Abstract
Lowest observable adverse effects level (LOAEL) is a standard point-of-departure dose in toxicology. However, first observable adverse effects level (FOAEL) was recently reported and is used, in this study, as one criterion to detect a mutagenic stimulus in a live imager. Fluorescence ubiquitinated cell cycle indicator (FUCCI) embryonic stem cells (ESC) are green in the S-G2-M phase of the cell cycle and not green in G1-phase. Standard media change here is a mild stress that delays G1-phase and media change increases green 2.5- to 5-fold. Since stress is mild, media change rapidly increases green cell number, but higher stresses of environmental toxicants and positive control hyperosmotic stress suppress increased green after media change. Perfluoro-octanoic acid (PFOA) and diethyl phthalate (DEP) previously suppressed progression of nongreen to green cell cycle progression. Here, bisphenol A (BPA), cortisol, and positive control hyperosmotic sorbitol also suppress green fluorescence, but benzo(a)pyrene (BaP) at high doses (10 μM) increases green fluorescence throughout the 74-h exposure. Since any stress can affect many cell cycle phases, messenger RNA (mRNA) markers are best interpreted in ratios as dose-dependent mutagens increase in G2/G1 and nonmutagens increase G1/G2. After 74-h exposure, RNAseq detects G1 and G2 markers and increasing BaP doses increase G2/G1 ratios but increasing hyperosmotic sorbitol and PFOA doses increase G1/G2 marker ratios. BaP causes rapid green increase in FOAEL at 2 h of stimulus, whereas retinoic acid caused significant green fluorescence increases only late in culture. Using a live imager to establish FOAEL and G2 delay with FUCCI ESC is a new method to allow commercial and basic developmental biologists to detect drugs and environmental stimuli that are mutagenic. Furthermore, it can be used to test compounds that prevent mutations. In longitudinal studies, uniquely provided by this viable reporter and live imager protocol, follow-up can be done to test whether the preventative compound itself causes harm.
Keywords: high-throughput screens, embryonic stem cells, hyperosmotic stress, mutagenic stress, toxicant stress, G0, G1, and G2 cell cycle stage markers
Introduction
Many stressors force differentiation of embryonic and placental stem cells related to cell cycle length
Heat shock stress, hypoxic stress, genotoxic stress, and hyperosmotic stress force differentiation of human and mouse embryonic stem cells (ESC) and trophoblast stem cell (TSC), often dependent on stress-activated protein kinase (SAPK) [1–13], although under certain circumstances pluripotency may be partially protected by stress [14]. Differentiation is accompanied by slowed cell growth due to diversion of energy from maintaining proliferation to homeostatic responses. For this reason, highest pluripotency in fluorescence ubiquitinated cell cycle indicator (FUCCI) ESC occurs when cell cycle and G1 transit is fastest, so that when G1 phase elongates imbalanced differentiation occurs [15,16].
FUCCI cell cycle reporter ESC can report delayed cell cycle, longer G1 phases related to toxicant and control stressors which induce mild stress due to infrequent media change
(FUCCI) ESCs are green in S-G2-M phase of the cell cycle and not green in G1. During normal culture of cells with leukemia inhibitory factor (LIF) that maintains naive pluripotency, FUCCI cells accumulate in nongreen G1 by lack of media change [17]. This can be used to measure general stress as the magnitude of cells released into cell cycle progression (eg, S-G2-M phase), by media change is suppressed in a dose-dependent manner by many stressors, such as perfluoro-octanoic acid (PFOA) and diethyl phthalate (DEP) in time lapse assays in a live imager.
Benzo[a]pyrene (BaP) is a five-ring polycyclic aromatic hydrocarbon that is mutagenic and carcinogenic [18,19]. BaP is a product of incomplete combustion of diesel motors and is highest near freeways and urban areas and in cigarette smoke. BaP induces cytochrome P4501A (CYP1A1) by binding to the AHR (aryl hydrocarbon receptor) in the cytosol [20–22]. Activated CYP1A1, together with other enzymes, catalyze BaP into benzopyrene diol epoxide (BPDE), which binds to several classes of macromolecules (DNA, RNA, and protein) to produce macromolecular adducts [23–25]. BPDE-DNA adducts can be detected by enzyme-linked immunosorbent assay or immunocytochemistry means using a polyclonal antibody against BPDE [22]. BPDE-DNA adducts disrupt DNA replication and are mutagenic.
BaP retarded ESC growth and causes mutagenesis and apoptosis at higher doses from 1 to 125 μM and suppressed endoderm, mesoderm, and ectoderm markers in embryoids that would normally be expressed at gastrulation [26]. Low-dose BaP exposures from 5 to 50 nM over a 96-h exposure of cultured embryos from zygote to late blastocyst induced significant increases in DNA damage as suggested by H2AX nuclear foci-increased apoptosis assayed by Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and reactive oxygen species. There were however no significant changes in the rate of development through zygotic genome activation (ZGA) or compaction nor in total cell number in the early blastocyst. However, BaP caused fewer Nanog and Oct4-positive cells in the inner cell mass (ICM) of the late blastocyst, the source of ESC [27]. Increase in H2AX foci occurred in culture ESC as well.
Cigarette smoke components are pathogenic for pregnancy. Adverse effects include decreased success rate of in vitro fertilization, miscarriage, intrauterine growth restriction, and low birth weight [28–30]. Of the thousands of cigarette smoke compounds, BaP is an important mutagenic one [31]. The primary cigarette tar benzo-(a)pyrene (BaP) blocks implantation of embryos from normal mice with an IC50 of 330 μM [32].
BaP causes G2 delay or arrest in placental cells and irradiation causes G2 delay in ESC, but use of FUCCI ESC in a live imager provides a novel means to screen large number of toxicants for G1 versus G2 mutagenic DevTox outcomes
BPDE-DNA adducts accumulate on the placenta and affect placental lineage proliferation and differentiation. BaP causes G2/M arrest in the JEG3 human placental cell line [33]. They can also cross the placenta barrier and are toxic to the developing conceptus [22,34–36]. BaP-DNA adducts in placenta and conceptus are considered to be a biomarker for maternal exposure to cigarette smoke or pollution [22]. BPDE-DNA adducts are detected in blastocysts from smoking parents, especially from smoking fathers [37]. G2 arrest caused by BaP has not been studied in mouse ESC but ESC does delay in S and G2 phases after irradiation [38] and both BaP and irradiation cause DNA damage, which can delay G2. Thus, BaP is a serious adverse pathogen to the blastocyst before implantation and to the early postimplantation through the earliest placenta.
We use BaP here to test a previously established ESC high throughput screen (HTS) for toxicants that delay cell cycle in G1 [9,17,39]. In this study, we show that a single assay using a live imager can use cell cycle G1 delay by infrequent media change to identify general stressors and simultaneously identify mutagenic stressors that delay in G2 and before G1. The live imager provides a real-time readout of viable FUCCI ESC reporters to detect mutagenic delay in G2 with confirmation by early first observable adverse effects level (FOAEL) and additional G2/G1 marker analysis. This model system should potentially also be useful in testing preventatives to identified mutagens and to assure the efficacy and lack of toxicity of the preventative compounds.
Materials and Methods
Materials
FUCCI mouse ESCs (muESCs) were a kind gift from Dr. Pierre Savatier [16]. FluroBright Dulbecco's modified Eagle's medium (DMEM) was obtained from Thermo Fisher–Gibco (Cat. No. A18967-01; Gibco). GlutaMAX and sodium pyruvate supplement solutions were from Life Technologies (Grand Island, NY). ESC-qualified EmbryoMax fetal bovine serum (FBS), 0.1% gelatin solution, and ESGRO™ Mouse LIF medium supplement were from EMD Millipore (Billerica, MA). MEM nonessential amino acid solution, sorbitol, 2-mercaptoethanol, and other chemicals were from Sigma (St. Louis, MO). PFOA, Benzo(a)pyrene (BaP), Bisphenol A (BPA), and Hydrocortisone were purchased from Sigma-Aldrich (Cat. Nos. 171468, SLCD4874, 239658, and SLCD6036, respectively). Retinoic acid (RA) and Sorbitol (Sor.) were from Sigma (Cat. Nos. SLCB4143 and s3889, respectively).
Methods
Embryonic stem cell culture
FUCCI mouse embryonic stem cells were cultured as described previously [9,39,40]. All ESCs were optimized at passage for exponential growth during the stimulus period, which began 18 h after passage at 25% confluence.
FUCCI muESCs were cultured in the absence of feeder cells in FluoroBright DMEM (Cat. No. A18967-01; Gibco) supplemented with 15% mESC-screened FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 0.1 mM 2-mercaptoethanol, and 1,000 U/mL murine LIF on 0.1% gelatin-coated dishes at 37°C in humidified air with 5% CO2 [41]. FUCCI muESCs were cultured overnight after passaging before stimulation with sorbitol-positive control stress or experimental stressor PFOA, BaP, BPA, and Hydrocortisone. Osmolality of ESC media with and without added 200–300 mM sorbitol was determined previously [6].
Fluorescent and confluence 96-well reading as an HTS for FUCCI mESCs
FUCCI mESCs were cultured on black-walled clear-bottom 96-well plates. The plates were pre-equilibrated in the hood for 1 h after passage to distribute the cells evenly in the wells [42]. Then they were moved to the Fisher dual CO2 incubator with ambient oxygen and 5% CO2 at 37°C overnight to reach ∼25% confluence by Tzero (HeraCell Vios 160i dual CO2 incubator; Thermo Fisher Scientific, Waltham, MA).
Then toxicants and Sorbitol were added at Tzero (∼18 h after passage) and the FUCCI mESCs were cultured for 72 h with ambient oxygen and 5% CO2 at 37°C in the BioSpa 8 Automated Incubator (BioTek, Winooski, VT). The BioSpa's robotic arm transferred the plates to the Cytation 5 Cell Imaging Multi-Mode Reader (BioTek) for imaging every 2 h. The previous toxicological study of FUCCI ESC used a IncuCyte Zoom live imager (Essen Biosciences, Inc., Ann Arbor, MI) [17].
Images were acquired by 4 × Objective for every well with cells. An image of the center of the well was taken with both Phase Contrast and GFP (EX 469/35, EM 525/39, PN 1225101). Gen5 software (Biotek) was used to process images: to measure cell confluence and to measure the area taken up by green fluorescing cells (green sum area). The media were changed every 24 h. The cells were treated with seven concentrations of toxicants in LIF+ media for 3 days: 0 and 1, 10, 100 nM and 1, 10, 100 μM. BaP, unlike the other toxicants, did not have the 100 μM dose due to imaging complications at that dose. The high dose created a completely green, fluorescent image, obstructing the view of the cells.
RNA isolation, complementary DNA library prep, and RNAseq
Lowest observable adverse effects level (LOAEL) and IC75 demonstration doses were determined for FUCCI ESC after HTS toxicant and control exposures (see below in Statistical and Graphical Analyses: Cytation Live Imager Data section). These and RA (−LIF, 0 stress), normal stemness (NS; +LIF, 0 dose stress), normal differentiation (ND; −LIF control, 0 stress) and 300 mM sorbitol stress (+LIF) were used to perform triplicated biological experiments to determine transcriptomic responses by bulk RNAseq.
To this end, messenger RNA (mRNA) expression analysis was done by the Wayne State University Genome Sciences by the following methods. An aliquot of the RNA was assessed by microfluidics using the ScreenTape for the Agilent 2200 TapeStation. The electrophoretogram, RNA Integrity Number, and the ratio of the 28S:18S RNA bands were optimized for overall quality of the RNA as done previously. RNA-seq was used to determine expression profiles. The Lexogen's QuantSeq 3′ mRNA-seq Library Prep Kit (FWD for Illumina) was used for building RNA-seq libraries from 0.1 to 200 ng of total RNA in 5 μL of nuclease-free ultrapure water.
Libraries were quantified on the Qubit and Agilent 2200 Tapestation using the DNA High-Sensitivity Screen tape. The barcoded libraries were multiplexed at equimolar concentrations and sequenced on an Illumina NovaSeq 6000. Data were demultiplexed using Illumina's CASAVA 1.8.2 software. After quality was assessed [43], reads were aligned to the mouse genome (Build mm9) [44] and tabulated for each gene region [45]. Significantly altered genes (log fold change ≥2; false discovery rate [FDR] ≤0.05) were used to identify affected pathways [46].
Statistical and graphical analyses: cytation live imager data
Initial data analysis was done using Gen5 (V3.10.06; BioTek). This software analyzed the micrographs to calculate the confluence and green sum area of each image at each time point. The micrographs were first cropped to circles, excluding the corners to reduce fluorescence background. For green fluorescence, the green nadirs were decided to be at 12 and 44 h with peaks at 32 and 56 h. Those times were the maxima or minima for the average ND green sum area line graph, and line up well with the maxima and minima for the other stimuli. For the line graphs, each time point is the average of all the replicates. For the histograms, the fold change was first calculated for each replicate well, then the average calculated. Data in all cases were from at least three independent biological experiments.
Data are presented as mean ± standard error of the mean (SEM). Graphs were initially composed and formatted using MS Excel (MS Office 365) and final formatting was done with Photoshop Elements Photoshop 13 Editor (Adobe Systems, Inc., San Jose, CA). Data from at least three independent biological experiments were analyzed using Microsoft Excel and presented as mean ± SEM. Statistical analysis was done by Student's t-test using GraphPad Prism 9.2.0. Adobe Photoshop Elements 13 (Adobe Systems, Inc.) was used to format graphs from Excel for publication.
Benchmark dose modeling
The benchmark dose (BMD) is a point of departure defined as a dose that elicits a specified response, and the Benchmark Dose Lower confidence limit (BMDL) is the lower limit at 95% confluence of the estimated BMD. The BMD and BMDLs for the toxicants' impacts on cell confluence were estimated using benchmark dose modeling software (BMDS) version 3.2 (USEPA). We selected a benchmark response of 1 standard deviation of the control samples, as recommended by the USEPA (Crump, 1995) [47]. We tested all models available in the default settings of BMDS, including linear, polynomial, exponential, and Hill models. The software-recommended model was used when available.
Otherwise, the optimal model was selected based on the visual assessment of the dose–response curve (so that the experimentally derived curve and curve fit were most similar), lowest Akaike's information criterion (AIC) (Akaike, 1974) [48], and largest goodness-of-fit P value (Dodge, 2010) [49].
Heatmaps
After obtaining triplicated bulk RNAseq data, heatmaps were used to visualize data. For the desired genes, the average counts of each stimulus were compiled. Heatmaps were created using ClustVis [50]. Rows were centered; unit variance scaling was applied to rows. Rows were clustered using correlation distance and average linkage for the FUCCI ESC data. The same color range was used for heatmaps in the same figure.
RNAseq data analysis: volcano plots
The Volcano Plots of the 2021 and 2019 RNAseq data were created by first using MS Excel (MS Office 365) to organize and transform the data, then importing the data into GraphPad Prism (9.2.0) to make the figures. All stimuli were compared with NS. Negative log of the P value was plotted against the log of the fold change. A line was drawn corresponding to P value at 0.05, so the dots above the line are significant. Red dots are P value significant and blue dots are FDR significant (<0.05). The top 5 most significant, the 3 highest fold change (FC), and the 3 lowest FC genes have their names displayed.
Results
High-throughput screening in a live imager using confluence assays and FUCCI cell cycle reporters identifies toxicant growth effects that associate G1 delay with some stressors and G2 delay by BaP
Our hypothesis is that FUCCI ESC, assayed for confluence and green fluorescence progression through S-G2-M phase of the cell cycle, can be analyzed to associate growth in the presence of toxicants with stemness/differentiation balance as previously suggested by researchers using FUCCI ESC [15,16]. We also hypothesize that general stressors slow all cell cycle phases but especially G1 [6,9,39,40,51]. In a live imager, NS grows most quickly, 100 μM PFOA and 10 μM BaP slightly less (P < 0.05 at 72 h), and 300 mM sorbitol has profound growth suppression (Fig. 1A). The mutagenic stressor BaP 10 μM quickly overrides LIF to produce significantly more green fluorescence sum area normalized to confluence (ie, cell number) than NS, indicating delay in S-G2-M phases and of the cell cycle (Fig. 1B, C).
FIG. 1.
Control normal stemness (NS) baseline culture with leukemia inhibitory factor (LIF), and three stimuli with LIF, control hyperosmotic stress at 300 mM sorbitol and an environmental general stress (PFOA) and a mutagenic stress (BaP) were tested in time lapse for confluence (A), green sum areas (B) and green sum area normalized to confluence/cell number (C). FUCCI1 embryonic stem cells (ESC) in a high throughput screen (HTS) with a live imager were used to calculate whether a stimulus produces significantly more (t-test, P < 0.05) (orange asterisk) or significantly less (t-test, P < 0.05; red asterisk) than NS. Confluence is compared at 72 h, and green fluorescence is compared with NS at 12, 32, 44, and 56 h during a 72 h culture. For (B, C), BaP after Tzero was significant at 2 h (in a statistical anomaly due to small variation BaP was statistically different at Tzero). Sample sizes are as follows: 36–38 for NS, 18–22 for 300 mM sorbitol, 4–6 for 10 μM BaP, and 18–20 for PFOA 100 μM. Some time points have up to 4 fewer N due to throwing out bad data. BaP, benzo(a)pyrene; FUCCI, fluorescence ubiquitinated cell cycle indicator; PFOA, perfluoro-octanoic acid.
Two other stressors, 300 mM sorbitol and 100 μM PFOA, override LIF to episodically produce significantly less green fluorescence normalized to confluence than NS. We hypothesize that PFOA and hyperosmotic sorbitol produce a G1 delay producing more nongreen cells and BaP produces a G2 delay those results in more green cells.
We next test the positive controls for ND and normal first-lineage induction RA, both with LIF removal. We also test 300 mM sorbitol control for override of LIF, which normally maintains NS. We compare confluence/growth and green fluorescence progression through cell cycle compared with culture of NS (LIF+) conditions. The ND, NS, and RA controls grow with similar rates by confluence assay throughout 72 h culture, as none of those has overt stress added. As expected, 300 mM sorbitol significantly decreases growth compared with NS (Fig. 2A). In comparison with NS, sorbitol also significantly suppresses green fluorescence with a FOAEL at 4 h, while ND suppresses growth only in the second Fed/unfed peak at 56 h. Interestingly, RA increases in green fluorescence compared with NS at 44 and 62 h and beyond (Fig. 2B, C).
FIG. 2.
Four Control stimuli for 72 h FUCCI ESC time lapse acquisition show that NS, ND, and RA grow similarly well, but 300 mM sorbitol suppresses growth/confluence accumulation (A) whereas green fluorescence (B) of green fluorescence/confluence (C). Significance compared with NS was calculated for times 12, 32, 44, 56, and 62 h. FUCCI1 ESC in an HTS in a live imager were used to calculate whether a stimulus produces significantly more (t-test, P < 0.05; orange asterisk) or significantly less (t-test P < 0.05; red asterisk) compared with NS. Confluence is compared at 72 h, and green fluorescence is compared with NS at 12, 32, 44, and 56 h during a 72 h culture. Also, for green fluorescence, RA was compared with NS at 62 h. At minimum, triplicate biological experiments were done. Sample sizes are as follows: 36–38 for NS, 17–19 for ND, 18–22 for RA, and 18–22 for sorbitol. Some time points have up to 4 fewer N due to throwing out bad data. ND, normal differentiation; RA, retinoic acid.
However, this is not as robust as the continuously upregulated green of BaP 10 μM, which has a FOAEL at 2 h (although it is significantly different at 0 h through a quirk of statistics) and persists through 72 h. FOAEL is a relatively new toxicology point of departure enabled by use of live imagers and viable reporters assayed in time lapse, but FOAEL has been used previously [17] to provide added toxicological risk analysis.
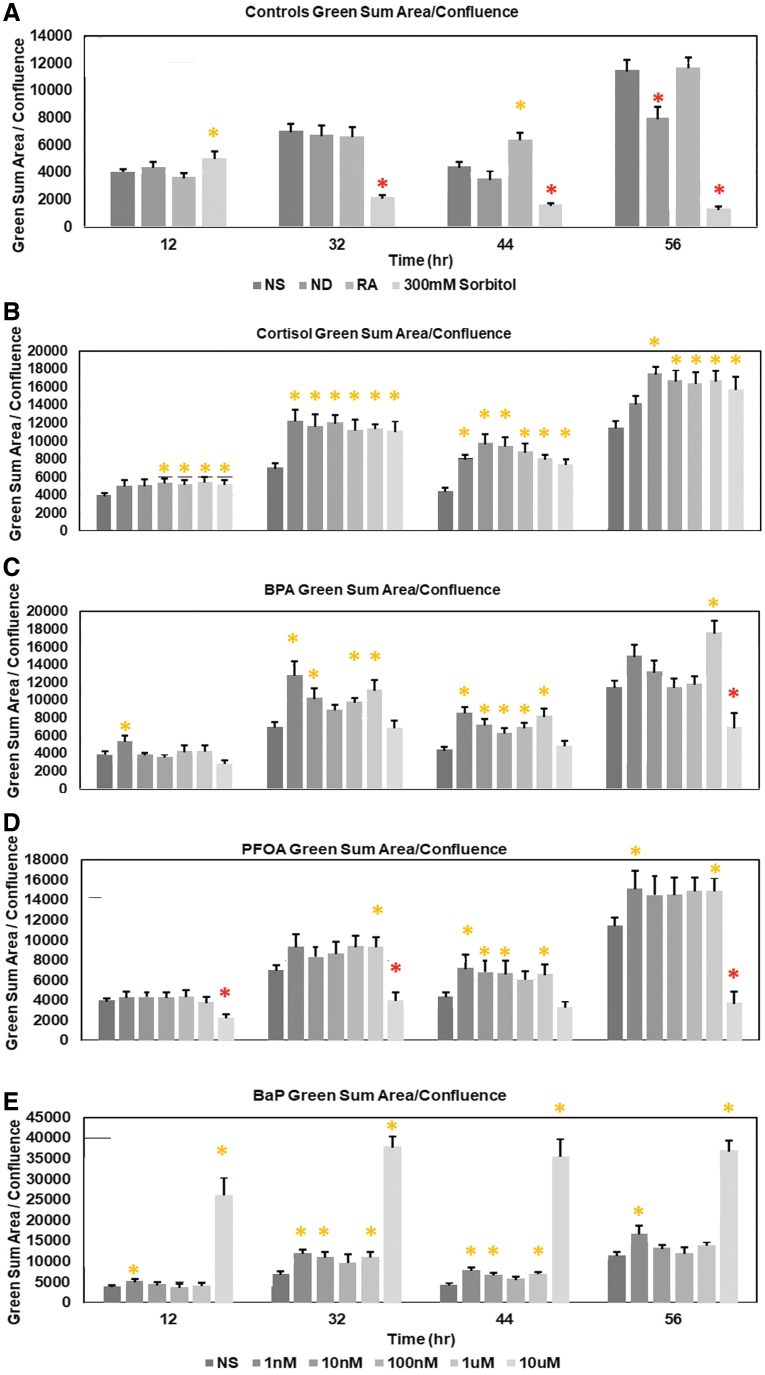
In vivo, BPA and cortisol may cosensitize to increase embryo loss early in pregnancy [52,53] in a time period modeled by the 3-day FUCCI ESC toxicant exposure here. Thus, we assay cortisol and BPA for effects on confluence/growth and green fluorescence to measure delay in G1 and progression through the cell cycle. In a further analysis of the confluence line graphs, it is apparent that among the toxicants, BPA has the lowest dose for a LOAEL (Fig. 3C), but that the sorbitol control has the highest fold change in growth suppression (Fig. 3A). Not including BPA, BaP, and PFOA have earliest effects with LOAELs at much higher levels (Fig. 3D, E). Cortisol (Fig. 3B) has later lesser effects on growth suppression than the other stimuli.
FIG. 3.
FUCCI ESC during a 72-h exposure, have significant confluence suppression by control stimuli only by 300 mM sorbitol (red asterisk) (A), but among experimental toxicants (C) BPA at lowest LOAEL/BMDL followed by (E) BaP, (D) PFOA, and least potent is (B) Cortisol. A minimum of triplicated biological experiments produces high sample sizes by using high replicate well number/biological replicate. The dose-dependent and time-dependent effects in Figs. 1 and 2 were re-interpreted for Confluence fold change for 3 days of culture: 24 h/Tzero, 48 h/Tzero, and 72 h/Tzero. Each stimulus produces significantly more (t-test, P < 0.05; orange asterisk) or significantly less (t-test P < 0.05; red asterisk) compared with NS's fold change (FC). Sample sizes are as follows: 38 for NS, 19 for ND, 22 for RA, and 300 mM sorbitol, 5–6 for each BaP dose, 5–6 for each BPA dose, 8–10 for each cortisol dose, and 8–20 for each PFOA dose. Note that cortisol, BPA, and PFOA have 7 doses (B–D), while BaP has 6 doses, lacking the highest dose (E). BPA, bisphenol A; BMDL, Benchmark Dose Lower confidence limit; LOAEL, lowest observable adverse effects level.
Is the rank order of the toxicants' suppression of confluence/growth also reflected in a suppression of the fed green peak after feeding with LIF+/NS media alone? It is apparent that sorbitol has the biggest, earliest, and most consistent daily suppression of the fed green peak compared with NS (Fig. 4A), and that the PFOA has the next largest suppression and most consistent daily suppression of the daily fed peak (Fig. 4D).
FIG. 4.
Like suppression of confluence (Fig. 3), suppression of Fed/unfed is greatest at most time points for 300 mM sorbitol (A) but ND also has suppressed Fed/unfed after media change compared with NS. However, unlike suppression of confluence, greatest and earliest suppression of Fed/unfed peak is by PFOA (D) and then to lesser extent BPA (C). Cortisol (B) and BaP (E) have significantly higher green per confluence than NS at most timepoints. A minimum of triplicated biological experiments produces high sample sizes by using high replicate well number/biological replicate. The dose-dependent and time-dependent effects in Figs. 1 and 2 were reinterpreted for green sum area normalized to confluence fold change for times corresponding to fluorescence peaks and nadirs: 12, 32, 44, and 56 h. Each stimulus produces significantly more (t-test, P < 0.05; orange asterisk) or significantly less (t-test P < 0.05; red asterisk) compared with 0 dose NS at that time point. Sample sizes are as follows: 38 for NS, 19 for ND, 22 for RA, 300 mM sorbitol, 5–6 for each BaP dose, 5–6 for each BPA dose, 8–10 for each cortisol dose, and 8–20 for each PFOA dose. Note that cortisol, BPA, and PFOA have 7 doses (Fig. 3B–D) while BaP has 6 doses, lacking the highest dose (Fig. 3E).
BaP and cortisol have no significant suppressive effects on green fluorescence at any of the four time points (see Materials and Methods: time points for green fluorescence are green nadirs at 12 and 44 h and peaks at 32 and 56 h) (Fig. 4B, E), and in contrast has a significantly higher level of green fluorescence than NS at several time points and doses. BPA has only one late suppression of green fluorescence (Fig. 4C) in contrast to its early and consistent suppression of confluence (Fig. 3C). In conclusion, except for BaP, the other toxicants are detected at lower levels for confluence/accumulated cell growth than for suppression of green fluorescence peak after media change.
Heatmap expression ratios for G1 and G2 transcriptional markers show BaP dose-dependent G2/G1 increases and PFOA and sorbitol dose-dependent G1/G2 increases
The elevated green fluorescence from early to late FUCCI ESC culture with BaP compared with NS, BPA, PFOA, sorbitol, and cortisol suggests the hypothesis that the known mutagen BaP leads to delays in G2 to repair DNA damage [33,54]. Known G2 [55–58] and G1 [56,59–63] transcriptional markers were tested to support or refute this hypothesis. The key outcomes are interpreted from the Heatmap of dose-dependent BaP (0 μM vs. 1 μM vs. 4 μM), sorbitol (0 mM vs. 300 mM), and PFOA (0 μM vs. 60 μM vs. 100 μM) in FUCCI ESC.
First, NS has the lowest markers for G1 and G2, suggesting transit through these cell cycle phases is rapid. Second, as G1 phase delaying stress PFOA increases in dose, G2 makers decrease and G1 markers are more than NS and do not decrease greatly. Third, as G2 delaying stress BaP increase in dose, G1 markers decrease greatly while G2 markers did not decrease as much (Fig. 5A, B). Previously characterized G1 and G2 transcriptional markers do not universally upregulate when ESC is stimulated by general stressors like sorbitol or mutagenic stressors, respectively.
FIG. 5.
NS does not cause increase in markers of G1 phase or G2 phase of the cell cycle but increasing BaP concentration causes more G2/G1 and increasing PFOA or sorbitol concentration causes more G1/G2 marker expression (A, B). FUCCI1 ESCs in HTS are used to calculate LOAEL and IC75 doses after 72 h or exposure of each toxicant, and these doses, as well as 0 dose/NS are used to re-expose at minimum triplicate biological experiments and assay global transcriptomic changes through RNAseq. Red-blue divergent scale is used with numbers indicating decreasing and increasing standard deviation from the mean for each gene.
Dose-dependent ratios indicate that sorbitol and PFOA cause more G1 delay, and BaP more G2 delay (Fig. 5A, B). For FUCCI1-ESC, NS culture in unstressed naive pluripotency has the lowest amount of delay in the two flexible parts of the cell cycle, G1 or G2, which respond to environmental signals that slow anabolism (G1) or require DNA repair (G2) [64,65]. Increasing doses of sorbitol and PFOA show increase/decrease in most genes corresponding to G1/G2 marker upregulation, and increasing BaP doses show increase in G2/G1 genes.
The effect of feeding versus no feeding on changing green fluorescence corresponding to S-G2-M phases of cell cycle
It was previously reported that standard media change for mouse ESC every 24 h at ambient 20% oxygen is a mild stress that delays cells in G1, but that 12 h feeding does not increase growth rate. In this study, FUCCI ESCs are tested for whether unfed FUCCI ESC escape G1 delay if not fed throughout 72 h and whether lack of feeding throughout the 72 h significantly decreases growth. We report that lack of feeding does not slow growth compared with daily feeding, when confluence is measured after 72 h (Fig. 6A, B). Net green fluorescence (Fig. 6C) and green fluorescence/confluence (ie, per cell, Fig. 6D) shows that feeding causes a period of relief of G1 delay, but that cells in unchanged media increase the fraction of green cells progressing through the cell cycle slowly for nearly the entire 72 h culture.
FIG. 6.
Although standard 24 h feeding creates a G1 delay this is a mild stress because (1) cells leave G1 delay without media change soon after 24 h and (2) the total confluence at Tfinal is not significantly different for cells with no media change or change every 24 h (#). FUCCI ESC were cultured in a live imager for 72 h with stimulants added at Tzero. There was either standard media change every 24 h or no feeding throughout the culture. Confluence was measured every 2 h. (A) Compares the confluence Fold Change (FC) for the controls and unfed cells. The rest compare the curves for the usual 24 hr feeding schedule to only feeding at 0 h (unfed), for confluence (B), green sum area (C), or green sum area rationated to confluence (D). At minimum triplicate biological experiments were done with a total of 36–38 wells measured for NS fed every 24 h (LIF+), 19 for ND (LIF−), 22 for RA 1 μM (LIF−), 22 for 300 mM sorbitol (LIF+), and 28 for 72 h no media change. (A, C, D) Each stimulus produces significantly more (t-test, P < 0.05; orange asterisk) or significantly less (t-test P < 0.05; red asterisk) compared with 0 dose NS at that time point compared with Tzero.
General characteristics of toxicants for points of departure and magnitude of growth suppression
Point of departures through Benchmark Dose Lower confidence limit (BMDL) were performed using Frequentist regression analysis of the dose–response confluence/cell growth curves of toxicants as shown in Fig. 3. FUCCI1 ESC are more sensitive to lowest concentrations of BaP, PFOA, and BPA (Fig. 7A, B, D) and are less sensitive to cortisol (Fig. 7A, C). The rank order of magnitude of effect is, for growth suppression at Tfinal highest dose, ∼32%; PFOA, ∼29%; BPA, ∼16%; Cortisol, and ∼13% growth suppression BaP.
FIG. 7.
LOAEL and BMDL are determined for several toxicants and controls compared with NS. Frequentist degree 3 for BaP and Cortisol (A, C) and degree 5 for BPA, PFOA (B, D) modeling of toxicant dose–response curves for accumulated growth at Tfinal to identify BMD, BMDL points of departure for lowest adverse-effect levels. BMDLs are calculated to be 8.0 μM for BaP, 10.3 μM for BPA, 59.7 μM for Cortisol, and 10.2 μM for PFOA. BMD, Benchmark Dose; BMDL, Benchmark Dose Lower confidence limit.
Confidence in copy number assignment per cell is enhanced when housekeeping genes used as loading controls are similar for lowest stress control NS and highest stress stimulus 300 mM sorbitol. As previously reported, traditional loading controls [10,66–68] actinB and Gapdh have FC <2 and no significance for changes in stimulation that create relatively low (ND vs. NS) and high amounts of stress (300 mM sorbitol), looking at P value and FDR (Fig. 8A, B). Thus, the input mRNA quantitation and QC steps in Methods produce consistent cell mRNA reads and traditional loading controls suggest that total mRNA counts, and genes expressed per cell are supported.
FIG. 8.
Loading controls from highest copy number (A) and from previously published reviews (B). For ESC we typically use GAPDH, ACTB, and 18S loading controls, which report high copy number RNA from ribosomes. Red shows decreasing expression from NS, green shows increasing expression from NS, and blue shows significance when comparing normal differentiation or 300 mM sorbitol compared with normal stemness.
Discussion
The use of FUCCI ESC in a live imager provided analysis of time- and dose-dependent growth and stress effects in early development
FUCCI ESC with their cell cycle-dependent fluorescence, examined with a live imager, allowed for unique integrated analysis of time- and dose-dependent responses from toxicants, including identifying G1 and G2 delay. The cell cycle has four phases; the two Gap phases (G1 and G2) are flexible in duration to mediate stress adaptation. While stress slowed cell growth in the early embryo and its stem cells, most stressors decreased growth by delaying cells in the G1 phase [69–71]. Fewer stressors such as mutagens delayed cells in G2 phase when DNA repair occurs. Some toxicants were examined to evaluate the ability of FUCCI cells with a live imager to determine mutagenicity.
In this study, 5 stimuli produced FUCCI green less than NS, showing a G1 delay. Sorbitol was the control general stressor causing G1 delay while PFOA was the toxicant with the greatest similar effect. Only 1 stimulus caused more green fluorescence than NS at early time points (RA) and another caused more green at later time points (BaP). BaP was the mutagen used to examine G2 delay.
Data from FUCCI ESC from a live imager and after global transcriptomic analysis of cell cycle markers suggested that BaP increased G2/G1 delay and PFOA and sorbitol increased G1/G2 delay
BaP 10 μM produced an immediate significant increase in green fluorescence compared with NS normalized to cell number after 2 h (although it was significantly different at 0 h through a quirk of statistics). This delay in G2 increased green fluorescence, which persisted throughout the 72 h exposure. Three stresses, PFOA, sorbitol, and BPA, overrode LIF and episodically decreased green fluorescence compared with NS culture. After media were changed, NS rapidly increased green S-G2-M phase cells, but these three stressors suppressed this increase. These toxicants showed a clear divide in effect from mutagenic and nonmutagenic stressors.
Muddying the waters was RA, which increased green fluorescence compared with NS like BaP but was not a mutagen. However, unlike BaP, this only happened at later time points: 42–48 and 60–72 h. For RA, green fluorescence was not increased above the peak, but stayed high after the peak level. The live imager data allowed RA and BaP to be distinguished from one another. However, other causes of increased green fluorescence other than mutagenicity need to be further examined, as before, time-lapse imaging FUCCI cells alone was enough to determine mutagenicity.
Bulk RNAseq was used to validate whether G1 and G2 delay were occurring (Fig. 5). Increasing BaP doses increased G2/G1 transcriptional marker ratios, while increasing doses of PFOA and sorbitol increased G1/G2 marker ratios. This along with the fluorescent data supported the conclusion that BaP delayed cell cycle in G2 while PFOA and sorbitol delayed cell cycle in G1. However, RA did not increase G2 markers appreciably while still having some increased fluorescence at later time points. Clearly the fluorescence assay was not enough to determine whether something was mutagenic. RNAseq was still needed to validate whether the green fluorescence was caused by a G2 delay or not. Clearly, no stress operated solely on only one part of the cell cycle and many analyses are needed to define criteria of DevTox mutagenesis.
ESC as guardians of DNA integrity
BaP had IC50 effects at 0.3–1.0 μM for growth, stemness loss in TSC, and blastocysts, which were about 80% TSC, showing far more sensitivity of effects in ESC, here [32,72–74]. However, several lines of evidence suggested that ESC operated at a much higher fidelity of DNA synthesis and repair, commensurate with their function in protecting genomic integrity in the first embryonic cells, which populate the entire later fetus and adult.
First, DNA polymerase k (aka PolK) is a less stringent Y family DNA polymerase that is induced by BaP [75]. BaP is metabolized by a diol esterase (DE) to produce BPDE that adduct DNA and was detected in early embryos and ESC [76,77]. Interestingly, although high-fidelity DNA polymerases with proofreading cannot read through BPDE adducted DNA, some lower fidelity Y family DNA polymerases can. Kappa Polymerase, PolK, reads through BPDE and in wild-type ESC creates fewer mutations than PolK null ESCs, which have slightly lower growth, much higher apoptosis, and mutations after culture with 5–40 μM BaP [75].
We did not find significant Gene Ontology groups mediating apoptosis (data not shown) and did not check for increased apoptosis during exposure or at Tfinal for BaP, but growth accumulation decreased a little due to BaP treatments, so apoptosis was not likely to be a major outcome. We also did not detect increases in PolK at any BaP dose after analysis of RNAseq data (data not shown), Whether existing PolK mRNA or protein are sufficient to media low-stringency read through, or existing high-stringency DNA polymerase fail to read through or read through and creates DNA damage to repair, remains to be tested.
One role of ESC is to preserve genomic integrity for the small early lineage that will populate huge later growth, and mouse ESCs have ∼100-fold lower mutation rate than somatic cells [78]. Another way the ESC preserves genomic integrity is to repair DNA by homologous recombination repair with sister chromosome without damage during double-strand break DSB repair [79,80], rather than low-fidelity nonhomologous end-joining repair common in somatic cells and adult stem cells [81].
ESC culture infrequent feeding was only a mild stress
It was clear that standard 24 h media change protocols for ESC shown here and previously [17], and placental TSC [5,82], create stress. When many of the stressors to culture are removed and major stimuli are optimized, such as optimization of oxygen to 2% for TSC, media change frequency becomes more important and adverse effects with low change frequency becomes profound. It is likely that for ESC culture infrequent media change was one of many stressors and lack of feeding appeared mild among the other stressors.
Three lines of evidence suggest that for current protocols for ESC culture, standard media change is a relatively mild stress. First, 12 h feeding did not increase accumulated growth in ESC compared with 24 h feeding [17], whereas with cultured TSC optimized at 2% oxygen grow nearly the same rapid rate as their lineage in vivo, but without 12 h feeding becoming morbid [5]. Second, accumulated cell number, measured by confluence at Tfinal, was the same for 24 h media change versus no media change. Third, FUCCI ESC cultured with no media change ESC began escaping nongreen G1 phase and entering green S-G2-M phase soon after the 24 h time point of standard that induced rapid exit of many G1-delayed cells.
We have not tested the global transcriptomic or epigenomic phenotypes of 24 h media change versus 72 h with media change, but this should be done. Ostensibly it would also be interesting to test knockout mice for changes in their epigenomic phenotype when the mice were made from the same germline D3 ESC with a long history of 25+ years of creating animals after standard ESC culture [83–85].
Decreased green due to contact inhibition
Although mouse ESC did not undergo complete cessation of cell cycle due to contact inhibition like other stem cells [86], they did undergo sizable G1 delays caused by stress and mediated G1 checkpoint proteins, such as by p27KIP1. As predicted by these reports, as FUCCI ESC neared confluence, green fluorescence/cell (eg, confluence) was at its lowest. In ESC, an accelerated G1 was caused by an increase in the rate of degradation of the p27KIP1 inhibitor of cell cycle progression [86] and an accelerated G1/S transition was accompanied by downregulation of p27KIP1 [87]. Thus, ESC may not cause contact inhibition, but they delayed in G1 as confluence was approached.
Relative growth decreases
Adding overt stress (sorbitol) to cultured FUCCI ESC decreased growth, but none of the powerful developmental signals like LIF removal by itself or along with the addition of XEN morphogen RA, diminished growth. Of the control stimuli, only 300 mM hyperosmotic sorbitol decreased growth compared with NS and caused imbalanced differentiation despite LIF [6,9,51]. Other control stimuli, such as ND and RA, affected pluripotency and anabolic transcriptomic programs (data not shown), but ND and RA 1 μM did not diminish growth. Thus, of the controls, only sorbitol forced decreased proliferation and increased differentiation.
Among the toxicants studied here, growth rate as measured by accumulation of cells was affected with the following rank order of sensitivity of ESC: PFOA, BPA > BaP, and cortisol. Highest growth suppression at Tfinal/Tzero was in the range of ∼32%; ∼29%; ∼16%; and ∼5% with rank order of growth suppression: PFOA, BPA, cortisol, and BaP, respectively. There was a general proportionality of highest sensitivity to growth suppression of these four toxic stress stimuli, to highest effect on growth, but the proportionality of growth decreases and other effects such as lineage choice and imbalance, glycolytic/Warburg effects anabolism, and epigenetic changes remain to be determined.
Analyzing growth: retrospective confluence versus prospective green
Alternate methods of analyzing growth here were retrospective accumulation of confluence and prospective increase in green fluorescence, which predicted future increase in cell number for cultured ESC. The retrospective accumulation of cells measured by confluence reports more robust suppressive effects than prospective green fluorescence measured as suppression of increased green after media change.
The two measures sometimes agreed as with sorbitol and BaP, or could be discordant as with BPA and to a lesser extent, PFOA. With BaP and PFOA, green suppression could have a point of departure at a lower dose or earlier time point, but for most stresses, suppression of accumulation was reported earlier or at a lower dose than suppression of green fluorescence. Sequelae of these more subtle differences should be studied in reference to epigenetic changes and more subtle changes in developmental decision making in terms of lineage or anabolic programming choices.
Relevance of the doses of BaP studied
BaP levels were tested in ovarian follicular fluid of women in In vitro fertilization therapy exposed to mainstream smoke and found to average 1.8 ng/mL [88]. For women who smoked 12–24 cigarettes/day, BaP was 4–10 ng/mL in follicular fluid and twice as high in serum. However, other BaP metabolites increased this range by as much as 10-fold (40–100 ng/mL). This was equivalent to 0.4/0.8/1.2 μM BaP metabolites for 1/2/3 pack/day smokers, respectively. This dose range of BaP had homeostatic effects at the low end and developmental effects at the high end as recently reported for TSC [72]. The lower sensitivity for BaP growth and mutagenesis in ESC was probably due to more stringent DNA repair, replication mechanisms, or apoptosis during the BaP response.
Loading controls
As in previous reports traditional loading controls like actinB and Gapdh had both low FC and were insignificantly different between NS and 300 mM sorbitol [17]. Other loading controls/housekeeping genes with consistent expression with low FC and no significance were Hsp90ab1 and Rplp0.
Conclusions
A step toward an ESC reporter of multiple different simultaneous toxicological stress categories for the speed and magnitude of DevTox responses
In 2007, the national research council released a recommendation that NRC Report, “Toxicity Testing in the 21st Century: A Vision and Strategy” [89]. This report was interpreted to recommend both the use of in vitro high-throughput screening to refine and reduce in vivo screening and to use promoter–reporter constructs that report stressors in seven major categories of toxicological stress [90,91].
One goal was to be able to use kinetics and magnitude of viable reporting stem cells, cultured as stem cells, to categorize general stressors diminishing growth and mutagenic stressors in real time. Ultimately a single ESC line could have all seven promoter–reporters simultaneously reading out the speed and magnitude of the developmental toxicology response. This would be followed up with pinpoint programmatic changes at key point of departure doses, for global transcriptomic and epigenomic programming that provide additional details on aberrant toxicological outcomes.
In conclusion, FUCCI ESC can be used to identify general G1-delaying stressors, or mutagenic G2-delaying stressors, where both data for magnitude of fold changes, fine-tuned by kinetic data for FOAEL and persistence of G2 delay were both important. Bulk, and in the future, scRNAseq are needed to confirm G1 and G2 delay and define subpopulation sizes and homeostatic and developmental reprogramming.
Since FUCCI ESC are based on ubiquitin-mediated degradation of fluorescent G1-specific Cdt1 at the start of S phase and S-G2-M phase-specific fluorescent geminin at the end of M phase [92], will be important to corroborate transcriptional markers here with immunofluorescence studies of population size changes dependent on BaP versus PFOA and sorbitol relative to NS. Important gaps to be filled are more complete dosimetries and a larger range of mutagens to test how relevant and consistent this HTS is in predicting various DevTox outcomes.
In summary, FUCCI ESC uniquely used in a live imager has revealed a surprising but mild stress caused by universally used daily feeding using culture at ambient, 20% oxygen [17]. It is likely that ESCs have a growth and potency maximum at 2% oxygen as previously shown for TSC, but only if media are changed frequently as stress rises rapidly without frequent media change [5,82,93]. Ideal stem cell culture is likely to be at 2% oxygen as this is normoxic for the ESC and TSC at the uterine implantation site [94]. Embryo, TSC, and ESC culture will likely be optimized using FUCCI viable reporters in a live imager to avoid early FOAEL reports of stress.
An additional advantage of FUCCI ESC in a live imager is that the protocol provides a real-time readout of viable FUCCI ESC reporters to detect mutagenic delay in G2 with confirmation by early FOAEL and additional transcriptomic markers. In addition, in the future, it can be used to then test preventative compounds that block mutagenic effects. Since FUCCI ESC and live imaging does not require invasive measurement, and provides viable ESC at the end of culture, these ESCs can be tested in longitudinal studies to confirm that exposures of compounds that block mutagenesis also cause no harm.
Acknowledgments
The authors thank members of their laboratory for analysis and comments on the article. The authors thank Wayne State University Biomedical Career Advancement Program (BCAP) interns Tanmai Nimmagadda and Syed Ali for analysis and assembly of Excel databases.
Author Disclosure Statement
The authors have no commercial associations that would cause a conflict of interest in connection with this review and that all funding sources supporting the work and all institutional or corporate affiliations are acknowledged.
Funding Information
This research was supported by grants to D.A.R. from NIH (1R41ES028991-01, 1R41ES031451-01A1, P30 ES020957, and Michigan Emerging Technology Fund), and to D.M.R. from NIH UH3 OD023285, S10 OD025170, and CURES Pilot. SMH was supported by the NIEHS grant P42ES017198. T.M. was supported by the ReBUILDetroit Project funded by NIH BUILD.
References
- 1. Wingert S, Thalheimer FB, Haetscher N, Rehage M, Schroeder T and Rieger MA. (2016). DNA-damage response gene GADD45A induces differentiation in hematopoietic stem cells without inhibiting cell cycle or survival. Stem Cells 34:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T, Iseki S, Hara E, Masunaga T, Shimizu H and Nishimura EK. (2009). Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 137:1088–1099. [DOI] [PubMed] [Google Scholar]
- 3. Byun K, Kim T-K, Oh J, Bayarsaikhan E, Kim D, Lee MY, Pack C-G, Hwang D and Lee B. (2013). Heat shock instructs hESCs to exit from the self-renewal program through negative regulation of OCT4 by SAPK/JNK and HSF1 pathway. Stem Cell Res 11:1323–1334. [DOI] [PubMed] [Google Scholar]
- 4. Zhong W, Xie Y, Abdallah M, Awonuga AO, Slater JA, Sipahi L, Puscheck EE and Rappolee DA. (2010). Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells. Reproduction 140:921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou S, Xie Y, Puscheck EE and Rappolee DA. (2011). Oxygen levels that optimize TSC culture are identified by maximizing growth rates and minimizing stress. Placenta 32:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slater JA, Zhou S, Puscheck EE and Rappolee DA. (2014). Stress-induced enzyme activation primes murine embryonic stem cells to differentiate toward the first extraembryonic lineage. Stem Cells Dev 23:3049–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie Y, Zhou S, Jiang Z, Dai J, Puscheck EE, Lee I, Parker G, Huttemann M and Rappolee DA. (2014). Hypoxic stress induces, but cannot sustain trophoblast stem cell differentiation to labyrinthine placenta due to mitochondrial insufficiency. Stem Cell Res 13:478–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang Y, Arenas-Hernandez M, Gomez-Lopez N, Dai J, Parker GC, Puscheck EE and Rappolee DA. (2016). Hypoxic stress forces irreversible differentiation of a majority of mouse trophoblast stem cells despite FGF4. Biol Reprod 95:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Q, Louden E, Zhou J, Drewlo S, Dai J, Puscheck EE, Chen K and Rappolee DA. (2019). Stress forces first lineage differentiation of mouse embryonic stem cells; validation of a high-throughput screen for toxicant stress. Stem Cells Dev 28:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abdulhasan MA, Ruden X, Rappolee B, Dutta S, Gurdziel K, Ruden DM, Awonuga AO, Korzeniewski SJ, Puscheck EE and Rappolee DA. (2021). Stress decreases host viral resistance and increases Covid susceptibility in embryonic stem cells. Stem Cell Rev Rep 17:2164–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo YL, Chakraborty S, Rajan S, Wang R and Huang F. (2010). Effects of oxidative stress on mouse embryonic stem cell proliferation, apoptosis, senescence, and self-renewal. Stem Cells Dev 19:1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toh YC and Voldman J. (2011). Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction. FASEB J 25:1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Casula E, Asuni GP, Sogos V, Fadda S, Delogu F and Cincotti A. (2017). Osmotic behaviour of human mesenchymal stem cells: implications for cryopreservation. PLoS One 12:e0184180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fan YL, Zhao HC and Feng XQ. (2021). Hypertonic pressure affects the pluripotency and self-renewal of mouse embryonic stem cells. Stem Cell Res 56:102537. [DOI] [PubMed] [Google Scholar]
- 15. Pauklin S and Vallier L. (2013). The cell-cycle state of stem cells determines cell fate propensity. Cell 155:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coronado D, Godet M, Bourillot PY, Tapponnier Y, Bernat A, Petit M, Afanassieff M, Markossian S, Malashicheva A, et al. (2013). A short G1 phase is an intrinsic determinant of naive embryonic stem cell pluripotency. Stem Cell Res 10:118–131. [DOI] [PubMed] [Google Scholar]
- 17. Abdulhasan M, Ruden X, You Y, Harris S, Ruden D, Awonuga A, Alvero A, Puscheck E and Rappolee D. (2021). Using live imaging and FUCCI embryonic stem cells (ESC) to rank Devtox risks: adverse growth effects of PFOA compared with DEP are 26times faster, 1,000 times more sensitive, and 13 times greater in magnitude. Front Toxicol 3:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brookes P. (1977). Mutagenicity of polycyclic aromatic hydrocarbons. Mutat Res 39:257–283. [DOI] [PubMed] [Google Scholar]
- 19. Aust AE, Falahee KJ, Maher VM and McCormick JJ. (1980). Human cell-mediated benzo(a)pyrene cytotoxicity and mutagenicity in human diploid fibroblasts. Cancer Res 40:4070–4075. [PubMed] [Google Scholar]
- 20. van Cantfort J and Gielen JE. (1981). Ontogenetic variation in rat liver, lung and kidney monooxygenase induction by low doses of benzo(A)pyrene and cigarette-smoke condensate. Br J Cancer 44:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Solhaug A, Ovrebo S, Mollerup S, Lag M, Schwarze PE, Nesnow S and Holme JA. (2005). Role of cell signaling in B[a]P-induced apoptosis: characterization of unspecific effects of cell signaling inhibitors and apoptotic effects of B[a]P metabolites. Chem Biol Interact 151:101–119. [DOI] [PubMed] [Google Scholar]
- 22. Sanyal MK and Li YL. (2007). Differential metabolism of benzo[alpha]pyrene in vitro by human placental tissues exposed to active maternal cigarette smoke. Birth Defects Res B Dev Reprod Toxicol 80:49–56. [DOI] [PubMed] [Google Scholar]
- 23. Shamsuddin AK and Gan R. (1988). Immunocytochemical localization of benzo(a)pyrene-DNA adducts in human tissue. Hum Pathol 19:309–315. [DOI] [PubMed] [Google Scholar]
- 24. Ginsberg GL and Atherholt TB. (1990). DNA adduct formation in mouse tissues in relation to serum levels of benzo(a)pyrene-diol-epoxide after injection of benzo(a)pyrene or the diol-epoxide. Cancer Res 50:1189–1194. [PubMed] [Google Scholar]
- 25. Mukherjee JJ, Gupta SK and Kumar S. (2008). Inhibition of benzopyrene diol epoxide-induced apoptosis by cadmium(II) is AP-1-independent: role of extracelluler signal related kinase. Chem Biol Interact 172:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang H, Zhu Y, Chi Y and Dong S. (2019). A human embryonic stem cell-based model for benzo[a]pyrene-induced embryotoxicity. Reprod Toxicol 85:26–33. [DOI] [PubMed] [Google Scholar]
- 27. Zhan S, Zhang X, Cao S and Huang J. (2015). Benzo(a)pyrene disrupts mouse preimplantation embryo development. Fertil Steril 103:815–825. [DOI] [PubMed] [Google Scholar]
- 28. Higgins S. (2002). Smoking in pregnancy. Curr Opin Obstet Gynecol 14:145–151. [DOI] [PubMed] [Google Scholar]
- 29. Zdravkovic T, Genbacev O, McMaster MT and Fisher SJ. (2005). The adverse effects of maternal smoking on the human placenta: a review. Placenta 26 (Suppl. A):S81–S86. [DOI] [PubMed] [Google Scholar]
- 30. Rappolee DA, Awonuga AO, Puscheck EE, Zhou S and Xie Y. (2010). Benzopyrene and experimental stressors cause compensatory differentiation in placental trophoblast stem cells. Syst Biol Reprod Med 56:168–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Witschi H. (2001). Tobacco toxicology revisited. Adv Exp Med Biol 500:471–478. [DOI] [PubMed] [Google Scholar]
- 32. Iannaccone PM, Fahl WE and Stols L. (1984). Reproductive toxicity associated with endometrial cell mediated metabolism of benzo[a]pyrene: a combined in vitro, in vivo approach. Carcinogenesis 5:1437–1442. [DOI] [PubMed] [Google Scholar]
- 33. Drukteinis JS, Medrano T, Ablordeppey EA, Kitzman JM and Shiverick KT. (2005). Benzo[a]pyrene, but not 2,3,7,8-TCDD, induces G2/M cell cycle arrest, p21CIP1 and p53 phosphorylation in human choriocarcinoma JEG-3 cells: a distinct signaling pathway. Placenta 26 (Suppl. A):S87–S95. [DOI] [PubMed] [Google Scholar]
- 34. Arnould JP, Verhoest P, Bach V, Libert JP and Belegaud J. (1997). Detection of benzo[a]pyrene-DNA adducts in human placenta and umbilical cord blood. Hum Exp Toxicol 16:716–721. [DOI] [PubMed] [Google Scholar]
- 35. Sanyal MK, Mercan D, Belanger K and Santella RM. (2007). DNA adducts in human placenta exposed to ambient environment and passive cigarette smoke during pregnancy. Birth Defects Res A Clin Mol Teratol 79:289–294. [DOI] [PubMed] [Google Scholar]
- 36. Everson RB, Randerath E, Santella RM, Avitts TA, Weinstein IB and Randerath K. (1988). Quantitative associations between DNA damage in human placenta and maternal smoking and birth weight. J Natl Cancer Inst 80:567–576. [DOI] [PubMed] [Google Scholar]
- 37. Zenzes MT. (2000). Smoking and reproduction: gene damage to human gametes and embryos. Hum Reprod Update 6:122–131. [DOI] [PubMed] [Google Scholar]
- 38. Wang X, Lui VC, Poon RT, Lu P and Poon RY. (2009). DNA damage mediated s and g(2) checkpoints in human embryonal carcinoma cells. Stem Cells 27:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Q, Yang Y, Louden E, Puscheck E and Rappolee D. (2016). High throughput screens for embryonic stem cells; stress-forced potency-stemness loss enables toxicological assays. In: Methods in Toxicology and Pharmacology. Faqi A ed. New York, NY: Springer. [Google Scholar]
- 40. Li Q, Gomez-Lopez N, Drewlo S, Sanchez-Rodriguez E, Dai J, Puscheck EE and Rappolee DA. (2016). Development and validation of a Rex1-RFP potency activity reporter assay that quantifies stress-forced potency loss in mouse embryonic stem cells. Stem Cells Dev 25:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masui S, Ohtsuka S, Yagi R, Takahashi K, Ko MS and Niwa H. (2008). Rex1/Zfp42 is dispensable for pluripotency in mouse ES cells. BMC Dev Biol 8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lundholt BK, Scudder KM and Pagliaro L. (2003). A simple technique for reducing edge effect in cell-based assays. J Biomol Screen 8:566–570. [DOI] [PubMed] [Google Scholar]
- 43. Wingett SW and Andrews S. (2018). FastQ Screen: A tool for multi-genome mapping and quality control. F1000Res 7:1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anders S, Pyl PT and Huber W. (2015). HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang W, Sherman BT and Lempicki RA. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. [DOI] [PubMed] [Google Scholar]
- 47. Crump KS. (1995). Calculation of Benchmark Doses from Continuous Data. Risk Analysis 15:79–89. [Google Scholar]
- 48. Akaike H. (1974). A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723. [Google Scholar]
- 49. Dodge Y. (2010). The concise encyclopedia of statistics. Springer. [Google Scholar]
- 50. Metsalu T and Vilo J. (2015). ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res 43:W566–W570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abdulhasan M, Ruden X, Rappolee B, Dutta S, Gurdziel K, Ruden DM, Awonuga AO, Korzeniewski SJ, Puscheck EE and Rappolee DA. (2021). Stress decreases host viral resistance and increases covid susceptibility in embryonic stem cells. Stem Cell Rev Rep 17:2164–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Borman ED, Foster WG and deCatanzaro D. (2016). Concurrent administration of diethylhexyl phthalate reduces the threshold dose at which bisphenol A disrupts blastocyst implantation and cadherins in mice. Environ Toxicol Pharmacol 49:105–111. [DOI] [PubMed] [Google Scholar]
- 53. Borman ED, Foster WG, Greenacre MKE, Muir CC and deCatanzaro D. (2015). Stress lowers the threshold dose at which bisphenol A disrupts blastocyst implantation, in conjunction with decreased uterine closure and e-cadherin. Chem Biol Interact 237:87–95. [DOI] [PubMed] [Google Scholar]
- 54. Tung EW, Philbrook NA, Belanger CL, Ansari S and Winn LM. (2014). Benzo[a]pyrene increases DNA double strand break repair in vitro and in vivo: a possible mechanism for benzo[a]pyrene-induced toxicity. Mutat Res Genet Toxicol Environ Mutagen 760:64–69. [DOI] [PubMed] [Google Scholar]
- 55. Cude K, Wang Y, Choi HJ, Hsuan SL, Zhang H, Wang CY and Xia Z. (2007). Regulation of the G2-M cell cycle progression by the ERK5-NF{kappa}B signaling pathway. J Cell Biol 177:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chaudhry MA, Chodosh LA, McKenna WG and Muschel RJ. (2002). Gene expression profiling of HeLa cells in G1 or G2 phases. Oncogene 21:1934–1942. [DOI] [PubMed] [Google Scholar]
- 57. Taylor WR and Stark GR. (2001). Regulation of the G2/M transition by p53. Oncogene 20:1803–1815. [DOI] [PubMed] [Google Scholar]
- 58. Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW and Vogelstein B. (1998). Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497–1501. [DOI] [PubMed] [Google Scholar]
- 59. Vaziri C, Stice L and Faller DV. (1998). Butyrate-induced G1 arrest results from p21-independent disruption of retinoblastoma protein-mediated signals. Cell Growth Differ 9:465–474. [PubMed] [Google Scholar]
- 60. Peverali FA, Ramqvist T, Saffrich R, Pepperkok R, Barone MV and Philipson L. (1994). Regulation of G1 progression by E2A and Id helix-loop-helix proteins. EMBO J 13:4291–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hara E, Yamaguchi T, Nojima H, Ide T, Campisi J, Okayama H and Oda K. (1994). Id-related genes encoding helix-loop-helix proteins are required for G1 progression and are repressed in senescent human fibroblasts. J Biol Chem 269:2139–2145. [PubMed] [Google Scholar]
- 62. Adrover MA, Zi Z, Duch A, Schaber J, Gonzalez-Novo A, Jimenez J, Nadal-Ribelles M, Clotet J, Klipp E and Posas F. (2011). Time-dependent quantitative multicomponent control of the G(1)-S network by the stress-activated protein kinase Hog1 upon osmostress. Sci Signal 4:ra63. [DOI] [PubMed] [Google Scholar]
- 63. Calder A, Roth-Albin I, Bhatia S, Pilquil C, Lee JH, Bhatia M, Levadoux-Martin M, McNicol J, Russell J, Collins T and Draper JS. (2013). Lengthened G1 phase indicates differentiation status in human embryonic stem cells. Stem Cells Dev 22:279–295. [DOI] [PubMed] [Google Scholar]
- 64. Dalton S. (2015). Linking the cell cycle to cell fate decisions. Trends Cell Biol 25:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Singh AM, Sun Y, Li L, Zhang W, Wu T, Zhao S, Qin Z and Dalton S. (2015). Cell-cycle control of bivalent epigenetic domains regulates the exit from pluripotency. Stem Cell Reports 5:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moein S, Javanmard SH, Abedi M, Izadpanahi MH and Gheisari Y. (2017). Identification of appropriate housekeeping genes for gene expression analysis in long-term hypoxia-treated kidney cells. Adv Biomed Res 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Curina A, Termanini A, Barozzi I, Prosperini E, Simonatto M, Polletti S, Silvola A, Soldi M, Austenaa L, et al. (2017). High constitutive activity of a broad panel of housekeeping and tissue-specific cis-regulatory elements depends on a subset of ETS proteins. Genes Dev 31:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Eisenberg E and Levanon EY. (2013). Human housekeeping genes, revisited. Trends Genet 29:569–574. [DOI] [PubMed] [Google Scholar]
- 69. Dalvai M, Schubart K, Besson A and Matthias P. (2010). Oct1 is required for mTOR-induced G1 cell cycle arrest via the control of p27(Kip1) expression. Cell Cycle 9:3933–3944. [DOI] [PubMed] [Google Scholar]
- 70. Truman AW, Kristjansdottir K, Wolfgeher D, Hasin N, Polier S, Zhang H, Perrett S, Prodromou C, Jones GW and Kron SJ. (2012). CDK-dependent Hsp70 Phosphorylation controls G1 cyclin abundance and cell-cycle progression. Cell 151:1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bertoli C, Skotheim JM and de Bruin RA. (2013). Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 14:518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xie Y, Abdallah ME, Awonuga AO, Slater JA, Puscheck EE and Rappolee DA. (2010). Benzo(a)pyrene causes PRKAA1/2-dependent ID2 loss in trophoblast stem cells. Mol Reprod Dev 77:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xie Y, Liu J, Proteasa S, Proteasa G, Zhong W, Wang Y, Wang F, Puscheck EE and Rappolee DA. (2008). Transient stress and stress enzyme responses have practical impacts on parameters of embryo development, from IVF to directed differentiation of stem cells. Mol Reprod Dev 75:689–697. [DOI] [PubMed] [Google Scholar]
- 74. Puscheck EE, Awonuga AO, Yang Y, Jiang Z and Rappolee DA. (2015). Molecular biology of the stress response in the early embryo and its stem cells. Adv Exp Med Biol 843:77–128. [DOI] [PubMed] [Google Scholar]
- 75. Ogi T, Shinkai Y, Tanaka K and Ohmori H. (2002). Polkappa protects mammalian cells against the lethal and mutagenic effects of benzo[a]pyrene. Proc Natl Acad Sci U S A 99:15548–15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Filler R and Lew KJ. (1981). Developmental onset of mixed-function oxidase activity in preimplantation mouse embryos. Proc Natl Acad Sci U S A 78:6991–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rappolee DA, Xie Y, Slater JA, Zhou S and Puscheck EE. (2012). Toxic stress prioritizes and imbalances stem cell differentiation: implications for new biomarkers and in vitro toxicology tests. Syst Biol Reprod Med 58:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Aladjem MI, Spike BT, Rodewald LW, Hope TJ, Klemm M, Jaenisch R and Wahl GM. (1998). ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr Biol 8:145–155. [DOI] [PubMed] [Google Scholar]
- 79. Stambrook PJ and Tichy ED. (2010). Preservation of genomic integrity in mouse embryonic stem cells. Adv Exp Med Biol 695:59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tichy ED, Pillai R, Deng L, Liang L, Tischfield J, Schwemberger SJ, Babcock GF and Stambrook PJ. (2010). Mouse embryonic stem cells, but not somatic cells, predominantly use homologous recombination to repair double-strand DNA breaks. Stem Cells Dev 19:1699–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mao Z, Bozzella M, Seluanov A and Gorbunova V. (2008). DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle 7:2902–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang Y, Jiang Z, Bolnick A, Dai J, Puscheck EE and Rappolee DA. (2017). Departure from optimal O2 level for mouse trophoblast stem cell proliferation and potency leads to most rapid AMPK activation. J Reprod Dev 63:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Doetschman TC, Eistetter H, Katz M, Schmidt W and Kemler R. (1985). The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 87:27–45. [PubMed] [Google Scholar]
- 84. Doetschman T, Gregg RG, Maeda N, Hooper ML, Melton DW, Thompson S and Smithies O. (1987). Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature 330:576–578. [DOI] [PubMed] [Google Scholar]
- 85. Doetschman T, Georgieva T, Li H, Reed TD, Grisham C, Friel J, Estabrook MA, Gard C, Sanford LP and Azhar M. (2012). Generation of mice with a conditional allele for the transforming growth factor beta3 gene. Genesis 50:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Burdon T, Smith A and Savatier P. (2002). Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol 12:432–438. [DOI] [PubMed] [Google Scholar]
- 87. Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X and Wu H. (1999). PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci U S A 96:6199–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Neal MS, Zhu J and Foster WG. (2008). Quantification of benzo[a]pyrene and other PAHs in the serum and follicular fluid of smokers versus non-smokers. Reprod Toxicol 25:100–106. [DOI] [PubMed] [Google Scholar]
- 89. National Research Council (U.S.). Committee on Toxicity Testing and Assessment of Environmental Agents. (2007). Toxicity Testing in the 21st Century: A Vision and a Strategy. National Academies Press, Washington, DC. [Google Scholar]
- 90. Krewski D, Westphal M, Al-Zoughool M, Croteau MC and Andersen ME. (2011). New directions in toxicity testing. Annu Rev Public Health 32:161–178. [DOI] [PubMed] [Google Scholar]
- 91. Kang KS and Trosko JE. (2011). Stem cells in toxicology: fundamental biology and practical considerations. Toxicol Sci 120 (Suppl. 1):S269–S289. [DOI] [PubMed] [Google Scholar]
- 92. Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, et al. (2008). Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132:487–498. [DOI] [PubMed] [Google Scholar]
- 93. Bolnick A, Awonuga AO, Yang Y, Abdulhasan M, Xie Y, Zhou S, Puscheck EE and Rappolee DA. (2017). Using stem cell oxygen physiology to optimize blastocyst culture while minimizing hypoxic stress. J Assist Reprod Genet 34:1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Houghton FD. (2021). Hypoxia and reproductive health: hypoxic regulation of preimplantation embryos: lessons from human embryonic stem cells. Reproduction 161:F41–F51. [DOI] [PubMed] [Google Scholar]