Abstract
Broca’s area is frequently implicated in sentence comprehension but its specific role is debated. Most lesion studies have investigated deficits at the chronic stage. We aimed (1) to use acute imaging to predict which left hemisphere stroke patients will recover sentence comprehension; and (2) to better understand the role of Broca’s area in sentence comprehension by investigating acute deficits prior to functional reorganization. We assessed comprehension of canonical and noncanonical sentences in 15 patients with left hemisphere stroke at acute and chronic stages. LASSO regression was used to conduct lesion symptom mapping analyses. Patients with more severe word-level comprehension deficits and a greater proportion of damage to supramarginal gyrus and superior longitudinal fasciculus were likely to experience acute deficits prior to functional reorganization. Broca’s area was only implicated in chronic deficits. We propose that when temporoparietal regions are damaged, intact Broca’s area can support syntactic processing after functional reorganization occurs.
Keywords: Language comprehension, Syntax, Broca’s area, sentence processing, Lesion-symptom mapping
1.0. Introduction
Left-hemisphere damage following stroke often results in sentence comprehension impairments. Damage to Broca’s area, the posterior portions of the inferior frontal gyrus (IFG) including both pars triangularis (IFGtri) (Brodmann Area 45) and pars opercularis (IFGop) (Brodmann Area 44), has frequently been implicated in sentence processing deficits. For example, Caramazza & Zurif (1976) found that participants with Broca’s aphasia had little difficulty comprehending sentences in canonical word order, but had significant difficulty comprehending semantically reversible sentences in noncanonical word order (e.g., The cat that the dog is biting is black). This asyntactic pattern of comprehension led researchers to conclude Broca’s area is likely involved in syntactic processing. Since this early research, many studies have investigated the role of Broca’s area in syntactic processing. Note that throughout this paper we are using the terms “sentence processing” and “syntactic processing” to refer to comprehension processes (Ferreira & Çokal, 2015). However, there is a debate regarding the specific role of Broca’s area. For example, Grodzinsky contends that it is primarily involved in syntactic movement (Grodzinsky & Santi, 2008; Godzinsky, 1986, 2000). According to some linguistic theories, syntactic movement refers to operations where sentences like passives (e.g., “The doctor with blonde hair is questioned by the soldier.”) are formed from movement of constituents in a syntactically simpler sentence (e.g., “The soldier questions the doctor with blonde hair.”) (Chomsky et al., 2017). Grodzinsky and colleagues hypothesize that Broca’s area is essential for processing noncanonical sentences like passives and object-relatives which involve syntactic movement. Other hypotheses propose that Broca’s area is essential for building syntactic hierarchies (Friederici, 2009, 2018), syntactic working memory processes (Fiebach et al., 2005; Matchin, 2018), cognitive control (Novick et al., 2005, 2010), or articulatory rehearsal (Caplan et al., 2000; Rogalsky et al., 2008). However, there is growing evidence that Broca’s area may not be critical for noncanonical sentence comprehension as was previously thought. Subsequent studies by Caramazza and colleagues contradicted their original conclusions regarding damage to Broca’s area causing asyntactic comprehension, and instead concluded that Broca’s aphasia is actually associated with several different comprehension patterns (Caramazza et al., 2001, 2005; Caramazza & Hillis, 1989).
Several models of the brain basis of syntactic comprehension exist. For example, Friederici (Friederici, 2011, 2012) proposed that complex syntactic processing engages posterior STG and Brodmann Area 44 (BA44) - pars opercularis of left IFG - connected via a dorsal pathway including superior longitudinal fasciculus and arcuate fasciculus. According to this model, syntactically complex sentences with syntactic movement first undergo phrase structure building processes in left IFG followed by thematic role analysis (i.e., understanding who is doing what to whom in the sentence) in left temporoparietal regions. More recently, Matchin and Hickok (2020) proposed a syntactic model where sentence comprehension is primarily subserved by posterior temporal regions. Specifically, auditory phonological representations in posterior STG (pSTG) are decoded in posterior MTG (pMTG). PMTG is linked to two essential hubs: an entity knowledge hub (e.g., object categories) in anterior temporal lobe, and an event knowledge hub in angular gyrus (AG) (knowledge of thematic relations between entities). According to this model, Broca’s area is essential for production but not for syntactic comprehension. Matchin and Hickok (2020) argue that Broca’s area is sometimes co-opted for comprehension when syntactic working memory and syntactic prediction processes are required. Syntactic working memory refers to working memory resources that may be specialized for sentence processing (Fiebach et al., 2005; Matchin, 2018; Rogalsky & Hickok, 2011). Syntactic prediction refers to facilitating structural top-down predictions of syntactic information that is likely to be encountered (e.g., phrasal nodes, tense) as a sentence is unfolding during online sentence processing (Friederici, 2012; Lau et al., 2006; Matchin et al., 2017; Rimmele et al., 2018; Sheppard et al., 2017, 2018). Matchin and Hickok (2020) maintain that activation of Broca’s area in functional neuroimaging studies of sentence comprehension is driven specifically by syntactic working memory and prediction, and is not the hub of all subprocesses related to syntactic processing.
Functional imaging studies in neurologically unimpaired populations indicate Broca’s area is part of a network of regions involved in sentence processing which includes frontal areas such as left IFG, middle frontal gyrus (MFG), insula, and posterior regions such as the middle temporal gyrus (MTG) and superior temporal gyrus (STG) (Europa et al., 2019; Mack et al., 2013; Meyer et al., 2002; Uddén et al., 2019; Walenski et al., 2019). Europa and colleagues (2019) investigated this model by examining regions activated by noncanonical sentences using a connectivity analysis in a group of neurotypicals. Two types of noncanonical sentences were investigated, those with wh-movement and those with NP-movement. Sentences with wh-movement, like object clefts, (e.g., It was the boy who the woman lectured), are more syntactically complex than sentences with NP-movement like passive sentences (e.g., The boy was lectured by the woman.). Both types of noncanonical sentences resulted in left IFG activation, along with posterior STG and left medial SFG activation. Similar networks were engaged by both types of movement, but more complex wh-movement elicited greater activity within the network.
Also, several lesion-symptom mapping studies have found that damage to regions besides Broca’s area are implicated in sentence comprehension impairments, particularly to temporoparietal regions (Dronkers et al., 2004; Fridriksson et al., 2018; Kristinsson et al., 2020; Magnusdottir et al., 2013; Rogalsky et al., 2018; Thothathiri et al., 2011). For example, in a large group of chronic stroke patients, Fridriksson (2018) found that damage to temporal regions including superior temporal gyrus (STG), temporal pole, as well as damage to IFG pars triangularis (IFGtri) predicted asyntactic comprehension patterns (more difficulty understanding noncanonical vs. canonical sentences) in a large group of chronic stroke patients. Rogalsky et al. (2018) also conducted a lesion-symptom mapping study where chronic stroke patients completed a sentence-picture matching task and a plausibility judgment task. Sentence comprehension patterns were equivalent between a group of patients with Broca’s area damage and a group of patients with damage to anterior temporal lobe. Neither group displayed asyntactic comprehension patterns. Furthermore, their analyses indicated that posterior temporal and inferior parietal regions were implicated in noncanonical sentence comprehension, but not Broca’s area. Broca’s area was not implicated in the sentence-picture matching task, but it was implicated in the plausibility judgment task. The authors interpreted this finding to mean that Broca’s area is used for task-related cognitive demands. Additional evidence for the role of temporoparietal regions in syntactic processing comes from investigating lesion patterns in individuals with chronic agrammatic aphasia, who have better comprehension of canonical than noncanonical sentences. Evidence suggests that for many individuals with agrammatic aphasia, their lesions are not constrained to Broca’s area and instead often encompass temporoparietal regions as well (Fridriksson et al., 2015; Mohr et al., 1978).
It is important to consider the timing of testing following stroke. As patients recover, functional reorganization occurs in the months following a stroke, which can result in perilesional as well as contralesional homologue areas subsuming functions from infarcted regions (Hartwigsen & Saur, 2019; Jarso et al., 2013; Ochfeld et al., 2010; Saur et al., 2006). For example, Saur et al. (2006) examined auditory sentence comprehension using functional neuroimaging in a group of left hemisphere stroke patients at acute (0–4 days post-stroke), subacute (~ 2 weeks post-stroke), and chronic (~ 4–12 months post-stroke). Distinct patterns of activation were discovered at each time point. Acutely, very little activation was observed in left hemisphere perilesional areas. While at the subacute stage a large amount of bilateral activation was found with the greatest activity in right IFG. Peak activation shifted back to the left hemisphere language areas at the chronic stage, which was associated with the greatest recovery of language skills. These results demonstrate how important it is to consider the time point at which language is tested in patients following a stroke.
Most sentence comprehension studies comparing canonical to noncanonical processing only include patients at the chronic stage of stroke. However, Magnusdottir and colleagues (2013) investigated a group of Icelandic patients at the acute stage, which they defined as within 20 days of stroke (note this would be considered the subacute stage in some research studies). The patients completed a sentence-picture matching task with both canonical and noncanonical sentences. Asyntactic comprehension (canonical > noncanonical) was associated with damage to middle and superior temporal gyrus, and temporal pole. Impaired noncanonical comprehension was associated with damage to Broca’s area and temporal regions. Newhart et al. (2012) also examined sentence comprehension at the acute stage. Specifically, they examined the comprehension of four types of sentence constructions in 53 patients at the acute stage, which was defined as within 48 hours of stroke. Patients completed a sentence-picture matching task as well as an enactment task where patients acted out each sentence using laminated paper figures and objects mentioned in the sentence. They found that asyntactic comprehension patterns were associated with damage to angular gyrus and not Broca’s area. However, damage to IFGtri was linked to difficulty comprehending passive sentences but not object-cleft sentences, which are both types of noncanonical sentences.
In a recent study by the same group as Magnusdottir et al. (2013), Kristinsson and colleagues (2020) investigated asyntactic comprehension patterns in a large group of individuals with acute aphasia who were also tested within 20 days of stroke. Similar to the results from Magnusdottir et al. (2013) and Rogalsky et al. (2018), damage to posterior temporal and temporoparietal regions was implicated in difficulty processing noncanonical sentences. Damage to Broca’s area was not strongly associated with impaired syntactic processing. The authors concluded that temporoparietal regions are essential for syntactic processing, while Broca’s area subserves complementary processes that aid sentence processing. Overall, the results of several lesion-symptom mapping studies in patients at both acute and chronic stages suggest that temporoparietal regions may play a greater role in the processing of complex syntax than Broca’s area.
Investigating patients at the acute stage is advantageous because it allows researchers to understand how damage to specific regions impacts sentence comprehension before any processes have reorganized and other brain regions have taken over some of the responsibilities of regions that were lost to stroke.
1.1. Current Study
In the current study, we aimed to better understand the longitudinal recovery of sentence comprehension following stroke. We conducted a longitudinal investigation of sentence comprehension recovery following stroke by assessing sentence comprehension at acute (within six days of stroke) and chronic (at least 6 months post-stroke) time points. Lesion symptom mapping was performed at both stages using lesions delineated on acute diffusion weighted imaging. This design serves two functions. First, it allows us to better make predictions at the acute time point about which patients will recover sentence comprehension skills, and which patients are likely to have a long-term deficit. Second, it allows us to better understand the role of Broca’s area by studying comprehension at the acute stage prior to any functional reorganization processes and then again at the chronic stage in the same patients after functional reorganization has potentially occurred.
2.0. Methods
2.1. Participants
2.1.1. Participants with Left Hemisphere Stroke
Fifteen patients (6 women, 9 men) at Johns Hopkins Hospital with ischemic left hemisphere stroke were enrolled in this study (Table 1). Participants did not have to be diagnosed with post-stroke aphasia to be included in the study. They had a mean age of 56.0 (SD = 15.9) years and a mean education level of 14.3 (SD = 2.8) years. Twelve participants were right-handed and three were left-handed. All participants were native speakers of English. Each participant had a unilateral left hemisphere infarct visualized on diffusion-weighted imaging (DWI), with no history of previous symptomatic stroke or other neurological disease affecting the brain. All of the patients provided informed consent or indicated a decision-maker to provide informed consent, and were able to complete testing within six days of stroke onset. Additionally, every patient had normal or corrected-to-normal hearing and vision. This study was approved by the Johns Hopkins Institutional Review Board.
Table 1.
Left hemisphere stroke patient demographic information and performance on language testing
| Participant | Age (Acute) | Sex | Handedness | Acute Interventions (tPA or Thrombectomy) | Time between MRI and Acute Language Testing (Days) | Aphasia Type | Single Word Comprehension Errors (SOAP Unrelated Errors/40 SOAP Items) | Noun Naming (BNT) | Verb Naming (HANA) | Semantic Memory (Nouns - PPTT) | Semantic Memory (Verbs - KDT) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
| Acute | Chronic | Acute | Chronic | Acute | Chronic | Acute | Chronic | Acute | Chronic | Acute | Chronic | ||||||
| P1 | 46 | M | Right | None | 3 | None | None | 8% | 0% | 93% | 87% | 71% | 77% | 100% | 100% | 100% | 100% |
| P2 | 50 | F | Right | Thrombectomy (prior to acute testing) | 6 | Moderate-severe Non-fluent | Mild Fluent | 15% | 0% | 0% | 70% | DNT | 71% | 43% | 100% | 47% | 93% |
| P3 | 74 | M | Right | None | 1 | Moderate-severe Non-fluent | Mild Non-fluent | 0% | 13% | 53% | 83% | 83% | 86% | 93% | 100% | 87% | 93% |
| P4 | 56 | M | Right | None | 2 | Moderate-severe Fluent | Mild Fluent | 35% | 13% | 7% | 73% | 0% | DNT | 53% | DNT | 53% | DNT |
| P5 | 65 | M | Left | None | 2 | Mild-moderate Fluent | None | 0% | 0% | 80% | 97% | 86% | 100% | 100% | 100% | 93% | 93% |
| P6 | 49 | M | Right | None | 1 | None | None | 0% | 0% | 87% | 87% | 89% | 87% | 100% | 100% | 100% | 100% |
| P7 | 87 | M | Right | None | 2 | Mild-moderate Fluent | None | 0% | 3% | 67% | 90% | 60% | 80% | 87% | 100% | 67% | 100% |
| P8 | 60 | M | Right | tPA (prior to acute testing) | 4 | Global | Moderate-severe Non-fluent | 20% | 5% | 0% | 0% | 0% | 0% | 87% | 100% | DNT | 100% |
| P9 | 32 | F | Right | None | 1 | Mild-moderate Non-fluent | Moderate Non-fluent | 0% | 0% | 90% | 53% | 89% | 31% | 100% | 86% | 100% | 80% |
| P10 | 77 | F | Right | None | 0 | None | None | 0% | 0% | 90% | 87% | 71% | 83% | 87% | 100% | 87% | 93% |
| P11 | 47 | M | Right | tPA (prior to acute testing) | 3 | None | None | 0% | 0% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 87% |
| P12 | 28 | F | Left | None | 4 | Severe Non-fluent | None | 18% | 0% | 17% | 83% | 6% | 74% | 100% | 93% | 60% | 100% |
| P13 | 49 | F | Left | None | 6 | Mild-moderate Non-fluent | None | 0% | 0% | 77% | 97% | 43% | 60% | 87% | 100% | 73% | 100% |
| P14 | 61 | F | Right | None | 2 | Moderate-severe Non-fluent | Mild Fluent | 0% | 0% | 50% | 53% | 66% | 63% | 93% | 93% | 87% | 73% |
| P15 | 60 | M | Right | None | 1 | Mild Fluent | None | 3% | 0% | 100% | 100% | 89% | 100% | 100% | 100% | 100% | 100% |
Note: BNT = Boston Naming Test (Kaplan et al., 2011); HANA = Hopkins Assessment of Naming Actions (Breining et al., 2021); PPTT = Pyramids & Palm Trees (Breining et al., 2015); KDT = Kissing & Dancing Test (Bak & Hodges, 2003); DNT = did not test.
Language functioning was assessed at acute (within six days of stroke) and chronic (≥ 6 months post-stroke) time points using a battery of language tests. Participants were an of average 2.5 days (SD = 1.8) post-stroke onset at the acute time point and an average of 21.4 months (SD = 22.3) post-stroke onset at the chronic time point. Testing was completed based on participant tolerance and included the following: the Subject-relative, Object-relative, Active, and Passive Test of Comprehension (SOAP) (Love & Oster, 2002), a modified version of the Boston Naming Test (BNT) (Kaplan et al., 2001) using 30 items to assess object naming; the Hopkins Assessment of Naming Actions (Breining et al., 2021)to assess action naming; the short form of the Pyramids and Palm Trees Test (Breining et al., 2015) to assess semantic memory for objects; and the short form of the Kissing and Dancing Test (Bak & Hodges, 2003) to assess semantic memory for actions. Percent accuracy on each of these tests is described in Table 1. Single word comprehension deficits are indicated on the SOAP (Love & Oster, 2002) when participants choose the unrelated foil picture with more frequency (see section 2.2 for more details on the SOAP). Thus, to assess single word comprehension the proportion of responses where the participant chose the unrelated foil out of the total number of items on the SOAP was calculated. This information is indicated in Table 1. A higher proportion of unrelated responses indicates a more severe single word comprehension deficit.
2.1.2. Healthy Age-matched Control Participants
A group of fourteen healthy age-matched controls with no history of stroke or other neurological disease were also enrolled. They had a mean age of 59.0 (SD = 12.9) years and a mean education level of 16.4 (SD = 2.85) years. Age matching was established statistically with a two-tailed independent samples t-test (t (27) = 0.54, p = 0.59).
2.2. Sentence Comprehension Test
Participants were given the SOAP test (Love & Oster, 2002) to assess their auditory sentence comprehension skills. This is a sentence-picture matching task testing the auditory comprehension of four types of sentences: active, passive, subject-relative, and object relative (Table 2). The test consists of 40 sentences (10 of each sentence type). The experimenter shows the participant a page with three color pictures, identifies the name of the characters in each picture (e.g., “This is the doctor, and this is the soldier”), and reads the relevant sentence aloud twice at a normal rate of speech. The participant is then asked to select the picture that matches the sentence they heard. The three choices consist of a target picture (correct), a thematic role reversal foil, and an unrelated foil. For example, for the sentence “The young doctor with blonde hair questions the soldier” the target picture depicts a doctor questioning a soldier, the thematic reversal foil depicts a soldier questioning a doctor, and the unrelated foil shows a man leading a little boy. Patients were tested at two time points. First, they were tested at the acute stage within six days of stroke onset and next they were tested at the chronic stage of recovery (at least 6 months post-stroke). Participants in the healthy control group were tested once. The percent accuracy for canonical sentences (active and subject-relative) and noncanonical sentences (passive and object-relative) was calculated for each participant at every testing time point. Asyntactic comprehension patterns (higher accuracy on canonical vs. noncanonical) were also calculated (noncanonical – canonical comprehension accuracy) to specifically investigate and isolate syntactic deficits.
Table 2.
Sentence examples from the SOAP test of comprehension (see Love & Oster, 2002 for full test)
| Sentence Type | Canonicity | Sentence |
|---|---|---|
|
| ||
| Active | Canonical | The young doctor with blonde hair questions the soldier. |
| Subject-relative | Canonical | The soldier in uniform that questions the doctor has blonde hair. |
| Passive | Noncanonical | The doctor with blonde hair is questioned by the soldier. |
| Object-relative | Noncanonical | The soldier in uniform that the doctor questions has blonde hair. |
2.3. Imaging
All patients were evaluated with MRI DWI, fluid attenuated inversion recovery (FLAIR) to rule out old lesions, susceptibility weighted imaging (SWI) to rule out hemorrhage, and T2-weighted imaging to check for any additional structural lesions. Scans were acquired clinically within 24 hours of admission for stroke on a 3.0T Siemens Trio scanner. The majority of MRIs were completed the same day as the stroke with an average of 0.27 days (SD = 0.70) between stroke onset and the MRI scan. Language testing was conducted within six days of stroke onset. Technicians blinded to the language evaluation results identified the presence or absence of ischemia on DWI images. Technicians blinded to the language evaluation results who were extensively trained and supervised by a neurologist manually traced stroke lesions slice-by-slice on the DWI trace images using MRIcron software (Rorden et al., 2007).
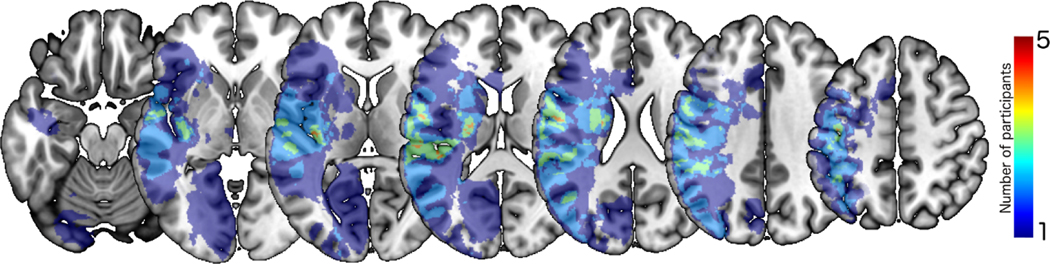
All images were subsequently normalized to standard space using SPM12 (Statistical Parametric Mapping; www.fil.ion.ucl.ac.uk/spm/software/spm12/). The normalization transforms were computed for the DWI b0 image to a template that was based on a group of age-matched controls (Rorden et al., 2012), and then these normalization parameters were applied to the lesion maps. Proportion of infarcted tissue was calculated for each participant in every anatomical parcel of the Johns Hopkins University (JHU) atlas (Faria et al., 2012; Mori et al., 2008). A lesion overlap map was created from individual participant lesion maps using the normalized DWI trace images and the corresponding lesion tracings (see lesion overlap map Figure 1). The percent damage to regions of interest (ROIs) that have been implicated in sentence comprehension in previous studies (Dronkers et al., 2004; Fridriksson et al., 2018; Magnusdottir et al., 2013; Newhart et al., 2012; Rogalsky et al., 2018; Thothathiri et al., 2011) was calculated. These regions included Broca’s area (IFGtri + IFGop), STG, MTG, pSTG, temporal pole, angular gyrus, supramarginal gyrus, superior longitudinal fasciculus (SLF), and inferior fronto-occipital fasciculus (IFOF). Analyses were conducted on ROIs where at least three participants had damage, which resulted in seven ROIs that were included in analyses: Broca’s area, STG, MTG, pSTG, angular gyrus, supramarginal gyrus, and SLF.
Figure 1.
Lesion overlap map. Depicts the number of patients with lesions in specific areas of the left hemisphere. The color scale indicates the number of patients with damage to each area.
2.4. Data Analysis
Mean accuracy on canonical and non-canonical sentences on the SOAP was computed for the group of healthy controls. Impaired performance for the patient group was defined as accuracy greater than two standard deviations below the mean of the healthy control group, with separate calculations for canonical and noncanonical sentences. For analyses in the patient group, Least Absolute Shrinkage and Selection Operator (LASSO) regression (Tibshirani, 1996) was used to evaluate whether damage to specific ROIs were related to sentence comprehension at acute and chronic time points. This method is useful when there are a large number of predictors and a small sample size (Meinshausen & Yu, 2009). It is also useful for situations where there is high multicollinearity, which was the case in this study because participants with damage in one brain region are very likely to have damage to neighboring brain regions. LASSO regression creates simple sparse models with few coefficients that have maximal prediction capacity by performing regularization that shrinks coefficients toward zero. The glmnet package (https://cran.r-project.org/web/packages/glmnet/index.html) (Friedman et al., 2010) using R software (R Core Team, 2020) was used to conduct leave-one-out cross validation LASSO using standardized features selecting the λ value that resulted in the minimum mean cross validation error. The selective Inference package in R (https://cran.rproject.org/web/packages/selectiveInference/selectiveInference.pdf) (Tibshirani et al., 2017) was used to conduct inference testing and calculate p-values associated with regions selected by the LASSO.
Accuracy was calculated separately for canonical and noncanonical sentences at acute and chronic time points. Analyses were conducted with sentence comprehension accuracy as the dependent variable. Asyntactic comprehension patterns were also investigated at each time point by subtracting the mean accuracy of canonical from noncanonical sentences. Recall that the SOAP test has three pictures to choose from: the target, a thematic reversal, and an unrelated picture that contains different referents from those mentioned in the target sentence. Participants with single-word comprehension difficulty are expected to have a high percentage of unrelated errors, whereas participants with intact single-word comprehension but impaired syntactic comprehension commonly make thematic reversal errors. In order to control for single-word comprehension deficits, the total number of unrelated errors made by each participant was calculated for canonical and noncanonical sentences. Predictors included age, overall lesion volume, number of unrelated errors on the SOAP out of the 40 total sentences (to control for word-level comprehension deficits), time since stroke onset, and percent damage to seven ROIs that previous studies have implicated in sentence comprehension (Broca’s area (IFGtri + IFGop), STG, MTG, pSTG, angular gyrus, supramarginal gyrus, and SLF).
3.0. Results
3.1. Behavioral Results
3.1.1. Healthy Controls
The controls attained a mean accuracy of 98.0% (SD = 1.88%) on overall sentence comprehension, 100.0% (SD = 0%) on canonical sentence comprehension, and 96.1% (SD = 3.76%) on noncanonical sentence comprehension on the SOAP. Impaired sentence comprehension in the patient group was defined as performance more than two standard deviations below the accuracy of the control group. Based on this definition, accuracy below 100% was considered to be impaired on canonical sentences, and accuracy below 88.6% was considered to be impaired on noncanonical sentences.
3.1.2. Individuals with Left-hemisphere Stroke
The behavioral results for individual participants are summarized in Table 3. At the acute time point the mean accuracy (SD) was 77.0% (25.7%) for overall sentence comprehension, 80.3% (27.9%) for canonical sentence comprehension, and 73.7% (23.9%) for noncanonical sentence comprehension. Of the 15 patients, eight patients exhibited impaired canonical sentence comprehension, and nine exhibited impaired noncanonical sentence comprehension at the acute time point. At the chronic time point, mean accuracy was 81.2% on overall sentence comprehension, 88.7% (18.8%) for canonical sentence comprehension, and 73.7% (26.0%) for noncanonical sentence comprehension. At the chronic time point, eight patients demonstrated impaired canonical sentence comprehension, and ten demonstrated impaired noncanonical sentence comprehension.
Table 3.
SOAP Accuracy for Individuals with Left Hemisphere Stroke
| Participant | Canonical Sentences (Mean Accuracy) | Noncanonical Sentences (Mean Accuracy) | ||
|---|---|---|---|---|
|
|
|
|
||
| Acute | Chronic | Acute | Chronic | |
| P1 | 100% | 100% | 95% | 75% |
| P2 | 45% | 70% | 40% | 50% |
| P3 | 100% | 95% | 85% | 80% |
| P4 | 25% | 35% | 45% | 25% |
| P5 | 100% | 100% | 65% | 100% |
| P6 | 100% | 100% | 90% | 95% |
| P7 | 100% | 95% | 85% | 85% |
| P8 | 45% | 95% | 35% | 35% |
| P9 | 95% | 65% | 90% | 35% |
| P10 | 90% | 100% | 100% | 85% |
| P11 | 100% | 100% | 100% | 100% |
| P12 | 35% | 80% | 35% | 65% |
| P13 | 95% | 100% | 80% | 100% |
| P14 | 75% | 95% | 70% | 75% |
| P15 | 100% | 100% | 90% | 100% |
| Mean (SD) | 80.3% (27.9%) | 88.7% (18.8%) | 73.7% (23.9%) | 73.7% (26.0%) |
Note: SD = Standard deviation; bold text indicates impaired performance relative to age-matched controls.
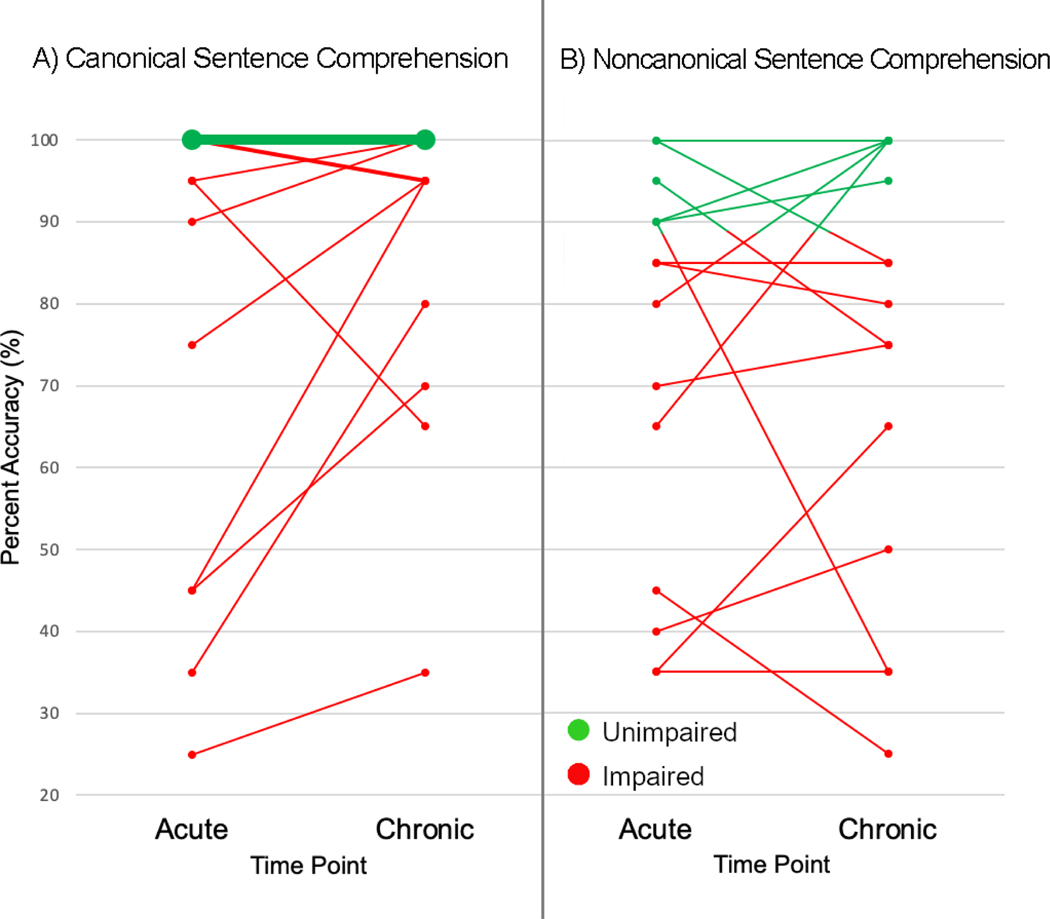
When comparing changes to performance from acute to chronic time points for canonical sentences, five patients were never impaired on comprehension of canonical sentences. When assessed chronically, two patients demonstrated a slight decline from 100% accuracy acutely to 95% accuracy (an error in one sentence) chronically. Of the eight patients who were classified as impaired at the acute time point on canonical sentences, two improved from impaired to unimpaired, five improved but remained impaired relative to healthy controls, and one experienced a significant decline of 30% accuracy. Examining changes to performance for noncanonical sentence comprehension between the acute and chronic time points revealed that three patients never displayed impaired noncanonical sentence comprehension performance, two patients improved from impaired to unimpaired performance, seven patients remained impaired, and three patients’ performance declined from unimpaired to impaired (Figure 2B). Of the seven patients who remained impaired on non-canonical sentences between acute and chronic time points, three patients improved (range of 5% - 30% improvement), two declined (range of 5% - 20% decline), and two patients’ performance remained the same. Of the 15 patients, three did not experience impaired sentence comprehension on any sentence type at any time point.
Figure 2.
Longitudinal changes to A) canonical and B) noncanonical sentence comprehension from acute to chronic time points. Line thickness is scaled so thicker lines indicate a greater number of participants with the same scores.
3.1.2.1. Error Analysis in Individuals with Left-hemisphere Stroke
An error analysis was conducted to investigate the types of errors (i.e., thematic reversal or unrelated errors) made by patients. Thematic reversal errors were more common than unrelated errors for both sentence types at both time points. Overall, for canonical sentences 64% of errors were thematic reversals and 36% were unrelated at the acute time point; 89% were thematic reversals and 11% were unrelated at the chronic time point. For errors on noncanonical sentences, 77% were thematic reversals and 23% were unrelated at the acute time point; 96% were thematic reversals and 4% were unrelated at the chronic time point. The proportion of errors by type are reported for each participant in Table 4.
Table 4.
Results of Error Analysis for Individuals with Left Hemisphere Stroke Comparing Proportion of Thematic Reversal and Unrelated Errors
| Acute | Chronic | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Canonical | Noncanonical | Canonical | Noncanonical | |||||||||
|
|
|
|
|
|||||||||
| Participant | # of Errors ( /20) | Thematic Reversal (%) | Unrelated (%) | # of Errors ( /20) | Thematic Reversal (%) | Unrelated (%) | # of Errors ( /20) | Thematic Reversal (%) | Unrela ted (%) | # of Errors ( /20) | Thematic Reversal (%) | Unrelated (%) |
| P1 | 2 | 50% | 50% | 2 | 0% | 100% | 0 | - | - | 5 | 100% | 0% |
| P2 | 12 | 75% | 25% | 11 | 73% | 27% | 6 | 100% | 0% | 10 | 100% | 0% |
| P3 | 0 | - | - | 3 | 100% | 0% | 1 | 100% | 0% | 4 | 100% | 0% |
| P4 | 15 | 40% | 60% | 11 | 55% | 45% | 13 | 69% | 31% | 15 | 93% | 7% |
| P5 | 0 | - | - | 7 | 100% | 0% | 0 | - | - | 0 | - | - |
| P6 | 0 | - | - | 2 | 100% | 0% | 0 | - | - | 1 | 100% | 0% |
| P7 | 0 | - | - | 3 | 100% | 0% | 1 | 100% | 0% | 4 | 75% | 25% |
| P8 | 11 | 64% | 36% | 13 | 69% | 31% | 11 | 91% | 9% | 12 | 92% | 8% |
| P9 | 1 | 100% | 0% | 2 | 100% | 0% | 7 | 100% | 0% | 13 | 100% | 0% |
| P10 | 1 | 100% | 0% | 0 | - | - | 0 | - | - | 3 | 100% | 0% |
| P11 | 0 | - | - | 0 | - | - | 0 | - | - | 0 | - | - |
| P12 | 11 | 64% | 36% | 12 | 75% | 25% | 4 | 100% | 0% | 7 | 100% | 0% |
| P13 | 1 | 100% | 0% | 5 | 100% | 0% | 0 | - | - | 0 | - | - |
| P14 | 4 | 100% | 0% | 5 | 100% | 0% | 1 | 100% | 0% | 5 | 100% | 0% |
| P15 | 0 | - | - | 2 | 50% | 50% | 0 | - | - | 0 | - | - |
3.2. Neuroimaging Results
We aimed to determine how percent damage to seven ROIs, single word comprehension deficits (measured using total number of unrelated errors on the SOAP), time post-stroke onset, age and overall lesion volume predicted performance on canonical and noncanonical sentence comprehension at acute and chronic time points. We used LASSO regression to determine which factors best predicted impaired performance in canonical sentences and noncanonical sentences at the acute and chronic time points. Asyntactic comprehension patterns were also investigated at both time points.
Results of the LASSO results are summarized in Table 5. At the acute time point, more time since stroke onset, more severe word-level comprehension deficits and greater percent damage to SMG were implicated in impaired canonical sentence comprehension. Word-level comprehension deficits were the only independent significant predictor of performance on canonical sentences at the acute time point; more severe word-level comprehension deficits predicted poorer canonical sentence comprehension. At the chronic time point the model for canonical sentence comprehension contained greater proportion of damage to pSTG and AG, but neither was an independent significant predictor. At the acute time point, more time since stroke onset, more severe word-level comprehension deficits, and greater damage to SMG and SLF predicted a more severe noncanonical comprehension impairment. Word-level comprehension deficits and proportion of damage to SMG were both significant independent predictors of noncanonical sentence comprehension at the acute time point. The model of noncanonical sentence comprehension at the chronic time point contained proportion of damage to Broca’s area and pSTG; proportion of damage to pSTG was the only significant independent predictor. The LASSO regression of asyntactic comprehension patterns (greater comprehension accuracy on canonical vs. noncanonical sentences) did not converge and therefore revealed no variables at the acute time point, but Broca’s area was implicated in more severe deficits at the chronic time point along with more time since stroke onset and greater word-level comprehension deficits. Damage to Broca’s area was the only significant independent predictor in the model of chronic asyntactic comprehension.
Table 5.
LASSO Regression Results in Participants with Left Hemisphere Stroke
| Acute Canonical | Acute Noncanonical | Chronic Canonical | Chronic Noncanonical | Acute Asyntactic (Canonical > Noncanonical) | Chronic Asyntactic (Canonical > Noncanonical) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| Adjusted Coefficient | p value | Adjusted Coefficient | p value | Adjusted Coefficient | p value | Adjusted Coefficient | p value | Adjusted Coefficient | p value | Adjusted Coefficient | p value | |
|
|
|
|
|
|
|
|||||||
| Model Intercept | 1.06 × 10−16 | −1.82 × 10−16 | −2.62 × 10−16 | −1.71 × 10−16 | - | −5.69 × 10−17 | ||||||
| Overall Lesion Volume | ||||||||||||
| Age | ||||||||||||
| Time Since Stroke Onset | −0.116 | 0.790 | −0.247 | 0.942 | −0.006 | 0.997 | ||||||
| Word Level Comprehension Deficits (Total Unrelated Errors) | −0.828 | 0.003 * | −4.98 | 0.013 * | −0.030 | 0.965 | ||||||
| % Damage to Left Hemisphere: | ||||||||||||
| Broca’s Area (IFGop + IFGtri) | −0.294 | 0.327 | −0.798 | <0.001 * | ||||||||
| Superior Temporal Gyrus | ||||||||||||
| Middle Temporal Gyrus | ||||||||||||
| Posterior Superior Temporal Gyrus | −0.684 | 0.167 | −0.661 | 0.019 * | ||||||||
| Angular gyrus | −0.167 | 0.794 | ||||||||||
| Supramarginal Gyrus | −0.073 | 0.632 | −0.257 | 0.038 * | ||||||||
| Superior Longitudinal Fasciculus | −0.070 | 0.980 | ||||||||||
Note
denotes significance at p ≤ 0.05
4.0. Discussion
In this longitudinal study, we examined sentence comprehension recovery in a group of 15 patients following left hemisphere stroke. We tested patients on simpler canonical and more complex noncanonical sentence comprehension acutely, within six days of stroke, and chronically, at least 6 months post-stroke. LASSO regression was used to create models for prediction of comprehension impairment for canonical and noncanonical sentences at each time point. Asyntactic comprehension patterns (canonical > noncanonical) were also investigated at each time point. We aimed to gain a better understanding of sentence comprehension recovery in left hemisphere stroke patients, and to examine the role of Broca’s area by studying sentence comprehension at the acute stage of recovery before functional reorganization processes have taken place.
For canonical sentences, the mean accuracy improved from 80.3% at the acute stage to 88.7% at the chronic stage of recovery in the patient group. Of the 15 patients, eight had impaired canonical sentence comprehension at the acute stage, and eight had impaired canonical comprehension at the chronic stage. Many patients experienced improvement between acute and chronic stages, but still remained impaired relative to the group of age-matched controls. One patient experienced significant decline from 95% accuracy acutely to 65% accuracy chronically on canonical sentences. This patient’s significant decline is discussed in more detail later in the discussion.
As expected, patients experienced more difficulty comprehending the more complex noncanonical sentences. The mean accuracy for noncanonical sentence comprehension was 73.7% at both the acute and chronic stages. While the mean accuracy was identical at each time point, only three patients achieved the same score at both time points (Figure 2; Table 3). At the acute stage, nine patients were classified as impaired, and at the chronic stage ten patients were impaired. However, seven patients’ noncanonical comprehension improved between acute and chronic time points even if they did not reach the level of unimpaired accuracy. Two patients improved from being classified as impaired to unimpaired and three patients declined from unimpaired to impaired.
The results from the error analysis show that participants were more likely to make unrelated errors on the SOAP at the acute time point, compared to the chronic time point. At the chronic time point the vast majority of errors were thematic reversals. This indicates that word-level comprehension did improve over time in most participants as they were much less likely to select the picture with unrelated referents at the chronic time point. Some participants’ overall accuracy declined, but the types of errors they made evolved from errors rooted in a word-level comprehension deficits to errors resulting from a syntactic deficit. In the five patients whose noncanonical comprehension declined over time (P1, P9, and P10 who declined from unimpaired to impaired performance; P3 and P4 whose performance was impaired at both time points but had a more severe chronic deficit) two participants (P1 and P4) had a higher proportion of unrelated errors at the acute vs. chronic time points but also had more noncanonical errors overall (almost all thematic reversals) at the chronic time point. Fewer chronic unrelated errors can be explained by resolution of word-level comprehension deficits during recovery in these two participants. The larger number of chronic thematic reversal errors may indicate these two participants adopted using an agent-first strategy (Caplan & Futter, 1986; Grodzinsky, 2000) during stroke recovery. This is a comprehension strategy where participants assume the first referent in a sentence is the agent and therefore leads to thematic reversal errors in noncanonical sentences (e.g., “The doctor was questioned by the soldier.”) where the theme (the doctor) precedes the agent (the soldier).
Recall that we had several aims in this study. Our first aim was to better understand sentence comprehension recovery patterns in left hemisphere stroke patients by investigating changes to structure-function relationships between the acute and chronic stages of recovery. More time since stroke onset, more severe word-level comprehension deficits, and greater damage to SMG predicted more severe deficits for both canonical and noncanonical sentences at the acute stage. For noncanonical sentences, greater damage to SLF was an additional predictor maintained in the model of impaired comprehension. Whereas chronic noncanonical sentence comprehension impairment was predicted by greater damage to IFG and pSTG, acute asyntactic sentence comprehension patterns (i.e., better comprehension of canonical vs. noncanonical sentences) were not predicted by damage to any particular region. However, chronic asyntactic sentence comprehension was predicted by greater damage to IFG as well as time since stroke onset and word-level comprehension deficits. It appears that patients with SMG and SLF damage are likely to recover noncanonical sentence comprehension skills as long as pSTG and IFG are intact.
Our results align with Fridriksson et al. (2018), where damage to IFG and temporal regions predicted more difficulty comprehending noncanonical sentences in chronic stroke patients. Yet, our results differ from Rogalsky et al. (2018), who also investigated chronic stroke and found that posterior temporal and inferior parietal damage was associated with chronic noncanonical sentence comprehension deficits, while damage to Broca’s area was not. We found the opposite pattern: for noncanonical sentence comprehension Broca’s area was implicated at the chronic stage of recovery and inferior parietal regions were implicated at the acute but not the chronic stage. Rogalsky et al. (2018) argued that Broca’s area may be erroneously linked to noncanonical sentence processing because many functional imaging studies have used written rather than auditory stimuli. They propose that the role of Broca’s area has been conflated because it is also important for articulatory rehearsal processes that are used to a greater extent in written vs. auditory language processing (Baddeley et al., 1975; Daneman & Newson, 1992). However, our study investigated auditory sentence processing, and we found that Broca’s area damage at the chronic stage was associated with both noncanonical and asyntactic comprehension. Intriguingly, our results also diverged from the Magnusdottir et al. (2013) study, where damage to both Broca’s area and temporal regions predicted impaired noncanonical comprehension in a group of (sub)acute stroke patients who were tested within 20 days of stroke. Instead, the results from Magnusdottir et al. (2013) align with our results at the chronic stage of recovery, where damage to both pSTG and Broca’s area were implicated in more severe noncanonical deficits. It is possible our results differed from Magnusdottir et al.’s results because we completed our testing within six days of stroke. Perhaps reorganization processes (whereby Broca’s area supports functioning for damaged temporoparietal regions) have already begun to take place in the few weeks following stroke. Recall that in a longitudinal functional neuroimaging study Saur et al. (2006) found different patterns of activation in the language network between acute (1–4 days post-stroke) and subacute (2 weeks post-stroke) phases in a group of left hemisphere stroke patients diagnosed with aphasia. Thus, it seems likely that structure-function relationships had already begun to change in the Magnusdottir et al. (2013) study, which could account for the discrepancy between their results and ours. In another study completed within 48 hours of stroke, Newhart et al. (2012) found that temporoparietal cortex, and not Broca’s area, was implicated in asyntactic comprehension patterns.
Based on our results, we propose that Broca’s area is essential for sentence comprehension recovery when temporoparietal regions are damaged. It appears that temporoparietal regions are more critical for sentence comprehension, including noncanonical sentence comprehension, at the acute stage before undergoing functional reorganization processes, even when controlling for word-level comprehension. Perhaps Broca’s area is able to support syntactic processing when these critical temporoparietal regions are damaged. This could explain why greater damage to SMG predicted acute noncanonical deficits, but damage to both Broca’s area and pSTG predicted chronic noncanonical deficits. Our results cannot speak to the specific role of Broca’s area in noncanonical sentence processing, but it is clear that it is involved in supporting noncanonical sentence processing. However, it should be noted that the majority of patients continued to experience difficulty comprehending both canonical and noncanonical sentences at the chronic stage of recovery. Comprehension deficits were more severe in noncanonical compared to canonical sentences. However, given that controls demonstrated no deficits on canonical sentence comprehension, comparatively the majority of patients remained impaired on canonical sentence comprehension at the chronic stage of recovery. Consequently, while Broca’s area may help support syntactic functioning, it is not able to fully compensate for temporoparietal damage.
It should also be noted that the majority of patients experienced canonical and noncanonical sentence comprehension deficits at both stages of recovery. Clinicians should be aware that both canonical and noncanonical sentence comprehension should be assessed acutely. Note that we tested semantically reversible sentences in this study, which are sentences where both the subject and direct object can perform the action in the sentence. For example, in the sentence “The young doctor with blonde hair questions the soldier” both the doctor and the soldier can question someone. Semantically reversible sentences are more difficult for individuals with a syntactic deficit because they cannot use semantic information to determine who is performing an action (Berndt et al., 1996). For this reason, clinicians should be sure to assess syntactic comprehension using semantically reversible sentences. Given that most patients were impaired chronically on canonical sentences, which are encountered more frequently than noncanonical sentences in daily interactions, it is likely that patients would experience many functional benefits from therapy targeting sentence comprehension. Even if patients do not have deficits with simple sentences, it appears that most patients would likely benefit from therapy targeting more syntactically complex language comprehension. Therapy would also be beneficial, as some patients’ performance declined from acute to chronic stages. Specifically, one patient (P9) experienced significant decline on both sentence types between acute and chronic time points. Note that this patient experienced significant decline despite beginning speech language therapy several weeks following her stroke. This result is not surprising given that several studies have indicated a subset of stroke patients will experience decline in language and cognitive skills following a stroke, which may occur when a stroke triggers the onset of vascular dementia (Brainin et al., 2015; Gottesman & Hillis, 2010; Ojala-Oksala et al., 2012). Furthermore, this particular participant was diagnosed with post-stroke major depressive disorder, which is associated with poorer outcomes following stroke (Baker et al., 2020; Brodaty et al., 2007) and likely contributed to her decline. Additionally, follow-up imaging indicated a large area of chronic hypoperfusion surrounding the lesioned area. This functionally lesioned tissue likely accounts for much of the language decline this participant experienced between acute and chronic stages, as chronic hypoperfusion can explain deficits that can’t be attributed to structural lesions alone (Abbott et al., 2021; Love et al., 2002; Robson et al., 2017; Thompson et al., 2017).
Research has demonstrated that speech language therapy between the acute and chronic phases of recovery can be very effective for boosting language recovery following stroke in many patients (Bakheit et al., 2007; Brady et al., 2016; Koyuncu et al., 2016). Speech language therapy is also associated with changes to brain regions recruited for language tasks for patients in both acute/subacute (Mattioli et al., 2014; van de Sandt-Koenderman et al., 2018) and chronic stages of recovery (Barbieri et al., 2019; Crosson et al., 2007; Fridriksson, 2010; Johnson et al., 2019; Kiran et al., 2015; Nardo et al., 2017; Thompson et al., 2010). We do not have detailed information about speech language therapy received by patients in the current study, but it will be important for future studies to consider how the type, duration, and intensity of therapy influence recovery along with the potential predictors investigated in the current study.
We were also interested in investigating the role of Broca’s area in sentence comprehension by examining the acute stage of recovery prior to the onset of functional reorganization recovery processes. Several functional imaging studies in healthy controls have implicated both Broca’s area and posterior temporal regions in noncanonical sentence processing (Bornkessel et al., 2005; Constable et al., 2004; Europa et al., 2019; Mack et al., 2013). Meta-analyses of healthy control functional imaging studies have also implicated IFG and posterior temporal regions (Meyer & Friederici, 2016; Walenski et al., 2019). For example, Meyer and Friederici (2016) found activation clusters in anterior left-hemisphere regions (IFG, insula, MFG) as well as left-hemisphere posterior regions (MTG and STG). Walenski et al. (2019) found activation clusters in similar regions including left-hemisphere anterior regions (IFG, MFG, SFG) and left-hemisphere posterior regions (posterior MTG, AG), as well as several right hemisphere regions (insula, paracingulate gyrus). Of note, activated regions in functional imaging studies in healthy controls do not necessarily indicate a particular region is vital for the process of interest (Price & Friston, 2002), and these results should be interpreted accordingly.
Another method that complements functional neuroimaging in healthy controls and lesion studies in patients is repetitive transcranial magnetic stimulation (rTMS), a form of neuromodulation that can be used for brain mapping through the creation of transient cortical lesions in healthy controls (Oliveri et al., 2004; Pobric et al., 2010). Specifically, rTMS can be applied to a specific cortical region of interest during a functional task (e.g., online sentence processing) to determine how a lesion in that location will impact task performance. Kuhnke et al (2017) recently reported that rTMS applied to left posterior IFG, but not to left temporoparietal cortex, resulted in significant performance decline for object-relative compared to subjectrelative sentences. They concluded that the left posterior IFG is a critical region for comprehending noncanonical sentences.
It is clear from research in healthy controls that Broca’s area is involved in sentence comprehension, but its specific function remains unclear. Thus, it is uncertain whether it is a critical syntactic processing hub. As we have discussed above, many lesion studies in stroke have suggested Broca’s area is essential for noncanonical sentence comprehension and have indicated damage to Broca’s area results in asyntactic sentence comprehension patterns (Fridriksson et al., 2018; Magnusdottir et al., 2013; Uddén et al., 2019). However, other lesions studies have found that Broca’s area is not implicated in asyntactic sentence comprehension deficits (Rogalsky et al., 2018; Thothathiri et al., 2011). Most lesion studies investigate the impact of left hemisphere lesions at the chronic stage of recovery. Our study design allowed for the unique opportunity to examine the role of Broca’s area damage to acute sentence comprehension deficits before functional reorganization processes have occurred. In the current study, damage to Broca’s area was not linked to asyntactic comprehension patterns or to comprehension deficits for canonical or noncanonical sentences at the acute stage. These results do not support the hypothesis that Broca’s area is a critical hub for noncanonical sentence processing in neurologically unimpaired populations. We propose Broca’s area is often implicated in studies of chronic left hemisphere stroke patients because patients with damage to both temporoparietal regions and Broca’s area cannot recover function to the same extent as patients without damage, not necessarily because it is a premorbid hub for complex syntactic processing.
While one strength of our study was the ability to follow patients longitudinally from acute to chronic stages of recovery, this study did have several limitations. First, we have a relatively small sample size. Future studies should incorporate our longitudinal design with a larger group of patients, particularly because of the inherent heterogeneity in the lesions and deficits experienced by stroke patients. Second, we did not test patients at a subacute stage of recovery, which would provide more information about the evolution of structure-function relationships during stroke recovery. Third, we do not have information about the speech language therapy some patients may have received between their acute and chronic time points. Future studies should incorporate this information to better understand recovery processes. Fourth, we did not assess hypoperfusion, which can significantly impair brain functioning beyond the borders of the lesion at acute and chronic stages of recovery (Abbott et al., 2021; Hillis et al., 2001, 2002b, 2002a; Love et al., 2002; Robson et al., 2017; Thompson et al., 2017). Fifth, the time between MRI and conclusion of acute language testing ranged between 0 – 6 days, and it is possible some brain changes occurred between neuroimaging and acute language testing. Finally, we did not collect functional neuroimaging data, which would allow us to examine patterns of functional activation during sentence comprehension at each time point.
Overall, our study suggests that when controlling for word-level comprehension deficits while patients with damage to SLF, and SMG are likely to experience acute sentence comprehension deficits, patients with Broca’s area and pSTG damage are more likely to experience long-term deficits. Broca’s area was only implicated in noncanonical comprehension or asyntactic comprehension at chronic stages after functional reorganization processes occurred. We propose that Broca’s area and pSTG may be able to partially subsume syntactic functioning from damaged temporal and parietal regions. Hence patients with damage to Broca’s area or pSTG will have difficulty regaining comprehension skills. Based on our findings Broca’s area does not appear as critical for noncanonical sentence processing as temporoparietal areas prior to stroke. Our results also suggest that the majority of patients will have chronic difficulties comprehending both canonical and noncanonical sentences. Assessing canonical and noncanonical comprehension acutely is recommended, and the majority of left hemisphere stroke patients would likely benefit from speech-language therapy to improve their auditory syntactic comprehension.
Highlights.
The role of Broca’s area in syntactic processing is debated
Most stroke brain mapping studies are after functional reorganization has occurred
Many left hemisphere stroke patients have chronic syntactic comprehension deficits
Canonical sentence comprehension is often impaired following left hemisphere stroke
Broca’s area is only implicated in chronic stage, and not acute, syntactic deficits
Acknowledgements
This work was supported by the National Institutes of Health [grant numbers R01DC05375, and R01DC015466].
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NT, Baker CJ, Chen C, Liu TT, & Love TE (2021). Defining Hypoperfusion in Chronic Aphasia: An Individualized Thresholding Approach. Brain Sciences, 11(4), 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Thomson N, & Buchanan M. (1975). Word length and the structure of short-term memory. Journal of Verbal Learning and Verbal Behavior, 14(6), 575–589. 10.1016/S0022-5371(75)80045-4 [DOI] [Google Scholar]
- Bak TH, & Hodges JR (2003). Kissing and dancing—A test to distinguish the lexical and conceptual contributions to noun/verb and action/object dissociation. Preliminary results in patients with frontotemporal dementia. Journal of Neurolinguistics, 16(2), 169–181. 10.1016/S0911-6044(02)00011-8 [DOI] [Google Scholar]
- Baker C, Worrall L, Rose M, & Ryan B. (2020). ‘It was really dark’: The experiences and preferences of people with aphasia to manage mood changes and depression. Aphasiology, 34(1), 19–46. [Google Scholar]
- Bakheit AMO., Shaw S., Barrett L., Wood J., Carrington S., Griffiths S., Searle K., & Koutsi F. (2007). A prospective, randomized, parallel group, controlled study of the effect of intensity of speech and language therapy on early recovery from poststroke aphasia. Clinical Rehabilitation, 21(10), 885–894. 10.1177/0269215507078486 [DOI] [PubMed] [Google Scholar]
- Barbieri E, Mack J, Chiappetta B, Europa E, & Thompson CK (2019). Recovery of offline and online sentence processing in aphasia: Language and domain-general network neuroplasticity. Cortex, 120, 394–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt RS, Mitchum CC, & Haendiges AN (1996). Comprehension of reversible sentences in “agrammatism”: A meta-analysis. Cognition, 58(3), 289–308. [DOI] [PubMed] [Google Scholar]
- Bornkessel I, Zysset S, Friederici AD, Von Cramon DY, & Schlesewsky M. (2005). Who did what to whom? The neural basis of argument hierarchies during language comprehension. Neuroimage, 26(1), 221–233. [DOI] [PubMed] [Google Scholar]
- Brady MC, Kelly H, Godwin J, Enderby P, & Campbell P. (2016). Speech and language therapy for aphasia following stroke. Cochrane Database of Systematic Reviews, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainin M, Tuomilehto J, Heiss W-D, Bornstein NM, Bath PMW, Teuschl Y, Richard E, Guekht A, & Quinn T. (2015). Post-stroke cognitive decline: An update and perspectives for clinical research. European Journal of Neurology, 22(2), 229–e16. 10.1111/ene.12626 [DOI] [PubMed] [Google Scholar]
- Breining BL, Faria AV, Caffo B, Meier EL, Sheppard SM, Sebastian R, Tippett DC, & Hillis AE (2021). Neural regions underlying object and action naming: Complementary evidence from acute stroke and primary progressive aphasia. Aphasiology, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breining BL., Lala T., Cuitiño MM., Manes F., Peristeri E., Tsapkini K., Faria AV., & Hillis AE. (2015). A brief assessment of object semantics in primary progressive aphasia. Aphasiology, 29(4), 488–505. 10.1080/02687038.2014.973360 [DOI] [Google Scholar]
- Brodaty H, Withall A, Altendorf A, & Sachdev PS (2007). Rates of depression at 3 and 15 months poststroke and their relationship with cognitive decline: The Sydney Stroke Study. The American Journal of Geriatric Psychiatry, 15(6), 477–486. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G, & Olivieri A. (2000). Activation of Broca’s area by syntactic processing under conditions of concurrent articulation. Human Brain Mapping, 9(2), 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, & Futter C. (1986). Assignment of thematic roles to nouns in sentence comprehension by an agrammatic patient. Brain and Language, 27(1), 117–134. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Capasso R, Capitani E, & Miceli G. (2005). Patterns of comprehension performance in agrammatic Broca’s aphasia: A test of the Trace Deletion Hypothesis. Brain and Language, 94(1), 43–53. 10.1016/j.bandl.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Caramazza A, Capitani E, Rey A, & Berndt RS (2001). Agrammatic Broca’s Aphasia Is Not Associated with a Single Pattern of Comprehension Performance. Brain and Language, 76(2), 158–184. 10.1006/brln.1999.2275 [DOI] [PubMed] [Google Scholar]
- Caramazza A, & Hillis AE (1989). The disruption of sentence production: Some dissociations. Brain and Language, 36(4), 625–650. 10.1016/0093-934X(89)90091-6 [DOI] [PubMed] [Google Scholar]
- Chomsky N, Gallego ÁJ, & Ott D. (2017). Generative Grammar and the Faculty of Language: Insights, Questions, and Challenges *. [Google Scholar]
- Constable RT, Pugh KR, Berroya E, Mencl WE, Westerveld M, Ni W, & Shankweiler D. (2004). Sentence complexity and input modality effects in sentence comprehension: An fMRI study. Neuroimage, 22(1), 11–21. [DOI] [PubMed] [Google Scholar]
- Crosson B., McGregor K., Gopinath KS., Conway TW., Benjamin M., Chang Y-L., Moore AB., Raymer AM., Briggs RW., & Shero MG. (2007). Functional MRI of language in aphasia: A review of the literature and the methodological challenges. Neuropsychology Review, 17(2), 157–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman M, & Newson M. (1992). Assessing the importance of subvocalization during normal silent reading. Reading and Writing, 4(1), 55–77. 10.1007/BF01027072 [DOI] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, & Jaeger JJ (2004). Lesion analysis of the brain areas involved in language comprehension. Cognition, 92(1), 145–177. 10.1016/j.cognition.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Europa E, Gitelman DR, Kiran S, & Thompson CK (2019). Neural Connectivity in Syntactic Movement Processing. Frontiers in Human Neuroscience, 13. 10.3389/fnhum.2019.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria AV., Joel SE., Zhang Y., Oishi K., van Zjil PCM., Miller MI., Pekar JJ., & Mori S. (2012). Atlas-based analysis of resting-state functional connectivity: Evaluation for reproducibility and multi-modal anatomy–function correlation studies. NeuroImage, 61(3), 613–621. 10.1016/j.neuroimage.2012.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira F, & Çokal D. (2015). Sentence processing. In Hickok G & Small SL(Eds.), Neurobiology of language (pp. 265–274). Academic Press. [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, & Friederici AD (2005). Revisiting the role of Broca’s area in sentence processing: Syntactic integration versus syntactic working memory. Human Brain Mapping, 24(2), 79–91. 10.1002/hbm.20070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J. (2010). Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. Journal of Neuroscience, 30(35), 11558–11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, den Ouden D-B, Hillis AE, Hickok G, Rorden C, Basilakos A, Yourganov G, & Bonilha L. (2018). Anatomy of aphasia revisited. Brain, 141(3), 848–862. 10.1093/brain/awx363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Fillmore P, Guo D, & Rorden C. (2015). Chronic Broca’s aphasia is caused by damage to Broca’s and Wernicke’s areas. Cerebral Cortex, 25(12), 4689–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2009). Pathways to language: Fiber tracts in the human brain. Trends in Cognitive Sciences, 13(4), 175–181. 10.1016/j.tics.2009.01.001 [DOI] [PubMed] [Google Scholar]
- Friederici AD (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91(4), 1357–1392. 10.1152/physrev.00006.2011 [DOI] [PubMed] [Google Scholar]
- Friederici AD (2012). The cortical language circuit: From auditory perception to sentence comprehension. Trends in Cognitive Sciences, 16(5), 262–268. 10.1016/j.tics.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Friederici AD (2018). The neural basis for human syntax: Broca’s area and beyond. Current Opinion in Behavioral Sciences, 21, 88–92. 10.1016/j.cobeha.2018.03.004 [DOI] [Google Scholar]
- Friedman J, Hastie T, & Tibshirani R. (2010). Regularization Paths for Generalized Linear Models via Coordinate Descent. Journal of Statistical Software, 33(1), 1–22. [PMC free article] [PubMed] [Google Scholar]
- Gottesman RF, & Hillis AE (2010). Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. The Lancet Neurology, 9(9), 895–905. 10.1016/S1474-4422(10)70164-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinsky Y. (2000). The neurology of syntax: Language use without Broca’s area. Behavioral and Brain Sciences, 23(01), 1–21. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, & Saur D. (2019). Neuroimaging of stroke recovery from aphasia – Insights into plasticity of the human language network. NeuroImage, 190, 14–31. 10.1016/j.neuroimage.2017.11.056 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, Wityk RJ, & Barker PB (2002a). Regions of neural dysfunction associated with impaired naming of actions and objects in acute stroke. Cognitive Neuropsychology, 19(6), 523–534. 10.1080/02643290244000077 [DOI] [PubMed] [Google Scholar]
- Hillis AE., Wityk RJ., Barker PB., Beauchamp NJ., Gailloud P., Murphy K., Cooper O., & Metter EJ. (2002b). Subcortical aphasia and neglect in acute stroke: The role of cortical hypoperfusion. Brain, 125(5), 1094–1104. 10.1093/brain/awf113 [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, & Selnes OA (2001). Hypoperfusion of Wernicke’s area predicts severity of semantic deficit in acute stroke. Annals of Neurology, 50(5), 561–566. 10.1002/ana.1265 [DOI] [PubMed] [Google Scholar]
- Jarso S, Li M, Faria A, Davis C, Leigh R, Sebastian R, Tsapkini K, Mori S, & Hillis AE (2013). Distinct mechanisms and timing of language recovery after stroke. Cognitive Neuropsychology, 30(7–8), 454–475. 10.1080/02643294.2013.875467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JP, Meier EL, Pan Y, & Kiran S. (2019). Treatment-related changes in neural activation vary according to treatment response and extent of spared tissue in patients with chronic aphasia. Cortex, 121, 147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S. (2001). Boston Naming Test. Pro-ed. [Google Scholar]
- Kiran S, Meier EL, Kapse KJ, & Glynn PA (2015). Changes in task-based effective connectivity in language networks following rehabilitation in post-stroke patients with aphasia. Frontiers in Human Neuroscience, 9, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu E, Çam P, Altınok N, Çallı DE, Duman TY, & Özgirgin N. (2016). Speech and language therapy for aphasia following subacute stroke. Neural Regeneration Research, 11(10), 1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristinsson S, Thors H, Yourganov G, Magnusdottir S, Hjaltason H, Stark BC, Basilakos A, den Ouden D-B, Bonilha L, Rorden C, Hickok G, Hillis AE, & Fridriksson J. (2020). Brain Damage Associated with Impaired Sentence Processing in Acute Aphasia. Journal of Cognitive Neuroscience, 32(2), 256–271. 10.1162/jocn_a_01478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnk P., Meye L., Friederic AD., & Hartwigse G. (2017). Left posterior inferior frontal gyrus is causally involved in reordering during sentence processing. Neuroimage, 148, 254–263. [DOI] [PubMed] [Google Scholar]
- Lau E, Stroud C, Plesch S, & Phillips C. (2006). The role of structural prediction in rapid syntactic analysis. Brain and Language, 98(1), 74–88. [DOI] [PubMed] [Google Scholar]
- Love T, & Oster E. (2002). On the categorization of aphasic typologies: The SOAP (a test of syntactic complexity). Journal of Psycholinguistic Research, 31(5), 503–529. [DOI] [PubMed] [Google Scholar]
- Love T, Swinney D, Wong E, & Buxton R. (2002). Perfusion imaging and stroke: A more sensitive measure of the brain bases of cognitive deficits. Aphasiology, 16(9), 873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JE, Meltzer-Asscher A, Barbieri E, & Thompson CK (2013). Neural Correlates of Processing Passive Sentences. Brain Sciences, 3(3), 1198–1214. 10.3390/brainsci3031198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusdottir S, Fillmore P, Ouden DB den, Hjaltason H, Rorden C, Kjartansson O, Bonilha L, & Fridriksson J(2013). Damage to left anterior temporal cortex predicts impairment of complex syntactic processing: A lesion-symptom mapping study. Human Brain Mapping, 34(10), 2715–2723. 10.1002/hbm.22096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchin WG (2018). A neuronal retuning hypothesis of sentence-specificity in Broca’s area. Psychonomic Bulletin & Review, 25(5), 1682–1694. 10.3758/s13423-017-1377-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchin W, Hammerly C, & Lau E. (2017). The role of the IFG and pSTS in syntactic prediction: Evidence from a parametric study of hierarchical structure in fMRI. Cortex, 88, 106–123. [DOI] [PubMed] [Google Scholar]
- Matchin W, & Hickok G. (2020). The Cortical Organization of Syntax. Cerebral Cortex, 30(3), 1481–1498. 10.1093/cercor/bhz180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli F, Ambrosi C, Mascaro L, Scarpazza C, Pasquali P, Frugoni M, Magoni M, Biagi L, & Gasparotti R. (2014). Early aphasia rehabilitation is associated with functional reactivation of the left inferior frontal gyrus: A pilot study. Stroke, 45(2), 545–552. [DOI] [PubMed] [Google Scholar]
- Meinshausen N, & Yu B. (2009). Lasso-type recovery of sparse representations for high-dimensional data. The Annals of Statistics, 37(1), 246–270. 10.1214/07-AOS582 [DOI] [Google Scholar]
- Meyer M, Alter K, Friederici AD, Lohmann G, & von Cramon DY (2002). FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Human Brain Mapping, 17(2), 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, & Davis KR (1978). Broca aphasia: Pathologic and clinical. Neurology, 28(4), 311–311. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, & Woods R. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage, 40(2), 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardo D, Holland R, Leff AP, Price CJ, & Crinion JT (2017). Less is more: Neural mechanisms underlying anomia treatment in chronic aphasic patients. Brain, 140(11), 3039–3054. 10.1093/brain/awx234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhart M, Trupe LA, Gomez Y, Cloutman L, Molitoris JJ, Davis C, Leigh R, Gottesman RF, Race D, & Hillis AE (2012). Asyntactic comprehension, working memory, and acute ischemia in Broca’s area versus angular gyrus. Cortex, 48(10), 1288–1297. 10.1016/j.cortex.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick JM., Trueswell JC., & Thompson-Schill SL. (2005). Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cogn Affect Behav Neurosci, 5(3 LB-r01284), 263–281. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, & Thompson‐Schill SL (2010). Broca’s Area and Language Processing: Evidence for the Cognitive Control Connection. Language and Linguistics Compass, 4(10), 906–924. 10.1111/j.1749-818X.2010.00244.x [DOI] [Google Scholar]
- Ochfeld E, Newhart M, Molitoris J, Leigh R, Cloutman L, Davis C, Crinion J, & Hillis AE (2010). Ischemia in broca area is associated with broca aphasia more reliably in acute than in chronic stroke. Stroke, 41(2), 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala-Oksala J, Jokinen H, Kopsi V, Lehtonen K, Luukkonen L, Paukkunen A, Seeck L, Melkas S, Pohjasvaara T, Karhunen P, Hietanen M, Erkinjuntti T, & Oksala N. (2012). Educational History Is an Independent Predictor of Cognitive Deficits and Long-Term Survival in Postacute Patients With Mild to Moderate Ischemic Stroke. Stroke. 10.1161/strokeaha.112.667618 [DOI] [PubMed] [Google Scholar]
- Oliveri M, Finocchiaro C, Shapiro K, Gangitano M, Caramazza A, & Pascual-Leone A. (2004). All Talk and No Action: A Transcranial Magnetic Stimulation Study of Motor Cortex Activation during Action Word Production. Journal of Cognitive Neuroscience, 16(3), 374–381. 10.1162/089892904322926719 [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, & Lambon Ralph MA (2010). Category-Specific versus Category-General Semantic Impairment Induced by Transcranial Magnetic Stimulation. Current Biology, 20(10), 964–968. 10.1016/j.cub.2010.03.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, & Friston KJ (2002). Degeneracy and cognitive anatomy. Trends in Cognitive Sciences, 6(10), 416–421. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing. https://www.Rproject.org/ [Google Scholar]
- Rimmele JM, Morillon B, Poeppel D, & Arnal LH (2018). Proactive sensing of periodic and aperiodic auditory patterns. Trends in Cognitive Sciences, 22(10), 870–882. [DOI] [PubMed] [Google Scholar]
- Robson H., Specht K., Beaumon H., Parkes LM., Sage K., Ralph MAL., & Zahn R. (2017). Arterial spin labelling shows functional depression of non-lesion tissue in chronic Wernicke’s aphasia. Cortex, 92, 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, & Hickok G. (2011). The role of Broca’s area in sentence comprehension. Journal of Cognitive Neuroscience, 23(7), 1664–1680. [DOI] [PubMed] [Google Scholar]
- Rogalsky C, LaCroix AN, Chen K-H, Anderson SW, Damasio H, Love T, & Hickok G. (2018). The neurobiology of agrammatic sentence comprehension: A lesion study. Journal of Cognitive Neuroscience, 30(2), 234–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Matchin W, & Hickok G. (2008). Broca’s area, sentence comprehension, and working memory: An fMRI study. Frontiers in Human Neuroscience, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, & Karnath H-O (2012). Age-specific CT and MRI templates for spatial normalization. NeuroImage, 61(4), 957–965. 10.1016/j.neuroimage.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, & Bonilha L. (2007). Improving Lesion-Symptom Mapping. Journal of Cognitive Neuroscience, 19(7), 1081–1088. 10.1162/jocn.2007.19.7.1081 [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, & Weiller C. (2006). Dynamics of language reorganization after stroke. Brain, 129(6), 1371–1384. 10.1093/brain/awl090 [DOI] [PubMed] [Google Scholar]
- Sheppard SM., Love T., Midgley KJ., Holcomb PJ., & Shapiro LP. (2017). Electrophysiology of prosodic and lexical-semantic processing during sentence comprehension in aphasia. Neuropsychologia, 107, 9–24. 10.1016/j.neuropsychologia.2017.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SM, Midgley KJ, Love T, Shapiro LP, & Holcomb PJ (2018). Electrophysiological evidence for the interaction of prosody and thematic fit during sentence comprehension. Language, Cognition and Neuroscience, 33(5), 547–562. 10.1080/23273798.2017.1390143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, den Ouden D-B, Bonakdarpour B, Garibaldi K, & Parrish TB (2010). Neural plasticity and treatment-induced recovery of sentence processing in agrammatism. Neuropsychologia, 48(11), 3211–3227. 10.1016/j.neuropsychologia.2010.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK., Walensk M., Chen Y., Caplan D., Kiran S., Rapp B., Grunewald K., Nunez M., Zinbarg R., & Parrish TB. (2017). Intrahemispheric perfusion in chronic stroke-induced aphasia. Neural Plasticity, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thothathiri M, Kimberg DY, & Schwartz MF (2011). The Neural Basis of Reversible Sentence Comprehension: Evidence from Voxel-based Lesion Symptom Mapping in Aphasia. Journal of Cognitive Neuroscience, 24(1), 212–222. 10.1162/jocn_a_00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. (1996). Regression Shrinkage and Selection Via the Lasso. Journal of the Royal Statistical Society: Series B (Methodological), 58(1), 267–288. 10.1111/j.2517-6161.1996.tb02080.x [DOI] [Google Scholar]
- Tibshirani R, Tibshirani R, Taylor J, Loftus J, Reid S, & Markovic J. (2017). selectiveInference: Tools for post-selection inference (R package version 1.2.5) [Computer software]. https://CRAN.R-project.org/package=selectiveInference [Google Scholar]
- Uddén J, Hultén A, Schoffelen J-M, Lam N, Harbusch K, Bosch A. van den, Kempen G, Petersson KM., & Hagoort P (2019). Supramodal Sentence Processing in the Human Brain: Fmri Evidence for the Influence of Syntactic Complexity in More Than 200 Participants. BioRxiv, 576769. 10.1101/576769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sandt-Koenderman MW, Mendez Orellana CP, van der Meulen I, Smits M, & Ribbers GM (2018). Language lateralisation after melodic intonation therapy: An fMRI study in subacute and chronic aphasia. Aphasiology, 32(7), 765–783. [Google Scholar]
- Walenski M, Europa E, Caplan D, & Thompson CK (2019). Neural networks for sentence comprehension and production: An ALE-based meta-analysis of neuroimaging studies. Human Brain Mapping, 40(8), 2275–2304. 10.1002/hbm.24523 [DOI] [PMC free article] [PubMed] [Google Scholar]