Abstract
Developing innovative agri-technologies is essential for the sustainable intensification of global food production. Seed dormancy is an adaptive trait which defines the environmental conditions in which the seed is able to germinate. Dormancy release requires sensing and integration of multiple environmental signals, a complex process which may be mimicked by seed treatment technologies. Here, we reveal molecular mechanisms by which non-thermal (cold) atmospheric gas plasma-activated water (GPAW) releases the physiological seed dormancy of Arabidopsis thaliana. GPAW triggered dormancy release by synergistic interaction between plasma-generated reactive chemical species (NO3–, H2O2, ·NO, and ·OH) and multiple signalling pathways targeting gibberellin and abscisic acid (ABA) metabolism and the expression of downstream cell wall-remodelling genes. Direct chemical action of GPAW on cell walls resulted in premature biomechanical endosperm weakening. The germination responses of dormancy signalling (nlp8, prt6, and dog1) and ABA metabolism (cyp707a2) mutants varied with GPAW composition. GPAW removes seed dormancy blocks by triggering multiple molecular signalling pathways combined with direct chemical tissue weakening to permit seed germination. Gas plasma technologies therefore improve seed quality by mimicking permissive environments in which sensing and integration of multiple signals lead to dormancy release and germination.
Keywords: Abscisic acid metabolism, Arabidopsis thaliana, endosperm weakening, gas plasma-activated water, nitrogen signalling, non-thermal atmospheric gas plasma technology, plant hormone signalling, reactive oxygen species, seed dormancy
Gas plasma-activated water agri-technologies mimick the multitude of environmental signals to enhance seed germination and growth required for the sustainable intensification of food production.
Introduction
‘Plasma agriculture’ is a rapidly emerging field in which pre-planting and post-harvest applications using non-thermal atmospheric gas plasma are developed into environment-friendly agri-technologies for the sustainable production of food (Bourke et al., 2018; Ito et al., 2018; Ranieri et al., 2021). Seeds are the beginning (sowing) and the end (harvesting) of many food chains important to human existence. High-quality crop seeds are the delivery systems of technological advances in plant breeding and seed treatment to agriculture and food chains (Finch-Savage and Leubner-Metzger, 2006; Nonogaki, 2017; Yan et al., 2014). Gas plasma is often defined as ‘the fourth state of matter’ due to its high energetic state. In non-thermal (cold, non-equilibrium) atmospheric plasma, reactive species including free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced at ambient temperature and atmospheric pressure (Graves, 2012; Park et al., 2013; Lukes et al., 2014; Takamatsu et al., 2014; Lu et al., 2016; Zhou et al., 2020; Ranieri et al., 2021). The formation of specific reactive species is dependent on the gas admixture, flow of the gas, plasma frequency, and temperature. The non-thermal plasma’s gaseous phase has demonstrated its potential as an effective method for decontaminating surfaces of heat-sensitive food products and to plant seeds by inactivating microbial pathogens (Bourke et al., 2018; Ito et al., 2018; Stepanova et al., 2018). During these gas-phase treatments of dry seeds, effects on germination performance were also noted and attributed to direct physical changes (etching) of the seed coat surface resulting in improved wetting, permeability, and water uptake (Bormashenko et al., 2015; Ling et al., 2015; Zhou et al., 2016; Bafoil et al., 2019). Progress in applying these as innovative sustainable seed technologies requires knowledge about the underpinning molecular mechanisms, as well as a clear distinction between the target seed quality traits dormancy, germination, and subsequent seedling growth.
A technological application distinct from using it in the ‘gaseous’ form is to utilize plasma chemistry for the treatment of biological materials through ‘activating’ water. Gas plasma-activated water (GPAW) is produced by a gas plasma discharge at the gas–liquid interface (Lukes et al., 2014; Takamatsu et al., 2014; Bruggeman et al., 2016; Liu et al., 2019; Zhou et al., 2020). In the case of air as carrier gas, this initiates the formation of transient ROS and RNS such as hydroxyl (·OH) and nitric oxide (·NO) radicals, which react to form more stable compounds such as hydrogen peroxide (H2O2), nitrite (NO2–), and nitrate (NO3–). Treatment of non-dormant seeds with GPAW enhanced their germination speed as well as the growth of the emerged seedlings (Park et al., 2013; Zhou et al., 2016; Bafoil et al., 2018; Liu et al., 2019). Works with Arabidopsis thaliana demonstrated that GPAW increased the permeability of the seed coat (testa) and slightly promoted testa rupture of non-dormant wild type (Col-0) seeds (Bafoil et al., 2018, 2019). However, neither were the chemical species in the GPAW which cause these effects identified, nor were the underpinning molecular mechanisms studied in these works with non-dormant A. thaliana seeds. Seed dormancy is an innate seed property that defines the environmental conditions in which a seed is able to germinate (Finch-Savage and Leubner-Metzger, 2006). Several well-distinguished seed dormancy classes are known and include the physiological dormancy of A. thaliana. The molecular mechanisms of physiological dormancy induction during seed maturation on the mother plant and its release after shedding are well known (Nambara et al., 2010; Graeber et al., 2012; Yan et al., 2014; Shu et al., 2016; Nonogaki, 2017; Steinbrecher and Leubner-Metzger, 2017; Duermeyer et al., 2018; Bailly, 2019), but it is not known if GPAW can release physiological dormancy of mature seeds of any species. Due to their larger size and similar seed structure, the seeds of Lepidium sativum, a Brassicaceae relative of Arabidopsis, provide a model system for the direct biomechanical measurement of endosperm weakening (Müller et al., 2009; Lee et al., 2012; Graeber et al., 2014; Steinbrecher and Leubner-Metzger, 2017).
In this study we compared the effects of GPAW (produced in a bubble reactor; Supplementary Fig. S1) treatment on physiologically dormant and after-ripened (non-dormant) A. thaliana seeds. We demonstrate that depending on the type of GPAW used, defined RNS and ROS in GPAW caused the dormancy release and stimulated germination via altering the metabolism and signalling of the antagonistically acting hormones abscisic acid (ABA) and gibberellins (GAs). We found that GPAW altered the expression of the associated genes, and mutant analysis revealed the signalling pathways involved. Further to this, biochemical and biomechanical analysis demonstrated that GPAW promoted micropylar endosperm (cap) weakening by direct and indirect mechanisms. Therefore, GPAW technology targets mechanisms conserved among plants, opening up an enticing prospect for the agricultural industry and the sustainable intensification of food production.
Materials and methods
Seed material and germination assays
Plant materials used in this study were A. thaliana (L.) Heynh. C24, Col-0, and mutants cyp707a2-1 (Kushiro et al., 2004), dog1-2 (Nakabayashi et al., 2012), nlp8-2 (Yan et al., 2016), and prt6-1 (Holman et al., 2009). The nlp8-2 mutant was kindly provided by Eiji Nambara (University of Toronto, Canada). The prt6-1 mutant (SAIL 1278_H11) was obtained from the Nottingham Arabidopsis Stock Centre, UK. All the mutants were in the Col-0 background. All plants were grown at 20 °C (16/8 h day/night cycle) until flowering, and were then transferred to 16 °C (16/8 h day/night cycle) to establish higher primary dormancy as previously described (Nakabayashi et al., 2012) except C24 which remained at 20 °C. Post-harvest, seeds were dried for 2 d on silica gel (15% relative humidity) before being stored at –20 °C to maintain primary dormancy. Seeds were defrosted at room temperature 1 h prior to assays. For after-ripening, seeds were stored at 21 °C and 33% relative humidity for up to 6 weeks. For germination assays, ~60 seeds in triplicate were imbibed in 600 µl of deionized water (dH2O; water purifier system Select Purewater 300, Purite Ltd, Trevose, PA, USA) and autoclaved water (control) or the specified GPAW (45 min discharged) within a 6 cm Petri dish with a single filter paper (MN 713, Macherey-Nagel, Düren, Germany), incubated in the growth chamber set at 20 °C in constant white light (~100 µmol m–2 s–1) (MLR-352 Environmental Test Chamber, Panasonic, Bracknell, UK). Radicle emergence was scored and germination percentage was graphed with the mean ±SEM and statistically compared through ANOVA and Tukey’s analysis using Prism 7.01 software (GraphPad Software, Inc., USA). Lepidium sativum L. FR14 seeds (‘Keimsprossen’, Juliwa) (Scheler et al., 2015) were propagated, and harvested seeds were dried under 15% relative humidity before storage at –20 °C until used in the experiment.
Dielectric barrier discharge gas plasma-activated water (GPAW) production
The plasma reactor engineered to produce GPAW (Supplementary Fig. S1) consists of 12 high voltage AC electrodes covered in a dielectric material fixed below a gas-permeable stainless-steel membrane (Wright et al., 2018). Above the membrane is a tank containing 100 ml of dH2O. A carrier gas (air or He–O2 admixture) flows past the electrodes at 1 SLPM (standard litre per minute), and then through the membrane and H2O. For activation, plasma is formed between the electrodes and the membrane within the carrier gas. The non-thermal atmospheric gas plasma after-glow then flows through the membrane bubbling up through the water, facilitating radical and ion diffusion into the water. To produce Air-GPAW, air was used as carrier gas with the plasma sustained at 15.8 kV and 27.1 kHz. To produce He/O2-GPAW, a helium (98%) and oxygen (2%) mixture was used as carrier gas with the plasma sustained at 8.5 kV and 29.3 kHz. In both cases, the plasma was modulated with an on-time of 100 ms and a duty cycle of 30%. Compressed air (UN1002, BOC Ltd, Guildford, UK), helium (UN1046, N4.6, BOC Ltd), and oxygen (UN1072, N5.0, BOC Ltd) gases were used for all treatments, and both their mixture and flow rate were controlled through Alicat MC-series mass flow controllers (Alicat Scientific, Tucson, AZ, USA). Voltage and frequency measurements were recorded using a Tektronix P6015A high voltage probe (Tektronix, OR, USA) and a TBS1102B digital oscilloscope (Tektronix, USA).
GPAW chemical analysis
H2O2 was quantified colorimetrically using the titanium sulfate method (Eisenberg, 1943). The peroxotitanium (IV) complex formed by the reaction of H2O2 with titanyl ions under acidic conditions was quantified by measuring the absorbance at 407 nm. A standard curve constructed using H2O2 solution (30%, Sigma-Aldrich, MO, USA) was used to calculate a molar extinction coefficient. NO2– and NO3– were quantified simultaneously using Griess and vanadium (III) chloride (VCl3) reagents in an assay described in detail by Garcia-Robledo et al. (2014). ·OH radicals were quantified through the hydroxylation of the chemical probe terephthalic acid; the resultant 2-hydroxy terephthalic acid (HTA) is fluorescent (excitation 315 nm, and emission at 425 nm). The ·OH radical synthetic rate was quantified during both plasma discharge and post-discharge. Standards of HTA (Sigma-Aldrich) were used for quantification and conversion to molar units (Sahni and Locke, 2006). For measurement during discharge, 100 ml of 2 mM HTA dissolved in 10 mM phosphate buffer (pH 6.8) was placed in the reactor chamber, and fluorescence was recorded from 300 µl samples at timed intervals. For post-discharge measurements, GPAW samples were removed from the reactor and combined with double concentration HTA reagent (4 mM HTA, 20 mM phosphate buffer, pH 6.8) in a 1:1 ratio, total volume 2 ml, sealed in a 6 cm Petri dish, incubated at 21 °C in constant light, and 300 µl samples were removed at timed intervals. For measurement, samples were placed in UV-transparent 96-well plates (UV transparent, Costar® 3635, Corning Inc., NY, USA) and fluorescence was measured (excitation 315 nm, and emission at 425 nm) using a Spark™ Multimode Plate Reader (Tecan Group Ltd, Männedorf, Switzerland).
RNA extraction, cDNA synthesis, and RT–qPCR analysis
For each sample, 10 mg of whole seeds were collected at the specified times, frozen in liquid nitrogen, and stored at –80 °C. RNA extraction was performed using the cetyltrimethylammonium bromide (CTAB) method (Chang et al., 1993) with the following modifications. Chloroform extraction was repeated three times before LiCl precipitation, additionally repeated three times following dissolving the RNA in SSTE buffer, and extracted RNA was treated with DNase (Qiagen, Manchester, UK) according to the manufacturer’s instructions. RNA quantity and purity were measured using a Spark™ Multimode Plate Reader (Tecan Group Ltd), and only samples with absorbance ratios of at least 2.0 (260/280 nm) and 2 (260/230 nm) were used for cDNA synthesis. Quantitative reverse transcription–PCR (RT–qPCR) analysis was conducted as described (Graeber et al., 2011). In brief, cDNA was synthesized with random pentadecamers from 1 µg of total RNA using Superscript III reverse transcriptase (Invitrogen, Paisley, UK) in a 20 µl volume and diluted 20-fold. qPCR was performed with ABsolute qPCR SYBR mix (Thermo Fisher Scientific, Oxford, UK) on a Biorad CFX96 system (Bio-Rad Laboratories, Watford, UK) with 140 nM gene-specific primer sets (Supplementary Table S1). The PCR program was as follows: 15 min at 95 °C, followed by 50 cycles of 15 s at 95 °C, 30 s at 60 °C or 64 °C, and 30 s at 72 °C, and then melt curve analysis was performed. Two technical replicates were performed on five biological replicates for each sample. The expression values were normalized against the geometric mean of two reference genes, HBT (At2g20000) and TIP41-Like (At4g34270), and relative expression values were shown as fold change against the indicated samples using the 2–ΔΔCt method (Livak and Schmittgen, 2001; Graeber et al., 2011).
Biomechanical analysis
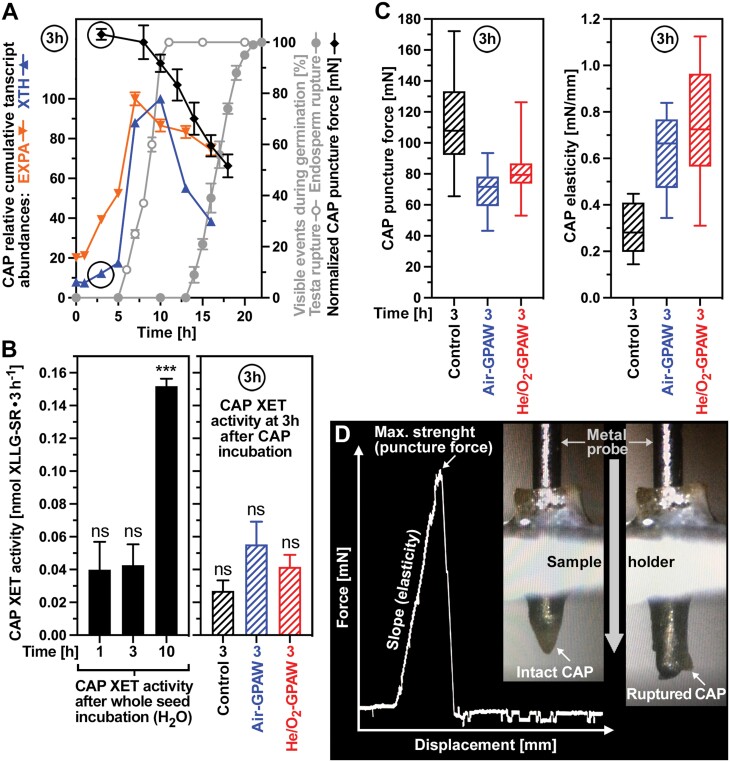
Puncture force measurements of L. sativum FR14 micropylar endosperm (cap) tissue was conducted using a custom-made machine as described earlier (Lee et al., 2012; Graeber et al., 2014; Steinbrecher and Leubner-Metzger, 2017). Seeds were imbibed for 1 h in 6 cm Petri dishes with two filter papers (4007130050 Macherey-Nagel) and 1.5 ml of H2O in constant light at 20 °C. The 1 h imbibed seeds were then dissected in moist conditions to obtain the micropylar endosperm (cap) tissues. The isolated caps were then incubated for 2 h in 1.5 ml of treatment solution [control (dH2O), Air-GPAW, or He/O2-GPAW] in constant light at 20 °C. The 3 h treated caps were then fixed into a metal mould before a metal probe (diameter 0.3 mm) was driven (0.7 mm min–1) through the caps. The force it took to rupture the caps was recorded with the displacement. The cap puncture force (tissue resistance) was determined as the maximal force from the displacement–force curve, and the cap tissue elasticity was calculated as the slope of the linear portion of the displacement-force curve (Fig. 5).
Fig. 5.
Biomechanical and biochemical analysis of GPAW-induced endosperm weakening. (A) Time courses of micropylar endosperm (cap) puncture force, testa and endosperm rupture, and expansin (EXPA) and xyloglucan endotransglycolases/hydrolase (XTH) transcript abundances in the cap of Lepidium sativum FR14 seeds during germination. For the cap puncture force, normalized values combined from two datasets (Linkies et al., 2009; Graeber et al., 2014) are presented. The cap-specific relative cumulative expression values of EXPA and XTH genes were from the spatiotemporal transcriptome dataset of L. sativum seed germination (Scheler et al., 2015); for individual genes and details, see Supplementary Fig. S4. (B) Xyloglucan endotransglycosylase (XET) enzyme activities of XTH proteins in the cap after incubation of whole L. sativum seeds in dH2O for the times indicated (left panel). Effects of incubating isolated caps in Air-GPAW (45 min) or He/O2-GPAW (45 min) on the XET enzyme activities at 3 h (right panel). Note that only the 10 h CAP XET activity was statistically different, while all of the other XET activity values were not significantly (‘ns’) different from each other. (C) Biomechanical analysis of the effects of Air-GPAW (45 min) or He/O2-GPAW (45 min) on cap endosperm weakening at 3 h. The cap puncture force (tissue resistance) at 3 h (left panel) was determined as the maximal force from the displacement–force curve, and the cap tissue elasticity at 3 h (right panel) was calculated as the slope of the linear portion of the displacement–force curve. (D) Example displacement–force curve and images of the biomechancial assay with cap prior to rupture (intact) and cap post-rupture by the metal probe of the biomechanics device.
Xyloglucan endotransglycosylase (XET) enzyme activity assays
Lepidium sativum FR14 seeds were imbibed in dH2O and incubated as described above. Cap tissues were prepared from 1, 3, and 10 h imbibed seeds for monitoring the XET activities in the caps in germination processes. Another set of cap tissues dissected from 1 h imbibed seeds (isolated cap) were further incubated for 2 h in 1.5 ml of treatment solution (water for the control, Air-GPAW, or He/O2-GPAW) in constant light at 20 °C for monitoring direct effects of GPAW on caps. Total protein was extracted from 22 caps per sample and the XET enzyme activities were assayed using the method of Fry (1997) with the modifications described in Holloway et al. (2021) except for the following changes. All protein samples were adjusted to 2 µg µl–1, 10 µg protein samples were used for each reaction, and were incubated in darkness at 20 °C for 4 h. Matrices were measured dry, twice, before native protein loading (fluorescence t=0) and post-incubation plus washing and drying (fluorescence t=4 h). The percentage of transglycosylated XLLG–SR (sulforhodamine-labelled xyloglucan nonasaccharide) relative to the xyloglucan substrate, after blank subtraction (extraction buffer loaded matrix), was used as the relative XET activity value.
Results
GPAW interferes with the seed hormone metabolism to release physiological dormancy and promote germination
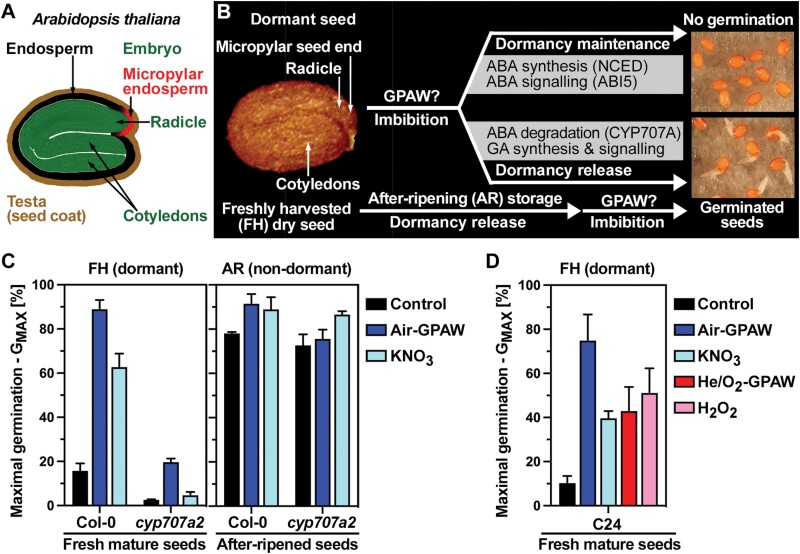
Freshly harvested (FH) mature seeds of A. thaliana (Fig. 1A) have physiological dormancy which means that they do not germinate when imbibed under favourable conditions (Graeber et al., 2012). The dormancy maintenance in imbibed FH seeds is achieved, at least in part, by enhanced ABA biosynthesis and signalling (Fig. 1B). In agreement with this, FH A. thaliana Col-0 and C24 seed populations were dormant in our experiments when imbibed in water (control). The maximum germination percentages (GMAX) remained low at 10–15% even after prolonged incubation (Fig. 1C, D). In contrast to water, imbibition in Air-GPAW caused dormancy release and resulted in high GMAX values of 80–90% (Fig. 1C, D). He/O2-GPAW also caused dormancy release of a fraction of the seed population, resulting in a GMAX of ~40% (Fig. 1D). The release of physiological seed dormancy in the imbibed state by cold stratification is known to be achieved by increased GA biosynthesis (Ogawa et al., 2003) and ABA degradation via ABA 8ʹ-hydroxlase encoded by the CYP707A2 gene (Kushiro et al., 2004). In agreement with a key role for CYP707A2 in the GPAW-mediated dormancy release, Air-GPAW treatment of non-germinating (FH) cyp707a2 mutant seeds did not result in full dormancy release as observed in the wild type (Fig. 1C). Release of physiological dormancy can also be achieved in the dry state during after-ripening (AR) storage (Fig. 1B). Non-dormant (AR) A. thaliana seed populations fully germinated when imbibed in water (control) with no or only small GMAX increases upon GPAW treatment (Fig. 1C). In contrast to the small effect on AR seed, the finding that GPAW treatment released the physiological dormancy of FH seeds resulting in a large increase in GMAX (Fig. 1) was intriguing and triggered follow-up experiments to identify the dormancy-releasing compounds in GPAW and to investigate the underpinning molecular mechanisms.
Fig. 1.
Seed dormancy release by gas plasma-activated water (GPAW). (A) Drawing of a mature Arabidopsis thaliana seed. (B) Visualization of A. thaliana seed states and dormancy release pathways. The freshly harvested (FH) dry seed has physiological dormancy, which can be released either during imbibition, such as by cold stratification, or by after-ripening (AR) storage. Abscisic acid (ABA) and gibberellin (GA) metabolism and signalling differ between dormancy maintenance and release to control germination in response to the environment. (C) The release of physiological dormancy of FH A. thaliana Col-0 seeds by treatment with Air-GPAW involves ABA degradation via the CYP707A2 gene. Air-GPAW treatment of AR seeds does not significantly improve the maximal germination (GMAX) of either wild-type or cyp707a2 mutant seeds. (D) The effects of Air-GPAW, He/O2-GPAW, 5 mM KNO3, and 300 µM H2O2 on the GMAX of FH (dormant) A. thaliana C24 seeds. Discharge times of 45 min were used for the GPAW production (Supplementary Fig. S1). Means ±SEM are presented for GMAX.
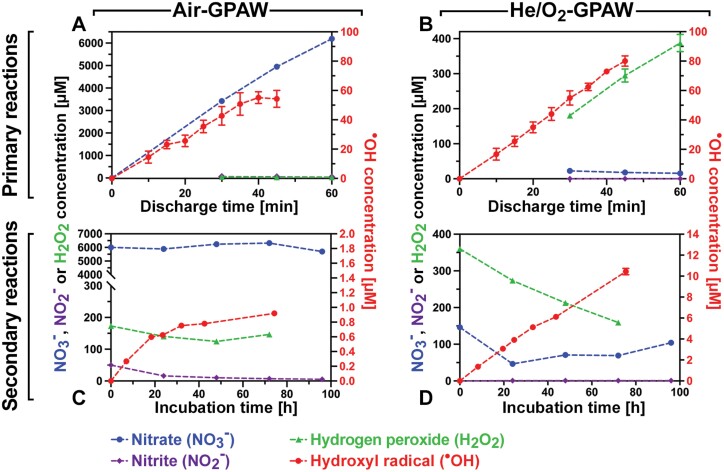
The GPAW produced for this study was standardized using a bubble reactor (Supplementary Fig. S1), and the active species generated under two preparation regimes, Air-GPAW and He/O2-GPAW, were quantified (Fig. 2). As the water was exposed to plasma during GPAW production, primary reactions led to an increase of the concentration of nitrate (NO3–) in Air-GPAW (Fig. 2A) and, as expected, negligible nitrate production in He/O2-GPAW (Fig. 2B). H2O2 increased to reach 388 µM in He/O2-GPAW (Fig. 2B) and was >10-fold lower in Air-GPAW (Fig. 2A). The highly reactive hydroxyl radical (·OH) increased to ~60 µM steady-state concentration in both Air-GPAW (Fig. 2A) and He/O2-GPAW (Fig. 2B), while nitrite (NO2–) was produced in Air-GPAW and absent in He/O2-GPAW. The two types of GPAW, therefore, shared ·OH production, but differed in that NO3– accumulated over discharge time only in Air-GPAW and H2O2 only in He/O2-GPAW. Post-discharge, the concentration of active species in the water evolved over time due to secondary reactions. This included initially a sharp drop in the very short-lived ·OH radical (half-life 1 ns), followed by secondary ·OH steady-state production. During a GPAW incubation period of up to 100 h, the concentration of H2O2 remained at 100–300 µM in both GPAWs (Fig. 2C,D). However, the two GPAWs differed in that NO3– remained high at ~6 mM, and ·OH secondary production was very slow (0.01 µM h–1), leading to <1 µM ·OH concentrations in Air-GPAW (Fig. 2C), while in He/O2-GPAW NO3– remained very low and a >10-fold faster (0.14 µM h–1) ·OH secondary production led to >10 µM ·OH concentrations. The observed seed dormancy release with GPAW (Fig. 1) may therefore be achieved by NO3–, H2O2, ·OH (Fig. 2), and other ROS/RNS derived from these (including ·NO radicals; Supplementary Fig. S2) produced in vitro (Lukes et al., 2014; Takamatsu et al., 2014; Zhou et al., 2020) and/or in planta (Müller et al., 2009; Albertos et al., 2015; Kolbert et al., 2019).
Fig. 2.
Chemical characterization of gas plasma-activated water (GPAW). (A) Air-GPAW. (B) He/O2-GPAW. Top panels: time evolution of the concentrations of major chemical species produced in GPAW during the discharge treatment in the plasma bubble reactor; primary reactions (Supplementary Fig. S1). Bottom panels: secondary chemical reactions after removal from the bubble reactor. Quantification of major chemical species as a function of the incubation time at ~22 °C after the plasma treatment. Note that Air-GPAW and He/O2-GPAW differ considerably in their composition; means ±SEM are presented.
GPAW-mediated dormancy release involves signalling of reactive species to induce genes for GA biosynthesis, ABA degradation, and cell wall remodelling
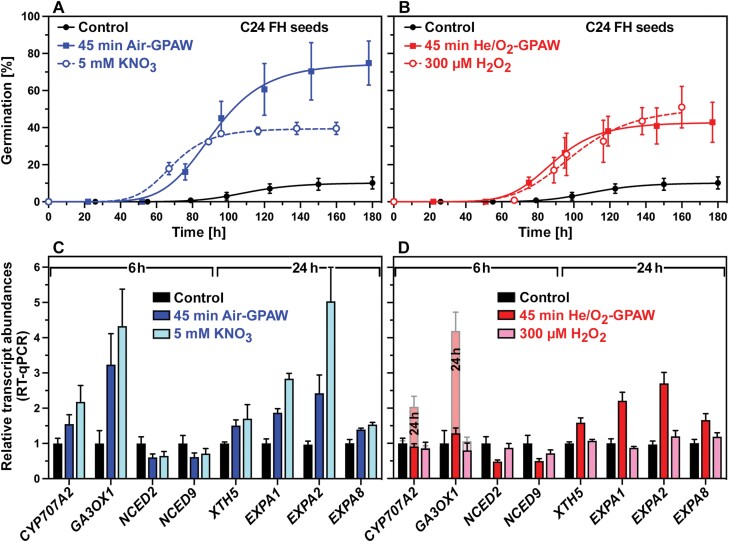
Figure 3 shows that GPAW-mediated dormancy release caused changes in the expression patterns of key genes in GA and ABA metabolism, as well as in downstream genes encoding cell wall-remodelling proteins (CWRPs) known to be required for germination (Finch-Savage and Leubner-Metzger, 2006; Nambara et al., 2010; Graeber et al., 2012). The full dormancy release of imbibed FH C24 seeds by Air-GPAW (Fig. 3A) was associated with the very early (6 h) up-regulation of GA 3-oxidase 1 (GA3OX1; biosynthesis of bioactive GA) and CYP707A2 (ABA degradation) and down-regulation of 9-cis-epoxycarotenoid dioxygenase NCED2 and NCED9 (ABA biosynthesis) transcript abundances (Fig. 3C). The partial dormancy release of imbibed FH C24 seeds by He/O2-GPAW (Fig. 3B) was also associated with the down-regulation of NCED2 and NCED9 at 6 h, but the up-regulation of GA3OX1 and CYP707A2 was slower and became evident only at 24 h (Fig. 3D). From studies of dormant and non-dormant A. thaliana seeds, it is known that these key genes are regulated early during imbibition to control the GA/ABA balance (Nakabayashi et al., 2005; Bethke et al., 2007; Preston et al., 2009; Liu et al., 2010; Graeber et al., 2014), which is decisive in the control of germination by dormancy maintenance or release (Fig. 1B). Nitrate treatment resulted in very similar expression patterns to Air-GPAW (Fig. 3C), suggesting that nitrate signalling may be involved in the dormancy release by the ~5 mM NO3– in the Air-GPAW (Fig. 2A). The dormancy release caused by nitrate treatment was, however, only partial (GMAX ~40%; Fig. 3A), suggesting that in addition ·OH (Fig. 2) and other ROS and RNS pathways may be involved (Supplementary Fig. S2) to achieve the full dormancy release.
Fig. 3.
GPAW-induced gene expression associated with dormancy release and germination. (A) Dormancy release of freshly harvested (FH) Arabidopsis thaliana C24 seeds by Air-GPAW (45 min discharge time) and 5 mM KNO3 mimicking the Air-GPAW’s NO3– concentration (see Fig. 2A) as compared with the control (dH2O). (B) Dormancy release of FH C24 seeds by He/O2-GPAW (45 min discharge time) and 300 µM H2O2 mimicking the He/O2-GPAW’s H2O2 concentration (see Fig. 2B). (C) RT–qPCR analyses of seed transcript abundances at 6 h for key genes encoding enzymes for ABA degradation (CYP707A2), GA biosynthesis (GA3OX1), ABA biosynthesis (NECD2/9), and at 24 h for CWRP genes known to be involved in endosperm weakening and germination. Relative mean ±SEM values for the Air-GPAW and NO3– treatments are compared with the 6 h and 24 h samples of the control series (set to 1 for each gene). (D) RT–qPCR analyses of seed transcript abundances for the He/O2-GPAW and H2O2 treatments. Relative mean ±SEM values compared with the 6 h and 24 h controls are presented.
In agreement with this, He/O2-GPAW treatment caused partial dormancy release associated with similar but slower changes in the GA3OX1, CYP707A2, and NCED2/9 transcript abundances (Fig. 3). The ~10-fold lower nitrate concentration in He/O2-GPAW is too low to alone trigger the dormancy release (Fig. 4), but ·OH, H2O2 (Fig. 2), or other ROS signalling molecules may be involved. It is known that high H2O2 concentrations (5–10 mM) fully release A. thaliana dormancy in association with very early up-regulation of GA3OX1 and CYP707A2 expression in imbibed seeds (Liu et al., 2010). In contrast to this, low H2O2 concentrations [<1 mM (Liu et al., 2010) or 300 µM (Fig. 3B)] are less effective. In addition, the treatments induced the expression of CWRP genes such as those encoding expansins and xyloglucan endotransglycolases/hydrolase (XTHs; XTH5, EXPA1, EXPA2, and EXPA8) responsible for endosperm weakening (Fig. 3C,D). Taken together, these findings demonstrate that GPAW treatment alters the expression of GA and ABA metabolism genes, and suggest that the expected change in the GA/ABA balance and resulting downstream CWRP gene expression may cause the GPAW-mediated dormancy release. To further investigate which of the known major ROS and RNS signalling pathways (Supplementary Fig. S2) are involved in these GPAW responses, we utilized specific A. thaliana mutants in dose–response experiments (Fig. 4).
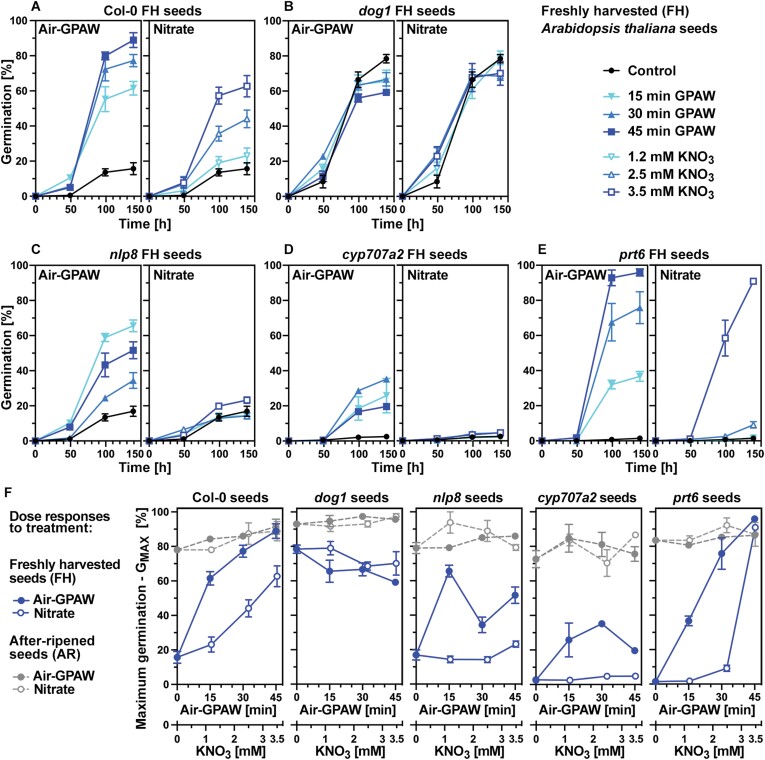
Fig. 4.
Germination responses of Arabidopsis thaliana mutant seeds to Air-GPAW treatment. (A) Germination responses of freshly harvested (FH) A. thaliana Col-0 seeds to Air-GPAW with increasing discharge time and the corresponding NO3– mimic with increasing concentration. (B) Germination responses of FH dog1 mutant seeds. (C) Germination responses of FH nlp8 mutant seeds. (D) Germination responses of FH cyp707a2 mutant seeds. (E) Germination responses of FH prt6 mutant seeds. (F) Dose responses to Air-GPAW and the corresponding NO3– mimic for the maximum germination (GMAX) of FH compared with AR wild-type and mutant seeds. Mean ±SEM germination values over time of seed populations incubated in constant white light at 20 °C are presented.
Mutant analysis reveals that GPAW acts to release dormancy via several key signalling pathways involved in the control of germination by physiological dormancy
Increased Air-GPAW discharge time leading to elevated production of major chemical species (Fig. 2) resulted in a dose-dependent increase in physiological dormancy release of FH A. thaliana seeds (Fig. 4A). This GPAW dose response resulted in a partial dormancy release (GMAX ~60%) for the 15 min discharge time to full dormancy release (GMAX ~90%) for the 45 min discharge time. A dose-dependent response was also evident for nitrate, which reached partial dormancy release (GMAX ~60%) at 3.5 mM KNO3 in FH Col-0 seeds (Fig. 4A). Using 5 mM KNO3 also only caused partial dormancy release (GMAX ~40%) in FH C24 seeds (Fig. 3A). This dormancy release by nitrate treatment in about half of the population’s seeds demonstrates that nitrate is indeed a major chemical involved in the GPAW-mediated seed dormancy release. However, other pathways must be involved in addition as for Air-GPAW full dormancy release of the seed population was achieved for C24 (Fig. 3A) and Col-0 (Fig. 4A). The control of germination by physiological dormancy includes the DELAY OF GERMINATION 1 (DOG1) gene, which encodes a dormancy-specific master regulator (Graeber et al., 2012; Nakabayashi et al., 2012; Nee et al., 2017; Nonogaki, 2017; Nishimura et al., 2018). Due to the absence of dormancy, FH dog1 mutant seeds fully germinated and no germination improvement was achieved by either GPAW or nitrate treatment (Fig. 4B, F). In agreement with GPAW not acting via altering the expression of DOG1 in imbibed FH seeds, no differences in the DOG1 transcript abundances were observed in response to GPAW or nitrate (Supplementary Fig. S3). Dormancy can also be released by AR storage (Fig. 1B) and, consequently, AR wild-type seed populations fully germinated (Fig. 4F). In contrast to FH Col-0 seeds and as for non-dormant dog1 seeds, treatment of AR Col-0 seeds with either GPAW or nitrate did not appreciably affect the GMAX responses of AR seeds.
Nitrate signalling in seeds to release physiological dormancy and promote germination is achieved via the NIN-LIKE PROTEIN 8 (NLP8) transcription factor which binds to the nitrate-responsive cis-element (NTR) in the promoter region of nitrate-responsive genes including CYP707A2 (Nambara et al., 2010; Nonogaki, 2017; Duermeyer et al., 2018). In agreement with this, FH seeds of the nlp8 mutant were dormant and did not respond to nitrate treatment with an increase in GMAX because they were nitrate insensitive (Fig. 4C). In contrast to their insensitivity to nitrate, the FH nlp8 seeds responded to Air-GPAW treatment with dormancy release, resulting in a maximum increase in GMAX of ~60% (Fig. 4C). This is direct evidence that the dormancy release by Air-GPAW is not only caused by nitrate signalling, but by additional mechanisms triggered by other chemical species (Supplementary Fig. S2). The dose response of the FH nlp8 seeds differs from that of FH wild-type (Col-0) seeds in that the maximum response (GMAX ~60%) was already achieved with 15 min discharge time (Fig. 4F). In contrast to the FH Col-0 seed dose response where GMAX further increased from ~60% (15 min) to ~90% (45 min), increased discharge time lowered the dormancy release response of the nlp8 mutant, suggesting that multiple positive and negative pathways are involved (Fig. 4F). The response of FH cyp707a2 mutant seeds to the treatments (Fig. 4D) has verified that the induction of the CYP707A2 gene expression (Fig. 3) and thereby ABA degradation is a major requirement for dormancy release. While nitrate was completely ineffective in releasing their dormancy, with Air-GPAW, a partial release of FH cyp707a2 seed dormancy was achieved (GMAX ~30%), suggesting that mechanisms independent of ABA degradation are also triggered by GPAW. After-ripening fully released the dormancy of nlp8 and cyp707a2 seeds, and the GMAX values of AR seeds were therefore not appreciably affected by GPAW or nitrate (Fig. 4F). The dormancy-releasing activity of the Air-GPAW is therefore not exclusively achieved by nitrate signalling via the NLP8 pathway, but via multiple pathways involving the concerted action of multiple chemical species in the GPAW.
Other major pathways involved in the control of physiological dormancy include RNS signalling (Supplementary Fig. S2) via ·NO, which is known to be generated in planta (Nambara et al., 2010; Nonogaki, 2017; Kolbert et al., 2019) and in GPAW (Lukes et al., 2014; Takamatsu et al., 2014; Zhou et al., 2020). Signalling of ·NO in seeds includes crosstalk with ABA to increase ABA degradation, to inhibit ABA biosynthesis, and to remove ABA sensitivity by triggering proteolysis of the transcription factor ABA INSENSITIVE 5 (ABI5) (Bethke et al., 2007; Holman et al., 2009; Albertos et al., 2015). The removal of the ABA sensitivity by ·NO signalling leading to proteasome-dependent ABI5 degradation can be achieved either by S-nitrosylation (Albertos et al., 2015) or via the N-end rule pathway (Holman et al., 2009). ABI5 degradation via the N-end rule pathway requires the E3 ubiquitin ligase PROTEOLYSIS 6 (PRT6) component, and prt6 mutant seeds of A. thaliana are therefore hypersensitive to ABA (Holman et al., 2009). In agreement with this, we found that FH prt6 seeds exhibited an altered sensitivity to nitrate, and dormancy release was only observed with the highest nitrate concentration used (Fig. 4E). This resulted in a very different nitrate dose response (GMAX) of the prt6 compared with Col-0 FH seeds (Fig. 4F). In contrast to this, the dose response of FH prt6 seeds to Air-GPAW treatment was very similar to that of Col-0 FH seeds (Fig. 4A, E). In addition, it appears that GPAW does not act on altering the expression of ABI5 in imbibed FH seeds (Supplementary Fig. S3). Air-GPAW-triggered dormancy release and germination in FH nlp8 and cyp707a2 seeds differed considerably from that of FH Col-0 seeds, whereas FH prt6 seeds displayed a wild-type-like dose response (Fig. 4F). The repression of germination through prevention of testa rupture in A. thaliana requires ABI5 for down-regulation of expansin (EXPA) gene expression (Barros-Galvao et al., 2019). Removal of ABA sensitivity by ABI5 proteolysis together with the accumulation of bioactive GA due to the GPAW-mediated induction of GA3OX1 (Fig. 3) and reduced expression of GA2OX2 (Supplementary Fig. S3) will therefore trigger CWRP gene expression to stimulate endosperm weakening and germination.
Micropylar endosperm weakening is caused directly by GPAW-generated ROS as well as indirectly by GPAW-induced expression of cell wall-remodelling genes
Endosperm weakening by cell wall loosening of the micropylar endosperm (cap) tissue precedes the completion of germination of non-dormant seeds by radicle emergence (Steinbrecher and Leubner-Metzger, 2017). The endosperm (aleurone layer) contributes to the coat-imposed dormancy of A. thaliana seeds (Fig. 1A), and the dormancy release, for example by ·NO and GA treatment, leads to A. thaliana cap weakening (Bethke et al., 2007). Testa rupture is known to be preceded by the induction of cell wall-remodelling genes in the endosperm of L. sativum (Scheler et al., 2015) and A. thaliana (Dekkers et al., 2013) to promote endosperm weakening and testa rupture (Steinbrecher and Leubner-Metzger, 2017). Due to their larger size, the seeds of the Arabidopsis close relative L. sativum are highly suited for the direct biomechanical measurement of cap weakening by the puncture force method (Müller et al., 2009; Graeber et al., 2014; Steinbrecher and Leubner-Metzger, 2017). Figure 5A shows that the L. sativum cap puncture force was >100 mN in GPAW seeds imbibed for 3–8 h and subsequently decreased to ~92 mN (10 h) and 70 mN (14 h) in association with the progression with testa rupture. This was associated with a rapid rise of the EXPA and XTH transcript abundances in the cap. Transcript accumulation of all EXPA and XTH genes in the cap was 5-fold (at 7 h) and 8-fold (at 10 h), respectively (Fig. 5A). Transcript accumulation of EXPA2 in the L. sativum cap was >60-fold (Supplementary Fig. S4) and EXPA2 is also induced exclusively in the endosperm in germinating A. thaliana seeds (Supplementary Fig. S5). Most LesaXTH genes are mainly cap expressed, and the cumulative transcript abundance of all LesaXTH genes was several-fold higher in the cap as compared with the other seed compartments (Supplementary Fig S4E, F). We found that the accumulation of XTH transcripts in the L. sativum cap (Fig. 5A) was accompanied by a 4-fold increase in XET (EC 2.4.1.207) enzyme activity in the cap between 3 h and 10 h (Fig. 5B). The increased cap XET activity at 10 h is consistent with a role for XTHs in the subsequent cap weakening and testa rupture (Fig. 3A). In contrast to the enhanced cap XET activity between 3 h and 10 h, there was no difference in the cap XET activity between the 1 h and 3 h time points (Fig. 5B). Consistent with this, the onset of cap weakening was evident between 8 h and 10 h, and no decrease in cap puncture force was evident until 8 h (Fig. 5A).
In addition to ROS signalling and CWRPs (Supplementary Fig. S2), apoplastic ROS (aROS) produced in the plant cell wall can also act directly by chemical scission of backbone polysaccharides, resulting in cell wall loosening to enhance embryo elongation growth (Müller et al., 2009; Bailly, 2019) and endosperm weakening (Müller et al., 2009; Zhang et al., 2014; Steinbrecher and Leubner-Metzger, 2017). Experimentally produced ·OH, for example, caused an ~50% decrease in the L. sativum cap puncture force within 1 h (Müller et al., 2009). To test if the ·OH and other ROS/RNS produced in Air-GPAW and He/O2-GPAW (Fig. 2) cause cap weakening, we incubated isolated L. sativum caps in GPAW and biomechanically analysed their responses (Fig. 5). Figure 5C shows that the puncture force of caps incubated for 3 h in water (control) remained high (113.1 ± 4.9 mN) and therefore no cap weakening had occurred at the 3 h time point. In contrast to this, incubation of isolated caps in GPAW resulted in a significant decrease in the cap puncture force at the 3 h time point, with 70.5 ± 3.6 mN for Air-GPAW and 80.9 ± 2.3 mN for He/O2-GPAW (Fig. 5C). The XET enzyme activities of these GPAW-treated caps did not, however, differ from the control at 3 h (Fig. 5B). We therefore conclude that GPAW can cause cap weakening very early in imbibed seeds by direct chemical action of the ROS produced in the GPAW. The observed premature cap weakening caused by direct chemical action of the GPAW was associated not only with a decreased cap puncture force, but also with an increased cap elasticity compared with the control (Fig. 5C). Biological materials are structurally complex composites, and a decrease in puncture force has been observed in many endospermic seeds (Steinbrecher and Leubner-Metzger, 2017), but a change in the slope of the strain–stress curves (Fig. 5D; i.e. the cap elasticity) has not been previously reported for seeds. Whether the increase in the cap elasticity caused by reactive species in the GPAW (direct chemical scission of cell wall polysaccharides) is specific for the GPAW or also occurs in seeds upon abiotic stress is not known. Taken together, the GPAW-induced dormancy release is caused by multiple biochemical and biomechanical mechanisms which include the induction of endosperm weakening and the control of radicle emergence by ROS/RNS-mediated signalling and direct action.
Discussion
GPAW is an emerging seed treatment agri-technology to release physiological dormancy and stimulate germination by multiple molecular signalling pathways
Most crop species and their wild relatives produce seeds which are either non-dormant or physiologically dormant in their mature state at harvest (Finch-Savage and Leubner-Metzger, 2006; Nambara et al., 2010; Holloway et al., 2021). Examples for the latter include Brassicaceae crops such as oilseed rape, the Brassica vegetables, and L. sativum sprouts, and A. thaliana as their wild relative. We demonstrate here using dormant seeds of A. thaliana that GPAW treatment releases the physiological dormancy by triggering multiple key signalling pathways which gear the hormonal control towards the germination programme and by direct chemical action resulting in premature endosperm weakening. Depending on the carrier gas (air or He/O2) and the discharge treatment time (bubble reactor; Supplementary Fig. S1), the GPAW used to imbibe the seeds differed qualitatively and quantitatively in the cocktail of reactive chemical species they contain (secondary reactions in Fig. 2). Major chemical species quantified included NO3– (only in Air-GPAW), and H2O2 and the ·OH radical (10-fold higher concentration in He/O2-GPAW compared with Air-GPAW). These, and in addition the ·NO generated from NO3– and H2O2, are known to be produced by seeds in response to environmental cues to release physiological dormancy via well-established molecular signalling pathways (Supplementary Fig. S2). We demonstrate here by using specific A. thaliana mutants that GPAW triggers many of these pathways which interact synergistically to cause the observed strong molecular and physiological responses. These include the NLP8-mediated nitrate signalling pathway that induces CYP707A2 gene expression as a key target (Kushiro et al., 2004; Okamoto et al., 2006; Matakiadis et al., 2009; Yan et al., 2016; Duermeyer et al., 2018). Non-NLP8 RNS (·NO) signalling pathways to remove ABA sensitivity by ABI5 degradation are also involved (Bethke et al., 2007; Holman et al., 2009; Albertos et al., 2015; Nonogaki, 2017; Kolbert et al., 2019), as well as ROS signalling pathways that up-regulate GA biosynthesis (GA3OX1) and CWRP genes from the EXPA and XTH families (Chen et al., 2002; Voegele et al., 2011; Graeber et al., 2014; Steinbrecher and Leubner-Metzger, 2017, 2022; Barros-Galvao et al., 2019; Sanchez-Montesino et al., 2019; Herburger et al., 2020).
GPAW-generated ROS cause micropylar endosperm (cap) weakening by direct chemical action and by inducing EXPA and XTH gene expression
Direct biomechanical quantification of the cap puncture force conducted in L. sativum seeds demonstrated that GPAW causes premature endosperm weakening that is detectable just 3 h after imbibition with GPAW (Fig. 5). Endosperm weakening is a prerequisite for the completion of germination by radicle protrusion and is blocked in physiologically dormant seeds as a component of the coat-imposed dormancy mechanism (Bethke et al., 2007; Graeber et al., 2014; Steinbrecher and Leubner-Metzger, 2017). In particular, endosperm weakening is inhibited by ABA, and promoted by GA and aROS (Bethke et al., 2007; Müller et al., 2009; Graeber et al., 2014; Yan et al., 2014; Zhang et al., 2014; Steinbrecher and Leubner-Metzger, 2017; Bailly, 2019). The aROS, including ·OH and O2·–, are produced in vivo in the cap cell walls of L. sativum and lettuce seeds, and direct ·OH attack of cell wall polysaccharides has been demonstrated to cause in vivo polysaccharide scission and therefore constitutes a more direct mechanism of cap weakening (Müller et al., 2009; Zhang et al., 2014; Steinbrecher and Leubner-Metzger, 2017). It is also not known if GPAW treatment of seeds causes a similar weakening of the non-micropylar endosperm. It is, however, known that micropylar (cap) and non-micropylar endosperm of L. sativum differ in the degree of the weakening in that it is only pronounced in the cap and comparatively small in other regions of the endosperm (Lee et al., 2012). Similar findings were made in endospermic seeds of other species (Steinbrecher and Leubner-Metzger, 2017). In addition, seeds contain seed coats and in many cases fruit coats which may differ locally in their permeability for compound uptake (Hermann et al., 2007; Scheler et al., 2015; Steinbrecher and Leubner-Metzger, 2017); this could also differ for GPAW.
We found that the GPAW-induced premature cap weakening at 3 h was not associated with the induction of XET enzyme activity (Fig. 5), but by the direct chemical action of ·OH radicals in Air-GPAW and He/O2-GPAW (Fig. 2). Further progression of cap weakening at ~10 h and later, however, involves the GPAW-enhanced up-regulation of XTH genes and accumulation of XET enzyme activity in the cap (Fig. 5). Consistent with this, the GA-regulated LeXET4 gene is induced during tomato cap weakening (Chen et al., 2002) and the XTH18 and XTH19 genes are expressed in A. thaliana and L. sativum in a GA-regulated manner (Voegele et al., 2011; Graeber et al., 2014). XTHs with XET enzyme activity can, however, also reinforce tissues and play roles in the coleorhiza-enforced dormancy in grasses, which is—together with the endosperm-imposed dormancy of eudicot seeds—an example for the convergent evolution of mechanical restraint by overlaying tissues (Yan et al., 2014; Holloway et al., 2021). Interestingly, and in agreement with a role in promoting endosperm weakening and testa rupture, most of the XTH genes are expressed in the endosperm upon GPAW treatment. About half of the XTH genes are differentially expressed in that they are up-regulated upon testa rupture in L. sativum (Supplementary Fig. S4) and A. thaliana (Supplementary Fig. S5). GPAW therefore acts by mimicking environmental cues which trigger the removal of the various layers of dormancy blocks to permit seed germination.
Practical applications
Innovations in seed treatment technologies support primary crop production and increasing yield potential by providing protection against pathogens and physiological enhancement to perform better in abiotic stress conditions. We demonstrated that the hallmark of GPAW action important for developing seed treatment and plant growth applications is the concerted action of the combined major chemical species produced to trigger multiple molecular direct (chemical weakening) and indirect (signalling pathways) mechanisms. GPAW thereby mimicks the multitude of environmental signals which are sensed and integrated by seeds. Among them are signalling pathways which are conserved among plants (Supplementary Fig. S2) and allow translation of our findings to seeds of other species for practical applications. The knowledge of the underpinning mechanisms derived from our work is therefore crucial for the emerging ‘plasma agriculture’ (Bourke et al., 2018; Ito et al., 2018; Ranieri et al., 2021) in which non-thermal atmospheric gas plasma is developed into environment-friendly agri-technologies for the sustainable global food production.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Diagram of the bubble reactor used to produce GPAW.
Fig. S2. Schematic presentation of ROS and RNS signalling pathways.
Fig. S3. GPAW-induced gene expression in seeds.
Fig. S4. Cell wall-remodelling genes in germinating L. sativum seeds.
Fig. S5. Cell wall-remodelling genes in germinating A. thaliana seeds.
Table S1. Primer sequences used for RT–qPCR.
Acknowledgements
We thank Eiji Nambara (University of Toronto, Canada) for the A. thaliana mutant seeds, and Michael Gasper for expert technical assistance (University of Münster, Germany). We also thank Gilbert Shama (Loughborough University) for early discussions on the use of plasma for agricultural applications, and Hemaka Bandulasena and Alec Wright for support with the development of the plasma reactor.
Contributor Information
Giles Grainge, Department of Biological Sciences, Royal Holloway University of London, Egham, Surrey TW20 0EX, UK.
Kazumi Nakabayashi, Department of Biological Sciences, Royal Holloway University of London, Egham, Surrey TW20 0EX, UK.
Tina Steinbrecher, Department of Biological Sciences, Royal Holloway University of London, Egham, Surrey TW20 0EX, UK.
Sue Kennedy, Elsoms Seeds Ltd, Spalding, Lincolnshire PE11 1QG, UK.
Junchen Ren, Wolfson School of Mechanical, Electrical and Manufacturing Engineering, Loughborough University, Leicestershire LE11 3TU, UK.
Felipe Iza, Wolfson School of Mechanical, Electrical and Manufacturing Engineering, Loughborough University, Leicestershire LE11 3TU, UK; Division of Advanced Nuclear Engineering, Pohang University of Science and Technology (POSTECH), Pohang, Gyeongbuk 790-784, South Korea.
Gerhard Leubner-Metzger, Department of Biological Sciences, Royal Holloway University of London, Egham, Surrey TW20 0EX, UK; Laboratory of Growth Regulators, Institute of Experimental Botany, Czech Academy of Sciences and Faculty of Science, Palacký University Olomouc, CZ-78371 Olomouc, Czech Republic.
Daniel Gibbs, University of Birmingham, UK.
Author contributions
GG, KN, TS, SK, FI, and GL-M: conceptualization and design; JR and FI: design and characterization of the GPAW generator; GG, JR, and TS: conducting the experiments; GG, KN, JR, TS, and GL-M: data analysis; GG and GL-M: writing with input from all authors. All authors read and approved the manuscript.
Conflict of interest
The authors declare no competing interests.
Funding
This work was supported by the UK Biotechnology and Biological Sciences Research Council (BBSRC) with an iCASE PhD training grant to GG (BB/M01651X/1) in collaboration with Loughborough University and Elsoms Seeds (Spalding, UK). The development of biomechanical and molecular methods was further supported by an AgriTech Catalyst BBSRC Research grant to GL-M and TS (BB/M005186/1) in collaboration with Elsoms Seeds, and the development of gas plasma reactors for treatment of seeds by BBSRC IPA to FI and GL-M (BB/S018441/1 and BB/S016112/1) in collaboration with Elsoms Seeds and Bejo Zaden.
Data availability
All data presented or analysed in this article are available online through figshare https://doi.org/10.17637/rh.19376306 and in the supplementary data.
References
- Albertos P, Romero-Puertas MC, Tatematsu K, Mateos I, Sanchez-Vicente I, Nambara E, Lorenzo O.. 2015. S-nitrosylation triggers ABI5 degradation to promote seed germination and seedling growth. Nature Communications 6, 8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafoil M, Jemmat A, Martinez Y, Merbahi N, Eichwald O, Dunand C, Yousfi M.. 2018. Effects of low temperature plasmas and plasma activated waters on Arabidopsis thaliana germination and growth. PLoS One 13, e0195512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafoil M, Le Ru A, Merbahi N, Eichwald O, Dunand C, Yousfi M.. 2019. New insights of low-temperature plasma effects on germination of three genotypes of Arabidopsis thaliana seeds under osmotic and saline stresses. Scientific Reports 9, 8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C. 2019. The signalling role of ROS in the regulation of seed germination and dormancy. Biochemical Journal 476, 3019–3032. [DOI] [PubMed] [Google Scholar]
- Barros-Galvao T, Vaistij FE, Graham IA.. 2019. Control of seed coat rupture by ABA-INSENSITIVE 5 in Arabidopsis thaliana. Seed Science Research 29, 143–148. [Google Scholar]
- Bethke PC, Libourel IGL, Aoyama N, Chung Y-Y, Still DW, Jones RL.. 2007. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiology 143, 1173–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormashenko E, Shapira Y, Grynyov R, Whyman G, Bormashenko Y, Drori E.. 2015. Interaction of cold radiofrequency plasma with seeds of beans (Phaseolus vulgaris). Journal of Experimental Botany 66, 4013–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke P, Ziuzina D, Boehm D, Cullen PJ, Keener K.. 2018. The potential of cold plasma for safe and sustainable food production. Trends in Biotechnology 36, 615–626. [DOI] [PubMed] [Google Scholar]
- Bruggeman PJ, Kushner MJ, Locke BR, et al. 2016. Plasma–liquid interactions: a review and roadmap. Plasma Sources Science & Technology 25, 053002. [Google Scholar]
- Chang S, Puryear J, Cairney J.. 1993. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter 11, 113–116. [Google Scholar]
- Chen F, Nonogaki H, Bradford KJ.. 2002. A gibberellin-regulated xyloglucan endotransglycosylase gene is expressed in the endosperm cap during tomato seed germination. Journal of Experimental Botany 53, 215–223. [DOI] [PubMed] [Google Scholar]
- Dekkers BJW, Pearce S, van Bolderen-Veldkamp RPM, et al. 2013. Transcriptional dynamics of two seed compartments with opposing roles in Arabidopsis seed germination. Plant Physiology 163, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duermeyer L, Khodapanahi E, Yan DW, Krapp A, Rothstein SJ, Nambara E.. 2018. Regulation of seed dormancy and germination by nitrate. Seed Science Research 28, 150–157. [Google Scholar]
- Eisenberg G. 1943. Colorimetric determination of hydrogen peroxide. Industrial and Engineering Chemistry Analytical Edition 15, 327–328. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G.. 2006. Seed dormancy and the control of germination. New Phytologist 171, 501–523. [DOI] [PubMed] [Google Scholar]
- Fry SC. 1997. Novel ‘dot-blot’ assays for glycosyltransferases and glycosylhydrolases: optimization for xyloglucan endotransglycosylase (XET) activity. The Plant Journal 11, 1141–1150. [Google Scholar]
- Garcia-Robledo E, Corzo A, Papaspyrou S.. 2014. A fast and direct spectrophotometric method for the sequential determination of nitrate and nitrite at low concentrations in small volumes. Marine Chemistry 162, 30–36. [Google Scholar]
- Graeber K, Linkies A, Steinbrecher T, et al. 2014. DELAY OF GERMINATION 1 mediates a conserved coat dormancy mechanism for the temperature- and gibberellin-dependent control of seed germination. Proceedings of the National Academy of Sciences, USA 111, E3571–E3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber K, Linkies A, Wood AT, Leubner-Metzger G.. 2011. A guideline to family-wide comparative state-of-the-art quantitative RT-PCR analysis exemplified with a Brassicaceae cross-species seed germination case study. The Plant Cell 23, 2045–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber K, Nakabayashi K, Miatton E, Leubner-Metzger G, Soppe WJ.. 2012. Molecular mechanisms of seed dormancy. Plant, Cell & Environment 35, 1769–1786. [DOI] [PubMed] [Google Scholar]
- Graves DB. 2012. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. Journal of Physics D: Applied Physics 45, 263001. [Google Scholar]
- Herburger K, Frankova L, Picmanova M, Loh JW, Valenzuela-Ortega M, Meulewaeter F, Hudson AD, French CE, Fry SC.. 2020. Hetero-trans-β-glucanase produces cellulose–xyloglucan covalent bonds in the cell walls of structural plant tissues and is sttimulated by expansin. Molecular Plant 13, 1047–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann K, Meinhard J, Dobrev P, Linkies A, Pesek B, Heß B, Machackova I, Fischer U, Leubner-Metzger G.. 2007. 1-Aminocyclopropane-1-carboxylic acid and abscisic acid during the germination of sugar beet (Beta vulgaris L.)—a comparative study of fruits and seeds. Journal of Experimental Botany 58, 3047–3060. [DOI] [PubMed] [Google Scholar]
- Holloway T, Steinbrecher T, Perez M, Seville A, Stock D, Nakabayashi K, Leubner-Metzger G.. 2021. Coleorhiza-enforced seed dormancy: a novel mechanism to control germination in grasses. New Phytologist 229, 2179–2191. [DOI] [PubMed] [Google Scholar]
- Holman TJ, Jones PD, Russell L, et al. 2009. The N-end rule pathway promotes seed germination and establishment through removal of ABA sensitivity in Arabidopsis. Proceedings of the National Academy of Sciences, USA 106, 4549–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Oh J-S, Ohta T, Shiratani M, Hori M.. 2018. Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Processes and Polymers 15, e1700073. [Google Scholar]
- Kolbert Z, Barroso JB, Brouquisse R, et al. 2019. A forty year journey: the generation and roles of NO in plants. Nitric Oxide-Biology and Chemistry 93, 53–70. [DOI] [PubMed] [Google Scholar]
- Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E.. 2004. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8ʹ-hydroxylases: key enzymes in ABA catabolism. The EMBO Journal 23, 1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJD, Dekkers BJW, Steinbrecher T, Walsh CT, Bacic A, Bentsink L, Leubner-Metzger G, Knox JP.. 2012. Distinct cell wall architectures in seed endosperms in representatives of the Brassicaceae and Solanaceae. Plant Physiology 160, 1551–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Jiangang L, Minchong S, Chunlei Z, Yuanhua D.. 2015. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Scientific Reports 5, 13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkies A, Müller K, Morris K, Turečková V, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, Leubner-Metzger G.. 2009. Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. The Plant Cell 21, 3803–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Honnorat B, Yang H, Arancibia J, Rajjou L, Rousseau A.. 2019. Non-thermal DBD plasma array on seed germination of different plant species. Journal of Physics D: Applied Physics 52, 025401. [Google Scholar]
- Liu Y, Ye N, Liu R, Chen M, Zhang J.. 2010. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. Journal of Experimental Botany 61, 2979–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lu X, Naidis GV, Laroussi M, Reuter S, Graves DB, Ostrikov K.. 2016. Reactive species in non-equilibrium atmospheric-pressure plasmas: generation, transport, and biological effects. Physics Reports 630, 1–84. [Google Scholar]
- Lukes P, Dolezalova E, Sisrova I, Clupek M.. 2014. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Science & Technology 23, 015019. [Google Scholar]
- Matakiadis T, Alboresi A, Jikumaru Y, Tatematsu K, Pichon O, Renou JP, Kamiya Y, Nambara E, Truong HN.. 2009. The Arabidopsis abscisic acid catabolic gene CYP707A2 plays a key role in nitrate control of seed dormancy. Plant Physiology 149, 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G.. 2009. In vivo cell wall loosening by hydroxyl radicals during cress (Lepidium sativum L.) seed germination and elongation growth. Plant Physiology 150, 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi K, Bartsch M, Xiang Y, Miatton E, Pellengahr S, Yano R, Seo M, Soppe WJ.. 2012. The time required for dormancy release in Arabidopsis is determined by DELAY OF GERMINATION1 protein levels in freshly harvested seeds. The Plant Cell 24, 2826–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E.. 2005. Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. The Plant Journal 41, 697–709. [DOI] [PubMed] [Google Scholar]
- Nambara E, Okamoto M, Tatematsu K, Yano R, Seo M, Kamiya Y.. 2010. Abscisic acid and the control of seed dormany and germination. Seed Science Research 20, 55–67. [Google Scholar]
- Nee G, Kramer K, Nakabayashi K, Yuan BJ, Xiang Y, Miatton E, Finkemeier I, Soppe WJJ.. 2017. DELAY OF GERMINATION1 requires PP2C phosphatases of the ABA signalling pathway to control seed dormancy. Nature Communications 8, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Tsuchiya W, Moresco JJ, et al. 2018. Control of seed dormancy and germination by DOG1–AHG1 PP2C phosphatase complex via binding to heme. Nature Communications 9, 2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki H. 2017. Seed biology update—highlights and new discoveries in seed dormancy and germination research. Frontiers in Plant Science 8, 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S.. 2003. Gibberellin biosynthesis and response during Arabidopsis seed germination. The Plant Cell 15, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E.. 2006. CYP707A1 and CYP707A2, which encode ABA 8ʹ-hydroxylases, are indispensable for a proper control of seed dormancy and germination in Arabidopsis. Plant Physiology 141, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DP, Davis K, Gilani S, Alonzo CA, Dobrynin D, Friedman G, Fridman A, Rabinovich A, Fridman G.. 2013. Reactive nitrogen species produced in water by non-equilibrium plasma increase plant growth rate and nutritional yield. Current Applied Physics 13, S19–S29. [Google Scholar]
- Preston J, Tatematsu K, Kanno Y, Hobo T, Kimura M, Jikumaru Y, Yano R, Kamiya Y, Nambara E.. 2009. Temporal expression patterns of hormone metabolism genes during imbibition of Arabidopsis thaliana seeds: a comparative study on dormant and non-dormant accessions. Plant & Cell Physiology 50, 1786–1800. [DOI] [PubMed] [Google Scholar]
- Ranieri P, Sponsel N, Kizer J, Rojas-Pierce M, Hernandez R, Gatiboni L, Grunden A, Stapelmann K.. 2021. Plasma agriculture: review from the perspective of the plant and its ecosystem. Plasma Processes and Polymers 18, e2000162. [Google Scholar]
- Sahni M, Locke BR.. 2006. Quantification of hydroxyl radicals produced in aqueous phase pulsed electrical discharge reactors. Industrial & Engineering Chemistry Research 45, 5819–5825. [Google Scholar]
- Sanchez-Montesino R, Bouza-Morcillo L, Marquez J, Ghita M, Duran-Nebreda S, Gomez L, Holdsworth MJ, Bassel G, Onate-Sanchez L.. 2019. A regulatory module controlling GA-mediated endosperm cell expansion is critical for seed germination in Arabidopsis. Molecular Plant 12, 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheler C, Weitbrecht K, Pearce SP, et al. 2015. Promotion of testa rupture during garden cress germination involves seed compartment-specific expression and activity of pectin methylesterases. Plant Physiology 167, 200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu K, Liu XD, Xie Q, He ZH.. 2016. Two faces of one seed: hormonal regulation of dormancy and germination. Molecular Plant 9, 34–45. [DOI] [PubMed] [Google Scholar]
- Steinbrecher T, Leubner-Metzger G.. 2017. The biomechanics of seed germination. Journal of Experimental Botany 68, 765–783. [DOI] [PubMed] [Google Scholar]
- Steinbrecher T, Leubner-Metzger G.. 2022. Xyloglucan remodelling enzymes and the mechanics of plant seed and fruit biology. Journal of Experimental Botany 73, 1253–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova V, Slavicek P, Kelar J, Prasil J, Smekal M, Stupavska M, Jurmanova J, Cernak M.. 2018. Atmospheric pressure plasma treatment of agricultural seeds of cucumber (Cucumis sativus L.) and pepper (Capsicum annuum L.) with effect on reduction of diseases and germination improvement. Plasma Processes and Polymers 15, 1700076. [Google Scholar]
- Takamatsu T, Uehara K, Sasaki Y, Miyahara H, Matsumura Y, Iwasawa A, Ito N, Azuma T, Kohno M, Okino A.. 2014. Investigation of reactive species using various gas plasmas. RSC Advances 4, 39901–39905. [Google Scholar]
- Voegele A, Linkies A, Müller K, Leubner-Metzger G.. 2011. Members of the gibberellin receptor gene family GID1 (GIBBERELLIN INSENSITIVE DWARF1) play distinct roles during Lepidium sativum and Arabidopsis thaliana seed germination. Journal of Experimental Botany 62, 5131–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A, Bandulasena H, Ibenegbu C, Leak D, Holmes T, Zimmerman W, Shaw A, Iza F.. 2018. Dielectric barrier discharge plasma microbubble reactor for pretreatment of lignocellulosic biomass. AIChE Journal 64, 3803–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Duermeyer L, Leoveanu C, Nambara E.. 2014. The functions of the endosperm during seed germination. Plant & Cell Physiology 55, 1521–1533. [DOI] [PubMed] [Google Scholar]
- Yan D, Easwaran V, Chau V, et al. 2016. NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis. Nature Communications 7, 13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen B, Xu Z, Shi Z, Chen S, Huang X, Chen J, Wang X.. 2014. Involvement of reactive oxygen species in endosperm cap weakening and embryo elongation growth during lettuce seed germination. Journal of Experimental Botany 65, 3189–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Zhou R, Zhang X, Zhuang J, Yang S, Bazaka K, Ken Ostrikov K.. 2016. Effects of atmospheric-pressure N2, He, air, and O2 microplasmas on mung bean seed germination and seedling growth. Scientific Reports 6, 32603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou RW, Zhou RS, Wang PY, Xian YB, Mai-Prochnow A, Lu XP, Cullen PJ, Ostrikov K, Bazaka K.. 2020. Plasma-activated water: generation, origin of reactive species and biological applications. Journal of Physics D: Applied Physics 53. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented or analysed in this article are available online through figshare https://doi.org/10.17637/rh.19376306 and in the supplementary data.