Abstract
Propolis is a natural compound collected by honeybees from different parts of plants. Honeybees produce a sticky component besides honey by mixing the tree resin and other botanical sources with saliva called propolis or bee glue. Propolis was traditionally used as a wound healing substance, cosmetic, medicine, and many other conditions. Till now, there is no definite curable treatment for most cancers and chemotherapeutic drugs and drugs used for targeted therapies have serious side effects. According to a recent research, natural products are becoming increasingly essential in cancer prevention. Natural products are a great source of potential therapeutic agents, especially in the treatment of cancer. Previous studies have reported that the presence of caffeic acid phenethyl ester (CAPE), artepillin C, and chrysin is responsible for the anticancer potential of propolis. Most of the previous studies suggested that propolis and its active compounds inhibit cancer progression by targeting multiple signaling pathways including phosphoinositide 3-kinases (PI3K)/Akt and mitogen-activated protein kinase (MAPK) signaling molecules, and induce cell cycle arrest. Induction of apoptosis by propolis is mediated through extrinsic and intrinsic apoptotic pathways. The aim of this review is to highlight and summarize the molecular targets and anticancer potential of propolis and its active compounds on cell survival, proliferation, metastasis, and apoptosis in cancer cells.
1. Introduction
Cancer is the world's second major cause of death and a serious global health issue. In 2020, an estimated 19.3 million new cancer cases were diagnosed worldwide, with over 10 million cancer deaths. An expected 2.3 million new cases of female breast cancer have surpassed lung cancer as the most commonly diagnosed malignancy, followed by lung, colorectal, prostate, and stomach cancers [1]. Cancer is a multifaceted disorder relating to abnormal cell growth, and cancer cells form aggressive unwanted masses of tissue called tumors, which are capable of spreading to other organs through the bloodstream or lymph system to form metastatic cancer [2]. Cancer cells dedifferentiate and are able to grow aggressively and escape from growth regulatory signals such as cell cycle control and apoptosis [3]. Currently, there are different methods for cancer treatment available based on cancer type and grade, such as radiation and/or chemotherapy and other options like including immunotherapy, targeted therapy, and hormone therapy employed in cancer patients [4–7]. The chemotherapeutic approach is used for the treatment of benign and malignant cancers; however, it has several limitations like side effects and off-target effects [8, 9].
Cancer tissue contains heterogeneous cell populations, and these cells activate multidrug resistance, thereby triggering tumor relapse. The available common cancer treatments have no complete cure and cause various side effects. Often chemotherapeutic drugs have poor bioavailability and a narrow therapeutic index. Therefore, there is an immediate requirement for the development of natural compound-based anticancer drugs with target-specific, cost-effective, and minimal side effects for cancer treatment. Previously, numerous research studies have been conducted to identify anticancer drugs from natural products [10, 11]. Several naturally obtained drugs like paclitaxel, vinblastine, vincristine, and several others are being clinically used as anticancer medicines. Therefore, natural products are developed as an essential research field, to identify the potential novel anticancer agent and also to understand the mechanism of action on cancer cells. Hence, this review summarizes the molecular targets and anticancer properties of propolis and its active compounds against various cancers.
2. Propolis
Propolis is a natural resinous substance produced by honeybees from plant-based natural source materials like tree buds, sap flowers, etc., by mixing with saliva secretions. It is used for the protection from infections, to seal gaps, openings in the hive, and toughen combs, and also, propolis is used to maintain the hive's internal temperature, leveling the beehive surface to protect from abnormal weather and predators [12]. Honeybees are flying insects, closely related to wasps and ants. The honeybee products like honey, beeswax, bee venom, and bee pollen have been used as traditional medicine and also exhibited antimicrobial, antioxidant, anti-inflammatory, and anticancer properties in several preclinical studies in cell line and animal experiments [13–17]. The word “Propolis” comes from Greek that means defense for “pro” and city or community for “polis,” or the beehive in other words. Propolis has been used by humans for many years as a powerful herbal and dietary supplement. The nature of propolis is like plastic material that protected the human body after death from bacteria, fungi, and viruses, and the Egyptians used it as preservative material to embalming the human body [18]. The presence of phytochemicals (polyphenols, flavonoid, phenolic acids, phenolic aldehydes, and ketones) in propolis is responsible for its biological properties, including antioxidant [19], anti-inflammatory [20], and anticancer potential [21–23]. The types of propolis are classified based on their origin, color, and texture. Generally, Asians and Europeans use propolis largely in the management of many diseases [24, 25].
2.1. Chemical Composition and Biological Properties of Propolis
The propolis is multifaceted, and the chemical composition differs according to bee species, geographical origin, and plants that stimulate the bee's biological activities [26, 27]. Different type of propolis has a variety of biological properties including antioxidant and anticancer effects based on their origin and chemical composition [28, 29]. The presence of polyphenols and flavonoids determines the therapeutic properties of propolis, and interestingly, there are 38 different types of flavonoids present in the propolis. The propolis contains 24 to 26% of fatty acids, 18 to 20% of flavonoids, 0.5 to 2% of microelements, 15 to 18% of sugars, 5 to 10% of aromatic acids, 2–6% of esters, 2 to 3.3% of alcohol and terpenes, and 2 to 4% of vitamins [30, 31]. Resins and balms are major components (50–60%) in raw propolis. Also, waxes (30–40%), essential oils (5–10%), pollen (5%), and vitamins (5%) are present in propolis [32, 33]. It has been reported that 300 different types of polyphenols compounds [34], terpenoids [35], amino acids, and others are found in propolis [32]. The HPLC-MS (high-performance liquid chromatography-mass spectrometry) and ultra-PLC-MS analyses have identified the predominant bioactive components in propolis such as chrysin, CAPE, artepillin C, nemorosone, galangin, cardanol, cardol, quercetin, kaempferol, and p-coumaric acid in Brazilian green propolis [36].
Propolis polyphenolic compounds are having anticarcinogenic activity, and in addition to this, well-known predominant bioactive components of propolis like CAPE, artepillin C, and chrysin can inhibit cancer cell growth in various malignancies [27, 37–39]. Artepillin C, another form of propolis, is reported to induce cytotoxicity through activating apoptosis and necrosis and inhibits mitosis in melanoma and carcinomas cells [1]. Chrysin has an anticancer property by inducing extrinsic and intrinsic apoptosis through activating caspases, and inhibiting antiapoptotic proteins in cancer cell lines [40]. The propolis composition differs based on the plant origin. For example, New Zealand propolis contains CAPE, Brazilian propolis contains artepillin C, and propolin G in Taiwan propolis [41]. Okinawa propolis (OP) originated from Macaranga tanarius comprises prenylated flavonoids [42]. Several recent studies also reported that the anticancer properties of propolis and its active principle compounds such as CAPE, artepillin C, and chrysin have shown anticancer potential by apoptosis induction and inhibition of cell growth in different cancer cells [43–46].
It has been established that prenylated flavonoids from OP could strongly suppress the albumin denaturation, thereby preventing the chronic inflammation-mediated cellular protein denaturation. Furthermore, OP treatment decreases the COX-2 activity and nitrite accumulation in lipopolysaccharide-induced inflammatory model, RAW264.7 cells [47]. Earlier reports elucidate that the antidiabetic, anti-inflammatory, and anti-Alzheimer's drug properties of OP are mediated through inhibition of PAK1 signaling. Overall, all of the earlier findings suggested that components of OP could be a harmless and novel drug for Alzheimer's, diabetes, and inflammation treatment [47]. Elnakady et al. [48] studied the importance of Saudi Arabia propolis in cancer cells, in which they have demonstrated that the propolis-treated cells showed inhibition of cell proliferation and induction of apoptosis. Furthermore, their gas chromatography-mass spectrometry (GC-MS) examination confirmed the occurrence of an active fraction, i.e., Saudi propolis L-acetate, and it was isolated from the region of Al Bahah. The occurrence of triterpenoids such as lupeol, ferruginol, β-amaryl acetate, β-amyrins, and lupeol acetate, sugiol, taraxasterol, and totarol is implicated in their beneficial effect on Saudi propolis. These fractions had the potential to inhibit the tubulin activity through cell cycle arrest at the G2M phase and to induce apoptosis in cancer cell lines.
Although propolis is a good source of minerals, amino acids, vitamins A, E, and B complex, and the biochemical compounds like bioflavonoids, phenols, aromatic compounds, and many other molecules, which possess hepatoprotective activity, anti-inflammatory, antiproliferative, antifungal, antibacterial, and antimicrobial qualities, the exact constituent of propolis is still unknown; hence, further research is required to find out the new compounds [31, 49–51]. The Brazilian red propolis is seen in red color due to pigmentation of Retusapurpurin B and Retusapurpurin A flavanols, and their chemical composition and pharmacological activities were thoroughly studied in a recent study [52]. By considering the above properties, propolis is utilized in manufacturing following cosmetics like soap, perfume, and sunscreen [31, 53]. It is also used in mouthwash for oral care for patients suffering from chemotherapy to improve oral hygiene [54].
Several studies have demonstrated that the antioxidant activity of flavonoids was achieved through the potential to decrease the formation of free radical and scavenging capacity [30, 55]. The antioxidant potential of propolis is well studied, and its powder is commercially available as a supplement that increases antioxidant enzymes like glutathione peroxidase, superoxide dismutase, and catalase [56, 57]. Recently, propolis was shown to have anti-inflammatory and immunomodulatory activity and inhibits the PAK1. In addition, propolis inhibits the interaction of ACE2 and SARS-CoV-2 virus. In silico approach reported that active components of propolis, such as CAPE, quercetin, rutin, kaempferol, and myricetin, possess a high affinity with ACE2. Furthermore, propolis does not affect the key enzymes and liver function; hence, it can be a better drug without any side effects [58]. Further investigations and studies are urgently needed to understand the exact mechanisms of propolis in the future. Figure 1 shows the biological properties of propolis and its bioactive components.
Figure 1.
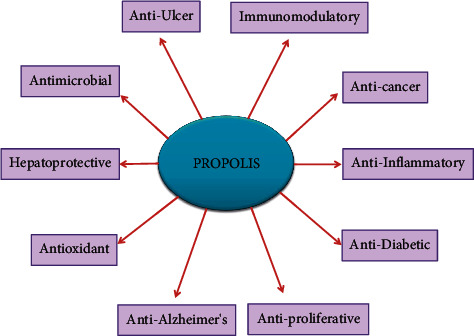
Overview of the biological properties of propolis and its bioactive components.
2.2. Bioavailability of Propolis
Poor gastrointestinal absorption and bioavailability hinder the effectiveness of numerous natural and synthetic anticancer drugs during the drug development process. The failure of drugs in clinical trials is frequently due to poor absorption and oral bioavailability of drugs [59].
It is crucial to understand which propolis components are bioavailable in the human body. Because most of the activity is given by free forms, and conjugation makes it harder to demonstrate activity, bioavailability is directly connected with sensitivity to conjugation when the components are integrated through the intestines [60]. To evaluate the in vivo antioxidant capabilities of propolis and its effect on human health, the researchers first investigated its bioavailability. These findings indicated that galangin may be adsorbed and promptly glucuronidated after oral treatment, and a brief bioavailability in mice for galangin, chrysin, and its derivatives [60].
Pharmacokinetic properties of compounds present in Okinawa propolis (OP) were analyzed by molinspiration online toolkit to consider them as potent drug candidates based on its Lipinski's 5th rule [61]. All the OP compounds have better absorption and bioavailability [47]. Several studies proved that the pharmacokinetics and toxicological properties of propolis approved them as potential drug sources [62]. According to Kimoto et al., [63, 64] an antioxidative dosage of artepillin C prevented renal and pulmonary malignancies in male mice developed by ferric nitrilotriacetate. As a result, artepillin C, which is difficult to conjugate and has a strong affinity for cell membranes, is a potential bioavailable factor for use in degenerative disease chemoprevention.
Polyphenols are known to have limited bioavailability as a result of their molecular weight, glycosylation level, metabolic conversion, and contact with the gut bacteria, which limits their use in vivo. The goal of the most recent study was to synthesize poly-(lactide-co-glycolide) acid (PLGA) nanoparticles for the encapsulation of Sonoran propolis (SP) as a matrix to increase the solubility of their polyphenolic compounds and improve delivery, and assess its antiproliferative activity on cancer cells. The SP might be possibly employed in antitumoral in vivo experiments after being encapsulated into PLGA nanoparticles, since the resulting delivery system displays suitable encapsulation features and antiproliferative activity for usage in the field of nanomedicine [65]. The activity of CYP450 enzymes is crucial for therapeutic effectiveness since it has a major impact on the drug's concentration in circulation and its metabolites. If a drug's efficacy is mostly determined by its original form (rather than its metabolites), inhibiting its corresponding CYP450 enzyme activity results in lower biotransformation activity, resulting in stronger drug effects or even overdose-induced toxicities. Induction of CYP450 enzyme activity, on the other hand, may result in a diminished drug effect and effectiveness [66]. Propolis is a combination of bioactive chemicals that are largely metabolized by the CYP450 family, and its impact on CYP450 is becoming more studied. As a result, it raises concerns about possible side effects from mixing propolis with other drugs owing to changes in CYP450 enzyme activity.
The activity levels of CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 in human hepatocytes were tested by Sasaki et al. [67]. A propolis-containing product, for instance, was shown to inhibit all five CYP450s by more than 50%. Naramoto et al. [68] examined studied blood concentrations of essential bioactive compounds in propolis to see whether they were high enough to elicit a clinical change in CYPs in the body. As the principal bioactive components, artepillin C, kaempferide, dihydrokaempferide, isosakuranetin, and kaempferol were shown to contribute to the CYP450 inhibitory activity of a standardized propolis extract (EEP-B55). The bioavailability of these main bioactive chemicals was then examined. Because of their low concentration in blood and hepatocytes, their ability to inhibit CYPs in the body was considered insignificant. Cusinato et al. [69] partially justified this hypothesis, revealing that the effects of propolis extract on CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A in healthy adult volunteers were not significant. Though multiple preclinical investigations showed that coincubation of propolis extract modified the CYP450 enzymes, the human trial revealed that the effect of propolis on CYP450 enzymes was modest due to the limited bioavailability of contributing compounds found in the propolis. Propolis phytoconstituents differ depending on the source. To guarantee consistent quality and effectiveness control of the propolis product, standardization of the chemical composition of propolis extract is required.
3. Molecular Mechanism of Anticancer Effect of Propolis and Its Bioactive Compounds
3.1. Effect of Propolis and Its Bioactive Compounds on Cancer Progression
Previous preclinical studies have reported that major compounds in the propolis such as flavonoids and polyphenols are responsible for its chemopreventive and anticancer effects [70, 71]. The anticarcinogenic activity of the flavonoid compounds in propolis can repress angiogenesis and induce apoptosis, thereby it act against tumor growth [72]. The chemopreventive effect of propolis in several cancer types is mediated by inhibiting tumor growth and induction of apoptosis [55] further, and in another way, it scavenges the reactive oxygen species and stimulates the activity of antioxidant enzymes [73]. Due to the side effects and drug resistance of anticancer drugs, the recent research on cancer is focusing on natural sources like propolis. The Algerian propolis extract-treated cells showed reduced cell proliferation and inhibited the cell adhesion by altering the fibrinogen in lung cancer [74].
The Wnt signaling is a primordial and evolutionarily conserved pathway that controls cell differentiation, proliferation, and migration [39]. The APC and Axin are Wnt pathway suppressors, and another downstream molecule β-catenin is involved in cancer progression. β-catenin gene mutation is found in many cancers. The CAPE administration in HCT16 and human colon cancer cell lines suppressed the Wnt signaling pathway [29]. Chrysin increases p21 protein expression by stimulating the p38 mitogen-activated protein kinase activity that led to the inhibition of the cell cycle and suppresses the cell proliferation in of C6 glioma [28]. Chrysin treatment in U937 cells (histiocytic lymphoma cells) showed induction of apoptosis by suppressing the PI3K/Akt signaling and inactivation of nuclear factor kappa B (NF-κB)/inhibitor of apoptosis (IAP), which led to the activation of caspase-3, which triggered apoptosis [75]. Another latest research reported that Turkey originated propolis extract can have antiproliferative, apoptotic, and cell cycle arrest potential against cancer cell lines. The presence of 3‐O‐methylquercetin, CAPE, galangin, chrysin, caffeic acid, and pinocembrin was implicated in the anticancer potential [76]. The CAPE exhibits cytotoxic and antiproliferative effects against breast cancer cell lines by increasing the endothelial nitric oxide synthases (e-NOS) and inducible nitric oxide synthase (i-NOS) levels [77]. The CAPE treatment showed the suppressed extracellular signal-regulated kinase (ERK) and Akt phosphorylation and decreased cyclin D expression in 3T3-L1 fibroblast cells [12]. Ethanol extract of propolis treatment significantly decreased the proliferation at 70 and 22 μg/ml in DU145 and PC3 cells. Also, it induces the cell cycle arrest by altering the cell cycle regulatory proteins including cyclins and cyclin-dependent kinases [78]. The effect of CAPE (75 μM/ml) on cell cycle-related gene expressions (CCND2, RB1, ATM, CDC34, and CDK5RAP1) was evaluated by real-time PCR in breast cancer cells. The CAPE significantly increased the expression of cell cycle regulatory genes when compared to control cells, thereby it may induce the cell cycle arrest in breast cancer [40].
The CAPE significantly repressed the activation of signal transducer and activator of transcription-3 (STAT-3) in HEp-2 cells. Further investigation using AG490, a selective STAT-3 inhibitor, demonstrated that CAPE not only suppressed STAT-3 but also suppressed the expression of PLK-1 in HEp-2 cells, indicating that STAT-3 was likely involved in the regulation of PLK-1 in HEp-2 cells. Furthermore, CAPE exposure caused a disruption of the cell cycle in the S phase in HEp-2 cells by inhibiting the STAT-3/PLK-1 pathway [45]. A recent study demonstrated that Chinese propolis (25, 50 & 100 μg/ml) treatment inhibits cell proliferation by targeting glycolysis enzymes and proinflammatory cytokines including TNF-α, IL-6, and NLRP3 in the breast cancer cells [79]. Reports from a recent study reveal that CAPE blocks the expression of the MALT1 gene to decrease the cell proliferation, invasion, and tumor growth of prostate carcinoma cells via the p53 and NF-κB signaling pathways, and they further verify that CAPE is an effective antitumor agent for human androgen-dependent and androgen-independent prostate carcinoma cells by inhibiting MALT1 expression in vitro and in vivo [80]. Overall, all of these studies suggested that propolis and its constituents inhibit cell proliferation, survival, and cell cycle by altering various signaling pathways (PI3K/Akt, MAPK, TNF-α/NF-κB, Wnt/β-catenin, and STAT-3/PLK-1) in several cancers (Figure 2).
Figure 2.
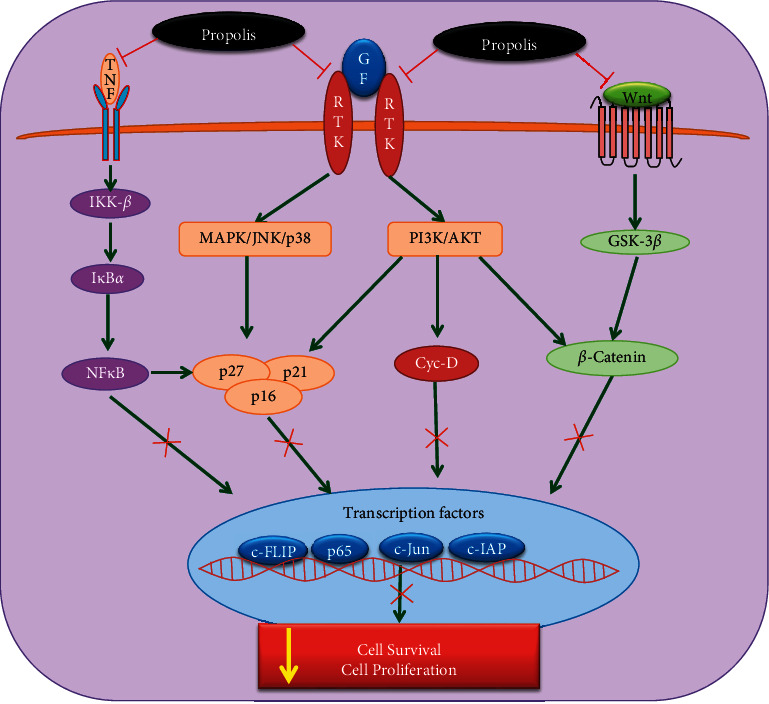
The molecular targets of propolis and its bioactive compounds in inhibition of cell proliferation and cell survival and induces the cell cycle arrest in cancer cells. The green arrow points out the upregulation of signaling molecules, while the red arrow indicates the downregulation of molecular targets. GF: growth factor, RTK: receptor tyrosine kinase, MAPK: mitogen-activated protein kinases, p38: p38 mitogen-activated protein kinases, JNK: c-Jun N-terminal kinase, PI3K: phosphoinositide 3-kinase, AKT: protein kinase B PAK1: p21-activated kinase-1, p16, p21, p27, and p16: cyclin-dependent kinase inhibitors, NFkB: nuclear factor-κB, IKK: inhibitor of NF-κB kinase, CDK: cyclin-dependent kinase, c-FLIP: cellular FLICE-inhibitory protein, c-IAP: cellular inhibitor of apoptosis protein 1.
The translation of preclinical data to a human application is the most critical aspect of any therapeutic's development. Propolis has been carefully studied for decades and has been utilized as a folk medicine for thousands of years. However, there is currently a lack of human clinical data, particularly in the field of cancer treatment. Propolis' possible preventive effect in breast cancer patients receiving chemotherapy and radiation was examined by Ebeid et al. [81]. This clinical trial comprised a total of 135 individuals. Propolis capsules (400 mg, 3 times daily) is consumed for 10 days before radiotherapy, 10 days during radiation treatment, and 10 days after irradiation. Propolis was reported to reduce the harmful effects of radiation in breast cancer patients, such as a rise in Comet tail parameters in peripheral blood mononuclear cells and serum malondialdehyde (MDA). Propolis also reduced radiotherapy-induced reductions in total antioxidant capacity, hemoglobin (Hb) concentration, white blood cells (WBCs), and platelet counts. Patients who used propolis supplements had a considerably longer median disease-free lifetime. This study found no adverse effects associated with propolis consumption [81].
Another pilot randomized clinical experiment was conducted to assess the safety, toxicities, and compliance with propolis in breast cancer patients receiving doxorubicin and cyclophosphamide, and test the preliminary clinical efficacy of propolis in the prevention of chemo-induced oral mucositis (OM) and prospectively assessing the incidence of OM. Sixty patients were randomly assigned to receive either a dry propolis extract containing 8%–12% galangin plus mouth rinsing with sodium bicarbonate or mouth rinsing with sodium bicarbonate. The incidence of OM was also studied throughout a 6-month period. Propolis with bicarbonate was shown to be safe, well tolerated, and effective in preventing OM in breast cancer patients [82].
Patients' nutritional health and quality of life suffer as a result of chemotherapy-related side effects. Propolis has been proposed as a coadjuvant nutritional supplement in cancer treatment due to its functional characteristics and biological activities, such as antitumoral activity, DNA protection, free radical scavenging, and immune stimulation; however, clinical trials to support these effects in cancer patients are required. The findings of a recent randomized, double-blind, and placebo-controlled clinical trial compared propolis to a placebo on nutritional status and quality of life in breast cancer patients after chemotherapy. Propolis has been indicated as a viable and safe therapeutic option for breast cancer patients receiving chemotherapy who want to improve their nutritional condition and quality of life [83]. Propolis, as an adjuvant therapy, may help to alleviate the symptoms of breast cancer. However, further clinical studies are urgently needed, particularly with a larger number of participants/subjects/patients.
3.2. Apoptosis-Inducing Potential of Propolis and Its Bioactive Compounds in Cancer Cells
Induction of apoptosis is a major mechanism proposed for the therapeutic effect of cancer drugs. Apoptosis is a programmed cell death regulated by two different pathways: 1. extrinsic pathway: activated by external factors like TNF-α (tumor necrosis factor-α), FasL (Fas ligand), and TNF-related apoptosis-inducing ligand (TRAIL); 2. intrinsic or mitochondrial-mediated pathway: this pathway is induced by ROS, which disrupts the mitochondrial membrane potential that leads to activation of proapoptotic proteins (p53, Bax/Bad, and cytochrome c) [84]. The anticancer potential of water-soluble derivatives of propolis (WSDP) from Brazil and Croatia was reported on MCF-7 (breast cancer), HeLa (cervical cancer), and V19 (lung fibroblast hamster) cells. Brazilian and Croatian propolis (50 μg/ml)-treated cells showed a significantly increased apoptotic percentage of MCF-7 and HeLa cells as compared to the control and V19 normal fibroblasts. The results from the above study demonstrated the effect of propolis in different cancer cells and normal fibroblast cells [85]. The antiproliferative/antitumor effect of propolis is studied in vitro and in vivo, where it showed its capability to suppress DNA synthesis in cancer cells and the capacity to promote apoptosis. The CAPE has the ability to promote apoptosis in breast cancer cell lines (MCF-7 and MDA-MB-231) [86]. Propolis extracts have shown apoptosis-promoting potential against diverse cancer cell lines such as HeLa [87], prostate adenocarcinoma [88], basophilic leukemia [89], and human breast [90], to study the activation of caspase and inhibition of mast cell granulation [91]. Ethanolic propolis extract (250 or 500 μg/ml) treatment induces apoptosis in C6 glioma cells by increasing the mRNA expression of caspase-3, caspase-8, and caspase-9. Also, it increases the total antioxidant levels and glutathione levels in glioma cells [92].
Propolis and CAPE inhibit the growth and migration of breast cancer cell lines. Furthermore, propolis modulates the inflammatory microenvironment and induces apoptosis by inhibiting TLR4 singling [93]. In another study, Turkish ethanolic propolis extract induces apoptosis by upregulating proapoptotic protein levels and alters the oncogenic and tumor suppressor gene expression in breast cancer cell lines [94]. A recent report suggested that red propolis along with L-lysine reduces tumor angiogenesis and tumor growth in hamster cheek pouch walker 256 cancer cell-inoculated cancer model [95]. Cuban propolis extracts and active compound nemorosone inhibit cell proliferation and induce apoptosis in doxorubicin-resistant colon cancer cells. These results showed that nemorosone alone was able to suppress cell proliferation. The combination of propolis extracts with nemorosone induces cell cycle arrest and decreases cell growth and also promotes apoptosis by ROS production and dissipation of mitochondrial membrane potential [96]. The chrysin acts as a possible replacement for the 5-FU and oxaliplatin combination to attain treatment strategy through autophagy for colorectal cancer therapy in the future [18]. The CAPE (1–30 μM) suppressed the growth of human multiple myeloma cells while leaving normal peripheral blood B cells unaffected. The CAPE induction of apoptosis in multiple myeloma cells was validated by flow cytometry, with up to 50% apoptotic cells induced by 50 μM CAPE within 24 h by activating the apoptosis executioner enzyme caspase-3 and corresponding cleavage of PARP. The oxidative stress generated by CAPE cytotoxicity in multiple myeloma cells was assessed using ROS and antioxidant levels. All of this evidence suggests that CAPE induces apoptosis in human multiple myeloma cells via oxidative stress [59].
The anticancer potential of Chinese propolis was studied on human breast cancer cells. Exposure to ethanolic Chinese propolis extract (EECP) significantly increased annexin A7 expression, ROS, NF-κB, and p65 expressions and dramatically altered the potential of mitochondrial membrane [86]. Propolis, chrysin, and CAPE promote apoptosis in various cancer cells through both extrinsic and intrinsic apoptotic signaling (Figure 3). Neurofibromatosis (NF) is a genetic disease due to the defects in the NF1/NF2 gene. It shows an abnormal activation of an oncogenic kinase named PAK1, where the growth of both NF1 and NF2 tumors requires PAK1, and all PAK1 blockers, synthetic chemicals (the ring peptide FK228, UnPAK309 (PF3758309), a combination of two tyrosine-kinase inhibitors, PP1 and AG 879/GL-2003, and ivermectin), or natural products like curcumin, propolis, bitter melon, and berberine suppress the growth of these NF tumor cells in vitro and in vivo mice model [97]. Out of this, propolis extracts showed a boost in their therapeutic effect for NF patients without any side effects [98–100]. The ethanolic extract of Brazilian red propolis exhibits a strong cytotoxicity effect on breast cancer cells (MCF-7) and triggers apoptosis in these cells [101]. The combination treatment of propolis with radiation therapy prevents the leukocyte's DNA damage during the ionizing radiation in patients [102]. CAPE's cytotoxic and apoptotic effects on the RKO colorectal cancer cell line and the CCD 841-CoN normal colorectal cell line were recently examined. They discovered that CAPE caused apoptotic cell death in around 40% of the RKO cells. Furthermore, CAPE exposure increased p53 phosphorylation at Serine 15 and Serine 46 while decreasing survivin expression. The study indicated that CAPE promoted apoptosis through modulating p53 phosphorylation, resulting in the reduction of survivin expression, and that CAPE may be considered as an alternative treatment in cancer therapy [43].
Figure 3.
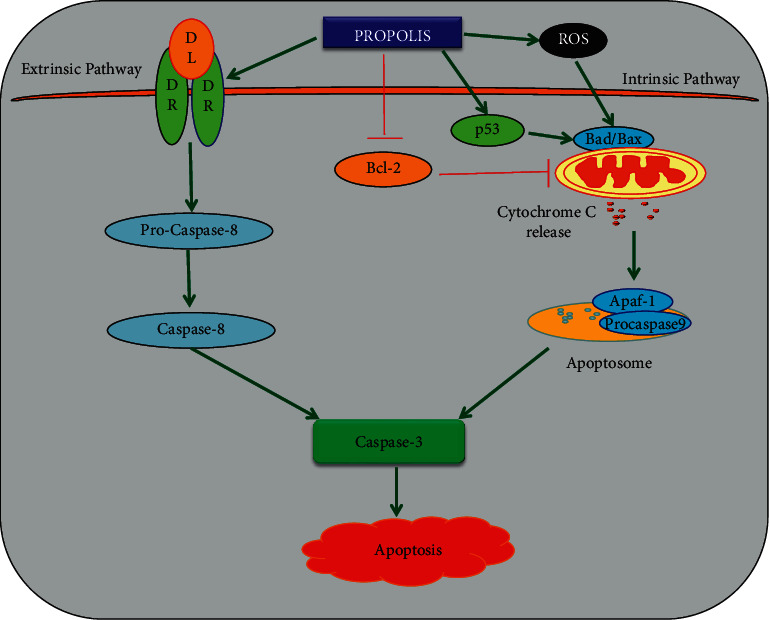
Propolis and its bioactive compounds induce apoptosis in various cancer cells through extrinsic and intrinsic apoptotic pathways. The green arrow points out the upregulation of signaling molecules, while the red arrow indicates the downregulation of molecular targets. DL: death ligand, DR: death receptor, TNF-α: tumor necrosis factor, FasL: Fas ligand, TRAIL: TNF-related apoptosis-inducing ligand, Bcl-2: B-cell lymphoma-2, Bad: Bcl-2-associated death promoter, Bax: Bcl-2-like protein, Apaf-1; apoptotic protease-activating factor 1.
The CAPE treatment showed strong cytotoxic activity on NPC cell lines. It also promotes apoptosis in NPC cells by downregulating the expression of antiapoptotic proteins Bcl-xL, elevating the expression of proapoptotic proteins Bax, and generating PARP cleavage [46]. Another study examined the anticancer properties of the CAPE analogue chemical 5A in nasopharyngeal carcinoma (NPC) cells. They discovered that chemical 5A effectively suppressed cell proliferation while having negligible cytotoxicity in normal cells. Moreover, compound 5A was discovered to promote cell cycle arrest and apoptosis in CNE2 cells by inhibiting the expression of EGFR downstream signaling molecules in NPC cells [44]. Egyptian propolis contains a high level of flavonoids, phenolics, and dihydroflavonoids in propolis extract, and it has anticancer effects in vitro studies and inhibits tumor growth in Ehrlich ascites carcinoma (EAC) in mice model. Propolis extract in combination with methotrexate stimulates the G0/G1 phase cell cycle arrest and induces apoptosis [8]. The latest study demonstrated that 25 to 100 μg/ml of Chinese propolis-treated cells showed increased ROS generation and altered mitochondrial membrane potential, thereby it induced apoptosis in breast cancer cells [79].
Docetaxel with CAPE inhibited the proliferation and survival of docetaxel-resistant prostate cancer cells via inhibiting Bcl-2 and c-Myc and induced metabolic interference and apoptosis. Patients with docetaxel-resistant prostate cancer may benefit from a combination therapy [103].
Overall, all of these above research reported that propolis and its constituents induce both death ligand-mediated (Extrinsic) apoptotic pathway and mitochondrial-mediated (intrinsic) apoptotic pathway in various cancer cells by modulating the several apoptosis-related signaling molecules such as TRAIL, FasL, TNF-α/DR, p53, Bax, Bcl-2, Bcl-xL, caspases, and PARP (Figure 3).
3.3. Effect of Propolis and Its Bioactive Compounds on Angiogenesis and Cancer Metastasis
Angiogenesis or neovascularization is the development of fresh blood vessels from present ones, and this process is very important to regulate the growth and maintenance of metastatic tumors [104]. Angiogenesis is a very complicated process, which stimulates cell proliferation, migration, and invasion [105]. Therefore, tumor development is mainly dependent on the angiogenesis process. Lately, numerous studies have reported that the inhibitor for angiogenesis is evolved to treat cancer by targeting the multiple molecules and signaling pathways, which are regulating the angiogenesis process [106]. However, the commercially existing drugs are only effective against certain types of tumor due to the complexity of cancer, particularly cancer signaling and angiogenesis [107]. Angiogenesis is regulated via various signaling pathways, which are extremely complex and involve numerous angiogenic mediators. The important growth factors and mediators of angiogenesis include VEGF (vascular endothelial growth factor), PDGF (platelet-derived growth factor), FGF (fibroblast growth factor), EGF (epidermal growth factor), integrins, angiopoietins, endothelins, notch, ephrins, and cadherins [108]. Cancer metastasis is a process involving cell invasion, cell migration, cell adhesion, extracellular matrix (ECM), and degradation of basement membrane by proteinase enzymes. Matrix metalloproteinases (MMPs) such as MMP-2 and MMP-9 and intercellular adhesion molecule (ICAM) play important role in proteolytic degradation. Many studies have already showed the overexpression of MMPs in cancer tissues including breast, colon, lung, and pancreatic cancer. Also, uPA (urokinase-type plasminogen activator) is a serine protease, involved in proteolytic degradation during cancer invasion, migration, and metastasis [109]. Recently, Li et al. [79] reported that Chinese propolis (25 to 100 μg/ml)-treated breast cancer cells showed decreased migration and invasion by targeting glycolysis enzymes and proinflammatory cytokines (TNF-α, IL-6, and NLRP3) in breast cancer cells.
Hwang et al. [110] have reported the impact of CAPE treatment on fibrosarcoma cells' (HT1080) invasion and metastasis. The results showed that CAPE treatment can suppress mRNA and protein expression of MMP-2 and MMP-9 in fibrosarcoma cells. Also, the mRNA expression of tissue inhibitor of matrix metalloproteinases (TIMPs) and MT-1 MMPs was decreased in CAPE-treated cells. Finally, it inhibits the colony formation potential, cell invasion, and migration. Cuban propolis (83 μg/ml) suppresses cell migration and invasion by inhibiting MMP-9 activity, β-catenin, vimentin expression, and decreased E-cadherin expression in human colorectal cancer cells [111]. Red propolis produces antioxidant effects, also inhibits angiogenesis through the modulation of angiogenic factors and inflammation, and reduces the levels of VEGF and HIF-1α, and this shows the relationship between angiogenesis, oxidative stress, and tumor hypoxia [112, 113]. The association between the antiangiogenic and antioxidant effects of propolis was evaluated in vitro using endothelial cells; unsurprisingly, the most antiangiogenic compounds also had antioxidant properties [114]. Brazilian red propolis, a water-insoluble resinous mixture of the saliva of bees (Apis mellifera) and vegetable exudate, mainly from Dalbergia ecastaphyllum (L) Taub, has strong antioxidant activity and has been investigated and proposed as an inhibitor of angiogenesis [34, 115].
Another study demonstrated that red propolis and active compounds can alter the progression of carcinogenesis and induce cytotoxicity to lineages of tumor cells in vitro [116]. However, a study with tumor cell lineages revealed that different concentrations of red propolis are associated with different profiles of cytotoxicity [117]. Previous research has reported that the WSDP may have many biological effects including antitumor [118, 119], antioxidant [120], immunomodulatory [121], anti-infectious, and anti-inflammatory [122], and also, they identified the constituents of propolis (artepillin C, caffeic acid, and caffeic acid phenethyl ester) and studied their anticarcinogenic effects [123, 124]. Dornelas et al. [125] have extracted WSDP from soluble propolis using 8% L-lysine. They have reported that green propolis WSDP may inhibit angiogenesis in N-butyl-(-4-hydroxybutyl) nitrosamine-stimulated bladder cancer model in rats, while L-lysine treatment induces angiogenesis if started simultaneously with N-butyl-(-4-hydroxybutyl) nitrosamine.
Jung and co-workers conducted an extensive study using caffeic acid, in which caffeic acid inhibited the tube formation in HUVEC (human umbilical vein endothelial) cells in 3D culture, and also, this treatment inhibited the angiogenesis of human kidney tumor implanted in nude mice. Furthermore, they found the reduction of VEGF and slowdown of tumor growth by inhibiting the STAT phosphorylation and decreasing the HIF-1-mediated expression of VEGF [126]. In another study, Brazilian green propolis and its active compound caffeic acid derivatives inhibited VEGF-induced cell migration and tube formation in HUVECs [127]. Caffeic acid inhibits human retinal tumor angiogenesis by blocking VEGF expression through STAT-3 [112]. All of the above studies suggest that the caffeoyl group's compounds in propolis may be the reason for its anticancer activities. Chinese red propolis and CAPE displayed a solid inhibitory effect in VEGF-mediated angiogenesis. The study suggested that Chinese red propolis and CAPE may act as a strong antiangiogenic therapeutic agents for human diseases [112]. Liao et al. [128] described that CAPE may be a potential inhibitor for angiogenesis and also suppress neovascularization. The VEGF expression significantly decreased in CAPE-treated colon cancer cells (CT26) and also inhibited the synthesis of prostaglandin E2. All of these studies have reported the anticancer activities of propolis and its well-known active components including caffeic acid, CAPE, chrysin, and artepillin C. Most in vitro and in vivo studies demonstrated that propolis targets various growth factor signaling, thereby it inhibits cell proliferation, cell cycle progression, cancer cell migration, and invasion and induces apoptosis in cancer cells. In a recent study, we demonstrate that a low-dose combination of Wi-A and CAPE causes reversal of EMT by the upregulation of E-cadherin and Claudin1, yielding inhibition of Wnt/β-catenin, vimentin, MMPs, VEGF, and VEGFR signaling pathways in cervical and breast cancer cells [129].
According to another recent study, the expression of GSK-3β suppressed Snail and the disruption of HDAC6-mediated vimentin protein stability inhibits the cell migration and invasion of TNBC by propolin G. Propolin G, which targets EMT, might be a contender for TNBC treatment [130]. Furthermore, Niyomtham et al. discovered that the ethyl acetate extract of propolis (EAEP) was cytotoxic and caused apoptosis in HNSCC cell lines. In addition, EAEP inhibited HNSCC cell invasion by lowering MMP-2 and MMP-9 activities. HPLC-ESI-TOF-MS was used to identify two flavonoids in EAEP: galangin and apigenin. The findings imply that EAEP causes apoptosis and has anti-invasion properties in HNSCC cell lines via reducing MMP-2 and MMP-9 activities. Galangin and apigenin may be responsible for these inhibitory actions [131]. In another study, CAPE hinders the migratory and invasive ability of NPC cells by reversing the epithelial-mesenchymal transition (EMT) pathway by modulating the EMT marker protein expression. The CAPE exposure upregulates the E-cadherin expression and downregulates the mesenchymal marker (vimentin, β-catenin, and MMP) in NPC cells [46]. Another recent study demonstrated that Chinese propolis (12.5 μg/ml) inhibited Panc-1 cell migration by modulating the epithelial-mesenchymal transition. Interestingly, after CP treatment, the Hippo pathway was stimulated in Panc-1 cells, providing as a mechanism for CP's antipancreatic cancer activity [132]. Overall, the studies have found that propolis and its constituents inhibit cell migration, epithelial-to-mesenchymal transition, and tumor metastasis by regulating several signaling molecules such as growth factors (EGF, VEGF, PDGF, HIF-1, and FGF), EMT markers (vimentin, E-cadherin, β-catenin, and Snail), and metastatic-related proteins (MMPs, uPA, and interleukin).
4. Summary and Conclusions
Overall, the present review highlights the anticancer activities and molecular targets of propolis in different cancer cells. The chemical composition of propolis differs greatly among species of bees and depends on geographical and climatic factors, plant resources, and collecting seasons, and it requires a different experimental approach to evaluate their biological potential. We summarized the inhibitory effect of propolis on cancer cell proliferation and metastasis-related signaling pathways, and also propolis induces apoptosis by targeting both extrinsic and mitochondrial-mediated apoptotic signaling pathways. Propolis has active ingredients such as CAPE, chrysin, and artepillin C. The antioxidant, antiproliferative, antiangiogenic, and antimetastatic potential of propolis were attributed to the presence of the above active compounds. Propolis' anticancer mechanisms are diverse, since it operates on a variety of cancer metabolic targets. Preventing metastatic spread, blocking nuclear localization of NF-κB, gene expression regulation, inactivation of MMP, and activation of tumor suppressors, and overcoming TRAIL resistance of cancer cells have all been identified as critical pathways. Clinical trials require standardized quality control and perfect design, and metabolomics is widely regarded to help achieve these goals. With the right research, novel, low-cost cancer therapies can be produced from propolis. The bioactive compounds of propolis, and the target molecules involved, must be investigated further in order to create novel cancer therapeutics and overcome the problem of chemotherapy resistance. Therefore, propolis, a natural compound, might be suggested for the combination therapy to reduce the adverse effect of chemotherapy. However, further clinical studies are needed to validate the ratio of propolis compounds in human subjects and also allergenic reactions to propolis, and its dosage merits require further research.
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Sung H., Ferlay J., Siegel R. L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians . 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadi A., Mohagheghi M., Karimi M., et al. Therapeutic effects of HESA-A in patients with end-stage metastatic cancers. Integrative Cancer Therapies . 2010;9(1):32–35. doi: 10.1177/1534735409357934. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z. M., Zhu X. Y., Song F. Z. Molecular mechanism of apoptosis induced by PsL5F in human anaplastic thyroid carcinoma FRO cells. Sichuan Da Xue Xue Bao Yi Xue Ban . 2009;40(6):1015–1020. [PubMed] [Google Scholar]
- 4.Bukovsky A. Involvement of blood mononuclear cells in the infertility, age-associated diseases and cancer treatment. World Journal of Stem Cells . 2016;8(12):p. 399. doi: 10.4252/wjsc.v8.i12.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdulridha M. K., Al-Marzoqi A. H., Al-Awsi G. R. L., Mubarak S. M. H., Heidarifard M., Ghasemian A. Anticancer effects of herbal medicine compounds and novel formulations: a literature review. Journal of Gastrointestinal Cancer . 2020;51(3):765–773. doi: 10.1007/s12029-020-00385-0. [DOI] [PubMed] [Google Scholar]
- 6.Cao B., Li S. T., Li Z., Deng W. L. Yiqi zhuyu decoction combined with FOLFOX-4 as first-line therapy in metastatic colorectal cancer. Chinese Journal of Integrative Medicine . 2011;17(8):593–599. doi: 10.1007/s11655-011-0822-z. [DOI] [PubMed] [Google Scholar]
- 7.Akaza H., Moore M. A., Chang S. J., et al. The 5th conference on asian trends in prostate cancer hormone therapy. Asian Pacific Journal of Cancer Prevention . 2007;8(1):3–12. [PubMed] [Google Scholar]
- 8.AkhavanKarbassi M. H., Yazdi M. F., Ahadian H., SadrAbad M. J. Randomized double blind placebo controlled trial of propolis for oral mucositis in patients receiving chemotherapy for head and neck cancer. Asian Pacific Journal of Cancer Prevention . 2016;17(7):3611–3614. [PubMed] [Google Scholar]
- 9.Ao M., Xiao X., Li Q. Efficacy and safety of compound Kushen injection combined with chemotherapy on postoperative patients with breast cancer: a meta-analysis of randomized controlled trials. Medicine (Baltimore) . 2019;98(3) doi: 10.1097/md.0000000000014024.e14024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan T., Gurav P. PhytoNanotechnology: enhancing delivery of plant based anti-cancer drugs. Frontiers in Pharmacology . 2017;8:p. 1002. doi: 10.3389/fphar.2017.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K. H. Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. Journal of Natural Products . 2010;73(3):500–516. doi: 10.1021/np900821e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi S. M., Tucker D. F., Gross D. N., et al. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Molecular and Cellular Biology . 2010;30(21):5009–5020. doi: 10.1128/mcb.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murtaza G., Sajjad A., Mehmood Z., Shah S. H., Siddiqi A. R. Possible molecular targets for therapeutic applications of caffeic acid phenethyl ester in inflammation and cancer. Journal of Food and Drug Analysis . 2015;23(1):11–18. doi: 10.1016/j.jfda.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta T., Kunimasa K., Kobayashi T., Sakamoto M., Kaji K. Propolis suppresses tumor angiogenesis by inducing apoptosis in tube-forming endothelial cells. Bioscience Biotechnology and Biochemistry . 2008;72(9):2436–2440. doi: 10.1271/bbb.80169. [DOI] [PubMed] [Google Scholar]
- 15.Song Y. S., Park E. H., Hur G. M., et al. Caffeic acid phenethyl ester inhibits nitric oxide synthase gene expression and enzyme activity. Cancer Letters . 2002;175(1):53–61. doi: 10.1016/s0304-3835(01)00787-x. [DOI] [PubMed] [Google Scholar]
- 16.Bouchelaghem S. Propolis characterization and antimicrobial activities against Staphylococcus aureus and Candida albicans: a review. Saudi Journal of Biological Sciences . 2022;29(4):1936–1946. doi: 10.1016/j.sjbs.2021.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnavacca A., Sangiovanni E., Racagni G., Dell’Agli M. The antiviral and immunomodulatory activities of propolis: an update and future perspectives for respiratory diseases. Medicinal Research Reviews . 2022;42(2):897–945. doi: 10.1002/med.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y. M., Chen C. I., Hsiang Y. P., et al. Chrysin attenuates cell viability of human colorectal cancer cells through autophagy induction unlike 5-fluorouracil/oxaliplatin. International Journal of Molecular Sciences . 2018;19(6):p. 1763. doi: 10.3390/ijms19061763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurek-Gorecka A., Keskin S., Bobis O., et al. Comparison of the antioxidant activity of propolis samples from different geographical regions. Plants . 2022;11(9):p. 1203. doi: 10.3390/plants11091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Seedi H. R., Eid N., Abd El-Wahed A. A., et al. Honey bee products: preclinical and clinical studies of their anti-inflammatory and immunomodulatory properties. Frontiers in Nutrition . 2021;8 doi: 10.3389/fnut.2021.761267.761267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omar H. A., Tolba M. F. Caffeic acid phenethyl ester guards against benign prostate hypertrophy in rats: role of IGF-1R/protein kinase-B (Akt)/β-catenin signaling. IUBMB Life . 2018;70(6):519–528. doi: 10.1002/iub.1743. [DOI] [PubMed] [Google Scholar]
- 22.Mehany A. B. M., Belal A., Mohamed A. F., Shaaban S., Abdelhamid G. Apoptotic and anti-angiogenic effects of propolis against human bladder cancer: molecular docking and in vitro screening. Biomarkers . 2022;27(2):138–150. doi: 10.1080/1354750x.2021.2020903. [DOI] [PubMed] [Google Scholar]
- 23.Cetin E. O., Salmanoglu D. S., Ozden I., et al. Preparation of ethanol extract of propolis loaded niosome formulation and evaluation of effects on different cancer cell lines. Nutrition and Cancer . 2022;74(1):265–277. doi: 10.1080/01635581.2021.1876889. [DOI] [PubMed] [Google Scholar]
- 24.Bankova V. Recent trends and important developments in propolis research. Evidence-Based Complementary and Alternative Medicine . 2005;2005:4. doi: 10.1093/ecam/neh059.108379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasupuleti V. R., Sammugam L., Ramesh N., Gan S. H., Honey P., Jelly R. Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxidative Medicine and Cellular Longevity . 2017;2017:21. doi: 10.1155/2017/1259510.1259510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akyol S., Ginis Z., Armutcu F., Ozturk G., Yigitoglu M. R., Akyol O. The potential usage of caffeic acid phenethyl ester (CAPE) against chemotherapy-induced and radiotherapy-induced toxicity. Cell Biochemistry and Function . 2012;30(5):438–443. doi: 10.1002/cbf.2817. [DOI] [PubMed] [Google Scholar]
- 27.Kurek-Gorecka A., Rzepecka-Stojko A., Gorecki M., Stojko J., Sosada M., Swierczek-Zieba G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules . 2013;19(1):78–101. doi: 10.3390/molecules19010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weng M. S., Ho Y. S., Lin J. K. Chrysin induces G1 phase cell cycle arrest in C6 glioma cells through inducing p21Waf1/Cip1 expression: involvement of p38 mitogen-activated protein kinase. Biochemical Pharmacology . 2005;69(12):1815–1827. doi: 10.1016/j.bcp.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Wang D., Xiang D. B., He Y. J., et al. Effect of caffeic acid phenethyl ester on proliferation and apoptosis of colorectal cancer cells in vitro. World Journal of Gastroenterology . 2005;11(26):p. 4008. doi: 10.3748/wjg.v11.i26.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dornelas C. A., Fechine-Jamacaru F. V., Albuquerque I. L., et al. Chemoprevention with green propolis green propolis extracted in L-lysine versus carcinogenesis promotion with L-lysine in N-Butyl-N-[4-hydroxybutyl] nitrosamine (BBN) induced rat bladder cancer. Acta Cirurgica Brasileira . 2012;27(2):185–192. doi: 10.1590/s0102-86502012000200015. [DOI] [PubMed] [Google Scholar]
- 31.Ahangari Z., Naseri M., Vatandoost F. Propolis: chemical composition and its applications in endodontics. Iranian Endodontic Journal . 2018;13(3):285–292. doi: 10.22037/iej.v13i3.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahinler N., Kaftanoglu O. Natural product propolis: chemical composition. Natural Product Research . 2005;19(2):183–188. doi: 10.1080/14786410410001704877. [DOI] [PubMed] [Google Scholar]
- 33.Thirugnanasampandan R., Raveendran S. B., Jayakumar R. Analysis of chemical composition and bioactive property evaluation of Indian propolis. Asian Pacific Journal of Tropical Biomedicine . 2012;2(8):651–654. doi: 10.1016/s2221-1691(12)60114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.da Silva Frozza C. O., Garcia C. S. C., Gambato G., et al. Chemical characterization, antioxidant and cytotoxic activities of Brazilian red propolis. Food and Chemical Toxicology . 2013;52:137–142. doi: 10.1016/j.fct.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Hsu T. H., Chu C. C., Hung M. W., Lee H. J., Hsu H. J., Chang T. C. Caffeic acid phenethyl ester induces E2F-1-mediated growth inhibition and cell-cycle arrest in human cervical cancer cells. FEBS Journal . 2013;280(11):2581–2593. doi: 10.1111/febs.12242. [DOI] [PubMed] [Google Scholar]
- 36.Hattori H., Okuda K., Murase T., et al. Isolation, identification, and biological evaluation of HIF-1-modulating compounds from Brazilian green propolis. Bioorganic & Medicinal Chemistry . 2011;19(18):5392–5401. doi: 10.1016/j.bmc.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 37.Popova M., Giannopoulou E., Skalicka-Wozniak K., et al. Characterization and biological evaluation of propolis from Poland. Molecules . 2017;22(7):p. 1159. doi: 10.3390/molecules22071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khacha-Ananda S., Tragoolpua K., Chantawannakul P., Tragoolpua Y. Antioxidant and anti-cancer cell proliferation activity of propolis extracts from two extraction methods. Asian Pacific Journal of Cancer Prevention . 2013;14(11):6991–6995. doi: 10.7314/apjcp.2013.14.11.6991. [DOI] [PubMed] [Google Scholar]
- 39.Cadigan K. M., Nusse R. Wnt signaling: a common theme in animal development. Genes & Development . 1997;11(24):3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 40.Balc-Okcanoglu T., Yilma-Susluer S., Kayabasi C., Ozme-Yelken B., Biray-Avci C., Gunduz C. The effect of caffeic acid phenethyl ester on cell cycle control gene expressions in breast cancer cells. Molecular Biology Research Communications . 2021;10(1):39–43. doi: 10.22099/mbrc.2020.38811.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taira N., Nguyen B. C. Q., Be Tu P. T., Tawata S. Effect of Okinawa propolis on PAK1 activity, Caenorhabditis elegans longevity, melanogenesis, and growth of cancer cells. Journal of Agricultural and Food Chemistry . 2016;64(27):5484–5489. doi: 10.1021/acs.jafc.6b01785. [DOI] [PubMed] [Google Scholar]
- 42.Kumazawa S., Ueda R., Hamasaka T., Fukumoto S., Fujimoto T., Nakayama T. Antioxidant prenylated flavonoids from propolis collected in Okinawa, Japan. Journal of Agricultural and Food Chemistry . 2007;55(19):7722–7725. doi: 10.1021/jf071187h. [DOI] [PubMed] [Google Scholar]
- 43.Sari C., Sümer C., Upoglu F. Caffeic acid phenethyl ester induces apoptosis in colorectal cancer cells via inhibition of survivin. Turkish Journal of Biology . 2020;44(5):264–274. doi: 10.3906/biy-2003-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ning X., Ren X., Xie X., Yan P., Wang D., Huang X. A caffeic acid phenethyl ester analog inhibits the proliferation of nasopharyngeal carcinoma cells via targeting epidermal growth factor receptor. Journal of Biochemical and Molecular Toxicology . 2020;34(7) doi: 10.1002/jbt.22491.e22491 [DOI] [PubMed] [Google Scholar]
- 45.Ren X., Liu J., Hu L., Liu Q., Wang D., Ning X. Caffeic acid phenethyl ester inhibits the proliferation of HEp2 cells by regulating stat3/plk1 pathway and inducing S phase arrest. Biological and Pharmaceutical Bulletin . 2019;42(10):1689–1693. doi: 10.1248/bpb.b19-00315. [DOI] [PubMed] [Google Scholar]
- 46.Liang Y., Feng G., Wu L., et al. Caffeic acid phenethyl ester suppressed growth and metastasis of nasopharyngeal carcinoma cells by inactivating the NF-κB pathway. Drug Design, Development and Therapy . 2019;13:1335–1345. doi: 10.2147/dddt.s199182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shahinozzaman M., Taira N., Ishii T., Halim M. A., Hossain M. A., Tawata S. Anti-inflammatory, anti-diabetic, and anti-alzheimer’s effects of prenylated flavonoids from okinawa propolis: an investigation by experimental and computational studies. Molecules . 2018;23(10):p. 2479. doi: 10.3390/molecules23102479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elnakady Y. A., Rushdi A. I., Franke R., et al. Characteristics, chemical compositions and biological activities of propolis from Al-Bahah, Saudi Arabia. Scientific Reports . 2017;7(1):p. 41453. doi: 10.1038/srep41453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gismondi A., Canuti L., Grispo M., Canini A. Biochemical composition and antioxidant properties of lavandula angustifolia miller essential oil are shielded by propolis against UV radiations. Photochemistry and Photobiology . 2014;90(5):p. 1214. doi: 10.1111/php.12339. [DOI] [PubMed] [Google Scholar]
- 50.Jonker J. W., Buitelaar M., Wagenaar E., et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proceedings of the National Academy of Sciences of the United States of America . 2002;99(24):15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrientos L., Herrera C. L., Montenegro G., et al. Chemical and botanical characterization of chilean propolis and biological activity on cariogenic bacteria streptococcus mutans and streptococcus sobrinus. Brazilian Journal of Microbiology . 2013;44(2):577–585. doi: 10.1590/s1517-83822013000200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dantas Silva R. P., Machado B. A. S., Barreto G. d. A., et al. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS One . 2017;12(3) doi: 10.1371/journal.pone.0172585.e0172585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saewan N., Jimtaisong A. Natural products as photoprotection. Journal of Cosmetic Dermatology . 2015;14(1):47–63. doi: 10.1111/jocd.12123. [DOI] [PubMed] [Google Scholar]
- 54.Kuo C. C., Wang R. H., Wang H. H., Li C. H. Meta-analysis of randomized controlled trials of the efficacy of propolis mouthwash in cancer therapy-induced oral mucositis. Supportive Care in Cancer . 2018;26(12):4001–4009. doi: 10.1007/s00520-018-4344-5. [DOI] [PubMed] [Google Scholar]
- 55.Valente M. J., Baltazar A. F., Henrique R., Estevinho L., Carvalho M. Biological activities of Portuguese propolis: protection against free radical-induced erythrocyte damage and inhibition of human renal cancer cell growth in vitro. Food and Chemical Toxicology . 2011;49(1):86–92. doi: 10.1016/j.fct.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Sobocanec S., Sverko V., Balog T., et al. Oxidant/antioxidant properties of Croatian native propolis. Journal of Agricultural and Food Chemistry . 2006;54(21):8018–8026. doi: 10.1021/jf0612023. [DOI] [PubMed] [Google Scholar]
- 57.Ramana K. V., Reddy A. B. M., Majeti N. V. R. K., Singhal S. S. Therapeutic potential of natural antioxidants. Oxidative Medicine and Cellular Longevity . 2018;2018:3. doi: 10.1155/2018/9471051.9471051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berretta A. A., Silveira M. A. D., Capcha J. M. C., De Jong D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: running title: propolis against SARS-CoV-2 infection and COVID-19. Biomedicine & Pharmacotherapy . 2020;131 doi: 10.1016/j.biopha.2020.110622.110622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marin E. H., Paek H., Li M., et al. Caffeic acid phenethyl ester exerts apoptotic and oxidative stress on human multiple myeloma cells. Investigational New Drugs . 2019;37(5):837–848. doi: 10.1007/s10637-018-0701-y. [DOI] [PubMed] [Google Scholar]
- 60.Curti V., Zaccaria V., Sokeng A. J. T., et al. Bioavailability and in vivo antioxidant activity of a standardized polyphenol mixture extracted from brown propolis. International Journal of Molecular Sciences . 2019;20(5):p. 1250. doi: 10.3390/ijms20051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews . 1997;23(1–3):3–25. doi: 10.1016/s0169-409x(96)00423-1. [DOI] [PubMed] [Google Scholar]
- 62.Wagh V. D. Propolis: a wonder bees product and its pharmacological potentials. Advances in Pharmacological Sciences . 2013;2013:11. doi: 10.1155/2013/308249.308249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimoto T., Koya S., Hino K., et al. Renal carcinogenesis induced by ferric nitrilotriacetate in mice, and protection from it by Brazilian propolis and artepillin C. Pathology International . 2000;50(9):679–689. doi: 10.1046/j.1440-1827.2000.01097.x. [DOI] [PubMed] [Google Scholar]
- 64.Kimoto T., Koya-Miyata S., Hino K., et al. Pulmonary carcinogenesis induced by ferric nitrilotriacetate in mice and protection from it by Brazilian propolis and artepillin C. Virchows Archiv . 2001;438(3):259–270. doi: 10.1007/s004280000350. [DOI] [PubMed] [Google Scholar]
- 65.Mendez-Pfeiffer P., Juarez J., Hernandez J., et al. Polymeric nanoparticles for the delivery of sonoran desert propolis: synthesis, characterization and antiproliferative activity on cancer cells. Colloids and Surfaces B: Biointerfaces . 2022;215 doi: 10.1016/j.colsurfb.2022.112475.112475 [DOI] [PubMed] [Google Scholar]
- 66.Zhou X., Fu L., Wang P., Yang L., Zhu X., Li C. G. Drug-herb interactions between Scutellaria baicalensis and pharmaceutical drugs: insights from experimental studies, mechanistic actions to clinical applications. Biomedicine & Pharmacotherapy . 2021;138 doi: 10.1016/j.biopha.2021.111445.111445 [DOI] [PubMed] [Google Scholar]
- 67.Sasaki T., Sato Y., Kumagai T., Yoshinari K., Nagata K. Effect of health foods on cytochrome P450-mediated drug metabolism. Journal of Pharmaceutical Health Care and Sciences . 2017;3(1) doi: 10.1186/s40780-017-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naramoto K., Kato M., Ichihara K. Effects of an ethanol extract of Brazilian green propolis on human cytochrome P450 enzyme activities in vitro. Journal of Agricultural and Food Chemistry . 2014;62(46):11296–11302. doi: 10.1021/jf504034u. [DOI] [PubMed] [Google Scholar]
- 69.Cusinato D. A. C., Martinez E. Z., Cintra M. T. C., et al. Evaluation of potential herbal-drug interactions of a standardized propolis extract (EPP-AF®) using an in vivo cocktail approach. Journal of Ethnopharmacology . 2019;245 doi: 10.1016/j.jep.2019.112174.112174 [DOI] [PubMed] [Google Scholar]
- 70.Doi K., Fujioka M., Sokuza Y., et al. Chemopreventive action by ethanol-extracted Brazilian green propolis on post-initiation phase of inflammation-associated rat colon tumorigenesis. In Vivo . 2017;31(2):187–198. doi: 10.21873/invivo.11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kasala E. R., Bodduluru L. N., Madana R. M., Gogoi R., Barua C. C. Chemopreventive and therapeutic potential of chrysin in cancer: mechanistic perspectives. Toxicology Letters . 2015;233(2):214–225. doi: 10.1016/j.toxlet.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Sun L. P., Xu X., Hwang H. H., Wang X., Su K. Y., Chen Y. L. S. Dichloromethane extracts of propolis protect cell from oxygen-glucose deprivation-induced oxidative stress via reducing apoptosis. Food & Nutrition Research . 2016;60(1) doi: 10.3402/fnr.v60.30081.30081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orsolic N., Car N., Lisicic D., et al. Synergism between propolis and hyperthermal intraperitoneal chemotherapy with cisplatin on ehrlich ascites tumor in mice. Journal of Pharmaceutical Sciences . 2013;102(12):4395–4405. doi: 10.1002/jps.23755. [DOI] [PubMed] [Google Scholar]
- 74.Teerasripreecha D., Phuwapraisirisan P., Puthong S., et al. In vitro antiproliferative/cytotoxic activity on cancer cell lines of a cardanol and a cardol enriched from thai Apis mellifera propolis. BMC Complementary and Alternative Medicine . 2012;12(1):p. 27. doi: 10.1186/1472-6882-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woo K. J., Jeong Y. J., Park J. W., Kwon T. K. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochemical and Biophysical Research Communications . 2004;325(4):1215–1222. doi: 10.1016/j.bbrc.2004.09.225. [DOI] [PubMed] [Google Scholar]
- 76.Coban E. A., Tecimel D., Sahin F., Deniz A. A. H. Targeting cancer metabolism and cell cycle by plant-derived compounds. Advances in Experimental Medicine & Biology . 2020;1247:125–134. doi: 10.1007/5584_2019_449. [DOI] [PubMed] [Google Scholar]
- 77.Samec M., Liskova A., Kubatka P., et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. Journal of Cancer Research and Clinical Oncology . 2019;145(7):1665–1679. doi: 10.1007/s00432-019-02940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zingue S., Maxeiner S., Rutz J., et al. Ethanol-extracted cameroonian propolis: antiproliferative effects and potential mechanism of action in prostate cancer. Andrologia . 2020;52(9) doi: 10.1111/and.13698.e13698 [DOI] [PubMed] [Google Scholar]
- 79.Li J., Liu H., Liu X., Hao S., Zhang Z., Xuan H. Chinese poplar propolis inhibits MDA-MB-231 cell proliferation in an inflammatory microenvironment by targeting enzymes of the glycolytic pathway. Journal of Immunology Research . 2021;2021:14. doi: 10.1155/2021/6641341.6641341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang K. S., Tsui K. H., Hsu S. Y., et al. The antitumor effect of caffeic acid phenethyl ester by downregulating mucosa-associated lymphoid tissue 1 via AR/p53/NF-κB signaling in prostate carcinoma cells. Cancers . 2022;14(2):p. 274. doi: 10.3390/cancers14020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ebeid A. E., El Moneim N. A., Moneim N. A., El-Benhawy S. A., Hussain N. G., Hussain M. I. Assessment of the radioprotective effect of propolis in breast cancer patients undergoing radiotherapy: new perspective for an old honey bee product. Journal of Radiation Research and Applied Sciences . 2016;9(4):431–440. doi: 10.1016/j.jrras.2016.06.001. [DOI] [Google Scholar]
- 82.Piredda M., Facchinetti G., Biagioli V., et al. Propolis in the prevention of oral mucositis in breast cancer patients receiving adjuvant chemotherapy: a pilot randomised controlled trial. European Journal of Cancer Care . 2017;26(6) doi: 10.1111/ecc.12757.e12757 [DOI] [PubMed] [Google Scholar]
- 83.Davoodi S. H., Yousefinejad V., Ghaderi B., et al. Oral propolis, nutritional status and quality of life with chemotherapy for breast cancer: a randomized, double-blind clinical trial. Nutrition and Cancer . 2021;8:1–9. doi: 10.1080/01635581.2021.1988118. [DOI] [PubMed] [Google Scholar]
- 84.Schuler M., Green D. R. Mechanisms of p53-dependent apoptosis. Biochemical Society Transactions . 2001;29(6):684–688. doi: 10.1042/bst0290684. [DOI] [PubMed] [Google Scholar]
- 85.Orsolic N., Bašić I. Water-soluble derivative of propolis and its polyphenolic compounds enhance tumoricidal activity of macrophages. Journal of Ethnopharmacology . 2005;102(1):37–45. doi: 10.1016/j.jep.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 86.Xuan H., Li Z., Yan H., et al. Antitumor activity of Chinese propolis in human breast cancer MCF-7 and MDA-MB-231 cells. Evidence-Based Complementary and Alternative Medicine . 2014;2014:11. doi: 10.1155/2014/280120.280120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tazawa S., Arai Y., Hotta S., Mitsui T., Nozaki H., Ichihara K. Discovery of a novel diterpene in Brown propolis from the state of parana, Brazil. Natural Product Communications . 2016;11(2) doi: 10.1177/1934578x1601100218. [DOI] [PubMed] [Google Scholar]
- 88.Barlak Y., Deger O., Colak M., Karatayli S. C., Bozdayi A. M., Yucesan F. Effect of Turkish propolis extracts on proteome of prostate cancer cell line. Proteome Science . 2011;9(1):p. 74. doi: 10.1186/1477-5956-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bestwick C. S., Milne L. Influence of galangin on HL-60 cell proliferation and survival. Cancer Letters . 2006;243(1):80–89. doi: 10.1016/j.canlet.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 90.Cragg G. M., Boyd M. R., Cardellina J. H., Newman D. J., Snader K. M., McCloud T. G. Ethnobotany and drug discovery: the experience of the US national cancer institute. Ciba Foundation Symposia . 1994;185:178–190. doi: 10.1002/9780470514634.ch13. [DOI] [PubMed] [Google Scholar]
- 91.Ahn J. C., Biswas R., Chung P. S. Synergistic effect of radachlorin mediated photodynamic therapy on propolis induced apoptosis in AMC-HN-4 cell lines via caspase dependent pathway. Photodiagnosis and Photodynamic Therapy . 2013;10(3):236–243. doi: 10.1016/j.pdpdt.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 92.Coskun Z. M., Ersoz M., Gecili M., Ozden A., Acar A. Cytotoxic and apoptotic effects of ethanolic propolis extract on C6 glioma cells. Environmental Toxicology . 2020;35(7):768–773. doi: 10.1002/tox.22911. [DOI] [PubMed] [Google Scholar]
- 93.Gaziano R., Moroni G., Bue C., Miele M. T., Sinibaldi-Vallebona P., Pica F. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World Journal of Gastrointestinal Oncology . 2016;8(1):p. 30. doi: 10.4251/wjgo.v8.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castro D. T. H., Campos J. F., Damiao M. J., et al. Ethanolic extract of senna velutina roots: chemical composition, in vitro and in vivo antitumor effects, and B16F10-nex2 melanoma cell death mechanisms. Oxidative Medicine and Cellular Longevity . 2019;2019:14. doi: 10.1155/2019/5719483.5719483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Juanes C. d. C., Souza S. M. d., Braga V. N. L., et al. Red propolis and L-lysine on angiogenesis and tumor growth in a new model of hamster cheek pouch inoculated with walker 256 tumor cells. Einstein (Sao Paulo) . 2019;17(2) doi: 10.31744/einstein_journal/2019ao4576.eAO4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frion-Herrera Y., Diaz-Garcia A., Ruiz-Fuentes J., Rodriguez-Sanchez H., Sforcin J. M. The cytotoxic effects of propolis on breast cancer cells involve PI3K/Akt and ERK1/2 pathways, mitochondrial membrane potential, and reactive oxygen species generation. Inflammopharmacology . 2019;27(5):1081–1089. doi: 10.1007/s10787-018-0492-y. [DOI] [PubMed] [Google Scholar]
- 97.Xuan H. Z., Zhang J. H., Wang Y. H., Fu C. L., Zhang W. Anti-tumor activity evaluation of novel chrysin-organotin compound in MCF-7 cells. Bioorganic & Medicinal Chemistry Letters . 2016;26(2):570–574. doi: 10.1016/j.bmcl.2015.11.072. [DOI] [PubMed] [Google Scholar]
- 98.Acikelli A. H., Gustmann S., Bardenheuer W., et al. Flavonoids isolated from Caribbean propolis show cytotoxic activity in human cancer cell lines. International Journal of Clinical Pharmacology & Therapeutics . 2013;51(1):51–53. doi: 10.5414/cpp51051. [DOI] [PubMed] [Google Scholar]
- 99.Diaz-Carballo D., Malak S., Bardenheuer W., Freistuehler M., Reusch H. P. The contribution of plukenetione A to the anti-tumoral activity of cuban propolis. Bioorganic & Medicinal Chemistry . 2008;16(22):9635–9643. doi: 10.1016/j.bmc.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 100.Shin S. A., Lee H. N., Choo G. S., Kim H. J., Che J. H., Jung J. Y. Ixeris dentata (thunb. Ex thunb.) nakai extract inhibits proliferation and induces apoptosis in breast cancer cells through Akt/NF-κB pathways. International Journal of Molecular Sciences . 2017;18(2):p. 275. doi: 10.3390/ijms18020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamiya T., Nishihara H., Hara H., Adachi T. Ethanol extract of Brazilian red propolis induces apoptosis in human breast cancer MCF-7 cells through endoplasmic reticulum stress. Journal of Agricultural and Food Chemistry . 2012;60(44):11065–11070. doi: 10.1021/jf303004n. [DOI] [PubMed] [Google Scholar]
- 102.Sulaiman G. M., Ad’hiah A. H., Al-Sammarrae K. W., et al. Assessing the anti-tumour properties of Iraqi propolis in vitro and in vivo. Food and Chemical Toxicology . 2012;50(5):1632–1641. doi: 10.1016/j.fct.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 103.Fu Y. K., Wang B. J., Tseng J. C., et al. Combination treatment of docetaxel with caffeic acid phenethyl ester suppresses the survival and the proliferation of docetaxel-resistant prostate cancer cells via induction of apoptosis and metabolism interference. Journal of Biomedical Science . 2022;29(1):p. 16. doi: 10.1186/s12929-022-00797-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ziyad S., Iruela-Arispe M. L. Molecular mechanisms of tumor angiogenesis. Genes & Cancer . 2011;2(12):1085–1096. doi: 10.1177/1947601911432334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bielenberg D. R., Zetter B. R. The contribution of angiogenesis to the process of metastasis. The Cancer Journal . 2015;21(4):267–273. doi: 10.1097/ppo.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gupta S. C., Kim J. H., Prasad S., Aggarwal B. B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer & Metastasis Reviews . 2010;29(3):405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Z., Dabrosin C., Yin X., et al. Broad targeting of angiogenesis for cancer prevention and therapy. Seminars in Cancer Biology . 2015;35:S224–S243. doi: 10.1016/j.semcancer.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dunn I. F., Heese O., Black P. M. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. Journal of Neuro-Oncology . 2000;50(1-2):121–137. doi: 10.1023/a:1006436624862. [DOI] [PubMed] [Google Scholar]
- 109.He Y., Liu X. D., Chen Z. Y., et al. Interaction between cancer cells and stromal fibroblasts is required for activation of the uPAR-uPA-MMP-2 cascade in pancreatic cancer metastasis. Clinical Cancer Research . 2007;13(11):3115–3124. doi: 10.1158/1078-0432.ccr-06-2088. [DOI] [PubMed] [Google Scholar]
- 110.Hwang H. J., Park H. J., Chung H. J., et al. Inhibitory effects of caffeic acid phenethyl ester on cancer cell metastasis mediated by the down-regulation of matrix metalloproteinase expression in human HT1080 fibrosarcoma cells. The Journal of Nutritional Biochemistry . 2006;17(5):356–362. doi: 10.1016/j.jnutbio.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 111.Frion-Herrera Y., Gabbia D., Scaffidi M., et al. The Cuban propolis component nemorosone inhibits proliferation and metastatic properties of human colorectal cancer cells. International Journal of Molecular Sciences . 2020;21(5):p. 1827. doi: 10.3390/ijms21051827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Izuta H., Shimazawa M., Tsuruma K., Araki Y., Mishima S., Hara H. Bee products prevent VEGF-induced angiogenesis in human umbilical vein endothelial cells. BMC Complementary and Alternative Medicine . 2009;9(1):p. 45. doi: 10.1186/1472-6882-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daleprane J. B., Schmid T., Dehne N., et al. Suppression of hypoxia-inducible factor-1α contributes to the antiangiogenic activity of red propolis polyphenols in human endothelial cells. Journal of Nutrition . 2012;142(3):441–447. doi: 10.3945/jn.111.150706. [DOI] [PubMed] [Google Scholar]
- 114.Ahn M. R., Kumazawa S., Hamasaka T., Bang K. S., Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of Korea. Journal of Agricultural and Food Chemistry . 2004;52(24):7286–7292. doi: 10.1021/jf048726s. [DOI] [PubMed] [Google Scholar]
- 115.Lima Cavendish R., de Souza Santos J., Belo Neto R., et al. Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. Journal of Ethnopharmacology . 2015;173:127–133. doi: 10.1016/j.jep.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 116.Ribeiro D. R., Alves Â. V. F., dos Santos E. P., et al. Inhibition of DMBA-induced oral squamous cells carcinoma growth by Brazilian red propolis in rodent model. Basic and Clinical Pharmacology and Toxicology . 2015;117(2):85–95. doi: 10.1111/bcpt.12374. [DOI] [PubMed] [Google Scholar]
- 117.de Mendonça I. C. G., Porto I. C. C. d. M., do Nascimento T. G., et al. Brazilian red propolis: phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complementary and Alternative Medicine . 2015;15(1):p. 357. doi: 10.1186/s12906-015-0888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Orsolic N., Bašić I. Antitumor, hematostimulative and radioprotective action of water-soluble derivative of propolis (WSDP) Biomedicine & Pharmacotherapy . 2005;59(10):561–570. doi: 10.1016/j.biopha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 119.Orsolic N., Sver L., Terzic S., Bašić I. Peroral application of water-soluble derivative of propolis (WSDP) and its related polyphenolic compounds and their influence on immunological and antitumour activity. Veterinary Research Communications . 2005;29(7):575–593. doi: 10.1007/s11259-005-3303-z. [DOI] [PubMed] [Google Scholar]
- 120.Orsolic N., Benkovic V., Lisicic D., Dikic D., Erhardt J., Horvat Knežević A. Protective effects of propolis and related polyphenolic/flavonoid compounds against toxicity induced by irinotecan. Medical Oncology . 2010;27(4):1346–1358. doi: 10.1007/s12032-009-9387-5. [DOI] [PubMed] [Google Scholar]
- 121.Orsolic N., Knezevic A. H., Sver L., Terzic S., Bašić I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. Journal of Ethnopharmacology . 2004;94(2-3):307–315. doi: 10.1016/j.jep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 122.Chan G. C. F., Cheung K. W., Sze D. M. Y. The immunomodulatory and anticancer properties of propolis. Clinical Reviews in Allergy and Immunology . 2013;44(3):262–273. doi: 10.1007/s12016-012-8322-2. [DOI] [PubMed] [Google Scholar]
- 123.Ahn M. R., Kunimasa K., Ohta T., et al. Suppression of tumor-induced angiogenesis by Brazilian propolis: major component artepillin C inhibits in vitro tube formation and endothelial cell proliferation. Cancer Letters . 2007;252(2):235–243. doi: 10.1016/j.canlet.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 124.Salmas R. E., Gulhan M. F., Durdagi S., Sahna E., Abdullah H. I., Selamoglu Z. Effects of propolis, caffeic acid phenethyl ester, and pollen on renal injury in hypertensive rat: an experimental and theoretical approach. Cell Biochemistry and Function . 2017;35(6):304–314. doi: 10.1002/cbf.3277. [DOI] [PubMed] [Google Scholar]
- 125.Dornelas C. A., Fechine-Jamacaru F. V., Albuquerque I. L., et al. Angiogenesis inhibition by green propolis and the angiogenic effect of L-lysine on bladder cancer in rats. Acta Cirurgica Brasileira . 2012;27(8):529–536. doi: 10.1590/s0102-86502012000800003. [DOI] [PubMed] [Google Scholar]
- 126.Jung J. E., Lee H. G., Cho I. H., et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. The FASEB Journal . 2005;19(10):1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- 127.Chikaraishi Y., Izuta H., Shimazawa M., Mishima S., Hara H. Angiostatic effects of Brazilian green propolis and its chemical constituents. Molecular Nutrition & Food Research . 2010;54(4):566–575. doi: 10.1002/mnfr.200900115. [DOI] [PubMed] [Google Scholar]
- 128.Liao H. F., Chen Y. Y., Liu J. J., et al. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. Journal of Agricultural and Food Chemistry . 2003;51(27):7907–7912. doi: 10.1021/jf034729d. [DOI] [PubMed] [Google Scholar]
- 129.Sari A. N., Dhanjal J. K., Elwakeel A., et al. A low dose combination of withaferin A and caffeic acid phenethyl ester possesses anti-metastatic potential in vitro: molecular targets and mechanisms. Cancers . 2022;14(3):p. 787. doi: 10.3390/cancers14030787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pai J. T., Chen X. H., Leu Y. L., Weng M. S. Propolin G-suppressed epithelial-to-mesenchymal transition in triple-negative breast cancer cells via glycogen synthase kinase 3β-mediated Snail and HDAC6-regulated vimentin degradation. International Journal of Molecular Sciences . 2022;23(3):p. 1672. doi: 10.3390/ijms23031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Niyomtham N., Koontongkaew S., Yingyongnarongkul B. E., Utispan K. Apis mellifera propolis enhances apoptosis and invasion inhibition in head and neck cancer cells. PeerJ . 2021;9 doi: 10.7717/peerj.12139.e12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tao L., Chen X., Zheng Y., et al. Chinese propolis suppressed pancreatic cancer panc-1 cells proliferation and migration via hippo-YAP pathway. Molecules . 2021;26(9):p. 2803. doi: 10.3390/molecules26092803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


