Abstract
(1) Background: Periimplantitis is an infectious condition that affects the periimplant tissue and is of bacterial etiology. However, to date, the exact bacterial flora involved in its occurrence is not known. The aim of this literature review was to summarize the articles published on this topic and to identify the main bacterial species isolated in periimplantitis. (2) Methods: The articles published in three databases were researched: Pubmed, Embase and Web of Science using Prisma guides and combinations of MeSH terms. We selected 25 items from the 980 found by applying the inclusion and exclusion criteria. (3) Results: We quantified the results of the 25 studies included in this review. In general, the most commonly identified bacterial species were Gram-negative anaerobic species, as Prevotella, Streptococcus, Fusobacterium and Treponema. (4) Conclusion: The most frequent bacteria in the periimplantitis sites identified in this review are Gram-negative anaerobic species, also involved in the pathogenesis of the periodontal disease.
Keywords: periimplantitis, dental implants, bacteria, bacterial species, bacterial strains, microorganisms
1. Introduction
The loss of dental units due to increased life expectancy, aging, lack of regular check-ups at the dentist, poor oral hygiene or various accidents leads to the installation of edentulousness. Once edentation appears, the functionality of the dento-maxillary apparatus, the homeostasis of the alveolar bone, as well as the balance of the digestive and nervous system are affected by the psychological and masticatory implications of tooth loss.
In recent years, the concern among dental practitioners for finding prosthetic rehabilitation solutions as functional as possible and for preserving the remaining dental structures has led to the widespread use of dental implants as a treatment solution of edentulous patients. There are several types of biomaterials from which dental implants can be made, the most used being titanium and titanium alloys, materials with high biocompatibility, high success rates over long periods of follow-up and good mechanical properties [1]. Although titanium implants are widely used, they also have drawbacks such as the gray color that can be visible in the frontal area with thin gingival tissue, which is why more aesthetic alternatives have been found. One of these is the use of Zirconia for dental implants, a material that has aesthetic properties as translucency, the color similar to natural teeth and biocompatibility with the surrounding soft and hard tissues [1,2,3]. The mechanical properties of Zirconia are encouraging, as it has high flexural strength, increased resistance to fracture and corrosion, as well as a low thermal conductivity [3]. Regarding osseointegration, most studies show that the two materials have similar properties [3,4]. Colonization of the surface of zirconium implants by pathogenic bacteria has been shown to be lower compared to implants made of titanium alloys, leading to optimal healing and a low rate of infectious complications [1,3,4].
Regarding the prosthetic rehabilitation on implants, several comparative studies were performed between the dental prostheses made of metal-ceramic and those of zirconia-ceramic, following the main causes of failure. Current information in the literature on the success of monolithic zirconia rehabilitation is limited. According to the studies, the most common causes of failure of crowns and bridges on implants are the fracture of materials, more common in the case of zirconia, either the fracture of the coating ceramics or the fracture of the zirconia structure. Biological complications such as periimplantitis are also more common in zirconia-ceramics than in metalo-ceramics [5,6].
Dental implants have improved oral rehabilitation in edentulous patients and have reported increased success rates over periods of time larger than 10 years [7,8]. However, once inserted into the oral cavity, the implant surfaces are rapidly colonized by commensal bacteria. Bacterial adhesion begins about 30 min after implantation, and after about one to three months the subgingival bacterial community is similar to those around natural teeth [9]. Once dental implants appeared as a new treatment alternative in dentistry, periimplant infections developed as new oral pathologies [10]. Periimplant infections are classified as perimucositis and periimplantitis. If in perimucositis the induced inflammation is limited to the soft tissues, in the case of periimplantitis the lesion progresses to the bone, causing osteolysis [10]. Both diseases have a bacterial etiology, being strongly correlated with the presence of bacterial biofilm, especially of Gram-negative anaerobic bacteria [11], their establishment occurring once the balance between bacterial and host communities is disturbed. Around healthy implants there are frequently found microorganisms from yellow and purple complexes (Streptococcus, Streptococcus oralis, Streptococcus mutans, Veillonella parvula and Actinomyces odontolyticus, etc.) [9]. Gram negative bacteria including Prevotella intermedia, Porphyromonas gingivalis, Prevotella nigrescens, and Campylobacter rectus are rarely found in healthy periimplantar sites. On the contrary, in case of periimplantitis, the main components of the bacterial community are represented by Gram-negative anaerobic bacteria. There are also some cases of periimplantitis induced by Gram-negative aerobic bacteria, but the main etiological factor is represented by Gram-negative anaerobic bacteria [9]. These bacteria communities include Porphyromonas gingivalis, Prevotella intermedia, Treponema denticola, Tannerella forsythia, and Fusobacterium nucleatum. In addition, the presence of fungi, e.g., Candida albicans and other microorganisms, such as Aggregatibacter actinomycetemcomitans and Staphylococcus aureus, has also been reported in places affected by periimplantitis [11]. Previous studies show that Staphylococcus aureus adheres around implant components in the first year after implantation and this bacteria has a major role in the development of periimplantitis. Staphylococcus aureus appears to play a predominant role in the development of periimplantitis. Dental implants that were affected by periimplantitis were found to be associated with low antibody titer and elevated Staphylococus aureus levels. In vitro studies have demonstrated that there is an increased affinity between Staphylococus aureus and titanium surfaces [12,13]. With the emergence of a new disease, COVID-19, caused by the infection with SARS-CoV-2, the possible link between this viral infection and periimplantitis has been studied. It is shown that SARS-CoV-2 is present in the saliva and crevicular fluid of patients with COVID-19 [14,15]. Patients with periodontal or periimplant diseases, such as periimplantitis, are at an increased risk of developing COVID-19-associated complications, and patients with COVID-19 risk worsening periodontal or periimplant lesions [16]. During the pandemic, due to the transmission of SARS-CoV-2 through aerosols, dental offices adapted to the new global situation, so that the access of patients suffering from chronic diseases, such as periimplantitis, to dental treatments was limited, this aggravating their condition [14]. At the same time, the increased level of stress and depression in the population, accentuated by the pandemic, caused the aggravation of periodontal diseases by increasing plaque accumulation and gingival bleeding due to neglect of oral hygiene, lack of regular visits to the dental office, as well as by decreasing the healing capacity of the tissues and affecting the body’s response to treatment [14,16,17]. Patients with periodontal disease or periimplantitis have a higher risk of developing complications associated with COVID-19, studies suggesting that lesions in the periodontal and periimplant pockets are an entry point for the virus into the general circulation [15,16]. Certain constituents of plaque in the periimplant sulcus, such as Treponema denticola, Porphyromonas gingivalis or Candida, release proteases that degrade the basement membrane facilitating viral and bacterial invasion [15]. Periodontal and periimplant pockets, rich in aggressive pathogens, can also be a starting point for microorganisms that can be aspirated by people infected with SARS-CoV-2, leading to various complications [16].
The information in the literature about the microbiota in periimplantitis is inaccurate and it is not specified exactly which bacteria are associated with periimplantitis. Recent data suggests that periimplantitis is a polymicrobial anaerobic infection that does not fully correspond to the severity of the disease [12].
The bacterial community located in the oral cavity is diverse, and information on microorganisms involved in the development of periimplant infectious diseases as well as standardized treatment protocols for these diseases are limited. This literature review aimed to highlight the main bacterial colonies isolated from periimplant sites affected by periimplantitis by synthesizing information published on this topic in specialized studies.
2. Materials and Methods
2.1. Search Strategy
The methodological design of this study is in line with the PRISMA 2020 criteria and guidelines [18]. The protocol of the review was registered within the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42022335476. In this study, we address the following question: “Which are the most common bacterial species in periimplantitis?” To answer this question, a systematic literature search was carried out in 3 databases: PubMed, Embase, and Web of Science. The search strategy consisted of different combinations of MeSH keywords: “periimplantitis”, “bacteria”, “biofilm”, “microorganisms”, “microbiota”, “dental implant”: (bacteria) AND (periimplantitis), (biofilm) AND (periimplantitis), (microorganisms) AND (periimplantitis), (microbiota) AND (periimplantitis), (biofilm) AND (dental implant), ((bacteria) AND (dental implant)) AND (periimplantitis), (microbiome) AND (periimplantitis), (“Bacterial strains and periimplantitis” or “biofilms and periimplantitis” or “bacterial cultures and periimplantitis” or “types of bacteria and periimplantitis”), the filters applied being: Clinical Study, Clinical Trial, Randomized Controlled Trial, Other Animals, Humans, in the last 10 years. Two researchers independently performed the database literature search, and subsequently the results were confronted.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria were the articles published in the three databases mentioned above, in the last ten years, between January 2012 until March 2022. We selected studies performed on patients with dental implants suffering from periimplantitis and those in which samples of bacterial plaque were collected from periimplant sulcus affected by periimplantitis, analyzed and provided results on the microbial flora involved in periimplantitis.
Among the exclusion criteria were: articles published in a language other than English or French, articles other than those mentioned above, such as systematic reviews or meta-analyzes, experimental or in vitro studies.
2.3. Study Selection and Data Collection
Initially, the titles and abstracts of the selected articles were checked for the relevance of the topic, in relation to the proposed research. Subsequently, a full text analysis of all eligible articles was performed based on the inclusion and exclusion criteria. All articles that met the eligibility criteria were selected, and a standardized document was used to collect information on authors, year of publication, bacterial isolation methods and bacteria identified from these studies. Initially, 980 items from the 3 databases were selected according to the search terms mentioned above. Subsequently, after the elimination of duplicates, 201 articles remained, which were analyzed in detail based on inclusion and exclusion criteria. Finally, 25 articles were selected that met the eligibility criteria and introduced in this review. The selected articles were analyzed and classified according to the methods of isolation and identification of bacterial species. To collect data from selected articles, a table was created that included information on the authors of the articles, the year of publication, the study design, population characteristics, the isolation techniques and microbiological methods of bacterial identification, as well as the main bacterial species identified in each study (Table 1).
Table 1.
Studies included in this review and the main bacterial species identified in each study.
| Article Title | Authors | Year of Publication | Study Design | Study Samples | Population Characteristics | Bacterial Isolation Technique | Isolated Bacteria |
|---|---|---|---|---|---|---|---|
| Diversity analysis of subgingival microbial bacteria in periimplantitis in Uygur population | Gao et al. [9] | 2018 | Observational Study | 40 samples of gingival crevicular fluid divided into two groups: healthy implants (Control group) and periimplantitis (Case group) | Uygur patients who had treatment with dental implants from 2013 to 2016 | DNA extraction, PCR amplification and 16S rRNA gene sequencing | Prevotella, Streptococcus, Acinetobacter, Fusobacterium, Neisseria, Porphyromonas, Treponema, Leptothrix, Capnocytophaga |
| Efficiency of photodynamic therapy in the treatment of periimplantitis—A three-month randomized controlled clinical trial | Rakašević et al. [19] | 2016 | Randomized controlled clinical trial | Samples from 52 periimplantitis sites divided into two groups (Study group and Control group) | Patients with periimplantitis who presented in two dental clinics in Belgrade between January 2014 until February 2015 | Bacterial culture for the diagnosis of aerobic and anaerobic pathogens | Veillonella spp., Prevotella intermedia, Peptostreptococcus spp., Peptostreptococcus asaccharolyticus, Porphyromonas gingivalis, Fusobacterium nucleatum, Actinomyces odontolyticus |
| Bacterial profiles and proteolytic activity in periimplantitis versus healthy sites | Neilands et al. [20] | 2015 | Non-randomized, controlled, clinical study | 50 samples (25 from healthy subjects, 25 from periimplantitis sites) | Patients with dental implants treatment in the past, attending maintenance appointments in a dental clinic in Sweden | Bacterial culture on Brucella agar | Porphyromonas/Prevotella, Fusobacterium, Tannerella, Streptococcus oralis, Streptococcus mitis, Streptococcus anginosus, Streptococcus constellatus, Streptococcus intermedius |
| Short-term effects of hyaluronic acid on the subgingival microbiome in periimplantitis: A randomized controlled clinical trial | Soriano-Lerma et al. [21] | 2020 | Randomized controlled trial | 108 samples divided into 3 groups (Test group, Control group 1, Control group 2) | Patients diagnosticated with periimplantitis in a private dental office in Spain | DNA isolation, PCR amplification and 16S rRNA gene sequencing | Fusobacterium, Prevotella, Porphyromonas, Ralstonia, Sphingomonas, Streptococcus, Treponema, Propionibacterium, Alloprevotella, Veillonella, Lactobacillus, Haemophilus, Staphylococcus, Campylobacter, Tannerella |
| A randomized clinical trial of an adjunct diode laser application for the nonsurgical treatment of periimplantitis | Arısan et al. [22] | 2015 | Randomized clinical trial | Samples collected from 24 implants affected by periimplantitis at baseline and 1 month after intervention | 10 patients diagnosticated with periimplantitis who went to the department clinic in Istanbul University between February 2010 and May 2013 | DNA extraction, PCR amplification and hybridization procedures | Actinomyces odontolyticus, Actinomyces viscosus, Aggregatibacteractinomycetemcomitans, Campylobacter concisus, Campylobacter gracilis, Campylobacterrectus/showae, Capnocytophaga gingivalis/sputigena/ochracea, Eikenella corrodens, Eubacterium nodatum, Fusobacterium nucleatum, Peptostreptococcus micros, Porphyromonas gingivalis, Prevotella intermedia, Prevotellanigrescens, Streptococcus constellatus group, Streptococcusgordonii group, Streptococcus mitis group, Tannerella forsythia (Bacteroides forsythus; Tannerella forsythensis), Treponema denticola, and Veillonella parvula. |
| The effects of Lactobacillus reuteri probiotics combined with azithromycin on periimplantitis: A randomized placebo-controlled study | Tada et al. [23] | 2017 | Randomized placebo-controlled study | Samples collected from periimplantitis sites at baseline and 4, 12, and 24 weeks after allocated treatment | 30 patients diagnosticated with periimplantitis from 7 different institutions including Kyushu Dental University Hospital, Japan, divided into 2 groups, placebo and probiotics | DNA isolation, PCR amplification and 16S rRNA gene sequencing | Treponema denticola, Fusobacterium nucleatum, Peptostreptococcus micros, Streptococcus constellatus, Prevotella nigrescens, Tannerella forsythia, Campylobacter gracilis. Prevotella intermedia, Campylobacter rectus, Porphyromonas gingivalis, Veillonella parvula, Streptococcus gordonii, Capnocytophaga, Streptococcus mitis |
| Effectiveness of enamel matrix derivative on the clinical and microbiological outcomes following surgical regenerativetreatment of periimplantitis. A randomized controlled trial | Isehed et al. [24] | 2016 | Randomized controlled trial | Samples collected from the deepest pocket of the each implant at baseline, 2 weeks, 3, 6, and 12 months after surgery treatment | 29 patients diagnosticated with periimplantitis from a periodontology clinic in Sweden | DNA extraction with Gen Elute Bacterial Geno-mic DNA kit (Sigma Aldrich, St. Louis, MO, USA), bacterial characterization by the HOMIM microarray | Fusobacteria (cluster probe), Parvimonas micra, Porphyromonas sp., Eubacterium nodatum, Porphyromonas gingivalis, Ochrobactrum anthropi, Tannerella forsythia and Campylobacter concisus/Campylobacter rectus. |
| Adjunctive Systemic and Local Antimicrobial Therapy in the Surgical Treatment of Periimplantitis: A Randomized Controlled Clinical Trial | Carcuac et al. [25] | 2016 | Randomized Controlled Clinical Trial | Samples collected from periimplantitis sites at baseline, 3, 6 and 12 months after surgery | 100 patients with severe periimplantitis who were referred to 2 clinics specialized in periodontics in Sweden | Culture and checkerboard DNA-DNA hybridization analyses | Fusobacterium nucleatum, Prevotella intermedia/Prevotella nigrescens, Campylobacter rectus, Porphyromonas gingivalis, Tannerella forsythia, Porphyromonas endodontalis, Parvimonas micra |
| Comparison of the effects of air-powder abrasion, chemical decontamination, or their combination in open-flap surface decontamination of implants failed for periimplantitis: an ex vivo study | Pranno et al. [26] | 2021 | Single-blind, randomized, controlled, ex vivo study | 80 samples collected from the retrieved implants | 20 patients from Oral Surgery Unit University of Rome with minimum 4 implants affected by periimplantitis which need to be explanted | Bacterial culture techniques: for aerobic bacteria—Columbia sheep blood agar plates and for anaerobic—Schaedler sheep blood agar | Staphylococcus aureus, Streptococcus mitis/oralis, Staphylococcus epidermidis and Streptococcus salivarius. Enterococcus faecalis, Candida albicans, Pseudomonas aeruginosa and Neisseria flavescens |
| The Efficacy of a Diode Laser on Titanium Implants for the Reduction of Microorganisms That Cause Periimplantitis | Wawrzyk et al. [27] | 2021 | Clinical study | Samples collected from saliva, the surfaces of the crowns and dental implants components | 3 patients with advanced periimplantitis | Bacterial culture technique of anaerobic using Schaedler horse blood agar | Staphylococcus aureus, Streptococcus constellatus, Streptococcus oralis, Streptococcus pneumoniae, Rothia mucilaginosa, and Rothia aeria, and the following Gram-negative bacteria: Haemophilus parainfluenzae, Klebsiella pneumoniae, Klebsiella oxytoca, and Veilonella parvula. Candida guilliermondii, Actinomyces odontolyticus |
| Investigation of antibiotic susceptibility of the bacterial isolates and local flora changes after complex therapy in chronic periodontitis and periimplantitis | Ciobanu et al. [28] | 2018 | Clinical study | Samples collected from sites with periimplantitis before and after therapy | Patients diagnosticated with chronic periimplantitis | Culture examination | Capnocytophaga spp., Prevotella oralis, S. intermedius, S. gordonii, Veillonella spp. |
| Shift of microbial composition of periimplantitis-associated oral biofilm as revealed by 16S rRNA gene cloning | Al-Ahmad et al. [29] | 2018 | Cross-sectional study | Samples collected from the deepest sites of periimplantitis and from the periimplantar healthy sulcus | 10 patients with at least one implant affected by periimplantitis and one healthy implant | DNA extraction and PCR amplification of 16S rRNA genes | Streptococcus spp., Prevotella spp., Fusobacterium spp., Eubacterium spp., Porphyromonas gingivalis, Treponema spp., Campylobacter spp., Filifactor alocis, Abitrophia defectiva, Alloprevotella tannarae, Neisseria spp., Parvimonas micra, Selenomonas spp., Capnocytophaga spp., Atopobium spp., Peptostreptococcus spp., Tannerella forsythia, Scadovia wiggisiae, Bacteroidetes bacterium, Eikenella Corodens, Fretibacterium fastidiosum, Johnsonella ignava, Synergistales bacterium, Dialister invisus, Raoultella sp. |
| Subgingival microbiome in patients with healthy and ailing dental implants | Zheng et al. [30] | 2015 | Clinical study | Samples collected from periimplantar sulcus and pockets | 10 patients with healthy implants, 8 patients with perimucositis and 6 with periimplantitis | Microbial DNA extraction, 16S rRNA gene library preparation, and pyrosequencing | Leptotrichia hofstadii, Eubacterium infirmum, Kingella denitrificans, Actinomycescardiffensis, Eubacterium minutum, Treponema lecithinolyticum, and Gemella sanguinis were higher in PI sites Streptococcus, Leptotrichia, Actinomyces, Capnocytophaga, Prevotella, Fusobacterium, Neisseria |
| Microbiota in Gingival Crevicular Fluid Before and After Mechanical Debridement With Antimicrobial Photodynamic Therapy in Periimplantitis | Wang et al. [31] | 2022 | Clinical study | 61 samples collected from all the implants: before treatment and 7, 14, 30, 60 and 180 days after treatment | 9 patients presented at Department of Stomatology in Beijing Hospital with 14 implants affected by periimplantitis | Bacterial 16S rRNA was amplified and sequenced using an Illumina MiSeq platform | Bacteroidetes, Proteobacteria, Firmicutes, Fusobacteria, Spirochaetes, Synergistetes, and Actinobacteria Prevotella, Neisseria, Fusobacterium, Porphyromonas, Treponema, Streptococcus, Haemophilus, Capnocytophaga, Leptotrichia, and Fretibacterium |
| Microbiological findings in early and late implant loss: an observational clinical case-controlled study | Korsch et al. [32] | 2021 | Observational clinical case–control study | Samples collected from implants affected by severe periimplantitis without any chance of preservation and from healthy implants as controls | 48 patients with 53 implants were introduced in the study | DNA extraction, PCR amplification and 16S rRNA gene sequencing | Treponema sp., Streptococcus, Fretibacterium, Anaerovoracaceae uncl, Desulfobulbus sp., Pseudoramibacter alactolyticus, Dialister pneumosintes, Streptococcus sanguinis, Shewanella sp., Pantoea sp., Haemophilus sp., Haemophilus parainfluenzae, Pseudomonas sp., Lautropia mirabilis, Actinomyces naeslundii |
| Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics | Ghensi et al. [33] | 2020 | Clinical study | Samples collected from each implant and from contralateral healthy implant or tooth for every patient included in the study | 80 patients enrolled in the study: 28 with healthy implants, 28 with mucositis and 24 with periimplantitis | DNA extraction | P. gingivalis, T. forsythia, Treponema denticola, P. endodontalis, F. fastidiosum, Filifactor alocis, Desulfobulbus spp., T. lecithinolyticum |
| Cluster of bacteria associated with periimplantitis | Persson et al. [12] | 2014 | Retrospective clinical study | Samples collected at one implant with periimplantitis in each of 166 patients and from 47 healthy implants | 166 patients with periimplantitis and 47 patients with healthy dental implants | Checkerboard DNA–DNA hybridization | Actinomyces odontolyticus, A. actinomycetemcomitans (a), Campylobacter gracilis, Campylobacter rectus, Campylobacter showae, Helicobacter pylori, Haemophilus influenzae, Leptothrichia buccalis, P. intermedia, Propionybacterium acnes, Porphyromonas endodontalis, P. gingivalis, Staph. aureus, Staph. anaerobius, Streptococcus intermedius, Streptococcus mitis, T. forsythia, T. denticola, and Treponema socranskii. |
| Microbiological diversity of periimplantitis biofilm by Sanger sequencing | da Silva et al. [34] | 2014 | Clinical study | Samples collected from the deepest pocket depth in the test group and from mesial site of healthy implants | 20 individuals, 10 with healthy implants and 10 with at least one implant with periimplantitis, both groups with minimum 10 periodontally healthy teeth | Extraction of DNA, PCR amplification of universal 16S rRNA | Fusobacterium nucleatum, Campylobacter gracilis, Dialister invisus, Streptococcus sp., Eubacterium infirmum, Filifactor alocis and Mitsuokella sp., Parvimonas micra and Prevotella intermedia |
| Analysis of bacterial flora associated with periimplantitis using obligate anaerobic culture technique and 16S rDNA gene sequence | Tamura et al. [35] | 2013 | Clinical study | Samples collected from the deepest sites of the both groups, test and control | 30 patients, 15 diagnosticated with periimplantitis, 15 with healthy implants | Culture technique and 16S rDNA gene sequence | Streptococcus, Eubacterium, Prevotella, Actinomyces, Fusobacterium, Eubacterium nodatum, Prevotella intermedia, Fusobacterium nucleatum, Filifactor alocis, E brachy, Parascardovia denticolenns, Parvimonas micra |
| Microbial profiles of peri-implant mucositis and periimplantitis: submucosal microbial dysbiosis correlates with disease severity | Shi et al. [36] | 2022 | Cross-sectional study | Samples collected from 64 patients, 27 with perimucositis and 37 with periimplantitis | Patients with periimplantitis or perimucositis presented in Dep. Of Oral Implantology in Zhejiang University School of Medicine, China | DNA extraction, PCR amplification and 16S rRNA gene sequencing | Porphyromonas, Fusobacterium, Treponema and Prevotella, Campylobacter, Filifactor, Alloprevotella |
| Exploring the microbiome of healthy and diseased peri-implant sites using Illumina sequencing | Sanz-Martin et al. [37] | 2017 | Clinical study | Sample collection from 32 healthy implants and from 35 implants affected by periimplantitis | Patients with healthy implants and with periimplantitis presented in center of Dental Medicine at the University of Zürich | Bacterial nucleic acids isolation, sample DNA analyzed by sequencing the 16S rRNA gene V3-V4 hypervariable region | Porphyromonas (phylum Bacteroidetes), Treponema (phylum Spirochetes), Filifactor (phylum Firmicutes), Fretibacterium (phylum Synergistetes) and Tannerella (phylum Bacteroidetes) |
| Intra-oral single-site comparisons of periodontal and peri-implant microbiota in health and disease | Yu et al. [38] | 2019 | Clinical study | Samples collected from 4 sites for each patient: Healthy implant, healthy tooth, periimplantitis site and periodontitis site | 18 Chinese partial dentate patients with both periimplantitis and periodontitis | DNA extraction, PCR amplification | Bacteroidetes and Prevotella taxa (including P. denticola, P. multiformis and P. fusca). |
| Identification of microbiota in periimplantitis pockets by matrix assisted laser desorption/ionization time-of-flight mass spectrometry | Yeh et al. [39] | 2019 | Clinical study | Samples collected from periimplantitis pockets | 12 patients with periimplantitis | Culture examination | Neisseria flavescen, Streptococcus constellatus, Slackia exigua, Streptococcus intermedius, Fusobacteriumnucleatum, Gemella morbillorum and Gram-positive anaerobic Bacillus |
| Intraindividual variation in core microbiota in periimplantitis and periodontitis | Maruyama et al. [40] | 2014 | Clinical study | Samples collected from the deepest pockets in periimplantitis sites and in periodontitis sites | 20 Patients with both periimplantitis and periodontitis | DNA extraction and PCR amplification of 16S rRNA genes | Olsenella, Sphingomonas, Peptostreptococcus, unclassified Neisseriaceae, genus Desulfomicrobium, Actinomyces johnsonii, Fusobacterium nucleatum, Porphyromonas gingivalis, Streptococcus oralis, Treponema denticola, and Treponema socranskii Achromobacter xylosoxidans, Actinomyces massiliensis, and Porphyromonas sp. |
| The severity of human periimplantitislesions correlates with the level of submucosal microbial dysbiosis | Kröger et al. [41] | 2018 | Cohort study or case–control study | Samples collected from all 45 implants affected by periimplantitis | 30 patients with at list one implant with periimplantitis | DNA extraction and PCR amplification of 16S rRNA genes | Eubacteriaceae, Fretibacterium sp., Fretibacterium fastidiosum, Peptostreptococcaceae, Alloprevotella sp., Fastidiosipila sanguinis, Filifactor alocis, Peptostreptococcaceae, Bacteriodetes bacterium, Treponema parvum, Clostridiales bacterium, and Orobacterium, Granulicatella elegans, Rothia aeria, Corynebacterium durum, Veillonella dispar, Acinetobacter |
The quality assessment of the included studies in this review was performed using the Newcastle-Ottawa Scale (NOS) [42]. The NOS scale quantifies three quality parameters (selection, comparability and outcome). These parameters are divided into eight specific categories. Each item on the scale is scored with a maximum of one point, except for comparability, which can be scored with up to two points. The maximum score that can be obtained for each study is 9 [43]. Studies with NOS scores 0–3 were considered as low quality, 4–6 moderate quality and 7–9 were considered as high quality (Table 2).
Table 2.
NOS scores for the included studies.
| Study | Selection | Comparability | Outcome | NOS Score |
|---|---|---|---|---|
| Case–control studies | ||||
| Gao et al. [9] | *** | * | *** | 7 |
| Rakašević et al. [19] | *** | ** | *** | 8 |
| Neilands et al. [20] | *** | * | *** | 7 |
| Soriano-Lerma et al. [21] | *** | * | ** | 6 |
| Arısan et al. [22] | *** | ** | *** | 8 |
| Tada et al. [23] | *** | ** | *** | 8 |
| Isehed et al. [24] | *** | ** | ** | 7 |
| Carcuac et al. [25] | *** | ** | ** | 7 |
| Pranno et al. [26] | *** | * | *** | 7 |
| Wawrzyk et al. [27] | ** | * | *** | 6 |
| Ciobanu et al. [28] | * | * | *** | 5 |
| Al-Ahmad et al. [29] | ** | * | *** | 6 |
| Zheng et al. [30] | ** | * | *** | 6 |
| Korsch et al. [32] | ** | * | *** | 6 |
| Persson et al. [12] | *** | * | *** | 7 |
| da Silva et al. [34] | *** | * | *** | 7 |
| Tamura et al. [35] | *** | * | *** | 7 |
| Shi et al. [36] | *** | * | *** | 7 |
| Sanz-Martin et al. [37] | *** | * | ** | 6 |
| Yeh et al. [39] | ** | * | 3 | |
| Maruyama et al. [40] | *** | * | *** | 7 |
| Kröger et al. [41] | ** | * | * | 4 |
| Cohort studies | ||||
| Wang et al. [31] | *** | * | *** | 7 |
| Ghensi et al. [33] | **** | * | *** | 8 |
| Yu et al. [38] | **** | * | *** | 8 |
NOS Scale star system for quality assessment of studies.The higher number of stars (*), the better quality. The maximum (*) possible are 9: **** for Selection, ** for Comparability and *** for Outcome.
3. Results
3.1. Bibliographic Documentation and Selection of Articles
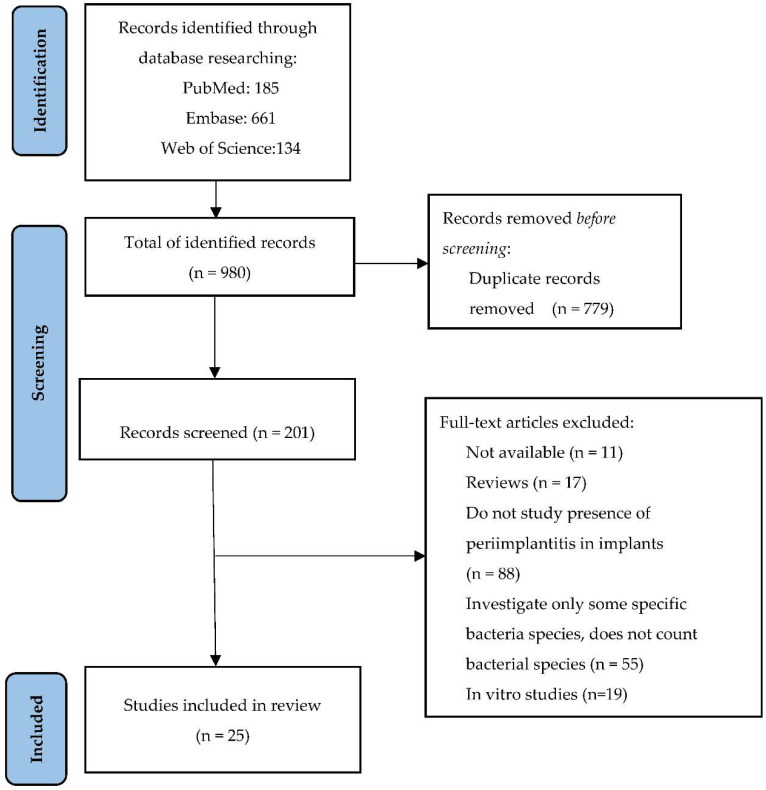
The process of searching and selecting the articles included in this literature review according to the PRISMA requirements is presented in Figure 1. Initially, 980 articles were selected from the three databases: PubMed, Embase and Web of Science that matched the search terms. Out of these, 779 duplicates were identified and removed. The remaining 201 articles were checked, 11 of them being removed because they were not available in full text, another 17 being literature reviews and 19 being in vitro studies. Other elimination criteria were the evaluation of specific bacteria, the lack of quantitative analysis of bacterial species or the absence of periimplantitis. Finally, after a detailed analysis, 25 articles that analyze the bacterial flora involved in periimplantitis were included in this study (Figure 1).
Figure 1.
PRISMA flow diagram. Study selection for review.
3.2. Clinical and Microbiological Characteristics of the Included Studies
Table 1 summarizes the most relevant information in the included studies, such as title, authors, year of publication, study design, population characteristics, methods of isolation and bacterial identification, and the main bacterial species identified. Out of a total of 25 articles, six of them used classical bacterial culture methods to identify microorganisms involved in the development of periimplantitis and 17 used modern molecular biology techniques, DNA-DNA hybridization or RNA sequencing, and two of them used combined bacterial culture and DNA hybridization techniques.
Quality assessment of the included studies in this review performed using NOS Scale emphasized that 16 studies were of high quality, eight studies were of moderate quality and one study was of low quality. The results are summarized in Table 2. The average NOS score for the quality assessment of the included studies in this review was 6.6.
A classification of the microorganisms identified in the 25 studies included in this review was made, and the frequency with which each microorganism was found in them was subsequently quantified. In addition, a separate classification was performed on studies that used the bacterial culture technique to identify microorganisms, analyzing the differences between the two classifications.
In general, the most commonly identified bacterial species were Prevotella, and Streptococcus, Gram-negative anaerobic species. Prevotella was found in 17 of the 25 studies, so with a frequency of 68%. In this bacterial species, Prevotella intermedia was isolated in 7 of the 17 studies (41.17%), Prevotella nigrescens in 4 of 17 (23.52%), Prevotella fusca, Prevotella multiformis and Prevotella denticola, in one of the 17 articles (5.88%).
Streptococcus was found in 17 of the 25 studies, so with a frequency of 68%. The following bacterial species were identified from the genus Streptococcus: Streptococcus oralis and Streptococcus constellatus in 5 out of 17 studies (29.41%), Streptococcus mitis, Streptococcus intermedius in 4 out of 17 studies (23.52%), Streptococcus gordonii in 3 out of 17 studies (17.64%), Streptococcus sanguinis, Streptococcus salivarius, Streptococcus pneumoniae, Streptococcus anginosus in 1 out of 17 studies (5.88%).
The genera Fusobacterium were isolated in 16 out of the 25 studies, so with a frequency of 64%. Of the genus Fusobacterium, Fusobacterium nucleatum was isolated in 8 of 16 cases, respectively, 50%.
The fourth most common bacterial genus was Treponema in 15 of the 25 studies, 60%, respectively. Treponema denticola was isolated from this species, 5 out of 15 studies (33.33%), Treponema socranski and Treponema lecithinolyticum, 2 out of 15 studies (13.33%), Treponema parvum and Treponema maltophilum in 1 out of 15 studies (6.66%).
The genus Porphyromonas, identified in 14 of the 25 studies (56%), includes the species Porphyromonas gingivalis, isolated in 10 of 14 studies (71.42%), respectively, Porphyromonas endodontalis, 3 of 14 studies (21.42%).
Tannerella was identified in 11 of the 25 studies (44%), with Tannerella forsythia being isolated in 9 of 11 (81.81%).
The genus Neisseria, identified in 9 of the 25 studies (36%), the species Neisseria flavescens being found in 2 of the 9 studies (22.22%).
Campylobacter, also identified in 9 of the 25 studies (36%), includes Campylobacter rectus, 5 out of 9 (55.55%), Campylobacter gracilis, 4 out of 9 (44.44%), Campylobacter concisus, 2 of 9 (22.22%) and Campylobacter showae 1 of 9 (11.11%).
The genus Fretibacterium, also identified in 7 of the 25 studies (28%), includes the species Fretibacterium fastidiosum, isolated in 4 of the 7 studies (57.14%).
Filifactor, isolated in 7 of the 25 studies (28%), includes the species Filifactor alocis, identified in 6 of the 7 studies (85.71%). Capnocytophaga was also isolated in 7 of the 25 studies (28%).
The species Parvimonas micra, genus Parvimonas, was identified in 6 of the 25 studies, respectively 24%.
Veillonella, identified in 5 of the 25 studies (20%), includes the species Veillonella parvula, identified in 3 of the 5 studies (60%).
The genus Peptostreptococcus, identified in 4 of the 25 studies (16%), includes the species Peptostreptococcus asaccharolyticus, isolated in 1 of the 4 studies (25%), respectively, Peptostreptococcus micros in one of the 4 studies (25%).
Haemophilus, identified in 5 of the 25 studies (20%), includes the species Haemophilus parainfluenzae, identified in 2 of the 5 studies (40%), respectively, Haemophilus influenzae in one of the 5 studies (20%).
The genus Eubacterium, identified in 5 out of 25 studies (20%), includes the species Eubacterium infirmum, 2 out of 5 studies (40%), respectively, Eubacterium minutum, 1 out of 5 studies (20%).
The genus Actinomyces includes the species Actinomyces odontolyticus, isolated in 4 of the 25 studies (16%), Actinomyces naeslundii, in 2 of the 25 studies (8%), respectively, Actinomyces massiliensis, Actinomyces johnsonii and Actinomyces cardiffensis in one of the 25 studies (4%).
Staphylococcus, identified in 4 of the 25 studies (16%), includes the species Staphylococcus aureus, identified in 3 of 4 studies (75%), Staphylococcus Anaerobius, in 2 of 4 studies (50%), respectively, Staphylococcus epidermidis, 1 of 4 studies (25%).
The genus Leptotrichia, identified in 4 of the 25 studies (16%), includes the species Leptotrichia hofstadii and Leptotrichia buccalis identified in one of the 25 studies (4%).
Alloprevotella, identified in 4 of the 25 studies (16%) includes the species Alloprevotella tannarae, identified in one of the 4 studies (25%).
Other bacterial species were sporadically identified in one or two studies out of 25, 4% and 8%, respectively. These include Synergistetes, Rothia aeria, Lautropia mirabilis, Johnsonella, Gemella, A. Actinomycetemcomitans, Dialister invisus, Eikenella Corodens, etc.
Regarding the species identified by microbial cultures, 6 of the 25 studies that used this method to isolate bacteria from periimplant sites were identified. The most common were the genera Veillonella, Prevotella and Fusobacterium, with a frequency of 3 out of 6 studies, respectively, 50%. Prevotella intermedia was identified in 1 of 3 studies (33.33%), Prevotella oralis in 1 of 3 (33.33%) and Fusobacterium nucleatum in 2 of 3 studies (66.66%).
Actinomyces odontolyticus, genus Actinomyces was isolated in 2 of 6 studies, respectively, 33.33%, and Actinomyces naeslundii in one of 6 studies, respectively, 16.66%.
Porphyromonas, identified in 3 of 6 studies, 50%, with the species Porphyromonas gingivalis, isolated in 2 of the 3 studies, 66.66%.
Streptococcus oralis, Streptococcus constellatus, Streptococcus intermedius identified in 3 of 6 studies (50%). The species Streptococcus mitis, Neisseria flavescens isolated in 2 out of 6 studies, respectively, 33.33%. The genus Peptostreptococcus spp., the species Peptostreptococcus asaccharolyticus in 1 out of 6 studies (16.66%).
Other species were sporadically identified in only one of the 6 studies (16.66%) in which the bacterial culture technique was used: Tannerella, Streptococcus anginosus, Staphylococcus epidermidis, Streptococcus salivarius, Enterococcus faecalis, Candida albicans Pseudomonas aeruginosa, Streptococcus, Rothia mucilaginosa, Rothia aeria, Haemophilus parainfluenzae, Klebsiella pneumoniae, Klebsiella oxytoca, Veilonella parvula, Actinomyces naeslundii, Capnocytophaga spp., Prevotella oralis, S. Gordonii, Slackia exigua, Gemella morbillorum.
4. Discussion
The complications related to dental implants are mainly inflammatory lesions of the bone and soft tissues surrounding the implants and their restorative components, induced by the accumulation of bacterial biofilm [8,44]. Periimplantitis is defined as an inflammatory disease of the mucosa surrounding an implant accompanied by progressive loss of periimplant support bone [44]. It is generally perceived that after implant insertion and initial loading, during the healing process, between 0.5 and 2 mm of crestal bone height is lost. Any radiographic evidence of additional bone loss suggests a periimplant condition [8]. Periimplantitis has an infectious etiology, and the diversity of bacteria in the oral cavity is increased. However, information on the microbiota involved in periimplantitis is limited and it is unclear whether there is a specific group of bacteria that may be associated with periimplantitis. Through this review, we wanted to summarize the results obtained in specialized studies, in order to identify the main pathogens involved in the development of periimplantitis. The main species identified in most studies included in this literature review are Gram-negative anaerobic bacteria such as those in the red complex, a highly virulent complex containing particularly aggressive species as Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola. These bacteria are also correlated with the loss of the gingivo-periodontal junction in periodontal diseases. At the same time, bacteria from the orange complex, Prevotella intermedia, Fusobacterium nucleatum, Campylobacter, Streptococcus constellatus, Peptostreptococcus and Eubacterium were identified in most studies in this review. These bacteria have the ability to make connections and enhance the destructive effect of bacteria in the red complex by promoting their colonization. These findings are consistent with other studies published in the literature [9,11], which also show that the main components of the bacterial community are Gram-negative anaerobic bacteria, as confirmed in this study. Perimucositis and periimplantitis have similar characteristics to gingivitis and parodontitis, that occur around natural dentition. These diseases have multifactorial etiologies and are strongly correlated with bacterial biofilm, especially with Gram-negative anaerobic bacteria [11]. Numerous studies have found the presence of a high number of periodontopathogenic bacteria of the red complex and those in the orange complex in the sites affected by periimplantitis and have highlighted the correlations between the two conditions and how they influence each other when coexisting within the same oral cavity [9,10,12,13,45].
In addition to Gram-negative anaerobic bacteria and those in the red and orange complexes, a high number of studies included in this review discovered Gram-negativeaerobic species such as Neisseria, yellow and purple complex species: Streptococcus, Veillonella, and Gram-positive anaerobic species: Filifactor, Parvimonas micra.
Therefore, the most common bacteria in the periimplantitis sites identified in this review are Gram-negative anaerobic species, also involved in the pathogenesis of the periodontal disease. This aspect is particularly important to establish new preventive measures and new therapeutic protocols, in order to reduce the incidence of this condition among patients.
The treatment of periimplant diseases is complex, the management of these diseases being difficult to achieve. The study of the bacterial flora involved in periimplantitis and the knowledge of the microorganisms incriminated in the pathogenesis of this disease can be a starting point in the research of new therapeutic means in order to achieve a personalized treatment targeted on the main periimplant pathogens. New preventive measures may also be developed in order to reduce the incidence of these conditions in patients or to prevent recurrence after treatment. All the studies included in this review were assessed for risk of bias with NOS Scale Tool [42], independently by two authors. Divergences in the assessment were solved by discussion and by re-evaluating the article. According to NOS Scale there are three groups of risk of bias: low risk of bias (7–9 NOS scores), high risk of bias (4–6 NOS scores) and very high risk of bias (0–3 NOS scores) [46]. The majority of the studies included in this review are at low risk of bias and only one study is considered at very high risk of bias.
The main limitation of this review is the relatively heterogeneity of the studies included in this research. The included articles have different study design, different methods of identifying the clusters of bacteria involved in periimplantitis and the studies are performed on different types of implants. Additionally, the results of counting bacteria are reported in different measurements units. Analysis of the differences in bacterial colonization regarding the type, material and surface design of the implants was impossible because this information was not available in all the articles included in this review.
5. Conclusions
Summarizing the results obtained in this review, we can state that there is a correlation between the germs involved in the pathogenesis of periodontal diseases and those that cause periimplant diseases. The bacteria identified in most of the analyzed studies: Prevotella, Streptococcus, Fusobacterium, Treponema, Tannerella, and Porphyromonas gingivalis are mostly anaerobic bacteria, pathogens with high virulence, also involved in the development of periodontal diseases, which require special treatment protocols.
Author Contributions
Conceptualization, S.A.L.I., O.P.L. and C.C.; methodology, S.A.L.I. and O.P.L.; validation, O.P.L., N.B.P., C.C. and S.A.; formal analysis, S.A.L.I., N.B.P. and I.C.M.; investigation, S.A.L.I. and N.B.P.; resources, O.P.L.; data curation, S.A.L.I., N.B.P. and I.C.M.; writing—original draft preparation, S.A.L.I. and O.P.L.; writing—review and editing, S.A.L.I., C.C., O.P.L., N.B.P., I.C.M. and D.-A.T.; visualization, S.A., C.C., O.P.L. and D.-A.T.; supervision, S.A., C.C. and O.P.L.; project administration, S.A. and O.P.L.; funding acquisition, O.P.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by a grant of the Ministry of Research, Innovation and Digitization, CNCS-UEFISCDI, project number PN-III-P1-1.1-TE-2021-0531, within PNCDI III.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Apratim A., Eachempati P., Krishnappa Salian K.K., Singh V., Chhabra S., Shah S. Zirconia in dental implantology: A review. J. Int. Soc. Prev. Community Dent. 2015;5:147–156. doi: 10.4103/2231-0762.158014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saini M., Singh Y., Arora P., Arora V., Jain K. Implant biomaterials: A comprehensive review. World J. Clin. Cases. 2015;16:52–57. doi: 10.12998/wjcc.v3.i1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cionca N., Hashim D., Mombelli A. Zirconia dental implants: Where are we now, and where are we heading? Periodontol. 2000. 2017;73:241–258. doi: 10.1111/prd.12180. [DOI] [PubMed] [Google Scholar]
- 4.Hanawa T. Zirconia versus titanium in dentistry: A review. Dent. Mater. J. 2020;31:24–36. doi: 10.4012/dmj.2019-172. [DOI] [PubMed] [Google Scholar]
- 5.Pjetursson B.E., Valente N.A., Strasding M., Zwahlen M., Liu S., Sailer I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implant. Res. 2018;29:199–214. doi: 10.1111/clr.13306. [DOI] [PubMed] [Google Scholar]
- 6.Sailer I., Strasding M., Valente N.A., Zwahlen M., Liu S., Pjetursson B.E. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic multiple-unit fixed dental prostheses. Clin. Oral Implant. Res. 2018;29:184–198. doi: 10.1111/clr.13277. [DOI] [PubMed] [Google Scholar]
- 7.Lafaurie G.I., Sabogal M.A., Castillo D.M., Rincón M.V., Gómez L.A., Lesmes Y.A., Chambrone L. Microbiome and Microbial Biofilm Profiles of Periimplantitis: A Systematic Review. J. Periodontol. 2017;88:1066–1089. doi: 10.1902/jop.2017.170123. [DOI] [PubMed] [Google Scholar]
- 8.Renvert S., Persson G.R., Pirih F.Q., Camargo P.M. Peri-implant health, peri-implant mucositis, and periimplantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018;45:278–285. doi: 10.1111/jcpe.12956. [DOI] [PubMed] [Google Scholar]
- 9.Gao X., Zhou J., Sun X., Li X., Zhou Y. Diversity analysis of subgingival microbial bacteria in periimplantitis in Uygur population. Medicine. 2018;97:e9774. doi: 10.1097/MD.0000000000009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belibasakis G.N., Manoil D. Microbial Community-Driven Etiopathogenesis of Periimplantitis. J. Dent. Res. 2021;100:21–28. doi: 10.1177/0022034520949851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadid-Zadeh R., Willis J., Forgo G., Haraszthy V. Comparative Analysis of Biofilm Formation on Materials Used for the Fabrication of Implant-Supported Prostheses. Braz. Dent. J. 2020;31:380–384. doi: 10.1590/0103-6440202003385. [DOI] [PubMed] [Google Scholar]
- 12.Persson G.R., Renvert S. Cluster of bacteria associated with periimplantitis. Clin. Implant Dent. Relat. Res. 2014;16:783–793. doi: 10.1111/cid.12052. [DOI] [PubMed] [Google Scholar]
- 13.Smeets R., Henningsen A., Jung O., Heiland M., Hammächer C., Stein J.M. Definition, etiology, prevention and treatment of periimplantitis—A review. Head Face Med. 2014;10:34. doi: 10.1186/1746-160X-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadkhodazadeh M., Amid R., Moscowchi A. Does COVID-19 Affect Periodontal and Peri-Implant Diseases? J. Long-Term Eff. Med. Implant. 2020;30:1–2. doi: 10.1615/JLongTermEffMedImplants.2020034882. [DOI] [PubMed] [Google Scholar]
- 15.Sorsa T., Sahni V., Buduneli N., Gupta S., Räisänen I.T., Golub L.M., Lee H.M., Pätilä T., Bostanci N., Meurman J., et al. Active matrix metalloproteinase-8 (aMMP-8) point-of-care test (POCT) in the COVID-19 pandemic. Expert Rev. Proteom. 2021;18:707–717. doi: 10.1080/14789450.2021.1976151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancini L., Americo L.M., Pizzolante T., Donati R., Marchetti E. Impact of COVID-19 on Periodontitis and Peri-Implantitis: A Narrative Review. Front. Oral Health. 2022;3:822–824. doi: 10.3389/froh.2022.822824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Ambrosio F., Caggiano M., Schiavo L., Savarese G., Carpinelli L., Amato A., Iandolo A. Chronic Stress and Depression in Periodontitis and Peri-Implantitis: A Narrative Review on Neurobiological, Neurobehavioral and Immune-Microbiome Interplays and Clinical Management Implications. Dent. J. 2022;10:49. doi: 10.3390/dj10030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakašević D., Lazić Z., Rakonjac B., Soldatović I., Janković S., Magić M., Aleksić Z. Efficiency of photodynamic therapy in the treatment of periimplantitis—A three-month randomized controlled clinical trial. Srp. Arh. Celok. Lek. 2016;144:478–484. doi: 10.2298/SARH1610478R. [DOI] [PubMed] [Google Scholar]
- 20.Neilands J., Wickström C., Kinnby B., Davies J.R., Hall J., Friberg B., Svensäter G. Bacterial profiles and proteolytic activity in periimplantitis versus healthy sites. Anaerobe. 2015;35:28–34. doi: 10.1016/j.anaerobe.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Soriano-Lerma A., Magán-Fernández A., Gijón J., Sánchez-Fernández E., Soriano M., García-Salcedo J.A., Mesa F. Short-term effects of hyaluronic acid on the subgingival microbiome in periimplantitis: A randomized controlled clinical trial. J. Periodontol. 2020;91:734–745. doi: 10.1002/JPER.19-0184. [DOI] [PubMed] [Google Scholar]
- 22.Arısan V., Karabuda Z.C., Arıcı S.V., Topçuoğlu N., Külekçi G. A randomized clinical trial of an adjunct diode laser application for the nonsurgical treatment of periimplantitis. Photomed. Laser Surg. 2015;33:547–554. doi: 10.1089/pho.2015.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tada H., Masaki C., Tsuka S., Mukaibo T., Kondo Y., Hosokawa R. The effects of Lactobacillus reuteri probiotics combined with azithromycin on periimplantitis: A randomized placebo-controlled study. J. Prosthodont. Res. 2018;62:89–96. doi: 10.1016/j.jpor.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Isehed C., Holmlund A., Renvert S., Svenson B., Johansson I., Lundberg P. Effectiveness of enamel matrix derivative on the clinical and microbiological outcomes following surgical regenerative treatment of periimplantitis. A randomized controlled trial. J. Clin. Periodontol. 2016;43:863–873. doi: 10.1111/jcpe.12583. [DOI] [PubMed] [Google Scholar]
- 25.Carcuac O., Derks J., Charalampakis G., Abrahamsson I., Wennström J., Berglundh T. Adjunctive Systemic and Local Antimicrobial Therapy in the Surgical Treatment of Periimplantitis: A Randomized Controlled Clinical Trial. J. Dent. Res. 2016;95:50–57. doi: 10.1177/0022034515601961. [DOI] [PubMed] [Google Scholar]
- 26.Pranno N., Cristalli M.P., Mengoni F., Sauzullo I., Annibali S., Polimeni A., La Monaca G. Comparison of the effects of air-powder abrasion, chemical decontamination, or their combination in open-flap surface decontamination of implants failed for periimplantitis: An ex vivo study. Clin. Oral Investig. 2021;25:2667–2676. doi: 10.1007/s00784-020-03578-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wawrzyk A., Łobacz M., Adamczuk A., Sofińska-Chmiel W., Rahnama M. The Efficacy of a Diode Laser on Titanium Implants for the Reduction of Microorganisms That Cause Periimplantitis. Materials. 2021;14:7215. doi: 10.3390/ma14237215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciobanu L., Bancescu G., Didilescu A.C., Băncescu A.A. Investigation of antibiotic susceptibility of the bacterial isolates and local flora changes after complex therapy in chronic periodontitis and periimplantitis. Farmacia. 2018;66:1064–1069. doi: 10.31925/farmacia.2018.6.21. [DOI] [Google Scholar]
- 29.Al-Ahmad A., Muzafferiy F., Anderson A.C., Wölber J.P., Ratka-Krüger P., Fretwurst T., Nelson K., Vach K., Hellwig E. Shift of microbial composition of periimplantitis-associated oral biofilm as revealed by 16S rRNA gene cloning. J. Med. Microbiol. 2018;67:332–340. doi: 10.1099/jmm.0.000682. [DOI] [PubMed] [Google Scholar]
- 30.Zheng H., Xu L., Wang Z., Li L., Zhang J., Zhang Q., Chen T., Lin J., Chen F. Subgingival microbiome in patients with healthy and ailing dental implants. Sci. Rep. 2015;5:10948. doi: 10.1038/srep10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Liu Y., Li W., Li W., Xu H., Niu G., Wang Z. Microbiota in Gingival Crevicular Fluid Before and After Mechanical Debridement with Antimicrobial Photodynamic Therapy in Periimplantitis. Front. Cell. Infect. Microbiol. 2022;11:1357. doi: 10.3389/fcimb.2021.777627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korsch M., Marten S.M., Stoll D., Prechtl C., Dötsch A. Microbiological findings in early and late implant loss: An observational clinical case-controlled study. BMC Oral Health. 2021;21:112. doi: 10.1186/s12903-021-01439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghensi P., Manghi P., Zolfo M., Armanini F., Pasolli E., Bolzan M., Bertelle A., Dell’Acqua F., Dellasega E., Waldner R., et al. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. NPJ Biofilms Microbiomes. 2020;6:47. doi: 10.1038/s41522-020-00155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Da Silva E.S., Feres M., Figueiredo L.C., Shibli J.A., Ramiro F.S., Faveri M. Microbiological diversity of periimplantitis biofilm by Sanger sequencing. Clin. Oral Implant. Res. 2014;25:1192–1199. doi: 10.1111/clr.12231. [DOI] [PubMed] [Google Scholar]
- 35.Tamura N., Ochi M., Miyakawa H., Nakazawa F. Analysis of bacterial flora associated with periimplantitis using obligate anaerobic culture technique and 16S rDNA gene sequence. Int. J. Oral Maxillofac. Implant. 2013;28:1521–1529. doi: 10.11607/jomi.2570. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y., Tong Z., Zhang Y., Si M., He F. Microbial profiles of peri-implant mucositis and periimplantitis: Submucosal microbial dysbiosis correlates with disease severity. Clin. Oral Implant. Res. 2022;33:172–183. doi: 10.1111/clr.13880. [DOI] [PubMed] [Google Scholar]
- 37.Sanz-Martin I., Doolittle-Hall J., Teles R.P., Patel M., Belibasakis G.N., Hämmerle C., Jung R.E., Teles F. Exploring the microbiome of healthy and diseased peri-implant sites using Illumina sequencing. J. Clin. Periodontol. 2017;44:1274–1284. doi: 10.1111/jcpe.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu X.L., Chan Y., Zhuang L., Lai H.C., Lang N.P., Keung Leung W., Watt R.M. Intra-oral single-site comparisons of periodontal and peri-implant microbiota in health and disease. Clin. Oral Implant. Res. 2019;30:760–776. doi: 10.1111/clr.13459. [DOI] [PubMed] [Google Scholar]
- 39.Yeh H.C., Lu J.J., Chang S.C., Ge M.C. Identification of microbiota in periimplantitis pockets by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Sci. Rep. 2019;9:774. doi: 10.1038/s41598-018-37450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maruyama N., Maruyama F., Takeuchi Y., Aikawa C., Izumi Y., Nakagawa I. Intraindividual variation in core microbiota in periimplantitis and periodontitis. Sci. Rep. 2014;4:6602. doi: 10.1038/srep06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kröger A., Hülsmann C., Fickl S., Spinell T., Hüttig F., Kaufmann F., Heimbach A., Hoffmann P., Enkling N., Renvert S., et al. The severity of human periimplantitis lesions correlates with the level of submucosal microbial dysbiosis. J. Clin. Periodontol. 2018;45:1498–1509. doi: 10.1111/jcpe.13023. [DOI] [PubMed] [Google Scholar]
- 42.Wells G.A., Shea B., O’Connell D., Peterson J., Welch Losos M., Tugwell P., Ga S.W., Zello G.A., Petersen J.A. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. The Ottawa Hospital Research Institute; Ottawa, ON, Canada: 2014. [Google Scholar]
- 43.Luchini C., Stubbs B., Solmi M., Veronese N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017;5:80–84. doi: 10.13105/wjma.v5.i4.80. [DOI] [Google Scholar]
- 44.Scarano A., Nardi G., Murmura G., Rapani M., Mortellaro C. Evaluation of the Removal Bacteria on Failed Titanium Implants After Irradiation with Erbium-Doped Yttrium Aluminium Garnet Laser. J. Craniofac. Surg. 2016;27:1202–1204. doi: 10.1097/SCS.0000000000002735. [DOI] [PubMed] [Google Scholar]
- 45.Misch C.E., Resnik R. Misch’s Avoiding Complications in Oral Implantology. 1st ed. Elsevier; Maryland Heights, MO, USA: 2017. pp. 771–826. [Google Scholar]
- 46.Lo C.K.L., Mertz D., Loeb M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]