Abstract
Introduction:
The goal of this review is to highlight the triumphs and frontiers in proteomic measurement of the lens proteome as it relates to onset of age-related nuclear cataract. As global life expectancy increases, so too does the frequency of age-related nuclear cataracts. Molecular therapeutics do not exist for delay or relief of cataract onset in humans. Since lens fiber cells are incapable of protein synthesis after initial maturation, age-related changes in proteome composition and post-translational modification accumulation can be measured with various techniques. Several of these modifications have been associated with cataract onset.
Areas covered:
We discuss the impact of long-lived proteins on the lens proteome and lens homeostasis as well as proteomic techniques that may be used to measure proteomes at various levels of proteomic specificity and spatial resolution.
Expert Opinion:
There is clear evidence that several proteome modifications are correlated with cataract formation. Past studies should be enhanced with cutting-edge, spatially resolved mass spectrometry techniques to enhance the specificity and sensitivity of modification detection as it relates to cataract formation.
Keywords: Lens, Cataract, Spatial Proteomics, Aging, Long Lived Proteins, Mass Spectrometry
1.0. Introduction to Lens Biology and Cataractogenesis
The ocular lens is a transparent tissue suspended behind the cornea and is responsible for transmission and focusing of light to the retina. Gradual opacification of the lens occurs with age and most frequently originates in the central nucleus of the lens, classified as an age-related nuclear cataract (ARNC) (Figure 1) [1]. When light passes through the cataractous lens, it is scattered and the degraded signal limits visual acuity and perception. Cataracts were first documented in ancient Egypt as the ‘white disease of the eye’, and the earliest known descriptions of the lens come from the Roman medical textbook De Medicina written AD 30 [2]. Despite a long history of lens research, cataract causes 51% of global blindness and affects >65% of people over the age of 80 [3,4]. As life expectancies increase, so too will the prevalence of ARNC. In 2010, twenty-four million Americans displayed cataract according to a LOCS II grading scale, but this number is expected to grow to more than fifty million by 2050 [4]. Cataract surgery costs more than $3.5 Billion per year in the US and delay of cataract may subsequently save Billions of dollars annually [5]. To date, no drug treatment has been developed for human cataract and surgical remediation is most often employed when access is available [6]. To reduce anticipated strain on the global medical system, it is desirable to characterize aging and pathology specific molecular changes in the lens for development of pharmaceutical delay or prevention of ARNC.
Figure 1 –
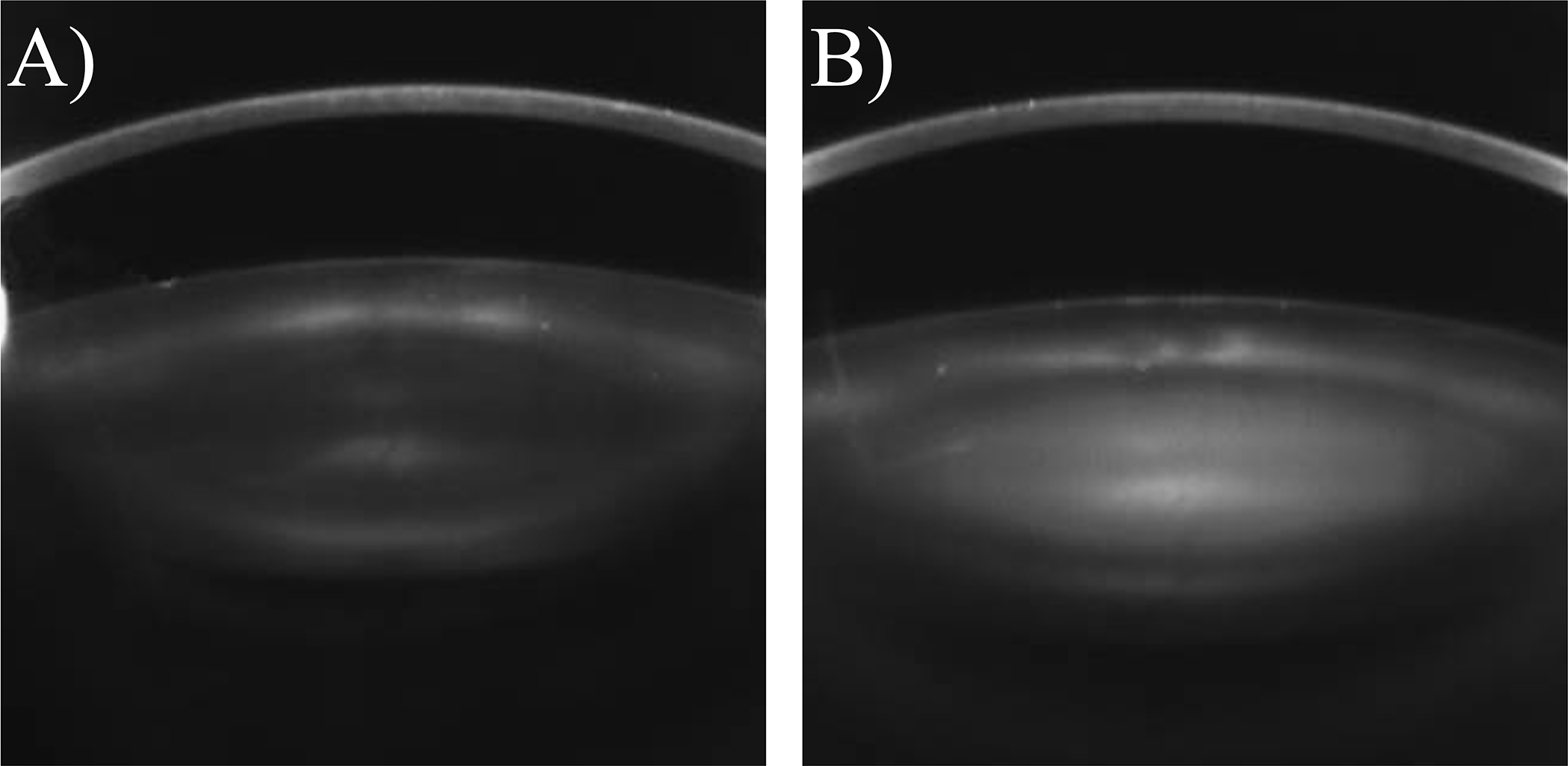
Black-and-white Scheimpflug image of two human lenses along axial plane: A) a healthy lens with limited light refraction, B) a lens with ARNC. Light is strongly scattered through the lens nucleus. Figure adapted from Yonova-Doing et al., 2016.
Lens development begins in embryogenesis with the formation of primary fiber cells and continues throughout life through differentiation of epithelial cells to secondary fiber cells [7,8]. Once differentiated, lens fibers extend towards the anterior and posterior poles to form complex sutures that do not refract light [9]. Each fiber cell is retained throughout life and is not degraded [10]. Continuous fiber cell differentiation and the oblate spheroid nature of the lens affords spatiotemporally organized growth rings wherein the oldest, primary fiber cells are in the centermost nucleus of the lens and recently formed fibers are at the peripheral cortex. Fiber cell organelles are degraded shortly after differentiation with a functional consequence of light-scattering prevention [11]. The molecular consequence of organelle degradation is the loss of protein synthetic ability in mature fiber cells, linking the spatiotemporal gradient of fiber cell positioning to both cell and protein age [12]. Therefore, proteomic techniques are uniquely posed to study all stages of fiber cell aging whereas genomic/transcriptomic techniques are only capable of measuring changes in epithelial cells and young fiber cells. In this review, the lens will be described in generalized age-related regions: the oldest inner nucleus region, the outer nucleus and younger outer cortex region (Figure 2). The outer nucleus region of the lens represents fibers formed during different stages of development based on subject age, but for this review will refer to fibers formed in late childhood and early adulthood.
Figure 2 -.

Cartoon of the axially sliced ocular lens. An enlarged depiction of the inner nucleus (yellow) is surrounded by growth rings of the outer nucleus (orange) and outer cortex (green). Peripheral fibers retain organelles, are differentiated from epithelial cells (grey), and elongate to sutures at each pole, here depicted as a black line. Light passes through the cornea anterior to the lens and is transmitted to the retina positioned posterior to the lens. The entire lens is retained in a collagenous capsule (blue). Lens fibers are not drawn to scale.
In humans, ARNC occurs over the course of decades, making establishment of aging models difficult. For researchers in fields where model systems are well established for in vivo phenotype characterization, this may not be obvious [13]. In contrast to human cataract which develops over several decades, animal models such as the Emory mouse exhibit cataract over a period of 5–8 months [14]. A result is that small molecule cofactor abundances are not modified in animal models and protein changes that occur over decades may not occur. This is true for glutathione (GSH) where high abundances are required in the lens nucleus to reduce the levels of reactive oxygen species [15]. In humans, a barrier to extracellular diffusion and water transport forms in the fifth decade of life at the divide between the outer nucleus and inner cortex region [16]. This barrier prevents GSH transport to the inner regions of the lens, leading to GSH depletion in the lens nucleus [17]. The resulting oxidation of lens nucleus proteins appears to be a precursor to a cascade of events for ARNC in humans [18]. In mouse models, this barrier is not reported, and inconsistent analogues to the human barrier are seen in bovine and rat models [19,20]. For oxidative stress simulation, hyperbaric oxygen (HBO) treated guinea pigs show the greatest similarities to human lens proteome aging and cataractogenesis [21], however, as with other model systems of human ARNC, there are other molecular inconsistencies of aging. We refer readers to the review of Lim et al. [22] for further discussion of cataract models.
In addition to complex suture formation, degradation of organelles and oxidation prevention, lens proteostasis networks establish and maintain transparency over the human lifespan and associated cellular stresses [23]. Cataract formation has been associated with protein aggregation, protein-protein crosslinking, protein insolubilization and proteome imbalance [24–27]. As much as 90% of all lens protein content is part of the highly ordered matrix of crystallin super-family proteins [28,29] believed to both protect against and participate in cataract formation. α-Crystallin is the predominant crystallin species and functions as a small heat shock protein (HSP) to prevent further misfolding of other proteins in the lens [24,30,31]. Larger molecular weight HSP proteins can then refold misfolded species in an ATP-dependent manner – a process that is critical for multi-decade proteostasis [32,33]. Structurally similar β- and γ-crystallin proteins constitute roughly 55% of lens cytoplasmic protein [34]. Each member of the βγ-crystallin family has been associated with transparency and establishment of refractive index [34–36]. Crystallin proteins are also associated with cytoskeletal stabilization, as evidenced by functional modification in other tissues: mutations of the α-crystallin HSPB5 gene in heart tissue are associated with several cardiomyopathies due to altered protein-protein interaction sites [37,38]. Aside from crystallin family proteins, significant components of the lens proteome include ion transporters, channels, gap junction and intermediate filament proteins [39]. Their concerted action maintains an ion gradient capable of generating avascular microcirculation, i.e. metabolite influx and efflux to and from the lens nucleus with essential assistance of aquaporin-0 (AQP0) for water transport [12,40–42].
As a consequence of lifelong protein maintenance, lens proteins undergo substantial modification in the form of both enzymatic and non-enzymatic post-translational modifications (PTMs) as well as protein abundance changes [43,44]. The compound effect of each modification manifests as ARNC; however, no studies have specifically studied the combined effect of multiple modifications in vitro or in vivo.
To characterize the lens proteome, HPLC based detection, two-dimensional gel electrophoresis (2DGE) and various mass spectrometry (MS) approaches have been implemented. However, several outstanding complications of the lens proteome make proteome analysis challenging. Complications include the overwhelming abundance of few crystallin species in the soluble proteome, sampling of the many critical function membrane proteins, the high number of proteoforms caused by compounding PTMs, and transition of proteins from soluble to insoluble due to modification. Each of these complications are significant in ARNC formation and when studied correctly, may uncover biological phenomena in lens aging and ARNC formation and that may lead to new therapeutic directions. In this review we will focus on research directed towards human ARN cataractogenesis using multiple proteomic technologies that address challenges and emphasize on opportunities in lens proteome measurement. We also offer our opinions for several exciting directions in lens proteome research.
2.0. Irreversible Post-Translational Modifications in Lens Aging and Cataractogenesis
In typical human cells, most proteins are maintained for <1–22 hours[45]. Several tissues such as the heart, lungs and brain are composed of proteins that are retained for months, years, or in the case of lens fiber cells for the duration of life. In mature lens fiber cells, in the absence of organelles, no new proteins are synthesized and metabolic function is decreased in the nucleus after the barrier to extracellular diffusion is established [16,46]. Lifelong protein retention allows many enzymatic and non-enzymatic PTMs to occur and, as discussed below, this complicates comprehensive lens proteome analysis. Reversible enzymatic modifications such as phosphorylation [47], ubiquitination [48], methylation [49], lipidation [50] and acetylation [51] occur throughout life, especially during early fiber cell maturation, and are essential for cellular processes including protein-protein interaction [52], cellular signaling [53] and membrane transport [47]. After fiber cells become less metabolically active, irreversible non-enzymatic PTMs occur including oxidation [1], truncation [12], racemization [54], deamidation [55], and cross-linking [25]. Each of these irreversible modifications are hypothesized to be linked to cataractogenesis due to deleterious or modified protein functionality. Each protein may be singly or multiply modified resulting in a complex network of proteoforms that are difficult to fully characterize in a high-throughput manner. Further, methods for age-related PTM characterization should be resolved to enhance understanding of fiber cell aging and proteoform distribution. Here we discuss several irreversible lens PTMs that are most effectively measured as a function of age and the implication of those PTMs towards ARNC formation.
2.1. Truncation
Fragmentation of proteins by N-terminal or C-terminal truncation to form multiple peptides is typically non-enzymatic and is one of the most common irreversible PTMs in the lens. Each major member of the crystallin super-family (α, β, γ) undergoes truncation at the N- and C-terminus [56,57]. Throughout maturation, α-crystallin undergoes successive C-terminal truncation [56] which is contrasted by N-terminal truncation in β-crystallins [58,59]. The majority of γ-crystallin proteins undergo proteolytic degradation throughout fiber maturation, but nearly all proteoforms remaining are truncated to various degrees [58]. In addition to crystallin proteins, truncation has been detected on most other lens proteins that have been examined including AQP0 [55]. C-terminal truncation at Asn259 and Asn246 is readily detected in 7-year old human lenses and by 21 years old, full length AQP0 is not detected in the lens nucleus (Figure 3) [55,60]. Other proteins of the lens that are truncated include connexins 46 and 50 [61] and beaded filament proteins filensin and phakinin [62,63].
Figure 3 –

The spatially resolved abundance of intact and C-terminal truncated (Asn259 and Asn246) AQP0 in aging human lenses is visualized with MALDI imaging mass spectrometry. Intact AQP0 is not detected in the nucleus by the age of 21. Figure adapted from Wenke et al., 2015.
The fragmentation of specific regions of lens proteins may confer additional functionality or have negative pathological effects. In the gap junction protein connexin 50, calpain mediated C-terminal truncation reduces the pH selectivity for gating [64]. As a byproduct of anaerobic glycolysis, high quantities of lactate from the inner nucleus of the lens must be transported of out of the cortex through gap junction networks [65]. The reduced pH selectivity allows for intracellular communication between cortical and nuclear fiber cells. Interestingly, limited C-terminal α-crystallin truncation at Ser172 was found to increase chaperone function [66], but more extensive truncation has been shown to degrade this function. Truncation products of α-crystallin have also been shown to aggregate, create hydrogen peroxide, and induce apoptosis in cultured cells [64]. Further, examination of low molecular weight peptides isolated from 3 whole human lenses indicated that peptides from a 72-year-old lens displayed a greater than two-fold increase in ability to produce hydrogen peroxide than peptides from 17 or 43-year-old lenses. Increased oxidation is believed to be a significant agent of ARNC formation and thus, the peroxide byproduct of α-crystallin truncation may be correlated with cataractogenesis[1,18,67]. The transition of potentially problematic α-crystallin over-chaperoning when mildly truncated to deleterious and damaging when more extensively truncated highlights the difficulty of functionally characterizing truncation PTMs.
2.2. Racemization and Deamidation
The most abundant PTM in the lens is racemization of amino acids from their L-forms to D-forms [68]. At synthesis, proteins are near completely composed of L-form amino acids, but by age 60, every protein in the lens has an average 2–3 D-form amino acids as determined by HPLC separation of hydrolyzed lens lysates [69]. Racemization of amino acids perturbs the tertiary structure of proteins and may change protein solubility and subsequent protein-protein interactions [70,71]. Thus, it is likely that racemization plays a key role in ARNC formation.
Racemization occurs in vitro via an α-proton abstraction followed by re-addition of a proton, and while not validated in vivo, kinetic favorability suggests this process occurs in the lens [72]. For Asn, a more complex pathway (Supplemental Figure 1) affords the joint racemization and deamidation of L-Asn to D-Asp via a cyclic succinimide intermediate. Racemization and isomerization can both be detected using mass spectrometry by radical chromophore modification of peptides followed by LC separation in front of serial CID and Radical Directed Dissociation fragment scans [73,74]. This method relies on a ratio calculation and does not allow complete separation of all products, especially in complex mixtures. Multiple Reaction Monitoring has also been used to identify targeted racemates and isomers of Asp residues in the human lens after LC separation [75]. Deamidation modifications are readily identified on high resolution instruments by simple mass shift (+0.98402 Da) in MS1 spectra and localization of the modification conferred in the MS2 spectra [76,77]. In vivo measurements have subsequently shown D-isoAsp is the most prevalent product of this racemization/deamidation reaction, especially as humans age [78].
Deamidation and racemization may be correlated with cataractogenesis, as demonstrated by the increased abundance of deamidated residues in the insoluble protein fraction of ARNC lenses as compared to cataract-free controls [79]. Abundance of insolubilization is of great importance for cataractogenesis because multiple studies have shown increased protein insolubility in cataractous lenses [26,27,79–82]. To date, no studies have investigated amino acid-specific isomerization in the whole lens proteome due to methodological challenges. Lehmann et al. suggest that the Asp immonium ion, m/z = 88, decreases in isoAsp and the intensity of complementary b and y ions from the X-(L-Asp/L-isoAsp) phosphodiester bond decreases in the isoAsp isomer species relative to Asp [83]. Alternative fragmentation by ECD has also been used to unambiguously delineate isoAsp from Asp [84,85]. Either method cannot separate D-Asp from L-Asp peptide racemates and requires manual interpretation. Alternatively, structure for lossless ion manipulation MS was able to readily separate L-Asp, L-isoAsp, D-Asp and D-isoAsp containing amyloid β peptides [86]. Altogether, racemization/deamidation are critical PTMs in lens aging and cataract formation; future work in isomer composition proteomics may reveal age and/or pathology specific dependencies in proteins outside of the crystallin super-family.
2.4. Cross-Linking
Protein aggregation and insolubilization are well-known processes related to lens aging and cataractogenesis [26,32,70,79]; protein-protein crosslinking plays a critical role in each of these processes [87]. Many groups have studied crosslinking by reversible oxidative disulfide formation [88–93], but irreversible crosslinks have also been detected in lysates treated with denaturing and reducing agents. Several different crosslinking mechanisms have been identified including through advanced glycation end products [94], oxidized ascorbate [95] and dehydroalanine (DHA) formation [96,97].
Among advanced glycation end products, crosslink by pentosidine formation between lysine and arginine residues is a result of a Maillard reaction in oxidized environments [94]. In the presence of a cellular diffusion barrier, reactive oxygen species are not reduced, putatively allowing pentosidine crosslinks to form [1,18,94]. Pentosidine crosslinks have only been studied by amino acid hydrolysis, but there is a positive correlation between age and the abundance of crosslinked pentosidine [94]. In both soluble and insoluble protein fractions, a significantly greater abundance of pentosidine was detected in ARNC lenses as compared to healthy and diabetic cataract lenses.
More recent efforts have identified significant crosslinking through DHA or dehydrobutyrine (DHB) intermediates (Supplemental Figure 2) [96,98]. An intermediate formed by β-elimination of 1) a phosphate group from phosphoserine to DHA or phosphothreonine to DHB, 2) water from serine to DHA and threonine to DHB and 3) hydrogen sulfide or disulfide from cysteine or cystine for DHA. DHA and DHB then react with nucleophilic amine or thiol side chains and metabolites, including GSH, to form irreversible crosslinks. At least 90 sites on 18 lens proteins are covalently modified by GSH [99], yet protein-protein crosslinks may still be formed. It is suggested that GSH forms a covalent bond as a preventative measure against protein-protein crosslinks which have increased abundance in cataractous lenses [97]. Quantitation of protein crosslinks from crystallin proteins do not show specific cataractous trends, but this may be due to many distinct proteoforms not considered in the analysis. A table of crosslinked peptides identified can be found in a recent review from Schey et al. [99].
Identification of lens protein crosslinking sites has been largely facilitated by manual interpretation of tandem MS[96,98,100]. Recent methodological and algorithmic advances in crosslinked peptide identification may facilitate a more extensive identification of cross-linked peptides [101]. To identify endogenous modification sites, bottom-up approaches have been used most frequently, and recent improvements to experimental preparation include strong cation exchange (SCX) enrichment of cross-linked peptides [102], O18 enrichment of cross-linked peptides facilitated by tryptic digest [103] and ultraviolet photodissociation assisted identification of dityrosine crosslinks after GC fractionation [104]. Mixed N14/N15 isotope labeling has also been used in vitro to differentiate intermolecular crosslinks from intramolecular [105]. Automated identification of cross-links has proven more difficult with multiple groups providing rapidly improving algorithms capable of identifying non-cleavable cross-links [106–110]. Altogether, there is a promising future for the identification of high-throughput, site-specific cross-linking sites in the lens with MS technologies. Future improvements in gas phase separations by ion mobility mass spectrometry and data searching improvements are promising directions for the future of cross-linking MS.
2.5. Other Modifications
So far, we have discussed several of the most important irreversible cataract-linked modifications in the lens. However, many other important modifications exist including lipidation, acetylation, carbamylation, oxidation, methylation, glycation and phosphorylation. Lipidation, a PTM process that covalently attaches lipids to proteins and may regulate specific targeting to membranes, was identified on AQP0 by LC-MS/MS and spatially characterized by IMS [50]. Lipidation by oleic acid and palmitic acid was especially prevalent at Lys 238 and the N-terminus in the cortical region after organelle degradation (Supplemental Figure 3) suggesting membrane remodeling as fiber cells mature in adolescence and adulthood. It is likely that AQP0 lipidation is a non-enzymatic process, however enzymatic lipidation by lipid transferases cannot be ruled out [111,112]. Myristoylation and palmitoylation have also been detected in lens fiber cells in global lens proteome analysis [113–115].
Acetylation has been well studied in other tissues but is not well studied in the lens. However, acetylation studies have shown that a majority of lens proteins including α-crystallins, β-crystallins and beaded filament proteins are acetylated at the N-terminus or at the N-terminus of truncated proteoforms, likely as co-translational and post-translational modifications respectively [63]. Lens protein carbamylation also occurs on lens proteins at the N-terminus or lysine residues, and effects α-crystallin activity [116]. Carbamylation appears to be more age-related than acetylation due to age-related distributions of each modification (Schey, unpublished results); however, neither modification has been conclusively linked to ARNC formation. It is clear that oxidation of the lens proteome plays a key role in ARNC formation [1,18,67,90,95], however, proteomic analysis of oxidized residues by either bottom-up or top-down methods may introduce artifacts which hinder oxidative PTM inference [117]. Multiple approaches to identify endogenous oxidized residues are highlighted in the review from Verrastro et al. [117]. Thiol oxidation has been studied in a glutathione deficient (LEGSKO) mouse model and human samples to show that intermediate filament proteins and several enzymes in cataract human lenses were similarly oxidized in the aging, glutathione deficient lens [90]. The abundance of oxidized thiols in this study were not mirrored in transparent lenses, implying pathological consequence of thiol oxidation. The functional consequence of these pathology-related modifications is not known, but enzymes identified in this study, including glutathione reductase, are known to have reduced function in the aging lens [91].
In contrast to the previously discussed modifications, reversible phosphorylation has been studied in depth in the lens with 2DGE, MS and/or extensive fractionation [44,47,118–123]. Proteins in the lens also undergo extensive phosphorylation. At least 271 proteins are phosphorylated at 855 sites and 54 proteins may be phosphorylated at least 5 times [44]. Reversible enzymatic phosphorylation of αA- and αB-crystallins occurs via a cAMP-dependent pathway, though autophosphorylation has also been reported [124]. Crystallin phosphorylation is pervasive with 35–50% of crystallin modified and phosphorylation identified in the lens shortly after birth [125]. The pathological role of lens phosphorylation has not been exhaustively studied in ARNC, however phosphorylation of αA-crystallin and αB-crystallin subunit proteins increases in ARNC lenses and expression is stimulated by oxidative stress [119,126]. In lenses of different age, Gutierrez et al. showed that AQP0 and MP20 phosphorylation decreases across the barrier when established, but remains constant in lenses where the barrier is not established [47]. The pathological role of phosphorylated MP20 is not well established, however AQP0 phosphorylation is known to increase channel water permeability [127]. Altogether, identification of PTMs and the resulting proteoforms, especially as they relate to lens fiber aging and cataractogenesis remains a significant challenge and opportunity in the lens community.
3.0. Global and Spatially Resolved Proteomic Characterization
It stands to reason that functional modification of more than one protein is responsible for ARNC formation due to a complex network of protein-protein interactions. Measurement of such complexity must quantify modified and unmodified proteoforms a measurement that is best executed by LC-MS/MS. Furthermore, in lens proteomics, the spatiotemporal organization of fibers such as fibers of the outer cortex, outer nucleus, and inner nucleus allows improved inference by division of tissue into cell age-specific sub-proteomes. These fibers may be collected either by manual, concentric dissection at lens sutures or equatorial sectioning of the frozen lens followed by concentric biopsy punches. To prevent crystallin products from suppressing the signal of low abundance proteins, solubility fractionation has been widely implemented in lens studies. This often results in fractions including water soluble (WSF), aqueous urea soluble (USF), and urea insoluble (UIF) fractions. The WSF retains the vast majority of crystallins in the lens and is composed of proteins that are less likely to be actively contributing to ARNC. The UIF in contrast is largely composed of membrane and cytoskeletal proteins and the proteins, including many crystallins, that have become insoluble or aggregated in the lens. Proteins in the UIF are most likely to contribute towards cataractogenesis. To further complicate data interpretation, lens proteomes naturally change as a function of age e.g., as fibers age, they lose enzymatic and metabolic activity as associated proteins are degraded.
While LC-MS/MS allows extensive coverage of the lens proteome, well-defined spatial analysis is critical for insights into proteome aging. Although the utility of IMS for spatial characterization of the lens has been demonstrated, several new spatially resolved technologies have emerged that may be useful in future lens studies. Here we describe the impact that whole proteome inference has had in identifying moderated protein networks in ARNC formation, spatial methodologies and their impact, and opportunities for enhanced lens proteomic methods in the future.
3.1. Whole Proteome Measurements
Unlike PTM specific measurements studied in section 2, measurements of the whole lens proteome seek to identify age-related changes in protein and proteoform abundances. Early whole proteome measurements were done with western blotting or 2DGE [46,80,128]. In the healthy lens of a 42-year-old, 2DGE facilitated the visualization of proteome differences in separated regions of the lens (Figure 4). It was clear that as fiber cells age, the majority of proteins are degraded, but the diversity of proteoforms increases as multiply modified proteins increase in abundance (Figure 4A). Crystallins in particular were examined and were found to undergo extensive modification after initial fiber maturation. As a limitation of 2DGE, relatively few proteins and proteoforms could be identified. This limitation was partially addressed by the use of Multi-Dimensional Protein Identification Technology (MuDPIT) LC-MS/MS on the UIF of a whole lens proteome of a 4-year-old congenital cataract patient in 2002 [129]. While not from an ARNC lens, this approach allowed identification of 253 proteins and facilitated the identification of 73 PTM sites. Initial investigation to age-related modifications was done in four lenses: a 3-day old, clear 70-year old, cataractous 70-year old, and cataractous 90-year old. [79]. As with similar studies, solubility fractionation was performed, and cation exchange was used as a second dimension of chromatography. With low resolution instruments operated in Data Dependent Acquisition (DDA) mode, 491 modification sites were identified. Importantly, deamidated residues were more abundant by spectral counting in the insoluble fractions and in older lenses further emphasizing the importance of PTMs in protein solubility and aging.
Figure 4 -.

2DGE of a 42-year old lens with manual dissection and separate analysis of A) the outer cortex, B) outer nucleus, C) inner nucleus, D) the innermost region of the inner nucleus, formed in embryogenesis. Several α-crystallin, β-crystallin and γ-crystallin species are annotated in A. Second dimension of focusing is from acidic (left) to basic (right). Figure from Garland et al., 1995.
By combining solubility fractionation, MuDPIT analysis and improved linear ion trap instrumentation and data analysis, significant improvements in protein and peptide group identifications were reported in 2013 [44]. In the UIF, 951 proteins were identified across 3 lenses (aged 25, 37 and 58 years). Separate phosphopeptide analysis by TiO2 enrichment afforded 855 phosphosites on 271 proteins. Finally, this study was among the first to implement Gene Ontology (GO) analysis in the lens (Table 1). GO analysis facilitates the identification of protein-network enrichment not easily identified with specific protein analyses often directed towards PTMs [130]. Other ontology networks including KEGG [131] have also been used for protein-network annotation [44,132]. This early ontology inference was able to identify several key processes in lens development and aging; however, the study did not employ spatial separation of the older nuclear fibers from the youngest fibers in the cortex and ontological implications were limited by non-quantitative overrepresentation analysis of differentially expressed proteins[133,134].
Table 1 -.
Enriched Gene Ontology IDs and KEGG Pathways estimated by DAVID Bioinformatic tools. Sample collected from 3 human lenses. Table adapted from Wang et al., 2013.
| Terms | Number of Genes | P Value | |
|---|---|---|---|
|
| |||
| Molecular function | Structural molecular activity | 130 | 5.66E-39 |
| GTPase activity | 58 | 6.53E-24 | |
| Protein Binding | 599 | 2.62E-22 | |
| Structural constituent of eye lens | 17 | 1.35E-19 | |
| GTP binding | 71 | 2.26E-19 | |
| Guanyl nucleotide binding | 72 | 2.53E-19 | |
| Catalytic activity | 414 | 6.29E-18 | |
| Nucleotide binding | 209 | 7.14E-14 | |
| Structural constituent of cytoskeleton | 26 | 1.28E-13 | |
| Pyrophosphate activity | 96 | 1.43E-13 | |
| Biological process | Translational elongation | 43 | 9.63E-26 |
| Cellular carbohydrate catabolic process | 38 | 1.12E-23 | |
| Alcohol catabolic process | 36 | 2.45E-22 | |
| Glucose catabolic process | 30 | 5.98E-21 | |
| Glucose metabolic process | 46 | 2.63E-20 | |
| Glycolysis | 16 | 4.27E-19 | |
| Small GTPase mediated signal transduction | 58 | 3.05E-15 | |
| Cellular component assembly | 110 | 4.35E-14 | |
| Actin filament-based process | 43 | 1.49E-10 | |
| Cytoskeleton organization | 62 | 1.55E-10 | |
| KEGG pathway | Ribosome | 40 | 7.96E-20 |
| Glycolysis/Gluconeogenesis | 29 | 4.61E-15 | |
| Pentose phosphate pathway | 14 | 2.27E-08 | |
| Proteasome | 17 | 8.07E-07 | |
| Focal adhesion | 39 | 1.83E-06 | |
| Pyruvate metabolism | 14 | 1.58E-05 | |
| Tight junction | 28 | 1.82E-05 | |
| Gap junction | 21 | 4.46E-05 | |
| Glutathione metabolism | 15 | 4.96E-05 | |
| Regulation of actin cytoskeleton | 36 | 1.03E-04 | |
Early MS proteome measurements were carried out on low-resolution mass spectrometers. The introduction of high-resolution orbitrap mass analyzers have greatly improved protein quantification for high dynamic range samples such as lens protein lysates [135,136]. To identify proteome differences between whole lenses of different disease state (age related cortical & nuclear cataract, congenital cataract, regenerative lenses with secondary cataract and healthy lenses), one study utilized protein extracts from eight lenses for MS analysis without fractionation or spatial segmentation[132]. Assisted by orbitrap instrumentation, 1,251 protein groups were identified alongside ontology pathways specific to the nuclear & cortical cataract lens including: endocytosis, salivary secretion, proximal tubule bicarbonate reclamation, vasopressin-regulated water reabsorption, and endocrine and other factor-related calcium reabsorption. Additionally, insignificant abundance differences of αA and αB-crystallin in cataractous lenses compared to healthy lenses was shown, while γ-crystallins and several β-crystallin subunits displayed near zero abundance in the age-related cataract lenses. These results added to the assumption that WSF and USF are not highly informative for studies of ARNC progression.
3.2. Opportunities for Improvements in Whole Proteome Measurement
Significant MS advances have been made that may facilitate the advancement of global lens proteomics. The first of these is high field orbitraps and high-resolution TOF instruments that facilitate Data Independent Acquisition (DIA) scan modes [137,138]. Many lens proteins, especially those in the UIF are of comparatively low abundance when co-eluted with crystallins and are not detected in Data Dependent Acquisition (DDA) scan modes with only one sample injection. This limitation of DDA has been previously overcome with basic reverse phase (BRP) pre-fractionation to identify >5,000 proteins in juvenile mouse lenses [39], but is not practical for large cohorts of samples where spatial separation, solubility fractionation, multiple pathologies and ages are of interest due to the long gradients employed and limited quantitative reproducibility [139]. Sequential small-mass window scanning limits the quantity of peptides co-isolated, especially when overlapping window strategies are employed [140] (Supplemental Figure 4). Advances in DIA analysis software include: library preparation with retention-time defined chromatography [138], machine learning developed libraries [141,142] and improved algorithms for searching of multiple proteoforms [143–145]. For an introduction to DIA methodology, we recommend the tutorial from Reubsaet et al. [146] and an article presented in the processing pipeline of Searle et al. [138]. In addition to improved identification over MuDPIT and BRP, DIA allows more accurate quantitation than LFQ and isobaric reporter tag technologies in DDA, especially when MS1 and MS2 spectra are considered simultaneously [147,148]. A second preparative advancement is on-membrane capture and tryptic digest for improved isolation of lens membrane proteins [149] (Schey, unpublished results). An ideal global proteomics experiment should employ lenses of different ages that are cataractous and non-cataractous, spatial separation of fiber cells of different age, solubility fractionation, effective protein isolation and digestion, and modern DIA detection and analysis. High throughput methods that maximize protein quantitation and bioinformatic analyses while limiting sample handling should also be used [150,151].
Bioinformatic analysis of whole-proteome MS datasets has not been performed in part due to limitations with cohort size and measurement approach used. To date, no whole-proteome datasets of human lenses have been analyzed in sufficient quantity or quantitative quality for network-based analysis of ARNC formation. Several studies in other tissues have highlighted effective methodology for detection of age-related or pathology-related changes [152–156]. Significant trends between these and similar reports include 1) Differential expression analysis, 2) Dimensional reduction, 3) Ontological overexpression, 4) Ontological enrichment, and 5) Protein-protein interaction network analysis. There is a significant positive impact for each of these analyses when a greater quantity of biological replicates is used. Successful application of each of these adjustments to previous studies of ARNC proteomic approaches will then enhance the quality of protein-protein interaction network analysis. It is possible that a successful study would provide a network-level interpretation of age-related changes and classification of those changes as normal processes in aging or pathological ARNC formative networks.
3.3. Spatially Resolved Lens Proteomics
As emphasized above, it is of great importance to spatially resolve findings to produce new ARNC directed insights regarding the spatiotemporally organized lens. In measurements of the entire lens, young fiber cells are not separated from metabolically inactive fibers that have sustained optical transparency for several decades which decreases the impact of the measurements. In a similar way, a discontinuous, non-gaussian boundary of fibers with and without organelles lies in the cortical region, adjacent to the barrier to extracellular diffusion that forms in the fifth decade of human life. Thus, even segmentation of the lens into one or a few concentric regions for LC-MS/MS analysis does not effectively capture the continuous fiber-cell aging processes that occur throughout life. Spatially resolved proteomics investigation must effectively coordinate spatial resolution, network coverage, and acquisition time. Here we discuss the impact that imaging mass spectrometry (IMS), micro liquid extraction surface analysis (microLESA), laser capture microdissection (LCM) coupled to nanodroplet processing in one pot for trace samples (nanoPOTS), and multi-isotope imaging mass spectrometry (MIMS) have had in spatial mapping of the lens and the future opportunities they provide.
Often called a “molecular microscope”, MALDI IMS has been used to detect protein, lipid, and metabolite relative abundances in a spatially resolved, untargeted manner [12,30,55,114,115]. In MALDI IMS, tissue sections are coated in a matrix that facilitates ionization upon laser ablation. Pulsed sampling in a rastered step allows for assembly of molecular information that correlates to the tissue localization. Heatmap plotting of selected signal abundance results in an image displaying molecular spatial distributions. Typical protein images are compiled at 50 um step/pixel size but spatial resolution may be reduced to 5 um with optimized laser ablation [157]. Intact protein IMS with time of flight mass analyzers effectively detect low weight proteins in the 5 – 25 kDa range and can discriminate modified from unmodified proteins [12,158]. In the lens, this allows measurement of AQP0 and crystallin family proteins, but prevents detection of large undigested proteins such as connexin and intermediate filament families of proteins (Figure 3). To circumvent this limitation, bottom-up approaches can be implemented with on-tissue tryptic digest prior to MALDI ionization. Subsequent products can then be detected by both time of flight and FT-ICR analyzers. Even with bottom-up approaches, MALDI IMS experiments do not capture as complete of a protein network as LC-MS/MS experiments, resulting in incomplete analysis of low and intermediate abundance products. To improve peptide isolation, tissue washing prior to digestion, increased pixel size and continuous accumulation of selected ion (CASI) in an ion trap have been implemented to improve signal intensity [159]. Finally, MS2 spectra are typically not collected in IMS experiments, so secondary MS/MS experiments are needed for definitive peptide identification [160]. MALDI IMS is then capable of multi-cell resolution, may achieve notable proteome depth, and can be performed in reasonable acquisition times.
To demonstrate the success of MALDI IMS in lens studies, Wang et al. confirmed LCM-LC-MS/MS findings from Wenke et al. that there is a transition of ubiquitous vimentin in the outer cortical fibers to lens specific beaded filament proteins filensin and phakinin in the inner cortex of the lens (Supplemental Figure 5) [115,160]. In this study of moderately abundant, larger molecular weight cytoskeletal proteins, successive washes were performed prior to on-tissue digest. A 50-um ablation spot was used with a 150-um raster to optimize signal intensity on a 15T FTICR instrument. Additionally, CASI was employed to enhance the signal of pre-selected modified peptides with validation by LC-MS/MS. While this study of a bovine lens was not targeted for detection of ARNC formation, it highlights the value of spatial mapping of the lens for detection of cell-age specific changes. IMS has been used to indicate where crystallin truncations and AQP0 modification occur in human lenses as a function of age [55,161,162].
Though low abundance proteins can be detected in IMS experiments with the assistance of CASI, network coverage is not comparable to the proteome identified by LC-MS/MS studies. MicroLESA [163] has been used in previous lens analyses to capture on-tissue digested lysates at >100 um radius [115]. In serial sections of bovine lenses from aforementioned study, the microLESA method allowed identification and relative quantitation of intermediate filament proteins and each truncation product. MicroLESA results enhanced the hypothesis of lipidation shortly after filament switch to lens specific proteins but was not used for analysis of the lens proteome at large. Since microLESA employs LC-MS/MS, it allows in-depth proteome network analysis, but relative to other technologies does so at low spatial resolution and with long acquisition times. Combining microLESA and IMS is a natural technological progression given the identification bottleneck posed by IMS and the spatial targeting desired in microLESA. Consequently, top-down IMS has previously been paired to ETD LC-MS where ETD samples are collected by 1 uL on-tissue extractions to unambiguously identify crystallin proteins including truncated βA3-crystallin which will not be identified in a bottom-up approach [164]. The combination of ETD LC-MS paired to IMS has been expanded on recently to map β and γ crystallin protein spatial distribution in the bovine lens [162]. There remains significant space for expansion of top-down MS lens studies – especially those evaluating spatial distribution of PTMs.
In the lens, LCM has been used to collect small, spatially targeted regions of the lens before trace LC-MS/MS sample analysis [165,166]. Recently, trace LCM-MS/MS was used to characterize proteins at either side of the extracellular diffusion barrier [166]. Developments in single-cell proteomics have ushered in new low-sample methodologies, such as nanoPOTS, capable of rapidly characterizing >2,000 protein groups on an LC-MS/MS platform [167–169]. Like other single-cell technologies, the nanoPOTS workflow begins with FACS for cell capture [167,168,170,171]. Subsequent deposition onto a lithographically created microwell plate and automated sub-microliter reagent additions for lysis, reduction, alkylation and digest is performed to isolate peptides in a reproducible fashion [167]. To reduce signal-to-noise, isobaric labeling strategies such as iBASIL and SCOPE-MS have been employed to maximize identifications [168,171]. A particular strength of the nanoPOTS platform introduced by Kelly et al. is label free trace sample preparations [167]. Recently, nanoPOTS was extended to imaging technologies by dissecting tissue with LCM directly onto microwell plate [172]. In mouse uterine tissue, this method facilitated cell-type specific identification of differentiation based on >2,000 identified protein groups at 100 um2 voxel area. Such technology applied to the lens may allow expansion of IMS network coverage and subsequent knowledge of fiber differentiation at fine spatial and molecular resolution. Relative to each previously described method, nanoPOTS is capable of moderate spatial resolution that exceeds current capabilities of microLESA and is one of the best methods for proteome network coverage but requires lengthy sample acquisition times.
Each of the previous methods is capable of proteome characterization of separate bundles of concentric fiber cells. However, these methods are incapable of mapping subcellular proteome organization or differentiating single fiber cells. Due to the 0.75 – 14 um cross section of fiber cells (depending on axis and cell age, older are smaller), the most effective technology for nanometer resolution definition is a microscopy-based approach [10]. Outside of standard immunohistochemistry experiments, CODEX microscopy offers an exciting method to spatially coordinate >100 proteins on a single tissue section but has not yet been applied to age-related tissue measurements. CODEX functions by conjugating unique nucleotide extenders to primary antibodies allowing simultaneous submicron resolution in a semiquantitative manner. [173]. Recent developments to hybridize secondary ion MS (SIMS) with scanning electron microscopy (SEM) yielded MIMS technology capable of proteomic characterization at 50 nm resolution [174]. Unlike other methods, MIMS does not allow identification of individual proteins because aged protein signatures are identified by diet supplemented N15 isotope. However, MIMS does allow measurement of aged proteins in a pulse chase-format of labeling. The best implementation of MIMS may be achieved with lenses of HBO guinea pigs to study the rate of cellular differentiation and migration towards the lens nucleus [21]. MIMS offers the best spatial resolution of any presented method but has the slowest sample collection rate and has the most limited proteome network coverage. Nonetheless, MIMS and similar microscopy techniques have important implications for lens proteome and ARNC characterization. The relative strengths and weaknesses of each discussed method is summarized in Figure 5.
Figure 5 -.

Summary of relative spatial resolution, proteome networks coverage and rate of detection for each of the discussed technologies. Differences are not uniform, especially in comparisons of spatial resolution.
4.0. Classification of Age and Pathology
The outstanding goal of ARNC proteomics research is to determine the impact that age-related single protein modifications and network-wide perturbations have on ARNC occurrence. Thus, the features in cataractous subjects that have clear abundance separation from the same feature in healthy lenses are of highest interest. Plotting feature intensity alongside subject age and calculating a linear regression is the most common method for identifying age-related changes. Pathological feature classification is presumptively simpler, comparing same age donors at various LOCS II grades to determine feature that are significantly differentiated with cataract intensity. However, limitations in cohort composition (e.g., size of cohort, subject age range, morbidity control, extent and type of cataract) reduce statistical power in past studies. Here we review studies that identify age-related features in the lens, identify features specific to ARNC and opportunities in the lens community.
4.1. Age-Related PTMs
As discussed, PTMs are inevitable in long-lived protein aging and play key roles in the functionality and metabolic activity of lens fibers. Racemization of L-amino acids to D-amino acids plays a key role in the functional modification of aged fiber cells [69,70,78,78]. Indeed, racemization has been analyzed in fossils to estimate age [175–177]. Racemization specific to individual amino acids in lenses of different age and pathology revealed a clear correlation of Asx (Asp and Asn) and Ser with age and pathology, but weaker trends with Thr, Phe, Glx (Glu and Gln), Leu, Ile, Val and Ala [69]. This study was expanded to look at α- and γ-crystallin racemization, deamidation, and isomerization in lenses of different age. Unlike the trends in Asx and Ser, no linear relationship was detected, but elevations in αA-crystallin D-Asp, deamidation, isoAsp and γs-crystallin deamidation, and isoAsp percent were seen in ARNC lenses, and increased with age [78].
4.2. Whole Proteome Measurement Correlation with Age
Classification of lens age or pathology based on a whole proteome measurement requires sufficient network coverage to minimize unmeasured feature, so-called “missing data”, impact on model development. Surprisingly, there are relatively few studies that correlate age with whole lens proteome changes. In the lens, an ontological protein analysis detected 19 ontologies differentially expressed by increased protein abundance, however this analysis was limited to one age-related cataract that encompassed cortical fibers and nuclear fibers[132]. Additionally, quantitative co-dependent enrichment was not calculated, which is now computationally realistic with GSEA or PSEA-quant algorithms [133]. In other long lived protein containing tissues, LC-MS/MS has been used for detection of proteins and protein networks which are most influential in aging and pathology by employing machine learning classification [154,156,178]. In cartilage, measurement of age-matched subjects revealed significant differences between Kashin-Beck disease and osteoarthritis tissues [156]. While each disease is similar, 375 proteins were found to be differentially expressed and were significantly associated with cell junctions, and signal transducer activity. In muscle fiber proteomics from 60 healthy patients, differences between patients along a continuous age gradient was readily detected by PCA feature extraction, yielding 361 proteins with reduced representation and 904 with increased representation as a function of age [178]. Overexpression analysis of the differentially expressed genes demonstrated that ribosomal proteins and proteins involved in energetic metabolism were downregulated with age, while proteins associated with immunity, proteostasis and alternative splicing were upregulated. Since strictly healthy patients were used in this cohort, no biomarker of disease was analyzed. The successes of biomarker and age-determination studies clearly paves a path for the future of ARNC proteomics research, and continuous improvements in the methods for analysis must be actively considered for effective investigation.
5.0. Expert Opinion
As humans age, the lens will inevitably undergo cataractogenesis requiring intervention to restore vision. The current lack of pharmacological cataract treatment is a growing problem as population size and life expectancy increase. Strain on the medical system already leads to a subset of the population not having access to care, and that subset is set to grow with increasing surgical needs. To develop a pharmacological intervention, it is necessary that a molecular understanding of ARNC formation be further developed.
Long-lived proteins in the lens undergo significant post translational and abundance modifications over the course of life, resulting in ARNC through protein aggregation, protein insolubilization, proteome instability and protein-protein crosslinking. No genomic or transcriptomic technique can measure the age-related changes leading to ARNC. The inherent spatiotemporal organization of the lens lends itself to age-related investigation, but the outstanding abundance of crystallin proteins limits detection of other lens proteins without prior sample fractionation. Past studies of the lens demonstrated that fiber cells of different age and lenses of different age display ongoing modification to proteoform composition and abundance. To measure these changes, various techniques have been used, but ongoing improvements to proteomics methodology are advantageous to effectively measure the spatially dispersed and heavily modified lens proteome. Ongoing efforts may contribute to the development of ARNC pharmaceuticals.
5.1. Strengths
The measurement of lens proteomes has been aided by the steady improvement in sensitivity and specificity of proteomic technologies. As emphasized above, the well-defined organization of lens fiber cells lends itself to spatially resolved proteome measurements of age-related changes that may be causal in ARNC formation. Solubility fractionation for membrane enrichment and on-tissue digests for spatially unbiased bottom-up IMS acquisition are just two of the many sample preparation advancements already employed. Instrumentally, advances in orbitrap and TOF mass analyzers have allowed improved quantitative measurement and IMS provides a clear example of spatially resolved data attainable by pairing MS to imaging principles (nanoPOTS, MIMS, SIM, microLESA). Improvements in computational algorithms have also allowed improved analysis of protein networks and more automatable identification of crosslinks and PTMs with static mass. Each of these strengths will undoubtedly continue to be utilized in future lens proteome research and ongoing developments will improve measurement of cataract-specific perturbations in lens aging.
5.2. Weaknesses
As a byproduct of the ongoing improvements in lens protein measurement, inherent weaknesses in past studies have reduced the potential impact of findings. These weaknesses can be summarized as inefficient identification of low abundance proteins, inefficient detection of proteins due to the overwhelming abundance of crystallins, PTM identification, lack of spatial resolution, clear differentiation of racemate and isomer products and limited quantitation. Statistical significance is diminished as a byproduct and clear variations between healthy and cataractous lenses are less likely. In addition to these weaknesses, the vast majority of lens measurements take into account the equatorial age-related architecture of lens fibers but omit measurements along the axis of light travel. In recent findings, there is a clear influx of metabolites at the anterior and posterior pole of the lens and efflux through the equatorial region of the lens. Studies that do not differentially measure fibers at different axial and equatorial positionings then reduce biological accessibility of insights and are subsequently less effective at describing the age-related changes. Finally, as is true in many bioinformatic analyses, ontology should not be inferred by Boolean detection of a protein. Even filtering of the n-most abundant proteins is a detriment to the mathematical basis of ontological assignment.
Even though weaknesses, such as cross-link identification are abundant in lens proteome measurement, developing methods for enrichment and interpretation algorithms also demonstrate the rapid improvements and future opportunities within the mass spectrometry community.
5.3. Opportunities
Exciting developments in proteomic analysis certainly extend to ongoing research in the lens community. Throughout this review, several cutting-edge methods have been highlighted to address current weaknesses and these should be applied to compare healthy lenses to ARNC lenses. In addition to described bottom-up techniques, there is a clear future for top-down proteomics implementation. Work from the Kelleher group [179] has demonstrated the ability of top-down platforms to identify multiply modified proteoforms in complex mixtures. It is also clear that native MS should be used to study protein oligomer complexes which are expected to change as a function of age – especially in the crystallin family of proteins. Additionally, measurement of proteome heterogeneity either by intact single-cell proteomics or microscopy hybridized techniques may lend insight into fiber cell maturation and age-related modifications that lead to cataractogenesis. Finally, it is critical that the lens is studied as a three-dimensional tissue. The long fiber cells of the lens display non-uniform protein distribution, thus subcellular organization of proteins must be studied alongside age-related changes at the lens equator. In future studies, it is advantageous to study larger cohorts to provide statistical power. Finally, statistical power should be capitalized on by using abundance dependent analyses of protein networks and related ontologies.
5.4. Outlook
In the next 5–10 years, we anticipate that many if not all of the emerging techniques be implemented in the lens. One of the limitations to sensitive measurement has been accessibility of instrumentation capable of advanced techniques such as DIA and the accessibility of software packages to search the data. In 2021, there is growing access to the next generation of high-resolution, rapid scanning analyzers and software packages are under constant development and improvement for identification of proteins, peptides and post-translational modifications. Implementation of these techniques will help expand the library of known modifications and potentially facilitate discovery of new modifications. Direct analyses of healthy and ARNC lenses may then be able to identify proteome modifications that can be approached by medicinal chemistry. Inside and outside the lens community it is expected that spatially resolved measurements will become prevalent in tissue-based measurements. Finally, mounting successes with single-cell proteomics leads the authors to believe that single-lens fiber proteomics will soon be possible to measure early fiber cell development and changes sustained over mammalian and/or human life.
There is a large and growing need for a non-surgical ARNC intervention. It is the hope of the authors that clear delineation of cataractogenic modifications to the lens proteome will enable such an intervention. In a broader context, it is expected that results from lens studies and age-related measurements could be extended to the brain, lung, heart and other tissues that host long-lived proteins to acquire and interpret data regarding other aging pathologies.
Supplementary Material
Article Highlights:
With age, all human lenses develop age-related cataract, but drug-based interventions do not exist.
Lens fiber cell organelles are degraded during maturation, but cells and their protein content are retained throughout life - reducing measurement of macromolecular aging to protein-centric techniques.
Spatiotemporal organization of the lens allows fiber cell age-resolved protein measurement of long-lived proteins.
Previous studies have highlighted the importance of post-translational modification accumulation and proteome composition for lens aging and cataractogenesis.
Future studies of cataract formation should implement cutting-edge techniques that coordinate fiber cell positioning with spatially-resolved high-resolution protein measurement.
Funding
This paper was, in part, supported by NIH grants R01 EY013463 (KLS), R01 EY024258 (KLS), P30 EY008126 and T32 GM065086 (LSC).
Glossary
- ARNC
Age Related Nuclear Cataract
- LOCS
Lens Opacity Classification System
- HSP
Heat Shock Protein
- PTMs
Post Translational Modifications
- MS
Mass Spectrometry
- HPLC
High Performance Liquid Chromatography
- AQP0
Aquaporin-0
- MS/MS
Tandem Mass Spectrometry
- DHA
Dehydroalanine
- DHB
Dehydrobutyrine
- IMS
Imaging Mass Spectrometry
- SCX
Strong Cation Exchange Chromatography
- GC-MS
Gas Chromatography Mass Spectrometry
- LC-MS/MS
Liquid Chromatography Tandem Mass Spectrometry
- WSF
Water Soluble Fraction
- USF
Urea Soluble Fraction
- UIF
Urea Insoluble Fraction
- 2DGE
2-Dimensional Gel Electrophoresis
- MuDPIT
Multi-Dimensional Proteomic Identification Technology
- DDA
Data Dependent Acquisition
- DIA
Data Independent Acquisition
- GO
Gene Ontology
- BRP
Basic Reverse Phase
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- microLESA
micro-Liquid Extraction Surface Analysis
- LCM
Laser Capture Microdissection
- nanoPOTS
nanodroplet Processing in One pot for Trace Samples
- MIMS
Multiple Ion Mass Spectrometry
- MALDI
Matrix Assisted Laser Desorption Ionization
- FT-ICR
Fourier Transform Ion Cyclotron Resonance
- CASI
Continuous Accumulation of Serial Ions
- ETD
Electron Transfer Dissociation
- iBASIL
improved Boosting to Amplify Signal with Isobaric Labeling
- SCOPE-MS
Single-Cell ProtEomics by Mass Spectrometry
- CODEX
CO-Detection by indEXing
- SEM
Scanning Electron Microscopy
- HBO
Hyperbaric Oxygen
- PCA
Principal Component Analysis
- GSEA
Gene Set Enrichment Analysis
- PSEA
Protein Set Enrichment Analysis
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- [1]. Hains PG, Truscott RJW. Proteomic analysis of the oxidation of cysteine residues in human age-related nuclear cataract lenses. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2008;1784:1959–1964. ** Highlights cataract specific oxidation as a proof of oxidation being a key feature of ARNC formation.
- [2].Albert DM, Edwards DD, editors. The history of ophthalmology. Cambridge, Mass., USA: Blackwell Science; 1996. [Google Scholar]
- [3].McCarty CA, Mukesh BN, Fu CL, et al. The epidemiology of cataract in Australia. Am J Ophthalmol. 1999;128:446–465. [DOI] [PubMed] [Google Scholar]
- [4].2010 Cataract Tables [Internet]. National Eye Institute; 2010. Available from: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics/cataract-tables.
- [5].Taylor A Role of Nutrients in Delaying Cataracts. Annals of the New York Academy of Sciences. 1992;669:111–123. [DOI] [PubMed] [Google Scholar]
- [6].Yonova-Doing E, Forkin ZA, Hysi PG, et al. Genetic and Dietary Factors Influencing the Progression of Nuclear Cataract. Ophthalmology. 2016;123:1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].O’Rahilly R, Müller F. Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs. 2010;192:73–84. [DOI] [PubMed] [Google Scholar]
- [8].Nagineni CN, Bhat SP. Lens fiber cell differentiation and expression of crystallins in Co-cultures of human fetal lens epithelial cells and fibroblasts. Experimental Eye Research. 1992;54:193–200. [DOI] [PubMed] [Google Scholar]
- [9].Kuszak JR, Sivak JG, Weerheim JA. Lens optical quality is a direct function of lens sutural architecture. Invest Ophthalmol Vis Sci. 1991;32:2119–2129. [PubMed] [Google Scholar]
- [10].Taylor VL, al-Ghoul KJ, Lane CW, et al. Morphology of the normal human lens. Invest Ophthalmol Vis Sci. 1996;37:1396–1410. [PubMed] [Google Scholar]
- [11].Lim JC, Walker KL, Sherwin T, et al. Confocal Microscopy Reveals Zones of Membrane Remodeling in the Outer Cortex of the Human Lens. Investigative Opthalmology & Visual Science. 2009;50:4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Korlimbinis A, Berry Y, Thibault D, et al. Protein aging: truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp Eye Res. 2009;88:966–973. *Excellent presentation of age-related AQP0 truncation and its putative role in barrier formation.
- [13].Truscott RJW, Friedrich MG. Molecular Processes Implicated in Human Age-Related Nuclear Cataract. Invest Ophthalmol Vis Sci. 2019;60:5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kuck JF, Kuwabara T, Kuck KD. The Emory mouse cataract: an animal model for human senile cataract. Curr Eye Res. 1981;1:643–649. [DOI] [PubMed] [Google Scholar]
- [15].Reddy VN. Glutathione and its function in the lens—An overview. Experimental Eye Research. 1990;50:771–778. [DOI] [PubMed] [Google Scholar]
- [16].Moffat BA, Landman KA, Truscott RJW, et al. Age-related Changes in the Kinetics of Water Transport in Normal Human Lenses. Experimental Eye Research. 1999;69:663–669. [DOI] [PubMed] [Google Scholar]
- [17].Sweeney MH, Truscott RJ. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp Eye Res. 1998;67:587–595. [DOI] [PubMed] [Google Scholar]
- [18].Truscott RJW. Age-related nuclear cataract—oxidation is the key. Experimental Eye Research. 2005;80:709–725. [DOI] [PubMed] [Google Scholar]
- [19].Grey AC, Jacobs MD, Gonen T, et al. Insertion of MP20 into lens fibre cell plasma membranes correlates with the formation of an extracellular diffusion barrier. Exp Eye Res. 2003;77:567–574. [DOI] [PubMed] [Google Scholar]
- [20].Vaghefi E, Walker K, Pontre BP, et al. Magnetic resonance and confocal imaging of solute penetration into the lens reveals a zone of restricted extracellular space diffusion. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2012;302:R1250–R1259. [DOI] [PubMed] [Google Scholar]
- [21].Giblin FJ, Padgaonkar VA, Leverenz VR, et al. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Experimental Eye Research. 1995;60:219–235. [DOI] [PubMed] [Google Scholar]
- [22].Lim JC, Umapathy A, Donaldson PJ. Tools to fight the cataract epidemic: A review of experimental animal models that mimic age related nuclear cataract. Experimental Eye Research. 2016;145:432–443. [DOI] [PubMed] [Google Scholar]
- [23].Wu S-Y, Zou P, Fuller AW, et al. Expression of Cataract-linked γ-Crystallin Variants in Zebrafish Reveals a Proteostasis Network That Senses Protein Stability. J Biol Chem. 2016;291:25387–25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rao PV, Huang Q, Horwitz J, et al. Evidence that α-crystallin prevents non-specific protein aggregation in the intact eye lens. Biochimica et Biophysica Acta (BBA) - General Subjects. 1995;1245:439–447. [DOI] [PubMed] [Google Scholar]
- [25].Buckingham RH. The behaviour of reduced proteins from normal and cataractous lenses in highly dissociating media: Cross-linked protein in cataractous lenses. Experimental Eye Research. 1972;14:123–129. [DOI] [PubMed] [Google Scholar]
- [26].Kopylova LV, Cherepanov IV, Snytnikova OA, et al. Age-related changes in the water-soluble lens protein composition of Wistar and accelerated-senescence OXYS rats. Mol Vis. 2011;17:1457–1467. [PMC free article] [PubMed] [Google Scholar]
- [27].Schmid PWN, Lim NCH, Peters C, et al. Imbalances in the eye lens proteome are linked to cataract formation. Nat Struct Mol Biol. 2021;28:143–151. [DOI] [PubMed] [Google Scholar]
- [28].Bloemendal H The vertebrate eye lens. Science. 1977;197:127–138. [DOI] [PubMed] [Google Scholar]
- [29].Trokel S The physical basis for transparency of the crystalline lens. Invest Ophthalmol. 1962;1:493–501. [PubMed] [Google Scholar]
- [30].Grey AC, Schey KL. Distribution of bovine and rabbit lens alpha-crystallin products by MALDI imaging mass spectrometry. Mol Vis. 2008;14:171–179. [PMC free article] [PubMed] [Google Scholar]
- [31].Horwitz J Alpha-crystallin can function as a molecular chaperone. Proceedings of the National Academy of Sciences. 1992;89:10449–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rao PV, Huang QL, Horwitz J, et al. Evidence that alpha-crystallin prevents non-specific protein aggregation in the intact eye lens. Biochim Biophys Acta. 1995;1245:439–447. [DOI] [PubMed] [Google Scholar]
- [33].Wang K, Spector A. α-Crystallin prevents irreversible protein denaturation and acts cooperatively with other heat-shock proteins to renature the stabilized partially denatured protein in an ATP-dependent manner: α-Crystallin chaperone activity. European Journal of Biochemistry. 2000;267:4705–4712. [DOI] [PubMed] [Google Scholar]
- [34].Bloemendal H, de Jong W, Jaenicke R, et al. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. [DOI] [PubMed] [Google Scholar]
- [35].Wang X, Garcia CM, Shui Y-B, et al. Expression and Regulation of α-, β-, and γ-Crystallins in Mammalian Lens Epithelial Cells. Investigative Opthalmology & Visual Science. 2004;45:3608. [DOI] [PubMed] [Google Scholar]
- [36].Slingsby C, Clout NJ. Structure of the crystallins. Eye (Lond). 1999;13 ( Pt 3b):395–402. [DOI] [PubMed] [Google Scholar]
- [37].Brodehl A, Gaertner-Rommel A, Klauke B, et al. The novel αB-crystallin (CRYAB) mutation p.D109G causes restrictive cardiomyopathy. Hum Mutat. 2017;38:947–952. [DOI] [PubMed] [Google Scholar]
- [38].Inagaki N, Hayashi T, Arimura T, et al. Alpha B-crystallin mutation in dilated cardiomyopathy. Biochem Biophys Res Commun. 2006;342:379–386. [DOI] [PubMed] [Google Scholar]
- [39].Zhao Y, Wilmarth PA, Cheng C, et al. Proteome-transcriptome analysis and proteome remodeling in mouse lens epithelium and fibers. Experimental Eye Research. 2019;179:32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schey KL, Petrova RS, Gletten RB, et al. The Role of Aquaporins in Ocular Lens Homeostasis. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sindhu Kumari S, Gupta N, Shiels A, et al. Role of Aquaporin 0 in lens biomechanics. Biochemical and Biophysical Research Communications. 2015;462:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mathias RT, Rae JL. Transport properties of the lens. American Journal of Physiology-Cell Physiology. 1985;249:C181–C190. [DOI] [PubMed] [Google Scholar]
- [43].MacCoss MJ, McDonald WH, Saraf A, et al. Shotgun identification of protein modifications from protein complexes and lens tissue. Proc Natl Acad Sci U S A. 2002;99:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Wang Z, Han J, David LL, et al. Proteomics and Phosphoproteomics Analysis of Human Lens Fiber Cell Membranes. Investigative Opthalmology & Visual Science. 2013;54:1135. ** This paper is the most-in depth analysis of lens fiber cell phospho/proteomes and highlights the ontological composition of the lens from a whole-tissue perspective
- [45].Eden E, Geva-Zatorsky N, Issaeva I, et al. Proteome half-life dynamics in living human cells. Science. 2011;331:764–768. [DOI] [PubMed] [Google Scholar]
- [46]. Garland DL, Duglas-Tabor Y, Jimenez-Asensio J, et al. The Nucleus of the Human Lens: Demonstration of a Highly Characteristic Protein Pattern by Two-Dimensional Electrophoresis and Introduction of a New Method of Lens Dissection. Experimental Eye Research. 1996;62:285–292. *An early 2DGE measurement demonstrates that fiber cell maturation results in proteomes that are unique as a function of spatial positioning.
- [47].Gutierrez DB, Garland DL, Schwacke JH, et al. Spatial distributions of phosphorylated membrane proteins aquaporin 0 and MP20 across young and aged human lenses. Exp Eye Res. 2016;149:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pereira P, Shang F, Hobbs M, et al. Lens fibers have a fully functional ubiquitin-proteasome pathway. Exp Eye Res. 2003;76:623–631. [DOI] [PubMed] [Google Scholar]
- [49].Truscott RJW, Mizdrak J, Friedrich MG, et al. Is protein methylation in the human lens a result of non-enzymatic methylation by S-adenosylmethionine? Exp Eye Res. 2012;99:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schey KL, Gutierrez DB, Wang Z, et al. Novel Fatty Acid Acylation of Lens Integral Membrane Protein Aquaporin-0. Biochemistry. 2010;49:9858–9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nahomi RB, Oya-Ito T, Nagaraj RH. The combined effect of acetylation and glycation on the chaperone and anti-apoptotic functions of human α-crystallin. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2013;1832:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tanimura S, Takeda K. ERK signalling as a regulator of cell motility. The Journal of Biochemistry. 2017;162:145–154. [DOI] [PubMed] [Google Scholar]
- [53].Betts MJ, Wichmann O, Utz M, et al. Systematic identification of phosphorylation-mediated protein interaction switches. Iakoucheva LM, editor. PLOS Computational Biology. 2017;13:e1005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fujii N, Takemoto LJ, Momose Y, et al. Formation of four isomers at the asp-151 residue of aged human alphaA-crystallin by natural aging. Biochem Biophys Res Commun. 1999;265:746–751. [DOI] [PubMed] [Google Scholar]
- [55].Wenke JL, Rose KL, Spraggins JM, et al. MALDI Imaging Mass Spectrometry Spatially Maps Age-Related Deamidation and Truncation of Human Lens Aquaporin-0. Invest Ophthalmol Vis Sci. 2015;56:7398–7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Takemoto LJ. Identification of the in vivo truncation sites at the C-terminal region of alpha-A crystallin from aged bovine and human lens. Current Eye Research. 1995;14:837–841. [DOI] [PubMed] [Google Scholar]
- [57].Srivastava OP, McEntire JE, Srivastava K. Identification of a 9 kDa γ-crystallin fragment in human lenses. Experimental Eye Research. 1992;54:893–901. [DOI] [PubMed] [Google Scholar]
- [58].Lampi KJ, Ma Z, Hanson SRA, et al. Age-related Changes in Human Lens Crystallins Identified by Two-dimensional Electrophoresis and Mass Spectrometry. Experimental Eye Research. 1998;67:31–43. [DOI] [PubMed] [Google Scholar]
- [59].Srivastava OP, Srivastava K. βB2-crystallin undergoes extensive truncation during aging in human lenses. Biochemical and Biophysical Research Communications. 2003;301:44–49. [DOI] [PubMed] [Google Scholar]
- [60].Gutierrez DB, Garland D, Schey KL. Spatial analysis of human lens aquaporin-0 post-translational modifications by MALDI mass spectrometry tissue profiling. Experimental Eye Research. 2011;93:912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Slavi N, Wang Z, Harvey L, et al. Identification and Functional Assessment of Age-Dependent Truncations to Cx46 and Cx50 in the Human Lens. Investigative Opthalmology & Visual Science. 2016;57:5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sandilands A, Prescott AR, Hutcheson AM, et al. Filensin is proteolytically processed during lens fiber cell differentiation by multiple independent pathways. Eur J Cell Biol. 1995;67:238–253. [PubMed] [Google Scholar]
- [63].Wang Z, Obidike JE, Schey KL. Posttranslational Modifications of the Bovine Lens Beaded Filament Proteins Filensin and CP49. Investigative Opthalmology & Visual Science. 2010;51:1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Raju M, Santhoshkumar P, Sharma KK. Lens Endogenous Peptide αA66–80 Generates Hydrogen Peroxide and Induces Cell Apoptosis. Aging and Disease. 2017;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kinoshita JH. PATHWAYS OF GLUCOSE METABOLISM IN THE LENS. Invest Ophthalmol. 1965;4:619–628. [PubMed] [Google Scholar]
- [66].Aziz A, Santhoshkumar P, Sharma KK, et al. Cleavage of the C-Terminal Serine of Human αA-Crystallin Produces αA 1–172 with Increased Chaperone Activity and Oligomeric Size †. Biochemistry. 2007;46:2510–2519. [DOI] [PubMed] [Google Scholar]
- [67]. Berthoud VM, Beyer EC. Oxidative stress, lens gap junctions, and cataracts. Antioxid Redox Signal. 2009;11:339–353. * This review demonstrates the complexity of oxidative stress response in lens aging and speaks at greater depth to the coordination of proteins in the microcirculatory system.
- [68].Truscott RJW, Schey KL, Friedrich MG. Old Proteins in Man: A Field in its Infancy. Trends Biochem Sci. 2016;41:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hooi MYS, Truscott RJW. Racemisation and human cataract. d-Ser, d-Asp/Asn and d-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. AGE. 2011;33:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Luthra M, Ranganathan D, Ranganathan S, et al. Racemization of tyrosine in the insoluble protein fraction of brunescent aging human lenses. J Biol Chem. 1994;269:22678–22682. [PubMed] [Google Scholar]
- [71].Magami K, Kim I, Fujii N. A single Asp isomer substitution in an αA-crystallin-derived peptide induces a large change in peptide properties. Exp Eye Res. 2020;192:107930. [DOI] [PubMed] [Google Scholar]
- [72].Bada JL, Protsch R. Racemization Reaction of Aspartic Acid and Its Use in Dating Fossil Bones. Proceedings of the National Academy of Sciences. 1973;70:1331–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Tao Y, Julian RR. Identification of Amino Acid Epimerization and Isomerization in Crystallin Proteins by Tandem LC-MS. Anal Chem. 2014;86:9733–9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tao Y, Quebbemann NR, Julian RR. Discriminating D -Amino Acid-Containing Peptide Epimers by Radical-Directed Dissociation Mass Spectrometry. Anal Chem. 2012;84:6814–6820. [DOI] [PubMed] [Google Scholar]
- [75].Fujii N, Takata T, Kim I, et al. Simultaneous and Rapid Detection of Multiple Epimers and Isomers of Aspartyl Residues in Lens Proteins Using an LC-MS-MRM Method. ACS Omega. 2020;5:27626–27632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hains PG, Truscott RJW. Age-dependent deamidation of lifelong proteins in the human lens. Invest Ophthalmol Vis Sci. 2010;51:3107–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Robinson NE, Lampi KJ, McIver RT, et al. Quantitative measurement of deamidation in lens betaB2-crystallin and peptides by direct electrospray injection and fragmentation in a Fourier transform mass spectrometer. Mol Vis. 2005;11:1211–1219. [PMC free article] [PubMed] [Google Scholar]
- [78].Hooi MYS, Raftery MJ, Truscott RJW. Racemization of Two Proteins over Our Lifespan: Deamidation of Asparagine 76 in γS Crystallin Is Greater in Cataract than in Normal Lenses across the Age Range. Investigative Opthalmology & Visual Science. 2012;53:3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wilmarth PA, Tanner S, Dasari S, et al. Age-Related Changes in Human Crystallins Determined from Comparative Analysis of Post-translational Modifications in Young and Aged Lens: Does Deamidation Contribute to Crystallin Insolubility? Journal of Proteome Research. 2006;5:2554–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].McFall-Ngai MJ, Ding LL, Takemoto LJ, et al. Spatial and temporal mapping of the age-related changes in human lens crystallins. Exp Eye Res. 1985;41:745–758. [DOI] [PubMed] [Google Scholar]
- [81].Forsythe HM, Vetter CJ, Jara KA, et al. Altered Protein Dynamics and Increased Aggregation of Human γS-Crystallin Due to Cataract-Associated Deamidations. Biochemistry. 2019;58:4112–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Vetter CJ, Thorn DC, Wheeler SG, et al. Cumulative deamidations of the major lens protein ГS -crystallin increase its aggregation during unfolding and oxidation. Protein Science. 2020;29:1945–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lehmann WD, Schlosser A, Erben G, et al. Analysis of isoaspartate in peptides by electrospray tandem mass spectrometry. Protein Sci. 2000;9:2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cournoyer JJ, Lin C, O’Connor PB. Detecting Deamidation Products in Proteins by Electron Capture Dissociation. Anal Chem. 2006;78:1264–1271. [DOI] [PubMed] [Google Scholar]
- [85].Sargaeva NP, Lin C, O’Connor PB. Identification of aspartic and isoaspartic acid residues in amyloid beta peptides, including Abeta1–42, using electron-ion reactions. Anal Chem. 2009;81:9778–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zheng X, Deng L, Baker ES, et al. Distinguishing d- and l-aspartic and isoaspartic acids in amyloid β peptides with ultrahigh resolution ion mobility spectrometry. Chem Commun (Camb) 2017;53:7913–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dilley KJ, Pirie A. Changes to the proteins of the human lens nucleus in cataract. Experimental Eye Research. 1974;19:59–72. [DOI] [PubMed] [Google Scholar]
- [88].Lou MF, Dickerson JE. Protein-thiol mixed disulfides in human lens. Experimental Eye Research. 1992;55:889–896. [DOI] [PubMed] [Google Scholar]
- [89].Lou MF, Dickerson JE, Garadi R. The role of protein-thiol mixed disulfides in cataractogenesis. Experimental Eye Research. 1990;50:819–826. [DOI] [PubMed] [Google Scholar]
- [90].Wang B, Hom G, Zhou S, et al. The oxidized thiol proteome in aging and cataractous mouse and human lens revealed by ICAT labeling. Aging Cell. 2017;16:244–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wei M, Xing K-Y, Fan Y-C, et al. Loss of Thiol Repair Systems in Human Cataractous Lenses. Investigative Ophthalmology & Visual Science. 2015;56:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Takemoto L Increase in the Intramolecular Disulfide Bonding of Alpha-A Crystallin During Aging of the Human Lens. Experimental Eye Research. 1996;63:585–590. [DOI] [PubMed] [Google Scholar]
- [93].Yu NT, DeNagel DC, Pruett PL, et al. Disulfide bond formation in the eye lens. Proceedings of the National Academy of Sciences. 1985;82:7965–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nagaraj RH, Sell DR, Prabhakaram M, et al. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proceedings of the National Academy of Sciences. 1991;88:10257–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Linetsky M, Shipova E, Cheng R, et al. Glycation by ascorbic acid oxidation products leads to the aggregation of lens proteins. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2008;1782:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wang Z, Lyons B, Truscott RJW, et al. Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging Cell. 2014;13:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Linetsky M, Hill JMW, LeGrand RD, et al. Dehydroalanine crosslinks in human lens. Experimental Eye Research. 2004;79:499–512. * This paper demonstrates the correlation of cross-linked proteins and cataract formation.
- [98].Friedrich MG, Wang Z, Schey KL, et al. Spontaneous cross-linking of proteins at aspartate and asparagine residues is mediated via a succinimide intermediate. Biochemical Journal. 2018;475:3189–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Schey KL, Wang Z, Friedrich MG, et al. Spatiotemporal changes in the human lens proteome: Critical insights into long-lived proteins. Progress in Retinal and Eye Research. 2020;76:100802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wang Z, Friedrich MG, Truscott RJW, et al. Cleavage C-terminal to Asp leads to covalent crosslinking of long-lived human proteins. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2019;1867:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hägglund P, Mariotti M, Davies MJ. Identification and characterization of protein cross-links induced by oxidative reactions. Expert Review of Proteomics. 2018;15:665–681. [DOI] [PubMed] [Google Scholar]
- [102].Fritzsche R, Ihling CH, Götze M, et al. Optimizing the enrichment of cross-linked products for mass spectrometric protein analysis: Enrichment of cross-linked products for MS protein analysis. Rapid Communications in Mass Spectrometry. 2012;26:653–658. [DOI] [PubMed] [Google Scholar]
- [103].Liu M, Zhang Z, Zang T, et al. Discovery of undefined protein cross-linking chemistry: a comprehensive methodology utilizing 18O-labeling and mass spectrometry. Anal Chem. 2013;85:5900–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Mukherjee S, Fang M, Kok WM, et al. Establishing Signature Fragments for Identification and Sequencing of Dityrosine Cross-Linked Peptides Using Ultraviolet Photodissociation Mass Spectrometry. Analytical Chemistry. 2019;91:12129–12133. [DOI] [PubMed] [Google Scholar]
- [105].Taverner T, Hall NE, O’Hair RAJ, et al. Characterization of an Antagonist Interleukin-6 Dimer by Stable Isotope Labeling, Cross-linking, and Mass Spectrometry. Journal of Biological Chemistry. 2002;277:46487–46492. [DOI] [PubMed] [Google Scholar]
- [106].Hoopmann MR, Zelter A, Johnson RS, et al. Kojak: Efficient Analysis of Chemically Cross-Linked Protein Complexes. Journal of Proteome Research. 2015;14:2190–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Rinner O, Seebacher J, Walzthoeni T, et al. Identification of cross-linked peptides from large sequence databases. Nature Methods. 2008;5:315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Götze M, Pettelkau J, Schaks S, et al. StavroX—A Software for Analyzing Crosslinked Products in Protein Interaction Studies. Journal of The American Society for Mass Spectrometry. 2012;23:76–87. [DOI] [PubMed] [Google Scholar]
- [109].Yang B, Wu Y-J, Zhu M, et al. Identification of cross-linked peptides from complex samples. Nature Methods. 2012;9:904–906. [DOI] [PubMed] [Google Scholar]
- [110].Chen Z-L, Meng J-M, Cao Y, et al. A high-speed search engine pLink 2 with systematic evaluation for proteome-scale identification of cross-linked peptides. Nat Commun. 2019;10:3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ismail VS, Mosely JA, Tapodi A, et al. The lipidation profile of aquaporin-0 correlates with the acyl composition of phosphoethanolamine lipids in lens membranes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2016;1858:2763–2768. [DOI] [PubMed] [Google Scholar]
- [112].Muszbek L, Haramura G, Cluette-Brown JE, et al. The pool of fatty acids covalently bound to platelet proteins by thioester linkages can be altered by exogenously supplied fatty acids. Lipids. 1999;34:S331–S337. [DOI] [PubMed] [Google Scholar]
- [113].Thinon E, Serwa RA, Broncel M, et al. Global profiling of co- and post-translationally N-myristoylated proteomes in human cells. Nat Commun. 2014;5:4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wang Z, Schey KL. Proteomic Analysis of S-Palmitoylated Proteins in Ocular Lens Reveals Palmitoylation of AQP5 and MP20. Investigative Opthalmology & Visual Science. 2018;59:5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Wang Z, Ryan DJ, Schey KL. Localization of the lens intermediate filament switch by imaging mass spectrometry. Exp Eye Res. 2020;198:108134. * A spatially resolved, high sensitivity approach to protein measurement that emphasizes the impact of proteome composition in fiber cell maturation.
- [116].Nagaraj RH, Nahomi RB, Shanthakumar S, et al. Acetylation of αA-crystallin in the human lens: effects on structure and chaperone function. Biochim Biophys Acta. 2012;1822:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Verrastro I, Pasha S, Jensen KT, et al. Mass spectrometry-based methods for identifying oxidized proteins in disease: advances and challenges. Biomolecules. 2015;5:378–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Aquilina JA, Benesch JLP, Ding LL, et al. Phosphorylation of αB-Crystallin Alters Chaperone Function through Loss of Dimeric Substructure. Journal of Biological Chemistry. 2004;279:28675–28680. [DOI] [PubMed] [Google Scholar]
- [119].Kamei A, Takamura S, Nagai M, et al. Phosphoproteome Analysis of Hereditary Cataractous Rat Lens α-Crystallin. Biological & Pharmaceutical Bulletin. 2004;27:1923–1931. [DOI] [PubMed] [Google Scholar]
- [120].Moroni M, Garland D. In vitro dephosphorylation of α-crystallin is dependent on the state of oligomerization. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 2001;1546:282–290. [DOI] [PubMed] [Google Scholar]
- [121].Schaefer H, Marcus K, Sickmann A, et al. Identification of phosphorylation and acetylation sites in alpha-A crystallin of the eye lens (mus musculus) after two-dimensional gel electrophoresis. Analytical and Bioanalytical Chemistry. 2003;376:966–972. [DOI] [PubMed] [Google Scholar]
- [122].Chiou S-H, Huang C-H, Lee I-L, et al. Identification of in vivo phosphorylation sites of lens proteins from porcine eye lenses by a gel-free phosphoproteomics approach. Mol Vis. 2010;16:294–302. [PMC free article] [PubMed] [Google Scholar]
- [123].Huang C-H, Wang Y-T, Tsai C-F, et al. Phosphoproteomics characterization of novel phosphorylated sites of lens proteins from normal and cataractous human eye lenses. Mol Vis. 2011;17:186–198. [PMC free article] [PubMed] [Google Scholar]
- [124].Kantorow M, Piatigorsky J. Phosphorylations of αA- and αB-crystallin. International Journal of Biological Macromolecules. 1998;22:307–314. [DOI] [PubMed] [Google Scholar]
- [125].Thornell E, Aquilina A. Regulation of αA- and αB-crystallins via phosphorylation in cellular homeostasis. Cell Mol Life Sci. 2015;72:4127–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wang K, Ma W, Spector A. Phosphorylation of alpha-crystallin in rat lenses is stimulated by H2O2 but phosphorylation has no effect on chaperone activity. Exp Eye Res. 1995;61:115–124. [DOI] [PubMed] [Google Scholar]
- [127].Lindsey Rose KM, Wang Z, Magrath GN, et al. Aquaporin 0−Calmodulin Interaction and the Effect of Aquaporin 0 Phosphorylation. Biochemistry. 2008;47:339–347. [DOI] [PubMed] [Google Scholar]
- [128].Delamere NA, Tamiya S. Expression, regulation and function of Na,K-ATPase in the lens. Prog Retin Eye Res. 2004;23:593–615. [DOI] [PubMed] [Google Scholar]
- [129].MacCoss MJ, McDonald WH, Saraf A, et al. Shotgun identification of protein modifications from protein complexes and lens tissue. Proceedings of the National Academy of Sciences. 2002;99:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wu X, Liu Z, Zhang X, et al. Proteomics analysis and proteogenomic characterization of different physiopathological human lenses. BMC Ophthalmol. 2017;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Lavallée-Adam M, Rauniyar N, McClatchy DB, et al. PSEA-Quant: a protein set enrichment analysis on label-free and label-based protein quantification data. J Proteome Res. 2014;13:5496–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Timmons JA, Szkop KJ, Gallagher IJ. Multiple sources of bias confound functional enrichment analysis of global -omics data. Genome Biol. 2015;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Hu Q, Noll RJ, Li H, et al. The Orbitrap: a new mass spectrometer. J Mass Spectrom. 2005;40:430–443. [DOI] [PubMed] [Google Scholar]
- [136].Krey JF, Wilmarth PA, Shin J-B, et al. Accurate label-free protein quantitation with high- and low-resolution mass spectrometers. J Proteome Res. 2014;13:1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Bruderer R, Bernhardt OM, Gandhi T, et al. Extending the Limits of Quantitative Proteome Profiling with Data-Independent Acquisition and Application to Acetaminophen-Treated Three-Dimensional Liver Microtissues. Molecular & Cellular Proteomics. 2015;14:1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138]. Searle BC, Pino LK, Egertson JD, et al. Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nat Commun. 2018;9. * For those interested in DIA, we recommend this article, which gives in-depth considerations to each step of DIA acquisition and analysis.
- [139].Washburn MP, Ulaszek RR, Yates JR. Reproducibility of Quantitative Proteomic Analyses of Complex Biological Mixtures by Multidimensional Protein Identification Technology. Analytical Chemistry. 2003;75:5054–5061. [DOI] [PubMed] [Google Scholar]
- [140].Amodei D, Egertson J, MacLean BX, et al. Improving Precursor Selectivity in Data-Independent Acquisition Using Overlapping Windows. J Am Soc Mass Spectrom. 2019;30:669–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Gessulat S, Schmidt T, Zolg DP, et al. Prosit: proteome-wide prediction of peptide tandem mass spectra by deep learning. Nature Methods. 2019;16:509–518. [DOI] [PubMed] [Google Scholar]
- [142].Bekker-Jensen DB, Bernhardt OM, Hogrebe A, et al. Rapid and site-specific deep phosphoproteome profiling by data-independent acquisition without the need for spectral libraries. Nat Commun. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Demichev V, Messner CB, Vernardis SI, et al. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nature Methods. 2020;17:41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Egertson JD, MacLean B, Johnson R, et al. Multiplexed peptide analysis using data-independent acquisition and Skyline. Nat Protoc. 2015;10:887–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Tsou C-C, Avtonomov D, Larsen B, et al. DIA-Umpire: comprehensive computational framework for data-independent acquisition proteomics. Nat Methods. 2015;12:258–264, 7 p following 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Reubsaet L, Sweredoski MJ, Moradian A. Data-Independent Acquisition for the Orbitrap Q Exactive HF: A Tutorial. Journal of Proteome Research. 2019;18:803–813. [DOI] [PubMed] [Google Scholar]
- [147].Muntel J, Kirkpatrick J, Bruderer R, et al. Comparison of Protein Quantification in a Complex Background by DIA and TMT Workflows with Fixed Instrument Time. Journal of Proteome Research. 2019;18:1340–1351. [DOI] [PubMed] [Google Scholar]
- [148].Huang T, Bruderer R, Muntel J, et al. Combining Precursor and Fragment Information for Improved Detection of Differential Abundance in Data Independent Acquisition. Molecular & Cellular Proteomics. 2020;19:421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Yang Y, Anderson E, Zhang S. Evaluation of six sample preparation procedures for qualitative and quantitative proteomics analysis of milk fat globule membrane. Electrophoresis. 2018;39:2332–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].McKetney J, Runde RM, Hebert AS, et al. Proteomic Atlas of the Human Brain in Alzheimer’s Disease. J Proteome Res. 2019;18:1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Overmyer KA, Shishkova E, Miller IJ, et al. Large-Scale Multi-omic Analysis of COVID-19 Severity. Cell Systems. 2021;12:23–40.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Dai Q, Escobar GP, Hakala KW, et al. The Left Ventricle Proteome Differentiates Middle-Aged and Old Left Ventricles in Mice. Journal of Proteome Research. 2008;7:756–765. [DOI] [PubMed] [Google Scholar]
- [153].Grant JE, Bradshaw AD, Schwacke JH, et al. Quantification of protein expression changes in the aging left ventricle of Rattus norvegicus. J Proteome Res. 2009;8:4252–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Angelidis I, Simon LM, Fernandez IE, et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat Commun. 2019;10:963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Schwarzer C, Siatkowski M, Pfeiffer MJ, et al. Maternal age effect on mouse oocytes: new biological insight from proteomic analysis. REPRODUCTION. 2014;148:55–72. [DOI] [PubMed] [Google Scholar]
- [156].Lei J, Amhare AF, Wang L, et al. Proteomic analysis of knee cartilage reveals potential signaling pathways in pathological mechanism of Kashin-Beck disease compared with osteoarthritis. Sci Rep. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Zavalin A, Yang J, Haase A, et al. Implementation of a Gaussian beam laser and aspheric optics for high spatial resolution MALDI imaging MS. J Am Soc Mass Spectrom. 2014;25:1079–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Chaurand P, Latham JC, Lane KB, et al. Imaging mass spectrometry of intact proteins from alcohol-preserved tissue specimens: bypassing formalin fixation. J Proteome Res. 2008;7:3543–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Fuchser J, Cornett S, Becker M. High Resolution Molecular Imaging of Pharmaceuticals at Therapeutic Levels. Bruker Daltonics. 2014; [Google Scholar]
- [160].Wenke JL, McDonald WH, Schey KL. Spatially Directed Proteomics of the Human Lens Outer Cortex Reveals an Intermediate Filament Switch Associated With the Remodeling Zone. Invest Ophthalmol Vis Sci. 2016;57:4108–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Grey AC, Schey KL. Age-related changes in the spatial distribution of human lens alpha-crystallin products by MALDI imaging mass spectrometry. Invest Ophthalmol Vis Sci. 2009;50:4319–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Anderson DM, Nye-Wood MG, Rose KL, et al. MALDI imaging mass spectrometry of β- and γ-crystallins in the ocular lens. J Mass Spectrom. 2020;55:e4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Ryan DJ, Patterson NH, Putnam NE, et al. MicroLESA: Integrating Autofluorescence Microscopy, In Situ Micro-Digestions, and Liquid Extraction Surface Analysis for High Spatial Resolution Targeted Proteomic Studies. Analytical Chemistry. 2019;91:7578–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Schey KL, Anderson DM, Rose KL. Spatially-Directed Protein Identification from Tissue Sections by Top-Down LC-MS/MS with Electron Transfer Dissociation. Anal Chem. 2013;85:6767–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Wang Z, Han J, Schey KL. Spatial Differences in an Integral Membrane Proteome Detected in Laser Capture Microdissected Samples. J Proteome Res. 2008;7:2696–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Wang Z, Schey KL. Spatially-Resolved Proteomic Analysis of the Lens Extracellular Diffusion Barrier [Internet]. Biochemistry; 2021. [cited 2021 Feb 25]. Available from: 10.1101/2021.02.23.432581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Zhu Y, Piehowski PD, Zhao R, et al. Nanodroplet processing platform for deep and quantitative proteome profiling of 10–100 mammalian cells. Nat Commun. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Budnik B, Levy E, Harmange G, et al. SCoPE-MS: mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biol. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Dou M, Clair G, Tsai C-F, et al. High-Throughput Single Cell Proteomics Enabled by Multiplex Isobaric Labeling in a Nanodroplet Sample Preparation Platform. Analytical Chemistry. 2019;91:13119–13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Specht H, Emmott E, Petelski A, et al. Single-cell mass-spectrometry quantifies the emergence of macrophage heterogeneity. bioRxiv [Internet]. 2019. [cited 2019 Dec 10]; Available from: 10.1101/665307. [DOI] [Google Scholar]
- [171].Tsai C-F, Zhao R, Williams SM, et al. An Improved Boosting to Amplify Signal with Isobaric Labeling (iBASIL) Strategy for Precise Quantitative Single-cell Proteomics. Mol Cell Proteomics. 2020;19:828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Piehowski PD, Zhu Y, Bramer LM, et al. Automated mass spectrometry imaging of over 2000 proteins from tissue sections at 100-μm spatial resolution. Nat Commun. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Goltsev Y, Samusik N, Kennedy-Darling J, et al. Deep Profiling of Mouse Splenic Architecture with CODEX Multiplexed Imaging. Cell. 2018;174:968–981.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Arrojo e Drigo R, Lev-Ram V, Tyagi S, et al. Age Mosaicism across Multiple Scales in Adult Tissues. Cell Metabolism. 2019;30:343–351.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Griffin RC, Chamberlain AT, Hotz G, et al. Age estimation of archaeological remains using amino acid racemization in dental enamel: A comparison of morphological, biochemical, and known ages-at-death. American Journal of Physical Anthropology. 2009;140:244–252. [DOI] [PubMed] [Google Scholar]
- [176].Ohtani S, Yamamoto T. Age estimation by amino acid racemization in human teeth. J Forensic Sci. 2010;55:1630–1633. [DOI] [PubMed] [Google Scholar]
- [177].Yasunaga G, Pastene LA, Bando T, et al. Age estimation of Antarctic minke whales Balaenoptera bonaerensis based on aspartic acid racemization technique. Fisheries Science. 2017;83:947–954. [Google Scholar]
- [178].Ubaida-Mohien C, Lyashkov A, Gonzalez-Freire M, et al. Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. eLife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Kafader JO, Melani RD, Durbin KR, et al. Multiplexed mass spectrometry of individual ions improves measurement of proteoforms and their complexes. Nat Methods. 2020;17:391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


