Abstract
Background
As the Coronavirus Disease-2019 (COVID-19) pandemic continues, healthcare providers struggle to manage both COVID-19 and non-COVID patients while still providing high-quality care. We conducted a systematic review/meta-analysis to describe the effects of the COVID-19 pandemic on patients with non-COVID illness and on healthcare systems compared to non-pandemic epochs.
Methods
We searched Ovid MEDLINE/EMBASE/Cochrane Database of Systematic Reviews/CENTRAL/CINAHL (inception to December 31, 2020). All study types with COVID-pandemic time period (after December 31, 2019) with comparative non-pandemic time periods (prior to December 31, 2019). Data regarding study characteristics/case-mix/interventions/comparators/ outcomes (primary: mortality; secondary: morbidity/hospitalizations/disruptions-to-care. Paired reviewers conducted screening and abstraction, with conflicts resolved by discussion. Effect sizes for specific therapies were pooled using random-effects models. Risk of bias was assessed by Newcastle-Ottawa Scale, with evidence rating using GRADE methodology.
Results
Of 11,581 citations, 167 studies met eligibility. Our meta-analysis showed an increased mortality of 16% during the COVID pandemic for non-COVID illness compared with 11% mortality during the pre-pandemic period (RR 1.38, 95% CI: 1.28–1.50; absolute risk difference: 5% [95% CI: 4–6%], p<0.00001, very low certainty evidence). Twenty-eight studies (17%) reported significant changes in morbidity (where 93% reported increases), while 30 studies (18%) reported no significant change (very low certainty). Thirty-nine studies (23%) reported significant changes in hospitalizations (97% reporting decreases), while 111 studies (66%) reported no significant change (very low certainty). Sixty-two studies (37%) reported significant disruptions in standards-to-care (73% reporting increases), while 62 studies (37%) reported no significant change (very low certainty).
Conclusions
There was a significant increase in mortality during the COVID pandemic compared to pre-pandemic times for non-COVID illnesses. When significant changes were reported, there was increased morbidity, decreased hospitalizations and increased disruptions in standards-of-care.
Systematic review registration
PROSPERO CRD42020201256 (Sept 2, 2020).
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease-19 (COVID-19), has spread globally to over 180 countries on 6 continents with over 500 million confirmed cases of COVID-19 worldwide, and over 6 million deaths [1, 2]. The COVID-19 pandemic has contributed to widespread disruption to the delivery of non-urgent healthcare services (e.g., scheduled surgical and elective procedure postponements/cancellations, delayed and missed cancer screening) [3] to create health system capacity and prioritize acute care access for patients with COVID-19. This has been further compounded by successive waves of surging case counts with incomplete opportunity for health systems recovery in between [4].
This shift in prioritization of the health system may have unintentional and underappreciated effects on patients without COVID, including altered access to health services and/or altered models of care. The pandemic may be contributing to substantial negative consequences for patients [5, 6] along with indirect and unintended harm reduction (e.g., reduced exposure to low-value healthcare). As an illustration, during the pandemic, patients have been found to have delayed presentations to hospital for several non-COVID urgent illnesses (e.g., stroke, acute coronary syndrome, intoxications, etc.), often due to patients’ perception to strictly adhere to public health interventions and/or fearing risk of contracting COVID-19 in hospitals [7–9]. Healthcare professionals and health systems have operated under considerable strain and may have struggled to maintain usual standards-of-care for patients admitted with non-COVID illnesses, while also having adapting to meet expanded care needs for patients with COVID-19 [10]. While the collateral damage on health systems of the COVID-19 pandemic has enormous potential global public health importance, it has remained largely unquantified.
Accordingly, to focus attention on this issue, we conducted a systematic review (SR) and meta-analysis (MA) to describe the effects of the COVID-19 pandemic on non-COVID outcomes with respect to patient mortality, morbidity, acute care hospitalizations and disruptions to standards-of-care (both at the population and healthcare system levels). Our SR serves to inform health care leaders, professionals and health policy makers, who have generated and implemented policy to prioritize resources throughout the COVID-19 pandemic, of the potential widespread impact of COVID-19 on capacity to sustainably provide standards-of-care and optimize outcomes for patients presenting with illnesses unrelated to COVID-19.
Methods
Searches and inclusion criteria
This SR was conducted and reported in accordance with the PRISMA guidelines [11], and was registered in PROSPERO (international prospective register of systematic reviews) on September 2, 2020 (CRD42020201256). The complete PRISMA checklist is included (S1 Table).
We systematically searched Ovid MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Controlled Trials Register (CENTRAL), and Cumulative Index to Nursing and Allied Health Literature (CINAHL) from inception 1948 to December 31, 2020. Last search was completed on Dec 31, 2020. Searches were performed by a research librarian (DKL), and were adjudicated by a second health information specialist (MS) using Peer Review Electronic Search Strategy (PRESS) criteria (S1 Appendix) [12].
We used a combination of subject headings and keywords: mortality; morbidity; pandemic; non-pandemic time periods; outcomes; healthcare disruption; healthcare system delivery; public health policy/measures; societal/public behaviour; acute care hospitalizations; occupancy rates; economics. We also screened reference lists of identified relevant individual studies and reviews.
Operational definitions
Exposure and study and control time periods were defined as during the COVID-19 pandemic (December 31, 2019 to December 31, 2020) compared to non-COVID-19 pandemic time periods (December 1948 to December 31, 2019).
Mortality was evaluated at the longest time interval provided for each study, and classified as increased or decreased relative to pre-pandemic epochs.
Morbidity was defined as the state of being symptomatic or unhealthy for a disease or condition [13], and as specifically defined in the individual studies relevant to the reported base health outcome.
A “disruption to standards-of-care” was defined as any change to a delivered health service (e.g., time to presentation or arrival, cancellation or delay to timely surgery or procedure, or diagnosis and/or treatment intervention, follow-up, etc.) which had a statistically significant change during the COVID-19 pandemic period as compared to a non-COVID pandemic historical control period (e.g., same months) [3, 14].
Eligibility criteria
Articles were considered eligible if they met the following criteria: (1) adult patients (≥18 years old); (2) randomized control trials (RCTs), observational studies and case series with control groups at any level (e.g., population level, healthcare facilities, etc.). We excluded all animal and pediatric studies. Conference abstracts and non-peer reviewed websites were excluded. We excluded case reports and case series without control groups. No language restrictions were applied.
Study selection and data abstraction
Paired reviewers (VL, SD, HC, PG, DL, BM, AVL, MS, KL, BK, DC, AA) independently screened the titles and abstracts of identified articles. Articles deemed potentially eligible by either or both reviewers advanced to the full-text review stage, and were screened for inclusion by paired reviewers (including pilot testing against eligibility criteria). Disagreements at this stage were resolved through discussion and consultation with a third reviewer, if necessary. We used Covidence (Veritas Health Innovation, Melbourne, Australia) to manage search results, screening, and selection of studies [15]. Our data abstraction is outlined in S2 Table.
An a priori data abstraction tool was piloted for all reviewers and was subsequently used to collect the following data from eligible articles: study characteristics (title, author), patient group demographic/clinical data, interventions and comparators, clinical outcome data (including morbidity and mortality, acute care hospitalizations/occupancy rates and disruptions to care), and jurisdiction(s) in which the study was performed.
Risk of bias assessment
We assessed risk of bias in observational cohort and case-control studies using the Newcastle-Ottawa Scale (NOS), examining the following domains: selection, comparability and exposure for cohort and case-control studies. Each of the criteria for the NOS scales for cohort/case-control studies are found in the footnotes [16]. Quality of the studies were based on either good (3–4 stars in selection domain and 1–2 stars in comparability domain and 2–3 stars in outcome/exposure domain), fair (2 stars in selection domain and 1–2 stars in comparability domain and 2–3 stars in outcome/exposure domain) or poor (0–1 star in selection domain or 0 stars in comparability domain or 0–1 stars in outcome/exposure domain) [16].
Grading of recommendations assessment, development and evaluation
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the following domains for each clinical outcome: individual study risk of bias, indirectness, imprecision, inconsistency and publication bias. Certainty in evidence from observational studies started as low, with RCTs starting as high. Final certainty was rated as high, moderate, low or very low [17–19].
Data synthesis and analysis
Continuous data was presented as means and standard deviations (SD), or medians and inter-quartile ranges (IQR), and were compared (where appropriate) using a t-test or Wilcoxon rank sum test. Categorical variables and proportions were compared using the Pearson’s Chi-Square or Fischer’s exact tests as appropriate. We summarized the eligible studies in terms of point estimates or proportions, with p-values or 95% confidence intervals [CIs], if available. Significance was set at 0.05.
We performed a meta-analysis of observational studies in this SR, with RevMan (Copenhagen: The Nordic Cochrane Centre, Cochrane Collaboration 2014) version 5.4 software for the outcome of mortality. We will use the method of DerSimonian and Laird to pool effect sizes for each outcome under a random-effects model for all outcomes of interest [20]. Study weights were measured using the inverse variance method. We presented the results as relative risk (RR) with 95% confidence intervals (CIs) for dichotomous outcomes [21]. We assessed heterogeneity using the I2 statistic, the χ2 test for homogeneity (p <0.1 for significance of substantial heterogeneity). We considered an I2 value greater than 50% indicative of substantial heterogeneity. We investigated further with subgroup analyses to assess clinical and methodological sources of heterogeneity. We assessed for publication bias using Begg’s funnel plots if there are 10 or more studies per outcome [21–23].
Given the heterogeneity, variation and disparate reporting for morbidity, hospitalizations/occupancy, disruptions in standards-of-care, we could not conduct a meta-analysis for these outcomes.
Subgroup analyses
Potential and expected clinical sources of heterogeneity were explored for selected outcomes (e.g. mortality). When a sufficient number of trials were available (e.g. >10 studies), we conducted the following pre-specified subgroup pooled analyses (hypothesized direction of effect in parentheses):
High vs. low risk of bias studies (hypothesis: high risk of bias studies would favour pre-pandemic usual care management outcomes).
High (HIC) vs. low-middle income (LMIC) countries, as defined by World Health Organization [2] (hypothesis: outcomes would favour HIC during both pandemic and pre-pandemic times)
Acute care hospital vs. jurisdictional/public health/population restrictions/interventions (hypothesis: acute care/public health interventions would be favoured during pandemic times)
Medical vs. surgical vs. medical/surgical case-mixes (hypothesis: surgical health care interventions would be favoured during pandemic times compared to medical cases)
If subgroups effects were credible, we presented the outcomes separately for each subgroup.
Dealing with missing data
If we encountered missing data, we attempted to contact the study authors for additional information or clarity. If we could not obtain additional data, we analyzed the available data and reported the potential impact of missing data in the discussion.
Results
Study characteristics
Of 11,581 records identified through our search, we reviewed 336 full-texts, and included 167 studies which fulfilled eligibility criteria (Fig 1). Summary of study characteristics are presented in Table 1. A complete list of all collected study data, demographics, baseline characteristics, subgroups and outcomes can be found in S2–S4 Tables [24–188].
Fig 1. COPES PRISMA flow diagram (non-COVID illness).
Table 1. Summary statistics of study design and characteristics for COPES Non-COVID illness during COVID pandemic (n = 168).
| Publication Status | n (%) | Country | n (%) |
| Peer-reviewed publication | 161 (96%) | Multinational | 4 (2%) |
| Pre-print | 6 (4%) | Single | 163 (98%) |
| Study Design | Primary Illness Category | ||
| Observational (cohort) | 164 (98%) | Cardiovascular | 51 (30%) |
| Observational (case-control) | 2 (1%) | Mixed multi-illness | 45 (27%) |
| Case-series with control group | 1 (1%) | Neurologic | 26 (16%) |
| Trauma | 12 (7%) | ||
| REB approval | Respiratory | 8 (5%) | |
| Yes | 91 (54%) | Gastrointestinal | 8 (5%) |
| Waived/not required | 46 (27%) | Infectious | 5 (3%) |
| Not reported | 25 (16%) | Musculoskeletal/skin and soft tissue | 5 (3%) |
| Not applicable | 5 (3.0%) | Urologic | 4 (2%) |
| Head and neck | 3 (2%) | ||
| Consent obtained | Transplant | 2 (1%) | |
| Yes | 22 (13%) | Metabolic/toxins | 1 (1%) |
| Waived/not required | 76 (45%) | Renal | 1 (1%) |
| Not reported | 59 (36%) | ||
| Not applicable | 10 (6.0%) | Subgroups: | |
| Risk of bias | |||
| Funding | Good (low risk of bias) | 25 (15%) | |
| Industry | 2 (1%) | Poor (high risk of bias) | 142 (85%) |
| Government | 23 (13%) | ||
| Institutional | 18 (11%) | High vs. low/middle income country | |
| Non-for-profit | 9 (5%) | High | 146 (88%) |
| Other | 6 (4%) | Low/middle | 21 (12%) |
| None | 75 (45%) | ||
| Not reported | 47 (28%) | Case-Mix | |
| Medical | 59 (36%) | ||
| Setting | Surgical | 40 (24%) | |
| Acute care hospital | 111 (67%) | Mixed (medical/surgical) | 68 (41%) |
| Emergency department | 26 (16%) | ||
| Ward | 20 (12%) | Level of healthcare intervention | |
| Intensive care unit | 15 (9%) | Acute care hospital level interventions | 134 (80%) |
| Other/Not applicable | 22 (13%) | Jurisdiction/public health/population level interventions | 33 (20%) |
COPES: Coronavirus Disease (COVID-19) and Outcomes Associated with Pandemic Effects Study (COPES), COVID-19: Coronavirus Disease-2019, REB: research ethics board
Of the 167 studies, there were 164 (98%) observational cohort studies and 2 (1%) case-control studies, and 1 (1%) case-series with control groups. The predominant setting for these studies was acute care hospitals (111 studies, 66%). These studies were largely conducted in a single country (163 studies, 97%) with 35 individual countries contributing (highest was the United States with 31 studies) (Table 1).
The top five primary illness categories were as follows: cardiovascular (51 studies, 30%); mixed multi-illness (45 studies, 27%); neurological (26 studies, 16%); trauma (12 studies, 7%); and, respiratory or gastrointestinal (8 studies, 5%), each (Table 1).
Risk of bias
The risks of bias (RoB) assessments using the Newcastle-Ottawa Scale tools for observational studies are shown in S5A and S5B Table cohort (5A) and case-control (5B), respectively.
For cohort studies (S5A Table), NOS tools revealed full scores for only 14 out of 163 studies (9%). Common deficiencies were found in 150 (92%) studies, with plurality in the following areas: lack of comparability of cohorts (111 studies, 68%), lack of long enough follow-up (113 studies, 69%), and lack of adequate follow-up (111 studies, 68%).
For case-control studies (S5B Table), NOS tools revealed full scores for 1 out of 3 (33%) of studies. Deficiencies were found in comparability of cases and controls, and non-response rate.
Data synthesis and analysis
Primary and secondary outcomes and GRADE assessments
Study outcomes are presented in S6 Table, with summary of significant changes in mortality (primary outcome), morbidity, acute care hospitalizations/occupancy and disruptions to care (secondary outcomes) presented in S7 Table. GRADE assessment is shown in Table 2. We found an overall “very low” certainty of evidence for non-COVID illnesses during the COVID-19 pandemic period for all outcomes (mortality, morbidity, acute care hospitalizations/occupancy, disruptions to care).
Table 2. Grading of Recommendations Assessment, Development and Evaluation (GRADE) of COPES outcomes: Mortality, morbidity, hospitalizations, disruptions to care.
| Certainty assessment | Study Measurements/Results/Impact | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design (sources) | Risk of bias | In-consistency | Indirect-ness | Im- precision | Other considerations a | |||
| Mortality | |||||||||
| 76 | Observational studies(74 cohort, 2 case-control) Sample size (76 studies): • 353,539 control patients (pre-pandemic) • 138,323 pandemic period patients |
very serious b | serious c | not serious d | not serious e | none f | • Study results (meta-analysis, 76 studies): • Absolute effect estimates—mortality events (76 studies): ○ Pandemic: 22,348 deaths/138,323 patients (16%) ○ Pre-pandemic: 40,768 deaths/354,539 patients (11%) ○ Absolute difference: 5% fewer deaths per 100 patients during pre-pandemic period ○ Mortality: RR 0.76 [95% CI: 0.70–0.82] favouring pre-pandemic period, p <0.00001, I2 = 97% (high heterogeneity) • Subgroup analyses: persistent statistical significance favouring pre-pandemic period for cardiovascular, respiratory, trauma/musculoskeletal, high & low risk of bias, high income countries, acute care hospital, medical, and surgical subgroups • The change in mortality outcome was reported in 97 studies, of which 50/97 (52%) studies reported a statistically significant change in mortality. • RoB was rated as “very serious”–given the high proportion of poor NOS vs. good NOS scores • There is serious inconsistency in this literature (given the discrepancies (48% of studies did not statistically significant mortality difference). However, this means publication bias is unlikely given the extensive and thorough search performed for this SR alongside the balanced findings of both significant and non-significant mortality outcomes • Imprecision was rated as “not serious” for imprecision, pooled 95% CI does not cross 1, and is significantly difference than null (p < 0.00001) • Given all observational studies start at a “low certainty rating”, plus downgrades for RoB, inconsistency and imprecision would consider the certainty in the evidence to be “very low” quality for mortality |
⨁◯◯◯ Very Low Quality | CRITICAL |
| Morbidity | |||||||||
| 58 | Observational studies (57 cohort, 1 case-control) |
very serious b | serious c | not serious d | serious e | none f | • No meta analyses possible given heterogeneity of morbidity outcomes • The change in morbidity outcome was reported in 58 studies, of which 28/58 (48%) studies reported a statistically significant change in morbidity. • RoB was rated as “very serious”–given the high proportion of poor NOS vs. good NOS scores • There is serious inconsistency in this literature (given the discrepancies (52% of studies did not statistically significant morbidity difference). However, this means publication bias is unlikely given the extensive and thorough search performed for this SR alongside the balanced findings of both significant and non-significant morbidity outcomes • Imprecision was rated as serious, given as many of the 95% CIs are still wide or cross 1, while many p-values or 95% CIs that are reported do not show significance in differences • Given all observational studies start at a “low certainty rating”, plus downgrades for RoB, inconsistency and imprecision would consider the certainty in the evidence to be “very low” quality for morbidity |
⨁◯◯◯ Very Low Quality | CRITICAL |
| Acute care hospitalizations/capacity/occupancy | |||||||||
| 150 | Observational studies (147 cohort, 3 case-control) | very serious b | serious c | not serious d | serious e | none f | • No meta analyses possible given heterogeneity of hospitalization outcomes • The change in acute care capacity outcome was reported in 150 studies, of which 39/150 (26%) studies reported a statistically significant change in acute care capacity. • RoB was rated as “very serious”–given the high proportion of poor NOS vs. good NOS scores • There is serious inconsistency in this literature (given the discrepancies (74% of studies did not statistically significant acute care capacity difference). However, this means publication bias is unlikely given the extensive and thorough search performed for this SR alongside the balanced findings of both significant and non-significant acute care capacity outcomes • Imprecision was rated as serious, given as many of the 95% CIs are still wide or cross 1, while many p-values or 95% CIs that are reported do not show significance in differences • Given all observational studies start at a “low certainty rating”, plus downgrades for RoB, inconsistency and imprecision would consider the certainty in the evidence to be “very low” quality for acute care capacity |
⨁◯◯◯ Very Low | IMPORTANT |
| Disruptions to care | |||||||||
| 124 | Observational studies (123 cohort, 1 case-control) |
very serious b | serious c | not serious d | serious e | none f | • No meta analyses possible given heterogeneity of disruptions in care outcomes • The change in disruptions to care outcome was reported in 124 studies, of which 62/125 (50%) studies reported a statistically significant change in disruptions to care. • RoB was rated as “very serious”–given the high proportion of poor NOS vs. good NOS scores • There is serious inconsistency in this literature (given the discrepancies (50% of studies did not statistically significant disruptions to care). However, this means publication bias is unlikely given the extensive and thorough search performed for this SR alongside the balanced findings of both significant and non-significant disruptions to care • Imprecision was rated as serious, given as many of the 95% CIs are still wide or cross 1, while many p-values or 95% CIs that are reported do not show significance in differences • Given all observational studies start at a “low certainty rating”, plus downgrades for RoB, inconsistency and imprecision would consider the certainty in the evidence to be “very low” quality for disruptions to care |
⨁◯◯◯ Very Low Quality | IMPORTANT |
CI: confidence interval, GRADE: Grading of Recommendations Assessment, Development and Evaluation, NOS: Newcastle-Ottawa Scale, RoB: risk of bias, SR: systematic review
a. Other considerations: e.g. publication bias, large magnitude of effect, dose-response gradient, all plausible confounding would reduce the demonstrated effect or increase the effect if no effect was observed
b. “Very serious” rating based on poor RoB in 85.2%, and only good RoB in 14.8% of all studies (n = 169)
c. “Serious” rating based on overall inconsistency (specifically there are large discrepancies for differences in all outcomes: mortality (51.0% statistically significant change vs. 49.0% not), morbidity (64.1% statistically significant change vs. 35.9% not), acute care hospitalizations/capacity/occupancy (25.8% statistically significant change vs. 74.2% not), and disruptions in care (50.0% statistically significant change vs. 50% not)
d. “Not serious” rating for indirectness, given all studies measured directly at the 4 a priori outcomes (mortality, morbidity, acute care hospitalizations/capacity/occupancy and disruptions to care)
e. “Not serious” for imprecision, pooled 95% CI does not cross 1, and is significantly difference than null (p < 0.00001)
f. There is unlikely to be any significant other considerations. Publication bias is unlikely to be present, given the extensive search during this SR, alongside finding which demonstrate both increases and decreases in various outcomes (mortality, morbidity, acute care hospitalizations/capacity/occupancy and disruptions to care). Furthermore, there is also no consistent large magnitude of effect, dose-response gradient, and many studies still have residual confounding.
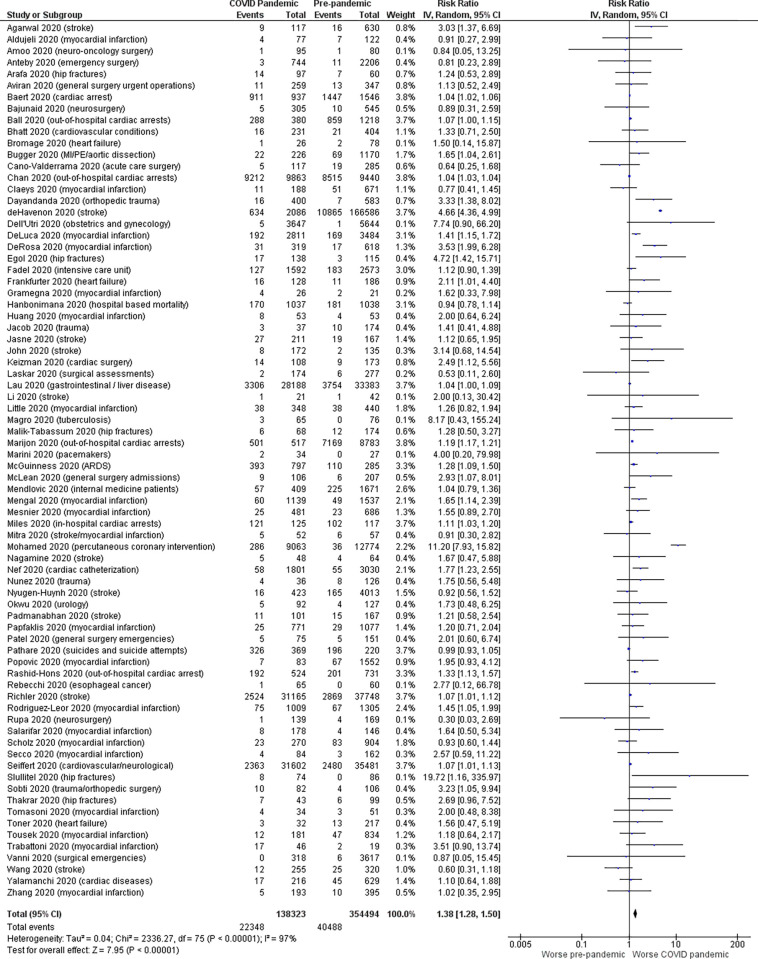
For overall mortality (Fig 2), our meta-analysis (74 observational studies reporting mortality counts, 491,862 patients) demonstrated an increase mortality of 16% during the COVID pandemic compared to 11% mortality during the pre-pandemic period for non-COVID illness (RR 1.38, 95% CI: 1.28–1.50; absolute risk difference: 5% [95% CI: 4–6%], p<0.00001, I2 = 97%). This observation was consistent for grouped systems including: cardiovascular (RR 1.27, 95% CI: 1.19–1.35; p<0.00001, 34 studies); respiratory (RR 1.28, 95% CI: 1.09–1.50; p = 0.003, 1 study); and trauma/musculoskeletal (RR 2.21, 95% CI: 1.50–3.24; p<0.0001, 9 studies).
Fig 2. Forest plot for overall mortality (meta-analysis).
Fifty studies (29.8%) reported a statistically significant change in mortality, while 47 studies (28.0%) reported no significant change, and 71 studies (42%) did not report on mortality. Of those 50 studies which reported a significant change in mortality, 49 studies (98.0%) reported an increase in mortality, while one study (2%) reported a decrease in mortality during the COVID-19 pandemic compared with non-COVID-19 pre-pandemic historical controls. Ninety-seven observational studies reporting mortality (starting at “low” quality of evidence) were downgraded for RoB (“very serious” RoB due to high proportion of poor NOS scores), and inconsistency (high heterogeneity). This led to a “very low” level of certainty in the quality of evidence.
Twenty-eight studies (17%) reported a statistically significant change in morbidity, while 30 studies (18%) reported no significant change, and 110 studies (66%) did not report on morbidity. Of those 28 studies which reported significant changes in morbidity, 26 studies (93%) reported an increase in morbidity, while two studies (7%) reported a decrease in morbidity during the COVID-19 pandemic compared with non-COVID-19 pre-pandemic historical controls. Morbidity was reported in 58 observational studies, where we similarly downgraded for RoB (“very serious” RoB due to high proportion of poor NOS scores), inconsistency (high heterogeneity), and imprecision (wide confidence intervals). This led to a “very low” level of certainty in the quality of evidence.
Thirty-nine studies (23%) reported a statistically significant change in acute care hospitalizations/occupancy, while 111 studies (66%) reported no significant change, and 18 studies (10%) did not report on hospitalizations/occupancy. Of those 39 studies which reported statistically significant change in hospitalizations/occupancy, one study (3%) reported an increase in hospitalizations/occupancy, while 38 studies (97%) reported a decrease in hospitalizations/occupancy during the COVID-19 pandemic compared with non-COVID-19 pre-pandemic historical controls. Hospitalizations and occupancy were reported in 150 observational studies, which were downgraded for RoB (“very serious” RoB due to high proportion of poor NOS scores), inconsistency (high heterogeneity), and imprecision (wide confidence intervals). This led to a “very low” level of certainty in the quality of evidence.
Sixty-two studies (37%) reported a statistically significant change in disruptions to care, while 62 studies (37%) reported no significant change, and 43 studies (26%) did not report on disruptions to care. Of those 62 studies which reported on disruptions to care, 47 studies (76%) reported an increase in disruptions to care, while 15 studies (24%) reported a decrease in disruptions to care (with surgical and elective procedure delays/cancellations and delays to presentation/treatment being the most common reasons) during the COVID-19 pandemic compared with non-COVID-19 pre-pandemic historical controls. Disruptions in standards-of-care were reported in 124 observational studies, where we downgraded for RoB (“very serious” RoB due to high proportion of poor NOS scores), inconsistency (high heterogeneity), and imprecision (wide confidence intervals). This led to a “very low” level of certainty in the quality of evidence.
Subgroups
Pre-specified subgroup analyses for mortality are shown S4 Table with subgroup Forest plots shown in S1–S5 Figs.
For RoB (S2 Fig), there was a similar increase in mortality for both studies with a high (RR 1.37, 95% CI: 1.19–1.54; p<0.00001, 62 studies) and low RoB (RR 1.46, 95% CI: 1.30–1.63; p<0.00001, 14 studies).
For HIC vs. LMIC countries (S3 Fig), there was a similar increase in mortality for HIC during the COVID-19 pandemic compared with non-COVID-19 pandemic historical controls (RR 1.42, 95% CI: 1.30–1.54; p<0.00001, 71 studies). However, LMIC showed no difference in mortality (RR 1.10, 95% CI: 0.87–1.38; p = 0.42, 5 studies).
For level of healthcare intervention (S4 Fig), there was a similar increased mortality for acute care hospital settings (RR 1.40, 95% CI: 1.29–1.52; p<0.00001, 75 studies) compared with jurisdictional/public health/population restrictions/interventions during the COVID-19 pandemic compared with non-COVID-19 pandemic historical controls. However jurisdictional settings showed no difference in mortality (RR 0.99, 95% CI: 0.93–1.05; p = 0.78, 1 study).
For case-mix (S5 Fig), there was a increase in mortality for both medical (RR 1.38, 95% CI: 1.26–1.51; p<0.00001, 50 studies) and surgical case-mix (RR 1.69, 95% CI: 1.27–2.24; p = 0.0003, 23 studies) during the COVID-19 pandemic compared with non-COVID-19 pandemic historical controls. However, mixed cases showed no difference in mortality (RR 1.12, 95% CI: 0.88–1.44; p = 0.36, 3 studies).
There was no significant change between inverse variance pooling and Mantel-Haenszel Random-Effects Forest Plot (S6 Fig). Mantel-Haenszel fixed-effects model are also shown (S7 Fig), although it is implausible that assumption that true effect was the same across all studies.
Publication bias
Visual inspection of Begg’s funnel plots did not reveal publication bias for the outcome of mortality (S8 Fig).
Discussion
In this systematic review of non-COVID illness occurring during the COVID-19 pandemic, patient outcomes were variably affected by the pandemic compared to historical non-pandemic epochs. However, our meta-analysis revealed a significant increase in mortality during the COVID pandemic for non-COVID illness as compared to pre-pandemic time periods (very low certainty evidence), which was consistent across most subgroups evaluated. A substantial proportion of studies reported changes in morbidity; health services and disruptions associated with the pandemic, although this was not universal. The following directional trends were observed: increased morbidity; decreased hospitalizations and lower occupancy; and increased disruptions in care in multiple jurisdictions from low certainty evidence (mainly due to the majority being observational studies with high risk of bias).
While this would preclude strong inferences or definitive recommendations on the nature of the public health interventions and health systems responses to the COVID-19 pandemic crisis, this analysis provides insight into the potential substantial trade-offs that have occurred for both patients with non-COVID illness and health systems capacity to meet standards-of-care. In multiple jurisdictions, excess all-cause mortality (USA: 72 deaths per 100,000, UK: 95 deaths/100,000, Spain: 102 deaths/100,000) has been reported over and above recorded COVID-19 deaths alone [44]. Therein lies the controversy of how the pandemic itself and public health policies around prioritization have had unintended damage to the normal functioning of our health systems and negatively impacted outcomes for non-COVID patients. This is further reinforced by the ethical dilemma of choosing between COVID versus non-COVID patients with scarce healthcare resources [188], especially if triage protocols are enacted [188–192].
Our systematic review adds new knowledge on the potential scope and magnitude of the effects of the COVID-19 pandemic on all non-COVID illness. There is emerging literature that excess mortality is not only driven by COVID-19 deaths [165], but there is also evidence of non-COVID excess mortality and morbidity [193], including in ICU settings [194], secondary to disruptions of global healthcare services by the COVID-19 pandemic [3]. The intensity of disruption (severity multiplied by duration) may have altered the apparent effects among non-COVID illness, leading to the variability observed for different jurisdictions and illnesses. For example, overwhelmed medical systems (e.g., Italy, United States, Brazil, India) may have had higher attributable excess mortality [44, 155], relative to initially less strained jurisdictions (e.g., Australia, New Zealand, Taiwan) by preserving existing healthcare capacity. Jurisdictions experiencing substantially strained healthcare capacity largely prioritized acute care hospitals and intensive care services for surges in COVID pandemic cases [4]. As such, to preserve and generate added capacity (e.g., redeployment of resources), healthcare policy was directed to postpone, delay or cancel elective and non-urgent procedures and scheduled surgeries [195], forced outpatient services to switch to virtual platforms [196], and required unprecedented compromise of entire healthcare systems to meet these challenges. Furthermore, there may be added unmeasured effects of the COVID-19 pandemic that we have not captured or may not be proximally seen (e.g., routine childhood immunization; cancer screening; intimate partner violence; mental health treatments; ethanol and substance abuse), with downstream effects not realized for years to come. Alternatively, it is also plausible that the disruptions caused by the COVID-19 pandemic to the health system have realized new efficiencies and reduced utilization of low-value care (e.g., discretionary diagnostics, imaging and procedures) [197], which may have led to risk of iatrogenic harm by the health care system.
There are fundamental trade-offs that occur when employing public health measures and policies during pandemics. Potential negative effects of the pandemic include affecting social determinants of health (e.g., social isolation, increases in domestic violence, unemployment rates, proportion of populations living in poverty, social security, etc.) alongside healthcare disruption that may have contributed to the overall excess mortality, morbidity, and disruptions in standards-of-care in the non-COVID population. As a society, do we continually tradeoff and prioritize COVID patients at the expense of non-COVID patients, especially those who continue to flaunt public health measures, refuse vaccines and spread misinformation? Are we willing to accept prolonged, sustained disruptions to healthcare systems and society, while continually delay care of non-COVID patients? This is all interwoven and extremely complex pieces of the puzzle within public health policy all need to be weighed such that both COVID and non-COVID patients are not harmed.
Anticipating ongoing global disruptions to healthcare is a key to weathering unanticipated short and long-term COVID-19 pandemic effects to non-COVID patients, which includes: (1) evidence-based, expedited vaccination where available, with mandates quickly implemented; (2) surge capacity planning aimed at: i) creating capacity as needed; ii) preserving acute health system capacity for non-pandemic illnesses; iii) attending to non-acute healthcare systems needs that were lower priority (e.g. social determinants, etc.). This systematic review highlights the potential unintended and collateral effects on health services access, care quality and outcomes for patients with non-COVID-related illness [10], and should spark further research and debate on how to achieve balance alongside determining healthcare policy between pandemic response and non-pandemic population health, particularly given the continued spread of emerging variants of concern contributing to prolongation of the pandemic [198].
The strengths of our SR include a comprehensive search strategy and a rigorous process for study selection and data abstraction based on an a priori protocol, with due consideration to study quality, risk of bias and overall certainty of the evidence using GRADE alongside our meta-analysis methodology.
This SR also has several limitations, most of which relate to limitations of the primary studies analyzed. As mentioned, given the heterogeneity and variable reporting, we could not conduct a meta-analysis for all outcomes. GRADE certainty of evidence was very low for all outcomes, driven primarily by many studies with high risk of bias (with the majority of included studies being observational in nature, without adjustment for baseline characteristics and illness severity) and inconsistency (high heterogeneity in jurisdictional responses to COVID). Delayed or lack of presentation to acute care hospitals may have resulted in increased death out of hospital with death upon arrival or no transfer to acute care facility, which may have biased findings due to under-reporting. Moreover, there is both likelihood of underreporting in the literature and temporal delays in further publications describing health systems effects of the COVID pandemic on non-COVID illnesses [199]. Furthermore, the time-horizon for mortality, morbidity and disruption will likely be far longer than has been captured in the studies to date, with the full scope of effects requiring longer periods for observation. Accordingly, these results must be interpreted carefully and within context.
Conclusion
The COVID-19 pandemic had variable associations with non-COVID illness patient outcomes (e.g., mortality, morbidity, acute care hospitalizations/occupancy and disruptions in standards-of-care) in multiple jurisdictions (very low certainty). Where significant changes were described, there was evidence of increased mortality, increased morbidity, decreased acute care hospitalizations/occupancy and increased disruptions in care across variations in case-mix and multiple jurisdictions (very low certainty). Informing healthcare policy and decision-makers of the potential pandemic effects is crucial to mitigate the impact of the COVID-19 pandemic on both COVID and non-COVID patients.
Supporting information
(DOCX)
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
We are grateful to Diane Keto-Lambert (Faculty of Medicine and Dentistry, University of Alberta) for her assistance with the SR search, as well as during the PRESS process.
Data Availability
All relevant data are within the manuscript and its Supporting Information files. Since this is a systematic review, all articles for which data were searched for are publicly available on search databases.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.COVID-19 Map. Johns Hopkins Coronavirus Resour. Cent. https://coronavirus.jhu.edu/map.html (accessed 27 Jul 2020).
- 2.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report—22 June 2021 (Edition 45). https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—22-june-2021 (accessed 28 Jun 2021). [Google Scholar]
- 3.Barach P, Fisher SD, Adams MJ, et al. Disruption of healthcare: Will the COVID pandemic worsen non-COVID outcomes and disease outbreaks? Prog Pediatr Cardiol Published Online First: 6 June 2020. doi: 10.1016/j.ppedcard.2020.101254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moghadas SM, Shoukat A, Fitzpatrick MC, et al. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc Natl Acad Sci 2020;117:9122–6. doi: 10.1073/pnas.2004064117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schull MJ, Stukel TA, Vermeulen MJ, et al. Effect of widespread restrictions on the use of hospital services during an outbreak of severe acute respiratory syndrome. CMAJ Can Med Assoc J J Assoc Medicale Can 2007;176:1827–32. doi: 10.1503/cmaj.061174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schull MJ, Stukel TA, Vermeulen MJ, et al. Surge capacity associated with restrictions on nonurgent hospital utilization and expected admissions during an influenza pandemic: lessons from the Toronto severe acute respiratory syndrome outbreak. Acad Emerg Med Off J Soc Acad Emerg Med 2006;13:1228–31. doi: 10.1197/j.aem.2006.04.011 [DOI] [PubMed] [Google Scholar]
- 7.Franchini S, Spessot M, Landoni G, et al. Stranger months: how SARS-CoV-2, fear of contagion, and lockdown measures impacted attendance and clinical activity during February and March 2020 at an urban Emergency Department in Milan. Disaster Med Public Health Prep 2020;:1–23. doi: 10.1017/dmp.2020.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myran DT, Cantor N, Pugliese M, et al. Sociodemographic changes in emergency department visits due to alcohol during COVID-19. Drug Alcohol Depend 2021;226:108877. doi: 10.1016/j.drugalcdep.2021.108877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes T, Kitchen SA, Murray R. Measuring the Burden of Opioid-Related Mortality in Ontario, Canada, During the COVID-19 Pandemic. JAMA Netw Open 2021;4:e2112865–e2112865. doi: 10.1001/jamanetworkopen.2021.12865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archer SL. Providing care for the 99.9% during the COVID-19 pandemic: How ethics, equity, epidemiology, and cost per QALY inform healthcare policy. Healthc Manage Forum 2020;:0840470420939854. doi: 10.1177/0840470420939854 [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 12.McGowan J, Sampson M, Salzwedel DM, et al. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol 2016;75:40–6. doi: 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 13.Hernandez JBR, Kim PY. Epidemiology Morbidity And Mortality. In: StatPearls. Treasure Island (FL):: StatPearls; Publishing 2021. http://www.ncbi.nlm.nih.gov/books/NBK547668/ (accessed 9 Aug 2021). [Google Scholar]
- 14.Czeisler MÉ. Delay or Avoidance of Medical Care Because of COVID-19–Related Concerns—United States, June 2020. MMWR Morb Mortal Wkly Rep 2020;69. doi: 10.15585/mmwr.mm6936a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veritas Health Innovation. Covidence systematic review software. 2019.www.covidence.org (accessed 3 Jan 2020).
- 16.Wells G, Shea B, O’Connell D, et al. Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. 2019.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 27 Jan 2019). [Google Scholar]
- 17.Guyatt GH, Oxman AD, Kunz R, et al. What is ‘quality of evidence’ and why is it important to clinicians? BMJ 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Published Online First: October 2013.https://gdt.gradepro.org/app/handbook/handbook.html (accessed 7 Mar 2019). [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Cochrane Collab 2011;:449–80. [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 23.Free C, Phillips G, Felix L, et al. The effectiveness of M-health technologies for improving health and health services: a systematic review protocol. BMC Res Notes 2010;3:250. doi: 10.1186/1756-0500-3-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelaziz HK, Abdelrahman A, Nabi A, et al. Impact of COVID-19 pandemic on patients with ST-segment elevation myocardial infarction: Insights from a British cardiac center. Am Heart J 2020;226:45–8. doi: 10.1016/j.ahj.2020.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal S, Scher E, Rossan-Raghunath N, et al. Acute stroke care in a New York City comprehensive stroke center during the COVID-19 pandemic. J Stroke Cerebrovasc Dis 2020;29:105068. doi: 10.1016/j.jstrokecerebrovasdis.2020.105068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal R, Sharma N, Patil A, et al. Impact of COVID-19 pandemic, national lockdown, and unlocking on an apex tertiary care ophthalmic institute. Indian J Ophthalmol 2020;68:2391. doi: 10.4103/ijo.IJO_2366_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aldujeli A, Hamadeh A, Briedis K, et al. Delays in Presentation in Patients With Acute Myocardial Infarction During the COVID-19 Pandemic. Cardiol Res 2020;11:386–91. doi: 10.14740/cr1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amaddeo G, Brustia R, Allaire M, et al. Impact of COVID-19 on the management of hepatocellular carcinoma in a high-prevalence area. JHEP Rep Innov Hepatol 2021;3:100199. doi: 10.1016/j.jhepr.2020.100199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amoo M, Horan J, Gilmartin B, et al. The provision of neuro-oncology and glioma neurosurgery during the SARS-CoV-2 pandemic: a single national tertiary centre experience. Ir J Med Sci 1971 —Published Online First: 5 November 2020. doi: 10.1007/s11845-020-02429-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amukotuwa SA, Bammer R, Maingard J. Where have our patients gone? The impact of COVID-19 on stroke imaging and intervention at an Australian stroke center. J Med Imaging Radiat Oncol 2020;64:607–14. doi: 10.1111/1754-9485.13093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlotte Andersson, Thomas Gerds, Emil Fosbøl, et al. Incidence of New-Onset and Worsening Heart Failure Before and After the COVID-19 Epidemic Lockdown in Denmark. Circ Heart Fail 2020;13:e007274. doi: 10.1161/CIRCHEARTFAILURE.120.007274 [DOI] [PubMed] [Google Scholar]
- 32.Anteby R, Zager Y, Barash Y, et al. The Impact of the Coronavirus Disease 2019 Outbreak on the Attendance of Patients with Surgical Complaints at a Tertiary Hospital Emergency Department. J Laparoendosc Adv Surg Tech A 2020;30:1001–7. doi: 10.1089/lap.2020.0465 [DOI] [PubMed] [Google Scholar]
- 33.Arafa M, Nesar S, Abu-Jabeh H, et al. COVID-19 pandemic and hip fractures: impact and lessons learned. Bone Jt Open 2020;1:530–40. doi: 10.1302/2633-1462.19.BJO-2020-0116.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Athiel Y, Civadier M-S, Luton D, et al. Impact of the outbreak of SARS-CoV-2 infection on urgent gynecological care. J Gynecol Obstet Hum Reprod 2020;49:101841. doi: 10.1016/j.jogoh.2020.101841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aviran E, Laks S, Benvenisti H, et al. The Impact of the COVID-19 Pandemic on General Surgery Acute Admissions and Urgent Operations: A Comparative Prospective Study. Isr Med Assoc J IMAJ 2020;11:673–9. [PubMed] [Google Scholar]
- 36.Baert V, Jaeger D, Hubert H, et al. Assessment of changes in cardiopulmonary resuscitation practices and outcomes on 1005 victims of out-of-hospital cardiac arrest during the COVID-19 outbreak: registry-based study. Scand J Trauma Resusc Emerg Med 2020;28:119. doi: 10.1186/s13049-020-00813-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bajunaid K, Alqurashi A, Alatar A, et al. Neurosurgical Procedures and Safety During the COVID-19 Pandemic: A Case-Control Multicenter Study. World Neurosurg 2020;143:e179–87. doi: 10.1016/j.wneu.2020.07.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ball J, Nehme Z, Bernard S, et al. Collateral damage: Hidden impact of the COVID-19 pandemic on the out-of-hospital cardiac arrest system-of-care. Resuscitation 2020;156:157–63. doi: 10.1016/j.resuscitation.2020.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barten DG, Latten GHP, van Osch FHM. Reduced Emergency Department Utilization During the Early Phase of the COVID-19 Pandemic: Viral Fear or Lockdown Effect? Disaster Med Public Health Prep 2020;:1–4. doi: 10.1017/dmp.2020.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batra TK, Tilak MR, Pai E, et al. Increased tracheostomy rates in head and neck cancer surgery during the COVID-19 pandemic. Int J Oral Maxillofac Surg Published Online First: 11 December 2020. doi: 10.1016/j.ijom.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becq A, Jais B, Fron C, et al. Drastic decrease of urgent endoscopies outside regular working hours during the Covid-19 pandemic in the paris area. Clin Res Hepatol Gastroenterol 2020;44:579–85. doi: 10.1016/j.clinre.2020.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benites-Goñi H, Pascacio-Fiori M, Valle FM-D, et al. Impact of the COVID-19 pandemic in the time to endoscopy in patients with upper gastrointestinal bleeding. Rev Gastroenterol Perú 2020;40:219–23. [PubMed] [Google Scholar]
- 43.Bhatt AS, Moscone A, McElrath EE, et al. Fewer Hospitalizations for Acute Cardiovascular Conditions During the COVID-19 Pandemic. J Am Coll Cardiol 2020;76:280–8. doi: 10.1016/j.jacc.2020.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bilinski A, Emanuel EJ. COVID-19 and Excess All-Cause Mortality in the US and 18 Comparison Countries. JAMA Published Online First: 12 October 2020. doi: 10.1001/jama.2020.20717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birkmeyer JD, Barnato A, Birkmeyer N, et al. The Impact Of The COVID-19 Pandemic On Hospital Admissions In The United States. Health Aff (Millwood) 2020;39:2010–7. doi: 10.1377/hlthaff.2020.00980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blangiardo M, Cameletti M, Pirani M, et al. Estimating weekly excess mortality at sub-national level in Italy during the COVID-19 pandemic. PLOS ONE 2020;15:e0240286. doi: 10.1371/journal.pone.0240286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyarsky BJ, Werbel WA, Durand CM, et al. Early national and center-level changes to kidney transplantation in the United States during the COVID-19 epidemic. Am J Transplant 2020;20:3131–9. doi: 10.1111/ajt.16167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bromage DI, Cannatà A, Rind IA, et al. The impact of COVID-19 on heart failure hospitalization and management: report from a Heart Failure Unit in London during the peak of the pandemic. Eur J Heart Fail 2020;22:978–84. doi: 10.1002/ejhf.1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bugger H, Gollmer J, Pregartner G, et al. Complications and mortality of cardiovascular emergency admissions during COVID-19 associated restrictive measures. PLOS ONE 2020;15:e0239801. doi: 10.1371/journal.pone.0239801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bustos Sierra N, Bossuyt N, Braeye T, et al. All-cause mortality supports the COVID-19 mortality in Belgium and comparison with major fatal events of the last century. Arch Public Health 2020;78:117. doi: 10.1186/s13690-020-00496-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butt AA, Kartha A, Asaad N, et al. Impact of COVID-19 upon changes in emergency room visits with chest pain of possible cardiac origin. BMC Res Notes 2020;13:539. doi: 10.1186/s13104-020-05381-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butt AA, Kartha AB, Masoodi NA, et al. Hospital admission rates, length of stay, and in-hospital mortality for common acute care conditions in COVID-19 vs. pre-COVID-19 era. Public Health 2020;189:6–11. doi: 10.1016/j.puhe.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calderon-Larranaga A, Vetrano DL, Rizzuto D, et al. High excess mortality during the COVID-19 outbreak in Stockholm Region areas with young and socially vulnerable populations. medRxiv 2020;:2020.07.07.20147983. doi: 10.1136/bmjgh-2020-003595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cannatà A, Bromage DI, Rind IA, et al. Temporal trends in decompensated heart failure and outcomes during COVID-19: a multisite report from heart failure referral centres in London. Eur J Heart Fail;n/a. 10.1002/ejhf.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cannavò A, Passamonti SM, Martinuzzi D, et al. The Impact of COVID-19 on Solid Organ Donation: The North Italy Transplant Program Experience. Transplant Proc 2020;52:2578–83. doi: 10.1016/j.transproceed.2020.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cano-Valderrama O, Morales X, Ferrigni CJ, et al. Acute Care Surgery during the COVID-19 pandemic in Spain: Changes in volume, causes and complications. A multicentre retrospective cohort study. Int J Surg Lond Engl 2020;80:157–61. doi: 10.1016/j.ijsu.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Casalino E, Choquet C, Bouzid D, et al. Analysis of Emergency Department Visits and Hospital Activity during Influenza Season, COVID-19 Epidemic, and Lockdown Periods in View of Managing a Future Disaster Risk: A Multicenter Observational Study. Int J Environ Res Public Health 2020;17. doi: 10.3390/ijerph17228302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cates J. Risk for In-Hospital Complications Associated with COVID-19 and Influenza—Veterans Health Administration, United States, October 1, 2018–May 31, 2020. MMWR Morb Mortal Wkly Rep 2020;69. doi: 10.15585/mmwr.mm6942e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cevallos-Valdiviezo H, Vergara-Montesdeoca A, Zambrano-Zambrano G. Measuring the impact of the COVID-19 outbreak in Ecuador using preliminary estimates of excess mortality, March 17–October 22, 2020. Int J Infect Dis 2020;0. doi: 10.1016/j.ijid.2020.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan KPF, Ma TF, Kwok WC, et al. Significant reduction in hospital admissions for acute exacerbation of chronic obstructive pulmonary disease in Hong Kong during coronavirus disease 2019 pandemic. Respir Med 2020;171:106085. doi: 10.1016/j.rmed.2020.106085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan PS, Girotra S, Tang Y, et al. Outcomes for Out-of-Hospital Cardiac Arrest in the United States During the Coronavirus Disease 2019 Pandemic. JAMA Cardiol 2021;6:296–303. doi: 10.1001/jamacardio.2020.6210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Claeys MJ, Argacha J-F, Collart P, et al. Impact of COVID-19-related public containment measures on the ST elevation myocardial infarction epidemic in Belgium: a nationwide, serial, cross-sectional study. Acta Cardiol 2020;0:1–7. doi: 10.1080/00015385.2020.1796035 [DOI] [PubMed] [Google Scholar]
- 63.D’Apolito R, Faraldi M, Ottaiano I, et al. Disruption of Arthroplasty Practice in an Orthopedic Center in Northern Italy During the Coronavirus Disease 2019 Pandemic. J Arthroplasty 2020;35:S6–9. doi: 10.1016/j.arth.2020.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davies PSE, Sinnerton RJH, MacInnes A, et al. Re-starting elective orthopaedic services in NHS Tayside during the COVID-19 pandemic. The Surgeon Published Online First: 19 November 2020. doi: 10.1016/j.surge.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dawoud BES, Kent P, Ho MWS. Impacts of lockdown during the SARS-CoV-2 pandemic on patients presenting with cervicofacial infection of odontogenic origin: a comparative study. Br J Oral Maxillofac Surg Published Online First: 11 September 2020. doi: 10.1016/j.bjoms.2020.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dayananda KSS, Mercer ST, Agarwal R, et al. A comparative review of 1,004 orthopaedic trauma patients before and during the COVID-19 pandemic. Bone Jt Open 2020;1:568–75. doi: 10.1302/2633-1462.19.BJO-2020-0121.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Havenon A, Ney JP, Callaghan B, et al. Impact of COVID-19 on Outcomes in Ischemic Stroke Patients in the United States. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 2021;30:105535. doi: 10.1016/j.jstrokecerebrovasdis.2020.105535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dell’Utri C, Manzoni E, Cipriani S, et al. Effects of SARS Cov-2 epidemic on the obstetrical and gynecological emergency service accesses. What happened and what shall we expect now? Eur J Obstet Gynecol Reprod Biol 2020;254:64–8. doi: 10.1016/j.ejogrb.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Luca G, Verdoia M, Cercek M, et al. Impact of COVID-19 Pandemic on Mechanical Reperfusion for Patients With STEMI. J Am Coll Cardiol 2020;76:2321–30. doi: 10.1016/j.jacc.2020.09.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.D’Urbano F, Fabbri N, Koleva Radica M, et al. Emergency surgery in COVID-19 outbreak: Has anything changed? Single center experience. World J Clin Cases 2020;8:3691–6. doi: 10.12998/wjcc.v8.i17.3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abi Fadel F, Al-Jaghbeer M, Kumar S, et al. The impact of the state of Ohio stay-at-home order on non-COVID-19 intensive care unit admissions and outcomes. Anaesthesiol Intensive Ther 2020;52:249–52. doi: 10.5114/ait.2020.98393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frankfurter C, Buchan TA, Kobulnik J, et al. Reduced Rate of Hospital Presentations for Heart Failure During the COVID-19 Pandemic in Toronto, Canada. Can J Cardiol 2020;36:1680–4. doi: 10.1016/j.cjca.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friedman J, Calderón-Villarreal A, Bojorquez I, et al. Excess Out-of-Hospital Mortality and Declining Oxygen Saturation: The Sentinel Role of Emergency Medical Services Data in the COVID-19 Crisis in Tijuana, Mexico. Ann Emerg Med 2020;76:413–26. doi: 10.1016/j.annemergmed.2020.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giannouchos TV, Biskupiak J, Moss MJ, et al. Trends in outpatient emergency department visits during the COVID-19 pandemic at a large, urban, academic hospital system. Am J Emerg Med 2021;40:20–6. doi: 10.1016/j.ajem.2020.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gluckman TJ, Wilson MA, Chiu S-T, et al. Case Rates, Treatment Approaches, and Outcomes in Acute Myocardial Infarction During the Coronavirus Disease 2019 Pandemic. JAMA Cardiol Published Online First: 7 August 2020. doi: 10.1001/jamacardio.2020.3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Göksoy B, Akça MT, Inanç ÖF. The impacts of the COVID-19 outbreak on emergency department visits of surgical patients. Ulus Travma Ve Acil Cerrahi Derg Turk J Trauma Emerg Surg TJTES 2020;26:685–92. doi: 10.14744/etd.2020.67927 [DOI] [PubMed] [Google Scholar]
- 77.Gramegna M, Baldetti L, Beneduce A, et al. ST-Segment-Elevation Myocardial Infarction During COVID-19 Pandemic: Insights From a Regional Public Service Healthcare Hub. Circ Cardiovasc Interv 2020;13:e009413. doi: 10.1161/CIRCINTERVENTIONS.120.009413 [DOI] [PubMed] [Google Scholar]
- 78.Grewal P, Pinna P, Hall JP, et al. Acute Ischemic Stroke and COVID-19: Experience From a Comprehensive Stroke Center in Midwest US. Front Neurol 2020;11. doi: 10.3389/fneur.2020.00910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gul M, Kaynar M, Yildiz M, et al. The Increased Risk of Complicated Ureteral Stones in the Era of COVID-19 Pandemic. J Endourol 2020;34:882–6. doi: 10.1089/end.2020.0658 [DOI] [PubMed] [Google Scholar]
- 80.Gupta R, Singhal A, Kapoor A, et al. Effect of COVID-19 on surgical management of open fractures and infection rates: A tertiary care experience in Indian set-up. J Clin Orthop Trauma Published Online First: 26 October 2020. doi: 10.1016/j.jcot.2020.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Habonimana D, Ouedraogo L, Ndirahisha E, et al. Understanding the influence of the COVID-19 pandemic on hospital-based mortality in Burundi: a cross-sectional study comparing two time periods. Epidemiol Infect 2020;148:e280. doi: 10.1017/S0950268820002770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang B, Xu C, Liu H, et al. In-Hospital Management and Outcomes of Acute Myocardial Infarction Before and During the Coronavirus Disease 2019 Pandemic. J Cardiovasc Pharmacol 2020;76:540–8. doi: 10.1097/FJC.0000000000000909 [DOI] [PubMed] [Google Scholar]
- 83.Jacob S, Mwagiru D, Thakur I, et al. Impact of societal restrictions and lockdown on trauma admissions during the COVID-19 pandemic: a single-centre cross-sectional observational study. ANZ J Surg 2020;90:2227–31. doi: 10.1111/ans.16307 [DOI] [PubMed] [Google Scholar]
- 84.Jacobson SH, Jokela JA. Non–COVID-19 excess deaths by age and gender in the United States during the first three months of the COVID-19 pandemic. Public Health 2020;189:101–3. doi: 10.1016/j.puhe.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jasne AS, Chojecka P, Maran I, et al. Stroke Code Presentations, Interventions, and Outcomes Before and During the COVID-19 Pandemic. Stroke 2020;51:2664–73. doi: 10.1161/STR.0000000000000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.John S, Hussain SI, Piechowski-Jozwiak B, et al. Clinical characteristics and admission patterns of stroke patients during the COVID 19 pandemic: A single center retrospective, observational study from the Abu Dhabi, United Arab Emirates. Clin Neurol Neurosurg 2020;199:106227. doi: 10.1016/j.clineuro.2020.106227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kastritis E, Tsitsimpis K, Anninos E, et al. Significant reduction in the visits to the emergency room department during the COVID-19 pandemic in a tertiary hospital in Greece: Indirect victims of the pandemic? Medicine (Baltimore) 2020;99:e23845. doi: 10.1097/MD.0000000000023845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katsouras C, Karapanayiotides T, Papafaklis M, et al. Greater decline of acute stroke admissions compared with acute coronary syndromes during COVID-19 outbreak in Greece: Cerebro/cardiovascular implications amidst a second wave surge. Eur J Neurol;n/a. doi: 10.1111/ene.14666 [DOI] [PubMed] [Google Scholar]
- 89.E K, E R, E K, et al. The impact of COVID-19 pandemic on cardiac surgery in Israel. J Cardiothorac Surg 2020;15:294–294. doi: 10.1186/s13019-020-01342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khalil A, von Dadelszen P, Draycott T, et al. Change in the Incidence of Stillbirth and Preterm Delivery During the COVID-19 Pandemic. JAMA 2020;324:705. doi: 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laskar NS, Hunt A, Karunaratne D, et al. Are there benefits to maintaining Covid-19 pandemic pathways for the long-term? A surgical assessment unit based study. The Surgeon Published Online First: 22 September 2020. doi: 10.1016/j.surge.2020.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lau LHS, Wong SH, Yip TCF, et al. Collateral Effect of Coronavirus Disease 2019 Pandemic on Hospitalizations and Clinical Outcomes in Gastrointestinal and Liver Diseases: A Territory-wide Observational Study in Hong Kong. Gastroenterology 2020;159:1979–1981.e3. doi: 10.1053/j.gastro.2020.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lauridsen MD, Butt JH, Østergaard L, et al. Incidence of acute myocardial infarction-related cardiogenic shock during corona virus disease 19 (COVID-19) pandemic. IJC Heart Vasc 2020;31:100659. doi: 10.1016/j.ijcha.2020.100659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leitinger M, Poppert K-N, Mauritz M, et al. Status epilepticus admissions during the COVID-19 pandemic in Salzburg-A population-based study. Epilepsia 2020;61:e198–203. doi: 10.1111/epi.16737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lerner EB, Newgard CD, Mann NC. Effect of the Coronavirus Disease 2019 (COVID-19) Pandemic on the U.S. Emergency Medical Services System: A Preliminary Report. Acad Emerg Med Off J Soc Acad Emerg Med 2020;27:693–9. doi: 10.1111/acem.14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leung WCY, Lau EHY, Kwan P, et al. Impact of COVID-19 on seizure-related emergency attendances and hospital admissions—A territory-wide observational study. Epilepsy Behav Published Online First: 21 September 2020. doi: 10.1016/j.yebeh.2020.107497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li S, Zeng M, Dong J, et al. Management of Endovascular Treatment for Acute Ischemic Stroke During the COVID-19 Pandemic at a Single Institution in Beijing, China: A Brief Report. J Neurosurg Anesthesiol Published Online First: 20 November 2020. doi: 10.1097/ANA.0000000000000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y-H, Huang W-C, Hwang J-J. No Reduction of ST-segment Elevation Myocardial Infarction Admission in Taiwan During Coronavirus Pandemic. Am J Cardiol 2020;131:133–4. doi: 10.1016/j.amjcard.2020.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Little CD, Kotecha T, Candilio L, et al. COVID-19 pandemic and STEMI: pathway activation and outcomes from the pan-London heart attack group. Open Heart 2020;7:e001432. doi: 10.1136/openhrt-2020-001432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luostarinen T, Virta J, Satopää J, et al. Intensive care of traumatic brain injury and aneurysmal subarachnoid hemorrhage in Helsinki during the Covid-19 pandemic. Acta Neurochir (Wien) 2020;162:2715–24. doi: 10.1007/s00701-020-04583-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lv H, Zhang Q, Yin Y, et al. Epidemiologic characteristics of traumatic fractures during the outbreak of coronavirus disease 2019 (COVID-19) in China: A retrospective & comparative multi-center study. Injury 2020;51:1698–704. doi: 10.1016/j.injury.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Madanelo M, Ferreira C, Nunes-Carneiro D, et al. The impact of the coronavirus disease 2019 pandemic on the utilisation of emergency urological services. BJU Int 2020;126:256–8. doi: 10.1111/bju.15109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Magnani C, Azzolina D, Gallo E, et al. How Large Was the Mortality Increase Directly and Indirectly Caused by the COVID-19 Epidemic? An Analysis on All-Causes Mortality Data in Italy. Int J Environ Res Public Health 2020;17. doi: 10.3390/ijerph17103452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Magro P, Formenti B, Marchese V, et al. Impact of the SARS-CoV-2 epidemic on tuberculosis treatment outcome in Northern Italy. Eur Respir J 2020;56. doi: 10.1183/13993003.02665-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Malik-Tabassum K, Crooks M, Robertson A, et al. Management of hip fractures during the COVID-19 pandemic at a high-volume hip fracture unit in the United Kingdom. J Orthop 2020;20:332–7. doi: 10.1016/j.jor.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mannucci E, Nreu B, Monami M. Factors associated with increased all-cause mortality during the COVID-19 pandemic in Italy. Int J Infect Dis IJID Off Publ Int Soc Infect Dis 2020;98:121–4. doi: 10.1016/j.ijid.2020.06.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marijon E, Karam N, Jost D, et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health 2020;5:e437–43. doi: 10.1016/S2468-2667(20)30117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marini M, Zilio F, Martin M, et al. COVID-19 pandemic and elderly: is the curtain dropped for urgent pacemaker implantations? Minerva Cardioangiol Published Online First: 1 December 2020. doi: 10.23736/S2724-5683.20.05451-1 [DOI] [PubMed] [Google Scholar]
- 109.Mariottini C, Ojanperä I, Kriikku P. Increase in drugs-of-abuse findings in post-mortem toxicology due to COVID-19 restrictions-First observations in Finland. Drug Test Anal Published Online First: 20 November 2020. doi: 10.1002/dta.2982 [DOI] [PubMed] [Google Scholar]
- 110.McGuinness G, Zhan C, Rosenberg N, et al. Increased Incidence of Barotrauma in Patients with COVID-19 on Invasive Mechanical Ventilation. Radiology 2020;297:E252–62. doi: 10.1148/radiol.2020202352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McLean RC, Young J, Musbahi A, et al. A single-centre observational cohort study to evaluate volume and severity of emergency general surgery admissions during the COVID-19 pandemic: Is there a “lockdown” effect? Int J Surg 2020;83:259–66. doi: 10.1016/j.ijsu.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mendlovic J, Weiss G, Da’as N, et al. Internal medicine patients admitted without COVID-19 during the outbreak. Int J Clin Pract 2020;74:e13630. doi: 10.1111/ijcp.13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mengal N, Saghir T, Rizvi SNH, et al. Acute ST-Elevation Myocardial Infarction Before and During the COVID-19 Pandemic: What is the Clinically Significant Difference? Cureus 2020;12. doi: 10.7759/cureus.10523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Merkler AE, Parikh NS, Mir S, et al. Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs Patients With Influenza. JAMA Neurol 2020;77:1366. doi: 10.1001/jamaneurol.2020.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mesnier J, Cottin Y, Coste P, et al. Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: a registry study. Lancet Public Health 2020;5:e536–42. doi: 10.1016/S2468-2667(20)30188-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meyer R, Levin G, Hendin N, et al. Impact of the COVID-19 Outbreak on Routine Obstetrical Management. Isr Med Assoc J IMAJ 2020;22:483–8. [PubMed] [Google Scholar]
- 117.Miles Jeremy A., Mejia Mateo, Rios Saul, et al. Characteristics and Outcomes of In-Hospital Cardiac Arrest Events During the COVID-19 Pandemic. Circ Cardiovasc Qual Outcomes 2020;13:e007303. doi: 10.1161/CIRCOUTCOMES.120.007303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mitra B, Mitchell RD, Cloud GC, et al. Presentations of stroke and acute myocardial infarction in the first 28 days following the introduction of State of Emergency restrictions for COVID-19. Emerg Med Australas EMA 2020;32:1040–5. doi: 10.1111/1742-6723.13621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohamed MO, Kinnaird T, Curzen N, et al. In-Hospital and 30-Day Mortality After Percutaneous Coronary Intervention in England in the Pre-COVID and COVID Eras. J Invasive Cardiol 2020. [DOI] [PubMed] [Google Scholar]
- 120.Mohammad MA, Koul S, Olivecrona GK, et al. Incidence and outcome of myocardial infarction treated with percutaneous coronary intervention during COVID-19 pandemic. Heart 2020;106:1812–8. doi: 10.1136/heartjnl-2020-317685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Monti S, Delvino P, Bellis E, et al. Impact of delayed diagnoses at the time of COVID-19: increased rate of preventable bilateral blindness in giant cell arteritis. Ann Rheum Dis 2020;79:1658–9. doi: 10.1136/annrheumdis-2020-217915 [DOI] [PubMed] [Google Scholar]
- 122.Mountantonakis SE, Saleh M, Coleman K, et al. Out-of-Hospital Cardiac Arrest and Acute Coronary Syndrome Hospitalizations During the COVID-19 Surge. J Am Coll Cardiol 2020;76:1271–3. doi: 10.1016/j.jacc.2020.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moustakis J, Piperidis AA, Ogunrombi AB. The effect of COVID-19 on essential surgical admissions in South Africa: A retrospective observational analysis of admissions before and during lockdown at a tertiary healthcare complex. South Afr Med J Suid-Afr Tydskr Vir Geneeskd 2020;110:910–5. doi: 10.7196/SAMJ.2020.v110i9.15025 [DOI] [PubMed] [Google Scholar]
- 124.Mulholland RH, Wood R, Stagg HR, et al. Impact of COVID-19 on accident and emergency attendances and emergency and planned hospital admissions in Scotland: an interrupted time-series analysis. J R Soc Med 2020;113:444–53. doi: 10.1177/0141076820962447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Naccarato M, Scali I, Olivo S, et al. Has COVID-19 played an unexpected “stroke” on the chain of survival? J Neurol Sci 2020;414:116889. doi: 10.1016/j.jns.2020.116889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagamine M, Chow DS, Chang PD, et al. Impact of COVID-19 on Acute Stroke Presentation at a Comprehensive Stroke Center. Front Neurol 2020;11. doi: 10.3389/fneur.2020.00850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nef HM, Elsässer A, Möllmann H, et al. Impact of the COVID-19 pandemic on cardiovascular mortality and catherization activity during the lockdown in central Germany: an observational study. Clin Res Cardiol Off J Ger Card Soc Published Online First: 21 November 2020. doi: 10.1007/s00392-020-01780-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nguyen-Huynh Mai N., Nan Tang Xian, Vinson David R., et al. Acute Stroke Presentation, Care, and Outcomes in Community Hospitals in Northern California During the COVID-19 Pandemic. Stroke 2020;51:2918–24. doi: 10.1161/STROKEAHA.120.031099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nuñez JH, Sallent A, Lakhani K, et al. Impact of the COVID-19 Pandemic on an Emergency Traumatology Service: Experience at a Tertiary Trauma Centre in Spain. Injury 2020;51:1414–8. doi: 10.1016/j.injury.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ogliari G, Lunt E, Ong T, et al. The impact of lockdown during the COVID-19 pandemic on osteoporotic fragility fractures: an observational study. Arch Osteoporos 2020;15. doi: 10.1007/s11657-020-00825-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okwu N, Ravindraanandan M, Davies R, et al. A Multi-Centre Snapshot Study Comparing Acute Urological Admissions during the COVID-19 Lockdown to a pre-COVID Period. J Endoluminal Endourol 2020;3:e32–6. doi: 10.22374/jeleu.v3i4.107 [DOI] [Google Scholar]
- 132.Orellana JDY, Cunha GM da, Marrero L, et al. Explosion in mortality in the Amazonian epicenter of the COVID-19 epidemic 19. Cad Saude Publica 2020;36:e00120020. doi: 10.1590/0102-311x00120020 [DOI] [PubMed] [Google Scholar]
- 133.Padmanabhan N, Natarajan I, Gunston R, et al. Impact of COVID-19 on stroke admissions, treatments, and outcomes at a comprehensive stroke centre in the United Kingdom. Neurol Sci 2020;:1–6. doi: 10.1007/s10072-020-04775-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pagotto VPF, Abbas L, Goldenberg DC, et al. The impact of COVID-19 on the plastic surgery activity in a high-complexity university hospital in Brazil: the importance of reconstructive plastic surgery during the pandemic. Eur J Plast Surg 2020;:1–6. doi: 10.1007/s00238-020-01729-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Papafaklis MI, Katsouras CS, Tsigkas G, et al. “Missing” acute coronary syndrome hospitalizations during the COVID-19 era in Greece: Medical care avoidance combined with a true reduction in incidence? Clin Cardiol 2020;43:1142–9. doi: 10.1002/clc.23424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Patel LG, Peck T, Starr MR, et al. Clinical Presentation of Rhegmatogenous Retinal Detachment during the COVID-19 Pandemic: A Historical Cohort Study. Ophthalmology 2020;0. doi: 10.1016/j.ophtha.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Patel R, Hainsworth AJ, Devlin K, et al. Frequency and severity of general surgical emergencies during the COVID-19 pandemic: single-centre experience from a large metropolitan teaching hospital. Ann R Coll Surg Engl 2020;:1–6. doi: 10.1308/rcsann.2020.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pathare S, Vijayakumar L, Fernandes TN, et al. Analysis of news media reports of suicides and attempted suicides during the COVID-19 lockdown in India. Int J Ment Health Syst 2020;14:88. doi: 10.1186/s13033-020-00422-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin Cancer Inform 2020;:1059–71. doi: 10.1200/CCI.20.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Perkin MR, Heap S, Crerar-Gilbert A, et al. Deaths in people from Black, Asian and minority ethnic communities from both COVID-19 and non-COVID causes in the first weeks of the pandemic in London: a hospital case note review. BMJ Open 2020;10:e040638. doi: 10.1136/bmjopen-2020-040638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Piccininni M, Rohmann JL, Foresti L, et al. Use of all cause mortality to quantify the consequences of covid-19 in Nembro, Lombardy: descriptive study. BMJ 2020;369. doi: 10.1136/bmj.m1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pintado JF, Gibaja W, Vallejos RA, et al. How COVID-19 has affected emergent visits to a Latin-American trauma department: Experience at a Peruvian national trauma referral center. Injury 2020;51:2834–9. doi: 10.1016/j.injury.2020.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pop R, Quenardelle V, Hasiu A, et al. Impact of the COVID-19 outbreak on acute stroke pathways—insights from the Alsace region in France. Eur J Neurol 2020;27:1783–7. doi: 10.1111/ene.14316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Popovic B, Varlot J, Metzdorf PA, et al. Changes in characteristics and management among patients with ST-elevation myocardial infarction due to COVID-19 infection. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv 2021;97:E319–26. doi: 10.1002/ccd.29114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Quaquarini E, Saltalamacchia G, Presti D, et al. Impact of COVID-19 Outbreak on Cancer Patient Care and Treatment: Data from an Outpatient Oncology Clinic in Lombardy (Italy). Cancers 2020;12. doi: 10.3390/cancers12102941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Muhammad Rashid (Hons), Gale (Hons) Chris P., Nick Curzen (Hons), et al. Impact of Coronavirus Disease 2019 Pandemic on the Incidence and Management of Out‐of‐Hospital Cardiac Arrest in Patients Presenting With Acute Myocardial Infarction in England. J Am Heart Assoc 2020;9:e018379. doi: 10.1161/JAHA.120.018379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rebecchi F, Arolfo S, Ugliono E, et al. Impact of COVID-19 outbreak on esophageal cancer surgery in Northern Italy: lessons learned from a multicentric snapshot. Dis Esophagus Published Online First: 27 November 2020. doi: 10.1093/dote/doaa124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Daniel Richter, Jens Eyding, Ralph Weber, et al. Analysis of Nationwide Stroke Patient Care in Times of COVID-19 Pandemic in Germany. Stroke 2021;52:716–21. doi: 10.1161/STROKEAHA.120.033160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Riemann S, Speck I, Gerstacker K, et al. Collateral damage of the COVID-19 pandemic: an alarming decline in critical procedures in otorhinolaryngology in a German university hospital. Eur Arch Otorhinolaryngol 2020;:1–7. doi: 10.1007/s00405-020-06519-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rodríguez-Leor O, Cid-Álvarez B, Pérez de Prado A, et al. Impact of COVID-19 on ST-segment elevation myocardial infarction care. The Spanish experience. Rev Espanola Cardiol Engl Ed 2020;73:994–1002. doi: 10.1016/j.rec.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rupa R, Sass B, Morales Lema MA, et al. The Demand for Elective Neurosurgery at a German University Hospital during the First Wave of COVID-19. Healthc Basel Switz 2020;8. doi: 10.3390/healthcare8040483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Russo V, Pafundi PC, Rapacciuolo A, et al. Arrhythmogenic syncope leading to cardiac rhythm management procedures during COVID-19 lockdown. Expert Rev Med Devices 2020;17:1207–10. doi: 10.1080/17434440.2020.1841632 [DOI] [PubMed] [Google Scholar]
- 153.Salarifar M, Ghavami M, Poorhosseini H, et al. The impact of a dedicated coronavirus disease 2019 primary angioplasty protocol on time components related to ST‑segment elevation myocardial infarction management in a 24/7 primary percutaneous coronary intervention-capable hospital. Kardiol Pol 2020;78:1227–34. doi: 10.33963/KP.15607 [DOI] [PubMed] [Google Scholar]
- 154.Scholz KH, Lengenfelder B, Thilo C, et al. Impact of COVID-19 outbreak on regional STEMI care in Germany. Clin Res Cardiol Off J Ger Card Soc 2020;109:1511–21. doi: 10.1007/s00392-020-01703-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scortichini M, Schneider dos Santos R, De’ Donato F, et al. Excess mortality during the COVID-19 outbreak in Italy: a two-stage interrupted time-series analysis. Int J Epidemiol Published Online First: 14 October 2020. doi: 10.1093/ije/dyaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Secco GG, Zocchi C, Parisi R, et al. Decrease and Delay in Hospitalization for Acute Coronary Syndromes During the 2020 SARS-CoV-2 Pandemic. Can J Cardiol 2020;36:1152–5. doi: 10.1016/j.cjca.2020.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Seiffert M, Brunner FJ, Remmel M, et al. Temporal trends in the presentation of cardiovascular and cerebrovascular emergencies during the COVID-19 pandemic in Germany: an analysis of health insurance claims. Clin Res Cardiol Published Online First: 4 August 2020. doi: 10.1007/s00392-020-01723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sharma M, Lioutas V-A, Madsen T, et al. Decline in stroke alerts and hospitalisations during the COVID-19 pandemic. Stroke Vasc Neurol 2020;:svn-2020-000441. doi: 10.1136/svn-2020-000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Silva GA e, Jardim BC, Santos CVB dos. Excess mortality in Brazil in times of Covid-19. Ciênc Saúde Coletiva 2020;25:3345–54. doi: 10.1590/1413-81232020259.23642020 [DOI] [PubMed] [Google Scholar]
- 160.Sinnathamby MA, Whitaker H, Coughlan L, et al. All-cause excess mortality observed by age group and regions in the first wave of the COVID-19 pandemic in England. Eurosurveillance 2020;25. doi: 10.2807/1560-7917.ES.2020.25.28.2001239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Slullitel PA, Lucero CM, Soruco ML, et al. Prolonged social lockdown during COVID-19 pandemic and hip fracture epidemiology. Int Orthop 2020;44:1887–95. doi: 10.1007/s00264-020-04769-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sobti A, Memon K, Bhaskar RRP, et al. Outcome of trauma and orthopaedic surgery at a UK District General Hospital during the Covid-19 pandemic. J Clin Orthop Trauma 2020;11:S442–5. doi: 10.1016/j.jcot.2020.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stang A, Standl F, Kowall B, et al. Excess mortality due to COVID-19 in Germany. J Infect Published Online First: 19 September 2020. doi: 10.1016/j.jinf.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stöhr E, Aksoy A, Campbell M, et al. Hospital admissions during Covid-19 lock-down in Germany: Differences in discretionary and unavoidable cardiovascular events. PloS One 2020;15:e0242653. doi: 10.1371/journal.pone.0242653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stokes AC, Lundberg DJ, Hempstead K, et al. Assessing the Impact of the Covid-19 Pandemic on US Mortality: A County-Level Analysis. medRxiv 2020;:2020.08.31.20184036. doi: 10.1101/2020.08.31.20184036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Strang P, Bergström J, Martinsson L, et al. Dying From COVID-19: Loneliness, End-of-Life Discussions, and Support for Patients and Their Families in Nursing Homes and Hospitals. A National Register Study. J Pain Symptom Manage 2020;60:e2–13. doi: 10.1016/j.jpainsymman.2020.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Strang P, Fürst P, Schultz T. Excess deaths from COVID-19 correlate with age and socio-economic status. A database study in the Stockholm region. Ups J Med Sci 2020;125:297–304. doi: 10.1080/03009734.2020.1828513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Strauss AT, Boyarsky BJ, Garonzik-Wang JM, et al. Liver transplantation in the United States during the COVID-19 pandemic: National and center-level responses. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg Published Online First: 27 October 2020. doi: 10.1111/ajt.16373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: A single tertiary center experience. Dermatol Ther 2020;33:e14136. doi: 10.1111/dth.14136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Teo K-C, Leung WCY, Wong Y-K, et al. Delays in Stroke Onset to Hospital Arrival Time During COVID-19. Stroke 2020;51:2228–31. doi: 10.1161/STROKEAHA.120.030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thakrar A, Chui K, Kapoor A, et al. Thirty-Day Mortality Rate of Patients With Hip Fractures During the COVID-19 Pandemic: A Single Centre Prospective Study in the United Kingdom. J Orthop Trauma 2020;:10.1097/BOT.0000000000001889. doi: 10.1097/BOT.0000000000001889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tomasoni D, Adamo M, Italia L, et al. Impact of COVID-2019 outbreak on prevalence, clinical presentation and outcomes of ST-elevation myocardial infarction. J Cardiovasc Med Hagerstown Md 2020;21:874–81. doi: 10.2459/JCM.0000000000001098 [DOI] [PubMed] [Google Scholar]
- 173.Toner L, Koshy AN, Ko J, et al. Clinical Characteristics and Trends in Heart Failure Hospitalizations. Jacc Heart Fail 2020;8:872–5. doi: 10.1016/j.jchf.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Toušek P, Kocka V, Masek P, et al. Modified Strategies for Invasive Management of Acute Coronary Syndrome during the COVID-19 Pandemic. J Clin Med 2020;10. doi: 10.3390/jcm10010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Trabattoni D, Montorsi P, Merlino L. Late STEMI and NSTEMI Patients’ Emergency Calling in COVID-19 Outbreak. Can J Cardiol 2020;36:1161.e7–1161.e8. doi: 10.1016/j.cjca.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Uchino K, Kolikonda MK, Brown D, et al. Decline in Stroke Presentations During COVID-19 Surge. Stroke 2020;51:2544–7. doi: 10.1161/STROKEAHA.120.030331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vandoros S. Excess mortality during the Covid-19 pandemic: Early evidence from England and Wales. Soc Sci Med 2020;258:113101. doi: 10.1016/j.socscimed.2020.113101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vanni G, Legramante JM, Pellicciaro M, et al. Effect of Lockdown in Surgical Emergency Accesses: Experience of a COVID-19 Hospital. Vivo Athens Greece 2020;34:3033–8. doi: 10.21873/invivo.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vestergaard LS, Nielsen J, Richter L, et al. Excess all-cause mortality during the COVID-19 pandemic in Europe–preliminary pooled estimates from the EuroMOMO network, March to April 2020. Eurosurveillance 2020;25:2001214. doi: 10.2807/1560-7917.ES.2020.25.26.2001214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vieira A, Peixoto VR, Aguiar P, et al. Rapid Estimation of Excess Mortality during the COVID-19 Pandemic in Portugal -Beyond Reported Deaths. J Epidemiol Glob Health 2020;10:209–13. doi: 10.2991/jegh.k.200628.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang J, Chaudhry SA, Tahsili-Fahadan P, et al. The impact of COVID-19 on acute ischemic stroke admissions: Analysis from a community-based tertiary care center. J Stroke Cerebrovasc Dis 2020;29:105344. doi: 10.1016/j.jstrokecerebrovasdis.2020.105344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Weinberger DM, Chen J, Cohen T, et al. Estimation of Excess Deaths Associated With the COVID-19 Pandemic in the United States, March to May 2020. JAMA Intern Med 2020;180:1336. doi: 10.1001/jamainternmed.2020.3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Westgard BC, Morgan MW, Vazquez-Benitez G, et al. An Analysis of Changes in Emergency Department Visits After a State Declaration During the Time of COVID-19. Ann Emerg Med 2020;76:595–601. doi: 10.1016/j.annemergmed.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wong JSH, Cheung KMC. Impact of COVID-19 on Orthopaedic and Trauma Service: An Epidemiological Study. J Bone Joint Surg Am 2020;102:e80. doi: 10.2106/JBJS.20.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Woolf SH, Chapman DA, Sabo RT, et al. Excess Deaths From COVID-19 and Other Causes, March-April 2020. JAMA 2020;324:510. doi: 10.1001/jama.2020.11787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yalamanchi R, Dasari BC, Narra L, et al. Cardiac Intensive Care Unit Admissions during COVID-19 Pandemic—A Single Center Experience. Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med 2020;24:1103–5. doi: 10.5005/jp-journals-10071-23660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang F, Song X, Dang Y. Experience of ST segment elevation myocardial infarction management during COVID-19 pandemic from the mainland of China. Cardiovasc Revascularization Med Mol Interv 2020;:S1553-8389(20)30453-X. doi: 10.1016/j.carrev.2020.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Huxtable R. COVID-19: where is the national ethical guidance? BMC Med Ethics 2020;21:32. doi: 10.1186/s12910-020-00478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Herreros B, Gella P, Asua DR de. Triage during the COVID-19 epidemic in Spain: better and worse ethical arguments. J Med Ethics 2020;46:455–8. doi: 10.1136/medethics-2020-106352 [DOI] [PubMed] [Google Scholar]
- 190.Feinstein MM, Niforatos JD, Hyun I, et al. Considerations for ventilator triage during the COVID-19 pandemic. Lancet Respir Med Published Online First: 28 April 2020. doi: 10.1016/S2213-2600(20)30192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vinay R, Baumann H, Biller-Andorno N. Ethics of ICU triage during COVID-19. Br Med Bull 2021;138:5–15. doi: 10.1093/bmb/ldab009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.White DB, Lo B. Mitigating Inequities and Saving Lives with ICU Triage during the COVID-19 Pandemic. Am J Respir Crit Care Med 2021;203:287–95. doi: 10.1164/rccm.202010-3809CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Karlinsky A, Kobak D. The World Mortality Dataset: Tracking excess mortality across countries during the COVID-19 pandemic. medRxiv 2021;:2021.01.27.21250604. doi: 10.1101/2021.01.27.21250604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zampieri FG, Bastos LSL, Soares M, et al. The association of the COVID-19 pandemic and short-term outcomes of non-COVID-19 critically ill patients: an observational cohort study in Brazilian ICUs. Intensive Care Med Published Online First: 13 September 2021. doi: 10.1007/s00134-021-06528-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg 2020;107:1440–9. doi: 10.1002/bjs.11746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gilbert AW, Billany JCT, Adam R, et al. Rapid implementation of virtual clinics due to COVID-19: report and early evaluation of a quality improvement initiative. BMJ Open Qual 2020;9:e000985. doi: 10.1136/bmjoq-2020-000985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Moynihan R, Sanders S, Michaleff ZA, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open 2021;11:e045343. doi: 10.1136/bmjopen-2020-045343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Skegg D, Gluckman P, Boulton G, et al. Future scenarios for the COVID-19 pandemic. The Lancet 2021;397:777–8. doi: 10.1016/S0140-6736(21)00424-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lau H, Khosrawipour T, Kocbach P, et al. Evaluating the massive underreporting and undertesting of COVID-19 cases in multiple global epicenters. Pulmonology 2021;27:110–5. doi: 10.1016/j.pulmoe.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files. Since this is a systematic review, all articles for which data were searched for are publicly available on search databases.