Abstract
3,3-Diphenylbenzo[f]chromene (1) represents an important architectural platform for photochromic systems. Since the practical utility of such chromophores is largely dependent upon the kinetics of coloration and decoloration, elucidating the mechanistic details of these processes is of great value. Toward this end, we studied the photochromic reaction of (3-(2-methoxyphenyl)-3-phenyl-3H-benzo[f]chromene (2) by both time-resolved UV–vis and mid-IR spectroscopies. We found that irradiation of 2 at 365 nm generates long-lived colored transoid-cis isomers with lifetimes of 17.1 s and 17.5 min (at 21 °C) and even longer-lived transoid-trans isomers with a lifetime of 16 h. These experimental results were supplemented with ab initio ground-state and excited-state calculations, and the resulting theoretical interpretation may be useful for the design of new photochromic systems with optimized photofunctionality.
Subject terms: Photochemistry, Physical chemistry, Optical materials
Introduction
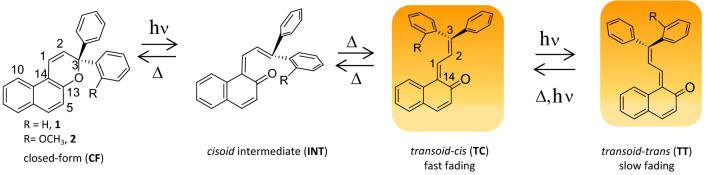
Among the known photochromic compounds, the family of 3,3-diphenyl-3H-naphtho[2,1-b]pyrans (1) has attracted great interest in the field of ophthalmic lens production1–4. Compound 1, exhibiting both P and T type photochromism, has been the subject of fundamental research5–13. In the photochromic reaction under typical conditions two colored species are formed (Fig. 1): transoid-cis TC (which fades rapidly) and transoid-trans TT (which fades more slowly)5. Synthetic changes in the structural pattern of 1 can tune its properties to fulfil the requirements of a particular application.
Figure 1.
UV excitation of 3,3-diphenyl-3H-naphtho[2,1-b]pyran (1) converts the closed form CF to color isomers TC (short-lived) and TT (long-lived), with the intermediacy of cisoid-cis form INT (ultrashort-lived)11. Compound 2 with a methoxy group implemented at one of the phenyl rings at ortho position is selected for studies of a strong photocoloration effect.
When the application requires a relatively fast decoloration rate, the presence of the long-lived TT form is unwanted. Efforts are focused then on designing derivatives of 1 with a minimized TT contribution in the photoreaction. Under typical experimental conditions with UV irradiation, the active channel for TT generation is TC → TT photoisomerization, which is a single-twist long amplitude motion11,13 occurring in the excited state (S1). Strong competition of the other TC excited state deactivation channels can minimize the TC → TT photoisomerization yield. For example, if the potential-energy landscape of the initially photoexcited TC S1 state favors a bicycle-pedal motion, the isomerization path might be quickly aborted through S1 → S0 internal conversion populating the form with the geometry close to that of the starting TC form12,13. Such a deactivation path can reduce the TC → TT photoisomerization yield, as reported for derivative of 1 with a methoxy group inserted at the position 1013,14.
The thermal fading rate of colored TC and TT isomers is also a key parameter of photochromic materials based on derivatives of 1. Appropriate substituents in the skeleton of 1 can be used to tune the TC color fading rate which depends on the ground state potential energy landscape15. A key role in this process is played by the cisoid-cis intermediate form (INT) lying midway between the colored TC form (see Fig. 2), and the colorless CF form15. The relationship between the energy barriers ΔEINT-TC and ΔEINT-CF separating the intermediate INT from nearby global and local minima may either decelerate the INT → CF process (favoring the return to the colored TC form) or accelerate the process (favoring the ring-closing reaction). For instance, the ΔEINT-TC barrier for 1 is lower than ΔEINT-CF (Fig. 2), which makes the apparent TC lifetime relatively long (9.3 s at 21 °C in cyclohexane).
Figure 2.
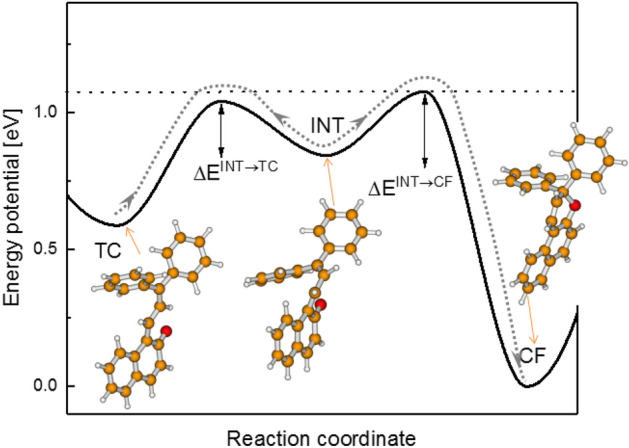
Thermal decoloration of TC → CF for compound 1 involves two reaction steps TC → INT and INT → CF, the first step is reversible15.
The opposite situation (ΔEINT-TC > ΔEINT-CF) has been reported for 3H-naphthopyrans with phenyl substitution at position 215. The apparent TC lifetime is remarkably short (only 30 μs) in solution at room temperature16. On the other hand, one can expect that a slow decoloration would help to achieve a strong photocoloration effect in the photostationary state. It is widely accepted that inclusion of a bulky substituent at the ortho position of one of the phenyl rings in 1 would result in a substantial extension of the thermal fading half-life 2,4,17–21. The half-life of the open forms gets longer with increasing size of the ortho substituent (in the order of H → F → MeO → Me → Cl → Br → I) in the phenyl ring of 3-aryl-3-(4-pyrrolidinophenyl)naphtho[2,1-b]pyrans22. A remarkably slow-decoloration rate remains an intriguing feature for mechanistic and spectroscopic investigations.
For our studies we selected 3-(2-methoxyphenyl)-3-phenyl-3H-benzo[f]chromene (2), Fig. 1, as a candidate for a material with the strongest UV activated coloration. The relatively simple structure of 2 facilitates ab initio quantum-chemical calculations for the excited state, while the stages of the photochromic reaction can be studied in detail with experimental techniques such as the time-resolved UV–vis and mid-IR spectroscopies.
Experimental
Materials
Compound 1 was purchased from TCI. Compound 2 was synthesized following the procedures described in the Supplementary Information. In the time-resolved spectroscopic investigation cyclohexane of spectroscopic grade from Sigma Aldrich was used for solution preparation.
Time-resolved UV–vis and mid-IR spectroscopies
Changes in UV–vis absorption spectra and kinetics were recorded using three configurations.
Jasco V-550 spectrophotometer with a modified cell compartment. The solution in 1 cm × 1 cm fused silica cuvette was placed into a temperature-controlled cuvette holder (Flash 300, Quantum Northwest) with stirring switched on. UV LED (λexc = 365 nm, Thorlabs M365LP1) was used to induce the photochromic reaction (as in the two other configurations below).
Similar arrangement with a temperature-controlled cuvette holder and white light generated by a xenon lamp (Applied Photophysics), equipped with a bundle fiber, as a probing beam. The probing beam was passed through an almost-closed iris to ensure low white light intensity. The UV–vis spectra were recorded by an Ocean Optics FLAME-T-VIS–NIR-ES USB spectrometer at the sampling rate of 10 spectra per second.
Acquisition mid-IR spectra upon simultaneous measurements in UV–vis spectral range has been described recently11. A Bruker Tensor 27 FT-IR spectrometer was equipped with an MCT detector, with a spectral resolution of 4 cm−1 and a sampling rate of 1.15 s−1. The UV–vis probing light was generated from the xenon lamp. An Ocean Optics spectrophotometer was used for recording of UV–vis spectra. A Harrick Scientific cell was used with 2 mm thick CaF2 window and a 0.63 mm spacer.
Theoretical calculations
The equilibrium geometries of the CF conformers and their isomers in the closed-shell ground state (S0) were obtained with the MP2 method23 with no imposed symmetry constrains. The energy of the most stable form CFc (Table S1, Supplementary Information) was assumed as the reference energy for higher energy structures. The excited-state (S1) equilibrium geometries were determined with the second-order algebraic diagrammatic construction ADC(2) method24–26. The correlation-consistent valence double zeta basis set with polarization functions on all atoms (cc-pVDZ)27 was used in these calculations as well as in the potential energy profiles and surfaces. The vertical excitation energies and response properties of the lowest singlet excited states were calculated using the CC2 methods28,29. The basis set augmented with the diffuse functions aug-cc-pVDZ was also used to compute vertical excitation energies of the molecular system. All calculations were performed using the TURBOMOLE program package30.
Results and discussion
The stationary UV–vis absorption spectrum of 2 in cyclohexane (Figure S1, Supplementary Information) is similar to that of the reference compound 111, reflecting structural similarities between the two derivatives. In the structure of 1, each phenyl ring has two ortho positions that can be substituted by a methoxy group, thus, four respective conformers of 2 are taken into account in the calculations. These conformers of the CF form are in thermal equilibrium in freshly prepared solution (indicated with subscripts: a, b, c, and d, see Table S1). The selection of wavelength at 365 nm induces the electronic transition S0 → S1(π,π*) separately in each CF form, which opens up along the C3–O4 photodissociation pathway, as in 111. The photoinduced pyran ring-opening process can lead to the colored-isomers CTC and TTC. All the CF, TC and TT conformers potentially involved in the photoreaction, along with their calculated vertical excitation energies (ΔEVE) simulating UV–vis absorption spectra, are collected in Table S1. The strong S0 → S2(π, π*) transitions for colored TC forms are slightly redshifted vs. those of TT forms; a similar trend was observed for the parent compound 111.
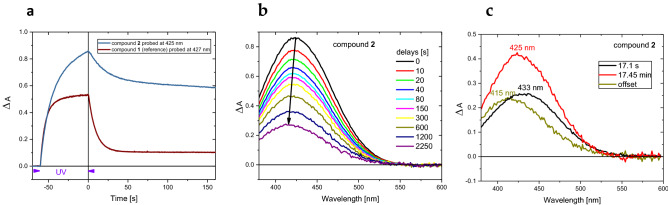
To study the photochromic reaction by changes in UV–vis absorption spectra, a UV LED light at 365 nm was used for sample excitation, while a xenon lamp was used for probing. Figure 3a shows the kinetics probed at 425 nm upon 60-s exposure a solution of 2 in cyclohexane to UV light.
Figure 3.
(a) Kinetics of the changes in absorption as a result of UV irradiation (4.1 mW/cm2) in the period of 60 s for 2 and the reference 1 in cyclohexane at 21 °C (both solutions prepared with the same absorbance A(365 nm, 1 cm) = 0.24). (b) Transient UV–vis absorption spectra for 2 after switching off UV irradiation. (c) Decay associated spectra of the TC (17.1 s and 17.5 min) and TT isomers (the offset, long-lived population) obtained by global analysis using a biexponential function.
Compared to 1, compound 2 produces a stronger absorption signal upon UV irradiation (Fig. 3a), and after cessation of UV irradiation its color fading occurs at a lower rate. The quantum yield of TC formation is similar for 2 and 1 (0.74511), since the early signal rise in the − 55 to − 60 s time window shows the same slope and the TC molar extinction coefficients are comparable (see Supplementary Information). Figure 3b shows the evolution of the transient UV–vis absorption spectra in the time window of 0–2250 s. Global analysis of data (Fig. 3c) reveals three distinct populations: TC1 with the absorption band peaking at 433 nm and a lifetime of 17.1 s, TC2 with the band maximum at 425 nm and a 17.5 min lifetime, and TT with a maximum at 415 nm and a long lifetime. The TT lifetime of 16 h was determined in an additional experiment performed in the time window of 40 h (Figure S2). The respective molar extinction coefficients, ≈ 18,900 M−1 cm−1, ≈ 18,700 M−1 cm−1 and ≈ 29,500 M−1 cm−1, are deduced from the simultaneously recorded changes in the UV–vis and mid-IR spectral ranges (see Figure S3, Supplementary Information). Thus, one can estimate from the data shown in Fig. 3 that at the moment of UV irradiation cessation, the conversion of CF population to colored forms is 69% and the concentrations of [TC1], [TC2] and [TT] are: 1.4 × 10–5 M, 2.2 × 10–5 M and 0.8 × 10–5 M, respectively. In addition, the FT-IR spectroscopy provides a confirmation of our signal assignment to TC and TT isomers, since the C=O stretching absorption band of TC is located at a lower frequency than that of TT, as has been already observed for the reference compound 1 (TC at 1644 cm−1 and TT at 1655 cm−111).
Photocycle reproducibility observed for 2 in cyclohexane is similar to that for 1, which is known to have high fatigue resistance (Fig. 4).
Figure 4.
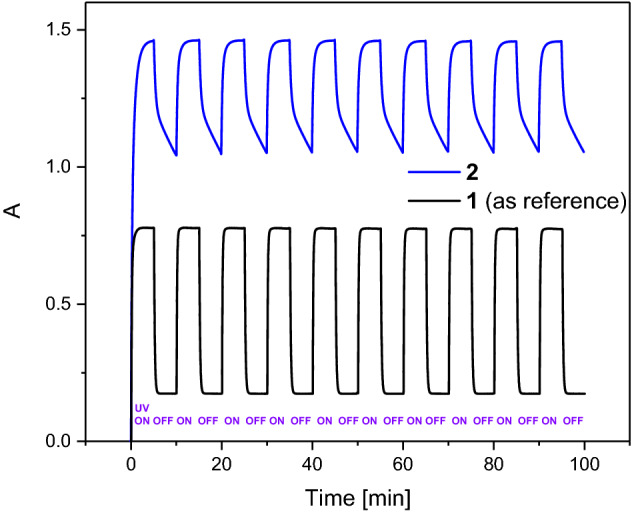
Kinetics of absorption upon switching on and off UV light at 365 nm (4.1 mW/cm2) for 2 and 1 in cyclohexane probed at 430 nm using a Jasco spectrophotometer, temperature set at 21 °C. Solutions prepared with the same absorbance A(365 nm, 1 cm) = 0.39.
Why do TC forms differ in decoloration kinetics?
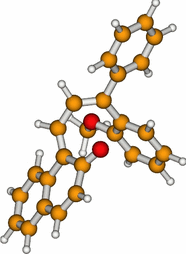
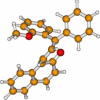
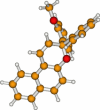
The TC form in 3H-naphthopyrans is photogenerated in a single-photon absorption process11. A single TC isomer is generated from compound 1, while four TC isomers from compound 2 should be taken into consideration. The geometries of these conformers are presented in the first column in Table S1. CTCa and CTCb are energetically more stable than TTCc and TTCd. Each of the four TC isomers is a colored species which, under thermal conditions, fades towards the respective closed CF form via a single cisoid-cis intermediate INT geometry located on the ground-state potential energy pathway. Each of the four S0-state pathways is characterized by a slightly different energy barriers separating the intermediate INT structure from the local TC and CF minima, which influence the apparent fading rate of each TC isomer. Energy barriers are shown in Table 1.
Table 1.
Energetics of the two-stepwise process of the TC form depopulation (TC ↔ INT → CF). Adiabatic S0-state energies, Ea (in eV), and dipole moment, μg (in Debye), for the relevant minima: TC, INT, CF, and transition states separating these minima: TS1 and TS2, calculated at the MP2/cc-pVDZ theory level.
| Form | ∆ETC-out | TS2 | ∆E INT-TC | INT | ∆EINT-CF | TS1 | CF | |
|---|---|---|---|---|---|---|---|---|
| CTC ↔ INT → CF, τ(CTC) = 17.5 min in cyclohexane at 21 °C | ||||||||
| CTCa |
|
|
|
|
|
|||
|
0.626 eV 3.51 D |
+ 0.380 |
1.005 4.02 |
+ 0.161 |
0.844 1.25 |
+ 0.341 |
1.185 1.22 |
0.056 1.79 |
|
| CTCb |
|
|
|
|
|
|||
|
0.610 eV 4.11 D |
+ 0.414 |
1.024 3.62 |
+ 0.166 |
0.858 2.93 |
+ 0.364 |
1.223 2.10 |
0.064 2.61 |
|
| TTC ↔ INT → CF, τ(TTC) = 17.1 s in cyclohexane at 21 °C | ||||||||
| TTCc |
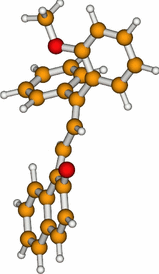
|
|
|
|
|
|||
|
0.655 eV 3.13 D |
+ 0.415 |
1.070 2.17 |
+ 0.164 |
0.906 1.79 |
+ 0.254 |
1.160 1.90 |
0.00 2.37 |
|
| TTCd |
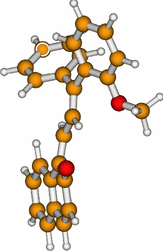
|
|
|
|
|
|||
|
0.669 eV 2.17 D |
+ 0.476 |
1.145 1.76 |
+ 0.207 |
0.938 1.53 |
+ 0.203 |
1.141 1.90 |
0.113 1.61 |
|
The first reaction step in the TC fading process is TC → INT isomerization, which must overcome a relatively high energy barrier ΔETC-OUT (~ 0.38−0.48 eV), to reach a highly energetic cisoid-cis INT intermediate in an endothermic process. In the second step, this intermediate can follow (1) an exothermic reaction towards the final closed-pyran ring CF form or (2) an alternative reverse process towards the TC form (Figure S4, Supplementary Information). As one can see in Table 1, the ΔEINT-CF barrier towards the final CF for each fading pathway is usually higher than the respective ΔEINT-TC barrier towards the initial TC form (except for the TTCd pathway, for which the TS2d structure (Ea = 1.145 eV) is slightly more destabilized due to O…O repulsion). Such a situation (ΔEINT-CF > ΔEINT-TC) favors deceleration of the TC fading process, since once INT is formed it would rather repopulate the TC form. This may explain long TC lifetimes observed in the experiment for 2 (17.1 s and 17.5 min) in comparison to that of 1 (9.3 s, ΔEINT-CF = 0.233 eV and ΔEINT-TC = 0.196 eV13,15).
In order to assign each of the two TC lifetimes (17.1 s and 17.5 min) to the respective CTC and TTC families, we should consider their depopulation following the S0-state energetic profile (Figure S4). While the ΔEINT-TC barrier of ~ + 0.16 eV is the same for all the considered TC isomers (expect for TTCd), it is the ΔEINT-CF barrier that seems to be critical. Since ΔEINT-CF is higher for CTC than for TTC (~ + 0.35 vs. 0.25 eV), the equilibrium of CTCa and CTCb is responsible for the long 17.5 min time-constant. The short time-constant (17.1 s) should be then ascribed to the TTC forms (equilibrium of TTCc and TTCd). The respective equilibria (CTCa − CTCb and TTCc − TTCd) are confirmed by the relatively low interconversion S0-state energy barriers of ~ + 0.2 eV.
To support the idea that the high value of the ΔEINT-CF barrier is a decisive parameter in longer fading time of CTC forms, we analyzed the geometries of the transition state TS1 structures. Indeed, the presence of a methoxy group is the cause of the O…O repulsion in TS1a (Ea = 1.185 eV, see Table 1) or 10H…OCH3 steric hindrance in TS1b (Ea = 1.223 eV). The ortho-methoxy substituent increases the electron density on that aromatic ring, which has a stabilizing effect on the cisoid-cis INT geometry through increased π-stacking of the aryl and naphthalenone moieties. This is responsible for a higher ΔEINT-CF barrier in the case of INTa and INTb (+0.341 eV and +0.364 eV) vs. INTc and INTd (+0.254 eV and +0.203 eV).
Mechanism of the TC → TT photoisomerization reaction in the singlet excited state
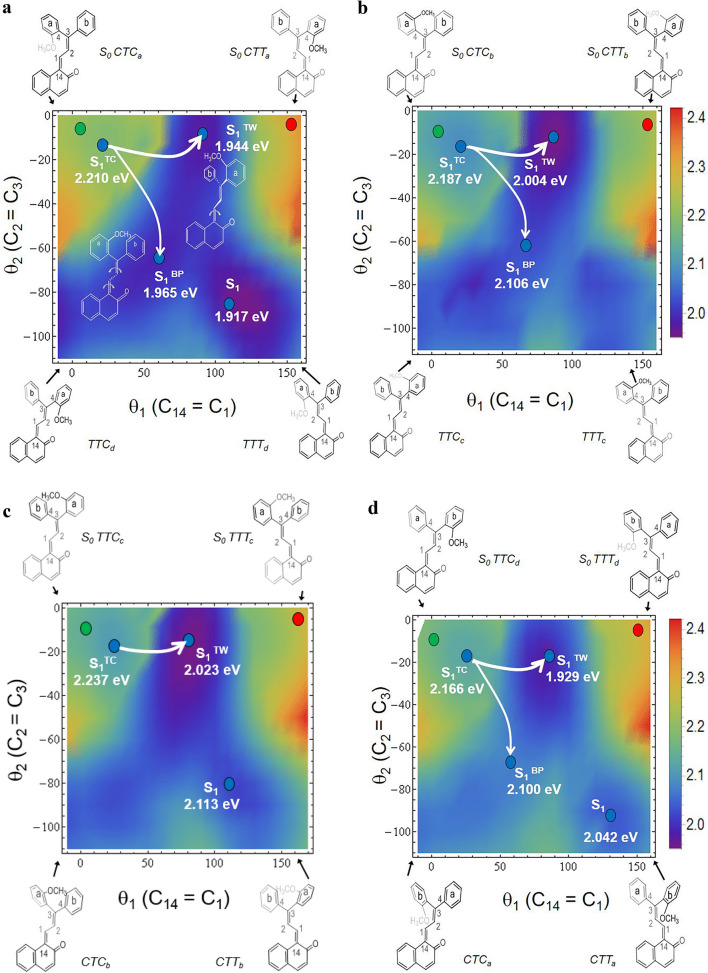
The act of photon absorption by each TC isomer activates the two double bonds present in the C14=C1–C2=C3 bridge linking the naphthalenone skeleton with the diphenylmethylene rotor. This double bond activation allows free rotation about these bonds in the singlet excited state. As already shown for 1 derivatives13, this rotation can be classified as a single-twist mechanism (rotation around the C14=C1 bond) or as a bicycle-pedal motion (if the concerted rotation about both double bonds takes place). Both mechanisms are visualized in the excited-state potential energy surface spanned over the two double bonds (Fig. 5). The single-twist motion can be seen as a motion along the X axes (C14=C1 rotation) from the green dot representing the initial TC geometry (upper left corner of the contour plot) towards the red dot (TT form, in the upper right corner). This motion meets the excited state S1TW minimum for which C13–C14=C1–C2 dihedral angle is usually a little less than 90°, but it should be considered as the active channel in TC → TT photoisomerization. Alternatively, the bicycle-pedal motion is followed along the diagonal of the plot—from the TC form (green dot, upper left corner) towards the bottom right corner. This path encounters the S1BP minimum midway through, which may deactivate through S1 → S0 internal conversion to repopulate eventually the initial TC geometry in the S0 state. Inspection of the contour plots in Fig. 5 and energetics in Table S2 shows that the relative energies of S1TW are below S1BP thus the photoisomerization TC → TT is expected to be efficient, as observed in the experiment.
Figure 5.
Minimum-potential-energy surface of the lowest excited electronic state of the TC-molecule: (a) CTCa, (b) CTCb, (c) TTCc and (d) TTCd, plotted as a function of θ1(C14=C1) and θ2(C2=C3) torsional angle coordinates. Green circle represents the Franck–Condon region of the ground-state S0TC local minimum and red circle represents the ground-state S0TT local minimum. Blue circles represent various types of the excited-state minima: S1TC—the minimum initially populated after S0TC photoexcitation, S1BP—achieved through the bicycle-pedal motion, and S1TW—reached by single-twist motion mechanism. The results were obtained with the aid of the ADC(2)/cc-pVDZ method, for the excited state, and with the MP2/cc-pVDZ, for the ground state.
According to theoretical calculations, the replacement of the methoxy group in 2 by a methyl substituent has a low impact since its potential energy surface is also tilted towards the S1TW minimum (see Table S3). Consequently, this system should also easily produce the TT form.
Conclusions
The synthesized compound 2 shows exceptional properties among the members of 3H-naphthopyran family. In the photochromic reaction its colored isomers are formed with longer lifetimes in comparison to that of the reference compound 1. Under UV irradiation a high accumulation of colored forms is observed in a photostationary state. The mechanism of the colored TC form fading process can be analyzed using the energetic landscape of the thermal TC → INT → CF reaction. The determined ΔEINT-CF energy barrier seems to hamper CF repopulation and extends the apparent TC lifetime. Thus, the experimentally determined long lifetime of 17.5 min can be assigned to CTC forms, while the short lifetime of 17.1 s to TTC conformers. The theory suggests also that all TC isomers can undergo photoisomerization by the single-twist mechanism around the C14=C1 angle. The photogenerated TT form is long-lived—the population decay occurs with a single time-constant of 16 h at 21 °C. The proposed photochromic reaction mechanism explains the strong photocoloration effect observed for 2. Theoretical investigations should be considered as an efficient tool for designing new 3H-naphthopyrans derivatives with optimized properties. In other words, the implementation of a promising substituent in 3,3-diphenyl-3H-naphtho[2,1-b]pyran skeleton can be first theoretically tested for desired photochromic properties prior to the synthesis efforts.
Supplementary Information
Acknowledgements
This work was performed with financial support from the National Science Centre (NCN), Poland, project 2017/27/B/ST4/00320. Calculations were performed at the PL-Grid Infrastructure.
Author contributions
The following co-authors contributed in particular with; B.G. synthesis, M.R. theoretical calculations, conceptualization, writing original draft and G.B. spectroscopic experiments, formal analysis, supervision, funding acquisition, conceptualization, writing original draft. All authors reviewed the manuscript.
Data availability
The datasets generated during the current study are available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michał F. Rode, Email: mrode@ifpan.edu.pl
Gotard Burdzinski, Email: gotardb@amu.edu.pl.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-14679-9.
References
- 1.Crano JC, Flood T, Knowles D, Kumar A, Van Gemert B. Photochromic compounds: Chemistry and application in ophthalmic lenses. Pure Appl. Chem. 1996;68:1395–1398. doi: 10.1351/pac199668071395. [DOI] [Google Scholar]
- 2.Hepworth JD, Heron BM. In: Functional Dyes. Kim SH, editor. Elsevier; 2006. pp. 85–135. [Google Scholar]
- 3.Corns SN, Partington SM, Towns AD. Industrial organic photochromic dyes. Color. Technol. 2009;125:249–261. doi: 10.1111/j.1478-4408.2009.00204.x. [DOI] [Google Scholar]
- 4.Towns A. In: Applied Photochemistry: When Light Meets Molecules. Giacomo B, Serena S, editors. Springer International Publishing; 2016. pp. 227–279. [Google Scholar]
- 5.Delbaere S, et al. Kinetic and structural studies of the photochromic process of 3H-naphthopyrans by UV and NMR spectroscopy. J. Chem. Soc. Perkin Trans. 1998;2:1153–1157. doi: 10.1039/A800906F. [DOI] [Google Scholar]
- 6.Ottavi G, Favaro G, Malatesta V. Spectrokinetic study of 2,2-diphenyl-5,6-benzo(2H) chromene: A thermoreversible and photoreversible photochromic system. J. Photochem. Photobiol. A. 1998;115:123–128. doi: 10.1016/S1010-6030(98)00254-8. [DOI] [Google Scholar]
- 7.Görner H, Chibisov AK. Photoprocesses in 2,2-diphenyl-5,6-benzo(2H)chromene. J. Photochem. Photobiol. A. 2002;149:83–89. doi: 10.1016/S1010-6030(02)00002-3. [DOI] [Google Scholar]
- 8.Gentili PL, Danilov E, Ortica F, Rodgers MAJ, Favaro G. Dynamics of the excited states of chromenes studied by fast and ultrafast spectroscopies. Photochem. Photobiol. Sci. 2004;3:886–891. doi: 10.1039/b407541b. [DOI] [PubMed] [Google Scholar]
- 9.Delbaere S, Vermeersch G. NMR characterization of allenyl-naphthol in the photochromic process of 3,3-diphenyl-[3H]-naphtho[2-1, b]pyran. J. Photochem. Photobiol. A. 2003;159:227–232. doi: 10.1016/S1010-6030(03)00191-6. [DOI] [Google Scholar]
- 10.Herzog TT, Ryseck G, Ploetz E, Cordes T. The photochemical ring opening reaction of chromene as seen by transient absorption and fluorescence spectroscopy. Photochem. Photobiol. Sci. 2013;12:1202–1209. doi: 10.1039/C3PP50020A. [DOI] [PubMed] [Google Scholar]
- 11.Brazevic S, Nizinski S, Szabla R, Rode MF, Burdzinski G. Photochromic reaction in 3H-naphthopyrans studied by vibrational spectroscopy and quantum chemical calculations. Phys. Chem. Chem. Phys. 2019;21:11861–11870. doi: 10.1039/C9CP01451A. [DOI] [PubMed] [Google Scholar]
- 12.Brazevic S, Baranowski M, Sikorski M, Rode MF, Burdziński G. Ultrafast dynamics of the transoid-cis isomer formed in photochromic reaction from 3H-naphthopyran. ChemPhysChem. 2020;21:1402–1407. doi: 10.1002/cphc.202000294. [DOI] [PubMed] [Google Scholar]
- 13.Brazevic S, et al. Control of the photo-isomerization mechanism in 3H-naphthopyrans to prevent formation of unwanted long-lived photoproducts. Int. J. Mol. Sci. 2020;21:7825. doi: 10.3390/ijms21217825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inagaki Y, Kobayashi Y, Mutoh K, Abe J. A simple and versatile strategy for rapid color fading and intense coloration of photochromic naphthopyran families. J. Am. Chem. Soc. 2017;139:13429–13441. doi: 10.1021/jacs.7b06293. [DOI] [PubMed] [Google Scholar]
- 15.Brazevic S, et al. Cisoid-cis intermediate plays a crucial role in decolouration rate in photochromic reaction of 8H-pyranoquinazolines and 3H-naphthopyrans. Dyes Pigm. 2022;201:110249. doi: 10.1016/j.dyepig.2022.110249. [DOI] [Google Scholar]
- 16.Brazevic S, Sliwa M, Kobayashi Y, Abe J, Burdzinski G. Disclosing whole reaction pathways of photochromic 3H-naphthopyrans with fast color fading. J. Phys. Chem. Lett. 2017;8:909–914. doi: 10.1021/acs.jpclett.6b03068. [DOI] [PubMed] [Google Scholar]
- 17.Gabbutt CD, Heron BM, Instone AC. The synthesis and electronic absorption spectra of 3-phenyl-3(4- pyrrolidino-2-substituted phenyl)-3H-naphtho[2,1-b]pyrans: Further exploration of the ortho substituent effect. Tetrahedron. 2006;62:737–745. doi: 10.1016/j.tet.2005.09.143. [DOI] [Google Scholar]
- 18.Alberti A, Teral Y, Roubaud G, Faure R, Campredon M. On the photochromic activity of some diphenyl-3H-naphtho[2,1-b]pyran derivatives: synthesis, NMR characterisation and spectrokinetic studies. Dyes Pigm. 2009;81:85–90. doi: 10.1016/j.dyepig.2008.09.018. [DOI] [Google Scholar]
- 19.Pardo R, Zayat M, Levy D. Effect of the chemical environment on the light-induced degradation of a photochromic dye in ormosil thin films. J. Photochem. Photobiol. A. 2008;198:232–236. doi: 10.1016/j.jphotochem.2008.03.013. [DOI] [Google Scholar]
- 20.de Azevedo ODCC, et al. Synthesis and photochromism of novel pyridyl-substituted naphthopyrans. J. Org. Chem. 2020;85:10772–10796. doi: 10.1021/acs.joc.0c01296. [DOI] [PubMed] [Google Scholar]
- 21.Zayat M, Levy D. Photochromic naphthopyrans in sol–gel ormosil coatings. J. Mater. Chem. 2003;13:727–730. doi: 10.1039/b211759b. [DOI] [Google Scholar]
- 22.Gabbutt C, Heron BM, Instone AC. Control of the fading properties of photochromic 3,3-diaryl-3H-naphtho[2,1-b]pyrans. Heterocycles. 2003;60:843–855. doi: 10.3987/COM-02-9692. [DOI] [Google Scholar]
- 23.Møller C, Plesset MS. Note on an aproximation treatment for many-electron systems. Phys. Rev. 1934;46:618–622. doi: 10.1103/PhysRev.46.618. [DOI] [Google Scholar]
- 24.Hättig C. In: Advances in Quantum Chemistry. Jensen HJÅ, editor. Academic Press; 2005. pp. 37–60. [Google Scholar]
- 25.Schirmer J. Beyond the random-phase approximation: A new approximation scheme for the polarization propagator. Phys. Rev. A. 1982;26:2395–2416. doi: 10.1103/PhysRevA.26.2395. [DOI] [Google Scholar]
- 26.Trofimov AB, Schirmer J. An efficient polarization propagator approach to valence electron excitation spectra. J. Phys. B: At. Mol. Opt. Phys. 1995;28:2299–2324. doi: 10.1088/0953-4075/28/12/003. [DOI] [Google Scholar]
- 27.Dunning TH., Jr Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989;90:1007–1023. doi: 10.1063/1.456153. [DOI] [Google Scholar]
- 28.Christiansen O, Koch H, Jørgensen P. The second-order approximate coupled cluster singles and doubles model CC2. Chem. Phys. Lett. 1995;243:409–418. doi: 10.1016/0009-2614(95)00841-Q. [DOI] [Google Scholar]
- 29.Hättig C, Weigend F. CC2 excitation energy calculations on large molecules using the resolution of the identity approximation. J. Chem. Phys. 2000;113:5154–5161. doi: 10.1063/1.1290013. [DOI] [Google Scholar]
- 30.TURBOMOLE V7.1 2016, a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, since 2007; available from http://www.turbomole.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on request.