs) [25]. MAbs are typically stored at low pH, and as a consequence, most stability data have been obtained under acidic conditions. Both cleavage at Asp and protein-protein crosslinking were observed. Crosslinking of stored MAbs involved reaction of the α-amino group of one protein with a postulated anhydride intermediate in a reaction analogous to that described in this manuscript. The pKa of an α–amino group is lower than that of ε-amino groups and therefore they will act as nucleophiles more readily under acidic conditions and therefore this will favour crosslink formation,
There is a body of literature on the cleavage of peptides at Asp. For example, Li and co-workers [58] examined 4 decapeptides at pH 4.0-5.5 and found that the major degradation pathway was cleavage at the Asp-Aaa bond. The process was consistent with first-order kinetics with Asp-Pro being more reactive. The kinetic mechanism of cleavage across a wide range of pHs and buffers was studied in greater detail by Oliyai and Borchardt [38] . Spontaneous cleavage of proteins at Asp, and other sites has been reviewed by Vlasek and Ionescu [25].
The major focus of the current study was to identify novel crosslinks in human lenses and to elucidate the mechanism of crosslink formation and, how this could be related to protein truncation at Asp residues. As summarised in Scheme 1, two major competing processes involving Asp residues occur in the pH range 5 – 7.4. The first is cleavage, coupled with anhydride formation, as outlined in this manuscript. This pathway results in peptide bond scission with the C-terminal anhydride itself undergoing two competing reactions: hydrolysis to yield a C-terminal Asp residue, or crosslinking. The second process involves succinimide formation and this has been extensively investigated. Racemisation/isomerisation and hydrolysis of the succinimides can yield four isomers of Asp e.g. [22].
Although not investigated in detail in this study, amino acid sequence, particularly on the C-terminal side of the Asp residue, will likely have a considerable influence on the nature of the products formed by breakdown of Asp in a protein. For example, an Asp site with a C-terminal Gly residue, is more readily able to form a cyclic succinimide and to racemise [23]. Because this process occurs relatively rapidly and isoAsp peptides that are formed cleave less readily [59], we predicted that little Asp cleavage is likely to be observed. This hypothesis was supported by a comparison of Ac-PDVF with Ac-PDGF (Fig 5).
Overall the percentage of Asp cleavage for each of the five peptide sequences studied at pH 5 was quite similar (Fig 5). The amount of cleavage at pH 6.7, the pH of the lens interior, was lower but still comparable. At both pH 6.7 and 7.4, succinimide formation (Pathway b) in Scheme 1) occurred more readily than cleavage (Pathway a) in Scheme 1).
The new crosslinking mechanism reported in this paper identifies a chemical reaction that links protein crosslinking with protein truncation. Previously we reported protein-protein crosslinking based on a DHA-mediated mechanism and all crosslinked peptides identified were from β-crystallins [7]. In the present study, the crosslinked peptides identified were mainly from α-crystallins and the lens major membrane protein AQP0. Crosslinked peptides containing the integral membrane protein AQP0 were, as expected, only detected in the urea-insoluble fractions where the amount increased with both age and cataract. Crosslinking levels of α-crystallins in the WSF, USF, and UIF fractions have not been systematically studied; however, those crosslinked peptides that were detected in 1D-LC-MS/MS analysis were either not detected in the WSF or showed clearly stronger signals in the USF (data not shown) suggesting a correlation between Lys-Asp protein-protein crosslinking and protein insolubilization. The crosslink between γS crystallin and AQP0 involves a covalent bond between a formerly soluble protein to a lens membrane protein. Such an event would sequester the crystallin to the plasma membrane, possibly creating a point of nucleation for protein membrane association that has been observed in the lens with age and cataract [60]
5. Conclusions
Overall this investigation has elucidated one mechanism responsible for spontaneous cleavage and crosslinking of proteins in the lens. Cleavage of the peptide bond adjacent to Asp via the formation of a C-terminal anhydride was a prerequisite. This is the first reported case where two of the main reactions of LLPs, crosslinking and cleavage, are linked directly. Asp residues in other long-lived proteins, should be considered as potential sites of cleavage and crosslinking.
Supplementary Material
Acknowledgements:
The authors acknowledge use of the UOW Mass Spectrometry User Resource and Research Facility (MSURRF), University of Wollongong.
Funding sources and disclosure of conflicts of interest
Funding for this study was provided by National Institutes of Health by grants R01 EY024258 and P30 EY008126. The authors declare no conflict of interest.
References
- [1].Truscott RJW, Schey KL, Friedrich MG, Old Proteins in Man: A Field in its Infancy, Trends in Biochemical Sciences, 41 (2016) 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Toyama Brandon H., Savas Jeffrey N., Park Sung K., Harris Michael S., Ingolia Nicholas T., Yates John R., Hetzer Martin W., Identification of Long-Lived Proteins Reveals Exceptional Stability of Essential Cellular Structures, Cell, 154 (2013) 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Heo S, Diering GH, Na CH, Nirujogi RS, Bachman JL, Pandey A, Huganir RL, Identification of long-lived synaptic proteins by proteomic analysis of synaptosome protein turnover, Proceedings of the National Academy of Sciences, 115 (2018) E3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clarke S, Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins, Int J Pept Protein Res, 30 (1987) 808–821. [DOI] [PubMed] [Google Scholar]
- [5].Hooi MYS, Truscott RJW, Racemisation and human cataract. d-Ser, d-Asp/Asn and d-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses, Age, 33 (2011) 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lyons B, Kwan AH, Truscott RJW, Spontaneous cleavage of proteins at serine and threonine is facilitated by zinc, Aging Cell, 15 (2016) 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang Z, Lyons B, Truscott RJW, Schey KL, Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates, Aging Cell, 13 (2014) 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Watanabe A, Hong W-K, Dohmae N, Takio K, Morishima-Kawashima M, Ihara Y, Molecular aging of tau: disulfide-independent aggregation and non-enzymatic degradation in vitro and in vivo, Journal of Neurochemistry, 90 (2004) 1302–1311. [DOI] [PubMed] [Google Scholar]
- [9].Fujimoto D, Aging and cross-linking in human aorta, Biochemical and Biophysical Research Communications, 109 (1982) 1264–1269. [DOI] [PubMed] [Google Scholar]
- [10].Tsamis A, Krawiec JT, Vorp DA, Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review, Journal of The Royal Society Interface, 10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Haus JM, Carrithers JA, Trappe SW, Trappe TA, Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle, Journal of Applied Physiology, 103 (2007) 2068–2076. [DOI] [PubMed] [Google Scholar]
- [12].Gautieri A, Redaelli A, Buehler MJ, Vesentini S, Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: Candidate amino acids involved in Advanced Glycation End-products, Matrix Biology, 34 (2014) 89–95. [DOI] [PubMed] [Google Scholar]
- [13].Verzijl N, Bank RA, TeKoppele JM, DeGroot J, AGEing and osteoarthritis: a different perspective, Current Opinion in Rheumatology, 15 (2003) 616–622. [DOI] [PubMed] [Google Scholar]
- [14].Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, Monnier VM, High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis, Proceedings of the National Academy of Sciences, 88 (1991) 10257–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Biemel KM, Friedl DA, Lederer MO, Identification and quantification of major maillard crosslinks in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound, The Journal of biological chemistry, 277 (2002) 24907–24915. [DOI] [PubMed] [Google Scholar]
- [16].Lorand L, Hsu LK, Siefring GE Jr., Rafferty NS, Lens transglutaminase and cataract formation, Proceedings of the National Academy of Sciences of the United States of America, 78 (1981) 1356–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Linetsky M, Hill JM, LeGrand RD, Hu F, Dehydroalanine crosslinks in human lens, Experimental eye research, 79 (2004) 499–512. [DOI] [PubMed] [Google Scholar]
- [18].Friedrich MG, Wang Z, Schey KL, Truscott RJW, Spontaneous crosslinking of proteins at aspartate and asparagine residues is mediated via a succinimide intermediate, Biochemical Journal, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Friedrich MG, Hancock SE, Raftery MJ, Truscott RJW, Isoaspartic acid is present at specific sites in myelin basic protein from multiple sclerosis patients: could this represent a trigger for disease onset?, Acta Neuropathologica Communications, 4 (2016) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hooi M, Truscott R, Racemisation and human cataract. d-Ser, d-Asp/Asn and d-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses, AGE, 33 (2011) 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fujii N, Takemoto LJ, Momose Y, Matsumoto S, Hiroki K, Akaboshi M, Formation of Four Isomers at the Asp-151 Residue of Aged Human αA-Crystallin by Natural Aging, Biochemical and Biophysical Research Communications, 265 (1999) 746–751. [DOI] [PubMed] [Google Scholar]
- [22].Geiger T, Clarke S, Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation, Journal of Biological Chemistry, 262 (1987) 785–794. [PubMed] [Google Scholar]
- [23].Stephenson RC, Clarke S, Succinimide formation from aspartyl and asparaginyl peptides as a model for the spontaneous degradation of proteins, The Journal of biological chemistry, 264 (1989) 6164–6170. [PubMed] [Google Scholar]
- [24].Riggs DL, Gomez SV, Julian RR, Sequence and Solution Effects on the Prevalence of d-Isomers Produced by Deamidation, ACS chemical biology, 12 (2017) 2875–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vlasak J, Ionescu R, Fragmentation of monoclonal antibodies, mAbs, 3 (2011) 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li A, Sowder RC, Henderson LE, Moore SP, Garfinkel DJ, Fisher RJ, Chemical Cleavage at Aspartyl Residues for Protein Identification, Analytical Chemistry, 73 (2001) 5395–5402. [DOI] [PubMed] [Google Scholar]
- [27].Cordoba AJ, Shyong B-J, Breen D, Harris RJ, Non-enzymatic hinge region fragmentation of antibodies in solution, Journal of Chromatography B, 818 (2005) 115–121. [DOI] [PubMed] [Google Scholar]
- [28].Joshi AB, Sawai M, Kearney WR, Kirsch LE, Studies on the Mechanism of Aspartic Acid Cleavage and Glutamine Deamidation in the Acidic Degradation of Glucagon, Journal of Pharmaceutical Sciences, 94 (2005) 1912–1927. [DOI] [PubMed] [Google Scholar]
- [29].Catak S, Monard G, Aviyente V, Ruiz-López MF, Computational Study on Nonenzymatic Peptide Bond Cleavage at Asparagine and Aspartic Acid, The Journal of Physical Chemistry A, 112 (2008) 8752–8761. [DOI] [PubMed] [Google Scholar]
- [30].Wang Z, Schey KL, Quantification of thioether-linked glutathione modifications in human lens proteins, Experimental eye research, 175 (2018) 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang Z, Schey KL, Aquaporin-0 interacts with the FERM domain of ezrin/radixin/moesin proteins in the ocular lens, Investigative ophthalmology & visual science, 52 (2011) 5079–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dasari S, Chambers MC, Slebos RJ, Zimmerman LJ, Ham A-JL, Tabb DL, TagRecon: High-Throughput Mutation Identification through Sequence Tagging, Journal of Proteome Research, 9 (2010) 1716–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gotze M, Pettelkau J, Schaks S, Bosse K, Ihling CH, Krauth F, Fritzsche R, Kuhn U, Sinz A, StavroX--a software for analyzing crosslinked products in protein interaction studies, Journal of the American Society for Mass Spectrometry, 23 (2012) 76–87. [DOI] [PubMed] [Google Scholar]
- [34].Kang HJ, Baker EN, Intramolecular isopeptide bonds: protein crosslinks built for stress?, Trends in Biochemical Sciences, 36 (2011) 229–237. [DOI] [PubMed] [Google Scholar]
- [35].Koretz JF, Cook CA, Kuszak JR, The zones of discontinuity in the human lens: development and distribution with age, Vision research, 34 (1994) 2955–2962. [DOI] [PubMed] [Google Scholar]
- [36].Mindell JA, Lysosomal Acidification Mechanisms, Annual Review of Physiology, 74 (2012) 69–86. [DOI] [PubMed] [Google Scholar]
- [37].Greiner JV, Kopp SJ, Sanders DR, Glonek T, Organophosphates of the crystalline lens: a nuclear magnetic resonance spectroscopic study, Investigative ophthalmology & visual science, 21 (1981) 700–713. [PubMed] [Google Scholar]
- [38].Oliyai C, Borchardt RT, Chemical pathways of peptide degradation. IV. Pathways, kinetics, and mechanism of degradation of an aspartyl residue in a model hexapeptide, Pharmaceutical research, 10 (1993) 95–102. [DOI] [PubMed] [Google Scholar]
- [39].Louis ELM Fieser F, Shriner RL, and Struck HC Succinic Anhydride, Organic Syntheses, 12 (1932) 66. [Google Scholar]
- [40].Gautieri A, Passini FS, Silván U, Guizar-Sicairos M, Carimati G, Volpi P, Moretti M, Schoenhuber H, Redaelli A, Berli M, Snedeker JG, Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue, Matrix Biology, 59 (2017) 95–108. [DOI] [PubMed] [Google Scholar]
- [41].Truscott RJW, Age-related nuclear cataract—oxidation is the key, Experimental eye research, 80 (2005) 709–725. [DOI] [PubMed] [Google Scholar]
- [42].Nagy I, Nagy K, On the role of cross-linking of cellular proteins in aging, Mechanisms of ageing and development, 14 (1980) 245–251. [DOI] [PubMed] [Google Scholar]
- [43].Snedeker JG, Gautieri A, The role of collagen crosslinks in ageing and diabetes - the good, the bad, and the ugly, Muscles, ligaments and tendons journal, 4 (2014) 303–308. [PMC free article] [PubMed] [Google Scholar]
- [44].Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J, Radiocarbon Dating of the Human Eye Lens Crystallines Reveal Proteins without Carbon Turnover throughout Life, PLoS ONE, 3 (2008) e1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kumari SS, Eswaramoorthy S, Mathias RT, Varadaraj K, Unique and Analogous Functions of Aquaporin 0 for Fiber Cell Architecture and Ocular Lens Transparency, Biochimica et biophysica acta, 1812 (2011) 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ortwerth BJ, Olesen PR, Studies on the solubilization of the water-insoluble fraction from human lens and cataract, Experimental eye research, 55 (1992) 777–783. [DOI] [PubMed] [Google Scholar]
- [47].Vázquez de la Torre A, Gay M, Vilaprinyó-Pascual S, Mazzucato R, Serra-Batiste M, Vilaseca M, Carulla N, Direct Evidence of the Presence of Cross-Linked Aβ Dimers in the Brains of Alzheimer’s Disease Patients, Analytical Chemistry, 90 (2018) 4552–4560. [DOI] [PubMed] [Google Scholar]
- [48].Grey AC, Schey KL, Age-related changes in the spatial distribution of human lens alpha-crystallin products by MALDI imaging mass spectrometry, Investigative ophthalmology & visual science, 50 (2009) 4319–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schey KL, Little M, Fowler JG, Crouch RK, Characterization of human lens major intrinsic protein structure, Investigative ophthalmology & visual science, 41 (2000) 175–182. [PubMed] [Google Scholar]
- [50].Slavi N, Wang Z, Harvey L, Schey KL, Srinivas M, Identification and Functional Assessment of Age-Dependent Truncations to Cx46 and Cx50 in the Human Lens, Investigative ophthalmology & visual science, 57 (2016) 5714–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Srivastava OP, Srivastava K, Chaves JM, Gill AK, Post-translationally modified human lens crystallin fragments show aggregation in vitro, Biochemistry and biophysics reports, 10 (2017) 94–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].David LL, Shearer TR, Role of proteolysis in lenses: a review, Lens and eye toxicity research, 6 (1989) 725–747. [PubMed] [Google Scholar]
- [53].Su S-P, Lyons B, Friedrich M, McArthur JD, Song X, Xavier D, Truscott RJW, Aquilina JA, Molecular signatures of long-lived proteins: autolytic cleavage adjacent to serine residues, Aging Cell, 11 (2012) 1125–1127. [DOI] [PubMed] [Google Scholar]
- [54].Ball LE, Garland DL, Crouch RK, Schey KL, Post-translational Modifications of Aquaporin 0 (AQP0) in the Normal Human Lens: Spatial and Temporal Occurrence, Biochemistry, 43 (2004) 9856–9865. [DOI] [PubMed] [Google Scholar]
- [55].Fujii N, Matsumoto S, Hiroki K, Takemoto L, Inversion and isomerization of Asp-58 residue in human alphaA-crystallin from normal aged lenses and cataractous lenses, Biochim Biophys Acta, 1549 (2001) 179–187. [DOI] [PubMed] [Google Scholar]
- [56].Barros TC, Yunes S, Menegon G, Nome F, Chaimovich H, Politi MJ, Dias LG, Cuccovia IM, Hydrolysis of 1,8- and 2,3-naphthalic anhydrides and the mechanism of cyclization of 1,8-naphthalic acid in aqueous solutions, Journal of the Chemical Society, Perkin Transactions 2, (2001) 2342–2350. [Google Scholar]
- [57].Panahi A, Brooks CL, Membrane Environment Modulates the pKa Values of Transmembrane Helices, The Journal of Physical Chemistry B, 119 (2015) 4601–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li N, Fort F, Kessler K, Wang W, Factors affecting cleavage at aspartic residues in model decapeptides, Journal of Pharmaceutical and Biomedical Analysis, 50 (2009) 73–78. [DOI] [PubMed] [Google Scholar]
- [59].Aki K, Okamura E, D-β-aspartyl residue exhibiting uncommon high resistance to spontaneous peptide bond cleavage, Scientific Reports, 6 (2016) 21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Friedrich MG, Truscott RJW, Membrane Association of Proteins in the Aging Human Lens: Profound Changes Take Place in the Fifth Decade of Life, Investigative Ophthalmology & Visual Science, 50 (2009) 4786–4793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.