Abstract
Infections caused by bacteria have a major impact on public health-related morbidity and mortality. Despite major advances in the prevention and treatment of bacterial infections, the latter continue to represent a significant economic and social burden worldwide. The WHO compiled a list of six highly virulent multidrug-resistant bacteria named ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) responsible for life-threatening diseases. Taken together with Clostridioides difficile, Escherichia coli, Campylobacter spp., (C. jejuni and C. coli), Legionella spp., Salmonella spp., and Neisseria gonorrhoeae, all of these microorganisms are the leading causes of nosocomial infections. The rapid and accurate detection of these pathogens is not only important for the early initiation of appropriate antibiotic therapy, but also for resolving outbreaks and minimizing subsequent antimicrobial resistance. The need for ever-improving molecular diagnostic techniques is also of fundamental importance for improving epidemiological surveillance of bacterial infections. In this review, we aim to discuss the recent advances on the use of molecular techniques based on genomic and proteomic approaches for the diagnosis of bacterial infections. The advantages and limitations of each of the techniques considered are also discussed.
Keywords: multidrug-resistant bacteria, antimicrobial resistance, ESKAPE, genotyping methods
1. Introduction
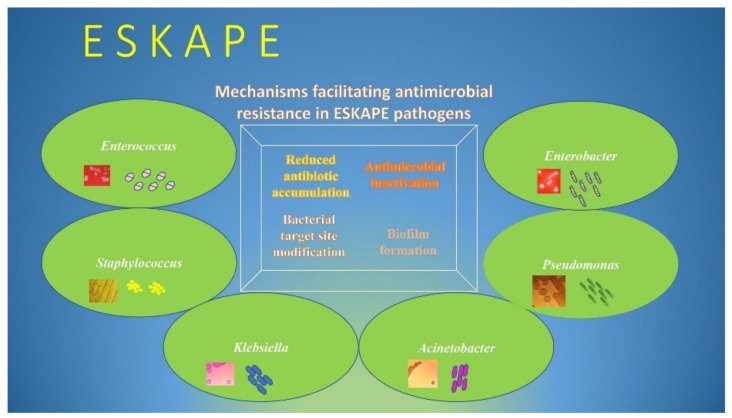
The rapid global spread of multidrug-resistant bacteria poses a serious threat to public health worldwide [1]. Critical-priority bacteria, as defined by the World Health Organization (WHO), include a group of life-threatening nosocomial pathogens known as “ESKAPE”, an acronym indicating the names of these bacteria and their ability to evade the antimicrobial activity of antibiotics [2]. The group of multidrug-resistant ESKAPE bacteria “for which new antibiotics are urgently needed”, as highlighted by the WHO, are: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. [3]. These pathogens, together with Clostridioides difficile, Escherichia coli, Campylobacter spp., (C. jejuni and C. coli), Legionella spp., and Salmonella spp., are the most common causes of nosocomial infections [4,5]. These pathogens exhibit drug resistance through several mechanisms, including active site modification, drug inactivation, efflux pump overexpression, and biofilm formation (Figure 1) [6,7,8]. As a result, these microorganisms are able to persist for long periods of time in hospital environments, becoming resistant to biocides or otherwise limiting their effects, spreading from person to person, and causing serious hospital infections [4,9,10]. The ability to produce enzymes such as ESBLs and carbapenemases, which can inactivate antibiotics of last resort, have contributed greatly to the rapid spread of the Gram-negative members of the ESKAPE group, especially in intensive care units (ICUs) [11,12]. For the same reason, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are the two members of the Gram-positive ESKAPE group that have been identified as two of the most important threats to patients in health-care settings [13,14]. Therefore, sensitive and specific microbe detection tests are needed to enable the rapid implementation of infection control practices. In recent decades, molecular diagnostics have developed considerably with the development of hybridization techniques that, through short sequences of nucleotide bases labeled with fluorescent tags (known as probes), are able to detect the presence of particular DNA sequences of interest (Supplementary Table S1). Numerous commercial kits are available for the rapid detection of different pathogens and their associated AMR genes directly from clinical specimens (Figure 2). However, as discussed below, all these molecular diagnostic techniques have both advantages and limitations compared to classical methods. According to the 2019 Centers for Disease Control and Prevention (CDC) report, resistance to essential antibiotics was increasing in seven of the eighteen germs originally reported in 2013 list [15]. The new report includes a new urgent threat posed by carbapenem-resistant Acinetobacter spp. causing pneumonia, bloodstream, wound, and urinary tract infections, primarily in hospitalized patients [15,16]. As mentioned above, Staphylococcus aureus is commonly found in the skin, but it is also a frequent cause of infection in catheterized patients [17]. Pseudomonas aeruginosa causes infections especially in hospital patients with weakened immune systems and can lead to worsening of previous lung diseases [18]. Enterobacter spp. can cause serious nosocomial infections, including blood and urinary tract infections, that are resistant to all antibiotics except for tigecycline and colistin [15,19]. These are also the last options against carbapenem-resistant hypervirulent Klebsiella pneumoniae [15,20]. Enterococci spp are major causes of hospital-acquired infections [21]. If these bacteria spread from the intestines, where they normally live harmlessly, to other parts of body, they can cause more serious infections, including bloodstream, surgical site, and urinary tract infections [22]. According to the 2019 CDC report, E. faecium caused 54,500 hospitalizations and 5400 deaths in 2017 in the US [23]. A broad range of enteric pathogens, including C. difficile, Campylobacter, and Salmonella species, are the most common causes of antibiotic-associated infectious diarrhea [24]. C. difficile is the pathogen responsible for the largest number of healthcare-associated infectious diarrheas and pseudomembranous colitis worldwide [24,25]. Cases of nosocomial legionellosis have increased substantially in recent years, with a lethality rate for healthcare cases exceeding 50% [26]. In addition, the risk of disease increases dramatically if the germ is found in certain areas within the hospital that are designed to closely monitor and treat patients with life-threatening conditions, such as intensive care units [27].
Figure 1.
Mechanisms of antimicrobial resistance in ESKAPE pathogens.
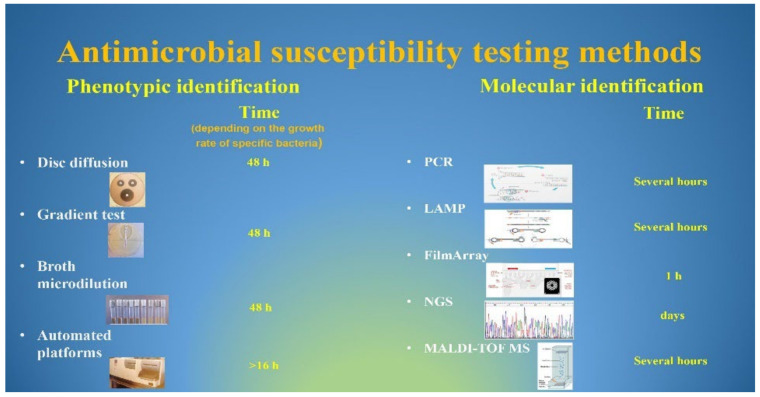
Figure 2.
Currently used methods for antimicrobial susceptibility testing.
2. Detection and Identification of Bacterial Pathogens
The difficulty in identifying bacterial pathogens and their associated AMR genes, due to the lack of rapid diagnostic methods, has long been a problem that, in the clinical setting, has often been overcome using broad-spectrum antibiotics [28]. Furthermore, the identification of bacterial pathogens for early aggressive antibiotic treatment is critical to prevent chronic infections that could lead to consequences such as wound infections, pneumonia, and catheter-related bloodstream infections that can lead to sepsis [1,29]. Therefore, rapid and sensitive molecular methods used for the detection of highly virulent drug-resistant bacteria in clinical samples are needed to guide antibiotic therapy and optimize control measures. In most cases, the gold standard for microbial identification relies on phenotypic approaches, including the use of enriched and selective culture media for the isolation of pathogenic bacteria, automated biochemical testing for identification, and antimicrobial susceptibility testing to guide therapy [30].
The main disadvantages of cultivation include the selection of a microbe-specific medium, the long incubation period for the microbe to spread on the selected medium, and the time required to obtain results [31]. In contrast, molecular diagnostics can be used to detect microbes directly from clinical specimens, thereby minimizing the occupational exposure of laboratory workers to infectious agents [12,32]. Improvements in molecular techniques, along with advances in technologies associated with bioinformatics tools, have led to the development of new technologies that have revolutionized the diagnosis of bacterial infections (Figure 3). This review aims to discuss the advantages and limitations of molecular methods, based on genomic and proteomic technologies, applied to the diagnosis of bacterial infections.
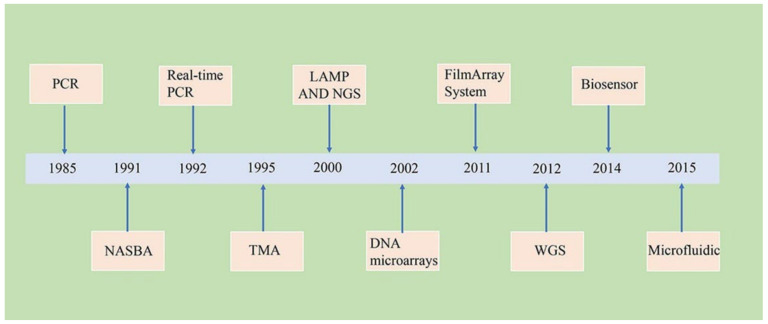
Figure 3.
Timeline of major molecular techniques for the diagnosis of bacterial infections.
3. Polymerase Chain Reaction (PCR) and Isothermal Amplification Methods
3.1. PCR
Nucleic-acid-based amplification technologies (NAATs) are high-performance tools used for the rapid and specific detection of pathogen-specific nucleic acids [33]. PCR was the first DNA amplification method, introduced by Mullis in 1985, that helps to make millions of copies of a DNA template starting from only a few molecules [34]. The PCR technique relies on thermal cycling, which consists of repeated heating and cooling cycles of the reaction to synthesize DNA. Short DNA fragments (known as primers) with sequences complementary to the target region are used to achieve exponential amplification of a target sequence, using a DNA polymerase that is stable at high temperatures (hence the name of the method) [35]. Because the identification of pathogens and their resistance mechanisms is a key challenge that must be achieved as quickly as possible to contain the further spread of antibiotic resistance, since its invention, many endpoint PCR variants have been developed to rapidly achieve these two goals [36].
3.2. Real-Time PCR
Due to its good sensitivity, specificity, and speed, real-time PCR, based on PCR techniques, is one of the most widely and clinically used techniques for the diagnosis of infectious diseases [36]. This technology allows real-time monitoring of the target amplification using either unspecifically intercalating fluorescent dyes or specific fluorescently labeled probes. The fluorescent signal is generated only after the probe hybridizes with its complementary target, and it is directly proportional to the number of PCR amplicons generated [28]. Although commercially distributed kits for the detection of pathogens and their resistance genes directly on biological samples are available, either false-positive or -negative results using different genes have also been previously reported [37,38,39]. This may be due to different factors, such as the rearrangement of genes within the genome and the acquisition of novel genes using horizontal transfer systems [40]. In addition, while mec gene identification signals methicillin-resistant Staphylococcus aureus (MRSA), the identification of resistance to β-lactam antibiotics in Gram-negative bacteria is more complex, because many of these bacteria contain more than one thousand β-lactamase-encoding genes [41,42]. Accordingly, different PCR platforms have focused on the most prevalent carbapenemases, such as blaKPC, blaOXA-48, blaOXA-23, blaOXA24/40, blaNDM, blaVIM, and blaIMP [43]. The main disadvantages of RT-PCR include a limited number of targets due to the limited availability of differentially fluorescent dyes. In addition, its sensitivity and specificity decrease with increasing levels of PCR multiplexing, due to the synthesis of nonspecific amplification products and the formation of primer dimers [44]. Moreover, the RT-PCR instrument requires high maintenance costs. In recent years, new developments in RT-PCR have led to the generation of a variety of isothermal amplification techniques including loop-mediated amplification (LAMP), helicase-dependent amplification (HDA), nucleic acid sequence-based amplification (NASBA), and transcription-mediated amplification (TMA) [28,35,45].
3.3. LAMP
LAMP is a valid and effective diagnostic test that does not require the use of expensive thermal cyclers or specialized personnel, and is cost-effective. LAMP is based on conventional PCR, but, unlike the latter, it uses a DNA polymerase with high strand displacement activity, with four primers that recognize six–eight distinct regions of the target DNA to produce a stem-loop structure of the DNA that facilitates subsequent rounds of amplification [46]. The release of pyrophosphate that follows the synthesis of the target DNA stem-loop can be detected by adding a DNA-binding dye, such as SYBR green. The ability of LAMP to generate up to 109 copies of products in less than an hour makes this technique more sensitive than conventional PCR [46,47]. Commercial LAMP kits are available for the detection of resistance genes, such as: blaKPC and blaNDM-1 in K. pneumonia and A. baumannii [47].
3.4. NASBA, TMA and HDA
NASBA and TMA are isothermal amplification reactions which, unlike PCR, typically use various mRNA’s as target sequences as the target sequence. These techniques are the gold standard in the diagnosis of gonorrhea and chlamydial infection [48]. HDA is another isothermal amplification method that uses the unwinding activity of a helicase to separate dsDNA into two single strands that can serve as a template for new DNA synthesis [49]. In fact, the two strands maintain a single-strand template due to the immediate binding of SSBs, which prevents the reconstitution of a double helix. HDA assays have been successfully applied to detect Clostridium difficile, S. aureus, and N. gonorrhoeae [45,49].
3.5. The BioFire FilmArray Panels
This system is a vitro diagnostic test platform that integrates nucleic acid extraction, reverse transcription, and nested multiplex PCR amplification, followed by a melting curve analysis [50]. This system is designed to be used with comprehensive panels that include assays for a variety of pathogens, causing respiratory viral infections, pneumonia, bloodstream, gastrointestinal infections, and meningitis–encephalitis, as well as antimicrobial resistance genes [51]. The FilmArray menu comprises: Gastrointestinal, Pneumonia, Meningitis Encephalitis, and Blood Culture Identification panels [52]. The FilmArray Gastrointestinal panel tests for 22 common pathogens, including viruses, bacteria, and protozoa, which cause gastrointestinal symptoms such as diarrheal diseases, i.e., a leading cause of child death in developing countries [53]. The FilmArray Pneumonia panel tests for 27 of the most common pathogens involved in lower respiratory tract infections and 7 genetic markers of antibiotic resistance [54]. This panel has a sensitivity and specificity for bronchoalveolar and sputum samples of >96%, enabling both the identification of the causative agents of hospital-associated respiratory infections, and also the prevention of the causative agents of secondary infections, including antibiotic-resistant strains of S. aureus and K. pneumoniae [55]. The FilmArray Blood Culture Identification panel detects 33 pathogen and 10 antimicrobial resistance genes associated with bloodstream infections [56]. For patients with sepsis, a leading cause of death in hospital patients, rapid identification of the organism from blood cultures in combination with the indication of pathogen-associated resistance genes is critical for reducing patient morbidity and mortality [57]. In addition, according to international guidelines for the management of sepsis, in order to reduce the occurrence of antimicrobial resistance, it is important to replace broad-spectrum therapy with appropriate antibiotic therapy as soon as pathogens are identified [58]. Finally, the FilmArray Meningitis Encephalitis panel is able to simultaneously test cerebral spinal fluid for the 14 most common pathogens responsible for community-acquired meningitis [59]. Meningitis affects more than one million people, and symptoms appear and progress rapidly; therefore, early diagnosis of meningitis is crucial to achieve the best treatment outcomes [59].
3.6. DNA Microarrays
PCR approaches are the gold standard for detecting and identifying a single gene or a limited number of genes [36]. In contrast, PCR is not a good choice for detecting a large spectrum of genes, such as those involved in antimicrobial resistance [36]. The high multiplexing ability of microarrays to detect and identify a vast range of genes simultaneously makes this technique noteworthy for the investigation of genetic AMR in bacteria [60]. This technology allows for the detection of genes encoding ESBLs and carbapenemases through the hybridization of oligonucleotide sequences that can select and amplify specific molecules from the sample of interest [60,61]. Some bacteria, including the ESKAPE pathogens, may have several resistance genes, which must be identified immediately to prevent the spread of multi-resistant strains. For example, ESBL genes are found on plasmids that can be easily transferred between enteric Gram-negative bacteria, such as E. coli and K. pneumoniae, which are among the most common uropathogenic bacteria that cause urinary tract infections [62,63]. A second type of multidrug resistance genes that must be recognized immediately is related to the presence of transposons carrying vancomycin resistance genes [64]. Vancomycin-resistant S. aureus (VRSA) is mediated by a cluster of vanA genes transferred from vancomycin-resistant enterococci [64]. These bacteria are often resistant to a wide range of antibiotics, including aminoglycosides, tetracyclines, and beta-lactam antibiotics [65]. The ability of the microarray to detect multiple multi-drug resistance genes present in a single strain can help clinicians direct antimicrobial therapy [66]. However, the microarray is not widely implemented in clinical microbiology laboratories due to the high cost of a single experiment and the complexity of the method [30,67].
3.7. Pulse-Field Gel Electrophoresis (PFGE), Multilocus Sequencing Typing (MLST), and Pyrosequencing
Different methods have been described for the characterization of ESBL and carbapenemase genes, and for epidemiological investigations such as pulsed-field gel electrophoresis (PFGE), multilocus sequencing typing (MLST), whole-genome sequencing (WGS), pyrosequencing, and next-generation sequencing (NGS) [30]. PFGE is a technique used for the separation of large fragments of DNA molecules, obtained after restriction enzyme digestion, by applying an electric field to a gel matrix that periodically changes direction. Although several PFGE protocols have been developed in the past for typing different bacteria and for outbreak investigations, this technique has recently been replaced by better-performing techniques such as NGS and MALDI [68]. MLST is a strain typing system that focuses exclusively on conserved housekeeping genes to derive a combination of alleles (known as sequence type (ST)) that can discriminate isolates of bacterial species without requiring whole-genome sequencing [69]. The high cost and low discrimination power of this technique limit its use to epidemiological investigations [69]. Pyrosequencing is a bioluminescence method that measures the release of inorganic pyrophosphate. Briefly, the release of inorganic pyrophosphate (PPi) following each nucleotide incorporation event is proportionally converted, by a series of enzymatic reactions, into visible light [70]. The main drawback of this technique is that it can only sequence a short nucleotide sequence [70]. In general, the main disadvantages of these techniques are their high cost and the availability of technicians capable of executing them.
3.8. Whole-Genome Sequencing (WGS)
Whole-genome sequencing (WGS) refers to the determination of the complete nucleotide sequence of a genome of a microorganism in a single assay [71]. Over the last two decades, WGS has been successfully applied in different fields including: (1) the identification of the virulence factors of some pathogens; (2) the identification of the disease transmission pathway in outbreak analysis; (3) AMR profiling; and (4) the identification of sources of recurrent infections and patient-to-patient transmissions [72]. Despite this enormous potential, the adoption of WGS in clinics is still slow, although new benchtop sequencing platforms have recently been introduced that, being cheaper, could facilitate the spread of bacterial WGS in the clinical setting [72].
3.9. Next-Generation Sequencing (NGS)
Next-generation sequencing (NGS) is a rapid and cost-effective massively parallel sequencing technology that, by focusing on the exome, is a viable alternative to whole-genome sequencing [73]. In fact, only second-generation NGS should be adopted in clinical microbiology, as it combines short-read technology for output and cost. Second-generation NGS workflows have three key phases: library preparation, template preparation, and sequencing [74]. The main difference between the different NGS platforms concerns the DNA amplification techniques used: sequencing by hybridization and sequencing by synthesis. Both platforms have been successfully applied to outbreak investigations and resistance gene mapping of several ESKAPE pathogens, including P. aeruginosa and A. baumanni [74].
3.10. Microfluidics
The microfluidic “lab-on-a-chip” technique represents a promising technology for the detection of antibiotic-resistant bacteria [75]. Compared to traditional macroscale methods, microfluidics offers many advantages such as: low cost, fast and high-throughput analysis, smaller sample volume, and automation. There are two main categories of microfluidic-based detection methods: genotypic and phenotypic assays [76]. Genotypic on-chip assays (e.g., PCR, LAMP) target genetic markers (e.g., 16S rRNA genes), thus circumventing bacterial growth and allowing rapid bacterial identification. However, this assay is not applicable for the determination of bacterial antibiotic susceptibility profiles. In contrast, phenotypic on-chip assays monitor bacterial growth in the presence of antibiotics, thus offering accurate AST results. Typically, bacterial cells are restricted to a small volume, such as a chamber or droplet, captured by antibodies on membranes or magnetic beads, or encapsulated in chambers containing agarose. The main drawbacks of these microfluidic platforms involve the use of expensive microscopes and the lack of antibodies targeting different bacterial strains. Because of these limitations, improvements are needed to make these systems commercially available [75,76].
3.11. Immunodetection of Pathogens
Immunodetection is a simple and specific method used for the identification of microbial pathogens that relies on the use of antibodies which are suitably immobilized on nanoparticles or strips and can bind specifically to a given target. The binding is visualized by labeling the antibody with fluorescent dyes or redox enzymes [28]. In contrast, the direct detection of antibiotic resistance proteins through this technology needs to be implemented.
3.12. Detection of Growth-Related Molecules
A wide range of devices detecting volatile compounds, so-called electronic noses (eNose), have been used in the diagnosis of bacterial infection diseases [77]. The eNose instruments can detect the smellprint patterns of a given bacterial species and discriminate among the complex mixtures of volatile metabolites associated with a specific disease. Several studies have revealed the efficacy of e-nose devices for the clinical diagnoses of many human diseases [78,79].
3.13. Biosensor Systems
A biosensor can be defined as a module that aids in detecting changes in physical quantities and thereby converts these changes to signals proportional to the concentration of an analyte in the reaction [80]. Sensors are classified into various categories, depending on the physical substance or analyte to be measured. Several studies showed that using microcalorimetry approaches, it was possible to identify bacteria directly from urine samples and vancomycin-resistant Staphylococcus aureus strains in less than 8 h [81,82]. The main disadvantages of these methods are the absence of clinically validated microcalorimetry systems for AST and the need to analyze pure cultures [80].
3.14. MALDI-TOF Mass Spectrometry
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) has become a reference method used for microbial identification in clinical microbiology laboratories [83]. In contrast to traditional methods of bacterial species identification and related susceptibility testing, which are labor- and time-intensive (for example, AST can usually be completed at least 24 h after cultures turn positive), MALDI-TOF is a fast, convenient, and low-cost method for this purpose [83]. The identification of microorganisms using this technique relies primarily on spectral libraries to identify the mass fingerprinting of the peptide generated by each microorganism. Briefly, the bacterial colony is suspended in a matrix solution and placed on the well of a stainless-steel plate. After evaporation of the solvent, the matrix desorbs and ionizes in the presence of a laser beam that transfers energy to the sample molecules, which are accelerated and separated under the action of an electric field according to their mass-to-charge ratio. Microorganisms are identified by comparing the resulting spectrum with a database of spectra of known organisms [83,84]. The most frequently used platforms are the Bruker Biotyper (Bruker Daltonik, Bremen, Germany) and the VitekMS (bioMérieux, Marcy l’Etoile, France). These two platforms have a single set of organisms in their database and both systems have a search-only module that allows the user to add organisms that are not in the database. Both platforms, being equally specific and reproducible, are able to identify the vast majority of organisms commonly isolated in the microbiology laboratory at the species level [85]. The high levels of identification of both techniques are based on the identification of low-molecular-weight spectra ranging from 2 to 20 KDa (i.e., ribosomal proteins). The Bruker Biotyper is better for identifying non-fermenting Gram-negative bacteria and yeasts, while the VitekMS is better suited for the identification of mycobacteria, actinomycetes, and filamentous fungi [85]. Despite the progress, a current challenge of both platforms is the identification of pathogens directly from samples. In addition, another aspect that can significantly develop this technology is related to the ability to identify multiple bacteria in complex polymicrobial samples [86]. In this regard, several processing methods used for the preparation of different clinical samples, such as urine, cerebrospinal fluid, and blood, suitable for MALDI-TOF MS analysis have been proposed and are still in the process of standardization [86,87]. Another critical challenge for MALDI-TOF MS in clinical microbiological diagnosis concerns the detection of antibiotic resistance [88] and the activation of drug efflux pumps [1]. In addition to disk diffusion susceptibility testing, current methods for antimicrobial susceptibility testing include broth dilution methods [1]. The latter are the reference methods for determining the minimum inhibitory concentrations (MICs) of antimicrobial agents (i.e., the lowest concentration at which the agent inhibits the growth of microorganisms). Although accurate, these methods are either labor-intensive or time-consuming, leading to long waiting times to obtain AST results [89].
The identification of AMR determinants, mostly based on molecular techniques such as PCR, also has several limitations, the most important of which are: (1) the expression of some resistance genes must be induced; (2) the discovery of new potential AMR determinants is difficult to achieve; (3) identifying the genetic mechanisms of AMR and predicting resistance phenotypes of bacterial pathogens requires a deep knowledge of the field; and (4) these techniques are expensive and time-consuming, which does not make them suitable for wide use in clinical microbiology laboratories [28]. Several approaches based on MALDI-TOF MS have been used for the rapid detection of antimicrobial resistance in bacteria. MALDI-TOF MS has been used to detect the presence of carbapenemases for the rapid detection of antimicrobial resistance [88,90]. The most promising applications include: (1) the determination of β-lactamase activity by visualizing the peak shift of β-lactam ring hydrolysis; (2) the detection of the specific peak for AMR determinants; (3) the detection of specific peaks for proteins co-expressed with AMR determinants; and (4) a comparison of the area under the curve of the MALDI spectra of bacteria incubated with or without antimicrobial drugs [88,90].
Although significant strides have been made toward susceptibility determination in antibiotics, several drawbacks need to be resolved such as (1) limited applications directly in clinical samples, (2) expensive equipment, and (3) specific extraction protocols for the detection of biomarkers of antimicrobial resistance [91].
4. Conclusions
The rapid diagnosis of serious infections is critical to initiate appropriate therapy as early as possible while simultaneously reducing the use of unnecessary antibiotics when they are not needed and associated morbidity and healthcare costs [1]. The ESKAPE pathogens and other serious health threats, through the rapid acquisition of AMR genes combined with the ability to form biofilms, have the greatest impact on healthcare-associated infections [12]. The main advantages of the molecular approach in the detection of bacterial pathogens and AMR genes are related to (1) obtaining results in reduced time; (2) the direct application of these methods to clinical samples resulting in reduced time-to-response; and (3) cost benefits in terms of reduced time to appropriate therapy and decreased hospitalization and risks associated with co-morbidity and mortality, [30]. Despite recent advances in molecular technologies in the field of microbiological diagnostics, it is not possible to identify a single diagnostic platform that fully meets the clinical need to (1) provide better treatments and care to patients, (2) reduce the use of broad-spectrum antibiotics, and (3) achieve better clinical outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11060663/s1, Table S1: Summary of available technologies. Refs [92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109] are cited in Supplementary Materials.
Author Contributions
Conceptualization: C.B.; manuscript writing: G.M., A.M., E.G., S.P., S.Z. and C.B.; review and editing: C.B., G.M., A.M. and E.G.; figure creation: S.Z. and S.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mancuso G., Midiri A., Gerace E., Biondo C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens. 2021;10:1310. doi: 10.3390/pathogens10101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Oliveira D.M.P., Forde B.M., Kidd T.J., Harris P.N.A., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020;33:e00181-19. doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y., Huang W.E., Yang Q. Clinical Perspective of Antimicrobial Resistance in Bacteria. Infect. Drug Resist. 2022;15:735–746. doi: 10.2147/IDR.S345574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sousa S.A., Feliciano J.R., Pita T., Soeiro C.F., Mendes B.L., Alves L.G., Leitao J.H. Bacterial Nosocomial Infections: Multidrug Resistance as a Trigger for the Development of Novel Antimicrobials. Antibiotics. 2021;10:942. doi: 10.3390/antibiotics10080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avershina E., Shapovalova V., Shipulin G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Front. Microbiol. 2021;12:707330. doi: 10.3389/fmicb.2021.707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair J.M., Webber M.A., Baylay A.J., Ogbolu D.O., Piddock L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 7.Asenjo A., Oteo-Iglesias J., Alos J.I. What’s new in mechanisms of antibiotic resistance in bacteria of clinical origin? Enferm. Infecc. Microbiol. Clin. 2021;39:291–299. doi: 10.1016/j.eimc.2020.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Uddin T.M., Chakraborty A.J., Khusro A., Zidan B.R.M., Mitra S., Emran T.B., Dhama K., Ripon M.K.H., Gajdacs M., Sahibzada M.U.K., et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health. 2021;14:1750–1766. doi: 10.1016/j.jiph.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Larsson D.G.J., Flach C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022;20:257–269. doi: 10.1038/s41579-021-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benko R., Gajdacs M., Matuz M., Bodo G., Lazar A., Hajdu E., Papfalvi E., Hannauer P., Erdelyi P., Peto Z. Prevalence and Antibiotic Resistance of ESKAPE Pathogens Isolated in the Emergency Department of a Tertiary Care Teaching Hospital in Hungary: A 5-Year Retrospective Survey. Antibiotics. 2020;9:624. doi: 10.3390/antibiotics9090624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulani M.S., Kamble E.E., Kumkar S.N., Tawre M.S., Pardesi K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019;10:539. doi: 10.3389/fmicb.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karaman R., Jubeh B., Breijyeh Z. Resistance of Gram-Positive Bacteria to Current Antibacterial Agents and Overcoming Approaches. Molecules. 2020;25:2888. doi: 10.3390/molecules25122888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y., Song G., Sun M., Wang J., Wang Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020;10:107. doi: 10.3389/fcimb.2020.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez M.S., Bonomo R.A., Tolmasky M.E. Carbapenemases: Transforming Acinetobacter baumannii into a Yet More Dangerous Menace. Biomolecules. 2020;10:720. doi: 10.3390/biom10050720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levi Y., Ben-David D., Estrin I., Saadon H., Krocker M., Goldstein L., Klafter D., Zilberman-Itskovich S., Marchaim D. The Impact of Differences in Surveillance Definitions of Hospital Acquired Urinary Tract Infections (HAUTI) Antibiotics. 2021;10:1262. doi: 10.3390/antibiotics10101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallabhaneni S., Huang J.Y., Grass J.E., Bhatnagar A., Sabour S., Lutgring J.D., Campbell D., Karlsson M., Kallen A.J., Nazarian E., et al. Antimicrobial Susceptibility Profiles to Predict the Presence of Carbapenemase Genes among Carbapenem-Resistant Pseudomonas aeruginosa Isolates. J. Clin. Microbiol. 2021;59:e02874-20. doi: 10.1128/JCM.02874-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y.F., Liu P., Zhang C.J., Liao X.P., Sun J., Liu Y.H. Colistin Combined with Tigecycline: A Promising Alternative Strategy to Combat Escherichia coli Harboring bla NDM-5 and mcr-1. Front. Microbiol. 2019;10:2957. doi: 10.3389/fmicb.2019.02957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J., Zeng Y., Zhang R., Cai J. In Vivo Emergence of Colistin and Tigecycline Resistance in Carbapenem-Resistant Hypervirulent Klebsiella pneumoniae during Antibiotics Treatment. Front. Microbiol. 2021;12:702956. doi: 10.3389/fmicb.2021.702956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkwirth S., Ayobami O., Eckmanns T., Markwart R. Hospital-acquired infections caused by enterococci: A systematic review and meta-analysis, WHO European Region, 1 January 2010 to 4 February 2020. Euro Surveill. Bull. Eur. Mal. Transm. Eur. Commun. Dis. Bull. 2021;26:45. doi: 10.2807/1560-7917.ES.2021.26.45.2001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krawczyk B., Wityk P., Galecka M., Michalik M. The Many Faces of Enterococcus spp.-Commensal, Probiotic and Opportunistic Pathogen. Microorganisms. 2021;9:1900. doi: 10.3390/microorganisms9091900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida-Santos A.C., Novais C., Peixe L., Freitas A.R. Enterococcus spp. as a Producer and Target of Bacteriocins: A Double-Edged Sword in the Antimicrobial Resistance Crisis Context. Antibiotics. 2021;10:1215. doi: 10.3390/antibiotics10101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkell M., Mysara M., Xavier B.B., van Werkhoven C.H., Monsieurs P., Lammens C., Ducher A., Vehreschild M., Goossens H., de Gunzburg J., et al. Microbiota-based markers predictive of development of Clostridioides difficile infection. Nat. Commun. 2021;12:2241. doi: 10.1038/s41467-021-22302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abad C.L.R., Safdar N. A Review of Clostridioides difficile Infection and Antibiotic-Associated Diarrhea. Gastroenterol. Clin. N. Am. 2021;50:323–340. doi: 10.1016/j.gtc.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Mazzotta M., Girolamini L., Pascale M.R., Lizzadro J., Salaris S., Dormi A., Cristino S. The Role of Sensor-Activated Faucets in Surgical Handwashing Environment as a Reservoir of Legionella. Pathogens. 2020;9:446. doi: 10.3390/pathogens9060446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falcone M., Russo A., Tiseo G., Cesaretti M., Guarracino F., Menichetti F. Predictors of intensive care unit admission in patients with Legionella pneumonia: Role of the time to appropriate antibiotic therapy. Infection. 2021;49:321–325. doi: 10.1007/s15010-020-01565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasala A., Hytonen V.P., Laitinen O.H. Modern Tools for Rapid Diagnostics of Antimicrobial Resistance. Front. Cell. Infect. Microbiol. 2020;10:308. doi: 10.3389/fcimb.2020.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha M., Jupe J., Mack H., Coleman T.P., Lawrence S.M., Fraley S.I. Emerging Technologies for Molecular Diagnosis of Sepsis. Clin. Microbiol. Rev. 2018;31:e00089-17. doi: 10.1128/CMR.00089-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsalik E.L., Bonomo R.A., Fowler V.G., Jr. New Molecular Diagnostic Approaches to Bacterial Infections and Antibacterial Resistance. Annu. Rev. Med. 2018;69:379–394. doi: 10.1146/annurev-med-052716-030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y.X., Wang C.Y., Li Y.Y., Li J., Wan Q.Q., Chen J.H., Tay F.R., Niu L.N. Considerations and Caveats in Combating ESKAPE Pathogens against Nosocomial Infections. Adv. Sci. 2020;7:1901872. doi: 10.1002/advs.201901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adzitey F., Huda N., Ali G.R. Molecular techniques for detecting and typing of bacteria, advantages and application to foodborne pathogens isolated from ducks. 3 Biotech. 2013;3:97–107. doi: 10.1007/s13205-012-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanoli L.M., Spoto G. Isothermal amplification methods for the detection of nucleic acids in microfluidic devices. Biosensors. 2013;3:18–43. doi: 10.3390/bios3010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantera J.L., White H., Diaz M.H., Beall S.G., Winchell J.M., Lillis L., Kalnoky M., Gallarda J., Boyle D.S. Assessment of eight nucleic acid amplification technologies for potential use to detect infectious agents in low-resource settings. PLoS ONE. 2019;14:e0215756. doi: 10.1371/journal.pone.0215756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolff N., Hendling M., Schroeder F., Schonthaler S., Geiss A.F., Bedenic B., Barisic I. Full pathogen characterisation: Species identification including the detection of virulence factors and antibiotic resistance genes via multiplex DNA-assays. Sci. Rep. 2021;11:6001. doi: 10.1038/s41598-021-85438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H., Zhang H., Xu Y., Lassakova S., Korabecna M., Neuzil P. PCR past, present and future. BioTechniques. 2020;69:317–325. doi: 10.2144/btn-2020-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J.W., Lau Y.Y., Krishnan T., Chan K.G., Chang C.Y. Recent Advances in Molecular Diagnosis of Pseudomonas aeruginosa Infection by State-of-the-Art Genotyping Techniques. Front. Microbiol. 2018;9:1104. doi: 10.3389/fmicb.2018.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan S.A., Ahmed S., Mushahid N., Anwer M., Saeed S., Khan F.A., Shamshad G.U., Joyia Z. Comparison of real time polymerase chain reaction with microscopy and antigen detection assay for the diagnosis of malaria. J. Coll. Physicians Surg.—Pak. JCPSP. 2013;23:787–792. [PubMed] [Google Scholar]
- 39.Espy M.J., Uhl J.R., Sloan L.M., Buckwalter S.P., Jones M.F., Vetter E.A., Yao J.D., Wengenack N.L., Rosenblatt J.E., Cockerill F.R., 3rd, et al. Real-time PCR in clinical microbiology: Applications for routine laboratory testing. Clin. Microbiol. Rev. 2006;19:165–256. doi: 10.1128/CMR.19.1.165-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunder W., Karch H. Genome plasticity in Enterobacteriaceae. Int. J. Med. Microbiol. IJMM. 2000;290:153–165. doi: 10.1016/S1438-4221(00)80084-3. [DOI] [PubMed] [Google Scholar]
- 41.McClure J.A., Conly J.M., Obasuyi O., Ward L., Ugarte-Torres A., Louie T., Zhang K. A Novel Assay for Detection of Methicillin-Resistant Staphylococcus aureus Directly from Clinical Samples. Front. Microbiol. 2020;11:1295. doi: 10.3389/fmicb.2020.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bush K. Past and Present Perspectives on beta-Lactamases. Antimicrob. Agents Chemother. 2018;62:e01076-18. doi: 10.1128/AAC.01076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerezales M., Biniossek L., Gerson S., Xanthopoulou K., Wille J., Wohlfarth E., Kaase M., Seifert H., Higgins P.G. Novel multiplex PCRs for detection of the most prevalent carbapenemase genes in Gram-negative bacteria within Germany. J. Med. Microbiol. 2021;70:001310. doi: 10.1099/jmm.0.001310. [DOI] [PubMed] [Google Scholar]
- 44.Elnifro E.M., Ashshi A.M., Cooper R.J., Klapper P.E. Multiplex PCR: Optimization and application in diagnostic virology. Clin. Microbiol. Rev. 2000;13:559–570. doi: 10.1128/CMR.13.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obande G.A., Banga Singh K.K. Current and Future Perspectives on Isothermal Nucleic Acid Amplification Technologies for Diagnosing Infections. Infect. Drug Resist. 2020;13:455–483. doi: 10.2147/IDR.S217571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mori Y., Notomi T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2009;15:62–69. doi: 10.1007/s10156-009-0669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poirier A.C., Kuang D., Siedler B.S., Borah K., Mehat J.W., Liu J., Tai C., Wang X., van Vliet A.H.M., Ma W., et al. Development of Loop-Mediated Isothermal Amplification Rapid Diagnostic Assays for the Detection of Klebsiella pneumoniae and Carbapenemase Genes in Clinical Samples. Front. Mol. Biosci. 2021;8:794961. doi: 10.3389/fmolb.2021.794961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muralidhar S. Molecular methods in the laboratory diagnosis of sexually transmitted infections. Indian J. Sex. Transm. Dis. AIDS. 2015;36:9–17. doi: 10.4103/0253-7184.156686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barreda-Garcia S., Miranda-Castro R., de-Los-Santos-Alvarez N., Miranda-Ordieres A.J., Lobo-Castanon M.J. Helicase-dependent isothermal amplification: A novel tool in the development of molecular-based analytical systems for rapid pathogen detection. Anal. Bioanal. Chem. 2018;410:679–693. doi: 10.1007/s00216-017-0620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoo I.Y., Huh K., Shim H.J., Yun S.A., Chung Y.N., Kang O.K., Huh H.J., Lee N.Y. Evaluation of the BioFire FilmArray Pneumonia Panel for rapid detection of respiratory bacterial pathogens and antibiotic resistance genes in sputum and endotracheal aspirate specimens. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;95:326–331. doi: 10.1016/j.ijid.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 51.Cortazzo V., D’Inzeo T., Giordano L., Menchinelli G., Liotti F.M., Fiori B., De Maio F., Luzzaro F., Sanguinetti M., Posteraro B., et al. Comparing BioFire FilmArray BCID2 and BCID Panels for Direct Detection of Bacterial Pathogens and Antimicrobial Resistance Genes from Positive Blood Cultures. J. Clin. Microbiol. 2021;59:e03163-20. doi: 10.1128/JCM.03163-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitton B., Rule R., Said M. Laboratory evaluation of the BioFire FilmArray Pneumonia plus panel compared to conventional methods for the identification of bacteria in lower respiratory tract specimens: A prospective cross-sectional study from South Africa. Diagn. Microbiol. Infect. Dis. 2021;99:115236. doi: 10.1016/j.diagmicrobio.2020.115236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torres-Miranda D., Akselrod H., Karsner R., Secco A., Silva-Cantillo D., Siegel M.O., Roberts A.D., Simon G.L. Use of BioFire FilmArray gastrointestinal PCR panel associated with reductions in antibiotic use, time to optimal antibiotics, and length of stay. BMC Gastroenterol. 2020;20:246. doi: 10.1186/s12876-020-01394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gastli N., Loubinoux J., Daragon M., Lavigne J.P., Saint-Sardos P., Pailhories H., Lemarie C., Benmansour H., d’Humieres C., Broutin L., et al. Multicentric evaluation of BioFire FilmArray Pneumonia Panel for rapid bacteriological documentation of pneumonia. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021;27:1308–1314. doi: 10.1016/j.cmi.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 55.Manohar P., Loh B., Nachimuthu R., Hua X., Welburn S.C., Leptihn S. Secondary Bacterial Infections in Patients with Viral Pneumonia. Front. Med. 2020;7:420. doi: 10.3389/fmed.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rule R., Paruk F., Becker P., Neuhoff M., Chausse J., Said M. Clinical utility of the BioFire FilmArray Blood Culture Identification panel in the adjustment of empiric antimicrobial therapy in the critically ill septic patient. PLoS ONE. 2021;16:e0254389. doi: 10.1371/journal.pone.0254389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berinson B., Both A., Berneking L., Christner M., Lutgehetmann M., Aepfelbacher M., Rohde H. Usefulness of BioFire FilmArray BCID2 for Blood Culture Processing in Clinical Practice. J. Clin. Microbiol. 2021;59:e0054321. doi: 10.1128/JCM.00543-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., Machado F.R., McIntyre L., Ostermann M., Prescott H.C., et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Domingues R.B., Santos M.V.D., Leite F., Senne C. FilmArray Meningitis/Encephalitis (ME) panel in the diagnosis of bacterial meningitis. Braz. J. Infect. Dis. Off. Publ. Braz. Soc. Infect. Dis. 2019;23:468–470. doi: 10.1016/j.bjid.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fishbain J.T., Sinyavskiy O., Riederer K., Hujer A.M., Bonomo R.A. Detection of extended-spectrum beta-lactamase and Klebsiella pneumoniae Carbapenemase genes directly from blood cultures by use of a nucleic acid microarray. J. Clin. Microbiol. 2012;50:2901–2904. doi: 10.1128/JCM.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cuzon G., Naas T., Bogaerts P., Glupczynski Y., Nordmann P. Evaluation of a DNA microarray for the rapid detection of extended-spectrum beta-lactamases (TEM, SHV and CTX-M), plasmid-mediated cephalosporinases (CMY-2-like, DHA, FOX, ACC-1, ACT/MIR and CMY-1-like/MOX) and carbapenemases (KPC, OXA-48, VIM, IMP and NDM) J. Antimicrob. Chemother. 2012;67:1865–1869. doi: 10.1093/jac/dks156. [DOI] [PubMed] [Google Scholar]
- 62.Overdevest I., Willemsen I., Rijnsburger M., Eustace A., Xu L., Hawkey P., Heck M., Savelkoul P., Vandenbroucke-Grauls C., van der Zwaluw K., et al. Extended-spectrum beta-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerg. Infect. Dis. 2011;17:1216–1222. doi: 10.3201/eid1707.110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rawat D., Nair D. Extended-spectrum beta-lactamases in Gram Negative Bacteria. J. Glob. Infect. Dis. 2010;2:263–274. doi: 10.4103/0974-777X.68531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed M.O., Baptiste K.E. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018;24:590–606. doi: 10.1089/mdr.2017.0147. [DOI] [PubMed] [Google Scholar]
- 65.Sparo M., Delpech G., Garcia Allende N. Impact on Public Health of the Spread of High-Level Resistance to Gentamicin and Vancomycin in Enterococci. Front. Microbiol. 2018;9:3073. doi: 10.3389/fmicb.2018.03073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dally S., Lemuth K., Kaase M., Rupp S., Knabbe C., Weile J. DNA microarray for genotyping antibiotic resistance determinants in Acinetobacter baumannii clinical isolates. Antimicrob. Agents Chemother. 2013;57:4761–4768. doi: 10.1128/AAC.00863-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedrich T., Rahmann S., Weigel W., Rabsch W., Fruth A., Ron E., Gunzer F., Dandekar T., Hacker J., Muller T., et al. High-throughput microarray technology in diagnostics of enterobacteria based on genome-wide probe selection and regression analysis. BMC Genom. 2010;11:591. doi: 10.1186/1471-2164-11-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neoh H.M., Tan X.E., Sapri H.F., Tan T.L. Pulsed-field gel electrophoresis (PFGE): A review of the “gold standard” for bacteria typing and current alternatives. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2019;74:103935. doi: 10.1016/j.meegid.2019.103935. [DOI] [PubMed] [Google Scholar]
- 69.Patino L.H., Camargo M., Munoz M., Rios-Chaparro D.I., Patarroyo M.A., Ramirez J.D. Unveiling the Multilocus Sequence Typing (MLST) Schemes and Core Genome Phylogenies for Genotyping Chlamydia trachomatis. Front. Microbiol. 2018;9:1854. doi: 10.3389/fmicb.2018.01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen S., Qin D. Pyrosequencing data analysis software: A useful tool for EGFR, KRAS, and BRAF mutation analysis. Diagn. Pathol. 2012;7:56. doi: 10.1186/1746-1596-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hasman H., Saputra D., Sicheritz-Ponten T., Lund O., Svendsen C.A., Frimodt-Moller N., Aarestrup F.M. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples. J. Clin. Microbiol. 2014;52:139–146. doi: 10.1128/JCM.02452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilchrist C.A., Turner S.D., Riley M.F., Petri W.A., Jr., Hewlett E.L. Whole-genome sequencing in outbreak analysis. Clin. Microbiol. Rev. 2015;28:541–563. doi: 10.1128/CMR.00075-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slatko B.E., Gardner A.F., Ausubel F.M. Overview of Next-Generation Sequencing Technologies. Curr. Protoc. Mol. Biol. 2018;122:e59. doi: 10.1002/cpmb.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu T., Chitnis N., Monos D., Dinh A. Next-generation sequencing technologies: An overview. Hum. Immunol. 2021;82:801–811. doi: 10.1016/j.humimm.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 75.Kaprou G.D., Bergspica I., Alexa E.A., Alvarez-Ordonez A., Prieto M. Rapid Methods for Antimicrobial Resistance Diagnostics. Antibiotics. 2021;10:209. doi: 10.3390/antibiotics10020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma L., Petersen M., Lu X. Identification and Antimicrobial Susceptibility Testing of Campylobacter Using a Microfluidic Lab-on-a-Chip Device. Appl. Environ. Microbiol. 2020;86:e00096-20. doi: 10.1128/AEM.00096-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson A.D. Application of Electronic-Nose Technologies and VOC-Biomarkers for the Noninvasive Early Diagnosis of Gastrointestinal Diseases. Sensors. 2018;18:2613. doi: 10.3390/s18082613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saviauk T., Kiiski J.P., Nieminen M.K., Tamminen N.N., Roine A.N., Kumpulainen P.S., Hokkinen L.J., Karjalainen M.T., Vuento R.E., Aittoniemi J.J., et al. Electronic Nose in the Detection of Wound Infection Bacteria from Bacterial Cultures: A Proof-of-Principle Study. Eur. Surg. Res. Eur. Chir. Forschung. Rech. Chir. Eur. 2018;59:1–11. doi: 10.1159/000485461. [DOI] [PubMed] [Google Scholar]
- 79.Lewis J.M., Savage R.S., Beeching N.J., Beadsworth M.B.J., Feasey N., Covington J.A. Identifying volatile metabolite signatures for the diagnosis of bacterial respiratory tract infection using electronic nose technology: A pilot study. PLoS ONE. 2017;12:e0188879. doi: 10.1371/journal.pone.0188879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naresh V., Lee N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors. 2021;21:1109. doi: 10.3390/s21041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Butini M.E., Gonzalez Moreno M., Czuban M., Koliszak A., Tkhilaishvili T., Trampuz A., Di Luca M. Real-Time Antimicrobial Susceptibility Assay of Planktonic and Biofilm Bacteria by Isothermal Microcalorimetry. Adv. Exp. Med. Biol. 2019;1214:61–77. doi: 10.1007/5584_2018_291. [DOI] [PubMed] [Google Scholar]
- 82.Entenza J.M., Betrisey B., Manuel O., Giddey M., Sakwinska O., Laurent F., Bizzini A. Rapid detection of Staphylococcus aureus strains with reduced susceptibility to vancomycin by isothermal microcalorimetry. J. Clin. Microbiol. 2014;52:180–186. doi: 10.1128/JCM.01820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singhal N., Kumar M., Kanaujia P.K., Virdi J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015;6:791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Croxatto A., Prod’hom G., Greub G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol. Rev. 2012;36:380–407. doi: 10.1111/j.1574-6976.2011.00298.x. [DOI] [PubMed] [Google Scholar]
- 85.Torres-Sangiao E., Leal Rodriguez C., Garcia-Riestra C. Application and Perspectives of MALDI-TOF Mass Spectrometry in Clinical Microbiology Laboratories. Microorganisms. 2021;9:1539. doi: 10.3390/microorganisms9071539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoon E.J., Jeong S.H. MALDI-TOF Mass Spectrometry Technology as a Tool for the Rapid Diagnosis of Antimicrobial Resistance in Bacteria. Antibiotics. 2021;10:982. doi: 10.3390/antibiotics10080982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen X.F., Hou X., Xiao M., Zhang L., Cheng J.W., Zhou M.L., Huang J.J., Zhang J.J., Xu Y.C., Hsueh P.R. Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) Analysis for the Identification of Pathogenic Microorganisms: A Review. Microorganisms. 2021;9:1536. doi: 10.3390/microorganisms9071536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Florio W., Baldeschi L., Rizzato C., Tavanti A., Ghelardi E., Lupetti A. Detection of Antibiotic-Resistance by MALDI-TOF Mass Spectrometry: An Expanding Area. Front. Cell. Infect. Microbiol. 2020;10:572909. doi: 10.3389/fcimb.2020.572909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benkova M., Soukup O., Marek J. Antimicrobial susceptibility testing: Currently used methods and devices and the near future in clinical practice. J. Appl. Microbiol. 2020;129:806–822. doi: 10.1111/jam.14704. [DOI] [PubMed] [Google Scholar]
- 90.Gato E., Anantharajah A., Arroyo M.J., Artacho M.J., Caballero J.D., Candela A., Chudejova K., Constanso I.P., Elias C., Fernandez J., et al. Multicenter Performance Evaluation of MALDI-TOF MS for Rapid Detection of Carbapenemase Activity in Enterobacterales: The Future of Networking Data Analysis with Online Software. Front. Microbiol. 2021;12:789731. doi: 10.3389/fmicb.2021.789731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brackmann M., Leib S.L., Tonolla M., Schurch N., Wittwer M. Antimicrobial resistance classification using MALDI-TOF-MS is not that easy: Lessons from vancomycin-resistant Enterococcus faecium. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020;26:391–393. doi: 10.1016/j.cmi.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 92.Mullis K.B. The unusual origin of the polymerase chain reaction. Sci. Am. 1990;262:56–65. doi: 10.1038/scientificamerican0490-56. [DOI] [PubMed] [Google Scholar]
- 93.Saiki R.K., Gelfand D.H., Stoffel S., Scharf S.J., Higuchi R., Horn G.T., Mullis K.B., Erlich H.A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 94.Deepak S., Kottapalli K., Rakwal R., Oros G., Rangappa K., Iwahashi H., Masuo Y., Agrawal G. Real-Time PCR: Revolutionizing Detection and Expression Analysis of Genes. Curr. Genom. 2007;8:234–251. doi: 10.2174/138920207781386960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Notomi T., Okayama H., Masubuchi H., Yonekawa T., Watanabe K., Amino N., Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomita N., Mori Y., Kanda H., Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008;3:877–882. doi: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- 97.Compton J. Nucleic acid sequence-based amplification. Nature. 1991;350:91–92. doi: 10.1038/350091a0. [DOI] [PubMed] [Google Scholar]
- 98.Ginocchio C.C. Life beyond PCR: Alternative target amplification technologies for the diagnosis of infectious diseases, part I. Clin. Microbiol. Newsl. 2004;26:121–128. doi: 10.1016/j.clinmicnews.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wroblewski J.K., Manhart L.E., Dickey K.A., Hudspeth M.K., Totten P.A. Comparison of transcription-mediated amplification and PCR assay results for various genital specimen types for detection of Mycoplasma genitalium. J. Clin. Microbiol. 2006;44:3306–3312. doi: 10.1128/JCM.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao Y., Kim H.J., Li Y., Kong H., Lemieux B. Helicase-dependent amplification of nucleic acids. Curr. Protoc. Mol. Biol. 2013;104:15.11.1–15.11.12. doi: 10.1002/0471142727.mb1511s104. [DOI] [PubMed] [Google Scholar]
- 101.Bridge S., Hullsiek K.H., Nerima C., Evans E.E., Nuwagira E., Stadelman A.M., Tran T., Kim G., Tadeo K.K., Kwizera R., et al. Evaluation of the BioFire(R) FilmArray(R) Meningitis/Encephalitis panel in an adult and pediatric Ugandan population. J. De Mycol. Med. 2021;31:101170. doi: 10.1016/j.mycmed.2021.101170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lade H., Kim J.M., Chung Y., Han M., Mo E.K., Kim J.S. Comparative Evaluation of Allplex Respiratory Panels 1, 2, 3, and BioFire FilmArray Respiratory Panel for the Detection of Respiratory Infections. Diagnostics. 2021;12:9. doi: 10.3390/diagnostics12010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koort J.M., Lukinmaa S., Rantala M., Unkila E., Siitonen A. Technical improvement to prevent DNA degradation of enteric pathogens in pulsed-field gel electrophoresis. J. Clin. Microbiol. 2002;40:3497–3498. doi: 10.1128/JCM.40.9.3497-3498.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maiden M.C. Multilocus sequence typing of bacteria. Annu. Rev. Microbiol. 2006;60:561–588. doi: 10.1146/annurev.micro.59.030804.121325. [DOI] [PubMed] [Google Scholar]
- 105.Perez-Losada M., Browne E.B., Madsen A., Wirth T., Viscidi R.P., Crandall K.A. Population genetics of microbial pathogens estimated from multilocus sequence typing (MLST) data. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2006;6:97–112. doi: 10.1016/j.meegid.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quainoo S., Coolen J.P.M., van Hijum S., Huynen M.A., Melchers W.J.G., van Schaik W., Wertheim H.F.L. Correction for Quainoo et al., “Whole-Genome Sequencing of Bacterial Pathogens: The Future of Nosocomial Outbreak Analysis”. Clin. Microbiol. Rev. 2018;31:e00082-17. doi: 10.1128/CMR.00082-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luna R.A., Fasciano L.R., Jones S.C., Boyanton B.L., Jr., Ton T.T., Versalovic J. DNA pyrosequencing-based bacterial pathogen identification in a pediatric hospital setting. J. Clin. Microbiol. 2007;45:2985–2992. doi: 10.1128/JCM.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gwinn M., MacCannell D., Armstrong G.L. Next-Generation Sequencing of Infectious Pathogens. JAMA. 2019;321:893–894. doi: 10.1001/jama.2018.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Y., Chau J., Yoon J., Hladky J. Rapid, label-free pathogen identification system for multidrug-resistant bacterial wound infection detection on military members in the battlefield. PLoS ONE. 2022;17:e0267945. doi: 10.1371/journal.pone.0267945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.