Abstract
Background and Objectives: Characterising the features of methodologies, clinical attributes and intervention protocols, of studies is valuable to advise directions for research and practice. This article reports the findings of a secondary analysis of the features from studies screened as part of a large systematic review of TENS (the meta-TENS study). Materials and Methods: A descriptive analysis was performed on information associated with methodology, sample populations and intervention protocols from 381 randomised controlled trials (24,532 participants) evaluating TENS delivered at a strong comfortable intensity at the painful site in adults with pain, irrespective of diagnosis. Results: Studies were conducted in 43 countries commonly using parallel group design (n = 334) and one comparator group (n = 231). Mean ± standard deviation (SD) study sample size (64.05 ± 58.29 participants) and TENS group size (27.67 ± 21.90 participants) were small, with only 13 of 381 studies having 100 participants or more in the TENS group. Most TENS interventions were ‘high frequency’ (>10 pps, n = 276) and using 100 Hz (109/353 reports that stated a pulse frequency value). Of 476 comparator groups, 54.2% were active treatments (i.e., analgesic medication(s), exercise, manual therapies and electrophysical agents). Of 202 placebo comparator groups, 155 used a TENS device that did not deliver currents. At least 216 of 383 study groups were able to access other treatments whilst receiving TENS. Only 136 out of 381 reports included a statement about adverse events. Conclusions: Clinical studies on TENS are dominated by small parallel group evaluations of high frequency TENS that are often contaminated by concurrent treatment(s). Study reports tended focus on physiological and clinical implications rather than the veracity of methodology and findings. Previously published criteria for designing and reporting TENS studies were neglected and this should be corrected in future research using insights gleaned from this analysis.
Keywords: transcutaneous electrical nerve stimulation (TENS), pain, pain management, therapeutic neuromodulation, secondary analysis
1. Introduction
Transcutaneous electrical nerve stimulation (TENS) is used to alleviate the intensity of pain and involves the delivery of pulsed electrical currents across the skin to stimulate peripheral nerves. Physiological research demonstrates that TENS reduces activity and excitability of central projection neurons reducing nociceptive input to the brain and modulating pain experience [1,2,3,4]. TENS has been used globally for symptomatic relief of pain since the 1970s and TENS equipment is available without prescription in many countries [5]. TENS treatment is usually self-administered as often as is needed with minimal risk of adverse effects or toxicity. TENS equipment and clinical support is inexpensive, and health economic analyses suggest TENS lowers costs for persistent pain [6], chronic low back pain [7,8] and knee osteoarthritis [9]. It is not possible to predict with certainty who is likely to respond to TENS, although a 30-min TENS treatment has been shown to forecast the likelihood of longer-term outcome in women with fibromyalgia [10].
The debate about the effectiveness of TENS is long-standing, despite a wealth of published research studies spanning over five decades [11]. The consequence is inconsistency in clinical recommendations about prescribing TENS in the U.K. National Health Service (NHS), or coverage by private healthcare insurance in the Unites States. Recently, we published a systematic review and meta-analysis of 381 studies that found moderate certainty evidence that strong non-painful TENS lowered pain intensity when compared with placebo (i.e., the Meta-TENS study [12]).
There have been no analyses of the features of clinical studies on TENS. An investigation of trends, strengths, weaknesses and gaps in study methodologies, including TENS intervention protocols would be valuable to inform directions for future research and clinical practice. The purpose of this article is to report a secondary analysis of the characteristics of studies that was not reported in our original systematic review and meta-analysis of TENS (the Meta-TENS study) [12].
2. Methods
The Meta-TENS study was registered on PROSPERO (CRD42019125054), published as a protocol in 2019 [13] and in the primary report of the findings in 2022 [12]. Readers are referred to these publications for methodological detail and findings of the Meta-TENS study. Ethical approval for the Meta-TENS study was given by Leeds Beckett University (Application Ref: 78097). Here we provide the methodology used in our secondary analysis of study characteristics beyond that provided in our primary report.
Electronic databases were searched from inception to 17 May 2020 for randomised controlled trials (RCTs) evaluating TENS at the site of pain versus placebo, no treatment, or other treatments in adults experiencing pain regardless of diagnosis. Two independent reviewers extracted a variety of information from study reports including:
Methodological characteristics of studies (e.g., overall risk of bias, study group study size, concurrent use of other treatments).
Pain characteristics (e.g., duration (acute, chronic), medical diagnosis (pain condition), mechanistic descriptor (nociceptive, neuropathic), physiological system (musculoskeletal, visceral, somatosensory).
Intervention characteristics (e.g., high-frequency TENS, low-frequency TENS, types of placebos, types of comparator treatments).
One reviewer (MIJ) performed descriptive analyses by plotting bar charts and histograms and reported summary measures as frequency counts, means or medians as appropriate.
3. Results
3.1. Observations: Searching
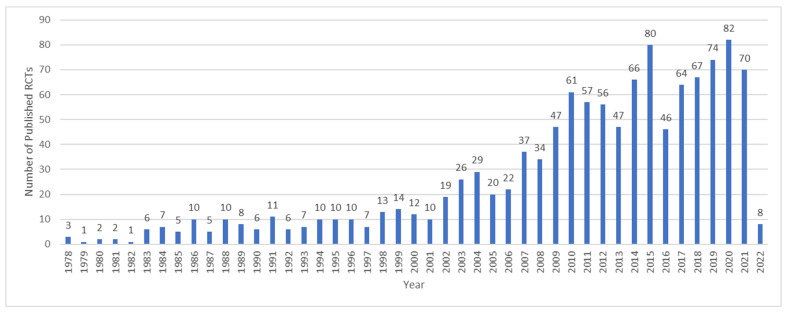
There has been a steady rise in the rate of publication of studies on the efficacy of TENS since the 1970s as reflected in a search of Pubmed.gov conducted on 31 March 2022 (Figure 1).
Figure 1.
Number of records identified by searching PubMed.gov for randomised controlled trials (RCTs) of TENS and pain: conducted on 31 March 2022. Search String: (transcutaneous electrical nerve stimulation) AND (pain) Filters: Randomized Controlled Trial (“transcutaneous electric nerve stimulation” (MeSH Terms) OR (“transcutaneous” (All Fields) AND “electric” (All Fields) AND “nerve” (All Fields) AND “stimulation” (All Fields)) OR “transcutaneous electric nerve stimulation” (All Fields) OR (“transcutaneous” (All Fields) AND “electrical” (All Fields) AND “nerve” (All Fields) AND “stimulation” (All Fields)) OR “transcutaneous electrical nerve stimulation” (All Fields) AND (“pain” (MeSH Terms) OR “pain” (All Fields))) AND (randomizedcontrolledtrial (Filter)). Note: These records have not been screened against eligibility criteria and will over-estimate the actual number of RCTs.
In our Meta-TENS study [12], a total of 381 studies with 383 distinct populations and 24,532 participants were eligible for inclusion [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367,368,369,370,371,372,373,374,375,376,377,378,379,380,381,382,383,384,385,386,387,388,389,390,391,392,393,394] (see our primary report for details [12], including studies awaiting classification [395,396,397,398,399,400,401,402,403,404,405,406,407,408,409,410,411,412,413] and studies excluded at full text screening with reasons [414,415,416,417,418,419,420,421,422,423,424,425,426,427,428,429,430,431,432,433,434,435,436,437,438,439,440,441,442,443,444,445,446,447,448,449,450,451,452,453,454,455,456,457,458,459,460,461,462,463,464,465,466,467,468,469,470,471,472,473,474,475,476,477,478,479,480,481,482,483,484,485,486,487,488,489,490,491,492,493,494,495,496,497,498,499,500,501,502,503,504,505,506,507,508,509,510,511,512,513,514,515,516,517,518,519,520,521,522,523,524,525,526,527,528,529,530,531,532,533,534,535,536,537,538,539,540,541,542,543,544,545,546,547,548,549,550,551,552,553,554,555,556,557,558,559,560,561,562,563,564,565,566,567,568,569,570,571,572,573,574,575,576,577,578,579,580,581,582,583,584,585,586,587,588,589,590,591,592,593,594,595,596,597,598,599,600,601,602,603,604,605,606,607,608,609,610,611,612,613,614,615,616,617,618,619,620,621]). It was noteworthy that an additional 36 studies were found and met our inclusion criteria between the initial search during July 2019 and the updated search on 17 May 2020. This demonstrates the high publication rate of studies on TENS, although only one of these additional studies had a study group sample size of at least 50 participants.
3.2. Observations: The Screening Process
3.2.1. Few Instances of Multiple Records (Secondary Reports)
There were five instances of multiple records (secondary reports) of one study:
A primary report of 70 participants with follow-up data at 1 year by Cherian et al. [79]; secondary reports of data of the first 23 participants [622] and 36 participants (presumably including the first 23 patients) [623] at 3 months.
A primary report by Chesterton et al. [80] of TENS added to usual care for tennis elbow; secondary report of an economic evaluation by Lewis et al. [624].
A primary report by Escortell-Mayor et al. [123] of TENS versus manual therapy for neck pain; additional Spanish language version equivalent [625].
A primary report by Oosterhof et al. [267] of short-term outcome of TENS for various chronic pains; secondary reports of predictors of TENS outcome [626], long-term outcomes [627] and physiological mechanisms [628].
A primary report by Pietrosimone et al. [281] on TENS for knee osteoarthritis and a secondary report of outcomes associated with knee kinematics and kinetics [629].
3.2.2. Few Instances of Multiple Samples within Study Reports
There were two instances of distinct samples in one study report:
Chia et al. [81] performed independent evaluations of nulliparous and multiparous participants combined (n = 101) and nulliparous only (n = 20).
Kayman-Kose et al. [184] performed distinct evaluations of caesarean section (n = 100) and vaginal delivery (n = 100).
In our systematic review, we managed this issue by categorising each report as having distinct sample populations within one study and analysed data from these samples separately, i.e., we identified 383 different samples from 381 studies.
3.2.3. No Instances of Duplication of Participants within Study Reports
Double counting of participant samples contributes to unit of analysis issues in meta-analyses. We did not detect any instances of data collected in published ‘pilot’ studies being included in ‘full’ study samples, although unclear reporting hindered our ability to detect duplication of participant data with certainty. For example, Lin et al. published a study of TENS for shoulder pain [217] and chronic shoulder tendonitis [218] but inspection of the reports suggested protocols and data were different. Thus, we considered these to be distinct study populations.
3.2.4. Few Instances of Inconsistencies of Extracted Data with Previous Meta-Analyses
There were few inconsistencies on study data extracted for our Meta-TENS study [12] and previously published meta-analyses that were included in an overview of systematic reviews [630]. There were occasional instances of double counting of groups in pooled data analyses in previous reviews [631,632,633,634] contributing to unit of analysis errors. We detected inclusion of data from the study by Bjersa and Andersson [56] that was area under the curve rather than 0–100 mm VAS in a meta-analysis by Zhou et al. [635]). These discrepancies did not affect conclusions of the previously published reviews.
3.3. Features of Excluded Studies
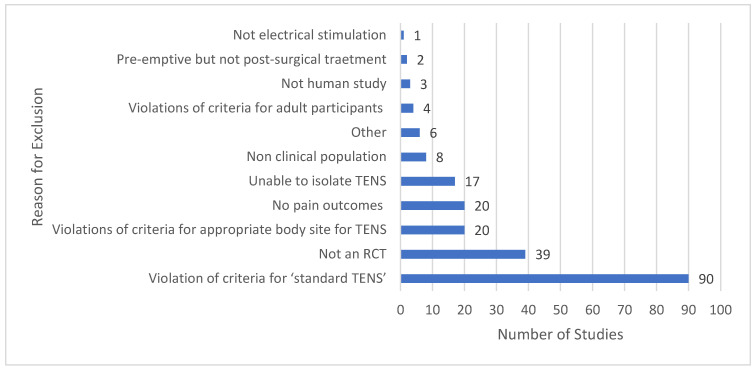
The main reasons that studies did not meet eligibility criteria for our Meta-TENS study is provided in Figure 2. Many reports stated that a study evaluated TENS, although the intervention violated our criteria for standard TENS or was applied to an inappropriate body site. It was not possible to isolate TENS effects from concurrent treatment in at least 17 studies.
Figure 2.
Summary of primary reason for excluding studies: RCT = randomised controlled trial.
3.4. Not Standard Electrical Characteristics
At least 90 studies used interventions that were not ‘standard TENS’ (i.e., type of stimulating device and accessories and electrical characteristics of stimulation): Codetron (acupuncture-like stimulation); auto-targeted neurostimulation; frequency rhythmic electrical modulation; H-wave therapy; high-voltage pulsed direct current; interferential current therapy; InterX (non-invasive interactive neurostimulation); microcurrent electrical stimulation; neuromuscular electrical stimulation; supraorbital transcutaneous stimulation; transcutaneous electric acupoint stimulation; transcutaneous spinal electroanalgesia; and 5 KHz sine wave currents.
Previous systematic reviews on TENS had considered some of these techniques as ‘TENS’ despite the electrical characteristics of currents deviating from those defined as standard TENS [630]. For example, Itoh et al. claimed to evaluate TENS for osteoarthritis of the knee [480] and for non-specific low back pain [481] yet devices were delivering interferential currents “… a single-channel portable TENS unit (model HVF3000, OMRON Healthcare Co Ltd., Japan), which sends between two electrodes a premixed amplitude-modulated frequency of 122 Hz (beat frequency) generated by two medium frequency sinusoidal waves of 4.0 and 4.122 kHz (feed frequency)” [481] p. 23. Both studies were included in systematic reviews on TENS [636,637].
3.5. Inappropriate Body Site
At least 20 studies applied TENS to acupuncture points that were not close to the painful site, commonly using transcutaneous electric acupoint stimulation (TEAS, TAES) and ‘dense-disperse’ currents alternating between 2 pps and 100 pps. Some studies administered transcutaneous electric acupoint stimulation before surgery to manage post-surgical pain. Often details about transcutaneous electric acupoint stimulation treatment protocols were unclear. Four studies were excluded for delivering TENS intravaginal [423,527,528] or intra-oral [519].
3.6. Features of Included Studies
The characteristics of the included studies are summarised in the primary report of the Meta-TENS study [12].
3.7. Study Design
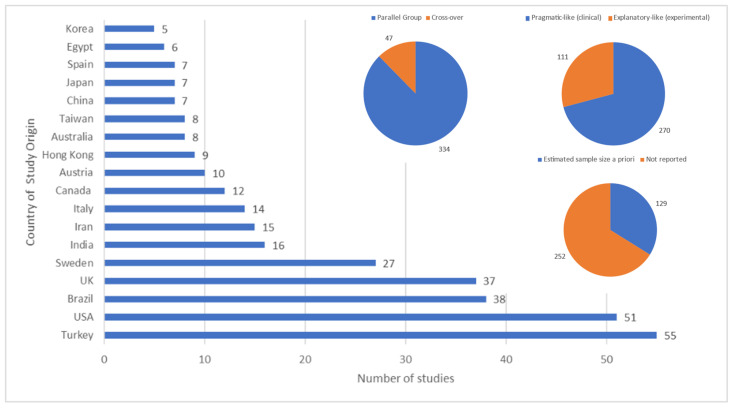
Studies were conducted in 43 countries with Turkey, the United States, Brazil, the United Kingdom and Sweden being commonest. The majority of studies were parallel-group, pragmatic (clinical) rather than exploratory (mechanistic) and did not estimate sample size a priori (Figure 3).
Figure 3.
Country of origin of the study; Parallel group or cross-over study design; Tending toward pragmatic or explanatory in focus; Report contained statement that a sample size had been estimated a priori.
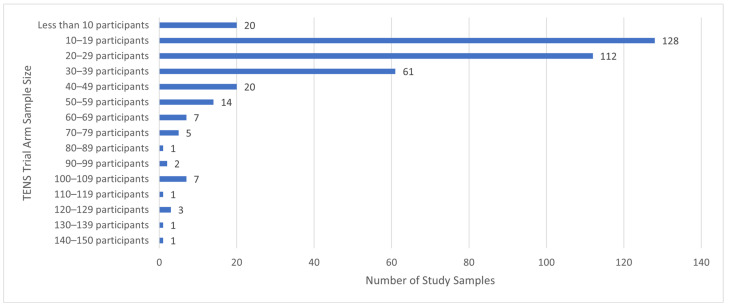
There were 381 studies, 383 population samples and 24,532 participants. Study sample size was 64.05 ± 58.29 participants (mean ± standard deviation (SD); maximum = 607 [63], minimum = 5 (370)) and TENS group size was 27.67 ± 21.90 participants (n = 10,596 participants, maximum = 144 [63]; minimum = 5 participants [26,93,101,370,382]). There were 13 studies with 100 or more participants in the TENS group (Figure 4), yet there was extractable data for only two studies (labour pain [352] and fibromyalgia [96]). There were 341 studies with less than 50 participants in the TENS group.
Figure 4.
Summary of study sample sizes.
3.8. Types of Pain
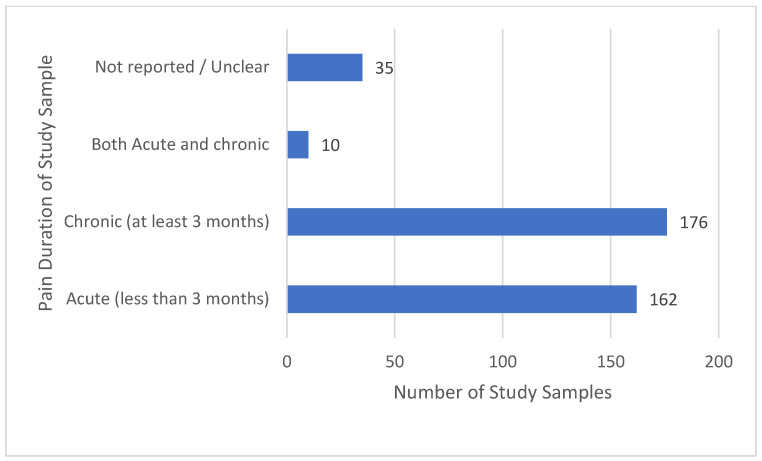
There were similar proportions of studies evaluating acute pain and chronic pain, and only a small proportion of studies evaluating both acute and chronic pain (Figure 5).
Figure 5.
Pain duration.
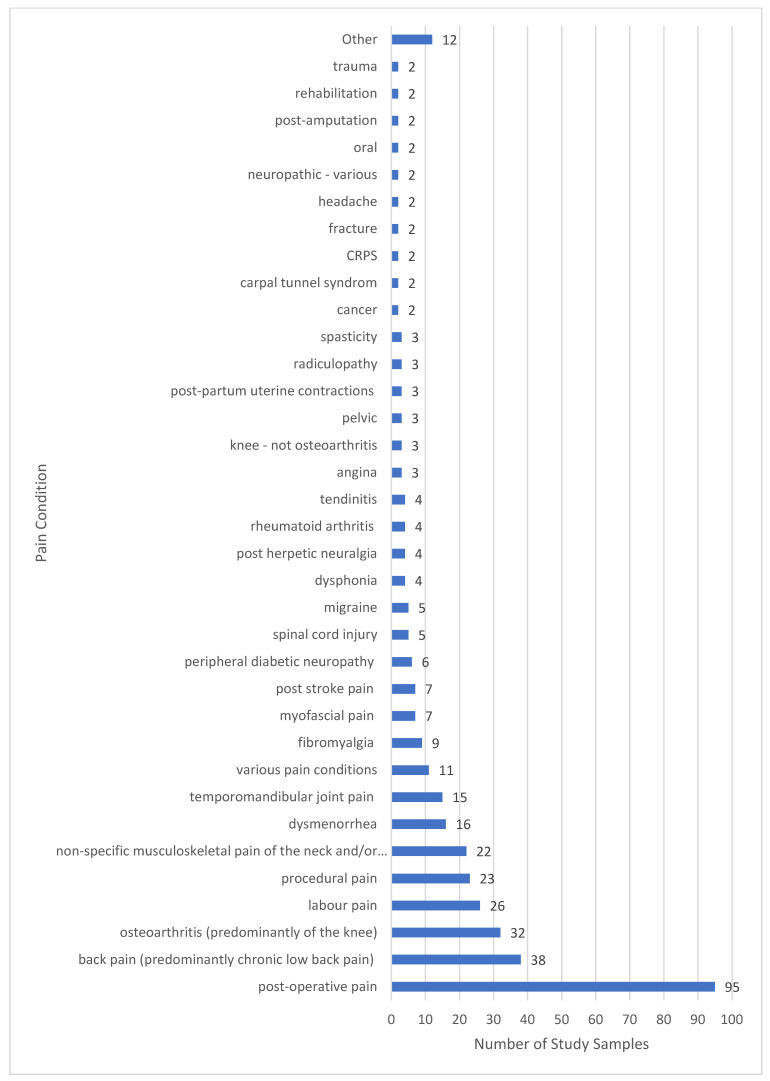
The commonest pain condition according to nomenclature used by study authors was post operative pain, followed by non-specific musculoskeletal pains and osteoarthritis (Figure 6). Challenges arose categorising pain condition because reports lacked detail about presenting features such as the presence of neuropathic elements, e.g., post-operative pain and post-stroke pain may present as primarily musculoskeletal, neuropathic or both. We created an operational aide memoire to improve the consistency of categorising pain conditions (medical diagnoses).
Figure 6.
Pain diagnosis categorised as stated by the study authors. Others represents n = 1 sample for each of the following: adhesive capsulitis, intercostobrachial pain, plantar fasciitis, haemophilia, ischemia, orchialgia, orofacial pain, pancreatitis, chronic breast cancer treatment pain, pressure ulcers, renal colic and trigeminal neuralgia.
3.9. Treatment Comparators
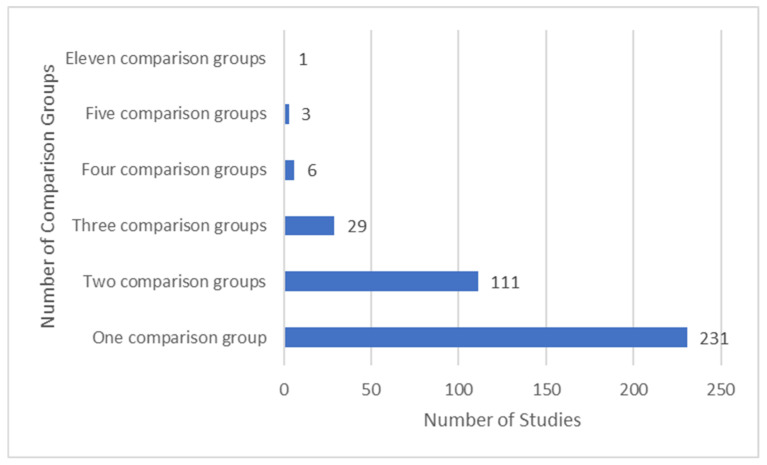
3.9.1. Most Studies Had No More Than Two Comparator Groups
Approximately two-thirds of studies had one comparison groups and one-third had two comparison groups (Figure 7).
Figure 7.
Summary of number of comparator groups.
3.9.2. Similar Proportions of Placebo or Active Treatment Comparators
Our definitions for comparison groups were as follows:
Placebo: an inactive intervention that looks the same as, and is given in the same way as, active TENS or active treatment (e.g., drug pill).
No treatment: participants did not receive any ‘active treatment’, including background or rescue medication or treatment.
Standard of care: intervention(s) that study authors stated to be routine, common or standard care or practice.
Other treatment(s): treatment not previously categorised as standard of care (SoC).
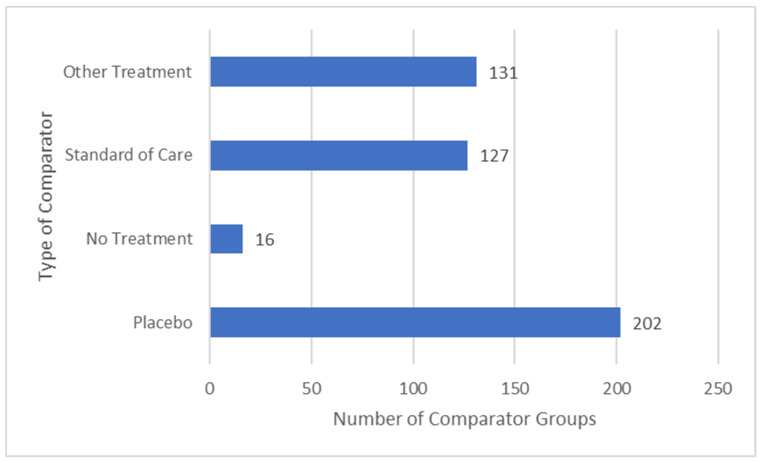
There were 476 comparators in 381 studies, 54.2% were an active treatment (i.e., a SoC (26.7%) or other treatment (27.5%)) and 42.4% were a placebo (Figure 8). Thirty-seven studies evaluated low versus high frequency TENS.
Figure 8.
Types of comparator groups.
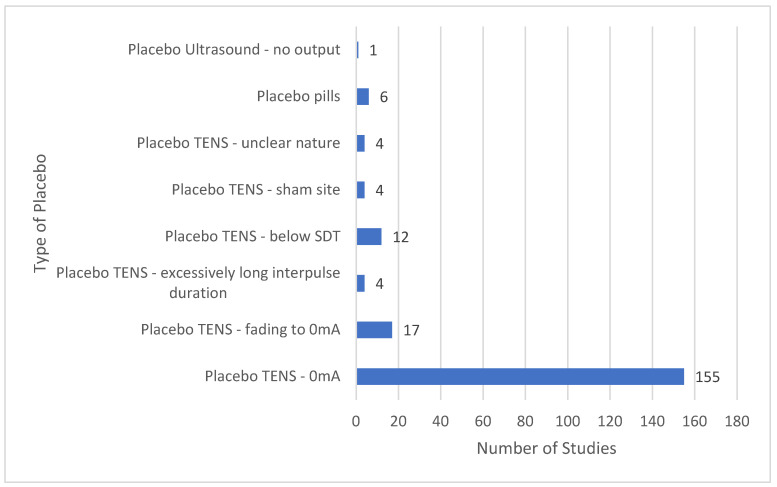
The majority of placebo comparators used a TENS device with a dead battery or with modified circuitry so that there was 0 mA current (Figure 9). Other placebo TENS interventions used TENS devices that delivered currents for less than one minute after which current amplitude declined to 0 mA, or long interpulse intervals claimed to be unlikely to produce physiological effects. Some placebo interventions administered TENS below the threshold for sensory detection or to locations unrelated to the painful site.
Figure 9.
Types of placebo comparators: SDT = sensory detection threshold; sham site = sites unrelated to pain.
3.10. Many Instances of Contamination from Concurrent Treatment
At least 216 of the 383 study samples were able to use other treatments whilst receiving TENS. Commonly, analgesic medication and/or exercise was combination treatment that was added as part of the study design or ongoing clinical care not considered to be part of study design. Often participants could access other treatments as background or rescue interventions in studies claiming that TENS was delivered as a sole treatment. Some studies monitored and/or standardised rescue medication but monitoring concurrent treatment was inadequate. Thus, unequal contamination from concurrent treatment between intervention groups could not be discounted. Improved monitoring and reporting of concurrent treatment are needed in future studies.
3.11. Features of TENS Intervention
3.11.1. Location of TENS and Pain
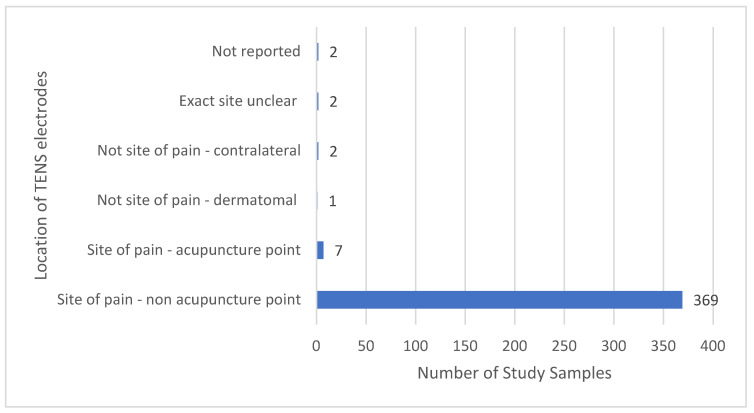
The majority of TENS interventions were located at the painful site; acupuncture points at the painful site were occasionally chosen [84,152,169,258,363,389,391] (Figure 10). TENS was not administered at the painful site if there was heightened sensitivity and/or poor integrity of the skin associated with painful diabetic neuropathy (TENS administered to the back-dermatomal [66,370]); and for phantom limb pain (TENS administered to the contralateral limb [355]). There were two reports with an unclear statement of TENS location [179,255] and two reports not stating TENS location; in both instances, information within reports confirmed that TENS was delivered at the painful site [171,323].
Figure 10.
Location of TENS relative to pain.
3.11.2. Intensity of TENS
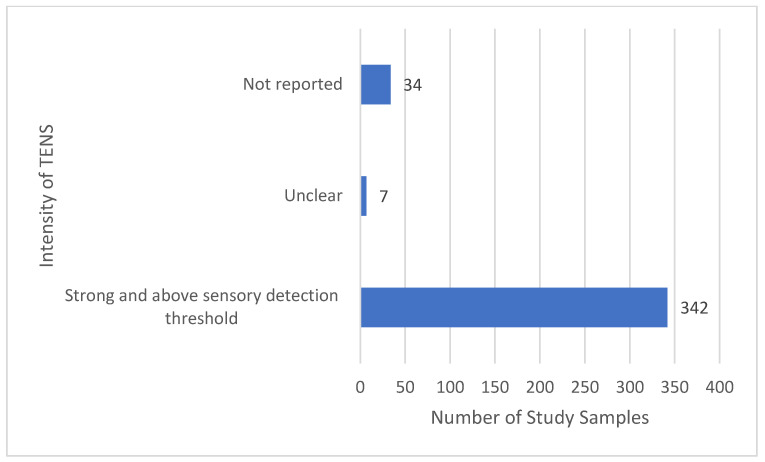
The majority of reports stated that TENS was delivered above the sensory detection threshold and at a strong non-painful intensity (Figure 11). The intensity of TENS was not reported for 34 samples and unclear for 7 samples, although it was possible to establish that the intensity of TENS was above sensory detection threshold from other information in reports (e.g., reporting of mA or statements in the main text).
Figure 11.
Intensity of TENS.
3.11.3. Electrical Characteristics of TENS—Pulse Frequency
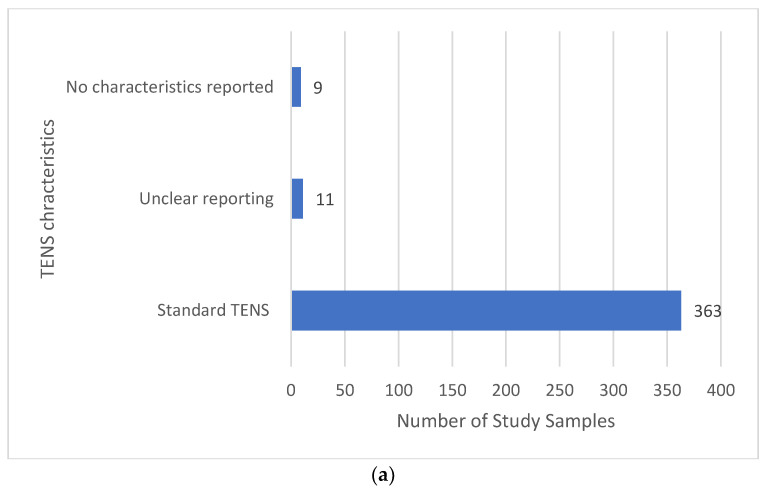
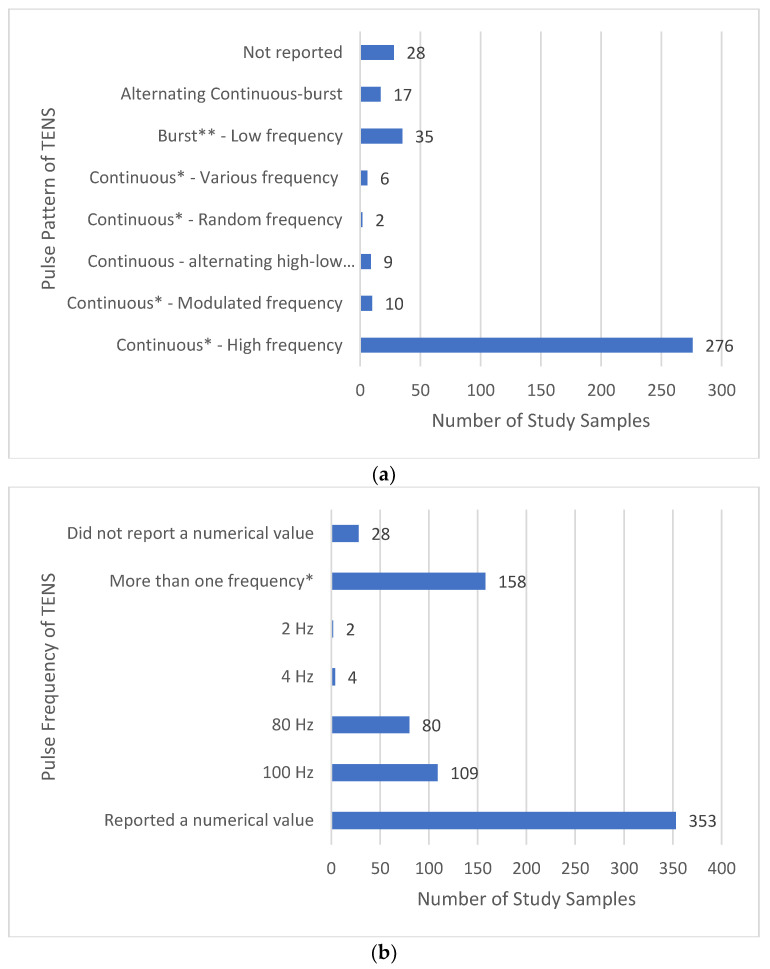
Most reports provided sufficient detail to confirm that the electrical characteristics of TENS were consistent with that available from a standard TENS device (i.e., pulsed currents, frequency ≤250 pulses per second (pps), pulse width (duration) ≤500 μs and amplitude ≤60 mA (peak-to-peak), Figure 12a). Extracting specific information about electrical characteristics was challenging with 9 reports not stating TENS parameters and 11 reports providing unclear information (other information such as device model was used to confirm that characteristics were consistent with standard TENS.
Figure 12.
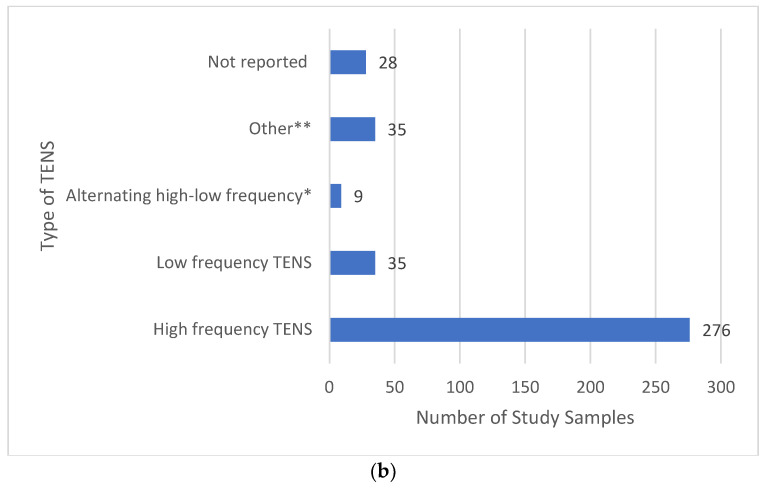
(a) Electrical characteristics of TENS; (b) type of TENS: * Using a continuous pulse pattern. ** Either unclear or prn.
Terms used to describe TENS technique were inconsistent and included conventional, acupuncture-like, brief intense, high- and low-frequency and acu-TENS. There is inconsistency in units of measure to describe pulse frequency with Hertz (Hz) used most frequently and pulses per second used occasionally. We will use Hz if study authors stated Hz in their study report and pulses per second (pps) when referring to our categorisation of pulse frequency.
We categorised the majority of TENS interventions as high-frequency TENS (>10 pps) and a small minority of 35 samples receiving low-frequency TENS (<10 pps and/or <10 trains of pulses (bursts) per second, Figure 12b). Often reports describing low-frequency stimulation did not differentiate bursts per second from pulses per second making it difficult to establish whether low-frequency pulses or low-frequency bursts of high-frequency pulses were used.
It was less common for reports to state the pattern (mode) of pulse delivery; we presumed high-frequency currents were administered using a continuous pattern of pulse delivery when pulse pattern statements were absent and confirmed this by checking the design of the device. There were 17 samples that received pulse patterns alternating (or switching) between burst and continuous patterns and 9 samples that received alternating (switching) between low and high-frequency pulses (Figure 13a). Ten samples received modulating pulse frequency between upper and lower boundaries, two samples received random pulse frequency and six samples received various pulse frequencies.
Figure 13.
(a) Pattern (mode) of pulse delivery of TENS: * We were able to infer that a continuous pattern of pulse delivery was used to deliver high frequency currents in instances where the pattern of pulse delivery was not clearly stated in the study report. ** We were able to infer that a low frequency bursts (trains) of high frequency pulses were used in instances where the pattern of pulse delivery was not clearly stated in the study report; (b) frequency of pulse delivery of TENS: * Including instances where modulated or alterating pulse frequencies were used or frequencies were prn.
Of the 353 reports that stated pulse frequency, 109 stated that 100 Hz was used and 11 stated that TENS was delivered at frequencies below 5 Hz (Figure 13b). A large proportion of reports stated numerous pulse frequencies than included situations where participants received instructions to adjust frequency as needed, or TENS was delivered using modulating or alternating frequencies. It was often unclear whether frequencies were static throughout treatment or whether participants could adjust frequency according to need.
3.11.4. Adequacy of TENS Intervention
There were 336 samples where the report clearly provided details about electrical characteristics, body site of TENS and the intensity of stimulation above sensory detection threshold, and 47 samples where this was not the case.
Regimens included treatments that delivered as a single dose or multiple doses. The shortest duration treatment was a few minutes (e.g., post-partum contraction pain [264], dysmenorrhea [265], surgical abortion [286] or laparoscopic surgery [287], procedural pain (e.g., carboxytherapy) [310]) to as long as needed (e.g., home treatment self-administered prn). One study advised participants to self-administer TENS for chronic pain until they no longer required TENS or until the study ended at 2 years [255].
3.12. Features of Outcome Measures
There were 352 studies that collected pain intensity data as the primary outcome and 29 studies that collected pain intensity data as a secondary outcome. The choice of other outcome measures depended on the clinical condition under investigation, e.g., WOMAC would only be selected in studies using population samples with osteoarthritis whereas analgesic consumption could be used for any study population. Analgesic consumption (127 studies) was most common. Other common outcome measures were 52 studies using range of motion, 26 studies using the McGill Pain Questionnaire, 23 studies using blunt pressure pain via algometry, 14 studies using WOMAC, 12 studies using Quality of Life and 8 studies using the Roland Morris Disability Questionnaire.
We explored outcome data collected as the last measurement during TENS or the first measurement post-TENS. There were 91/202 studies (92/203 samples, 4841 participants) with extractable pain intensity (continuous) data and 3 of these studies were crossover studies [96,248,293]. Only two of these samples had 100 participants or more in the primary TENS group (Thomas et al. [352]—labour pain and Dailey et al. [96]—fibromyalgia); and nine samples had at least 50 participants in the primary TENS group with extractable data.
We detected two instances where study reports described outcomes in favour of TENS, yet data analyses suggested otherwise [184,225]. It appeared in both instances that the inconsistencies were transcriptional errors. For the study by Kayman-Kose et al. [184], the inconsistency was for vaginal delivery but the caesarean section data. For the study by Luchesa et al. [225]), crossing checking data with a systematic review [638] established that that TENS data had been ascribed to the placebo group and vice versa.
Of the 127 evaluations of TENS versus a SoC intervention, 71 were versus medication (pharmacological), 40 versus exercise/manual therapy, 3 versus medication combined with exercise/manual therapy and 13 studies were not exercise/physiotherapy or pharmacological or were unclear. There were 61 studies (61 samples, 3155 participants) with extractable data. Of the 131 evaluations with other treatment not categorised as SoC, the commonest comparators were interferential therapy, other electrotherapies and manual therapies. There were 67 studies and 131 samples (3327 participants) that had extractable data, although this included duplicate data from some primary TENS groups.
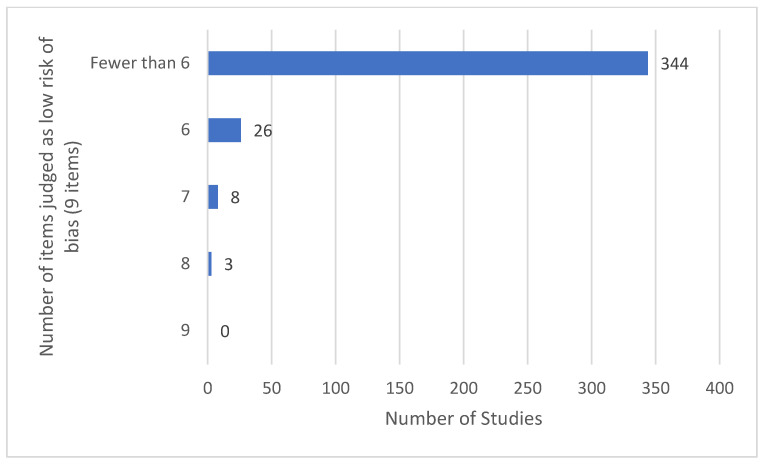
3.13. Features of Risk of Bias Assessment
No studies had low risk of bias (RoB) across all 9 RoB items and only 3/381 studies were assessed as having a low RoB across 8 of the 9 items [95,294,362] (Figure 14). Many study reports were sparce on methodological operational details and were categorised as unclear RoB. Insufficient numbers on TENS participants were a serious problem, with 341 out of 381 studies having a high RoB due to having less than 50 participants in the TENS trial arm. No studies met criteria for low RoB (≥200 participants in the TENS group).
Figure 14.
Scores of overall low risk of bias for the 381 studies.
Reasons for high or unclear risk of bias across the nine items were:
Reports stated participants were randomised to intervention groups but did not specify whether randomisation was constrained or unconstrained or provided operational details of randomisation (e.g., coin toss, random number sequence generation) or allocation concealment (e.g., sequentially numbered, opaque, sealed envelopes or containers, centrally controlled procedures).
Partial or unclear reporting meant that it was not possible to determine with certainty whether all participants completed the study.
Only partial descriptions for analyses of outcome measures leading to the possibility of overestimation or underestimation of treatment effects (Selective Reporting (Reporting Bias)).
Inadequate descriptions for methods of blinding of participants, personnel or assessor.
Inadequate sample sizes.
Absence of calculation used to estimate study sample size.
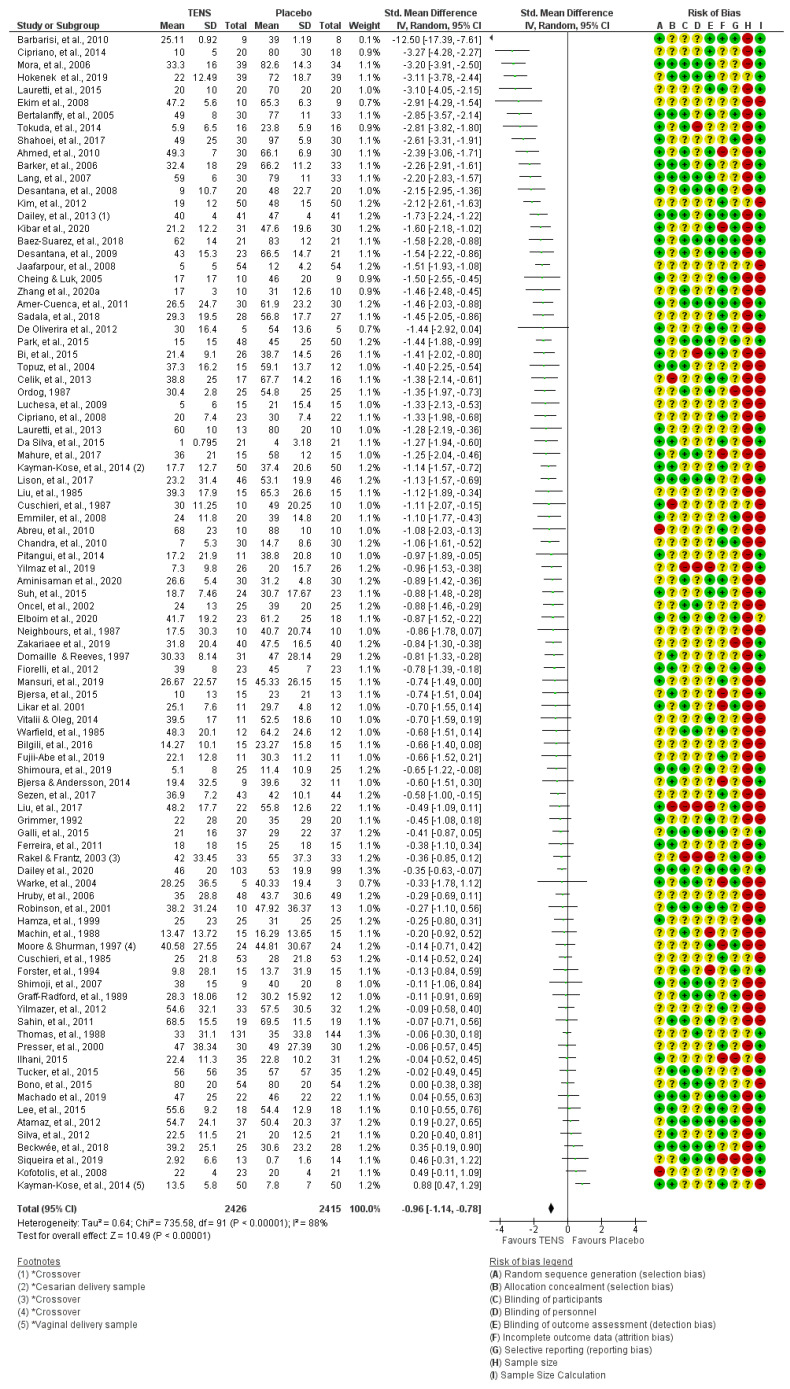
It was notable that only 130 described adequate methods of blinding of assessors (i.e., low risk of detection bias) and only 94 described adequate blinding procedures for participants and 48 for personnel (i.e., low risk of performance bias). Few studies assessed blinding leakage or the plausibility and credibility of interventions, especially whether active and placebo TENS devices were considered to be ‘functioning correctly’ [96,221,282,294]. High quality design, delivery and success of blinding of placebo TENS interventions was observed in studies by Dailey et al. [95,96,294]. There were 129 reports that claimed to have estimated sample size and those that included a calculation often estimated study sample size rather than study group sample size. A summary of individual study RoB judgements for individual studies is provided in Figure 15.
Figure 15.
TENS versus placebo for pain intensity (continuous data) as standardised mean difference (SMD), including individual risk of bias (RoB) judgements. Green + circles = low RoB, Yellow ? circles = unclear RoB, Red circles = high RoB, * = calls attention to the footnote: ◂ = SMD and 95% confidence intervals lie outside the range of the horizontal axis −12.5 (−17.39, −7.61): ♦ = Overall measure of effect, the lateral points indicate confidence intervals for this estimate. The sequence of the in-figure reference citations from top to bottom are [43], [86], [249], [166], [201], [115], [52], [356], [321], [19], [44], [198], [104], [187], [95], [186], [39], [105], [176], [75], [393], [30], [310], [101], [277], [53], [358], [71], [268], [225], [85], [200], [94], [230], [184], [221], [222], [92], [118], [16], [73], [285], [386], [31], [346], [266], [116], [258], [392], [112], [135], [237], [57], [213], [377], [381], [54], [140], [325], [56], [320], [223], [152], [141], [132], [293], [96], [382], [168], [305], [156], [229], [248], [91], [139], [324], [149], [387], [311], [352], [289], [173], [364], [60], [228], [206], [36], [329], [48], [332], [192] and [184].
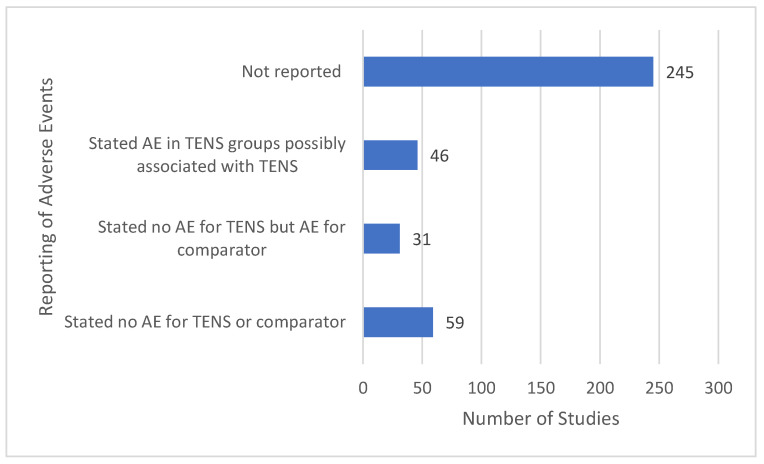
3.14. Features of Adverse Events
Generally, reporting of evaluation and reporting of adverse events was inadequate with only 136 out of 381 reports including a statement of adverse events (Figure 16).
Figure 16.
Tally of adverse event statements.
Evaluation of adverse events was neglected and when present, methodology and reporting were inadequate. Reports failed to distinguish adverse events associated with study conditions (e.g., resulting from interventions or measurement procedures) or a general worsening of a medical condition (e.g., natural fluctuation of symptoms). Ninety reports acknowledged no adverse events associated with TENS. Forty-six reports acknowledged an adverse event in the TENS group although often it was not possible to determine whether adverse events in the TENS group were directly attributable to TENS. TENS-related adverse events were irritation, soreness or tenderness of the skin that were mild and infrequent. Some reports inappropriately ascribed TENS discomfort or worsening pain as an adverse event, although this could be considered a lack of response to treatment. Only one adverse event was deemed serious “There was a possible relationship between the treatment and spontaneous abortion. A 36-year-old woman had a spontaneous abortion that occurred 21 days after BTX-A injection and electrical stimulation.” [318] p. 414.
4. Discussion
Our descriptive analysis of studies evaluating the efficacy of TENS provides a visual synopsis of key features of TENS studies. TENS literature is characterised by small, parallel group studies conducted in over 40 countries that evaluate high-frequency TENS at a strong non-painful intensity located at the painful area compared with placebo, analgesic medication(s), exercise, manual therapies and electrophysical agents. There were very few instances of duplicate publications or inconsistencies between data within study reports and that were extracted for meta-analyses.
The main weakness of studies is inadequate sample sizes and the escalating rate of inadequately powered studies on TENS is troubling. Ioannidis raised concern about the proliferation of the publication of useless and meaningless research associated with inadequate sample sizes, “Most clinical research therefore fails to be useful not because of its findings but because of its design” [639] p. 1. No studies aligned with criteria for undertaking TENS studies based on previous work by Bennett et al. [640] and Sluka et al. [641], and in doing so, the design and execution of TENS would markedly improve. In addition, study reports should focus much more on the robustness of methods, data and analyses than the physiological and clinical plausibility of findings. In future, authors should make better use of supplemental material to overcome restrictions in manuscript word counts.
4.1. Considerations for Future TENS Studies
Adequate Sample Size
Inadequate sample size is the most serious shortcoming in TENS studies. Calls for large multicentre studies date back to the 1970s and have been a consistent conclusion of systematic reviews (see Johnson 2021 for review [11]). Readers should be mindful of apparently large study sample sizes that are distributed across multiple intervention groups, seriously compromising statistical power and the likelihood of detecting a true effect [642]. Excessive use of comparison groups and outcome measures complicate interpretation of findings. We discourage the use of multiple intervention groups at the expense of group sample size in future studies.
4.2. Reframing Blinding of TENS
Performance bias is central to debates about the quality of studies on TENS. Strategies to judge blinding of participants and therapists have varied in previous reviews with some reviewers arguing that TENS studies always have a high RoB because blinding participants to TENS sensations is not possible as participants will know whether they are receiving an active or placebo intervention. We counter-argue that whether participants are uncertain about the intervention ‘functioning properly’ is the crucial factor, as this creates the belief that a placebo intervention may be a credible and therefore potentially active treatment. Pre-study briefings can be used to generate uncertainty in participants and personnel about whether a device needs to generate a sensation to be functioning properly and a TENS device that has been adapted so that there is no current output can be used [643,644]. This was the case for many of the studies included in this review. However, at present there is a paucity of studies that evaluate the outcome of blinding.
4.3. Managing Contamination from Concurrent Treatment
There were many instances of concurrent treatment and inadequate monitoring and/or reporting of concurrent treatment. Contamination from concurrent treatment is an issue in clinical trials [645]. In pain studies, differential adjustment of concurrent treatment (analgesics) between groups may generate comparable pain intensity outcomes [646] and undervalue the extent of beneficial effects [640]. Improved monitoring and reporting of concurrent treatment are needed in future studies.
4.4. Evaluating Adverse Events
Most TENS studies focus on benefits, and it was rare for adverse effects to be pre-specified as an outcome. Commonly, adverse event data were captured by ad hoc observation resulting in a high risk of selective reporting bias. This is not unique to TENS [647]. In future, methodologies to obtain data for adverse events should be formalised with clear criteria to ascribe the occurrence and seriousness of adverse events.
4.5. Reframing Outcomes
There were similar proportions of studies evaluating acute pain and chronic pain; the commonest evaluations were for post-operative pain with a paucity of studies with extractable data for prevalent chronic pain conditions, including non-specific neck and/or back pain, osteoarthritis and neuropathic pain. At present, there is inconsistency in the National Institute for Health and Care Excellence (NICE) guidelines in the United Kingdom where TENS is recommended as an addition to primary treatment for osteoarthritis [648] and rheumatoid arthritis [649], but not recommended for non-specific chronic low back pain [650], chronic pain [651] and intrapartum care (labour pain) [652].
Our Meta-TENS study found moderate-certainty evidence of pain relief irrespective of diagnosis [12].
We argued that pathological indicators that underpin medical diagnoses (pain conditions) may be of little relevance for treatments that alleviate pain using soothing sensations (e.g., TENS, warmth, cooling, touch). This is because the complex nature of the lived experience of pain results from much more than pathological indicators that may or may not be generating nociceptive input—everything matters for pain. Pain is a warning signal to protect tissue and is not always a dependable monitor of tissue damage (status), i.e., hurt does not always mean harm. Thus, we suggest that it may be more appropriate to select such treatments based on the quality of pain sensation.
Our descriptive analysis found few instances of studies allowing participants to select outcomes that were important to them. In clinical practice, TENS is selected following a holistic evaluation of a person’s lived experience of pain, irrespective of medical diagnosis, and in line with a biopsychosocial self-management framework. Long-term users of TENS report using it to relieve sensations of pain and muscle spasm, thus reducing the negative impact of an ‘overprotective brain’ by enhancing function, sleep, psychological well-being and medication reduction [653,654,655]. TENS can promote activities of daily living and quality of life when used in combination with pain education, lifestyle adjustments, and movement and psychological-based interventions. Thus, outcome measures should document immediate relief of pain and function ‘in-the-moment’ during TENS, and longer-term effects over a prolonged course of TENS treatment. Careful consideration should be given to techniques used to measure follow-up effects after a course of treatment because, for example, a participant may stop using TENS because pain has resolved or because of lack of benefit or intolerable adverse events.
Research supports advice to personalise TENS treatment to personal need using systematic trial and error to find electrical characteristics and electrode positions that maximise benefit and minimise problems [654]. Gladwell et al. argue that future TENS studies need to be designed to align potential benefits of using TENS with patient reported outcome measures (PROMS) using the International Classification of Functioning, Disability and Health (ICF) [656]. This should be underpinned by foundational research to improve evaluations of TENS [657].
4.6. Reframing the Active Ingredient of TENS
The TENS study literature is contaminated by TENS-like interventions using non-standard currents or techniques including interferential therapy, transcutaneous electrical acupoint stimulation or microcurrent and therefore it is important to distinguish TENS from TENS-like interventions including administering TENS to acupuncture points remote to the site of pain. The eligibility criteria for our review were optimised to improve the likelihood of beneficial effects from TENS and were based on a pragmatic assumption that the effects of TENS are optimal when a standard TENS device administers strong non-painful TENS sensation close to the painful site. This is consistent with the physiological rationale of TENS to selectively stimulate non-noxious low threshold cutaneous peripheral afferents because this has been shown to reduce activity in nociceptive transmission cells in the central nervous system [2,3,4,5,658]. Studies using animal models of nociceptive processing suggest that central neuropharmacological actions of TENS are influenced by the pulse current frequency [659], yet this may not translate into clinically predictable outcomes in humans [660]. We argue that searching for optimal TENS parameters for specific pain conditions should be abandoned [11].
Our descriptive analysis revealed that the vast majority of TENS interventions were administered using high-frequency currents (>10 pps). However, reporting on electrical characteristics of TENS used in studies needs to be improved. Many reports did not state the pattern (mode) of pulse delivery, which is necessary to differentiate bursts per second and pulses per second when referring to low-frequency TENS. Likewise, the terms ‘alternating’, ‘varying’ and ‘modulating’ were not accompanied with sufficient detail to ascertain the precise nature of currents such as frequencies that switch between upper and lower boundaries at a single time point (i.e., 6 s at 10 pps and 6 s at 100 pps) or over a period of time (e.g., 6 s linear increase in frequency from 10 pps to 100 pps).
In most instances, TENS settings were determined by the researcher with insufficient information to ascertain whether settings could subsequently be adjusted by participants according to need. In most instances, the variability in treatment regimens reflected clinical context, e.g., a single five-minute dose during procedural pains to regular 30–60 min doses as needed over a period of weeks. This level of detail is important to ascertain exactly how TENS was administered throughout a study and whether participants adhered to clinical practice guidelines.
Much debate has focused around optimal and appropriate electrical characteristics of TENS. We have argued that differential neurophysiological and pharmacological pulse frequency effects of TENS observed in animal models of nociception may not moderate clinical outcome, i.e., frequency-dependent physiological effects do not translate to clinically meaningful outcomes. We speculate that the critical factor for success of TENS is the ‘comfortability’ of the sensory experience of TENS as this will ‘soothe’ the intensity and quality of and reduce perceived bodily threat, akin to rubbing, warming or cooling the skin for pain relief. One advantage of TENS is that electrical characteristics can be adjusted to alter the quality of sensations such as pulsate and paraesthesiae, and such moment-to-moment adjustment may be beneficial to combat the dynamic nature of pain.
It may be prudent to consider the ‘active ingredient’ of TENS as a pleasant bodily-TENS sensation irrespective of the electrical characteristics needed to achieve this. It is likely that electrical characteristics of TENS need to be regularly adjusted within and between treatment sessions to maintain this sensory comfortability on a moment-to-moment basis. Studies exploring the lived experiences of successful TENS users are few [653,654] and more are needed.
4.7. Monitoring Adherence and TENS Usage Patterns
Most study measurements obtained for in-patient populations reflected real-world situations where TENS was administered and outcome was measured during routine care, e.g., for procedural or post-surgical pain. However, this was apparent for out-patient populations self-administering TENS at home. Often participants were required to attend a ‘study visit’ to the clinic or laboratory to measure TENS effects at that one instance in time, occasionally supported with pain diary data. There was a paucity of formal monitoring of the pattern of TENS usage or whether participants had adhered to instructions for use in out-patient and in-patient settings. The use of sophisticated TENS devices that record patterns of treatment should be encouraged in future studies. Likewise, there is an urgent need for TENS education packages to support the intricate behaviours and choices facing TENS users so that they can optimise TENS benefits both in clinical practice and research studies [657].
4.8. Consideration of Novel Study Designs
The majority of TENS studies were parallel group randomised controlled trials conducted in hospital settings and using short-term outcomes. Two-thirds of the studies were pragmatic (clinical) rather than explanatory (mechanistic) with a large proportion of evaluations for post-operative pain and chronic musculoskeletal pain (e.g., back pain and osteoarthritis). The paucity of large studies with long-term courses of treatment and long-term follow-up for common chronic pain conditions should be addressed in future research.
There were no enriched enrolment randomised withdrawal studies. Previously, we have argued the need for a large multicentred enriched enrolment randomised withdrawal study that includes an observational ‘run-in’ phase of at least two weeks to enable participants to tailor treatment to their needs (i.e., optimise treatment and troubleshoot problems) and facilitate the assessment of adverse effects [11]. Group sample sizes should be 200 participants or more to ensure adequate statistical power [11,642], although we suspect the effect size estimate of such a study would be of a similar magnitude to that reported in our Meta-TENS study (i.e., pain intensity was lower for TENS versus placebo with a standardised mean difference of −0.96 (95% CI −1.14 to −0.78; 91 RCTs, 92 samples, n = 4841 [12]).
There is a need for real-world data so that educational packages can be developed to support patients to self-administer TENS and to integrate TENS within public health service settings [655,657]. Research is also needed to determine appropriate, contextualised TENS patient-reported outcomes [656].
5. Conclusions
The analyses offer insights into the factors influencing the fidelity of studies on TENS and offers avenues to improve the direction and design of future research. The conduct and publication of small-sized studies claiming to evaluate efficacy should be discouraged, as should new systematic reviews without the inclusion of large studies that are likely to change outcome or improve the level of certainty of evidence. Our descriptive analysis should be considered alongside the moderate-certainty evidence that TENS alleviates ‘pain-in-the-moment’ from our Meta-TENS study [12].
Author Contributions
Conceptualisation: M.I.J.; data curation: M.I.J., P.G.W. and C.A.P. (G.J. cross-checking); formal analysis: M.I.J., P.G.W., C.A.P., M.R.M. and G.J.; funding acquisition: M.I.J.; investigation: M.I.J., P.G.W., C.A.P., M.R.M. and G.J.; methodology (protocol development): M.I.J., P.G.W., C.A.P. and G.J.; project administration: M.I.J.; resources: M.I.J.; software: M.I.J. and M.R.M.; supervision: M.I.J.; validation: M.I.J.; visualisation: M.I.J.; writing—original draft: M.I.J.; writing—review and editing: M.I.J., P.G.W., C.A.P., G.J. and M.R.M. All authors had access to the data and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical approval for the review was granted by Leeds Beckett University (Application Ref: 78097).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available as yet due to the investigating team continuing to undertake secondary analyses.
Conflicts of Interest
All authors have completed the ICMJE uniform disclosure form and declare the following: In the previous 5 years, Mark I. Johnson’s employer has received income for expert consultancy activities from GlaxoSmithKline, TENSCare, and LifeCare Ltd. that lie outside of the submitted work. Mark I. Johnson declares book royalties from Oxford University Press. Carole A. Paley, Gareth Jones, Mathew R. Mulvey and Priscilla G. Wittkopf declare no conflicts of interests. There was no patient or public involvement in any aspect of this study or its write-up.
Funding Statement
GlaxoSmithKline (GSK) Consumer Healthcare, Nyon, Switzerland provided funding for the study (Investigator Sponsored Study grant, award/grant number N/A). The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Johnson M. Transcutaneous Electrical Nerve Stimulation (TENS). Research to Support Clinical Practice. Oxford University Press; Oxford, UK: 2014. [Google Scholar]
- 2.Garrison D.W., Foreman R.D. Decreased activity of spontaneous and noxiously evoked dorsal horn cells during transcutaneous electrical nerve stimulation (TENS) Pain. 1994;58:309–315. doi: 10.1016/0304-3959(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 3.Garrison D.W., Foreman R.D. Effects of transcutaneous electrical nerve stimulation (TENS) on spontaneous and noxiously evoked dorsal horn cell activity in cats with transected spinal cords. Neurosci. Lett. 1996;216:125–128. doi: 10.1016/0304-3940(96)13023-8. [DOI] [PubMed] [Google Scholar]
- 4.Peng W.W., Tang Z.Y., Zhang F.R., Li H., Kong Y.Z., Iannetti G.D., Hu L. Neurobiological mechanisms of TENS-induced analgesia. Neuroimage. 2019;195:396–408. doi: 10.1016/j.neuroimage.2019.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson M.I. Transcutaneous electrical nerve stimulation. In: Watson T., Nussbaum E.L., editors. Electrophysical Agents. 13th ed. Elsevier; Amsterdam, The Netherlands: 2020. pp. 264–295. [Google Scholar]
- 6.Chabal C., Fishbain D.A., Weaver M., Heine L.W. Long-term transcutaneous electrical nerve stimulation (TENS) use: Impact on medication utilization and physical therapy costs. Clin. J. Pain. 1998;14:66–73. doi: 10.1097/00002508-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Pivec R., Stokes M., Chitnis A.S., Paulino C.B., Harwin S.F., Mont M.A. Clinical and economic impact of TENS in patients with chronic low back pain: Analysis of a nationwide database. Orthopedics. 2013;36:922–928. doi: 10.3928/01477447-20131120-04. [DOI] [PubMed] [Google Scholar]
- 8.Pivec R., Minshall M.E., Mistry J.B., Chughtai M., Elmallah R.K., Mont M.A. Decreased Opioid Utilization and Cost at One Year in Chronic Low Back Pain Patients Treated with Transcutaneous Electric Nerve Stimulation (TENS) Surg. Technol. Int. 2015;27:268–274. [PubMed] [Google Scholar]
- 9.Woods B., Manca A., Weatherly H., Saramago P., Sideris E., Giannopoulou C., Rice S., Corbett M., Vickers A., Bowes M., et al. Cost-effectiveness of adjunct non-pharmacological interventions for osteoarthritis of the knee. PLoS ONE. 2017;12:e0172749. doi: 10.1371/journal.pone.0172749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vance C.G., Zimmerman M.B., Dailey D.L., Rakel B.A., Geasland K.M., Chimenti R.L., Williams J.M., Golchha M., Crofford L.J., Sluka K.A. Reduction in movement-evoked pain and fatigue during initial 30-minute TENS treatment predicts TENS responders in women with fibromyalgia. Pain. 2020;162:1545–1555. doi: 10.1097/j.pain.0000000000002144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson M.I. Resolving Long-Standing Uncertainty about the Clinical Efficacy of Transcutaneous Electrical Nerve Stimulation (TENS) to Relieve Pain: A Comprehensive Review of Factors Influencing Outcome. Medicina. 2021;57:378. doi: 10.3390/medicina57040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson M.I., Paley C.A., Jones G., Mulvey M.R., Wittkopf P.G. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: A systematic review and meta-analysis of 381 studies (the meta-TENS study) BMJ Open. 2022;12:e051073. doi: 10.1136/bmjopen-2021-051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson M.I., Jones G., Paley C.A., Wittkopf P.G. The clinical efficacy of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain: A protocol for a meta-analysis of randomised controlled trials (RCTs) BMJ Open. 2019;9:e029999. doi: 10.1136/bmjopen-2019-029999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbasi A.A., Shehzad M., Hussain A. Transcutaneous Electrical Nerve Encouragement by Way of the Adjunct to Non-Steroidal Unwilling-Inflammatory Medicines for Aching Management throughout Pleurodesis. Indo Am. J. Pharm. Sci. 2019;6:12943–12947. doi: 10.5281/zenodo.3256559. [DOI] [Google Scholar]
- 15.Abelson K., Langely G.B., Sheppeard H. Transcutaneous electrical nerve stimulation in rheumatoid arthritis. N. Z. Med. J. 1983;96:156–158. [PubMed] [Google Scholar]
- 16.Abreu E.A., Santos J.D.M.D., Ventura P.L. Efetividade da eletroestimulação nervosa transcutânea no alívio da dor durante o trabalho de parto: Um ensaio clínico controlado. Rev. Dor. 2010;11:313–318. [Google Scholar]
- 17.Acedo A.A., Antunes A.C.L., dos Santos A.B., de Olveira C.B., dos Santos C.T., Colonezi G.L.T., Fontana F.A.M., Yukio Fukuda T. Upper trapezius relaxation induced by tens and interferential current in computer users with chronic nonspecific neck discomfort: An electromyographic analysis. J. Back Musculoskelet. Rehabil. 2015;28:19–24. doi: 10.3233/BMR-140482. [DOI] [PubMed] [Google Scholar]
- 18.Adedoyin R.A., Olaogun M.O.B., Oyeyemi A.L. Transcutaneous Electrical Nerve Stimulation and Interferential Current Combined with Exercise for the Treatment of Knee Osteoarthritis: A Randomised Controlled Trial. Hong Kong Physiother. J. 2005;23:13–19. doi: 10.1016/S1013-7025(09)70054-5. [DOI] [Google Scholar]
- 19.Ahmed M.T. Effect of Transcutaneous Electrical Nerve Stimulation on Postoperative Pain after Inguinal Hernia Repair: A Randomized Placebo-Controlled Trial. Turk. Fiz. Tip Rehabil. Derg. 2010;56:170–176. doi: 10.4274/tftr.56.170. [DOI] [Google Scholar]
- 20.Ahmed G.M., Maher E.A., Elnassag B.A.E.M.R., Sayed H.M., Kabbash S.I. Effects of repetitive transcranial magnetic stimulation versus transcutaneous electrical nerve stimulation to decrease diabetic neuropathic pain. Int. J. Ther. Rehabil. 2020;27:1–10. doi: 10.12968/ijtr.2018.0037. [DOI] [Google Scholar]
- 21.Alcidi L., Beneforti E., Maresca M., Santosuosso U., Zoppi M. Low power radiofrequency electromagnetic radiation for the treatment of pain due to osteoarthritis of the knee. Reumatismo. 2007;59:140–145. doi: 10.4081/reumatismo.2007.140. [DOI] [PubMed] [Google Scholar]
- 22.Ali J., Yaffe C.S., Serrette C. The effect of transcutaneous electric nerve stimulation on postoperative pain and pulmonary function. Surgery. 1981;89:507–512. doi: 10.1097/00132586-198204000-00071. [DOI] [PubMed] [Google Scholar]
- 23.Alizade M.H., Ahmadizad S. A comparison of exercise therapy and transcutaneous electrical nerve stimulation for the treatment of chronic low back pain. World J. Sport Sci. 2009;2:43–47. [Google Scholar]
- 24.Allais G., De Lorenzo C., Quirico P.E., Lupi G., Airola G., Mana O., Benedetto C. Non-pharmacological approaches to chronic headaches: Transcutaneous electrical nerve stimulation, laser therapy and acupuncture in transformed migraine treatment. Neurol. Sci. 2003;24((Suppl. 2)):S138–S142. doi: 10.1007/s100720300062. [DOI] [PubMed] [Google Scholar]
- 25.Alm W., Gold M., Weil L. Evaluation of transcutaneous electrical nerve stimulation (TENS) in podiatric surgery. J. Am. Podiatry Assoc. 1979;69:537–542. doi: 10.7547/87507315-69-9-537. [DOI] [PubMed] [Google Scholar]
- 26.Al-Smadi J., Warke K., Wilson I., Cramp A., Noble G., Walsh D., Lowe-Strong A. A pilot investigation of the hypoalgesic effects of transcutaneous electrical nerve stimulation upon low back pain in people with multiple sclerosis. Clin. Rehabil. 2003;17:742–749. doi: 10.1191/0269215503cr672oa. [DOI] [PubMed] [Google Scholar]
- 27.Altay F., Durmus D., Cantürk F. Effects of TENS on pain, disability, quality of life and depression in patients with knee osteoarthritis. Turk. J. Rheumatol. 2010;25:116–121. doi: 10.5152/tjr.2010.14. [DOI] [Google Scholar]
- 28.Alvarez-Arenal A., Junquera L.M., Fernandez J.P., Gonzalez I., Olay S. Effect of occlusal splint and transcutaneous electric nerve stimulation on the signs and symptoms of temporomandibular disorders in patients with bruxism. J. Oral. Rehabil. 2002;29:858–863. doi: 10.1046/j.1365-2842.2002.00923.x. [DOI] [PubMed] [Google Scholar]
- 29.Alves Silverio K.C., Brasolotto A.G., Thais Donalonso Siqueira L., Carneiro C.G., Fukushiro A.P., Roberto de Jesus Guirro R. Effect of application of transcutaneous electrical nerve stimulation and laryngeal manual therapy in dysphonic women: Clinical trial. J. Voice. 2015;29:200–208. doi: 10.1016/j.jvoice.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Amer-Cuenca J.J., Goicoechea C., Girona-Lopez A., Andreu-Plaza J.L., Palao-Roman R., Martinez-Santa G., Lison J.F. Pain relief by applying transcutaneous electrical nerve stimulation (TENS) during unsedated colonoscopy: A randomized double-blind placebo-controlled trial. Eur. J. Pain. 2011;15:29–35. doi: 10.1016/j.ejpain.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 31.AminiSaman J., Karimpour H., Hemmatpour B., Mohammadi S., Darvishi S., Kawyannejad R. Effect of TENS on the Pain Intensity during Insertion of Needle in Patients Undergoing Spinal anesthesia: A Randomized Controlled Study. J. Acupunct. Meridian Stud. 2020;13:83–86. doi: 10.1016/j.jams.2020.03.062. [DOI] [PubMed] [Google Scholar]
- 32.Angulo D.L., Colwell C.W. Use of Postoperative TENS and Continuous Passive Motion Following Total Knee Replacement. J. Orthop. Sports Phys. 1990;11:599–604. doi: 10.2519/jospt.1990.11.12.599. [DOI] [PubMed] [Google Scholar]
- 33.Ardic F., Sarhus M., Topuz O. Comparison of two different techniques of electrotherapy on myofascial pain. J. Back Musculoskelet. Rehabil. 2002;16:11–16. doi: 10.3233/BMR-2002-16103. [DOI] [PubMed] [Google Scholar]
- 34.Arvidsson I., Eriksson E. Postoperative TENS pain relief after knee surgery: Objective evaluation. Orthopedics. 1986;9:1346–1351. doi: 10.3928/0147-7447-19861001-06. [DOI] [PubMed] [Google Scholar]
- 35.Asgari Z., Tavoli Z., Hosseini R., Nataj M., Tabatabaei F., Dehghanizadeh F., Haji-Amoo-Assar H., Sepidarkish M., Montazeri A. A Comparative Study between Transcutaneous Electrical Nerve Stimulation and Fentanyl to Relieve Shoulder Pain during Laparoscopic Gynecologic Surgery under Spinal Anesthesia: A Randomized Clinical Trial. Pain Res. Manag. 2018;2018:9715142. doi: 10.1155/2018/9715142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atamaz F.C., Durmaz B., Baydar M., Demircioglu O.Y., Iyiyapici A., Kuran B., Oncel S., Sendur O.F. Comparison of the efficacy of transcutaneous electrical nerve stimulation, interferential currents, and shortwave diathermy in knee osteoarthritis: A double-blind, randomized, controlled, multicenter study. Arch. Phys. Med. Rehabil. 2012;93:748–756. doi: 10.1016/j.apmr.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 37.Aydin G., Tomruk S., Keles I., Demir S.O., Orkun S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity: Clinical and electrophysiologic comparison. Am. J. Phys. Med. Rehabil./Assoc. Acad. Physiatr. 2005;84:584–592. doi: 10.1097/01.phm.0000171173.86312.69. [DOI] [PubMed] [Google Scholar]
- 38.Azatcam G., Atalay N.S., Akkaya N., Sahin F., Aksoy S., Zincir O., Topuz O. Comparison of effectiveness of Transcutaneous Electrical Nerve Stimulation and Kinesio Taping added to exercises in patients with myofascial pain syndrome. J. Back Musculoskelet. Rehabil. 2017;30:291–298. doi: 10.3233/BMR-150503. [DOI] [PubMed] [Google Scholar]
- 39.Báez-Suárez A., Martín-Castillo E., García-Andújar J., García-Hernández J., Quintana-Montesdeoca M.P., Loro-Ferrer J.F. Evaluation of different doses of transcutaneous nerve stimulation for pain relief during labour: A randomized controlled trial. Trials. 2018;19:652. doi: 10.1186/s13063-018-3036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai H.Y., Bai H.Y., Yang Z.Q. Effect of transcutaneous electrical nerve stimulation therapy for the treatment of primary dysmenorrheal. Medicine. 2017;96:e7959. doi: 10.1097/MD.0000000000007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baki E.D., Oz G., Kokulu S., Ulasli A.M., Ela Y., Sivaci R.G., Senay H., Dogan G. Comparison of Transcutaneous Electrical Nerve Stimulation and Paravertebral Block for Postthoracotomy Pain Relief. Thorac. Cardiovasc. Surg. 2015;63:514–518. doi: 10.1055/s-0035-1544212. [DOI] [PubMed] [Google Scholar]
- 42.Ballegaard S., Christophersen S., Dawids S., Hesse J., Olsen N. Acupuncture and transcutaneous electric nerve stimulation in the treatment of pain associated with chronic pancreatitis. A randomized study. Scand. J. Gastroenterol. 1985;20:1249–1254. doi: 10.3109/00365528509089285. [DOI] [PubMed] [Google Scholar]
- 43.Barbarisi M., Pace M.C., Passavanti M.B., Maisto M., Mazzariello L., Pota V., Aurilio C. Pregabalin and transcutaneous electrical nerve stimulation for postherpetic neuralgia treatment. Clin. J. Pain. 2010;26:567–572. doi: 10.1097/AJP.0b013e3181dda1ac. [DOI] [PubMed] [Google Scholar]
- 44.Barker R., Lang T., Steinlechner B., Mora B., Heigel P., Gauss N., Zimpfer M., Kober A. Transcutaneous electrical nerve stimulation as prehospital emergency interventional care: Treating acute pelvic pain in young women. Neuromodulation. 2006;9:136–142. doi: 10.1111/j.1525-1403.2006.00053.x. [DOI] [PubMed] [Google Scholar]
- 45.Barker K.L., Elliott C.J., Sackley C.M., Fairbank J.C. Treatment of chronic back pain by sensory discrimination training. A Phase I RCT of a novel device (FairMed) vs. TENS. BMC Musculoskelet. Disord. 2008;9:97. doi: 10.1186/1471-2474-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Başkurt Z., Başkurt F., Özcan A., Yilmaz Ö. The immediate effects of heat and TENS on pressure pain threshold and pain intensity in patients with Stage I shoulder impingement syndrome. Pain Clin. 2006;18:81–85. doi: 10.1163/156856906775249839. [DOI] [Google Scholar]
- 47.Bayindir O., Paker T., Akpinar B., Erenturk S., Askin D., Aytac A. Use of transcutaneous electrical nerve stimulation in the control of postoperative chest pain after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 1991;5:589–591. doi: 10.1016/1053-0770(91)90012-I. [DOI] [PubMed] [Google Scholar]
- 48.Beckwée D., Bautmans I., Lefeber N., Lievens P., Scheerlinck T., Vaes P. Effect of Transcutaneous Electric Nerve Stimulation on Pain after Total Knee Arthroplasty: A Blind Randomized Controlled Trial. J. Knee Surg. 2018;31:189–196. doi: 10.1055/s-0037-1602134. [DOI] [PubMed] [Google Scholar]
- 49.Benedetti F., Amanzio M., Casadio C., Cavallo A., Cianci R., Giobbe R., Mancuso M., Ruffini E., Maggi G. Control of postoperative pain by transcutaneous electrical nerve stimulation after thoracic operations. Ann. Thorac. Surg. 1997;63:773–776. doi: 10.1016/S0003-4975(96)01249-0. [DOI] [PubMed] [Google Scholar]
- 50.Bennett M.I., Johnson M.I., Brown S.R., Radford H., Brown J.M., Searle R.D. Feasibility study of Transcutaneous Electrical Nerve Stimulation (TENS) for cancer bone pain. J. Pain. 2010;11:351–359. doi: 10.1016/j.jpain.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Bergeron-Vezina K., Filion C., Couture C., Vallee E., Laroche S., Leonard G. Adjusting Pulse Amplitude During Transcutaneous Electrical Nerve Stimulation Does Not Provide Greater Hypoalgesia. J. Altern. Complement. Med. 2018;24:262–267. doi: 10.1089/acm.2016.0184. [DOI] [PubMed] [Google Scholar]
- 52.Bertalanffy A., Kober A., Bertalanffy P., Gustorff B., Gore O., Adel S., Hoerauf K. Transcutaneous electrical nerve stimulation reduces acute low back pain during emergency transport. Acad. Emerg. Med. 2005;12:607–611. doi: 10.1197/j.aem.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Bi X., Lv H., Chen B.L., Li X., Wang X.Q. Effects of transcutaneous electrical nerve stimulation on pain in patients with spinal cord injury: A randomized controlled trial. J. Phys. Ther. Sci. 2015;27:23–25. doi: 10.1589/jpts.27.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bilgili A., Cakir T., Dogan S.K., Ercalik T., Filiz M.B., Toraman F. The effectiveness of transcutaneous electrical nerve stimulation in the management of patients with complex regional pain syndrome: A randomized, double-blinded, placebo-controlled prospective study. J. Back Musculoskelet. Rehabil. 2016;29:661–671. doi: 10.3233/BMR-160667. [DOI] [PubMed] [Google Scholar]
- 55.Binder P., Gustafsson A., Uvnas-Moberg K., Nissen E. Hi-TENS combined with PCA-morphine as post caesarean pain relief. Midwifery. 2011;27:547–552. doi: 10.1016/j.midw.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Bjersa K., Andersson T. High frequency TENS as a complement for pain relief in postoperative transition from epidural to general analgesia after pancreatic resection. Complement. Ther. Clin. Pract. 2014;20:5–10. doi: 10.1016/j.ctcp.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Bjersa K., Jildenstaal P., Jakobsson J., Egardt M., Fagevik Olsen M. Adjunct High Frequency Transcutaneous Electric Stimulation (TENS) for Postoperative Pain Management during Weaning from Epidural Analgesia Following Colon Surgery: Results from a Controlled Pilot Study. Pain Manag. Nurs. 2015;16:944–950. doi: 10.1016/j.pmn.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Bloodworth D.M., Nguyen B.N., Garver W., Moss F., Pedroza C., Tran T., Chiou-Tan F.Y. Comparison of stochastic vs. conventional transcutaneous electrical stimulation for pain modulation in patients with electromyographically documented radiculopathy. Am. J. Phys. Med. Rehabil./Assoc. Acad. Physiatr. 2004;83:584–591. doi: 10.1097/01.PHM.0000133439.28817.51. [DOI] [PubMed] [Google Scholar]
- 59.Bolat M.S., Cinar O., Asci R., Buyukalpelli R. A novel method for pain control: Infiltration free local anesthesia technique (INFLATE) for transrectal prostatic biopsy using transcutaneous electrical nerve stimulation (TENS) Int. Urol. Nephrol. 2019;51:2119–2126. doi: 10.1007/s11255-019-02277-0. [DOI] [PubMed] [Google Scholar]
- 60.Bono F., Salvino D., Mazza M.R., Curcio M., Trimboli M., Vescio B., Quattrone A. The influence of ictal cutaneous allodynia on the response to occipital transcutaneous electrical stimulation in chronic migraine and chronic tension-type headache: A randomized, sham-controlled study. Cephalalgia Int. J. Headache. 2015;35:389–398. doi: 10.1177/0333102414544909. [DOI] [PubMed] [Google Scholar]
- 61.Börjesson M., Eriksson P., Dellborg M., Eliasson T., Mannheimer C. Transcutaneous electrical nerve stimulation in unstable angina pectoris. Coron. Artery Dis. 1997;8:543–550. [PubMed] [Google Scholar]
- 62.Börjesson M., Pilhall M., Eliasson T., Norssell H., Mannheimer C., Rolny P. Esophageal visceral pain sensitivity: Effects of TENS and correlation with manometric findings. Dig. Dis. Sci. 1998;43:1621–1628. doi: 10.1023/A:1018886309364. [DOI] [PubMed] [Google Scholar]
- 63.Borup L., Wurlitzer W., Hedegaard M., Kesmodel U.S., Hvidman L. Acupuncture as pain relief during delivery: A randomized controlled trial. Birth. 2009;36:5–12. doi: 10.1111/j.1523-536X.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- 64.Breit R., Van der Wall H. Transcutaneous electrical nerve stimulation for postoperative pain relief after total knee arthroplasty. J. Arthroplast. 2004;19:45–48. doi: 10.1016/S0883-5403(03)00458-3. [DOI] [PubMed] [Google Scholar]
- 65.Buchmuller A., Navez M., Milletre-Bernardin M., Pouplin S., Presles E., Lanteri-Minet M., Tardy B., Laurent B., Camdessanche J.P. Value of TENS for relief of chronic low back pain with or without radicular pain. Eur. J. Pain. 2012;16:656–665. doi: 10.1002/j.1532-2149.2011.00061.x. [DOI] [PubMed] [Google Scholar]
- 66.Bulut M., Özcan A., Çakan T., Mektas M., Çulha C. The Comparison of Effectiveness of TENS and Placebo TENS in Peripheral Neuropathic Pain in Patients with Type II Diabetes Mellitus. Turk. Klin. J. Med. Sci. 2011;31:913–918. doi: 10.5336/medsci.2010-18141. [DOI] [Google Scholar]
- 67.Bundsen P., Ericson K., Peterson L., Thiringer K. Pain relief in labor by transcutaneous electrical nerve stimulation. Testing of a modified stimulation technique and evaluation of the neurological and biochemical condition of the newborn infant. Acta Obstet. Gynecol. Scand. 1982;61:129–136. doi: 10.3109/00016348209156543. [DOI] [PubMed] [Google Scholar]
- 68.Can F., Tandogan R., Yilmaz I., Dolunay E., Erden Z. Rehabilitation of patellofemoral pain syndrome: TENS versus diadynamic current therapy for pain relief. Pain Clin. 2003;15:61–68. doi: 10.1163/156856903321196519. [DOI] [Google Scholar]
- 69.Casale R., Damiani C., Maestri R., Wells C.D. Pain and electrophysiological parameters are improved by combined 830-1064 high-intensity LASER in symptomatic carpal tunnel syndrome versus Transcutaneous Electrical Nerve Stimulation. A randomized controlled study. Eur. J. Phys. Rehabil. Med. 2013;49:205–211. [PubMed] [Google Scholar]
- 70.Cebi A.-T. Effects of transcutaneous electrical nerve stimulation on pain after impacted third molar surgery. Med. Oral Patol. Oral Cir. Bucal. 2019;24:E404–E408. doi: 10.4317/medoral.22871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Celik E.C., Erhan B., Gunduz B., Lakse E. The effect of low-frequency TENS in the treatment of neuropathic pain in patients with spinal cord injury. Spinal Cord. 2013;51:334–337. doi: 10.1038/sc.2012.159. [DOI] [PubMed] [Google Scholar]
- 72.Cetin N., Aytar A., Atalay A., Akman M. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: A single-blind, randomized, controlled trial. Am. J. Phys. Med. Rehabil. 2008;87:443–451. doi: 10.1097/PHM.0b013e318174e467. [DOI] [PubMed] [Google Scholar]
- 73.Chandra A., Banavaliker J.N., Das P.K., Hasti S. Use of transcutaneous electrical nerve stimulation as an adjunctive to epidural analgesia in the management of acute thoracotomy pain. Indian J. Anaesth. 2010;54:116–120. doi: 10.4103/0019-5049.63648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheing G.L., Hui_Chan C.W. Transcutaneous electrical nerve stimulation: Nonparallel antinociceptive effects on chronic clinical pain and acute experimental pain. Arch. Phys. Med. Rehabil. 1999;80:305–312. doi: 10.1016/S0003-9993(99)90142-9. [DOI] [PubMed] [Google Scholar]
- 75.Cheing G.L., Luk M.L. Transcutaneous electrical nerve stimulation for neuropathic pain. J. Hand Surg. 2005;30:50–55. doi: 10.1016/J.JHSB.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Cheing G.L., Hui-Chan C.W., Chan K.M. Does four weeks of TENS and/or isometric exercise produce cumulative reduction of osteoarthritic knee pain? Clin. Rehabil. 2002;16:749–760. doi: 10.1191/0269215502cr549oa. [DOI] [PubMed] [Google Scholar]
- 77.Cheing G.L., Tsui A.Y., Lo S.K., Hui-Chan C.W. Optimal stimulation duration of TENS in the management of osteoarthritic knee pain. J. Rehabil. Med. 2003;35:62–68. doi: 10.1080/16501970306116. [DOI] [PubMed] [Google Scholar]
- 78.Chellappa D., Thirupathy M. Comparative efficacy of low-Level laser and TENS in the symptomatic relief of temporomandibular joint disorders: A randomized clinical trial. Indian J. Dent. Res. 2020;31:42–47. doi: 10.4103/ijdr.IJDR_735_18. [DOI] [PubMed] [Google Scholar]
- 79.Cherian J.J., Harrison P.E., Benjamin S.A., Bhave A., Harwin S.F., Mont M.A. Do the Effects of Transcutaneous Electrical Nerve Stimulation on Knee Osteoarthritis Pain and Function Last? J. Knee Surg. 2016;29:497–501. doi: 10.1055/s-0035-1566735. [DOI] [PubMed] [Google Scholar]
- 80.Chesterton L., Van Der Windt D.A., Sim J., Lewis M., Mallen C.D., Mason E., Hay E. Transcutaneous electrical nerve stimulation for the management of tennis elbow: A pragmatic randomized controlled trial. Rheumatology. 2013;1:i38. doi: 10.1186/1471-2474-10-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chia Y., Arulkumaran S., Chua S., Ratnam S. Effectiveness of transcutaneous electric nerve stimulator for pain relief in labour. Asia Ocean. J. Obstet. Gynaecol. 1990;16:145–151. doi: 10.1111/j.1447-0756.1990.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 82.Chiou Y.F., Yeh M.L., Wang Y.J. Transcutaneous Electrical Nerve Stimulation on Acupuncture Points Improves Myofascial Pain, Moods, and Sleep Quality. Rehabil. Nurs. J. 2020;45:225–233. doi: 10.1097/RNJ.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 83.Chitsaz A., Janghorbani M., Shaygannejad V., Ashtari F., Heshmatipour M., Freeman J. Sensory complaints of the upper extremities in multiple sclerosis: Relative efficacy of nortriptyline and transcutaneous electrical nerve stimulation. Clin. J. Pain. 2009;25:281–285. doi: 10.1097/AJP.0b013e318190862b. [DOI] [PubMed] [Google Scholar]
- 84.Chiu T.T., Hui-Chan C.W., Chein G. A randomized clinical trial of TENS and exercise for patients with chronic neck pain. Clin. Rehabil. 2005;19:850–860. doi: 10.1191/0269215505cr920oa. [DOI] [PubMed] [Google Scholar]
- 85.Cipriano G., Jr., de Camargo Carvalho A.C., Bernardelli G.F., Tayar Peres P.A. Short-term transcutaneous electrical nerve stimulation after cardiac surgery: Effect on pain, pulmonary function and electrical muscle activity. Interact. Cardiovasc. Thorac. Surg. 2008;7:539–543. doi: 10.1510/icvts.2007.168542. [DOI] [PubMed] [Google Scholar]
- 86.Cipriano G., Jr., Neder J.A., Umpierre D., Arena R., Vieira P.J., Chiappa A.M., Ribeiro J.P., Chiappa G.R. Sympathetic ganglion transcutaneous electrical nerve stimulation after coronary artery bypass graft surgery improves femoral blood flow and exercise tolerance. J. Appl. Physiol. 2014;117:633–638. doi: 10.1152/japplphysiol.00993.2013. [DOI] [PubMed] [Google Scholar]
- 87.Coelho de Amorim J.S., Braz Rossetti M.R., Mendes Braga N.L.H. Effects of manual therapy and electrotherapy on knee osteoarthritis. ConScientiae Saude. 2014;13:11–20. doi: 10.5585/ConsSaude.v13n1.4492. [DOI] [Google Scholar]
- 88.Cooperman A.M., Hall B., Mikalacki K., Hardy R., Sardar E. Use of transcutaneous electrical stimulation in the control of postoperative pain. Am. J. Surg. 1977;133:185–187. doi: 10.1016/0002-9610(77)90077-0. [DOI] [PubMed] [Google Scholar]
- 89.Coyne P., MacMurren M., Izzo T., Kramer T. Transcutaneous electrical nerve stimulator for procedural pain associated with intravenous needlesticks. J. Intraven. Nurs. 1995;18:263–267. [PubMed] [Google Scholar]
- 90.Crompton A.C., Johnson N., Dudek U., Batra N., Tucker A. Is transcutaneous electrical nerve stimulation of any value during cervical laser treatment? Br. J. Obstet. Gynaecol. 1992;99:492–494. doi: 10.1111/j.1471-0528.1992.tb13788.x. [DOI] [PubMed] [Google Scholar]
- 91.Cuschieri R.J., Morran C.G., McArdle C.S. Transcutaneous electrical stimulation for postoperative pain. Ann. R. Coll. Surg. Engl. 1985;67:127–129. [PMC free article] [PubMed] [Google Scholar]
- 92.Cuschieri R.J., Morran C.G., Pollock J.G. Transcutaneous electrical stimulation for ischaemic pain at rest. Br. Med. J. (Clin. Res. Ed.) 1987;295:306. doi: 10.1136/bmj.295.6593.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Da Silva T.F.G., Suda E.Y., Marçulo C.A., Paes F.H.S., Pinheiro G.T. Comparison of transcutaneous electrical nerve stimulation and hydrotherapy effects on pain, flexibility and quality of life in patients with fibromyalgia. Fisioter. Pesqui. 2008;15:118–124. [Google Scholar]
- 94.Da Silva M.P., Liebano R.E., Rodrigues V.A., Abla L.E.F., Ferreira L.M. Transcutaneous Electrical Nerve Stimulation for Pain Relief After Liposuction: A Randomized Controlled Trial. Aesthetic. Plast. Surg. 2015;39:262–269. doi: 10.1007/s00266-015-0451-6. [DOI] [PubMed] [Google Scholar]
- 95.Dailey D.L., Rakel B.A., Vance C.G., Liebano R.E., Amrit A.S., Bush H.M., Lee K.S., Lee J.E., Sluka K.A. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154:2554–2562. doi: 10.1016/j.pain.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dailey D.L., Vance C.G.T., Rakel B.A., Zimmerman M.B., Embree J., Merriwether E.N., Geasland K.M., Chimenti R., Williams J.M., Golchha M., et al. Transcutaneous Electrical Nerve Stimulation Reduces Movement-Evoked Pain and Fatigue: A Randomized, Controlled Trial. Arthritis Rheumatol. 2020;72:824–836. doi: 10.1002/art.41170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davies J.R. Ineffective transcutaneous nerve stimulation following epidural anaesthesia. Anaesthesia. 1982;37:453–454. doi: 10.1111/j.1365-2044.1982.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 98.Dawood M.Y., Ramos J. Transcutaneous electrical nerve stimulation (TENS) for the treatment of primary dysmenorrhea: A randomized crossover comparison with placebo TENS and ibuprofen. Obstet. Gynecol. 1990;75:656–660. [PubMed] [Google Scholar]
- 99.De Angelis C., Perrone G., Santoro G., Nofroni I., Zichella L. Suppression of pelvic pain during hysteroscopy with a transcutaneous electrical nerve stimulation device. Fertil. Steril. 2003;79:1422–1427. doi: 10.1016/S0015-0282(03)00363-7. [DOI] [PubMed] [Google Scholar]
- 100.De Giorgi I., Castroflorio T., Sartoris B., Deregibus A. The use of conventional transcutaneous electrical nerve stimulation in chronic facial myalgia patients. Clin. Oral. Investig. 2017;21:275–280. doi: 10.1007/s00784-016-1787-2. [DOI] [PubMed] [Google Scholar]
- 101.De Oliveira G.C.Q., Silva R.C., de Almeida J.F., de Araajo A.C.R., Pitangui C.R. High and low frequency TENS for primary dysmenorrhea: Preliminary study. ConScientiae Saude. 2012;11:149–158. doi: 10.5585/ConsSaude.v11n1.2722. [DOI] [Google Scholar]
- 102.Orange F.A.D., Amorim M.M.R.D., Lima L. Uso da eletroestimulação transcutânea para alívio da dor durante o trabalho de parto em uma maternidade-escola: Ensaio clínico controlado. Rev. Bras. Ginecol. Obs. 2003;25:45–52. doi: 10.1590/S0100-72032003000100007. [DOI] [Google Scholar]
- 103.De Sousa L., Gomes-Sponholz F.A., Nakano A.M. Transcutaneous electrical nerve stimulation for the relief of post-partum uterine contraction pain during breast-feeding: A randomized clinical trial. J. Obstet. Gynaecol. Res. 2014;40:1317–1323. doi: 10.1111/jog.12345. [DOI] [PubMed] [Google Scholar]
- 104.DeSantana J.M., Santana-Filho V.J., Guerra D.R., Sluka K.A., Gurgel R.Q., da Silva W.M., Jr. Hypoalgesic effect of the transcutaneous electrical nerve stimulation following inguinal herniorrhaphy: A randomized, controlled trial. J. Pain. 2008;9:623–629. doi: 10.1016/j.jpain.2008.01.337. [DOI] [PubMed] [Google Scholar]
- 105.DeSantana J., Sluka K., Rocha L.G. High and low frequency TENS reduce postoperative pain intensity after laparoscopic tubal ligation: A randomized controlled trial. Clin. J. Pain. 2009;25:12–28. doi: 10.1097/AJP.0b013e31817d1070. [DOI] [PubMed] [Google Scholar]
- 106.Dewan A., Sharma R. Effectiveness of transcutaneous electrical nerve stimulation and interferential electrotherapy in adhesive capsulitis. Pb J. Orthop. 2011;21:64. [Google Scholar]
- 107.Deyo R., Walsh N., Martin D., Schoenfeld L., Ramamurthy S. A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. N. Engl. J. Med. 1990;322:1627–1634. doi: 10.1056/NEJM199006073222303. [DOI] [PubMed] [Google Scholar]
- 108.Dibenedetto P., Iona L.G., Zidarich V. Clinical-evaluation of s-adenosyl-l-methionine versus transcutaneous electrical nerve-stimulation in primary fibromyalgia. Curr. Ther. Res. Clin. Exp. 1993;53:222–229. doi: 10.1016/S0011-393X(05)80250-4. [DOI] [Google Scholar]
- 109.Dilekci E., Alpayci M., Bayram K.B., Bal S., Kocyigit H., Gurgan A., Kaplan S. The efficacy of TENS in patients with lateral epicondylitis: A randomized controlled study. Turk. Fiz. Tip Ve Rehabil. Derg. 2016;62:297–302. doi: 10.5606/tftrd.2016.05763. (In Turkish) [DOI] [Google Scholar]
- 110.Dissanayaka T.D., Pallegama R.W., Suraweera H.J., Johnson M.I., Kariyawasam A.P. Comparison of the Effectiveness of Transcutaneous Electrical Nerve Stimulation and Interferential Therapy on the Upper Trapezius in Myofascial Pain Syndrome: A Randomized Controlled Study. Am. J. Phys. Med. Rehabil./Assoc. Acad. Physiatr. 2016;95:663–672. doi: 10.1097/PHM.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 111.Dogu B., Yilmaz F., Karan A., Ergoz E., Kuran B. Comparative the effectiveness of occlusal splint and TENS treatments on clinical findings and pain threshold of temporomandibular disorders secondary to bruxism. Turk. Fiz. Tip Ve Rehabil. Derg. 2009;55:1–7. (In Turkish) [Google Scholar]
- 112.Domaille M., Reeves B. TENS and pain control after coronary artery bypass surgery. Physiotherapy. 1997;83:510–516. doi: 10.1016/S0031-9406(05)65606-4. [DOI] [Google Scholar]
- 113.Ebadi S., Ansari N., Ahadi T., Fallah E., Forogh B. No immediate analgesic effect of diadynamic current in patients with nonspecific low back pain in comparison to TENS. J. Bodyw. Mov. Ther. 2018;22:693–699. doi: 10.1016/j.jbmt.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 114.Ekblom A., Hansson P. Thermal sensitivity is not changed by acute pain or afferent stimulation. J. Neurol. Neurosurg. Psychiatry. 1987;50:1216–1220. doi: 10.1136/jnnp.50.9.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ekim A., Armagan O., Oner C. Efficiency of TENS treatment in hemiplegic shoulder pain: A placebo controlled study. Agri Agri (Algoloji) Dern. Yayin. Organidir. 2008;20:41–46. doi: 10.1016/S1090-3801(06)60842-2. [DOI] [PubMed] [Google Scholar]
- 116.Elboim-Gabyzon M., Andrawus Najjar S., Shtarker H. Effects of transcutaneous electrical nerve stimulation (TENS) on acute postoperative pain intensity and mobility after hip fracture: A double-blinded, randomized trial. Clin. Interv. Aging. 2019;14:1841–1850. doi: 10.2147/CIA.S203658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Elserty N., Kattabei O., Elhafez H. Effect of Fixed Versus Adjusted Transcutaneous Electrical Nerve Stimulation Amplitude on Chronic Mechanical Low Back Pain. J. Altern. Complement. Med. 2016;22:557–562. doi: 10.1089/acm.2015.0063. [DOI] [PubMed] [Google Scholar]
- 118.Emmiler M., Solak O., Kocogullari C., Dundar U., Ayva E., Ela Y., Cekirdekci A., Kavuncu V. Control of acute postoperative pain by transcutaneous electrical nerve stimulation after open cardiac operations: A randomized placebo-controlled prospective study. Heart Surg. Forum. 2008;11:E300-3. doi: 10.1532/HSF98.20081083. [DOI] [PubMed] [Google Scholar]
- 119.Engen D.J., Carns P.E., Allen M.S., Bauer B.A., Loehrer L.L., Cha S.S., Chartrand C.M., Eggler E.J., Cutshall S.M., Wahner-Roedler D.L. Evaluating efficacy and feasibility of transcutaneous electrical nerve stimulation for postoperative pain after video-assisted thoracoscopic surgery: A randomized pilot trial. Complement. Ther. Clin. Pract. 2016;23:141–148. doi: 10.1016/j.ctcp.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 120.Erden S., Senol Celik S. The effect of transcutaneous electrical nerve stimulation on post-thoracotomy pain. Contemp. Nurse. 2015;51:163–170. doi: 10.1080/10376178.2016.1166971. [DOI] [PubMed] [Google Scholar]
- 121.Erdogan M., Erdogan A., Erbil N., Karakaya H., Demircan A. Prospective, randomized, placebo-controlled study of the effect of TENS on postthoracotomy pain and pulmonary function. World J. Surg. 2005;29:1563–1570. doi: 10.1007/s00268-005-7934-6. [DOI] [PubMed] [Google Scholar]
- 122.Erkkola R., Pikkola P., Kanto J. Transcutaneous nerve stimulation for pain relief during labour: A controlled study. Ann. Chir. Gynaecol. 1980;69:273–277. [PubMed] [Google Scholar]
- 123.Escortell-Mayor E., Riesgo-Fuertes R., Garrido-Elustondo S., Asunsolo-Del Barco A., Diaz-Pulido B., Blanco-Diaz M., Bejerano-Alvarez E. Primary care randomized clinical trial: Manual therapy effectiveness in comparison with TENS in patients with neck pain. Man. Ther. 2011;16:66–73. doi: 10.1016/j.math.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 124.Esteban Gonzalez P., Novoa N.M., Varela G. Transcutaneous Electrical Nerve Stimulation Reduces Post-Thoractomy Ipsilateral Shoulder Pain. A Prospective Randomized Study. Arch. Bronconeumol. 2015;51:621–626. doi: 10.1016/j.arbres.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 125.Eyigor S., Karapolat H., Ibisoglu U., Durmaz B. Does transcutaneous electrical nerve stimulation or therapeutic ultrasound increase the effectiveness of exercise for knee osteoarthritis: A randomized controlled study. Agri Agri (Algoloji) Dern. Yayin. Organidir. 2008;20:32–40. [PubMed] [Google Scholar]
- 126.Eyigor C., Eyigor S., Kivilcim Korkmaz O. Are intra-articular corticosteroid injections better than conventional TENS in treatment of rotator cuff tendinitis in the short run? A randomized study. Eur. J. Phys. Rehabil. Med. 2010;46:315–324. [PubMed] [Google Scholar]
- 127.Facci L.M., Nowotny J.P., Tormem F., Trevisani V.F. Effects of transcutaneous electrical nerve stimulation (TENS) and interferential currents (IFC) in patients with nonspecific chronic low back pain: Randomized clinical trial. Sao Paulo Med. J. 2011;129:206–216. doi: 10.1590/S1516-31802011000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Farahani D.M., Tavallaie S.A., Ahmadi K., Ashtiani A.F. Comparison of Neurofeedback and Transcutaneous Electrical Nerve Stimulation Efficacy on Treatment of Primary Headaches: A Randomized Controlled Clinical Trial. Iran. Red Crescent Med. J. 2014;16:e17799. doi: 10.5812/ircmj.17799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Farina S., Casarotto M., Benelle M., Tinazzi M., Fiaschi A., Goldoni M., Smania N. A randomized controlled study on the effect of two different treatments (FREMS AND TENS) in myofascial pain syndrome. Eura Med. 2004;40:293–301. [PubMed] [Google Scholar]
- 130.Fatima N., Sarfraz N. Comparison of High Versus Low Transcutaneous Electrical Nerve Stimulation for Pain Management After Caesarean Section: A Clinical Trial. Ann. King Edw. Med. Univ. Lahore Pak. 2019;25:1–6. [Google Scholar]
- 131.Ferraz F.S., Moreira C.M.C. Electroanalgesia com utilizacao de TENS no pos-operatorio de cirurgia cardiaca. Fisioter. Mov. 2009;22:133–139. [Google Scholar]
- 132.Ferreira F.C., Issy A.M., Sakata R.K. Assessing the effects of transcutaneous electrical nerve stimulation (TENS) in post-thoracotomy analgesia. Rev. Bras. Anestesiol. 2011;61:561–567. doi: 10.1016/S0034-7094(11)70067-8. [DOI] [PubMed] [Google Scholar]
- 133.Ferreira A.P., Costa D.R., Oliveira A.I., Carvalho E.A., Conti P.C., Costa Y.M., Bonjardim L.R. Short-term transcutaneous electrical nerve stimulation reduces pain and improves the masticatory muscle activity in temporomandibular disorder patients: A randomized controlled trial. J. Appl. Oral Sci. 2017;25:112–120. doi: 10.1590/1678-77572016-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Finsen V., Persen L., Lovlien M., Veslegaard E.K., Simensen M., Gasvann A.K., Benum P. Transcutaneous electrical nerve stimulation after major amputation. J. Bone Jt. Surg. Br. 1988;70:109–112. doi: 10.1302/0301-620X.70B1.3257494. [DOI] [PubMed] [Google Scholar]
- 135.Fiorelli A., Morgillo F., Milione R., Pace M.C., Passavanti M.B., Laperuta P., Aurilio C., Santini M. Control of post-thoracotomy pain by transcutaneous electrical nerve stimulation: Effect on serum cytokine levels, visual analogue scale, pulmonary function and medication. Eur. J. Cardiothorac. Surg. 2012;41:861–868. doi: 10.1093/ejcts/ezr108. [DOI] [PubMed] [Google Scholar]
- 136.Fodor-Sertl B., Miller K., Hohenfellner B. (Transcutaneous electric nerve block in postoperative pain-therapy) [German] Z. Fuer Phys. Med. Balneol. Med. Klimatol. 1990;19:132–137. [Google Scholar]
- 137.Forogh B., Aslanpour H., Fallah E., Babaei-Ghazani A., Ebadi S. Adding high-frequency transcutaneous electrical nerve stimulation to the first phase of post anterior cruciate ligament reconstruction rehabilitation does not improve pain and function in young male athletes more than exercise alone: A randomized single-blind clinical trial. Disabil. Rehabil. 2019;41:514–522. doi: 10.1080/09638288.2017.1399294. [DOI] [PubMed] [Google Scholar]
- 138.Forst T., Nguyen M., Forst S., Disselhoff B., Pohlmann T., Pfutzner A. Impact of low frequency transcutaneous electrical nerve stimulation on symptomatic diabetic neuropathy using the new Salutaris device. Diabetes Nutr. Metab. 2004;17:163–168. [PubMed] [Google Scholar]
- 139.Forster E.L., Kramer J.F., Lucy S.D., Scudds R.A., Novick R.J. Effect of TENS on pain, medications, and pulmonary function following coronary artery bypass graft surgery. Chest. 1994;106:1343–1348. doi: 10.1378/chest.106.5.1343. [DOI] [PubMed] [Google Scholar]
- 140.Fujii-Abe K., Umino M., Kawahara H., Terada C., Satomura K., Fukayama H. New method for postoperative pain relief using a combination of noxious and non-noxious stimuli after impacted wisdom tooth extraction. J. Oral Sci. 2019;61:364–369. doi: 10.2334/josnusd.18-0187. [DOI] [PubMed] [Google Scholar]
- 141.Galli T.T., Chiavegato L.D., Liebano R.E. Effects of TENS in living kidney donors submitted to open nephrectomy: A randomized placebo-controlled trial. Eur. J. Pain. 2015;19:67–76. doi: 10.1002/ejp.521. [DOI] [PubMed] [Google Scholar]
- 142.Galloway D., Boyle P., Burns H., Davidson P., George W. A clinical assessment of electroanalgesia following abdominal operations. Surg. Gynecol. Obstet. 1984;159:453–456. doi: 10.1097/00132586-198508000-00026. [DOI] [PubMed] [Google Scholar]
- 143.Garcia-Perez S., Garcia-Rios M.C., Perez-Marmol J.M., Tapia-Haro R.M., Albornoz-Cabello M., Valenza M.C., Aguilar-Ferrandiz M.E. Effectiveness of Transcutaneous Electrical Nerve Stimulation Energy in Older Adults: A Pilot Clinical Trial. Adv. Ski. Wound Care. 2018;31:462–469. doi: 10.1097/01.ASW.0000544614.18501.b4. [DOI] [PubMed] [Google Scholar]
- 144.Gerson G.R., Jones R.B., Luscombe D.K. Studies on the concomitant use of carbamazepine and clomipramine for the relief of post-herpetic neuralgia. Postgrad. Med. J. 1977;53((Suppl. 4)):104–109. [PubMed] [Google Scholar]
- 145.Ghoname E.A., Craig W.F., White P.F., Ahmed H.E., Hamza M.A., Henderson B.N., Gajraj N.M., Huber P.J., Gatchel R.J. Percutaneous electrical nerve stimulation for low back pain: A randomized crossover study. JAMA. 1999;281:818–823. doi: 10.1001/jama.281.9.818. [DOI] [PubMed] [Google Scholar]
- 146.Ghoname E.S.A., White P.F., Ahmed H.E., Hamza M.A., Craig W.F., Noe C.E. Percutaneous electrical nerve stimulation: An alternative to TENS in the management of sciatica. Pain. 1999;83:193–199. doi: 10.1016/S0304-3959(99)00097-4. [DOI] [PubMed] [Google Scholar]
- 147.Gilbert J., Geldhill T., Law N., George C. Controlled trial of transcutaneous electrical nerve stimulation (TENS) for postoperative pain relief following inguinal herniorrhaphy. Br. J. Surg. 1986;73:749–751. doi: 10.1002/bjs.1800730923. [DOI] [PubMed] [Google Scholar]
- 148.Grabianska E., Lesniewicz J., Pieszynski I., Kostka J. Comparison of the analgesic effect of interferential current (IFC) and TENS in patients with low back pain. Wiad. Lek. 2015;68:13–19. [PubMed] [Google Scholar]
- 149.Graff-Radford S., Reeves J., Baker R., Chiu D. Effects of transcutaneous electrical nerve stimulation on myofascial pain and trigger point sensitivity. Pain. 1989;37:1–5. doi: 10.1016/0304-3959(89)90146-2. [DOI] [PubMed] [Google Scholar]
- 150.Grant D.J., Bishop_Miller J., Winchester D.M., Anderson M., Faulkner S. A randomized comparative trial of acupuncture versus transcutaneous electrical nerve stimulation for chronic back pain in the elderly. Pain. 1999;82:9–13. doi: 10.1016/S0304-3959(99)00027-5. [DOI] [PubMed] [Google Scholar]
- 151.Gregorini C., Cipriano G., Jr., De Aquino L.M., Rodrigues Branco J.N., Bernardelli G.F. Short-duration transcutaneous electrical nerve stimulation in the postoperative period of cardiac surgery. Arq. Bras. Cardiol. 2010;94:325–331, 345–351. doi: 10.1590/s0066-782x2010000300011. [DOI] [PubMed] [Google Scholar]
- 152.Grimmer K. A controlled double blind study comparing the effects of strong burst mode TENS and High Rate TENS on painful osteoarthritic knees. Aust. J. Physiother. 1992;38:49–56. doi: 10.1016/S0004-9514(14)60551-1. [DOI] [PubMed] [Google Scholar]
- 153.Gschiel B., Kager H., Pipam W., Weichart K., Likar R. Analgesic efficacy of TENS therapy in patients with gonarthrosis A prospective, randomized, placebo-controlled, double-blind study. Schmerz. 2010;24:494–500. doi: 10.1007/s00482-010-0957-4. [DOI] [PubMed] [Google Scholar]
- 154.Gunay Ucurum S., Kaya D.O., Kayali Y., Askin A., Tekindal M.A. Comparison of different electrotherapy methods and exercise therapy in shoulder impingement syndrome: A prospective randomized controlled trial. Acta Orthop. Traumatol. Turc. 2018;52:249–255. doi: 10.1016/j.aott.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo X.J., Jia J. Comparison of therapeutic effects on fibromyalgia syndrome between dermal-neurological electric stimulation and electric acupuncture. Chin. J. Clin. Rehabil. 2005;9:171–173. [Google Scholar]
- 156.Hamza M.A., White P.F., Ahmed H.E., Ghoname E.A. Effect of the frequency of transcutaneous electrical nerve stimulation on the postoperative opioid analgesic requirement and recovery profile. Anesthesiology. 1999;91:1232–1238. doi: 10.1097/00000542-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 157.Hanfy H.M., El-Bigawy A. Effects of transcutaneous electrical nerve stimulation versus acupressure in the treatment of primary dysmenorrhea. Bull. Fac. Phys. Ther. Cairo Univ. 2004;9:2. [Google Scholar]
- 158.Hansson P., Ekblom A. Transcutaneous electrical nerve stimulation (TENS) as compared to placebo TENS for the relief of acute oro-facial pain. Pain. 1983;15:157–165. doi: 10.1016/0304-3959(83)90015-5. [DOI] [PubMed] [Google Scholar]
- 159.Hansson P., Ekblom A., Thomsson M., Fjellner B. Influence of naloxone on relief of acute oro-facial pain by transcutaneous electrical nerve stimulation (TENS) or vibration. Pain. 1986;24:323–329. doi: 10.1016/0304-3959(86)90118-1. [DOI] [PubMed] [Google Scholar]
- 160.Hargreaves A., Lander J. Use of transcutaneous electrical nerve stimulation for postoperative pain. Nurs. Res. 1989;38:159–161. doi: 10.1097/00006199-198905000-00014. [DOI] [PubMed] [Google Scholar]
- 161.Harrison R., Woods T., Shore M., Mathews G., Unwin A. Pain relief in labour using transcutaneous electrical nerve stimulation (TENS). A TENS/TENS placebo controlled study in two parity groups. Br. J. Obstet. Gynaecol. 1986;93:739–746. doi: 10.1111/j.1471-0528.1986.tb08061.x. [DOI] [PubMed] [Google Scholar]
- 162.Hart J.M., Kuenze C.M., Pietrosimone B.G., Ingersoll C.D. Quadriceps function in anterior cruciate ligament-deficient knees exercising with transcutaneous electrical nerve stimulation and cryotherapy: A randomized controlled study. Clin. Rehabil. 2012;26:974–981. doi: 10.1177/0269215512438272. [DOI] [PubMed] [Google Scholar]
- 163.Hazneci B., Tan A.K., Özdem T., Dinçer K., Kalyon T.A. The effects of transcutaneous electroneurostimulation a ultrasound in the treatment of reflex sympathetic dystrophy syndrome. Turk. J. Phys. Med. Rehabil. 2005;51:83–89. [Google Scholar]
- 164.Herrera-Lasso I., Mobarak L., Fernández-Dominguez L., Cardiel M.H., Alarcón-Segovia D. Comparative Effectiveness of Packages of Treatment Including Ultrasound or Transcutaneous Electrical Nerve Stimulation in Painful Shoulder Syndrome. Physiotherapy. 1993;79:251–253. doi: 10.1016/S0031-9406(10)60708-0. [DOI] [Google Scholar]
- 165.Hershman M.C.W., Swift R., Reilly D., Gompertz H., Wood C. Transcutaneous electrical nerve stimulation as adjunctive analgesia in patients undergoing abdominal procedures. Surg. Res. Commun. 1989;7:65–69. [Google Scholar]
- 166.Hokenek N.M., Erdogan M.O., Hokenek U.D., Algin A., Tekyol D., Seyhan A.U. Treatment of migraine attacks by transcutaneous electrical nerve stimulation in emergency department: A randomize controlled trial. Am. J. Emerg. Med. 2020;39:80–85. doi: 10.1016/j.ajem.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 167.Hou C., Tsai L., Cheng K., Chung K., Hong C. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity. Arch. Phys. Med. Rehabil. 2002;83:1406–1414. doi: 10.1053/apmr.2002.34834. [DOI] [PubMed] [Google Scholar]
- 168.Hruby G., Ames C., Chen C., Yan Y., Sagar J., Baron P., Landman J. Assessment of efficacy of transcutaneous electrical nerve stimulation for pain management during office-based flexible cystoscopy. Urology. 2006;67:914–917. doi: 10.1016/j.urology.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 169.Hsieh R.L., Lee W.C. One-shot percutaneous electrical nerve stimulation vs. transcutaneous electrical nerve stimulation for low back pain: Comparison of therapeutic effects. Am. J. Phys. Med. Rehabil./Assoc. Acad. Physiatr. 2002;81:838–843. doi: 10.1097/00002060-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 170.Hsueh T.C., Cheng P.T., Kuan T.S., Hong C.Z. The immediate effectiveness of electrical nerve stimulation and electrical muscle stimulation on myofascial trigger points. Am. J. Phys. Med. Rehabil./Assoc. Acad. Physiatr. 1997;76:471–476. doi: 10.1097/00002060-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 171.Hughes S., Dailey P., Partridge C. Transcutaneous electrical nerve stimulation for labour analgesia. Anesth. Analg. 1988;67:S99. doi: 10.1213/00000539-198802001-00099. [DOI] [Google Scholar]
- 172.Husch H.H., Watte G., Zanon M., Pacini G.S., Birriel D., Carvalho P.L., Kessler A., Sbruzzi G. Effects of Transcutaneous Electrical Nerve Stimulation on Pain, Pulmonary Function, and Respiratory Muscle Strength After Posterolateral Thoracotomy: A Randomized Controlled Trial. Lung. 2020;198:345–353. doi: 10.1007/s00408-020-00335-4. [DOI] [PubMed] [Google Scholar]
- 173.Ilhanli I. Conventional, Acupuncture-Like or Brief-Intense: Is There Any Difference Between TENS Modalities According to Outcomes of Chronic Low Back Pain with Lumbar Disc Herniation. Clin. Med. Res. 2015;4:143–150. doi: 10.11648/j.cmr.20150405.14. [DOI] [Google Scholar]
- 174.Inal E.E., Eroglu P., Yucel S.H., Orhan H. Which is the Appropriate Frequency of TENS in Managing Knee Osteoarthritis: High or Low Frequency? J. Clin. Anal. Med. 2016;7:339–344. doi: 10.4328/jcam.3387. [DOI] [Google Scholar]
- 175.Isik M., Ugur M., Yakisan R.S., Sari T., Yilmaz N. Comparison of the effectiveness of medicinal leech and TENS therapy in the treatment of primary osteoarthritis of the knee: A randomized controlled trial. Z. Rheumatol. 2017;76:798–805. doi: 10.1007/s00393-016-0176-1. [DOI] [PubMed] [Google Scholar]
- 176.Jaafarpour M., Khani A., Javadifar N., Taghinejad H., Mahmoudi R., Saadipour K.H. The analgesic effect of transcutaneous electrical nerve stimulation (TENS) on caesarean under spinal anaesthesia. J. Clin. Diagn. Res. 2008;2:815–819. [Google Scholar]
- 177.Jamison R.N., Wan L., Edwards R.R., Mei A., Ross E.L. Outcome of a High-Frequency Transcutaneous Electrical Nerve Stimulator (hfTENS) Device for Low Back Pain: A Randomized Controlled Trial. Pain Pract. 2019;19:466–475. doi: 10.1111/papr.12764. [DOI] [PubMed] [Google Scholar]
- 178.Jarzem P., Harvey E., Arcaro N., Kaczorowski J. Transcutaneous electrical nerve stimulation (TENS) for short-term treatment of low back pain—randomized double blind crossover study of sham versus conventional TENS. J. Musculoskelet. Pain. 2005;13:11–17. doi: 10.1300/J094v13n02_03. [DOI] [Google Scholar]
- 179.Jensen J.E., Conn R.R., Hazelrigg G., Hewett J.E. The use of transcutaneous neural stimulation and isokinetic testing in arthroscopic knee surgery. Am. J. Sports Med. 1985;13:27–33. doi: 10.1177/036354658501300105. [DOI] [PubMed] [Google Scholar]
- 180.Jensen H., Zesler R., Christensen T. Transcutaneous electrical nerve stimulation (TNS) for painful osteoarthrosis of the knee. Int. J. Rehabil. Res. 1991;14:356–358. doi: 10.1097/00004356-199112000-00013. [DOI] [PubMed] [Google Scholar]
- 181.Jones A.Y.M., Hutchinson R.C. A comparison of the analgesic effect of transcutaneous electrical nerve stimulation and entonox. Physiotherapy. 1991;77:526–530. doi: 10.1016/S0031-9406(10)61868-8. [DOI] [Google Scholar]
- 182.Kara B., Baskurt F., Acar S., Karadibak D., Ciftci L., Erbayraktar S., Gokmen A.N. The effect of TENS on pain, function, depression, and analgesic consumption in the early postoperative period with spinal surgery patients. Turk. Neurosurg. 2011;21:618–624. doi: 10.5137/1019-5149.JTN.4985-11.0. [DOI] [PubMed] [Google Scholar]
- 183.Kararmaz A., Kaya S., Karaman H., Turhanoglu S. Effect of the frequency of transcutaneous electrical nerve stimulation on analgesia during extracorporeal shock wave lithotripsy. Urol. Res. 2004;32:411–415. doi: 10.1007/s00240-004-0438-2. [DOI] [PubMed] [Google Scholar]
- 184.Kayman-Kose S., Arioz D.T., Toktas H., Koken G., Kanat-Pektas M., Kose M., Yilmazer M. Transcutaneous electrical nerve stimulation (TENS) for pain control after vaginal delivery and cesarean section. J. Matern.-Fetal Neonatal. Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2014;27:1572–1575. doi: 10.3109/14767058.2013.870549. [DOI] [PubMed] [Google Scholar]
- 185.Keskin E.A., Onur O., Keskin H.L., Gumus I.I., Kafali H., Turhan N. Transcutaneous electrical nerve stimulation improves low back pain during pregnancy. Gynecol. Obstet. Investig. 2012;74:76–83. doi: 10.1159/000337720. [DOI] [PubMed] [Google Scholar]
- 186.Kibar S., Konak H.E., Av S., Erdogan B.D., Evcik D. The Effectiveness of Combined Transcutaneous Electrical Nerve Stimulation and Interferential Current Therapy on Chronic Low Back Pain: A Randomized, Double-Blind, Sham-Controlled Study. J. Phys. Med. Rehabil. Sci./Fiz. Tup Ve Rehabil. Bilimleri Derg. 2020;23:32–40. doi: 10.31609/jpmrs.2019-71464. [DOI] [Google Scholar]
- 187.Kim S., Park K., Son B., Jeon Y. The effect of transcutaneous electrical nerve stimulation on pain during venous cannulation. Curr. Ther. Res. Clin. Exp. 2012;73:134–139. doi: 10.1016/j.curtheres.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim D.H., Yoon K.B., Park S., Jin T.E., An Y.J., Schepis E.A., Yoon D.M. Comparison of NSAID patch given as monotherapy and NSAID patch in combination with transcutaneous electric nerve stimulation, a heating pad, or topical capsaicin in the treatment of patients with myofascial pain syndrome of the upper trapezius: A pilot study. Pain Med. 2014;15:2128–2138. doi: 10.1111/pme.12611. [DOI] [PubMed] [Google Scholar]
- 189.Kirupa K., Divya Mary S.M., Vaishnavi G., Nithya Nisha R., Rennie Mercy J., Jaiganesh G. A comparative study of ultrasound therapy and transcutaneous electrical nerve stimulation in reducing pain for temporomandibular joint disorder. Drug Invent. Today. 2019;12:515–517. [Google Scholar]
- 190.Knobel R., Radünz V., Carraro T.E.J.T. Use of transcutaneous electric nerve stimulation for pain relief in labour: A possible way to care the child-bearer. Texto Contexto-Enferm. 2005;14:229–236. doi: 10.1590/S0104-07072005000200010. [DOI] [Google Scholar]
- 191.Koca I., Boyaci A., Tutoglu A., Ucar M., Kocaturk O. Assessment of the effectiveness of interferential current therapy and TENS in the management of carpal tunnel syndrome: A randomized controlled study. Rheumatol. Int. 2014;34:1639–1645. doi: 10.1007/s00296-014-3005-3. [DOI] [PubMed] [Google Scholar]
- 192.Kofotolis N.D., Vlachopoulos S.P., Kellis E. Sequentially allocated clinical trial of rhythmic stabilization exercises and TENS in women with chronic low back pain. Clin. Rehabil. 2008;22:99–111. doi: 10.1177/0269215507080122. [DOI] [PubMed] [Google Scholar]
- 193.Koke A.J., Schouten J.S., Lamerichs-Geelen M.J., Lipsch J.S., Waltje E.M., van Kleef M., Patijn J. Pain reducing effect of three types of transcutaneous electrical nerve stimulation in patients with chronic pain: A randomized crossover trial. Pain. 2004;108:36–42. doi: 10.1016/j.pain.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 194.Korkmaz O., Capaci K., Eyigor C., Eyigor S. Pulsed radiofrequency versus conventional transcutaneous electrical nerve stimulation in painful shoulder: A prospective, randomized study. Clin. Rehabil. 2010;24:1000–1008. doi: 10.1177/0269215510371417. [DOI] [PubMed] [Google Scholar]
- 195.Kumar S., Raje A. Effect of progressive muscular relaxation exercises versus transcutaneous electrical nerve stimulation on tension headache: A comparative study. Hong Kong Physiother. J. 2014;32:86–91. doi: 10.1016/j.hkpj.2014.06.002. [DOI] [Google Scholar]
- 196.Labrecque M., Nouwen A., Bergeron M., Rancourt J.F. A randomized controlled trial of nonpharmacologic approaches for relief of low back pain during labor. J. Fam. Pract. 1999;48:259–263. [PubMed] [Google Scholar]
- 197.Laitinen J., Nuutinen L. Failure of transcutaneous electrical nerve stimulation and indomethacin to reduce opiate requirement following cholecystectomy. Acta Anaesthesiol. Scand. 1991;35:700–705. doi: 10.1111/j.1399-6576.1991.tb03375.x. [DOI] [PubMed] [Google Scholar]
- 198.Lang T., Barker R., Steinlechner B., Gustorff B., Puskas T., Gore O., Kober A. TENS relieves acute posttraumatic hip pain during emergency transport. J. Trauma. 2007;62:184–188. doi: 10.1097/01.ta.0000197176.75598.fc. [DOI] [PubMed] [Google Scholar]
- 199.Langley G.B., Sheppeard H., Johnson M., Wigley R.D. The analgesic effects of transcutaneous electrical nerve stimulation and placebo in chronic pain patients. A double-blind non-crossover comparison. Rheumatol. Int. 1984;4:119–123. doi: 10.1007/BF00541180. [DOI] [PubMed] [Google Scholar]
- 200.Lauretti G.R., Chubaci E.F., Mattos A.L. Efficacy of the use of two simultaneously TENS devices for fibromyalgia pain. Rheumatol. Int. 2013;33:2117–2122. doi: 10.1007/s00296-013-2699-y. [DOI] [PubMed] [Google Scholar]
- 201.Lauretti G.R., Oliveira R., Parada F., Mattos A.L. The New Portable Transcutaneous Electrical Nerve Stimulation Device Was Efficacious in the Control of Primary Dysmenorrhea Cramp Pain. Neuromodulation. 2015;18:522–527. doi: 10.1111/ner.12269. [DOI] [PubMed] [Google Scholar]
- 202.Law P.P., Cheing G.L. Optimal stimulation frequency of transcutaneous electrical nerve stimulation on people with knee osteoarthritis. J. Rehabil. Med. 2004;36:220–225. doi: 10.1080/16501970410029834. [DOI] [PubMed] [Google Scholar]
- 203.Law P.P.W., Cheing G.L.Y., Tsui A.Y.Y. Does transcutaneous electrical nerve stimulation improve the physical performance of people with knee osteoarthritis? J. Clin. Rheumatol. 2004;10:295–299. doi: 10.1097/01.rhu.0000147047.77460.b0. [DOI] [PubMed] [Google Scholar]
- 204.Leandri M., Parodi C., Corrieri N., Rigardo S. Comparison of TENS treatments in hemiplegic shoulder pain. Scand. J. Rehabil. Med. 1990;22:69–71. [PubMed] [Google Scholar]
- 205.Lee E., Chung I., Lee J., Lam P., Chin R. The role of transcutaneous electrical nerve stimulation in management of labour in obstetric patients. Asia Ocean. J. Obstet. Gynaecol. 1990;16:247–254. doi: 10.1111/j.1447-0756.1990.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 206.Lee C.H., Lee T.Y., Her J.S., Liao W.L., Hsieh C.L. Single-Blinded, Randomized Preliminary Study Evaluating the Effect of Transcutaneous Electrical Nerve Stimulation on Postoperative Pain in Patients with Colles’ Fracture. J. Altern. Complement. Med. 2015;21:754–758. doi: 10.1089/acm.2015.0119. [DOI] [PubMed] [Google Scholar]
- 207.Lee J.E., Anderson C.M., Perkhounkova Y., Sleeuwenhoek B.M., Louison R.R. Transcutaneous electrical nerve stimulation reduces resting pain in head and neck cancer patients: A randomized and placebo-controlled double-blind pilot study. Cancer Nurs. 2019;42:218–228. doi: 10.1097/NCC.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 208.Leo K., Dostal W., Bossen D., Eldridge V., Fairchild M., Evans R. Effect of transcutaneous electrical nerve stimulation characteristics on clinical pain. Phys. Ther. 1986;66:200–205. doi: 10.1093/ptj/66.2.200. [DOI] [PubMed] [Google Scholar]
- 209.Leonard G., Cloutier C., Marchand S. Reduced Analgesic Effect of Acupuncture-like TENS but Not Conventional TENS in Opioid-Treated Patients. J. Pain. 2011;12:213–221. doi: 10.1016/j.jpain.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 210.Lewers D., Clelland J., Jackson J., Varner R., Bergman J. Transcutaneous electrical nerve stimulation in the relief of primary dysmenorrhea. Phys. Ther. 1989;69:3–9. doi: 10.1093/ptj/69.1.3. [DOI] [PubMed] [Google Scholar]
- 211.Lewis D., Lewis B., Sturrock R. Transcutaneous electrical nerve stimulation in osteoarthrosis: A therapeutic alternative? Ann. Rheum. Dis. 1984;43:47–49. doi: 10.1136/ard.43.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lewis B., Lewis D., Cumming G. The comparative analgesic efficacy of transcutaneous electrical nerve stimulation and a non-steroidal anti-inflammatory drug for painful osteoarthritis. Br. J. Rheumatol. 1994;33:455–460. doi: 10.1093/rheumatology/33.5.455. [DOI] [PubMed] [Google Scholar]
- 213.Likar R., Molnar M., Pipam W., Koppert W., Quantschnigg B., Disselhoff B., Sittl R. Postoperative transcutaneous electrical nerve stimulation (TENS) in shoulder surgery (randomized, double blind, placebo controlled pilot trial) Schmerz. 2001;15:158–163. doi: 10.1007/s004820170017. [DOI] [PubMed] [Google Scholar]
- 214.Lim A.T., Edis G., Kranz H., Mendelson G., Selwood T., Scott D.F. Postoperative pain control: Contribution of psychological factors and transcutaneous electrical stimulation. Pain. 1983;17:179–188. doi: 10.1016/0304-3959(83)90141-0. [DOI] [PubMed] [Google Scholar]
- 215.Lima P.M., Farias R.T., Carvalho A.C., da Silva P.N., Ferraz Filho N.A., de Brito R.F. Transcutaneous electrical nerve stimulation after coronary artery bypass graft surgery. Rev. Bras. Cir. Cardiovasc. 2011;26:591–596. doi: 10.5935/1678-9741.20110049. [DOI] [PubMed] [Google Scholar]
- 216.Limoges M.F., Rickabaugh B. Evaluation of TENS during screening flexible sigmoidoscopy. Gastroenterol. Nurs. 2004;27:61–68. doi: 10.1097/00001610-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 217.Lin M.L., Luo Y.J., Hsu Y.M., Guo C.H., Lin S.C., Lin K.Y., Lin S.H., Chiu H.W. IEEE Randomized controlled trial Comparing of Analgesic Effectiveness of TPRF and TENs in Clinical and Endocrinological Changes for Chronic Shoulder Pain; Proceedings of the 2015 International Symposium on Bioelectronics and Bioinformatics (ISBB); Beijing, China. 14–17 October 2015; pp. 23–26. [Google Scholar]
- 218.Lin M.-L., Chiu H.-W., Shih Z.-M., Lee P.-Y., Li P.-Z., Guo C.-H., Luo Y.-J., Lin S.-C., Lin K.-Y., Hsu Y.-M., et al. Two Transcutaneous Stimulation Techniques in Shoulder Pain: Transcutaneous Pulsed Radiofrequency (TPRF) versus Transcutaneous Electrical Nerve Stimulation (TENS): A Comparative Pilot Study. Pain Res. Manag. 2019;2019:2823401. doi: 10.1155/2019/2823401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Linde C., Isacsson G., Jonsson B. Outcome of 6-week treatment with transcutaneous electric nerve stimulation compared with splint on symptomatic temporomandibular joint disk displacement without reduction. Acta Odontol. Scand. 1995;53:92–98. doi: 10.3109/00016359509005953. [DOI] [PubMed] [Google Scholar]
- 220.Linn S.L., Granat M.H., Lees K.R. Prevention of shoulder subluxation after stroke with electrical stimulation. Stroke. 1999;30:963–968. doi: 10.1161/01.STR.30.5.963. [DOI] [PubMed] [Google Scholar]
- 221.Lison J.F., Amer-Cuenca J.J., Benavent-Caballer V., Bivia-Roig G., Piquer-Marti S., Marin-Buck A. Transcutaneous Nerve Stimulation for Pain Relief During Office Hysteroscopy: A Randomized Controlled Trial In Reply. Obstet. Gynecol. 2017;129:1141. doi: 10.1097/AOG.0000000000001842. [DOI] [PubMed] [Google Scholar]
- 222.Liu Y., Liao W., Lien I. Effect of transcutaneous electrical nerve stimulation for post-thoracotomic pain. Taiwan Yi Xue Hui Za Zhi. J. Formos. Med. Assoc. 1985;84:801–809. [PubMed] [Google Scholar]
- 223.Liu Y., Dong Z., Wang R., Ao R., Han X., Tang W., Yu S. Migraine Prevention Using Different Frequencies of Transcutaneous Occipital Nerve Stimulation: A Randomized Controlled Trial. J. Pain. 2017;18:1006–1015. doi: 10.1016/j.jpain.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 224.Lofgren M., Norrbrink C. Pain relief in women with fibromyalgia: A cross-over study of superficial warmth stimulation and transcutaneous electrical nerve stimulation. J. Rehabil. Med. 2009;41:557–562. doi: 10.2340/16501977-0371. [DOI] [PubMed] [Google Scholar]
- 225.Luchesa C.A., Greca F.H., Guarita-Souza L.C., dos Santos J.L., Aquim E.E. The role of electroanalgesia in patients undergoing coronary artery bypass surgery. Rev. Bras. Cir. Cardiovasc. 2009;24:391–396. doi: 10.1590/S0102-76382009000400020. [DOI] [PubMed] [Google Scholar]
- 226.Lundeberg T. A comparative study of the pain alleviating effect of vibratory stimulation, transcutaneous electrical nerve stimulation, electroacupuncture and placebo. Am. J. Chin. Med. 1984;12:72–79. doi: 10.1142/S0192415X84000088. [DOI] [PubMed] [Google Scholar]
- 227.Lundeberg T., Bondesson L., Lundstrom V. Relief of primary dysmenorrhea by transcutaneous electrical nerve stimulation. Acta Obstet. Gynecol. Scand. 1985;64:491–497. doi: 10.3109/00016348509156727. [DOI] [PubMed] [Google Scholar]
- 228.Machado A.F.P., Perracini M.R., Rampazo É.P., Driusso P., Liebano R.E. Effects of thermotherapy and transcutaneous electrical nerve stimulation on patients with primary dysmenorrhea: A randomized, placebo-controlled, double-blind clinical trial. Complement. Ther. Med. 2019;47:102188. doi: 10.1016/j.ctim.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 229.Machin D., Lewith G.T., Wylson S. Pain Measurement in Randomized Clinical Trials: A Comparison of Two Pain Scales. Clin. J. Pain. 1988;4:161–168. doi: 10.1097/00002508-198809000-00006. [DOI] [Google Scholar]
- 230.Mahure S.A., Rokito A.S., Kwon Y.W. Transcutaneous electrical nerve stimulation for postoperative pain relief after arthroscopic rotator cuff repair: A prospective double-blinded randomized trial. J. Shoulder Elbow Surg. 2017;26:1508–1513. doi: 10.1016/j.jse.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 231.Manigandan J.B., Ganesh G.S., Pattnaik M., Mohanty P. Effect of electrical stimulation to long head of biceps in reducing gleno humeral subluxation after stroke. Neurorehabilitation. 2014;34:245–252. doi: 10.3233/NRE-131041. [DOI] [PubMed] [Google Scholar]
- 232.Mannheimer C., Carlsson C. The analgesic effect of transcutaneous electrical nerve stimulation (TNS) in patients with rheumatoid arthritis. A comparative study of different pulse pattern. Pain. 1979;6:329–334. doi: 10.1016/0304-3959(79)90051-4. [DOI] [PubMed] [Google Scholar]
- 233.Mannheimer J., Whalen E. The efficacy of transcutaneous electric nerve stimulation in dysmenorrhea. Clin. J. Pain. 1985;1:75–83. doi: 10.1097/00002508-198501020-00004. [DOI] [Google Scholar]
- 234.Mannheimer C., Lund S., Carlsson C. The effect of transcutaneous electrical nerve stimulation (TNS) on joint pain in patients with rheumatoid arthritis. Scand. J. Rheumatol. 1978;7:13–16. [PubMed] [Google Scholar]
- 235.Mannheimer C., Carlsson C., Emanuelsson H., Vedin A., Waagstein F., Wilhelmsson C. The effects of transcutaneous electrical nerve stimulation in patients with severe angina pectoris. Circulation. 1985;71:308–316. doi: 10.1161/01.CIR.71.2.308. [DOI] [PubMed] [Google Scholar]
- 236.Mansourian A., Pourshahidi S., Sadrzadeh-Afshar M.S., Ebrahimi H. A Comparative Study of Low-Level Laser Therapy and Transcutaneous Electrical Nerve Stimulation as an Adjunct to Pharmaceutical Therapy for Myofascial Pain Dysfunction Syndrome: A Randomized Clinical Trial. Front. Dent. 2019;16:256–264. doi: 10.18502/fid.v16i4.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mansuri B., Torabinezhad F., Jamshidi A.A., Dabirmoghadam P., Vasaghi-Gharamaleki B., Ghelichi L. Application of High-Frequency Transcutaneous Electrical Nerve Stimulation in Muscle Tension Dysphonia Patients With the Pain Complaint: The Immediate Effect. J. Voice. 2019;34:657–666. doi: 10.1016/j.jvoice.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 238.Mansuri B., Torabinejhad F., Jamshidi A.A., Dabirmoghaddam P., Vasaghi-Gharamaleki B., Ghelichi L. Transcutaneous Electrical Nerve Stimulation Combined with Voice Therapy in Women With Muscle Tension Dysphonia. J. Voice. 2020;34:490.e11–490.e21. doi: 10.1016/j.jvoice.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 239.Marchand S., Charest J., Li J., Chenard J.R., Lavignolle B., Laurencelle L. Is TENS purely a placebo effect? A controlled study on chronic low back pain. Pain. 1993;54:99–106. doi: 10.1016/0304-3959(93)90104-W. [DOI] [PubMed] [Google Scholar]
- 240.Mascarin N.C., Vancini R.L., Andrade M.D., Magalhaes E.D., de Lira C.A.B., Coimbra I.B. Effects of kinesiotherapy, ultrasound and electrotherapy in management of bilateral knee osteoarthritis: Prospective clinical trial. BMC Musculoskelet. Disord. 2012;13:182. doi: 10.1186/1471-2474-13-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.McCallum M., Glynn C., Moore R., Lammer P., Phillips A. Transcutaneous electrical nerve stimulation in the management of acute postoperative pain. Br. J. Anaesth. 1988;61:308–312. doi: 10.1093/bja/61.3.308. [DOI] [PubMed] [Google Scholar]
- 242.Melzack R., Vetere P., Finch L. Transcutaneous electrical nerve stimulation for low back pain. A comparison of TENS and massage for pain and range of motion. Phys. Ther. 1983;63:489–493. doi: 10.1093/ptj/63.4.489. [DOI] [PubMed] [Google Scholar]
- 243.Merrill D. Clinical evaluation of FasTENS, an inexpensive, disposable transcutaneous electrical nerve stimulator designed specifically for postoperative electroanalgesia. Urology. 1989;33:27–30. doi: 10.1016/0090-4295(89)90061-7. [DOI] [PubMed] [Google Scholar]
- 244.Miller L., Mattison P., Paul L., Wood L. The effects of transcutaneous electrical nerve stimulation (TENS) on spasticity in multiple sclerosis. Mult. Scler. 2007;13:527–533. doi: 10.1177/1352458506071509. [DOI] [PubMed] [Google Scholar]
- 245.Milsom I., Hedner N., Mannheimer C. A comparative study of the effect of high-intensity transcutaneous nerve stimulation and oral naproxen on intrauterine pressure and menstrual pain in patients with primary dysmenorrhea. Am. J. Obstet. Gynecol. 1994;170:123–129. doi: 10.1016/S0002-9378(13)70292-8. [DOI] [PubMed] [Google Scholar]
- 246.Moharic M., Marincek C., Vidmar G., Burger H. Transcutaneous electrical nerve stimulation, pregabalin and their combination in patients with painful diabetic neuropathy: Effects on pain and quality of life. Zdr. Vestn.-Slov. Med. J. 2009;78:371–380. [Google Scholar]
- 247.Mondal P., Biswas M., Saha J., Middhya A.K. Effect of Trigger Point Injection vs. Ultrasonic Therapy vs. Transcutaneous Electrical Nerve Stimulation in Rehabilitation of Cervical and Peri-Scapular Myofascial Pain Syndrome—A Randomized Clinical Trial. J. Evol. Med. Dent. Sci. 2019;8:430–436. doi: 10.14260/jemds/2019/95. [DOI] [Google Scholar]
- 248.Moore S., Shurman J. Combined neuromuscular electrical stimulation and transcutaneous electrical nerve stimulation for treatment of chronic back pain: A double-blind, repeated measures comparison. Arch. Phys. Med. Rehabil. 1997;78:55–60. doi: 10.1016/S0003-9993(97)90010-1. [DOI] [PubMed] [Google Scholar]
- 249.Mora B., Giorni E., Dobrovits M., Barker R., Lang T., Gore C., Kober A. Transcutaneous electrical nerve stimulation: An effective treatment for pain caused by renal colic in emergency care. J. Urol. 2006;175:1737–1741. doi: 10.1016/S0022-5347(05)00980-8. [DOI] [PubMed] [Google Scholar]
- 250.Morgan B., Jones A.R., Mulcahy K.A., Finlay D.B., Collett B. Transcutaneous electric nerve stimulation (TENS) during distension shoulder arthrography: A controlled trial. Pain. 1996;64:265–267. doi: 10.1016/0304-3959(95)00107-7. [DOI] [PubMed] [Google Scholar]
- 251.Møystad A., Krogstad B.S., Larheim T.A. Transcutaneous nerve stimulation in a group of patients with rheumatic disease involving the temporomandibular joint. J. Prosthet. Dent. 1990;64:596–600. doi: 10.1016/0022-3913(90)90135-Y. [DOI] [PubMed] [Google Scholar]
- 252.Murray S., Collins P.D., James M.A. An investigation into the ‘carry over’ effect of neurostimulation in the treatment of angina pectoris. Int. J. Clin. Pract. 2004;58:669–674. doi: 10.1111/j.1368-5031.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 253.Mutlu B., Paker N., Bugdayci D., Tekdos D., Kesiktas N. Efficacy of supervised exercise combined with transcutaneous electrical nerve stimulation in women with fibromyalgia: A prospective controlled study. Rheumatol. Int. 2013;33:649–655. doi: 10.1007/s00296-012-2390-8. [DOI] [PubMed] [Google Scholar]
- 254.Nabi B.N., Sedighinejad A., Haghighi M., Biazar G., Hashemi M., Haddadi S., Fathi A. Comparison of transcutaneous electrical nerve stimulation and pulsed radiofrequency sympathectomy for treating painful diabetic neuropathy. Anesthesiol. Pain Med. 2015;5:e29280. doi: 10.5812/aapm.29280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nash T., Williams J., Machin D. TENS: Does the type of stimulus really matter? Pain Clin. 1990;3:161–168. [Google Scholar]
- 256.Navarathnam R.G., Wang I.Y., Thomas D., Klineberg P.L. Evaluation of the transcutaneous electrical nerve stimulator for postoperative analgesia following cardiac surgery. Anaesth. Intensive Care. 1984;12:345–350. doi: 10.1177/0310057X8401200411. [DOI] [PubMed] [Google Scholar]
- 257.Neary J. Transcutaneous electrical nerve stimulation for the relief of post-incisional surgical pain. AANA J. 1981;49:151–155. [PubMed] [Google Scholar]
- 258.Neighbours L.E., Clelland J., Jackson J.R., Bergman J., Orr J. Transcutaneous electrical nerve stimulation for pain relief in primary dysmenorrhea. Clin. J. Pain. 1987;3:17–22. doi: 10.1097/00002508-198703010-00004. [DOI] [Google Scholar]
- 259.Nesheim B. The use of transcutaneous electrical nerve stimulation for pain relief during labour. A controlled clinical study. Acta Obstet. Gynecol. 1981;60:13–16. doi: 10.3109/00016348109154102. [DOI] [PubMed] [Google Scholar]
- 260.Neumark J., Pauser G., Scherzer W. Pain relief in childbirth; an analysis of the analgesic effects of transcutaneous nerve stimulation (TNS), pethidine and placebos (author’s transl) Prakt. Anaesth. 1978;13:13–20. [PubMed] [Google Scholar]
- 261.Ng M., Leung M., Poon D. The effects of electro-acupuncture and transcutaneous electrical nerve stimulation on patients with painful osteoarthritic knees: A randomized controlled trial with follow-up evaluation. J. Altern. Complement. Med. 2003;9:641–649. doi: 10.1089/107555303322524490. [DOI] [PubMed] [Google Scholar]
- 262.Nordemar R., Thorner C. Treatment of acute cervical pain—A comparative group study. Pain. 1981;10:93–101. doi: 10.1016/0304-3959(81)90050-6. [DOI] [PubMed] [Google Scholar]
- 263.Norrbrink C. Transcutaneous electrical nerve stimulation for treatment of spinal cord injury neuropathic pain. J. Rehabil. Res. Dev. 2009;46:85–93. doi: 10.1682/JRRD.2008.04.0051. [DOI] [PubMed] [Google Scholar]
- 264.Olsen M.F., Elden H., Janson E.D., Lilja H., Stener-Victorin E. A comparison of high- versus low-intensity, high-frequency transcutaneous electric nerve stimulation for painful postpartum uterine contractions. Acta Obstet. Gynecol. Scand. 2007;86:310–314. doi: 10.1080/00016340601040928. [DOI] [PubMed] [Google Scholar]
- 265.Fagevik Olsen M., Bjorndahl S., Stahl A., Borjesson S., Sundemo A., Gutke A. Effects of high-intensity high-frequency transcutaneous electric nerve stimulation in primary dysmenorrhea-a randomised cross-over pilot study. Eur. J. Physiother. 2019;22:248–252. doi: 10.1080/21679169.2019.1585945. [DOI] [Google Scholar]
- 266.Oncel M., Sencan S., Yildiz H., Kurt N. Transcutaneous electrical nerve stimulation for pain management in patients with uncomplicated minor rib fractures. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2002;22:13–17. doi: 10.1016/S1010-7940(02)00206-3. [DOI] [PubMed] [Google Scholar]
- 267.Oosterhof J., De Boo T.M., Oostendorp R.A., Wilder-Smith O.H., Crul B.J. Outcome of transcutaneous electrical nerve stimulation in chronic pain: Short-term results of a double-blind, randomised, placebo-controlled trial. J. Headache Pain. 2006;7:196–205. doi: 10.1007/s10194-006-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ordog G.J. Transcutaneous electrical nerve stimulation versus oral analgesic: A randomized double-blind controlled study in acute traumatic pain. Am. J. Emerg. Med. 1987;5:6–10. doi: 10.1016/0735-6757(87)90281-6. [DOI] [PubMed] [Google Scholar]
- 269.Ozkaraoglu D.K., Tarakci D., Algun Z.C. Comparison of two different electrotherapy methods in low back pain treatment. J. Back Musculoskelet. Rehabil. 2020;33:193–199. doi: 10.3233/BMR-181199. [DOI] [PubMed] [Google Scholar]
- 270.Ozkul C., Kilinc M., Yildirim S.A., Topcuoglu E.Y., Akyuz M. Effects of visual illusion and transcutaneous electrical nerve stimulation on neuropathic pain in patients with spinal cord injury: A randomised controlled cross-over trial. J. Back Musculoskelet. Rehabil. 2015;28:709–719. doi: 10.3233/BMR-140573. [DOI] [PubMed] [Google Scholar]
- 271.Oztas B., Iyigun E. The effects of two different electrical stimulation methods on the pain intensity of the patients who had undergone abdominal surgery with a midline incision: Randomized Controlled Clinical Trial. Contemp. Nurse. 2019;55:122–138. doi: 10.1080/10376178.2019.1628650. [DOI] [PubMed] [Google Scholar]
- 272.Ozturk N.K., Baki E.D., Kavakli A.S., Sahin A.S., Ayoglu R.U., Karaveli A., Emmiler M., Inanoglu K., Karsli B. Comparison of Transcutaneous Electrical Nerve Stimulation and Parasternal Block for Postoperative Pain Management after Cardiac Surgery. Pain Res. Manag. 2016;2016:4261949. doi: 10.1155/2016/4261949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Padma, Prasanna A., Urala Transcutaneous electrical nerve stimulation and labor pain. Bahrain Med. Bull. 2000;22:13–17. [Google Scholar]
- 274.Paker N., Tekdos D., Kesiktas N., Soy D. Comparison of the therapeutic efficacy of TENS versus intra-articular hyaluronic acid injection in patients with knee osteoarthritis: A prospective randomized study. Adv. Ther. 2006;23:342–353. doi: 10.1007/BF02850139. [DOI] [PubMed] [Google Scholar]
- 275.Palmer S., Domaille M., Cramp F., Walsh N., Pollock J., Kirwan J., Johnson M.I. Transcutaneous electrical nerve stimulation as an adjunct to education and exercise for knee osteoarthritis: A randomized controlled trial. Arthritis Care Res. 2014;66:387–394. doi: 10.1002/acr.22147. [DOI] [PubMed] [Google Scholar]
- 276.Pan P., Chou C., Chiou H., Ma H., Lee H., Chan R. Extracorporeal shock wave therapy for chronic calcific tendinitis of the shoulders: A functional and sonographic study. Arch. Phys. Med. Rehabil. 2003;84:988–993. doi: 10.1016/S0003-9993(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 277.Park C., Choi J.B., Lee Y.S., Chang H.S., Shin C.S., Kim S., Han D.W. The effect of intra-operative transcutaneous electrical nerve stimulation on posterior neck pain following thyroidectomy. Anaesthesia. 2015;70:434–439. doi: 10.1111/anae.12933. [DOI] [PubMed] [Google Scholar]
- 278.Patil S.R., Aileni K.R. Effect of transcutaneous electrical nerve stimulation versus home exercise programme in management of temporomandibular joint disorder. J. Clin. Diagn. Res. 2017;11:ZC19–ZC22. doi: 10.7860/JCDR/2017/32761.10967. [DOI] [Google Scholar]
- 279.Peacock K.S., Stoerkel E., Libretto S., Zhang W., Inman A., Schlicher M., Cowsar J.D., Jr., Eddie D., Walter J. A randomized trial comparing the Tennant Biomodulator to transcutaneous electrical nerve stimulation and traditional Chinese acupuncture for the treatment of chronic pain in military service members. Mil. Med. Res. 2019;6:37. doi: 10.1186/s40779-019-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Pietrosimone B.G., Hart J.M., Saliba S.A., Hertel J., Ingersoll C.D. Immediate effects of transcutaneous electrical nerve stimulation and focal knee joint cooling on quadriceps activation. Med. Sci. Sports Exerc. 2009;41:1175–1181. doi: 10.1249/MSS.0b013e3181982557. [DOI] [PubMed] [Google Scholar]
- 281.Pietrosimone B.G., Saliba S.A., Hart J.M., Hertel J., Kerrigan D.C., Ingersoll C.D. Effects of transcutaneous electrical nerve stimulation and therapeutic exercise on quadriceps activation in people with tibiofemoral osteoarthritis. J. Orthop. Sports Phys. Ther. 2011;41:4–12. doi: 10.2519/jospt.2011.3447. [DOI] [PubMed] [Google Scholar]
- 282.Pietrosimone B., Luc-Harkey B.A., Harkey M.S., Davis-Wilson H.C., Pfeiffer S.J., Schwartz T.A., Nissman D., Padua D.A., Blackburn J.T., Spang J.T. Using TENS to Enhance Therapeutic Exercise in Individuals with Knee Osteoarthritis. Med. Sci. Sports Exerc. 2020;52:2086–2095. doi: 10.1249/MSS.0000000000002353. [DOI] [PubMed] [Google Scholar]
- 283.Pike P.M. Transcutaneous electrical stimulation. Its use in the management of postoperative pain. Anaesthesia. 1978;33:165–171. doi: 10.1111/j.1365-2044.1978.tb08344.x. [DOI] [PubMed] [Google Scholar]
- 284.Pitangui A.C., de Sousa L., Gomes F.A., Ferreira C.H., Nakano A.M. High-frequency TENS in post-episiotomy pain relief in primiparous puerpere: A randomized, controlled trial. J. Obstet. Gynaecol. Res. 2012;38:980–987. doi: 10.1111/j.1447-0756.2011.01824.x. [DOI] [PubMed] [Google Scholar]
- 285.Pitangui A.C., Araujo R.C., Bezerra M.J., Ribeiro C.O., Nakano A.M. Low and high-frequency TENS in post-episiotomy pain relief: A randomized, double-blind clinical trial. Braz. J. Phys. Ther. 2014;18:72–78. doi: 10.1590/S1413-35552012005000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Platon B., Andrell P., Raner C., Rudolph M., Dvoretsky A., Mannheimer C. High-frequency, high-intensity transcutaneous electrical nerve stimulation as treatment of pain after surgical abortion. Pain. 2010;148:114–119. doi: 10.1016/j.pain.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 287.Platon B., Mannheimer C., Andrell P. Effects of high-frequency, high-intensity transcutaneous electrical nerve stimulation versus intravenous opioids for pain relief after gynecologic laparoscopic surgery: A randomized controlled study. Korean J. Anesthesiol. 2018;71:149–156. doi: 10.4097/kjae.2018.71.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Prabhakar R., Ramteke G. Cervical Spinal Mobilization Versus TENS in the Management of Cervical Radiculopathy: A Comparative, Experimental, Randomized controlled trial. Indian J. Physiother. Occup. Ther. 2011;5:128–133. [Google Scholar]
- 289.Presser M., Birkhan J., Adler R., Hanani A., Eisenberg E. Transcutaneous electrical nerve stimulation (TENS) during epidural steroids injection: A randomized controlled trial. Pain Clin. 2000;12:77–80. doi: 10.1163/156856900750229816. [DOI] [Google Scholar]
- 290.Rainov N.G., Heidecke V., Albertz C., Burkert W. Transcutaneous electrical nerve stimulation (TENS) for acute postoperative pain after spinal surgery. Eur. J. Pain. 1994;15:44–49. [Google Scholar]
- 291.Rajfur J., Pasternok M., Rajfur K., Walewicz K., Fras B., Bolach B., Dymarek R., Rosinczuk J., Halski T., Taradaj J. Efficacy of Selected Electrical Therapies on Chronic Low Back Pain: A Comparative Clinical Pilot Study. Med. Sci. Monit. 2017;23:85–100. doi: 10.12659/MSM.899461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Rajpurohit B., Khatri S., Metgud D., Bagewadi A. Effectiveness of transcutaneous electrical nerve stimulation and microcurrent electrical nerve stimulation in bruxism associated with masticatory muscle pain—A comparative study. Indian J. Dent. Res. 2010;21:104–106. doi: 10.4103/0970-9290.62816. [DOI] [PubMed] [Google Scholar]
- 293.Rakel B., Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J. Pain. 2003;4:455–464. doi: 10.1067/S1526-5900(03)00780-6. [DOI] [PubMed] [Google Scholar]
- 294.Rakel B.A., Zimmerman M.B., Geasland K., Embree J., Clark C.R., Noiseux N.O., Callaghan J.J., Herr K., Walsh D., Sluka K.A. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: A randomized, blinded, placebo-controlled trial. Pain. 2014;155:2599–2611. doi: 10.1016/j.pain.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ramanathan D., Saleh A., Klika A., Higuera C., Barsoum W.J.S.T.I. The Use of Transcutaneous Electrical Nerve Stimulation After Total Knee Arthroplasty: A Prospective Randomized Controlled Trial. Surg. Technol. Int. 2017;30:425–434. [PubMed] [Google Scholar]
- 296.Ramos L.A.V., Callegari B., Franca F.J.R., Magalhaes M.O., Burke T.N., Carvalho e Silva A.P.D.M.C., Almeida G.P.L., Comachio J., Marques A.P. Comparison Between Transcutaneous Electrical Nerve Stimulation and Stabilization Exercises in Fatigue and Transversus Abdominis Activation in Patients with Lumbar Disk Herniation: A Randomized Study. J. Manip. Physiol. Ther. 2018;41:323–331. doi: 10.1016/j.jmpt.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 297.Rani P., Kalyani V., Goyal T., Yadav R., Mishra R. Effect of Transcutaneous Electrical Nerve Stimulation Therapy on Pain and Functional Disability Level among Patients with Rotator Cuff Disease—A Randomized Controlled Trial. Int. J. Physiother. 2020;7:7–13. doi: 10.15621/ijphy/2020/v7i1/193667. [DOI] [Google Scholar]
- 298.Ratajczak B., Hawrylak A., Demidas A., Kuciel-Lewandowska J., Boerner E. Effectiveness of diadynamic currents and transcutaneous electrical nerve stimulation in disc disease lumbar part of spine. J. Back Musculoskelet. Rehabil. 2011;24:155–159. doi: 10.3233/BMR-2011-0289. [DOI] [PubMed] [Google Scholar]
- 299.Rawat B., Genz A., Fache J.S., Ong M., Coldman A.J., Burhenne H.J. Effectiveness of transcutaneous electrical nerve stimulation (TENS) for analgesia during biliary lithotripsy. Investig. Radiol. 1991;26:866–869. doi: 10.1097/00004424-199110000-00004. [DOI] [PubMed] [Google Scholar]
- 300.Renovato Franca F.J., Callegari B., Vidal Ramos L.A., Burke T.N., Magalhaes M.O., Comachio J., Moura Campos CarvalhoSilva A.P., Leao Almeida G.P., Marques A.P. Motor Control Training Compared with Transcutaneous Electrical Nerve Stimulation in Patients with Disc Herniation With Associated Radiculopathy A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2019;98:207–214. doi: 10.1097/PHM.0000000000001048. [DOI] [PubMed] [Google Scholar]
- 301.Reuss R., Cronen P., Abplanalp L. Transcutaneous electrical nerve stimulation for pain control after cholecystectomy: Lack of expected benefits. South. Med. J. 1988;81:1361–1363. doi: 10.1097/00132586-198908000-00026. [DOI] [PubMed] [Google Scholar]
- 302.Revadkar M.T., Bhojwani T.M. Comparison of the Effectiveness of Transcutaneous Electrical Nerve Stimulation (Tens) Vs. Interferential Therapy (Ift) for Relief of Pain in Primary Dysmenorrhea. Int. J. Physiother. 2019;6:263–267. doi: 10.15621/ijphy/2019/v6i6/190223. [DOI] [Google Scholar]
- 303.Ringel G., Taubert K. Zur effektivitat der transkutanen elektrischen nervenstimulation bei migrane (The efficacy of transcutaneous nerve stimulation in migraine) Z. Physiother. 1991;43:8–11. (In German) [Google Scholar]
- 304.Robb K., Newham D., Williams J. Transcutaneous Electrical Nerve Stimulation vs. Transcutaneous Spinal Electroanalgesia for Chronic Pain Associated with Breast Cancer Treatments. J. Pain Symptom Manag. 2007;33:410–419. doi: 10.1016/j.jpainsymman.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 305.Robinson R., Darlow S., Wright S.J., Watters C., Carr I., Gadsby G., Mayberry J. Is transcutaneous electrical nerve stimulation an effective analgesia during colonoscopy? Postgrad. Med. J. 2001;77:445–446. doi: 10.1136/pmj.77.909.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Roche P.A., Gijsbers K., Belch J.J., Forbes C.D. Modification of haemophiliac haemorrhage pain by transcutaneous electrical nerve stimulation. Pain. 1985;21:43–48. doi: 10.1016/0304-3959(85)90075-2. [DOI] [PubMed] [Google Scholar]
- 307.Rooney S.M., Jain S., Goldiner P.L. Effect of transcutaneous nerve stimulation on postoperative pain after thoracotomy. Anesth. Analg. 1983;62:1010–1012. doi: 10.1213/00000539-198311000-00010. [DOI] [PubMed] [Google Scholar]
- 308.Rosenberg M., Curtis L., Bourke D. Transcutaneous electrical nerve stimulation for the relief of postoperative pain. Pain. 1978;5:129–133. doi: 10.1016/0304-3959(78)90034-9. [DOI] [PubMed] [Google Scholar]
- 309.Rutgers M., Van-Romunde L., Osman P. A small randomised comparative trial of acupuncture verses transcutaneous electrical neurostimulation in postherpetic neuralgia. Pain Clin. 1988;2:87–89. [Google Scholar]
- 310.Sadala A.Y., Machado A.F.P., Liebano R.E. Effects of transcutaneous electrical nerve stimulation on pain intensity during application of carboxytherapy in patients with cellulite: A randomized placebo-controlled trial. J. Cosmet. Dermatol. 2018;17:1175–1181. doi: 10.1111/jocd.12489. [DOI] [PubMed] [Google Scholar]
- 311.Sahin N., Albayrak I., Ugurlu H. Effect of Different Transcutaneous Electrical Stimulation Modalities on Cervical Myofascial Pain Syndrome. J. Musculoskelet. Pain. 2011;19:18–23. doi: 10.3109/10582452.2010.538825. [DOI] [Google Scholar]
- 312.Samadzadeh S., Rezavand N., Yari M., Rezaei M., Faizmahdavi H., Hematti M. Comparison of Entonox and Transcutaneous Electrical Nerve Stim-ulation (TENS) in labor pain. J. Med. Biomed. Sci. 2017;6:11–16. doi: 10.4314/jmbs.v6i2.2. [DOI] [Google Scholar]
- 313.Sangtong K., Chupinijrobkob C., Putthakumnerd W., Kuptniratsaikul V. Does adding transcutaneous electrical nerve stimulation to therapeutic ultrasound affect pain or function in people with osteoarthritis of the knee? A randomized controlled trial. Clin. Rehabil. 2019;33:1197–1205. doi: 10.1177/0269215519838017. [DOI] [PubMed] [Google Scholar]
- 314.Santamato A., Notarnicola A., Panza F., Ranieri M., Micello M.F., Manganotti P., Moretti B., Fortunato F., Filoni S., Fiore P. SBOTE study: Extracorporeal shock wave therapy versus electrical stimulation after botulinum toxin type a injection for post-stroke spasticity-a prospective randomized trial. Ultrasound Med. Biol. 2013;39:283–291. doi: 10.1016/j.ultrasmedbio.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 315.Santana L.S., Gallo R.B., Ferreira C.H., Duarte G., Quintana S.M., Marcolin A.C. Transcutaneous electrical nerve stimulation (TENS) reduces pain and postpones the need for pharmacological analgesia during labour: A randomised trial. J. Physiother. 2016;62:29–34. doi: 10.1016/j.jphys.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 316.Saranya B., Ahmed J., Shenoy N., Ongole R., Sujir N., Natarajan S. Comparison of Transcutaneous Electric Nerve Stimulation (TENS) and Microcurrent Nerve Stimulation (MENS) in the Management of Masticatory Muscle Pain: A Comparative Study. Pain Res. Manag. 2019;2019:8291624. doi: 10.1155/2019/8291624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sayilir S., Yildizgoren M.T. The medium-term effects of diadynamic currents in chronic low back pain; TENS versus diadynamic currents: A randomised, follow-up study. Complement. Ther. Clin. Pract. 2017;29:16–19. doi: 10.1016/j.ctcp.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 318.Seo H.G., Bang M.S., Chung S.G., Jung S.H., Lee S.-U. Effect of Electrical Stimulation on Botulinum Toxin A Therapy in Patients With Chronic Myofascial Pain Syndrome: A 16-Week Randomized Double-Blinded Study. Arch. Phys. Med. Rehabil. 2013;94:412–418. doi: 10.1016/j.apmr.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 319.Serry Z., Mossa G., Elhabashy H., Elsayed S., Elhadidy R., Azmy R., Mokhtar A. Transcutaneous nerve stimulation versus aerobic exercise in diabetic neuropathy. Egypt. J. Neurol. Psychiatry Neurosurg. 2016;53:124–129. doi: 10.4103/1110-1083.183449. [DOI] [Google Scholar]
- 320.Sezen C.B., Akboga S.A., Celik A., Kalafat C.E., Tastepe A.I. Transcutaneous electrical nerve stimulation effect on postoperative complications. Asian Cardiovasc. Thorac. Ann. 2017;25:276–280. doi: 10.1177/0218492317703838. [DOI] [PubMed] [Google Scholar]
- 321.Shahoei R., Shahghebi S., Rezaei M., Naqshbandi S. The effect of transcutaneous electrical nerve stimulation on the severity of labor pain among nulliparous women: A clinical trial. Complement. Ther. Clin. Pract. 2017;28:176–180. doi: 10.1016/j.ctcp.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 322.Shehab D., Adham N. Comparative effectiveness of ultrasound and transcutaneous electrical stimulation in treatment of periarticular shoulder pain. Physiother. Can. 2000;52:208–214. [Google Scholar]
- 323.Sherry E., Kitchener P., Smart R. A prospective randomized controlled study of VAX-D and TENS for the treatment of chronic low back pain. Neurol. Res. 2001;23:780–784. doi: 10.1179/016164101101199180. [DOI] [PubMed] [Google Scholar]
- 324.Shimoji K., Takahashi N., Nishio Y., Koyanagi M., Aida S. Pain relief by transcutaneous electric nerve stimulation with bidirectional modulated sine waves in patients with chronic back pain: A randomized, double-blind, sham-controlled study. Neuromodulation. 2007;10:42–51. doi: 10.1111/j.1525-1403.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- 325.Shimoura K., Iijima H., Suzuki Y., Aoyama T. Immediate Effects of Transcutaneous Electrical Nerve Stimulation on Pain and Physical Performance in Individuals With Preradiographic Knee Osteoarthritis: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2019;100:300–306. doi: 10.1016/j.apmr.2018.08.189. [DOI] [PubMed] [Google Scholar]
- 326.Shoukry R.A., Al-Ansary A.M. Transcutaneous Electric Nerve Stimulation (TENS) for pain relief during Extracorporeal Shock-Wave Lithotripsy (ESWL) Egypt. J. Anaesth. 2019;35:65–70. doi: 10.1080/11101849.2019.1655202. [DOI] [Google Scholar]
- 327.Siemens W., Boehlke C., Bennett M.I., Offner K., Becker G., Gaertner J. Transcutaneous electrical nerve stimulation for advanced cancer pain inpatients in specialist palliative care—A blinded, randomized, sham-controlled pilot cross-over trial. Supportive Care Cancer. 2020;28:5323–5333. doi: 10.1007/s00520-020-05370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Sikiru L., Shmaila H., Muhammed S.A. Transcutaneous electrical nerve stimulation (TENS) in the symptomatic management of chronic prostatitis/chronic pelvic pain syndrome: A placebo-control randomized trial. Int. Braz. J. Urol. 2008;34:708–714. doi: 10.1590/s1677-55382008000600005. [DOI] [PubMed] [Google Scholar]
- 329.Silva M.B., de Melo P.R., de Oliveira N.M., Crema E., Fernandes L.F. Analgesic effect of transcutaneous electrical nerve stimulation after laparoscopic cholecystectomy. Am. J. Phys. Med. Rehabil. 2012;91:652–657. doi: 10.1097/PHM.0b013e318246638f. [DOI] [PubMed] [Google Scholar]
- 330.Silva J.G., Santana C.G., Inocencio K.R., Orsini M., Machado S., Bergmann A. Electrocortical Analysis of Patients with Intercostobrachial Pain Treated with TENS after Breast Cancer Surgery. J. Phys. Ther. Sci. 2014;26:349–353. doi: 10.1589/jpts.26.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Sim D.T. Effectiveness of transcutaneous electrical nerve stimulation following cholecystectomy. Physiotherapy. 1991;77:715–722. doi: 10.1016/S0031-9406(10)60447-6. [DOI] [Google Scholar]
- 332.Siqueira L.T.D., Ribeiro V.V., Moreira P.A.M., Brasolotto A.G., de Jesus Guirro R.R., Alves Silverio K.C. Effects of transcutaneous electrical nervous stimulation (TENS) associated with vocal therapy on musculoskeletal pain of women with behavioral dysphonia: A randomized, placebo-controlled double-blind clinical trial. J. Commun. Disord. 2019;82:105923. doi: 10.1016/j.jcomdis.2019.105923. [DOI] [PubMed] [Google Scholar]
- 333.Sloan J., Muwanga C., Waters E., Dove A., Dave S. Multiple rib fractures: Transcutaneous nerve stimulation versus conventional analgesia. J. Trauma. 1986;26:1120–1122. doi: 10.1097/00005373-198612000-00012. [DOI] [PubMed] [Google Scholar]
- 334.Smania N., Corato E., Fiaschi A., Pietropoli P., Aglioti S.M., Tinazzi M. Repetitive magnetic stimulation: A novel therapeutic approach for myofascial pain syndrome. J. Neurol. 2005;252:307–314. doi: 10.1007/s00415-005-0642-1. [DOI] [PubMed] [Google Scholar]
- 335.Smedley F., Taube M., Wastell C. Transcutaneous electrical nerve stimulation for pain relief following inguinal hernia repair: A controlled trial. Eur. Surg. Res. Eur. Chir. Forschung. Rech. Chir. Eur. 1988;20:233–237. doi: 10.1159/000128766. [DOI] [PubMed] [Google Scholar]
- 336.Smith C., Lewith G., Machin D. Preliminary study to establish a controlled method of assessing transcutaneous nerve stimulation as a treatment for the pain caused by osteoarthritis of the knee. Physiotherapy. 1983;69:266–268. [PubMed] [Google Scholar]
- 337.Smith C.M., Guralnick M.S., Gelfand M.M., Jeans M.E. The effects of transcutaneous electrical nerve stimulation on post-cesarean pain. Pain. 1986;27:181–193. doi: 10.1016/0304-3959(86)90209-5. [DOI] [PubMed] [Google Scholar]
- 338.Sodipo J., Adedeji S., Olumide O. Postoperative pain relief by transcutaneous electrical nerve stimulation (TENS) Am. J. Chin. Med. 1980;8:190–194. doi: 10.1142/S0192415X80000153. [DOI] [PubMed] [Google Scholar]
- 339.Solak O., Turna A., Pekcolaklar A., Metin M., Sayar A., Gurses A. Transcutaneous electric nerve stimulation for the treatment of postthoracotomy pain: A randomized prospective study. Thorac. Cardiovasc. Surg. 2007;55:182–185. doi: 10.1055/s-2006-924631. [DOI] [PubMed] [Google Scholar]
- 340.Solak O., Emmiler M., Ela Y., Dundar U., Kocogullar C.U., Eren N., Gokce I.Y., Cekirdekci A., Kavuncu V. Comparison of continuous and intermittent transcutaneous electrical nerve stimulation in postoperative pain management after coronary artery bypass graft ing: A randomized, placebo-controlled prospective study. Heart Surg. Forum. 2009;12:E266–E271. doi: 10.1532/HSF98.20081139. [DOI] [PubMed] [Google Scholar]
- 341.Sonde L., Gip C., Fernaeus S.E., Nilsson C.G., Viitanen M. Stimulation with low frequency (1.7 Hz) transcutaneous electric nerve stimulation (low-tens) increases motor function of the post-stroke paretic arm. Scand. J. Rehabil. Med. 1998;30:95–99. doi: 10.1080/003655098444192. [DOI] [PubMed] [Google Scholar]
- 342.Stepanovic A., Kolsek M., Kersnik J., Erculj V. Prevention of post-herpetic neuralgia using transcutaneous electrical nerve stimulation. Wien. Klin. Wochenschr. 2015;127:369–374. doi: 10.1007/s00508-014-0669-3. [DOI] [PubMed] [Google Scholar]
- 343.Steptoe P., Bo J.O. Pain-relieving effect of transcutaneous nerve stimulation during delivery. A study among primiparas. Ugeskr. Laeger. 1984;146:3186–3188. [PubMed] [Google Scholar]
- 344.Stratton S., Smith M. Postoperative thoracotomy. Effect of transcutaneous electrical nerve stimulation on forced vital capacity. Phys. Ther. 1980;60:45–47. doi: 10.1093/ptj/60.1.45. [DOI] [PubMed] [Google Scholar]
- 345.Stubbing J.F., Jellicoe J.A. Transcutaneous electrical nerve stimulation after thoracotomy. Pain relief and peak expiratory flow rate—A trial of transcutaneous electrical nerve stimulation. Anaesthesia. 1988;43:296–298. doi: 10.1111/j.1365-2044.1988.tb08977.x. [DOI] [PubMed] [Google Scholar]
- 346.Suh H.R., Kim T.H., Han G.S. The Effects of High-Frequency Transcutaneous Electrical Nerve Stimulation for Dental Professionals with Work-Related Musculoskeletal Disorders: A Single-Blind Randomized Placebo-Controlled Trial. Evid.-Based Complement. Altern. Med. 2015;2015:327486. doi: 10.1155/2015/327486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Talbot L.A., Solomon Z., Webb L., Morrell C., Metter E.J. Electrical Stimulation Therapies for Active Duty Military with Patellofemoral Pain Syndrome: A Randomized Trial. Mil. Med. 2020;185:e963–e971. doi: 10.1093/milmed/usaa037. [DOI] [PubMed] [Google Scholar]
- 348.Tantawy S.A., Kamel D.M., Abdelbasset W.K. Does transcutaneous electrical nerve stimulation reduce pain and improve quality of life in patients with idiopathic chronic orchialgia? A randomized controlled trial. J. Pain Res. 2018;11:77–82. doi: 10.2147/JPR.S154815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Taylor P., Hallett M., Flaherty L. Treatment of osteoarthritis of the knee with transcutaneous electrical nerve stimulation. Pain. 1981;11:233–240. doi: 10.1016/0304-3959(81)90008-7. [DOI] [PubMed] [Google Scholar]
- 350.Taylor A., West B., Simon B., Skelton J., Rowlingson J. How effective is TENS for acute pain? Am. J. Nurs. 1983;83:1171–1174. doi: 10.1097/00000446-198383080-00022. [DOI] [PubMed] [Google Scholar]
- 351.Thakur R., Patidar R. Comparative study of Transcutaneous Electrical Nerve Stimulation (TENS) and Tramadol Hydrochloride for Pain Relief in Labour. J. Obstet. Gynecol. India. 2004;54:346. [Google Scholar]
- 352.Thomas I.L., Tyle V., Webster J., Neilson A. An evaluation of transcutaneous electrical nerve stimulation for pain relief in labour. Aust. N. Z. J. Obstet. Gynaecol. 1988;28:182–189. doi: 10.1111/j.1479-828X.1988.tb01660.x. [DOI] [PubMed] [Google Scholar]
- 353.Thomas M., Lundeberg T., Bjork G., Lundstrom-Lindstedt V. Pain and discomfort in primary dysmenorrhea is reduced by preemptive acupuncture or low frequency TENS. Eur. J. Phys. Med. Rehabil. 1995;5:71–76. [Google Scholar]
- 354.Thorsteinsson G., Stonnington H.H., Stillwell G.K., Elveback L.R. The placebo effect of transcutaneous electrical stimulation. Pain. 1978;5:31–41. doi: 10.1016/0304-3959(78)90022-2. [DOI] [PubMed] [Google Scholar]
- 355.Tilak M., Isaac S.A., Fletcher J., Vasanthan L.T., Subbaiah R.S., Babu A., Bhide R., Tharion G. Mirror Therapy and Transcutaneous Electrical Nerve Stimulation for Management of Phantom Limb Pain in Amputees—A Single Blinded Randomized Controlled Trial. Physiother. Res. Int. 2016;21:109–115. doi: 10.1002/pri.1626. [DOI] [PubMed] [Google Scholar]
- 356.Tokuda M., Tabira K., Masuda T., Nishiwada T., Shomoto K. Effect of modulated-frequency and modulated-intensity transcutaneous electrical nerve stimulation after abdominal surgery: A randomized controlled trial. Clin. J. Pain. 2014;30:565–570. doi: 10.1097/AJP.0b013e31829ea151. [DOI] [PubMed] [Google Scholar]
- 357.Tonella R.M., Araujo S., Da Silva A.M.O. Transcutaneous electrical nerve stimulation in the relief of pain related to physical therapy after abdominal surgery. Rev. Bras. Anestesiol. 2006;56:630–642. doi: 10.1590/s0034-70942006000600007. [DOI] [PubMed] [Google Scholar]
- 358.Topuz O., Ozfidan E., Ozgen M., Ardic F. Efficacy of transcutaneous electrical nerve stimulation and percutaneous neuromodulation therapy in chronic low back pain. J. Back Musculoskelet. Rehabil. 2004;17:127–133. doi: 10.3233/BMR-2004-173-407. [DOI] [Google Scholar]
- 359.Tosato J., Biasotto-Gonzalez D., Caria P.J.F.P. Effect of massage therapy and of transcutaneous eletrical nerve stimulation on pain and electrotromyographic activity in patients with temporomandibular dysfunction. Fisioter. Pesq. 2007;14:21–26. [Google Scholar]
- 360.Treacy K. Awareness/relaxation training and transcutaneous electrical neural stimulation in the treatment of bruxism. J. Oral Rehabil. 1999;26:280–287. doi: 10.1046/j.1365-2842.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- 361.Tsen L.C., Thomas J., Segal S., Datta S., Bader A.M. Transcutaneous electrical nerve stimulation does not augment combined spinal epidural labour analgesia. Can. J. Anaesth. 2000;47:38–42. doi: 10.1007/BF03020729. [DOI] [PubMed] [Google Scholar]
- 362.Tsen L.C., Thomas J., Segal S., Datta S., Bader A.M. Transcutaneous electrical nerve stimulation does not augment epidural labor analgesia. J. Clin. Anesth. 2001;13:571–575. doi: 10.1016/S0952-8180(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 363.Tsukayama H., Yamashita H., Amagai H., Tanno Y. Randomised controlled trial comparing the effectiveness of electroacupuncture and TENS for low back pain: A preliminary study for a pragmatic trial. Acupunct. Med. 2002;20:175–180. doi: 10.1136/aim.20.4.175. [DOI] [PubMed] [Google Scholar]
- 364.Tucker D.L., Rockett M., Hasan M., Poplar S., Rule S.A. Does transcutaneous electrical nerve stimulation (TENS) alleviate the pain experienced during bone marrow sampling in addition to standard techniques? A randomised, double-blinded, controlled trial. J. Clin. Pathol. 2015;68:479–483. doi: 10.1136/jclinpath-2015-202908. [DOI] [PubMed] [Google Scholar]
- 365.Tugay N., Akbayrak T., Demirturk F., Karakaya I.C., Kocaacar O., Tugay U., Karakaya M.G. Effectiveness of transcutaneous electrical nerve stimulation and interferential current in primary dysmenorrhea. Pain Med. 2007;8:295–300. doi: 10.1111/j.1526-4637.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 366.Tulgar M., McGlone F., Bowsher D., Miles J.B. Comparative effectiveness of different stimulation modes in relieving pain. Part I. A pilot study. Pain. 1991;47:151–155. doi: 10.1016/0304-3959(91)90199-8. [DOI] [PubMed] [Google Scholar]
- 367.Tulgar M., McGlone F., Bowsher D., Miles J.B. Comparative effectiveness of different stimulation modes in relieving pain. Part II. A double-blind controlled long-term clinical trial. Pain. 1991;47:157–162. doi: 10.1016/0304-3959(91)90200-H. [DOI] [PubMed] [Google Scholar]
- 368.Unterrainer A.F., Friedrich C., Krenn M.H., Piotrowski W.P., Golaszewski S.M., Hitzl W. Postoperative and preincisional electrical nerve stimulation TENS reduce postoperative opioid requirement after major spinal surgery. J. Neurosurg. Anesthesiol. 2010;22:1–5. doi: 10.1097/ANA.0b013e3181b7fef5. [DOI] [PubMed] [Google Scholar]
- 369.Unterrainer A.F., Uebleis F.X., Gross F.A., Werner G.G., Krombholz M.A., Hitzl W. TENS compared to opioids in postoperative analgesic therapy after major spinal surgery with regard to cognitive function. Middle East J. Anesthesiol. 2012;21:815–821. [PubMed] [Google Scholar]
- 370.Upton G.A., Tinley P., Al-Aubaidy H., Crawford R. The influence of transcutaneous electrical nerve stimulation parameters on the level of pain perceived by participants with painful diabetic neuropathy: A crossover study. Diabetes Metab. Syndr. 2017;11:113–118. doi: 10.1016/j.dsx.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 371.Vaidya S.M. Sacroiliac joint mobilisation versus transcutaneous electrical nerve stimulation for pregnancy induced posterior pelvic pain-a randomised clinical trial. J. Clin. Diagn. Res. 2018;12:YC04–YC07. doi: 10.7860/JCDR/2018/26696.10781. [DOI] [Google Scholar]
- 372.Vaillancourt S., Coulombe-Lévêque A., Fradette J., Martel S., Naour W., da Silva R.A., Léonard G. Combining transcutaneous electrical nerve stimulation with therapeutic exercise to reduce pain in an elderly population: A pilot study. Disabil. Rehabil. 2019;43:1–8. doi: 10.1080/09638288.2019.1693639. [DOI] [PubMed] [Google Scholar]
- 373.Valenza M.C., Torres-Sanchez I., Cabrera-Martos I., Valenza-Demet G., Cano-Cappellacci M. Acute Effects of Contract-Relax Stretching vs. TENS in Young Subjects with Anterior Knee Pain: A Randomized Controlled Trial. J. Strength Cond. Res. 2016;30:2271–2278. doi: 10.1097/JSC.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 374.Van der Ploeg J., Vervest H., Liem A., Schagen van Leeuwen J. Transcutaneous nerve stimulation (TENS) during the first stage of labour: A randomized clinical trial. Pain. 1996;68:75–78. doi: 10.1016/S0304-3959(96)03141-7. [DOI] [PubMed] [Google Scholar]
- 375.Van der Spank J.T., Cambier D.C., De Paepe H.M., Danneels L.A., Witvrouw E.E., Beerens L. Pain relief in labour by transcutaneous electrical nerve stimulation (TENS) Arch. Gynecol. Obstet. 2000;264:131–136. doi: 10.1007/s004040000099. [DOI] [PubMed] [Google Scholar]
- 376.Vance C., Rakel B., Blodgett N., DeSantana J., Amendola A., Zimmerman M., Walsh D., Sluka K. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: A randomized controlled trial. Phys. Ther. 2012;92:898–910. doi: 10.2522/ptj.20110183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Vitalii C., Oleg P. The efficiency of transcutaneous electrical nerve stimulation in association with gabapentin in the treatment of neuropathic pain in patients with spinal cord injury. Rom. J. Neurol. 2014;13:193–196. [Google Scholar]
- 378.Vrouva S., Batistaki C., Paraskevaidou E., Chanopoulos K., Kostopoulos D., Stamoulis E., Kostopanagiotou G. Comparative study of pain relief in two non-pharmacological treatments in patients with partial rotator cuff tears: A randomized trial. Anesthesiol. Pain Med. 2019;9:e88327. doi: 10.5812/aapm.88327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Walker R., Morris B., Angulo D., Schneider J., Colwell C. Postoperative use of continuous passive motion, transcutaneous electrical nerve stimulation and continuous cooling pad following total knee arthroplasty. J. Arthroplast. 1991;6:151–156. doi: 10.1016/S0883-5403(11)80010-0. [DOI] [PubMed] [Google Scholar]
- 380.Wang S.F., Lee J.P., Hwa H.L. Effect of transcutaneous electrical nerve stimulation on primary dysmenorrhea. Neuromodulation. 2009;12:302–309. doi: 10.1111/j.1525-1403.2009.00226.x. [DOI] [PubMed] [Google Scholar]
- 381.Warfield C., Stein J., Frank H. The effect of transcutaneous electrical nerve stimulation on pain after thoracotomy. Ann. Thorac. Surg. 1985;39:462–465. doi: 10.1016/S0003-4975(10)61957-1. [DOI] [PubMed] [Google Scholar]
- 382.Warke K., Al-Smadi J., Baxter G.D., Walsh D.M., Lowe-Strong A.S. A double blind, placebo controlled, randomised, clinical trial investigating the efficacy of transcutaneous electrical nerve stimulation for low back pain in a multiple sclerosis population. Mult. Scler. 2004;10:S107. doi: 10.1097/01.ajp.0000210935.73686.79. [DOI] [PubMed] [Google Scholar]
- 383.Warke K., Al-Smadi J., Baxter D., Walsh D.M., Lowe-Strong A.S. Efficacy of transcutaneous electrical nerve stimulation (TENS) for chronic low-back pain in a multiple sclerosis population—A randomized, placebo-controlled clinical trial. Clin. J. Pain. 2006;22:812–819. doi: 10.1097/01.ajp.0000210935.73686.79. [DOI] [PubMed] [Google Scholar]
- 384.Yameen F., Shahbaz N.N., Hasan Y., Fauz R., Abdullah M. Efficacy of transcutaneous Electrical Nerve Stimulation and its different modes in patients with Trigeminal Neuralgia. J. Pak. Med. Assoc. 2011;61:437–439. [PubMed] [Google Scholar]
- 385.Yesil H., Hepguler S., Dundar U., Taravati S., Isleten B. Does the Use of Electrotherapies Increase the Effectiveness of Neck Stabilization Exercises for Improving Pain, Disability, Mood, and Quality of Life in Chronic Neck Pain? Spine. 2018;43:E1174–E1183. doi: 10.1097/BRS.0000000000002663. [DOI] [PubMed] [Google Scholar]
- 386.Yilmaz M., Tarakci D., Tarakci E. Comparison of high-intensity laser therapy and combination of ultrasound treatment and transcutaneous nerve stimulation on cervical pain associated with cervical disc herniation: A randomized trial. Complement. Ther. Med. 2020;49:102295. doi: 10.1016/j.ctim.2019.102295. [DOI] [PubMed] [Google Scholar]
- 387.Yilmazer M., Kose S., Arioz D.T., Koken G., Ozbulut O. Efficacy of transcutaneous electrical nerve stimulation for pain relief in women undergoing office endometrial biopsy. Arch. Gynecol. Obstet. 2012;285:1059–1064. doi: 10.1007/s00404-011-2111-7. [DOI] [PubMed] [Google Scholar]
- 388.Yokoyama M., Sun X., Oku S., Taga N., Sato K., Mizobuchi S., Takahashi T., Morita K. Comparison of percutaneous electrical nerve stimulation with transcutaneous electrical nerve stimulation for long-term pain relief in patients with chronic low back pain. Anesth. Analg. 2004;98:1552–1556. doi: 10.1213/01.ANE.0000112312.94043.DF. [DOI] [PubMed] [Google Scholar]
- 389.Yoshimizu M., Teo A.R., Ando M., Kiyohara K., Kawamura T. Relief of chronic shoulder and neck pain by electro-acupuncture and transcutaneous electrical nervous stimulation: A randomized crossover trial. Med. Acupunct. 2012;24:97–103. doi: 10.1089/acu.2011.0824. [DOI] [Google Scholar]
- 390.Yuksel M., Ayas S., Cabioglu M.T., Yilmaz D., Cabioglu C. Quantitative Data for Transcutaneous Electrical Nerve Stimulation and Acupuncture Effectiveness in Treatment of Fibromyalgia Syndrome. Evid.-Based Complement. Alternat. Med. 2019;2019:9684649. doi: 10.1155/2019/9684649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Yurtkuran M., Kocagil T. TENS, electroacupuncture and ice massage: Comparison of treatment for osteoarthritis of the knee. Am. J. Acupunct. 1999;27:133–140. [PubMed] [Google Scholar]
- 392.Zakariaee S.S., Shahoei R., Nosab L.H., Moradi G., Farshbaf M. The Effects of Transcutaneous Electrical Nerve Stimulation on Post-Episiotomy Pain Severity in Primiparous Women: A Randomized, Controlled, Placebo Clinical Trial. Galen Med. J. 2019;8:UNSP e1404. doi: 10.31661/gmj.v8i0.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Zhang Y., Zhang J., Wang L., Wang K., Svensson P. Effect of transcutaneous electrical nerve stimulation on jaw movement-evoked pain in patients with TMJ disc displacement without reduction and healthy controls. Acta Odontol. Scand. 2020;78:309–320. doi: 10.1080/00016357.2019.1707868. [DOI] [PubMed] [Google Scholar]
- 394.Zhou M., Li F., Lu W., Wu J., Pei S. Efficiency of Neuromuscular Electrical Stimulation and Transcutaneous Nerve Stimulation on Hemiplegic Shoulder Pain: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018;99:1730–1739. doi: 10.1016/j.apmr.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 395.Aiyejusunle C.B., Kola-Korolo T.A., Ajiboye O.A. Comparison of the effects of tens and sodium salicylate iontophoresis in the management of osteoarthritis of the knee. Niger. Q. J. Hosp. Med. 2007;17:30–34. doi: 10.4314/nqjhm.v17i1.12539. [DOI] [PubMed] [Google Scholar]
- 396.Chen L., Zhang X.L., Ding H., Tao Y.Q., Zhan H.S. Comparative study on effects of manipulation treatment and transcutaneous electrical nerve stimulation on patients with cervicogenic headache. J. Chin. Integr. Med. 2007;5:403–406. doi: 10.3736/jcim20070408. [DOI] [PubMed] [Google Scholar]
- 397.Houshyar A.E., Rezaie H.H., Jahani Y., Kazemi M., Monfared S. Comparison of two methods of aromatherapy with lavender essence and Transcutaneous Electrical Nerve Stimulation (TENS) on cesarean postoperative pain. Iran. J. Obstet. Gynecol. Infertil. 2015;18:6–12. [Google Scholar]
- 398.Kim B., Lohman E., Yim J. Acupuncture-like Transcutaneous Electrical Nerve Stimulation for Pain, Function, and Biochemical Inflammation After Total Knee Arthroplasty. Altern. Ther. Health Med. 2020;27:28–34. [PubMed] [Google Scholar]
- 399.Kumar S.S., Abdul Rahim A. A study on effectiveness of conventional mode TENS on trismus. Res. J. Pharm. Technol. 2019;12:1193–1196. doi: 10.5958/0974-360X.2019.00198.7. [DOI] [Google Scholar]
- 400.Mehlhorn G., Beckmann M.W., Schild R.L., Binder H. Analgesia of afterpains with transcutaneous nerve stimulation (TENS) vs. metamizole. A prospective, randomized placebo controlled double-blind study. Geburtshilfe Frauenheilkd. 2005;65:266–271. doi: 10.1055/s-2005-837560. [DOI] [Google Scholar]
- 401.Pourmomeny A.A., Amini M., Safaei H., Hassanzadeh A. The effect of electroanalgsia [sic] on pain relief in patient with diabetic neuropathy type II. Iran. J. Endocrinol. Metab. 2009;11:472. [Google Scholar]
- 402.Renklitepe N., Dogan N., Kayhan O., Ozaras N. Effects of different TENS electrode types in degenerative joint disease of the hands. Fiz. Tedavi Rehabil. Derg. 1995;19:204–208. [Google Scholar]
- 403.Sakai T., Tsutani K., Tsukayama H. Multi center randomized controlled trial of acupuncture with electric stimulation and acupuncture-like transcutaneous electrical nerve stimulation for lumbago. J. Jpn. Soc. Acupunct. Moxibust. 2001;51:175–184. [Google Scholar]
- 404.Tokuda M., Tabira K., Masuda T., Nishiwada T., Shomoto K. Effect of Transcutaneous Electrical Nerve Stimulation after Abdominal Surgery-A Randomized Controlled Trial. Rigakuryoho Kagaku. 2013;28:415–421. doi: 10.1589/rika.28.415. [DOI] [PubMed] [Google Scholar]
- 405.Tunc M., Gunal H., Bilgili T., Ulus F., Tunc H., Savkilioglu E. The effect of TENS on epidural patient controlled analgesia with tramadol for postthoracotomy pain relief. Turk Anesteziyoloji Ve Reanimasyon. 2002;30:315–321. [Google Scholar]
- 406.Van D.P.I., Smits A., Verwer J. Elektrodeplaatsing bij TENS: Een pilot-studie. Ned. Tijdschr. Fysioter. 1998;108:128–131. [Google Scholar]
- 407.Wang N.X.B., Wei X., Li M., Xu Y. A clinical controlled study of immediate analgesia effect of TENS on post-operative pain after total knee replacement. Chin. J. Rehabil. Med. 2005;20:188–190. [Google Scholar]
- 408.Xiao H., She S.Z., Xu L.X. Effects of transcutaneous electric nerve stimulation on the postoperative analgesia with PCEA and recovery after surgery [Chinese-simplified characters] Chin. J. Clin. Rehabil. 2002;6:1784–1785. [Google Scholar]
- 409.Zati A., Fortuna D., Valent A., Pulvirenti F., Bilotta T.W. Treatment of low back pain caused by intervertebral disk displacement: Comparison between high power laser, TENS and NSAIDs. Med. Dello Sport. 2004;57:77–82. [Google Scholar]
- 410.Zheng G., Huang X., Zhao X. Influence of neuromuscular electrical stimulation of musculi quadriceps femoris on motor function rehabilitation after total knee replacement. Chin. J. Rehabil. Med. 2011;26:1126–1130. [Google Scholar]
- 411.Zhang Q., Zhang J.H., Tong P.J. Application of transcutaneous electrical nerve stimulation to multimodal analgesia after total knee arthroplasty. China J. Orthop. Traumatol. 2014;27:283–286. [PubMed] [Google Scholar]
- 412.Zhong J., Zhang L. Transcutaneous electrical acupoint stimulation for pregnancy of in vitro fertilization-embryo transfer. Zhongguo Zhen Jiu—Chin. Acupunct. Moxibust. 2017;37:253–255. doi: 10.13703/j.0255-2930.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 413.Zhou G.Y., Zhou G.S., Jian-hong J. Clinical observation on transcutaneous electrical acupoint stimulation for treatment of functional dyspepsia. Zhongguo Zhen Jiu—Chin. Acupunct. Moxibust. 2009;29:436–440. [PubMed] [Google Scholar]
- 414.Aguilar Ferrandiz M.E., Nijs J., Gidron Y., Roussel N., Vanderstraeten R., Van Dyck D., Huysmans E., De Kooning M. Auto-Targeted Neurostimulation Is Not Superior to Placebo in Chronic Low Back Pain: A Fourfold Blind Randomized Clinical Trial. Pain Physician. 2016;19:E707–E719. [PubMed] [Google Scholar]
- 415.Albayrak I., Apiliogullari S., Dal C.N., Levendoglu F., Ozerbil O.M. Efficacy of Pulsed Radiofrequency Therapy to Dorsal Root Ganglion Adding to TENS and Exercise for Persistent Pain after Total Knee Arthroplasty. J. Knee Surg. 2017;30:134–142. doi: 10.1055/s-0036-1583268. [DOI] [PubMed] [Google Scholar]
- 416.Alhusaini A.A., Fallatah S., Melam G.R., Buragadda S. Efficacy of transcutaneous electrical nerve stimulation combined with therapeutic exercise on hand function in children with hemiplegic cerebral palsy. Somatosens. Mot. Res. 2019;36:49–55. doi: 10.1080/08990220.2019.1584555. [DOI] [PubMed] [Google Scholar]
- 417.Altas E.U., Demirdal U. The effect of physical therapy and rehabilitation modalities on sleep quality in patients with primary knee osteoarthritis: A single-blind, prospective, randomized-controlled study. Turk. J. Phys. Med. Rehabil. 2020;66:73–83. doi: 10.5606/tftrd.2020.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Al Zamil M., Kulikova N., Bezrukova O., Volkova I., Stahurlova V. Comparative analysis between transcutaneous electroneurostimulation and acupuncture in treatment of carpal tunnel syndrome. Eur. J. Neurol. 2019;26:729. doi: 10.1111/ene.14019. [DOI] [Google Scholar]
- 419.Askin A., Savas S., Koyuncuoglu H.R., Baloglu H.H., Inci M.F. Low dose high frequency ultrasound therapy for stellate ganglion blockade in complex regional pain syndrome type I: A randomised placebo controlled trial. Int. J. Clin. Exp. Med. 2014;7:5603–5611. [PMC free article] [PubMed] [Google Scholar]
- 420.Atalay C., Yilmaz K.B. The effect of transcutaneous electrical nerve stimulation on postmastectomy skin flap necrosis. Breast Cancer Res. Treat. 2009;117:611–614. doi: 10.1007/s10549-009-0335-z. [DOI] [PubMed] [Google Scholar]
- 421.Augustinsson L., Bohlin P., Bundsen P., Carlsson C., Forssman L., Sjoberg P., Tyreman N. Pain relief during delivery by transcutaneous electrical nerve stimulation. Pain. 1977;4:59–65. doi: 10.1016/0304-3959(77)90087-2. [DOI] [PubMed] [Google Scholar]
- 422.Avramidis K., Strike P.W., Taylor P.N., Swain I.D. Effectiveness of electric stimulation of the vastus medialis muscle in the rehabilitation of patients after total knee arthroplasty. Arch. Phys. Med. Rehabil. 2003;84:1850–1853. doi: 10.1016/S0003-9993(03)00429-5. [DOI] [PubMed] [Google Scholar]
- 423.Aydın S., Arıoğlu Aydın Ç., Batmaz G., Dansuk R. Effect of vaginal electrical stimulation on female sexual functions: A randomized study. J. Sex. Med. 2015;12:463–469. doi: 10.1111/jsm.12788. [DOI] [PubMed] [Google Scholar]
- 424.Aydogan S., Er U., Ozlu O. Effectiveness of preemptive analgesia using a frequency rhythmic electrical modulation system in patients having instrumented fusion for lumbar stenosis. Asian Spine J. 2014;8:190–196. doi: 10.4184/asj.2014.8.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Ayyildiz A., Nuhoglu B., Huri E., Huri M., Gurdal M., Germiyanoglu C. Transcutaneous electrical nerve stimulation (TENS): Decreased of pain when extracorporeal shock wave lithotripsy. Turk Uroloji Derg. 2004;30:446–450. [Google Scholar]
- 426.Bai W.Y., Yang Y.C., Teng X.F., Wan Y.X., Wei W., Zhu J.C. Effects of Transcutaneous Electrical Acupoint Stimulation on the Stress Response During Extubation After General Anesthesia in Elderly Patients Undergoing Elective Supratentorial Craniotomy: A Prospective Randomized Controlled Trial. J. Neurosurg. Anesthesiol. 2018;30:337–346. doi: 10.1097/ANA.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 427.Behm D.G., Colwell E.M., Power G.M.J., Ahmadi H., Behm A.S.M., Bishop A., Murph C., Pike J., McAssey B., Fraser K., et al. Transcutaneous electrical nerve stimulation improves fatigue performance of the treated and contralateral knee extensors. Eur. J. Appl. Physiol. 2019;119:2745–2755. doi: 10.1007/s00421-019-04253-z. [DOI] [PubMed] [Google Scholar]
- 428.Belmonte R., Tejero M., Ferrer M., Muniesa J.M., Duarte E., Cunillera O., Escalada F. Efficacy of low-frequency low-intensity electrotherapy in the treatment of breast cancer-related lymphoedema: A cross-over randomized trial. Clin. Rehabil. 2012;26:607–618. doi: 10.1177/0269215511427414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Bouafif L., Ellouze N. Design and evaluation of a modulated TENS stimulation in medical pain therapy. Curr. Signal Transduct. Ther. 2019;14:75–83. doi: 10.2174/1574362413666180723153648. [DOI] [Google Scholar]
- 430.Bundsen P., Peterson L., Selstam U. Pain relief in labor by transcutaneous electrical nerve stimulation. A prospective matched study. Acta Obstet. Gynecol. Scand. 1981;60:459–468. doi: 10.3109/00016348109155461. [DOI] [PubMed] [Google Scholar]
- 431.Burch F.X., Tarro J.N., Greenberg J.J., Carroll W.J. Evaluating the benefits of patterned stimulation in the treatment of osteoarthritis of the knee: A multi-center, randomized, single-blind, controlled study with an independent masked evaluator. Osteoarthr. Cartil. 2008;16:865–872. doi: 10.1016/j.joca.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 432.Burssens P., Forsyth R., Steyaert A., Van Ovost E., Praet M., Verdonk R. Influence of burst TENS stimulation on the healing of Achilles tendon suture in man. Acta Orthop. Belg. 2003;69:528–532. [PubMed] [Google Scholar]
- 433.Carbonario F., Matsutani L., Yuan S., Marques A. Effectiveness of high-frequency transcutaneous electrical nerve stimulation at tender points as adjuvant therapy for patients with fibromyalgia. Eur. J. Phys. Rehabil. Med. 2013;49:197–204. [PubMed] [Google Scholar]
- 434.Chao A.-S., Chao A., Wang T.-H., Chang Y.-C., Peng H.-H., Chang S.-D., Chao A., Chang C.-J., Lai C.-H., Wong A.M.K. Pain relief by applying transcutaneous electrical nerve stimulation (TENS) on acupuncture points during the first stage of labor: A randomized double-blind placebo-controlled trial. Pain. 2007;127:214–220. doi: 10.1016/j.pain.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 435.Chee E., Walton H. Treatment of trigger points with microamperage transcutaneous electrical nerve stimulation (TENS)—(The Electro-Acuscope 80) J. Manip. Physiol. Ther. 1986;9:131–134. [PubMed] [Google Scholar]
- 436.Cheing G.L., Hui-Chan C.W. Would the addition of TENS to exercise training produce better physical performance outcomes in people with knee osteoarthritis than either intervention alone? Clin. Rehabil. 2004;18:487–497. doi: 10.1191/0269215504cr760oa. [DOI] [PubMed] [Google Scholar]
- 437.Chen W.-L., Hsu W.-C., Lin Y.-J., Hsieh L.-F. Comparison of Intra-articular Hyaluronic Acid Injections With Transcutaneous Electric Nerve Stimulation for the Management of Knee Osteoarthritis: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2013;94:1482–1489. doi: 10.1016/j.apmr.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 438.Chen M.Y., Pu Q.Q., Liu S.Y., Jiang Z.Y. Efficacy comparison of different stimulation therapies for periarthritis of shoulder. Zhongguo Zhen Jiu—Chin. Acupunct. Moxibust. 2013;33:109–112. [PubMed] [Google Scholar]
- 439.Chen Y., Wu W., Yao Y., Yang Y., Zhao Q., Qiu L. Transcutaneous electric acupoint stimulation at Jiaji points reduce abdominal pain after colonoscopy: A randomized controlled trial. Int. J. Clin. Exp. Med. 2015;8:5972–5977. [PMC free article] [PubMed] [Google Scholar]
- 440.Chen Y., Yao Y., Wu Y., Dai D., Zhao Q., Qiu L. Transcutaneous electric acupoint stimulation alleviates remifentanil-induced hyperalgesia in patients undergoing thyroidectomy: A randomized controlled trial. Int. J. Clin. Exp. Med. 2015;8:5781–5787. [PMC free article] [PubMed] [Google Scholar]
- 441.Chen Y., Yang Y., Yao Y., Dai D., Qian B., Liu P. Does transcutaneous electric acupoint stimulation improve the quality of recovery after thyroidectomy? A prospective randomized controlled trial. Int. J. Clin. Exp. Med. 2015;8:13622–13627. [PMC free article] [PubMed] [Google Scholar]
- 442.Chen J., Zhang Y., Li X., Wan Y., Ji X., Wang W., Kang X., Yan W., Fan Z. Efficacy of transcutaneous electrical acupoint stimulation combined with general anesthesia for sedation and postoperative analgesia in minimally invasive lung cancer surgery: A randomized, double-blind, placebo-controlled trial. Thorac. Cancer. 2020;11:928–934. doi: 10.1111/1759-7714.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Cheng R.S.S., Pomeranz B. Electrotheraphy of chronic musculoskeletal pain: Comparison of electroacupuncture and acupuncture-like transcutaneous electrical nerve stimulation. Clin. J. Pain. 1986;2:143–149. doi: 10.1097/00002508-198602030-00001. [DOI] [Google Scholar]
- 444.Chiu J.H., Chen W.S., Chen C.H., Jiang J.K., Tang G.J., Lui W.Y., Lin J.K. Effect of transcutaneous electrical nerve stimulation for pain relief on patients undergoing hemorrhoidectomy: Prospective, randomized, controlled trial. Dis. Colon Rectum. 1999;42:180–185. doi: 10.1007/BF02237124. [DOI] [PubMed] [Google Scholar]
- 445.Coletta R., Maggiolo F., Di Tizio S. Etofenamate and transcutaneous electrical nerve stimulation treatment of painful spinal syndromes. Int. J. Clin. Pharmacol. Res. 1988;8:295–298. [PubMed] [Google Scholar]
- 446.Conn I.G., Marshall A.H., Yadav S.N., Daly J.C., Jaffer M. Transcutaneous electrical nerve stimulation following appendicectomy: The placebo effect. Ann. R. Coll. Surg. Engl. 1986;68:191–192. [PMC free article] [PubMed] [Google Scholar]
- 447.Cornell P., Lopez A., Malofsky H. Pain reduction with transcutaneous electrical nerve stimulation after foot surgery. J. Foot Surg. 1984;23:326–333. [PubMed] [Google Scholar]
- 448.Demidas A., Zarzycki M. Touch and Pain Sensations in Diadynamic Current (DD) and Transcutaneous Electrical Nerve Stimulation (TENS): A Randomized Study. Biomed Res. Int. 2019;2019:9073073. doi: 10.1155/2019/9073073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Duzyj C.M., Simonds A., Jones I., Hill J.M., Khan S., Parrott J.S. Transcutaneous electrical nerve stimulation to reduce pain and opioid use after cesarean: A pilot study. Am. J. Obstet. Gynecol. 2020;222:S190. doi: 10.1016/j.ajog.2019.11.297. [DOI] [Google Scholar]
- 450.Dodick D.W., Lipton R.B., Goadsby P.J., Tfelt-Hansen P., Ferrari M.D., Diener H., Almas M., Albert K.S., Parsons B. Predictors of Migraine Headache Recurrence: A Pooled Analysis From the Eletriptan Database. Headache J. Head Face Pain. 2008;48:184–193. doi: 10.1111/j.1526-4610.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 451.Eidy M., Fazel M.R., Janzamini M., Haji Rezaei M., Moravveji A.R. Preemptive Analgesic Effects of Transcutaneous Electrical Nerve Stimulation (TENS) on Postoperative Pain: A Randomized, Double-Blind, Placebo-Controlled Trial. Iran. Red Crescent Med. J. 2016;18:e35050. doi: 10.5812/ircmj.35050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Ertzgaard P., Alwin J., Sorbo A., Lindgren M., Sandsjo L. Evaluation of a self-administered transcutaneous electrical stimulation concept for the treatment of spasticity: A randomized placebo-controlled trial. Eur. J. Phys. Rehabil. Med. 2018;54:507–517. doi: 10.23736/S1973-9087.17.04791-8. [DOI] [PubMed] [Google Scholar]
- 453.Fagade O.O., Obilade T.O. Therapeutic effect of TENS on post-IMF trismus and pain. Afr. J. Med. Med. Sci. 2003;32:391–394. [PubMed] [Google Scholar]
- 454.Fargas-Babjak A., Rooney P., Gerecz E. Randomized trial of Codetron for pain control in osteoarthritis of the hip/knee. Clin. J. Pain. 1989;5:137–141. doi: 10.1097/00002508-198906000-00002. [DOI] [PubMed] [Google Scholar]
- 455.Fargas-Babjak A.M., Pomeranz B., Rooney P.J. Acupuncture-like stimulation with codetron for rehabilitation of patients with chronic pain syndrome and osteoarthritis. Acupunct. Electrother. Res. 1992;17:95–105. doi: 10.3727/036012992816357828. [DOI] [PubMed] [Google Scholar]
- 456.Fary R.E., Carroll G.J., Briffa T.G., Briffa N.K. The effectiveness of pulsed electrical stimulation in the management of osteoarthritis of the knee: Results of a double-blind, randomized, placebo-controlled, repeated-measures trial. Arthritis Rheum. 2011;63:1333–1342. doi: 10.1002/art.30258. [DOI] [PubMed] [Google Scholar]
- 457.Fletcher-Smith J.C., Walker D.M., Allatt K., Sprigg N., James M., Ratib S., Boadu J., Richardson C., Pandyan A.D. The ESCAPS study: A feasibility randomized controlled trial of early electrical stimulation to the wrist extensors and flexors to prevent post-stroke complications of pain and contractures in the paretic arm. Clin. Rehabil. 2019;33:1919–1930. doi: 10.1177/0269215519868834. [DOI] [PubMed] [Google Scholar]
- 458.Gadsby G., Franks A., Jarvis P., Dewhurst F. Acupuncture-like transcutaneous electrical nerve stimulation within palliative care: A pilot study. Complement. Ther. Med. 1997;5:13–18. doi: 10.1016/S0965-2299(97)80084-2. [DOI] [Google Scholar]
- 459.Gao Y.Q., Jia Q., Xie S., Yin L.W., Xue J.X., Kou L.H., Xue B., Liu J.L., Shi J.H. Clinical Trials for Thyroidectomy Under Acupuncture-aided Anesthesia by Using Electroacupuncture or Transcutaneous Acupoint Electrical Stimulation of Different Acupoints. Zhen Ci Yan Jiu—Acupunct. Res. 2017;42:332–337. [PubMed] [Google Scholar]
- 460.Garland D., Holt P., Harrington J.T., Caldwell J., Zizic T., Cholewczynski J. A 3-month, randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of a highly optimized, capacitively coupled, pulsed electrical stimulator in patients with osteoarthritis of the knee. Osteoarthr. Cartil. 2007;15:630–637. doi: 10.1016/j.joca.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 461.Garaud T., Gervais C., Szekely B., Michel-Cherqui M., Dreyfus J.-F., Fischler M., Ertem D.H. Randomized study of the impact of a therapeutic education program on patients suffering from chronic low-back pain who are treated with transcutaneous electrical nerve stimulation. Medicine. 2018;97:e13782. doi: 10.1097/MD.0000000000013782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Gaul C., Diener H.C., Silver N., Magis D., Reuter U., Andersson A., Liebler E.J., Straube A. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): A randomised controlled study. Cephalalgia Int. J. Headache. 2016;36:534–546. doi: 10.1177/0333102415607070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Geirsson G., Wang Y., Lindstrom S., Fall M. Traditional acupuncture and electrical stimulation of the posterior tibial nerve. A trial in chronic interstitial cystitis. Scand. J. Urol. Nephrol. 1993;27:67–70. doi: 10.3109/00365599309180416. [DOI] [PubMed] [Google Scholar]
- 464.Ghoname E.A., Craig W.F., White P.F., Ahmed H.E., Hamza M.A., Gajraj N.M., Vakharia A.S., Noe C.E., Stone E.G. Effectiveness of PENS for lower back pain. Integr. Med. 1999;2:19–21. [Google Scholar]
- 465.Gokce A.H., Gokce F.S. Effects of bilateral transcutaneous tibial nerve stimulation on constipation severity in geriatric patients: A prospective clinical study. Geriatr. Gerontol. Int. 2020;20:101–105. doi: 10.1111/ggi.13822. [DOI] [PubMed] [Google Scholar]
- 466.Gorodetskyi I.G., Gorodnichenko A.I., Tursin P.S., Reshetnyak V.K., Uskov O.N. Non-invasive interactive neurostimulation in the post-operative recovery of patients with a trochanteric fracture of the femur. A randomised, controlled trial. J. Bone Jt. Surg. Br. 2007;89:1488–1494. doi: 10.1302/0301-620X.89B11.19352. [DOI] [PubMed] [Google Scholar]
- 467.Gottfried A., Adler E.P., Fernandez-Becker N., Clarke J.O., Habtezion A., Nguyen L.A.B. Transcutaneous vagal nerve stimulation improves symptoms, pain, and gastric emptying in patients with idiopathic gastroparesis. Neurogastroent. Motil. 2019;156:S789–S790. doi: 10.1111/nmo.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Govil M., Mukhopadhyay N., Holwerda T., Sluka K., Rakel B., Schutte D.L. Effects of genotype on TENS effectiveness in controlling knee pain in persons with mild to moderate osteoarthritis. Eur. J. Pain. 2020;24:398–412. doi: 10.1002/ejp.1497. [DOI] [PubMed] [Google Scholar]
- 469.Gu S., Lang H., Gan J., Zheng Z., Zhao F., Tu Q. Effect of transcutaneous electrical acupoint stimulation on gastrointestinal function recovery after laparoscopic radical gastrectomy—A randomized controlled trial. Eur. J. Integr. Med. 2019;26:11–17. doi: 10.1016/j.eujim.2019.01.001. [DOI] [Google Scholar]
- 470.Harrison R., Shore M., Woods T., Mathews G., Gardiner J., Unwin A. A comparative study of transcutaneous electrical nerve stimulation (TENS), entonox, pethidine + promazine and lumbar epidural for pain relief in labor. Acta Obstet. Gynecol. Scand. 1987;66:9–14. doi: 10.3109/00016348709092945. [DOI] [PubMed] [Google Scholar]
- 471.Herman E., Williams R., Stratford P., Fargas-Babjak A., Trott M. A randomized controlled trial of transcutaneous electrical nerve stimulation (CODETRON) to determine its benefits in a rehabilitation program for acute occupational low back pain. Spine. 1994;19:561–568. doi: 10.1097/00007632-199403000-00012. [DOI] [PubMed] [Google Scholar]
- 472.Hedner N., Milsom I., Eliasson T., Mannheimer C. TENS is effective in painful menstruation. Lakartidningen. 1996;93:1219–1222. [PubMed] [Google Scholar]
- 473.Hettrick H.H., O’Brien K., Laznick H., Sanchez J., Gorga D., Nagler W., Yurt R. Effect of transcutaneous electrical nerve stimulation for the management of burn pruritus: A pilot study. J. Burn Care Rehabil. 2004;25:236–240. doi: 10.1097/01.BCR.0000124745.22170.86. [DOI] [PubMed] [Google Scholar]
- 474.Hsieh C.Y.J., Phillips R.B., Adams A.H., Pope M.H. First prize: Functional outcomes of low back pain: Comparison of four treatment groups in a randomized controlled trial. J. Manip. Physiol. Ther. 1992;15:4–9. [PubMed] [Google Scholar]
- 475.Huang S., Peng W., Tian X., Liang H., Jia Z., Lo T., He M., Feng Y. Effects of transcutaneous electrical acupoint stimulation at different frequencies on perioperative anesthetic dosage, recovery, complications, and prognosis in video-assisted thoracic surgical lobectomy: A randomized, double-blinded, placebo-controlled trial. J. Anesth. 2017;31:58–65. doi: 10.1007/s00540-015-2057-1. [DOI] [PubMed] [Google Scholar]
- 476.Huang W., Yu T.Y., Long W.F., Xiao J.B. Application of Transcutaneous Electrical Acupoint Stimulation Combined with Transversus Abdominis Plane Block to Enhanced Recovery After Surgery in Patients Undergoing Laparoscopic Colorectal Cancer Resection: A Randomized Controlled Clinical Trial. Zhen Ci Yan Jiu. 2018;43:611–615. doi: 10.13702/j.1000-0607.180005. [DOI] [PubMed] [Google Scholar]
- 477.Huang Y., Bian W.W., Hou L.L. Effects of transcutaneous electrical acupoint stimulation on pain of patients in expansion process of skin soft tissue dilator on forehead by water injection. Zhonghua Shao Shang Za Zhi Chin. J. Burn. 2019;35:193–197. doi: 10.3760/cma.j.issn.1009-2587.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 478.Ing M.R., Hellreich P.D., Johnson D.W., Chen J.J. Transcutaneous electrical nerve stimulation for chronic post-herpetic neuralgia. Int. J. Dermatol. 2015;54:476–480. doi: 10.1111/ijd.12385. [DOI] [PubMed] [Google Scholar]
- 479.Issenman J., Nolan M., Rowley J., Hobby R. Transcutaneous electrical nerve stimulation for pain control after spinal fusion with Harrington rods. A clinical report. Phys. Ther. 1985;65:1517–1520. doi: 10.1093/ptj/65.10.1517. [DOI] [PubMed] [Google Scholar]
- 480.Itoh K., Hirota S., Katsumi Y., Ochi H., Kitakoji H. A pilot study on using acupuncture and transcutaneous electrical nerve stimulation (TENS) to treat knee osteoarthritis (OA) Chin. Med. 2008;3:2. doi: 10.1186/1749-8546-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Itoh K., Itoh S., Katsumi Y., Kitakoji H. A pilot study on using acupuncture and transcutaneous electrical nerve stimulation to treat chronic non-specific low back pain. Complement. Ther. Clin. Pract. 2009;15:22–25. doi: 10.1016/j.ctcp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 482.Jardem P. Transcutaneous electrical nerve stimulation for non-acute low back pain: A randomized double-blind study of conventional, nu-waveform, acupuncture-type and sham therapies; Proceedings of the American Academy of Orthopaedic Surgeons Annual Meeting; San Francisco, CA, USA. 13–17 February 1997. [Google Scholar]
- 483.Jeans M. Relief of chronic pain by brief, intense transcutaneous electrical stimulation—A double blind study. In: Bonica J., Liebeskind J., Albe-Fessard D., editors. Advances in Pain Research and Therapy. Volume 3. Raven Press; New York, NY, USA: 1979. pp. 601–606. [Google Scholar]
- 484.Jiang L., Yuan D.L., Li M., Liu C., Liu Q., Zhang Y., Tan G. Combination of flunarizine and transcutaneous supraorbital neurostimulation improves migraine prophylaxis. Acta Neurol. Scand. 2019;139:276–283. doi: 10.1111/ane.13050. [DOI] [PubMed] [Google Scholar]
- 485.Juárez-Albuixech M.L., Redondo-González O., Tello I., Collado-Vázquez S., Jiménez-Antona C. Vojta Therapy versus transcutaneous electrical nerve stimulation for lumbosciatica syndrome: A quasi-experimental pilot study. J. Bodyw. Mov. Ther. 2020;24:39–46. doi: 10.1016/j.jbmt.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 486.Jünger M., Arnold A., Zuder D., Stahl H.W., Heising S. Local therapy and treatment costs of chronic, venous leg ulcers with electrical stimulation (Dermapulse): A prospective, placebo controlled, double blind trial. Wound Repair Regen. 2008;16:480–487. doi: 10.1111/j.1524-475X.2008.00393.x. [DOI] [PubMed] [Google Scholar]
- 487.Kaplan B., Peled Y., Pardo J., Rabinerson D., Hirsh M., Ovadia J., Neri A. Transcutaneous electrical nerve stimulation (TENS) as a relief for dysmenorrhea. Clin. Exp. Obstet. Gynecol. 1994;21:87–90. [PubMed] [Google Scholar]
- 488.Katz J., Melzack R. Auricular transcutaneous electrical nerve stimulation (TENS) reduces phantom limb pain. J. Pain Symptom Manag. 1991;6:73–83. doi: 10.1016/0885-3924(91)90521-5. [DOI] [PubMed] [Google Scholar]
- 489.Kempf K., Rohling M., Darwish E., Martin S., Jander S., Herdmann J., Stehr-Zirngibl S. High-tone external muscle stimulation for the treatment of chronic sciatica—A randomized controlled crossover trial. Open Pain J. 2018;11:21–30. doi: 10.2174/1876386301811010021. [DOI] [Google Scholar]
- 490.Kho H., Eijk R., Kapteijns W., van Egmond J. Acupuncture and transcutaneous stimulation analgesia in comparison with moderate-dose fentanyl anaesthesia in major surgery. Clinical efficacy and influence on recovery and morbidity. Anaesthesia. 1991;46:129–135. doi: 10.1111/j.1365-2044.1991.tb09359.x. [DOI] [PubMed] [Google Scholar]
- 491.Kocyigit F., Akalin E., Gezer N.S., Orbay O., Kocyigit A., Ada E. Functional magnetic resonance imaging of the effects of low-frequency transcutaneous electrical nerve stimulation on central pain modulation: A double-blind, placebo-controlled trial. Clin. J. Pain. 2012;28:581–588. doi: 10.1097/AJP.0b013e31823c2bd7. [DOI] [PubMed] [Google Scholar]
- 492.Kolen A.F., de Nijs R.N.J., Wagemakers F.M., Meier A.J.L., Johnson M.I. Effects of spatially targeted transcutaneous electrical nerve stimulation using an electrode array that measures skin resistance on pain and mobility in patients with osteoarthritis in the knee: A randomized controlled trial. Pain. 2012;153:373–381. doi: 10.1016/j.pain.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 493.Kolu E., Buyukavci R., Akturk S., Eren F., Ersoy Y. Comparison of high-intensity laser therapy and combination of transcutaneous nerve stimulation and ultrasound treatment in patients with chronic lumbar radiculopathy: A randomized single-blind study. Pak. J. Med. Sci. 2018;34:530–534. doi: 10.12669/pjms.343.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Koo C.C., Lin R.S., Wang T.G., Tsauo J.Y., Yang P.C., Yen C.T., Biswal S. Novel Noxipoint Therapy versus Conventional Physical Therapy for Chronic Neck and Shoulder Pain: Multicentre Randomised Controlled Trials. Sci. Rep. 2015;5:16342. doi: 10.1038/srep16342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Kumar D., Alvaro M.S., Julka I.S., Marshall H.J. Diabetic peripheral neuropathy. Effectiveness of electrotherapy and amitriptyline for symptomatic relief. Diabetes Care. 1998;21:1322–1325. doi: 10.2337/diacare.21.8.1322. [DOI] [PubMed] [Google Scholar]
- 496.Kumar D., Marshall H.J. Diabetic peripheral neuropathy: Amelioration of pain with transcutaneous electrostimulation. Diabetes Care. 1997;20:1702–1705. doi: 10.2337/diacare.20.11.1702. [DOI] [PubMed] [Google Scholar]
- 497.Labrunée M., Boned A., Granger R., Bousquet M., Jordan C., Richard L., Garrigues D., Gremeaux V., Sénard J.M., Pathak A., et al. Improved Walking Claudication Distance with Transcutaneous Electrical Nerve Stimulation: An Old Treatment with a New Indication in Patients with Peripheral Artery Disease. Am. J. Phys. Med. Rehabil. 2015;94:941–949. doi: 10.1097/PHM.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 498.Lan F., Ma Y.H., Xue J.X., Wang T.L., Ma D.Q. Transcutaneous electrical nerve stimulation on acupoints reduces fentanyl requirement for postoperative pain relief after total hip arthroplasty in elderly patients. Minerva Anestesiol. 2012;78:887–895. [PubMed] [Google Scholar]
- 499.Lanham R.H., Jr., Powell S., Hendrix B.E. Efficacy of hypothermia and transcutaneous electrical nerve stimulation in podiatric surgery. J. Foot Surg. 1984;23:152–158. [PubMed] [Google Scholar]
- 500.Lee B., Hong S.H., Kim K., Kang W.C., No J.H., Lee J.R., Jee B.C., Yang E.J., Cha E.-J., Kim Y.B. Efficacy of the device combining high-frequency transcutaneous electrical nerve stimulation and thermotherapy for relieving primary dysmenorrhea: A randomized, single-blind, placebo-controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;194:58–63. doi: 10.1016/j.ejogrb.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 501.Lee J.C., Lin D.T., Hong C.Z. The effectiveness of simultaneous thermotherapy with ultrasound and electrotherapy with combined AC and DC current on the immediate pain relief of myofascial trigger points. J. Musculoskelet. Pain. 1997;5:81–90. doi: 10.1300/J094v05n01_06. [DOI] [Google Scholar]
- 502.Lehmann T., Russell D., Spratt K. The impact of patients with nonorganic physical findings on a controlled trial of transcutaneous electrical nerve stimulation and electroacupuncture. Spine. 1983;8:625–634. doi: 10.1097/00007632-198309000-00008. [DOI] [PubMed] [Google Scholar]
- 503.Lehmann T., Russell D., Spratt K., Colby H., Liu Y., Fairchild M., Christensen S. Efficacy of electroacupuncture and TENS in the rehabilitation of chronic low back pain patients. Pain. 1986;26:277–290. doi: 10.1016/0304-3959(86)90057-6. [DOI] [PubMed] [Google Scholar]
- 504.Lerma K., Goldthwaite L.M., Blumenthal P.D., Shaw K.A. Transcutaneous electrical nerve stimulation (TENS) for pain control during first-trimester abortion: A single-blinded randomized controlled trial. Contraception. 2020;101:357. doi: 10.1016/j.contraception.2020.03.015. [DOI] [Google Scholar]
- 505.Li M., Xu F., Liu M., Li Y., Lin L., Chen J. Promoting effects and autonomic mechanisms of transcutaneous electrical acustimulation on the postoperative recovery after caesarean sections. Gastroenterology. 2019;156:S-584–S-585. doi: 10.1016/S0016-5085(19)38354-4. [DOI] [Google Scholar]
- 506.Lin Y., Yang W., Li Y., Li Y., Tong Q., Ma W., Li Y., Shen W. Mechanism of acupoint transcutaneous electric stimulation on analgesic anesthesia in the patients undergoing general anesthesia anorectal operation. Zhongguo Zhen Jiu—Chin. Acupunct. Moxibust. 2017;37:747–752. doi: 10.13703/j.0255-2930.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 507.Liu X., Li S., Wang B., An L., Ren X., Wu H. Intraoperative and postoperative anaesthetic and analgesic effect of multipoint transcutaneous electrical acupuncture stimulation combined with sufentanil anaesthesia in patients undergoing supratentorial craniotomy. Acupunct. Med. 2015;33:270–276. doi: 10.1136/acupmed-2014-010749. [DOI] [PubMed] [Google Scholar]
- 508.Loeser J., Black R., Christman A. Relief of pain by transcutaneous electrical nerve stimulation. J. Neurosurg. 1975;42:308–314. doi: 10.3171/jns.1975.42.3.0308. [DOI] [PubMed] [Google Scholar]
- 509.Lone A.R., Wafai Z.A., Buth B.A., Wani T.A., Koul P.A., Khan S.H. Analgesic efficacy of transcutaneous electrical nerve stimulation compared with diclofenac sodium in osteo-arthritis of the knee. Physiotherapy. 2003;89:478–485. doi: 10.1016/S0031-9406(05)60005-3. [DOI] [Google Scholar]
- 510.Lorenzana F.D. A randomized controlled trial of the efficacy of transcutaneous electrical nerve stimulation (TENS) versus lidocaine in the relief of episiotomy pain. Philipp. J. Obstet. Gynecol. 1999;23:135–142. [PubMed] [Google Scholar]
- 511.Lourenzi V.D.G.C.M., Jones A., Lourenzi F.M., Jennings F., Natour J. Effectiveness of the transcutaneous electrical nerve stimulation in pain control of patients with acute low back pain: A randomized controlled trial. Ann. Rheum. Dis. 2015;2:1322. doi: 10.1136/annrheumdis-2015-eular.4989. [DOI] [Google Scholar]
- 512.Lv Y., He H., Xie J., Jin W., Shou C., Pan Y., Wang L., Mo Y., Dai Q., Geng W., et al. Effects of transcutaneous acupoint electrical stimulation combined with low-dose sufentanil pretreatment on the incidence and severity of etomidate-induced myoclonus: A randomized controlled trial. Medicine. 2018;97:e10969. doi: 10.1097/MD.0000000000010969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Macdonald A.J.R., Coates T.W. The discovery of transcutaneous spinal electroanalgesia and its relief of chronic pain. Physiotherapy. 1995;81:653–661. doi: 10.1016/S0031-9406(05)66616-3. [DOI] [Google Scholar]
- 514.Malmir K., Ghotbi N., Mir S.M., Moradi B. Comparing effects of cryotherapy and transcutaneous electrical nerve stimulation on signs and symptoms of delayed onset muscle soreness in amateur athletes. Open Pain J. 2017;10:73–80. doi: 10.2174/1876386301710010073. [DOI] [Google Scholar]
- 515.Maria Fernandez-Seguin L., Marcos Heredia-Rizo A., Antonio Diaz-Mancha J., Gonzalez-Garcia P., Ramos-Ortega J., Munuera-Martinez P.V. Immediate and short-term radiological changes after combining static stretching and transcutaneous electrical stimulation in adults with cavus foot A randomized controlled trial. Medicine. 2019;98:e18018. doi: 10.1097/MD.0000000000018018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Matsuse H., Segal N.A., Rabe K.G., Shiba N. The Effect of Neuromuscular Electrical Stimulation During Walking on Muscle Strength and Knee Pain in Obese Women With Knee Pain: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2020;99:56–64. doi: 10.1097/PHM.0000000000001319. [DOI] [PubMed] [Google Scholar]
- 517.McGough J.J., Sturm A., Cowen J., Tung K., Salgari G.C., Leuchter A.F., Cook I.A., Sugar C.A., Loo S.K. Double-Blind, Sham-Controlled, Pilot Study of Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2019;58:403–411.e403. doi: 10.1016/j.jaac.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Meade C.S., Lukas S.E., McDonald L.J., Fitzmaurice G.M., Eldridge J.A., Merrill N., Weiss R.D. A randomized trial of transcutaneous electric acupoint stimulation as adjunctive treatment for opioid detoxification. J. Subst. Abuse Treat. 2010;38:12–21. doi: 10.1016/j.jsat.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Meechan J.G., Gowans A.J., Welbury R.R. The use of patient-controlled transcutaneous electronic nerve stimulation (TENS) to decrease the discomfort of regional anaesthesia in dentistry: A randomised controlled clinical trial. J. Dent. 1998;26:417–420. doi: 10.1016/S0300-5712(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 520.Melzack R. Prolonged relief of pain by brief, intense transcutaneous somatic stimulation. Pain. 1975;1:357–373. doi: 10.1016/0304-3959(75)90073-1. [DOI] [PubMed] [Google Scholar]
- 521.Melzack R., Jeans M., Stratford J., Monks R. Ice massage and transcutaneous electrical stimulation: Comparison of treatment for low-back pain. Pain. 1980;9:209–217. doi: 10.1016/0304-3959(80)90008-1. [DOI] [PubMed] [Google Scholar]
- 522.Mi Z., Gao J., Chen X., Ge Y., Lu K. Effects of transcutaneous electrical acupoint stimulation on quality of recovery during early period after laparoscopic cholecystectomy. Zhongguo Zhen Jiu—Chin. Acupunct. Moxibust. 2018;38:256–260. doi: 10.13703/j.0255-2930.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 523.Miller Jones C.M. Transcutaneous nerve stimulation in labour. Anaesthesia. 1980;35:372–375. doi: 10.1111/j.1365-2044.1980.tb05121.x. [DOI] [PubMed] [Google Scholar]
- 524.Monaco A., Sgolastra F., Pietropaoli D., Giannoni M., Cattaneo R. Comparison between sensory and motor transcutaneous electrical nervous stimulation on electromyographic and kinesiographic activity of patients with temporomandibular disorder: A controlled clinical trial. BMC Musculoskelet. Disord. 2013;14:168. doi: 10.1186/1471-2474-14-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Mucuk S., Baser M. Effects of noninvasive electroacupuncture on labour pain and duration. J. Clin. Nurs. 2014;23:1603–1610. doi: 10.1111/jocn.12256. [DOI] [PubMed] [Google Scholar]
- 526.Mummolo S., Nota A., Tecco S., Caruso S., Marchetti E., Marzo G., Cutilli T. Ultra-low-frequency transcutaneous electric nerve stimulation (ULF-TENS) in subjects with craniofacial pain: A retrospective study. Cranio. 2018;38:1–6. doi: 10.1080/08869634.2018.1526849. [DOI] [PubMed] [Google Scholar]
- 527.Murina F., Bianco V., Radici G., Felice R., Di Martino M., Nicolini U. Transcutaneous electrical nerve stimulation to treat vestibulodynia: A randomised controlled trial. BJOG—Int. J. Obstet. Gynaecol. 2008;115:1165–1170. doi: 10.1111/j.1471-0528.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 528.Murina F., Felice R., Di Francesco S., Oneda S. Vaginal diazepam plus transcutaneous electrical nerve stimulation to treat vestibulodynia: A randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018;228:148–153. doi: 10.1016/j.ejogrb.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 529.Mysliwiec A., Saulicz E., Kuszewski M., Kokosz M., Wolny T. Assessment of the influence of Saunders traction and transcutaneous electrical nerve stimulation on hand grip force in patients with neck pain. Ortop. Traumatol. Rehabil. 2011;13:37–44. doi: 10.5604/15093492.933786. [DOI] [PubMed] [Google Scholar]
- 530.Naeser M.A., Hahn K.-A.K., Lieberman B.E., Branco K.F. Carpal tunnel syndrome pain treated with low-level laser and microamperes transcutaneous electric nerve stimulation: A controlled study. Arch. Phys. Med. Rehabil. 2002;83:978–988. doi: 10.1053/apmr.2002.33096. [DOI] [PubMed] [Google Scholar]
- 531.Nakano J., Ishii K., Fukushima T., Ishii S., Ueno K., Matsuura E., Hashizume K., Morishita S., Tanaka K., Kusuba Y. Effects of transcutaneous electrical nerve stimulation on physical symptoms in advanced cancer patients receiving palliative care. Int. J. Rehabil. Res. 2020;43:62–68. doi: 10.1097/MRR.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 532.Ngai S.P., Jones A.Y., Hui-Chan C.W., Ko F.W., Hui D.S. Effect of 4 weeks of Acu-TENS on functional capacity and beta-endorphin level in subjects with chronic obstructive pulmonary disease: A randomized controlled trial. Respir. Physiol. Neurobiol. 2010;173:29–36. doi: 10.1016/j.resp.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 533.Noehren B., Dailey D.L., Rakel B.A., Vance C.G., Zimmerman M.B., Crofford L.J., Sluka K.A. Effect of transcutaneous electrical nerve stimulation on pain, function, and quality of life in fibromyalgia: A double-blind randomized clinical trial. Phys. Ther. 2015;95:129–140. doi: 10.2522/ptj.20140218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Nourbakhsh M.R., Fearon F.J. An alternative approach to treating lateral epicondylitis. A randomized, placebo-controlled, double-blinded study. Clin. Rehabil. 2008;22:601–609. doi: 10.1177/0269215507088447. [DOI] [PubMed] [Google Scholar]
- 535.Okonkwo U.P., Ibeneme S.C., Ihegihu E.Y., Egwuonwu A.V., Ezema I.C., Maruf A.F., Okoye E.C., Ibikunle O.P., Ezekwu A.O. Effects of transcutaneous electrical nerve stimulation in the Management of Post-Injection Sciatic Pain in a non-randomized controlled clinical trial in Nnewi, Nigeria. BMC Complement. Altern. Med. 2018;18:310. doi: 10.1186/s12906-018-2373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Oyibo S.O., Breislin K., Boulton A.J.M. Electrical stimulation therapy through stocking electrodes for painful diabetic neuropathy: A double blind, controlled crossover study. Diabet. Med. 2004;21:940–944. doi: 10.1111/j.1464-5491.2004.01243.x. [DOI] [PubMed] [Google Scholar]
- 537.Ozen S., Cosar S.N.S., Cabioglu M.T., Cetin N. A Comparison of Physical Therapy Modalities Versus Acupuncture in the Treatment of Fibromyalgia Syndrome: A Pilot Study. J. Altern. Complement. Med. 2019;25:296–304. doi: 10.1089/acm.2018.0330. [DOI] [PubMed] [Google Scholar]
- 538.Park J., Seo D., Choi W., Lee S.W. The effects of exercise with TENS on spasticity, balance, and gait in patients with chronic stroke: A randomized controlled trial. Med. Sci. Monit. 2014;20:1890–1896. doi: 10.12659/MSM.890926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Patel J.I., Kumar B.N.P., Ravish V.N. Effect of mckenzie method with tens on lumbar radiculopathy—A randomized controlled trial. Int. J. Physiother. 2016;3:94–99. doi: 10.15621/ijphy/2016/v3i1/88919. [DOI] [Google Scholar]
- 540.Peng T., Li X.T., Zhou S.F., Xiong Y., Kang Y., Cheng H.D. Transcutaneous electrical nerve stimulation on acupoints relieves labor pain: A non-randomized controlled study. Chin. J. Integr. Med. 2010;16:234–238. doi: 10.1007/s11655-010-0234-9. [DOI] [PubMed] [Google Scholar]
- 541.Polat C.S., Dogan A., Ozcan D.S., Koseoglu B.F., Akselim S.K., Onat S.S. The Effectiveness of Transcutaneous Electrical Nerve Stimulation in Knee Osteoarthritis with Neuropathic Pain Component: A Randomized Controlled Study. Turk Osteoporoz Derg.-Turk. J. Osteoporos. 2017;23:47–51. doi: 10.4274/tod.47113. [DOI] [Google Scholar]
- 542.Pope M., Phillips R., Haugh L., Hsieh C., MacDonald L., Haldeman S. A prospective randomized three-week trial of spinal manipulation, transcutaneous muscle stimulation, massage and corset in the treatment of subacute low back pain. Spine. 1994;19:2571–2577. doi: 10.1097/00007632-199411001-00013. [DOI] [PubMed] [Google Scholar]
- 543.Pour N.H., Kaviani M., Razeghi M. Comparison of effect of transcutaneous electrical nerve stimulation and acupressure in decreasing labor pain in primiparous women. Iran. J.Obstet. Gynecol. Infertil. 2012;15:27–33. [Google Scholar]
- 544.Quinton D.N., Sloan J.P., Theakstone J. Transcutaneous electrical nerve stimulation in acute hand infections. J. Hand Surg. Br. Eur. Vol. 1987;12:267–268. doi: 10.1016/0266-7681_87_90030-1. [DOI] [PubMed] [Google Scholar]
- 545.Radhakrishna N.R.V., Chouhan R.S., Singh S., Pandia M.P. Effect of preoperative transcutaneous electrical nerve stimulation on intraoperative anesthetic drug consumption and pain scores in patients undergoing lumbar discectomy under general anesthesia. Indian J. Pain. 2020;34:22–26. [Google Scholar]
- 546.Rapoport A.M., Bonner J.H., Lin T., Harris D., Gruper Y., Ironi A., Cowan R.P. Remote electrical neuromodulation (REN) in the acute treatment of migraine: A comparison with usual care and acute migraine medications. J. Headache Pain. 2019;20:83. doi: 10.1186/s10194-019-1033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Razavi M., Jansen G.B. Effects of acupuncture and placebo TENS in addition to exercise in treatment of rotator cuff tendinitis. Clin. Rehabil. 2004;18:872–878. doi: 10.1191/0269215504cr849oa. [DOI] [PubMed] [Google Scholar]
- 548.Reich B.A. Non-invasive treatment of vascular and muscle contraction headache: A comparative longitudinal clinical study. Headache. 1989;29:34–41. doi: 10.1111/j.1526-4610.1989.hed2901034.x. [DOI] [PubMed] [Google Scholar]
- 549.Reichstein L., Labrenz S., Ziegler D., Martin S. Effective treatment of symptomatic diabetic polyneuropathy by high-frequency external muscle stimulation. Diabetologia. 2005;48:824–828. doi: 10.1007/s00125-005-1728-0. [DOI] [PubMed] [Google Scholar]
- 550.Rodriguez-Fernandez A.L., Garrido-Santofimia V., Geita-Rodriguez J., Fernandez-De-Las-Peas C. Effects of burst-type transcutaneous electrical nerve stimulation on cervical range of motion and latent myofascial trigger point pain sensitivity. Arch. Phys. Med. Rehabil. 2011;92:1353–1358. doi: 10.1016/j.apmr.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 551.Rooney S.M., Jain S., McCormack P., Bains M.S., Martini N., Goldiner P.L. A comparison of pulmonary function tests for postthoracotomy pain using cryoanalgesia and transcutaneous nerve stimulation. Ann. Thorac. Surg. 1986;41:204–207. doi: 10.1016/S0003-4975(10)62670-7. [DOI] [PubMed] [Google Scholar]
- 552.Roth P.M., Thrash W.J. Effect of transcutaneous electrical nerve stimulation for controlling pain associated with orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 1986;90:132–138. doi: 10.1016/0889-5406(86)90045-4. [DOI] [PubMed] [Google Scholar]
- 553.Santiesteban A.J., Burnham T.L., George K.L. Primary spasmodic dysmenorrhea: The use of TENS on acupuncture points. Am. J. Acupunct. 1985;13:35–42. [Google Scholar]
- 554.Sari Z., Aydoğdu O., Demirbüken İ., Yurdalan S.U., Polat M.G. A Better Way to Decrease Knee Swelling in Patients with Knee Osteoarthritis: A Single-Blind Randomised Controlled Trial. Pain Res. Manag. 2019;2019:8514808. doi: 10.1155/2019/8514808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Schuster G.D., Infante M.C. Pain relief after low back surgery: The efficacy of transcutaneous electrical nerve stimulation. Pain. 1980;8:299–302. doi: 10.1016/0304-3959(80)90075-5. [DOI] [PubMed] [Google Scholar]
- 556.Schoenen J., Jensen R.H., Lantéri-Minet M., Láinez M.J.A., Gaul C., Goodman A.M., Caparso A., May A. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: A randomized, sham-controlled study. Cephalalgia Int. J. Headache. 2013;33:816–830. doi: 10.1177/0333102412473667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Schomburg F., Carter-Baker S. Transcutaneous electrical nerve stimulation for postlaparotomy pain. Phys. Ther. 1983;63:188–193. doi: 10.1093/ptj/63.2.188. [DOI] [PubMed] [Google Scholar]
- 558.Selfe T.K., Bourguignon C., Taylor A.G. Effects of noninvasive interactive neurostimulation on symptoms of osteoarthritis of the knee: A randomized, sham-controlled pilot study. J. Altern. Complement. Med. 2008;14:1075–1081. doi: 10.1089/acm.2008.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Shirazi Z.R., Shafaee R., Abbasi L. The effects of transcutaneous electrical nerve stimulation on joint position sense in patients with knee joint osteoarthritis. Physiother. Theory Pract. 2014;30:495–499. doi: 10.3109/09593985.2014.903547. [DOI] [PubMed] [Google Scholar]
- 560.Silberstein S.D., Mechtler L.L., Kudrow D.B., Calhoun A.H., McClure C., Saper J.R., Liebler E.J., Rubenstein Engel E., Tepper S.J., Group O.B.O.T.A.S. Non-Invasive Vagus Nerve Stimulation for the ACute Treatment of Cluster Headache: Findings From the Randomized, Double-Blind, Sham-Controlled ACT1 Study. Headache. 2016;56:1317–1332. doi: 10.1111/head.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Silberstein S.D., Calhoun A.H., Lipton R.B., Grosberg B.M., Cady R.K., Dorlas S., Simmons K.A., Mullin C., Liebler E.J., Goadsby P.J., et al. Chronic migraine headache prevention with noninvasive vagus nerve stimulation: The EVENT study. Neurology. 2016;87:529–538. doi: 10.1212/WNL.0000000000002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Simon C.B., Riley J.L., 3rd, Fillingim R.B., Bishop M.D., George S.Z. Age Group Comparisons of TENS Response Among Individuals With Chronic Axial Low Back Pain. J. Pain. 2015;16:1268–1279. doi: 10.1016/j.jpain.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Simpson K.H., Ward J. A randomized, double-blind, crossover study of the use of transcutaneous spinal electroanalgesia in patients with pain from chronic critical limb ischemia. J. Pain Symptom Manag. 2004;28:511–516. doi: 10.1016/j.jpainsymman.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 564.Solomon S., Guglielmo K.M. Treatment of headache by transcutaneous electrical stimulation. Headache. 1985;25:12–15. doi: 10.1111/j.1526-4610.1985.hed2501012.x. [DOI] [PubMed] [Google Scholar]
- 565.Solomon S., Elkind A., Freitag F., Gallagher R.M., Moore K., Swerdlow B., Malkin S. Safety and effectiveness of cranial electrotherapy in the treatment of tension headache. Headache. 1989;29:445–450. doi: 10.1111/j.1526-4610.1989.hed2907445.x. [DOI] [PubMed] [Google Scholar]
- 566.Sonde L., Kalimo H., Fernaeus S.E., Viitanen M. Low TENS treatment on post-stroke paretic arm: A three-year follow-up. Clin. Rehabil. 2000;14:14–19. doi: 10.1191/026921500673534278. [DOI] [PubMed] [Google Scholar]
- 567.Stralka S.W., Jackson J.A., Lewis A.R. Treatment of hand and wrist pain. A randomized clinical trial of high voltage pulsed, direct current built into a wrist splint. AAOHN J. 1998;46:233–236. doi: 10.1177/216507999804600502. [DOI] [PubMed] [Google Scholar]
- 568.Strayhorn G. Transcutaneous electrical nerve stimulation and postoperative use of narcotic analgesics. J. Natl. Med. Assoc. 1983;75:811–816. [PMC free article] [PubMed] [Google Scholar]
- 569.Sun K., Xing T., Zhang F., Liu Y., Li W., Zhou Z., Fang L., Yu L., Yan M. Perioperative Transcutaneous Electrical Acupoint Stimulation for Postoperative Pain Relief Following Laparoscopic Surgery: A Randomized Controlled Trial. Clin. J. Pain. 2017;33:340–347. doi: 10.1097/AJP.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 570.Sunshine W., Field T.M., Quintino O., Fierro K., Kuhn C., Burman I., Schanberg S. Fibromyalgia benefits from massage therapy and transcutaneous electrical stimulation. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 1996;2:18–22. doi: 10.1097/00124743-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 571.Takla M.K.N. Low-frequency high-intensity versus medium-frequency low-intensity combined therapy in the management of active myofascial trigger points: A randomized controlled trial. Physiother. Res. Int. 2018;23:e1737. doi: 10.1002/pri.1737. [DOI] [PubMed] [Google Scholar]
- 572.Takla M.K.N., Rezk-Allah S.S. Immediate Effects of Simultaneous Application of Transcutaneous Electrical Nerve Stimulation and Ultrasound Phonophoresis on Active Myofascial Trigger Points: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil./Assoc. Acad. Physiatr. 2018;97:332–338. doi: 10.1097/PHM.0000000000000876. [DOI] [PubMed] [Google Scholar]
- 573.Thiese M.S., Hughes M., Biggs J. Electrical stimulation for chronic non-specific low back pain in a working-age population: A 12-week double blinded randomized controlled trial. BMC Musculoskelet. Disord. 2013;14:117. doi: 10.1186/1471-2474-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Thompson J.W., Bower S., Tyrer S.P. A double blind randomised controlled clinical trial on the effect of transcutaneous spinal electroanalgesia (TSE) on low back pain. Eur. J. Pain. 2008;12:371–377. doi: 10.1016/j.ejpain.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 575.Tok F., Aydemir K., Peker F., Safaz I., Taskaynatan M.A., Ozgul A. The effects of electrical stimulation combined with continuous passive motion versus isometric exercise on symptoms, functional capacity, quality of life and balance in knee osteoarthritis: Randomized clinical trial. Rheumatol. Int. 2011;31:177–181. doi: 10.1007/s00296-009-1263-2. [DOI] [PubMed] [Google Scholar]
- 576.Tousignant-Laflamme Y., Laroche C., Beaulieu C., Bouchard A.J., Boucher S., Michaud-Létourneau M. A randomized trial to determine the duration of analgesia following a 15- and a 30-minute application of acupuncture-like TENS on patients with chronic low back pain. Physiother. Theory Pract. 2017;33:361–369. doi: 10.1080/09593985.2017.1302540. [DOI] [PubMed] [Google Scholar]
- 577.Tu Q., Gan J., Shi J., Yu H., He S., Zhang J. Effect of transcutaneous electrical acupoint stimulation on postoperative analgesia after ureteroscopic lithotripsy: A randomized controlled trial. Urolithiasis. 2019;47:279–287. doi: 10.1007/s00240-018-1056-8. [DOI] [PubMed] [Google Scholar]
- 578.Vance C.G., Chimenti R.L., Dailey D.L., Hadlandsmyth K., Zimmerman M.B., Geasland K.M., Williams J.M., Merriwether E.N., Alemo Munters L., Rakel B.A., et al. Development of a method to maximize the transcutaneous electrical nerve stimulation intensity in women with fibromyalgia. J. Pain Res. 2018;11:2269–2278. doi: 10.2147/JPR.S168297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.VanderArk G.D., McGrath K.A. Transcutaneous electrical stimulation in treatment of postoperative pain. Am. J. Surg. 1975;130:338–340. doi: 10.1016/0002-9610(75)90397-9. [DOI] [PubMed] [Google Scholar]
- 580.Vincenti E., Cervellin A., Mega M. Comparative study between patients treated with transcutaneous electric stimulation and controls during labour. Clin. Exp. Obstet. Gynaecol. 1982;9:95–97. [PubMed] [Google Scholar]
- 581.Vinterberg H., Donde R., Andersen R.B. Transcutaneous nerve-stimulation for relief of pain in patients with rheumatoid arthritis. Ugeskr. Laeger. 1978;140:1149–1150. [PubMed] [Google Scholar]
- 582.Wang W., George S., Wilimas J. Transcutaneous electrical nerve stimulation treatment of sickle cell pain crises. Acta Haematol. 1988;80:99–102. doi: 10.1159/000205612. [DOI] [PubMed] [Google Scholar]
- 583.Wang B., Tang J., White P.F., Naruse R., Sloninsky A., Kariger R., Gold J., Wender R.H. Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesth. Analg. 1997;85:406–413. doi: 10.1097/00000539-199708000-00029. [DOI] [PubMed] [Google Scholar]
- 584.Wang B., Xiong X., Li W. Study on Transcutaneous Electrical Nerve Stimulation (TENS) Applied to Acupoints for Relieving Labor Pain. J. Int. J. Clin. Acupunct. 2007;16:7. [Google Scholar]
- 585.Wang Z.L., Chen L.F., Zhu W.M. Observation on the transient analgesic effect of abdominal acupuncture TENS on pain of neck, shoulder, loin and legs. Zhongguo Zhen Jiu—Chin. Acupunct. Moxibust. 2007;27:657–659. [PubMed] [Google Scholar]
- 586.Wang K., Svensson P., Arendt-Nielsen L. Effect of acupuncture-like electrical stimulation on chronic tension-type headache: A randomized, double-blinded, placebo-controlled trial. Clin. J. Pain. 2007;23:316–322. doi: 10.1097/AJP.0b013e318030c904. [DOI] [PubMed] [Google Scholar]
- 587.Wang J.L., Ren Q.S., Shen C.C., Xie W.X., Zheng R.X., Ni J.W. Effect of transcutaneous acupoint electrical stimulation on blood bioactive compounds involving cerebral injury during craniotomy. Zhen Ci Yan Jiu. 2008;33:26–30. [PubMed] [Google Scholar]
- 588.Wang Z.X. Clinical observation on electroacupuncture at acupoints for treatment of senile radical sciatica. Zhongguo Zhen Jiu—Chin. Acupunct. Moxibust. 2009;29:126–128. [PubMed] [Google Scholar]
- 589.Wang H., Xie Y., Zhang Q., Xu N., Zhong H., Dong H., Liu L., Jiang T., Wang Q., Xiong L. Transcutaneous electric acupoint stimulation reduces intra-operative remifentanil consumption and alleviates postoperative side-effects in patients undergoing sinusotomy: A prospective, randomized, placebo-controlled trial. Br. J. Anaesth. 2014;112:1075–1082. doi: 10.1093/bja/aeu001. [DOI] [PubMed] [Google Scholar]
- 590.Ward A.R., Lucas-Toumbourou S., McCarthy B. A comparison of the analgesic efficacy of medium-frequency alternating current and TENS. Physiotherapy. 2009;95:280–288. doi: 10.1016/j.physio.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 591.Wattrisse G., Leroy B., Dufossez F., Bui Huu Tai R. Transcutaneous electric stimulation of the brain: A comparative study of the effects of its combination with peridural anesthesia using bupivacaine-fentanyl during obstetrical analgesia. Cah. Anesthesiol. 1993;41:489–495. [PubMed] [Google Scholar]
- 592.Weng C., Shu S., Chen C. The evaluation of two modulated frequency modes of acupuncture-like TENS on the treatment of tennis elbow pain. Biomed. Eng. Appl. Basis Comm. 2005;17:236–242. doi: 10.4015/S1016237205000354. [DOI] [Google Scholar]
- 593.Whitehair V.C., Chae J., Hisel T., Wilson R.D. The effect of electrical stimulation on impairment of the painful post-stroke shoulder. Top. Stroke Rehabil. 2019;26:544–547. doi: 10.1080/10749357.2019.1633796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Wieselmann-Penkner K., Janda M., Lorenzoni M., Polansky R. A comparison of the muscular relaxation effect of TENS and EMG-biofeedback in patients with bruxism. J. Oral Rehabil. 2001;28:849–853. doi: 10.1046/j.1365-2842.2001.00748.x. [DOI] [PubMed] [Google Scholar]
- 595.Williams A.E., Miller M.M., Bartley E.J., McCabe K.M., Kerr K.L., Rhudy J.L. Impairment of Inhibition of Trigeminal Nociception via Conditioned Pain Modulation in Persons with Migraine Headaches. Pain Med. 2019;20:1600–1610. doi: 10.1093/pm/pny305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Williams K.M. Is TENS a Feasible Non-Pharmacologic Adjunctive Treatment for Post-operative Orthopedic Pain? Pain Manag. Nurs. 2020;21:219. doi: 10.1016/j.pmn.2020.02.057. [DOI] [Google Scholar]
- 597.Wilson C.M., Stanczak J.F. Palliative Pain Management Using Transcutaneous Electrical Nerve Stimulation (TENS) Rehabil. Oncol. 2020;38:E1–E6. doi: 10.1097/01.REO.0000000000000188. [DOI] [Google Scholar]
- 598.Wong R.K., Jones G.W., Sagar S.M., Babjak A.F., Whelan T. A Phase I–II study in the use of acupuncture-like transcutaneous nerve stimulation in the treatment of radiation-induced xerostomia in head-and-neck cancer patients treated with radical radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003;57:472–480. doi: 10.1016/S0360-3016(03)00572-8. [DOI] [PubMed] [Google Scholar]
- 599.Wong R.K., James J.L., Sagar S., Wyatt G., Nguyen-Tan P.F., Singh A.K., Lukaszczyk B., Cardinale F., Yeh A.M., Berk L. Phase 2 results from Radiation Therapy Oncology Group Study 0537: A phase 2/3 study comparing acupuncture-like transcutaneous electrical nerve stimulation versus pilocarpine in treating early radiation-induced xerostomia. Cancer. 2012;118:4244–4252. doi: 10.1002/cncr.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Wu L.L., Su C.H., Liu C.F. Effects of noninvasive electroacupuncture at Hegu (LI4) and Sanyinjiao (SP6) acupoints on dysmenorrhea: A randomized controlled trial. J. Altern. Complement. Med. 2012;18:137–142. doi: 10.1089/acm.2010.0506. [DOI] [PubMed] [Google Scholar]
- 601.Xie J., Chen L.H., Ning Z.Y., Zhang C.Y., Chen H., Chen Z., Meng Z.Q., Zhu X.Y. Effect of transcutaneous electrical acupoint stimulation combined with palonosetron on chemotherapy-induced nausea and vomiting: A single-blind, randomized, controlled trial. Chin. J. Cancer. 2017;36:6. doi: 10.1186/s40880-016-0176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Xu G., Feng Y., Tang W.Z., Lv Z.W. Transcutaneous electrical nerve stimulation in combination with cobalamin injection for postherpetic neuralgia: A single-center randomized controlled trial. Am. J. Phys. Med. Rehabil./Assoc. Acad. Physiatr. 2014;93:287–298. doi: 10.1097/PHM.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 603.Yarnitsky D., Volokh L., Ironi A., Weller B., Shor M., Shifrin A., Granovsky Y. Nonpainful remote electrical stimulation alleviates episodic migraine pain. Neurology. 2017;88:1250–1255. doi: 10.1212/WNL.0000000000003760. [DOI] [PubMed] [Google Scholar]
- 604.Yang G., Su Y.X., Guo Y.X. Acupuncture like transcutaneous electrical nerve stimulation (Tens) on knee osteoarthritis (KOA) with low pain: A pilot study. Int. J. Clin. Exp. Med. 2017;10:1059–1065. [Google Scholar]
- 605.Yang Y., Yim J., Choi W., Lee S. Improving slow-transit constipation with transcutaneous electrical stimulation in women: A randomized, comparative study. Women Health. 2017;57:494–507. doi: 10.1080/03630242.2016.1176098. [DOI] [PubMed] [Google Scholar]
- 606.Yao Y., Zhao Q., Gong C., Wu Y., Chen Y., Qiu L., Wu X., Chen Y. Transcutaneous Electrical Acupoint Stimulation Improves the Postoperative Quality of Recovery and Analgesia after Gynecological Laparoscopic Surgery: A Randomized Controlled Trial. Evid.-Based Complement. Alternat. Med. 2015;2015:324360. doi: 10.1155/2015/324360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Yarnitsky D., Dodick D.W., Grosberg B.M., Burstein R., Ironi A., Harris D., Lin T., Silberstein S.D. Remote Electrical Neuromodulation (REN) Relieves Acute Migraine: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Headache J. Head Face Pain. 2019;59:1240–1252. doi: 10.1111/head.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Yeh M.L., Chung Y.C., Chen K.M., Tsou M.Y., Chen H.H. Acupoint electrical stimulation reduces acute postoperative pain in surgical patients with patient-controlled analgesia: A randomized controlled study. Altern. Ther. Health Med. 2010;16:10–18. [PubMed] [Google Scholar]
- 609.Yeh M.-L., Chung Y.-C., Hsu L.-C., Hung S.-H. Effect of transcutaneous acupoint electrical stimulation on post-hemorrhoidectomy-associated pain, anxiety, and heart rate variability: A randomized-controlled study. Clin. Nurs. Res. 2018;27:450–466. doi: 10.1177/1054773816685745. [DOI] [PubMed] [Google Scholar]
- 610.Yip Y.B., Sonny Tse H.M., Wu K.K. An experimental study comparing the effects of combined transcutaneous acupoint electrical stimulation and electromagnetic millimeter waves for spinal pain in Hong Kong. Complement. Ther. Clin. Pract. 2007;13:4–14. doi: 10.1016/j.ctcp.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 611.Youssef T., Youssef M., Thabet W., Lotfy A., Shaat R., Abd-Elrazek E., Farid M. Randomized clinical trial of transcutaneous electrical posterior tibial nerve stimulation versus lateral internal sphincterotomy for treatment of chronic anal fissure. Int. J. Surg. 2015;22:143–148. doi: 10.1016/j.ijsu.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 612.Yu X., Zhang F., Chen B. The effect of TEAS on the quality of early recovery in patients undergoing gynecological laparoscopic surgery: A prospective, randomized, placebo-controlled trial. Trials. 2020;21:43. doi: 10.1186/s13063-019-3892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Zeb A., Arsh A., Bahadur S., Ilyas S.M. Effectiveness of transcutaneous electrical nerve stimulation in management of neuropathic pain in patients with post traumatic incomplete spinal cord injuries. Pak. J. Med. Sci. 2018;34:1177–1180. doi: 10.12669/pjms.345.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Zhan W., Tian W. Addition of transcutaneous electric acupoint stimulation to transverse abdominis plane block for postoperative analgesia in abdominal surgery: A randomized controlled trial. Eur. J. Integr. Med. 2020;35:101087. doi: 10.1016/j.eujim.2020.101087. [DOI] [Google Scholar]
- 615.Zhang Q., Gao Z., Wang H., Ma L., Guo F., Zhong H., Xiong L., Wang Q. The effect of pre-treatment with transcutaneous electrical acupoint stimulation on the quality of recovery after ambulatory breast surgery: A prospective, randomised controlled trial. Anaesthesia. 2014;69:832–839. doi: 10.1111/anae.12639. [DOI] [PubMed] [Google Scholar]
- 616.Zhang H., Lei C., Zhang T.Y., Hou L.H., Wang Q., Dong H.L., Han J.G., Xiong L.Z. Randomized controlled trial of TEAS with different acupoints combination on opioids consumption in patients undergoing off-pump coronary artery bypass grafting. Int. J. Clin. Exp. Med. 2016;9:23060–23071. [Google Scholar]
- 617.Zhang C., Xiao Z., Zhang X., Guo L., Sun W., Tai C., Jiang Z., Liu Y. Transcutaneous electrical stimulation of somatic afferent nerves in the foot relieved symptoms related to postoperative bladder spasms. BMC Urol. 2017;17:58. doi: 10.1186/s12894-017-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Zhang X.Y., Fu Y., Zhang X.D., Ding J.X., Hua K.Q. The effect of transcutaneous electrical stimulation treatment in combination with intraoperative nerve staining on sexual function after radical hysterectomy: A pilot study. Eur. J. Gynaecol. Oncol. 2020;41:188–191. doi: 10.31083/j.ejgo.2020.02.4954. [DOI] [Google Scholar]
- 619.Zhao W., Wang C., Li Z., Chen L., Li J., Cui W., Ding S., Xi Q., Wang F., Jia F., et al. Efficacy and safety of transcutaneous electrical acupoint stimulation to treat muscle spasticity following brain injury: A double-blinded, multicenter, randomized controlled trial. PLoS ONE. 2015;10:e0116976. doi: 10.1371/journal.pone.0116976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Zhou D., Hu B., He S., Li X., Gong H., Li F., Wang Q. Transcutaneous electrical acupoint stimulation accelerates the recovery of gastrointestinal function after cesarean section: A randomized controlled trial. Evid.-Based Complement. Med. 2018;2018:7341920. doi: 10.1155/2018/7341920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Zizic T.M., Hoffman K.C., Holt P.A., Hungerford D.S., O’Dell J.R., Jacobs M.A., Lewis C.G., Deal C.L., Caldwell J.R., Cholewczynski J.G., et al. The treatment of osteoarthritis of the knee with pulsed electrical stimulation. J. Rheumatol. 1995;22:1757–1761. [PubMed] [Google Scholar]
- 622.Cherian J.J., Kapadia B.H., Bhave A., McElroy M.J., Cherian C., Harwin S.F., Mont M.A. Use of Transcutaneous Electrical Nerve Stimulation Device in Early Osteoarthritis of the Knee. J. Knee Surg. 2015;28:321–327. doi: 10.1055/s-0034-1389160. [DOI] [PubMed] [Google Scholar]
- 623.Cherian J.J., Kapadia B.H., McElroy M.J., Johnson A.J., Bhave A., Harwin S.F., Mont M.A. Knee Osteoarthritis: Does Transcutaneous Electrical Nerve Stimulation Work? Orthopedics. 2016;39:e180–e186. doi: 10.3928/01477447-20151222-02. [DOI] [PubMed] [Google Scholar]
- 624.Lewis M., Chesterton L.S., Sim J., Mallen C.D., Hay E.M., Van Der Windt D.A., Griffiths U.K. An economic evaluation of TENS in addition to usual primary care management for the treatment of tennis elbow: Results from the TATE randomized controlled trial. PLoS ONE. 2015;10:e0135460. doi: 10.1371/journal.pone.0135460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Escortell Mayor E., Lebrijo Perez G., Perez Martin Y., Asunsolo del Barco A., Riesgo Fuertes R., Saa Requejo C. Randomized clinical trial for primary care patients with neck pain: Manual therapy versus electrical stimulation. Aten. Primaria/Soc. Esp. Med. Fam. Comunitaria. 2008;40:337–343. doi: 10.1157/13124126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Oosterhof J., Samwel H.J., de Boo T.M., Wilder-Smith O.H., Oostendorp R.A., Crul B.J. Predicting outcome of TENS in chronic pain: A prospective, randomized, placebo controlled trial. Pain. 2008;136:11–20. doi: 10.1016/j.pain.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 627.Oosterhof J., Wilder-Smith O.H., de Boo T., Oostendorp R.A., Crul B.J. The Long-Term Outcome of Transcutaneous Electrical Nerve Stimulation in the Treatment for Patients with Chronic Pain: A Randomized, Placebo-Controlled Trial. Pain Pract. 2012;12:513–522. doi: 10.1111/j.1533-2500.2012.00533.x. [DOI] [PubMed] [Google Scholar]
- 628.Oosterhof J., Wilder-Smith O.H., Oostendorp R.A., Crul B.J. Different mechanisms for the short-term effects of real versus sham transcutaneous electrical nerve stimulation (TENS) in patients with chronic pain: A pilot study. J. Pain Palliat. Care Pharmacother. 2012;26:5–12. doi: 10.3109/15360288.2011.650352. [DOI] [PubMed] [Google Scholar]
- 629.Pietrosimone B.G., Saliba S.A., Hart J.M., Hertel J., Kerrigan D.C., Ingersoll C.D. Effects of disinhibitory transcutaneous electrical nerve stimulation and therapeutic exercise on sagittal plane peak knee kinematics and kinetics in people with knee osteoarthritis during gait: A randomized controlled trial. Clin. Rehabil. 2010;24:1091–1101. doi: 10.1177/0269215510375903. [DOI] [PubMed] [Google Scholar]
- 630.Paley C.A., Wittkopf P.G., Jones G., Johnson M.I. Does TENS Reduce the Intensity of Acute and Chronic Pain? A Comprehensive Appraisal of the Characteristics and Outcomes of 169 Reviews and 49 Meta-Analyses. Medicina. 2021;57:1060. doi: 10.3390/medicina57101060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Johnson M., Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: A meta-analysis of randomized controlled trials. Pain. 2007;130:157–165. doi: 10.1016/j.pain.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 632.Sawant A., Dadurka K., Overend T., Kremenchutzky M. Systematic review of efficacy of TENS for management of central pain in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2015;4:219–227. doi: 10.1016/j.msard.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 633.Kroeling P., Gross A., Goldsmith C.H., Burnie S.J., Haines T., Graham N., Brant A. Electrotherapy for neck pain. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD004251.pub5. [DOI] [PubMed] [Google Scholar]
- 634.Zhu J., Xu Q., Zou R., Wu W., Wang X., Wang Y., Ji F., Zheng Z., Zheng M. Distal acupoint stimulation versus peri-incisional stimulation for postoperative pain in open abdominal surgery: A systematic review and implications for clinical practice. BMC Complement. Altern. Med. 2019;19:192. doi: 10.1186/s12906-019-2583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Zhou J., Dan Y., Yixian Y., Lyu M., Zhong J., Wang Z., Zhu Y., Liu L. Efficacy of Transcutaneous Electronic Nerve Stimulation in Postoperative Analgesia After Pulmonary Surgery A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2020;99:241–249. doi: 10.1097/PHM.0000000000001312. [DOI] [PubMed] [Google Scholar]
- 636.Rutjes A.W., Nuesch E., Sterchi R., Kalichman L., Hendriks E., Osiri M., Brosseau L., Reichenbach S., Juni P. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2009:CD002823. doi: 10.1002/14651858.CD002823.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Wu L.C., Weng P.W., Chen C.H., Huang Y.Y., Tsuang Y.H., Chiang C.J. Literature Review and Meta-Analysis of Transcutaneous Electrical Nerve Stimulation in Treating Chronic Back Pain. Reg. Anesth. Pain Med. 2018;43:425–433. doi: 10.1097/AAP.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Sbruzzi G., Silveira S.A., Silva D.V., Coronel C.C., Plentz R.D. Transcutaneous electrical nerve stimulation after thoracic surgery: Systematic review and meta-analysis of 11 randomized trials. Rev. Bras. Cir. Cardiovasc. Orgao Of. Soc. Bras. Cir. Cardiovasc. 2012;27:75–87. doi: 10.5935/1678-9741.20120012. [DOI] [PubMed] [Google Scholar]
- 639.Ioannidis J.P. Why Most Clinical Research Is Not Useful. PLoS Med. 2016;13:e1002049. doi: 10.1371/journal.pmed.1002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Bennett M.I., Hughes N., Johnson M.I. Methodological quality in randomised controlled trials of transcutaneous electric nerve stimulation for pain: Low fidelity may explain negative findings. Pain. 2011;152:1226–1232. doi: 10.1016/j.pain.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 641.Sluka K.A., Bjordal J.M., Marchand S., Rakel B.A. What Makes Transcutaneous Electrical Nerve Stimulation Work? Making Sense of the Mixed Results in the Clinical Literature. Phys. Ther. 2013;93:1397–1402. doi: 10.2522/ptj.20120281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Moore R.A., Gavaghan D., Tramer M.R., Collins S.L., McQuay H.J. Size is everything—Large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78:209–216. doi: 10.1016/S0304-3959(98)00140-7. [DOI] [PubMed] [Google Scholar]
- 643.Rakel B., Cooper N., Adams H.J., Messer B.R., Frey Law L.A., Dannen D.R., Miller C.A., Polehna A.C., Ruggle R.C., Vance C.G., et al. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. J. Pain. 2010;11:230–238. doi: 10.1016/j.jpain.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Deyo R.A., Walsh N.E., Schoenfeld L.S., Ramamurthy S. Can trials of physical treatments be blinded? The example of transcutaneous electrical nerve stimulation for chronic pain. Am. J. Phys. Med. Rehabil./Assoc. Acad. Physiatr. 1990;69:6–10. doi: 10.1097/00002060-199002000-00003. [DOI] [PubMed] [Google Scholar]
- 645.Grovle L., Hasvik E., Haugen A.J. Rescue and concomitant analgesics in placebo-controlled trials of pharmacotherapy for neuropathic pain and low back pain. Pain. 2020;161:3–10. doi: 10.1097/j.pain.0000000000001690. [DOI] [PubMed] [Google Scholar]
- 646.Bjordal J.M., Johnson M.I., Ljunggreen A.E. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur. J. Pain. 2003;7:181–188. doi: 10.1016/S1090-3801(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 647.Hunsinger M., Smith S.M., Rothstein D., McKeown A., Parkhurst M., Hertz S., Katz N.P., Lin A.H., McDermott M.P., Rappaport B.A., et al. Adverse event reporting in nonpharmacologic, noninterventional pain clinical trials: ACTTION systematic review. Pain. 2014;155:2253–2262. doi: 10.1016/j.pain.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 648.NICE Osteoarthritis: Care and Management. [(accessed on 29 August 2019)]. Available online: http://nice.org.uk/guidance/cg177.
- 649.NICE Rheumatoid Arthritis in Adults. [(accessed on 17 October 2019)]. Available online: www.nice.org.uk/NG100.
- 650.National Institute for Health and Care Excellence . Low Back Pain and Sciatica in Over 16s: Assessment and Management. Clinical Guideline [NG59] National Institute for Health and Care Excellence (NICE); London, UK: 2016. pp. 1–18. [Google Scholar]
- 651.National Institute for Health and Care Excellence . Chronic Pain (Primary and Secondary) in Over 16s: Assessment of All Chronic Pain and Management of Chronic Primary Pain (NG193) National Institute for Health and Care Excellence (NICE); London, UK: 2021. pp. 1–36. [PubMed] [Google Scholar]
- 652.National Institute for Health and Clinical Excellence . NICE Clinical Guideline 55 Intrapartum Care: Care of Healthy Women and Their Babies during Childbirth. NICE; London, UK: 2007. pp. 1–65. [Google Scholar]
- 653.Gladwell P.W., Badlan K., Cramp F., Palmer S. Direct and Indirect Benefits Reported by Users of Transcutaneous Electrical Nerve Stimulation for Chronic Musculoskeletal Pain: Qualitative Exploration Using Patient Interviews. Phys. Ther. 2015;95:1518–1528. doi: 10.2522/ptj.20140120. [DOI] [PubMed] [Google Scholar]
- 654.Gladwell P.W., Badlan K., Cramp F., Palmer S. Problems, Solutions, and Strategies Reported by Users of Transcutaneous Electrical Nerve Stimulation for Chronic Musculoskeletal Pain: Qualitative Exploration Using Patient Interviews. Phys. Ther. 2016;96:1039–1048. doi: 10.2522/ptj.20150272. [DOI] [PubMed] [Google Scholar]
- 655.Gozani S.N., Ferree T.C., Moynihan M., Kong X. Impact of transcutaneous electrical nerve stimulation on sleep in chronic low back pain: A real-world retrospective cohort study. J. Pain Res. 2019;12:743–752. doi: 10.2147/JPR.S196129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Gladwell P.W., Cramp F., Palmer S. Matching the perceived benefits of Transcutaneous Electrical Nerve Stimulation (TENS) for chronic musculoskeletal pain against Patient Reported Outcome Measures using the International Classification of Functioning, Disability and Health (ICF) Physiotherapy. 2020;106:128–135. doi: 10.1016/j.physio.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 657.Gladwell P.W., Cramp F., Palmer S. Foundational Research Could Improve Future Transcutaneous Electrical Nerve Stimulation Evaluations. Medicina. 2022;58:149. doi: 10.3390/medicina58020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Sluka K., Walsh D. Mechanisms and Management of Pain for the Physical Therapist. 2nd ed. IASP Press; Philidelphia, PA, USA: 2016. Chapter 8: Transcutaneous Electrical Nerve Stimulation and Interferential Therapy; pp. 203–224. [Google Scholar]
- 659.Sluka K.A., Vance C.G., Lisi T.L. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. J. Neurochem. 2005;95:1794–1801. doi: 10.1111/j.1471-4159.2005.03511.x. [DOI] [PubMed] [Google Scholar]
- 660.Johnson M.I., Ashton C.H., Thompson J.W. An in-depth study of long-term users of transcutaneous electrical nerve stimulation (TENS). Implications for clinical use of TENS. Pain. 1991;44:221–229. doi: 10.1016/0304-3959(91)90089-G. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available as yet due to the investigating team continuing to undertake secondary analyses.